20
Grape Must Composition Overview
20.1 Sampling
A typical winegrape berry sugar content is in the range of 18–25% w/w, but the standard deviation among berries in a vineyard, and even within a cluster, is often ± 2% w/w (Figure 20.1), likely due to a range of flowering dates and subsequent fruit‐set dates for individual berries [1]. This variation in sugar can even be seen within a single berry [2]. The result is that not all berries are at the same degree of ripeness at any one time. Similar variations have been reported for other compositional aspects like color [1], and some aroma compounds (e.g., rotundone, Chapter 8) are reported to vary by 10‐fold within a vineyard [3]. Predicting the concentrations of sugars and other parameters in a population of berries, that is, a vineyard block, based on a small or non‐representative subsample is thus challenging for researchers and winemakers alike. Berry sample sizes (either as individual berries or whole clusters) for any particular component must be evaluated to determine if they will lead to adequate precision, and sampling must be appropriately representative to achieve acceptable accuracy [4, 5].
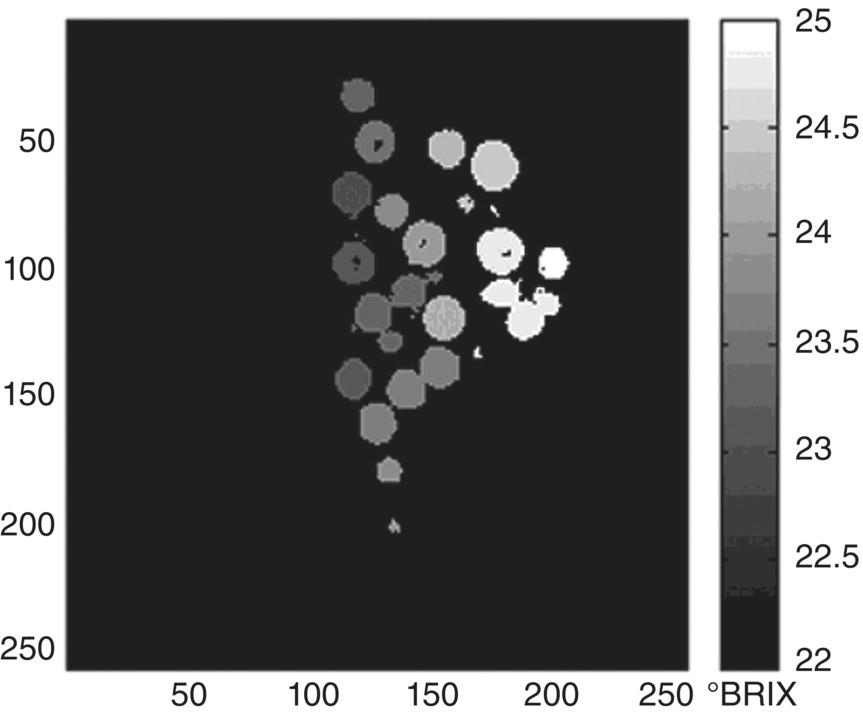
Figure 20.1 Magnetic resonance image of berry sugar levels (°Brix) in a grape cluster.
Source: Andaur 2004 [2]. Reproduced with permission of American Chemical Society
20.2 Sugars
After water, the most abundant substances in grapes at ripeness are sugars, in the form of fructose and glucose. These are found in near equimolar amounts since they arise from hydrolysis of the disaccharide, sucrose, produced through leaf photosynthesis. Small amounts of pentoses and other sugars are also detected (Chapter 2). Sugar concentrations are very low prior to veraison, but accumulate rapidly afterwards, and may reach 25% w/w or higher by harvest time. Sugar concentration is arguably the most widely used parameter for assessing grape composition and ripeness, in part because the amount of sugar present determines the eventual alcohol concentration. Within a region, the sugar attained during ripening can be predicted by the ratio of vegetative growth (a proxy for leaf area and photosynthetic activity) to the quantity of grapes to be ripened [6], and warmer temperatures and longer growing seasons will result in more photosynthesis. Thus, a limiting factor in achieving adequate sugar in winegrapes are temperatures during the growing season (degree‐days is one such measure) [7]; hence, sugar additions are more common in cool regions.
Related to sugars are the polysaccharides, carbohydrate‐containing polymers originating from berry cell wall material (Chapter 2). Although pectins and related structural polysaccharides may approach 1% in V. labruscana grapes, they are generally at values closer to 0.1–0.2% in vinifera juices. Enzymatic treatments can hydrolyze some polysaccharides and will increase their release into must and the resulting wine [8] (Chapters 21 and 23.1). Common pectinases will yield as much as 1 g/L of galacturonic acid from pectin hydrolysis (Chapter 2), which is unfermentable and can persist into finished wine. Aside from pectins, grape skin contains hemicellulose and cellulose, with some of the former being extracted into wine. The combination of polysaccharides is sometimes referred to as fiber [9] and is a major constituent (a few percent) of the pomace on a fresh weight basis (Figure 20.2).

Figure 20.2 Components of grape juice (g/L) versus seeds and skin (g/kg)
20.3 Acids
Grapes contain substantial quantities of organic acids (Chapter 3), 10 grams or more per kg, and these are largely retained in the wine.1 The presence of organic acids is essential to the taste of wine and juice, and the resulting low pH results in exclusion of many spoilage and pathogenic microbes. Tartaric acid is generally the major acid and a key marker for grape juice, while malic acid will also contribute substantially to acidity. Minor acids in grapes include citric and ascorbic, although the former will also be produced through yeast metabolism, and traces of other acids have also been documented (Chapter 3) [10]. Both malic acid and citric acid can be metabolized by lactic acid bacteria if they are present during winemaking, such as when inoculated for malolactic fermentation (Chapter 22.5). Malic and tartaric acids are accumulated pre‐veraison. The acid concentration decreases during ripening and sugar accumulation, primarily due to respiration and loss of malic acid, but also because of berry expansion (and dilution of both acids). The rate of malic acid degradation increases at higher temperatures – thus grapes grown in cooler climates or harvested at earlier dates generally yield higher acidity levels in the must and wine [11]. These factors explain why acid additions are common in warm regions, whereas levels of acidity are often lowered by various treatments when using fruit from cooler environments (Chapter 3).
20.4 Phenolics
Phenolic compounds are found largely in the skins and seeds of grape berries (Chapter 11), though additional material can be extracted from the rachis if whole cluster fermentation is practiced. While considerable variation exists among varieties, a representative study reported red berries to have a total phenolic content of 5.6 g/kg, with one‐third (1.9 g/kg) in the skins, two‐thirds (3.5 g/kg) in the seeds and less than 5% in the pulp and juice. White berries have about 3.8 g/kg, with less than 1 g/kg in skins due to the absence of the anthocyanins, but also about 2.8 g/kg in the seeds, with a similarly small amount in the pulp and juice [12]. Conventional red winemaking only extracts about half of the phenolic substances, with the maceration protocol having a significant impact on their extraction into wine (Chapter 21).
The phenolics are comprised of four major classes and numerous minor ones.
- The skin of grapes contains flavonols, whose formation is induced by sunlight as a UV protective material [13] with typical concentrations in the range of 1–80 mg/kg (Chapter 15) [14].
- Red grape skins are pigmented by the presence of anthocyanins, with 5–15 or more constituent compounds, varying by B‐ring substitution, glucosylation, and further acylation of the glucose moiety (Chapter 16). Anthocyanin concentrations in red varieties vary widely, from 200 to 6000 mg/kg [14], and darker grapes or those with red flesh (teinturier) having higher levels.
- Flavan‐3‐ol monomers and their polymers (proanthocyanidins, also called condensed tannin) are a major phenolic constituent. These are found in both skin and seed, and comprise approximately half the phenolics in grape berries (Chapter 14). During ripening, extractable seed tannin (and flavan‐3‐ol monomers) declines as the result of oxidative reactions in the seed coat – this manifests as a change in the seed color from green to brown [15]. There are many ways to measure tannin (Chapters 14 and 33), but a chromatographic method comparing 37 varieties showed an average of 1.3 g tannin per kg of fruit [16].
- Finally, the hydroxycinnamates are the major phenolic compounds in the pulp and juice of the berry and are the dominant phenolics in white grape juice and wine; one survey of 28 varieties (both red and white) reported an average of 178 mg/kg, with a range from 85 to 400 mg/kg [17].
20.5 Nitrogen species
Amino acids and ammonium salts are the major nitrogenous compounds present in grapes, although their concentrations can vary considerably (300–5000 mg/L in juice or 40–700 mg/L as N) (Chapter 5). These compounds are necessary for yeast nitrogen metabolism and synthesis of proteins and other key macromolecules. However, only about half of this pool are α‐amino acids – which can be metabolized during fermentation (yeast assimilable nitrogen (YAN)) – while the remainder is proline and cannot be utilized (Chapter 22.3). The amino acid profile varies depending on grape variety and environmental factors [18], and musts may be supplemented with nitrogen, typically as diammonium phosphate, if available YAN levels are too low for a successful fermentation (Chapter 22.3).
Proteomics analyses of grape berries shows many different enzymes and other proteins [19], but these are usually present only at low concentrations (<50 mg/kg). Several berry proteins have enzymatic activity, including oxidases that will affect juice browning upon grape berry damage or crushing at the winery [20], as well as chitinases, esterases, glucosidases, pectinases, and glucanases [21], some of which are discussed elsewhere (Chapters 21 and 23.1). Thaumatin‐like proteins and chitinases are produced in response to fungal infections (i.e., pathogenesis‐related), and when induced these become the major proteins present [22], with amounts approaching 300 mg/kg fresh weight (FW) of berry (Chapter 5). These proteins can cause white wine haze (Chapter 26.2) and may also bind to grape tannins and decrease their extractability (Chapter 21). Grapes also contain oligopeptides, and one of importance is glutathione, a key antioxidant due to its thiol functional group (Chapter 5). One report describes levels in grapes as varying from approximately 15 to 100 mg/L with an average of 44 mg/L [23]. Finally, grapes contain small amounts of biogenic amines, including isopentylamine, ethylamine, agmatine, diaminopropane, spermidine, and spermine, at approximately 3–5 mg/kg [24].
20.6 Lipids and waxes
Lipids are a key constituent of grape seeds, but are found in low amounts in skins and pulp. Totals vary from 1.5 to 2.5 g/kg FW in must, with the major lipids in Cabernet Sauvignon being glycolipids and phospholipids containing palmitic, stearic, linoleic, and linolenic acids [25]. However, over 20 different fatty acids have been detected in the neutral and polar lipid fractions, and a V. vinifera cultivar (Cabernet Sauvignon) had much higher levels of unsaturated fatty acids than hybrids, where saturated fatty acids dominate. While they are poorly extracted, the unsaturated fatty acids – for example, oleic, linolenic, and linoleic acids – are of particular importance because (i) they can serve as substrates for formation of the C6 alcohols and aldehydes (Chapter 23.3) and (ii) they are critical for the yeast cell membrane and thus yeast growth (Chapter 22.2).
The exterior of the grape skin is covered with a waxy cuticle, which is a protective water vapor barrier several μm thick composed of triterpenoids (sometimes called saponins or phytosterols). The mixture is complex and includes mostly oleanolic acid and related compounds such as ursolic acid, α‐amyrin, and others [26]. In addition, the wax also contains long‐chain alcohols, esters, aldehydes, hydrocarbons, and other substances [27]. The amount of wax on grape berries is quite high, 1–2 g/kg in fresh fruit, but owing to their hydrophobicity these compounds are weakly soluble in wine and only low amounts are extracted, even in red wine.
20.7 Minerals and vitamins
Minerals found in wine serve primarily as counter ions to deprotonated acids (Chapter 3).
- The dominant juice cation is potassium (1–2 g/kg in grapes), with much lower amounts of calcium (100 mg/kg), magnesium (70 mg/kg), sodium (20 mg/kg), and iron (3 mg/kg) [28].
- Grape juices also contain several anions – phosphate (200 mg/L), sulfate (260 mg/L as K2SO4) and chloride (232 mg/L as NaCl) [29] and small amounts of nitrate. Minerals in juice can vary over 10‐fold with NaCl levels as high as 1800 mg/L and K2SO4 as high as 1200 mg/L resulting from saline soil [29].
- Grapes contain many vitamins, most of which are utilized by yeast but are then returned to the wine at similar levels [30]. The amounts of vitamins as reported in the USDA nutritional analysis of fresh grapes include ascorbic acid (32 mg/kg), niacin (1.8 mg/kg), vitamin B6 (0.86 mg/kg), riboflavin (0.7 mg/kg), thiamin (0.69 mg/kg), folate (20 μg/kg), and vitamin A (30 μg/kg) [28]. Data on winegrape juice shows that levels are similar, with biotin reported at 1–3 μg/L and pantothenic acid at 0.5 mg/L [31].
20.8 Isoprenoids
Carotenoids are found in most plants, but only at low levels in grape berries. They are of interest because of their role as precursors to C13–norisoprenoid aroma compounds (Chapters 8 and 23.1). Grapes contain 5 major carotenoids, and at ripeness have total carotenoid levels in the range of 0.4 to 2.5 mg/kg [32].
Isoprenoids are a large class of substances that include monoterpenoids, sesquiterpenoids, and C13–norisoprenoids, and many are important aroma contributors in wine (Chapter 8). C13–Norisoprenoid accumulation starts shortly after veraison, or shortly after carotenoid degradation completes, and peaks within a few weeks [33]. Accumulation of other isoprenoids starts a few weeks after veraison and can continue well past commercial ripeness. While many isoprenoids – particularly C13–norisoprenoids – exist as non‐volatile glycosides, a fraction of monoterpenoids exists in free form, accounting for the distinctive floral aroma of Muscat‐type grapes (Chapter 8) [34]. The amounts of these substances vary widely depending on grape variety for vinifera vines. For instance, grapes of the Muscat family have high levels, in the range of 1–6 mg/kg of bound and free monoterpenoids at ripeness [35], with free linalool and geraniol well in excess of sensory thresholds (Chapter 8). Other varieties, such as Riesling, have perithreshold concentrations (0.05–0.2 mg/kg total), and most vinifera varieties have levels that are too low to affect sensory perception.
20.9 Insoluble materials
Insoluble grape tissues, including the skin and seeds, are present in grapes but these components are not extracted into wine. On average, seeds comprise approximately 4% of grape weight and skins about 11% [16]. A total analysis of grape skins showed that on a dry weight basis, 22.6% of the grape skin was insoluble in strong sulfuric acid [36]. The authors studied the residue by solid phase NMR and suggested that it was composed of cellulose and waxy material, and did not contain lignin. Other studies of pomace have yielded similar results, showing that in skins from white pomace, soluble sugars compose a large fraction of the mass, approximately 50%, while the insoluble polysaccharides are about 25% of the dry matter. On the other hand, in red pomace, the soluble sugars are only a few percent of the total while insoluble polysaccharides are 50% of the dry matter. Tannins and other polyphenols comprise most of the remaining characterized material [37]. About half of the mass of seeds results from polysaccharides, referred to as neutral detergent fiber (NDF), but due to the highly lignified state of this material, it is not very useful as a source of calories (in feeding animals, for example). The seeds also contain about 12% crude protein, 12% fat, and 5% ash, largely composed of minerals including potassium, calcium, phosphate, sulfate, and magnesium [38].
References
- 1. Pagay, V. and Cheng, L. (2010) Variability in berry maturation of Concord and Cabernet franc in a cool climate. American Journal of Enology and Viticulture, 61 (1), 61–67.
- 2. Andaur, J.E., Guesalaga, A.R., Agosin, E.E., et al. (2004) Magnetic resonance imaging for nondestructive analysis of wine grapes. Journal of Agricultural and Food Chemistry, 52 (2), 165–170.
- 3. Zhang, P., Barlow, S., Krstic, M., et al. (2015) Within‐vineyard, within‐vine, and within‐bunch variability of the rotundone concentration in berries of Vitis vinifera L. cv. Shiraz. Journal of Agricultural and Food Chemistry, 63 (17), 4276–4283.
- 4. Wolpert, J.A. and Howell, G.S. (1984) Sampling Vidal Blanc grapes. II. Sampling for precise estimates of soluble solids and titratable acidity of juice. American Journal of Enology and Viticulture, 35 (4), 242–246.
- 5. Meyers, J.M. and Vanden Heuvel, J.E. (2014) Use of normalized difference vegetation index images to optimize vineyard sampling protocols. American Journal of Enology and Viticulture, 65 (2), 250–253.
- 6. Bravdo, B., Hepner, Y., Loinger, C., et al. (1984) Effect of crop level on growth, yield and wine quality of a high yielding carignane vineyard. American Journal of Enology and Viticulture, 35 (4), 247–252.
- 7. McIntyre, G.N., Kliewer, W.M., Lider, L.A. (1987) Some limitations of the degree day system as used in viticulture in California. American Journal of Enology and Viticulture, 38 (2), 128–132.
- 8. Ducasse, M.A., Canal‐Llauberes, R.M., de Lumley, M., et al. (2010) Effect of macerating enzyme treatment on the polyphenol and polysaccharide composition of red wines. Food Chemistry, 118 (2), 369–376.
- 9. Diaz‐Rubio, M.E. and Saura‐Calixto, F. (2006) Dietary fiber in wine. American Journal of Enology and Viticulture, 57 (1), 69–72.
- 10. Kliewer, W.M. (1966) Sugars and organic acids of Vitis vinifera. Plant Physiology, 41 (6), 923.
- 11. Lakso, A.N. and Kliewer, W.M. (1975) Influence of temperature on malic acid metabolism in grape berries. 1. Enzyme responses. Plant Physiology, 56 (3), 370–372.
- 12. Singleton, V.L. and Esau, P. (1969) Phenolic substances in grapes and wine, and their significance, Academic, New York.
- 13. Price, S.F., Breen, P.J., Valladao, M., Watson, B.T. (1995) Cluster sun exposure and quercetin in Pinot noir grapes and wine. American Journal of Enology and Viticulture, 46, 187–194.
- 14. Mattivi, F., Guzzon, R., Vrhovsek, U., et al. (2006) Metabolite profiling of grape: flavonols and anthocyanins. Journal of Agricultural and Food Chemistry, 54 (20), 7692–7702.
- 15. Kennedy, J.A., Matthews, M.A., Waterhouse, A.L. (2000) Changes in grape seed polyphenols during fruit ripening. Phytochemistry, 55 (1), 77–85.
- 16. Travaglia, F., Bordiga, M., Locatelli, M., et al. (2011) Polymeric proanthocyanidins in skins and seeds of 37 Vitis vinifera L. cultivars: a methodological comparative study. Journal of Food Science, 76 (5), C742–C749.
- 17. Cheynier, V., Souquet, J.M., Moutounet, M. (1989) Glutathione content and glutathione to hydroxycinnamic acid ratio in Vitis vinifera grapes and musts. American Journal of Enology and Viticulture, 40, 320–324.
- 18. Huang, Z. and Ough, C.S. (1991) Amino‐acid profiles of commercial grape juices and wines. American Journal of Enology and Viticulture, 42 (3), 261–267.
- 19. Negri, A.S., Prinsi, B., Scienza, A., et al. (2008) Analysis of grape berry cell wall proteome: a comparative evaluation of extraction methods. Journal of Plant Physiology, 165 (13), 1379–1389.
- 20. Macheix, J.J., Sapis, J.C., Fleuriet, A. (1991) Phenolic‐compounds and polyphenoloxidase in relation to browning in grapes and wines. Critical Reviews in Food Science and Nutrition, 30 (4), 441–486.
- 21. Vincenzi, S., Tolin, S., Cocolin, L., et al. (2012) Proteins and enzymatic activities in Erbaluce grape berries with different response to the withering process. Analytica Chimica Acta, 732, 130–136.
- 22. Salzman, R.A., Tikhonova, I., Bordelon, B.P., et al. (1998) Coordinate accumulation of antifungal proteins and hexoses constitutes a developmentally controlled defense response during fruit ripening in grape. Plant Physiology, 117 (2), 465–472.
- 23. Fracassetti, D., Lawrence, N., Tredoux, A.G.J., et al. (2011) Quantification of glutathione, catechin and caffeic acid in grape juice and wine by a novel ultra‐performance liquid chromatography method. Food Chemistry, 128 (4), 1136–1142.
- 24. Smit, I., Pfliehinger, M., Binner, A., et al. (2014) Nitrogen fertilisation increases biogenic amines and amino acid concentrations in Vitis vinifera var. Riesling musts and wines. Journal of the Science of Food and Agriculture, 94 (10), 2064–2072.
- 25. Gallander, J.F. and Peng, A.C. (1980) Lipid and fatty‐acid compositions of different grape types. American Journal of Enology and Viticulture, 31 (1), 24–27.
- 26. Pensec, F., Paczkowski, C., Grabarczyk, M., et al. (2014) Changes in the triterpenoid content of cuticular waxes during fruit ripening of eight grape (Vitis vinifera) cultivars grown in the Upper Rhine Valley. Journal of Agricultural and Food Chemistry, 62 (32), 7998–8007.
- 27. Grncarevic, M. and Radler, F. (1971) Review of surface lipids of grapes and their importance in drying process. American Journal of Enology and Viticulture, 22 (2), 80–86.
- 28. US Department of Agriculture (2014) A.R.S. USDA National Nutrient Database for Standard Reference, Release 27, http://www.ars.usda.gov/ba/bhnrc/ndl.
- 29. Leske, P.A., Sas, A.N., Coulter, A.D., et al. (1997) The composition of Australian grape juice: chloride, sodium and sulfate ions. Australian Journal of Grape and Wine Research, 3 (1), 26–30.
- 30. Ough, C.S. and Amerine, M.A. (1988) Methods for analysis of musts and wines, 2nd edn, Wiley‐Interscience, New York.
- 31. Hagen, K.M., Keller, M., Edwards, C.G. (2008) Survey of biotin, pantothenic acid, and assimilable nitrogen in winegrapes from the Pacific Northwest. American Journal of Enology and Viticulture, 59 (4), 432–436.
- 32. Oliveira, C., Ferreira, A.C.S., Pinto, M.M., et al. (2003) Carotenoid compounds in grapes and their relationship to plant water status. Journal of Agricultural and Food Chemistry, 51 (20), 5967–5971.
- 33. Ryona, I. and Sacks, G.L. (2013) Behavior of glycosylated monoterpenes, C13‐norisoprenoids, and benzenoids in Vitis vinifera cv. Riesling during ripening and following hedging, in Carotenoid cleavage products, American Chemical Society, pp. 109–124.
- 34. Hjelmeland, A.K. and Ebeler, S.E. (2015) Glycosidically bound volatile aroma compounds in grapes and wine: a review. American Journal of Enology and Viticulture, 66 (1), 1–11.
- 35. Mateo, J.J. and Jimenez, M. (2000) Monoterpenes in grape juice and wines. Journal of Chromatography A, 881 (1–2), 557–567.
- 36. Mendes, J.A.S., Prozil, S.O., Evtuguin, D.V., Lopes, L.P.C. (2013) Towards comprehensive utilization of winemaking residues: characterization of grape skins from red grape pomaces of variety Touriga Nacional. Industrial Crops and Products, 43 (0), 25–32.
- 37. Deng, Q., Penner, M.H., Zhao, Y. (2011) Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Research International, 44 (9), 2712–2720.
- 38. Spanghero, M., Salem, A.Z.M., Robinson, P.H. (2009) Chemical composition, including secondary metabolites, and rumen fermentability of seeds and pulp of Californian (USA) and Italian grape pomaces. Animal Feed Science and Technology, 152 (3–4), 243–255.