25
Topics Related to Aging
25.1 Introduction
Wine is a chemically dynamic system, and even after fermentation is complete wine composition contiunes to evolve during storage [1–9]. These post‐fermentation changes are associated with the general term aging, but a useful distinction can be made between those changes that transpire during the maturation phase (e.g., bulk storage of wine in a tank or barrel), during which winemaker intervention can still readily occur, and an aging phase (post‐packaging), where wine is sealed in its container and intervention is essentially limited to selection of storage conditions. Changes can be further distinguished by whether they will happen under completely anaerobic conditions or whether they require trace levels of oxygen. This chapter will examine some compositional and sensory effects of maturation and aging as a result of oxidation, the presence of nucleophiles and electrophiles, and acid‐catalyzed reactions.
25.2 Reactions involving red wine pigments
One of the most dramatic chemical changes in red wine involves grape‐derived monomeric anthocyanins. These can be a major component of red wine (approaching 1 g/L), but disappear as new pigments are produced (Figure 25.1a), resulting in a change in wine color from red‐purple to brick‐ or orange‐red [10–12]. This process begins during fermentation and continues during storage, such that, within two years, the majority of wine color derives from so‐called “polymeric pigments” that are resistant to bleaching by SO2. Mechanisms for the formation of derived pigments from monomeric anthocyanins, tannins, and other wine components were covered in Chapter 16, and these important reactions continue during maturation and aging [13]. As a common thread, the reactions that lead to the most stable forms of red wine color require trace amounts of oxygen and the presence of tannins. To summarize, the key types of compounds formed from reactions of anthocyanins are:
- Pigmented species resistant to SO2 bleaching (O2 usually required). The flavylium form of anthocyanins can act as an electrophile to react with nucleophiles (Chapter 16), including the enol form of aldehydes (to yield pyranoanthocyanins), or tannins (to yield A‐T type pigmented polymers). The former reactions will occur more rapidly at low pH (greater proportion of flavylium form, Figure 25.1b), and the latter will be favored at higher pH [14, 15]. However, all of these reactions will be favored in the presence of oxygen. In the case of A‐T pigments, it is proposed that the flavene intermediate requires oxidation to regenerate the flavylium (colored) form of the anthocyanin [16, 17], which may cyclize to form a xanthylium cation (Figure 25.2) [15], while formation of aldehydes requires oxidation of other wine components (Chapter 24). Additionally, the presence of acetaldehyde (from fermentation or oxidation) can lead to the generation of ethyl‐linked pigments (also favored at lower pH), which are not stable in the long term but are more resistant to bleaching than monomeric grape anthocyanins [18, 19].
- Pigmented species that are not resistant, or are less resistant, to SO2 bleaching (O2 usually not required). The major pathway for formation of pigmented polymers involves direct condensation of electrophilic flavan‐3‐ol cations (from tannin hydrolyis) with anthocyanins to form T‐A adducts. This pathway does not require O2, but will occur faster at lower wine pH due to faster hydrolysis of tannin interflavan bonds [14] (Chapters 14 and 16).
- Non‐pigmented or insoluble species (no O2 or excess O2 required). Anthocyanins that do not form stable adducts can undergo acid‐catalyzed transformation resulting in a loss of color, through opening of the heterocyclic C ring; hydrolysis of the glucoside also destablizes anthocyanins [18]. A‐T adducts (flavene form, Chapter 16) can also be a source of color loss through intramolecular cyclization (to an A‐type dimer) under anaerobic conditions or at low pH (Figure 25.2) [15, 20]. Alternatively, excessive oxidation and production of aldehydes and quinones (Chapter 24) can lead to formation of insoluble complexes, as well as high levels of browning [21].

Figure 25.1 Changes occurring in red wine due to (a) age for a vertical series of Cabernet Sauvignon wines from Coonawarra, South Australia, showing a decline in anthocyanins, and increases in pigmented polymer (high molecular weight pigmented compounds measured by HPLC, expressed as malvidin‐3‐glucoside equivalents) and hue (A420/A520; a higher value indicates more brick red versus red‐purple color), and (b) pH adjustment and addition of oxygen at different rates (held constant over a 4 month period) for a 2011 Merlot wine from Bordeaux, France, showing increases in a pyranoanthocyanin pigment upon greater exposure to oxygen and as a result of a lower pH.
Data obtained from References [22] and [23]

Figure 25.2 A colorless A‐T flavene formed from malvidin‐3‐glucoside and (–)‐epicatechin can react to form an A‐type ether linkage (also colorless), or may oxidize to regenerate the flavylium cation (red color), which can undergo cyclization to produce a xanthylium cation (yellow/brown color)
The effects of oxygen on anthocyanins have also been studied through work on microoxygenation (MOX; for reviews on this technique see References [24] and [25]) 1 and closures with differing oxygen transmission rates (OTRs) for wines at different pH values, as seen with the following examples. These studies highlight not only the continued evolution of phenolic composition and color, but also the impact of different closures and the prospect of choosing a closure OTR based on wine composition, expected storage conditions and time. For example, at 3 months post‐bottling, Aglianico red wines (pH 3.46 and 3.64) that underwent a single MOX treatment at 2 mL of O2/L for 8 weeks, or an additional MOX treatment of 1.5 mL O2/L for another 8 weeks, had higher total anthocyanins (measured at pH 1) and color intensity compared to the control wines (Figure 25.3a and b) [26]. After 42 months of bottle storage the differences between MOX treatments and controls were no longer significant for color intensity (and hue), and total anthocyanins in the case of the pH 3.64 wine (Figure 25.3a and b). This moderating effect with time has been observed in other studies (e.g., References [27] and [28]), likely due to alternative degradation pathways of pigments during storage obscuring the early effects of the oxygen‐dependent reactions. It is worth noting that while all treatments likely lost pigmented anthocyanins as compared to the initial wine (not reported in the study), the MOX‐treated wines had proportionally higher concentrations of stable pigmented forms [26]. Such a decrease in total anthocyanins and increase in stable pigments can readily be seen with data from a study of Cabernet Sauvignon wine at different pH values that underwent MOX (15 mg/L per month for 3 months) followed by a period of storage (Figure 25.3c and d) [28].

Figure 25.3 Impact of pH and oxygen combined with storage time (months) on (a) total anthocyanins and (b) color intensity for Aglianico red wines differening in pH treated with two different MOX regimes (left panel), and (c) total anthocyanins and (d) PVPP index (a measure of polymeric pigments) for Cabernet Sauvignon wine (pH 3.5) adjusted to pH 3.1 and 3.9 (right panel).
Data obtained from References [26] and [28]
The effect of synthetic closure OTR 2 was also studied for the Aglianico red wines mentioned above sealed with three different closures (low, medium, and high OTR), and having 6.5 or 9.8 mg/L of total package oxygen (TPO) at bottling [26]. After 10 months, the wine with 9.8 mg/L TPO revealed a decrease in total monomeric anthocyanins of about 40% for the closures with medium and high OTR, likely due to their incorporation into stable pigments, but the wine sealed with 6.5 mg/L TPO showed no signficant effect due to closure, likely because the differences were small compared to variations among treatments. Similar impacts of closure OTR and wine pH were also seen for pH‐adjusted Cabernet Sauvignon stored under SaranTin (impermeable to oxygen) and Saranex (slightly permeable) screw cap closures [29], with greater formation of stable pigments attributed to oxygen exposure and increased incorporation of anthocyanins into derived (non‐bleachable) pigments at a lower pH. As with the effect of MOX over time, the impact of screw cap OTR appeared not to be a factor when evaluated after 24 months.
25.3 Hydrolytic and pH‐dependent reactions
Several pH‐dependent reactions have been discussed throughout the book; their relevance to aging is summarized as follows:
- Lower pH affects the equilibria of anthocyanin species (Chapter 16) and increases the hydrolytic cleavage rate of tannin interflavan bonds, which in turn impacts formation of different derived pigments as described above.
- Lower pH increases the rate of ester hydrolysis and esterification reactions (Chapters 7, 8, and 23.1), as discussed below.
- Through protonation of the carbonyl oxygen, lower pH increases the electrophilicity of carbonyl carbons and the overall reactivity of carbonyl species with nucleophiles such as phenolics (Chapter 9).
- Lower pH increases the rate of glycoside hydrolysis, 3 as well as the rate of isoprenoid rearrangements observed in the resulting aglycone intermediates (Chapter 23.1), as discussed below.
- Higher pH will increase the rate of dimethyl sulfide (DMS) formation, although this increase is negligible over the pH range typically observed in wine (Chapter 23.3).
25.3.1 Glycoside hydrolysis and aglycone rearrangements
Many wine odorants exist as bound precursors that will liberate free, volatile forms during storage (Chapter 23.1). Notably, 1,1,6‐trimethyl‐1,2‐dihydronapthalene (TDN) in Riesling and monoterpenoid‐related compounds in aromatic varieties such as Muscat both arise from hydrolysis and rearrangement of glycosidic precursors (Chapters 8 and 23.1). Proposed acid‐catalyzed reaction mechanisms for the formation of TDN from Riesling acetal (itself an intermediate of C13‐norisoprenoid glycoconjugates from grapes) and wine lactone from monoterpenoid (linalool‐8‐carboxylate) precursors are shown in Figure 25.4. As is expected based on the mechanism, the rate of formation of these compounds will increase with decreasing pH (Figure 25.5) [30, 31].

Figure 25.4 Proposed acid‐catalyzed reaction mechanisms for conversion of (a) Riesling acetal into TDN via other known intermediates [32] and (b) precursor glucose ester and acid derivatives of linalool into wine lactone, invoking an interesting 1,3‐hydride shift [33]

Figure 25.5 Acid‐catalyzed formation of wine aroma compounds over time showing (a) concentration of TDN arising from Riesling acetal over 60 days in model wine at pH 3.0 and 3.2 stored at 45 °C and (b) concentration of wine lactone formed from precursor glucose ester or acid derivatives of linalool over 16 weeks in model wine at pH 3.0 and 3.4, and at room temperature (RT) and 45 °C. Note the precursor ester did not form wine lactone at room temperature over 12 weeks.
Data derived from [30] and [31]
25.3.2 Ester hydrolysis and esterification
Esters have a critical role in wine aroma (Chapter 7), and their formation and degradation by acid‐catalyzed esterification and ester hydrolysis show strong pH dependence (Figure 25.6). As mentioned earlier, the ester/acid ratio will approach equilibrium during aging, which may result in either an increase or decrease of individual esters (Chapter 7). Typically, initial ester/acid ratios are such that ethyl esters of branched‐chain fatty acids and fixed (non‐volatile) acids increase, acetate esters decrease precipitously, and straight‐chain fatty acids decrease slightly [34–36] (Figure 25.7). Notably, the hydrolysis of 3‐mercaptohexyl acetate can be especially influential on the aroma of Sauvignon Blanc wines (Chapter 10), 4 and acetate esters in general hydrolyze faster than fatty acid ethyl esters of similar molecular weight (by around 2–4 times [37]). Overall, the storage temperature in conjunction with wine pH can have a strong influence on ester composition and wine sensory properties during aging, especially for young white wines reliant on the aroma contributions of volatile acetate esters, and ethyl esters to a lesser extent, derived from fermentation.

Figure 25.6 Relative changes in concentration of volatiles 16 weeks after bottling for Colombard wine with altered pH values kept at different storage temperatures (10, 20, 30 °C), showing (a) sizeable decreases in acetate esters with increased temperature (as initial concentrations were far away from equilibrium concentrations), as well as an effect of lower pH, and (b) minimal changes in straight‐chain ethyl esters (ignoring the aberrant results at 30 °C and pH 3.72) but a general decrease with increased temperature is relatively evident. Note the different y‐axis scales.
Data from Reference [38]

Figure 25.7 Relative changes in concentration of volatiles one year after bottling for Sauvignon Blanc wine kept at different storage temperatures, showing (a) decrease in acetate esters, (b) decrease in straight‐chain ethyl esters, and (c) increase in branched‐chain ethyl esters. Room temperature (RT) fluctuated between 13 and 26 °C, with a mean of 19.5 °C. Despite the mean for RT being higher than the other treatments, the x axes are ordered according to the trends in the data. Note the different y‐axis scales.
Data from References [35] and [39]
25.3.3 Other pH‐dependent reactions
Apart from releasing grape‐derived aroma volatiles, acid‐catalyzed reactions can result in a loss of odor, such as in the conversion of linalool to the less potent monoterpenoid, α‐terpineol (Chapter 8). Glycosidic precursors of oak lactones, present in wine from maturation in oak wood, can undergo similar transformations to their grape‐derived counterparts (Chapter 7 and Chapter 23.1) [40]. Finally, sugars (pentoses and hexoses) present in wine may be converted to furfural, 5‐(hydroxymethyl)furfural (HMF), and other volatiles (as well as brown colored polymers, i.e., caramelization) through acid‐catalyzed degradation (Chapter 2), a pathway of particular importance to sweet wines or wines stored at higher temperatures [41]. This occurs through sugar enolization and elimination of water (dehydration), producing unsaturated dicarbonyl compounds, which can cyclize and further dehydrate to yield furans (and pyrans) (Figure 25.8) [42].
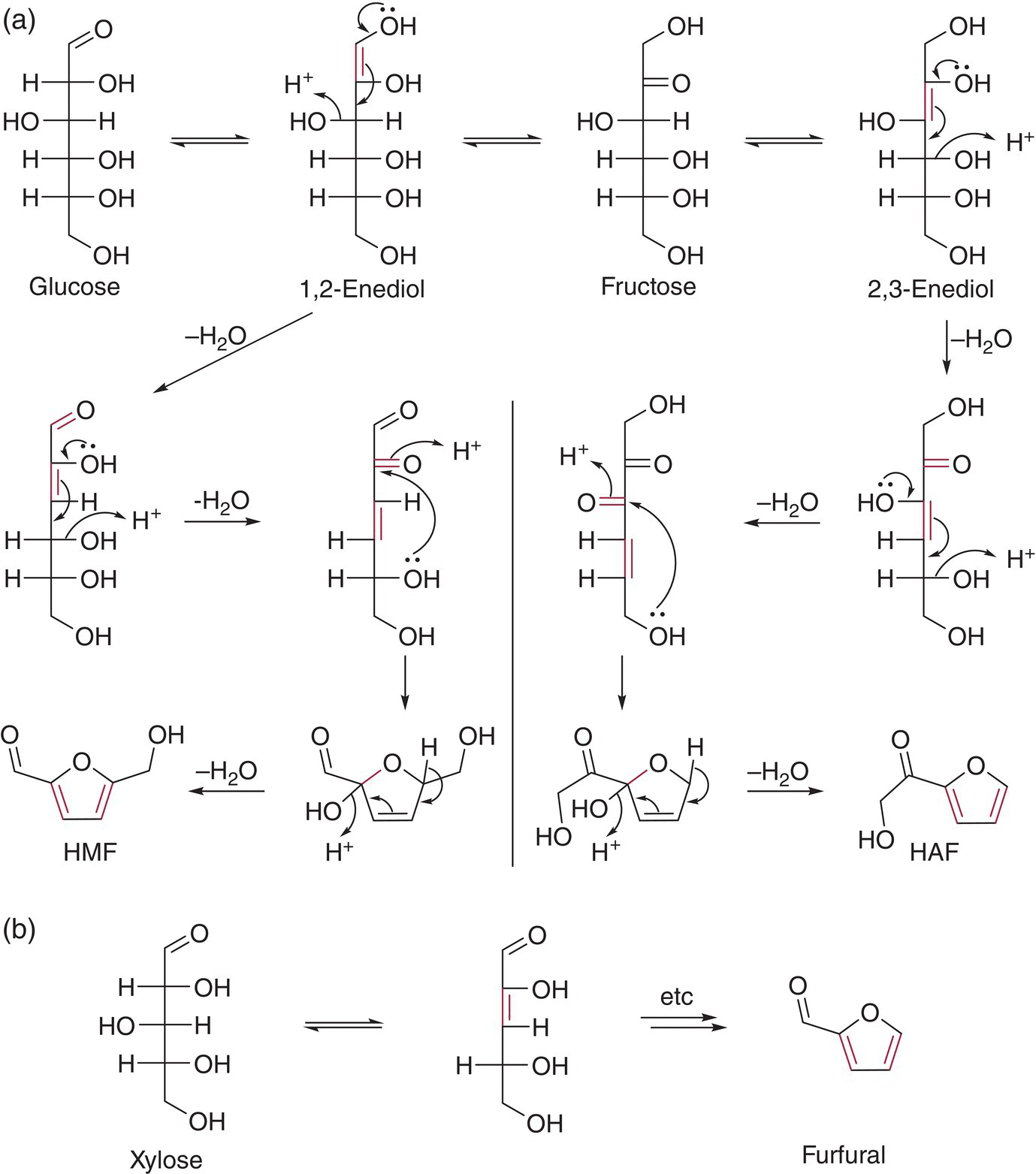
Figure 25.8 Acid‐catalyzed enolization, dehydration, and cyclization reaction pathways of sugars leading to aroma compounds such as (a) HMF and 2‐(hydroxyacetyl)furan (HAF) from hexoses including glucose and fructose and (b) furfural from pentoses such as xylose
25.4 Activation energy and temperature effects on aging
The rates of chemical reactions, including those observed in wine during storage, increase with increasing temperature. This arises due to the dependence of the rate constant (k) on temperature, as expressed by the Arrhenius equation:
where A is the Arrhenius constant (the proportion of molecules that will collide and react), E a is the activation energy (the energy barrier to overcome for a reaction to occur), R is the gas constant (8.314 J/mol K), and T is the temperature in Kelvin. The logarithmic form of the Arrhenius equation can be used to calculate E a and A when rate constants at different temperatures are known (Figure 25.9). Alternatively, E a can be calculated from the logarithmic form of the Arrhenius equation, after subtraction and rearrangement, using rate constants determined at two temperatures as follows:

where k 1 and k 2 are the rate constants at temperatures T 1 and T 2, respectively.

Figure 25.9 Determination of Ea and A from a plot of ln k versus 1/T for hydrolysis of two esters (hexyl acetate and ethyl hexanoate) based on the logarithmic form of Equation (25.1). Values of Ea are 62 and 53 kJ/mol for hexyl acetate and ethyl hexanoate, respectively.
Data from Reference [43]
For E
a values in the range of 40–70 kJ/mol (e.g., hydrolysis of acetate esters), the fold difference in reaction rates at two different temperatures can be approximated as  , which relates to the often‐quoted rule of thumb that each 10 °C increase in temperature leads to roughly a doubling of the reaction rate. However, because of the exponential nature of the Arrhenius equation, this heuristic is less appropriate for reaction rates with higher E
a values, which will usually proceed slowly at room temperature but demonstrate a larger increase in rate upon heating. As shown in Table 25.1, increasing the storage temperature increases all reaction rates. However, the increase in acid hydrolysis of esters and tannins upon increasing storage temperature from 12 °C to 50 °C is about a factor of 10, while the same temperature increase results in almost a 20 000‐fold increase in HMF. Notably, the reactions that increase most dramatically at high temperatures (high E
a) are undesirable in most table wines, including anthocyanin hydrolysis, and formation of dimethyl sulfide (DMS), HMF, and ethyl carbamate. This observation helps explain why expediting wine maturation by high‐temperature storage will lead to different (and often inferior) outcomes than longer‐term, lower‐temperature storage.
5
, which relates to the often‐quoted rule of thumb that each 10 °C increase in temperature leads to roughly a doubling of the reaction rate. However, because of the exponential nature of the Arrhenius equation, this heuristic is less appropriate for reaction rates with higher E
a values, which will usually proceed slowly at room temperature but demonstrate a larger increase in rate upon heating. As shown in Table 25.1, increasing the storage temperature increases all reaction rates. However, the increase in acid hydrolysis of esters and tannins upon increasing storage temperature from 12 °C to 50 °C is about a factor of 10, while the same temperature increase results in almost a 20 000‐fold increase in HMF. Notably, the reactions that increase most dramatically at high temperatures (high E
a) are undesirable in most table wines, including anthocyanin hydrolysis, and formation of dimethyl sulfide (DMS), HMF, and ethyl carbamate. This observation helps explain why expediting wine maturation by high‐temperature storage will lead to different (and often inferior) outcomes than longer‐term, lower‐temperature storage.
5
Table 25.1 Variation in activation energies and increases in reaction rate at elevated temperatures for a representative sample of reactions relevant to wine
| Reaction | Conditions | Reference | Activation energy, E a (kJ/mol) | Fold‐increase in reaction rate as compared to 12 °C a | |
| At 30 °C | At 50 °C | ||||
| Acid hydrolysis of hexyl acetate | Model wine, pH 2.95–4.10 | [43] | 62 | 5 | 22 |
| Hydrolysis of S‐methylmethionine to dimethyl sulfide | Model beer, pH 5.2 | [44] | 186 | 106 | 10 250 |
| Acid degradation of fructose to HMF | Model orange juice, pH 2.5–4.5 | [45] | 199 | 147 | 19 546 |
| Formation of ethyl carbamate from urea and ethanol | White wines, pH 3.1–3.5 | [46] | 118 | 19 | 350 |
| Acid hydrolysis of proanthocyanidins (tannins) | Model wine, pH 3.2 | [47] | 45 | 3 | 9 |
| Acid hydrolysis of malvidin‐3‐glucoside | Model wine, pH 3.5 | [48] | 118 | 19 | 350 |
a Calculated from E a and the Arrhenius equation.
25.5 Effects of oak storage
The storage and transport of wine in wooden barrels has been practiced since antiquity. Historically, wooden barrels provided a robust (compared to an amphora), watertight, lightweight, and relatively convenient means to carry liquids, even across land – with the added benefit that the barrels could be broken down into staves for easy storage when not in use [49]. While different wood sources can be used for barrel production, oak heartwood is favored because of its large concentration of “tyloses” – plugs within the xylem that render it watertight.
25.5.1 Production of oak barrels and oak alternatives
Excellent technical discussions of the barrel production process have been published [50, 51], and the major steps can be summarized as follows:
- Mature white oak trees (genus Quercus) are harvested. The most commonly utilized species are native to Europe (Q. robur and Q. petraea) and North America (Q. alba).
- Planks are cut from the oak heartwood and dried. Classically, this is done by stacking planks outside and air‐drying (“seasoning”) for long periods, typically 1–2 years. Alternate drying strategies that involve partial or complete usage of elevated temperatures also exist.
- Planks are cut into barrel staves.
- A cooper (barrel‐maker) positions staves in metal hoops to form the barrel. The staves are heated during barrel production to increase their pliability. Traditionally, heating was done over a fire, although in modern production alternate heat sources (e.g., infrared lamps, steam) can be used.
- Once the barrel is formed, the cooper may continue to heat (“toast”) the inside of the barrel to further alter flavor chemistry, as described below.
- The barrel is finished by addition of circular top and bottom pieces (“heads”), and cutting of a bung hole.
Alternative forms of oak – such as chips, shavings, cubes, and spiral rods – can be produced from waste material generated during stave production. Because of their smaller size, toasting of alternatives is often done in ovens as opposed to a point heat source, and is thus more uniform.
The most notable effect of maturing wine in contact with oak is the extraction of aroma compounds (Chapters 7, 10 and 12). The key compound classes and their precursors in oak (where relevant) are listed in Table 25.2.
Table 25.2 Major components of oak heartwood and their role as precursors to key aroma compounds in oak barrels
| Compound class |
Concentration (dry weight) [50, 51] |
Derived compounds | Aromas | Notes on derived compounds |
| Lignin | 25–30% | Volatile phenols and phenolic aldehydes (e.g., guaiacol, vanillin) | Smoky, spicy, vanilla | Via toasting |
| Polysaccharides (cellulose, hemicellulose) | 60–70% | Carbohydrate degradation products (e.g., furaneol) | Toast, caramel | Via toasting |
| Lipids | 1–2% | Oak lactones Unsaturated aldehydes (e.g., (E)‐2‐nonenal) |
Coconut, sweet Rancid oil, cardboard |
Native, or via glycoside or hydroxyacid precursors Via unsaturated fatty acid oxidation |
| Hydrolyzable tannin | 5–10% | – | – | – |
Like other woods, oak is primarily composed of lignocellulose consisting of two classes of structural polymers (lignin and polysaccharides). These polymer classes have poor solubility in wine, but can serve as precursors for hundreds of volatiles under pyrolytic toasting conditions [52, 53]; these include several key aroma compounds (Table 25.2):
- Lignin, a highly crosslinked polymer of phenols, can pyrolyze to form volatile phenols and aldehydes with smoky, spicy, and vanilla aromas (Figure 25.10).
- Polysaccharides, particularly hemicellulose, can pyrolyze to form heterocyclic compounds with caramel and toasty aromas. The other major polysaccharide in wood, cellulose, appears to be of less importance to odorant formation due to its greater thermal stability [54].
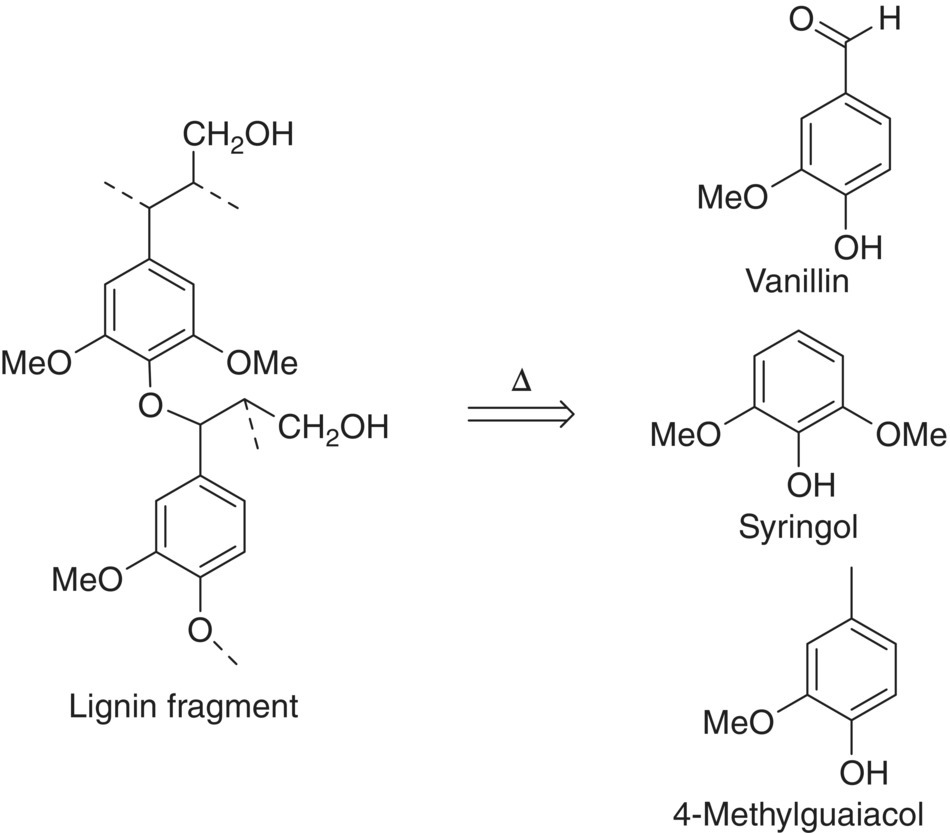
Figure 25.10 Thermal degradation of lignin yielding representative volatile phenols
Several factors will affect the concentrations of oak‐derived volatiles for wine stored in contact with oak, but the three most important are wood source, barrel toasting protocols, and extraction conditions.
25.5.2 Variation due to tree source
Several volatiles – particularly the oak lactones and their precursors – can vary considerably from tree to tree. Variation may also exist for the toast‐derived compounds, but any source variation is usually overwhelmed by differences caused by the toasting process (see below). For the oak lactones:
- Species has a profound effect on concentrations in oak and resulting wines. In a survey of individual trees, Q. petraea averaged over 15‐fold higher in total oak lactones than Q. robur (10.78 versus 0.61 µg/g) [55]. Similarly high concentrations of oak lactones have been observed for Q. alba [56], which likely contributes to American oak’s anecdotal reputation as possessing more noticeable “coconut” and “woody” aromas.
- The localized growing environment will also have an effect, with standard deviations >100% and evidence of “high” and “low” regions within the same forest. The reason for higher oak lactone production in certain zones is unknown, although insect or disease pressure may contribute [55].
Grain tightness – that is, the spacing between tree growth rings – was historically believed to affect oak extractables, with a finer grain wood putatively having lower tannins and higher volatile content, although comparisons were often confounded by locational or species differences. More recent large‐scale studies of hundreds of oak trees within and across sites have shown negligible correlations between volatiles and ring width [55, 57]. Similarly, negliglible changes were observed in sensory profiles of wines aged in oak barrels produced from different grain sizes [58].
25.5.3 Variation due to toasting
Toasting protocols vary in their length of time and in the temperatures achieved by the wood surface. Typically, the lexicon of “light,” “medium,” and “heavy” is used to describe increasing toast levels, although defining these conditions precisely is a challenge. One text has suggested a criteria of 120–180 °C surface temperature and a 5 min toast time for light, 200 °C and 10 min for medium, and 230 °C and 15 min for heavy [59], but other authors have suggested lower or higher maximum temperatures for medium and heavy toasts [58]. Furthermore, these reports do not generally account for the time the barrel is held below the maximum, during which toasting may still occur. Finally, the degree of toasting is typically heterogenous across a barrel, with the top and the bottom experiencing higher temperatures and more toasting than the middle of the barrel [58]. Detailed information about temperature profiles during toasting can be achieved with embedded thermocouples, but this is not a widespread practice. In sum, comparing absolute numbers on toast‐derived volatiles across literature reports is challenging due to variations in cooperage methods.
As reported earlier in this book, both lignin‐derived volatile phenols (Chapter 12) and carbohydrate degradation products (Chapter 2) are sensorially subthreshold in untoasted wood and only accumulate during toasting. The former (volatile phenols) are proportionally favored at higher toast temperatures or times (Figure 25.11). Carbohydrate degradation products appear to reach a maximum at lower toast temperatures, and in some cases they decline at high toast temperatures. The reason for this may be due to volatilization at high temperatures, but also because the majority of hemicellulose pyrolysis occurs at temperatures that are ~50 °C lower than lignin pyrolysis [54].

Figure 25.11 The correlation of final wood temperature with concentrations of a lignin degradation product (guaiacol) and a carbohydrate degradation product (furfural) during barrel toasting. Temperatures were measured by thermocouples positioned within the barrel staves. Concentrations in untoasted portions of the wood averaged 0.03 µg/g for guaiacol and 0.003 mg/g for furfural.
Data from Reference [60]
The large effects of the toast temperature on volatiles can help rationalize the high variability in toast‐derived volatiles among barrels. High variability in toast profiles is seen not only among coopers but by the same cooper. A recent study of barrel toast patterns within a cooperage showed standard deviations of ±15 °C at various time points – in other words, the difference between medium and heavy toast levels [60].
In combination, the biological variability among trees and production variability during the toasting process explains the high variability in volatiles observed among barrels – for 10 barrel lots, standard deviations ranging from 15 to 40% were observed for several representative compounds (oak lactones, volatile phenols, furfural derivatives) [61]. Based on this, standard deviations of 50–125% are to be expected for key aroma compounds across individual barrels. 6
25.5.4 Variation due to extraction
Volatiles are not extracted instantaneously from the barrel surface, and several factors vary the rate and amount of extraction:
- Many (but not all) phenols and carbohydrate degradation products obey roughly first‐order kinetics, with concentrations eventually reaching an apparent equilibrium. In one study using traditional 225 L barrels, concentrations of guaiacol and syringol reached 50% of maximum within 3 weeks and furfural/5‐methylfurfural within 2 months.
- Precursor‐derived compounds, particularly oak lactone (Chapter 7), will continue to increase even once oak barrel contact ceases. For example, oak lactone increased by 25% during 3 months of bottle storage after an initial 9 month barrel storage period [62]. Glycosylated precursors of vanillin and other phenols have also been detected in oak wood [63], which may explain why vanillin increases linearly even after 2 years of barrel storage of a model wine [64].
- The amount of volatiles extracted will, of course, be lower in reused barrels – in one report, about 2‐fold lower for guaiacol and 10‐fold lower for furfuryl compounds after 180 days of storage [65]. The extraction rate of volatiles from reused barrels may also appear linear rather than asymptotic, presumably because volatiles must diffuse to the wood surface from the barrel interior.
- Oak alternatives with a small particle size, such as oak shavings, are reported to reach equilibrium faster than larger oak formats like chips [66]. In very general terms, this is credited to “a greater surface area‐to‐volume ratio.” More specifically, this could be because small particles decrease the size of the boundary layer around the oak particle.
Finally, volatiles extracted from oak can undergo further (bio)chemical reactions in the wine. Aldehydes like vanillin and furfural are particularly prone to reactions, for example, reduction to alcohols by yeast (Chapter 22.1), reaction with H2S to yield thiols during barrel‐fermentation (Chapter 10), or reaction with anthocyanins or other phenolics during storage [67].
25.5.5 Other effects of oak
Beyond volatiles, oak storage can result in several other changes to wine, although the last two are only relevant to barrel storage as opposed to oak alternatives:
- Extraction of hydrolyzable tannins (Chapter 13) and other non‐volatile components from the wood [50]. As mentioned earlier, tannins are usually extracted at concentrations far below the sensory threshold. However, recent reports have identified bitter tasting lignans [68] (a class of polyphenols) and sweet tasting triterpenoids [69] at concentrations around threshold.
- Adsorption of wine components by the wood [3, 70].
- Oxygen ingress through diffusion, either due to the porosity of the wood, the gaps between staves, or ullage when the bung is removed. In combination, these typically lead to oxygen pickup on the order of a few mg/L/month in a standard 225 L barrel, but the relative importance of these effects is contentious [50, 71, 72].
- A concentrating effect over time due to diffusion through staves and evaporation (around –5% per annum), along with slight changes in ethanol concentration (either an increase or decrease) as a function of relative humidity and factors (e.g., temperature, air speed) related to the storage environment [50, 73] – the angels’ share.
25.6 Sensory effects of different aging conditions
As a general rule, bottle or tank aging of a wine results in a loss of fruity, floral, and fresh vegetable aromas and an increase in dried fruit, earthy, canned vegetable, woody, and either sulfurous (e.g., due to hydrolysis of S‐methylmethionine, Chapter 23.3) or oxidation aromas (Figure 25.12). Oak‐derived flavors may also increase in the case of barrel storage, among other changes as specified above. Greater oxygen exposure will increase concentrations of odorants with “oxidized” aromas (e.g., carbonyl compounds, Chapters 9 and 24), but also result in loss of thiols with “reduced” and “fruity” aromas (Chapter 10).

Figure 25.12 Effect of storage temperature (10, 20, 40 °C) on aroma attribute ratings (mean values for significant attributes) for (a) a Chardonnay wine stored in bottle for 3 months and (b) a Cabernet Sauvignon wine stored in bottle for 6 months (Molas/soy = molasses/soy sauce).
Data from References [76] and [77]
Higher storage temperatures and shorter times will partially simulate the effects of longer storage at cooler temperatures, particularly for the loss of primary and secondary characters observed for white wines, but also for some developed characters in red wines [74]. This information is relevant in a global market place where wines are shipped internationally, and by some estimates wine may appear to be aged an additional year or more during transit compared to isothermal storage at ordinary cellar temperatures [75]. However, as described in section 25.4, the relative rates of reactions important to wine flavor will vary with changes in temperature.
Tannin maturation in tank or barrel and aging in bottle leads not only to stabilization of color (as outlined above) but also results in decreased intensity of astringency (Chapters 14 and 24; also see References [78] and [79], and citations therein). Red wine quality is well correlated with having appropriate levels of astringency [80, 81]. Bottle aging is a classic approach to decrease the astringency of tannic young red wines, and some high tannin (and high price point wines) will be allowed to cellar‐age for several years to allow the mouthfeel to “soften.” For example, Cabernet Sauvignon wines produced by a single chateau in France and stored under similar conditions show a linear correlation (R 2 = 0.598) between vintage year and astringency scores [82] (Figure 25.13). 7 These changes arise from transformations of proanthocyanidins during storage that decrease their ability to react with proteins and, thus, decrease their astringency [22, 29, 83]. Many reactions involving proanthocyanidins will happen more readily in the presence of O2 and at lower pH (as outlined above), including:
- Reactions with anthocyanins (to form T‐A or A‐T adducts).
- Reactions with aldehydes, vinylphenols, or other electrophiles.
- Acid hydrolysis, to yield smaller tannins (lower degree of polymerization) with decreased astringency.

Figure 25.13 Links between wine age (vintage), mean astringency rating, and proanthocyanidin (PA) concentration (g/L), determined by depolymerization with acid and measuring absorbance at 550 nm, for a series of Cabernet Sauvignon wines from a single French chateau that were prepared and cellared under similar conditions.
Data from Reference [82]
Interestingly, the correlation of astringency with total proanthocyanidins was much weaker (R 2 = 0.36, Figure 25.13), which is evidence that most chemical measurements of tannins are imperfectly correlated with protein‐binding capacity (Chapter 33).
Decisions about wine packaging are some of the most crucial to be made, in part because the winemaker typically has little recourse once a wine is packaged. The effects of material including glass and PET bottles, and bag‐in‐box pouches, along with natural cork, synthetic, and screw cap closures, have been investigated in several studies [7, 8, 29, 76, 77, 84–94]:
- Permeability of the packaging has a strong influence on sensory properties over time, as demonstrated with closure OTR. While there are reasonable differences within a closure type, they tend to obey the following pattern in increasing OTR:
Screw caps < Technical < Natural < Synthetic
Increased closure OTR can facilitate beneficial reactions, as highlighted above with red wine color and mouthfeel, but it also leads to losses of SO2. This can have particularly stark effects on white wine once the protective effects of SO2 are diminished, with an increase in browning (A420), loss of fruit characters, and an increase in “oxidized” and “developed” aromas (Figure 25.14). Conversely, “reduced” aroma attributes are more often ascribed to screw cap closures with a low OTR (Chapter 30), yet these closures preserve SO2 and fruit characteristics. Similar considerations regarding permeability apply to the choice of container material; that is, whether it is made of glass or plastic (as in PET bottles or bag‐in‐box pouches).
- Packaging can cause taints due to migration of odorants from the packaging into the wine, or scalping due to absorption (or adsorption) of odorants into the packaging [95]. The best known of these conditions is “cork taint” (Chapter 18), but other packaging materials (particularly plastic‐based) can also cause this. For example, after 3–6 months, sensory changes in white wine can be readily noted for certain plastics due to scalping or tainting [89, 91].

Figure 25.14 Mean aroma ratings for a Clare Valley Semillon wine bottled under a variety of closures, cellared at an average temperature of 17 °C and assessed by a sensory panel after (a) 6 months and (b) 12 months. ROTE = roll on tamper evident (i.e., screw cap).
Selected data from Reference [84]
Finally, the role of lees aging (sur lie) deserves mention for its ability to influence wine sensory properties [3, 5, 96, 97]. Lees in this instance describes the autolysis of yeast (primarily) and bacteria cells, which releases a range of components into the wine. Among other things, these include:
- Enzymes that lead to glycoside hydrolysis (Chapter 23.1).
- Nitrogenous compounds such as amino acids, proteins and peptides, and mannoproteins, which can affect perceived sweetness and body (Chapter 6), stabilize foam in sparkling wines, as well as increase tartrate stability (Chapter 26.1).
- Aroma compounds (e.g., fatty acids, alcohols, and esters), which can lead to perception of the “yeasty” and “bread‐like” aroma of sur lie aged wine.
- Polysaccharides that can impact mouthfeel (Chapter 2).
Additionally, storage in contact with lees under reductive conditions is implicated in the formation of sulfurous off aromas (Chapters 10 and 22.4) but lees can also be used to bind these compounds and remove them from wine. The differences between these roles of lees presumably relates to their relative degrees of oxidation. Similarly, lees can also adsorb other compounds, particularly non‐polar volatiles and phenolic components, and, overall, aging on lees is aimed at improving wine quality through compositional changes that modify aroma, flavor, body and mouthfeel, and stabilize foam in sparkling wines. Commercial yeast autolysates (or other inactive dry yeast preparations) are available to complement or replace the lees arising from winery fermentations, potentially allowing a more rapid incorporation of beneficial components in a shorter time than would occur with ordinary storage on lees.
References
- 1. Singleton, V.L. (2000) A survey of wine aging reactions, especially with oxygen. Proceedings of the 50th Anniversary Annual Meeting, Seattle, Washington, pp. 323–336.
- 2. Ebeler, S.E. (2001) Analytical chemistry: unlocking the secrets of wine flavor. Food Reviews International , 17 (1), 45–64.
- 3. Garde‐Cerdán, T. and Ancín‐Azpilicueta, C. (2006) Review of quality factors on wine ageing in oak barrels. Trends in Food Science and Technology , 17 (8), 438–447.
- 4. Alexandre, H. and Guilloux‐Benatier, M. (2006) Yeast autolysis in sparkling wine – a review. Australian Journal of Grape and Wine Research , 12 (2), 119–127.
- 5. Pérez‐Serradilla, J.A. and de Castro, M.D.L. (2008) Role of lees in wine production: a review. Food Chemistry , 111 (2), 447–456.
- 6. Jackson, R.S. (2009) Nature and origins of wine quality, in Wine tasting: a professional handbook , 2nd edn, Academic Press, San Diego, CA, pp. 387–426.
- 7. Silva, M., Julien, M., Jourdes, M., Teissedre, P.‐L. (2011) Impact of closures on wine post‐bottling development: a review. European Food Research and Technology , 233 (6), 905–914.
- 8. Ugliano, M. (2013) Oxygen contribution to wine aroma evolution during bottle aging. Journal of Agricultural and Food Chemistry , 61 (26), 6125–6136.
- 9. Tao, Y., García, J.F., Sun, D.‐W. (2014) Advances in wine aging technologies for enhancing wine quality and accelerating wine aging process. Critical Reviews in Food Science and Nutrition , 54 (6), 817–835.
- 10. Cheynier, V., Remy, S., Fulcrand, H. (2000) Mechanisms of anthocyanin and tannin changes during winemaking and aging, in Proceedings of the ASEV 50th Anniversary Annual Meeting (ed. Rantz, J.M.), June 19–23, 2000, Seattle, WA, American Society of Enology and Viticulture, Davis, CA, pp. 337–344.
- 11. Cheynier, V., Duenas‐Paton, M., Salas, E., et al. (2006) Structure and properties of wine pigments and tannins. American Journal of Enology and Viticulture , 57 (3), 298–305.
- 12. He, F., Liang, N.‐N., Mu, L., et al. (2012) Anthocyanins and their variation in red wines. II. Anthocyanin derived pigments and their color evolution. Molecules, 17 (2), 1483–1519.
- 13. de Freitas, V.A.P. and Mateus, N. (2010) Updating wine pigments, in Recent advances in polyphenol research , Wiley‐Blackwell, pp. 59–80.
- 14. Salas, E., Fulcrand, H., Meudec, E., Cheynier, V. (2003) Reactions of anthocyanins and tannins in model solutions. Journal of Agricultural and Food Chemistry , 51 (27), 7951–7961.
- 15. Duenas M., Fulcrand H., Cheynier V. (2006) Formation of anthocyanin‐flavanol adducts in model solutions. Analytica Chimica Acta , 563 (1–2), 15–25.
- 16. Somers, T.C. (1971) The polymeric nature of wine pigments. Phytochemistry , 10, 2175–2189.
- 17. Malien‐Aubert, C., Dangles, O., Amiot, M.J. (2002) Influence of procyanidins on the color stability of oenin solutions. Journal of Agricultural and Food Chemistry , 50 (11), 3299–3305.
- 18. Escribano‐Bailón, T., Álvarez‐García, M., Rivas‐Gonzalo, J.C., et al. (2001) Color and stability of pigments derived from the acetaldehyde‐mediated condensation between malvidin 3‐O‐glucoside and (+)‐catechin. Journal of Agricultural and Food Chemistry , 49 (3), 1213–1217.
- 19. Atanasova, V., Fulcrand, H., Cheynier, W., Moutounet, M. (2002) Effect of oxygenation on polyphenol changes occurring in the course of wine‐making. Analytica Chimica Acta , 458 (1), 15–27.
- 20. Remy‐Tanneau, S., Le Guerneve, C., Meudec, E., Cheynier, V. (2003) Characterization of a colorless anthocyanin‐flavan‐3‐ol dimer containing both carbon–carbon and ether interflavanoid linkages by NMR and mass spectrometry. Journal of Agricultural and Food Chemistry , 51 (12), 3592–3597.
- 21. Romero, C. and Bakker, J. (2000) Effect of acetaldehyde and several acids on the formation of vitisin A in model wine anthocyanin and colour evolution. International Journal of Food Science and Technology , 35 (1), 129–140.
- 22. McRae, J.M., Dambergs, R.G., Kassara, S., et al. (2012) Phenolic compositions of 50 and 30 year sequences of Australian red wines: the impact of wine age. Journal of Agricultural and Food Chemistry , 60 (40), 10093–10102.
- 23. Pechamat, L., Zeng, L., Jourdes, M., et al. (2014) Occurrence and formation kinetics of pyranomalvidin–procyanidin dimer pigment in Merlot red wine: impact of acidity and oxygen concentrations. Journal of Agricultural and Food Chemistry , 62 (7), 1701–1705.
- 24. Gómez‐Plaza, E. and Cano‐López, M. (2011) A review on micro‐oxygenation of red wines: claims, benefits and the underlying chemistry. Food Chemistry , 125 (4), 1131–1140.
- 25. Anli, R.E. and Cavuldak, Ö.A. (2012) A review of microoxygenation application in wine. Journal of the Institute of Brewing , 118 (4), 368–385.
- 26. Gambuti, A., Rinaldi, A., Ugliano, M., Moio, L. (2013) Evolution of phenolic compounds and astringency during aging of red wine: effect of oxygen exposure before and after bottling. Journal of Agricultural and Food Chemistry , 61 (8), 1618–1627.
- 27. Gonzalez‐del Pozo, A., Arozarena, I., Noriega, M.J., et al. (2010) Short‐ and long‐term effects of micro‐oxygenation treatments on the colour and phenolic composition of a Cabernet Sauvignon wine aged in barrels and/or bottles. European Food Research and Technology , 231 (4), 589–601.
- 28. Kontoudakis, N., González, E., Gil, M., et al. (2011) Influence of wine pH on changes in color and polyphenol composition induced by micro‐oxygenation. Journal of Agricultural and Food Chemistry , 59 (5), 1974–1984.
- 29. McRae, J.M., Kassara, S., Kennedy, J.A., et al. (2013) Effect of wine pH and bottle closure on tannins. Journal of Agricultural and Food Chemistry , 61 (47), 11618–11627.
- 30. Daniel, M.A., Capone, D.L., Sefton, M.A., Elsey, G.M. (2009) Riesling acetal is a precursor to 1,1,6‐trimethyl‐1,2‐dihydronaphthalene (TDN) in wine. Australian Journal of Grape and Wine Research , 15 (1), 93–96.
- 31. Giaccio, J., Capone, D.L., Håkansson, A.E., et al. (2011) The formation of wine lactone from grape‐derived secondary metabolites. Journal of Agricultural and Food Chemistry , 59 (2), 660–664.
- 32. Winterhalter, P. (1991) 1,1,6‐Trimethyl‐1,2‐dihydronaphthalene (TDN) formation in wine. 1. Studies on the hydrolysis of 2,6,10,10‐tetramethyl‐1‐oxaspiro[4.5]dec‐6‐ene‐2,8‐diol rationalizing the origin of TDN and related C13 norisoprenoids in Riesling wine. Journal of Agricultural and Food Chemistry , 39 (10), 1825–1829.
- 33. Luan, F., Degenhardt, A., Mosandl, A., Wust, M. (2006) Mechanism of wine lactone formation: demonstration of stereoselective cyclization and 1,3‐hydride shift. Journal of Agricultural and Food Chemistry , 54 (26), 10245–10252.
- 34. Diaz‐Maroto, M.C., Schneider, R., Baumes, R. (2005) Formation pathways of ethyl esters of branched short‐chain fatty acids during wine aging. Journal of Agricultural and Food Chemistry , 53 (9), 3503–3509.
- 35. Makhotkina, O. and Kilmartin, P.A. (2012) Hydrolysis and formation of volatile esters in New Zealand Sauvignon Blanc wine. Food Chemistry , 135 (2), 486–493.
- 36. Antalick, G., Perello, M.‐C., de Revel, G. (2014) Esters in wines: new insight through the establishment of a database of French wines. American Journal of Enology and Viticulture , 65 (3), 293–304.
- 37. Rayne, S. and Forest, K. (2011) Estimated carboxylic acid ester hydrolysis rate constants for food and beverage aroma compounds. Nature Precedings. doi:10.1038/npre.2011.6471.1. Available from Nature Precedings, http://dx.doi.org/10.1038/npre.2011.6471.1.
- 38. Marais, J. (1978) Effect of pH on esters and quality of colombar wine during maturation. Vitis , 17 (4), 396–403.
- 39. Makhotkina, O., Pineau, B., Kilmartin, P.A. (2012) Effect of storage temperature on the chemical composition and sensory profile of Sauvignon Blanc wines. Australian Journal of Grape and Wine Research , 18 (1), 91–99.
- 40. Wilkinson, K.L., Prida, A., Hayasaka, Y. (2013) Role of glycoconjugates of 3‐methyl‐4‐hydroxyoctanoic acid in the evolution of oak lactone in wine during oak maturation. Journal of Agricultural and Food Chemistry , 61 (18), 4411–4416.
- 41. Pereira, V., Albuquerque, F.M., Ferreira, A.C., et al. (2011) Evolution of 5‐hydroxymethylfurfural (HMF) and furfural (F) in fortified wines submitted to overheating conditions. Food Research International , 44 (1), 71–76.
- 42. Belitz, H.‐D., Grosch, W., Schieberle, P. (2009) Carbohydrates, in Food chemistry (eds Belitz, H.‐D., Grosch, W., Schieberle, P.), Springer, Berlin, pp. 248–339.
- 43. Ramey, D.D. and Ough, C.S. (1980) Volatile ester hydrolysis or formation during storage of model solutions and wines. Journal of Agricultural and Food Chemistry , 28 (5), 928–934.
- 44. Scheuren, H., Tippmann, J., Methner, F.J., Sommer, K. (2014) Decomposition kinetics of dimethyl sulphide. Journal of the Institute of Brewing , 120 (4), 474–476.
- 45. Arena, E., Fallico, B., Maccarone, E. (2001) Thermal damage in blood orange juice: kinetics of 5‐hydroxymethyl‐2‐furancarboxaldehyde formation. International Journal of Food Science and Technology , 36 (2), 145–151.
- 46. Kodama, S., Suzuki, T., Fujinawa, S., et al. (1994) Urea contribution to ethyl carbamate formation in commercial wines during storage. American Journal of Enology and Viticulture , 45 (1), 17–24.
- 47. Dallas, C., Hipólito‐Reis, P., Ricardo‐da‐Silva, J.M., Laureano, O. (2003) Influence of acetaldehyde, pH, and temperature on transformation of procyanidins in model wine solutions. American Journal of Enology and Viticulture , 54 (2), 119–124.
- 48. Baranowski, E.S. and Nagel, C.W. (1983) Kinetics of malvidin‐3‐glucoside condensation in wine model systems. Journal of Food Science , 48 (2), 419–421.
- 49. McGovern, P.E. (2003) Ancient wine: the search for the origins of viniculture , Princeton University Press, Princeton, NJ.
- 50. Singleton, V.L. (1995) Maturation of wines and spirits: comparisons, facts, and hypotheses. American Journal of Enology and Viticulture , 46 (1), 98–115.
- 51. Schahinger, G. (2005) Cooperage for winemakers: a manual on the construction, maintenance, and use of oak barrels (ed. Rankine, B.C.), Winetitles, Adelaide, SA, Australia.
- 52. Faix, O., Fortmann, I., Bremer, J., Meier, D. (1991) Thermal degradation products of wood. Holz als Roh‐ und Werkstoff , 49 (7–8), 299–304.
- 53. Faix, O., Meier, D., Fortmann, I. (1990) Thermal degradation products of wood. Holz als Roh‐ und Werkstoff , 48 (7–8), 281–285.
- 54. Brebu, M. and Vasile, C. (2010) Thermal degradation of lignin – a review. Cellulose Chemistry and Technology , 44 (9), 353.
- 55. Prida, A., Ducousso, A., Petit, R., et al. (2007) Variation in wood volatile compounds in a mixed oak stand: strong species and spatial differentiation in whisky‐lactone content. Annals of Forest Science , 64 (3), 313–320.
- 56. Masson, G., Guichard, E., Fournier, N., Puech, J.‐L. (1995) Stereoisomers of ß‐methyl‐γ‐octalactone. II. Contents in the wood of French (Quercus robur and Quercus petraea) and American (Quercus alba) oaks. American Journal of Enology and Viticulture , 46 (4), 424–428.
- 57. Mosedale, J.R. and Savill, P.S. (1996) Variation of heartwood phenolics and oak lactones between the species and phenological types of Quercus petraea and Q. robur. Forestry , 69 (1), 47–55.
- 58. Collins, T.S. (2012) The impact of variability in the toasting process at a commercial cooperage on the volatile composition of oak barrels and barrel aged wines, PhD Thesis, University of California, Davis.
- 59. Ribereau‐Gayon, P., Glories, Y., Maujean, A., Dubourdieu, D. (2006) Handbook of enology , Vol. 2, The chemistry of wine stabilization and treatments, 2nd edn, John Wiley & Sons, Chichester, UK.
- 60. Collins, T.S., Miles, J.L., Boulton, R.B., Ebeler, S.E. (2015) Targeted volatile composition of oak wood samples taken during toasting at a commercial cooperage. Tetrahedron , 71 (20), 2971–2982.
- 61. Towey, J.P. and Waterhouse, A.L. (1996) Barrel‐to‐barrel variation of volatile oak extractives in barrel‐fermented Chardonnay. American Journal of Enology and Viticulture , 47 (1), 17–20.
- 62. Perez‐Prieto, L.J., Lopez‐Roca, J.M., Martinez‐Cutillas, A., et al. (2003) Extraction and formation dynamic of oak‐related volatile compounds from different volume barrels to wine and their behavior during bottle storage. Journal of Agricultural and Food Chemistry , 51 (18), 5444–5449.
- 63. Slaghenaufi, D., Marchand‐Marion, S., Richard, T., et al. (2013) Centrifugal partition chromatography applied to the isolation of oak wood aroma precursors. Food Chemistry , 141 (3), 2238–2245.
- 64. Spillman, P.J., Iland, P.G., Sefton, M.A. (1998) Accumulation of volatile oak compounds in a model wine stored in American and Limousin oak barrels. Australian Journal of Grape and Wine Research , 4 (2), 67–73.
- 65. Gómez‐Plaza, E., Pérez‐Prieto, L.J., Fernández‐Fernández, J.I., López‐Roca, J.M. (2004) The effect of successive uses of oak barrels on the extraction of oak‐related volatile compounds from wine. International Journal of Food Science and Technology , 39 (10), 1069–1078.
- 66. Campbell, J., Pollnitz, A., Sefton, M., et al. (2006) Factors affecting the influence of oak chips on wine flavour. Australian and New Zealand Wine Industry Journal , 21 (4), 38–42.
- 67. Nonier, M.‐F., Vivas, N., De Freitas, V., et al. (2011) A kinetic study of the reaction of (+)‐catechin and malvidin‐3‐glucoside with aldehydes derived from toasted oak. The Natural Products Journal , 1 (1), 47–56.
- 68. Marchal, A., Cretin, B.N., Sindt, L., et al. (2015) Contribution of oak lignans to wine taste: chemical identification, sensory characterization and quantification. Tetrahedron , 71 (20), 3148–3156.
- 69. Marchal, A., Waffo‐Teguo, P., Genin, E., et al. (2011) Identification of new natural sweet compounds in wine using centrifugal partition chromatography–gustatometry and Fourier transform mass spectrometry. Analytical Chemistry , 83 (24), 9629–9637.
- 70. Jarauta, I., Cacho, J., Ferreira, V. (2005) Concurrent phenomena contributing to the formation of the aroma of wine during aging in oak wood: an analytical study. Journal of Agricultural and Food Chemistry , 53 (10), 4166–4177.
- 71. Nevares, I., Crespo, R., Gonzalez, C., del Alamo‐Sanza, M. (2014) Imaging of oxygen transmission in the oak wood of wine barrels using optical sensors and a colour camera. Australian Journal of Grape and Wine Research , 20 (3), 353–360.
- 72. Nevares, I. and del Alamo‐Sanza, M. (2015) Oak stave oxygen permeation: a new tool to make barrels with different wine oxygenation potentials. Journal of Agricultural and Food Chemistry , 63 (4), 1268–1275.
- 73. Blazer, R.M. (1991) Influence of environment – wine evaporation from barrels. Practical Winery and Vineyard , 11, 20–22.
- 74. Robinson, A.L., Mueller, M., Heymann, H., et al. (2010) Effect of simulated shipping conditions on sensory attributes and volatile composition of commercial white and red wines. American Journal of Enology and Viticulture , 61 (3), 337–347.
- 75. Butzke, C.E., Vogt, E.E., Chacón‐Rodríguez, L. (2012) Effects of heat exposure on wine quality during transport and storage. Journal of Wine Research , 23 (1), 15–25.
- 76. Hopfer, H., Ebeler, S.E., Heymann, H. (2012) The combined effects of storage temperature and packaging type on the sensory and chemical properties of Chardonnay. Journal of Agricultural and Food Chemistry , 60 (43), 10743–10754.
- 77. Hopfer, H., Buffon, P.A., Ebeler, S.E., Heymann, H. (2013) The combined effects of storage temperature and packaging on the sensory, chemical, and physical properties of a Cabernet Sauvignon wine. Journal of Agricultural and Food Chemistry , 61 (13), 3320–3334.
- 78. del Carmen Llaudy, M., Canals, R., Gonzalez‐Manzano, S., et al. (2006) Influence of micro‐oxygenation treatment before oak aging on phenolic compounds composition, astringency, and color of red wine. Journal of Agricultural and Food Chemistry , 54 (12), 4246–4252.
- 79. Oberholster, A., Elmendorf, B.L., Lerno, L.A., et al. (2015) Barrel maturation, oak alternatives and micro‐oxygenation: influence on red wine aging and quality. Food Chemistry , 173, 1250–1258.
- 80. Fanzone, M., Peña‐Neira, A., Gil, M., et al. (2012) Impact of phenolic and polysaccharidic composition on commercial value of Argentinean Malbec and Cabernet Sauvignon wines. Food Research International , 45 (1), 402–414.
- 81. Saenz‐Navajas, M.P., Martin‐Lopez, C., Ferreira, V., Fernandez‐Zurbano, P. (2011) Sensory properties of premium Spanish red wines and their implication in wine quality perception. Australian Journal of Grape and Wine Research , 17 (1), 9–19.
- 82. Chira, K., Jourdes, M., Teissedre, P.‐L. (2012) Cabernet Sauvignon red wine astringency quality control by tannin characterization and polymerization during storage. European Food Research and Technology , 234 (2), 253–261.
- 83. Chira, K., Pacella, N., Jourdes, M., Teissedre, P.‐L. (2011) Chemical and sensory evaluation of Bordeaux wines (Cabernet‐Sauvignon and Merlot) and correlation with wine age. Food Chemistry , 126 (4), 1971–1977.
- 84. Godden, P., Francis, L., Field, J., et al. (2001) Wine bottle closures: physical characteristics and effect on composition and sensory properties of a Semillon wine. 1. Performance up to 20 months post‐bottling. Australian Journal of Grape and Wine Research , 7 (2), 64–105.
- 85. Capone, D., Sefton, M., Pretorius, I., Høj, P. (2003) Flavour “scalping” by wine bottle closures – the “winemaking” continues post vineyard and winery. Australian and New Zealand Wine Industry Journal , 18 (5), 16, 18–20.
- 86. Skouroumounis, G.K., Kwiatkowski, M.J., Francis, I.L., et al. (2005) The impact of closure type and storage conditions on the composition, colour and flavour properties of a Riesling and a wooded Chardonnay wine during five yearsʼ storage. Australian Journal of Grape and Wine Research , 11 (3), 369–384.
- 87. Brajkovich, M., Tibbits, N., Peron, G., et al. (2005) Effect of screwcap and cork closures on SO2 levels and aromas in a Sauvignon Blanc wine. Journal of Agricultural and Food Chemistry , 53 (26), 10006–10011.
- 88. Sefton, M.A. and Simpson, R.F. (2005) Compounds causing cork taint and the factors affecting their transfer from natural cork closures to wine – a review. Australian Journal of Grape and Wine Research , 11 (2), 226–240.
- 89. Ghidossi, R., Poupot, C., Thibon, C., et al. (2012) The influence of packaging on wine conservation. Food Control , 23 (2), 302–311.
- 90. Wirth, J., Caillé, S., Souquet, J.M., et al. (2012) Impact of post‐bottling oxygen exposure on the sensory characteristics and phenolic composition of Grenache rosé wines. Food Chemistry , 132 (4), 1861–1871.
- 91. Revi, M., Badeka, A., Kontakos, S., Kontominas, M.G. (2014) Effect of packaging material on enological parameters and volatile compounds of dry white wine. Food Chemistry , 152, 331–339.
- 92. Karbowiak, T., Gougeon, R.D., Alinc, J.B., et al. (2010) Wine oxidation and the role of cork. Critical Reviews in Food Science and Nutrition , 50 (1), 20–52.
- 93. Guaita, M., Petrozziello, M., Motta, S., et al. (2013) Effect of the closure type on the evolution of the physical‐chemical and sensory characteristics of a Montepulciano dʼAbruzzo rosé wine. Journal of Food Science , 78 (2), C160–C169.
- 94. Silva, M.A., Jourdes, M., Darriet, P., Teissedre, P.‐L. (2012) Scalping of light volatile sulfur compounds by wine closures. Journal of Agricultural and Food Chemistry , 60 (44), 10952–10956.
- 95. Sajilata, M.G., Savitha, K., Singhal, R.S., Kanetkar, V.R. (2007) Scalping of flavors in packaged foods. Comprehensive Reviews in Food Science and Food Safety , 6 (1), 17–35.
- 96. Torresi, S., Frangipane, M.T., Anelli, G. (2011) Biotechnologies in sparkling wine production. Interesting approaches for quality improvement: a review. Food Chemistry , 129 (3), 1232–1241.
- 97. Gambetta, J.M., Bastian, S.E.P., Cozzolino, D., Jeffery, D.W. (2014) Factors influencing the aroma composition of Chardonnay wines. Journal of Agricultural and Food Chemistry , 62 (28), 6512–6534.
