11
Introduction to Phenolics
11.1 Introduction
The grape berry is the source of the vast majority of the many phenolic compounds found in wine, although oak or other woods used in the production or aging of the wine may be a minor source. Because of their importance to wine stability and organoleptic properties, reviews of grape and wine phenolics are common in the literature [1–7]. Most phenolic compounds are non‐volatile, but a few volatile and odorous phenols exist, such as 4‐ethylphenol, and are addressed separately in Chapter 12. As phenolics are ubiquitous in the plant world, all plant foods contain at least small quantities. Aside from grapes and wine, other major dietary sources are coffee, tea, chocolate, fruits, and, in lesser quantities, vegetables. Distilled spirits will lack phenolics unless there has been an infusion process after distillation. For instance, whiskeys contain some phenolics from the barrel used in aging.
Like aliphatic alcohols and water, phenols partake in hydrogen bonding and are weak acids. However, due to the inductive electron withdrawing effect of the benzene ring, a phenolic proton is more acidic, with a pKa of about 10 (cf. ethanol pKa = 15.9), but at wine pH 3–4, the ionization of phenolics is too small to be a factor in reactions. Oxidation of phenols to quinones is facile for ortho‐diphenols, and this important chemistry is detailed elsewhere (Chapter 24). The aromatic ring of phenols is susceptible to electrophilic aromatic substitution (Chapter 10 for overview of wine electrophiles and nucleophiles). For example, electrophiles such as chlorine (Figure 11.1) can add to the ring to yield halophenols. Subsequent microbial activity can methylate the phenolic groups to form haloanisoles, for example, 2,4,6‐trichloroanisole, the well‐known wine taint (Chapter 18). The electron‐rich phloroglucinol moiety in the flavanols is quite reactive with electrophiles, as detailed in Chapter 14.

Figure 11.1 Electrophilic aromatic substitution reaction of phenol with sources of halogens such as chlorine contained in bleach used for cleaning
The naming system is rather complex, so a few definitions are in order.
- The terms phenols and phenolics encompass all compounds with hydroxyl groups attached to aromatic rings. The simplest phenols are those substances that have a single aromatic ring with one or more hydroxyl groups, and examples include guaiacol and caffeic acid.
- Polyphenols (or polyphenolics) are a class of compounds with multiple phenol rings within a single structure. This would include most of the phenolic substances in wine; epicatechin and resveratrol are two such compounds. Flavonoids are polyphenols with a very specific C6–C3–C6 3‐ring structure elaborated below (Section 11.3).
- Tannin is widely used (including in this text) to describe all polymeric polyphenols. However, from a strict chemical perspective, the term tannin does not define a chemical structure but instead declares a specific function for a substance – the ability to tan animal hide (through crosslinking) into leather. These are generally high molecular weight (molecular weight > 1200 daltons) polyphenolic compounds traditionally obtained from plants. These extracts typically contain a complex combination of flavan‐3‐ol oligomeric and polymeric polyphenols, correctly described as condensed tannin, found in grapes, or hydrolyzable tannin, found in oak. Condensed tannins are also called proanthocyanidins, since they will produce anthocyanidins following acid hydrolysis. The hydrolyzable tannins (e.g., tannic acid) are oligomeric forms of gallic acid and can be specified as gallotannins or ellagitannins depending on whether they are constituted of gallic or ellagic acid moieties. These oligomers are not found in V. vinifera, but are in oak wood and V. rotundifolia (muscadine grapes). The root names for many phenolic moieties encountered in grapes and wine phenolics are listed in Table 11.1.
Table 11.1 The simple phenolic structural motifs found in wine phenolic compounds. Their nomenclature provides guidance to understanding more complex names
| Structural moiety | Name | Notes |

|
Phenol | Root name for phenolics; functional group found only in a few classes of phenolics in grapes |

|
Catechol or pyrocatechol | Catechol functional group – common; susceptibility to oxidation makes this group very important (Chapter 24) |
|
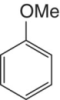
Anisole | Not generally found in wine‐related phenolics |
|
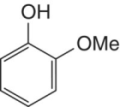
Guaiacol | Common in most classes of phenolics found in grapes and wine |
|
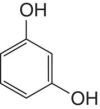
Resorcinol | The term res is commonly applied to compounds that include this functional group, such as resveratrol |

|
Hydroquinone | Not found in grapes and wine |

|
Pyrogallol | Common in grape and wine phenolics; easily oxidized like catechol |
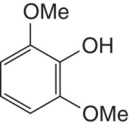
|
Syringol | Found on the B‐ring of anthocyanins, in malvidin |
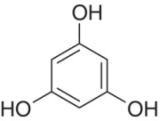
|
Phloroglucinol | Present in the A‐ring of flavonoids; strong nucleophilic character due to π‐donation by oxygens |
The more detailed descriptions below and in following chapters are based on the compounds and levels found in grapes and wine from V. vinfera, the European wine grape, which dominates global wine production. The factors that alter phenolic content include [8–10]:
- Grape variety (genetics)
- Site, particularly climate, soil, aspect, sun exposure, elevation (environment)
- Vine, cluster, berry (within and between plants on the same site, there is high variability).
Grape phenolics are largely found in the skin and seed of the berry, while the juice and pulp have much lower concentrations. Red grapes have higher total phenolic concentrations than white grapes, due to the presence of the red anthocyanins in the skin (Figure 11.2). Typically, white wines are made by quickly pressing the juice away from the grape solids, so have lower concentrations of phenolics than reds, which are made by fermenting the juice in the presence of the grape skins and seeds (Chapter 21). Due to the sensory impact of phenolics, winemakers attempt to control the amount and relative extraction of different phenolic classes during winemaking. Control is exercised by manipulation of extraction, called maceration (Chapter 21), as well as additions of protein and other agents to bind and precipitate tannins, a process called fining (Chapter 26.2). Overall, wine phenolics are grouped into two categories, the flavonoids and non‐flavonoids.

Figure 11.2 Tissue sources of grape phenolics. Source: Coombe 1987 [11]. Reproduced with permission of American Journal of Enology and Viticulture
Many grape phenolics have their structures altered by fermentation reactions and subsequent chemical reactions during storage, for example, glycoside hydrolysis, acid‐catalyzed rearrangements, oxidation reactions, and reactions with oxidation products. All phenolic compounds are susceptible to oxidation reactions, and in the presence of iron react with oxygen to produce quinones and hydrogen peroxide, both of which continue the oxidation process (Chapter 24). The reactivity of phenolics towards oxidation products contributes to their complex role as both pro‐oxidant and antioxidant – their presence accelerates the rate of oxygen consumption and formation of some oxidation products, but can concurrently decrease the occurrence of some (often undesirable) oxidative changes (Chapter 24).
The total phenolic content of wines varies widely, but is generally about 200 mg/L of gallic acid equivalents (GAEs) in white wines and 2000 mg/L in reds that are ready to drink. Reds destined for long aging will have as much as 3500 mg/L or more, but at the high end of the range, the wines are very astringent until aging reduces the perceived tannin (Chapter 33). While analyses can be done chromatographically, the analysis of total phenolics is typically conducted by the Folin–Ciocalteu method or by UV absorbance at 280 nm [12, 13].
- Measurement of absorbance at 280 nm (Abs 280) is very quick and is blind to sugar, and is thus useful during fermentations or for routine authenticity checks. Quantitative comparison of Abs 280 values across wines is challenging because there is considerable variation in molar absorbance (extinction coefficients) across phenolic compounds.
- The Folin method gives quantitative results that better reflect true phenolic concentrations and are more useful for cross‐wine comparisons. However, sugar is an interference.
Both methods have problems with interferences for wines with low phenolic concentrations, which includes many white wines.
11.2 Non‐flavonoids
Hydroxycinnamates are the major phenols in grape juice and the major class of phenolics in white wine; similar levels are found in red and white wine (Chapter 13). These components – particularly caffeic acid – are also the first to be enzymatically oxidized during crushing and subsequently initiate browning in must (Chapters 13 and 24). There are three common hydroxycinnamates in grapes and wine, those based on coumaric acid (4‐hydroxycinnamic acid, derived from phenol), caffeic acid (catechol substitution in place of phenol), and ferulic acid (guaiacol substitution).
Benzoic acids are a minor component in most newly fermented wines. The major benzoic acid – gallic acid – can appear during storage via extraction from oak or by hydrolysis of oak hydrolyzable tannins or grape proanthocyanidins during aging.
Stilbenes include the most famous individual wine phenolic compound, resveratrol. These are produced in response to fungal infection and other stresses and are found throughout the grapevine, as well as in the berry skin. Resveratrol is produced as a glucoside and the aglycone appears to oligomerize to the active antifungal viniferins.
11.3 Flavonoids
Flavonoids contain multiple aromatic rings possessing hydroxyl groups and are thus polyphenols. Flavonoids are characterized by a three‐ring system with a central oxygen‐containing ring (C ring) that has different oxidation states defining the flavonoid class. It is fused to an aromatic ring (A ring) along one bond and attached to another aromatic ring with a single bond (B ring), as seen in Figure 11.3. The flavonoids found in grapes and wine all have the same hydroxyl substitution groups on ring A, at positions 5 and 7. Differences in the oxidation state and substitution on ring C define the different classes of flavonoids.
- Flavan‐3‐ols are the most abundant class of flavonoids. The term “flavan” indicates a saturated C ring, and the “‐3‐ol” suffix indicates an –OH group at the C3 position. They include simple monomeric catechins, but most exist in the oligomeric and polymeric proanthocyanidin forms from the skins and seeds. These larger compounds constitute about half the phenolics in red wine but negligible concentrations in white wines (Chapter 14).
- Flavonols possess a keto group at position C4 and unsaturation between C2 and C3 (a “flavone”) as well as an –OH group at C3. Flavonols are found in the berry skin, where they appear to function as sun screen, and are increased by high sunlight exposure of pre‐veraison fruit (Chapter 15).
- Anthocyanins are red‐colored polyphenols characterized by a fully aromatic, positively charged ring. While their direct effect on taste is minimal, better quality red wines often have higher levels of anthocyanins, and their reactions with the condensed tannins create stable red wine pigments (Chapter 16).

Figure 11.3 Core flavonoid ring system with a C6–C3–C6 structure
The substitution pattern on ring B defines the member of the class. Normal substitution patterns are a hydroxyl at the 4ʹ position with additional oxygen substitution at 3ʹ and/or 5ʹ, and those oxygens can be hydroxyls or methoxyls. Thus the number of class members is relatively short; however, the “free” flavonoid structure can also be substituted further (usually with sugar conjugation on the oxygens), which gives rise to many additional compounds.
The flavonoids are derived from extraction of grape skins and seeds during fermentation and other maceration steps, and comprise the majority of the phenols in red wine (Chapter 21). Ethanol is a good solvent for polyphenol extraction, and over a typical 4–10 day fermentation a good portion of polyphenols are extracted into red wine. As white wine is derived solely from juice, flavonoid levels are very low, less than 5% that of red wine. In general, about half the polyphenols from the skins and seeds are extracted into red wine during the maceration process, leaving the remainder behind in the pomace.
References
- 1. Singleton, V.L. and Esau, P. (1969) Phenolic substances in grapes and wine, and their significance, Academic, New York.
- 2. Singleton, V.L. (1982) Grape and wine phenolics; background and prospects, in Proceedings of the Symposium of the University of Calififornia, Davis, Grape and Wine Centennial, 1980, Department of Viticulture and Enology, University of California, Davis, CA, pp. 215–227.
- 3. Somers, T.C. and Vérette, E. (1988) Phenolic composition of natural wine types, in Modern methods of plant analysis, wine analysis (eds Liskens, H.G. and Jackson, J.F.), Springer‐Verlag, Berlin, pp. 219.
- 4. Ribereau‐Gayon, P. and Glories, Y. (1991) Phenolics in grapes and wines, in Proceedings of the Sixth Australian Wine Industry Technical Conference, pp. 247–256.
- 5. Fulcrand, H., Duenas, M., Salas, E., Cheynier, V. (2006) Phenolic reactions during winemaking and aging. American Journal of Enology and Viticulture, 57 (3), 289–297.
- 6. Kennedy, J.A. (2008) Grape and wine phenolics: observations and recent findings. Ciencia e Investigacion Agraria, 35 (2), 107–120.
- 7. Casassa, L.F. and Harbertson, J.F. (2014) Extraction, evolution, and sensory impact of phenolic compounds during red wine maceration. Annual Review of Food Science and Technology, 5 (1), 83–109.
- 8. Reynolds, A.G. (2010) Viticultural and vineyard management practices and their effects on grape and wine quality, in Managing wine quality, Vol. 1, Viticulture and wine quality (ed. Reynolds, A.G.), Woodhead Publishing and CRC Press, Oxford and Boca Raton, FL.
- 9. Young, P.R. and Vivier, M.A. (2010) Genetics and genomic approaches to improve wine quality, in Managing wine quality, Vol. 1, Viticulture and wine quality (ed. Reynolds, A.G.), Woodhead Publishing and CRC Press, Oxford and Boca Raton, FL.
- 10. Reynolds, A.G. and Heuvel, J.E.V. (2009) Influence of grapevine training systems on vine growth and fruit composition: a review. American Journal of Enology and Viticulture, 60 (3), 251–268.
- 11. Coombe, B.G. (1987) Distribution of solutes within the developing grape berry in relation to its morphology. American Journal of Enology and Viticulture, 38 (2), 120–127.
- 12. Zoecklein, B.W., Fugelsang, K.C., Gump, B.H., Nury, F.S. (1999) Wine analysis and production, Springer, New York.
- 13. Harbertson, J.F. and Spayd, S. (2006) Measuring phenolics in the winery. American Journal of Enology and Viticulture, 57 (3), 280–288.