10
Thiols and Related Sulfur Compounds
10.1 Introduction
Along with oxygen and nitrogen, sulfur is a common heteroatom found in organic molecules derived from natural sources. Sulfur sits below oxygen on the periodic table so has a similar electronic configuration and forms analogous compounds, including many organic ones (cf. alcohol, ROH, and thiol, RSH), but displays dissimilar properties due to the presence of d‐orbitals and ability to form multiple bonds as a result (i.e., sulfur can expand its octet). In addition, sulfur is larger and less electronegative than oxygen, which influences the relative reactivity of sulfur compounds as compared to oxygen analogs. This chapter will discuss sulfur‐containing species with redox states ≤0, also called “reduced” forms of sulfur, which have relevance to both wine aroma and wine redox chemistry. The chemistry of sulfur dioxide, SO2, will be discussed in Chapter 17.
10.1.1 Key chemical properties and reactions
The chemistry of sulfur compounds is most conveniently discussed in contrast to their oxygen analogs. Like oxygen, the prevalent oxidation state for sulfur is –2, but a range of other oxidation states are possible (up to +6, Table 10.1). As a result, many of the reactions that sulfur compounds partake in are redox reactions that involve a change in oxidation state. The key chemistry of reduced sulfur compounds in wine is summarized below:
- Thiol nucleophilicity. Thiols are better nucleophiles than alcohols, as sulfur is more polarizable than oxygen due to the greater distance of the valence electrons from the nucleus, and the lower electronegativity of sulfur means its lone pair electrons are more available for bond formation. As nucleophiles, thiols can participate in reactions with electrophiles like aldehydes and quinones.
- Thiols and hydrogen bonding. S–H bonds are less polar than O–H bonds, which results in a poorer hydrogen‐bonding capability, as evident in the respective properties of H2O (liquid) and H2S (gas) at room temperature (see Chapter 1 for more on this).
- Acidity of thiols. S–H bonds are weaker than O–H bonds and there is greater delocalization of negative charge on the larger sulfur atom of the thiolate, such that H2S and thiols are stronger acids than H2O and alcohols (cf. ethanethiol, pKa = 8.5 versus ethanol, pKa = 15.9).
- Thiol/disulfide redox chemistry. C–S bonds are similar in strength to C–O bonds, and C = S bonds are weaker than C = O bonds; however, S–S bonds are much stronger than O–O bonds (250 versus 150 kJ/mol).1 S–S bonds can be formed by oxidation of thiols to yield disulfides (RSSR),2 with disulfide bridges being important to the structure and function of proteins.
- Reactions with transition metals. Metal ions (e.g., copper and iron) contribute to redox reactions such as formation of disulfides, and are involved in the formation of metal sulfides and sulfur–metal complexes.
Table 10.1 Reactions of sulfur compounds particularly relevant to wine chemistry
| Oxidation statea | Compound type | Example reactionb |
| –2 | Sulfide Thiol Alkyl sulfide Thioacetate |
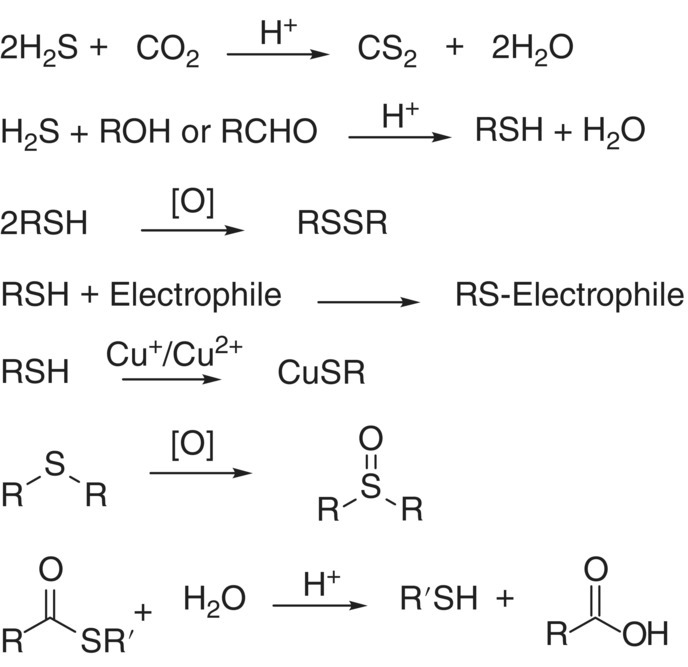 |
| –1 | Alkyl disulfide | 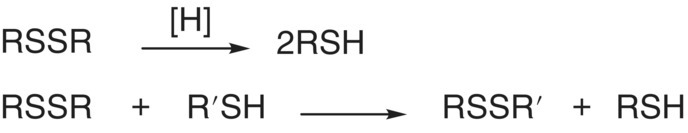 |
| 0 | Sulfoxide | 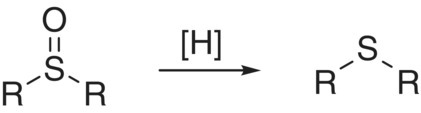 |
a Oxidation states of +4 (e.g., sulfur dioxide, bisulfite, and sulfite) and +6 (e.g., sulfate) are also possible; reactions involving these compounds are described elsewhere.
b May involve chemical and/or enzymatic transformations, some of which are putative; [O] = oxidation, [H] = reduction.
10.1.2 Sulfur compounds and wine aroma
Sulfur‐containing compounds relevant to wine aroma are mainly ascribed to microbial metabolic activities acting on grape constituents or other substrates. There are effects of viticulture, winemaking, and storage on the sulfur compounds present in different wine styles and their resulting diversity contributes significantly to wine sensory properties and quality. Various classes of volatile sulfur compounds (primarily those with reduced forms of sulfur, that is, an oxidation state of –1 or –2, Table 10.1) are especially important to aroma, as well as in other foods (e.g., garlic, cabbage, roasted meat) and beverages (e.g., coffee, beer) [1]. Volatile sulfur compounds possess a wide range of aroma descriptors and detection thresholds, and contribute aromas to wine that can be perceived as positive or negative, depending on the nature of the compound and its concentration. Table 10.2 outlines some aspects of the types of volatile sulfur compounds found in wine.
Table 10.2 Indicative odor descriptors, detection thresholds, and odor activity values for classes of volatile sulfur compounds found in wine [2–8]
| Compound type | Examplesa | Structure | Odor descriptor | Threshold (ng/L) | OAV (max.) |
| Sulfide | Hydrogen sulfide (H2S) | 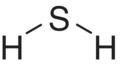 |
Rotten egg | 1000 | 35 |
| Alkyl thiol | Methanethiol (MeSH) Ethanethiol (EtSH) |
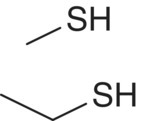 |
Putrefaction Onion, rubber |
2000 1000 |
8 19 |
| Alkyl sulfide | Dimethyl sulfide (DMS) Diethyl sulfide (DES) 3‐(Methylsulfanyl)propan‐1‐ol (methionol) |
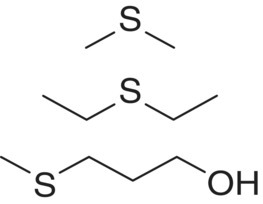 |
Cabbage, asparagus, truffle Garlic, rubber Potato, cauliflower |
25 000 1000 1 000 000 |
30 30 6 |
| Alkyl disulfide | Dimethyl disulfide (DMDS) Diethyl disulfide (DEDS) |
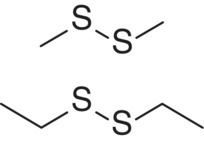 |
Onion, cabbage, asparagus Onion |
29 000 4000 |
<1 22 |
| Thioacetate | Methyl thioacetate (MeSAc) Ethyl thioacetate (EtSAc) |
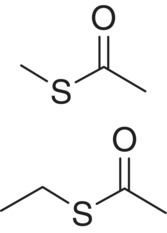 |
Cheese, egg Garlic, onion |
50 000 10 000 |
2 18 |
| Polyfunctional thiol | 3‐Mercaptohexan‐1‐ol (3‐MH) 3‐Mercaptohexyl acetate (3‐MHA) 4‐Mercapto‐4‐methylpentan‐2‐one (4‐MMP) 4‐Mercapto‐4‐methylpentan ‐2‐ol (4‐MMPOH) |
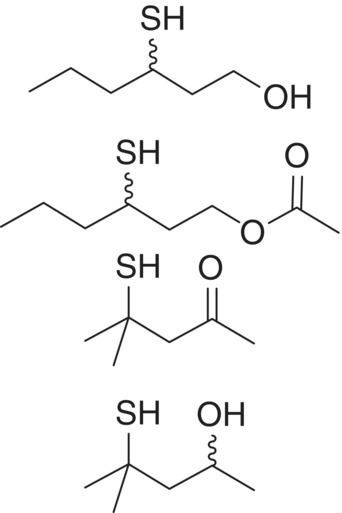 |
Grapefruit, passionfruit Passionfruit, box tree Box tree, guava Citrus |
60 4 3 55 |
310 625 30 2 |
| S‐Heterocycle | Benzothiazole (BT) 2‐Methyltetrahydrothiophen‐3‐one (MTHT) |
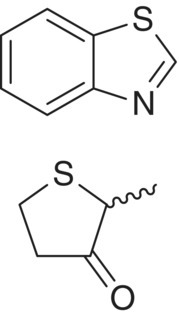 |
Rubber Natural gas, sulfurous |
50 90 |
<1 5 |
| Aryl thiol | Benzenemethanethiol (BMT) 2‐Furanmethanethiol (2‐furfurylthiol (FFT)) 2‐Methyl‐3‐furanthiol (MFT) |
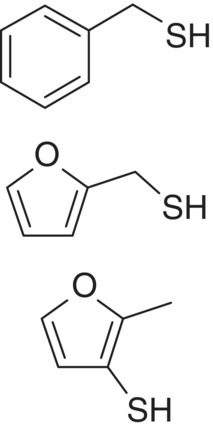 |
Smoke, struck flint Roasted coffee Cooked meat |
0.3 0.4 3 |
440 (1330)b 560 (13 750)b 66 |
a The IUPAC terms “sulfanyl” (i.e., RS–, R ≠ H) and “thiol” (i.e., RSH, R ≠ H) are preferably used in place of obsolete “mercapto” and “mercaptan” in the systematic naming of these compounds, although the older terms are still used interchangeably due to their familiarity; for example, 3‐mercaptohexan‐1‐ol can be found in the literature as 3‐sulfanylhexan‐1‐ol (3‐SH) and methanethiol as methyl mercaptan. The IUPAC prefix “thio” denotes the replacement of oxygen with sulfur (as in thioacetate, for example), so “sulfanyl” is used in the systematic name 3‐(methylsulfanyl)propan‐1‐ol (methionol) rather than “thio”; both prefixes are found in the literature and 3‐(methylthio)propan‐1‐ol is also used to refer to methionol. Chiral compounds are presented as their racemates.
b From one study of aged Champagne wines (up to 27 years in bottle) [9].
The odorous sulfur compounds listed in Table 10.2 are typically undetectable in grapes, but may be classified based on their origins during the winemaking process. Some compounds have precursors that can be traced to the grape (varietal), whereas others are yeast metabolites (fermentative) or arise during storage of wine in bulk vessels (e.g., oak barrels) or bottles. In many cases, the precursors are non‐volatile organic sulfur compounds in the form of amino acids, peptides, and their conjugates, although inorganic sulfur compounds (such as sulfite, sulfate, or elemental sulfur) can strongly impact wine aroma when metabolized by yeast. The non‐volatile precursors are introduced below where relevant to description of the associated sulfur aroma compounds, although bisulfite/sulfur dioxide is treated separately (Chapter 17) due to its overall significance in winemaking. Aspects related to conversion of sulfur compounds during fermentation are presented in Chapter 22.4.
10.2 Varietal sulfur aroma compounds – polyfunctional thiols
Some thiols that contribute to wine aroma have their origins in the grape berry. Varietal thiols such as 3‐MH, 3‐MHA, and 4‐MMP, which impart desirable citrus and tropical fruit notes (Table 10.2), are of particular importance to certain grape varieties. Such thiols are denoted polyfunctional due to the presence of additional functional groups containing oxygen, and are notable for their very low sensory thresholds (and generally high OAVs) and major impact on wine aroma. The range of polyfunctional thiols continues to grow as new compounds are identified and assessed (e.g., 3‐sulfanyl forms of 2‐methylbutan‐1‐ol, pentan‐1‐ol, and heptan‐1‐ol), but most of the understanding has come from studies on key odorants 4‐MMP and 3‐MH/3‐MHA, which are particularly abundant in Sauvignon Blanc [8,10,11]. These and similar thiols have also been identified as important contributors to the aroma of other fruits and plants including grapefruit, passionfruit, guava, box tree and broom. With the exception of 3‐MHA and other O‐acetylated derivatives arising from acetylation of 3‐sulfanyl alcohols, varietal thiols can be released from non‐volatile, grape‐derived precursors (amino acid S‐conjugates) during fermentation [8,11–13] (Chapter 23.2).
4‐MMP and the more widespread 3‐MH and 3‐MHA have low ng/L odor detection thresholds and are frequently present at concentrations in excess of those thresholds (Table 10.2). These three aroma impact compounds, which are particularly important to the varietal aroma of young Sauvignon Blanc wines, have been identified in a range of white (including botrytized), red, and rosé wines from grape varietals such as Scheurebe, Colombard, Riesling, Semillon, Petit and Gros Manseng, Gewurztraminer, Muscat, Grenache, Merlot, Syrah, and Cabernet Sauvignon [8,10,11] (e.g., Table 10.3). Note that 3‐MH and 3‐MHA (and other chiral thiols) exist as pairs of enantiomers whose profiles are not necessarily 50:50 (i.e., a racemic mixture). Studies on the individual enantiomers reveal slightly different odor detection thresholds and aroma qualities in hydroalcoholic media [14], as could be expected of these chiral aroma molecules. (R)‐3‐MH has a reported threshold of 50 ng/L and an aroma of “citrus peel” and “passionfruit,” whereas (S)‐3‐MH has a threshold of 60 ng/L and a “grapefruit” aroma. For the acetates, (R)‐3‐MHA has a slightly higher reported threshold of 9 ng/L and a “passionfruit” aroma when compared to (S)‐3‐MHA with its threshold of 2.5 ng/L and aroma of “box wood”. The profile of 3‐MH and 3‐MHA enantiomers can therefore influence the overall fruit, vegetal, and tropical aromas of wine [15]. Furthermore, there is good correlation between the concentration of 3‐MH/3‐MHA and tropical/passionfruit characters for Sauvignon Blanc [3,16].
Table 10.3 Effect of grape variety on typical concentrations of 3‐MH, 3‐MHA, and 4‐MMP in wines [3,5,17–19]
| Grape varietal | Concentration range in wine (ng/L) | ||
| 3‐MH | 3‐MHA | 4‐MMP | |
| Sauvignon Blanc | 25.8–18 700 | NDa–2510 | <0.6–87.9 |
| Red blendsb | 678–11 500 | 4.62–154 | 4.83–54.2 |
| Botrytized winec | 2330–9650 | ND | 8.5–40 |
| Gewurztraminer | 1340–3280 | 0.5–5.7 | ND–15 |
| Riesling | 407–562 | ND–6.4 | ND–7.6 |
a ND, not detected.
b Different proportions of Syrah, Grenache, Mourvedre, Cinsault, and Carignan.
c Sauvignon Blanc or Semillon.
Varietal thiols will participate in many of the reactions described in Table 10.1, of which nucleophilic addition to o‐quinones is particularly well studied [e.g., 11, 20, 21]. Varietal thiols may therefore be lost due to reaction with o‐quinones formed through polyphenol oxidation, in an analogous manner to the reaction of glutathione (GSH) in the formation of GRP (Chapters 11 and 24). In fact, as described in Chapter 24, GSH may help preserve varietal thiols in wine [22].
Thiols can also participate in some unique thiol‐disulfide chemistry following oxidation [8]; for instance, 3,3′‐dithiobis(hexan‐1‐ol) and related chiral oxidation products (3‐propyl‐1,2‐oxathiolane and its 2‐oxide, 3‐propyl‐γ‐sultine, Figure 10.1), identified in botrytized Sauternes wines, can arise from 3‐MH oxidation [23]. Such disulfides and oxidation products (which can also be artifacts from aroma compound extraction and analysis [24]) are expected to have different detection thresholds and altered aroma qualities compared to the varietal thiols themselves.
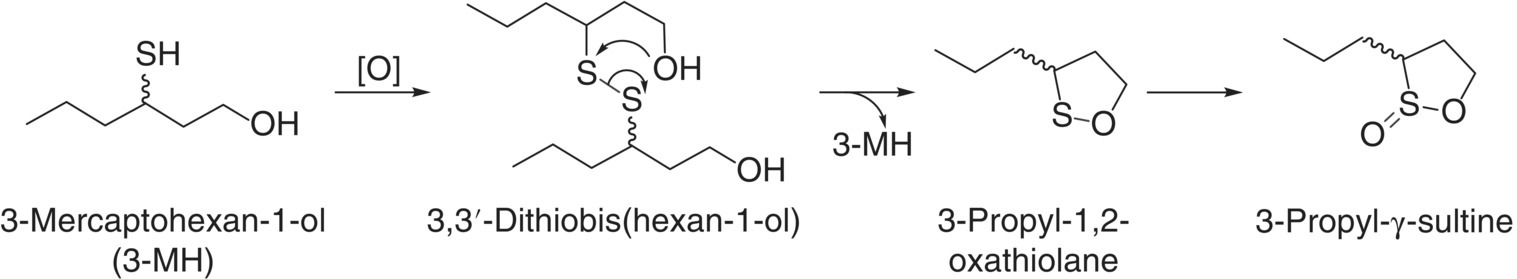
Figure 10.1 Sulfur compounds resulting from oxidation of 3‐MH and thermal disproportionation of its disulfide
Beyond oxidative reactions, hydrolysis of thiol‐containing acetate esters (e.g. 3‐MHA to 3‐MH and acetic acid) will occur during storage, with an important effect of higher temperature increasing the rate of 3‐MHA hydrolysis (as with other acetate esters) [25]. Since the acetate esters are more potent than their corresponding hydrolysis products, this will result in a loss of odor intensity. Factors governing ester hydrolysis are discussed in more detail in Chapter 7.
10.3 Fermentative sulfur aroma compounds
A range of sulfur‐containing aroma compounds are produced as general products of fermentation, including H2S, MeSH, DMS, DMDS, thioacetates, methionol, and heterocycles (Table 10.2); polysulfides such as dimethyl tri‐ and tetrasulfide have also been identified. These compounds typically have a negative impact on wine aroma and are often linked to H2S production by yeast. Yeast may utilize a variety of grape‐derived sources of sulfur (organic or inorganic, including sulfur fungicides, GSH and SO2, but primarily SO42–) to produce H2S for incorporation into sulfur‐containing amino acids [26]. Fermentation conditions, nutrients, and yeast strain can therefore have a large impact on the biosynthesis of these sulfur compounds (Chapter 22.4), with suboptimal conditions leading to greater production. Such sulfur aroma compounds are important to the aromas of other foods and beverages (particularly those involving fermentation) such as beer, cider, cheese, heated foods, and vegetables [1].
Of particular relevance to wine aroma are the low molecular weight, low boiling point fermentative sulfur compounds, which have thresholds in the low μg/L range and may commonly be encountered at these concentrations or higher in wine (Table 10.2). At low levels, relatively more abundant compounds such as H2S and DMS may contribute positively to wine aroma; H2S forms part of a young wine’s fermentation bouquet whereas DMS has been shown to increase perceived fruitiness of wines, up to around 100 μg/L [4,26,27]. When concentrations of fermentative sulfur compounds in wine are excessive (i.e., OAV >1), especially where they occur in combination, it leads to perceptions that the wine is “reduced,” due to the “reductive” aromas associated with rotten egg, putrefaction (sewage), onion, garlic, and cooked/canned vegetables from compounds containing reduced forms of sulfur (i.e., –1 or –2 oxidation states, Table 10.1). The presence of particular sulfur compounds (e.g., H2S, MeSH, and DMS) can provide some idea of whether a wine will be perceived as “reduced,” as indicated in Table 10.4 for a range of commercial bottled wines with evident reductive sensory characteristics. Concentrations of other fermentative sulfur compounds are also elevated under conditions that favor H2S formation, such that CS2, EtSH, DEDS, and thioacetates are also implicated in “reduced” aromas. As indicated below, there are aroma defects that may only become evident after bottling and storage [28] (e.g., increases in H2S or DMS) but more research on fermentative sulfur compounds is required to properly understand the influences of bottle closure, redox status of wine, and additive or masking sensory effects, among other factors.
Table 10.4 Volatile sulfur compounds determined in commercial bottled white and red wines that had evident reductive sensory characteristics. DMDS, EtSH, EtSAc, and DEDS have been omitted due to not being detected in the majority of these wines.
Data from Reference [4]
| Variety (no. assessed) | Concentration range (μg/L) | |||||
| H2S | MeSH | MeSAc | DMS | CS2 | DES | |
| White wine | ||||||
| Chardonnay (4) | 1.5–5.0 | 3.0–8.0 | NDa–7.0 | 20.0–185.0 | 0.5–5.0 | ND |
| Pinot Gris (1) | 2.0 | 3.0 | ND | 11.0 | 0.5 | ND |
| Riesling (10) | 0.5–35.0 | ND–3.0 | ND | 11.0–37.1 | ND–21.1 | ND–0.4 |
| Sauvignon Blanc (6) | 0.8–4.0 | 1.7–6.0 | ND | 25.0–118.2 | 1.0–13.5 | ND–0.4 |
| Sauvignon Blanc Semillon (4) | 2.0–13.0 | 1.0–4.0 | ND–2.1 | 25.0–76.0 | 0.5–14.8 | ND–0.4 |
| Verdelho (1) | 1.0 | 1.6 | ND | 47.7 | 18.6 | 0.4 |
| Viognier (1) | 0.5 | 3.0 | ND | 78.0 | 6.0 | ND |
| Red wine | ||||||
| Cabernet Merlot (2) | 0.5–0.8 | 0.4–1.0 | ND | 102.5–106.0 | 3.5–15.6 | ND–0.4 |
| Cabernet Sauvignon (5) | ND–1.6 | ND–1.5 | ND–10.0 | 88.0–379.5 | 3.0–20.0 | ND–0.4 |
| Durif (1) | 2.0 | 2.0 | 18.0 | 61.0 | 1.0 | ND |
| Grenache Shiraz Merlot (1) | 0.7 | 0.7 | ND | 111.0 | 18.0 | 0.4 |
| Merlot (3) | 0.5–1.2 | ND–1.6 | 3.0–8.0 | 48.0–235.0 | 8.0–17.0 | ND–0.4 |
| Sangiovese (1) | ND | ND | ND | 68.0 | 4.0 | ND |
| Shiraz (22) | ND–8.7 | ND–5.0 | ND–12.5 | 28.0–765.0 | 2.0–45.1 | ND–0.5 |
| Shiraz blends (4) | ND–1.0 | 1.0–1.2 | ND | 57.0–228.4 | 2.0–17.4 | ND–0.4 |
a ND, not detected.
Fermentative sulfur compounds such as thiols and thioacetates can enter into similar reactions [26, 29] to those described previously for varietal thiols. Methyl or ethyl thioacetate can hydrolyze over time [30] to release more potent thiols. Thiols can also potentially be oxidized to their corresponding disulfides, trisulfides, and presumably mixed forms (remembering that artifact formation is possible upon analysis). These compounds may constitute a latent “reservoir” of malodorous thiols that could be formed during anaerobic wine storage – this hypothesis is discussed in more detail in Chapter 30. Similar to varietal thiols, low molecular weight thiols and H2S can be lost through reaction with o‐quinones [31], and these losses will be limited in the presence of other thiols like GSH [32]. As an alkyl sulfide the chemistry of DMS is distinct from the other species discussed in this chapter. DMS does not appear to be susceptible to oxidation, and its concentration will rise during wine storage as a result of hydrolysis of S‐methyl methionine (Chapter 23.3) [33].
Because most low‐molecular weight sulfur compounds have undesirable “reductive” aromas, winemakers employ several tools to remove them from wine when present. Most commonly, Cu2+ (in the form of copper sulfate) is used to remove H2S and thiols3 from wine as their insoluble sulfides, but not dialkyl sulfides, di‐ (or poly‐)sulfides, or thioacetates (Chapter 26.2). Storage of wine on yeast lees may decrease concentrations of fermentative sulfur compounds through adsorption, although care must be taken with additions of SO2 if storing on lees, since sulfite reductases can produce H2S for some time after fermentation has ceased (Chapter 22.4).
10.4 Other sulfur‐containing aroma compounds
Apart from fermentative and varietal sulfur compounds, a number of other sulfur aroma compounds can form during storage. The aryl thiols BMT, FFT, and MFT are particularly important odorants with low ng/L sensory thresholds (and high OAV in some wines) and are responsible for smoke/struck flint, roasted coffee and meat (or “toasty”) aromas, respectively (Table 10.2). Naturally, BMT, MFT, and FFT are significant contributors to the aroma of other foods and beverages such as garden cress, meat and seafood, fruit juice, beer, roasted hazelnuts, and coffee [1]. Aged wines are commonly associated with higher concentrations of these thiols, where they have been detected in Champagne and white (e.g., Chardonnay, Sauvignon Blanc, Semillon) and red (e.g. Cabernet Sauvignon, Merlot) wines [10].
FFT is believed to be formed primarily by reaction of H2S (from fermentation) with furfural extracted from oak barrels (Chapter 25), and is thus at particularly high concentrations in barrel fermented wines [34]. In support of this, furfural and FFT are typically well correlated with each other and with the extent of wood toasting and age of oak barrels [34]. In addition, toasted oak furanyl precursors may also be formed through acid‐catalyzed sugar degradation [35]. In contrast, BMT and benzaldehyde (from benzyl alcohol) are not well correlated, and BMT shows strong varietal dependence, suggesting that a different (unknown) pathway exists for its formation [36]. MFT has no formation pathway proposed for wine, but it is thermally generated in other foodstuffs, along with FFT, from Maillard reactions involving cysteine (or H2S) and pentoses [37]. Interestingly, cysteine S‐conjugates of FFT and MFT (analogous to those that act as varietal thiol precursors, Chapter 23.2) have been identified in Maillard reactions between xylose (which gives furfuryl alcohol) and cysteine [38] (Figure 10.2). Whether such conjugates have a role in the formation of FFT and MFT in wine is yet to be determined, but this would likely require an enzymatic thiolysis process, which is harder to rationalize considering the effect of storage.
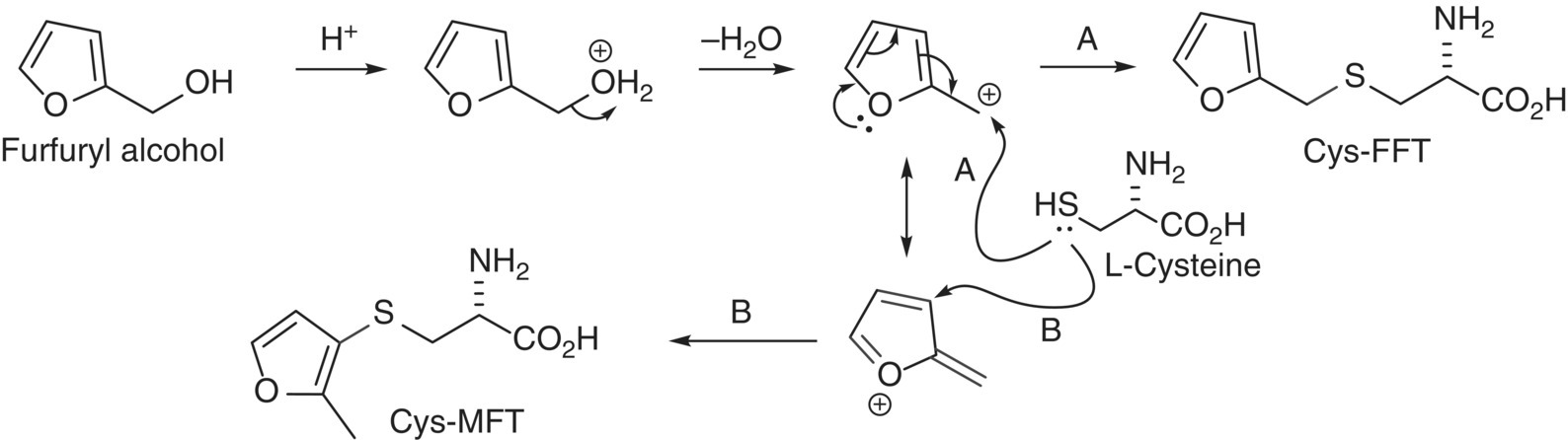
Figure 10.2 Proposed mechanisms for formation of odorless cysteine‐S‐conjugates of FFT (Cys‐FFT, pathway A) and MFT (Cys‐MFT, pathway B) isolated and identified from the reaction of xylose (or furfuryl alcohol) with L‐cysteine
Although there are no specific studies to draw on, the reactivity of BMT, MFT, and FFT in wine is envisaged to be comparable to other thiols, with similar guiding principles about subsequent contributions to wine aroma. Oxidation is expected to lead to disulfide formation (again, analytical artifacts should be considered), and BMT, FFT, and MFT could conceivably react with o‐quinones in the same manner as 3‐MH, H2S, and GSH.
References
- 1. McGorran, R.J. (2011) The significance of volatile sulfur compounds in food flavors, in Volatile sulfur compounds in food (eds Qian, M.C., Fan, X., Mahattanatawee, K.), American Chemical Society, Washington, DC, pp. 3–31.
- 2. Mestres, M., Busto, O., Guasch, J. (2000) Analysis of organic sulfur compounds in wine aroma. Journal of Chromatography A, 881 (1–2), 569–581.
- 3. Lund, C.M., Thompson, M.K., Benkwitz, F., et al. (2009) New Zealand Sauvignon Blanc distinct flavor characteristics: sensory, chemical, and consumer aspects. American Journal of Enology and Viticulture, 60 (1), 1–12.
- 4. Siebert, T.E., Solomon, M.R., Pollnitz, A.P., Jeffery, D.W. (2010) Selective determination of volatile sulfur compounds in wine by gas chromatography with sulfur chemiluminescence detection. Journal of Agricultural and Food Chemistry, 58 (17), 9454–9462.
- 5. Mateo‐Vivaracho, L., Zapata, J., Cacho, J., Ferreira, V. (2010) Analysis, occurrence, and potential sensory significance of five polyfunctional mercaptans in white wines. Journal of Agricultural and Food Chemistry, 58 (18), 10184–10194.
- 6. Tominaga, T. and Dubourdieu, D. (2006) A novel method for quantification of 2‐methyl‐3‐furanthiol and 2‐furanmethanethiol in wines made from Vitis vinifera grape varieties. Journal of Agricultural and Food Chemistry, 54 (1), 29–33.
- 7. Bailly, S., Jerkovic, V., Marchand‐Brynaert, J., Collin, S. (2006) Aroma extraction dilution analysis of Sauternes wines. Key role of polyfunctional thiols. Journal of Agricultural and Food Chemistry, 54 (19), 7227–7234.
- 8. Roland, A., Schneider, R., Razungles, A., Cavelier, F. (2011) Varietal thiols in wine: discovery, analysis and applications. Chemical Reviews, 111 (11), 7355–7376.
- 9. Tominaga, T., Guimbertau, G., Dubourdieu, D. (2003) Role of certain volatile thiols in the bouquet of aged Champagne wines. Journal of Agricultural and Food Chemistry, 51 (4), 1016–1020.
- 10. Dubourdieu, D. and Tominaga, T. (2009) Polyfunctional thiol compounds, in Wine chemistry and biochemistry (eds Moreno‐Arribas, M.V. and Polo, M.C.), Springer, New York, pp. 275–293.
- 11. Coetzee, C. and du Toit, W.J. (2012) A comprehensive review on Sauvignon Blanc aroma with a focus on certain positive volatile thiols. Food Research International, 45 (1), 287–298.
- 12. Capone, D.L., Sefton, M.A., Jeffery, D.W. (2012) Analytical investigations of wine odorant 3‐mercaptohexan‐1‐ol and its precursors, in Flavor chemistry of wine and other alcoholic beverages, American Chemical Society, pp. 15–35.
- 13. Peña‐Gallego, A., Hernández‐Orte, P., Cacho, J., Ferreira, V. (2012) S‐cysteinylated and S‐glutathionylated thiol precursors in grapes. A review. Food Chemistry, 131 (1), 1–13.
- 14. Tominaga, T., Niclass, Y., Frerot, E., Dubourdieu, D. (2006) Stereoisomeric distribution of 3‐mercaptohexan‐1‐ol and 3‐mercaptohexyl acetate in dry and sweet white wines made from Vitis vinifera (Var. Sauvignon Blanc and Semillon). Journal of Agricultural and Food Chemistry, 54 (19), 7251–7255.
- 15. King, E.S., Osidacz, P., Curtin, C., et al. (2011) Assessing desirable levels of sensory properties in Sauvignon Blanc wines – consumer preferences and contribution of key aroma compounds. Australian Journal of Grape and Wine Research, 17 (2), 169–180.
- 16. Benkwitz, F., Tominaga, T., Kilmartin, P.A., et al. (2012) Identifying the chemical composition related to the distinct aroma characteristics of New Zealand Sauvignon Blanc wines. American Journal of Enology and Viticulture, 63 (1), 62–72.
- 17. Tominaga, T., Baltenweck‐Guyot, R., Peyrot des Gachons, C., Dubourdieu, D. (2000) Contribution of volatile thiols to the aromas of white wines made from several Vitis vinifera grape varieties. American Journal of Enology and Viticulture, 51 (2), 178–181.
- 18. Rigou, P., Triay, A., Razungles, A. (2014) Influence of volatile thiols in the development of blackcurrant aroma in red wine. Food Chemistry, 142, 242–248.
- 19. Sarrazin, E., Shinkaruk, S., Tominaga, T., et al. (2007) Odorous impact of volatile thiols on the aroma of young botrytized sweet wines: identification and quantification of new sulfanyl alcohols. Journal of Agricultural and Food Chemistry, 55 (4), 1437–1444.
- 20. Nikolantonaki, M., Chichuc, I., Teissedre, P.‐L., Darriet, P. (2010) Reactivity of volatile thiols with polyphenols in a wine‐model medium: impact of oxygen, iron, and sulfur dioxide. Analytica Chimica Acta, 660 (1–2), 102–109.
- 21. Laurie, V.F., Zúñiga, M.C., Carrasco‐Sánchez, V., et al. (2012) Reactivity of 3‐sulfanyl‐1‐hexanol and catechol‐containing phenolics in vitro. Food Chemistry, 131 (4), 1510–1516.
- 22. Kritzinger, E.C., Bauer, F.F., du Toit, W.J. (2013) Role of glutathione in winemaking: a review. Journal of Agricultural and Food Chemistry, 61 (2), 269–277.
- 23. Sarrazin, E., Shinkaruk, S., Pons, M., et al. (2010) Elucidation of the 1,3‐sulfanylalcohol oxidation mechanism: an unusual identification of the disulfide of 3‐sulfanylhexanol in Sauternes botrytized wines. Journal of Agricultural and Food Chemistry, 58 (19), 10606–10613.
- 24. Hofmann, T., Schieberle, P., Grosch, W. (1996) Model studies on the oxidative stability of odor‐active thiols occurring in food flavors. Journal of Agricultural and Food Chemistry, 44 (1), 251–255.
- 25. Makhotkina, O. and Kilmartin, P.A. (2012) Hydrolysis and formation of volatile esters in New Zealand Sauvignon Blanc wine. Food Chemistry, 135 (2), 486–493.
- 26. Rauhut, D. (2009) Usage and formation of sulphur compounds, in Biology of microorganisms on grapes, in must and in wine (eds König, H., Unden, G., Fröhlich, J.), Springer‐Verlag, Berlin and Heidelberg, pp. 181–207.
- 27. Ugliano, M. and Henschke, P.A. (2009) Yeasts and wine flavour, in Wine chemistry and biochemistry (eds Moreno‐Arribas, M.V. and Polo, M.C.), Springer, New York, pp. 313–392.
- 28. Limmer, A. (2005) Suggestions for dealing with post‐bottling sulfides. The Australian and New Zealand Grapegrower and Winemaker, 503, 67–76.
- 29. Vermeulen, C., Gijs, L., Collin, S. (2005) Sensorial contribution and formation pathways of thiols in foods: a review. Food Reviews International, 21 (1), 69–137.
- 30. Leppänen, O.A., Denslow, J., Ronkainen, P.P. (1980) Determination of thiolacetates and some other volatile sulfur compounds in alcoholic beverages. Journal of Agricultural and Food Chemistry, 28 (2), 359–362.
- 31. Nikolantonaki, M. and Waterhouse, A.L. (2012) A method to quantify quinone reaction rates with wine relevant nucleophiles: a key to the understanding of oxidative loss of varietal thiols. Journal of Agricultural and Food Chemistry, 60 (34), 8484–8491.
- 32. Ugliano, M., Henschke, P.A., Waters, E.J. (2012) Fermentation and post‐fermentation factors affecting odor‐active sulfur compounds during wine bottle storage, in Flavor chemistry of wine and other alcoholic beverages, American Chemical Society, Washington, DC, pp. 189–200.
- 33. Segurel, M.A., Razungles, A.J., Riou, C., et al. (2005) Ability of possible DMS precursors to release DMS during wine aging and in the conditions of heat‐alkaline treatment. Journal of Agricultural and Food Chemistry, 53 (7), 2637–2645.
- 34. Blanchard, L., Tominaga, T., Dubourdieu, D. (2001) Formation of furfurylthiol exhibiting a strong coffee aroma during oak barrel fermentation from furfural released by toasted staves. Journal of Agricultural and Food Chemistry, 49 (10), 4833–4835.
- 35. Pereira, V., Albuquerque, F.M., Ferreira, A.C., et al. (2011) Evolution of 5‐hydroxymethylfurfural (HMF) and furfural (F) in fortified wines submitted to overheating conditions. Food Research International, 44 (1), 71–76.
- 36. Tominaga, T., Guimbertau, G., Dubourdieu, D. (2003) Contribution of benzenemethanethiol to smoky aroma of certain Vitis vinifera L. wines. Journal of Agricultural and Food Chemistry, 51 (5), 1373–1376.
- 37. Mottram, D.S. (2007) The Maillard reaction: source of flavour in thermally processed foods, in Flavours and fragrances (ed. Berger, R.G.), Springer, Berlin, pp. 269–283.
- 38. Cerny, C. and Guntz‐Dubini, R. (2013) Formation of cysteine‐S‐conjugates in the Maillard reaction of cysteine and xylose. Food Chemistry, 141 (2), 1078–1086.