21
Maceration and Extraction of Grape Components
21.1 Introduction
Winemaking results in the incomplete and variable extraction of components from the skin, pulp and seed – thus, even when fermentation and its resultant production of ethanol and other metabolites are ignored, wine composition differs from initial grape composition. Winemakers refer to the extraction process as maceration, a description that includes the physical manipulation of the must as well as the chemical extraction that results from it. Maceration may also be referred to as “skin contact,” especially when discussing short‐term, pre‐fermentation maceration often used for aromatic varieties (Chapter 23.1). For our purposes, we will focus on the extraction products that are unmodified by microbial metabolism, although discussion of some transformed products is unavoidable. In addition, while this section deals with the impact of the extraction process on wine composition, in many cases initial grape composition will have a greater effect than altering maceration practices, for example, for explaining differences in varietal aroma compounds between cultivars.
There are a number of variables that have an important impact on the extraction process, with time and temperature having the largest influences [1]. Beyond these basic parameters, extraction of components from the grape solids can be enhanced by a range of chemical and physical processes, such as enzymatic treatments, mixing, pressing, heating, freezing, high‐pressure pulses, and application of microwave or even pulsed electric field (PEF) treatments. These processes are rarely selective and will result in increased extraction of a range of compounds. The following pages focus on the major constituents of grapes that largely remain unchanged by a simple extraction step, although the maceration/extraction process can also increase the yield of flavor precursors that are transformed later in winemaking (Chapter 23).
The principal components studied in maceration investigations are the phenolics, especially condensed tannins and anthocyanins. As seen in Figure 21.1, an extended maceration process following fermentation will increase condensed tannin, but decrease the anthocyanin levels, and by doing so the total phenolics increase or stay level. Polysaccharides – an often overlooked but important factor – also increase with increasing maceration time. The extraction of other substances will also be dependent on maceration conditions, and many of these cases are mentioned below where data are available.
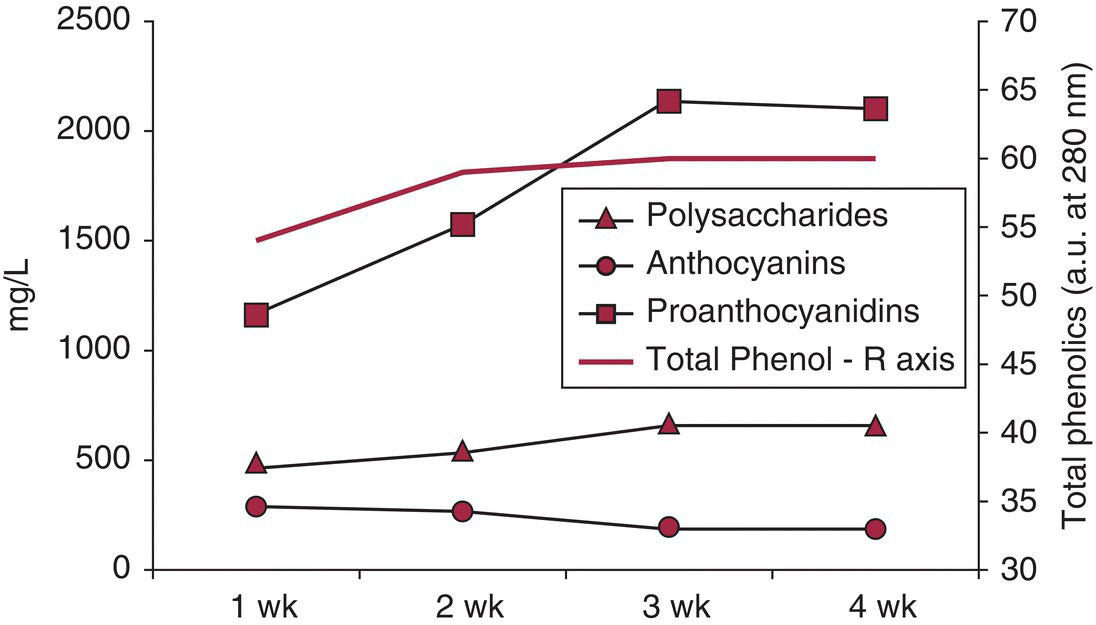
Figure 21.1 Extraction of key components during extended maceration.
Data from Gil et al., 2012 [2]
21.1.1 General features of extraction during maceration
Some general principles should be recognized in interpreting extraction data from winemaking. It is possible to derive a fractional extraction coefficient (F) for compounds during maceration, where F = mwine∕mgrape, or the ratio of the amount of a compound in the wine to the initial amount in the grape. If mwine is measured at multiple time points during maceration it is possible to derive an extraction rate function, and to model these by appropriate equations (e.g., Fick’s Law of diffusion). Fitting the exponential model to the data can reveal an extraction constant and equilibrium tannin concentration (Figure 21.2) [3].
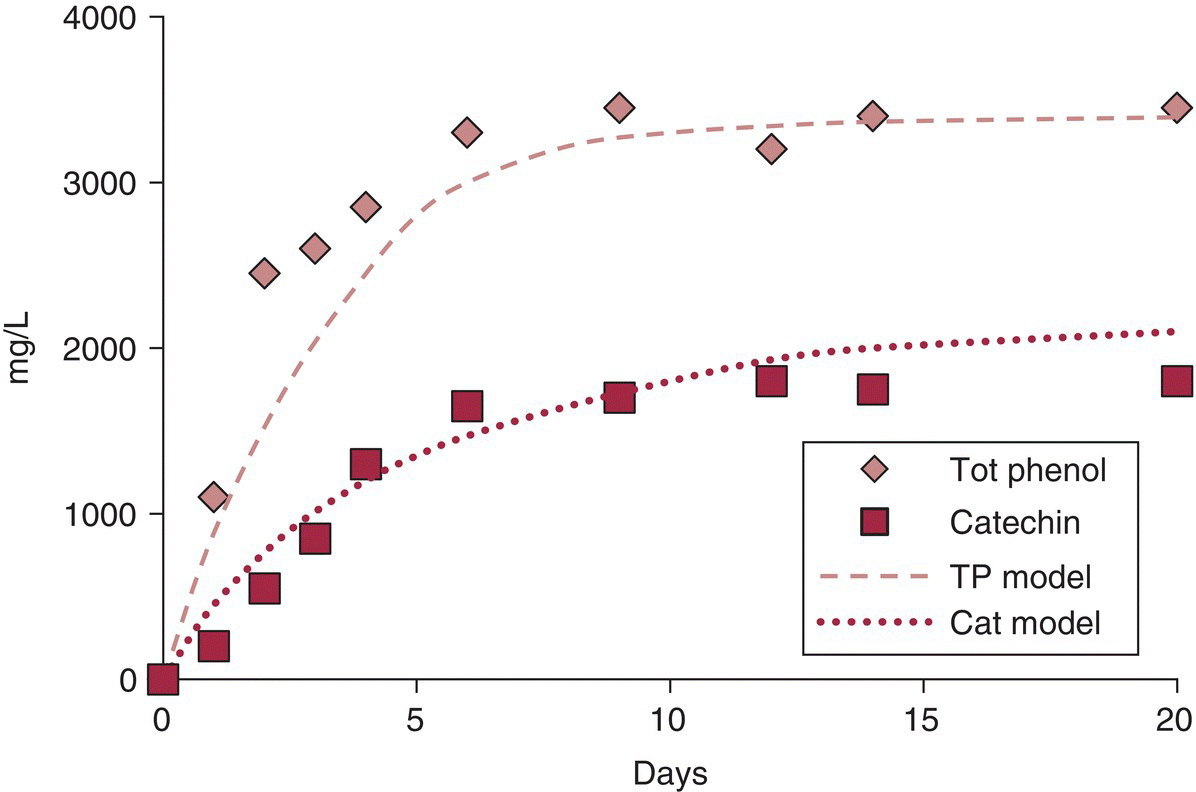
Figure 21.2 Measured extraction of total phenolics and catechin versus modeling.
Data from Andrich et al., 2005 [3]
As described later, the extraction coefficient generally increases with increasing maceration time, temperature, and other parameters. However, assuming identical maceration conditions, the extraction rate and extraction coefficient for a given compound will depend largely on three factors:
- Location. Typically, compounds in the pulp are extracted immediately after crushing, while compounds from the skins will reach a maximum after several days, and compounds from the seeds may take weeks.
- Polarity. Hydroalcoholic solutions like wine are relatively polar solvents and readily dissolve polar substances such as sugars, acids, phenolics, alcohols, and so on. Non‐polar substances can dissolve in wine, but will not be extracted as quickly, will have low limits of solubility, and the amount dissolved is generally much less than that limit. For some compounds, extraction will be minimal until sufficient ethanol is produced to solubilize the compound.
- Molecular size.
The apparent extraction coefficient will also be modified by these factors:
- Re‐adsorption on to skin and other tissues.
- Reactive losses, for example, hydrolysis of bound forms, that is, glycosides, or reactions with other wine components such as acetaldehyde.
21.1.2 Extraction of phenolics
The grape components for which maceration is of greatest interest are the phenolic compounds (Chapter 11), and a number of reviews have addressed this topic [4]. In the berry, most of the phenolics are in the grape solids, skins, and seeds, and an extractive step is essential to their incorporation into wine. Of those, condensed tannins and pigments receive the most attention because they are recognized as essential factors in red wine quality (Chapter 11), and are typically the targets when altering maceration regimes (Figure 21.1). Extraction of flavonols from skin is generally ignored, but some studies show the effects of particular maceration techniques. On the other hand, ~50% of hydroxycinnamates are found in juice (Chapter 13) and thus are rarely measured in maceration studies.
Condensed tannins are part of the flavan‐3‐ol family (Chapter 14), all of which are extracted together, so increases in monomeric catechins and oligomeric proanthocyanidins are coincident with any process that enhances tannin extraction [5]. While seed tannin extraction can extend over weeks, the extraction of tannin from the skin is very rapid, typically reaching a maximum within a few days [3]. Quantifying the extraction of phenolics (as well as other grape components) as a result of maceration is complicated by reactions that occur subsequent to extraction; for instance, the increase in anthocyanins during winemaking is less than the amount extracted from the solids due to the wide range of reactions in which these compounds can participate (Chapters 16 and 25). However, it is possible to compare the amount of a particular substance in wine to the amount in the grapes and derive a fractional extraction coefficient. Singleton first reported the grape phenolic content per mass of tissue at about 40 mg/kg (fresh berry weight) in pulp, 1800 mg/kg in red skin (and half as much for white), and 3500 mg/kg in seeds [6]. In typical red winemaking, 30–50% of these substances (2000–3500 mg/L of total phenols) end up in wine, assuming the mass of wine is 80% that of the grapes used [7].
While anthocyanins in grapes tend to be well correlated with anthocyanins in wine, assuming similar maceration conditions, grape tannin is often poorly correlated with wine tannin, especially across cultivars (Figure 21.3) [8]. Potential explanations for the variation in tannin extraction coefficients across grape varieties include:
- Grape tannin measurements do not always distinguish between seed and skin tannin, and the former is more slowly extracted than the latter.
- The “amount” of phenolic material present in the grape is generally determined by an aqueous acetone extraction (e.g., 70% v/v), but recent studies have shown some phenolics may be released via acid treatment [9], and the acidic environment of wine could promote their extraction.
- Grape cell wall material can re‐bind tannins after it is initially extracted into the fermenting must [10].
- Anthocyanins can complex with tannins (Chapter 16), increasing the latter’s extractability [11].
- The major soluble proteins in grapes can bind tannin and limit their extraction [12].
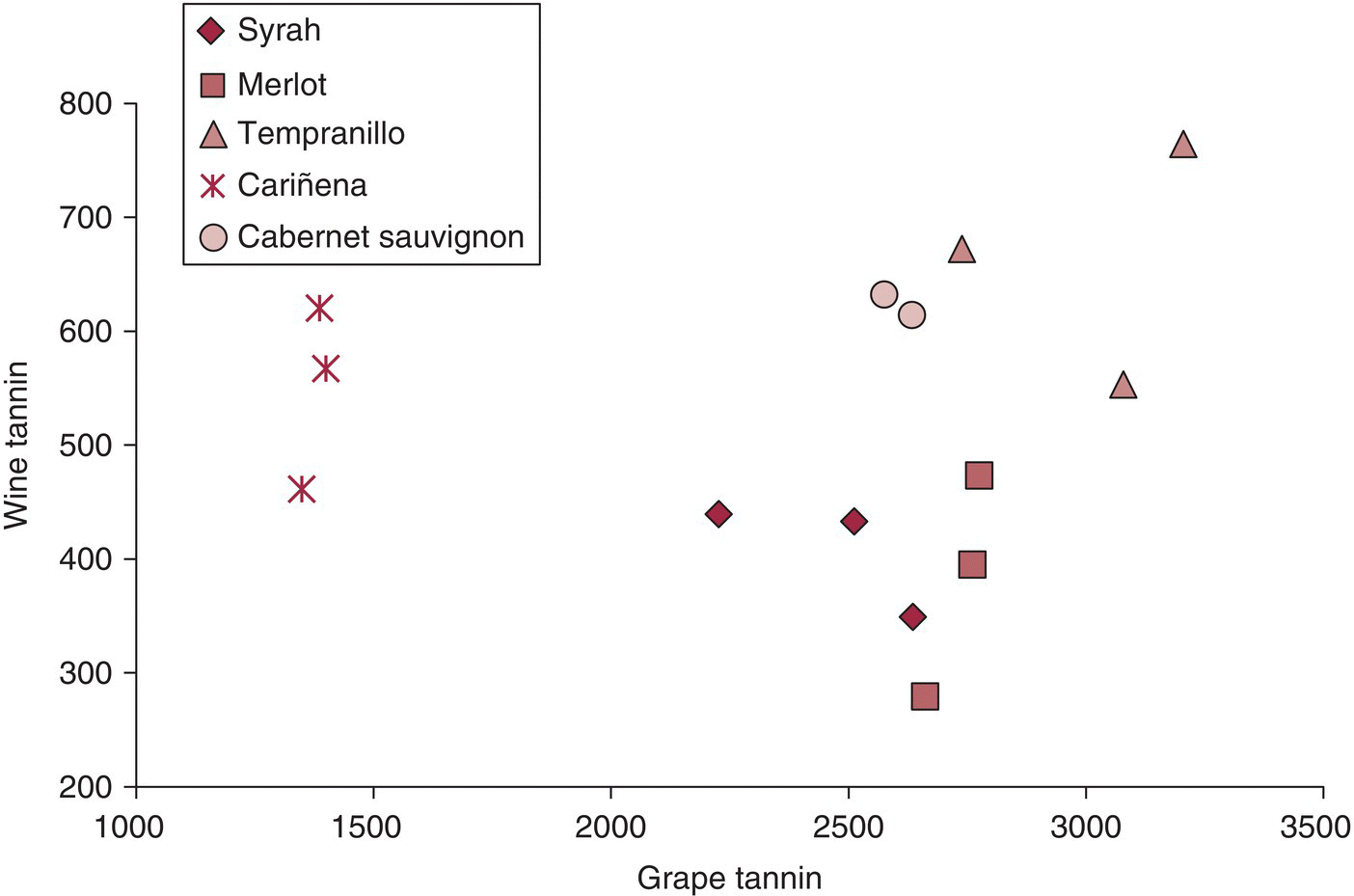
Figure 21.3 Data (mg/L and mg/kg) from Tables S2, S4 in Fragoso et al., 2011 [8].
Source: Casassa 2013 [8]. Reproduced with permission of John Wiley and Sons
In contrast to current winemaking practices, in the distant past white grapes were frequently vinified with substantial maceration of the skin throughout fermentation. The resulting “orange wines” would have better longevity imparted by a higher antioxidant phenolic content (important considering the poor oxygen exclusion of the containers used at the time). However, enhanced phenolics in white wines cuts both ways, enhancing oxidative browning potential, and scavenging volatile thiols via the formation of quinones1 (Chapter 24) [13]. Orange wines possess high astringency (more akin to red wines than white), but are rare in the current wine marketplace, and thus are unfamiliar to many white wine drinkers today.2
21.1.3 Extraction of polysaccharides
In comparison to phenolics, the effects of maceration on polysaccharides are often overlooked. Grape polysaccharides are derived from the cell walls of the berries, and appear to have minor effects on the physical sensation of wine in the mouth (i.e., mouthfeel) [14] as well as the volatility of some aroma compounds (Chapter 2). Polysaccharides, particularly pectin, hemicellulose, and cellulose, constitute the vast majority of grape cell wall structural compounds (Chapter 20), but the majority of these components are poorly recovered, with only a small portion of pectic substances (~0.1% w/w) extracted during fermentation. These are primarily rhamnogalacturonans (RGs) and arabinogalactan proteins (AGPs), whose effect and concentration in wine are described in Chapter 2. These two classes of polysaccharides share the properties of being both sufficiently soluble as well as being resistant to acid and enzymatic degradation during fermentation, and thus can persist into the finished wines. Similar to seed phenolics, extraction of both classes increases throughout maceration (Figure 21.1) [2]. Since most grape polysaccharides are retained in the pomace, the surface of the skins can act as an adsorbent and retain polyphenolics that would otherwise be solubilized into wine. Hydrolyzing the polysaccharides of the grape skin also increases the yield of juice from pressing, and alters juice clarity.
21.1.4 Extraction of odorants and their precursors
Most primary odorants in grapes (e.g. monoterpenoids, methyl anthranilate, rotundone, IBMP) are found predominantly in the skins and thus will increase with increasing maceration time, although maximal concentrations will usually be reached quickly (with 2–3 days) because of these compounds’ low molecular weight and fast diffusivity. Maceration will also increase concentrations of volatile compound precursors, for example, glycosides, hydroxycinnamic acids, S‐conjugates (Chapter 23). Similar to primary odorants, concentrations of their precursors will usually reach a maximum soon after fermentation commences.
21.1.5 Extraction of cations
Maceration will result in the exchange of metal cations for protons. In many literature reports, this results in an increase in both metal cations and pH (see Chapter 3). However, high potassium juices may show an initial decrease in potassium during skin contact, followed by stable levels. Testing at different pH levels suggested an ion‐exchange effect with pectins in the skins and higher ethanol suppressed potassium extraction [15].
21.2 Pre‐fermentative treatments
Many of the pre‐fermentation maceration techniques involve physical or chemical changes to the structure of the grape tissue that are designed to enhance release of desired phytochemicals or increase juice yield. The treatments often respond differently depending on grape source. For example, Gonzáles‐Neves et al. investigated the effects of enzymes and “cold soak” on multiple varieties (Merlot, Tannat, and Syrah) [16] – the large standard deviations indicate the variable response across the cultivars (Figure 21.4).
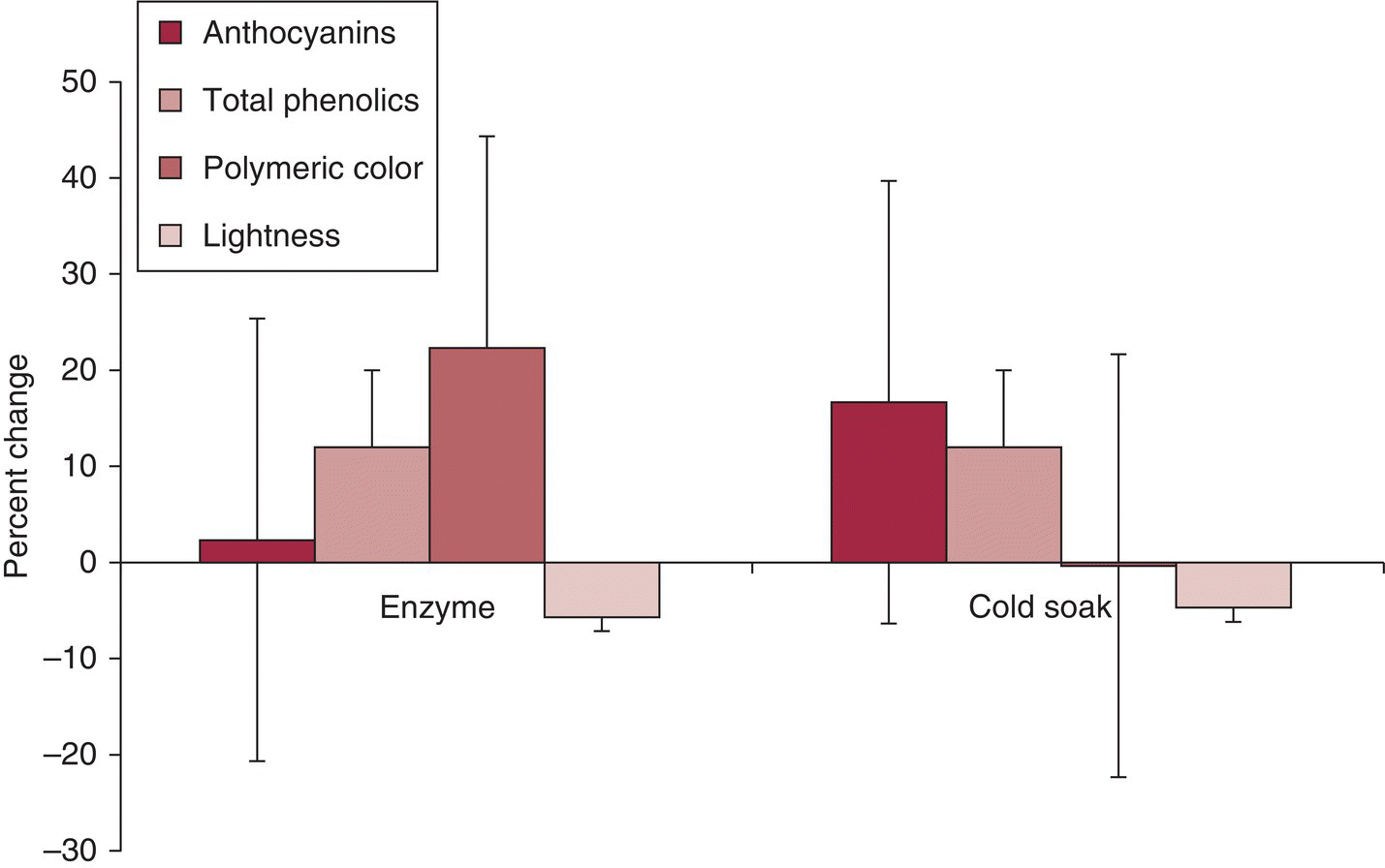
Figure 21.4 Comparison of average and standard deviation of wine phenolic and color parameters affected by cold soak and enzyme addition on three varieties.
Data from Gonzáles‐Neves et al., 2013 [16].
21.2.1 Use of maceration enzymes
The most common treatment to enhance juice yield is the use of enzymes to chemically degrade cell wall polysaccharides, with reported increases of 10–30% [17, 18]. These processing enzymes include polygalacturonase, pectin‐esterase and pectin‐lyase activity, but as these are industrial preparations, they include other activities (Chapter 23.1), which can have a major impact on aroma and/or phenolics (sometimes intentionally) [19] (Chapter 27). A key side‐effect of pectinase treatments is the release of methanol from the methyl ester in pectin slightly enhancing methanol levels (Chapter 6), which can be concentrated on distillation (Chapter 26.4).
While the total picture is partially obscured by the presence of yeast polysaccharides (Chapter 2), pectinase results in a significant decrease in the high molecular weight fractions of the grape‐sourced arabinogalactan proteins (AGPs) and arabinans, and these are replaced by lower molecular weight substances, including rhamnogalacturonan II (RGII) and other smaller molecules [20]. The composition of the polysaccharides is also affected by enzyme treatment – in other words, the constituent subunit composition changes. The use of an enzymatic treatment generally decreases the amount of arabinose and galactose in the remaining polysaccharides [21].
Enzymatic treatments of red grapes increase wine polyphenol content. Ducasse et al. showed total polyphenol concentration increased modestly, by about 10%, while color increased between 10 and 20%, largely from an increase in polymeric wine pigments. Tannin rose consistently by approximately 20%, explaining the common perception of increased astringency from these treatments [20]. There are at least two possible explanations (not mutually exclusive) for these observations: one is that polysaccharides, particularly those high in arabinose and galactose, have been shown to moderate astringency in wine [22], so degrading these by enzymes should increase astringency and give a higher response in protein‐binding assays for tannin. Alternatively, degrading the polysaccharides may expose tannin‐containing tissue to more facile extraction, thereby increasing tannin extraction.
21.2.2 Application of heat
Thermovinification involves heating either white or red must and then pressing before fermentation, and is a common treatment in some areas. One of its major applications is for decreasing the microbial load or inhibiting browning enzymes on mold‐contaminated musts, and inactivating enzymes can also result in lower production of C6 alcohols (Chapter 6) [23], although others have reported that thermal inactivation can preserve C6 aldehydes by inactivating ADH enzymes that would reduce the aldehydes to alcohols (Chapter 22.1). Thermovinification also has a profound effect on phenolic extraction as compared to traditional maceration, leading to reductions in tannin when pressing is performed pre‐fermentation – this approach also increases anthocyanin content, presumably by limiting reactions with tannins that would result in color loss [24]. On the other hand, significant increases are observed when pressing is delayed until after fermentation. A side‐effect of thermal treatments as compared to conventional macerations is a fruitier aroma due to an increase in both acetate and ethyl esters (up to an order of magnitude) [23], although it is not clear if this is due to lower solids content or due to lower oxygen exposure during fermentation (Chapters 22.2 and 22.3).
Flash release (flash détente) is a related, modern maceration technique that involves heating the must and releasing it into a vacuum, causing grape tissue cells to rupture. This technique increased arabinose‐ and galactose‐rich polysaccharides in the finished wine, while a similar thermal‐only treatment had little effect [21]. This technique was very effective in increasing the amounts of polyphenolics in the juice (>5 times more) and in the subsequent wine by 1.2–2 times for some seed components [25]. As expected, there was no increase in juice‐derived hydroxycinnamates or the anthocyanins as these are readily extracted by normal maceration, but the harder to extract seed‐derived tannins and catechins did increase. Flash release is also reported to increase juice yield as compared to conventional maceration [18].
21.2.3 Extraction by other physical means
Several other physical treatments of grape musts have been explored for their ability to generally or selectively enhance extraction. One recent innovation in fruit and vegetable processing is the use of pulsed electric field (PEF) treatments to damage cell walls; in the case of grapes, this increases both yield [26] and extraction of tannins and anthocyanins [27]. Some reports suggest that tannin extraction can be higher but still yield wines with less astringency [28, 29], perhaps due to greater polysaccharide extraction, while others report effects on aromas [30]. Other related technologies that have been studied include ultrasound [31], microwave [32], and freezing (cryomaceration) [33], with varying and modest effects in wine and model systems. However, cryomaceration of the whole or crushed fruit has been shown to increase the extraction of volatile aroma substances [34].
21.2.4 Simple pre‐fermentation skin contact
One basic approach to increasing extraction is to simply allow for solids/juice contact before fermentation occurs. In the case of white wine, this skin‐contact step usually involves allowing the crushed grapes to stay in contact with the skins for several hours before pressing and fermenting. A major reason to carry out skin contact with white grapes is to increase extraction of aroma compounds and their precursors, but a few investigations have looked at phenolics from white grapes, showing that a cold soak increased flavan‐3‐ols and flavonols [35], as well as total polyphenolics and polysaccharides. However, the resulting differences had only a small impact on taste or mouthfeel [36]. There appears to be a pronounced effect of skin contact on grape‐derived volatiles. A study of Airén showed that this approach increased monoterpenoids (Chapter 8) in the wine in addition to skin flavonols and minor increases of seed phenolics [37].
Several studies have investigated the effects of various pre‐fermentation treatments of varietal thiols, particularly in Sauvignon Blanc. As described earlier, it is now well‐established that machine harvesting followed by some hours of skin maceration time yields far more of the varietal thiol 3‐mercaptohexanol (3'MH) in finished wine [38] (Chapter 10). This is likely due to extraction of precursors from grape skin and not from direct extraction of free volatiles, as grapes contain negligible 3‐MH [39]. It is possible that enzymatic oxidation [40] encountered during mechanical harvesting and/or pre‐fermentative skin contact converts α‐linolenic acid in the berries to (E)‐2‐hexenal (Chapter 23.3) as the introduction of oxygen during the maceration step increases 3‐MH yield [41]. The subsequent steps from 2‐hexenal and leading to 3‐MH are covered in Chapter 23.2. Regardless of the mechanism, both skin contact and pressing have been shown to increase the ultimate amount of 3‐MH precursor in different Sauvignon Blanc juices [42], and several odorous varietal thiols were enhanced four‐fold after 48 hours of skin contact using Muscadine grapes [43].
For red grapes, pre‐fermentative skin contact (i.e., cold soak) is sometimes done prior to fermentation on skins. Cold soak may last several days, and is purportedly done to alter phenolic composition or sensory properties of finished wines. The practice is controversial among winemakers, with advocates and detractors [44]. A review of numerous literature reports concluded that at the low temperatures employed (10–15 °C), a cold soak had little to no measureable effect on the finished wine [4], Subsequent reports showed varying effects, with Alvarez et al. observing modest effects depending on variety [33], Apolinar‐Valiente et al. making similar observations (+10% in only one case of four), and also noting little impact on polysaccharides [45], while Koyama saw lower seed extracton [46]. In contrast, other investigators showed that a cold soak increased the amount of seed and skin proanthocyanidins extracted in the finished wine [47, 48].
Finally, freezing, dry ice treatment, or cold maceration can have modest effects on fermentation‐derived esters and higher alcohols (particularly C6 alcohols like 1‐hexanol) in several V. vinifera wines [49]. These factors are discussed in more detail in Chapters 22.2, 22.3, and 23.3.
Overall, pre‐fermentation maceration includes many different techniques to alter extraction. However, the presence of constitutive and added enzymes, and the potential for non‐enzymatic chemical reactions or unintended microbial activity, ensure that the process is not a simple chemical extraction but a complex interactive system.
21.3 Maceration treatments during fermentation
In red winemaking, the maceration process generally targets the extraction of anthocyanins and tannins. During fermentation anthocyanin extraction typically peaks at about 3–5 days and then declines, due to re‐adsorption on to skin tissue or reactions to form wine pigments (Figure 21.5). Tannin extraction is also limited by re‐adsorption on to grape skin tissues, with the larger proanthocyanidins tightly bound by flesh cell wall material [10].
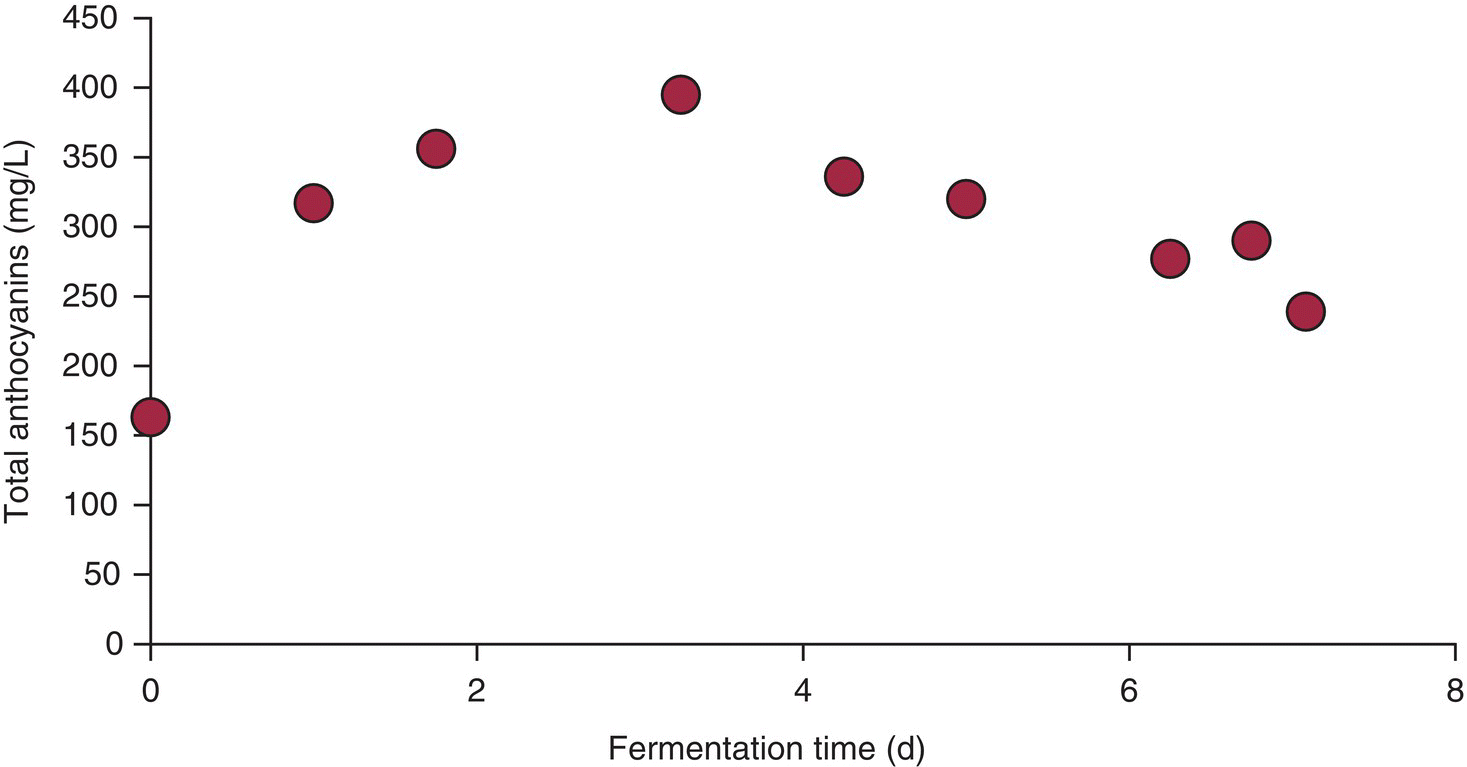
Figure 21.5 Typical extraction curves for anthocyanin during fermentation.
Data from Zimman and Waterhouse, 2004 [50]
21.3.1 Time and temperature
As with pre‐fermentation maceration, increasing time and temperature will generally increase the extraction coefficient and rate. However, a complicating issue is that the formation of ethanol will also favor greater extraction. Since fermentation rate (and thus ethanol production) can be accelerated by higher temperatures and other factors, it is often difficult to decouple the variables of fermentation conditions on extraction rates. Generally, extraction of both total and individual polyphenolic species increases with increasing temperature (Table 21.1), although both increases and decreases are reported for anthocyanins in response to increasing fermentation temperature. The variable behavior of anthocyanins likely arises because (i) anthocyanin extraction is rapid in comparison to tannins and thus increasing temperature may have negligible effect on extraction per se and (ii) anthocyanins can be lost to side reactions that are promoted at higher temperatures. For example, new wine pigments can be formed due to increased production of carbonyl metabolites by the yeast, resulting in the reaction of these aldehydes with the anthocyanins and tannins (Chapter 24).
Table 21.1 Fermentation maceration effects on red wine composition
| Treatment | Controla | Change relative to controlc | Analysis timee | Notesf | Reference | ||||
| Total phenol | Tannin | Anthocyanin | Wine pigmentd | Color | |||||
| Temperature, 27 °C | 12 °C | – | – | – | – | +100% | 130 d | 38 L, PN | [51] |
| Temperature, 25 °C | 15 °C | –23% | – | – | – | –1% | ~1 mo | 3 L, 6 da, CS | [52] |
| Temperature, 32 °C | 24 °C | – | +11% | NSb | NS | NS | 18 mo | 22,800 L, 7 d, CS | [1] |
| Temperature, 30 °C | 20 °C | – | – | NS | +50% | –50% | 225 d | 40 kg, 7, 5 d, PN | [53] |
| Temperature, 30 °C | 15 °C | +32% | – | +21% | – | +20% | 2.5 y | 35 L, press at dryness, Pn | [54] |
| Delestageg | Conventional | –5% | +9% | NS | –9% | +26% | 4 mo | 250 L, 12 d, Mencia | [55] |
| Ganimedeh | Conventional | –13% | –23% | NS | –9% | +16% | 4 mo | 250 L, 12 d, Mencia | [55] |
| Press 3 versus 6 d | 3 d | –5% | – | – | – | +6% | ~1 mo | 3 L, 25 °C, CS | [52] |
| Press 7 versus 10 d | 7 d | – | +13% | – | – | +19% | 10 mo | 38 L, 25 °C, CS | [56] |
| Press 4 versus 10 d | 4 d | – | – | +13% | +88% | +54% | 12 mo | 120 L, Monastrell | [57] |
| Pumpover | Punchdown | –10% | –11% | – | – | 180 kg, 25 °C, Pinotage | [58] | ||
| Rotary | Pumpover | – | – | –4% | – | –8% | 24 mo | 5 °C, Vinhão | [59] |
a Control treatment.
b NS = No significant difference.
c Various methods.
d Non‐bleachable pigments.
e Elapsed time after fermentation.
f Scale, time or temperature of fermentation, and grape variety (CS = Cabernet Sauvignon, PN = Pinot Noir).
g Delestage is draining the fermentation tank and pouring the liquid back over the cap as one large addition.
h Ganimede is a complex semi‐continuous extraction device generally used for Port wine production.
As shown in Figure 21.5, anthocyanin concentration increases and then decreases during the fermentation. By introducing 14C labeled malvidin‐3‐glucoside at day three, Zimman and Waterhouse [50] showed that even at maximum extraction, half the anthocyanins were not in solution, but apparently bound to the grape solids. After pressing, anthocyanin levels continued to decrease but the constant level of radioactivity in solution indicated that this was due to formation of soluble anthocyanin derivatives (Chapters 16 and 25) [50].
21.3.2 Mixing solids and liquid
Techniques for mixing the grape solids into the fermenting must are diverse and very creative, and are designed to enhance or otherwise alter the extraction of tannins, color, and other phenolics (Chapter 19). As with fermentation temperature, little attention has been directed to understanding how these techniques might alter the extraction of non‐phenolic compounds from grape solids. New techniques are typically compared to the standard punchdown (pigeage), a physical mixing of the cap solids with the must, or pumpover, pumping the liquid must over the cap to facilitate an extraction process. Most variants have modest effects on overall phenolic extraction [1], and the observed effects are highly variable, presumably due to varying composition of the berries or difficulties in controlling these mechanical processes. However, extreme techniques, like the continuous maceration (i.e., Ganimede system) used in Port production, where extraction is limited to a few days early in the fermentation, can enhance the color and tannin extraction rate and coefficients [60]. It is most difficult to generalize the effects of specific treatments – appropriate use of these various fermentation protocols is obtained empirically by experimenting with specific fruit sources. One typical observation showed that different maceration techniques resulted in large differences immediately after fermentation or extended maceration, but during bottle aging the differences diminish [59], so observations just after the treatment are of questionable value. Table 21.1 shows the variation in response to a number of typical treatments and the time between fermentation and analysis.
Because the seeds and skins have different polyphenol compositions, altering the proportions of these tissues during maceration can have measureable effects. In a Cabernet Sauvignon fermentation, repeatedly racking the wine and returning it to the same tank (delestage) decreased the seed content by ~80%. The treatment also decreased the proanthocyanidin content and epicatechin gallate to epigallocatechin ratio, made the wine darker while diminishing the anthocyanin content, and moderated bitterness and astringency [61]. A similar study on Merlot showed little change in color, but the proanthocyanidins contained more epigallocatechin subunits [62]. Apart from phenolic extraction, removing the solids during fermentation of a red grape must has dramatic effects on some volatiles, and both increases and decreases have been observed [63].
21.3.3 Carbonic maceration
Aside from physical maceration methods, the practice of carbonic maceration (CM) is a well‐known technique that alters extraction of red grape must. In CM, a small fraction of the grapes are crushed into a fermentation tank with the balance of the fruit added as whole clusters. The tank is sealed under a carbon dioxide atmosphere, which induces the fruit to ferment its sugars anaerobically, in addition to alcoholic fermentation brought about by yeast. After about one week, the fruit is crushed and pressed into another tank to complete fermentation by yeast. Wines made with this technique have a lower anthocyanin content and thus are lighter, and while the hue is similar, the CM wines have higher chroma values [64]. These wines also have very different aroma profiles due to high ester levels that contribute a strong fruity aroma [65], similar to what is observed with thermovinified wines.
21.4 Post‐fermentation maceration
21.4.1 Extended maceration (EM)
The practice of continuing maceration after alcoholic fermentation is complete is common with particular wine styles where high levels of tannin are desired; seed tannin continues to dissolve into the wine, as the alcohol from fermentation is at the maximum concentration at this point (Figure 21.6). After alcoholic fermentation has ended, however, the wine is no longer protected from oxygen by carbon dioxide evolution. As such, this maceration step is typically conducted in a closed system with little air exposure because any solids floating on the wine can facilitate Acetobacter growth and acetic acid formation under aerobic conditions (Chapter 22.5).
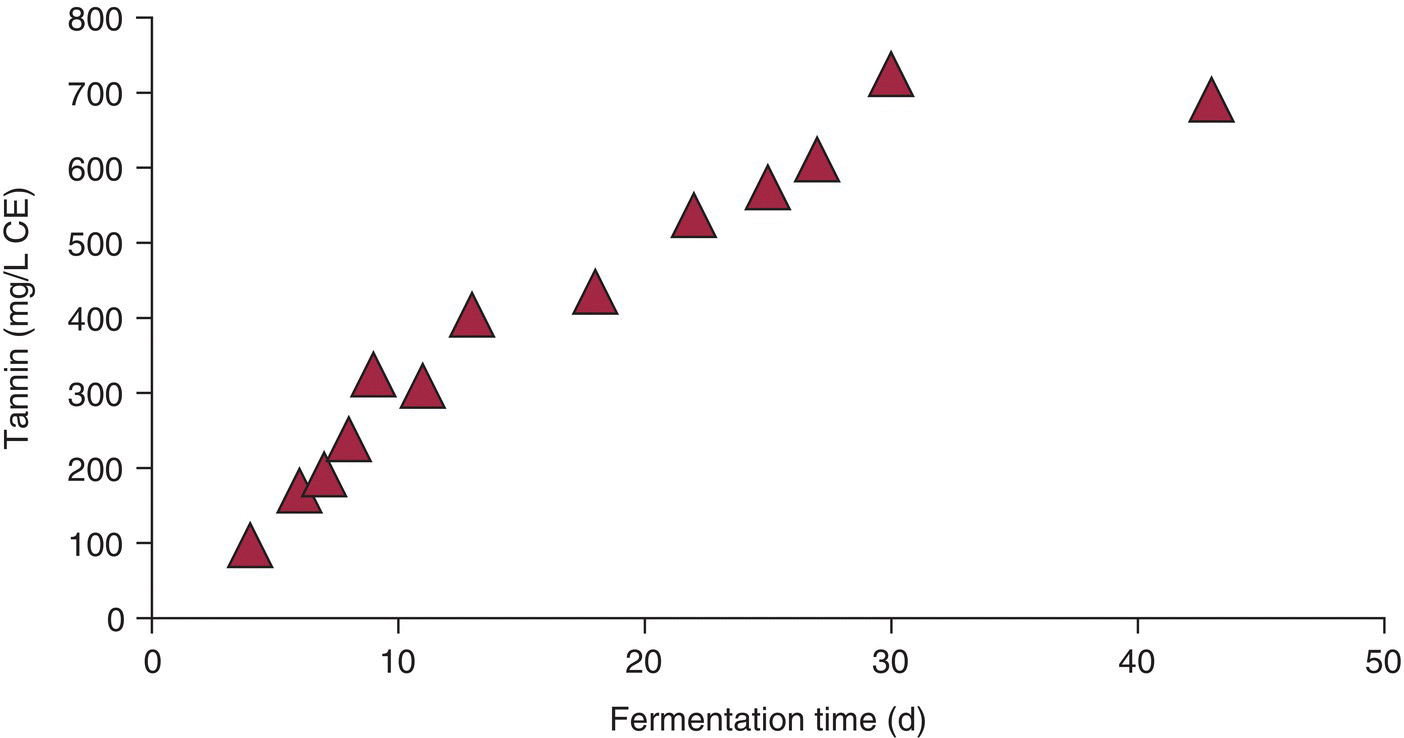
Figure 21.6 Tannin extraction during extended maceration.
Data from Casassa et al., 2013 [66]
A remarkably detailed study compared pressing at 10 days versus 30 days total maceration time. Of the tannin available in seeds and skins, extended maceration (EM) increased seed extraction from 11 to 18%, but skin tannin remained constant, and the extracted levels in the wine did not change up to 540 days later. EM resulted in much poorer recovery of anthocyanins, and while it accelerated polymeric pigment formation during the first year of aging, after 540 days the amount was similar to the control, so the fraction of color from polymeric pigment was higher in the EM wine at that time [66]. Similar results were observed by Yokotsuka et al. [67]. Yet another study also found that EM leads to increases in color and proanthocyanidins, but a lower yield from thiolysis, suggesting that more irregular linkages arise during the extended maceration and that EM was related to increases in astringency [68].
These results suggest that EM is primarily a physiochemical extraction process, with little effect from yeast or grape enzymatic activity. Components in the grape solids slowly equilibrate with the wine, although reactions with dissolved phenolics and aldehydes/ketones generated during fermentation will continue.
As for other components, EM time substantially increased the concentration of polysaccharides (Figure 21.1) in wine in nearly all cases, across a range of grape maturities [2]. Finally, extended skin contact was also suggested as a means to increase rotundone levels for varieties that contain this “peppery” compound, since rotundone accumulates predominantly in skins, is very non‐polar, and so is poorly extracted (~10%) [69].
References
- 1. Zimman, A., Joslin, W.S., Lyon, M.L., et al. (2002) Maceration variables affecting phenolic composition in commercial‐scale Cabernet Sauvignon winemaking trials. American Journal of Enology and Viticulture, 53 (2), 93–98.
- 2. Gil, M., Kontoudakis, N., Gonzalez, E., et al. (2012) Influence of grape maturity and maceration length on color, polyphenolic composition, and polysaccharide content of Cabernet Sauvignon and Tempranillo wines. Journal of Agricultural and Food Chemistry, 60 (32), 7988–8001.
- 3. Andrich, G., Zinnai, A., Venturi, E., Fiorentini, R. (2005) A tentative mathematical model to describe the evolution of phenolic compounds during the maceration of Sangiovese and Merlot grapes. Italian Journal of Food Science, 17 (1), 45–58.
- 4. Sacchi, K.L., Bisson, L.F., Adams, D.O. (2005) A review of the effect of winemaking techniques on phenolic extraction in red wines. American Journal of Enology and Viticulture, 56 (3), 197–206.
- 5. Sun, B.S., Pinto, T., Leandro, M.C., et al. (1999) Transfer of catechins and proanthocyanidins from solid parts of the grape cluster into wine. American Journal of Enology and Viticulture, 50 (2), 179–184.
- 6. Singleton, V.L. and Esau, P. (1969) Phenolic substances in grapes and wine, and their significance, Academic, New York.
- 7. Singleton, V.L. (1982) Grape and wine phenolics; background and prospects, in Proceedings of the Symposium of the University of California, Davis, Grape and Wine Centennial, 1980, Department of Viticulture and Enology, University of California, Davis, CA, pp. 215–227.
- 8. Fragoso, S., Guasch, J., Aceña, L., et al. (2011) Prediction of red wine colour and phenolic parameters from the analysis of its grape extract. International Journal of Food Science and Technology, 46 (12), 2569–2575.
- 9. Rustioni, L., Fiori, S., Failla, O. (2014) Evaluation of tannins interactions in grape (Vitis vinifera L.) skins. Food Chemistry, 159, 323–327.
- 10. Bindon, K.A., Smith, P.A., Holt, H., Kennedy, J.A. (2010) Interaction between grape‐derived proanthocyanidins and cell wall material. 2. Implications for vinification. Journal of Agricultural and Food Chemistry, 58 (19), 10736–10746.
- 11. Singleton, V.L. and Trousdale, E.K. (1992) Anthocyanin–tannin interactions explaining the differenced in polymeric phenols between white and red wines. American Journal of Enology and Viticulture, 43 (1), 63–70.
- 12. Springer, L.E., Stahlecker, A.C., Sherwood, R.W., Sacks, G.L. (2015) Limits on red wine tannin extraction and addition. Part II: Role of pathogenesis related proteins in Terroir, in American Society for Enology and Viticulture 66th National Conference, Portland, Oregon, p. 61.
- 13. Merida, J., Moyano, L., Millan, C., Medina, M. (1991) Extraction of phenolic compounds in controlled macerations of Pedro Ximenez grapes. Vitis, 30, 117–127.
- 14. Vidal, S., Francis, L., Williams, P., et al. (2004) The mouth‐feel properties of polysaccharides and anthocyanins in a wine like medium. Food Chemistry, 85 (4), 519–525.
- 15. Harbertson, J.F. and Harwood, E.D. (2009) Partitioning of potassium during commercial‐scale red wine fermentations and model wine extractions. American Journal of Enology and Viticulture, 60 (1), 43–49.
- 16. Gonzalez‐Neves, G., Gil, G., Favre, G., et al. (2013) Influence of winemaking procedure and grape variety on the colour and composition of young red wines. South African Journal of Enology and Viticulture, 34 (1), 138–146.
- 17. Haight, K.G. and Gump, B.H. (1994) The use of macerating enzymes in grape juice processing. American Journal of Enology and Viticulture, 45 (1), 113–116.
- 18. Paranjpe, S.S., Ferruzzi, M., Morgan, M.T. (2012) Effect of a flash vacuum expansion process on grape juice yield and quality. LWT‐Food Science and Technology, 48 (2), 147–155.
- 19. Bisson, L.F. and Butzke, C.E. (1996) Technical enzymes for wine production. Agro Food Industry Hi‐Tech, 7 (3), 11–14.
- 20. Ducasse, M.A., Canal‐Llauberes, R.M., de Lumley, M., et al. (2010) Effect of macerating enzyme treatment on the polyphenol and polysaccharide composition of red wines. Food Chemistry, 118 (2), 369–376.
- 21. Doco, T., Williams, P., Cheynier, V. (2007) Effect of flash release and pectinolytic enzyme treatments on wine polysaccharide composition. Journal of Agricultural and Food Chemistry, 55 (16), 6643–6649.
- 22. Quijada‐Morin, N., Williams, P., Rivas‐Gonzalo, J.C., et al. (2014) Polyphenolic, polysaccharide and oligosaccharide composition of Tempranillo red wines and their relationship with the perceived astringency. Food Chemistry, 154, 44–51.
- 23. Fischer, U., Strasser, M., Gutzler, K. (2000) Impact of fermentation technology on the phenolic and volatile composition of German red wines. International Journal of Food Science and Technology, 35 (1), 81–94.
- 24. Gao, L., Girard, B., Mazza, G., Reynolds, A.G. (1997) Changes in anthocyanins and color characteristics of Pinot noir wines during different vinification processes. Journal of Agricultural and Food Chemistry, 45 (6), 2003–2008.
- 25. Morel‐Salmi, C., Souquet, J.M., Bes, M., Cheynier, V. (2006) Effect of flash release treatment on phenolic extraction and wine composition. Journal of Agricultural and Food Chemistry, 54 (12), 4270–4276.
- 26. Praporscic, I., Lebovka, N., Vorobiev, E., Mietton‐Peuchot, M. (2007) Pulsed electric field enhanced expression and juice quality of white grapes. Separation and Purification Technology, 52 (3), 520–526.
- 27. Lopez, N., Puertolas, E., Hernandez‐Orte, P., et al. (2009) Effect of a pulsed electric field treatment on the anthocyanins composition and other quality parameters of Cabernet Sauvignon freshly fermented model wines obtained after different maceration times. LWT‐Food Science and Technology, 42 (7), 1225–1231.
- 28. Delsart, C., Ghidossi, R., Poupot, C., et al. (2012) Enhanced extraction of phenolic compounds from Merlot grapes by pulsed electric field treatment. American Journal of Enology and Viticulture, 63 (2), 205–211.
- 29. Luengo, E., Franco, E., Ballesteros, F., et al. (2014) Winery trial on application of pulsed electric fields for improving vinification of Garnacha grapes. Food and Bioprocess Technology, 7 (5), 1457–1464.
- 30. Garde‐Cerdan, T., Gonzalez‐Arenzana, L., Lopez, N., et al. (2013) Effect of different pulsed electric field treatments on the volatile composition of Graciano, Tempranillo and Grenache grape varieties. Innovative Food Science and Emerging Technologies, 20, 91–99.
- 31. Tiwari, B.K., Patras, A., Brunton, N., et al. (2010) Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrasonics Sonochemistry, 17 (3), 598–604.
- 32. Ghassempour, A., Heydari, R., Talebpour, Z., et al. (2008) Study of new extraction methods for separation of anthocyanins from red grape skins: analysis by HPLC and LC‐MS/MS. Journal of Liquid Chromatography and Related Technologies, 31 (17), 2686–2703.
- 33. Alvarez, I., Aleixandre, J.L., Garcia, M.J., Lizama, V. (2006) Impact of prefermentative maceration on the phenolic and volatile compounds in Monastrell red wines. Analytica Chimica Acta, 563 (1–2), 109–115.
- 34. Bavcar, D., Cesnik, H.B., Cus, F., et al. (2011) Impact of alternative skin contact procedures on the aroma composition of white wine. South African Journal of Enology and Viticulture, 32 (2), 190–203.
- 35. Caceres‐Mella, A., Pena‐Neira, A., Parraguez, J., et al. (2013) Effect of inert gas and prefermentative treatment with polyvinylpolypyrrolidone on the phenolic composition of Chilean Sauvignon blanc wines. Journal of the Science of Food and Agriculture, 93 (8), 1928–1934.
- 36. Gawel, R., Day, M., Van Sluyter, S.C., et al. (2014) White wine taste and mouthfeel as affected by juice extraction and processing. Journal of Agricultural and Food Chemistry, 62 (41), 10008–10014.
- 37. Cejudo‐Bastante, M.J., Castro‐Vazquez, L., Hermosin‐Gutierrez, I., Perez‐Coello, M.S. (2011) Combined effects of prefermentative skin maceration and oxygen addition of must on color‐related phenolics, volatile composition, and sensory characteristics of Airen white wine. Journal of Agricultural and Food Chemistry, 59 (22), 12171–12182.
- 38. Allen, T., Herbst‐Johnstone, M., Girault, M., et al. (2011) Influence of grape‐harvesting steps on varietal thiol aromas in Sauvignon blanc wines. Journal of Agricultural and Food Chemistry, 59 (19), 10641–10650.
- 39. Capone, D.L., Sefton, M.A., Jeffery, D.W. (2012) Analytical investigations of wine odorant 3‐mercaptohexan‐1‐ol and its precursors, in Flavor chemistry of wine and other alcoholic beverages, American Chemical Society, Washington, DC, pp. 15–35.
- 40. Podolyan, A., White, J., Jordan, B., Winefield, C. (2010) Identification of the lipoxygenase gene family from Vitis vinifera and biochemical characterisation of two 13‐lipoxygenases expressed in grape berries of Sauvignon Blanc. Functional Plant Biology, 37 (8), 767–784.
- 41. Larcher, R., Nicolini, G., Tonidandel, L., et al. (2013) Influence of oxygen availability during skin‐contact maceration on the formation of precursors of 3‐mercaptohexan‐1‐ol in Muller‐Thurgau and Sauvignon Blanc grapes. Australian Journal of Grape and Wine Research, 19 (3), 342–348.
- 42. Maggu, M., Winz, R., Kilmartin, P.A., et al. (2007) Effect of skin contact and pressure on the composition of Sauvignon blanc must. Journal of Agricultural and Food Chemistry, 55 (25), 10281–10288.
- 43. Gurbuz, O., Rouseff, J., Talcott, S.T., Rouseff, R. (2013) Identification of Muscadine wine sulfur volatiles: pectinase versus skin‐contact maceration. Journal of Agricultural and Food Chemistry, 61 (3), 532–539.
- 44. Cutler, L. (2009) Industry Roundtable: Fermentation Techniques. Wine Communications Group [cited December 20, 2013]; Available from: http://www.winebusiness.com/wbm/?go=getArticle&dataId=62843.
- 45. Apolinar‐Valiente, R., Williams, P., Romero‐Cascales, I., et al. (2013) Polysaccharide composition of Monastrell red wines from four different Spanish Terroirs: effect of wine‐making techniques. Journal of Agricultural and Food Chemistry, 61 (10), 2538–2547.
- 46. Koyama, K., Goto‐Yamamoto, N., Hashizume, K. (2007) Influence of maceration temperature in red wine vinification on extraction of phenolics from berry skins and seeds of grape (Vitis vinifera). Bioscience Biotechnology and Biochemistry, 71 (4), 958–965.
- 47. Des Gachons, C.P. and Kennedy, J.A. (2003) Direct method for determining seed and skin proanthocyanidin extraction into red wine. Journal of Agricultural and Food Chemistry, 51 (20), 5877–5881.
- 48. Favre, G., Peña‐Neira, Á., Baldi, C., et al. (2014) Low molecular‐weight phenols in Tannat wines made by alternative winemaking procedures. Food Chemistry, 158 (0), 504–512.
- 49. Moreno‐Perez, A., Vila‐Lopez, R., Fernandez‐Fernandez, J.I., et al. (2013) Influence of cold pre‐fermentation treatments on the major volatile compounds of three wine varieties. Food Chemistry, 139 (1–4), 770–776.
- 50. Zimman, A. and Waterhouse, A.L. (2004) Incorporation of malvidin‐3‐glucoside into high molecular weight polyphenols during fermentation and wine aging. American Journal of Enology and Viticulture, 55 (2), 139–146.
- 51. Ough, C.S. and Amerine, M.A. (1961) Studies on controlled fermentation. V. Effects on color, composition, and quality of red wines. American Journal of Enology and Viticulture, 12 (1), 9–19.
- 52. Sener, H. and Yildirim, H.K. (2013) Influence of different maceration time and temperatures on total phenols, colour and sensory properties of Cabernet Sauvignon wines. Food Science and Technology International, 19 (6), 523–533.
- 53. Girard, B., Yuksel, D., Cliff, M.A., et al. (2001) Vinification effects on the sensory, colour and GC profiles of Pinot noir wines from British Columbia. Food Research International, 34 (6), 483–499.
- 54. Girard, B., Kopp, T.G., Reynolds, A.G., Cliff, M. (1997) Influence of vinification treatments on aroma constituents and sensory descriptors of Pinot noir wines. American Journal of Enology and Viticulture, 48 (2), 198–206.
- 55. Vazquez, E.S., Segade, S.R., Fernandez, I.O. (2010) Effect of the winemaking technique on phenolic composition and chromatic characteristics in young red wines. European Food Research and Technology, 231 (5), 789–802.
- 56. Ough, C.S. and Amerine, M.A. (1961) Studies with controlled fermentation. VI. Effects of temperature and handling on rates, composition and quality of wines. American Journal of Enology and Viticulture, 12 (3), 117–128.
- 57. Gomez‐Plaza, E., Gil‐Munoz, R., Lopez‐Roca, J.M., et al. (2001) Phenolic compounds and color stability of red wines: effect of skin maceration time. American Journal of Enology and Viticulture, 52 (3), 266–270.
- 58. Marais, J. (2003) Effect of different wine‐making techniques on the composition and quality of Pinotage wine. II. Juice/skin mixing practices. South African Journal of Enology and Viticulture, 24 (2), 76–79.
- 59. Castillo‐Sanchez, J.J., Mejuto, J.C., Garrido, J., Garcia‐Falcon, S. (2006) Influence of wine‐making protocol and fining agents on the evolution of the anthocyanin content, colour and general organoleptic quality of Vinhao wines. Food Chemistry, 97 (1), 130–136.
- 60. Garde‐Cerdan, T., Jarauta, I., Salinas, M.R., Ancin‐Azpilicueta, C. (2008) Comparative study of the volatile composition in wines obtained from traditional vinification and from the Ganimede method. Journal of the Science of Food and Agriculture, 88 (10), 1777–1785.
- 61. Canals, R., Llaudy, M.D.C., Canals, J.M., Zamora, F. (2008) Influence of the elimination and addition of seeds on the colour, phenolic composition and astringency of red wine. European Food Research and Technology, 226 (5), 1183–1190.
- 62. Lee, J.M., Kennedy, J.A., Devlin, C., et al. (2008) Effect of early seed removal during fermentation on proanthocyanidin extraction in red wine: a commercial production example. Food Chemistry, 107 (3), 1270–1273.
- 63. Callejon, R.M., Margulies, B., Hirson, G.D., Ebeler, S.E. (2012) Dynamic changes in volatile compounds during fermentation of Cabernet Sauvignon grapes with and without skins. American Journal of Enology and Viticulture, 63 (3), 301–312.
- 64. Gomez‐Miguez, M. and Heredia, F.J. (2004) Effect of the maceration technique on the relationships between anthocyanin composition and objective color of Syrah wines. Journal of Agricultural and Food Chemistry, 52 (16), 5117–5123.
- 65. Etaio, I., Elortondo, F.J.P., Albisu, M., et al. (2008) Effect of winemaking process and addition of white grapes on the sensory and physicochemical characteristics of young red wines. Australian Journal of Grape and Wine Research, 14 (3), 211–222.
- 66. Casassa, L.F., Beaver, C.W., Mireles, M.S., Harbertson, J.F. (2013) Effect of extended maceration and ethanol concentration on the extraction and evolution of phenolics, colour components and sensory attributes of Merlot wines. Australian Journal of Grape and Wine Research, 19 (1), 25–39.
- 67. Yokotsuka, K., Sato, M., Ueno, N., Singleton, V.L. (2000) Colour and sensory characteristics of Merlot red wines caused by prolonged pomace contact. Journal of Wine Research, 11 (1), 7–18.
- 68. Cadot, Y., Caillé, S., Samson, A., et al. (2012) Sensory representation of typicality of Cabernet franc wines related to phenolic composition: impact of ripening stage and maceration time. Analytica Chimica Acta, 732, 91–99.
- 69. Caputi, L., Carlin, S., Ghiglieno, I., et al. (2011) Relationship of changes in rotundone content during grape ripening and winemaking to manipulation of the “peppery” character of wine. Journal of Agricultural and Food Chemistry, 59 (10), 5565–5571.