13
Non‐flavonoid Phenolics
13.1 Introduction
Non‐flavonoid phenolics include several subclasses of importance to wine, in particular the hydroxycinnamates, stilbenes, and benzoic acids. The hydroxycinnamates and stilbenes are found in the grape, while the benzoic acids are found in the grape and in oak, so oak‐treated wines will include additional phenolics from that source.
13.2 Hydroxycinnamates
The hydroxycinnamates are phenolic acids that include a conjugated double bond between the phenolic ring and the carboxylate group. Three acids are commonly found – coumaric, caffeic, and ferulic acids – but these simple hydroxycinnamic acids are not found in grape berries; they instead exist as their tartaric acid esters. Enologists have adopted trivial names for these esters, those being p‐coutaric acid, caftaric acid, and fertaric acid, respectively (Table 13.1). These substances are found in the flesh of the fruit, and thus are found in all grape juices and consequently in all wines. Plants predominantly produce the trans form of hydroxycinnamic acids, but isomerization to the cis form is induced by light and thus varying amounts are found in wine [1, 2]. All plant foods contain hydroxycinnamates, though the tartrate esters are relatively unusual – their presence is evidence that a juice was sourced from grapes.
Table 13.1 The hydroxycinnamic acids shown in their tartrate ester form as found in the grape. Enzymatic hydrolysis of the ester leads to the free acid form in wine
| Name | Structure | Representative levels (juice/wine, mg/L) |
| Coutaric acid, coumaric acid, tartrate ester |
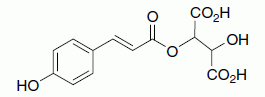 |
20/15 |
| Caftaric acid, caffeic acid, tartrate ester |
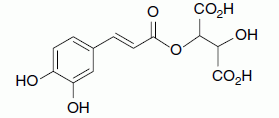 |
170/40 |
| Fertaric acid, ferulic acid, tartrate ester |
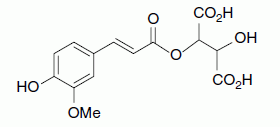 |
5/4 |
Considering their functionality, the grape‐derived tartrate esters are susceptible to hydrolysis, which is accelerated via hydroxycinnamate ester hydrolase enzymes produced by lactic acid bacteria and other organisms and continues, albeit very slowly, in the aqueous acid environment of finished wine (Chapter 23.3). The action of hydrolysis releases the simple hydroxycinnamic acids, which can be readily detected in newly fermented wines [3]. Conversely, the free acids and tartrate derivatives (also bearing free carboxylic acids) will partially esterify with the ethanol in wine [4] (Chapter 7 and 25). In addition, caffeic acid and its tartrate ester in both juice and wine are susceptible to oxidation and follow‐on reactions that lead to browning and many other outcomes (Chapter 24).
The hydroxycinnamates in various forms have been reported to have bitterness and astringency in water [5], but work by Noble and others [6] showed that the levels of these compounds were below the threshold in wine.
13.3 Hydroxybenzoic acids
Gallic acid is not found in grapes, but is generated in wine by the hydrolysis of the gallate esters found in condensed and hydrolyzable tannins (Figure 13.1) [7]. On long aging, gallic acid is persistent and can be observed in older wines. In typical red table wines it can be found at about 70 mg/L, but white wines contain less, at about 10 mg/L [8]. Small amounts of syringic, protocatechuic, and vanillic acids are also found. The presence of syringic acids has been used to identify the prior presence of malvidin (from grape wine) in archeological samples [9]. As carboxylic acids, these can undergo acid catalyzed esterification in the presence of ethanol to yield a fraction of the ethyl ester (Chapters 7 and 25). In a manner similar to the hydroxycinnamates, the acids with catechol or galloyl functionality (Chapter 11) are also susceptible to oxidation.
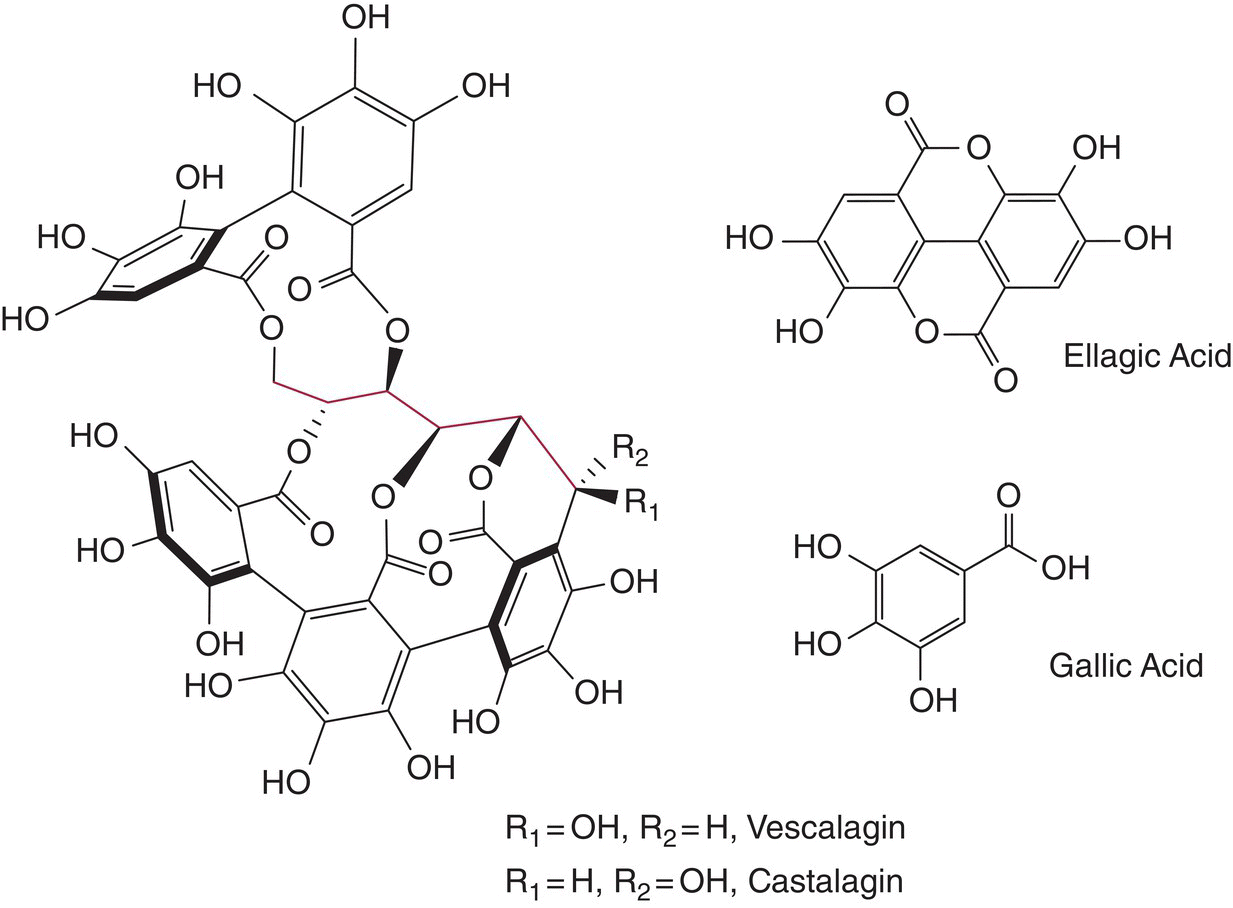
Figure 13.1 Oak tannins, vescalagin and castalagin, and ellagic acid and gallic acid. The sugar moiety is highlighted
13.3.1 Hydrolyzable tannins
The hydrolyzable tannins are composed of gallic acid and ellagic acid esters of glucose (Figure 13.1) or related sugars. Hydrolyzable tannins are further categorized as gallotannins or ellagitannins based on their constituent phenolic acid, but most plant sources consist of a mixture of the two. The term “hydrolyzable” results from the fact that the ester linkage is more susceptible to hydrolysis under mild conditions than the interflavan linkages of condensed tannins,1 and hydrolyzable tannins will form their constituent gallic and ellagic acids on aging. These compounds, largely castalagin, vescalagin and the roburins, are extracted into wine from oak, affording levels near a few mg/L for white wines after 6 months in new barrels, while red wines will have levels in the range of 2–20 mg/L after aging two or more years [10] (Chapter 25). Hydrolyzable tannins are not found in V. vinifera, but can be found in other fruits, such as raspberries or muscadine (V. rotundifolia) grapes. The taste impact of oak‐derived tannins in wine is likely to be minor [11]. When hydrolyzed in wine from ellagitannins, ellagic acid will precipitate if it is at high levels, as in muscadine wine.
13.4 Stilbenes
The principal stilbene in grapes, resveratrol, is produced by vines, as glucosides, in response to Botrytis infection and other fungal attacks [12]. The actual antifungal compounds appear to be the oligomers of resveratrol, called the viniferins. Several forms of resveratrol exist including the cis and trans isomers as well as their glucosides (Figure 13.2). Grapes appear to biosynthesize the trans form and light causes cis/trans isomerization [13]. Resveratrol derivatives are found largely in the skin of the grape, and consequently greater amounts are found in red wine, and although significant amounts can also be found in the vine shoots, this is largely irrelevant to winemaking. The total levels of all forms average about 7 mg/L for reds [14], 2 mg/L for rosés, and 0.5 mg/L for white wines [15]. Resveratrol has been implicated as a wine component that may reduce heart disease or cancer, with interest greatly stimulated by a 1997 report in Science [16], and it is a known marker in urine for wine consumption [17]. Numerous follow‐on studies have shown other benefits as well, but generally the therapeutic effects (in animals) occur only at doses 10–100 times that found in a glass of wine, in part because absorption is poor. More important to winemaking, resveratrol, and its O‐methylated derivative pterostilbene in particular (Figure 13.2), have potent antimicrobial activity against wild yeasts and Acetobacter [18].
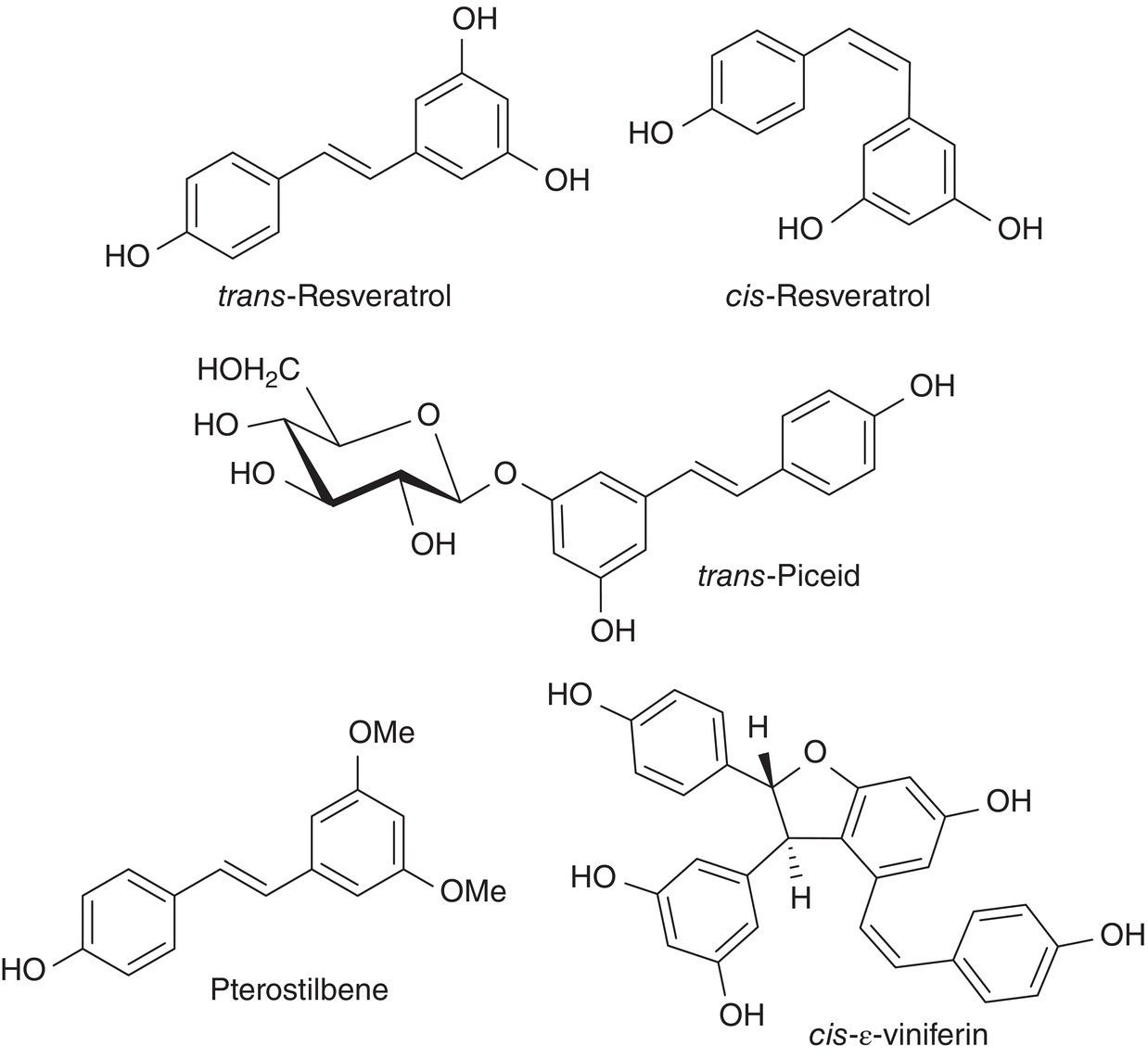
Figure 13.2 Selected stilbenes in wine, including the more abundant resveratrol isomers, along with an example of a resveratrol glucoside (trans‐piceid), a dimethoxylated analog (pterostilbene), and a dimeric viniferin
References
- 1. Baranowski, J.D. and Nagel, C.W. (1981) Isolation and identification of the hydroxycinnamic acid‐derivatives in white Riesling wine. American Journal of Enology and Viticulture, 32 (1), 5–13.
- 2. Ritchey, J.G. and Waterhouse, A.L. (1999) A standard red wine: monomeric phenolic analysis of commercial Cabernet Sauvignon wines. American Journal of Enology and Viticulture, 50 (1), 91–100.
- 3. Rentzsch, M., Schwarz, M., Winterhalter, P., Hermosin‐Gutierrez, I. (2007) Formation of hydroxyphenyl‐pyranoanthocyanins in Grenache wines: precursor levels and evolution during aging. Journal of Agricultural and Food Chemistry, 55 (12), 4883–4888.
- 4. Somers, T.C., Vérette, E., Pocock, K.F. (1987) Hydroxycinnamate esters of V. vinifera: changes during white vinification and effects of exogenous enzyme hydrolysis. Journal of the Science of Food and Agriculture, 40 (1), 67–78.
- 5. Hufnagel, J.C. and Hofmann, T. (2008) Orosensory‐directed identification of astringent mouthfeel and bitter‐tasting compounds in red wine. Journal of Agricultural and Food Chemistry, 56 (4), 1376–1386.
- 6. Verette, E., Noble, A.C., Somers, C.T. (1988) Hydroxycinnamates of Vitis vinifera: sensory assessment in relation to bitterness in white wine. Journal of the Science of Food and Agriculture, 45 (3), 267–272.
- 7. Chira, K., Pacella, N., Jourdes, M., Teissedre, P.L. (2011) Chemical and sensory evaluation of Bordeaux wines (Cabernet‐Sauvignon and Merlot) and correlation with wine age. Food Chemistry, 126 (4), 1971–1977.
- 8. Teissedre, P.L., Frankel, E.N., Waterhouse, A.L., et al. (1996) Inhibition of in vitro human LDL oxidation by phenolic antioxidants from grapes and wine. Journal of the Science of Food and Agriculture, 70 (1), 55–61.
- 9. Guasch‐Jane, M.R., Andres‐Lacueva, C., Jauregui, O., Lamuela‐Raventos, R.M. (2006) The origin of the ancient Egyptian drink Shedeh revealed using LC/MS/MS. Journal of Archaeological Science, 33 (1), 98–101.
- 10. Garcia‐Estevez, I., Escribano‐Bailon, M.T., Rivas‐Gonzalo, J.C., Alcalde‐Eon, C. (2012) Validation of a mass spectrometry method to quantify oak ellagitannins in wine samples. Journal of Agricultural and Food Chemistry, 60 (6), 1373–1379.
- 11. Glabasnia, A. and Hofmann, T. (2006) Sensory‐directed identification of taste‐active ellagitannins in American (Quercus alba L.) and European oak wood (Quercus robur L.) and quantitative analysis in bourbon whiskey and oak‐matured red wines. Journal of Agricultural and Food Chemistry, 54 (9), 3380–3390.
- 12. Joshi, V.K. and Devi, M.P. (2009) Resveratrol: importance, role, contents in wine and factors influencing its production. Proceedings of the National Academy of Sciences India Section B‐Biological Sciences, 79, 212–226.
- 13. Trela, B.C. and Waterhouse, A.L. (1996) Resveratrol: isomeric molar absorptivities and stability. Journal of Agricultural and Food Chemistry, 44 (5), 1253–1257.
- 14. Lamuela‐Raventos, R.M., Romero Perez, A.I., Waterhouse, A.L., de la Torre‐Boronat, M.C. (1995) Direct HPLC analysis of cis‐ and trans‐resveratrol and piceid isomers in Spanish Red Vitis vinifera wines. Journal of Agricultural and Food Chemistry, 42 (2), 281–283.
- 15. Romero‐Perez, A.I., Lamuela‐Raventos, R.M., Waterhouse, A.L., de la Torre‐Boronat, M.C. (1996) Levels of cis‐ and trans‐resveratrol and their glycosides in white and rose Vitis vinifera wines from Spain. Journal of Agricultural and Food Chemistry, 44 (8), 2124–2128.
- 16. Jang, M., Cai, L., Udeani, G.O., et al. (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science, 275 (5297), 218–220.
- 17. Zamora‐Ros, R., Urpi‐Sarda, M., Lamuela‐Raventos, R.M., et al. (2009) Resveratrol metabolites in urine as a biomarker of wine intake in free‐living subjects: the PREDIMED Study. Free Radical Biology and Medicine, 46 (12), 1562–1566.
- 18. Pastorkova, E., Zakova, T., Landa, P., et al. (2013) Growth inhibitory effect of grape phenolics against wine spoilage yeasts and acetic acid bacteria. International Journal of Food Microbiology, 161 (3), 209–213.