24
Wine Oxidation
24.1 Introduction
Oxidation is generally the limiting factor in the preservation of wine. In other words, most wines lose their palatability during long‐term storage due to excessive oxidation rather than other causes like microbial spoilage. However, a small amount of oxygen can improve qualities of some wines, and large amounts of oxygen exposure are critical for the flavor of certain wines, such as Madeira, Sherry, Vino Santo, and Jura Vin Jaune. Several important historical wine authors have reflected on the significance of oxidation. In the earliest US text on wine production Rixford states, “[i]n a few instances, where the wines are strong enough to bear it, aging may be hastened by some exposure to the air, but great care must be taken that they are not left too long under its influence or disorganization may ensue”[1]. Almost 100 years later, in the early 1970s Amerine et al. wrote that “the principal changes in flavor and bouquet during aging in the wood are generally believed to be due to slow oxidation” [2]. Numerous other reviews address the importance of wine oxidation to wine stability, flavor and color, including those by Singleton [3], Cheynier [4], Danilewicz [5], du Toit et al. [6], Waterhouse and Laurie [7], Li et al. [8], Karbowiak [9], Oliveira et al. [10] Danilewicz [11], and Ugliano [12].
In summary, although there is now a greater awareness of other non‐oxidative reactions that can occur during wine aging, for example, acid hydrolysis (Chapter 25), Louis Pasteur’s nineteenth century observation, “C’est l’oxygene qui fait le vin” is still largely true [13].
24.2 Redox reactions
Redox refers to chemical reactions that involve the gain (reduction) and loss (oxidation) of electrons by the reactants. In the context of wine chemistry, oxidation is usually associated with an increase in the number of covalent bonds to oxygen and/or decrease in bonds made to hydrogen or other less electronegative atoms.
The tendency of compounds to be oxidized or reduced is described by their reduction potential, E. Tables of reduction potentials are compiled as half‐reactions (see Table 24.1), which will include the oxidized form, reduced form, and electrons and/or protons gained during the reduction process. Reduction potentials for species are usually reported under standard conditions (e.g., E0 values are reported for 1 M solute concentration or 101 325 Pa (1 atm) gas concentration, 25 °C, pH 0). Half‐reactions that include protons will have pH‐dependent reduction potentials; typically, reduction potentials that involve protons will be about 0.2 V lower at wine pH than at pH 0. Higher values (more positive) for E indicate that the oxidized form is a stronger oxidizer, and the reduced form is a weaker reducer (weak antioxidant). Further information on this topic can be found in an introductory chemistry book.
Table 24.1 Half‐reactions for the reduction of some wine components
| Oxidized form | Reduced form | E0 (pH 0), 25 °C | E3.5 (pH 3.5), 25 °C | |
| SO42− | +2e− + 2H+ – H2O | HSO3− | −0.4 V | –0.6 V |
| Disulfides | +2e− + 2H+ | Mercaptans | ~0.1 | ~ –0.1 |
| Acetaldehyde | +2e− + 2H+ | Ethanol | 0.2 | 0 |
| Cu2+ | +e− | Cu+ | 0.15 | 0.15 |
| Dehydro‐ascorbic acid | +2e− + 2H+ | Ascorbic acid | 0.5 | 0.3 |
| o‐Quinone | +2e− + 2H+ | o‐Diphenol (catechol) | 0.8 | 0.6 |
| Fe3+ | +e− | Fe2+ | 0.77 | 0.77 |
| H2O2 | +e− + H+ | ·OH + H2O | 0.38 | 0.28 |
| ½ O2 | +2e− + 2H+ | H2O | 1.23 | 1.0 |
The oxidizing or reducing nature of a solution is described by its redox potential, E, and under equilibrium conditions the ratio of oxidized to reduced ([Ox]/[Red]) forms of a species can be related to E by the Nernst equation, where F is Faraday’s constant:
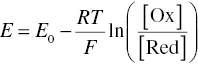
In principle, redox potential measurements could be useful for evaluating whether wine is excessively reduced or oxidized. In practice, this is challenging because redox measurements are generally performed using platinum electrodes, and the redox potential is defined as the voltage applied across electrodes where there is no net current (reduction and oxidation reactions occur at the same rate). Danilewicz clarifies that this requires the presence of all participating substances at the electrode, but the key first step in wine oxidation, the reaction of oxygen and phenols, produces unstable o‐quinone intermediates (Figure 24.1) [11]. Thus no equilibrium condition is present, so the Nernst equation cannot be utilized, and these measurements do not reveal a true redox potential. Instead, these platinum electrode measurements on wine primarily measure the direct reduction of oxygen, that is, the measured value is related only to the concentration of dissolved oxygen [14]. In addition, the redox potentials of metals are strongly influenced by the presence of metal chelators, one of which is tartrate, a strong Fe3+ chelator, so the use of redox potentials to assess thermodynamics must take such effects into account [15].
24.2.1 Thermodynamics of redox reactions
Full redox reactions involve combinations of half‐reactions to balance out the exchange of electrons. For example, taking into account the reduction of oxygen and oxidation of ethanol, the overall reaction to form acetaldehyde under standard conditions would be:
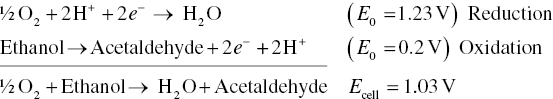
The electrochemical cell potential, Ecell, is related to the equilibrium constant for a reaction, Keq, by the following equation, where n = number of electrons involved in the redox reaction:
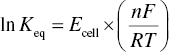
Positive values of Ecell, as is the case for the aforementioned reaction of oxygen with ethanol, describe reactions that are thermodynamically favorable, and in the case of the full reaction shown above, Keq > 1034. The data shown in Table 24.1 can thus be used to determine what redox reactions could feasibly occur, which provides a very crude understanding of the events that will occur during wine oxidation, that is, sulfited wines exposed to oxygen should accumulate sulfate and/or acetaldehyde and lose oxygen over time. However, a strictly thermodynamic interpretation of wine oxidation is by itself inadequate. Thermodynamics alone would incorrectly predict that reducing compounds should be lost in reverse order of their reduction potentials; that is, nearly all sulfites must be converted to sulfate before diphenols could be oxidized to quinones or alcohols are oxidized to aldehydes, etc. Furthermore, thermodynamics alone can also not explain why vodka can sit on a shelf for years without displaying any signs of oxidation. Thus, in addition to a thermodynamic analysis, a kinetic, mechanistic understanding is necessary to rationalize the rate and products of real oxidations.
24.3 The central tenets of wine oxidation
The last decade has seen a growing consensus on the primary mechanisms involved in wine oxidation, which we will refer to as “The Central Tenets of Wine Oxidation.”
- Molecular oxygen (O2) does not directly react with most wine components. The initial steps of oxygen consumption requires the presence of transition metal catalysts and good hydrogen donor molecules, particularly Fe(II) and o‐diphenols.
- Metal‐catalyzed oxidation of o‐diphenol groups results in the formation of quinones and H2O2. However, the thermodynamics of this reaction are unfavorable, and product consumption is essential to enable the reaction.
- Quinones are strongly electrophilic and will react with wine nucleophiles including bisulfite, thiols, and flavan‐3‐ols. Antioxidants act in part by consuming the quinones, thus preserving flavors.
- H2O2 reacts with bisulfite and oxidation reactions end. Alternatively, if there is insufficient bisulfite, H2O2 participates in the Fe(II)‐catalyzed Fenton reaction to produce aldehydes and other oxidized species.
- Aldehydes and related species react with phenolics, bisulfite, and other nucleophilic species. When the nucleophiles are exhausted, the aldehydes and related compounds accumulate, making the wine “oxidized.”
This model has been particularly successful in describing why the rate of oxidation and product distribution changes with wine chemistry. Oxidation rates may be defined in several ways, including:
- The rate of loss of oxygen once introduced into wine.
- The rate of loss of reduced compounds, particularly SO2.
- The rate of formation of compounds associated with oxidation, for example, brown pigments or acetaldehyde.
Early studies of wine chemistry observed that O2 consumption roughly obeyed first‐order kinetics. The half‐life of O2 in wine is usually on the order of several hours or days at room temperatures, or 100–1000‐fold slower than oxygen consumption by polyphenol oxidase (PPO) activity in juice. Oxygen consumption was observed to be faster in red than in white wines, but there was little understanding as to how non‐enzymatic oxidation occurred [16].
In 1974, Wildenradt and Singleton published one of the first papers discussing a reaction mechanism for oxidation. Using model wines, the authors observed that aldehyde formation and browning were only observed when polyphenolic compounds containing the o‐diphenol moiety were added to the model system (e.g., caffeic acid, catechin); model systems containing only ethanol, water, and tartaric acid showed no aldehyde production [17].1 The authors then hypothesized several important parts of the central tenets, namely that catechols react with oxygen to yield quinones and hydrogen peroxide, and that the peroxide subsequently reacts with ethanol to generate acetaldehyde [17] (Figure 24.1).
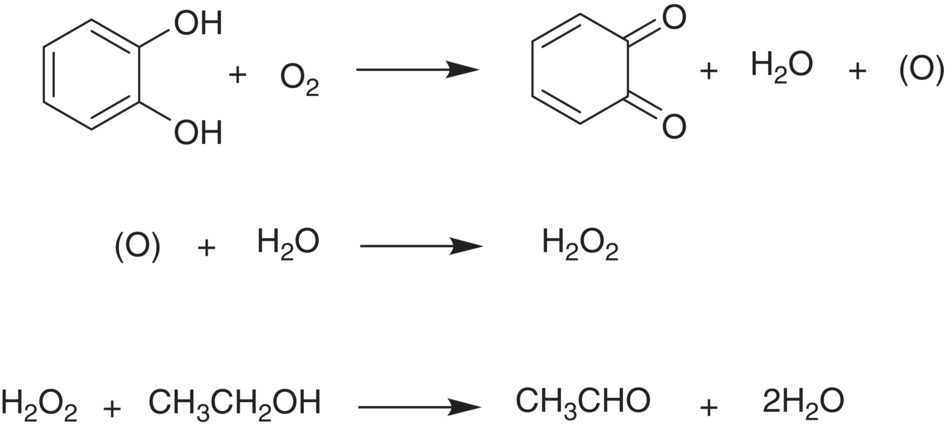
Figure 24.1 Formation of an o‐quinone from an o‐diphenol (top) and concurrent production of hydrogen peroxide (H2O2) through coupled oxidation (middle) in wine oxidation as proposed by Wildenradt and Singleton [17]. The resulting H2O2 can subsequently oxidize ethanol to form acetaldehyde (CH3CHO, bottom)
The discovery of quinone formation was an important milestone in rationalizing wine oxidation. The simultaneous formation of hydrogen peroxide was named coupled oxidation by Wildenradt and Singleton [17].
24.3.1 The role of metal catalysts in quinone formation
One problem with the scheme in Figure 24.1 is that the favored ground‐state triplet form of O2 cannot directly react with other ground‐state organic molecules, as it is spin forbidden by Pauli’s exclusion principle. In food systems, ground‐state oxygen is typically activated to a reactive singlet state through one of two pathways:
- UV light exposure
- Transition metal catalysts.
Subsequent research has shown that transition metal catalysts, and particularly iron, appear to be critical to wine oxidation [5] (Chapter 4). Iron primarily exists as the reduced Fe(II) species in wine due to the presence of stronger reducing agents (Table 24.1). However, the precise mechanism by which the Fe(III)/Fe(II) couple facilitates oxidation is still difficult to discern, with the possibility of two one‐electron steps, or one two‐electron step, or some hybrid. A two‐step scheme is shown in Figure 24.2.
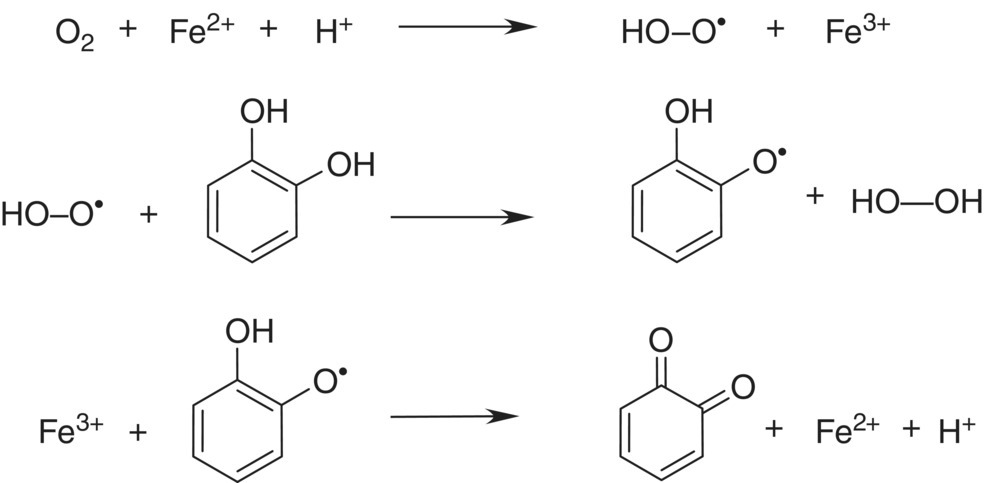
Figure 24.2 Hypothetical mechanism forming quinone and peroxide via the hydroperoxyl radical step, adapted from [4]
Fe(II) can be rapidly oxidized to Fe(III), yielding a hydroperoxy radical capable of oxidizing o‐diphenol to a semiquinone. The semiquinone can then be oxidized by Fe(III) to an o‐quinone. Cu(I) may have a synergistic role in increasing oxidation rates in wine by regenerating Fe(II) (Table 24.1). This two‐step process would involve the formation of the hydroperoxyl radical (protonated superoxide O2−·), but an attempt to detect this species was not successful [18]. An alternative mechanism has been proposed that reduces oxygen with two Fe(II) species to generate hydrogen peroxide (Figure 24.3) and quinone [19]. Regardless of the mechanism, phenolic compounds and metal catalysts have a synergistic effect on the rate of wine oxidation [20] (Figure 24.4). Simple phenols have a much higher oxidation potential than catechols (Chapter 11), rendering them inaccessible to oxidation via this mechanism.
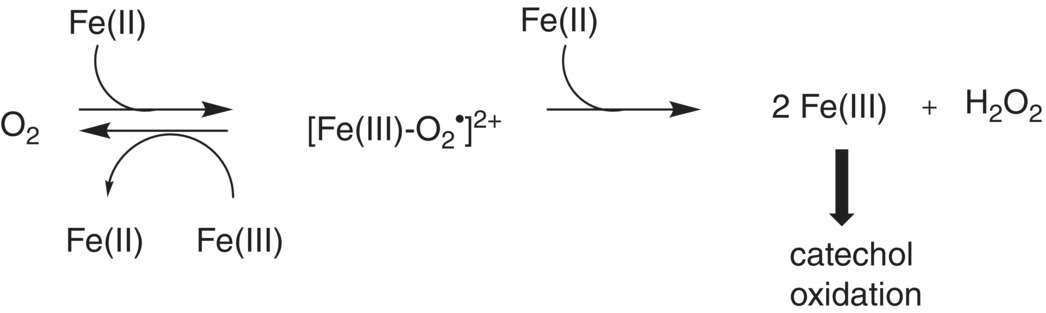
Figure 24.3 A two‐step process for formation of o‐quinone from an o‐diphenol, showing potential roles of metal catalysts.
Source: Danilewicz 2013 [19]. Reproduced with permission of AJEV
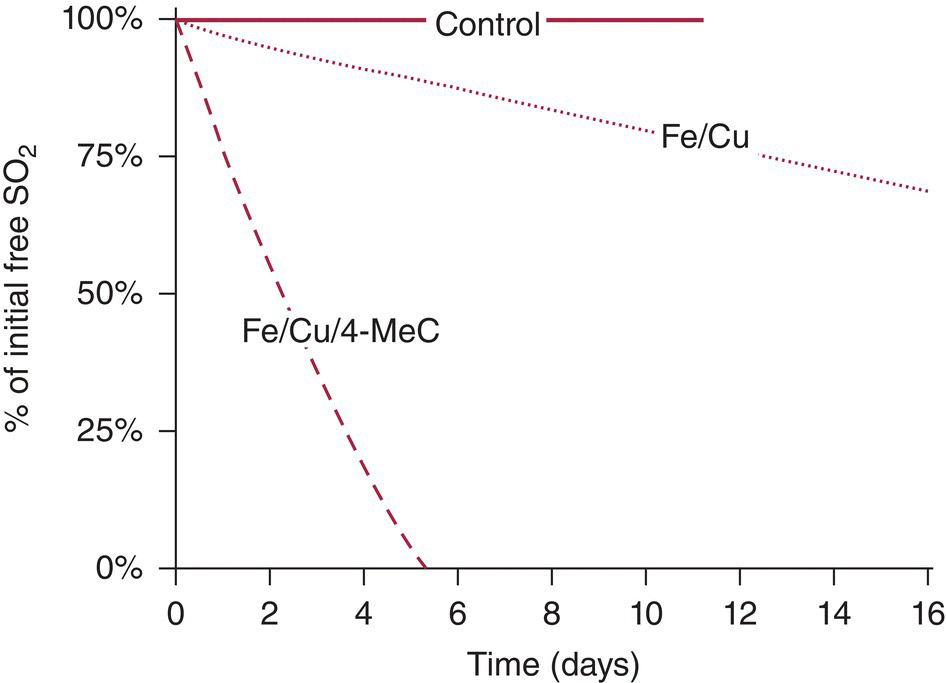
Figure 24.4 Effect of metals and phenolics on SO2 consumption in model wine. 4‐MeC is 4‐methylcatechol.
Source: Danilewicz 2007 [20]. Reproduced with permission of AJEV
The reactivity of Fe(II) towards oxygen is determined by the redox potential of the Fe(III)/Fe(II) couple, which in an ideal solution will be E0 = 0.77 V. However, this reduction potential can be altered by complexation of one or both of these two Fe forms.
- The major wine acid, tartaric, complexes Fe(III) and decreases the reduction potential of the Fe(III)/Fe(II) couple to 0.34 V, below that of the O2/H2O2 couple. This favors Fe(III) and H2O2 formation and increases the rate of oxidation [15]. Similar pro‐oxidative effects can be seen following addition of the Fe(III) chelating agent, ethylenediaminetetraacetic acid (EDTA) [21].
- Conversely, the decrease in Fe(III)/Fe(II) reduction potential brought about by tartrate chelation of Fe(III) makes it less able to directly oxidize o‐diphenols to quinones, slowing oxidation [19]. This effect can be negated by the presence of strong Fe(II) chelators, which brings back the redox potential closer to the literature value for an Fe(III)/Fe(II) couple [22]. In addition, if a nucleophile is available to react with the quinone, the reaction can proceed even in systems with stabilized Fe(III), that is, tartrate buffered wine, based on Le Châtelierʼs principle.
- Strong Fe(II) complexing reagents, for example, ferrozine, slow oxidation reactions [21].2
In summary, if a wine contained no iron or related transition metals, oxygen would accumulate in barrels and bottles, and the wine would never oxidize!
24.4 The central tenets of quinone reactions
Subsequent investigations have shown that o‐diphenols and metals are essential but not sufficient for wine oxidation. The driving force for the initial oxidation reactions of oxygen and catechol to quinone is not, surprisingly, the oxidation of catechol. The redox potential of the H2O2/O2 couple at wine pH (E3.5 ~ 0.45 V) is lower than a typical quinone/diphenol couple (0.6 V, Table 24.1) and equilibrium will strongly favor the 1,2‐diphenol. Thus, little oxidation occurs unless a suitable nucleophile is available to consume the quinone (Figure 24.5) [19]. It is the consumption of the quinone by nucleophiles and the application of Le Chatelier’s principle that leads to oxygen consumption [23].
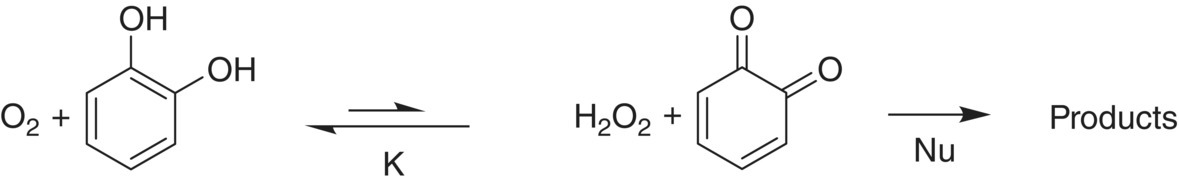
Figure 24.5 Equilibrium reaction of oxygen and catechol and the subsequent reaction with various nucleophiles. K of the equilibrium reaction is very small
In most cases, wine contains sufficient nucleophiles or other reducing agents and oxidation will proceed (Figure 24.5). Potential quinone reactions include:
- Condensation reaction with nucleophilic thiols
- Condensation reaction with other phenolics, particularly flavan‐3‐ol nucleophiles
- Condensation reaction with other nucleophiles, particularly bisulfite
- Reaction with reducing agents (ascorbic acid, bisulfite) to regenerate the original diphenol.
24.4.1 Quinone‐thiol adducts
Thiols are very good nucleophiles and will react rapidly with quinones (Chapter 10). The first quinone‐thiol adduct discovered in enology, and in fact the first specific wine oxidation product that was well characterized, resulted from an initial observation that caftaric acid is lost during must oxidation [24]. This was subsequently shown to be a result of the reaction between caftaric acid quinone and glutathione (GSH) [25]. The oxidation was proposed to be enzymatic, with grape polyphenol oxidase (PPO) catalyzing the oxidation of caftaric acid and its quinone reacting chemically with the thiol. This adduct, called the grape reaction product (GRP), is found in most commercial wines as a result of the oxygen exposure encountered during grape crushing and pressing. This outcome is not overly surprising given that GSH is the major thiol in grape juice, and considering that thiols are one of the most reactive nucleophiles present in wine. Others have reported that GSH can react with oxidized catechin to form a bond to the B‐ring [26], in a manner similar to that shown for the thiol in Figure 24.6.
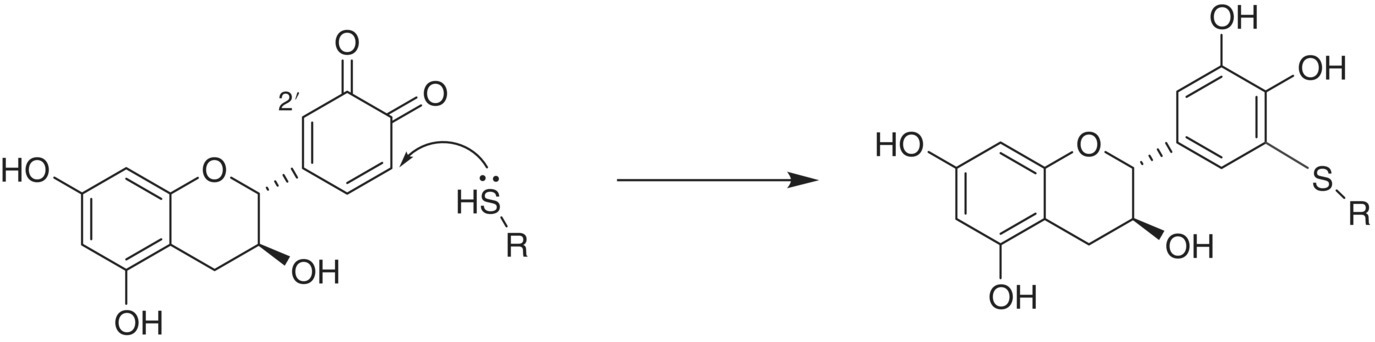
Figure 24.6 Thiol reaction with catechin quinone. All sites (2′, 5′, and 6′) are reactive and product ratios depend on the specific nucleophile and quinone
Although GSH is the most quantitatively important thiol in wine, there are several low‐concentration odorous thiols that can also react with quinones [27] in an analogous manner.
24.4.2 Quinone‐phenol adducts and browning in juice
The appearance of brown color in plant‐based products is of broad interest to many different areas of food science [28]. In grape juice, browning is initiated largely by enzymatic oxidation, not chemical oxidation as observed in bottle aging [29], and particularly through PPO and related enzymes acting on hydroxycinnamates (Chapter 13). It can also be facilitated by a process called hyperoxidation, in which oxygen is intentionally added to the must to induce browning reactions [30]. The brown insoluble material is then separated from the must (e.g. by racking) prior to fermentation the finished wine is less susceptible to browning in the bottle. The first step of enzymatic browning is formation of the caftaric acid quinone via enzymatic oxidation by PPO. Browning will be decreased in musts containing high relative amounts of GSH as compared to hydroxycinnamates, since GSH can form GRP and arrest oxidation [31]. However, when solutions containing only hydroxycinnamates are oxidized, no browning appears, and, instead, some colorless dimeric products are formed (Figure 24.7) [32].
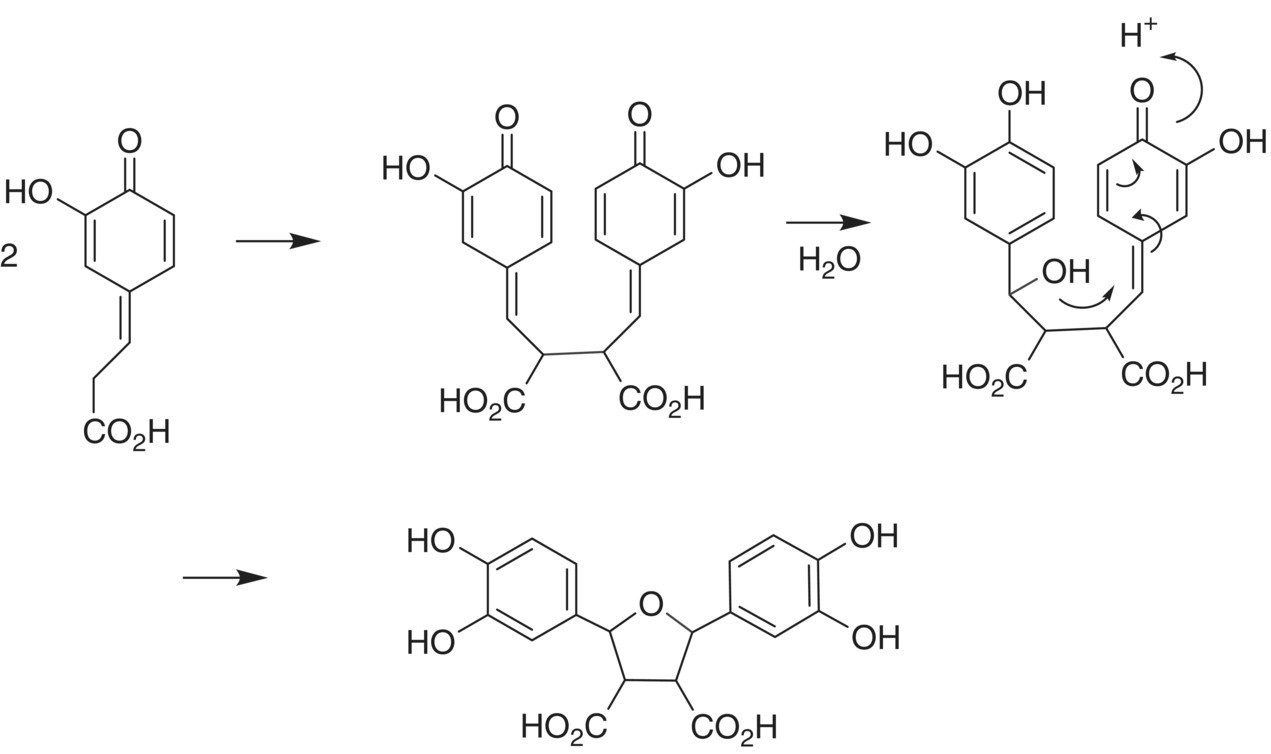
Figure 24.7 Hydroxycinnamate quinone dimerization producing a colorless product as reported by Fulcrand et al. [32]
Browning in juice is, however, well correlated with flavan‐3‐ol concentration [33–35]. The pathway to enzymatic browning appears to follow coupled oxidation reactions where hydroxycinnamate quinones oxidize catechol groups on the flavan‐3‐ols to quinones, regenerating the hydroxycinnamates [36]. The electrophilic flavan‐3‐ol quinones are reactive and can couple with nucleophilic phloroglucinol A‐rings on another flavanol (Chapter 10, boxed section). In the simplest example, one catechin quinone reacts with a catechin to form a dimer as reported by Guyot et al. [37] and shown in Figure 24.8. These authors also report related reactions with oxygen on the A‐ring acting as a nucleophile. This type of reaction has since been confirmed by others [38].

Figure 24.8 Flavan‐3‐ol condensation product arising from a nucleophilic attack of C8 of one catechin monomer on to the o‐quinone of another. Reaction at C6 is also possible.
Adapted from Reference [37]
The products shown in Figure 24.8 have no pigmentation, and formation of yellow or brown pigmented substances involves a second oxidation of a B‐ring catechol on an initial dimeric product to form a new quinone. This is followed by in intramolecular reaction at the 1′ position on the quinone with a nucleophilic A‐ring oxygen from the other flavanol unit. This product has no hydrogen left at the addition site, so the product cannot rearrange via H isomerization back to an aromatic benzene structure, locking in a conjugated keto form as shown in Figure 24.9. These products can have a large number of conjugated double bonds, shifting the absorbance of light into the visible region3 and thus yellow color is observed.

Figure 24.9 Formation of pigmented products of flavan‐3‐ols as described by Guyot et al. [37]. Products arising from the initial reaction at C6 are also observed
ortho‐Quinone groups may also isomerize to the pseudo‐para‐quinone form (i.e., quinone methide), providing an electrophilic reaction center at the C2 on the C‐ring, creating linkages at that position [39]. This can involve hydroxyl groups on a phloroglucinol ring, as these are also reactive as nucleophiles. Thus, in sterically constrained situations an intramolecular bond at the 2‐position can be formed. Such products can also have extended π‐bond conjugation, and thus be pigmented.
While such products are expected as a result of enzymatic oxidation in juice, in finished wine PPO is no longer active and oxidative browning occurs by other routes. In model wine solutions, the main yellow‐brown compounds do not arise from a quinone electrophile, but appear to be xanthylium reaction products of aldehydes and flavan‐3‐ols, described in more detail below [40].
24.4.3 Reactions of quinones and other antioxidants: sulfur dioxide and ascorbic acid
Sulfur dioxide is well known to suppress enzymatic browning in a range of food systems, and early reports established that bisulfite reduced quinones and consequently lowered the level of oxidation products [41]. The general chemistry of bisulfite reactions with quinones was demonstrated some time ago with p‐quinone, revealing that at low pH there are two reaction pathways: nucleophilic addition to the quinone, yielding about 25% of the sulfonate, and reduction to regenerate hydroquinone (p‐dihydroxybenzene) as the major product. However, at higher pH values (neutral or slightly basic) the amount of hydroquinone decreased until the only product was the sulfonate adduct [42]. More recently, analogous results were observed with an o‐quinone at wine pH values; that is, most of the quinone was reduced back to an o‐diphenol, but the reaction yielded a small fraction of the sulfonate (Figure 24.10) [23].
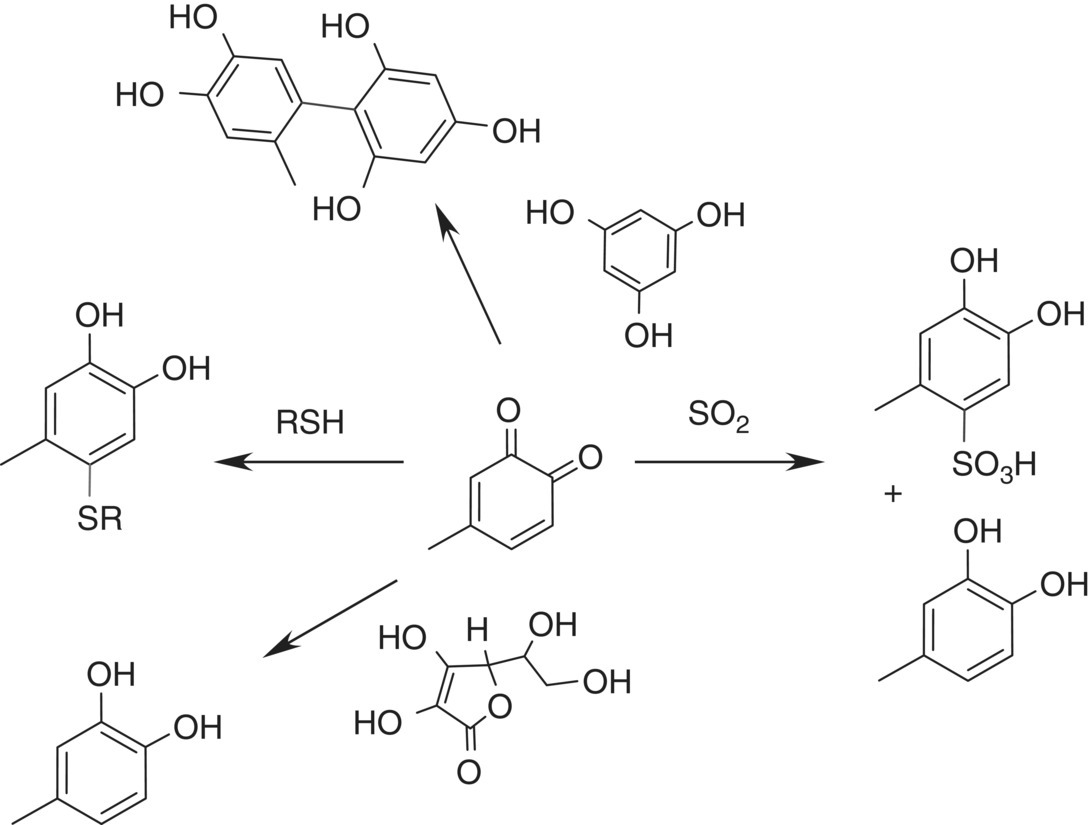
Figure 24.10 Alternate pathways for reactions of quinones with known nucleophiles and reducing agents
Another common antioxidant used in winemaking, ascorbic acid, is not as strong an antioxidant as SO2, but can reduce o‐quinones back to their catechol form just as quickly [43] (Figure 24.10). There is a small amount in grapes that is quickly lost during fermentation so that new wine has a negligible ascorbic acid content, but it can be added as a preservative, typically prior to bottling. Because of its ability to scavenge quinones, ascorbic acid can be used as an antioxidant adjuvant to SO2, and its proper use can allow for lower usage of SO2 in finished wine. However, ascorbic acid does not readily consume the other major product of wine oxidation (H2O2, see below) and its use without SO2 can lead to enhanced browning [44].
24.4.4 Comparison of quinone scavenging reactions
When comparing the benefits of any particular antioxidant, a chemical comparison could entail studying the relative reaction rates of the antioxidant versus a desirable flavor substance, to show whether the antioxidant could prevent the loss of varietal flavor. Put another way, oxidation contributes reactive quinone electrophiles, so what important nucleophiles react with the quinones? A key category is the volatile thiols that contribute varietal aroma, such as 3‐mercaptohexanol (3‐MH) (Chapter 10). Knowledge of the relative reaction rates of the varietal thiols compared to potential protective antioxidants or sacrificial nucleophiles, which would consume quinones before the desirable thiols could react, has obvious importance. Some typical reactions of a model quinone with antioxidants and nucleophiles are summarized in Figure 24.10.
While this model ignores other oxidation‐related reactions, the antioxidants SO2, ascorbate, and GSH all react very quickly, and at a rate similar to hydrogen sulfide [43]. This was approximately six times faster than 3‐MH at the same concentration. Based on these rate differentials, antioxidants should be fairly effective in protecting wines from loss of 3‐MH. Furthermore, the concentration of GSH in juices is reported to be 50–320 μM [45], higher than cysteine, which is reported to be 8–60 μM [46]. Both these substances are found at orders of magnitude higher concentrations than the varietal thiols in wine, with those reported at about 100 nM (Chapter 10). Because GSH and SO2 are more concentrated compared to the volatile thiols, and react more quickly, they should be effective in preventing the reaction of the varietal thiols with quinones until they are exhausted through oxidation. Alternatively, in the absence of SO2 and other antioxidants, an oxidized flavan‐3‐ol should react with thiols, including 3‐MH, H2S, methanethiol, and so on.
24.5 The central tenets of the Fenton reaction and byproducts
24.5.1 Hydrogen peroxide reactions
The hydrogen peroxide formed via coupled oxidation of diphenols to quinones has two potential fates. The first, termed the Fenton reaction, involves Fe(II) catalysts again; hydrogen peroxide is decomposed to a hydroxyl radical (Figure 24.11), which can subsequently oxidize a large number of substrates, particularly alcohols to carbonyls.
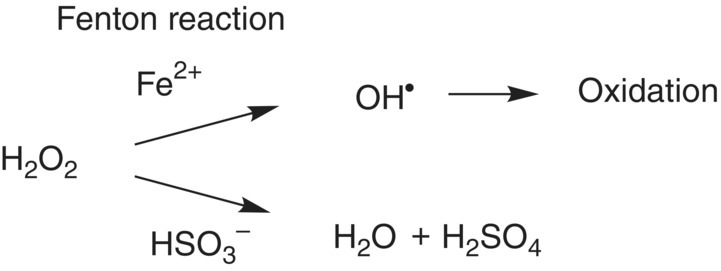
Figure 24.11 Key branch point for hydrogen peroxide
The second pathway involves direct reaction of hydrogen peroxide with sulfur dioxide (in the form of bisulfite) [47]. This second pathway will avoid the Fenton step, but requires sufficient concentrations of bisulfite (Chapter 17). Other wine antioxidants, such as ascorbic acid [48] and GSH [49], can react with hydrogen peroxide at acid pH, but current information suggests their reaction rates with hydrogen peroxide are not competitive with Fe(II), and thus are unlikely to prevent the Fenton reaction.
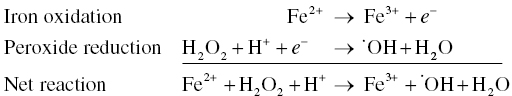
The hydroxyl radical formed by the Fenton reaction is highly reactive and once formed it is not feasible to use antioxidants to slow or prevent its reaction with the majority of wine components. Instead, this radical will readily abstract a hydrogen or add to a substrate in a very non‐selective manner [50]. Reaction of ˙OH with water simply regenerates ˙OH, but reaction with the next highest concentration component of wine, ethanol, generates the major detectable product of the Fenton reaction, the 1‐hydroxyethyl radical [18]. This radical goes on to produce acetaldehyde unless it can be quenched by other processes, such as reaction with hydroxycinnamates [51].
The indiscriminant reactivity of the hydroxyl radical towards wine compounds results in the Fenton reaction generating a large range of aldehydes and ketones, with acetaldehyde being the major product [17]. Other major oxidation products roughly parallel the concentrations of potential substrates in wine, include glyoxylic acid from tartaric acid [52], pyruvic acid from the oxidation of malic or lactic acid [53], and glyceraldehyde from the oxidation of glycerol [54]. In fact, as the hydroxyl radical is not selective, virtually all wine constituents will be oxidized in rough proportion to their concentration (see the Introduction chapter) so many additional oxidation products are formed [50].
Other aldehydes and ketones that arise from oxidation can have a major impact on wine aroma. In particular, these include methional and phenylacetaldehyde [55], sotolon [56], and 3‐methyl‐2,4‐nonanedione [57] (Chapter 9). However, the origins of these aldehydes are not always clear and they do not necessarily arise by oxidation of the related alcohol. For instance, a route via the Strecker reaction is addressed elsewhere (Chapter 9). In addition, during grape crushing, lipid oxidase enzymes release (E)‐2‐alkenals [58] via lipid oxidation, as discussed earlier (Chapter 23.3). Finally, while not strictly a wine reaction, if a wine high in iron is consumed with foods high in omega‐3 fatty acids, that is, fish, the ensuing oxidation reactions in the oral cavity (which include the Fenton reaction) can yield odorous mid‐chain aldehydes that cause an off‐flavor.4
24.5.2 Reactions of carbonyls
After the Fenton reaction creates an inventory of aldehydes and ketones, these reactive electrophiles go on to combine with nucleophilic substances in the wine. The well‐known nucleophiles include thiols, alcohols, SO2, and the phloroglucinol moiety (A ring) on flavonoids (Figure 24.12 and Chapter 10).
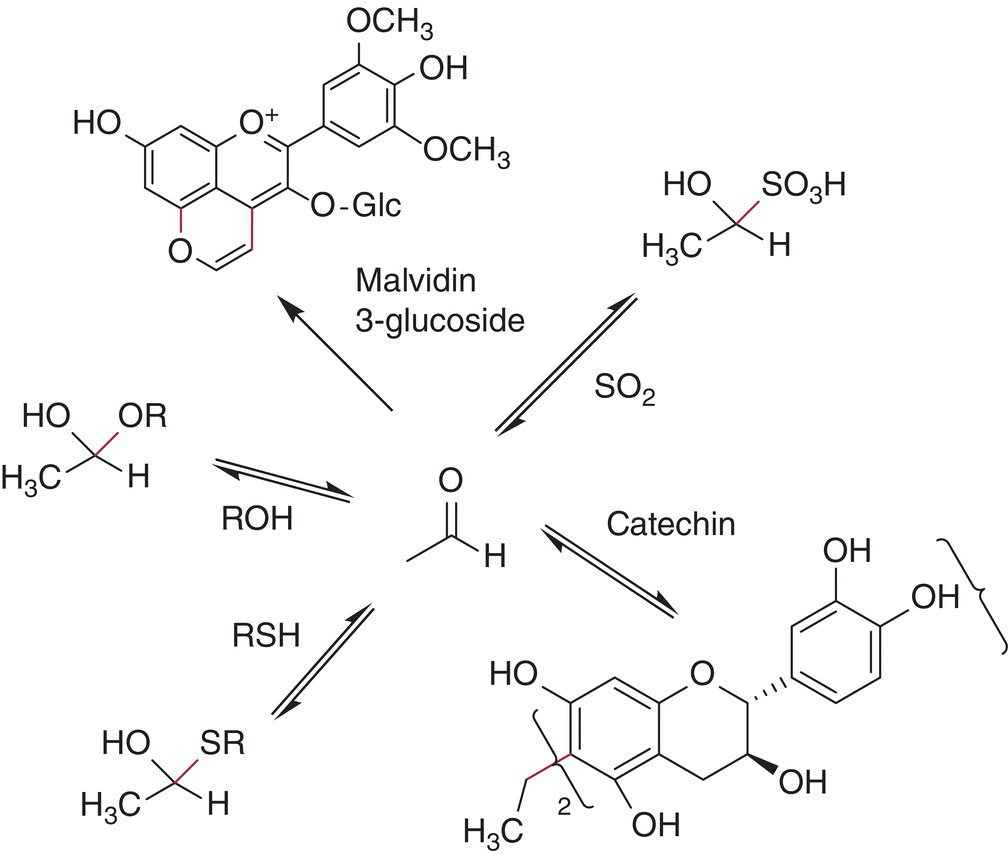
Figure 24.12 Alternative reaction pathways for acetaldehyde
The reaction of aldehydes and ketones with SO2 is well documented (see Chapters 9 and 17) and, notably, acetaldehyde forms a very stable hydroxysulfonate, while other carbonyls form weaker adducts. Acetaldehyde also reacts with alcohols under acidic conditions to form acetals (Chapter 9). These compounds have been particularly well documented in fortified wines as a result of extensive aging [60], and they appear to be important contributors to Port and Madeira aroma.
All flavonoids can react with electrophilic carbonyls and numerous products have been characterized. Desirable aging reactions dependent on oxidation involve the conversion of anthocyanins to derived wine pigments that arise in part from reactions with oxidation products. For instance, vitisin B is a product formed by reaction of malvidin‐3‐glucoside with acetaldehyde, and it is notably stable against bleaching by SO2 or pH changes (Figure 24.12). These compounds and reactions are described in more detail in Chapters 16 and 25.
Reactions of oxidation products with other flavonoids are well known, and some of the products are pigmented due to unique cyclization and aromatization pathways. Condensation of flavan‐3‐ols with acetaldehyde results in colorless structures, with two flavan‐3‐ols bridged by an ethylene group (i.e., ethyl‐linked). However, when tartaric acid is oxidized to glyoxal (also a yeast metabolite), the resulting bridged product continues to react, creating a xanthylium product (Figure 24.13) that absorbs in the visible region, and may contribute to the yellow hue of oxidized wines [61].

Figure 24.13 Reaction of catechin and glyoxal to colored xanthylium product as suggested in Reference [61]
The quantities of flavonoid‐acetaldehyde condensation products could potentially serve as a marker for the amount of oxygen to which a wine has been exposed. One approach to quantify these acetaldehyde‐addition products is to hydrolyze them to release acetaldehyde in the presence of phloroglucinol, which forms the ethylene‐bridged phloroglucinol dimer as a single substance that can be quantified [62]. The presence of condensation products increases as a wine ages, as might be expected from the generation of acetaldehyde by oxidation during aging [63]. However, the fraction of all bridging that could be detected with this method was small, at around 4–5%, suggesting that other substances such as glyoxal or pyruvate may be involved as well. Similar such reactions are expected to lead to higher molecular weight tannins in the wine and a study by Poncet‐Legrand using X‐ray scattering shows some confirmatory data [64]. The formation of these bridged species can be interrupted by the capture of the intermediate carbocation species by other nucleophiles, and this has been documented for GSH using NMR spectroscopy [26].
Studies describing the reaction of carbonyls with thiols are limited. An early study of acetaldehyde and a number of primary thiols observed equilibrium constants ranging from 20 to 36 M−1. The pH had little effect on the equilibria but did affect product formation rate, which were generally not very fast [65]. One study of the effect of GSH on acetaldehyde initiated reactions in wine observed little GSH inhibition of reactions of acetaldehyde with other wine components, and reported a relatively weak equilibrium binding constant of 20 M−1 [26]. These results suggest that thiols would not be capable of binding acetaldehyde adequately to diminish aroma or reactivity of either substrate, but further work could clarify the situation.
References
- 1. Rixford, E. (2008) The wine press and the cellar, Robert Mondavi Institute, Davis, CA.
- 2. Amerine, M.A., Berg, H.W., Cruess, W. (1972) The technology of wine making, 3rd edn, AVI, Westport, CT.
- 3. Singleton, V.L. (2001) A survey of wine aging reactions, especially with oxygen, in Proceedings of the ASEV 50th Anniversary Annual Meeting (ed. Rantz, J.M.), American Society for Enology and Viticulture, Seattle, WA, pp. 323–336.
- 4. Cheynier, V. (2002) Oxygen in wine and its role in phenolic reactions during aging, in Uses of gases in winemaking (eds Allen, M., Bell, S., Rowe, N., Wall, G.), Australian Society of Viticulture and Enology, Adelaide, SA, Australia, pp. 23–27.
- 5. Danilewicz, J.C. (2003) Review of reaction mechanisms of oxygen and proposed intermediate reduction products in wine: central role of iron and copper. American Journal of Enology and Viticulture, 54 (2), 73–85.
- 6. du Toit, W.J., Marais, J., Pretorius, I.S., du Toit, M. (2006) Oxygen in must and wine. South African Journal of Enology and Viticulture, 27 (1), 76–94.
- 7. Waterhouse, A.L. and Laurie, V.F. (2006) Oxidation of wine phenolics: a critical evaluation and hypotheses. American Journal of Enology and Viticulture, 57 (3), 306–313.
- 8. Li, H., Guo, A., Wang, H. (2008) Mechanisms of oxidative browning of wine. Food Chemistry, 108 (1), 1–13.
- 9. Karbowiak, T., Gougeon, R.D., Alinc, J.B., et al. (2010) Wine oxidation and the role of cork. Critical Reviews in Food Science and Nutrition, 50 (1), 20–52.
- 10. Oliveira, C.M., Ferreira, A.C.S., De Freitas, V., Silva, A.M.S. (2011) Oxidation mechanisms occurring in wines. Food Research International, 44 (5), 1115–1126.
- 11. Danilewicz, J.C. (2012) Review of oxidative processes in wine and value of reduction potentials in enology. American Journal of Enology and Viticulture, 63 (1), 1–10.
- 12. Ugliano, M. (2013) Oxygen contribution to wine aroma evolution during bottle aging. Journal of Agricultural and Food Chemistry, 61 (26), 6125–6136.
- 13. Pasteur, M.L. (1875) Etudes sur le vin, 2nd edn, Librairie F. Savy, Paris.
- 14. Kilmartin, P.A. (2010) Understanding and controlling non‐enzymatic wine oxidation, in Managing wine quality oenology and wine quality (ed. Reynolds, A.G.), Woodhead Publishing, Oxford, pp. 432–458.
- 15. Danilewicz, J.C. (2014) Role of tartaric and malic acids in wine oxidation. Journal of Agricultural and Food Chemistry, 62 (22), 5149–5155.
- 16. Rossi, J.A.J. and Singleton, V.L. (1966) Contributions of grape phenols to oxygen absorption and browning of wines. American Journal of Enology and Viticulture, 17 (4), 231–239.
- 17. Wildenradt, H.L. and Singleton, V.L. (1974) The production of aldehydes as a result of oxidation of polyphenolic compounds and its relation to wine aging. American Journal of Enology and Viticulture, 25 (2), 119–126.
- 18. Elias, R.J., Andersen, M.L., Skibsted, L.H., Waterhouse, A.L. (2009) Identification of free radical intermediates in oxidized wine using electron paramagnetic resonance spin trapping. Journal of Agricultural and Food Chemistry, 57 (10), 4359–4365.
- 19. Danilewicz, J.C. (2013) Reactions involving iron in mediating catechol oxidation in model wine. American Journal of Enology and Viticulture, 64 (3), 316–324.
- 20. Danilewicz, J.C. (2007) Interaction of sulfur dioxide, polyphenols, and oxygen in a wine‐model system: central role of iron and copper. American Journal of Enology and Viticulture, 58 (1), 53–60.
- 21. Kreitman, G.Y., Cantu, A., Waterhouse, A.L., Elias, R.J. (2013) Effect of metal chelators on the oxidative stability of model wine. Journal of Agricultural and Food Chemistry, 61 (39), 9480–9487.
- 22. Elias, R.J. and Waterhouse, A.L. (2010) Controlling the Fenton reaction in wine. Journal of Agricultural and Food Chemistry, 58 (3), 1699–1707.
- 23. Danilewicz, J.C., Seccombe, J.T., Whelan, J. (2008) Mechanism of interaction of polyphenols, oxygen, and sulfur dioxide in model wine and wine. American Journal of Enology and Viticulture, 59 (2), 128–136.
- 24. Singleton, V.L., Zaya, J., Trousdale, E., Salgues, M. (1984) Caftaric acid in grapes and conversion to a reaction product during processing. Vitis, 23, 113–120.
- 25. Singleton, V.L., Salgues, M., Zaya, J., Trousdale, E. (1985) Caftaric acid disappearance and conversion to products of enzymic oxidation in grape must and wine. American Journal of Enology and Viticulture, 36 (1), 50–56.
- 26. Sonni, F., Moore, E.G., Clark, A.C., et al. (2011) Impact of glutathione on the formation of methylmethine‐ and carboxymethine‐bridged (+)‐catechin dimers in a model wine system. Journal of Agricultural and Food Chemistry, 59 (13), 7410–7418.
- 27. Nikolantonaki, M., Chichuc, I., Teissedre, P.L., Darriet, P. (2010) Reactivity of volatile thiols with polyphenols in a wine‐model medium: impact of oxygen, iron, and sulfur dioxide. Analytica Chimica Acta, 660 (1–2), 102–109.
- 28. Lee, C.Y. and Whitaker, J.R. (1995) Enzymatic browning and its prevention, ACS Symposium Series, Vol. 600, American Chemical Society, Washington, DC.
- 29. Cheynier, V., Basire, N., Rigaud, J. (1989) Mechanism of trans‐caffeoyltartaric acid and catechin oxidation in model solutions containing grape polyphenoloxidase. Journal of Agricultural and Food Chemistry, 37 (4), 1069–1071.
- 30. Schneider, V. (1998) Must hyperoxidation: a review. American Journal of Enology and Viticulture, 49 (1), 65–73.
- 31. Cheynier, V., Rigaud, J., Souquet, J.M., et al. (1990) Must browning in relation to the behavior of phenolic compounds during oxidation. American Journal of Enology and Viticulture, 41 (4), 346–349.
- 32. Fulcrand, H., Cheminat, A., Brouillard, R., Cheynier, V. (1994) Characterization of compounds obtained by chemical oxidation of caffeic acid in acidic conditions. Phytochemistry, 35 (2), 499–505.
- 33. Simpson, R.F. (1982) Factors affecting oxidative browning of white wine. Vitis, 21 (3), 233–239.
- 34. Cheynier, V., Rigaud, J., Souquet, J.M., et al. (1989) Effect of pomace contact and hyperoxidation on the phenolic composition and quality of Grenache and Chardonnay wines. American Journal of Enology and Viticulture, 40 (1), 36–42.
- 35. Fernandez‐Zurbano, P., Ferreira, V., Escudero, A., Cacho, J. (1998) Role of hydroxycinnamic acids and flavanols in the oxidation and browning of white wines. Journal of Agricultural and Food Chemistry, 46 (12), 4937–4944.
- 36. Rigaud, J., Cheynier, V., Souquet, J.M., Moutounet, M. (1991) Influence of must composition on phenolic oxidation‐kinetics. Journal of the Science of Food and Agriculture, 57 (1), 55–63.
- 37. Guyot, S., Vercauteren, J., Cheynier, V. (1996) Structural determination of colourless and yellow dimers resulting from (+)‐catechin coupling catalysed by grape polyphenoloxidase. Phytochemistry, 42 (5), 1279–1288.
- 38. Jimenez‐Atienzar, M., Cabanes, J., Gandia‐Herrero, F., Garcia‐Carmona, F. (2004) Kinetic analysis of catechin oxidation by polyphenol oxidase at neutral pH. Biochemical and Biophysical Research Communications, 319 (3), 902–910.
- 39. Mouls, L. and Fulcrand, H. (2012) UPLC‐ESI‐MS study of the oxidation markers released from tannin depolymerization: toward a better characterization of the tannin evolution over food and beverage processing. Journal of Mass Spectrometry, 47 (11), 1450–1457.
- 40. Es‐Safi, N.E., Le Guerneve, C., Cheynier, V., Moutounet, M. (2000) New phenolic compounds formed by evolution of (+)‐catechin and glyoxylic acid in hydroalcoholic solution and their implication in color changes of grape‐derived foods. Journal of Agricultural and Food Chemistry, 48 (9), 4233–4240.
- 41. Embs, R.J. and Markakis, P. (1965) Mechanism of sulfite inhibition of browning caused by polyphenol oxidase. Journal of Food Science, 30 (5), 753.
- 42. Luvalle, J.E. (1952) The reaction of quinone and sulfite. 1. Intermediates. Journal of the American Chemical Society, 74 (12), 2970–2977.
- 43. Nikolantonaki, M. and Waterhouse, A.L. (2012) A method to quantify quinone reaction rates with wine relevant nucleophiles: a key to the understanding of oxidative loss of varietal thiols. Journal of Agricultural and Food Chemistry, 60 (34), 8484–8491.
- 44. Barril, C., Clark, A.C., Scollary, G.R. (2012) Chemistry of ascorbic acid and sulfur dioxide as an antioxidant system relevant to white wine. Analytica Chimica Acta, 732, 186–193.
- 45. Cheynier, V., Souquet, J.M., Moutounet, M. (1989) Glutathione content and glutathione to hydroxycinnamic acid ratio in Vitis vinifera grapes and musts. American Journal of Enology and Viticulture, 40 (4), 320–324.
- 46. Bell, S.J. and Henschke, P.A. (2005) Implications of nitrogen nutrition for grapes, fermentation and wine. Australian Journal of Grape and Wine Research, 11 (3), 242–295.
- 47. McArdle, J.V. and Hoffmann, M.R. (1983) Kinetics and mechanism of the oxidation of aquated sulfur‐dioxide by hydrogen‐peroxide at low pH. Journal of Physical Chemistry, 87 (26), 5425–5429.
- 48. Deutsch, J.C. (1998) Ascorbic acid oxidation by hydrogen peroxide. Analytical Biochemistry, 255 (1), 1–7.
- 49. Pirie, N.W. (1931) The oxidation of sulphydryl compounds by hydrogen peroxide. I. Catalysis of oxidation of cysteine and glutathione by iron and copper. Biochemical Journal, 25 (5), 1565–1579.
- 50. Kaur, H., Halliwell, B., Lester, P. (1994) Detection of hydroxyl radicals by aromatic hydroxylation, in Methods in enzymology, Academic Press, pp. 67–82.
- 51. Gislason, N.E., Currie, B.L., Waterhouse, A.L. (2011) Novel antioxidant reactions of cinnamates in wine. Journal of Agricultural and Food Chemistry, 59 (11), 6221–6226.
- 52. Fulcrand, H., Cheynier, V., Oszmianski, J., Moutounet, M. (1997) An oxidized tartaric acid residue as a new bridge potentially competing with acetaldehyde in flavan‐3‐ol condensation. Phytochemistry, 46 (2), 223–227.
- 53. Fulcrand, H., Benabdeljalil, C., Rigaud, J., et al. (1998) A new class of wine pigments generated by reaction between pyruvic acid and grape anthocyanins. Phytochemistry, 47 (7), 1401–1407.
- 54. Laurie, V.F. and Waterhouse, A.L. (2006) Glycerol oxidation in wine and reactions with flavonoids. American Journal of Enology and Viticulture, 57 (3), 394A–395A.
- 55. Ferreira, A.C.S., Hogg, T., de Pinho, P.G. (2003) Identification of key odorants related to the typical aroma of oxidation‐spoiled white wines. Journal of Agricultural and Food Chemistry, 51 (5), 1377–1381.
- 56. Cutzach, I., Chatonnet, P., Henry, R., et al. (1998) Study in aroma of sweet natural non Muscat wines: 2nd Part. Quantitative analysis of volatile compounds taking part in aroma of sweet natural wines during ageing. Journal International des Sciences de la Vigne et du Vin, 32 (4), 211–221.
- 57. Pons, A., Lavigne, V., Darriet, P., Dubourdieu, D. (2013) Role of 3‐methyl‐2,4‐nonanedione in the flavor of aged red wines. Journal of Agricultural and Food Chemistry, 61 (30), 7373–7380.
- 58. Cullere, L., Cacho, J., Ferreira, V. (2007) An assessment of the role played by some oxidation‐related aldehydes in wine aroma. Journal of Agricultural and Food Chemistry, 55 (3), 876–881.
- 59. Tamura, T., Taniguchi, K., Suzuki, Y., et al. (2009) Iron is an essential cause of fishy aftertaste formation in wine and seafood pairing. Journal of Agricultural and Food Chemistry, 57 (18), 8550–8556.
- 60. Ferreira, A.C.D., Barbe, J.C., Bertrand, A. (2002) Heterocyclic acetals from glycerol and acetaldehyde in port wines: evolution with aging. Journal of Agricultural and Food Chemistry, 50 (9), 2560–2564.
- 61. Es‐Safi, N.E., Cheynier, V., Moutounet, M. (2003) Effect of copper on oxidation of (+)‐catechin in a model solution system. International Journal of Food Science and Technology, 38 (2), 153–163.
- 62. Drinkine, J., Lopes, P., Kennedy, J.A., et al. (2007) Analysis of ethylidene‐bridged flavan‐3‐ols in wine. Journal of Agricultural and Food Chemistry, 55 (4), 1109–1116.
- 63. Drinkine, J., Lopes, P., Kennedy, J.A., et al. (2007) Ethylidene‐bridged flavan‐3‐ols in red wine and correlation with wine age. Journal of Agricultural and Food Chemistry, 55 (15), 6292–6299.
- 64. Poncet‐Legrand, C., Cabane, B., Bautista‐Ortin, A.B., et al. (2010) Tannin oxidation: intra‐ versus intermolecular reactions. Biomacromolecules, 11 (9), 2376–2386.
- 65. Lienhard, G.E. and Jencks, W.P. (1966) Thiol addition to carbonyl group. Equilibria and kinetics. Journal of the American Chemical Society, 88 (17), 3982–3995.