23.3
Conversion of Variety Specific Components, Other
23.3.1 Introduction
Along with glycosides (Chapter 23.1) and S‐conjugates (Chapter 23.2), grape‐derived polyunsaturated fatty acids (PUFAs), hydroxycinnamic acids (HCAs), and S‐methylmethionine (SMM) can also serve as odorant precursors. As with glycosides and S‐conjugates, volatiles formed from these precursors would not be formed through fermentation of a simple sugar + ammonium medium, and are strongly dependent on processing or storage conditions (e.g., maceration conditions, microbial populations, wine age) as well as precursor concentrations.
Several other odorants found in wine appear to have poorly defined relationships with non‐odorous grape‐derived precursors, but are not considered in detail in this chapter. For example, ortho‐aminoacetophenone (o‐AAP, “dirty dishrag, foxy” aroma) is correlated with the “atypical aging” off‐flavor found in some wines (Chapter 5). Although o‐AAP is plausibly formed via oxidation of plant hormone, indole acetic acid (IAA), the correlation between the two components is weak [1], and the proposed mechanism has not been demonstrated in wine or wine‐like conditions. Similarly, indole and skatole in wine are likely to be related to tryptophan (Chapter 5), but it is unclear if this is connected to grape tryptophan content.
23.3.2 Polyunsaturated fatty acid precursors of C6 compounds
The grassy‐smelling C6 alcohols – namely hexan‐1‐ol (hexanol), (Z)‐3‐hexen‐1‐ol (cis‐3‐hexen‐1‐ol), and (E)‐2‐hexen‐1‐ol (trans‐2‐hexenol) – were introduced earlier in this book (Chapter 6). These alcohols are present at negligible concentrations in most intact plant tissues including grapes,1 but instead are formed from enzymatic oxidation of the major fatty acids in grape berries – α‐linolenic and α‐linoleic acids, both PUFAs – following disruption of grape tissue. A key difference between PUFA precursors and other enzymatically transformed precursors discussed elsewhere in Chapter 23 is that PUFAs are enzymatically degraded prior to fermentation through grape enzymes released when berries are crushed, rather than during or after fermentation through microbial action.
The lipid oxidation pathway is outlined in Figure 23.3.1 [2]. In intact berry tissues, PUFAs are predominantly found as glycerolipids, where they have key roles in forming cell membranes (Chapter 22.2), or as triacylglycerides with roles as energy stores. Disruption of the plant tissue (i.e., by crushing grapes) brings the glycerolipids into contact with the critical enzymes in the lipid oxidation pathway. Firstly, a lipase enzyme hydrolyzes the glycerolipid, releasing free PUFAs. These can be stereospecifically converted to their corresponding 13‐hydroperoxides in the presence of oxygen by lipoxygenase enzyme (13‐LOX).2 In the case of 13‐hydroperoxylinolenic acid, the hydroperoxide lyase enzyme (HPL) then catalyzes the formation of (Z)‐3‐hexenal, which can subsequently be isomerized and/or reduced by additional enzymes to form other related C6 compounds, including (Z)‐3‐hexen‐1‐ol, (E)‐2‐hexenal, and (E)‐2‐hexen‐1‐ol. An analogous pathway is responsible for the formation of hexanal and 1‐hexanol from α‐linoleic acid.
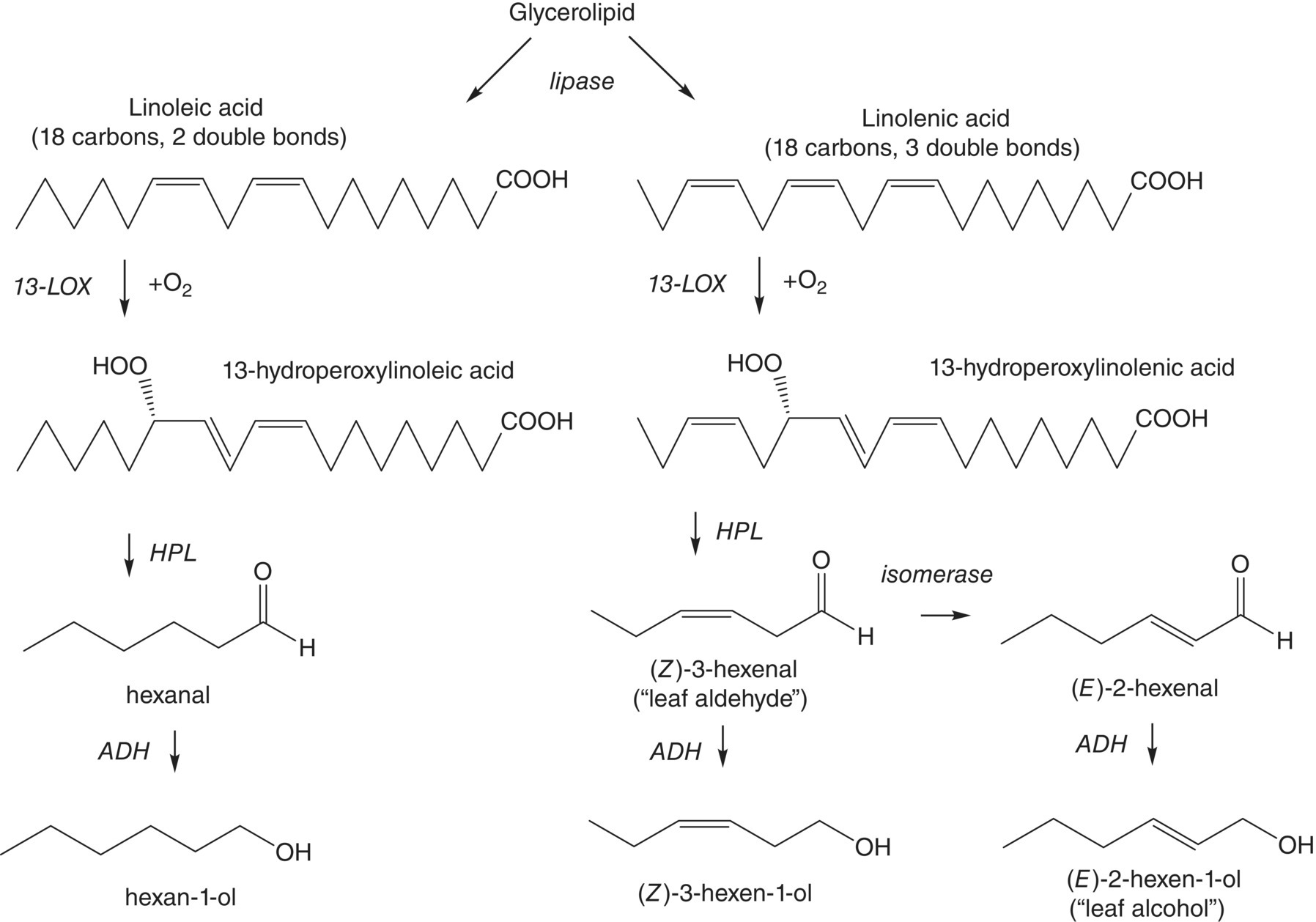
Figure 23.3.1 Enzymatic pathway for formation of C6 alcohols and aldehydes in grapes and other plants. HPL = hydroperoxide lyase, LOX = lipoxgenase, ADH = alcohol dehydrogenase
Lipid‐derived oxidation products are responsible for the major detectable volatiles in crushed grapes – in one report, 16 of the 27 volatiles identified in a grape macerate were direct products or derivatives of unsaturated fatty acids [3]. Of these volatiles, the C6 aldehydes, particularly (E)‐2‐hexenal (sensory threshold in water = 17 µg/L), hexanal (4.5 µg/L), and (Z)‐3‐hexenal (0.25 µg/L), are likely the major contributors to the green/grassy aromas of freshly crushed grapes. Blender macerated Cabernet Sauvignon berries reportedly have 8000 µg/kg of (E)‐2‐hexenal, 2400 µg/kg of hexanal, and 910 µg/kg of (Z)‐3‐hexenal, which translates into OAV > 470 for all three compounds [4]. In real musts, which hopefully undergo less thorough maceration, C6 aldehyde concentrations following crushing are generally about 10% of the aforementioned values [5], but still above sensory threshold values. Considering these high OAVs, it is likely that the major retronasal aroma character perceived when chewing on grape skins is due to these “green” aldehydes, particularly for non‐aromatic grape varieties.
23.3.2.1 Changes during fermentation
During fermentation, hexanol and (Z)‐3‐hexenol remain relatively stable or increase, while the C6 aldehydes and 2‐alkenols decrease by 95% or more to nearly undetectable concentrations within the first 24–48 hours of fermentation [6]. There are several reasons for this phenomenon:
- During fermentation, alkenals and 2‐alkenols will be reduced to corresponding saturated alcohols (Figure 23.3.2). This is the major pathway accounting for loss of (E)‐2‐hexen‐1‐ol, hexanal, and (E)‐2‐hexenal [7, 8] (Chapter 22.1).
- (E)‐2‐Hexenal can react with grape‐derived glutathione to form a precursor for 3‐mercaptohexan‐1‐ol (Chapter 23.2).
- A small portion of hexanol (i.e., <1%), and possibly other lipid derived alcohols, may be enzymatically esterified to their corresponding acetate esters [7], as described earlier (Chapters 7 and 22.3).
- Other possible fates for C6 compounds during fermentation include volatilization, binding to lees, or partial oxidation to hexanoic acid.
![Schematic flows depicting the fate of unstable C6 compounds in the reductive fermentation environment. [H] represents a reducing equivalent, typically NAD(P)H.](images/c23f003_2.gif)
Figure 23.3.2 Fate of unstable C6 compounds in the reductive fermentation environment. [H] represents a reducing equivalent, typically NAD(P)H
23.3.2.2 Factors affecting C6 production and formation from precursors
Under controlled maceration conditions, C6 aldehyde formation from grapes is reported to reach a maximum around veraison [9] and in some instances will decrease with increasing grape maturity – by a factor of two in the case of Spanish Macabeo [10]. As a result, higher concentrations of C6 alcohols are often found in wines produced from early‐harvest grapes [11]. PUFA content in grapes decreases by about a factor of two between veraison and maturity although the timing of this decline is not fully synchronized with the decrease in C6 aldehyde production [10]. Surprisingly, LOX activity increases during berry ripening, suggesting that activity of other enzymes in the lipid oxidation pathway may be more important for explaining changes in C6 production and relative concentrations [9, 10]. As a caveat, comparing quantitative results for grapes across studies is often challenging because of differences in maceration conditions. For example, in some reports whole berries are processed to a fine powder [9]; this approach is expected to yield higher C6 compounds than typical wine maceration conditions, both because of the greater degree of tissue damage, especially to seeds (a rich source of PUFAs).
Because formation of C6 compounds requires maceration, pre‐fermentation conditions can have a profound effect on their eventual concentrations in wine. Factors affecting C6 formation during winemaking have been studied [12–14] and include:
- Extent of maceration and solids contact time prior to alcoholic fermentation (e.g., extent of clarification, temperature, time, degree of crushing). This parameter is of particular importance to white wine production. For example, avoiding skin contact or clarifying white juice by settling results in lower PUFAs in must and lower concentrations of C6 volatiles (e.g., 1‐hexanol, Figure 23.3.3) in must and wine [15]. Similarly, mechanical harvesting, crushing, and hard pressing can result in up to 10‐fold increases in final C6 concentrations in wine as compared to hand‐harvesting and hand‐pressing [16] (Figure 23.3.4).
- Oxygen availability. The LOX enzyme requires O2, and saturation of a must with air prior to fermentation (“hyperoxidation”) results in a two‐fold increase in hexanol in finished wines [17].
- Inhibition of enzyme activity, for example, through SO2 addition [18] or thermovinification [19], will decease C6 formation
- Presence of material other than grape berries (MOG). For example, on a weight‐by‐weight basis, macerated grape leaf produces 100‐fold more (Z)‐3‐hexenal than grape berries, as well as higher concentrations of several other C6 compounds [4].
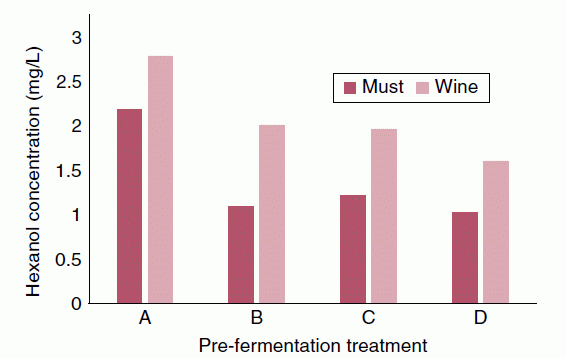
Figure 23.3.3 Effect of pre‐fermentation clarification treatments on 1‐hexanol, a C6 alcohol, in must and in wine. A, control treatment; Airen white wine grapes, 16 h skin contact following crushing, no clarification following pressing. B, must clarified by settling for 24 h at 20 °C following pressing. C, must clarified by settling for 24 h at 15 °C following pressing. D, must clarified by settling with added pectinase enzymes for 24 h at 15 °C following pressing. Data from Reference [15]
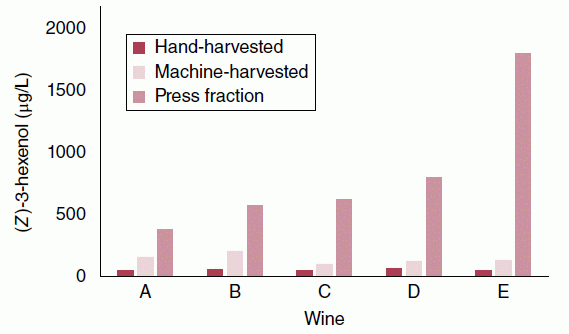
Figure 23.3.4 Effect of pre‐fermentation grape handling on concentrations of (Z)‐3‐hexenol in five different wines (A–E). Wines were produced from Sauvignon Blanc grapes that were either (i) hand‐harvested and hand‐pressed, (ii) machine‐harvested and hand‐pressed, or (iii) machine‐harvested, crushed, and mechanically pressed at 1 bar. Data from Reference [16]
23.3.3 Hydroxycinnamic acids, Brettanomyces, and volatile phenols
Wines possess modest concentrations of HCAs (~60 mg/L total), which in grapes exist almost exclusively as their tartaric acid esters (Chapter 13). HCAs are synthesized pre‐veraison, and will decline by a factor of 2 on a weight‐by‐weight (but not per berry) basis during ripening due to the increase in berry size [20]. Concentrations of HCA tartrate esters will vary considerably among cultivars, as discussed in Chapter 13. Although HCAs (particularly caffeic acid) have important roles in oxidation reactions (Chapter 24), they are also of interest because of their ability to serve as precursors for odor‐active volatile phenols. The conversion of HCA tartrate esters to volatile phenols is summarized in Figure 23.3.5.
23.3.3.1 Tartrate ester hydrolysis
Formation of volatile phenols requires initial hydrolysis of the tartrate ester group. Cinnamic acid esters undergo very slow hydrolysis at wine pH and at ambient temperature due to resonance stabilization of the carbonyl (Figure 23.3.5, top right) – estimated half‐lives are on the order of decades for the hydrolysis of methyl and ethyl cinnamate at pH 3.4 and room temperature [21]. Average concentrations of caftaric acid in commercial Pinotage wines were inversely correlated with wine age and were 34% lower in wines from the 1998 vintage versus 2001 vintage (53 mg/L versus 79 mg/L) [22], which can be extrapolated to a half‐life of ~7 years in wine. Ratios of esterified and free forms of HCA in wine can be as high as 9:1 even after ~15 years of storage [23]. Thus, acid hydrolysis of HCA tartrate esters is probably negligible for most wines and release of free HCAs through cinnamic acid ester hydrolase activity is of much greater importance.
- Some yeasts have modest cinnamic acid ester hydrolase (also called cinnamic acid esterase, CE, or hydroxycinnamate ester hydrolase, HCEH) activity. One report on Chardonnay showed a steady increase in trans‐caffeic acid from undetectable to 11.6 mg/L caffeic acid equivalents (CAE) over the course of fermentation, and a corresponding decrease in its ester form (trans‐caftaric acid) from a maximum of 41.8 mg/L CAE to 30.2 mg/L CAE by the end of fermentation [24], or a conversion of approximately 25% ignoring oxidative losses. Greater conversion (~75%) was observed for coutaric acid to coumaric acid.
- Lactic acid bacteria possess widely variable CE activity – for the same wine, the VFO strain of O. oeni resulted in near‐quantitative conversion of caftaric to caffeic acid, while other strains (Alpha, VP71) had no significant impact [25]. Thus, wines that undergo malolactic fermentation (e.g., many reds, Chapter 19) are expected to have greater concentrations of free HCAs.
- Finally, pectinase enzyme preparations added to increase juice yield (Chapters 21, 23.1, and 27) can demonstrate unintended CE side activity (Figure 23.3.6). Similar to LAB strains, the degree of CE activity varies considerably – in one study, degradation of coutaric acid ranged from negligible to >70% [26]. High CE activity is of concern to winemakers since it results in high free HCAs before fermentation, precursors for the malodorous vinyl‐ or ethylphenols, as described in the next sections. As a result, many commercial pectinases are selected to have (and advertised as possessing) low CE activity.
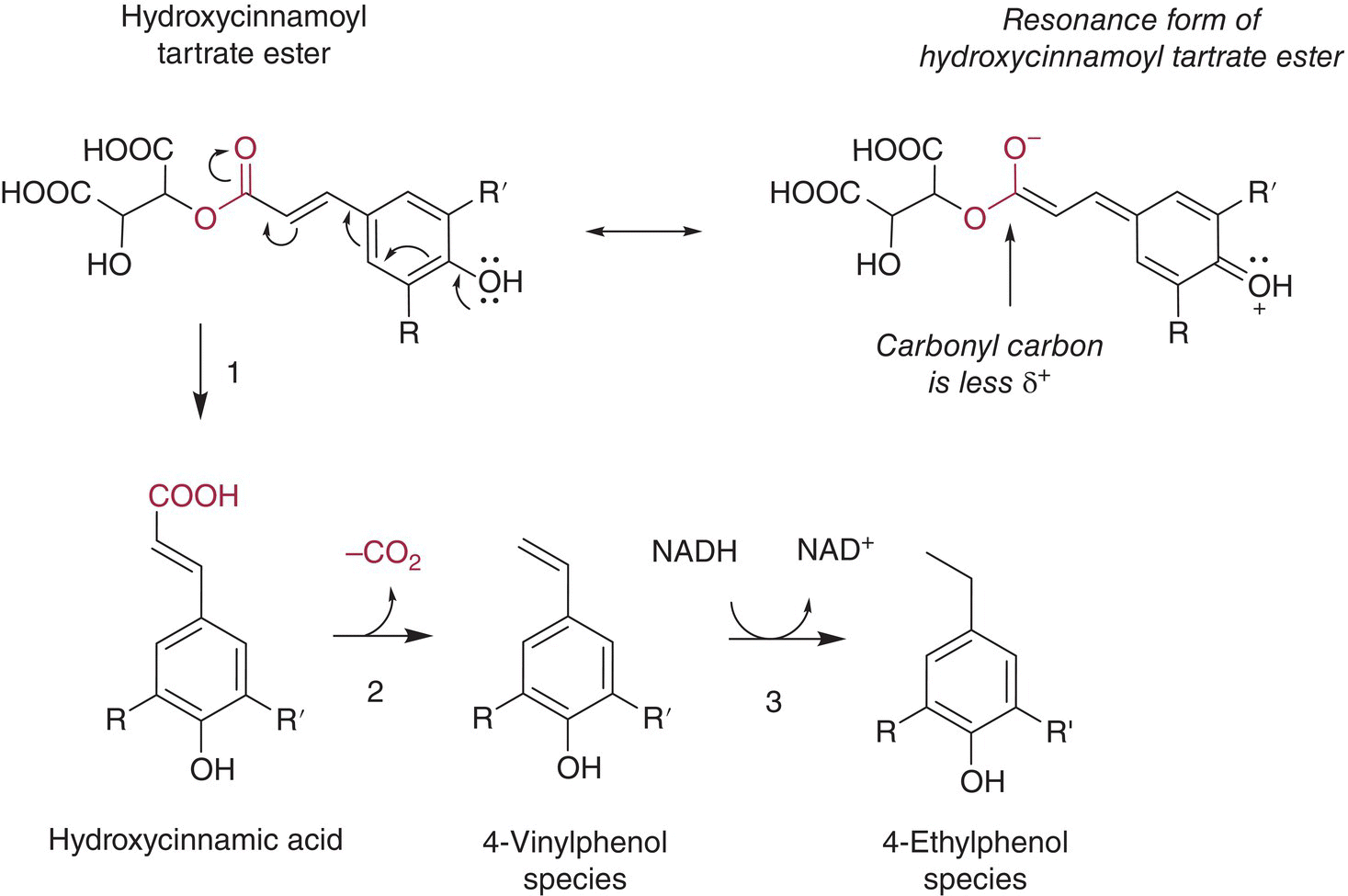
Figure 23.3.5 Reaction pathway to form odorous vinylphenols and ethylphenols from hydroxycinnamoyl (HCA) tartrate esters in grapes. The resonance stabilization of HCA tartrate esters (top right) decreases the rate of non‐enzymatic acid hydrolysis as compared to simpler esters. 1, Hydroxycinnamoyl esterase (CE); certain lactic acid bacteria, pectinase preparations, and to a lesser extent yeast. 2, Hydroxyxcinnamate decarboxylase (HCD); S. cerevesiae, Brettanomyces and other yeasts. 3, Vinylphenol reductase (VPR); Brettanomyces
23.3.3.2 Vinylphenol formation
Free HCAs and their ethyl esters possess some astringent and bitter properties (Chapter 13). During fermentation, the trans forms of HCAs can be decarboxylated to yield volatile vinylphenols with “smoky” and “medicinal” odors (Chapter 12). In particular, ferulic acid can be converted to 4‐vinylguaiacol (4‐VG) and coumaric acid can be converted to 4‐vinylphenol (4‐VP). This transformation is carried out through hydroxycinnamic decarboxylase (HCD) enzymes, also referred to as cinnamic acid decarboxylases (Figure 23.3.5). Most S. cerevesiae wine yeast strains possess decarboxylase ability [27, 28], as do Brettanomyces spoilage yeasts (described in more detail below) [29].3 Disruption of either one of two genes (FDC1 or PAD1) results in a loss of HCD activity in yeast [27]. Cinnamic acid derivatives have antimicrobial activities, and the function of the decarboxylase genes in yeasts is apparently to protect them against these common plant secondary metabolites [30].
Although most wine yeasts possess decarboxylase activity, the extent of HCA decarboxylation can vary considerably among strains – from <10% to 75% in a model juice system supplemented with 12 mg/L coumaric acid and fermented with one of three different strains [31]. As a result, vinylphenol concentrations can differ by an order of magnitude or more as a function of yeast strain, although initial concentrations of free HCAs will play an equally critical role (Figure 23.3.6 [26]).
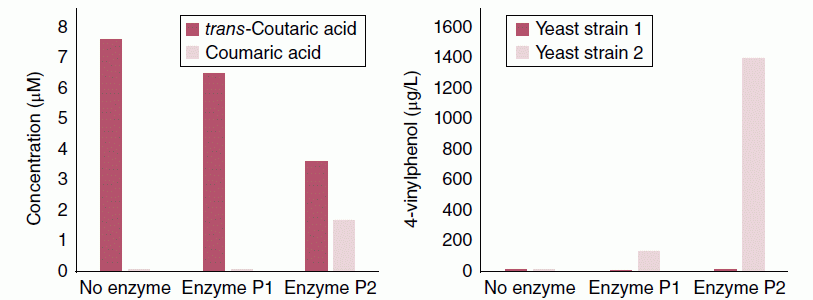
Figure 23.3.6 (Left) Demonstration of CE side activity in two commercial pectinase enzymes, P1 and P2, as evidenced by a decrease in coutaric acid and an increase in coumaric acid in Muscat of Frontignan juice samples incubated for 20 h at 20 °C. (Right) 4‐Vinylphenol (4‐VP) concentration in wines following fermentation of control and enzyme‐treated juices. The highest 4‐VP accumulation required both high free coumaric acid (from enzyme preparation P2) and a yeast with high cinnamic acid decarboxylase activity (Strain 2). Data from Reference [26]
As a caveat, interpreting differences among vinylphenol concentrations in real wine fermentations is often not straightforward, since formation will depend on not only initial hydroxycinnamate concentrations but also the rate of release of free HCAs from tartrate esters, and the reactivity of vinylphenols. 4‐VG and 4‐VP decrease in aged wines due to formation of 1‐ethoxyethanol adducts (Figure 23.3.7, [26]), and the half‐life of vinylphenol in white wines is approximately 6 months at 16–18 °C. Vinylphenols may decrease at faster rates in red wines due to their reactivity with anthocyanins to yield vinylphenolic pyranoanthocyanins (Chapter 16), or through further metabolism by Brettanomyces, as described next.
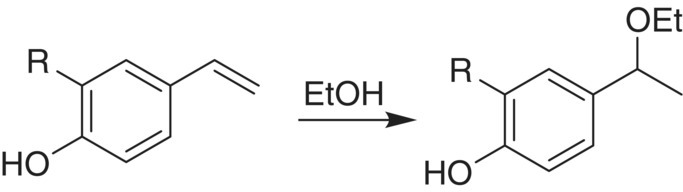
Figure 23.3.7 Reaction of vinylphenol (left) to form a 4‐(1‐ethoxyethyl)phenol [26]
23.3.3.3 Ethylphenol formation
Although 4‐VG and 4‐VP are detectable in many wines, particularly whites, they rarely appear to contribute to wine aroma due to their high reported sensory thresholds (770 µg/L) [32]. However, these vinylphenols can be enzymatically reduced to their more potent alkyl analogs – 4‐ethylguaiacol (4‐EG) and 4‐ethylphenol (4‐EP) – by the action of vinylphenol reductase (VPR) enzymes. In contrast to CE and HCD activity, which are found in a wide range of wine microorganisms, VPR activity is found almost exclusively in Brettanomyces yeasts [33], and a VPR enzyme from Brettanomyces has recently been purified and the protein sequenced [34]. Although Brettanomyces metabolism can result in several changes to wine organoleptic properties, including an increase in acetic acid and a loss of anthocyanins due to glycosidases [35], the most characteristic aspect of “Brett” flavor is an increase in “phenolic” or “medicinal” odor arising from 4‐EP and related volatile phenols (Chapter 12) [36]. This formation of ethylphenols, and associated aromas, are generally (but not universally) considered undesirable [35, 44].
Brettanomyces grows at a much slower rate than Saccharomyces and has a negligible presence during alcoholic fermentation, in large part because alcoholic fermentation by “Brett” is slowed under anaerobic conditions (due to the “negative Pasteur effect”) [37]. However, Brettanomyces can remain viable even in the harsh conditions of a finished wine (i.e., high ethanol, low pH, low O2, low nutrients, SO2) [38, 39], and requires minimal amounts of sugars to sustain growth (<1 g/L) [40]. Finally, in contrast to Saccharomyces, some Brettanomyces can use ethanol as a carbon source with minimal oxygen present, and most strains have the ability to utilize cellobiose, a β(1 → 4) disaccharide of two glucose molecules and a byproduct of cellulose degradation, that is, from toasting oak [39]. Viable 4‐EP‐producing Brettanomyces have been found several mm deep in the interior of used oak barrels [41], and thus can withstand many common surface cleaning and sanitation protocols [42]. Oak storage also introduces more oxygen, which stimulates Brettanomyces growth. As a consequence, barrels in wineries often serve as sites of Brettanomyces growth as well as reservoirs for further contamination [35, 42].
Almost all of ethylphenol production occurs towards the end of the growth phase and start of the stationary phase, and production is reported to cease when suitable carbon sources (e.g., glucose) are no longer detectable [43]. Brettanomyces can convert free vinylphenols to ethylphenols, but the majority of ethylphenol production occurs via the two‐step enzyme‐catalyzed processes of HCA → vinylphenol → ethylphenol [45]. Conversion of HCA precursors can be highly efficient under model conditions – one strain was able to convert 90% of coumaric acid to 4‐EP in a model wine containing 2% glucose [43]. However, this conversion efficiency has a strong strain dependence, with variation in production of ethylphenols across 37 Brettanomyces strains ranging from undetectable to >2500 µg/L of both 4‐EP and 4‐EG using the same wine substrate [36] (Figure 23.3.8). Interestingly, 4‐EP and 4‐EG appear to be highly correlated, indicating that there is relatively little variation in VPR selectivity towards the main vinylphenols, and their relative concentrations ultimately appear to be related to that of the precursor HCAs (Chapter 12). Caffeic acid can also be reduced by Brettanomyces to form 4‐ethylcatechol (4‐EC) [45], although this conversion has not been as well studied as for 4‐EP and 4‐EG due to its lower efficiency, the higher olfactory threshold of 4‐EC, and challenges associated with analysis of 4‐EC.
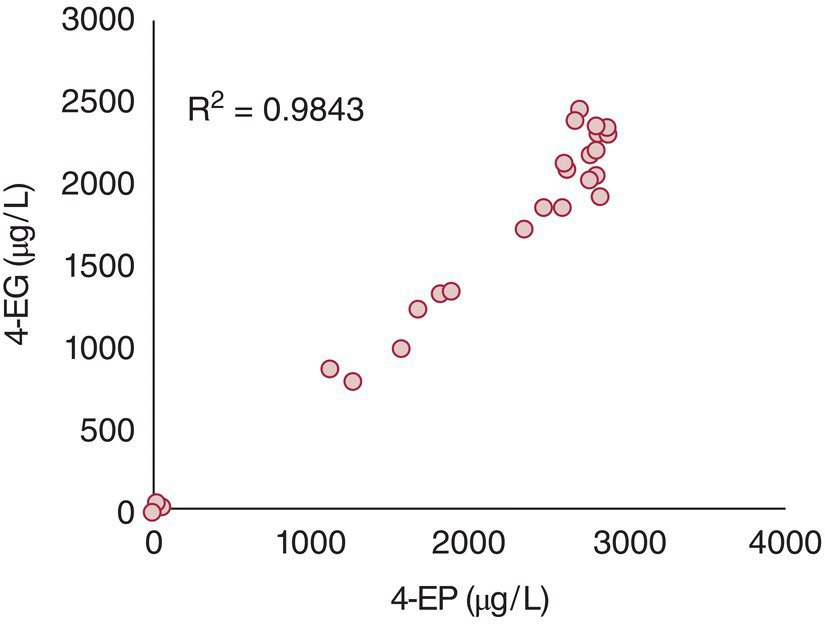
Figure 23.3.8 Correlation of 4‐EG and 4‐EP production for 37 Brettanomyces strains inoculated into an off‐dry Grenache rosé wine. Note that 13 of the strains (37%) produced negligible amounts (<150 µg/L total) of ethylphenols, whereas around half of the strains were high producers (>2000 g/L total). Data from Conterno et al. [39]
In summary, factors that will affect final ethylphenol species concentrations include:
- Initial hydroxycinnamate ester concentration, which can be strongly affected by cultivar and winemaking conditions (Chapter 13).
- Extent of conversion of hydroxycinnamate esters to free HCAs, for example, by microbial enzymatic activity.
- Stability of HCAs during fermentation and storage and prior to Brettanomyces growth.
- Most importantly, the introduction and growth of Brettanomyces strains with VPR activity.
The physiological conditions that promote Brettanomyces growth include lower SO2, higher O2, and avoidance of filtration or other stabilization approaches [35]. These are also conditions that are more widely encountered in red winemaking and, along with the more widespread use of extended oak aging, can rationalize the more frequent appearance of the “Brett” character in red wines.
23.3.4 S‐methylmethionine and dimethyl sulfide
Dimethyl sulfide (DMS, “truffle”, “canned corn”) can contribute both to the aroma of aged wine (Chapter 25) and, at high concentrations, to sulfurous off‐aromas (Chapter 10). DMS is generally present at low µg/L concentrations in young wines (<1 year old), but will be formed during storage from grape‐derived precursors [46]. The most important of these precursors appears to be S‐methylmethionine (SMM) [47], which can form DMS upon hydrolysis. At pH values above 5 (as is found in beer wort and many vegetables), this hydrolysis appears to be base‐catalyzed and will increase with increasing pH [48], but at pH < 5 the release appears to proceed through nucleophilic substitution by water [49], and no pH dependence is expected (Figure 23.3.9).
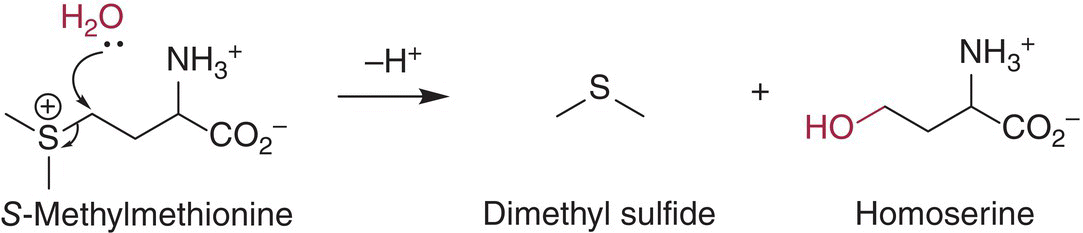
Figure 23.3.9 Hydrolytic formation of dimethyl sulfide (DMS) from S‐methylmethionine (SMM) precursor to yield DMS [49]
SMM is widespread in the plant kingdom, where it can be formed by methylation of methionine. The metabolic purpose of SMM is somewhat unclear but its primary role seems to be as a convenient means to transport methionine from its place of origin (vegetative tissues like leaves) into sinks (like seeds) [50]. The ability of SMM to form DMS during the thermal processing and storage of plant‐based foods and beverages has been well studied [51]. Higher concentrations of SMM are typically observed in vegetative tissues rather than fleshy fruits, with SMM concentrations in cabbage and asparagus in the range of 0.1–0.2% w/w [45], or about 1000‐fold higher than what is typically observed in grapes [52] based on “potential DMS” measurements (see the next paragraph). The typical concentrations of DMS in these vegetables following cooking (10–20 mg/kg) are also two to three orders of magnitude higher than the highest concentrations reported in wine (Chapter 10).4
SMM can be quantified in grape juice [53], but more commonly “potential DMS” (pDMS) is determined by heating a juice or wine sample under alkaline conditions and quantifying the DMS formed [47]. One study has reported a wide range of pDMS in grapes of different varieties and growing locations, from low µg/L to > 4000 µg/L [53]. A particularly high concentration of pDMS was observed in a late harvest Petit Manseng and higher DMS concentrations are correlated with later harvest times [11], but the factors that control SMM in grapes are generally not well studied. Regardless, the pDMS concentration of grape juice is an excellent predictor of wine DMS following storage under controlled conditions [52]. From a limited number of Syrah grape samples, total wine DMS (pDMS + free) was about 70% of grape pDMS, indicating that SMM is mostly stable during fermentation. However, some yeast can reportedly use SMM as a nitrogen source during beer fermentations [48], and variation among wine yeasts has not been characterized.
Yeast do not appear to enzymatically hydrolyze SMM to DMS during fermentation. As mentioned above, the primary reaction will involve water as a nucleophile at wine pH and therefore will not show strong pH dependence. However, storage time and temperature will be critically important. The hydrolytic release of DMS from SMM has a high activation energy barrier, 186 kJ/mol [54] (Chapter 25). While SMM hydrolysis can occur rapidly around the boiling point of water (half‐life = 30 min in beer wort, 98.5 °C), the release will be much slower under typical wine storage temperatures (half‐life ~5 years, Figure 23.3.10).
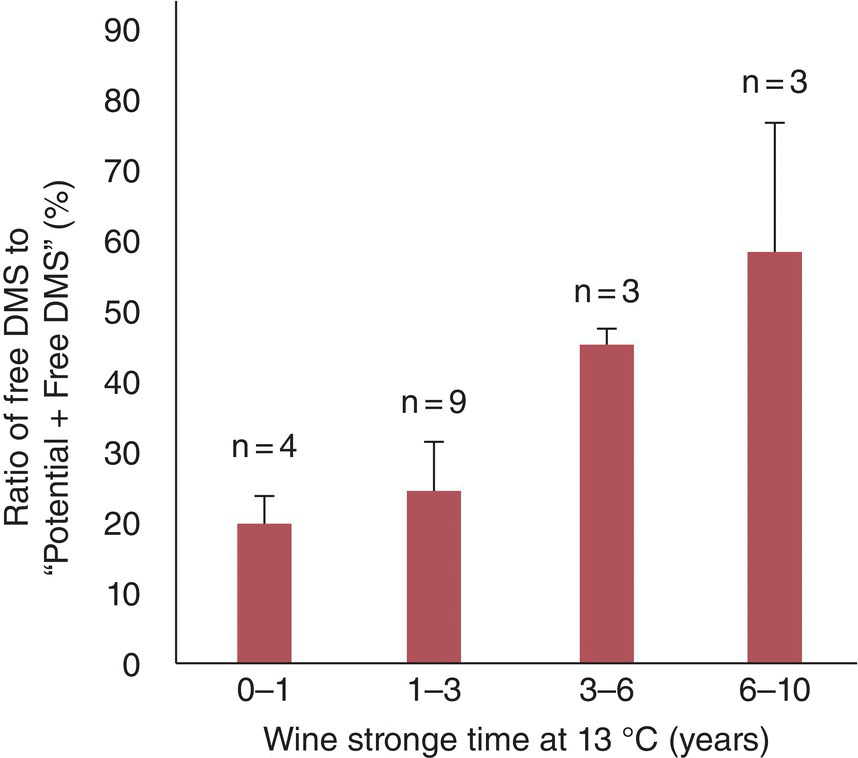
Figure 23.3.10 Mean percentage of DMS in free form as compared to the total DMS pool (pDMS + free DMS) as a function of wine age for Grenache and Syrah wines. Values over top of bars indicate number (n) of wines in an age category. Error bars represent standard deviations. Data from Reference [52]
References
- 1. Linsenmeier, A., Rauhut, D., Sponholz, W.R. (2010) Ageing and flavour deterioration in wine, in Managing wine quality, Vol. 2, Oenology and wine quality (ed. Reynolds, A.G.), Woodhead Publishing and CRC Press, Oxford and Boca Raton, FL.
- 2. Matsui, K. (2006) Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Current Opinion in Plant Biology, 9 (3), 274–280.
- 3. Canuti, V., Conversano, M., Calzi, M.L., et al. (2009) Headspace solid‐phase microextraction–gas chromatography–mass spectrometry for profiling free volatile compounds in Cabernet Sauvignon grapes and wines. Journal of Chromatography A, 1216 (15), 3012–3022.
- 4. Hashizume, K. and Samuta, T. (1997) Green odorants of grape cluster stem and their ability to cause a wine stemmy flavor. Journal of Agricultural and Food Chemistry, 45 (4), 1333–1337.
- 5. Iyer, M.M., Sacks, G.L., Padilla‐Zakour, O.I. (2010) Impact of harvesting and processing conditions on green leaf volatile development and phenolics in Concord grape juice. Journal of Food Science, 75 (3), C297–C304.
- 6. Harsch, M.J., Benkwitz, F., Frost, A., et al. (2013) New precursor of 3‐mercaptohexan‐1‐ol in grape juice: thiol‐forming potential and kinetics during early stages of must fermentation. Journal of Agricultural and Food Chemistry, 61 (15), 3703–3713.
- 7. Dennis, E.G., Keyzers, R.A., Kalua, C.M., et al. (2012) Grape contribution to wine aroma: production of hexyl acetate, octyl acetate, and benzyl acetate during yeast fermentation is dependent upon precursors in the must. Journal of Agricultural and Food Chemistry, 60 (10), 2638–2646.
- 8. Herraiz, T., Herraiz, M., Reglero, G., et al. (1990) Changes in the composition of alcohols and aldehydes of C6 chain length during the alcoholic fermentation of grape must. Journal of Agricultural and Food Chemistry, 38 (4), 969–972.
- 9. Kalua, C.M. and Boss, P.K. (2010) Comparison of major volatile compounds from Riesling and Cabernet Sauvignon grapes (Vitis vinifera L.) from fruitset to harvest. Australian Journal of Grape and Wine Research, 16 (2), 337–348.
- 10. Iglesias, J.L.M., Dabila, F.H., Marino, J.I.M., et al. (1991) Biochemical aspects of the lipids in Vitis vinifera grapes (Macabeo variety). 1. Linoleic and linolenic acids as aromatic precursors. Nahrung‐Food, 35 (7), 705–710.
- 11. Bindon, K., Varela, C., Kennedy, J., et al. (2013) Relationships between harvest time and wine composition in Vitis vinifera L. cv. Cabernet Sauvignon. 1. Grape and wine chemistry. Food Chemistry, 138 (2–3), 1696–1705.
- 12. Ramey, D., Bertrand, A., Ough, C.S., et al. (1986) Effects of skin contact temperature on Chardonnay must and wine composition. American Journal of Enology and Viticulture, 37 (2), 99–106.
- 13. Joslin, W.S. and Ough, C.S. (1978) Cause and fate of certain C6 compounds formed enzymatically in macerated grape leaves during harvest and wine fermentation. American Journal of Enology and Viticulture, 29 (1), 11–17.
- 14. Capone, D.L., Black, C.A., Jeffery, D.W. (2012) Effects on 3‐mercaptohexan‐1‐ol precursor concentrations from prolonged storage of Sauvignon Blanc grapes prior to crushing and pressing Journal of Agricultural and Food Chemistry, 60 (13), 3515–3523.
- 15. Ferreira, B., Hory, C., Bard, M.H., et al. (1995) Effects of skin contact and settling on the level of the C18:2, C18:3 fatty‐acids and C6 compounds in Burgundy Chardonnay musts and wines. Food Quality and Preference, 6 (1), 35–41.
- 16. Herbst‐Johnstone, M., Araujo, L., Allen, T., et al. (2012) Effects of mechanical harvesting on “Sauvignon blanc” aroma, in 1st International Workshop on Vineyard mechanization and grape and wine quality (ed. Poni, S.), ISHS Acta Horticulturae, pp. 179–186.
- 17. Cejudo‐Bastante, M.J., Castro‐Vázquez, L., Hermosín‐Gutiérrez, I., Pérez‐Coello, M.S. (2011) Combined effects of prefermentative skin maceration and oxygen addition of must on color‐related phenolics, volatile composition, and sensory characteristics of Airén white wine. Journal of Agricultural and Food Chemistry, 59 (22), 12171–12182.
- 18. Makhotkina, O., Herbst‐Johnstone, M., Logan, G., et al. (2013) Influence of sulfur dioxide additions at harvest on polyphenols, C6‐compounds, and varietal thiols in Sauvignon blanc. American Journal of Enology and Viticulture, 64 (2), 203–213.
- 19. Fischer, U., Strasser, M., Gutzler, K. (2000) Impact of fermentation technology on the phenolic and volatile composition of German red wines. International Journal of Food Science and Technology, 35 (1), 81–94.
- 20. Ong, B.Y. and Nagel, C.W. (1978) Hydroxycinnamic acid–tartaric acid ester content in mature grapes and during the maturation of white Riesling grapes. American Journal of Enology and Viticulture, 29 (4), 277–281.
- 21. Rayne, S. and Forest, K. (2011) Estimated carboxylic acid ester hydrolysis rate constants for food and beverage aroma compounds. Nature Precedings. doi: 10.1038/npre.2011.6471.1.
- 22. Schwarz, M., Hofmann, G., Winterhalter, P. (2004) Investigations on anthocyanins in wines from Vitis vinifera cv. Pinotage: factors influencing the formation of Pinotin A and its correlation with wine age. Journal of Agricultural and Food Chemistry, 52 (3), 498–504.
- 23. Cheynier, V.F., Trousdale, E.K., Singleton, V.L., et al. (1986) Characterization of 2‐S‐glutathionyl caftaric acid and its hydrolysis in relation to grape wines. Journal of Agricultural and Food Chemistry, 34 (2), 217–221.
- 24. Somers, T.C., Vérette, E., Pocock, K.F. (1987) Hydroxycinnamate esters of Vitis vinifera: changes during white vinification, and effects of exogenous enzymic hydrolysis. Journal of the Science of Food and Agriculture, 40 (1), 67–78.
- 25. Burns, T.R. and Osborne, J.P. (2013) Impact of malolactic fermentation on the color and color stability of Pinot noir and Merlot wine. American Journal of Enology and Viticulture, 64 (3), 370–377.
- 26. Dugelay, I., Gunata, Z., Sapis, J.C., et al. (1993) Role of cinnamoyl esterase activities from enzyme preparations on the formation of volatile phenols during winemaking. Journal of Agricultural and Food Chemistry, 41 (11), 2092–2096.
- 27. Mukai, N., Masaki, K., Fujii, T., et al. (2010) PAD1 and FDC1 are essential for the decarboxylation of phenylacrylic acids in Saccharomyces cerevisiae. Journal of Bioscience and Bioengineering, 109 (6), 564–569.
- 28. Chatonnet, P., Dubourdieu, D., Boidron, J.‐n., Lavigne, V. (1993) Synthesis of volatile phenols by Saccharomyces cerevisiae in wines. Journal of the Science of Food and Agriculture, 62 (2), 191–202.
- 29. Edlin, D.A.N., Narbad, A., Gasson, M.J., et al. (1998) Purification and characterization of hydroxycinnamate decarboxylase from Brettanomyces anomalus. Enzyme and Microbial Technology, 22 (4), 232–239.
- 30. Clausen, M., Lamb, C.J., Megnet, R., Doerner, P.W. (1994) PAD1 encodes phenylacrylic acid decarboxylase which confers resistance to cinnamic acid in Saccharomyces cerevisiae. Gene, 142 (1), 107–112.
- 31. Benito, S., Palomero, F., Morata, A., et al. (2009) Minimization of ethylphenol precursors in red wines via the formation of pyranoanthocyanins by selected yeasts. International Journal of Food Microbiology, 132 (2–3), 145–152.
- 32. Nikfardjam, M.P., May, B., Tschiersch, C. (2009) Analysis of 4‐vinylphenol and 4‐vinylguaiacol in wines from the Wuerttemberg region (Germany). Mitteilungen Klosterneuburg, Rebe und Wein, Obstbau und Früchteverwertung, 59 (2), 84–89.
- 33. Chatonnet, P., Viala, C., Dubourdieu, D. (1997) Influence of polyphenolic components of red wines on the microbial synthesis of volatile phenols. American Journal of Enology and Viticulture, 48 (4), 443–448.
- 34. Granato, T., Romano, D., Vigentini, I., et al. (2015) New insights on the features of the vinyl phenol reductase from the wine‐spoilage yeast Dekkera/Brettanomyces bruxellensis. Annals of Microbiology, 65 (1), 321–329.
- 35. Conterno, L. and Henick‐Kling, T. (2010) Brettanomyces/Dekkera off‐flavours and other wine faults associated with microbial spoilage, in Managing wine quality (ed. Reynolds, A.G.), Woodhead Publishing, pp. 346–387.
- 36. Chatonnet, P., Dubourdie, D., Boidron, J.‐N., Pons, M. (1992) The origin of ethylphenols in wines. Journal of the Science of Food and Agriculture, 60 (2), 165–178.
- 37. Scheffers, W.A. and Wiken, T.O. (1969) The Custers effect (negative Pasteur effect) as a diagnostic criterion for the genus Brettanomyces. Antonie Van Leeuwenhoek, 35 (Suppl. Yeast Symp.), A31–A32.
- 38. Suárez, R., Suárez‐Lepe, J.A., Morata, A., Calderón, F. (2007) The production of ethylphenols in wine by yeasts of the genera Brettanomyces and Dekkera: a review. Food Chemistry, 102 (1), 10–21.
- 39. Conterno, L., Joseph, C.M.L., Arvik, T.J., et al. (2006) Genetic and physiological characterization of Brettanomyces bruxellensis strains isolated from wines. American Journal of Enology and Viticulture, 57 (2), 139–147.
- 40. Coulon, J., Perello, M.C., Lonvaud‐Funel, A., et al. (2010) Brettanomyces bruxellensis evolution and volatile phenols production in red wines during storage in bottles. Journal of Applied Microbiology, 108 (4), 1450–1458.
- 41. Barata, A., Laureano, P., D’Antuono, I., et al. (2013) Enumeration and identification of 4‐ethylphenol producing yeasts recovered from the wood of wine ageing barriques after different sanitation treatments. Journal of Food Research, 2 (1), 140–149.
- 42. Aguilar Solis, M. (2014) Evaluation of common and novel sanitizers against spoilage yeasts found in wine environments. PhD Thesis, Cornell Unversity, Ithaca, NY.
- 43. Dias, L., Pereira‐da‐Silva, S., Tavares, M., et al. (2003) Factors affecting the production of 4‐ethylphenol by the yeast Dekkera bruxellensis in enological conditions. Food Microbiology, 20 (4), 377–384.
- 44. Juan, F.S., Cacho, J., Ferreira, V., Escudero, A. (2012) Aroma chemical composition of red wines from different price categories and its relationship to quality. Journal of Agricultural and Food Chemistry, 60 (20), 5045–5056.
- 45. Harris, V., Ford, C., Jiranek, V., Grbin, P. (2008) Dekkera and Brettanomyces growth and utilisation of hydroxycinnamic acids in synthetic media. Applied Microbiology and Biotechnology, 78 (6), 997–1006.
- 46. Marais, J. (1979) Effect of storage time and temperature on the formation of dimethyl sulfide and on white wine quality. Vitis, 18 (3), 254–260.
- 47. Segurel, M.A., Razungles, A.J., Riou, C., et al. (2005) Ability of possible DMS precursors to release DMS during wine aging and in the conditions of heat‐alkaline treatment. Journal of Agricultural and Food Chemistry, 53 (7), 2637–2645.
- 48. Anness, B.J. and Bamforth, C.W. (1982) Dimethyl sulfide – a review. Journal of the Institute of Brewing, 88 (4), 244–252.
- 49. Cremer, D.R. and Eichner, K. (2000) Formation of volatile compounds during heating of spice paprika (Capsicum annuum) powder. Journal of Agricultural and Food Chemistry, 48 (6), 2454–2460.
- 50. Giovanelli, J., Mudd, S.H., Datko, A.H. (1980) Sulfur amino acids in plants, in Amino acids and derivatives (ed. Miflin, B.J.), Academic Press, pp. 453–505.
- 51. Scherb, J., Kreissl, J., Haupt, S., Schieberle, P. (2009) Quantitation of S‐methylmethionine in raw vegetables and green malt by a stable isotope dilution assay using LC‐MS/MS: comparison with dimethyl sulfide formation after heat treatment. Journal of Agricultural and Food Chemistry, 57 (19), 9091–9096.
- 52. Segurel, M.A., Razungles, A.J., Riou, C., et al. (2004) Contribution of dimethyl sulfide to the aroma of Syrah and Grenache noir wines and estimation of its potential in grapes of these varieties. Journal of Agricultural and Food Chemistry, 52 (23), 7084–7093.
- 53. Loscos, N., Segurel, M., Dagan, L., et al. (2008) Identification of S‐methylmethionine in Petit Manseng grapes as dimethyl sulphide precursor in wine. Analytica Chimica Acta, 621 (1), 24–29.
- 54. Scheuren, H., Tippmann, J., Methner, F.J., Sommer, K. (2014) Decomposition kinetics of dimethyl sulphide. Journal of the Institute of Brewing, 120 (4), 474–476.