7
Esters
7.1 Introduction
In nature, esters are well‐known contributors to the aroma of flowers and ripe fruits. Despite their low total concentration (<0.1% w/w), esters are also characteristic products of alcoholic fermentation and are critical to the aroma of most alcoholic beverages, and wine is no exception.1 Some esters may contribute to bitterness, serve as flavor precursors, or participate in reactions with phenolics. In wines, the majority of esters are secondary or tertiary flavor compounds (see Introduction chapter) that are largely absent from grapes but are instead formed during fermentation and storage, respectively. The two main classes of esters important to wine are ethyl esters and acetate esters, although cyclic esters with 5‐membered rings (γ‐lactones) may also contribute to wine aroma (Figure 7.1).

Figure 7.1 General structures for major classes of esters that contribute to wine flavor
7.2 Chemistry of esters
The ester functional group is characterized by the presence of a carbonyl group (C = O) in which the carbonyl carbon is covalently bound to an alkoxy group (–OR, Figure 7.1). The carbonyl group of an ester is relatively stable due to resonance stabilization through the –OR group. As a result, reactions of the ester carbonyl group with nucleophiles like bisulfite (HSO3−) are less favorable than for aldehyde and ketone carbonyl groups.
In wine and related beverages, esters are formed through reaction of a carboxylic acid (R–COOH) and an alcohol (R′–OH) (Figure 7.2).2 The ester‐forming reaction is referred to as esterification and the reverse reaction is referred to as ester hydrolysis. In wine, these reactions can occur during fermentation through enzymatic processes (Chapter 22.2 and 22.3), and can also occur post‐fermentation through non‐enzymatic, acid‐catalyzed reactions (Chapter 25). The reaction mechanism for acid‐catalyzed esterification is shown in Figure 7.3.
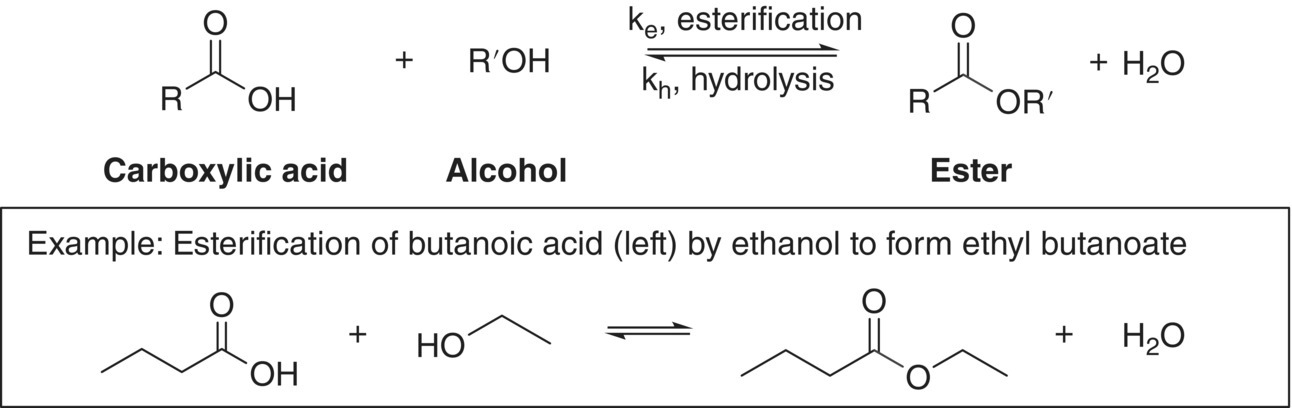
Figure 7.2 General reaction for the formation and hydrolysis of esters (top) and esterification reaction to form ethyl butanoate (bottom)
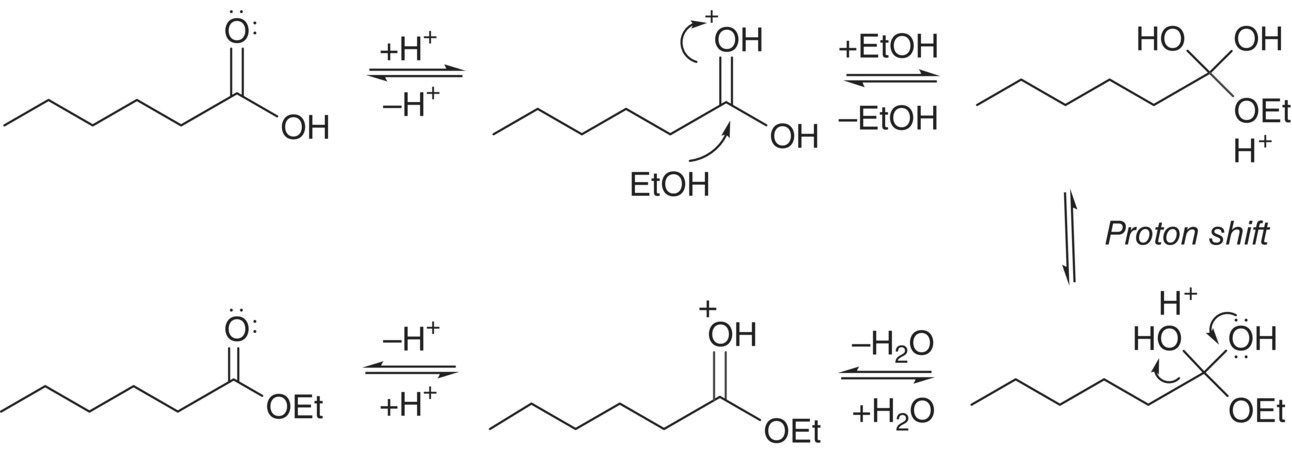
Figure 7.3 Reaction mechanism for acid‐catalyzed esterification of an alcohol (ethanol) and a carboxylic acid (hexanoic acid) to yield an ester (ethyl hexanoate). All steps are reversible, and the reverse reaction represents ester hydrolysis
Esterification reactions are typically reversible – that is, both hydrolysis and esterification are always occurring at measurable rates. The relative proportions of carboxylic acid, alcohol, and ester forms will move towards equilibrium during wine storage. The equilibrium expression for ester formation is determined as the ratio of the esterification reaction rate constant (ke) to the reverse hydrolysis reaction (kh), and can also be defined in terms of the ratios of the reaction constituents:
For most acyclic esters, Keq = 4 is a commonly accepted value [1]. Because systems will move towards equilibrium (the “Law of Mass Action”), the concentration of an individual ester can increase or decrease during storage, depending on the initial concentrations of acid, ester, and alcohol. Water is generally constant, around 50 M. Equation (7.1) can be used to predict equilibrium concentrations of esters in wine and changes that will occur during storage. For example:
- Aliphatic ethyl esters, for example, ethyl hexanoate, tend to increase or decrease only slightly during storage. Using ethyl hexanoate as an example: in a typical wine, [Ethanol] = 100 g/L or 2 M. From Equation (7.1) and assuming K = 4, the ratio of [Ester]/[Acid] should be approximately 1:6. After fermentation, a typical wine may have 0.01 mM (1.44 mg/L) ethyl hexanoate and 0.05 mM (5.8 mg/L) hexanoic acid, or a ratio of 1:5. Thus, ethyl hexanoate would decrease only slightly during storage.
- Acetate esters, for example, isoamyl acetate, tend to be at much greater concentrations than equilibrium following fermentation, and thus will decrease during storage. Using isoamyl acetate as an example: in a typical wine, [Acetic acid] = 0.5 g/L or 8.3 mM, and thus the ratio of [Ester]/[Alcohol] should be approximately 1:1600. However, after fermentation, a typical wine may have 0.01 mM (1.3 mg/L) isoamyl acetate and 1 mM (90 mg/L) isoamyl alcohol, or a ratio of 1:100. Thus, isoamyl acetate is expected to decrease by over an order of magnitude during storage.
- Esters of organic acids and other more complex acids can increase by over an order of magnitude during storage, for the reason that these esters tend to be at negligible concentrations immediately after fermentation.
- Lactones, particularly oak lactones, can increase during storage because the closed‐ring form is strongly favored thermodynamically (see Section 7.4).
In wine‐like solutions, ester hydrolysis will obey pseudo‐first‐order reaction kinetics:3

where C represents the concentration at a given time t and C0 is the initial concentration of the ester. The time necessary for the ester to decrease by 50% (the half‐life, t½) is inversely related to k:

Assuming that the ester is in large excess of its corresponding acid, that is esterification is negligible, it is possible to calculate k and t½ from the slope of a plot of log [C] versus t (see Figure 7.4). These values have been tabulated for a wide range of esters at different pH values and ethanol concentrations [2].

Figure 7.4 Isoamyl acetate hydrolysis over time in a model wine as a function of temperature.
Data were adapted from Reference [1]
At a normal wine pH of 3.4–3.7 and room temperature, the apparent half‐life of most aliphatic esters will be several months, but many factors are known to affect the kinetics of ester formation and hydrolysis [1]. Importantly, these factors will not dramatically alter the equilibrium constant. This is reviewed in further detail in Chapter 25.
- pH. Esterification in wine is an acid‐catalyzed reaction (Figure 7.3), so decreasing pH will increase the rate of esterification or hydrolysis at a factor roughly proportional to the change in [H+]. For example, increasing the pH from 2.9 to 4.1 in a model wine solution increases the half‐life for isoamyl acetate hydrolysis from 60 days to 570 days [1]. A review of predicted and calculated pH‐dependent ester hydrolysis values can be found in Reference [2].
- Temperature. Higher temperatures will also increase reaction rates (Figure 7.4), with a 10 °C increase in temperature roughly doubling the rate of hydrolysis (Ea = 45–65 kJ/mol; see Chapter 25 for more on activation energy). Similar increases in reaction rates are expected for esterification reactions.
- Substrate. Esters with longer carbon chains tend to approach equilibrium faster. For example, the hydrolysis rate of ethyl decanoate is about 3‐fold faster than ethyl octanoate and 15‐fold faster than ethyl hexanoate under similar model wine conditions. Conjugated acids, for example, sorbic acid, have been observed to have very slow esterification kinetics [3], presumably due to resonance stabilization of the carbonyl group.
7.3 Esters in grapes
Many fruits accumulate suprathreshold concentrations of volatile esters during ripening, presumably to attract frugivores.4 By comparison, most grapes accumulate negligible concentrations of volatile esters: in one study of Cabernet Sauvignon and Riesling berries, only methyl hexanoate and (Z)‐3‐hexenyl butanoate were detectable during ripening, and the total ester concentration was < 1 mg/kg [5]. These grape‐derived esters would be expected to be hydrolyzed during fermentation and storage, and to subsequently have a negligible impact on wine flavor. One notable exception is methyl anthranilate (MA), responsible for the “foxy, Concord” aromas of mature V. labruscana and V. rotundifolia grapes (Chapter 5).
Hydroxycinnamic acids (HCA, e.g., coumaric acid) exist in grapes primarily as their tartrate esters. These esters have no aroma and their role is discussed in Chapter 13. Some flavan‐3‐ols are found as gallate esters, as described in Chapter 14.
7.4 Esters formed during winemaking and storage
The majority of odorous wine esters are formed during fermentation and storage via enzymatic or non‐enzymatic esterification of carboxylic acids. The factors affecting these reactions are discussed later in this chapter.
7.4.1 Ethyl and acetate esters
All carboxylic acids and alcohols in wine can potentially esterify. Since there are dozens (if not hundreds) of both of these compound classes in wines, one could expect to find thousands of different esters in wine, if only at vanishingly small concentrations. Because ethanol is the dominant alcohol in wine, the majority of esters formed in wine are ethyl esters, with the most potent typically being fatty acid ethyl esters (FAEE). Fatty acids are formed as part of yeast lipid metabolism (Chapter 22.2), and their concentrations and sensory properties are discussed in Chapter 3. A list of FAEE with high OAV in wine are reported in Table 7.1.
Table 7.1 Odor descriptors, structures, detection thresholds, and concentrations for key fatty acid ethyl esters (FAEE) in wines. The ethoxy group is highlighted
| Examples | Structure | Odor descriptora | Odor threshold (μg/L)b |
Typical range (μg/L)c |
| Ethyl acetate | 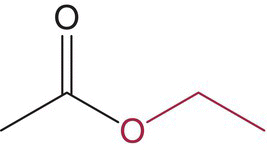 |
Nail polish remover, fruity | 12 000 | 5000–63 000d |
| Ethyl butanoate |  |
Apple, fruity | 20 | 70–540 |
| Ethyl hexanoate (ethyl caproate) |
 |
Green apple | 14 | 150–1500 |
| Ethyl octanoate (ethyl caprylate) |
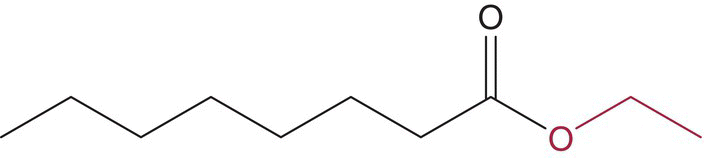 |
Fruity, peach | 5 | 140–2500 |
| Ethyl decanoate | 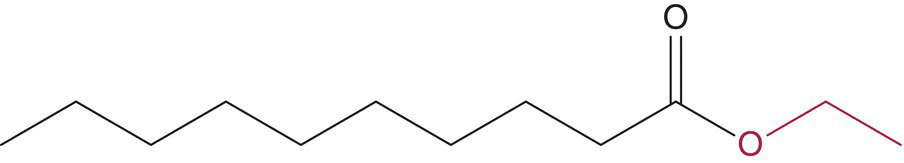 |
Fruity | 200 | 14–910 |
| Ethyl 2‐methylpropanoate (ethyl isobutyrate) |
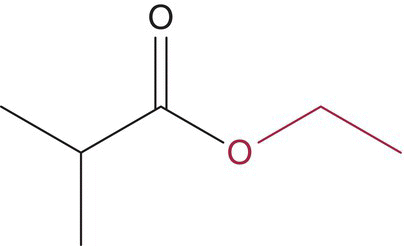 |
Sweet | 15 | 10–120 |
| Ethyl 2‐methylbutanoate | 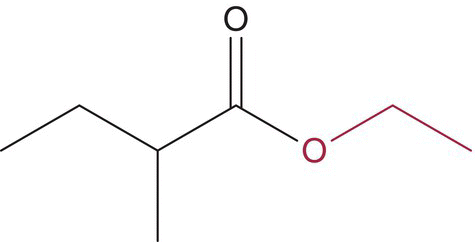 |
Apple | 18 | n/a |
| Ethyl 3‐methylbutanoate (ethyl isovalerate) |
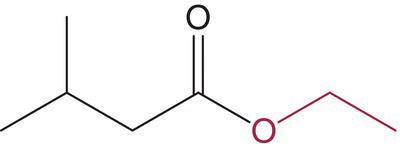 |
Fruity | 3 | 2–30 |
| Ethyl 3‐methylpentanoate |  |
Strawberry | 0.5c | 30 ng/Le |
| Ethyl 4‐methylpentanoate |  |
Strawberry | 0.75c | 230 ng/Le |
a Descriptors from Flavornet (www.flavornet.org) and Reference [6].
b Thresholds in model wine from References [7] and [8].
c Ranges summarized from Spanish red wines [9] and New Zealand Sauvignon Blanc white wines [10]. Mean values from Reference [10].
d Higher concentrations possible in wines that are microbially spoiled, for example, by Acetobacter.
e Concentrations in two aged red wines (>5 years old). Concentrations of all but ethyl 4‐methylpentanoate were undetectable in young wines.
Beyond FAEE, several other ethyl esters can form during storage and have been detected in wine:
- Ethyl lactate, ethyl hydrogen malate, diethyl malate, and other esters of the major wine organic acids
- Ethyl cinnamate, ethyl vanillate, and related ethyl esters of cinnamic and phenolic acids
- Ethyl sorbate and ethyl phenylacetate, implicated in some wine faults, as described below
- Ethyl leucinate, ethyl valinate, and other ethyl esters of amino acids.
In wine, volatile esters other than ethyl esters are generally at concentrations well below their sensory thresholds. One exception to this rule is the acetate esters, which are distinguished by the presence of an acetyl moiety (Table 7.2). Acetate esters are formed by enzymatic acetylation of alcohols during fermentation [11], discussed in more detail in Chapter 22.3. The alcohols that participate in these reactions are often byproducts of amino acid biosynthesis (e.g., isoamyl alcohol, 2‐phenylethanol) or can be grape‐derived (e.g., 1‐hexanol, cis‐3‐hexen‐1‐ol), described in Chapter 6. 3‐Mercaptohexanol, a varietal thiol released during fermentation, can also be acetylated to form its corresponding O‐acetate ester (Chapter 10).
Table 7.2 Odor descriptors, structures, detection thresholds, and concentrations for key acetate esters in wines. The acetate moiety is highlighted
| Examples | Structure | Odor descriptora | Odor threshold in wine (μg/L) [11] | Typical range (μg/L) [11] |
| 2‐Methylpropyl acetate (isobutyl acetate) |
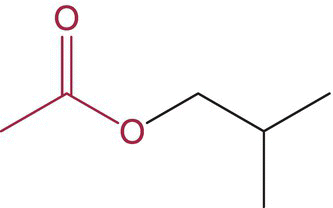 |
Banana, cherry | 1600 | ND–170b |
| 3‐Methylbutyl acetate (isoamyl acetate) |
 |
Banana | 160 | 30–5500 |
| Hexyl acetate |  |
Green apple, sweet | 1800 | ND–260 |
| 2‐Phenylethyl acetate |  |
Honey, rose | 2400 | ND–260 |
a Descriptors from Flavornet (www.flavornet.org).
b ND = Not detectable.
Formation of the FAEE and acetate esters during fermentation is governed by several factors, which will be discussed in more detail in later chapters. Briefly, factors include:
- Concentrations of FAEE are affected by the free fatty acid concentration formed during fermentation, which is influenced by must composition, insoluble solids concentration, oxygen availability, temperature, and yeast strain, as well as the degree of enzymatic esterification (Chapter 22.2).
- Formation of the acetate esters is governed by the availability of alcohol substrates and the activity of acetyltransferase enzymes capable of esterifying higher alcohols to acetate esters (Chapter 22.3).
As mentioned above in Section 7.1 and Chapter 25, acetate esters tend to decrease during storage, FAEE stay roughly constant, and esters of other wine carboxylic acids like organic acids tend to increase. The rate of increase or decrease (i.e., the rate at which equilibrium is approached) will be faster at lower pH and higher temperature, for reasons previously discussed.
7.4.2 Aliphatic γ‐lactones
Cyclic esters formed by intramolecular condensation of carboxylic acid and alcohol groups (hydroxycarboxylic acids) are referred to as lactones. In principle, the lactone ring can range from three atoms to infinitely large, but in wine the most important lactones are those that are the most thermodynamically stable rings, specifically those containing five atoms (γ‐lactone) and to a lesser extent those containing six atoms (δ‐lactones).
One important category is the unsubstituted γ‐lactones, shown in Table 7.3. Except for γ‐butyrolactone (where R = H), these lactones exist as enantiomers with slightly different sensory properties. In most wines, the distribution of enantiomers is nearly racemic (60:40) [12], and stereochemical distinctions can often be ignored. Relatively little work has gone into understanding factors that affect production of these unsubstituted lactones. Butyrolactone appears to be formed by yeast catabolism of glutamic acid [13]. The source of the γ‐hydroxycarboxylic acid precursors for the other lactones have not been well studied. Several of the other lactones (e.g., octalactone, nonalactone, decalactone) are also found in beer, so it is possible that they are formed de novo during fermentation. Red wines generally have higher concentrations of longer‐chain lactones than white wines and botrytized wines can have up to 59 μg/L of nonalactone, but explanations for these effects are unclear [12].
Table 7.3 Odor descriptors, structures, detection thresholds, and concentrations for key lactones in wines
| Examples | Structure | Odor descriptora | Odor threshold (μg/L) [14,15]b | Typical range (μg/L) [8,12,14,16] |
| γ–Butyrolactone (C4) | 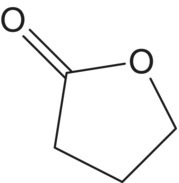 |
Caramel, sweet | Unknown (high) |
4100–21 400 |
| γ–Octalactone (C8) | 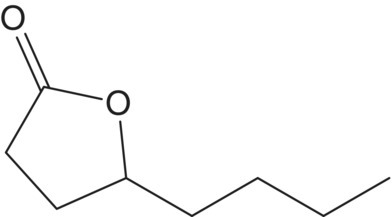 |
Coconut | 7 | <0.1–5 |
| γ–Nonalactone (C9) | 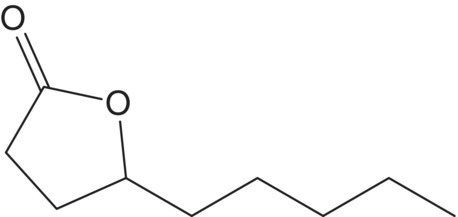 |
Peach | 25 | 2–30 |
| γ–Decalactone (C10) |  |
Fatty, peach | 0.7 | <0.1–1.5 |
| γ–Dodecalactone (C12) | 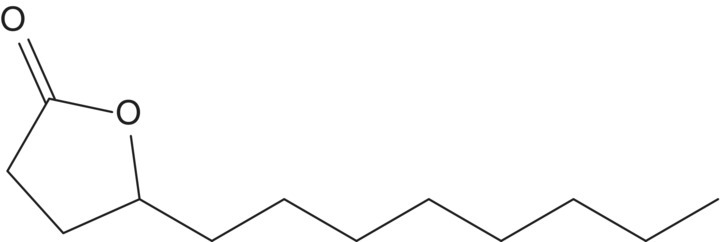 |
Coconut | 7 | <0.1–20 |
| (4S,5S)‐3‐Methyl‐γ‐octalactone (cis‐oak lactone) |
 |
Coconut | 25 | <0.1–700 |
| (4S,5R)‐3‐Methyl‐γ‐octalactone (trans‐oak lactone) |
 |
Coconut | 110 | <0.02–350 |
a From www.flavornet.org and Reference [15].
b In model wine, for racemic mixture if not specified.
A second important class of aliphatic γ‐lactones are the oak‐derived 3‐methyl‐γ‐octalactones, also referred to as oak lactones or whiskey lactones (Table 7.3). These compounds can exist as four stereoisomers, of which two are the dominant forms detected in wines: the (4S,5S)‐isomer, referred to as cis‐oak lactone, and the (4S,5R)‐isomer, referred to as trans‐oak lactone. These compounds are usually at undetectable concentrations in wines made without oak contact, but can be extracted during contact of wine with oak barrels or barrel alternatives (e.g., oak staves, oak chips).
The factors affecting cis‐oak lactone variation in oak and oaked wines is discussed in more detail in Chapter 25. Briefly, oak lactones are present in untoasted oak at total concentrations ranging from 10 to 100 mg/kg, with the majority (>75%) existing as the cis‐isomer [17]. In wine, the cis‐isomer can be detected at concentrations close to 700 μg/L, and is usually present at two‐fold higher concentrations (and 10‐fold greater odor activity) than the trans‐isomer.
Similar to some monoterpenoids (Chapter 23.1), oak lactones can be derived from non‐volatile precursors, one of which is the lactone open‐ring form, 3‐(S)‐hydroxy‐4‐methylcarboxylic acid (Figure 7.5). Formation of the oak lactone from the precursor is acid‐catalyzed, similar to that for other esters, and also appears to be irreversible. The rate of lactonization is over 10‐fold faster for the trans‐isomer formation than for the cis‐isomer (half‐life = 3 h versus 40 h at pH 2.9 and room temperature), likely due to greater thermodynamic stability of the trans‐isomer [18]. The lactonization rate is such that oak lactones can continue to form and oak aromas continue to intensify even once the wine is no longer in contact with oak, and the faster rate of trans‐isomer formation explains the decrease in the cis/trans rate during storage. These carboxylic acid precursors appear likely to form either through pyrolysis (during toasting) or hydrolysis of glycoconjugates of 3‐hydroxy‐4‐methylcarboxylic acid in oak, notably the galloylglucoside (present at up to 500 mg/kg in oak), rutinoside, and glucoside forms (Figure 7.5) [19]. The glucoside is more stable than the free 3‐hydroxy‐4‐methylcarboxylic acid, and conversion to oak lactones can take over 1 year under model conditions [19]. Other odorous lactones including wine lactone (Chapter 8 and Chapter 25 ) and sotolon (Chapter 9) are discussed later.

Figure 7.5 Formation of cis‐ and trans‐oak lactones from lactonization of 3‐(S)‐hydroxy‐4‐methylcarboxylic acid in aqueous acidic environments.
(adapted from Reference [19])
The carboxylic acid precursor is detectable in oak or may be formed during storage from glycoconjugate precursors (X = gallolylglucosyl, rutinosyl, or glucosyl group)
7.5 Sensory effects
In most gas chromatography–olfatometry studies, the straight chain and branched‐chain FAEE are invariably included in the list of the most potent compounds (highest OAV), in particular ethyl butanoate, ethyl hexanoate, ethyl octanoate, and ethyl 2‐ and 3‐methylbutanoate. Elimination of any individual FAEE generally has minor effects on the overall aroma, but in combination these appear to be responsible for red‐ and dark‐fruit aromas in wines [20]. The esters of most other organic acids, e.g., diethyl tartrate or diethyl succinate, have low volatility and sensory thresholds well above their concentrations in wine, although an additive contribution cannot be ruled out.
A handful of ethyl esters and their corresponding acids may be responsible for off‐aromas (also see Chapter 18) in some wines, such as:
- High concentrations of ethyl acetate and acetic acid are well known to contribute to perception of “volatile acidity,” a wine fault characterized by pungent “nail polish remover” and “vinegar” aromas [21].
- Ethyl phenylacetate and phenylacetic acid are reported to be characteristic odorants in wines produced from “sour rot” afflicted grapes [22].
- Ethyl sorbate, formed by esterification of sorbic acid (a preservative) is reported to yield a “candy, pineapple” aroma during storage [3].
In contrast to ethyl esters, which appear to act in combination, acetate esters (particularly isoamyl acetate) may exert an effect on the fruity aromas of young wines in isolation. Removal of isoamyl acetate from a reconstituted Grenache rosé wine is reported to diminish fruitiness [7], and spiking a Maccabeo white wine to yield a 200% increase is reported to increase “banana” aroma [23]. However, since acetate ester concentrations are often well correlated, these experiments are not very realistic. Reconstitution studies in which all acetate esters are selectively omitted have not been reported. One acetate ester that deserves special consideration is the polyfunctional thiol 3‐mercaptohexyl acetate (3‐MHA), whose concentration is strongly correlated with “passionfruit” aromas (Chapter 10). As described previously, acetate ester concentrations will decrease during wine storage through acid hydrolysis to well below threshold, and thus will have a minimal effect on the aroma of aged wines.
The C8–C12 lactones have similar “peach, fruity, coconut” aromas, and may have a minor effect on wine aroma even at subthreshold concentrations through additive effects [24]. Since they are present in most wines and arise from fermentation, they may contribute to the typical vinous character of wine. Despite its relatively high concentration, γ‐butyrolactone likely does not affect wine aroma.5
Because of its lower threshold and higher concentrations in most wines, the cis‐isomer of oak lactone has a more profound effect on the aroma of wine and other oak‐aged beverages. The cis‐isomer is reported to correlate positively with “coconut” aroma in Chardonnay, and with “coconut”, “vanilla”, “berry,” and “dark chocolate” aromas in Cabernet Sauvignon wine [17]. Oak lactone concentrations correlate with consumer liking of oaked wines, but only to a point – a racemic mixture of cis‐ and trans‐isomers in red wine was reported to decrease consumer preference at concentrations above 235 μg/L [25].
References
- 1. Ramey, D.D. and Ough, C.S. (1980) Volatile ester hydrolysis or formation during storage of model solutions and wines. Journal of Agricultural and Food Chemistry, 28 (5), 928–934.
- 2. Rayne, S. and Forest, K. (2011) Estimated carboxylic acid ester hydrolysis rate constants for food and beverage aroma compounds. Nature Proceedings. doi: 10.1038/npre.2011.6471.1.
- 3. Derosa, T., Margheri, G., Moret, I., et al. (1983) Sorbic acid as a preservative in sparkling wine – its efficacy and adverse flavor effect associated with ethyl sorbate formation. American Journal of Enology and Viticulture, 34 (2), 98–102.
- 4. Nogueira, J.M.F., Fernandes, P.J.P., Nascimento, A.M.D. (2003) Composition of volatiles of banana cultivars from Madeira Island. Phytochemical Analysis, 14 (2), 87–90.
- 5. Kalua, C.M. and Boss, P.K. (2009) Evolution of volatile compounds during the development of Cabernet Sauvignon grapes (Vitis vinifera L.). Journal of Agricultural and Food Chemistry, 57 (9), 3818–3830.
- 6. Campo, E., Ferreira, V., Escudero, A., Cacho, J. (2005) Prediction of the wine sensory properties related to grape variety from dynamic‐headspace gas chromatography–olfactometry data. Journal of Agricultural and Food Chemistry, 53 (14), 5682–5690.
- 7. Ferreira, V., Ortin, N., Escudero, A., et al. (2002) Chemical characterization of the aroma of Grenache rose wines: aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. Journal of Agricultural and Food Chemistry, 50 (14), 4048–4054.
- 8. San Juan, F., Cacho, J., Ferreira, V., Escudero, A. (2012) Aroma chemical composition of red wines from different price categories and its relationship to quality. Journal of Agricultural and Food Chemistry, 60 (20), 5045–5056.
- 9. Ferreira, V., Lopez, R., Cacho, J.F. (2000) Quantitative determination of the odorants of young red wines from different grape varieties. Journal of the Science of Food and Agriculture, 80 (11), 1659–1667.
- 10. Benkwitz, F., Tominaga, T., Kilmartin, P.A., et al. (2012) Identifying the chemical composition related to the distinct aroma characteristics of New Zealand Sauvignon Blanc wines. American Journal of Enology and Viticulture, 63 (1), 62–72.
- 11. Sumby, K.M., Grbin, P.R., Jiranek, V. (2010) Microbial modulation of aromatic esters in wine: current knowledge and future prospects. Food Chemistry, 121 (1), 1–16.
- 12. Cooke, R.C., Capone, D.L., van Leeuwen, K.A., et al. (2009) Quantification of several 4‐alkyl substituted gamma‐lactones in Australian wines. Journal of Agricultural and Food Chemistry, 57 (2), 348–352.
- 13. Wurz, R.E.M., Kepner, R.E., Webb, A.D. (1988) The biosynthesis of certain gamma‐lactones from glutamic acid by film yeast activity on the surface of flor sherry. American Journal of Enology and Viticulture, 39 (3), 234–238.
- 14. Ferreira, V., Jarauta, I., Ortega, L., Cacho, J. (2004) Simple strategy for the optimization of solid‐phase extraction procedures through the use of solid–liquid distribution coefficients. Application to the determination of aliphatic lactones in wine. Journal of Chromatography A, 1025 (2), 147–156.
- 15. Spillman, P.J., Sefton, M.A., Gawel, R. (2004) The contribution of volatile compounds derived during oak barrel maturation to the aroma of a Chardonnay and Cabernet Sauvignon wine. Australian Journal of Grape and Wine Research, 10 (3), 227–235.
- 16. Elliott, S. and Burgess, V. (2005) The presence of gamma‐hydroxybutyric acid (GHB) and gamma‐butyrolactone (GBL) in alcoholic and non‐alcoholic beverages. Forensic Science International, 151 (2–3), 289–292.
- 17. Pollnitz, A.P., Jones, G.P., Sefton, M.A. (1999) Determination of oak lactones in barrel‐aged wines and in oak extracts by stable isotope dilution analysis. Journal of Chromatography A, 857 (1–2), 239–246.
- 18. Wilkinson, K.L., Elsey, G.M., Prager, R.H., et al. (2004) Rates of formation of cis‐ and trans‐oak lactone from 3‐methyl‐4‐hydroxyoctanoic acid. Journal of Agricultural and Food Chemistry, 52 (13), 4213–4218.
- 19. Wilkinson, K.L., Prida, A., Hayasaka, Y. (2013) Role of glycoconjugates of 3‐methyl‐4‐hydroxyoctanoic acid in the evolution of oak lactone in wine during oak maturation. Journal of Agricultural and Food Chemistry, 61 (18), 4411–4416.
- 20. Lytra, G., Tempere, S., de Revel, G., Barbe, J.C. (2012) Impact of perceptive interactions on red wine fruity aroma. Journal of Agricultural and Food Chemistry, 60 (50), 12260–12269.
- 21. Fugelsang, K.C. and Edwards, C.G. (2007) Wine microbiology practical applications and procedures, Springer, New York.
- 22. Campo, E., Saenz‐Navajas, M.P., Cacho, J., Ferreira, V. (2012) Consumer rejection threshold of ethyl phenylacetate and phenylacetic acid, compounds responsible for the sweet‐like off odour in wines made from sour rotten grapes. Australian Journal of Grape and Wine Research, 18 (3), 280–286.
- 23. Escudero, A., Gogorza, B., Melus, M.A., et al. (2004) Characterization of the aroma of a wine from Maccabeo. Key role played by compounds with low odor activity values. Journal of Agricultural and Food Chemistry, 52 (11), 3516–3524.
- 24. Ferreira, V. (2010) Volatile aroma compounds and wine sensory attributes, in Managing wine quality, Vol. 1, Viticulture and wine quality (ed. Reynolds, A.G.), Woodhead Publishing and CRC Press, Oxford and Boca Raton, FL.
- 25. Chatonnet, P., Boidron, J.N., Pons, M. (1990) Maturation of red wines in oak barrels – evolution of some volatile compounds and their aromatic impact. Sciences Des Aliments, 10 (3), 565–587.
