29
Optimizing White Wine Aromas
29.1 Introduction
Wine producers often speak about making the “best wine,” but such statements obscure the fact that consumers (and critics) do not have uniform opinions in their wine preferences. For example, Sauvignon Blanc wines can possess a diverse range of aromas (e.g., tropical/fruity versus green/herbaceous), and controlled studies have identified specific consumer segments that prefer each of these styles [1]. “Optimization” of aromas or other sensory attributes of a wine – whether by changing vineyard practices, the winemaking process, or even packaging decisions – first requires defining a desired outcome, which is often one that the winemaker believes will lead to better sales.
Because of legal restrictions regarding additives and production practices (Chapter 27) the chemistry of a finished wine is closely related to the initial grape composition, which will in turn be dependent on grape variety, growing region, and viticultural practices. Despite the marketing parlance that the best winemakers “let the fruit speak for itself,” winemakers have a high level of influence over wine quality and style, and research has been conducted into all manner of aspects, from harvest decisions to pre‐fermentation operations and adjustment of juice/must composition, and then to fermentation and storage conditions (Chapters 21 to 25).
Several techniques for optimizing wine aromas – particularly white wine aromas – have been described throughout the book, such as the practice of fermenting at low temperatures to increase “fruity” aromas due to esters (Chapter 22.1) and minimizing oxygen exposure post‐fermentation to avoid oxidation of varietal thiols that impart tropical aromas (Chapter 24). Selection of yeast (and bacteria) will also critically affect many white wine sensory attributes (e.g., References [2] to [8]). This chapter focuses on one emerging research area within this broad topic – optimization of white wine aromas through yeast selection, with particular emphasis on varietal thiols.
29.2 Enhancement of varietal thiols
The highly potent odorants known as polyfunctional (varietal) thiols contribute to the tropical aromas of many wines, and particularly Sauvignon Blanc (Chapter 10). As discussed previously, these thiols appear to be formed by microbial metabolism of grape‐derived, non‐volatile precursors (S‐conjugates) in the juice (Chapter 23.2). Certain consumer segments prefer Sauvignon Blanc with strong tropical aromas and less intense green/herbaceous aromas [1, 9]. As noted by Swiegers et al., vineyard management is effective at controlling the primary odorants responsible for herbaceous aromas (particularly methoxypyrazines, Chapter 5), but the secondary aromas of “tropical”‐smelling varietal thiols and other compounds are controlled through fermentation parameters [10]. Because only a small fraction of the S‐conjugate pool is liberated during a typical fermentation (Chapter 23.2), there has been considerable interest in characterizing the ability of various yeast strains to release different varietal thiols during fermentation [10–12].
As shown in Figure 29.1, yeast strains can vary both in the total amount of key varietal thiols they produce (3‐mercaptohexan‐1‐ol [3‐MH], 3‐mercaptohexyl acetate [3‐MHA], and 4‐mercapto‐4‐methylpentan‐2‐one [4‐MMP]), but also in their relative concentrations. This information can allow winemakers to optimize production of wines like Sauvignon Blanc with certain characteristics, that is, more or less 3‐MHA (“passionfruit”) as compared to 4‐MMP (“cat pee”), depending on the targeted style. Due to the relative stability of many of the thiols under reductive conditions, the sensory effects of yeast strain can still be evident after some years of bottle aging [13].
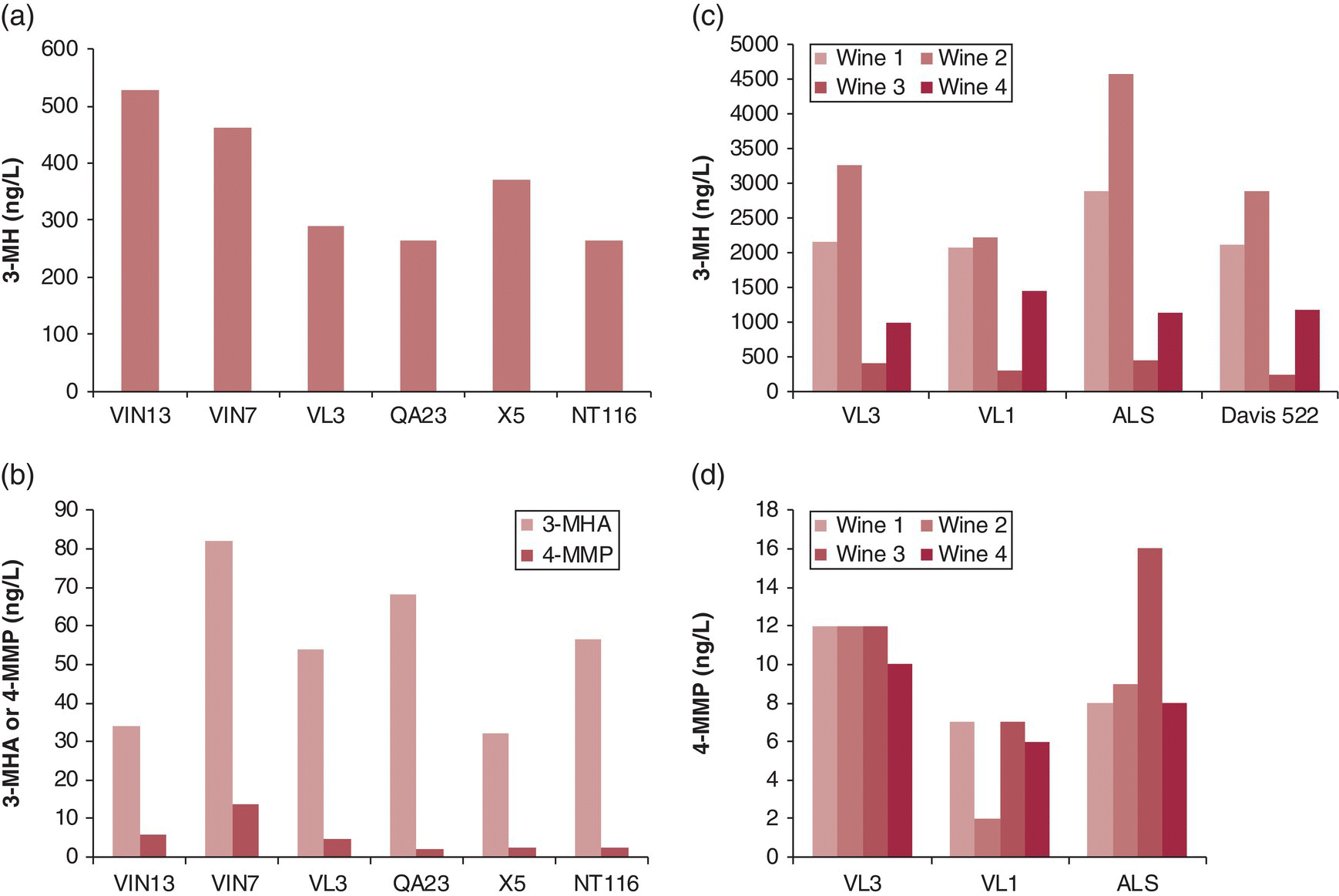
Figure 29.1 Influence of yeast strain on varietal thiol concentrations for (a and b) a single Sauvignon Blanc juice fermented on a 20 L scale (left panel), and (c and d) four different Sauvignon Blanc juices fermented on a 225 L scale (4‐MMP was undetectable in each wine using Davis 522 strain, right panel).
Data from References [10] and [12]
Key enzymes involved in the liberation of varietal thiols during fermentation have been identified through studies of genetically modified (GM) yeasts, and non‐commercial GM yeasts overexpressing certain enzymes can produce greater amounts of varietal thiols (Chapter 23.2). This knowledge has helped drive research in molecular breeding1 to produce yeasts with superior varietal thiol production in commercial wine strains [14, 15].
29.3 Cofermentation and spontaneous fermentation
The genetic diversity of commercial S. cerevisiae yeast strains provides tools to optimize wine aroma attributes. However, an even greater diversity may be accessible through the myriad indigenous yeasts (including non‐Saccharomyces) present in and around wineries and vineyards. Winemakers may choose not to inoculate with a commercial yeast, but instead execute a spontaneous (or wild) fermentation (more properly, a spontaneous cofermentation, since multiple species/strains will be present).2 Alternatively, commercial non‐Saccharomyces yeasts – or hybrids of Saccharomyces and non‐Saccharomyces yeasts – may be employed alone or in cofermentation with conventional yeasts [16–20].
The use of spontaneous fermentation or commercial non‐Saccharomyces yeasts often produces sensorially distinct wines.3 These wines are not necessarily appealing to a broad range of consumers – the use of “native” Saccharomyces isolated from oak trees yielded wines with strong sulfurous aromas and low fruitiness [21]. However, other work with spontaneous or non‐Saccharomyces fermentations has shown characteristics that would be appealing to particular segments, such as greater wine complexity or increased intensity of certain fruity aromas [18, 20, 22]. Unsurprisingly, sensory differences often appear to be related to differences in common fermentation‐derived metabolites (e.g., fatty acid ethyl esters, acetate esters, higher alcohols, fatty acids), but grape‐derived aroma compounds can also be influenced. A comparison of three indigenous yeast strains (inoculated separately) and a spontaneous fermentation using Albariño juice showed that one strain in particular produced substantially higher amounts of C13‐norisoprenoids (β‐damascenone and β‐ionone) and monoterpenoids (geraniol and linalool), yielding a wine that was rated highest in quality by a sensory panel [23].
Cofermentations have also been demonstrated to result in unexpected outcomes due to interactions between different yeasts. In one study, Sauvignon Blanc wines produced by coinoculated commercial S. cerevisiae and non‐Saccharomyces natural isolates were compared to the same wines produced from yeast monocultures (Figure 29.2). Concentrations of grape‐derived compounds (C13‐norisoprenoids, monoterpenoids, and varietal thiols) produced from cofermentation were not necessarily intermediary to other values, for example, β‐damascenone production was double in an Mp‐Sc cofermentation as compared to monocultures of Mp or Sc. However, in some cases monocultures stood out as higher producers, especially Mp and Sc for 3‐MH and 3‐MHA, and Cz for linalool, geraniol, and β‐damascenone (Figure 29.2) [24]. Significantly, differences in varietal thiol production (and sensory characters) have also been observed for Sauvignon Blanc fermented with combinations of two or three commercial S. cerevisiae strains [25]. Considering that many yeast suppliers offer over 30 strains, winemakers potentially have thousands of possible combinations to explore even if they limit themselves to no more than three strains per cofermentation. Future work is expected to lead to a better molecular understanding of why yeast strain interactions occur, and how they can be employed by winemakers to optimize for particular outcomes.
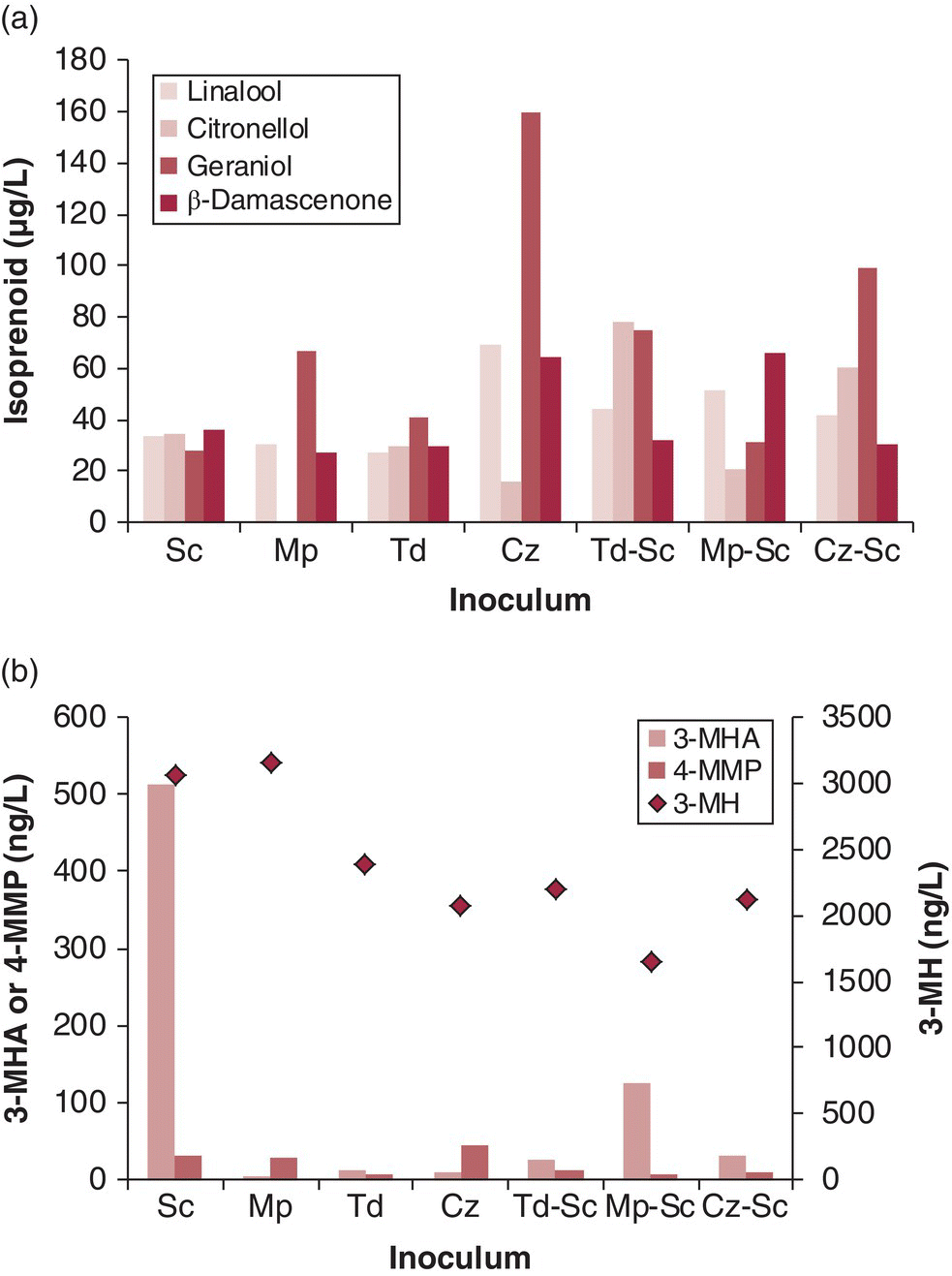
Figure 29.2 Influence of yeast species and coinoculation on concentrations of (a) monoterpenoids and β‐damascenone, and (b) varietal thiols, in fermentations of Sauvignon Blanc juice. Sc, Saccharomyces cerevisiae; Mp, Metchnikowia pulcherrima; Td, Torulaspora delbrueckii; Cz, Candida zemplinina.
Selected data from Reference [24]
References
- 1. King, E.S., Osidacz, P., Curtin, C., et al. (2011) Assessing desirable levels of sensory properties in Sauvignon Blanc wines – consumer preferences and contribution of key aroma compounds. Australian Journal of Grape and Wine Research , 17 (2), 169–180.
- 2. Lambrechts, M.G. and Pretorius, I.S. (2000) Yeast and its importance to wine aroma – a review. South African Journal for Enology and Viticulture , 21, 97–129.
- 3. Swiegers, J.H., Bartowsky, E.J., Henschke, P.A., Pretorius, I.S. (2005) Yeast and bacterial modulation of wine aroma and flavour. Australian Journal of Grape and Wine Research , 11 (2), 139–173.
- 4. Swiegers, J.H. and Pretorius, I.S. (2007) Modulation of volatile sulfur compounds by wine yeast. Applied Microbiology and Biotechnology , 74 (5), 954–960.
- 5. Lerm, E., Engelbrecht, L., du Toit, M. (2010) Malolactic fermentation: the ABCʼs of MLF. South African Journal of Enology and Viticulture , 31 (2), 186–212.
- 6. Cordente, A., Curtin, C., Varela, C., Pretorius, I. (2012) Flavour‐active wine yeasts. Applied Microbiology and Biotechnology , 96 (3), 601–618.
- 7. Steyer, D., Ambroset, C., Brion, C., et al. (2012) QTL mapping of the production of wine aroma compounds by yeast. BMC Genomics , 13 (1), 573.
- 8. Styger, G., Jacobson, D., Prior, B., Bauer, F. (2013) Genetic analysis of the metabolic pathways responsible for aroma metabolite production by Saccharomyces cerevisiae. Applied Microbiology and Biotechnology , 97 (10), 4429–4442.
- 9. Lund, C.M., Thompson, M.K., Benkwitz, F., et al. (2009) New Zealand Sauvignon Blanc distinct flavor characteristics: sensory, chemical, and consumer aspects. American Journal of Enology and Viticulture , 60 (1), 1–12.
- 10. Swiegers, J.H., Kievit, R.L., Siebert, T., et al. (2009) The influence of yeast on the aroma of Sauvignon Blanc wine. Food Microbiology , 26 (2), 204–211.
- 11. Dubourdieu, D., Tominaga, T., Masneuf, I., et al. (2006) The role of yeasts in grape flavor development during fermentation: the example of Sauvignon Blanc. American Journal of Enology and Viticulture , 57 (1), 81–88.
- 12. Murat, M.‐L., Masneuf, I., Darriet, P., et al. (2001) Effect of Saccharomyces cerevisiae yeast strains on the liberation of volatile thiols in Sauvignon Blanc wine. American Journal of Enology and Viticulture , 52 (2), 136–139.
- 13. King, E.S., Francis, I.L., Swiegers, J.H., Curtin, C. (2011) Yeast strain‐derived sensory differences retained in Sauvignon Blanc wines after extended bottle storage. American Journal of Enology and Viticulture , 62 (3), 366–370.
- 14. Dufour, M., Zimmer, A., Thibon, C., Marullo, P. (2013) Enhancement of volatile thiol release of Saccharomyces cerevisiae strains using molecular breeding. Applied Microbiology and Biotechnology , 97 (13), 5893–5905.
- 15. Pretorius, I.S., Curtin, C.D., Chambers, P.J. (2015) Designing wine yeast for the future, in Advances in fermented foods and beverages (ed. Holzapfel, W.), Woodhead Publishing, Cambridge, UK, pp. 197–226.
- 16. Varela, C., Siebert, T., Cozzolino, D., et al. (2009) Discovering a chemical basis for differentiating wines made by fermentation with “wild” indigenous and inoculated yeasts: role of yeast volatile compounds. Australian Journal of Grape and Wine Research , 15 (3), 238–248.
- 17. Saberi, S., Cliff, M.A., van Vuuren, H.J.J. (2012) Impact of mixed S. cerevisiae strains on the production of volatiles and estimated sensory profiles of Chardonnay wines. Food Research International , 48 (2), 725–735.
- 18. Medina, K., Boido, E., Fariña, L., et al. (2013) Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co‐fermentation with Hanseniaspora vineae. Food Chemistry , 141 (3), 2513–2521.
- 19. Bellon, J.R., Schmid, F., Capone, D.L., et al. (2013) Introducing a new breed of wine yeast: interspecific hybridisation between a commercial Saccharomyces cerevisiae wine yeast and Saccharomyces mikatae. PLoS One , 8 (4), e62053.
- 20. Azzolini, M., Tosi, E., Lorenzini, M., et al. (2015) Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World Journal of Microbiology and Biotechnology , 31 (2), 277–293.
- 21. Hyma, K.E., Saerens, S.M., Verstrepen, K.J., Fay, J.C. (2011) Divergence in wine characteristics produced by wild and domesticated strains of Saccharomyces cerevisiae. FEMS Yeast Research , 11 (7), 540–551.
- 22. Soden, A., Francis, I.L., Oakey, H., Henschke, P.A. (2000) Effects of co‐fermentation with Candida stellata and Saccharomyces cerevisiae on the aroma and composition of Chardonnay wine. Australian Journal of Grape and Wine Research , 6 (1), 21–30.
- 23. Carrascosa, A.V., Bartolome, B., Robredo, S., et al. (2012) Influence of locally‐selected yeast on the chemical and sensorial properties of Albariño white wines. LWT – Food Science and Technology , 46 (1), 319–325.
- 24. Sadoudi, M., Tourdot‐Maréchal, R., Rousseaux, S., et al. (2012) Yeast–yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co‐culture of non‐Saccharomyces and Saccharomyces yeasts. Food Microbiology , 32 (2), 243–253.
- 25. King, E.S., Kievit, R.L., Curtin, C., et al. (2010) The effect of multiple yeasts co‐inoculations on Sauvignon Blanc wine aroma composition, sensory properties and consumer preference. Food Chemistry , 122 (3), 618–626.