9
Aldehydes, Ketones, and Related Compounds
9.1 Introduction
Aldehydes and ketones originate as fermentation metabolites as well as oxidation products and in many situations the source is difficult to discern. However, once a wine is microbially stable, additional aldehydes or ketones arise from non‐enzymatic oxidation pathways, generally from oxidation of the analogous alcohol, but also from less direct routes such as reaction of amino acids with α‐dicarbonyls [1] (Figure 9.1). Grape‐derived aldehydes are also detectable in juice following crushing, and particularly six‐carbon aldehydes formed by enzymatic oxidation of grape lipids (Chapters 6 and 23.3). However, these compounds will be reduced to their corresponding alcohols during winemaking.
The critical phenomena associated with these compounds include:
- The carbonyl carbon of aldehydes, α‐diketones, and, to a lesser extent, ketones is electropositive, especially in acid, and these compounds are fairly reactive electrophiles (Chapter 10). Because wine is rich in nucleophiles (e.g., phenolics, sulfites), the most reactive carbonyl compounds will not accumulate in wine, but instead react with other compounds as a part of wine aging; significant accumulation of these carbonyls is a mark of a highly oxidized wine (Chapter 24).1
- Volatile aldehydes and ketones often have 100–10000‐fold lower sensory thresholds than their corresponding alcohols and thus small degrees of oxidation can affect wine aroma.
- Aldehydes and ketones can form reversible adducts with bisulfite (yielding sulfonates, Chapter 17), alcohols (yielding hemiacetals and acetals), or create adducts or bridges between two phenolic structures through addition to the A‐ring of flavonoids (Chapter 24).2
- The sensory perception of odorous carbonyl compounds can be attenuated by the reversible formation of sulfonates, so sensory studies should control for the concentration of sulfites.
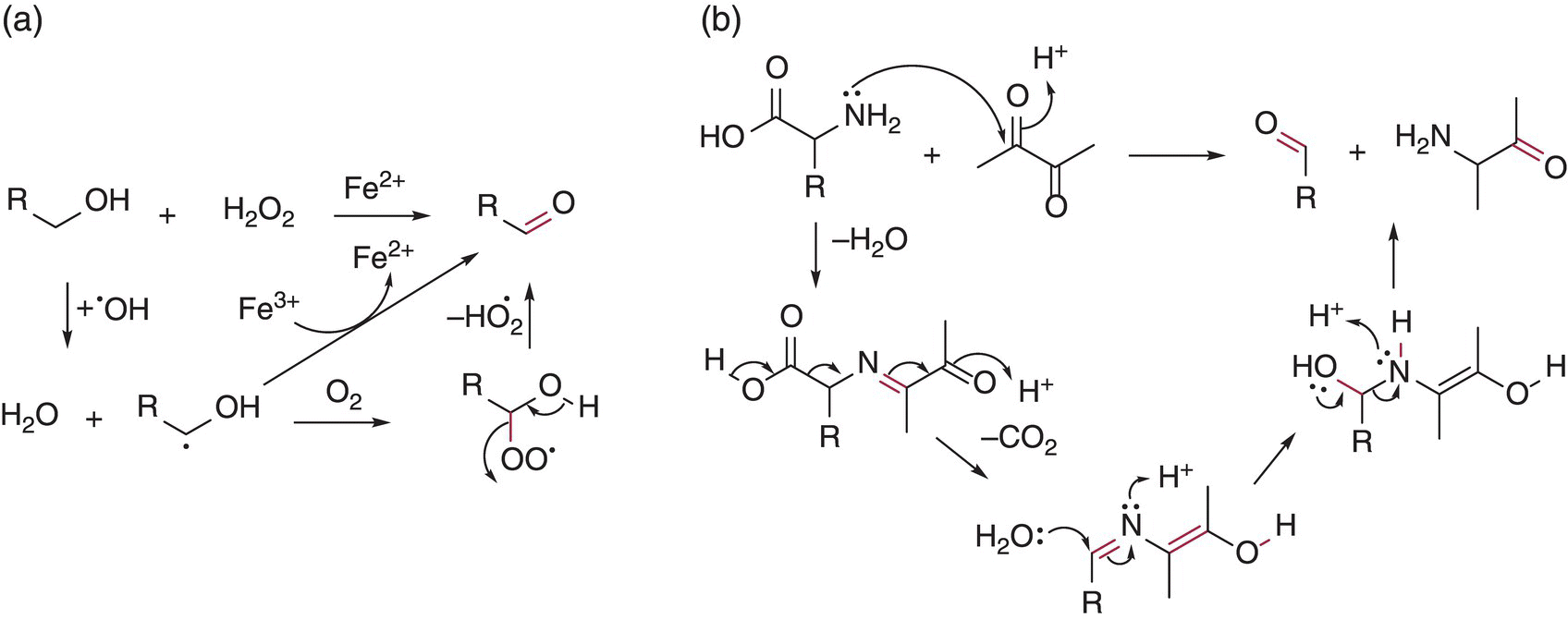
Figure 9.1 Examples of two pathways to form aldehydes and ketones via (a) Fenton oxidation of alcohols and (b) Strecker reaction of an amino acid with diacetyl, a common wine component (note that the enol tautomerization step to give the α‐aminoketone as the other product is not shown)
9.2 Acetaldehyde
Acetaldehyde, the best documented of the wine aldehydes and certainly the most abundant, is chemically related to ethanol, from which much is derived by oxidation. Acetaldehyde concentrations in standard table wine are in the 20–100 mg/L range, and often at levels described as the sensory threshold (40–100 mg/L). However, it is also a very strong SO2 binder (Kd = 1 × 10−6, Chapter 17) such that <1% of acetaldehyde typically exists in the free, volatile form in wines with free SO2 present. Consequently, acetaldehyde may be of negligible importance to table wine aroma but could contribute to wines with low SO2 such as Sherries [2]. The sensory descriptors of acetaldehyde vary with concentration; at low levels, it enhances fruitiness, but with higher concentrations, it is reminiscent of nuts, and at still higher levels smells like rotten apple (Table 9.1).
Table 9.1 Aldehydes and ketones in wines. Table derived from References [3] to [14]
| Name | Structure | Odor descriptor | Threshold (μg/L) | Range (μg/L) |
| Acetaldehyde (Ethanal) | CH3CHO | Fruity to rotting apple | 100000 | ND–211 000 |
| 2‐Methylpropanal (Isobutyraldehyde) | 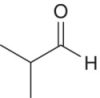
|
Banana, melon, varnish, cheese | 6 | 1–200 |
| 2‐Methylbutanal | 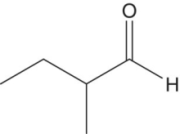
|
Green grass, fruity | 16 | 3–100 |
| 3‐Methylbutanal (Isovaleraldehyde) | 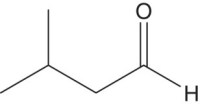
|
Unripe banana, apple, cheese, amylic | 4 | 40–250 |
| Octanal | CH3(CH2)6CHO | Citrus | 2.5 | 0.04–6 |
| Nonanal | CH3(CH2)7CHO | Citrus | 2.5 | 0.05–9 |
| Decanal | CH3(CH2)8CHO | Citrus | 1.25 | 0.07–1.6 |
| (E)‐2‐octenal | 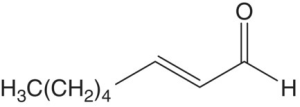
|
3 | 0.04–4 | |
| (E)‐2‐nonenal | 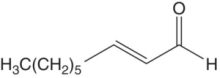
|
Greasy | 0.6 | 0.1–9 |
| Methional [9] | 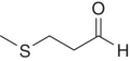
|
Cooked potato, cabbage | 0.5 | 0.5–80 |
| Phenylacetaldehyde | 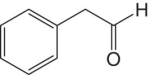
|
Floral | 1 | 2.5–130 |
| Diacetyl | 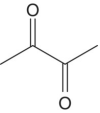
|
Butter | 100 | 5–7500 |
| Acetoin | 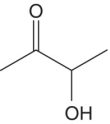
|
Butter, cream | 150 000 | 100–60 000 |
| Furfural | 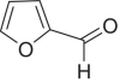
|
Caramel | 15,000 | 0–5000 |
| Sotolon | 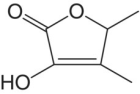
|
Maple syrup | 8 | 1–6 |
| 3‐Methyl‐2,4‐nonanedione | 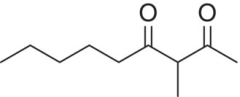
|
Prune, minty, anise | 0.016 | 0.004–0.3 |
| 1,1‐Diethoxyethane | CH3CH(OCH2CH3)2 | Licorice, green fruit | 1400 | 500–70 000 |
| 2‐Methyl‐4‐hydroxymethyl‐dioxolane and dioxane isomers | 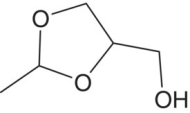
|
Sweet, port‐like | 100 000 (mix of 4 isomers) | 200–1400 |
| Non‐volatile | Average level | |||
| Pyruvic acid | 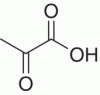
|
14 000 reds, 25 000 whites | ||
| Glyoxylic acid | 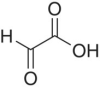
|
NRa | ||
| Glyoxal | 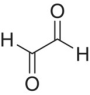
|
100–2000 | ||
| Methylglyoxal | 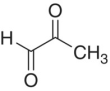
|
100–1000 |
a Not reported, presence inferred by reaction products.
Acetaldehyde is produced by yeast during alcoholic fermentation as the penultimate step before ethanol formation (Chapter 22.1). As a result, the total amount of acetaldehyde produced during fermentation is nearly equivalent to the molar amount of ethanol produced (~2 M or 90 g/L). However, since most acetaldehyde is immediately reduced to ethanol by yeast, the final wine concentration is reported to average 25 mg/L in reds and 40 mg/L in whites [3], with variations due to yeast strain and initial must SO2 concentration (Chapter 17). After fermentation, additional acetaldehyde will appear due to the oxidation of ethanol via the Fenton reaction (Figure 9.1).
9.3 Short and medium chain aldehydes
9.3.1 Higher alcohol‐derived aldehydes
Higher alcohols are readily oxidized to aldehydes, and due to their relatively high concentrations (i.e., hundreds of mg/L, Chapter 6), oxidation can yield suprathreshold concentrations of some odorous aldehydes. Short branched‐chain aldehydes (C4 and C5), including 2‐methylpropanal and 2‐ and 3‐methylbutanal, are associated with dried fruit, woody, and sweet and fusel type aromas (Table 9.1). These compounds are found in many wines and are particularly high in aged ports and Sherries, although they are found in any aged wine. The amounts of these aldehydes in young wines are between 5 to 10 μg/L, but in older oxidized wines, the levels are much higher, at 20–80 μg/L [4]. These aldehydes have been shown to be involved in reactions with flavonoids, analogous to acetaldehyde [15].
Methional (cooked potato aroma) and phenylacetaldehyde (floral aroma) are very potent aldehydes arising from the oxidation of corresponding alcohols, which are derived from amino acids methionine and phenylalanine, respectively (Chapter 6). These have the aroma of cooked vegetables, honey, and other fermented foods. Higher concentrations of these compounds are more often observed in older wines, and are strongly associated with quality degradation [4] and oxidized character [9].
9.3.2 Medium chain aldehydes (C8–C10)
These are found in many foods, and impart citrus fruit type aromas to wine (Table 9.1). These compounds are added to many foods, cosmetics, and cleaning agents, so they are ubiquitous in the modern environment.3 Their concentrations are usually correlated in a given wine and their sensory effect appears to be additive. However, due to their low levels they are thought to only rarely impact sensory attributes, and even in their absence wines can still present similar citrus fruit nuances [7].
9.3.3 (E)‐2‐Alkenals
These are α,β‐unsaturated aldehydes with an (E)‐ (trans‐) double bond at the 2 position. The most potent (E)‐2‐alkenals in wine have medium chain length (C6–C9), of which (E)‐2‐nonenal is the most potent. The C6 form ((E)‐2‐hexenal, “grassy”) is formed at high concentrations in must by enzymatic oxidation of linolenic acid (Chapter 23.3), but is largely metabolized during fermentation. The other (E)‐2‐alkenals have aromas of mushroom, mold, earth, and dust (Table 9.1). In finished wines, these compounds are likely formed through non‐enzymatic degradation of unsaturated fatty acids. The (E)‐2‐alkenals are generally correlated with negative sensory attributes and low price points, and most wines do not have suprathreshold levels unless they are oxidized or long‐aged [5].
9.3.4 1,2‐ or α‐dicarbonyl compounds
These compounds contain two adjacent carbonyl groups. Many of these species are produced by microbial metabolism. For example, diacetyl (“butter”) primarily arises from lactic acid bacterial activity (Chapter 22.5) and is associated with the buttery aromas of wine (Table 9.1). It is closely related to the less potent α‐hydroxycarbonyl compound, acetoin, via reduction of one of the carbonyl moieties, and 2,3‐butanediol via further reduction. The proportion of diacetyl versus acetoin is diagnostic for the status of lactic acid oxidation [16] and these compounds are thought to react with thiols to yield potent heterocyclic products with roasted and herbaceous aromas [17]. Diacetyl is typically present at 0.2–2.5 mg/L in commercial wine but can be 2–3 times higher during malolactic fermentations. As noted above in Figure 9.1, diacetyl and other 1,2‐dicarbonyls can react in a Strecker reaction with amino acids to produce other odor‐active aldehydes.
Glyoxal and methylglyoxal can be produced both by microbial metabolism and by abiotic oxidative processes such as the Maillard reaction [18]. These are present in wine at concentrations ranging from 0.1 to 2 mg/L. As noted for other aldehydes, levels are expected to decrease with time due to the reaction of glyoxal with flavonoids. Glyoxal is near odorless, but it appears that some of its secondary reaction products may form odorous heterocyclic reaction products, such as thiazoles [19]. In addition to the more abundant α‐dicarbonyl compounds such as diacetyl and glyoxal, there is also the homologous pentane‐2,3‐dione as well as phenylglyoxal. All of these compounds increase during lactic acid fermentations, diminish if wine is subsequently aged on lees due to enzymatic reduction (Chapter 22.5), but remain relatively static if the wine is racked [8].
The medium‐chain β‐diketone, 3‐methyl‐2,4‐nonanedione has recently been identified in red wines. It was found in a search for the prune character of oxidatively aged red wines, although its descriptors in model wine are concentration dependent and also include mint and anise [20]. It has an exceptionally low aroma detection threshold of 16 ng/L in model wine, and appears to be present in aged red Bordeaux wines well above threshold. This diketone may be a general contributor to aged wine character Its origin is unclear, although its formation in soybean oil is linked to unsaturated fatty acids.
9.4 Complex carbonyls
A significant number of carbonyls originate from oxidation of complex precursor molecules such as organic acids, which result in highly functionalized products. Many of these precursors are themselves produced by oxidation, but also exist as microbial metabolites from fermentation, so their source in a particular wine is difficult to discern. In general, these compounds have no aroma due to the multiple oxygenated groups, but their importance lies in their reactivity and ability to modify wine color, and possibly astringency, through reactions with anthocyanins and/or tannins (Chapters 14, 16, and 25).
Several α‐keto‐carboxylic acids are reported in wine (i.e. α‐keto acids, Chapter 22.3) and may contribute to SO2 binding due to their relatively high concentrations (Chapter 17). Pyruvic acid is produced by yeast metabolism (Chapter 22.1) as well as by Fenton oxidation of lactic or malic acid. Pyruvic acid is of particular interest for its ability to react with anthocyanins to yield stabilized pigments (Chapter 16). Published values for typical pyruvic acid levels cover a wide range, with concentrations after fermentation reportedly about 100 mg/L, and mean values of 14 mg/L in commercial reds and 25 mg/l in whites [21]. The higher concentrations in white wines likely reflect lower concentrations of phenolic species that would react with the pyruvic acid. Glyoxylic acid has rarely been directly measured in wine [22], but many products of its reaction with flavonoids have been observed [23] (Chapter 24). It is presumed to arise from the Fenton oxidation of tartaric acid, as reported by Fenton himself [24]. α‐Ketoglutaric acid is a key intermediate in nitrogen metabolism (Chapter 22.3), and typical wine concentrations are 22 mg/L in whites and 74 mg/L in reds [21].
Sotolon is a potent aroma compound that is reminiscent of maple syrup and is an important aroma component of oxidized wine styles, such as port, vin jaune or sherry, whereas in white wine it appears to diminish varietal character.4 Studies have shown that sotolon arises in dry wines from the reaction between 2‐keto‐butyric acid, formed by either yeast metabolism or ascorbic acid degradation, and acetaldehyde [25]. Levels in table wines are usually below the threshold of 8 μg/L in dry white wines, but much higher in oxidized styles [26]. Concentrations in sweet, thermally oxidized wines like Madeira can approach 2000 μg/L. The mechanism for sotolon formation in wine under high sugar and high temperature conditions is not established.
Furfural and related compounds are found in styles of wines that are heated, such as Madeira,5 or in oak aged wines, where the aldehydes are formed during oak toasting (Chapter 25). In cooked wines like Madeira, these compounds can be formed through acid‐catalyzed degradation of sugars (Chapter 25 for the mechanism). Such wines contain related aldehydes such as maltol and cyclotene, which can also arise from sugar degradation [27]. Extraction of furfural into Chardonnay wine from barrels was observed at 2–6 mg/L in the first cycle of use (6 months aging) and, in the same barrels, only 0.2–1.1 mg/L on the second use [28]. 5‐Methylfurfural was also observed at 0.4–1 mg/L in the first year of use and 0.1–0.4 mg/L in the second year of use (Chapter 25 for more on oak extractives). In Madeira, levels of furfural between 1 and 24 mg/L and between 1 and 74 mg/L of hydroxymethylfurfural have been reported [29].
Several other carbonyls have been historically implicated as important to wine aroma, but occur at concentrations well below threshold, for example, solerone in sherry. Benzaldehyde (“marzipan”, “cherry”) may exceed its threshold in wines contaminated by epoxy‐resin tanks, but is usually found far below its detection threshold of 2000 μg/L [30], although it may contribute to aromas that are characteristic of wines produced with carbonic maceration.
9.5 Carbonyl reactivity
Due to the electrophilic nature of aldehydes and ketones, one common reaction of these substances is the addition of water and/or alcohols to create hydrates (i.e., R2C(OH)2), hemiacetals (i.e., R2C(OH)OR', R' ≠ H) or acetals (i.e., R2C(OR')2, R' ≠ H, and R' groups may be different, affording a mixed acetal) (Chapter 2). Hydrates and hemiacetals rapidly equilibrate with their parent carbonyl compounds in the presence of aqueous acid, but acetals can have slow equilibration times with their respective hemiacetal. Common examples of mixed acetals in wine include the glycosidic forms of alcohols and phenols, for instance geraniol or quercetin glucosides (Chapters 8 and 15), but here the focus is on the reactions of the simpler, volatile compounds.
The simplest of the acetals is 1,1‐diethoxyethane, derived from the reaction of ethanol and acetaldehyde. (Figure 9.2) It has been reported at above threshold concentrations in some wines, and has an aroma of apple and licorice in model wine [31] (Table 9.1). Its initial concentration will be dependent on the amount of acetaldehyde produced, for example, by ethanol oxidation, and will be depleted as oxidation slows and the acetaldehyde is consumed by other reactions, for example, with flavanoids (Figure 9.3) or by microbial metabolism.
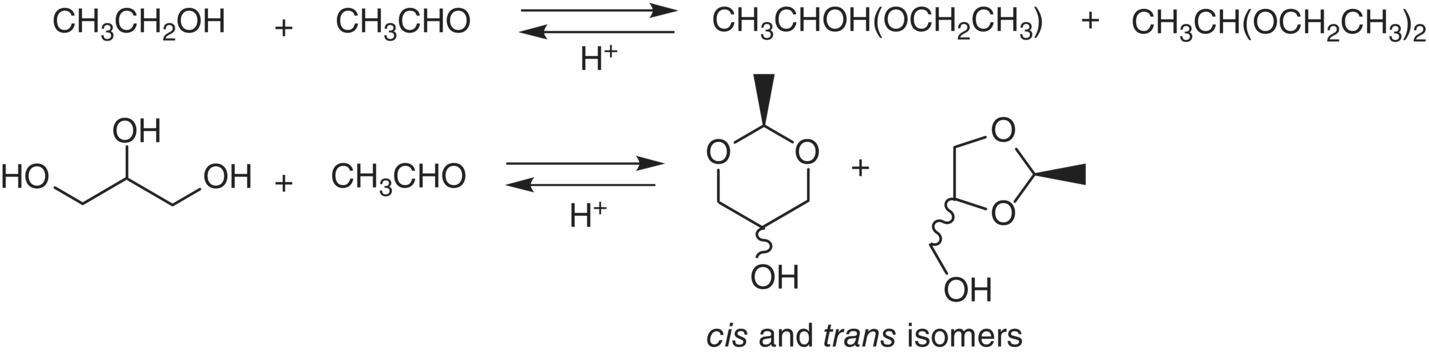
Figure 9.2 Acetal equilibria in the presence of acid in hydroalcoholic media
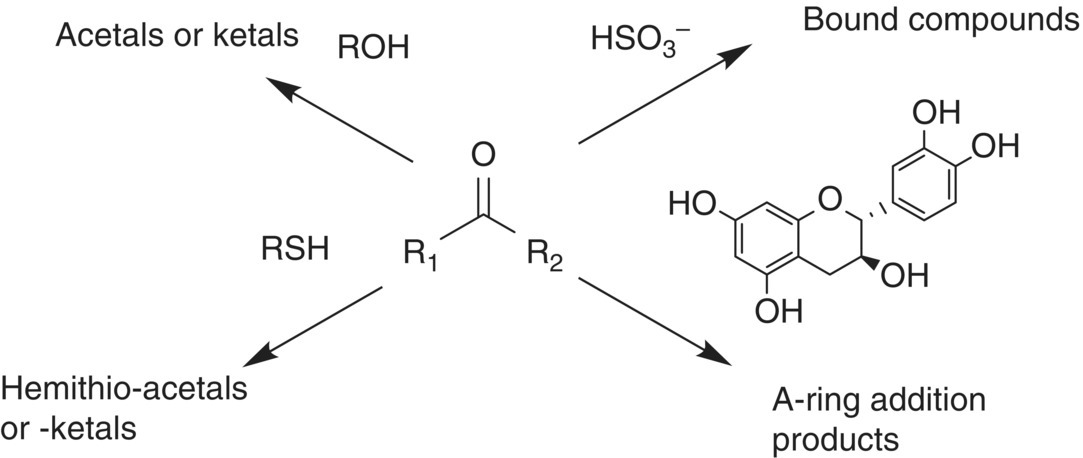
Figure 9.3 Possible reactions of carbonyls in wine
Acetaldehyde also reacts with other alcohols such as glycerol, an abundant polyol in wine (Chapters 2 and 22.1). With alcohols at three positions, glycerol can form two different cyclic acetals, a dioxolane and a dioxane (Figure 9.2) [32]. These cyclic structures have cis‐ and trans‐ (i.e., (Z)‐ and (E)‐) isomers, each with two stereoisomers, for a total of four compounds with aromas described as “sweet” and “old port” (Table 9.1). These products are relatively polar and have weak aroma impact unless the wine has been oxidized quite a bit, but they have been proposed as markers of wine oxidation or poor storage conditions [11]. Another related cyclic acetal is the one formed by acetaldehyde and 2,3‐butanediol, the reduced form of diacetyl (and acetoin), yielding 2,4,5‐trimethyl‐1,3‐dioxolane. This compound has been reported a few times in studies of oxidized wine [33], where it appears to contribute green and aldehydic characters. Similar aromas were attributed to acetals arising from acetaldehyde and other diols found in wine.
In addition to these acetals, aldehydes and ketones generally exist in wine partially as their hydrated forms. As there is a rapid equilibrium between the forms the hydrates are often not observed analytically, for example, by gas chromatography, as dehydration occurs during injection.
Carbonyl compounds also react reversibly with other wine nucleophiles, including bisulfite, the phloroglucinol ring of flavonoids, and thiols (Figure 9.3), since carbonyls represent a major class of SO2 binders, and these bound forms of SO2 have diminished antimcrobial and antioxidant activity (Chapter 17) [34]. Reactions with flavonoids are also well documented [35] (Chapters 14 and 16), while reactions with thiols are not as well studied [36] (Chapter 24). Any analysis of aldehydes or ketones should account for the possibility of these various addition products, releasing the carbonyls in the course of the analysis.
References
- 1. Pripis‐Nicolau, L., de Revel, G., Bertrand, A., Maujean, A. (2000) Formation of flavor components by the reaction of amino acid and carbonyl compounds in mild conditions. Journal of Agricultural and Food Chemistry, 48 (9), 3761–3766.
- 2. Escudero, A., Asensio, E., Cacho, J., Ferreira, V. (2002) Sensory and chemical changes of young white wines stored under oxygen. An assessment of the role played by aldehydes and some other important odorants. Food Chemistry, 77 (3), 325–331.
- 3. Jackowetz, J.N. and Mira de Orduña, R. (2013) Survey of SO2 binding carbonyls in 237 red and white table wines. Food Control, 32 (2), 687–692.
- 4. Cullere, L., Cacho, J., Ferreira, V. (2007) An assessment of the role played by some oxidation‐related aldehydes in wine aroma. Journal of Agricultural and Food Chemistry, 55 (3), 876–881.
- 5. San Juan, F., Cacho, J., Ferreira, V., Escudero, A. (2012) Aroma chemical composition of red wines from different price categories and its relationship to quality. Journal of Agricultural and Food Chemistry, 60 (20), 5045–5056.
- 6. Bartowsky, E.J., Francis, I.L., Bellon, J.R., Henschke, P.A. (2002) Is buttery aroma perception in wines predictable from the diacetyl concentration? Australian Journal of Grape and Wine Research, 8 (3), 180–185.
- 7. Cullere, L., Ferreira, V., Cacho, J. (2011) Analysis, occurrence and potential sensory significance of aliphatic aldehydes in white wines. Food Chemistry, 127 (3), 1397–1403.
- 8. de Revel, G., Pripis‐Nicolau, L., Barbe, J.C., Bertrand, A. (2000) The detection of alpha‐dicarbonyl compounds in wine by formation of quinoxaline derivatives. Journal of the Science of Food and Agriculture, 80 (1), 102–108.
- 9. San‐Juan, F., Ferreira, V., Cacho, J., Escudero, A. (2011) Quality and aromatic sensory descriptors (mainly fresh and dry fruit character) of Spanish red wines can be predicted from their aroma‐active chemical composition. Journal of Agricultural and Food Chemistry, 59 (14), 7916–7924.
- 10. Lavigne, V., Pons, A., Darriet, P., Dubourdieu, D. (2008) Changes in the sotolon content of dry white wines during barrel and bottle aging. Journal of Agricultural and Food Chemistry, 56 (8), 2688–2693.
- 11. Ferreira, A.C.D., Barbe, J.C., Bertrand, A. (2002) Heterocyclic acetals from glycerol and acetaldehyde in port wines: evolution with aging. Journal of Agricultural and Food Chemistry, 50 (9), 2560–2564.
- 12. Pons, A., Lavigne, V., Darriet, P., Dubourdieu, D. (2011) Determination of 3‐methyl‐2,4‐nonanedione in red wines using methanol chemical ionization ion trap mass spectrometry. Journal of Chromatography A, 1218 (39), 7023–7030.
- 13. Guth, H. (1997) Quantitation and sensory studies of character impact odorants of different white wine varieties. Journal of Agricultural and Food Chemistry, 45 (8), 3027–3032.
- 14. Cutzach, I., Chatonnet, P., Dubourdieu, D. (2000) Influence of storage conditions on the formation of some volatile compounds in white fortified wines (vins doux naturels) during the aging process. Journal of Agricultural and Food Chemistry, 48 (6), 2340–2345.
- 15. Pissarra, J., Lourenco, S., Gonzalez‐Paramas, A.M., et al. (2004) Structural characterization of new malvidin 3‐glucoside‐catechin aryl/alkyl‐linked pigments. Journal of Agricultural and Food Chemistry, 52 (17), 5519–5526.
- 16. Nielsen, J.C. and Richelieu, M. (1999) Control of flavor development in wine during and after malolactic fermentation by Oenococcus oeni. Applied and Environmental Microbiology, 65 (2), 740–745.
- 17. Marchand, S., de Revel, G., Bertrand, A. (2000) Approaches to wine aroma: release of aroma compounds from reactions between cysteine and carbonyl compounds in wine. Journal of Agricultural and Food Chemistry, 48 (10), 4890–4895.
- 18. Wang, Y. and Ho, C.T. (2012) Flavour chemistry of methylglyoxal and glyoxal. Chemical Society Reviews, 41 (11), 4140–4149.
- 19. de Revel, G., Marchand, S., Bertrand, A. (2004) Identification of Maillard‐type aroma compounds in winelike model systems of cysteine–carbonyls: cccurrence in wine, in Nutraceutical beverages: chemistry, nutrition, and health effects (eds Shahidi, F. and Weerasinghe, D.K.), American Chemical Society, Washington, pp. 353–364.
- 20. Pons, A., Lavigne, V., Eric, F., et al. (2008) Identification of volatile compounds responsible for prune aroma in prematurely aged red wines. Journal of Agricultural and Food Chemistry, 56 (13), 5285–5290.
- 21. Jackowetz, J.N. and de Orduna, R.M. (2013) Improved sample preparation and rapid UHPLC analysis of SO2 binding carbonyls in wine by derivatisation to 2,4‐dinitrophenylhydrazine. Food Chemistry, 139 (1–4), 100–104.
- 22. Blouin, J. (1966) Contribution to study of binding of sulphur dioxide in musts and wines. I. Annales de Technologie Agricole, 15 (3), 223–287.
- 23. Es‐Safi, N.E., Le Guerneve, C., Cheynier, V., Moutounet, M. (2000) New phenolic compounds formed by evolution of (+)‐catechin and glyoxylic acid in hydroalcoholic solution and their implication in color changes of grape‐derived foods. Journal of Agricultural and Food Chemistry, 48 (9), 4233–4240.
- 24. Fenton, H. (1894) Oxidation of tartaric acid in the presence of iron. Journal of the Chemical Society, 75, 1–11.
- 25. Pons, A., Lavigne, V., Landais, Y., et al. (2010) Identification of a Sotolon Pathway in dry white wines. Journal of Agricultural and Food Chemistry, 58 (12), 7273–7279.
- 26. Moyano, L., Zea, L., Moreno, J.A., Medina, M. (2010) Evaluation of the active odorants in Amontillado sherry wines during the aging process. Journal of Agricultural and Food Chemistry, 58 (11), 6900–6904.
- 27. Belitz, H.D., Grosch, W., Schieberle, P. (2009) Food Chemistry, Springer‐Verlag, Berlin.
- 28. Towey, J.P. and Waterhouse, A.L. (1996) The extraction of volatile compounds from French and American oak barrels in Chardonnay during three successive vintages. American Journal of Enology and Viticulture, 47, 163–172.
- 29. Camara, J.S., Alves, M.A., Marques, J.C. (2006) Changes in volatile composition of Madeira wines during their oxidative ageing. Analytica Chimica Acta, 563 (1–2), 188–197.
- 30. Peinado, R.A., Moreno, J., Bueno, J.E., et al. (2004) Comparative study of aromatic compounds in two young white wines subjected to pre‐fermentative cryomaceration. Food Chemistry, 84 (4), 585–590.
- 31. Moyano, L., Zea, L., Moreno, J., Medina, M. (2002) Analytical study of aromatic series in sherry wines subjected to biological aging. Journal of Agricultural and Food Chemistry, 50 (25), 7356–7361.
- 32. Peterson, A.L., Gambuti, A., Waterhouse, A.L. (2015) Rapid analysis of heterocyclic acetals in wine by stable isotope dilution gas chromatography–mass spectrometry. Tetrahedron, 71 (20), 3032–3038.
- 33. Escudero, A., Cacho, J., Ferreira, V. (2000) Isolation and identification of odorants generated in wine during its oxidation: a gas chromatography–olfactometric study. European Food Research and Technology, 211 (2), 105–110.
- 34. Han, G.M., Wang, H., Webb, M.R., Waterhouse, A.L. (2015) A rapid, one step preparation for measuring selected free plus SO2‐bound wine carbonyls by HPLC‐DAD/MS. Talanta, 134, 596–602.
- 35. Drinkine, J., Lopes, P., Kennedy, J.A., et al. (2007) Analysis of ethylidene‐bridged flavan‐3‐ols in wine. Journal of Agricultural and Food Chemistry, 55 (4), 1109–1116.
- 36. Sonni, F., Clark, A.C., Prenzler, P.D., et al. (2011) Antioxidant action of glutathione and the ascorbic acid/glutathione pair in a model white wine. Journal of Agricultural and Food Chemistry, 59 (8), 3940–3949.