22.5
Bacterial Fermentation Products
22.5.1 Introduction
While yeast is necessary for alcoholic fermentation during wine production, several types of bacteria may also affect wine composition. Although the number of bacterial species on Earth may number in the millions, only a small percentage can grow under conditions of high osmotic stress (juice), high alcohol (wine), and/or low pH (both grape juice and wine). Those with the best established role in wine production are lactic acid bacteria (LAB) and two members of the acetic acid bacteria (AAB) family: Acetobacter and Gluconobacter. The changes imparted by these microflora can be desirable (and encouraged) in some circumstances, as is frequently the case for Oenococcus oeni (an LAB) in conducting malolactic fermentation (MLF). However, modifications induced by AAB as well as some LAB are often associated with spoilage [1–3]. Because some metabolic transformations by these bacteria are analogous to those already described for yeast, for example, LAB are capable of hydrolyzing glycosides and reducing aldehydes to alcohols, the focus of this chapter will be on transformations that are associated most strongly with bacteria and have not been addressed elsewhere, as follows:
- Wine deacidification due to MLF (Chapter 3)
- Production of “lactic” volatile compounds such as diacetyl and acetoin (Chapter 9)
- Production of spoilage compounds such as mannitol and β‐glucans (Chapter 2), acetic acid (Chapter 3), biogenic amines and imines (Chapter 5), and 2‐ethoxyhexa‐3,5‐diene (Chapter 18).
22.5.2 Lactic acid bacteria
22.5.2.1 Malolactic fermentation
As the name implies, MLF involves the conversion of the diprotic grape‐derived l‐malic acid into the monoprotic l‐lactic acid due to LAB metabolism, although strictly speaking this is an enzymatic decarboxylation rather than fermentation.1 Oenococcus oeni (formerly Leuconostoc oenos) is the LAB species most adapted to wine conditions (low pH, high ethanol) and is the dominant LAB present at the end of alcoholic fermentation [4,5]. Strains of O. oeni are therefore preferred for conducting MLF, which results in a decrease in wine titratable acidity (TA), with deacidification of approximately 1–3 g/L as tartaric acid equivalents2 and an increase in pH of around 0.1–0.3 units3 (Table 22.5.1), as well as a decrease in sourness (Chapter 3). In spite of the pH increase, the removal of malic acid substrate and other nutrients can improve the microbial stability of the resulting wine. MLF is desired for some white wines (often to affect flavor) and most red wines (for flavor and/or stability). Usually, this is achieved through inoculation with a selected commercial strain of O. oeni after alcoholic fermentation is complete, although spontaneous or inoculated MLF coincident with alcoholic fermentation can also occur.
Table 22.5.1 Mean values for pH and selected MLF‐related compounds in wines from different grape varieties inoculated with two strains of Oenococcus oeni. Data from Reference [6]
| Wine composition | Grape variety | ||||||||
| Syrah | Cabernet Sauvignon | Merlot | |||||||
| Pre‐MLF | Strain 1a | Strain 2b | Pre‐MLF | Strain 1a | Strain 2b | Pre‐MLF | Strain 1a | Strain 2b | |
| pH | 3.71 | 4.01 | 4.06 | 3.21 | 3.27 | 3.24 | 3.13 | 3.17 | 3.17 |
| Non‐volatiles (g/L) | |||||||||
| Titratable acidityc | 6.74 | 3.90 | 3.86 | 6.17 | 5.21 | 5.56 | 5.66 | 5.18 | 5.19 |
| Malic acid | 3.78 | 0.03 | 0.10 | 1.41 | 0.09 | 0.11 | 0.93 | 0.08 | 0.11 |
| Lactic acid | 0.07 | 2.04 | 2.19 | 0.04 | 1.00 | 0.88 | 0.02 | 0.62 | 0.56 |
| Citric acid | 0.38 | 0.02 | 0.10 | 0.32 | 0.04 | 0.19 | 0.18 | 0.04 | 0.12 |
| Volatiles (mg/L) | |||||||||
| Diaectyl | 6.01 | 10.88 | 4.59 | 5.06 | 13.21 | 6.95 | 3.70 | 6.92 | 3.66 |
| Acetoin | 1.29 | 3.26 | 0.45 | 1.34 | 15.39 | 4.83 | 1.44 | 5.29 | 1.30 |
| Volatile acidityd | 0.21 | 0.37 | 0.38 | 0.26 | 0.36 | 0.31 | 0.30 | 0.35 | 0.36 |
| Acetaldehyde | 22.06 | 6.46 | 4.20 | 25.69 | 8.27 | 7.87 | 20.28 | 11.14 | 4.87 |
| Ethyl lactate | 3.27 | 48.14 | 52.34 | 7.13 | 48.00 | 40.94 | 5.76 | 28.06 | 28.57 |
a Commercial strain O. oeni PN4.
b Indigenous strain O. oeni C22L9.
c Expressed as tartaric acid.
d Expressed as acetic acid (g/L).
L‐Malic acid is transported into the cell as monovalent malate HM‐ [7] where the decarboxylation reaction (Figure 22.5.1, pathway A) is catalyzed by the malolactic enzyme (L‐malate:NAD+ carboxylase) in the presence of NAD+ and Mn2+ as cofactors [4, 8]. The resulting L‐lactic acid and CO2 are excreted from the cell by diffusion. Unlike the reactions involved in glycolysis (Chapter 22.1), the enzymatic decarboxylation of malic to lactic acid does not directly yield ATP and the process is energetically unfavorable. However, this reaction consumes a proton, increasing intracellular pH and resulting in a proton gradient (i.e., proton‐motive force, PMF) across the cell membrane. The PMF facilitates malate transport and, in combination with cell membrane ATPases, can be used to generate energy in the form of ATP [5].

Figure 22.5.1 Formation of L‐lactic acid from L‐malic acid metabolism (pathway A) and MLF‐associated volatile compounds, diacetyl and acetoin, from citric acid metabolism (pathway B) by Oenococcus oeni. Other metabolites arising from citrate, via pyruvate, are also shown. Numbers for the steps refer to the following: 1, carboxylate transporter; 2, malolactic enzyme; 3, citrate lyase; 4, oxaloacetate decarboxylase; 5, pyruvate decarboxylase; 6, α‐acetolactate synthase, 7, oxidative decarboxylation (nonenzymatic), 8, α‐acetolactate decarboxylase, 9, diacetyl reductase, 10, acetoin reductase
22.5.2.2 Metabolism of citric acid by LAB
Another important outcome of MLF is the metabolism of citric acid to form diacetyl, acetoin, and 2,3‐butanediol (Figure 22.5.1, pathway B) [4, 5, 9, 10], of which the “buttery” smelling diacetyl has the lowest sensory threshold and the greatest impact on wine flavor (Chapter 9) [2]. As with malate metabolism, citrate is transported into the cell as a singly charged anion and cleaved to yield acetate and oxaloactetate. Although alternate pathways exist, the majority of oxaloacetate will eventually be transformed into α‐acetolactate via reactions that produce pyruvate and acetaldehyde‐thiamine pyrophosphate (TPP). Under semi‐aerobic conditions, α‐acetolactate can spontaneously react with O2 to yield diacetyl (Figures 22.5.1 and 22.5.2). However, under anaerobic conditions typical to wine production, α‐acetolactate can be sequentially converted to less odorous acetoin and then reduced to 2,3‐butanediol. This process helps to maintain redox balance by regenerating NAD+ from NADH, and is analogous to the role of acetaldehyde reduction to ethanol during alcoholic fermentation (Chapter 22.1). After reaching a maximum, diacetyl can be enzymatically reduced by LAB to acetoin, and subsequently 2,3‐butanediol. Factors that affect diacetyl production4 and stability in wine are reviewed elsewhere [11], but major effects include the following [4, 12]:
- Citrate is almost fully consumed after malate degradation is complete5 and higher initial concentrations of citric acid in wine will result in greater diacetyl (and acetate) production.
- Semi‐aerobic conditions will favor the accumulation of diacetyl through its non‐enzymatic formation.
- Differences among bacterial strains can alter metabolite profiles; for example, higher diacetyl reductase activity in a strain can result in greater conversion of diacetyl to acetoin. Additionally, storage in the presence of LAB or yeast lees will result in lower diacetyl concentrations due to the presence of reductases.
- Conditions that favor faster MLF (due to higher temperature and pH) tend to produce greater amounts of acetic acid at the expense of diacetyl/acetoin.
- Diacetyl can be partially and reversibly bound by SO2 (Chapters 9 and 17), decreasing its volatility and masking the intensity of its buttery aroma.
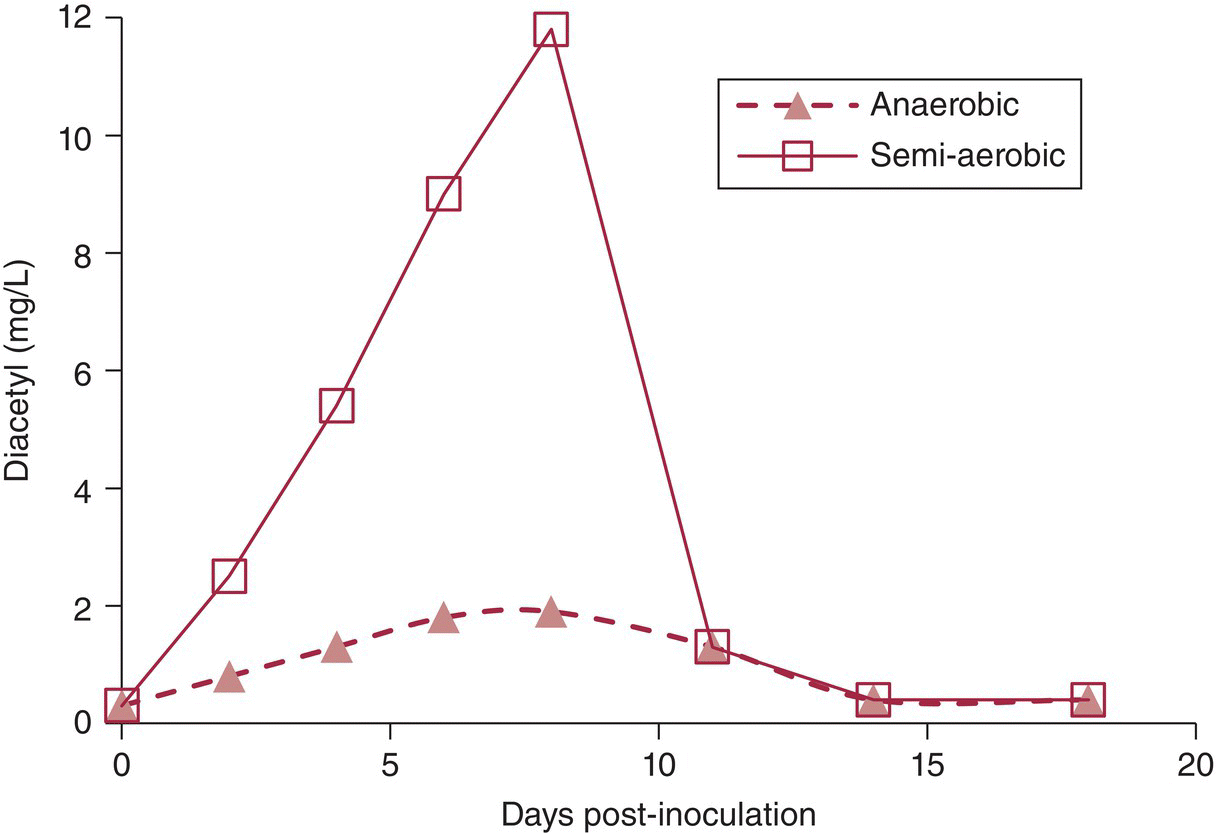
Figure 22.5.2 Diacetyl concentrations (mg/L) during MLF as a function of time after inoculation, showing that semi‐aerobic conditions promote greater formation of diacetyl than anaerobic conditions. In both situations, diacetyl will eventually be reduced to acetoin and 2,3‐butanediol through LAB metabolism.
Data from Reference [12]
Metabolism of citric acid will also yield lactate, ethanol, ethyl lactate (from esterification of lactate), and at least one molar equivalent of acetic acid; hence volatile acidity (VA) typically increases during MLF (Table 22.5.1).
22.5.2.3 Fermentation of sugars by LAB
L‐Malic acid and citric acid cannot be used as sole carbon sources for growth of LAB [13], and biomass production requires fermentable sugars or amino acids [5]. Co‐metabolism of glucose and citric acid is energetically favorable for O. oeni and leads to an increased growth rate and enhanced biomass production [10]. LAB can be distinguished by their ability to ferment sugars and are classified as homofermentative or heterofermentative [1, 14]:
- Homofermentative LAB produce lactic acid6 as the primary metabolite by reduction of pyruvate formed through glycolysis (EMP pathway, Chapter 22.1). In wine, homofermentative LAB are typically associated with lactic spoilage, and include Pediococcus spp. and some Lactobacillus spp.
- Heterofermentative LAB such as O. oeni and a number of Lactobacillus spp. metabolize hexoses and pentoses (and other carbohydrates) using the pentose‐phosphate (phosphoketolase) pathway (Figure 22.5.3) to produce not only D‐lactic acid (in addition to L‐ and DL‐lactic acid in the case of some Lactobacillus spp.), but also acetic acid, ethanol, CO2, and other products such as glycerol and erythritol [15, 16].
- Heterofermentative Lactobacilli can be subdivided into strict (e.g., L. brevis and L. hilgardii) and facultative heterofermenters (e.g., L. casei and L. plantarum) based on their metabolism of hexoses; facultative heterofermenters produce only lactic acid by the EMP pathway and the strict heterofermenters produce the array of products mentioned above. In all cases, heterofermentative LAB ferment pentoses to lactic acid and acetic acid (or ethanol under reductive conditions) as the main products by the phosphoketolase pathway. Since pyruvate is an intermediate in the pathway, volatile compounds such as diacetyl and acetoin can also be formed (see Figure 22.5.1).

Figure 22.5.3 Heterofermentative pathways for LAB based on hexose and pentose sugars (shown in the β‐pyranose conformation), yielding a range of metabolites. Numbers for the steps refer to the following: 1, sugar transporter; 2, hexokinase; 3, glucose‐6‐phosphate dehydrogenase; 4, 6‐phosphogluconate dehydrogenase; 5, ribokinase; 6, ribose 5‐phosphate ketol‐isomerase; 7, ribulose‐5‐phosphate 3‐epimerase; 8, phosphoketolase
22.5.2.4 Effects of LAB on wine composition and stability
Similar to yeast, LAB have the ability to reduce aldehydes and other carbonyls to their corresponding alcohols (Chapter 22.1). As a result, concentrations of several key SO2 binders, particularly acetaldehyde, will decrease during MLF (see Table 22.5.1). The concentrations of other volatile compounds can also be altered during MLF due to the production of glycosidases (Chapter 23.1) and esterases (Chapter 22.2). The presence of residual sugars in a wine (whether intentional or not) can encourage the growth of spoilage organisms and the formation of unwanted metabolites (see Section 22.5.3 below). Wine pH less than 3.5 favors the presence of O. oeni (with its often desirable organoleptic consequences) and helps to eliminate the potential for spoilage by other bacteria [17]; however, if the pH is higher than 3.5, Pediococcus spp. can flourish and undertake MLF. Even after the addition of SO2, some LAB (particularly Lactobacillus spp. and Pediococcus spp.) can remain viable post‐MLF, producing undesirable organoleptic changes over time. In general, LAB are much more susceptible to SO2 than yeasts, and sulfiting of wine is usually avoided if MLF is to be encouraged.
The changes in major indigenous LAB species have been mapped from the grapes/must through to the end of MLF, revealing that most Lactobacillus spp., O. oeni, and Pedioccocus spp. cells present in the must do not survive alcoholic fermentation, which is attributed to increases in ethanol, SO2, and other yeast metabolites. Assuming no LAB inoculation occurs, the few surviving cells of O. oeni develop and conduct MLF [1, 4, 17]. Ultimately, apart from the use of SO2 and control of wine pH, sterile filtration (Chapter 26.3) is necessary to remove these bacteria and render a wine microbiologically stable.
22.5.3 Spoilage of wine by bacteria
Bacterial spoilage is defined as the unintended growth of bacteria and the subsequent production (or accumulation to objectionable levels) of compounds that negatively impact on wine quality due to undesirable organoleptic and/or health effects [1–4, 18]. Several compounds formed during LAB spoilage have been described:
- An increase of acetic acid and diacetyl, as described above.
- Formation of mannitol from d‐fructose (Figure 22.5.3), which causes a viscous texture, sweet taste, and irritating finish (Chapter 2).
- Metabolism of glycerol (amertune) to acrolein (i.e., 2‐propenal, Figure 22.5.4). In addition to being toxic, acrolein can induce bitterness following reaction with phenolics, a more likely issue in red wine.
- Ropiness, where the production of exopolysaccharides (β‐d‐glucan from fermentation of residual glucose) by Pediococcus spp. leads to an abnormally viscous wine texture.
- Heterofermentative LAB can produce a mousy off‐flavor due to heterocyclic imines such as 2‐acetyl‐3,4,5,6‐tetrahydropyridine (Chapter 5) from ornithine or lysine metabolism, in conjunction with acetyl‐CoA production from acetyl‐P during fermentation of sugars (Figure 22.5.3).
- Biogenic amines such as histamine and putrescine can arise enzymatically from decarboxylation of the corresponding amino acids due primarily to the presence of Pediococcus spp. and Lactobacillus spp. (Chapter 5).
- Metabolism of arginine produces citrulline and carbamoyl‐P through the arginine deaminase pathway, and these intermediates can react with ethanol to yield carcinogenic ethyl carbamate (Figure 22.5.4), although production of urea by yeast is the main source of this compound (Chapter 5).

Figure 22.5.4 Formation of (a) acrolein under acidic wine conditions from glycerol produced by heterofermentative LAB, where Enz. refers to glycerol hydro‐lyase and Δ (i.e., heat) indicates an impact from temperature, and (b) ethyl carbamate from citrulline or carbamyl‐P by ethanolysis
LAB also possess cinnamoyl decarboxylase activity, which can provide a source of 4‐vinylphenol/4‐vinylguaiacol from hydroxycinnamic acids present in wine, thereby increasing the pool of compounds that can be metabolized by Brettanomyces (Chapters 12 and 23.3). Furthermore, the presence of reductases means care should be taken when employing sorbic acid (or potassium sorbate) as a preservative to prevent refermentation in sweet wines. Enzymatic reduction of the acid functional group to a primary alcohol by LAB provides an activated dienol, which can undergo rearrangement to produce 2‐ethoxyhexa‐3,5‐diene, a compound responsible for geranium taint (Chapter 18).
AAB in the form of Acetobacter spp. and Gluconobacter spp. only serve as spoilage bacteria in wine, in contrast to the potentially desirable role that LAB can play. The presence of AAB contributes to production of acetaldehyde, acetic acid, and ethyl acetate due to oxidation of ethanol, leading to increases in VA and vinegary characteristics in wine (Chapter 3). The risks from Acetobacter spp. tend to arise post‐fermentation, during extended maturation in barrels, storage in tank, or post‐bottling in conjunction with exposure to air. Rotting grapes present higher populations of AAB and a greater potential for spoilage before fermentation starts. Gluconobacter is typically found on grapes and in musts but does not withstand alcoholic fermentation; thus, its primary role in wine spoilage is from damaged grapes, where it generates high concentrations of both gluconic acid (Chapter 2) and acetic acid.
22.5.3.1 Protecting against spoilage
Disease in the vineyard and operations in the winery will affect the presence, viability, and growth of microorganisms. Aside from controlling these aspects, a number of other compositional and winemaking parameters influence bacterial activity [2, 18]. Some have been mentioned in the last paragraph of Section 22.5.2 above with respect to LAB, namely pH control (by addition of tartaric acid or other permitted acids), maintaining effective levels of SO2 (at each stage of the winemaking process), and sterile filtration (especially prior to bottling). Other precautionary measures include the following:
- Minimizing residual sugar and nitrogen requires healthy fermentations, fermenting to dryness to avoid the risk of refermentation, and avoiding the overuse of diammonium phosphate.
- Pasteurization, high pressure, or ultrasonic treatments are used to reduce the load of viable bacteria.
- Appropriate temperature control, such as cooler temperatures, for example, 15 °C, reduces the rate of bacterial growth.
- Inoculating with a commercial LAB strain with known characteristics; spontaneous MLF by indigenous LAB can produce the range of undesirable results mentioned above.
- The use of preservatives other than SO2, for example, dimethyldicarbonate, depending on local regulations (Chapter 27).
- Limiting exposure to oxygen by eliminating ullage in tanks and using inert gas coverage for wine transfers (Chapter 19).
- Using clean wine for topping up vessels.
- Routine quality control, such as testing of filter integrity and microbial populations.
References
- 1. Costantini, A., García‐Moruno, E., Moreno‐Arribas, M.V. (2009) Biochemical transformations produced by malolactic fermentation, in Wine Chemistry and Biochemistry (eds Moreno‐Arribas, M.V. and Polo, M.C.), Springer, New York, pp. 27–57.
- 2. Bartowsky, E.J. (2009) Bacterial spoilage of wine and approaches to minimize it. Letters in Applied Microbiology, 48 (2), 149–156.
- 3. Bartowsky, E.J. and Pretorius, I.S. (2009) Microbial formation and modification of flavor and off‐flavor compounds in wine, in Biology of microorganisms on grapes, in must and in wine, (eds König, H., Unden, G., Fröhlich, J.), Springer‐Verlag, Berlin and Heidelberg, pp. 209–231.
- 4. Lonvaud‐Funel, A. (1999) Lactic acid bacteria in the quality improvement and depreciation of wine. Antonie van Leeuwenhoek, 76 (1–4), 317–331.
- 5. Versari, A., Parpinello, G.P., Cattaneo, M. (1999) Leuconostoc oenos and malolactic fermentation in wine: a review. Journal of Industrial Microbiology and Biotechnology, 23 (6), 447–455.
- 6. Ruiz, P., Izquierdo, P.M., Seseña, S., et al. (2012) Malolactic fermentation and secondary metabolite production by Oenoccocus oeni strains in low pH wines. Journal of Food Science, 77 (10), M579–M585.
- 7. Salema, M., Poolman, B., Lolkema, J.S., et al. (1994) Uniport of monoanionic L‐malate in membrane vesicles from Leuconostoc oenos. European Journal of Biochemistry, 225 (1), 289–295.
- 8. Radler, F. (1986) Microbial biochemistry. Experientia, 42 (8), 884–893.
- 9. Swiegers, J.H., Bartowsky, E.J., Henschke, P.A., Pretorius, I.S. (2005) Yeast and bacterial modulation of wine aroma and flavour. Australian Journal of Grape and Wine Research, 11 (2), 139–173.
- 10. Ramos, A. and Santos, H. (1996) Citrate and sugar cofermentation in Leuconostoc oenos, a 13C nuclear magnetic resonance study. Applied and Environmental Microbiology, 62 (7), 2577–2585.
- 11. Bartowsky, E.J. and Henschke, P.A. (2004) The “buttery” attribute of wine – diacetyl – desirability, spoilage and beyond. International Journal of Food Microbiology, 96 (3), 235–252.
- 12. Nielsen, J.C. and Richelieu, M. (1999) Control of flavor development in wine during and after malolactic fermentation by Oenococcus oeni. Applied and Environmental Microbiology, 65 (2), 740–745.
- 13. Liu, S.Q., Davis, C.R., Brooks, J.D. (1995) Growth and metabolism of selected lactic acid bacteria in synthetic wine. American Journal of Enology and Viticulture, 46 (2), 166–174.
- 14. Zúñiga, M., Pardo, I., Ferrer, S. (1993) An improved medium for distinguishing between homofermentative and heterofermentative lactic acid bacteria. International Journal of Food Microbiology, 18 (1), 37–42.
- 15. Unden, G. and Zaunmüller, T. (2009) Metabolism of sugars and organic acids by lactic acid bacteria from wine and must, in Biology of microorganisms on grapes, in must and in wine (eds König, H., Unden, G., Fröhlich, J.), Springer‐Verlag, Berlin and Heidelberg, pp. 135–147.
- 16. Kandler, O. (1983) Carbohydrate metabolism in lactic acid bacteria. Antonie van Leeuwenhoek, 49 (3), 209–224.
- 17. Wibowo, D., Eschenbruch, R., Davis, C.R., et al. (1985) Occurrence and growth of lactic acid bacteria in wine: a review. American Journal of Enology and Viticulture, 36 (4), 302–313.
- 18. du Toit, M. and Pretorius, I.S. (2000) Microbial spoilage and preservation of wine: using weapons from nature’s own arsenal – a review. South African Journal for Enology and Viticulture, 21 (Special Issue), 74–96.