Chapter 9
Animal Memory
Morphic resonance and memory
The hypothesis of formative causation provides a radical reinterpretation of the nature of memory. It proposes that memory is inherent in all organisms in two related ways. First, all organisms inherit a collective memory of their species by morphic resonance from previous organisms. Second, individual organisms are subject to morphic resonance from themselves in the past (see above), and this self-resonance provides the basis for their own individual memories and habits.
As we have seen in Chapter 8, according to this hypothesis patterns of behaviour are organized by nested hierarchies of behavioural fields, just as patterns of morphogenesis are organized by nested hierarchies of morphogenetic fields. These behavioural fields organize the activities of the nervous system by imposing spatio-temporal patterns on its inherently indeterminate or probabilistic functioning.
Just as appropriate genes are necessary for normal morphogenesis, so an appropriate nervous system is necessary for normal behaviour. Chemical or physical disturbances of the nerves can affect behaviour, just as disturbances of genes and proteins can affect morphogenesis. But behaviour is no more programmed in the nervous system than morphogenesis is programmed in the genes.
According to the hypothesis of formative causation there is only a difference of degree, not of kind, between inherited and learned behaviour. Both depend on morphic fields stabilized by morphic resonance. In instinctive behaviour, such as the building of nests by Paralastor wasps (Fig. 8.9), the influence of many other similar insects predominates; whereas in learned behaviour, such as the learning of the way out of a maze by a rat, resonance from an animal’s own past is more important. Usually both play a part: instinctive behaviour involves an element of adaptation to the animal’s particular circumstances, and learned behaviour takes place within the framework of potentialities provided by the species’ morphic fields.
Learning inevitably involves memory; the influence of past experience on present behaviour would not be possible if the experience were not in some way retained. There is of course no need for memory to involve consciousness: we ourselves are influenced by many unconscious memories that are expressed in our habits. We remember how to swim, write, or ride bicycles, but these habit memories are not conscious. There is no reason to assume that the habit memories we see at work in animals are any more conscious than our own.
Memory is conventionally believed to be explicable in terms of physico-chemical modifications of the nervous system, the ‘traces’ of past experience. Attempts to locate such traces within the brain and to analyse them have so far been unsuccessful; but from the point of view of the mechanistic theory memory must depend on material traces of some kind. This is an a priori assumption. As Steven Rose expressed it:
Memories are in some way ‘in’ the mind, and therefore, for a biologist, also ‘in’ the brain. But how? The term memory must include at least two separate processes. It must involve, on the one hand, that of learning something new about the world around us; and on the other, at some later date, recalling, or remembering that thing. We infer that what lies between the learning and the remembering must be some permanent record, a memory trace, within the brain.1
By contrast, through formative causation, memory depends on morphic resonance between patterns of activity within the nervous system now and similar patterns of activity in the past. It need not depend on physico-chemical modifications of the nerves. Memory need not be stored in material memory traces if it results from morphic resonance; the past can exert a direct influence on the present.
In this chapter, we first examine the evidence for memory storage within the brain; we then consider different types of learning, comparing the orthodox mechanistic interpretations with those of morphic resonance. Finally, we discuss what kinds of experiments could be done to find out which of these alternative approaches is in closer accordance with the way memory really works.
Are memories stored inside the brain?
The traditional idea of memory storage within the brain goes back to classical times. Stimuli falling on the sense organs produce disturbances in the brain, which cause the perception of the stimuli. The disturbances leave behind traces, minute changes in the structure of the brain. As a result of these changes, brain activity becomes more likely to follow the same paths again in response to stimuli that are similar or whose traces are intermingled or ‘associated’ with those of the first stimulus.
In the seventeenth century, Descartes proposed a hydraulic version of this theory, based on the assumption that nerves are hollow and conduct a flow of ‘animal spirits’: sensory nerves contain delicate threads attached to valves within the brain, the opening of which releases animal spirits, which pass through the nerves to appropriate muscles. Descartes in fact invented the concept of the reflex: animal spirits are ‘reflected’ in the brain and pass back to the muscles (Fig. 9.1).2 The memory traces ‘are nothing else than the circumstances that the pores of the brain through which the spirits have already taken their course on presentation of the object, have thereby acquired a greater facility than the rest to be opened again the same way by the spirits which come to them; so that these spirits coming upon the pores enter therein more readily than into the others.’3 This idea has the great attraction of simplicity, and is echoed in the modern theories of synaptic modification.
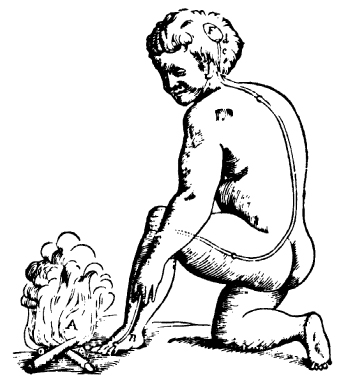
Figure 9.1 The diagram of a kneeling man by a fire used by Descartes to illustrate his idea of reflex action. (As reproduced by Boakes, 1984)
Pavlov’s famous researches on conditioned reflexes greatly strengthened the traditional concept of memory traces. Pavlov himself was reluctant to claim that reflex arcs depended on specifically localized traces within the cerebral cortex because he found that the conditioning could survive considerable surgical damage to the brain.4 But some of those who followed him were less cautious. In the first few decades of the twentieth century it was assumed by many biologists that all psychological activity, including the phenomena of the human mind, could ultimately be reduced to simple associations and chains of reflexes. The path of the reflex circuits was supposed to run from the sense organs to the sensory areas of the brain, thence through associative areas to the motor cortex, and finally to the motor cells, which conducted impulses to the muscles.5 These definite pathways of connection were often conceived by analogy with a telephone system, with the nerve fibres as wires and the brain as the exchange where appropriate connections were made.
Modern theories usually rely on computer analogies. The central model is of coding, storage, and retrieval. Incoming nerve impulses from the sense organs are usually said to ‘encode’ the external stimulus, and these then change the properties of other nerve cells within the brain so that these changes encode or ‘represent’ the stimulus, but in some different way. These changes constitute the process of memory storage. Retrieval is the supposed process by which the stored pattern is revived as needed.
The complexity of computers means that this model can be developed in a more sophisticated way than the telephone model, but it still depends on definite memory traces, even if there are ‘back-up’ storage systems. In computers the ‘traces’ are carried either in the solid-state memory or on discs. If in a real computer the memory store is destroyed, then the memory is, of course, lost.
Much effort has been expended in an attempt to locate memory traces within the brain, and vast numbers of animals have been used up in the process. The classic investigations on the subject were made by Karl Lashley with rats, monkeys, and chimpanzees. For over thirty years he tried to trace conditioned reflex paths through the brain and to find the locus of specific memory traces, or ‘engrams’. To do this, he trained the animals in a variety of tasks, ranging from simple conditioned reflexes to the solution of difficult problems. Either before or after the training, he surgically cut nerve tracts within the brain or removed portions of the brain, and then measured the effects on initial learning or post-operative retention.
Lashley first became sceptical of the supposed path of conditioned reflex arcs through the motor cortex when he found that rats trained to respond in particular ways to light showed no reduction in accuracy of performance when nearly the entire motor cortex was cut out. In similar experiments with monkeys, he removed most of the motor cortex after they had been trained to open various latch boxes. This operation resulted in a temporary paralysis, but after eight to twelve weeks the monkeys recovered sufficiently to be able to make the movements required to open the latches. They were then exposed to the puzzle boxes, and opened them promptly without random exploratory movements.
Lashley then showed that learned habits were retained if the associative areas of the brain were destroyed. Habits also survived a series of deep incisions into the cerebral cortex that destroyed cross-connections within it. Moreover, if the cerebral cortex was intact, removal of subcortical structures such as the cerebellum did not destroy the memory either.
Lashley started as an enthusiastic supporter of the reflex theory of learning, but was forced by his results to abandon it:
The original programme of research looked toward the tracing of conditioned-reflex arcs throughout the cortex, as the spinal paths of simple reflexes seemed to have been traced through the cord. The experimental findings have never fitted into such a scheme. Rather, they have emphasized the unitary character of every habit, the impossibility of stating any learning as concatenations of reflexes, and the participation of large masses of nervous tissue in the functions rather than the development of restricted conduction paths.6
In reviewing the types of human memory loss that follow brain damage, he came to a similar conclusion:
I believe that the evidence strongly favours the view that amnesia from brain injury rarely, if ever, is due to the destruction of specific memory traces. Rather, the amnesias represent a lowered level of vigilance, a greater difficulty in activating the organized pattern of traces, or a disturbance of some broader system of organized functions.7
Lashley did not consider the possibility that memories might not be stored inside the brain at all. He suggested that rather than localized traces, there must be multiple memory traces throughout entire functional areas of the brain. He thought that this indicated that ‘the characteristics of the nervous network are such that when it is subject to any pattern of excitation, it may develop a pattern of activity, reduplicated through an entire functional area by spread of excitations, such as the surface of a liquid develops an interference pattern of spreading waves when it is disturbed at several points.’ He suggested that recall involved ‘some sort of resonance among a very large number of neurons’.8 These ideas were carried further by his former student Karl Pribram in his proposal that memories are stored in a distributed manner analogous to the interference patterns in a hologram.9
Analogous experiments have shown that even in invertebrates such as the octopus, specific memory traces cannot be localized. Observations on the survival of learned habits after destruction of various parts of the brain have led to the seemingly paradoxical conclusion that ‘memory is both everywhere and nowhere in particular’.10
The conventional response to such findings is that there must be multiple memory-storage systems distributed throughout various regions of the brain: if some are lost, back-up systems can take over. This hypothesis, invented to account for the failure of attempts to find localized memory traces, follows naturally from the assumption that memories must be stored somehow inside the brain; but in the continuing absence of any direct evidence, it remains more a matter of faith than of fact.
There is, however, good evidence that changes can occur in the brains of young animals as a result of the way they grow up. In one experiment, for example, young rats were raised either in solitary confinement in featureless cages, or in groups in larger cages furnished with a variety of playthings, which were regularly replaced with novel ones. After various periods of time, rats from both environments were killed and their brains examined. Those that had grown up in the enriched environment had bigger brains than those in solitary confinement, and the individual nerve cells and synapses were larger.11 These results show that the development of the nervous system was influenced by its activity.
Experiments with young monkeys led to a similar conclusion. They were deprived of the use of one eye by stitching the eyelids together to find out how this would affect the development of their brains. In normal adults, the right and left visual cortices of the brain receive nervous input from both eyes. Thus in the left visual cortex there are two orderly maps of the right half of the visual field, one received from the right eye and the other from the left; likewise in the right visual cortex there are two maps of the left half of the visual field. The input from the two eyes is segregated in a pattern of alternating cortical strips about 0.4 millimetres wide. Young monkeys with one eye stitched up became blind in that eye after several weeks, and the strips connected with it became very narrow while those connected with the other eye expanded to take up almost all the space. Experiments on kittens gave similar results.
These changes appeared to be due to competition between the nerves connected to the two eyes: inactive nerves linked to the closed eye made fewer connections with the cortical cells than the electrically active nerves from the other eye.12 As with rats raised in an enriched environment, these results show that the development of the nervous system depended on the activity of the nerves within it.
It is not surprising that changes in the functioning of the nervous system are associated with changes in the nerve cells themselves; we all know that changes in muscles occur as a result of use or disuse. Body-builders show us how far such changes can go. The fact that such changes occur in developing brains emphasizes that the nervous system, like the muscular system, is dynamic in its structure.
Steven Rose and his colleagues carried out some of the most careful and thorough attempts to study changes in the brain as learning took place in young chicks. A day after hatching, the chicks underwent simple forms of training, and Rose and his team found that nerve cells in a region of the left forebrain underwent more active growth and development when learning took place than when it did not.13 In chicks, as in the growing brains of young rats, kittens, and monkeys, nerve cells that were active developed more than those that were inactive. But this greater development of active cells did not prove that they contained specific memory traces. When the region of the left forebrain associated with the learning process was removed a day after training, the chicks could still remember what they had learned. Therefore the cells that involved in the learning process were not necessary for the retention of memory. Again, the hypothetical memory traces proved to be elusive, and once more those who searched for them were forced to postulate unidentified ‘storage systems’ somewhere else in the brain.14
In mice and other mammals, the formation of memories when they learn to negotiate a maze involves activity in median temporal lobes of the brain, particularly in the region known as the hippocampus. The ability to form long-term memories depends on a process called long-term potentiation, involving protein synthesis in the nerve cells of the hippocampus. This process has been described in impressive detail. But yet again, the memories proved elusive. The destruction of the hippocampus on both sides of the brain prevented the formation of new long-term memories, but older memories remained. Thus it had to be assumed that the hypothetical memory traces somehow moved from one part of the brain to another.
Eric Kandel, who was awarded the Nobel Prize in 2000 for his work on memory storage, summarized the problem as follows:
How do different regions of the hippocampus and the median temporal lobe … interact in the storage of explicit memory? How is information in these regions transferred for ultimate consolidation in the neocortex? We do not, for example, understand why the initial storage of memory requires the hippocampus, whereas the hippocampus is not required once a memory has been stored for weeks or months. What critical information does the hippocampus convey to the neo-cortex? We also know very little about the recall of explicit (declarative) memory… These systems properties of the brain will require more than the bottom-up approach of molecular biology.15
Not only are the hypothetical memory traces elusive, but their physical nature is obscure. The idea of specific RNA ‘memory molecules’ was fashionable in the 1960s but has now been more or less abandoned. The theory of reverberating circuits of electrical activity, giving a kind of echo, may help to account for short-term memory over periods of seconds or minutes, but cannot plausibly explain long-term memory. The most popular hypothesis remains the old favourite that memory depends on modifications of the synaptic connections between nerve cells in a manner still unknown.
If memories are somehow stored in synapses, then the synapses themselves must remain stable over long periods of time: indeed, the nervous system as a whole must be stable if it is to act as a memory store. Until recently this was generally assumed to be the case, even though it has long been known that there is a continuous process of cell death within the brain. But recent evidence suggests that the nervous systems of mature animals are more dynamic than previously supposed.
Studies on canaries’ brains, and in particular those parts involved in the learning of song, have shown that many new connections between nerve cells continue to develop, and many new nerve cells appear even in older birds. In males the number of neurons increases in the spring, and decreases by about 40 per cent by the autumn. As the new mating season approaches, the number of nerve cells increases again. Such changes also occur in other parts of canaries’ brains. In adults of other species too there is a turnover of neurons in the forebrain, the ‘seat’ of complex behaviour and learning, with new cells being formed while others die.16
Brains are also more functionally dynamic than once thought. Studies on monkeys have shown that sensory areas of the brain that ‘map’ different parts of the body are not ‘hard wired’ or anatomically frozen, but are unexpectedly fluid. In one series of experiments the regions of the sensory cortex connected with touch sensations from the monkeys’ hands were localized. The ‘map’ in the brain was subdivided into regions for each of the five fingers and for other surfaces of the hand. After one or more of the fingers was amputated, the sensory input from the remaining adjacent fingers shifted over a period of weeks into the missing finger’s hitherto exclusive brain region (Fig. 9.2). The increased areas of brain connected to the adjacent fingers were associated with an increased acuity of sensation in these fingers.17
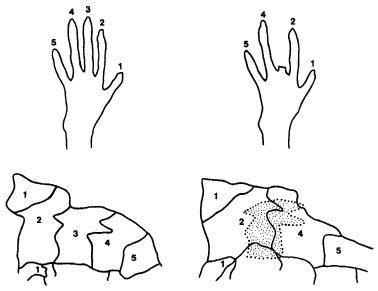
Figure 9.2 Brain maps of the area in the cortex of adult owl monkeys where the tactile inputs from the hand are received. Within several weeks of the amputation of the third finger, the area of the cortex in which it used to be represented is taken over by expanded areas representing the adjacent fingers. These brain maps were worked out by microelectrode analysis. (After Fox, ‘The Brain’s Dynamic Way of Keeping in Touch,’ in Science 225:820–821, 24 August 1984; copyright 1984 by the AAAS)
The dynamism of the nervous system is also shown when the brain itself is damaged. For example, if a portion of the sensory cortex is injured, the appropriate sensory ‘map’ that used to be in the injured region can shift to the region surrounding it, albeit with some loss in acuity. The movement of the ‘map’ probably does not depend on the growth of nerve cells, but rather on a spatial shift of nerve-cell activity.18
Changes in the structure and function of the nervous system present great difficulties for the memory-trace theory. At the molecular level too, as Francis Crick pointed out, the long-term storage of memory traces is problematic. The time span of human memory is often years or tens of years. ‘Yet it is believed that almost all the molecules in our bodies, with the exception of DNA, turn over in a matter of days, weeks, or at the most a few months. How then is memory stored in the brain so that its trace is relatively immune to molecular turnover?’ Crick suggested a mechanism whereby ‘molecules in the synapse interact in such a way that they can be replaced by new material, one at a time, without altering the overall state of the structure.’ His ingenious hypothetical scheme involved protein molecules endowed with a number of unusual properties. But there is no evidence that such molecules exist.19
An interpretation of memory in terms of morphic resonance offers a new approach to these problems. If memories depend on morphic fields, then they need not be stored within the brain at all, but may be given by morphic resonance from the organism’s own past. After damage to parts of the brain, these fields may be capable of organizing the nerve cells in other regions to carry out the same functions as before. The ability of learned habits to survive substantial brain damage may be due to the self-organizing properties of the fields – properties that are expressed in the realm of morphogenesis in regeneration and embryonic regulation.
This alternative to the conventional interpretation of memory can be distinguished from it by experiment, as discussed below. If the hypothesis of formative causation is correct, then it should be possible for the habit memories of one organism to influence another by morphic resonance, facilitating the acquisition of the same habits. Such an effect would not be expected on the basis of mechanistic theories of memory storage.
We now consider how the interpretation of memory in terms of morphic resonance from an animal’s own past applies in the context of learning. We start with the simplest and most basic type of learning: habituation.
Habituation
If a stimulus is harmless and not followed by anything of interest, less and less response occurs as it is repeated. This is known as habituation. We ourselves become habituated in many different ways: we cease to notice the contact of our clothes with our skin; we usually become unaware of background noises, background smells, or background objects; we get used to new environments and settle down in new situations.
Animals too become accustomed to their environments. They generally react to the appearance of something new precisely because they are not used to it, often with alarm or avoidance. But if the new stimulus is harmless they soon cease to respond. Probably everyone has observed this kind of habituation taking place in pets, as well as in wild mammals and birds.
Habituation also occurs in lower animals such as snails, and even in single-celled organisms. Stentor, for example, an inhabitant of marshy pools, is a trumpet-shaped cell covered with rows of fine, beating hairs called cilia. Ciliary activity sets up currents around the cell, carrying suspended particles to the mouth, which is at the bottom of a tiny vortex (Fig. 9.3). The response of these creatures to various stimuli was studied in detail by Herbert Jennings over a century ago and is described in his classic work The Behavior of the Lower Organisms (1906). When the object it is attached to is slightly jarred, ‘like a flash it contracts into its tube. In about half a minute it extends again, and the cilia resume their activity.’ If the same stimulus is repeated, it does not contract but continues its normal activities. This is not due to fatigue, since the animal still responds to a new stimulus, such as being touched. If this new stimulus is repeated, once again it does not react.
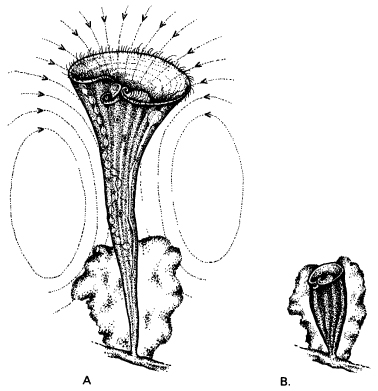
Figure 9.3 The single-celled organism Stentor raesilii, showing the currents in the water around it caused by the beating of its cilia. In response to an unfamiliar stimulus, it rapidly contracts into its tube (B). (After Jennings, 1906)
Habituation implies that harmless and irrelevant stimuli are recognized when they recur. In a unicellular organism like Stentor there are no nerves, so this kind of learning does not depend on a nervous system. It may well depend on the resonance of the organism with its own past patterns of activity, especially those in the recent past. These past patterns would include its return to normal following the response to the harmless stimulus. Repeated irrelevant stimuli are assimilated into the organism’s own background resonance; they become, as it were, part of itself. Conversely, any new kind of stimulus stands out precisely because it is new and unfamiliar. In more complex organisms habituation involves the nervous system, and has been studied in great detail in the giant marine slug Aplysia, which grows to be over a foot long. Normally the gill is extended, but it is withdrawn if the slug is touched (Fig. 9.4). This reflex soon ceases to take place if weak and harmless stimuli are repeated. (With stronger stimuli, the slug responds in an octopus-like way by releasing a brilliant purple ink that conceals it within an opaque cloud.)
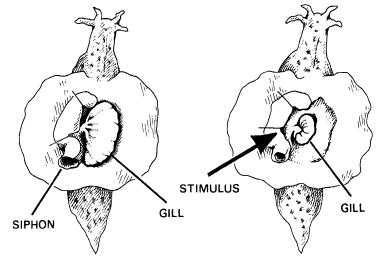
Figure 9.4 Aplysia, a marine slug. On the left, its gill and siphon are extended. When the siphon is touched, both the siphon and the gill contract in a defensive reflex (right). (After Kandel, ‘Nerve Cells and Behavior’, Scientific American, July 1970)
The nervous system is very similar from slug to slug; identifiable cells occur in predictable places. The sensory and motor cells involved in the gill reflex have been located; only four motor cells are responsible for the withdrawal response.20 (In higher organisms the ‘wiring diagrams’ are much more complex than in slugs, and much more variable from individual to individual.) Electrical measurements from single nerve cells have shown that as habituation occurs, the sensory cells cease to excite the motor cells. This happens because they release fewer and fewer packets or ‘quanta’ of chemical transmitter at the junctions or synapses with the motor cells.21 This change in the functioning of the sensory cells persists for minutes or hours, depending on how often the stimulus is repeated. Four training sessions of ten stimuli each result in a profound habituation that lasts for weeks. This means that some kind of memory of the stimulus can affect the sensory cells for long periods.
Since habituation can occur in a single cell such as Stentor, it is not surprising that cells within the nervous system of Aplysia can exhibit it as well. But there is no need to assume that it depends on physical or chemical memory traces within these cells. It may be due to morphic fields maintained by resonance with the organism’s own past. These fields, modified by morphic resonance from the previous activity of the nervous system in response to the harmless stimuli, organize the physical and chemical activities of the cells, including the release of chemical transmitters at the synapses. There are indeed changes within the cells as a result of their activity, but this does not mean that the memory is stored within them as a material trace.
In higher animals, behavioural fields may embrace many millions of nerve cells. But here again, there is no need for habituation to involve memory traces; it may depend, as in Stentor and Aplysia, on morphic resonance with the organisms’ own patterns of activity in the past.
Learning
From the point of view of the hypothesis of formative causation, inherited behavioural units or fixed action patterns are associated with particular morphic fields, such as the fields of attacking behaviour in robins or web-spinning behaviour in spiders. Morphic resonance from countless past members of the species gives these fields their probability structures, which organize the general expression of instinctive patterns of behaviour. However, actual experience within the framework of a behavioural field influences the way the actions are performed in similar circumstances on subsequent occasions, owing to self-resonance. Thus an animal acquires its own particular way of behaving instinctively.
There are many examples of such learning within the framework of instinct. Most young animals move rather clumsily at first, but become far better co-ordinated as time goes on. In part this improvement is due to maturation of the nervous system and the body in general; but it is due partly to practice as well.22 An animal learns to carry out an inherited action pattern in a way that is appropriate to its particular body and environment.
Many bees and wasps instinctively go out on foraging or hunting expeditions, yet show a remarkable ability to memorize the terrain around their nests and are able to home by means of a variety of landmarks.23 This kind of spatial learning is very widespread in the animal kingdom and makes possible a detailed adaptation of instinctive behaviour to the place where it is performed.
Perhaps the most dramatic type of instinctive learning is imprinting. Young birds such as chickens, goslings, and ducklings show an inherited behaviour pattern of following, and normally follow their mother. In his famous studies on geese, Konrad Lorenz got broods of newly hatched goslings to treat him as a mother figure and to follow him. Indeed, young goslings imprint on almost anything that moves, including objects such as balloons.24 After an imprinting period of only fifteen to thirty minutes, the young birds recognize and approach the moving object when they are exposed to it again. This ability to recognize the moving object is conventionally attributed to memory traces; but morphic resonance provides a direct connection. The object is recognized because through the senses it sets up specific patterns of activity within the nervous system, and these enter into morphic resonance with those previously set up by the same object.
The kind of learning on which experimental psychologists have concentrated is called associative learning. In Pavlovian conditioning, an automatic or unconditioned response such as the salivation of a hungry dog at the sight of meat is linked to another stimulus, such as the ringing of a bell, through repeated association; a conditioned reflex is established; the dog will salivate when the bell is rung even if no meat is provided.
Another kind of associative learning depends on an animal’s own activities. This was called operant conditioning by B.F. Skinner and the behaviourist school of psychology, and is also known as instrumental learning. For instance, if a cat finds how to open a door by trial and error and reaches a supply of food as a consequence, then it will sooner or later associate opening the door with receiving food; a conditioned response is established.
In conventional mechanistic terms, associative learning depends on the formation of new patterns of nervous connection within the brain. From the perspective of formative causation, by contrast, it results from the establishment of morphic fields that embrace previously separate patterns of activity within the nervous system. Such higher-level fields jump into being: they synthesize previously disparate parts and emerge as wholes. And indeed, associative learning often seems to involve definite discontinuities; it occurs in steps or stages. In trial-and-error learning, for instance, animals quite suddenly seem to grasp a connection, and we ourselves are familiar with jumps in learning: new patterns of connection suddenly ‘dawn on us’ or come ‘in a flash.’ (The origin of new fields is discussed in Chapter 18.)
This may happen even without overt trial-and-error behaviour, by insight. Ethologists commonly use this word when animals solve problems more rapidly than would be expected by trial and error. Wolfgang Köhler’s studies of chimpanzees over eighty years ago provided a classic example. Presented with a banana too high to reach, after some time the chimpanzees piled up boxes to make a stand for themselves so they could reach it, or they fitted two sticks together to pull the banana down. Often they arrived at the solution quite suddenly, although they benefited from previous experience of playing with the boxes and sticks and showed considerable trial-and-error learning when actually building a stable pile of boxes.25
Such examples are evidence for mental activity.26 At the moment of insight a potential pattern of organized behaviour comes into being. This can be regarded as a new morphic field. If the behavioural pattern is repeated, the field will be increasingly stabilized by morphic resonance. This behaviour will become more probable, more habitual, and increasingly unconscious.
The transmission of learning by morphic resonance
The nineteenth- and early-twentieth-century literature abounds in anecdotes about the apparent hereditary transmission of acquired behaviour, especially in dogs.27 Charles Darwin took a great interest in such stories, and he published an account in Nature of a mastiff’s violent antipathy to butchers and butchers’ shops, supposedly due to its mistreatment at the hands of a butcher, which was transmitted to at least two generations of its offspring.28
However, it was not until the 1920s that attempts were made to investigate experimentally the hereditary transmission of acquired habits. Some experiments provided evidence that such a transmission did in fact occur.29 Pavlov, for instance, trained white mice to run to a feeding place when an electric bell was rung. The first generation required an average of 300 trials to learn, the second only 100, the third 30, and the fourth ten.30 His last statement on the subject was that ‘the question of the hereditary transmission of conditioned reflexes and of the hereditary facilitation of their acquirement must be left completely open.’31
William McDougall began the most thorough investigation of the hereditary transmission of learning at Harvard in 1920. His own experiments, together with their sequels in Scotland and Australia, lasted for over 30 years and are one of the longest series of experiments in the history of experimental psychology. McDougall used standard white laboratory rats and trained them in a water maze, a tank of water from which they could escape only by swimming to a gangway and climbing up it. There were two exits, one on either side of the tank. One exit was illuminated, and if the rats chose it they received an electric shock as they left the water. The other exit was safe. The next time they were put into the tank, the gangway that was previously illuminated was now dim and safe. The rats had to learn that it was painful to leave by the illuminated exit but safe to take the dim one.
The first generation of rats made an average of 165 errors before learning to take the dim exit. Subsequent generations learned more and more quickly, until by the thirtieth generation the rats made an average of only 20 errors. McDougall showed that this striking improvement was not due to genetic selection for more intelligent rats, because even if he selected the most stupid rats in each generation as parents of the next, there was still a progressive increase in the rate of learning. 32 He interpreted these results in terms of Lamarckian inheritance, in other words in terms of the modification of the rats’ genes.
This conclusion was unpalatable to many biologists. The only recourse was to repeat McDougall’s experiments. When Francis Crew did so in Edinburgh, the very first generation of his rats learned very quickly, with an average of only 25 errors, and some got it right the first time.33 His rats seemed to be taking up where McDougall’s left off. Neither he nor McDougall was able to account for this effect.
In Melbourne, Wilfred Agar and his colleagues also found that the first generation they tested learned far quicker than McDougall’s original rats. They continued to test fifty successive generations of rats over a period of twenty years, and like McDougall found an increase in the rate of learning in subsequent generations. But unlike McDougall, they also repeatedly tested control rats that were not descended from trained parents. These too showed a similar improvement.34 The investigators very reasonably concluded that the changes they observed were not due to Lamarckian inheritance; if they had been, quicker learning should have been confined to the progeny of the trained rats. But then why did the improvement occur in both lines of rats? This effect is just what would be expected on the basis of morphic resonance.
Other experimental psychologists have, to their surprise, found very similar effects. They were not looking for such improvements; they cropped up in the course of experiments carried out for a different purpose. For example, at the University of California R.C. Tryon bred rats with the intention of establishing ‘bright’ and ‘dull’ strains. He used a special kind of maze into which rats were released automatically, greatly reducing any influence of handling by the experimenter.35 As expected, he found that the offspring of ‘bright’ parents were more often ‘bright’ than ‘dull,’ and the offspring of ‘dull’ ones more ‘dull’ than ‘bright.’ But he also found something he did not expect: both strains became quicker at learning the maze.36
According to the hypothesis of formative causation, an acceleration in learning should occur whenever animals are trained to do new tricks or adjust to new conditions: the average rate of learning should increase as the training is repeated, other things being equal. However, other things are rarely equal, if only because trainers themselves tend to improve with experience. Nevertheless, there seems to be a wealth of anecdotal evidence that such changes occur. Over the last twenty years, I have received fascinating accounts from dog owners, horse trainers, falconers, cattle ranchers, and dairy farmers about progressive improvements in the ease with which new generations of animals could be trained or adapted to new methods. They all felt that only some of this improvement could be explained by their own experience; some real change seemed to be happening in the animals as well.
Comparable changes may well be occurring all the time in psychology laboratories, but they are rarely, if ever, systematically documented. I explored this possibility with two of the most ingenious experimental psychologists in Britain. When they devised new tricks for rats to perform, both found that in general the first rats tended to learn very slowly, but that as the experiments proceeded, new batches of rats usually picked up the tricks faster and faster. However, both believed that these improvements reflected improvements in their own performance as experimenters.37 There is no doubt that experimenters can influence the performance of animals they are working with, and ‘experimenter effects’ have been well documented.38 But the animals themselves may also improve as a result of morphic resonance from their predecessors. These influences are complementary, not mutually exclusive.
Clearly these two kinds of influence need to be distinguished in experiments specifically designed to test the hypothesis of formative causation. One possible design is as follows. Several new tricks are devised for rats to perform, and appropriate pieces of apparatus are constructed in duplicate. The duplicate set is sent to a second laboratory, where experimenters are asked to test rats with each of the tasks and record their rates of learning. They are requested to do the same thing again six months later with fresh batches of rats. Meanwhile in the first laboratory, one of these tasks is selected at random and thousands of rats are trained to perform it. Experimenters at the second laboratory are not told which task has been selected. If, six months after the first tests, they find a striking increase in the rate of learning in this task but not in the others, this result would support the hypothesis of morphic resonance.
Such experiments by their very nature would not be exactly repeatable because of morphic resonance from previous experiments, but they could be replicated indefinitely with new species of experimental animals or with new sets of tricks.
In 1990, Steven Rose and I jointly designed an experiment to test for morphic resonance in day-old chicks. This test took place after Rose and I had a prolonged debate in the Guardian newspaper about the nature of memory.39 He supported the trace theory, and I the morphic resonance hypothesis. In this debate he publicly challenged me to test ‘this seemingly absurd hypothesis’ in his laboratory at the Open University. I accepted. We agreed on a conditioned aversion experiment based on a one-shot kind of learning that Rose and his colleagues were using in their attempt to find memory traces. Conditioned aversion is a scientific name for the revulsion animals and humans feel towards foods that once made them sick.
Chicks instinctively peck at small bright objects in their environment. Rose’s standard procedure was to expose chicks to a test stimulus, for example a small green light-emitting diode (LED), which they pecked. Soon afterwards, they were made mildly sick with an injection of lithium chloride. As a result they avoided pecking the same kind of bead again.
Control chicks were exposed to a control stimulus, a chrome-plated bead. After pecking it, they were injected with a harmless saline solution, and developed no aversion to it. This form of learning is different from conditioned taste aversion in that it involved a visual stimulus, but like taste aversion provided a rapid form of learning that needed only one trial.
Rose and I designed an experiment to test for morphic resonance with a new stimulus, a yellow LED, not previously used in experiments of this kind, to avoid any carry-over of morphic resonance from previous aversion experiments with green LEDs. Indeed, we found that the chicks pecked a yellow LED much more readily than a green LED: there was an average delay of 4.1 seconds before they pecked the yellow LED and 19.0 seconds with green.40 For the control stimulus we used a chrome bead.
Every day for 37 days the same tests were performed with fresh batches of day-old chicks. Half the batch of chicks, selected at random, was tested with the yellow LED, the other half with the chrome bead. Then the chicks exposed to the yellow LED were made mildly sick. Three hours later they were tested again. Most avoided pecking the yellow LED, but had no aversion to the chrome bead. The control chicks that had pecked at the chrome bead were injected with saline solution, and they too were tested three hours later with both the chrome bead and the yellow LED.
I predicted that if morphic resonance was taking place, successive batches of day-old chicks should show an increasing aversion to the yellow LED when first exposed to it. No such aversion would be expected with the control chicks. Rose was very sceptical about morphic resonance and predicted that there would be no increase in aversion in the control or the test chicks.
To start with, there was an effect that neither of us had predicted, though in retrospect we should have done. The student carrying out the tests, Amanda Harrison, had never worked with chicks before, and took several days to learn how to handle the chicks and carry out the tests properly. The data from the first few days showed a big learning effect – not by the chicks but by the student. From then on, after she had learned the techniques, there was a consistent pattern. Relative to the controls, the test chicks exposed for the first time to the yellow LED became progressively averse to pecking it (Fig. 9.5). This effect was statistically significant. In my view the data were consistent with the operation of morphic resonance.41 In Rose’s view they were not, and he tried to explain them away as artefacts.42 Unfortunately he based his case on an incomplete data file that excluded results from the chicks that were most averse to pecking the test stimulus. For this and other reasons, in my opinion, Rose’s arguments were flawed.43
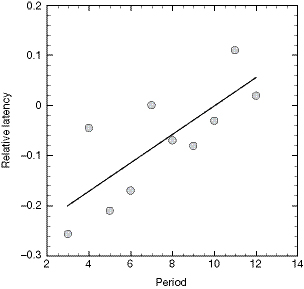
Figure 9.5 Data from an experiment on conditioned aversion with day-old chicks. ‘Test’ chicks were exposed to a yellow light-emitting diode (LED) and control chicks to a chrome bead. There was an increased delay in pecking at the LED relative to the control stimulus in successive three-day periods. The measure of delay, or ‘latency’, was the proportion of chicks that did not peck at the stimulus within 10 seconds. (Data from Sheldrake, 1992a).
Perhaps the best opportunity for further research on the transmission of aversion is with rats. Conditioned aversion is an important practical problem for the rat control industry. If rats are fed bait laced with a quick-acting toxin, the poison kills a few rats to start with, but the other rats soon avoid it. They rapidly become ‘bait shy’. For this reason, the most effective rat poisons are slow acting and do not cause illness soon after being eaten, like warfarin, first licensed as a rodenticide in 1952. Warfarin is an anticoagulant that works slowly because it kills rats through internal bleeding, while some bleed to death after being bitten by other rats.44
Here is a simple experimental design to test for the transfer of bait shyness by morphic resonance. Two kinds of food, A and B, are given unusual flavours that rats are unlikely to have encountered before. Six colonies are selected for this experiment, located miles away from each other. Three are picked at random and both A and B are made available to them. The rates at which the rats eat them are recorded. Next, one of the foods – say B – is poisoned with low doses of zinc phosphide. The rats become sick, bait shy and avoid B. Now rats in the other three colonies are given unpoisoned A and B to eat. If morphic resonance is at work, the rats should show a tendency to avoid B but not A.
Similar experiments could be done under more controlled conditions with captive colonies of rats or mice, but to minimize unnecessary suffering it would be better to do these experiments in situations where the animals are going to be poisoned anyway.
The evolution of new patterns of behaviour
The best-documented example of the spontaneous spread of a new habit concerns the opening of milk bottles by birds. In Britain, fresh supplies of milk were (and still are) delivered to the doorsteps of houses every morning except Sunday. In the 1920s, blue tits (Parus caeruleus) and several related species of birds began to steal cream by removing the caps and drinking from the tops of the bottles (Fig. 9.6). The bottles were usually attacked within a few minutes of delivery, and there were even reports of parties of tits following the milkman down the street and drinking from bottles on the cart while he was busy delivering milk. Occasionally birds were found drowned head first inside the bottles.
Figure 9.6 A blue tit opening a milk bottle by tearing the foil cap. (After Hinde, 1982)
The first record of this behaviour was from Southampton in 1921, and its spread was recorded at regular intervals from 1930 to 1947 (Fig. 9.7). It was observed in eleven species, but most frequently in great tits, coal tits, and blue tits. Once discovered in any particular place, the habit spread locally, presumably by imitation and other forms of cultural transmission, for example by tits feeding from bottles already opened by other birds and hence learning about this new food source.45
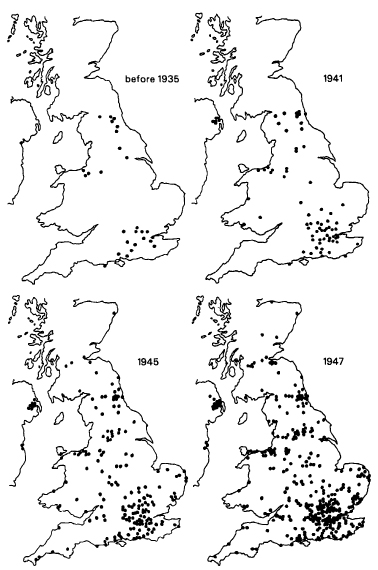
Figure 9.7 Distribution of recorded opening of milk bottles by tits up to and including the years indicated. (After Fisher and Hinde, 1949. Reproduced, by permission, from the monthly magazine British Birds.)
British tits do not usually venture more than a few miles from their breeding place, and a movement of as much as fifteen miles is exceptional. Hence new appearances of this behaviour more than fifteen miles from where it had previously been recorded probably represented new discoveries by individual birds. Once discovered, the habit spread locally by observational learning. A detailed analysis of the records by researchers at Cambridge University, Robert Hinde and J. Fisher, showed that the spread of the habit accelerated as time went on, and that individual tits discovered it independently at least 89 times in the British Isles.46 A more sophisticated mathematical analysis of the data confirmed the probability of multiple, independent innovations.47
The habit also appeared in Sweden, Denmark, and Holland. The Dutch records are particularly interesting. Milk bottles practically disappeared during the Second World War, and became common again only in 1947 or 1948. Few if any tits that had learned the habit before the war could have survived to this date, but nevertheless attacks on bottles began again rapidly, and ‘it seems certain that the habit was started in many different places by many individuals.’48
Hinde and Fisher pointed out that bottle opening is related to the instinctive behaviour of tits: ‘The initial discovery of the bottle as a source of food may be a logical consequence of the feeding habits of tits. They appear to have an inborn tendency to inspect a great variety of conspicuous objects which contrast with their surroundings, and to test their palatability.’ As for the opening of the bottles, ‘the hammering action with which foil caps are punctured is very similar to a motor pattern used in opening nuts, and the tearing action often used on cardboard tops is similar to a movement used in tearing bark from a twig’.49
An explanation in terms of formative causation complements this suggestion. These instinctive motor patterns, themselves organized by morphic fields, did not automatically give rise to the bottle-opening habit; they only did so when they were embraced within a higher-level behavioural field, the field of bottle opening. The field was progressively reinforced by the cumulative effects of morphic resonance from previous milk-drinking tits, and consequently enabled both the discovery and the passing on of the habit by cultural transmission to take place ever more readily. Morphic resonance would therefore help to explain the spread of the bottle-opening habit as well as its rapid reappearance in Holland after the war.
In North America, close relatives of the tits found in Europe are called chickadees. In the 1980s some Canadian researchers tested captive black-capped chickadees (Parus atricapillus) with closed tubs of cream in the laboratory to study how this behaviour spread from bird to bird. They found that birds learned to open the tubs either by observing other birds doing so, or by encountering tubs that had already been opened. But their most surprising finding was that a quarter of the birds they tested opened the tubs spontaneously on their first exposure to them.50 Unfortunately, no one knows whether they would have done this before the British tits had learned to steal cream, or whether this is an example of cross-species morphic resonance.
In the 1980s many people switched to semi-skimmed milk, and the habit died out because there was so little cream to steal.
The case of the tits is just one example of a rapid evolutionary change in behaviour in response to human activity. Many others have been observed, but none were systematically documented.
According to an eminent Texas naturalist, Roy Bedechek, when barbed wire was first introduced in the late nineteenth century, sceptics predicted that it would never be suitable for horse pastures. Horses dashed right into it and ‘cut their own throats, tore great slugs of flesh from their breasts, while wounds not fatal or mere scratches became infested with screw worms’. In 1947 Bedechek wrote: ‘I can remember the time when there was hardly a horse to be found in Texas farming or ranching sections that was not scarred from encounters with barbed wire.’51 Yet by the middle of the twentieth century, this was no longer a serious problem: ‘In half a century the horse has learned to avoid barbed wire. Colts rarely dash into it. The whole species has been taught a new fear.’
Bedechek also commented on the changed reactions of horses to cars:
When automobiles first appeared, horse-drawn traffic was disorganized. The more considerate autoist would drive out of the road and cut off the motor immediately a team of horses hove in sight. Not only that, the motorist would get out of his car and help the driver lead the rearing, snorting horses by it. Many the vehicle wrecked and many the neck broken in making the introduction of horse to automobile and establishing his tolerance for it. Loud were the demands for laws to keep automobiles in their place … We no longer have breakneck runaways every time a team of horses meet an automobile.52
Another example of behavioural evolution in farm animals concerns cattle grids (known as cattle guards in the United States), which are pits with a series of parallel steel tubes or rails over the top. They were invented in the United States in the nineteenth century to stop animals wandering onto railway lines. They began to be used on American roads around 1905,53 and are now widely used in many other countries. They make it physically impossible for cattle to walk across them, but allow vehicles and people to cross freely.
When cattle grids were first introduced, animals may have had to learn the hard way that they could not pass. But this is no longer the case. Farm animals seem to avoid these grids instinctively and do not even try to cross them.
By the 1950s, some ranchers in the American West found that they could save money on cattle grids by using fake grids instead, consisting of stripes painted across the road. The painted grids worked because the animals did not even try to cross them. There are now hundreds of thousands of phoney cattle grids on ranches and on public roads in states such as Texas, Nevada and California. The fake grids are confined to drier regions; in wetter climates, like England, they would soon become covered with mud and cease to work.
In response to my enquiries, several ranchers in the western United States told me that there is no need for herds to be exposed to real cattle grids first. Animals that have never seen a real cattle grid avoid the fake ones. When young cattle approach a painted grid, they ‘put on brakes with all four feet’ as one rancher expressed it. I corresponded with researchers in the Departments of Animal Science at the Colorado State University and Texas Agricultural and Mechanical (A&M) University who confirmed this observation.54
Perhaps painted cattle grids work simply because they create the illusion of a drop. In this case, they should have worked all along, and ranchers need not have used real grids in the first place. It would be interesting to find out if wild species never before exposed to cattle grids show a comparable aversion to crossing them. It would also be good to find out whether cattle respond equally well to a variety of stripy patterns, or just stripes that look like cattle grids.
Interestingly, a new response to cattle grids is currently evolving. In 1985, sheep near Blaenau Ffestiniog in Wales started escaping from their pastures by rolling over grids. So did sheep in Sweden, around Malmoehus. An editorial in The Guardian in 1985 commented:
To the best of our knowledge the sheep in the Yorkshire Dales, which are mostly Swaledales or Dalesbred, have yet to master the technique of crossing cattle grids by curling up and rolling over them. Yet the sheep of Blaenau Ffestiniog, which are a different breed, have learned how to do it (to the annoyance of the town, which may have to put up a fence) and so have the lowland sheep of southern Sweden. Among the questions which immediately arise, are how long will it take the Swaledales to learn and whether, when they do, they will be demonstrating the theory of formative causation.55
Twelve years later, sheep started crossing cattle grids in Hampshire, in southern England. To start with they used a ‘commando’ technique with one of them lying on the cattle grid while others scrambled across her. But then they started crossing by rolling across the bars of the grid, like the Welsh sheep.56 Similar behaviour was observed in the Valais region of Switzerland.57
In 2004, 19 years after the editors of the Guardian had anticipated the possibility, sheep on the Yorkshire moors began escaping by rolling over cattle grids and grazing on the nearby gardens of villagers.58 Will this behaviour spread further, and show up in parts of the world, like New Zealand, where it has not yet been observed?
Behavioural evolution is currently occurring on a large scale in places where towns have only recently been established. In Papua New Guinea, for instance, where the first town was founded in the 1870s, a number of local species of birds have adapted to living in towns, as Jared Diamond has observed:
Such shifts in behaviour do not occur instantly and newly acquired behaviours take time to spread. In several cases the timetable is known. It was not until 1971–74 that a flock of black kites settled in the town of Lae to feed on road kills, although the town was founded in the 1930s. Only since 1983 have brahminy kites learned to feed on road-killed toads in Port Moresby. Goldie’s lorikeet, which was formerly a rare species occurring in primary forest, arrived in the 1970s to feed on casuarina seeds in highland towns, where it is now the most abundant bird. Lemon-bellied flycatchers expanded from the savanna into the town of Wau between 1976 and 1978, 50 years after Wau was founded.59
Such situations would provide a good opportunity to study the spread of new habits of behaviour and to investigate the possible role of morphic resonance.
As we have seen in this chapter, the hypothesis of formative causation and the mechanistic theory provide radically different views of memory and learning in animals.
From a mechanistic point of view, memory depends on memory traces, as yet unidentified, which function in a manner that remains obscure. The inheritance of instinctive patterns of behaviour is different in kind from the ability of individual animals to acquire new habits of behaviour: inherited behaviour is programmed in the genes, and acquired patterns of behaviour cannot be inherited because there is no known way in which they could modify the genetic program.
By contrast, according to the hypothesis of formative causation, behaviour is organized by morphic fields associated with the activities of the nervous system. The inheritance of instincts and the building up of an animal’s own habits both depend on morphic resonance, and there is no radical difference in kind between them. For this reason, habits acquired by some animals can facilitate the acquisition of the same habits by similar animals elsewhere, even in the absence of any known means of connection or communication. These effects may play an important part in the evolution of new patterns of behaviour.