6
Higher Alcohols
6.1 Introduction
The term “higher alcohols” refers to volatile alcohols with more than two carbon atoms. These compounds are produced as a byproduct of yeast amino acid metabolism and are common to all products of alcoholic fermentation (e.g., beer, wine, cider, etc.). The higher alcohol category excludes polyols like glycerol and other sugar alcohols (Chapter 2), volatile phenols (Chapter 12), and grape‐derived varietal aroma compounds like monoterpene alcohols (Chapter 8). In addition to fermentation‐derived higher alcohols, this chapter will also discuss grape‐derived six carbon alcohols (e.g., 1‐hexanol) and methanol since they share some chemical properties with the fermentation‐derived higher alcohols.
6.2 Properties of higher alcohols
The higher alcohols are amphiphilic, possessing a non‐polar hydrocarbon group and a polar (and H‐bonding) –OH group. Most of the higher alcohols discussed in this chapter are moderately non‐polar, have low volatility, and, except for the fully water‐miscible n‐propanol, are only somewhat soluble in water.
Higher alcohols are not strongly reactive under wine conditions, although the alcohol group is a weak nucleophile at wine pH. They represent the most reduced form of oxygen‐containing compounds and are generally stable in the reducing environment of wine. The most important reactions that the higher alcohols participate in are as follows:
- Esterification. Higher alcohols can combine with carboxylic acids to form esters (Chapter 7). During fermentation, this can result in suprathreshold concentrations of acetate esters as a result of acetyltransferase enzymes (Chapter 22.3). Esterification of higher alcohols can occur non‐enzymatically during storage through an acid‐catalyzed mechanism but the concentrations of resulting esters formed are typically well below threshold and not of clear sensory importance.
- Substrate for oxidation. Higher alcohols can be oxidized to form their corresponding aldehydes, many of which are contributors to “oxidized aromas.” The mechanism for this reaction is discussed later (Chapter 24).
6.3 Origins and concentrations of higher alcohols
The majority of the higher alcohols are formed as a byproduct of yeast amino acid metabolism. Amino acids are formed and degraded via α‐keto acid carbon skeletons. Under certain conditions, such as the case of nitrogen limitation, these skeletons will be decarboxylated and reduced to form higher alcohols. This process may be anabolic, in which the carbon skeleton is biosynthesized by yeast from sugars, or catabolic, in which an existing amino acid is broken down. This will be discussed in more detail later (Chapter 22.3).
Representative concentrations of the several higher alcohols, which are largely absent from grapes and are almost exclusively formed during fermentation, appear in Table 6.1. Most of the higher alcohols have a clear structural relationship with a specific amino acid.
Table 6.1 Summary of characteristics of major fermentation‐derived higher alcohols
| Name/structure | Related amino acid | Typical range (mg/L) | Threshold in model wine (mg/L)[2] | Odor descriptor |
| 2‐Methyl‐1‐propanol (isobutanol) 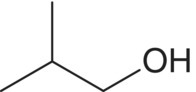
|
Valine | 25–87a | 40 | Solvent |
| 2‐Methyl‐1‐butanol (active amyl alcohol)c,d 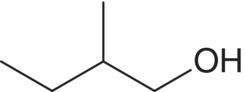
|
Isoleucine | 16–31b | 1.2 [3]e | Solvent, fusel |
| 3‐Methyl‐1‐butanol (isoamyl alcohol)c 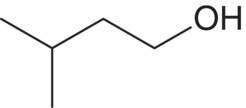
|
Leucine | 84–333b | 30 | Solvent, fusel |
| 3‐Methylsulfanyl‐1‐propanol (methionol) 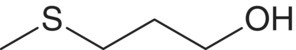
|
Methionine | 0.16–2.4a | 1 | Boiled potato |
| 2‐Phenylethanol (β‐phenylethanol) 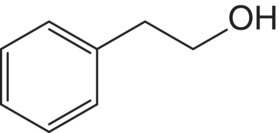
|
Phenylalanine | 40–153a | 14 | Rose, honey |
a Spanish red wines [2].
b New York State white wines [4].
c “Amyl” is the common name for a pentyl (five‐carbon) hydrocarbon chain.
d Exists as a mixture of R/S enantiomers. Threshold reported for racemic mixture.
e Threshold determined in water.
Because the higher alcohols are formed during fermentation as part of basic yeast nitrogen metabolism, their production is dependent on both fermentation conditions and nutrient availability. Factors that increase production of higher alcohols relate to metabolism of amino acids and include [5]:
- Low yeast assimilable nitrogen or other nutrient deficiencies related to amino acid biosynthesis
- Higher concentrations of suspended solids
- Higher temperatures
- Certain yeast strains.
In addition, the relative concentrations of these higher alcohols will be dependent on the initial distribution of amino acids. All of these factors are discussed in more detail later (Chapter 22.3). Small amounts of 2‐phenylethanol may be formed from glycosylated precursors (Chapter ), although it is not a major source in wine [6]. After fermentation, the higher alcohols in Table 6.1 appear to be stable during storage. For example, there is no significant change in isoamyl alcohol, isobutyl alcohol, and 2‐phenylethanol after 1 year of bottle storage at temperatures ranging from 5 to 18 °C [7].
With the exception of 2‐phenylethanol (“rose, honey”) the higher alcohols do not typically have desirable odors in isolation. However, reconstitution studies indicate that the individual higher alcohols have only minor effects. For example, removal of select higher alcohols resulted in a detectable but indescribable difference in reconstituted model Grenache rosé [8] and had no significant effect on a model Gewurztraminer [9]. Furthermore, no correlation was observed between concentrations of isoamyl alcohol, 2‐phenylethanol, or methionol and red wine quality scores [10]. In all likelihood, higher alcohols make a modest contribution to the vinous nature of all wines (and fermented beverages more generally), but are unlikely to serve as impact odorants. Their more important role may be as substrates in the formation of more potent odorants, e.g. acetate esters and aldehydes (Chapters 7 and 9).
6.4 Six‐carbon (C6) alcohols
Several C6 alcohols and aldehydes are formed enzymatically by grapes and other plants following mechanical damage, for example, crushing grapes, mowing the lawn, or chewing on fresh parsley. These compounds are largely absent from the intact grape berry (or other plant tissues), but are instead formed by enzymatic oxidation of polyunsaturated fatty acids. Because they typically have “herbaceous” and “green grass” aromas, these compounds are also referred to as green leaf volatiles (GLVs). Formation of C6 compounds appears to be a ubiquitous response of plants to mechanical wounding, possibly for inter‐ or intraplant signaling to result in an upregulation of antiherbivory compounds like tannins [11].
The enzymatic lipid oxidation pathway responsible for formation of C6 compounds in grapes and many other plants is discussed in more detail later in the book (Chapter 23.3). This pathway yields C6 aldehydes and alcohols as primary products, but the C6 aldehydes are mostly lost during fermentation (Chapters 22.1 and 23.3). Typical C6 compound concentrations are shown in Table 6.2. The primary C6 compounds that persist at concentrations around or above their sensory thresholds following fermentation are 1‐hexanol and cis‐3‐hexenol. Addition of these compounds at typical red wine concentrations of (1.48 mg/L of 1‐hexanol and 234 μg/L of cis‐3‐hexenol) to both dearomatized and neutral wines had no significant effect on aroma. Addition of the same compounds to neutral wines containing spikes of perithreshold concentrations of IBMP (Chapter 5) changed perception of the wines from “earthy” to “green pepper” [13], suggesting that they may contribute additively. Multivariate analyses also show a correlation between C6 compounds and “green” or “leafy” aromas [12], although this may be an indirect relationship resulting from the fact that fewer C6 compounds are formed during crushing with increased grape maturity.
Table 6.2 Typical concentrations of C6 alcohols in wine
| Name | Wine source | Typical range (μg/L)a | Thresholdb (μg/L) [9] |
| 1‐Hexanol (n‐hexanol)c |
Spanish red wines [2] Marlborough (NZ) Sauvignon Blanc [12] |
2100–13800 1327–3739 |
8000 |
| cis‐3‐Hexenol ((Z)‐3‐hexenol) |
Spanish red wines Marlborough (NZ) Sauvignon Blanc |
8–651 331–711 |
400 |
| trans‐3‐Hexenol ((E)‐3‐hexenol) |
Marlborough (NZ) Sauvignon Blanc | 66–130 | 1550d |
| cis‐2‐Hexenol ((Z)‐2‐hexenol) |
Marlborough (NZ) Sauvignon Blanc | 6–18 | Unknown |
| trans‐2‐Hexenol ((E)‐2‐hexenol) |
Marlborough (NZ) Sauvignon Blanc | ND–8 | 400d |
a 95% confidence interval reported for NZ Sauvignon Blanc.
b Threshold determined in 10% ethanol.
c All C6 alcohols in the table have the –OH group at the 1‐position, for example, cis‐3‐hexenol is cis‐3‐hexen‐1‐ol.
d Threshold in water (Leffingwell)
6.5 Methanol
In terms of origin and wine chemistry, methanol does not fit neatly into any other chapter, and is included in the discussion of higher alcohols because of its shared –OH functional group and volatility. Unlike higher alcohols, methanol is not a yeast fermentation metabolite, but is instead formed by acidic or enzymatic hydrolysis of methylated galacturonic acid residues of pectin, an important grape polysaccharide (Chapter 2). Enzymes capable of hydrolyzing methanol from esterified pectin are referred to as pectin esterases (PE) or pectin methylesterases (PME). These are present in fruits like grapes [14],2 which can be released by S. cerevesiae and other yeasts during fermentation [15], or may be a part of exogenous enzymes added by winemakers to increase extraction of components. Methanol can thus be found at mg/L concentrations in a wide range of fruit juices and wines, particularly those with high pectin or that are processed with pectinases.
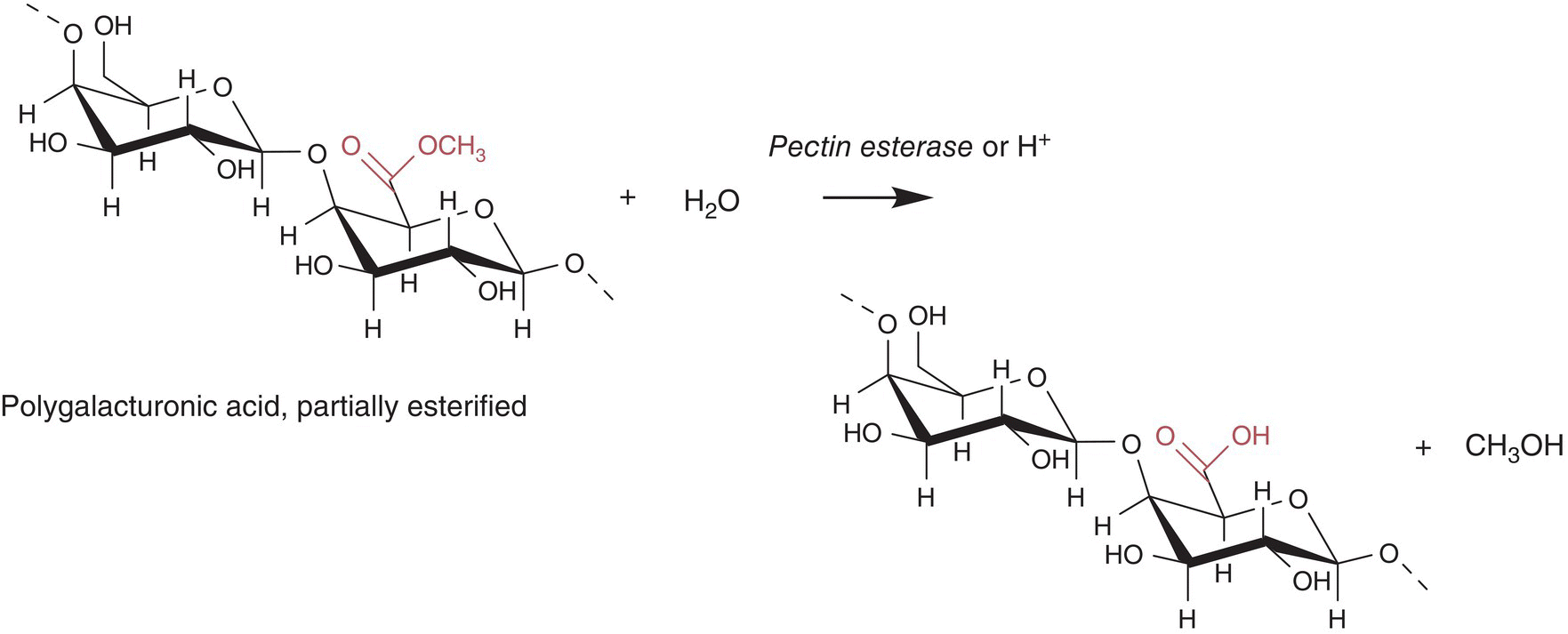
Figure 6.1 Hydrolytic reaction of esterified polygalacturonic acid to yield methanol [17]
In humans, methanol is metabolized to formaldehyde by alcohol dehydrogenase, which can lead to neurotoxicological effects (e.g., blindness) and death. The maximum safe amount of methanol that can be ingested is estimated to be 2 grams, and for acute toxicity is estimated to be 8 grams [16].3 Although most alcohol‐producing nations impose limits on methanol, in many countries (including the US) these limits are only explicitly stated for distilled spirits. The European Union limits methanol in red wine to 400 mg/L and in rosé and white wine to 250 mg/L. This would equate to consumption of 20 L of a red wine at the legal limit to reach toxic levels of methanol, and it appears that commercial wines rarely approach this limit. A review of ~20 surveys of methanol content in commercial wines was published in 1976 (Figure 6.1) [17].4 The survey reported that average methanol values ranged from 21 to 194 mg/L in red table wines and from 16 to 80 mg/L in white table wines, with a high concentration of 635 mg/L in an Italian red table wine.
Because it is derived from pectin hydrolysis, methanol content will start at negligible concentrations and increase during fermentation. Factors that affect methanol concentration in finished wines have been explored in the literature [17–19]:
- Grape variety. Grapes with high concentrations of pectin, for example, V. labruscana grapes such as Concord (Chapter 20) will result in wines with higher methanol concentrations (up to 500 mg/L) [18]. Analogously, wines produced from high pectin fruits like plums can yield wines with 0.15% v/v methanol [20].
- Pectolytic enzymes. While these enzymes are typically selected for their ability to cleave bonds between sugars (e.g., galacturonase, Chapter 2), most commercial pectinase preparations also possess PME activity, and use of pectinases will increase methanol content by >50% [19].
- Maceration time and fermentation temperature. Most pectin will be bound to the pomace and longer contact with grape cell wall material during fermentation increases methanol content. In one report, an increase of 50–60 mg/L was seen when pressing occurred after 8 hours (presumably, before fermentation commenced) as opposed to 64 hours [17]. Generally, higher temperatures will also increase hydrolysis, although very high temperature, for example, thermovinification, can inactivate PME [18].
The effects of maceration time and fermentation temperature likely explain the differences in average methanol content observed between red and white table wines, described above. Finally, methanol will be further concentrated during distillation, as described later (Chapter 26.4).
References
- 1. Watts, H. (1864) A dictionary of chemistry and the allied branches of other sciences, Vol. 2, William Wood & Co., New York.
- 2. Ferreira, V., Lopez, R., Cacho, J.F. (2000) Quantitative determination of the odorants of young red wines from different grape varieties. Journal of the Science of Food and Agriculture, 80 (11), 1659–1667.
- 3. Czerny, M., Christlbauer, M., Christlbauer, M., et al. (2008) Re‐investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. European Food Research and Technology, 228 (2), 265–273.
- 4. Lee, C.Y. and Cooley, H.J. (1981) Higher‐alcohol contents in New York wines. American Journal of Enology and Viticulture, 32 (3), 244–246.
- 5. Bell, S.J. and Henschke, P.A. (2005) Implications of nitrogen nutrition for grapes, fermentation and wine. Australian Journal of Grape and Wine Research, 11 (3), 242–295.
- 6. Ugliano, M., Bartowsky, E.J., McCarthy, J., et al. (2006) Hydrolysis and transformation of grape glycosidically bound volatile compounds during fermentation with three Saccharomyces yeast strains. Journal of Agricultural and Food Chemistry, 54 (17), 6322–6331.
- 7. Makhotkina, O. and Kilmartin, P.A. (2012) Hydrolysis and formation of volatile esters in New Zealand Sauvignon blanc wine. Food Chemistry, 135 (2), 486–493.
- 8. Ferreira, V., Ortin, N., Escudero, A., et al. (2002) Chemical characterization of the aroma of Grenache rose wines: aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. Journal of Agricultural and Food Chemistry, 50 (14), 4048–4054.
- 9. Guth, H. (1997) Quantitation and sensory studies of character impact odorants of different white wine varieties. Journal of Agricultural and Food Chemistry, 45 (8), 3027–3032.
- 10. Ferreira, V., San Juan, F., Escudero, A., et al. (2009) Modeling quality of premium Spanish red wines from gas chromatography − olfactometry data. Journal of Agricultural and Food Chemistry, 57 (16), 7490–7498.
- 11. Matsui, K. (2006) Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Current Opinion in Plant Biology, 9 (3), 274–280.
- 12. Benkwitz, F., Tominaga, T., Kilmartin, P.A., et al. (2012) Identifying the chemical composition related to the distinct aroma characteristics of New Zealand Sauvignon blanc wines. American Journal of Enology and Viticulture, 63 (1), 62–72.
- 13. Escudero, A., Campo, E., Farina, L., et al. (2007) Analytical characterization of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. Journal of Agricultural and Food Chemistry, 55 (11), 4501–4510.
- 14. Barnavon, L., Doco, T., Terrier, N., et al. (2001) Involvement of pectin methyl‐esterase during the ripening of grape berries: partial cDNA isolation, transcript expression and changes in the degree of methyl‐esterification of cell wall pectins. Phytochemistry, 58 (5), 693–701.
- 15. Jayani, R.S., Saxena, S., Gupta, R. (2005) Microbial pectinolytic enzymes: a review. Process Biochemistry, 40 (9), 2931–2944.
- 16. Paine, A. and Davan, A.D. (2001) Defining a tolerable concentration of methanol in alcoholic drinks. Human and Experimental Toxicology, 20 (11), 563–568.
- 17. Gnekow, B. and Ough, C.S. (1976) Methanol in wines – source and amounts. American Journal of Enology and Viticulture, 27 (1), 1–6.
- 18. Lee, C.Y., Robinson, W.B., Van Buren, J.P., et al. (1975) Methanol in wines in relation to processing and variety. American Journal of Enology and Viticulture, 26 (4), 184–187.
- 19. Cabaroglu, T. (2005) Methanol contents of Turkish varietal wines and effect of processing. Food Control, 16 (2), 177–181.
- 20. Zhang, H., Woodams, E.E., Hang, Y.D. (2012) Factors affecting the methanol content and yield of plum brandy. Journal of Food Science, 77 (4), T79–T82.