2
Carbohydrates
2.1 Introduction
Carbohydrates are the most abundant class of biological molecules on Earth. Colloquially, “sugar” is used as either a synonym for carbohydrate or to refer to sucrose (table sugar, a disaccharide composed of glucose and fructose). In food chemistry and in this text, the term sugar will refer to low molecular weight carbohydrates, especially those with a sweet taste (e.g. glucose, sucrose).
Sugars are biologically important because they represent a water‐soluble energy source. In most organisms glucose is used as a major energy substrate as part of glycolysis, and in humans and most other animals, glucose is used to circulate energy throughout the body. In grapes and many other plants, sugars are transported in the form of sucrose, and will accumulate in the grape berry during ripening in the form of glucose and fructose (Chapter 20). These sugars are the primary substrate for yeast to produce ethanol during alcoholic fermentation (Chapter 22.1) and sugars in wine can contribute to perceived sweetness. Higher molecular weight polymers of sugars (polysaccharides) are major components of the cell walls in grapes and other plants. Finally, covalent bonding between non‐sugar compounds (aglycones) and sugars to form glycosides will result in increased solubility and other chemical changes to the original compound (Chapter 23.1)
2.2 Nomenclature, representation, and occurrence of sugars
The simplest carbohydrates are the monosaccharides, which include the aldoses (polyhydroxylated aldehydes) and ketoses (polyhydroxylated methyl ketones). The name, carbohydrate, refers to the empirical formula Cx(H2O)x of aldoses and ketoses, whose general structures are shown in Figure 2.1.

Figure 2.1 General structures for aldose and ketose sugars
These sugars can be further distinguished by the following properties:
- Carbon number. Pentoses (5 carbons) and hexoses (6 carbons) are the most common in wine.
- Fermentability. Sugars may be distinguished by whether they can be fermented by wine yeast under ordinary circumstances.
- Derivatives. The basic monosaccharide structure may be modified by reduction, oxidation, etc.
In grapes, the most quantitatively important sugars are hexoses: fructose (a ketose) and glucose (an aldose). These compounds are reducing sugars, that is, they are capable of reducing Cu(II), Fe(III), and other transition metals under alkaline conditions. The reaction involves oxidation of the aldehyde group of aldoses, but ketoses are also measured because they can isomerize to form aldoses under basic conditions. Importantly, many oligosaccharides (e.g., sucrose) lack a reactive carbonyl group and will not be included in measurements of reducing sugars.
In aqueous solution, hexoses and pentoses predominantly exist as hemiacetal rings, formed by the reversible intramolecular reaction of the carbonyl group with an –OH group. The resulting forms are referred to as either furanoses (5‐member rings) or pyranoses (6‐membered rings). For each ring form, the hemiacetal group can exist as one of two stereoisomers, referred to as anomers (α‐ or β‐; Figure 2.2). The different forms exist in equilibrium, and at room temperature the most abundant isomers of glucose and fructose are the β‐pyranose forms. The hemiacetal form of sugars can react with alcohols to form acetals (see Chapter 9). The resulting compounds are called glycosides. Glycosidic bonds between the hemiacetal of one sugar ring and the –OH group of another sugar result in the formation of disaccharides, trisaccharides, and larger polysaccharides. Sucrose, for example, is a disaccharide of glucose and fructose. A more complete description of sugar nomenclature can be found in an introductory biochemisty or food chemistry textbook [1].
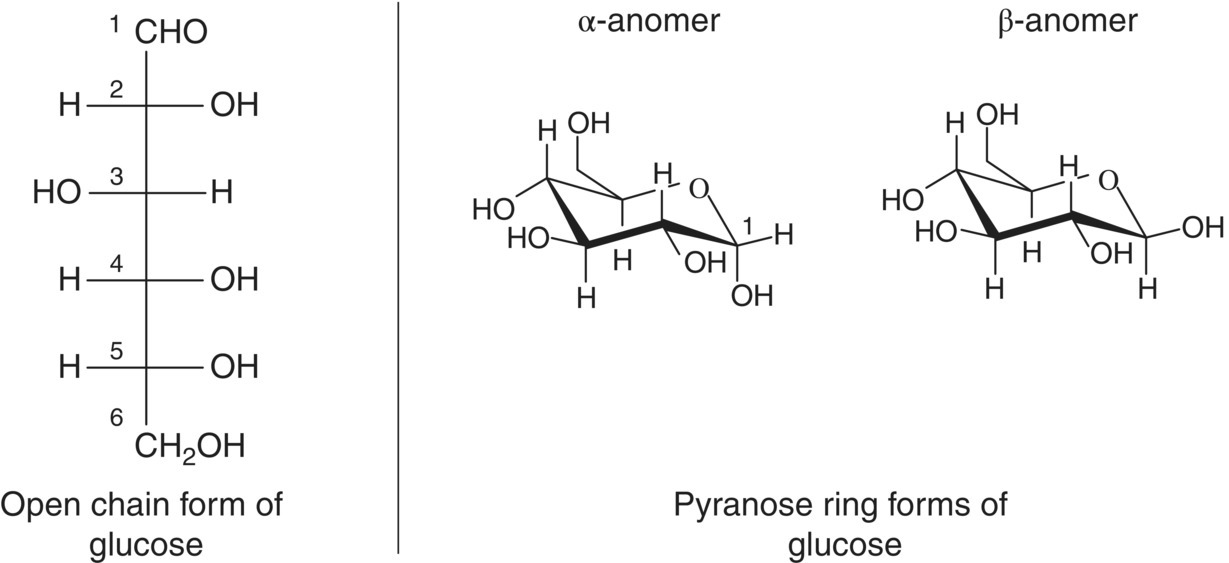
Figure 2.2 Glucose, a hexose sugar, represented as an open‐chain Fischer projection (left) and as a chair conformation of its pyranose form (right). The C1–C6 numbering system for hexoses is also shown on the left
Typical concentrations of sugars in wine are reported in Table 2.1, and their structures are shown in Figure 2.3. In grapes, fructose and glucose increase during ripening from near‐undetectable before veraison to a total concentration in the range of 180–250 g/kg at harvest (Chapter 20). In the wine industry, it is more common to report total soluble solids (TSS) rather than individual sugars in juices. Typically, TSS measurements are performed by density or refractometry, and are calibrated against sucrose standards. Because sugars represent only 90–95% of soluble solids, these measurements are more accurately described as “apparent sugars”. TSS can be reported in units of Brix, where 1 Brix = 1% w/w soluble solids as sucrose, but may also be reported as Baumé, calculated as Brix ÷ 1.8. Baumé is a rough measure of the “potential alcohol” of a wine: that is, the % v/v ethanol concentration that would be achieved if the juice was fermented to dryness.
Table 2.1 Concentrations and taste properties of major sugars in wine
| Sugar or sugar derivatives | Typical concentration in dry wine (g/L)a | Taste threshold in H2O (g/L) [4–6] | Sweet intensity of a 10% w/w solution [5]b | Notes |
| Sugars | ||||
| Fructose | 0.2–4 | 1.8–2.4 | 114 | Major grape sugar, hexose |
| Glucose | 0.5–1 | 3.6–12 | 69 | Major grape sugar, hexose |
| Sucrose | 0–0.2 | 3.6 | 100 | Disaccharide of glucose and fructose |
| Arabinose | 0.5–1 | 2.5 | Non‐fermentable pentose, component of glycosides and pectin | |
| Galactose | 0.1 | 9.0 | Isomer of glucose, component of pectin | |
| Rhamnose | 0.2–0.4 | Deoxy sugar Forms glycosides | ||
| Sugar alcohols | ||||
| Glycerol | 7–10 | 5.2–7.7 | Fermentation metabolite | |
| Mannitol | 0.01–0.05 | 7.3 | 69 | Indicative of grape rot or wine spoilage |
| Arabitol | 6.5 | |||
| Sorbitol (glucitol) | 0–0.05 | 6.2 | 51 | |
| Inositols, total | 0.2–0.7 | 3.2 | ||
| Sugar acids | ||||
| Gluconic acid | Up to 2c | Sour | Indicative of grape rot | |
| Galacturonic acid | 0.1–1 | Sour/astringent | Major component of pectin | |
| 2‐Oxogluconic acid | Up to 0.1c | Indicative of grape rot | ||
a Sugars and sugar alcohol concentrations compiled from multiple sources from Reference [7]. Data on sugar acids compiled from Reference [8].
b Reference is sucrose = 100.
c High values in grapes afflicted by rot.
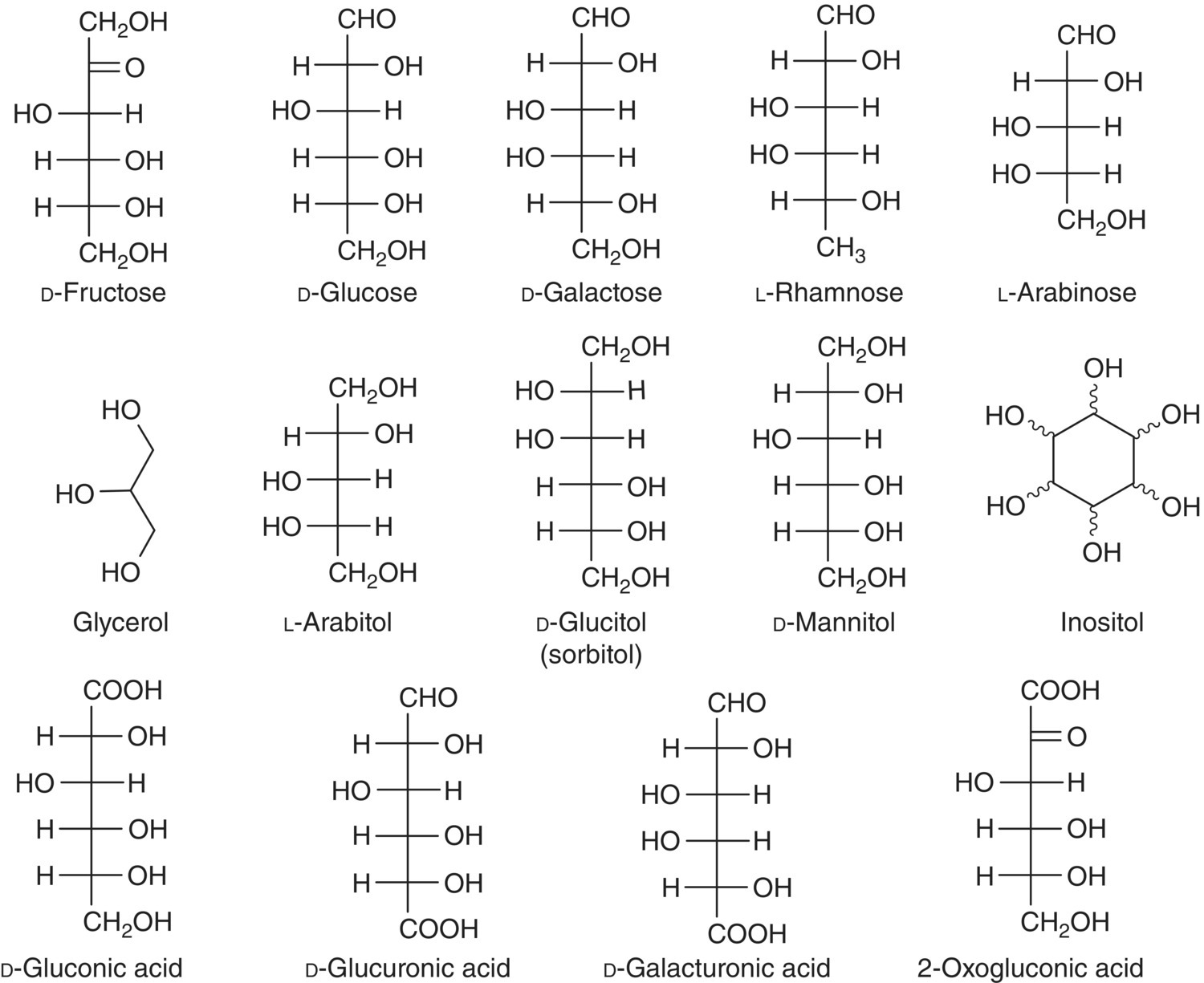
Figure 2.3 Structures of major sugars and sugar derivatives found in wine
During alcoholic fermentation, hexose sugars are largely converted to ethanol and CO2, but some residual sugar (RS) will still be detectable in wines. Potential sources of residual sugars in finished wines include:
- Incomplete fermentation, either because fermentation is stopped or because sugars are non‐fermentable (e.g. arabinose, xylose)
- Back‐sweetening of wine with sucrose, grape must, or other sources after fermentation is complete
- Hydrolysis of glycosides during storage (Chapter 23.1)
- Extraction from oak [2]
The species included in RS measurements will vary with methodology. For example, enzymatic methods will generally only measure fructose, glucose, and possibly sucrose. Methods based on copper reduction assays will include other reducing sugars (e.g., arabinose), but not sucrose. Typical dry table wines will have residual sugar concentrations between 1 and 4 g/L, but sweet wines may have greater than 100 g/L (Chapter 19). Because yeast are glucophilic, fructose is usually at higher concentrations than glucose [3], although this difference will be obscured by back‐sweetening. Several other monosaccharides are present in wine at concentrations ranging from 0.1 g/L to 1 g/L, including arabinose, galactose, and rhamnose (Table 2.1). Most of these sugars cannot be fermented by wine yeast strains.
2.3 Physical, chemical, and sensory properties of sugars
Sugars are hydrophilic due to their large number of –OH groups (Figure 2.2). Monosaccharides are soluble in both ethanol and water, but solubility (particularly in ethanol) decreases with increasing chain length [1]. Sugars can participate in a range of reactions in aqueous solutions, including wine.
Enzymatic reactions of sugars in grape must or non‐stabilized wine include:
- Glycolysis – the metabolism of sugars (Chapter 22.1)
- Enzymatic oxidation to form sugar acids or enzymatic reduction to form sugar alcohols
- Enzymatic hydrolysis of glycoside bonds, for example, hydrolysis of sucrose to yield glucose and fructose by invertase.
Important non‐enzymatic reactions in finished wine include:
- Acid hydrolysis of glycoside bonds – for example, sucrose added to wine will be ~50% hydrolyzed to fructose and glucose when stored at room temperature for two months at pH 3.0 [9].
- Sugar degradation – under acidic conditions, sugars can be degraded to furanic carbonyl compounds (furfural, 5‐(hydroxymethyl)furfural (HMF), sotolon) and related products, particularly at higher temperatures (Chapter 25).
- Carbonyl chemistry – the carbonyl group of reducing sugars exists primarily as a hemiacetal and thus is less available to react than analogous carbonyls discussed in Chapter 9. Nonetheless, the carbonyl group can react with bisulfite to yield an adduct (Chapter 17). Reactions with amine groups to yield browning products, as occurs in the thermal processing of many foods (Maillard reactions) is expected to be negligible due to the low pH and low amino acid concentrations of wine (Chapter 5).
The notable flavor property of most sugars is sweetness (Table 2.1). Most monosaccharides have sweet detection thresholds in the range of 10–50 mM (0.2–1.0% w/w). Of the sugars found in wine, the most potent is fructose, which is almost twice as sweet as glucose and 15% sweeter than sucrose at a concentration of 10% w/w [5]. The sweet taste of sugars will increase perception of body and mask other taste and tactile sensations like sourness, bitterness, astringency, and pungency, and vice versa [10]. For example, increasing the sugar concentration of a citric acid solution results in a decrease in perceived acidity [11]. Similar observations have been made in wine reconstitution and omission studies near the sensory thresholds of sugars, in which increasing the concentration of sugars decreases astringency and sourness [6]. The sweetness threshold of sugars also provides a rationale for labeling or classifying wines as “dry” versus “sweet” based on quantitative analysis of residual sugars. For example, in EU regulations, “dry” (or its translation) indicates <4 g/L of residual sugar, corresponding roughly to the detection threshold for sugars in wines.
Sugars are non‐volatile and have no aroma – the sweet caramel aroma of cooked sugar is due to degradation products like sotolon, discussed later (Chapter 9). However, the presence of sugar has well‐known cognitive effects on non‐taste attributes of food flavor. For example, in coffee, addition of sweeteners increases the retronasal intensity of “caramel,” while decreasing “roasty” and “coffee” flavors [12]. The reverse can also happen, where sweet aromas can increase perception of sweetness by taste [13]. This phenomenon of combining information from two different sensory modalities is referred to as synesthesia [14]. High sugar concentrations can also have quantifiable effects on the volatility of odorant compounds, by decreasing the availability of water for solvation (“salting‐out”). The magnitude of these effects are usually small, with <20% increase in volatility observed in a 15% w/v sucrose solution versus water for three representative odorants (isoamyl acetate, ethyl hexanoate, eugenol) [15].
2.3.1 Sugar alcohols
Polyalcohols (polyols) describe aliphatic compounds with multiple –OH groups and no other functional groups (Figure 2.2). The term is used synonymously with sugar alcohols in some texts, since they are often derived from sugars and usually have a sweet taste. The major class of sugar alcohols in wine are the alditols, which are formed by reduction of the sugar carbonyl group to an alcohol during fermentation. They are named by replacing the –ose suffix of the corresponding aldose sugar with –itol.
The major sugar alcohol in found in wine is glycerol, which is usually the most abundant compound in dry wines after water and ethanol. Glycerol is common to all alcoholic fermentations, where it has several important physiological functions as an osmoregulant and in redox balancing (Chapter 22.1). Glycerol concentrations are usually in the range of 7–10 g/L [16], but may be over 15 g/L in high sugar fermentations like ice wines [17]. Other alditols, such as mannitol, arabitol, and sorbitol, are present in the range of 20–300 mg/L (total < 1 g/L, Table 2.1). Higher concentrations of sugar alcohols may be a sign of microbial spoilage; for example, high concentrations of mannitol formed by enzymatic reduction of fructose are indicative of lactic spoilage [18]. Other quantitatively important polyalcohols are 1,2‐propanediol (propylene glycol) and 2,3‐butanediol, formed by reduction of lactic acid and acetoin, respectively. Another class of polyalcohols found in wines is the inositols (cyclitols), which have perhydroxylated cyclohexane rings. The inositols are components of phosphoinositols, used by yeast and other eukaryotes in cell membranes and signal transduction. One major (myo‐) and two minor (scyllo‐ and chiro‐) inositols are observed, and typical total concentrations are ~300 mg/L [19].
Sugar alcohols are chemically and microbiologically stable under reductive wine conditions. Sugar alcohols lack a reactive carbonyl group, and thus will not participate in electrophilic reactions as is observed with sugars. Sugar alcohols do not appear to be metabolized by commercial yeast or LAB strains under normal wine conditions, although some rare spoilage LAB can degrade glycerol [18].
Sugar alcohols have about 50% of the sweet intensity of sucrose on a w/w basis (Table 2.1). In wine, only glycerol is usually present at concentrations above its taste threshold (5.2 g/L) [4]. Historically, glycerol was implicated as an important contributor to the mouthfeel of a wine, but the actual impact on mouthfeel appears to be minor. For example, addition of >25 g/L of glycerol to a model wine was necessary to cause a detectable change in mouthfeel [4], and in a non‐targeted study on white wines, no correlation was observed between glycerol and wine body [20]. Although these results cast doubt on the impact of glycerol in isolation, a reconstitution study on dry red wines observed that elimination of all alditols (glycerol, sorbitol, mannitol, and others) results in a significant decrease in wine mouthfulness/body [6].
2.3.2 Sugar acids
Aldonic acids have a carboxylic acid group in place of the aldehyde group and are named by replacing the –ose suffix of the corresponding aldose sugar with –onic acid, for example, glucose and gluconic acid. Uronic acids have a carboxyl acid group in place of the terminal hydroxyl group. Uronic acid derivatives of aldoses are named by replacing the –ose suffix of the corresponding sugar with –uronic acid, e.g. glucose and glucuronic acid. Confusingly, uronic acid derivatives of ketoses are named as oxo derivatives of aldonic acids, for example, 2‐oxogluconic acid. Uronic acids possess an electrophilic aldehyde group and at high concentrations can contribute to SO2 binding (Chapter 17). These sugar acids (Figure 2.2, Table 2.1) are generally formed by enzymatic oxidation and high concentrations, particularly of gluconic acid, are associated with grape spoilage organisms such as Gluconobacter [21]. One exception is galacturonic acid, which is typically present at several hundred mg/L in dry wines, and is likely formed by pectin hydrolysis during or after fermentation.
The impact of uronic and aldonic acids in wine is poorly studied. They will contribute to titratable acidity (pKa = ~3.5), but at typical concentrations found in wine (<0.5 g/L as tartaric acid equivalents) they are likely to be of negligible sensory importance. Although higher concentrations are found in wines produced from grapes compromised by Acetobacter and Gluconobacter, it is likely that other off‐flavors (e.g. volatile acidity) will have a more noticeable role.
2.4 Polysaccharides
Polysaccharides are polymers of carbohydrates. The major polysaccharides in grapes are structural components:
- Cellulose, a water‐insoluble β‐(1 → 4) polymer of d‐glucopyranose
- Pectin, a water‐soluble heteropolymer that consists primarily of α‐(1 → 4) d‐galacturonic acid linkages
- Hemicellulose, a diverse class of heteropolymers related to pectin that consists of xylose, arabinose, galactose, glucose, and several other sugars.
In wine, polysaccharides may be derived from both grape and yeast cell walls (Table 2.2). Because of their polymeric nature, polysaccharides are not ordinarily characterized as individual molecules. Instead, it is common to isolate and quantify fractions based on size (e.g., by gel permeation chromatography) or charge (ion exchange chromatography). The fractions may then be hydrolyzed to determine the constituent sugars [22].
Table 2.2 Typical concentrations and sources of major polysaccharides in wine. Adapted from Reference [23]
| Concentration (mg/L) | |||||
| Class | Source | Red wine | White wine | Typical MW (kDa) | Charge |
| Rhamnogalacturonans (RG‐I and ‐II) |
Grape | 50–250 | 10–50 | 10 | Acidic |
| Arabinogalactan proteins (AGPs) | Grape | 100–150 | 50–150 | 100–250 | Neutral |
| Mannoproteins | Yeast | 100–150 | 100–150 | 50–500 | Neutral |
Grape polysaccharides can decrease yield and extraction during winemaking (Chapters 19 and 21) and decrease the efficiency of filtration (Chapter 26.3), and winemakers will often use carbohydrase enzymes to decrease these impacts. Grape polysaccharides will be further degraded by microbial enzymes during fermentation and are not efficiently transferred to wine during fermentation either due to degradation or poor solubility.
Two exceptions are rhamnogalacturonans I and II (RG‐I and RG‐II), pectin fragments that contain a high degree of branching from several classes of sugar residues. Arabinogalactan proteins (AGPs) consist primarily of arabinose, galactose, and glucuronic acid, and also contain about 10% of hydroxyproline‐rich protein. Both appear to be less susceptible to hydrolysis than other pectic or hemicellulosic substances. The major yeast‐derived polysaccharides are mannoproteins, the primary constituents of yeast cell walls. They are released during yeast autolysis and thus are expected to be at higher concentrations in wines aged on lees. They consist primarily of mannose, with smaller amounts of protein. More detailed information on polysaccharide composition can be found elsewhere [22, 23].
Polysaccharides are widely used in the food industry to increase perceived viscosity and mouthfeel in beverages, although typically at higher concentrations than those in wine [1]. The effects of wine polysaccharides on mouthfeel and other flavor properties are an active area of investigation. Addition of isolated polysaccharide fractions to model wine are reported to slightly increase body, and the acidic RG‐II fraction also decreased perceived astringency [24]. Polysaccharides may also have a modest indirect effect on wine aroma by decreasing volatility of some odorants [25], although the effect appears to be small. Mannoproteins can inhibit potassium bitartrate crystallization (Chapters 26.1 and 27) and stabilize the foam of sparkling wines [26]. Finally, some polysaccharides are associated with wine faults. For example, β‐glucans produced by Pediococcus (and other bacteria) can cause a visible fault called “ropiness” (viscous and oily texture) [18] which can also affect mouthfeel and filterability.
References
- 1. Brady, J.W. (2013) Introductory food chemistry, Comstock Pub. Associates, Ithaca, NY.
- 2. del Alamo, M., Bernal, J.L., del Nozal, M.J., Gomez‐Cordoves, C. (2000) Red wine aging in oak barrels: evolution of the monosaccharides content. Food Chemistry, 71 (2), 189–193.
- 3. Fugelsang, K.C. and Edwards, C.G. (2007) Wine microbiology practical applications and procedures, Springer, New York.
- 4. Noble, A.C. and Bursick, G.F. (1984) The contribution of glycerol to perceived viscosity and sweetness in white wine. American Journal of Enology and Viticulture, 35 (2), 110112.
- 5. Belitz, H.D., Grosch, W., Schieberle, P. (2009) Food chemistry, Springer‐Verlag, Berlin.
- 6. Hufnagel, J.C. and Hofmann, T. (2008) Quantitative reconstruction of the nonvolatile sensometabolome of a red wine. Journal of Agricultural and Food Chemistry, 56 (19), 9190–9199.
- 7. Liu, S.‐Q. and Davis, C.R. (1994) Analysis of wine carbohydrates using capillary gas liquid chromatography. American Journal of Enology and Viticulture, 45 (2), 229–234.
- 8. Luz Sanz, M. and Martinez‐Castro, I. (2009) Carbohydrates, in Wine chemistry and biochemistry (eds Moreno‐Arribas M.V. and Polo M.C.), Springer, New York, pp. xv, 735 pp.
- 9. Wilker, K.L. (1992) Hydrolysis of sucrose in Eastern US table wines. American Journal of Enology and Viticulture, 43 (4), 381–383.
- 10. Lawless, H.T. and Heymann, H. (2010) Sensory evaluation of food principles and practices. Springer, New York.
- 11. McBride, R.L. and Johnson, R.L. (1987) Perception of sugar–acid mixtures in lemon juice drink. International Journal of Food Science and Technology, 22 (4), 399–408.
- 12. Chiralertpong, A., Acree, T.E., Barnard, J., Siebert, K.J. (2008) Taste–odor integration in espresso coffee. Chemosensory Perception, 1 (2), 147–152.
- 13. Stevenson, R.J., Prescott, J., Boakes, R.A. (1999) Confusing tastes and smells: how odours can influence the perception of sweet and sour tastes. Chemical Senses, 24 (6), 627–635.
- 14. Auvray, M. and Spence, C. (2008) The multisensory perception of flavor. Consciousness and Cognition, 17 (3), 1016–1031.
- 15. Friel, E.N., Linforth, R.S.T., Taylor, A.J. (2000) An empirical model to predict the headspace concentration of volatile compounds above solutions containing sucrose. Food Chemistry, 71 (3), 309–317.
- 16. Mattick, L.R. and Rice, A.C. (1970) Survey of the glycerol content of New York State wines. American Journal of Enology and Viticulture, 21 (4), 213–215.
- 17. Pigeau, G.M., Bozza, E., Kaiser, K., Inglis, D.L. (2007) Concentration effect of Riesling Icewine juice on yeast performance and wine acidity. Journal of Applied Microbiology, 103 (5), 1691–1698.
- 18. Bartowsky, E.J. (2009) Bacterial spoilage of wine and approaches to minimize it. Letters in Applied Microbiology, 48 (2), 149–156.
- 19. Carlavilla, D., Villamiel, M., Martínez‐Castro, I., Moreno‐Arribas, M.V. (2006) Occurrence and significance of quercitol and other inositols in wines during oak wood aging. American Journal of Enology and Viticulture, 57 (4), 468–473.
- 20. Skogerson, K., Runnebaum, R., Wohlgemuth, G., et al. (2009) Comparison of gas chromatography‐coupled time‐of‐flight mass spectrometry and 1H nuclear magnetic resonance spectroscopy metabolite identification in white wines from a sensory study investigating wine body. Journal of Agricultural and Food Chemistry, 57 (15), 6899–6907.
- 21. Sponholz, W.R. and Dittrich, H.H. (1985) Origin of gluconic, 2‐oxogluconic, and 5‐oxogluconic, glucuronic and galacturonic acids in musts and wines. Vitis, 24 (1), 51–58.
- 22. Vidal, S., Williams, P., Doco, T., et al. (2003) The polysaccharides of red wine: total fractionation and characterization. Carbohydrate Polymers, 54 (4), 439–447.
- 23. Cheynier, V. and Sarni‐Manchado, P. (2010) Wine taste and mouthfeel, in Managing wine quality, Vol. 1, Viticulture and wine quality (ed. Reynolds, A.G.), Woodhead Publishing and CRC Press, Oxford and Boca Raton, FL.
- 24. Vidal, S., Francis, L., Williams, P., et al. (2004) The mouth‐feel properties of polysaccharides and anthocyanins in a wine like medium. Food Chemistry, 85 (4), 519–525.
- 25. Villamor, R.R. and Ross, C.F. (2013) Wine matrix compounds affect perception of wine aromas, in Annual review of food science and technology, Vol. 4 (eds Doyle, M.P. and Klaenhammer, T.R.), Annual Reviews, Palo Alto, CA, pp. 1–20.
- 26. Blasco, L., Vinas, M., Villa, T.G. (2011) Proteins influencing foam formation in wine and beer: the role of yeast. International Microbiology, 14 (2), 61–71.