12
Volatile Phenols
12.1 Introduction
Volatile phenols contribute characteristic odors to various foods and beverages, including wine. They are of particular importance to the flavor and appeal of smoked foods [1] and historically the application of wood smoke was a useful way of preventing food from spoiling.1 In wine and alcoholic beverages, barrels or tanks made from wood (particularly oak) are frequently used for maturation and storage, and volatile phenols are among the most important organoleptic compounds extracted from toasted oak wood [2] (Chapter 25). Volatile phenols in wines may also arise from precursors originating in the grapes that are transformed by microbiological or chemical processes. A number of highly potent, halogenated volatile phenols, which are detrimental to wine aroma are derived from additional sources and are presented elsewhere (Chapter 18).
12.2 Structure and chemical properties
Volatile phenols are low molecular weight, aromatic2 alcohols with some similarities in reactivity to other phenols (Chapter 11). The term encompasses phenol and its derivatives containing alkyl, methoxyl, vinyl, allyl, aldehyde (Table 12.1), and halide substituents (Chapter 18). Volatile phenols have hydrogen bonding capability due to the hydroxyl group, but in general are of intermediate hydrophobicity, with influences from various ring substituents (e.g., guaiacol has log P = 1.3 and water solubility = 15.3 g/L whereas 4‐vinylguaiacol has log P = 2.2 and water solubility = 3.0 g/L). Compared to other phenolic compounds in wine (e.g., polyphenols and hydroxycinnamic acids (HCAs)), volatile phenols are much lower in concentration and simple o‐diphenols (i.e., catechols) are rare, meaning this class of compounds is less likely to play a role in oxidation phenomenon (via quinone formation, Chapter 24). Apart from electrophilic aromatic substitution reactions (Chapter 11), volatile phenols are generally stable molecules, although reactive functional groups can undergo the usual enzymatic transformations (i.e., reduction of aldehyde or alkene), and they can be bound as phenolic O‐glycosides (which may undergo hydrolysis, Chapter ). Based on the extent of hydrophobicity of the different volatiles phenols, they may be adsorbed onto hydrophobic surfaces such as wood [3], yeast cell walls [4] or lees [5], or solid‐phase polymers used for amelioration [6, 7].
Table 12.1 Indicative odor descriptors, detection thresholds, and odor activity values of selected volatile phenols encountered in wine [8, 11–18]
| Compounda | Structure | Odor descriptorb | Threshold (μg/L)b | OAV (max.) | Major sourcec |
| Phenol | 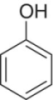
|
Chemical, ink | 30 | 4 | L |
| Guaiacol (2‐methoxyphenol) |
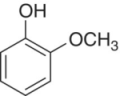
|
Smoke, sweet | 23 | 3 | L |
| 4‐Methylguaiacol (2‐Methoxy‐4‐methylphenol) |

|
Smoke, ash | 21 | 3 | L |
| Syringol (2,6‐dimethoxyphenol) |

|
Smoke, medicinal | 57 | 8 | L |
| Eugenol (4‐Allyl‐2‐methoxyphenol) |

|
Spice, clove | 6 | 15 | L |
| Vanillin (4‐hydroxy‐3‐methoxybenzaldehyde) |

|
Vanilla | 200 | 5 | L |
| m‐Cresol (3‐methylphenol) |
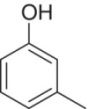
|
Leather | 20 | 8 | L |
| 4‐Ethylphenol (4‐EP) |
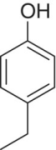
|
Leather, horse stable | 440 | 6 | B |
| 4‐Vinylphenol (4‐VP) |
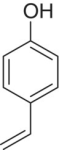
|
Medicinal, phenolic, | 180 | 27 | S |
| 4‐Ethylguaiacol (4‐EG) |

|
Spice, clove | 33 | 13 | B |
| 4‐Vinylguaiacol (4‐VG) |
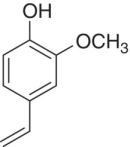
|
Smoke, phenolic | 40 | 10 | S |
a Common names are widely used in the literature for these compounds; systematic names (or common abbreviations) can also be encountered and are given in parentheses.
b Descriptor and threshold data refer to different matrices, including water and red or white wine.
c L, lignin degradation (i.e., oak or smoke); B, Brettanomyces yeast activity; S, Saccharomyces yeast activity.
12.3 Concentrations in wine and sensory effects
Volatile phenols can be found at μg/L–mg/L (i.e., suprathreshold) concentrations in wine and impart odors reminiscent of smoke, medicinal/cleaning products, vanilla, spice, and leather (Table 12.1). The presence of the phenolic hydroxyl group has a major impact on the odor of benzenoid substances; the hydroxylated phenols have lower thresholds compared to the methyl (toluene) analogs. The position of ring substituents relative to the hydroxyl group plays an important role in the aroma characteristics and detection thresholds of these compounds. For instance, monoalkyl groups meta to the hydroxyl (e.g. m‐cresol) result in lower odor thresholds compared to their ortho and para analogues, and odor descriptors differ for the various isomers and as a function of alkyl chain length [8] (e.g., 4‐methylguaiacol versus eugenol). In comparison, ortho halogenated compounds (Chapter 18) tend to have lower thresholds than their isomeric or methylated counterparts, with odor qualities again dependent on substitution pattern [9, 10].
12.4 Origins in wine and effects on volatile phenol profile
12.4.1 Oak storage
Several volatile phenols, including phenol, guaiacol, syringol, vanillin, cresols, and eugenol (Table 12.1), are extracted when storing wine in contact with toasted oak wood (e.g., oak barrels, chips, or staves). These compounds arise from thermal degradation of lignin and are generally positive contributors to wine aroma when present at appropriate concentrations. Analogously, heating other lignin‐containing foodstuffs (e.g. roasting coffee or peanuts, kilning of malt for beer and whiskey) will generate volatile phenols that contribute to the characteristic odors of these products.3 The extent of heating (i.e., amount of oak barrel toasting, Table 12.2) and time in contact with toasted oak influences the level of lignin degradation compounds in wine, as does wine alcohol content, barrel age, oak origin, and seasoning (Chapter 25) [19, 20]. Typically, dry red wines will be subjected to oak contact but few white wines receive such treatment, with Chardonnay, Semillon, and botrytised white wines being the main exceptions. Table 12.3 provides a guide to the concentrations that can be encountered for a range of volatile phenols in oaked wines.
Table 12.2 Volatile phenols extracted into model wine (12% v/v/ethanol, 5 g/L tartaric acid, adjusted to pH 3.5) after two weeks of maceration of French oak wood shavings obtained from light, medium, and high toasting of barrels. Data from Reference [21]
| Compound | Average μg/g (% RSD) of dry wood at toasting level | ||
| Light | Medium | High | |
| Phenol | 0.47 (102) | 0.83 (17) | 0.41 (101) |
| Guaiacol | 0.45 (72) | 1.26 (13) | 0.40 (60) |
| 4‐Methylguaiacol | 0.80 (28) | 1.72 (23) | 0.74 (20) |
| Syringol | 0.94 (74) | 3.66 (10) | 0.83 (61) |
| Eugenol | 1.87 (84) | 1.27 (58) | 1.65 (75) |
| Vanillin | 27.17 (18) | 49.81 (47) | 25.45 (12) |
| 4‐Ethylphenol | 0.30 (78) | 0.29 (13) | 0.26 (72) |
| 4‐Ethylguaiacol | 0.04 (55) | 0.14 (23) | 0.04 (38) |
| 4‐Vinylguaiacol | 0.13 (42) | 0.17 (66) | 0.12 (37) |
Table 12.3 Concentrations of selected volatile phenols in a number of wine varieties aged in contact with toasted oak wood [14, 18, 22, 23]
| Compound | Concentration range (μg/L) | |||
| Chardonnaya | Tempranillob | Cabernet Sauvignonc | Merlotd | |
| Guaiacol | 3–28 | 25–76 | 5–44 | 16–139 |
| 4‐Methylguaiacol | 1–8 | 16–66 | <1–16 | 5–32 |
| Syringol | –e | – | – | 68–488 |
| Eugenol | 10–26 | 30–96 | 13–52 | NDf–19g |
| Vanillin | 198–388 | 347–854 | 9–369 | 77–236 |
| 4‐Ethylphenol | ND–1 | 8–251 | 630–1850 | – |
| 4‐Vinylphenol | 7–76 | 1406–2521 | 1–5 | – |
| 4‐Ethylguaiacol | ND–5 | 5–105 | 22–306 | – |
| 4‐Vinylguaiacol | 5–30 | 178–409 | 1–3 | – |
a Aged in medium toasted French and American oak barrels for 55 weeks.
b Aged in medium toasted Spanish, French, and American oak barrels for 52 weeks.
c Aged in medium toasted French and American oak barrels for 52 and 93 weeks.
d Aged in contact with light to heavy toasted French oak wood staves in stainless steel tanks for 52 weeks.
e –, not reported.
f ND, not detected.
g Eugenol + isoeugenol.
12.4.2 Fermentation – release from grape‐derived glycosides
Compounds formed by lignin degradation in toasted oak are also naturally present in wood smoke. Volatile phenols can therefore give rise to undesirable aroma and flavor characters (medicinal, smoky, ashy‐aftertaste [24]) in wine when grapes become contaminated due to bushfire (wildfire) smoke in the vicinity of vineyards [25]. This smoke taint is of most concern in winegrape growing regions in proximity to areas where bushfires or forest fires are common, such as parts of Australia, North America, and South Africa. Originally, the focus was on the volatile compounds themselves, and guaiacol and 4‐methylguaiacol were used as indicators of contamination by smoke, but these compounds can be found at higher concentrations in oaked wines [17] (e.g., compare Tables 12.3 and 12.4). Further research discovered that other volatile phenols from smoke were also involved (Table 12.4), and that volatile phenols could be glycosylated in grape leaves or berries. These non‐volatile glycosides could be stored in grapes [26] and hydrolyzed by acid or enzymes during fermentation or storage, subsequently releasing the volatile compounds into wine [27] (Chapter ). These glycosides can also be released in‐mouth due to oral microflora (and therefore perceived retronasally) [17, 28], and the phenomenon of glycosylation of exogenous volatiles by grape berries and release during fermentation and storage (or enzymatically) has since been extended to studies involving the deliberate application of oak extracts to grapevines in the vineyard [29–31].
Table 12.4 Average concentration (and standard deviation, SD) of volatile phenols in unoaked wines elaborated from grapes affected by bushfire smoke. Data from Reference [17]
| Compound | Average concentration (SD) (μg/L) | |||
| Control wines (N = 3)a |
Pinot Noir (N = 9) |
Cabernet Sauvignon (N = 2) |
Shiraz (N = 3) | |
| Guaiacol | 5.0 (1.7) | 18.2 (14.7) | 23.5 (10.6) | 32.7 (4.9) |
| 4‐Methylguaiacol | 2.3 (3.2) | 3.8 (2.7) | 5.0 (0) | 4.7 (4.0) |
| 4‐Vinylguaiacol | NDb | 4.9 (4.6) | 2.0 (2.8) | 5.7 (3.1) |
| Syringol | 9.7 (5.5) | 16.9 (6.0) | 19.5 (4.9) | 19.3 (4.0) |
| 4‐Methylsyringol | 2.0 (2.0) | 5.1 (2.3) | 7.5 (3.5) | 6.0 (5.2) |
| 4‐Allylsyringol | 8.3 (9.1) | 10.7 (5.6) | 4.0 (2.8) | 8.7 (7.4) |
| Phenol | 2.7 (3.1) | 24.3 (15.9) | 34.5 (7.8) | 28.7 (13.2) |
| o‐Cresol | 2.7 (3.1) | 10.1 (6.4) | 7.5 (2.1) | 5.0 (1.7) |
| m‐Cresol | 2.0 (1.7) | 7.1 (2.9) | 7.5 (0.7) | 2.3 (0.6) |
| p‐Cresol | 0.7 (1.2) | 5.0 (0.9) | 3.5 (0.7) | 2.0 (1.0) |
a N, number of wines; the control wines were Pinot Noir (N = 2) and Cabernet Sauvignon (N = 1).
b ND, not detected.
12.4.3 Fermentation – metabolism of hydroxycinnamic acids
Volatile phenols can also arise from yeast activity acting on grape‐derived components, presenting a significant challenge to winemakers. Metabolism of HCAs in wine (Chapter 13) gives rise to “Brett” off‐flavor compounds such as 4‐ethylphenol (4‐EP) and 4‐ethylguaiacol (4‐EG, Table 12.1) from their 4‐vinyl analogs [32], which contrasts with the small amounts formed during lignin pyrolysis. The route from precursor acids involves enzymatic decarboxylation (by Saccharomyces or Brettanomyces) of HCAs to give 4‐vinylphenol (4‐VP) and 4‐vinylguaiacol (4‐VG, Table 12.1) from p‐coumaric and ferulic acids, respectively, and subsequent reduction of the vinyl group by Brettanomyces to form 4‐EP/4‐EG (Chapter 23.3). Although dependent on grape variety and wine style, the relative concentrations of Brett spoilage compounds tend to reflect the concentrations of the precursor acids (Chapter ). Typically, this leads to an approximately 8:1 ratio of 4‐EP to 4‐EG [33]. These compounds demonstrate additive effects and are major contributors to “Brett” aromas, although predicting sensory effects can be complicated by the presence of other volatile phenols, or due to masking, for example, by isovaleric and isobutyric acids [34]. Table 12.3 shows representative concentrations of Brett‐related volatile phenols in different wines, with the higher levels of ethyl analogs in particular being an indication of Brettanomyces activity during barrel aging. Wines are particularly susceptible to Brett spoilage during this time (and also while undergoing MLF) due to the low levels of SO2, slow ingress of O2 and potential for poor sanitation, especially of older barrels.
References
- 1. Maga, J.A. (1987) The flavor chemistry of wood smoke. Food Reviews International, 3 (1–2), 139–183.
- 2. Maga, J.A. (1989) The contribution of wood to the flavor of alcoholic beverages. Food Reviews International, 5 (1), 39–99.
- 3. Barrera‐García, V.D., Gougeon, R.D., Voilley, A., Chassagne, D. (2006) Sorption behavior of volatile phenols at the oak wood/wine interface in a model system. Journal of Agricultural and Food Chemistry, 54 (11), 3982–3989.
- 4. Jiménez‐Moreno, N. and Ancín‐Azpilicueta, C. (2009) Sorption of volatile phenols by yeast cell walls. International Journal of Wine Research, 1, 11–18.
- 5. Chassagne, D., Guilloux‐Benatier, M., Alexandre, H., Voilley, A. (2005) Sorption of wine volatile phenols by yeast lees. Food Chemistry, 91 (1), 39–44.
- 6. Fudge, A.L., Ristic, R., Wollan, D., Wilkinson, K.L. (2011) Amelioration of smoke taint in wine by reverse osmosis and solid phase adsorption. Australian Journal of Grape and Wine Research, 17 (2), S41–S48.
- 7. Larcher, R., Puecher, C., Rohregger, S., et al. (2012) 4‐Ethylphenol and 4‐ethylguaiacol depletion in wine using esterified cellulose. Food Chemistry, 132 (4), 2126–2130.
- 8. Czerny, M., Brueckner, R., Kirchhoff, E., et al. (2011) The Influence of molecular structure on odor qualities and odor detection thresholds of volatile alkylated phenols. Chemical Senses, 36 (6), 539–553.
- 9. Strube, A., Buettner, A., Czerny, M. (2012) Influence of chemical structure on absolute odour thresholds and odour characteristics of ortho‐ and para‐halogenated phenols and cresols. Flavour and Fragrance Journal, 27 (4), 304–312.
- 10. Capone D.L., Van Leeuwen K.A., Pardon K.H., et al. (2010) Identification and analysis of 2‐chloro‐6‐methylphenol, 2,6‐dichlorophenol and indole: causes of taints and off‐flavours in wines. Australian Journal of Grape and Wine Research, 16 (1), 210–217.
- 11. Chatonnet, P., Dubourdieu, D., Boidron, J.‐N., Lavigne, V. (1993) Synthesis of volatile phenols by Saccharomyces cerevisiae in wines. Journal of the Science of Food and Agriculture, 62 (2), 191–202.
- 12. López, R., Aznar, M., Cacho, J., Ferreira, V. (2002) Determination of minor and trace volatile compounds in wine by solid‐phase extraction and gas chromatography with mass spectrometric detection. Journal of Chromatography A, 966 (1–2), 167–177.
- 13. Culleré L., Escudero A., Cacho J., Ferreira V. (2004) Gas chromatography–olfactometry and chemical quantitative study of the aroma of six premium quality Spanish aged red wines. Journal of Agricultural and Food Chemistry, 52 (6), 1653–1660.
- 14. Fernandez de Simon, B., Cadahia, E., Sanz, M., et al. (2008) Volatile compounds and sensorial characterization of wines from four Spanish denominations of origin, aged in Spanish Rebollo (Quercus pyrenaica Willd.) oak wood barrels. Journal of Agricultural and Food Chemistry, 56 (19), 9046–9055.
- 15. Prida, A. and Chatonnet, P. (2010) Impact of oak‐derived compounds on the olfactory perception of barrel‐aged wines. American Journal of Enology and Viticulture, 61 (3), 408–413.
- 16. Fernández de Simón, B., Cadahía, E., Muiño, I., et al. (2010) Volatile composition of toasted oak chips and staves and of red wine aged with them. American Journal of Enology and Viticulture, 61 (2), 157–165.
- 17. Parker, M., Osidacz, P., Baldock, G.A., et al. (2012) Contribution of several volatile phenols and their glycoconjugates to smoke‐related sensory properties of red wine. Journal of Agricultural and Food Chemistry, 60 (10), 2629–2637.
- 18. Chira, K. and Teissedre, P.‐L. (2013) Extraction of oak volatiles and ellagitannins compounds and sensory profile of wine aged with French winewoods subjected to different toasting methods: behaviour during storage. Food Chemistry, 140 (1–2), 168–177.
- 19. Garde‐Cerdán, T. and Ancín‐Azpilicueta, C. (2006) Review of quality factors on wine ageing in oak barrels. Trends in Food Science and Technology, 17 (8), 438–447.
- 20. Pérez‐Coello, M.S. and Díaz‐Maroto, M.C. (2009) Volatile compounds and wine aging, in Wine chemistry and biochemistry (eds Moreno‐Arribas, M.V. and Polo, M.C.), Springer, New York, pp. 295–311.
- 21. Chatonnet, P., Cutzach, I., Pons, M., Dubourdieu, D. (1999) Monitoring toasting intensity of barrels by chromatographic analysis of volatile compounds from toasted oak wood. Journal of Agricultural and Food Chemistry, 47 (10), 4310–4318.
- 22. Spillman, P.J., Sefton, M.A., Gawel, R. (2004) The effect of oak wood source, location of seasoning and coopering on the composition of volatile compounds in oak‐matured wines. Australian Journal of Grape and Wine Research, 10 (3), 216–226.
- 23. Garde Cerdán, T., Rodrı&c.acute;guez Mozaz, S., Ancı&c.acute;n Azpilicueta, C. (2002) Volatile composition of aged wine in used barrels of French oak and of American oak. Food Research International, 35 (7), 603–610.
- 24. Ristic, R., Boss, P., Wilkinson, K. (2015) Influence of fruit maturity at harvest on the intensity of smoke taint in wine. Molecules, 20 (5), 8913.
- 25. Jiranek V. (2011) Smoke taint compounds in wine: nature, origin, measurement and amelioration of affected wines. Australian Journal of Grape and Wine Research, 17 (2), S2–S4.
- 26. Hayasaka, Y., Baldock, G.A., Parker, M., et al. (2010) Glycosylation of smoke‐derived volatile phenols in grapes as a consequence of grapevine exposure to bushfire smoke. Journal of Agricultural and Food Chemistry, 58 (20), 10989–10998.
- 27. Kennison, K.R., Gibberd, M.R., Pollnitz, A.P., Wilkinson, K.L. (2008) Smoke‐derived taint in wine: the release of smoke‐derived volatile phenols during fermentation of Merlot juice following grapevine exposure to smoke. Journal of Agricultural and Food Chemistry, 56 (16), 7379–7383.
- 28. Mayr, C.M., Parker, M., Baldock, G.A., et al. (2014) Determination of the importance of in‐mouth release of volatile phenol glycoconjugates to the flavor of smoke‐tainted wines. Journal of Agricultural and Food Chemistry, 62 (11), 2327–2336.
- 29. Martínez‐Gil, A.M., Garde‐Cerdán, T., Martínez, L., et al. (2011) Effect of oak extract application to Verdejo grapevines on grape and wine aroma. Journal of Agricultural and Food Chemistry, 59 (7), 3253–3263.
- 30. Martínez‐Gil, A.M., Garde‐Cerdán, T., Zalacain, A., et al. (2012) Applications of an oak extract on Petit Verdot grapevines. Influence on grape and wine volatile compounds. Food Chemistry, 132 (4), 1836–1845.
- 31. Martínez‐Gil, A.M., Angenieux, M., Pardo‐García, A.I., et al. (2013) Glycosidic aroma precursors of Syrah and Chardonnay grapes after an oak extract application to the grapevines. Food Chemistry, 138 (2–3), 956–965.
- 32. Oelofse, A., Pretorius, I.S., du Toit, M. (2008) Significance of Brettanomyces and Dekkera during winemaking: a synoptic review. South African Journal for Enology and Viticulture, 29 (2), 128–144.
- 33. Chatonnet, P., Dubourdieu, D., Boidron, J.N., Pons, M. (1992) The origin of ethylphenols in wines. Journal of the Science of Food and Agriculture, 60 (2), 165–178.
- 34. Romano, A., Perello, M.C., Lonvaud‐Funel, A., et al. (2009) Sensory and analytical re‐evaluation of “Brett character.” Food Chemistry, 114 (1), 15–19.