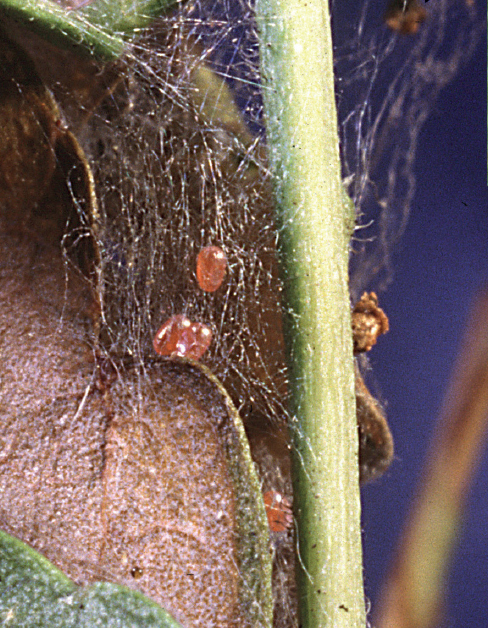
Eggs of mimosa webworm scattered among webbing. DAVID SHETLAR
CATERPILLARS THAT PRODUCE LARGE SILKEN SHELTERS AND TENTS
Caterpillars of several families of Lepidoptera and sawfly larvae of one Hymenoptera family collectively produce a large silken structure within which they rest and/or feed. Perhaps best known are the tent caterpillars (Lasiocampidae), a few of which may be locally common on trees and shrubs in midspring. Many other caterpillars that produce large silken webs may be known as “webworms,” a term that is more loosely applied and includes several caterpillars that are normally solitary (pages 122 and 128).
MIMOSA WEBWORM (Homadaula anisocentra)1

Eggs of mimosa webworm scattered among webbing. DAVID SHETLAR
HOSTS Honeylocust, mimosa.
DAMAGE Larvae feed on leaf surfaces of honeylocust and mimosa, producing skeletonizing injuries that cause leaves to turn dull gray or appear scorched brown. Individual larvae web together several leaflets and may feed together in groups, creating large areas of unsightly webs. Isolated trees, particularly adjacent to buildings, tend to be most consistently damaged.
DISTRIBUTION Mid-Atlantic and southeastern states west to eastern Kansas and Nebraska.
APPEARANCE Caterpillars are grayish to dark brown, sometimes tinged with rose or pink. Full grown they are about ⅝ inch and extremely active, wriggling and dropping on a silken strand when disturbed. Adults are undistinguished gray moths, rarely seen, with a wingspan of ½ inch; wings have a silver sheen and dark blue spots.
LIFE HISTORY AND HABITS Adults begin to lay rosy-colored eggs in late May into June on flowers and foliage. The young larvae produce silk and skeletonize adjacent foliage and flowers. As they get older, their webbing bcomes more extensive and may coalesce with that of adjacent larvae. By midsummer the larvae descend to the ground on silken threads and pupate in cocoons in bark cracks, on adjacent buildings, and among ground cover. A second generation with peak feeding injury in August is often more conspicuously damaging. A third generation is reported in the southeastern area of the range. The overwintering stage is a pupa.
OTHER WEBWORMS
JUNIPER WEBWORM (Dichomeris marginella)2 develops on most Juniperus species and occasionally on arborvitae. It is a European species that apparently has been introduced on multiple occasions and can be found in many areas of the U.S. and southern Canada. Young larvae mine needles. Later-stage larvae often feed in small groups, producing a shelter of webbing that ties needles together and incorporates bits of chewed needles and frass into the “nest.” Winter is spent in the shelter in the form of late-stage larvae that pupate in spring. One generation is produced per year.

B. Adult of the mimosa webworm. DAVID SHETLAR

C. Mimosa webworm. DAVID SHETLAR

D. Pupae of mimosa webworm within bark cracks of a honeylocust tree. DAVID SHETLAR

E. Larva of the juniper webworm. CONNECTICUT AGRICULTURAL EXPERIMENT STATION, BUGWOOD.ORG

F. Juniper webworm adult. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

G. Webbing mixed with frass and needle fragments produced by juniper webworm. CONNECTICUT AGRICULTURAL EXPERIMENT STATION, BUGWOOD.ORG
COTTON EASTER WE BWORM (Athrips rancidella)2 is also a European species currently established in northern California and the Pacific Northwest. During spring the larvae skeletonize leaves and make dense webs on cotoneaster that are filled with leaf fragments and frass. Adults are typically present in June and July, when females lay eggs in small groups on leaves. After egg hatch the young larvae feed for about a month then move to branches where they construct a silken shelter, usually in the axils of branches. They remain dormant through winter, resuming activity in March. There is one generation per year. Rock cotoneaster (Cotoneaster horizontalis) is most commonly damaged.

Cotoneaster webworm. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY
UGLYNEST CATERPILLAR (Archips cerasivorana)3 is found across the northern U.S. and southern Canada. It feeds on a wide range of trees and shrubs, although cherry and chokecherry are most commonly infested. Uglynest caterpillars feed in groups, creating large, loose silken shelters that cover foliage in a manner similar to that of fall webworms. One generation is produced per year, with the olive green caterpillars present from the time of egg hatch in late spring through much of the summer. A large cluster of leaves in the terminals of black and red oaks may be similarly tied in by OAK WEBWORM (A. fervida), a species that occurs from the upper Midwest and eastern Canada into North Carolina.
AILANTHUS WEBWORM (Atteva punctella)4 is one of the few insects commonly associated with tree-of-heaven. It feeds on leaves and loosely covers them with silk.
PINE WEBWORM (Pococerca robustella)5 feeds on several species of pines throughout the eastern U.S. and Canada. In northern areas young caterpillars are present in late spring and originally feed as needleminers. As they get older, they feed in groups, loosely webbing together needles of pine terminals. One generation is produced annually in northern states, with winter spent as a pupa in a cocoon in soil. In the southeast two generations may be produced.
BARBERRY WEBWORM (Omphalocerca cariosa)5 is an eastern species associated with barberry (Berberis spp.) and pawpaw. The larvae are black, about 1½ inches long when full grown, and feed on leaves and new shoots in a loose silken shelter. It occurs across the southeastern U.S., from Texas to Florida and as far north as Illinois.
MAHOGANY WEBWORM (Macalla thrysisalis)5 feed on the spring flush of growth of West Indies mahogany (Swietenia mahagoni) in southern Florida and other areas where this plant grows. Adults lay eggs shortly after the spring flush of growth is produced and the larvae subsequently tie together a small number of leaves on which they feed. The larvae are brightly colored, predominantly yellow with alternating black and white stripes. Feeding occurs on the new growth and is usually concluded within a couple of weeks. One generation is produced per year.
1 Lepidoptera: Glyphipterygidae
2 Lepidoptera: Gelechiidae
3 Lepidoptera: Tortricidae
4 Lepidoptera: Yponomeutidae
5 Lepidoptera: Pyralidae

B. Uglynest caterpillar. WHITNEY CRANSHAW

C. Webbing and larvae of the oak webworm. STEVEN KATOVICH, USDA FOREST SERVICE, BUGWOOD.ORG

D. Adult of the ailanthus webworm. DAVID SHETLAR

E. Ailanthus webworm. DAVID SHETLAR

F. Webbing and frass associated with pine webworm. CONNECTICUT AGRICULTURAL EXPERIMENT STATION, BUGWOOD.ORG

G. Pine webworm larva. CONNECTICUT AGRICULTURAL EXPERIMENT STATION, BUGWOOD.ORG

H. Barberry webworm within a sheath of silk. J. A. DAVIDSON, UNIVERSITY OF MARYLAND, BUGWOOD.ORG
FALL WEBWORM (Hyphantria cunea)1
HOSTS Fall webworm has one of the widest host ranges of any caterpillar and may feed on more than 100 species of deciduous trees and shrubs. Some plants tend to be preferred, including cottonwood, chokecherry, mountain-ash, pecan, elm, willow, and various fruit and nut trees.
DAMAGE The larvae feed on leaves and build unsightly silken tents. Heavy infestations can defoliate trees. Wandering larvae are sometimes a serious nuisance. Fall webworm is the most common tent-making caterpillar in much of North America.
DISTRIBUTION Widely distributed through most of North America, extending from southern Canada to northern Mexico.
APPEARANCE Larvae of fall webworm are hairy caterpillars with distinct paired dark spots on each segment of the back. They can be variable in color, however, with both black- and red-headed races present, sometimes together. The general color of the caterpillars is also variable, with the black-headed form tending to be yellow or pale green with light-colored hairs. The red-headed form is usually darker with reddish brown hairs. Caterpillars are about 1 inch long when mature. The adults are attractive satiny white moths, sometimes with brown or black spots. The wingspan is about 1½ inches.
LIFE HISTORY AND HABITS Fall webworm spends the winter as a pupa in a flimsy light-colored cocoon found in protected areas such as bark furrows, crevices along the sides of buildings, or mixed among debris around the soil surface. In southern areas of its distribution, adults begin to emerge in early spring, whereas emergence usually occurs in late June or July in northern areas. Eggs are laid in masses on the underside of leaves, and the young larvae feed together, first skeletonizing the leaf and then incorporating leaves and ultimately entire branches in their loosely spun tent of silk. Unlike in tent caterpillars (below), all larval development and feeding occur in the tents, into which leaf fragments, droppings, and cast skins become incorporated. The caterpillars, when disturbed, often twitch in an effort to deter potential predators.
When full grown the larvae usually wander from the plant and search for a protected location to pupate. A single generation occurs in northern areas of the range; in southern states, there may be up to four generations per year.
1 Lepidoptera: Erebidae (Arctiinae)

B. Late-instar larvae of fall webworm, brown morph. WHITNEY CRANSHAW

C. Early-instar tent produced by fall webworm, black headed morph. DAVID SHETLAR

D. Mating pair of fall webworm moths. DAVID SHETLAR

E. Fall webworm egg mass. DAVID SHETLAR

F. Fall webworm larvae at egg hatch. DAVID SHETLAR
EASTERN TENT CATERPILLAR (Malacosoma americana)1
HOSTS Rosaceous plants including most fruit trees (apple, crabapple, plum, cherry, hawthorn); sometimes found on poplar, willow, ash, and birch.
DAMAGE Caterpillars chew leaves early in the season and can cause significant defoliation. Developing fruit may be chewed incidentally. Conspicuous tents of tightly woven silk in branch crotches attract attention and may be considered unsightly.
DISTRIBUTION Throughout the eastern U.S. and southern Canada, to the Rocky Mountains.
APPEARANCE Larvae are overall dark, primarily black, with a distinct light stripe on the back and a series of blue markings on the sides. Adults are rather stout moths with reddish brown wings marked with a pair of wavy light bands.
LIFE HISTORY AND HABITS Eggs are laid on twigs as distinct masses, shiny black and covered with varnish-like material (spumaline). They hatch shortly after bud break, and the young larvae immediately begin to spin a silken mat in a branch crotch near the egg mass. The tent is gradually expanded as the caterpillars get older. Most often the caterpillars feed at night on nearby foliage, returning to rest together on or in the tent during the day. Late-stage caterpillars tend to disperse through the plant and feed in a more solitary manner. When full grown they migrate from plants and produce white or creamy white cocoons on nearby trunks or rocks, under eaves, or in other sheltered locations. Adults emerge in early summer; after mating, females attach the overwintering egg masses to branches of host trees. One generation is produced per year.
OTHER TENT CATERPILLARS
WESTERN TENT CATERPILLAR (Malacosoma californica) is found in much of the U.S. from the Great Plains westward. It is rarely a pest of ornamental plantings but is common on wild hosts such as mountain-mahogany, wax currant, and aspen. Plum, other fruit trees, willow, and several other deciduous woody plants are less common hosts. The caterpillars can be distinguished from other species by being slightly hairy and having a typically light brown general coloration with powdery blue markings along the sides and a blue head; however, several races are recognized with slightly different appearances.

Western tent caterpillars. WHITNEY CRANSHAW

Early stage colony of western tent caterpillar, shortly after egg hatch. WHITNEY CRANSHAW

B. Eastern tent caterpillar. DAVID SHETLAR

C. Early stage colony of eastern tent caterpillar. DAVID SHETLAR

D. Egg mass of eastern tent caterpillar at egg hatch. DAVID SHETLAR

E. Egg mass of western tent caterpillar. WHITNEY CRANSHAW

F. Cocoon of the western tent caterpillar. WHITNEY CRANSHAW

G. Mating pair of western tent caterpillar moths. WHITNEY CRANSHAW
SOUTHWESTERN TENT CATERPILLAR (M. incurva) can be an important pest west of the Rockies, most commonly feeding on poplar and cottonwood along riverways. Eggs hatch very early, in late March or early April, and feeding is completed by mid-May. In the southwestern U.S., SONORAN TENT CATERPILLAR (M. tigris) feeds on native stands of oak, especially Gambel oak. In the Pacific States oak is the host for PACIFIC TENT CATERPILLAR (M. constricta).
FOREST TENT CATERPILLAR (M. disstria) is the most widespread and perhaps most frequently damaging tent caterpillar in North America. Ash, aspen, various fruit trees, oak, poplar, and willow are among the many deciduous trees this insect feeds on. In many areas it has short-lived outbreaks during which there is extensive defoliation, particularly to aspen or oak. At these times large numbers of wandering caterpillars may be present, leading to them being locally called “armyworms.”
Although this is often the most common of the tent caterpillars (Malacosoma spp.), it does not make a permanent silken tent in branch crotches. Instead, colonies make several resting mats of lightly spun silk during a season on trunks and larger branches. The forest tent caterpillar is also distinctive in having an electric blue color and a series of light “keyhole” patterns along its back.
1 Lepidoptera: Lasiocampidae
OTHER TENT-MAKING CATERPILLARS
POPLAR TENTMAKER (Clostera inclusa)1 occurs over a wide area of southern Canada and the eastern half of the U.S., ranging south to Georgia. It develops on aspen, poplar, and willow. Larvae feed gregariously and construct a structure of leaves loosely tied with silk. Two generations are produced, with eggs from the overwintered generation laid in March and April, followed by a summer generation with eggs laid mostly in July and August. Winter is spent in a cocoon around the base of previously infested trees.

Poplar tentmaker. STANTON GILL, UNIVERSITY OF MARYLAND COOPERATIVE EXTENSION, BUGWOOD.ORG
SILVERSPOTTED TIGER MOTH (Lophocampaargentata)2 is a tent-producing species present in forested areas of the Pacific Northwest North America, where it feeds primarily on Douglas-fir. Eggs are laid in clusters on twigs and needles during midsummer. In about 3 weeks the larvae emerge and begin to produce loose webbing. They feed together and continue to expand the silken tent. They remain in the tent through the winter and resume feeding with the return of warm weather. Late-stage larvae then disperse and feed as individuals. Pupation occurs in late spring in a brown cocoon attached to plants or among debris on the ground. There is one generation per year. In the Rocky Mountain region a related species, L. ingens, feeds on pinyon and juniper in a similar manner. Lophocampa sobrina occurs on Monterey pine in California.
1 Lepidoptera: Notodontidae
2 Lepidoptera: Erebidae (Arctiinae)
A. Pacific tent caterpillar. DONALD OWEN, CALIFORNIA DEPARTMENT OF FORESTRY AND FIRE PROTECTION, BUGWOOD.ORG

B. Eastern tent caterpillar (left) and forest tent caterpillar (right). DAVID SHETLAR

C. Southwestern tent caterpillar. DAVID LEATHERMAN

D. Forest tent caterpillars. GERALD J. LENHARD, LOUISIANA STATE UNIVERSITY, BUGWOOD.ORG

E. Mass of forest tent of forest tent caterpillars resting on silk mat. DAVID SHETLAR

F. Forest tent caterpillar, adult female. DAVID SHETLAR

G. Tent produced by Lophocampa ingens. DAVID LEATHERMAN

H. Forest tent caterpillar, adult male. DAVID SHETLAR

I. Late-instar larva of Lophocampa ingens. FRANK PEAIRS
The webspinning sawflies (Pamphiliidae) are the only web-producing larvae in the order Hymenoptera one may find associated with plants. Unlike other sawflies, the larval stages in members of this family have very reduced legs and prolegs and move little about the plants on which they feed. Instead, most construct shelters of webbing, of a form similar to those produced by certain webworms and tent-making caterpillars, and spend much of the time resting on or within the silken shelter. Although webspinnng sawflies occasionally attract some attention and interest because of the tents they construct, they are not common insects and none are considered serious plant pests.
PINE FALSE WEBWORM (Acantholyda erythrocephala)1 is found in much of the northern U.S. and southern Canada. Adults insert small groups of eggs into needles in spring, and the newly hatched larvae spin webs at the base of needles. They feed in this shelter, cutting adjacent needles, which they pull into the web. Often several feed in a loose group, creating a messy “nest” of silk incorporated with needle fragments and frass. When full grown they drop to the ground and spend winter as full-grown larvae in cocoons in the soil at the base of previously infested plants. Eastern white pine and red pine are favored hosts. Other species of Acantholyda also feed on pine.
Cephalcia species1 also feed on pines as well as spruce. Some feed gregariously, producing messy nests. Most have solitary habits and live inconspicuously in a silken tube at the base of needles.
Some sixteen species of webspinning sawflies in the genus Pamphilius1 occur in North America. Most are solitary leafrollers of various deciduous trees and shrubs, curling leaves and fastening them with silk. P. phyllisae feeds on northern red oak and curls a new leaf after each molt. Its life cycle may take more than a year to complete under certain conditions. Larvae normally are present on plants for about 3 weeks, after which they move to the soil and produce overwintering cocoons. Other species include PEACH SAWFLY (P. persicus) and BLACKBERRY SAWFLY (P. dentatus).
PLUM WEBSPINNING SAWFLY (Neurotoma inconspicua)1 is found in plum and sand cherry throughout much of the midwestern and northeastern U.S. and southern Canada. It feeds in groups, creating nests of large, loose webbing that enclose the tips of branches in a manner similar to that of uglynest caterpillar. Larvae are grayish yellow. One generation is produced annually. In the southern U.S., CHERRY WEBSPINNING SAWFLY 1 Hymenoptera: Pamphiliidae (N. asciata) has similar habits and is associated with black cherry.

Adult of Oncyolyda sitkensis, a webspinning sawfly that feeds on blackberry. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

Leaf tying and frass associated with a webspinning sawfly on blackberry. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

Young larva of Oncyolyda sitkensis, a webspinning sawfly that feeds on blackberry. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY
1 Hymenoptera: Pamphiliidae

B. Pine false webworm larva. DAVID SHETLAR

C. Male of the pine false webworm. DAVID SHETLAR

D. Female of the pine false webworm. DAVID SHETLAR

E. Mixture of frass and silk produced by a webspinning sawfly on pine. DAVID LEATHERMAN

F. Webspinning sawflies on cherry. DAVID SHETLAR
In the diverse order Hymenoptera (ants, bees, wasps) few species feed diretly on leaves of plants, and many are critically important in pollination or as natural enemies of plant-feeding insects. Among those that do chew on leaves, the sawflies are by far the most commonly encountered. Larval stages of these insects often have an appearance very similar to that of many caterpillars (larvae of Lepidoptera) and similarly possess prolegs on the abdomen. They can be distinguished from caterpillars by having 6–8 pairs of prolegs (versus 2–5), none of which are tipped by the hooks (crochets) found on caterpillars. Most sawfly larvae are hairless, but some species may be covered with short spines. Adult sawflies are heavy-bodied, nonstinging wasps, uncommonly noticed, that insert eggs into foliage with a sawlike ovipositor.
The two main families of sawflies are the conifer sawflies (Diprionidae) and the common sawflies (Tenthredinidae). About three dozen species of conifer sawflies occur in North America, all of which feed on needles of conifers. Larvae of the conifer sawflies often feed in groups and often have bizarre defensive behaviors such as mass twitching, arching, and disgorging of sticky fluids when disturbed. The common sawflies (Tenthredinidae), constitute a much larger insect family (more than 700 North American species) and occur on a wide range of broadleaf plants.
EUROPEAN PINE SAWFLY (Neodiprion sertifer)1
HOSTS Pines, particularly mugho and tabletop pines.
DAMAGE Larvae consume the older foliage in spring, before bud break. Seriously affected plants have a tufted “bottle brush” appearance with only new needles present.
DISTRIBUTION Generally distributed throughout the Midwest, Northeast, and parts of southern Canada.
APPEARANCE Larvae are grayish green and about 1 inch when full grown. A light stripe runs down the back, and a light stripe followed by a dark green stripe runs along the sides. Adults are about ⅓ inch long. Males are nearly black and have highly feathered antennae. Females are reddish brown and somewhat larger.
LIFE HISTORY AND HABITS European pine sawfly spends winter in the egg stage, inserted in needles. Eggs hatch in midspring and the larvae feed on needles, often in small groups, with three or four per needle being common. When disturbed, they raise both their head and tail in defense. In late spring or early summer, full-grown larvae move to soil or bark cracks where they form a cocoon and pupate. Adults emerge in late August and September. Females insert six to eight of the overwintering eggs per needle and may lay eggs in about a dozen individual needles. Egg-laying scars may be quite visible, particularly after frosts cause the wounded areas to yellow.
OTHER CONIFER SAWFLIES
Almost three dozen Neodiprion species develop on conifers in North America, mostly on pines. REDHEADED PINE SAWFLY (N. lecontei) is an important species in parts of the midwestern U.S. and southeastern Canada. It feeds on two- and three-needle pines, including Scotch, jack, shortleaf, loblolly, slash, red, and mugho. It produces two generations over much of its range, with the spring generation stripping old needles before bud break and the summer generation consuming new needles. Defoliated pines can be killed or have dead branches. Redheaded pine sawfly is gregarious and may form feeding groups that include dozens of larvae that twitch in unison when disturbed. Winter is spent as a prepupa in the cocoon, which is formed among leaf litter or dug shallowly in soil. Redheaded pine sawfly may remain dormant for a few years in this stage. Adults lay eggs in slits in rows along the needle edge.

B. Damage produced by European pine sawfly. DAVID SHETLAR

C. European pine sawfly, adult female. DAVID SHETLAR

D. European pine sawfly, adult male. DAVID SHETLAR

E. Eggs of European pine sawfly, inserted into needles. DAVID SHETLAR

F. European pine sawfly larvae shortly after egg hatch. DAVID SHETLAR

G. Redheaded pine sawflies. DAVID SHETLAR
Among the other Neodiprion species of importance, almost all occur in forest sites:
— N. swainei, SWAINE JACK PINE SAWFLY, a common species in eastern Canada that develops primarily on Jack pine;
— N. autumnalis, a common “pine sawfly” of western forests, particularly damaging to ponderosa pine. Feeding damage occurs in mid- to late summer;
— N. pinetum, WHITE PINE SAWFLY, a species that occurs throughout the natural range of its primary host, eastern white pine. Both new and old needles are consumed by this species, which can be seriously damaging during outbreaks;
— N. excitans, BLACKHEADED PINE SAWFLY, common on loblolly and shortleaf pines in the southeastern and Gulf states. Three to four generations per year may occur in southern areas of its range;
— N. edulicolis, PINYON SAWFLY, a common and sometimes outbreak species of pinyon in the southwestern states;
— and N. tsugae, HEMLOCK SAWFLY, found across the northern U.S. and Canada in association with the native range of either western or, possibly, eastern hemlock.
INTRODUCED PINE SAWFLY (Diprion similis)1 feeds primarily on white pine throughout the northeastern quadrant of the U.S. Scotch, jack, red, and Swiss mountain pines are less commonly infested. Adults lay about 10 eggs, in a row, on needles, and the young larvae feed gregariously in groups. The first-generation feeding occurs before bud break and confines feeding to the previous year’s needles, but a midsummer generation consumes both old and newly produced needles. Pupation occurs in a cocoon, often on the host tree.

Damage caused by pinyon sawflies. DAVID LEATHERMAN
The most important sawfly affecting spruce is YELLOWHEADED SPRUCE SAWFLY (Pikonema alaskensis),1 a species restricted to western North America. Adults lay eggs in late spring on current-season needles or tender twigs, and the larvae consume the new growth. Larvae have a reddish yellow to chestnut brown head and an olive green body. A single generation is produced, with winter spent as a full-grown larva in a cocoon at the base of previously infested trees.
The genus Monoctenus1 contains several species that are minor pests of conifers. JUNIPER SAWFLY (M. fulvus) feeds on the tips of junipers, sometimes checking the new growth and causing a thinning of the shrub; however, the larvae do not appear to feed heavily on shrubs, even when in high populations. Larvae are generally gray-green with an orange head. ARBORVITAE SAWFLY (M. melliceps) occasionally damages arborvitae and juniper in the northeastern states.
BULL PINE SAWFLY (Zadiprion townsendi)1 feeds on ponderosa pine needles in the western U.S. In an unusual life history, the larvae continue to feed on warm days throughout winter, becoming full grown in spring. Adults appear in summer to lay eggs, and the newly hatched larvae begin to feed on needles by early fall.
1 Hymenoptera: Diprionidae

B. Adult Neodiprion autumnalis ovipositing into needles. DAVID LEATHERMAN

C. Cocoon of the white pine sawfly. DAVID SHETLAR

D. White pine sawfly larva. DAVID SHETLAR

E. Imported pine sawfly. DAVID SHETLAR

F. Pinyon sawflies. DAVID LEATHERMAN

G. Mating pair of imported pine sawfly. JOHN GHENT, BUGWOOD.ORG

H. Eggs of imported pine sawfly inserted in needle. JOHN GHENT, BUGWOOD.ORG

I. Yellowheaded spruce sawfly. STEVEN KATOVICH, USDA FOREST SERVICE, BUGWOOD.ORG

J. Juniper sawfly. DAVID SHETLAR

K. Bull pine sawflies. WHITNEY CRANSHAW
IMPORTED CURRANTWORM/CURRANT SAWFLY (Nematus ribesii)1
HOSTS Currants, gooseberry.
DAMAGE The larvae chew the leaves of currants and gooseberries, often extensively defoliating plants early in the season. Foliage in the interior is damaged first, but all leaves may be eaten. Yield and quality of fruit can be affected by this injury.
DISTRIBUTION Northern half of U.S. and southern Canada.
APPEARANCE Larvae are generally light green-gray with numerous black spots; newly molted, they are uniformly light green. Adult females are thick-bodied wasps, about ⅓ inch long with a dark head and thorax and yellowish abdomen. Males are slightly smaller and more generally dark.
LIFE HISTORY AND HABITS Imported currantworm spends the winter in a cocoon in the soil around previously infested currants and gooseberries. The adults usually emerge shortly after the first leaves have expanded, and females insert eggs along the main veins of the leaf underside. The larvae hatch about 7–10 days after eggs are laid and at first chew small shotholes in the leaf interior. Later they disperse throughout the plant and feed along the leaf margins, becoming full grown in about 3 weeks. Young larvae are pale green but develop distinctive dark spots as they grow and reach a size of about ¾ inch.
The full-grown larvae drop to the ground and form a cocoon. Some pupate and emerge in late June and July, producing a small second generation. The majority remain dormant and emerge the following year.

Larvae of imported currantworm shortly after egg hatch, producing holes in the leaf interior. WHITNEY CRANSHAW
OTHER COMMON SAWFLIES1
WILLOW SAWFLY (Nematus ventralis) is occasionally abundant on willow in the central states and Prairie Provinces. Larvae are dark green and commonly feed in groups. Multiple generations may be produced. Larvae may remain dormant for two winters before completing development and emerging as adults in spring.
Two species of AZALEA SAWFLIES (Amauronematus azalae, Nematus lipvskyi) are damaging to azaleas in much of the Midwest and Northeast. The larvae are green and feed along the leaf edge, often consuming the entire leaf except the larger veins. One generation is produced annually.
BROWNHEADED ASH SAWFLY (Tomostethus multicinctus) is a sporadic but important early-season defoliator of ash in Colorado and many eastern states. Green ash is particularly damaged, but all species are susceptible.
Brownheaded ash sawfly spends the winter as a full-grown larva in a cocoon around the base of a previously infested ash tree. Pupation occurs in early spring. Adults are small black wasps that emerge in April and can sometimes be found in swarms around the tree. Females insert eggs into young leaves, usually around the edge, resulting in a slight distortion of the leaves. Early-stage larvae feed on the interior of the leaf, producing small pinhole feeding wounds. Older larvae feed extensively on the leaf, avoiding only the main veins. Larval development and feeding occur throughout May, and by early June individuals are full grown. Full-grown larvae shed a papery larval skin that remains attached to the leaf and then crawl to the ground, where they form protective cocoons.

B. Adult female of the imported currantworm/currant sawfly. WHITNEY CRANSHAW

C. Eggs of imported currantworm inserted into midribs of currant leaf. WHITNEY CRANSHAW

D. Adult of the willow sawfly. DAVID SHETLAR

E. Willow sawfly larvae. DAVID SHETLAR

F. Larva of the azalea sawfly. DAVID SHETLAR

G. Larvae of the brownheaded ash sawfly. JIM KALISCH, UNIVERSITY OF NEBRASKA

H. Pinhole feeding wounds produced by young larvae of the brownheaded ash sawfly. JIM KALISCH, UNIVERSITY OF NEBRASKA
BLACKHEADED ASH SAWFLY (Tethida cordigera) is associated with red, green, and white ash through much of the eastern U.S. It is also found in California. Habits are similar to those of brownheaded ash sawfly.
MOUNTAIN-ASH SAWFLY (Pristophora geniculata) is a common sawfly on mountain-ash east of the Mississippi River. Adults emerge in late spring and insert eggs in small groups along the leaf edge. The larvae usually feed gregariously, clinging to the leaf edge and consuming the entire leaf except the midrib before moving to the next leaf. Like many sawflies that feed as a group, they rear in unison in an S-shape when disturbed. Full-grown larvae drop to the soil and form a cocoon. Some complete pupation and produce a second generation in late summer. Others remain as prepupae in the cocoon and winter in this stage.
COLUMBINE SAWFLY (Pristophora aquiligae) defoliates columbine in late spring in areas of the Midwest, the Rocky Mountain region, and some eastern states. Larvae are pale green and apparently only a single generation is produced. CALIFORNIA PEAR SAWFLY (P. abbreviata) makes a semicircular cut, somewhat resembling that of leafcutter bees, in pear leaves. The larvae lie along the cut and blend well. There is only one generation produced, with peak feeding in late April and May. CHOKECHERRY SAWFLY (P. serrula) is a common species on chokecherry in the Rocky Mountain States. LARCH SAWFLY (P. erichsonii) is a European species that feeds on larch. It is now present in many areas of southern Canada and the northern U.S.
Larvae of RASPBERRY SAWFLY (Monophadnoides geniculatus) feed on the interior of raspberry leaves, cutting irregular holes between veins. When they are abundant, foliage may become very lacy, but raspberry sawflies develop rapidly and the full-grown larvae begin to crawl to the ground within about 2 weeks after hatching. They dig a small cell in the soil and spin a cocoon in which they remain until the following spring.
Several sawflies develop on leaves of rose (also see ROSESLUG, page 166). Larvae of the BRISTLY ROSE SAWFLY (Cladius difformis) have a pale green body covered with short bristles. CURLED ROSE SAWFLY (Allantus cinctus) is somewhat darker, with small white bristles. Both of these are European species now widely distributed in North America. More recently introduced are A. nigritibialis and A. viennensis, presently known to be present in parts of the Mid-Atlantic region and Ontario. In early stages, larvae of all of these sawflies skeletonize the leaves, but older larvae more generally chew foliage and can extensively defoliate plants. When full grown, larvae may move into the pith of stems to form a pupal chamber. Two generations are likely normal.
SPINY OAK SAWFLY (Periclista albicollis) develops on the leaves of several species of both red and white oaks. It ranges widely across the eastern half of North America in association with its hosts.
DUSKY BIRCH SAWFLY (Croesus latitarsus) is a common defoliator of birch and found widely in North America. Gray birch is the favored host. Larvae strongly hold a rather unusual S-shaped form while they feed along the leaf margin. One or two generations may occur annually. Larvae of another sawfly associated with birch, the FRINGED BIRCH SAWFLY (Dimorphopteryx melanognathus), are less commonly observed but have an unusual appearance with fringed projections along the sides of the body.
GRAPE SAWFLY (Erythraspides vitis) feeds on wild grape in the southern U.S. The larvae are distinctly marked with a double row of black tubercles on each segment and feed as groups on the leaf underside.
VIOLET SAWFLY (Ametastegia pallipes) feeds on wild and cultivated pansies and violets. The larvae are dark olive green with a black head. Several generations may occur, with injury most evident in late summer. Larvae skeletonize the underside of leaves, then feed more generally, later leaving the food host plant to pupate in the pith of larger nearby plants.

B. Mating pair of blackheaded ash sawfly. DAVID SHETLAR

C. Mountain ash sawflies. DAVID SHETLAR

D. Columbine sawflies. DAVID SHETLAR

E. Leaf injury produced by raspberry sawfly. WHITNEY CRANSHAW

F. Bristly roseslug larva. DAVID SHETLAR

G. California pear sawfly larva and damage. WHITNEY CRANSHAW

H. Dusky birch sawfly larva. DAVID SHETLAR
Young larvae of POPLAR LEAFFOLDING SAWFLY (Phyllocolpa bozemani) induce an unusual leaf fold along the edge of poplar, cottonwood, and willow. The young larvae wound the expanding leaf, causing it to curl around them. Later they move out and feed along the leaf edge. There is normally only one generation per year, completed by early summer.
DOGWOOD SAWFLIES (Macremphytus tarsatus, M. testaceus) feed on leaves of various dogwood, particularly gray dogwood. They are most common in the northeastern U.S. and Great Lakes area but known as far west as Colorado. Larvae appear white due to a thick waxy coating in early stages and become prominently spotted in the last larval instar. When full grown the larvae move away from the plant and excavate a chamber within which they pupate, often boring into wood of nearby posts, retaining walls, or building siding.
Monostegia abdominalis is also generally whitish, with some pale green coloration that is present in areas of eastern Canada and the northeastern U.S. It feeds on various Lysimachia (loosestrife, creeping jenny). Two generations are produced per year.
Several GRASS SAWFLIES in the genera Dolerus and Pachynematus develop on grasses, including some that may occur in turfgrass. Dolerus nitens is the most common turfgrass species in Ohio. Adults of both genera appear in very early spring, sometimes in numbers that alarm homeowners who see them on sidewalks and siding of their homes. The adults are primarily black, although some may have reddish markings.
The larvae are present from April to early June. They are rarely noticed as they usually don’t feed during the day, although they may be active on overcast days. The body is solid color, ranging from shades of gray to green, with a light brown head. Occasionally they may be observed on sidewalks as they migrate in search of pupation sites; pupation occurs in the soil.
Grass sawflies are more common on unmowed grasses, in pastures, and in small grains. The damage they produce is generally negligible, although Pachynematus setator (GRASS SEED SAWFLY) can be a serious pest of grass seed production as they may chew through the stems.

Last-instar larvae of dogwood sawfly resting on underside of leaf. WHITNEY CRANSHAW
One of the sawflies of most unusual appearance is BUTTERNUT WOOLLYWORM (Eriocampa juglandis). Larvae are covered with long threads of whitish wax and develop on the foliage of nut trees in the eastern states.
1 Hymenoptera: Tenthredinidae

B. Young larvae of dogwood sawfly shortly after egg hatch. DAVID SHETLAR

C. Dogwood sawfly larva. WHITNEY CRANSHAW

D. Larva of Monostegia abdominalis on Lysmachia. DAVID SHETLAR

E. Adult of the grass sawfly. DAVID SHETLAR

F. Grass sawfly. DAVID SHETLAR

G. Butternut woollyworm. BRUCE WATT, UNIVERSITY OF MAINE, BUGWOOD.ORG
OTHER SAWFLIES THAT CHEW ON LEAVES
BIRCH SAWFLY (Arge pectoralis)1 is the most commonly encountered of the argid sawflies, a small family of sawflies marked by dusky wings, a dark body, and unusually developed antennae on the males. They are found in the northeastern U.S. and southern Canada, where they feed primarily on birches, occasionally on alder and willow. Larvae feed during summer and are yellow with six dark spots along the side.
HOLLYHOCK SAWFLY (Neoptilia malvacearum)1 can be very damaging to hollyhock in Ohio and some Mid-Atlantic States. Young larvae first feed on the leaf undersurface and produce windowpane injuries on the leaves. They later skeletonize extensively. The larvae are light green with dark spots. Adults are all black or black with an orange thorax. Two generations normally occur in Ohio, with a small third generation in warm seasons. HIBISCUS SAWFLY (Atomacerca decepta)1 has been reported to damage hibiscus, marshmallow, buttonbush, and some other perennial plants in the Mid-Atlantic States. Schizocerella lineata1 chews on the leaves of purslane. It is closely related to another purslane feeding species, S. pilicornis, which develops as a leafminer and does not feed externally as a defoliator.
ELM SAWFLY (Cimbex americana)2 is a large sawfly, widely distributed with a host range that includes willow, elm, and occasionally basswood, birch, maple, poplar, and alder. When feeding, the larvae often coil the hind end around an adjacent twig. Elm sawfly larvae can be distinguished by their development of unusual pebbly skin when nearly full grown, pale yellow-green coloration, and dark stripe down the back. They feed during the summer and are rarely abundant enough to cause significant damage, although isolated outbreaks are reported. Trichiosoma triangulum2 is a related species that feeds on ash, birch, poplar, willow, and wild cherry. T. viminalis is common on willow and poplar in the Midwest.
1 Hymenoptera: Argidae
2 Hymenoptera: Cimbicidae
A. Arge quidia, feeding on northern red oak. BRUCE WATT, UNIVERSITY OF MAINE, BUGWOOD.ORG

B. Hollyhock sawfly larva. DAVID SHETLAR

C. Hollyhock sawfly adult. DAVID SHETLAR

D. Elm sawfly. HERBERT A. “JOE” PASE III, TEXAS A&M FOREST SERVICE, BUGWOOD.ORG

E. Elm sawfly adult. DAVID SHETLAR

F. Hibiscus sawfly larva. WHITNEY CRANSHAW
PEARSLUG (PEAR SAWFLY, CHERRY SLUG) (Caliroa cerasi)1
HOSTS Sweet and ornamental varieties of cherry, plum, hawthorn, pear, and cotoneaster.
DAMAGE Larvae feed on the upper surface of leaves, producing distinctive skeletonizing wounds. Heavily damaged leaves turn brown and drop early.
DISTRIBUTION Throughout most of the northern half of the U.S. and southern Canada.
APPEARANCE Pearslug larvae are shiny and sluglike in general appearance but can be somewhat variable in color. Many are a dark olive green, but later instars tend to be lighter and may even have orange tones. Adults are shiny black stout-bodied wasps, about ⅓ inch long.
LIFE HISTORY AND HABITS Adults emerge in late June or early July. Females insert their eggs singly in circular slits on the upper surface of leaves. Eggs hatch in about 2 weeks, and larvae chew small pits in the upper surface of leaves during early development. Later they more extensively chew the leaves but always avoid feeding on the larger veins and lower leaf surface, producing a typical skeletonizing injury pattern.
When larvae are full grown they wander off the plant and dig a shallow cell in the soil to pupate. Pupation occurs in a small cocoon encrusted with soil particles. In about 2 weeks, many of the insects continue development and emerge as adults to produce a second generation. Larvae of these typically cause peak injury in early September. Populations of this second generation are usually smaller than those of the first generation, since many produced in the first generation remain dormant until the following spring. The full-grown larvae from the September generation drop to the soil and spin a cocoon in which they spend the winter. They pupate the following spring.
OTHER SLUG SAWFLIES1
Larvae of ROSESLUG (Endelomyia aethiops) are smooth and pale green, largely lacking the shiny moist surface of the pearslug. They feed as skeletonizers on the upper surface of rose leaves, typically leaving the lower epidermis intact, giving a windowpane appearance to leaves. Most injury is completed by late spring, and there is only one generation produced per year.
SCARLET OAK SAWFLY (Caliroa quercuscoccineae) develops on scarlet and pin oak, producing skeletonizing injuries to the lower leaf surface. Eggs are inserted into leaves; upon hatch the larvae migrate to the underside of the leaf. Younger larvae, which are pale green, feed gregariously, typically lined up next to each other. Older larvae turn green and they are solitary in the late stages. Two to three generations may occur annually, with damage most evident in late summer. Other oak-infesting slug sawflies include C. petiolata on pin oak; C. lobata on pin and black oak; C. fasciata on black, pin, and red oak; and C. obsoleta on white oak.
1 Hymenoptera: Tenthredinidae

B. Adult pearslug. WHITNEY CRANSHAW

C. Young pearslug next to egg scar. WHITNEY CRANSHAW

D. Roseslug larva. DAVID SHETLAR

E. Damage typical of roseslug. DAVID SHETLAR

F. Damage produced by larvae of scarlet oak sawfly. JIM KALISCH, UNIVERSITY OF NEBRASKA

G. Oakslugs. DAVID SHETLAR
TEXAS LEAFCUTTING ANT (Atta texana)1
HOSTS A wide variety of plants, including grasses, small fruit, plum and peach, nut trees, certain ornamentals, and weeds.
DAMAGE Leafcutting ants remove plant buds and cut leaf fragments, causing potentially serious foliage loss. Young plants are at particular risk and may be entirely defoliated in a short period. Leafcutting ants may travel over 600 feet from the colony to locate forage.
DISTRIBUTION Western Louisiana, east to south-central Texas and northern Mexico.
APPEARANCE Leafcutting ant workers are rusty brown or dark brown. On close inspection they have three pairs of spines on the thorax and one pair on the head. Size varies considerably, from 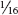 to ½ inch long. Reproductive stages (males, potential queens) are larger and have dusky-colored wings.
to ½ inch long. Reproductive stages (males, potential queens) are larger and have dusky-colored wings.
LIFE HISTORY AND HABITS Leafcutting ants are fungus gardeners. The leaf fragments they cut from plants are not eaten but instead used to cultivate a fungus that grows in special chambers deep in the colony. Parts of this fungus provide the sole food source of the leafcutting ant.
Crater-shaped mounds around the nest entrance are external evidence of leafcutting ant colonies. These mounds may be from 5 to 14 inches high and 1 to 1½ feet in diameter. The belowground colony can be very large, extending 15–20 feet below the surface. Very large colonies may survive for several years and contain up to 2 million individuals.
Winged reproductive forms emerge for mating flights on clear, moonless nights in late spring following heavy rains. Females that mate during this flight subsequently attempt to establish new colonies, with little chance of success. They carry with them a small packet of the essential fungus and will use it to establish fungal gardens.
1 Hymenoptera: Formicidae
LEAFCUTTER BEES (Megachile spp.)1
HOSTS Many broadleaf plants, particularly rose, ash, lilac, and Virginia creeper.
DAMAGE Leafcutter bees do not eat plants, but adults do make smooth semicircular cuts along the edges of leaves and flowers. Damage is usually cosmetic, but serious defoliation is sometimes reported, particularly in desert areas of western states where isolated plantings of favorable plants are grown.
DISTRIBUTION Throughout North America, common. Approximately 140 native species occur in North America.
APPEARANCE Adults are usually black or gray and slightly smaller than a honey bee. Pollen is carried on hairs on the underside of the abdomen rather than in pollen baskets on the legs as in some other bees.
LIFE HISTORY AND HABITS Winter is spent as a pupa in a cell lined with leaf fragments fastened together by the adult females. In late spring and early summer the adults emerge. Females seek natural hollows for nest sites or excavate tunnels in rotten wood or the pith of plant stems. They then create nest cells lined with pieces of cut leaves or flower petals. When complete, these somewhat resemble a cigar butt, and individual cells may involve more than two dozen leaf fragments cemented together. The nest cells are then packed with a mixture of pollen and nectar, and an egg is laid in each one. Larvae develop in the cells. One generation is produced per year.
1 Hymenoptera: Megachilidae
A. Texas leafcutting ants cutting leaf. SUSAN ELLIS, BUGWOOD.ORG

B. Texas leafcutting ants carrying leaf fragments. SUSAN ELLIS, BUGWOOD.ORG

C. Trail produced by Texas leafcutting ants. DAVID SHETLAR

D. Leafcutter bee damage to rose. JIM KALISCH, UNIVERSITY OF NEBRASKA

E. Drilled “bee board” exposing nest cells produced by leafcutter bees. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

F. Leafcutter bee cutting a leaf disk. DAVID SHETLAR

G. Larvae of leafcutter bees exposed within nest cells. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

H. Leafcutter bee collecting nectar from sweet pea. WHITNEY CRANSHAW

I. Contents of nest cell produced by a leafcutter bee. The large oblong object in the center is the cocoon within which a developing bee spends the winter. WHITNEY CRANSHAW
Leaf beetles (Chrysomelidae family) are among the largest insect families in North America, containing about 1,500 species, several of which are serious plant pests. All species feed on plants in both adult and larval stages. Among the different species there is a wide range in the size of the adults (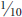 to nearly ½ inch), and most are oval or elongate oval in form. Many leaf beetles are brightly colored, others have banded or striped patterning. Larvae are varied in shape, with grub to sluglike, thin-elongate, and/or spiny larvae being common forms. Larvae can feed on plant foliage, mine leaves (page 218), or bore into roots (page 482).
to nearly ½ inch), and most are oval or elongate oval in form. Many leaf beetles are brightly colored, others have banded or striped patterning. Larvae are varied in shape, with grub to sluglike, thin-elongate, and/or spiny larvae being common forms. Larvae can feed on plant foliage, mine leaves (page 218), or bore into roots (page 482).
COLORADO POTATO BEETLE (Leptinotarsa decemlineata)1
HOSTS Colorado potato beetle is most common and damaging to potato but may also be found on eggplant, nicotiana, petunia, and certain nightshade weeds.
DAMAGE Both the adult beetles and the larvae chew on foliage. Notching wounds along the leaf margin are more typical of adults, whereas larvae produce more ragged injuries and may soil foliage with excrement. Although plants can usually tolerate low levels of leaf loss (at least 25%), extensive and damaging defoliation occurs during outbreaks. Colorado potato beetle is considered the most important potato insect pest worldwide, being very damaging throughout much of Europe. Management is often complicated by high levels of pesticide resistance that have developed in many populations.
DISTRIBUTION Colorado potato beetle has spread dramatically over the past 150 years, and it is now generally found east of the Rockies, excluding some areas around the Gulf of Mexico and the Maritime Provinces. It is most damaging in the eastern and central states.
APPEARANCE Adults are about ⅜ inch long, generally oval in shape and of convex body form. Alternating yellow and black stripes run along the back. Larvae are reddish or red-orange grubs with a dark head and some dark spotting. Colorado potato beetle produces masses of orange-yellow eggs that are somewhat similar to those of lady beetles but larger and darker in color.
Other arthropods are sometimes confused with Colorado potato beetle. Certain blister beetles were sometimes known as “old-fashioned potato beetles” because they were conspicuous on potato before Colorado potato beetle was present. Pillbugs (page 530) are sometimes called “potato bugs” because of their habit of attacking potato starts that may get too wet in the spring. In some areas Jerusalem cricket (page 514) is also called a “potato bug.”
LIFE HISTORY AND HABITS Colorado potato beetles winter in the adult stage under cover near plantings infested the previous season. They are capable of flying over moderate distances and move back to fields and gardens in late spring as potatoes and other susceptible plants emerge. Eggs begin to be laid in late spring as potatoes first emerge. The orange-red larvae feed on leaves, originally in groups but later dispersing individually throughout the plant. After becoming full grown, they drop from the plant and pupate in the soil. In about two weeks, adult beetles emerge and feed on plants. A second generation is typically produced in southern areas of the range; one generation predominates in the north. After feeding for a few weeks, adults disperse to overwintering sheltering areas and remain dormant until the following season.
RELATED INSECTS In eastern North America the closely related FALSE POTATO BEETLE (Leptinotarsa juncta) is present. Adults and larvae feed on horsenettle, ground cherry, and bittersweet, but not potato.

B. Colorado potato beetle egg mass. WHITNEY CRANSHAW

C. Colorado potato beetle larvae hatching from eggs. WHITNEY CRANSHAW

D. Colorado potato beetle larvae. WHITNEY CRANSHAW

E. Damage to potato by Colorado potato beetle. WHITNEY CRANSHAW

F. Pupa of the Colorado potato beetle. WHITNEY CRANSHAW
C2g False potato beetle, Leptinotarsa juncta. JOHNNY N. DELL, BUGWOOD.ORG

G. False potato beetle, Leptinotarsa juncta. JOHNNY N. DELL, BUGWOOD.ORG
ASPARAGUS BEETLE (Crioceris asparagi)1
HOSTS Asparagus.
DAMAGE Adult beetles chew pits in emerging spears, causing distortions of growth, and large numbers of the dark eggs may be laid on spears. Most injury, however, is caused by the larvae that chew the ferns, giving the plant a bleached appearance. Extreme infestations can reduce subsequent yields and sometimes even kill plants. Moderate asparagus beetle injury is tolerated by plants with little or no effect. Incidence of parasitism by the tiny eulophid wasp, Tetrastichus asparagi, is often very important in regulating abundance of asparagus beetle.
DISTRIBUTION This insect of European origin is most common in the Midwest and in states along the Atlantic Coast; however, it can now be found in Colorado, California, and Oregon.
APPEARANCE The adult beetle is about ¼ inch long, of general metallic blue-black color; however, it is also marked with yellowish square spots and has some red along the margins of the wing cover and prothorax. Larvae are generally gray with a dark head and rather sluglike in appearance.
LIFE HISTORY AND HABITS Asparagus beetles overwinter in the adult stage around asparagus plantings. They become active in spring and fly to emerging spears and mate. The beetles chew on spears, and females lay upright dark eggs on the plant. Eggs hatch in about 1 week, and the gray, grublike larvae feed on the ferns. The larvae become full grown in about 2–3 weeks and drop to the soil to pupate. Adults emerge and repeat the cycle. Two or three generations are completed in a season, with some larvae present into September.
RELATED SPECIES
SPOTTED ASPARAGUS BEETLE (Crioceris duodecimpunctata)1 is often as common as asparagus beetle but is far less damaging to asparagus. The adult is bright orange with black spots. Larvae of this beetle develop in asparagus berries but do not damage the ferns, causing little injury. Minor chewing on ferns and spears is done by adults.
TOMATILLO LEAF BEETLE (Lema trivittata)1 is widely distributed west of the Mississippi. Rarely seriously damaging, the grayish grublike larvae feed on leaves of various Physalis spp. nightshade family plants, particularly tomatillo and Chinese lantern. The larvae are further distinguished by their use of a “fecal shield,” created by piling moist feces on the back. Adults are generally yellow with some reddish markings and have distinct black stripes that give a fair resemblance to striped cucumber beetle and western corn rootworm. A very closely related species, Lema daturaphila, is also associated with nightshade family plants, particularly Datura spp. The taxonomy of the various Lema species is presently unsettled and major questions remain about the identity of the species present in North America and their host range.
The RED LILY LEAF BEETLE (Lilioceris lilii)1 is a European species that has become established in areas of the Northeast. It feeds on the foliage of true lilies (Lilium spp.) and Fritillaria spp. and can extensively defoliate plants. Adults are bright red with a black head and appendages and about ¼ to ⅜ inches long. The larvae are generally sluglike in appearance and cover themselves with their feces; their overall color is variable but they are most often orange or orange-brown and have a black head.
CEREAL LEAF BEETLE (Oulema melanopus)1 is an accidentally introduced European species first detected in Michigan in 1962. Since then it has steadily increased its range and can be found in most states in the northern half of the U.S. Both adults and larvae feed on the leaves of grasses, with larvae producing extensive skeletonizing. Cereal leaf beetle is primarily a concern for small grain crops (e.g., wheat, rye, barley, oats), but some ornamental grasses found in gardens are also hosts.

B. Asparagus beetle eggs. WHITNEY CRANSHAW

C. Asparagus beetle damage. WHITNEY CRANSHAW

D. Asparagus beetle larvae. WHITNEY CRANSHAW

E. Spotted asparagus beetle. WHITNEY CRANSHAW

F. Tomatillo leaf beetle. WHITNEY CRANSHAW

G. Larvae of the tomatillo leaf beetle. WHITNEY CRANSHAW

H. Tomatillo beetle larva. WHITNEY CRANSHAW

I. Red lily leaf beetle. BRUCE WATT, UNIVERSITY OF MAINE, BUGWOOD.ORG

J. Red lily leaf beetle eggs. BRUCE WATT, UNIVERSITY OF MAINE, BUGWOOD.ORG

K. Red lily leaf beetle larvae. TOM MURRAY

L. Cereal leaf beetle. DAVID SHETLAR
STRIPED CUCUMBER BEETLE (Acalymma vittatum)1
HOSTS A wide variety of vegetables, although cucumber, melon, squash, and other cucurbits are preferred and most severely damaged.
DAMAGE Adult beetles feed on emergent seedlings and can retard development or even kill young plants. Later they may be found in large numbers in flowers of squash or melon and may chew pits in fruit. Larvae feed on the roots, causing little apparent injury, but may move into the rind of ripening melon fruit that rests on soil. Adult beetles can transmit a bacterium (Erwinia tracheiphila) that produces bacterial wilt in cucurbits. Striped cucumber beetle is also a vector of cucumber mosaic virus.
DISTRIBUTION Broadly distributed east of the Rockies. A closely related species, WESTERN STRIPED CUCUMBER BEETLE (A. trivittatum), is found in the Pacific States. The latter is yellowish orange with three black stripes and has a similar life history but is less often damaging.
APPEARANCE Adult beetles are about ⅕ inch long and bright yellow with three even, dark black stripes. The brighter yellow color, uniform width of the stripes, and presence of rows of minute indentations on the wing cover separate this insect from western corn rootworm, with which it is often confused.
LIFE HISTORY AND HABITS Adult beetles spend winter under debris in the vicinity of gardens and fields. As temperatures warm in spring, they become active and feed on leaves and flowers (pollen) of several different trees and shrubs. When squash-family host plants emerge, the beetles move to these plants and chew seedlings. Eggs are laid in cracks around the base of the plants, and the hatching larvae feed on the roots for about a month. They then pupate in the soil, later emerging as adult beetles. There typically are two generations per season, but this can range from one to three depending on location and weather.
RELATED SPECIES
Diabrotica undecimpunctata howardi,1 known as both SPOTTED CUCUMBER BEETLE and SOUTHERN CORN ROOTWORM, feeds on a very wide range of plants, notably cucurbits and corn. Adults may be found in almost any type of flower. Most damage is done by adults that chew on flowers, foliage, and rinds of ripening fruit. Larvae may tunnel roots and can stunt plants and sometimes kill seedlings. It occurs east of the Rockies and ranges deep into Mexico. In the Pacific States a subspecies occurs known as WESTERN SPOTTED CUCUMBER BEETLE (D. u. undecimpunctata).
1 Coleoptera: Chrysomelidae
A. Striped cucumber beetles. CLEMSON UNIVERSITY–USDA COOPERATIVE EXTENSION SLIDE SERIES, BUGWOOD.ORG

B. Seedling damage by striped cucumber beetle. WHITNEY CRANSHAW

C. Bacterial wilt symptoms in pumpkin. JIM JASINSKI, OHIO STATE UNIVERSITY EXTENSION, BUGWOOD.ORG

D. Striped cucumber beetle larva underneath melon. WHITNEY CRANSHAW

E. Larval tunneling injury in base of squash. WHITNEY CRANSHAW

F. Striped cucumber beetles, and one western corn rootworm, in discarded melon. WHITNEY CRANSHAW

G. Mating pair of western striped cucumber beetles. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

H. Spotted cucumber beetle/southern corn rootworm. WHITNEY CRANSHAW

I. Western spotted cucumber beetle. WHITNEY CRANSHAW

J. Spotted cucumber beetle and striped cucumber beetle on squash fruit. WHITNEY CRANSHAW
In most areas, winter is usually spent in the adult stage in plant debris and particularly among plants that have not been completely killed by frosts. Spotted cucumber beetle may be active year-round in warmer climates, whereas in northern areas it may die out and infestations may result from migration from southern states during the growing season. Adults feed on pollen, corn silks, tender foliage, and other plant parts. Eggs are laid in soil cracks near seedlings of host plants in the spring, and larvae feed on roots for about 1 month. One to three generations may be produced during a growing season, but they overlap considerably and are difficult to distinguish.
Two other insects are also known as “corn rootworms”: the WESTERN CORN ROOTWORM (Diabrotica virgifera virgifera) and the NORTHERN CORN ROOTWORM (D. barberi). Both are primarily important in the larval stage (page 482), which feeds on the roots of corn, and they are the most important insect pests of field corn grown in the Corn Belt states. Adults, which are present throughout summer, can be found feeding primarily on leaves and silk of corn and on pollen and flowers of squash family plants. Incidental damage may also occur on leaves of a wide range of other plants, particularly leafy vegetables.
BANDED CUCUMBER BEETLE (D. balteata) is originally from the southwestern U.S. and Mexico but has extended its range throughout the southern U.S. Adults feed on foliage and flowers of a wide range of vegetables. Larvae chew roots. Squash, melon, bean, and some crucifers are commonly damaged. Larval injury may also be severe in tubers of sweetpotato. The life history is similar to that of spotted cucumber beetle, and there can be nearly continual breeding in areas of warm climate.
Also found feeding on various squash family plants in the southwestern states is the CHECKERED MELON BEETLE (Paranapiacaba tricincta),1 a colorful leaf beetle with black and white spotting. Large masses of adults can sometimes seriously damage young plants of squash and melons, with honeydew being particularly susceptible to injury.
OTHER LEAF BEETLE DEFOLIATORS
RED TURNIP BEETLE (Entomoscelis americana)1 can be a common vegetable garden pest in the northwestern U.S. and western Canada. In spring it feeds on most of the crucifers, including cabbage, broccoli, mustard, radish, watercress, and horseradish, and it can be damaging in both larval and adult stages. Larvae hatch from overwintered eggs and chew foliage, stems, and cotyledons of seedlings and young plants. Pupation occurs in the soil, and the adults may also feed on foliage for a brief period before undergoing a period of summer dormancy. Adults become active again in late summer, feed on foliage of crucifers, and lay eggs, which are the wintering stage.
YELLOWMARGINED LEAF BEETLE (Microtheca ochroloma)1 feeds on crucifers in some of the Gulf States. Both adults and larvae feed on foliage during spring. Only a single generation appears to be produced, with overwintered adults moving to plants in spring and laying eggs on the leaves. The life history of this introduced species is poorly understood in the U.S., but adults appear to go through a period of dormancy during the summer months.
SWEETPOTATO LEAF BEETLE (Typophorus nigritus)1 occurs in most of the southeastern U.S. Adults feed on foliage of sweetpotato and morning glory, often in groups, and cause some localized defoliation; however, most damage to sweetpotato is produced by the larvae, which develop on the roots and stems. They may burrow deeply into the sweetpotato tubers or limit feeding to scarring of the surface. One generation is produced, with adults emerging from their overwintering pupal cells beginning in late May and early June.

B. Western corn rootworm leaf feeding injury to corn leaf. DAVID KEITH, UNIVERSITY OF NEBRASKA

C. Comparison of western corn rootworm (left, center) and striped cucumber beetle. WHITNEY CRANSHAW

D. Comparison of western corn rootworm and northern corn rootworm. JIM KALISCH, UNIVERSITY OF NEBRASKA

E. Checkered melon beetles damaging honeydew. WHITNEY CRANSHAW

F. Banded cucumber beetle. RUSS OTTENS, UNIVERSITY OF GEORGIA, BUGWOOD.ORG

G. Yellowmargined leaf beetle. JOHN CAPINERA, UNIVERSITY OF FLORIDA

H. Yellowmargined leaf beetle larva. JOHN CAPINERA, UNIVERSITY OF FLORIDA
BEAN LEAF BEETLE (Ceratoma trifucata)1 is common in the eastern U.S. It feeds on a wide variety of legumes and is most damaging to soybean but will damage garden beans, although it is far less common than Mexican bean beetle. It is particularly abundant on early planted beans, and adults may cause serious defoliation of small plantings. Larvae develop on the roots of beans but apparently cause little injury. Two or three generations may occur annually.
SUNFLOWER BEETLE (Zygogramma exclamationis)1 is a common insect associated with sunflower in parts of the Midwest and High Plains. Adults are somewhat similar to Colorado potato beetle in shape and patterning, although a bit smaller. Sunflower beetle larvae, which are pale green and grublike, feed on the leaves of sunflower and some related plants.
MILKWEED LEAF BEETLE (Labidomera clivicollis)1 feeds on the leaves and sometimes the flowers of milkweeds. The bright orange and black markings of the adults somewhat resemble those of lady beetles. Eggs are laid on leaves and the plump-bodied larvae feed for a few weeks until full grown, when they drop to the ground and pupate in the soil. Two generations are produced annually.
DOGBANE BEETLE (Chrysochus auratus)1 is a brilliantly colored, iridescent beetle associated with dogbane (Apocynum spp.), uncommonly found on a few other broadleaf plants. It is not considered a pest species but often attracts attention because of its vivid coloration and moderately large size (¾–½ inch). Larvae develop on the roots of dogbane. Dogbane beetle is widely distributed east of the Rocky Mountains.
The RUBBER RABBITBRUSH BEETLE (Trirhabda nitidicollis)1 is an early season defoliator of Ericameria nauseosa in the western U.S. Winter is spent as eggs laid in small masses in the soil, and they are stimulated to hatch by spring rains associated with production of new growth. The larvae climb the plant to feed on leaves, and they are an unusual metallic dark blue color. Pupation occurs in the soil and later the adults emerge, feed for a brief period, then lay the overwintering eggs. Other Trirhabda in the western U.S. are associated with yellow rabbitbrush, big sagebrush, coyote brush, and brittlebush. In eastern North America Trirhabda spp. are most often observed associated with goldenrods. At least five goldenrod-feeding species occur, with T. virgata often the most commonly observed species in the Mid-Atlantic States. T. bacharidis commonly defoliates eastern baccharis (sea or salt myrtle) in Gulf States.
STRAWBERRY ROOTWORM (Paria fragariae)1 is a common insect associated with strawberry, and less commonly blueberry, in much of eastern North America and California. Larvae feed on roots, but most damage is in the form of leaf chewing by adults, which feed at night. Feeding injuries may conspicuously riddle leaves, although effects on plant growth are usually minor.
Adult beetles are about ⅛ inch long and shiny black, with four light patches on the wing covers. They emerge from overwintering cover in spring and alternate between feeding and egg-laying at this time. Larvae develop during late spring and early summer. Adults are present again in late summer and also feed at this time before moving to winter shelters.
Adults of CRANBERRY ROOTWORM (Rhabdopterus picipes)1 produce conspicuous feeding injuries to rhododendron, camellia, photinia, and other shrubs. They feed at night on the emerging leaves, causing elongated, often crescent-shaped feeding holes in the mature leaves. Larvae develop on the roots of cranberry and blueberry but rarely cause much injury.

Damage by cranberry rootworm. CHAZZ HESSELEIN, ALABAMA COOPERATIVE EXTENSION SYSTEM, BUGWOOD.ORG
1 Coleoptera: Chrysomelidae

B. Bean leaf beetle damage. JIM KALISCH, UNIVERSITY OF NEBRASKA

C. Sunflower beetle. WHITNEY CRANSHAW

D. Sunflower beetle larva. WHITNEY CRANSHAW

E. Milkweed leaf beetle. TOM MURRAY

F. Milkweed leaf beetle larva. DAVID SHETLAR

G. Dogbane leaf beetles. DAVID SHETLAR

H. Rubber rabbitbrush beetle. WHITNEY CRANSHAW

I. Larva of the rubber rabbitbrush beetle. WHITNEY CRANSHAW

J. Larva of the goldenrod leaf beetle. DAVID SHETLAR

K. Strawberry rootworm adult. CHAZZ HESSELEIN, ALABAMA COOPERATIVE EXTENSION SYSTEM, BUGWOOD.ORG

L. Damage by strawberry rootworm. CHAZZ HESSELEIN, ALABAMA COOPERATIVE EXTENSION SYSTEM, BUGWOOD.ORG

M. Goldenrod leaf beetle. DAVID SHETLAR
Tortoise beetles are a subfamily (Cassidinae) of the leaf beetles. Adults have a broad body form and are sometimes mistaken for lady beetles; some can produce brilliant metallic coloration. Larvae are rather flattened and spiny but most are marked by their habit of carrying a shield of old cast skins and feces attached to the tip of the abdomen but covering much of the body. Adults characteristically chew large holes in the interior of leaves; larvae skeletonize leaves.
GOLDEN TORTOISE BEETLE (Charidotella sexpunctata)1
HOSTS Morning glory, sweetpotato, field bindweed, and related plants in the morning-glory family (Convulvulaceae).
DAMAGE Adults and larvae feed on foliage, producing shothole-type wounds in the leaf interior. Damage is insignificant for plant health, but the unusual appearance of the insects and the leaf riddling they produce may attract attention.
DISTRIBUTION Tortoise beetles are most common in the eastern U.S., particularly the southeast. Several species have ranges extending to the Rocky Mountains, Texas, and into Mexico.
APPEARANCE Adults are generally oval, somewhat flattened, with the head tucked under the shield of the prothorax; however, what attracts attention is their bright metallic gold coloration. Because of this feature they are sometimes mistaken for golden lady beetles and have also been known as “gold bugs.” Larvae are generally flattened and spiny but are shielded on top by their old cast skins and feces, so they look gray, brown, or black when viewed from above.
LIFE HISTORY AND HABITS Tortoise beetles winter as adults, emerging in late spring when they begin to feed on leaves. Females subsequently lay eggs, in a series of small clusters, on the underside of leaves.
Immature stages chew the underside of leaves. Upon molting they subsequently carry the previous cast skin, held by a pair of movable forked spines on the hind end. The skins, plus feces, create a shield that arches over the body. The habit of carrying this debris on their backs, common among tortoise beetles, leads to their sometimes being called “peddlers.”
Larval development is completed within a month. Pupation occurs on leaves and stems to which they attach by the hind end. The pupae are dark colored, somewhat spiny, and often still covered with debris. Adults emerge in 7–10 days and feed on leaves for a short while before going into dormancy. Although relatively little is known about the life history, one generation per year seems normal.
The bright coloration of this insect is what attracts most attention. The coloration can vary depending on angle of view, being brightest when viewed from above and more reddish brown from a side angle. Coloration also varies depending on condition of the beetle, which can rapidly lose much of its reflection when disturbed. This effect is achieved by differences in the hydration of the exoskeleton, which is largely translucent but constructed of minute channels that can carry moisture. When hydrated, the grooves cause the exoskeleton to reflect light in a mirror-like fashion, producing the brilliant metallic coloration. The underlying darker pigments become exposed when the insect reduces movement of water into the surface channels of the exoskeleton, or when it dies.

B. Golden tortoise beetle. DAVID SHETLAR

C. Late-instar larva of the golden tortoise beetle. WHITNEY CRANSHAW

D. Young larva of the golden tortoise beetle. WHITNEY CRANSHAW

E. Mottled tortoise beetle. DAVID SHETLAR

F. Argus tortoise beetle. DAVID SHETLAR
OTHER COMMON TORTOISE BEETLES
Several other tortoise beetles are associated with morning-glory family plants. The MOTTLED TORTOISE BEETLE (Deloyala guttata)1 also has extensive areas of metallic gold coloration but is more extensively patterned with black than the golden tortoise beetle. Four broad dark stripes on the wings are characteristic of the STRIPED TORTOISE BEETLE (Agrioconota bivittata),1 which also can show reflected metallic coloration. The BLACK-LEGGED TORTOISE BEETLE (Jonthonota nigripes)1 is more generally reddish or brown, with black spotting. More extensive black spotting is present on the ARGUS TORTOISE BEETLE (Chelymorpha cassidea),1 which is primarily yellow or orange-brown. Chelymorpha cribraria is a species recently established in southern Florida that can show wide range in coloration.
EGGPLANT TORTOISE BEETLE (Gratiana pallidula)1 is found throughout the eastern U.S. but is abundant only in the southern states. It often has spotted or striped markings. It makes small holes in the foliage of eggplant, potato, and various nightshade family weeds but does not cause serious injury. CLAVATE TORTOISE BEETLE (Plagiometriona clavata) also develops on nightshade family plants. SUNFLOWER TORTOISE BEETLE (Physonota helianthi) develops on sunflowers in the midwestern and northeastern states. PALMETTO TORTOISE BEETLE, Hemisphaerota cyanea,1 is found in coastal areas of the southeastern U.S. to Texas, where it feeds on foliage of Washington palms. It is dark gun-metal blue to purple and about 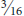 inch in length. The yellowish-white larvae have conspicuous projections along the sides and cover themselves with fecal matter.
inch in length. The yellowish-white larvae have conspicuous projections along the sides and cover themselves with fecal matter.
The THISTLE TORTOISE BEETLE (Cassida rubiginosa)1 is a green-colored beetle that feeds on leaves of Canada thistle, sometimes causing extensive defoliation. It is a European species that was purposely introduced in Virginia as a potential biological control of Canada thistle and has subsequently spread widely (sometimes with intentional human assistance) so that it can be found throughout much of North America where its host plant is present.
1 Coleoptera: Chrysomelidae (Cassidinae)
CASE-BEARING LEAF BEETLES
SYCAMORE LEAF BEETLE (Neochlamisus platani)1 feeds on foliage of American sycamore in both the adult and larval stages. River birch, hazel, and elm are additional, but infrequent, host plants. Eggs are laid singly on leaves and covered by a bit of fecal material. Upon hatch the larvae feed on leaves and almost immediately begin constructing a case that covers the abdomen. This case is constructed primarily of feces produced by the insect, mixed with some plant material, and it tapers at the tip. The larvae expand the case as they grow and feed on all of the leaf except for major veins. Pupation also occurs in the case, which is often attached to twigs and resembles a bud. Adults emerge in summer, feed for a period, and then move to winter shelter. Adults, a type of “warty leaf beetle,” are small, bronze to reddish-bronze beetles of irregular rounded body form, somewhat resembling a caterpillar dropping.
At least 17 Neochlamisus species occur in North America, almost all of which occur east of the Rocky Mountains. Most are quite host specific: BLUEBERRY CASEBEETLE (N. cribripennis) feeds on native lowland blueberry in eastern Canada and the northeastern U.S., whereas caneberries (Rubus spp.) host N. bimaculatus. Within the genus, N. bebbianae is unusual in having a fairly wide host range that includes specific plants that occur in five plant families. In addition, there are nine species of North American warty leaf beetles in the genus Exema that develop on aster family plants.
1 Coleoptera: Chrysomelidae

B. Clavate tortoise beetle. TOM MURRAY

C. Sunflower tortoise beetle. TOM MURRAY

D. Sunflower tortoise beetle larva. TOM MURRAY

E. Thistle tortoise beetle. WHITNEY CRANSHAW

F. Palmetto tortoise beetle. JOHNNY H. DELL, BUGWOOD.ORG

G. Thistle tortoise beetle larva. WHITNEY CRANSHAW

H. Thistle tortoise beetle pupa. WHITNEY CRANSHAW

I. Thistle tortoise beetle egg mass. WHITNEY CRANSHAW

J. Larva of a case bearer beetle, Neochlamisus sp. TOM MURRAY

K. A “warty leaf beetle,” Neochlamisus bebbianae. TOM MURRAY

L. Pupa of a case bearer beetle, Neochlamisus sp. TOM MURRAY

M. Larva of a case bearer beetle. DAVID SHETLAR
LEAF BEETLES THAT SKELETONIZE LEAVES
ELM LEAF BEETLE (Xanthogaleruca luteola)1 feeds on the leaves of various elms (Ulmus). Adults chew large shotholes in leaves and larvae develop as skeletonizers of the lower leaf surface. It is generally found throughout North America wherever elm hosts are grown, but in recent years populations have declined in many areas across the central U.S.

Elm leaf beetle prepupae and pupae at base of tree. WHITNEY CRANSHAW
Winter is spent in the adult stage in protected areas, including nearby buildings. During this period the adults are in reproductive diapause and a dark khaki-green color. In late April and early May the beetles emerge and move to elm trees to feed and mate, and coloration changes to a yellow-green. After a period of several weeks, females begin to lay masses of bright yellow eggs, typically attached to veins on the lower leaves.
Larvae hatch after about 10–14 days and feed for about 3 weeks, molting three times in the course of development. Larvae are generally black, developing some yellow striping in later stages, and feed primarily on the underside of leaves. They then crawl down the tree trunk in search of pupation sites. Most work their way to the base of the tree to pupate at the base, but some may rest in folds of bark furrows. Adults emerge in about 10–15 days, and in most areas of the U.S. there is a second generation. In northern areas the beetles may feed briefly and then move to winter shelter, deferring reproduction to the next season.
LARGER ELM LEAF BEETLE (Monocesta coryli)1 is one of largest leaf beetles in North America, about ½ inch in length. Much less damaging to elm than elm leaf beetle, it also feeds on foliage. It is currently found from Pennsylvania to Florida and west to Kansas.

Elm leaf beetle eggs. WHITNEY CRANSHAW

Elm leaf beetle larvae at egg hatch. WHITNEY CRANSHAW

B. Elm leaf beetle adult, summer coloration. WHITNEY CRANSHAW

C. Elm leaf beetle larvae. WHITNEY CRANSHAW

D. Elm leaf beetle adult with dormant season coloration. JOSEPH BERGER, BUGWOOD.ORG

E. Larvae of the larger elm leaf beetle. GERALD J. LENHARD, LOUISIANA STATE UNIVERSITY, BUGWOOD.ORG

F. Larger elm leaf beetle. GERALD J. LENHARD, LOUISIANA STATE UNIVERSITY, BUGWOOD.ORG
VIBURNUM LEAF BEETLE (Pyrrhalta viburni)1 is a European species recently introduced into North America and currently found in New York, northern New England, and Ontario south to central Pennsylvania and Ohio. Larvae and adults feed on leaves of several types of viburnum and have been very damaging to some species; other viburnum species show resistance. Winter is spent as eggs glued into pits chewed in small twigs. Larvae hatch in early May and initially feed in groups, skeletonizing the lower leaf surface. Older larvae feed more generally. Adults, which appear similar to drab-colored elm leaf beetles, appear in July and are present through September. Adult feeding produces oblong holes in the interior of leaves. One generation is produced annually.

Viburnum leaf beetle adult. DAVID SHETLAR
IMPORTED WILLOW LEAF BEETLE (Plagiodera versicolor)1 is a small species of leaf beetle (ca. ⅛ inch), metallic black to dark greenish blue. Eggs are laid in clusters on willow, particularly black and white willows. The young larvae feed as a group, skeletonizing the underside of leaves. Older larvae disperse, and adults may chew small holes in leaves. Two to three generations may occur through the range, which currently extends from New England to Virginia and west to Michigan. PACIFIC WILLOW LEAF BEETLE (Tricholochmaea decora carbo)1 develops on willow, poplar, and alder in the Pacific Northwest. Adults closely resemble elm leaf beetle but are dull black. A grayish or yellow-brown subspecies (T. d. decora) is present in the Prairie Provinces. The flea beetle Altica suplicata occurs on willow in the Rocky Mountain region.

Imported willow leaf beetle larvae and damage. DAVID SHETLAR
COTTONWOOD LEAF BEETLE (Chrysomela scripta)1 is found throughout much of North America and can be very damaging to cottonwood and poplar, particularly in the midwestern states. During early stages, larvae feed in groups and produce ragged skeletonizing injuries; later they feed more generally on leaves. Succulent new growth is favored. Adults feed on tender twigs and also skeletonize leaves, but they do much less damage than the larvae.
Cottonwood leaf beetle is a light tan, oval beetle about ⅜ inch long marked with elongate black spots. Widely distributed in North America, it is most common in the northern U.S. and southern Canada. Cottonwood leaf beetles winter as adults, scattered around previously infested trees in protected locations such as under leaf litter or clumps of grass. Shortly after leaves emerge, the adults begin to move back to trees and feed. After a few weeks females begin to lay eggs, which are deposited in clusters of a few dozen or more, on the undersurface of leaves. The larvae are first yellow then black and grublike, with whitish spotting appearing as they age. When disturbed they can produce defensive chemicals that ooze from the light-colored glands on the body. Cottonwood leaf beetles pupate attached to the leaf, the old larval skin conspicuously present at the base of the black pupa. Adults from this first generation emerge in early summer and produce a second generation.

B. Viburnum leaf beetle egg masses on twig. DAVID SHETLAR

C. Defoliation produced by viburnum leaf beetle. DAVID SHETLAR

D. Imported willow leaf beetle. DAVID CAPPAERT, BUGWOOD.ORG

E. Imported willow leaf beetle larvae. WHITNEY CRANSHAW

F. Cottonwood leaf beetle larvae. WHITNEY CRANSHAW

G. Cottonwood leaf beetle larvae. DAVID LEATHERMAN

H. Cottonwood leaf beetle egg mass. WHITNEY CRANSHAW

I. Cottonwood leaf beetle larvae at egg hatch. WHITNEY CRANSHAW

J. Cottonwood leaf beetle pupae. WHITNEY CRANSHAW
Chrysomela knabi (KNAB’S LEAF BEETLE) produces similar injuries primarily to willow and is found throughout much of North America. Adults are highly variable in both coloration and patterning. Chrysomela aenicollis also develops on willow as well as aspen but has a much more restricted distribution within parts of the western U.S. and Canada.
ELM CALLIGRAPHA (Calligrapha scalaris),1 sometimes known as the “linden leaf beetle,” feeds on foliage of elm, linden, alder, and willow in areas of the Midwest and Northeast. Several other common species develop on willows and C. philadelphica on dogwood. COREOPSIS BEETLE (C. californica coreopsivora)2 skeletonizes foliage of coreopsis and ambrosia.
1 Coleoptera: Chrysomelidae
LEAF BEETLES OF AQUATIC PLANTS
WATERLILY LEAF BEETLE (Xanthogaleruca nymphaeae)1 is associated with water lilies particularly yellow water lily, in eastern North America. Both larvae and adults feed on the upper leaf surface of floating lily leaves. Injuries by the insect also provide entry for leaf pathogens, contributing to decay. The overwintering stage is an adult that spends winter under protective debris in the vicinity of ponds. Adults are dark brown to yellow brown with a yellow underside. They become active in late spring and lay masses of eggs on the upper leaf surface. Eggs hatch in 4–5 days, and subsequent larval development is rapid, being completed in about 9 days. Younger larvae usually feed in small groups and skeletonize leaf surfaces. Older larvae chew holes in leaves. Pupation occurs on the leaf surface, and the pupal stage takes about 5 days to complete. In South Carolina, continual overlapping generations are produced into October.
Another common beetle associated with yellow water lily in the eastern U.S. is Donacia piscatrix,1 a type of “false longhorn beetle.” Damage is caused by the larvae, which are adapted to aquatic life. They feed on the underwater stems and petioles, deriving oxygen from a special spiracle on the end of the body that is inserted into the plant stems. Pupation also occurs in water. Adults are bronzy colored beetles, about ¼ inch long. One generation is produced annually.
About 30 Donacia species are present in North America, most found in the northeastern U.S. and southeastern Canada. All feed on water lilies in the genera Nymphaea, Brasenia, and/or Nuphar. Another closely related group of leaf beetles are in the genus Plateumaris, representing 17 North American species. Host plants of these aquatic leaf beetles include Acorus, Carex, Eleocharis, Phragmites, Scirpus, Juncus, Caltha, and Iris versicolor.
1 Coleoptera: Chrysomelidae

B. Larvae of Knab’s leaf beetle. WHITNEY CRANSHAW

C. Calligrapha beetle associated with willow. SUSAN ELLIS, BUGWOOD.ORG

D. Calligrapha beetle associated with dogwood. BRUCE WATT, UNIVERSITY OF MAINE, BUGWOOD.ORG

E. Coreopsis beetle. ROBIN ROSETTA, OREGON STATE UNIVERSITY

F. Waterlily leaf beetle. TOM MURRAY

G. A Donacia species of false longhorn leaf beetle associated with waterlily. TOM MURRAY

H. Mating pair of a Plateumaris species of leaf beetle. TOM MURRAY
Flea beetles are a subfamily (Alticinae) of the leaf beetles. Many are among the smallest of the beetles that feed on leaves, typically 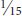 to ⅙ inch. Characteristically they possess very large rear legs that enable them to jump, and the combination of small size and jumping ability lends them their name. Many flea beetles are uniformly metallic colored; others may have stripes or spots. Several species are commonly encountered in yards and gardens and a few are important plant pests.
to ⅙ inch. Characteristically they possess very large rear legs that enable them to jump, and the combination of small size and jumping ability lends them their name. Many flea beetles are uniformly metallic colored; others may have stripes or spots. Several species are commonly encountered in yards and gardens and a few are important plant pests.
There are two general patterns of life history among the flea beetles. Adults of all feed on foliage, but the larvae of some species are also leaf feeders; these include the larger flea beetles. Plant injury produced is typical of that produced by other leaf beetles: a generalized defoliation or feeding in a skeletonizing pattern.
Most flea beetles have larval stages that develop on plant roots. These are small beetles, and the most conspicuous injuries are produced by adults which chew small pits in leaves (shotholes).
FLEA BEETLES WITH LARVAE THAT DEVELOP ON FOLIAGE
A few flea beetles, including some of the largest species, develop on foliage. Eggs are laid in masses on the leaves, and young larvae often feed gregariously, producing skeletonizing injuries typical of many other leaf beetles. Older larvae and adults chew larger holes in foliage.
Probably the most commonly encountered of these flea beetles with larvae that feed on foliage are the various “spinach flea beetles” of the genus Disonycha.1 Three species occur in the U.S, SPINACH FLEA BEETLE (D. xanthomelas), THREESPOTTED FLEA BEETLE (D. triangularis), and YELLOWNECKED FLEA BEETLE (D. mellicollis), and they are all about ¼ inch long. Eggs are laid in small groups on leaves and stems of host plants, and the larvae initially feed gregariously. As they get older, they disperse throughout the plant and produce a generalized defoliation. A wide range of plants are hosts, and many weedy plants, notably pigweeds and lambsquarters, are much more commonly damaged than spinach. In addition, there are more than two dozen other Disonycha species, many of which with prominent striping. Some chew on leaves of perennial plants such as willow and penstemon, but the majority feed on herbaceous annuals, including many common weedy hosts.

Larva and feeding damage by spinach flea beetle. WHITNEY CRANSHAW
Shiny metallic flea beetles found on various trees and shrubs are in the genera Macrohaltica and Altica. GRAPE FLEA BEETLE (Altica chalybea)1 is found throughout the eastern U.S. on grape, Virginia creeper, apple, beech, elm, and plum. Buds are tunneled by adults of the first generation, causing the most damage. Later in the season leaves are skeletonized by larvae. A. litigata is an occasional leaf-chewing pest of crape myrtle and evening primrose in the southern states. Evening primrose and strawberry are hosts for STRAWBERRY FLEA BEETLE (A. ignata) in the eastern U.S., and willow is the host of A. bimarginata in the west. APPLE FLEA BEETLE (A. foliacea) is a metallic bright green or shiny blue species found throughout most of North America. Adults and larvae feed on the foliage of apple, crabapple, grape, evening primrose, willow, Gaura, rose, sedum, and fuchsia. In the southwest, STEEL-BLUE GRAPEVINE FLEA BEETLE (A. torquata) feeds on grape and evening primrose. ALDER FLEA BEETLE (Macrohaltica ambiens) is a transcontinental species associated with alder.

B. Threespotted flea beetle. WHITNEY CRANSHAW

C. A Disonycha species associated with willow. WHITNEY CRANSHAW

D. Grape flea beetle. DAVID SHETLAR

E. Apple flea beetles. WHITNEY CRANSHAW

F. Egg mass of the apple flea beetle. WHITNEY CRANSHAW

G. Apple flea beetle larvae on evening primrose. WHITNEY CRANSHAW

H. Apple flea beetles on evening primrose. WHITNEY CRANSHAW

I. Alder flea beetle. WHITNEY CRANSHAW
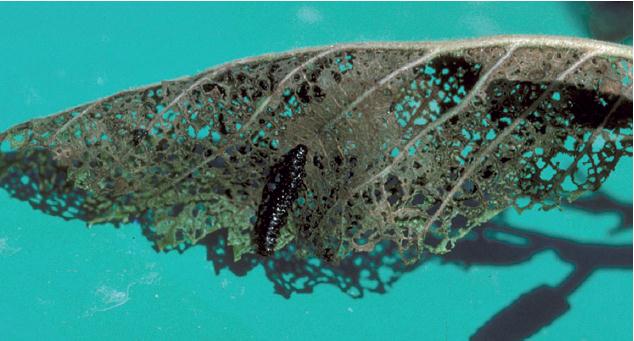
J. Alder flea beetle larvae and damage. KENNETH R. GIBSON, USDA-FOREST SERVICE, BUGWOOD.ORG
SUMAC FLEA BEETLE (Blepharida rhois) is a rather large species common in northern areas east of the Rockies. Adults have striped patterning and a general shape similar to that of a small Colorado potato beetle. They develop on Rhus (sumac, skunkbrush).
1 Coleoptera: Chrysomelidae (Alticinae)
CRUCIFER FLEA BEETLE (Phyllotreta cruciferae)1
HOSTS Virtually all members of the mustard family (Brassicaceae). This includes cabbage, turnip, cauliflower, kale, Brussel sprouts, horseradish, radish, and canola
DAMAGE Crucifer flea beetles make shotholes wounds in leaves of host plants. In high populations they can kill seedlings and seriously affect quality of plants, particularly those produced for leaves (e.g., Chinese cabbage, mustard greens, arugula). The larvae feed on the roots of host plants which can cause minor to severe stunting of growth.
DISTRIBUTION Crucifer flea beetle is a non-native species accidentally introduced from Europe in the 1920s and now common across Canada and northern North America.
APPEARANCE Adults are small black beetles, 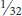 –⅛ inch long, with a distinctive metallic blue sheen. Like most flea beetles, this species quickly jumps when approached or disturbed. The larvae are about ⅛ inch long, wormlike and cream colored.
–⅛ inch long, with a distinctive metallic blue sheen. Like most flea beetles, this species quickly jumps when approached or disturbed. The larvae are about ⅛ inch long, wormlike and cream colored.
LIFE HISTORY AND HABITS Successfully wintered adults emerge from leaf litter and grassy areas when temperatures first begin to reach 60° F. These adults feed on leaves of wild mustard and other cruciferous plants. By June, host plants and soil temperatures are suitable for females to lay eggs. The females lay eggs until July in the soil around potential host plants. Eggs take two weeks to hatch, and the larvae quickly dig into the soil to feed on the roots of cruciferous plants. Larvae take 25–30 days to complete development, then form a pupa in a soil cell. A week or so later, the new adults begin to emerge. These continue feeding on host leaves until fall weather coaxes the adults to find shelter. One generation is completed per year.
OTHER FLEA BEETLES WITH LARVAE THAT FEED ON ROOTS
Cabbage family plants host several additional flea beetles in the genus Phyllotreta, some of which can be extremely damaging, particularly to seedlings. WESTERN BLACK FLEA BEETLE (P. pusilla) is a small, shiny black species that is injurious to a wide range of crucifers throughout much of the western half of the northern U.S. and southern Canada. It has a considerably wider host range than other members of this genus and is also found on other vegetables such as beet, potato, and lettuce. Other members of this genus are generally pale brown with dark striping of some pattern. These include WESTERN STRIPED FLEA BEETLE (P. ramosa) in California, HORSERADISH FLEA BEETLE (P. armoraciae), and STRIPED FLEA BEETLE (P. striolata).

B. Sumac flea beetle egg masses. WHITNEY CRANSHAW

C. Sumac flea beetle larvae. WHITNEY CRANSHAW

D. Crucifer flea beetle. JEFF HAHN, UNIVERSITY OF MINNESOTA

E. Western black flea beetle on broccoli. WHITNEY CRANSHAW

F. Western black flea beetle damage to Chinese cabbage. WHITNEY CRANSHAW

G. Western black flea beetle damage to seedling kohlrabi. WHITNEY CRANSHAW

H. Western black flea beetle larva. WHITNEY CRANSHAW

I. Horseradish flea beetle. WHITNEY CRANSHAW

J. Striped flea beetle and crucifer flea beetle. DAVID SHETLAR
Several flea beetles in the genus Epitrix1 are associated with nightshade family plants. They are among the smallest flea beetles (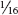 inch) and uniformly colored from black to dark brown. Most widespread is the POTATO FLEA BEETLE (E. cucumeris), which very commonly produces shothole injuries to potato and tomato. Others of similar appearance but with more western distribution include WESTERN POTATO FLEA BEETLE (E. subcrinita) and TUBER FLEA BEETLE (E. tuberis). The latter species is also damaging in the larval stage as it will tunnel into tubers. TOBACCO FLEA BEETLE (E. hirtipennis) commonly feeds on eggplant, tobacco, and potato. It has bronze coloration and is predominant in the southern half of the U.S. In much of the eastern U.S., eggplant is also a favored host of EGGPLANT FLEA BEETLE (E. fuscula).
inch) and uniformly colored from black to dark brown. Most widespread is the POTATO FLEA BEETLE (E. cucumeris), which very commonly produces shothole injuries to potato and tomato. Others of similar appearance but with more western distribution include WESTERN POTATO FLEA BEETLE (E. subcrinita) and TUBER FLEA BEETLE (E. tuberis). The latter species is also damaging in the larval stage as it will tunnel into tubers. TOBACCO FLEA BEETLE (E. hirtipennis) commonly feeds on eggplant, tobacco, and potato. It has bronze coloration and is predominant in the southern half of the U.S. In much of the eastern U.S., eggplant is also a favored host of EGGPLANT FLEA BEETLE (E. fuscula).

Western potato flea beetle. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

Tobacco flea beetle. RUSS OTTENS, UNIVERSITY OF GEORGIA, BUGWOOD.ORG
Also among the smallest flea beetles are Chaetocnema species.1 Most feed strictly on grasses, and CORN FLEA BEETLE (C. pulicaria) is an important species in parts of the Midwest, where it may transmit the bacteria that produce the disease Stewart’s wilt of corn. TOOTHED FLEA BEETLE (C. denticulata) also occurs in the midwestern and northeastern states on corn and other grasses. SWEETPOTATO FLEA BEETLE (C. confinis) is a common species, particularly in the southern U.S., where it develops on sweetpotato, dichondra, morning glory, and raspberry. In southern California DICHONDRA FLEA BEETLE (C. repens) can be a pest where dichondra is grown as a groundcover and in lawns. Adults cut small crescent-shaped holes in leaves. More damage is done by larvae, which feed on roots and burrow into the crown of the plants, causing wilting and even death.
Striping is also found among flea beetles in the genus Systena,1 which tend to be slightly larger species (⅛ inch). PALESTRIPED FLEA BEETLE (S. blanda) is not only one of the most widely distributed flea beetles of North America but has the broadest host range. It commonly damages squash family plants, bean, corn, sunflower, lettuce, potato, and many weeds. Closely resembling it is ELONGATE FLEA BEETLE (S. elongata) which overlaps considerably in geographic range and host range. REDHEADED FLEA BEETLE (S. frontalis) is common in the areas of the midwestern and Mid-Atlantic States. It has a very wide host range that includes cabbage, bean, beet, corn, potato, forsythia, dogwood, sedums, zinnia, hibiscus, aster, as well as several common weeds such as smartweed, lambsquarters, and pigweeds. Adults are about ⅙ inch long and black with a reddish-yellow head.

Elongate flea beetle. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY
HOP FLEA BEETLE (Psylliodes punctulata)1 occurs throughout the northern U.S. and southern Canada. It also can be found on a wide range of plants, including cabbage, cucumber, hops, cannabis, strawberry, tomato, and beet. Adults feed on the underside of leaves. Hop flea beetle is one of the first flea beetles to appear in spring, and larvae feed on root hairs of the host plants. A single generation is produced with new-generation adults emerging in August. After a brief period of feeding, the adults move to overwintering sites among sheltering debris.
1 Coleoptera: Chrysomelidae (Alticinae)

B. Potato flea beetle damage to leaves and fruit of tomato. WHITNEY CRANSHAW

C. Corn flea beetle. JIM KALISCH, UNIVERSITY OF NEBRASKA

D. Corn flea beetle damage. JIM KALISCH, UNIVERSITY OF NEBRASKA

E. A Chaetocnema species of flea beetle. TOM MURRAY

F. Palestriped flea beetles. WHITNEY CRANSHAW

G. Redheaded flea beetle. DAVID SHETLAR

H. Hop flea beetle. TOM MURRAY
Weevils are the largest family of insects and have a wide range of feeding habits. The chewing mouthparts of the adults tip an elongate “snout” that can allow some species to chew into stems, seeds and developing fruit. Adults that feed on leaves typically confine feeding along the leaf edge, producing angular notching wounds. Few species of weevil larvae feed on leaves, the great majority instead developing within seeds or fruit. (These are discussed in chapters 4 and 7). Others, notably the bark beetles, develop as borers in stems or trunks, and a few occur in roots. (These are discussed in chapters 5 and 6.)
Adults of weevils that chew along the edges of leaves are usually active at night and rarely observed. Often the most common species involved in such injuries are various ROOT WEEVILS of the genus Otiorhynchus, many of which are widespread in North America. Adults of the BLACK VINE WEEVIL (O. sulcatus)1 chew on foliage of hemlock, yew, rhododendron, euonymus, other broadleaf evergreens, and many perennials. The STRAWBERRY ROOT WEEVIL (O. ovatus), ROUGH STRAWBERRY ROOT WEEVIL (O. rugosostriatus), and CLAY-COLORED WEEVIL (O. singularis) also chew notches in the leaves of a variety of landscape plants and small fruits. LILAC ROOT WEEVIL (O. meridionalis) feeds on leaves of lilac, privet, and peony, among other plants. CRIBATE ROOT WEEVIL (O. cribricollis) is a pest of olive, grape, citrus, and various fruit trees in California. These species are often more damaging as larvae, which develop on roots and are further discussed in chapter 6.
Nemocestes incomptus,1 known variously as the “woods weevil” and “raspberry bud weevil,” is a common member of the root weevil complex associated with landscape plants in Washington and British Columbia. Most adults begin to emerge in late summer and may feed throughout the winter months as temperatures allow. Distinct generations do not occur, and all life stages may be present throughout the year. Adults are sooty dark brown, covered with small hairs, and about ⅓ inch long.
Another native forest species in the region is Dyslobus granicollis,1 which occasionally damages landscape plantings and strawberries. Like most root weevils, adults are flightless, so damage usually occurs only in limited sites adjacent to woodlands. Dyslobus decoratus, sometimes known as the “decorated strawberry root weevil,” is another leaf-notching species, native to the Pacific Northwest, which has expanded its range into the Rocky Mountain States, apparently through human-assisted movement of plant material. Buckthorn is a particularly favored plant.
Adults of the OBSCURE ROOT WEEVIL (Sciopithes obscures)1 chew notches on the leaf margins of rhododendron. This insect is also more damaging in the larval stage and an important nursery pest in the Pacific Northwest and northern California.
At least three European Polydrusus species1 have become established in North America. Polydrusus impressifrons is a pale green weevil that chews buds and notches leaves of many deciduous trees and shrubs in late spring. It occurs in many areas of the northern U.S. and southern Canada and is most often associated with birch, black locust, blueberry, pear, plum, poplar, rose, strawberry and willow. P. formosus, known as the GREEN IMMIGRANT WEEVIL, is brightly colored green and also feeds on leaves and open blossoms of many woodland trees and shrubs and some fruit trees. It has also been steadily extending its range and can be found in many of the northern forested areas. More recently introduced, with present range limited to northeastern areas, is P. cervinus. This weevil is predominantly brown colored, but greenish scales may be present.

B. Black vine weevil notching of euonymus. WHITNEY CRANSHAW

C. Strawberry root weevil and leaf injury. DAVID SHETLAR

D. Rough strawberry root weevil. WHITNEY CRANSHAW

E. Clay-colored root weevil. TOM MURRAY

F. Lilac root weevil. WHITNEY CRANSHAW

G. Leaf notching characteristic of lilac root weevil. WHITNEY CRANSHAW

H. A Nemocestes species of root weevil. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

I. Obscure root weevil. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

J. Leaf injury produced by Dyslobus decoratus. WHITNEY CRANSHAW

K. Dyslobus decoratus. WHITNEY CRANSHAW

L. Polydrusus formosus, the “green immigrant weevil.” DAVID SHETLAR
Myllocerus undecimpustulatus undata,1 sometimes known as the “Sri Lanka weevil,” was first detected in southern Florida in 2000 and has since spread to include most of the state. Adults notch the leaves of a great number of plants, including fruit trees (e.g., citrus, lychee, peach) various ornamentals (e.g., crape myrtle, green buttonwood, cocoplum, pygmy date palm, eucalyptus) and some vegetables (e.g., pepper, eggplant, sweetpotato). Larvae have been observed to feed on the roots of several herbaceous plants.
TWOBANDED JAPANESE WEEVIL (Callirhopalus bifasciatus)1 can cause extensive defoliation of many deciduous shrubs in the northeastern quadrant of the U.S. Adult feeding is typical of that of many other weevils, producing notched cuts that extend in from the leaf margin. Privet, azalea, forsythia, dogwood, holly, spirea, and ash are among the plants injured by adults. Larvae have been observed feeding on roots of forsythia and privet. One generation is produced annually, but all stages may be present throughout the year. Adults are about ¼ inch and light brown with two darker bands across the back.
FULLER ROSE BEETLE (Naupactus cervinus)1 is the most widely distributed of the four species of introduced “whitefinged weevils” (Naupactus spp.). Larvae girdle roots, but the most conspicuous damage is caused by adults, which make notching wounds on foliage and consume buds and blossoms. Unsightly dark fecal matter is left around feeding sites. Fuller rose beetle is flightless, and only females occur. Overwintered weevils crawl to nearby plants in spring and feed on leaves. Eggs are laid in small, sticky masses, usually in soil but also in bark crevices or between leaves. Larvae enter the soil and chew on roots. Pupation occurs near the soil surface. There are one to two generations per year.
Ithycerus noveboracensis,2 sometimes referred to as the “New York weevil,” occurs in wooded areas of the northeastern U.S. and southern Canada. Adults chew on the new growth, leaf petioles, and buds of many different trees but are most noticed on white oak and American beech. Eggs are laid in the soil and larvae feed on the roots of the same host plants.
ASIATIC OAK WEEVIL (Cyrtepistomus castaneus)1 feeds on leaves of many woody plants, including oak, beech, red maple, dogwood, willow, sycamore, redbud, persimmon, and viburnum. It has been particularly damaging to chestnuts. Its present range extends to Missouri from its original point of introduction in New Jersey. When abundant it may be a significant nuisance invader of homes.
HAIRY SPIDER WEEVIL (Barypeithes pellucidus),1 also known as “juniper root weevil” and “hairy broadnosed weevil,” is very similar in size and shape to the strawberry root weevils, but brown with thick hairs scattered over the entire body. This is a native of Europe, but it is now generally spread across northern North America. The adults feed primarily on the leaf buds and foliage of various landscape and garden plants. It is assumed that the larvae feed on plant roots, but they have not been reported to produce significant damage; this insect attracts attention primarily when it wanders into buildings. Another small weevil of European origin that has the habit of incidentally entering buildings is Trachyphloeus asperatus. Life history of this species, including host plants, is very poorly known.

Hairy spider weevil in strawberry. ROBIN ROSETTA, OREGON STATE UNIVERSITY
Adults of the PEA LEAF WEEVIL (Sitona lineatus)1 chew notches along the edges of leaves at night. Various legumes are hosts, but it is most often observed on garden peas and fava beans. Larvae develop on roots of perennial legumes such as clover and alfalfa. Pea leaf weevil presently occurs in parts of the Pacific Northwest and extends into the Prairie Provinces.
A. Damage to lychee by Myllocerus undecimpustulatus undata, the “Sri Lanka weevil.” DOUG CALDWELL, UNIVERSITY OF FLORIDA

B. Mating pair of “Sri Lanka weevil.” DOUG CALDWELL, UNIVERSITY OF FLORIDA

C. Twobanded Japanese weevil. DAVID SHETLAR

D. Fuller rose weevil. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

E. A Naupactus species of whitefringed weevil feeding on blueberry. JERRY A. PAYNE, USDA AGRICULTURAL RESEARCH SERVICE, BUGWOOD.ORG

F. Asiatic oak weevil and damage. DAVID SHETLAR

G. Trachyphloeus asperatus. TOM MURRAY

H. Hairy spider weevil. ROBIN ROSETTA, OREGON STATE UNIVERSITY

I. Pea leaf weevil notching pea leaf. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY
VEGETABLE WEEVIL (Listroderes costirostris)1 also damages carrot as well as turnip, potato, and other root crops in the Gulf States. It is also reported as a pest of dichondra lawns in California. Adults feed on foliage and buds. Feeding injury by larvae can occur on roots but is often aboveground, concentrated around the soil surface, but also involving buds and foliage. Feeding activity of both adults and larvae occurs primarily at night. Vegetable weevil has only one generation a year, with peak adult activity and egg-laying occurring in late summer and early fall.
Clover, particularly red clover, is host to two leaf-feeding weevils. Larvae of the CLOVER LEAF WEEVIL (Hypera zoilus)1 mature in spring, when they feed on the buds and emerging leaves, producing ragged foliage. Adults emerge late May through July but do not lay eggs until autumn. Young larvae are the primary overwintering stage. The LESSER CLOVER LEAF WEEVIL (Hypera nigrirostris) overwinters as adults. Peak feeding by this species is from late May through July. Larvae of a related species, the ALFALFA WEEVIL (Hypera postica), chew the leaves of alfalfa early in the season; this is the most important insect pest of the alfalfa in North America.

Alfalfa weevil larva and damage. WHITNEY CRANSHAW
An unusual group of weevils associated with foliage are the FLEA WEEVILS. These are small weevils with enlarged hind legs that allow them to jump in a manner similar to flea beetles (page 190). Most are in the genus Orchestes1 and the larvae of these develop as leafminers, producing small blotch mines in leaves of their host plant (page 218). Adults produce shothole wounds in leaves, similar to flea beetles.
APPLE FLEA WEEVIL (Orchestes pallicornis)1 is an occasional pest of apple but may also attack wild cherry and hornbeam. The adults produce shotholes in leaves, both before and after larval development. Adults lay eggs in the midvein of host leaves and the larvae mine to the leaf margin to make a small blotch mine. These mines are often mistaken for late frost damage or disease. EUROPEAN ELM FLEA WEEVIL (O. alni) can be locally common on elm, particularly Siberian elm. Overwintered adults move to emerging leaves in spring and chew small holes in leaves, which produce shothole wounds. The subsequent larval generation makes leaf mines, but adults then emerge in early summer and feed heavily on leaves at this time, often producing extensive shothole wounding. Another common species is the ALDER FLEA WEEVIL (O. testaceus).
Another group of weevils of odd habit are the LEAFROLLING WEEVILS.3 Adults tightly roll the tips of leaves in a process of strategic leaf cuts, laying an egg in the center. A specific name is given to the leaf roll “nest” they produce, a nidus, and the process of making a single nidus (nidification) takes about two hours to complete. Homeolababus analis is a common species in the southern states, using various oaks as host plants. Synobabus bipustulatus is more widely distributed in eastern North America and develops on oak, walnut, pecan, and hornbeam. The three other North American species are primarily associated with oaks.
1 Coleoptera: Curculionidae
2 Coleoptera: Brentidae
3 Attelabidae (Attelabinae)

B Vegetable weevil larva. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

C. Clover leaf weevil larva. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

D. Clover leaf weevil. WHITNEY CRANSHAW

E. Lesser clover leaf weevil. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

F. Shothole injuries produced by European elm flea weevil. WHITNEY CRANSHAW

G. European elm flea weevils on new growth of elm. WHITNEY CRANSHAW

H. Leaf rolled nest cells of a leafrolling weevil. DAVID SHETLAR

I. Leafrolling weevils making leafroll nest cell. DAVID SHETLAR

J. Leafrolling weevil making cuts prior to leafroll. LACY L. HYCHE, AUBURN UNIVERSITY, BUGWOOD.ORG

K. The leafrolling weevil Synobabus bipustulatus. DAVID CAPPAERT, BUGWOOD.ORG
JAPANESE BEETLE (Popillia japonica)1
HOSTS Adults feed on foliage of more than 300 species, including rose, mountain-ash, willow, linden, elm, grape, Virginia creeper, bean, Japanese and Norway maples, birch, pin oak, horse chestnut, rose of Sharon, sycamore, ornamental apple, plum, and cherry. Larvae develop on roots of various grasses.
DAMAGE Japanese beetle is one of the few beetles that is highly damaging in both the adult and larval stages. Adults feed on foliage and flower petals, producing skeletonizing injuries that cause leaves to appear lacelike. In high populations they may completely consume flower petals and more tender foliage. The larva, a type of white grub that feeds on grass roots (page 468), is among the most damaging pests of turfgrass in the northeastern quadrant of the U.S.
DISTRIBUTION Originally introduced into New Jersey and now important in the northeastern U.S. and parts of southern Canada. This species ranges into Colorado to the west, Arkansas to the south, and is found in parts of northern Alabama, northern Georgia, and South Carolina. Its range continues to expand, with localized infestations present in many other states.
APPEARANCE Adults are generally metallic green with bronze wing covers. A row of white hair brushes is present along each side. The overall form is broadly oval, and length ranges from about ⅓ to nearly ½ inch. Larvae are typical white grubs, C-shaped when at rest and a translucent creamy white, and feed on organic matter in the soil or under turf.
LIFE HISTORY AND HABITS Winter is spent in soil as a nearly full-grown grub that moves deeper into the soil for winter. As soils warm, the grubs resume feeding on grass roots and pupate 2–4 inches below the surface, in a tamped earthen cell. Adults emerge in late June and early summer, feed on foliage, and mate, returning to lawn near sunset. The aggregation pheromones these insects produce combined with attractive odors produced by their food plants often result in large numbers feeding together.
Females lay eggs in small masses in soil cavities they excavate 2–4 inches deep. Most eggs are usually laid by early August, but some are laid into September. Over most of its range Japanese beetle has a 1-year life cycle, although it may extend to 2 years in the extreme northern areas where it occurs.
OTHER LEAF-FEEDING SCARABS
Some other scarab beetles feed on foliage, although feeding damage is much less conspicuous than that by the Japanese beetle. Various species of MAY/JUNE BEETLES (Phyllophaga spp.)1 occur east of the Rocky Mountains; adults feed on foliage during the night. Often certain broadleaf trees are preferred, with oaks and maples being especially attractive. A few chew needles of pines. The LINED JUNE BEETLES (Polyphylla spp.) tend to be more western in distribution and similarly feed on leaves at night. Both develop as white grubs that feed on roots (page 464), but adults sometimes emerge en masse, accumulating by the hundreds in landscape trees at night to mate and feed. By morning, they return to the soil and the trees appear mysteriously defoliated. If these beetles are suspected, going out at night with a flashlight will soon disclose their activities. Other scarab beetles that may cause limited damage to foliage include the ROSE CHAFER (Macrodactylus subspinosus)1 and SPOTTED PELIDNOTA (Pelidnota punctata).1
1 Coleoptera: Scarabaeidae

B. Japanese beetle injury to Virginia creeper. WHITNEY CRANSHAW

C. Japanese beetle injury to rose. WHITNEY CRANSHAW

D. Life stages of Japanese beetle. DAVID SHETLAR

E. Japanese beetles flying to trap. WHITNEY CRANSHAW

F. Japanese beetle mating ball on lawn. DAVID SHETLAR

G. Damage to pinyon by the June beetle Phyllophaga falsa. WHITNEY CRANSHAW

H. June beetle feeding on oak at night. DAVID SHETLAR

I. Tenlined June beetle, Polyphylla decemlineata. WHITNEY CRANSHAW

J. Spotted pelidnota. DAVID SHETLAR

K. Rose chafer. DAVID SHETLAR
MEXICAN BEAN BEETLE (Epilachna varivestis)1
HOSTS Garden beans and field beans primarily; soybean and cowpea are less commonly damaged.
DAMAGE Mexican bean beetle is a plant-feeding lady beetle. Larvae feed on the underside of bean leaves in a typical skeletonizing manner, producing a lacy appearance. Adults primarily chew leaves but may also feed on green pods.
DISTRIBUTION Originally restricted to the southeastern U.S. and Mexico but has greatly extended its range and is now common in most areas east of the Rockies. It tends to be more abundant and damaging in southern areas of this range.
APPEARANCE Adults are a typical oval form, similar to that of other lady beetles, and slightly larger than most. General color ranges from nearly mustard yellow to copper, with those present late in the season often reddish brown. Each wing cover is marked with eight dark spots, arranged in three rows (3-3-2). Larvae are yellow or orange-yellow and quite spiny.
LIFE HISTORY AND HABITS Mexican bean beetles winter in the adult stage in protected areas along the edges of plantings and emerge over an extended period in late spring. Females lay masses of yellow eggs on the lower leaf surface. The larvae feed for about 3 weeks, skeletonizing the lower surface of the leaf. When full grown they pupate underneath a leaf, often in small groups. One generation is typical in the western and northern states, but up to three may occur in the southeast. Greatest feeding and injury typically occur in late July and August.
OTHER LEAF-FEEDING LADY BEETLES
A few other lady beetles also develop by feeding on plants. SQUASH BEETLE (Epilachna borealis)1 is a minor pest of squash family plants and found in the eastern U.S., particularly in the Atlantic States. Adults and larvae chew foliage in a manner similar to that of the Mexican bean beetle but are rarely seriously damaging. Two generations occur annually in southern areas, one in the north. The 24-SPOT LADY BEETLE (Subcoccinella vigintiquatuorpunctata) is a European species that is now present over a large area of the eastern U.S. Larvae rarely damage garden plants, but they have a wide host range that includes campion, dianthus, and many legumes.
1 Coleoptera: Coccinellidae

B. Mexican bean beetle adult recently emerged from pupa. WHITNEY CRANSHAW

C. Mexican bean beetle egg mass. WHITNEY CRANSHAW

D. Mexican bean beetle damage. WHITNEY CRANSHAW

E. Mexican bean beetle pupae. WHITNEY CRANSHAW

F. Mexican bean beetle larvae and damage. WHITNEY CRANSHAW

G. Squash beetle. CLEMSON UNIVERSITY–USDA COOPERATIVE EXTENSION SLIDE SERIES, BUGWOOD.ORG

H. 24-spot lady beetle. TOM MURRAY
Blister beetles (Meloidae) are elongated beetles of moderate size. They have relatively soft wing covers that do not cover the tip of the abdomen. More than 300 species occur in North America, but few are commonly associated with gardens. The common name of the family is related to production of a defensive compound in their blood, cantharidin, which can be highly irritating and capable of producing blisters in high concentration. Fortunately, most blister beetles found in gardens contain relatively low amounts of cantharidin and pose little threat to gardeners who may come in contact with them.
Only the adult blister beetles feed on plants. Larvae develop as predators of other insects. Most (all Epicauta species) feed on eggs of grasshoppers. Others (Lytta spp., Meloe spp., Nemognatha spp.) develop as parasites of ground-nesting bees.
Epicauta is an extremely large genus, with more than 150 North American species. All develop as predators on grasshopper egg pods. Adults of many feed on pollen, but some chew flowers and leaves. STRIPED BLISTER BEETLE (Epicauta vittata)1 feeds on leaves of a wide range of garden and landscape plants such as Amaranthus, bean, beet, carrot, cabbage, corn, eggplant, melon, mustard, pea, pepper, potato, radish, spinach, squash, sweetpotato, and tomato. Adults will also chew on developing fruit of peas, peppers, and tomatoes. Adults of ASHGRAY BLISTER BEETLE (E. fabricii) and MARGINED BLISTER BEETLE (E. funebris) feed primarily on flower petals. but they will also feed on leaves, producing a very ragged appearance to plants. The BLACK BLISTER BEETLE (E. pennsylvanica) seems primarily a pollen-feeding species but it will also feed some on flowers. The CLEMATIS BLISTER BEETLE (E. cinerea) looks like a small version of the margined or gray blister beetle and the adults feed on the leaves of clematis, sometimes causing extensive defoliation. These species are generally distributed across the Prairie States eastward. Western species of Epicauta include the SPOTTED BLISTER BEETLE (E. maculata), CARAGANA BLISTER BEETLE (E. subglabra), and E. ferruginea.
Blister beetles in the genus Lytta are often quite brightly colored with bright orange and yellow colors or a metallic blue or green sheen, and antennae consisting of a row of beads. The larval habits of this genus differ from those of Epicauta spp. in that the larvae develop as parasites within the nests of ground-nesting solitary bees (rather than in egg pods of grasshoppers). NUTTALL BLISTER BEETLE (L. nuttalli) feeds on lupines, other legumes, and occasionally canola in the western states. Adults are brilliant iridesent blue-green, often with violet coloration of the wings. SAY BLISTER BEETLE (L. sayi) is a metallic green with bright yellow-orange legs that is common over eastern North America. The adults are found on flowers of willow, rose, locusts, lupines, and wild cherry in late spring.
The OIL BEETLES (Meloe spp.) are very large, black blister beetles. They have a distended abdomen that is only partially covered by the wing covers and are incapable of flight. Adults chew leaves and flowers of plants and on occasion some species have been reported to damage potatoes, turnips, Ranunculus, and some other garden plants. Adults of the most commonly observed species (e.g., M. laevis, M. niger, M. impressus) are usually active in late summer and autumn.
Larvae of the oil beetles develop as parasites of ground-nesting bees. Adults lay masses of eggs in soil cavities and the active first-stage larvae (triungulins) climb to flowers upon emergence, where they wait to attach to visiting bees. If transported by a bee back to its the nest, the oil beetle larvae will then consume the food provided for the larval bees and usually eat the developing bee larvae.
1 Coleoptera: Meloidae
A. Striped blister beetle. CLEMSON UNIVERSITY–USDA COOPERATIVE EXTENSION SLIDE SERIES, BUGWOOD.ORG

B. Ashgray blister beetle. WHITNEY CRANSHAW

C. Margined blister beetle. DAVID SHETLAR

D. Black blister beetle. DAVID SHETLAR

E. Clematis blister beetle. TOM MURRAY

F. An oil beetle, Meloe species. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

G. Spotted blister beetle. JOHN CAPINERA, UNIVERSITY OF FLORIDA

H. Nuttall’s blister beetle. WHITNEY CRANSHAW
Slugs and snails are types of animals known as gastropods, fairly close relatives of clams, mussels, and other mollusks. As such, they have several features that differ from those of the arthropods (insects, mites, spiders, crabs, and the like), such as lack of distinct segmentation and an external skeleton (when present) that grows with the mollusk rather than being shed on a periodic basis. The body is soft and moves by means of a broad, muscular “foot” (sole) that covers the underside. On slugs, a large lobe called the mantle is present on the front half of the back; this is covered by a hard shell in snails. Two pairs of tentacles are present in the front, a short pair for sensing odors and a longer pair tipped with eyes. Many slugs and snails are hermaphrodites, possessing both sex organs. In some species, being male or female can occur at different times, causing the slug to pass through male and female phases. Other slugs may be permanently hermaphroditic, as are most land snails.
Slugs and snails typically feed at night or during heavily overcast periods, avoiding sunny, drying conditions. During the day most slugs and snails migrate to sheltered areas, such as under debris, in soil cracks, or within shaded areas of dense vegetation.
GRAY GARDEN SLUG (Deroceras reticulatum)1
HOSTS Almost all garden plants, although lettuce, bean, corn, and hosta are among those particularly favored. The gray garden slug is particularly damaging to seedlings.
DAMAGE Slugs feed by using a rasplike structure called a radula that scrapes away plant tissues. They typically produce irregular damaged areas on foliage and leave slime trails on areas where they have been active.
DISTRIBUTION Of European origin, the gray garden slug is now widely present through North America but most common in the northern states and Canada.
APPEARANCE Adults are moderate size, ranging from 1 to 1½ inches long. Color varies from cream to gray or pinkish gray, with some dark flecks. The mantle area is large, covering a third of the body or more, and on close inspection has concentric folds. The mucous slime is normally clear but will turn milky if the slug is disturbed, leading to another common name, “milky garden slug.”
LIFE HISTORY AND HABITS Gray garden slug is quite tolerant of low temperatures and may be active at fairly cool temperatures. Most activity occurs during spring and fall, with masses of clear eggs laid in soil cracks. Development may be suspended during very warm temperatures. The slugs develop rapidly, becoming full grown in 3–6 months. Life span rarely exceeds one year.
OTHER GARDEN SLUGS
MARSH SLUG (Deroceras laeve)1 is a relatively small slug, about ¾–1 inch long. Color ranges from dark brown or yellowish to nearly black but the slug may be distinguishable by tentacles that are smoky blue-black. It is one of the very few slugs commonly encountered that are native to North America and occurs in fairly moist sites, including many gardens. The marsh slug can reproduce year-round where temperatures allow and can often be the earliest active slug in gardens in spring.

B. Gray garden slugs. WHITNEY CRANSHAW

C. Brown garden snail. BOB HAMMON, COLORADO STATE UNIVERSITY

D. Marsh slug. LYLE BUSS, UNIVERSITY OF FLORIDA

E. Gray garden slug and associated leaf injury. JIM KALISCH, UNIVERSITY OF NEBRASKA

F. Slug damage to hosta. DAVID SHETLAR

G. Slug eggs under garden timber. DAVID SHETLAR
Ten Arion species2 of slugs, all native to Europe, have become established in areas of the northern U.S. and southern Canada. Most are omnivores that feed on a variety of materials, including living and dead plant material, fungi, animal feces, and carrion; many can be seriously damaging to ornamental and vegetable plants. Two of the larger species are Arion rufus (EUROPEAN RED SLUG or CHOCOLATE ARION) and A. ater (BLACK ARION), which may reach 3–6 inches when full grown. Both show a considerable range of colors, from dark brown or black to orange or reddish, and they have been known to interbreed. A. subfuscus (DUSKY SLUG) is a slightly smaller species, ranging up to 3 inches when full grown, and variably gray-brown to orange brown. It has established over a broad area of the U.S., including some southern states, and in some sites appears particularly well adapted, displacing other slug species. Other species known to range at least as far south as Kentucky include A. hortensis (BLACK FIELD SLUG, YELLOW-SOLED SLUG, SOUTHERN GARDEN SLUG), A. circumscriptus (BROWNBANDED ARION), and A. fasciatus (ORANGEBANDED ARION). These are gray-colored slugs with the underside (“sole”) light-colored yellow or white. Arion slugs have a 1-year life cycle.
The TAWNY GARDEN SLUG (Limacus flavus)3 is a moderately large species, ranging 3–4½ inches, with yellowish coloration and gray-green mottling. Of approximately similar size is the THREEBANDED SLUG (Lehmannia valentiana),3 a somewhat translucent slug, of yellow-gray or yellow-violet color, and often having two darker bands on the back. It feeds primarily on decaying plant matter and fungi but can be a pest of living plants in very moist sites, including greenhouses. Considerably larger is Limax maximus,3 known as the SPOTTED LEOPARD SLUG or GIANT GARDEN SLUG, which can range to 6 inches long. It is generally gray but often has conspicuous dark spotting. Limax maximus feeds primarily on decaying plant matter, animal feces, and fungi and prefers shaded areas, and only infrequently damages garden plants. It is a long-lived species that may live 3–4 years.
Most slugs tend to confine feeding damage to aboveground parts of plants, but the GREENHOUSE SLUG (Milax gagates)4 burrows more extensively and may damage belowground parts of plants, including roots and tubers. It ranges up to about 2½ inches and is gray-brown to black, with a ridge (keel) running along the back. The greenhouse slug is well established and often very damaging in many areas in the Pacific States and Hawaii and has been introduced into some areas of the eastern U.S. Greenhouse slugs can also develop more rapidly than most other slugs, completing a generation within 6 months.
FLORIDA LEATHERLEAF (Leidyula floridana)5 is a mottled brown slug, more flattened in cross section than other slugs, and has a very narrow sole. It is a moderately important garden pest species in Florida and has more recently been introduced into areas of Louisiana and Texas.
1, 2, 3, 4, 5 Class Gastropoda. With very few exceptions (e.g., Veroncellidae), all terrestrial slugs and snails are in the clade Stylommatophora.
1 Agriolimacidae
2 Arionidae
3 Limacidae
4 Milacidae
5 Veronicellidae
A. European red slug. GARY BERNON, USDA APHIS, BUGWOOD.ORG

B. European red slug. GARY BERNON, USDA APHIS, BUGWOOD.ORG

C. Dusky slug. GARY BERNON, USDA APHIS, BUGWOOD.ORG

D. Yellow-soled slug. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

E. Tawny garden slug. DAVID CAPPAERT, BUGWOOD.ORG

F. Tawny garden slug. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

G. Spotted garden slug. JIM KALISCH, BUGWOOD.ORG
BROWN GARDEN SNAIL (Cornu aspersum)6
HOSTS Brown garden snail feeds on a wide range of commonly grown vegetables, flowers, shrubs, and fruit trees. Boxwood, rose, hibiscus, lettuce, peach, magnolia, and citrus are among the plants most commonly damaged. Dead plant matter is also commonly consumed.
The brown garden snail is also one of the escargot species that is cultured for human consumption. It is sometimes referred to as the “petit gris” and said to taste best in spring when levels of calcium salts are lowest.
DAMAGE Brown garden snail feeds primarily on leaves but may rasp the bark of twigs and small branches.
DISTRIBUTION Purposefully introduced as a potentially edible species in the San Francisco area in the 1850s and currently common throughout the Pacific States. It is found in localized infestations elsewhere, particularly in the southern U.S., although it has been eradicated from Florida.
APPEARANCE The globular brown shell is slightly wrinkled and often flecked with yellow. Full grown, the shell may be 1½ inches in diameter with four or five whorls.
LIFE HISTORY AND HABITS Brown garden snail remains dormant in winter, sealing itself in the shell with a thin membrane (epiphragm). Activity resumes in spring, and adults lay masses of eggs, up to six dozen at a time, in soil. Eggs hatch in a few weeks, and the snails grow slowly, becoming full grown in one to two years. This species can be long lived, living up to five years.
OTHER GARDEN SNAILS
The CUBAN BROWN SNAIL (Zachrysia provisoria)7 is a species introduced into parts of Florida that is approximately similar in size and shape to the brown garden snail. It is reportedly very damaging to many ornamental plants. The ASIAN TRAMP SNAIL (Bradybaena similaris)8 is a smaller species, now established in much of the southeastern U.S., reported to feed on cucurbits, legumes, hibiscus, and several ornamental plants. The WHITE GARDEN SNAIL (Theba pisana)6 occurs in coastal areas of California. It is particularly well adapted to dry sites, burrowing deeply into soil when laying eggs. During dormant periods it may be seen conspicuously attached to fences, walls, and tree trunks.
GLASS SNAILS (Oxychilus spp.)9 are rather small snails, with a shell about ½ inch in diameter. The shell is translucent, pale brown, and depressed, so that it is only about ⅙ inch wide, allowing the snails to move under logs and rocks, where they sometime aggregate in large numbers. These snails feed on living and dead plant matter but rarely cause noticeable injury. The body of the GARLIC GLASS-SNAIL (Oxychilus alliarus) is bluish and it can emit a garlicky odor when disturbed. The CELLAR GLASS-SNAIL (Oxychilus cellarius) has a gray body. These snails feed on a wide variety of materials, including both living and dead plant matter as well as soft-bodied animals such as small slugs, snails, and earthworms. They can be found year-round, with peak breeding in autumn.
A few snails are primarily predatory, feeding on soft-bodied invertebrates (slugs, snails, earthworms). The ROSY PREDATOR SNAIL (Euglandina rosae)10 is a predatory snail native to the southeastern U.S. The DECOLLATE SNAIL (Rumina decollata)11 is a Mediterranean species that has been introduced into North America and subsequently spread, often purposefully, so now it can be found broadly across the southern U.S.
6, 7, 8, 9, 10, 11 Class Gastropoda, All terrestrial snails are in the clade Stylommatophora.
6 Helicidae
7 Pleurodontidae
8 Bradybaenidae
9 Zonitidae
10 Spiraxidae
11 Subulinidae

B. Brown garden snail at egg hatch. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

C. White garden snail. DAVID ROSEN, COURTESY UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

D. Brown garden snail laying eggs. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

E. Glass snail. WHITNEY CRANSHAW

F. Shells of glass snails. JIM KALISCH, UNIVERSITY OF NEBRASKA
Some of the most discriminating feeders among the insects are the leafminers and needleminers. These insects tunnel between the upper and lower leaf surfaces, feeding on the soft inner tissue and avoiding the tough epidermis. Immature stages of many different groups of insects share the leafmining habit, including the larvae of various flies, small moths, beetles, and sawflies. They are often classified by the pattern of the mine they create. Serpentine leaf mines meander across the leaf, gradually increasing in width as the insect grows. More common are various blotch leaf mines, which are an irregular but generally round form. One subgroup of these are the tentiform leaf mines, blotchlike mines produced by some types of moth larvae that tie the interior mine with silk in a manner that causes it to pucker (like a tent) as it dries. Many of the fly leafminers in the family Agromyzidae make a serpentine mine as first-instar maggots, but they dramatically enlarge the mine into a blotch when in the second and third instars. These mines are often called comma leaf mines. In addition, small pinholes are usually present that result from feeding wounds by adult female agromyzid flies. These are produced when the female uses her ovipositor to mash underlying cells to release plant juices on which she will feed. As leaves expand, the pinhole wounds can expand into larger shotholes.
VEGETABLE LEAFMINER (Liriomyza sativae)1
HOSTS Many vegetables, including bean, tomato, potato, onion, pepper, squash, and melon.
DAMAGE Larvae make thin, meandering serpentine mines. Puncture wounds (pinholes) are made by the ovipositor of the female during egg-laying or to produce fluid for feeding, and these may give the leaves a stippled appearance. Serious problems are often associated with insecticide use that adversely affects the many natural enemies that attack this insect.
DISTRIBUTION Predominantly a problem in warmer areas of the southern U.S. but occasionally ranges northward into the Midwest.
APPEARANCE Adults are small (ca.  inch) yellow and black flies. Larvae are pale, slightly greenish maggots found within serpentine-type leaf mines.
inch) yellow and black flies. Larvae are pale, slightly greenish maggots found within serpentine-type leaf mines.
LIFE HISTORY AND HABITS Eggs are inserted into foliage, producing small puncture wounds. Larvae develop rapidly under warm temperatures and may complete development in under 2 weeks. Often they then cut the leaf surface, drop to the soil, and pupate; pupation occasionally occurs in the mined leaf. Numerous overlapping generations occur during the year.
RELATED SPECIES
AMERICAN SERPENTINE LEAFMINER (Liriomyza trifolii),1 also known as CHRYSANTHEMUM LEAFMINER, is closely related to vegetable leafminer and can cause many similar injuries. It also has a wide range of hosts but is particularly damaging to flower crops, notably chrysanthemum. Primarily limited to warmer climates, it has spread widely among greenhouse crops. Problems are particularly severe following certain pesticide-use practices that devastate natural enemies. A species of similar habit is PEA LEAFMINER (L. huidobrensis). Widely distributed in South and Central America, it is found in areas of the western U.S. SERPENTINE LEAFMINER (L. brassicae) is also primarily a tropical species but may be common in the southern U.S. Cabbage, broccoli, mustard, and nasturtium are among the reported hosts.

B. Extreme leafminer damage to melon leaves. DAVID RILEY, UNIVERSITY OF GEORGIA, BUGWOOD.ORG

C. Adult serpentine leafminer, close-up. DAVID SHETLAR

D. Adult vegetable leafminer on onion leaf showing relative size. WHITNEY CRANSHAW

E. Vegetable leafminer larva outlined with leaf mine in onion. WHITNEY CRANSHAW

F. Pupa of a Liriomyza sp. leafminer. DAVID SHETLAR

G. Leafmines produced by the columbine leafminer Phytomyza aquilegivora. JIM KALISCH, UNIVERSITY OF NEBRASKA

H. Adult columbine leafminer. DAVID SHETLAR
OTHER SERPENTINE-TYPE LEAFMINING FLIES AND CATERPILLARS
The COLUMBINE LEAFMINER (Phytomyza aquilegivora)1 produces serpentine mines in leaves of columbine. Winter is spent in the pupal stage at the base of previously infested plants, and adults emerge in early spring. Females insert eggs into the foliage but also use their ovipositor to make pinhole wounds that provide plant fluids on which they feed. These oviposition injuries appear as small white spots on leaves. The larvae develop within the mines, which gradually widen as the larvae grow. Pupation also occurs in the mines. As many as five generations may be produced per season. Other Phytomyza spp. leafminers make blotch-type mines in columbine and are discussed below.
DAYLILY LEAFMINER (Ophiomyia kwanosis)1 is a newly established insect in North America that has spread widely across the eastern and southern states in the past decade. Adults typically insert eggs into the upper leaf tissues of daylilies, and larvae create serpentine mines. Pupation occurs within the mines and multiple generations are produced annually.
ASPEN LEAFMINER (Phyllocnistis populiella)2 caterpillars make meandering mines just under the upper leaf surface of aspen and other Populus species. The mines are distinctively silvery with a dark line of excrement in the center. Phyllocnistis vitifoliella produces serpentine mines just under the surface of grape and Virginia creeper. In southern California, CITRUS LEAFMINER (P. citrella) makes meandering tunnels in the leaves of citrus.
PECAN SERPENTINE LEAFMINER (Stigmella juglandifoliella)3 caterpillars produce serpentine mines between the leaf veins on the upper surface of pecan and black walnut. A large number of other Stigmella species also occur, producing serpentine mines in leaves of various roses, caneberries, birch, sumac, oak, elm and many other plants.
EUROPEAN ELM FLEA WEEVIL (Orchestes alni)4
HOSTS Elm, particularly Asian species
DAMAGE Adult feeding produces small shothole wounds in the leaf interior, resulting in a lacey appearance when injury is extensive. Larvae develop as leafminers that create a serpentine mine originating from a main leaf vein and terminating in a blotch leaf mine at the edge of the leaf.
DISTRIBUTION Broadly distributed across the midwestern and Rocky Mountain states.
APPEARANCE Larvae are cream-colored grubs that develop in leaf mines. The mines typically originate near the midrib and produce a serpentine course before terminating in a large blotch mine at the edge of the leaf. The adults are small (⅛ inch) reddish-brown beetles with black spots. They have a short snout on the head and their hind legs are enlarged to allow them to jump.
LIFE HISTORY AND HABITS Winter is spent in the adult stage, in the vicinity of previously infested elm trees. Adults resume activity in early spring and females lay eggs in larger veins of new leaves. Upon egg hatch, the larvae tunnel through the leaf as they feed, producing a serpentine mine that gradually enlarges in diameter as the insect gets older. The mines terminate at the leaf edge where the larvae remain and produce a blotchy area of mined tissue. Pupation occurs in the mine and adults emerge in late May and June.
Adults feed mainly on the underside of leaves, producing shothole injuries to the leaf interior. Where abundant, the insects will make foliage of affected trees appear lacy. Adults are present through early August then move to sheltering cover for winter. One generation is produced annually.
1 Diptera: Agromyzidae
2 Lepidoptera: Gracillariidae
3 Lepidoptera: Nepticulidae
4 Coleoptera: Curculionidae
A. Daylily leafminer larva in tunnel. GARY STECK, FLORIDA DEPARTMENT OF AGRICULTURE AND CONSUMER SERVICES

B. Daylily leafminer adult. GARY STECK, FLORIDA DEPARTMENT OF AGRICULTURE AND CONSUMER SERVICES
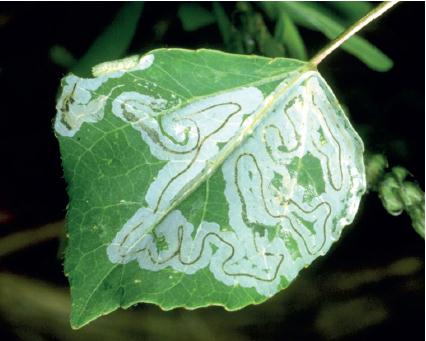
C. Leafmine produced by the aspen leafminer. WILLIAM CIESLA, FOREST HEALTH MANAGEMENT INTERNATIONAL, BUGWOOD.ORG
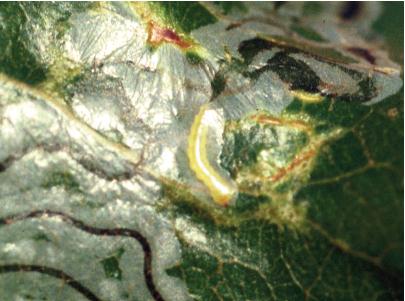
D. Aspen leafminer larva outlined within mine. WHITNEY CRANSHAW

E. Serpentine leafmine produced by Phyllocnistis vitifoliella. WHITNEY CRANSHAW

F. Leaf injury produced by citrus leafminer. WHITNEY CRANSHAW

G. Leaf injuries produced by pecan serpentine leafminer. JERRY A PAYNE, USDA-ARS, BUGWOOD.ORG

H. Leafmine produced by European elm flea weevil. DAVID LEATHERMAN

I. Adult European elm flea weevils following emergence from leaf mine. WHITNEY CRANSHAW

J. Larva of a European flea weevil (left) and elm leafminer (right). WHITNEY CRANSHAW

K. Pupa of a European elm flea weevil exposed within blotch leaf mine. DAVID SHETLAR
About a half-dozen other Orchestes species occur in North America. APPLE FLEA WEEVIL (O. pallicornis) is an eastern species with similar habits and develops on apple, quince, hawthorn, winged elm, and hazelnut. Others develop on various trees and shrubs, including birch, alder, elm, and various rosaceous hosts. Two other genera of North American flea weevils, Isochnus1 and Tachyerges,1 include about a dozen species that also produce similar leaf mines on woody plant hosts. WILLOW FLEA WEEVIL (Isochnus rufipes)1 is a small ( inch) black weevil that moves in spring to feed on the buds and newly emergent leaves of willow, birch, elm, red maple, aspen, red oak, cherry, and serviceberry. They lay eggs on the underside of leaves, and the larvae develop as leafminers, pupating in the leaf. If infested plants are nearby, the adults may migrate in nuisance numbers into buildings during late summer.
inch) black weevil that moves in spring to feed on the buds and newly emergent leaves of willow, birch, elm, red maple, aspen, red oak, cherry, and serviceberry. They lay eggs on the underside of leaves, and the larvae develop as leafminers, pupating in the leaf. If infested plants are nearby, the adults may migrate in nuisance numbers into buildings during late summer.
YELLOW POPLAR WEEVIL (Odontopus calceatus)1 develops on magnolia, tuliptree, and sassafras in several midwestern and Mid-Atlantic states. Primary damage is caused by larvae, which often feed as groups and produce large blotchlike leaf mines early in the season. Adults feed initially on buds, then move to emerging leaves, chewing small bean-shaped areas. Chewing also occurs on the main veins and can cause tips of leaves to wilt. Eggs are inserted into pits chewed in the main veins of the leaf, and a dozen or more larvae may feed together. When feeding is completed, they pupate in spherical cocoons in the mined leaf. Adults that emerge in midsummer feed for a brief period and then move to winter shelter and go into dormancy. One generation is produced annually.
1 Coleoptera: Curculionidae
LOCUST LEAFMINER (Odontota dorsalis)1
HOSTS Larvae develop in black locust and some other leguminous trees, including false indigo and golden chain tree. Adults feed on a wider range of plants that include oak, dogwood, elm, and hawthorn.
DAMAGE Larvae feed in the leaves, making irregular blotchlike mines. These mines, which during development may not be readily observed, can be seen most easily from the bottom. Adults feed on the underside of leaves and produce a skeletonizing injury.
DISTRIBUTION Most common in the Mid-Atlantic area, extending into Ohio, but can be found from Alabama and Georgia north into southern Canada.
APPEARANCE Adults are moderate-sized beetles, about ¼ inch. They are primarily yellow or yellow-orange with black patterning running down the center of the body and covering most of the hind end. The larvae, found in the leaf mines, are yellowish white, flattened, with dark appendages.
LIFE HISTORY AND HABITS Locust leafminer overwinters as an adult, under fallen leaves and in other protective cover. It may feed on foliage of other trees, notably oak, before moving to black locust. Eggs are laid on the underside of leaves, and the larvae subsequently tunnel. They create a gradually expanding blotch-type mine, usually on the outer edge of leaves. Pupation occurs in the mine. In southern areas of the species’ distribution, a second generation occurs.

B. Leaf mine on hornbeam produced by apple flea weevil larva. DAVID SHETLAR

C. Leafmine produced by yellow poplar weevil. DAVID SHETLAR

D. Cocoons surround the pupae of yellow poplar weevil exposed from leaf mine. DAVID SHETLAR

E. weevils and typical leaf injuries. DAVID SHETLAR

F. Locust leafminer adults and associated skeletonizing injury produced. DAVID SHETLAR

G. Adult locust leafminer and two blotch mines produced by larvae. In the mine at the top, the outline of two larvae can be seen. DAVID SHETLAR
OTHER BLOTCH LEAFMINING BEETLES
POPLAR BLACKMINE BEETLE (Zeugophora scutellaris)1 makes large dark blotch mines in poplar and cottonwood. Adults feed on the surface of leaves, making meandering skeletonizing injuries. BASSWOOD LEAFMINER (Baliosus nervosus)1 occurs in much of eastern Canada and the U.S., where it makes blotch leaf mines on basswood and several other deciduous trees. Both species have a single generation, with winter spent in the adult stage.
Larvae of the GOLDENROD LEAFMINER (Microrhapala vittata)1 produces blotch leaf mines in goldenrod. Adult feeding on leaves typically produces numerous shothole or windowpane-type injuries. Eggs are laid in small batches on the leaf underside, each batch typically including about 3 eggs that are covered with feces. The larvae feed gregariously and may completely mine a leaf.
1 Coleoptera: Chrysomelidae
HOLLY LEAFMINERS2
HOSTS Four Phytomyza species of leafminers are associated with hollies (Ilex spp.). NATIVE HOLLY LEAFMINER (Phytomyza ililocola) prefers American holly and may also attack English and Chinese hollies. HOLLY LEAFMINER (P. ilicis) only attacks English holly. INKBERRY LEAFMINER (P. glabricola) and WINTERBERRY LEAFMINER (P. verticillatae) exclusively attack their respective hosts.
DAMAGE Larvae of most of these “holly leafminers” produce comma-shaped mines. Each larva starts the mine as a thin linear trail (the first-instar larva) and then forms a blotch mine (the second and third larval instars). Damaged leaves may drop prematurely, especially during the winter and early spring.
Adults of many holly leafminers also cause conspicuous injuries. Adult females create puncture wounds with their ovipositor, which is used not only when laying eggs but also more commonly to acquire plant fluids on which to feed. These puncture wounds result in pinhole damage and affected leaves can become distorted and stunted. The inkberry leafminer produces fewer pinholes.
DISTRIBUTION Primarily important in the eastern and southern U.S. where Ilex hosts occur. Some species are also present in the Pacific Northwest.
APPEARANCE Larvae are tiny, pale yellow maggots found in the mines. Adults are small gray flies.
LIFE HISTORY AND HABITS The native holly and inkberry leafminer life cycles have been studied, but the others are poorly known. Native holly leafminer adults emerge in mid-May, when the new leaves are about ½ inch long. Females, which are about the size of a fruit fly, mate and begin maturation feeding by making pinholes in new leaves. These females often make a series of pinholes that can severely distort the expanding leaves. Eventually the females will insert eggs into the pinholes. After 1–2 weeks, the egg hatches and the larva makes a small ¼-inch-long mine. This first-instar larva then enters a dormant state and does not resume feeding until October or November. At this time the second-instar larva begins to expand the width of the mine. During freezing temperatures, the larvae stop feeding. By mid- to late April the third-instar larva finishes its development and cuts a small hole in the upper leaf epidermis. Under this hole, the larva pupates. The adults emerge a couple of weeks later. One generation occurs each season. The holly leafminer is suspected to have the same life cycle. Inkberry and winterberry leafminers appear to have two generations per season.

B. Adults of the basswood leafminer. DAVID SHETLAR

C. Dark blotch mine produced by larvae of the poplar blackmine beetle. WHITNEY CRANSHAW

D. Adult of the goldenrod leafminer. DAVID CAPPAERT, BUGWOOD.ORG

E. Leaf mine produced by goldenrod leafminer. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

F. Leafmines produced by a holly leafminer. DAVID SHETLAR

G. Adult holly leafminer and feeding wounds on new growth. DAVID SHETLAR

H. Pinhole wounds resulting from feeding punctures by holly leafminer adult. DAVID SHETLAR

I. Early stage mines on holly. DAVID SHETLAR
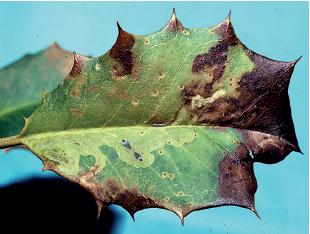
J. Extensive leafmining damage to holly. Also present are numerous pinhole feeding wounds and two pupae in the lower center of the photo. DAVID SHETLAR

K. Leafmining damage by inkberry leafminer. DAVID SHETLAR
SPINACH LEAFMINER (Pegomya hyoscyami)1
HOSTS Spinach, chard, beet, lambsquarter, pigweed
DISTRIBUTION Spinach leafminer is a species of European origin that is now generally distributed in the northern U.S. and Canada. In the western areas the closely related BEET LEAFMINER (P. betae) may be more abundant.
DAMAGE The immature maggots tunnel through older leaves. The large, dark, blotchy mines that are produced destroy the leaves for use as greens, although effects on plant growth appear to be minimal.
APPEARANCE The larvae are pale-colored, generally cylindrical maggots found in the leaf mines. Adults are small (ca. ⅕–⅓ inch) gray or grayish brown flies.
LIFE HISTORY AND HABITS The insect overwinters in the soil as a pupa, emerging in midspring. The adult flies lay small masses of white eggs on the underside of older leaves. Upon hatching, the young maggots tunnel into the leaves, where they feed, typically for 2–3 weeks. When full grown, they cut through the leaf, drop to the ground, and pupate in the soil. Several generations may be completed during the season, but activity largely ceases in midsummer, with most damage occurring on the cooler sides of the growing season.
OTHER LEAFMINING FLIES
BEET LEAFMINER (Pegomya betae)1 has a host range that largely overlaps that of the spinach leafminer. It is also native to Europe, but appears more restricted in distribution in North America and is more common in western states and provinces. Life history and feeding injuries to garden plants are similar to spinach leafminer.
Several species of leafminers in the genus Phytomyza2 produce blotch mines in various garden flowers. Phytomyza aquilegiana and P. columbinae produce blotch mines in columbine. Phytomyza delphinivora and other species develop in larkspur, delphinium, and aconite. Little is known about the biology of these species, but it appears two generations are normally produced annually. Chrysanthemum, marguerite, daisy, and several other composites may be hosts for P. atricornis or P. chrysanthemi. Blotch mines on many of the same hosts are also produced by Amauromyza maculosa,2 a common species in Florida. Three to six larvae may commonly occur in each mine. Adults make feeding punctures with their ovipositor, and these pinhole spots on leaves can be useful indicators of adult activity.
CORN BLOTCH LEAFMINER (Agromyza parvicornis)2 develops in leaves of corn, producing irregular blotch mines, particularly on lower leaves. Several generations can be produced annually but natural controls, particularly several species of parasitic wasps, normally keep populations low so plant damage is insignificant. Grassy weeds, including barnyardgrass and crabgrass, are occasional hosts for this insect.

B. Adult spinach leafminer. WHITNEY CRANSHAW

C. Egg mass of the spinach leafminer. WHITNEY CRANSHAW

D. Spinach leafminer larva exposed from mine on spinach. WHITNEY CRANSHAW

E. Blotch leafmines in columbine. WHITNEY CRANSHAW

F. Adult columbine leafminer and feeding puncture wounds. WHITNEY CRANSHAW

G. Columbine leafminer larva exposed from blotch mine. WHITNEY CRANSHAW

H. Columbine leafminer pupa exposed from blotch mine. WHITNEY CRANSHAW

I. Corn blotch leafminer damage. Numerous small feeding wounds produced by adults are present. JIM KALISCH, UNIVERSITY OF NEBRASKA

J. Larva of a corn blotch leafminer outlined within mine. JIM KALISCH, UNIVERSITY OF NEBRASKA
BOXWOOD LEAFMINER (Monarthropalpus flavus)3 produces small blistered blotches in areas of the leaf where larvae feed. Most species of boxwood are affected, and this is often considered the most serious pest of this ornamental plant. This is an introduced insect, currently found in areas of New England and the Mid-Atlantic States. Adults are tiny orange gnatlike flies that lay eggs in mid- to late spring. There is one generation per year. In the spring, leafmining damage can cause the plants to look as though they have received severe winter burn, and damaged leaves drop early.

Boxwood leafminer larvae and pupae exposed from within mine. DAVID SHETLAR
ASPARAGUS MINER (Ophiomyia simplex)2 is an introduced species that has spread through most of the East Coast States and is found in some areas of the Midwest. Larvae develop at the base of asparagus stems, mining the tissues. In large numbers they may girdle the stem, causing yellowing and dieback. More damage is thought to occur from incidental spread of Fusarium root-rotting fungi. Adults are tiny, shiny black flies about ⅛ inch long.
Conspicuous holes are produced in the foliage of red oak by the OAK SHOTHOLE LEAFMINER (Japanagromyza viridula).2 The adult females make puncture wounds with their sharp ovipositor in newly emergent leaves less than 2 inches expanded to produce fluids on which the flies feed; eggs are eventually inserted into leaves. The developing fly larva develops as a leafminer, producing a small blotch mine that later dries and drops out. As the leaves continue to expand, the holes produced by adult punctures and larval feeding continue to expand, making distinctive shotholes and giving leaves a tattered appearance. Larvae of the ELM AGROMYZID LEAFMINER (Agromyza aristata)2 develop in leaves of American elm. They produce a more serpentine mine than those of other leafminers associated with elms (e.g., European elm flea weevil, elm leafminer).
1 Diptera: Anthomyiidae
2 Diptera: Agromyzidae
3 Diptera: Cecidomyiidae

B. Boxwood leafminer adult next to pupal skin. DAVID SHETLAR

C. Mass emergence of boxwood leafminer adults. DAVID SHETLAR

D. Adult of the oak shothole leafminer. TOM MURRAY.

E. Leafmine and shothole injuries produced by oak shothole leafminer. DAVID SHETLAR

F. Shotholes in oak leaves resulting from expansion of feeding puncture wounds by adult oak shothole leafminer. DAVID SHETLAR

G. Leafmines produced by the elm agromyzid leafminer. DAVID SHETLAR
BIRCH LEAFMINER (Fenusa pusilla)1
HOSTS Birch.
DAMAGE Larvae develop in leaves, creating large brown blotch mines. After the insects exit, the mined areas wrinkle and can be confused with late frost or sun scald damage. Defoliation occurs on heavily damaged leaves. Stresses from repeated defoliation can increase risk of infestation by bronze birch borer.
DISTRIBUTION Birch leafminer is an introduced European sawfly species currently found in the northern half of the U.S. and southern Canada, east of the Rockies. It is particularly damaging in the Midwest.
APPEARANCE The mature larvae are ¼ inch long, slightly flattened, and yellowish white in color. They, and their dark droppings, are found in the mined leaf. Adults are black, thick-waisted wasps, about ¼ inch long.
LIFE HISTORY AND HABITS Birch leafminer winters in the soil in the prepupal stage, pupating in spring as soils warm (April). Adults first appear in spring, coincident with when the first leaves are about half grown, and prefer to mate and oviposit on upper leaves in sunny areas. Small oviposition punctures are visible as dark spots, although eggs are not always laid. Larvae hatch and mine out the middle layers of the leaf; several mines may grow together to form one large blotch mine. After 2–3 weeks, the larvae cut exit holes, drop to the ground, and burrow into the soil to make a pupal chamber. After another 2–3 weeks, the second-generation adults emerge and the cycle repeats. Normally there are two generations per year, although if conditions are dry many of the first-generation wasps remain dormant and do not emerge until the second year. As attacks are limited to new foliage, subsequent generations are less damaging and develop primarily in the crown of the tree.
OTHER LEAFMINING SAWFLIES
EUROPEAN ALDER LEAFMINER (Fenusa dohrnii) is a closely related species that produces blotch leaf mines on alder. HAWTHORN LEAFMINER (Profenusa canadensis) produces blotchlike mines in the tips of leaves and is particularly common on Crateagus crus-galli, C. persimilis, and C. erectus. ELM LEAFMINER (Kaliofenusa ulmi) is associated primarily with English elm, American elm, and hybrids. Eggs are inserted in foliage in late spring. Early-stage larvae produce serpentine mines that gradually enlarge and turn blotchlike as they mature. Hawthorn leafminer and elm leafminer have only a single generation per year; European alder leafminer may have multiple generations.
BLACKBERRY LEAFMINER (Metallus rubi) produces blotchlike mines in blackberry and dewberry leaves. Two generations occur in Connecticut, with the first adults present in May and early June and second-generation adults present in August.
PURSLANE LEAFMINER (Schizocerella pilicornis)2 produces blotch mines in leaves of purslane. Development of the larvae within the mines is rapid, typically taking about 1 week, and pupation occurs in the soil. A half-dozen or more generations may occur annually.
1 Hymenoptera: Tenthredinidae
2 Hymenoptera: Argidae

B. Adult birch leafminer ovipositing into leaf. DAVID SHETLAR
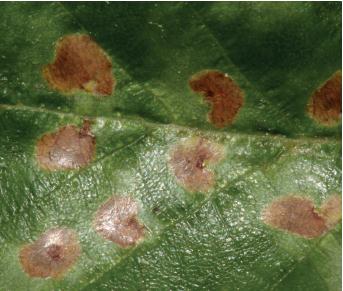
C. Early stage leafmines of birch leafminer. DAVID SHETLAR

D. Birch leafminer larva exposed from mine. WHITNEY CRANSHAW

E. Leafmines produced by European alder leafminer. WHITNEY CRANSHAW

F. Female hawthorn leafminer ovipositing into leaf. DAVID SHETLAR

G. Typical leafmines of hawthorn leafminer. DAVID SHETLAR

H. Elm leafminer larvae feeding within elm leaf. WHITNEY CRANSHAW

I. Blotch leafmine produced by purslane sawfly. WHITNEY CRANSHAW

J. Purslane sawfly larvae exposed from mine. WHITNEY CRANSHAW
LILAC LEAFMINER (Caloptilia syringella)1
HOSTS Lilac, privet. Rarely ash, euonymus.
DAMAGE Larvae first produce a blotch mine on the foliage, then tie and feed on the leaves. The ragged leaves and associated leaf rolling can seriously detract from a plant’s appearance.
DISTRIBUTION Primarily a western species found in northern states and southern Canada.
APPEARANCE Adults are small moths (ca. ½ inch long) that fold their wings tubelike over the body at rest. The wings are generally gray with reddish-brown patches, and there is some light striping across the body. Larvae are glossy green and found in mines or folded leaves. Younger larvae are more flattened than older larvae.
LIFE HISTORY AND HABITS Lilac leafminer spends the winter as a pupa or full-grown larva in the mines of dropped leaves. The adult moths emerge in spring after leaves emerge and lay eggs in small groups on the leaves. Newly hatched larvae tunnel directly into the leaf, entering underneath the egg. As they develop and grow larger, they excavate a blotchlike mine, and tunneling by several larvae may coalesce. As the larvae become nearly mature, they leave the leaf mine and tie the edge of the leaf with silk. They continue to feed in the folded leaf and later pupate. There are probably two generations per season.
OTHER LEPIDOPTERAN LEAFMINERS
BOXELDER LEAFMINER (Caloptilia negundella)1 feeds on boxelder and causes injuries similar to those of lilac leafminer. There are two generations per year, and the life cycle is likely similar to that of lilac leafminer. AZALEA LEAFMINER (C. azaleella) is found commonly in the eastern and southeastern U.S. and incidentally in the Pacific States. It is associated with low-growing azalea, and two generations are reported from North Carolina.
Nepticula slingerlandella2 is an infrequent pest of cherry and plum but has had historical outbreaks in cherry orchards of the Great Lakes States. Adults emerge from overwintered pupae and lay eggs on the underside of leaves in June. The larvae tunnel under the upper epidermis, so their meandering mines are most visible from the top surface. Caterpillars are about ⅕ inch when full grown and opaque greenish white. One generation is produced annually, with winter spent as a pupa in debris around the base of infested trees. In the Pacific States, leaves of hollyleaf cherry are commonly mined by larvae of Paraleucoptera heinrichi.3 Full-grown larvae emerge from the mine, construct a white H-shaped tent of silk on the upper leaf surface, and pupate in this protective structure. In the Pacific Northwest, blotch mines in Laburnum are produced by Leucoptera laburnella.3
Blotchy leaf mines of oak in eastern North America are commonly produced by OAK LEAFMINERS (Cameraria spp.).1 Depending on the species, mines may be produced by a single larva or by larvae feeding in small groups. Several generations may be produced annually, with the overwintering generation staying in mines of dropped leaves. Cameraria caryaefoliella is a common leafminer of pecan, mining the upper leaf surface. Cameraria species produce blotch mines in other woody plants including hickory, sugar maple, and Ohio buckeye.
Throughout the continental U.S. and parts of southern Canada, MORNING GLORY LEAFMINER (Bedellia somnulentella)3 mines the leaves of sweetpotato, morning glory, and bindweed. Initial mines are serpentine and later enlarge to become blotchlike. Two generations are produced in the northern U.S.
LYONETIA LEAFMINER (Lyonetia speculella)3 has become increasingly common in the mid-Atlantic region since the 1980s. It produces small blotchy mines in young leaves of apple, plum, cherry, birch, and grape. Probably little damage is done since older leaves are not suitable hosts. Larvae are light green, and pupation occurs in a silken “hammock” on the undersurface of the leaf. Multiple generations occur during the year.

B. Lilac leafminer larvae exposed from leafmine. WHITNEY CRANSHAW

C. Lilac leafminer adult. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

D. Azalea leafminer larva. JOHN A. DAVIDSON, UNIVERSITY OF MARYLAND, BUGWOOD.ORG

E. Adult of a Caloptilia sp. leafminer DAVID SHETLAR

F. Blotch mines produced by oak leafminers. DAVID SHETLAR

G. Larva of an oak leafminer exposed from mine. DAVID SHETLAR

H. Extensive leafmining by Cameraria sp. leafminers in oak. ERIC R. DAY, VIRGINIA POLYTECHNIC INSTITUTE AND STATE UNIVERSITY, BUGWOOD.ORG

I. A larva of a shield bearer, Coptodisca cercocarpella, covered with a circular leaf piece. ROBERT HAMMON, COLORADO STATE UNIVERSITY
SHIELD BEARERS (Coptodisca spp.)4 are tiny moths that develop as leafminers, producing small blotch mines. When feeding is completed, they cut a round section of leaf and fashion a small shielded case around themselves in which they pupate. Heavily infested plants may have numerous small holes, similar to those produced by a hole punch. RESPLENDENT SHIELD BEARER (C. splendoriferella) is widespread in the U.S. and feeds on apple and cherry. MADRONE SHIELD BEARER (C. arbutiella) is a common Pacific Coast species associated with madrone and manzanita.
APPLELEAF TRUMPET LEAFMINER (Coptotriche malifoliella)5 makes distinctive mines in apple, hawthorn, and crabapple. From the initial point of entry into the leaf, the mine expands to a large blotch, giving the appearance of a trumpet. Apple trumpet leafminer is a native species found in much of the northeastern quadrant of the U.S. and some areas of southeastern Canada.
1 Lepidoptera: Gracillariidae
2 Lepidoptera: Nepticulidae
3 Lepidoptera: Lyonetiidae
4 Lepidoptera: Heliozelidae
5 Lepidoptera: Tischeriidae
TENTIFORM LEAFMINERS (Phyllonorycter spp.)1
HOSTS Tentiform leafminers are moths associated with many kinds of woody plants, and more than 80 species occur in North America. At least eight native species are associated with either Populus (aspen, poplar, cottonwood) or Salix (willow). Apple and crabapple (Malus) host several species, including some of European origin that are common orchard pests. Celtis (hackberry), Acer (maple), Carya (pecan), and Tilia (basswood) host other commonly encountered tentiform leafmining species.
DAMAGE Early instar larvae are sap feeders that produce patches of leaf spotting where mesophyll cells have been gouged and destroyed. Later stage larvae produce a blotch leaf mine on the leaf underside. Silk is spun over the epidermis of the mined area, which later causes the affected area to contract, producing a tentlike pucker.
DISTRIBUTION Among apple infesting species, the SPOTTED TENTIFORM LEAFMINER (P. blancardella), an introduced species, appears to have the widest distribution and is found in almost all apple producing regions. In western orchards other Phyllonorycter1 species predominate. WESTERN TENTIFORM LEAFMINER (P. elmaella) develops in leaves of apple and cherry; P. mispilella feeds on apple, pear, and cherry. Some 17 species of rosaceous plants support APPLE BLOTCH LEAFMINER (P. crataegella), a species found in the northern U.S. and southern Canada.
Species associated with Populus (Phyllonorycter apparella, P. nipigon, P. deserticola, P. populiella) and Salix (P. salicifoliella, P. acanthus, P. mildredae, P. scudderella) can be found across southern Canada and the northern U.S., but are more common in the western regions. MAPLE LEAFMINER (P. aceriella), which mines the upper leaf surface of red and sugar maples, and P. lucetiella, which makes squarish mines on basswood, are found in eastern North America. Tentiform mines in hackberry result from the activity of P. celtisella. Phyllonorycter caryaealbella produces blotch mines in the lower leaf surface of pecan.
APPEARANCE Adults are tiny moths with a wingspan of about ⅓ inch. The wings are patterned with gold, black, and white. Larvae are cream colored and flattened during early stages, developing a more typical caterpillar form and functional legs in later stages.
LIFE HISTORY AND HABITS Life histories of most species have been poorly studied, but a generalized biology is known, based largely on observations of species affecting apple. Adults emerge in spring at bud break, mate, and deposit eggs singly on the underside of young leaves. The first three larval stages feed on the leaf surface, taking sap from parenchyma cells that produce leaf spotting. At the end a small blotch mine is initiated and the last two larval stages enter the leaf and extend the mine as they feed on tissues. A thin layer of silk is produced that covers the outside of the mined area.
By mid- to late June, the larvae pupate within the mine. If a second generation is produced, just prior to adult emergence, the pupae wiggle halfway out of the mine, leaving the pupal skin partially extended from the leaf. The number of generations produced is unknown for most species, but two generations are probably typical, with adult flights in late spring and late summer; some species associated with apple have three generations and up to four generations are reported with a species on pecan. All Phyllonorycter spp. spend winter as a pupa within the mines of fallen leaves.
Tentiform leafminers are heavily parasitized by many insects. Bird predation of late-instar larvae can also be locally common. Outbreaks are typically of short duration, one to two years at most. Inappropriate insecticide use can prolong outbreaks through adverse effects on biological controls.
1 Lepidoptera: Gracillariidae
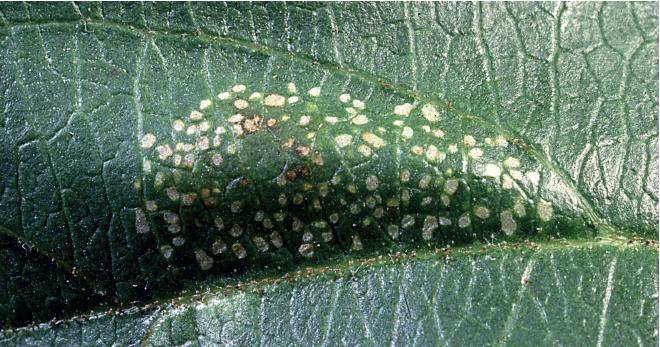
B. Upper surface symptom of tentiform leafminer in apple. DAVID SHETLAR

C. Lower surface symptom of tentiform leafminer in apple. DAVID SHETLAR

D. Tentiform leafminer larva in apple leaf. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

E. Tentiform leafminer injuries to cottonwood. WHITNEY CRANSHAW

F. Tentiform leafmines in hackberry. WHITNEY CRANSHAW

G. Adult of a tentiform leafminer. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

H. Larval stage of a parasitic wasp that attacks tentiform leafminers. DAVID SHETLAR
Larvae of the SPRUCE NEEDLEMINER (Taniva albolineana)1 hollow out the base of spruce needles. Groups of needles are often cut and later webbed together in a small mass; often, several larvae may feed together. Infestation of large trees is usually confined to lower branches, but entire crowns of small trees can be infested and defoliated. Spruce needleminer is widespread through the northern half of the U.S. and southern Canada. Larvae are light greenish-brown caterpillars with a dark head, about ⅜ inch long, and found associated with damaged needles.
Spruce needleminer spends the winter as a nearly full-grown larva in a cocoon constructed within mats of webbing and dead needles. It resumes feeding in early spring, becoming full grown in April or May. Adult moths emerge and lay eggs on the needles in late May and June. Eggs are laid in rows, on the underside of year-old needles. Larvae initially mine the interior of needles, and damaged needles are later cut off and bound to the twigs with webbing. Larvae feed throughout the summer, suspending feeding with the onset of cold weather. There is one generation per year.
PINE NEEDLE SHEATHMINER (Zelleria haimbachi)2 is primarily a western species but does occur in the Great Lakes States and Ontario. Larvae tunnel into the fascicle at the base of needles of several kinds of pines, including lodgepole, jack, ponderosa, and white. Individual larvae may destroy 6–10 bundles of needles in the course of development and may cause dieback of terminals in sustained outbreaks.
Larvae overwinter as miners in needles. After shoot elongation, they move to the new growth and tunnel into the base of needles. As they get older, they form a silken tube outside the needles and cut them as they feed. Infested plants can be detected by drooping and faded needles hanging by the silk. The full-grown larvae are tan colored and about ½ inch. They pupate in late spring and early summer. Adults then lay eggs, and the newly hatched larvae then tunnel into needles to overwinter. One generation is produced annually.
Various species of Argyresthia are often collectively known as “arborvitae leafminers”3 and mine the needles of arborvitae and cypress, causing tips to brown and die back. In most species, adults emerge in late spring and early summer. Eggs are laid in the tips of 1- or 2-year-old twigs, and the larvae tunnel under leaf scales. They feed as miners for the remainder of their lives and winter as partially grown larvae. Visible symptoms of damage usually appear in winter and are commonly mistaken for winterkill or fungal tip blights, although the presence of tunneling activity is diagnostic. Pupation occurs in spring, usually in the tunnels, although CYPRESS TIPMINER (A. cupressella), a western species, exits the needle and spins a cocoon on the foliage. Small holes indicate the emergence of Argyresthia adults or the exiting larvae.
Several Coleotechnites4 species develop as needleminers of various conifers and are particularly common in western forests. Most important is PONDEROSA PINE NEEDLEMINER (C. ponderosae), which has produced several widespread outbreaks in the Mountain States. Adults lay eggs in August, usually in older, previously mined needles. After egg hatch, the larvae move to the new needles and bore near the tip. Larval development and feeding continue until the following spring, when pupation occurs. In eastern North America a species that may attract attention is C. picealla, which occasionally damages spruce and produces small “nests” of mined needles that are webbed together. Other Coleotechnites spp. occur in pines, cypress, junipers, and arborvitae.
1 Lepidoptera: Tortricidae
2 Lepidoptera: Yponomeutidae
3 Lepidoptera: Argyresthiidae
4 Lepidoptera: Gelechiidae

B. Larva of a spruce needleminer. THE CONNECTICUT AGRICULTURAL EXPERIMENT STATION, BUGWOOD.ORG

C. Adult of the spruce needleminer. DAVID SHETLAR

D. Damage produced by pine needle sheathminer. DONALD OWEN/CALIFORNIA DEPARTMENT OF FORESTRY AND FOREST PROTECTION, BUGWOOD.ORG.

E. Larva of the pine needle sheathminer within silken retreat. DONALD OWEN/CALIFORNIA DEPARTMENT OF FORESTRY AND FOREST PROTECTION, BUGWOOD.ORG.

F. Damage produced by arborvitae leafminer. DAVID SHETLAR

G. Adult exit hole at base of needles mined by arborvitae leafminer. BRUCE WATT, UNIVERSITY OF MAINE, BUGWOOD.ORG

H. Adult arborvitae leafminer. BRUCE WATT, UNIVERSITY OF MAIN, BUGWOOD.ORG

I. Needlemining of pine by a Coleotechnites sp. in Ohio. Exit holes are noticeable at the base of some needles. DAVID SHETLAR

J. Adult of a Coleotechnites sp. associated with pine in Ohio. DAVID SHETLAR

K. Larva of Coleotechnites picealla, a needleminer of spruce. CONNECTICUT AGRICULTURAL EXPERIMENT STATION, BUGWOOD.ORG

L. Small “nest” of webbed together needles produced by Coleotechnites picealla, a needleminer of spruce. CONNECTICUT AGRICULTURAL EXPERIMENT STATION, BUGWOODO.ORG
GALL WASPS THAT DEVELOP ON LEAVES
The gall wasp family Cynipidae contains hundreds of North American species, almost all of which make galls on oaks and related plants or roses. All aboveground plant parts are distorted into galls by one or more gall wasps, with galls produced in leaves and twigs often being most conspicuous. Furthermore, a great majority of gall wasps have complex life cycles that involve two different forms, each of which makes a different kind of gall. Several examples of these are included in chapter 4, where gall wasps affecting twigs and shoots are discussed.
The types of galls produced by gall wasps can be of extraordinary form, with changes in plant growth resulting in growths that are completely different from the original tissues from which they arose. A great many can have very bizarre forms, with complicated architecture, and these galls often attract attention; however, none of the galls on leaves are considered to cause significant effects on plant health.
Perhaps the most spectacular form of galls produced on oak are the oak apples, large parchment-covered galls that are large with spongy or fibrous material in the interior. In the center is a chamber in which the gall wasp larva develops. The largest of the oak apple galls are produced by Amphibolips confluenta1 and A. quercusspongifica, which form galls on the leaves or petioles of red, black, scarlet, and some other oaks in the eastern U.S. The galls may be up to 2 inches in diameter and are dark greenish or brownish, drying to light brown.
The related TRANSLUCENT OAK GALL WASP (A. nubilipennis) makes large round, succulent galls—somewhat resembling a grape—on various red oaks. Other large leaf galls of round form, known variously as “oak apples” or “roly-poly galls” are produced by wasps in the genera Andricus and Atrusca. Examples include SUCCULENT OAK GALL WASP (Andricus palustris), common in the northeastern U.S. on red oak, and ANT-LIKE GALLFLY (A. lasius), which makes rounded galls covered by filaments on live oak leaves.
One of the galls that attracts particular attention in the northwestern states and parts of the Midwest is produced by JUMPING OAK GALL WASPS (Neuroterus saltatorius and probably related species).1 They make seedlike galls on the leaves of various white oaks, and the galls later drop from the leaf. The developing wasps in the fallen galls are active and cause the galls to jump about until they lodge in a protected crevice, in a manner similar to a tiny Mexican jumping bean. On the underside of bur oak, N. quercusverrucum produces small galls covered by a dense mat of white plant hairs.
Phylloteras poculum1 produces a cluster of pale or red saucerlike galls (“spangle galls”) attached to a slender stalk on the underside of oak leaves. Other Phylloteras species produce galls of more cupped form on the upper surface of oak leaves. Galls of similar form are also produced by some Andricus species of gall wasps.
Short spiny hairs emerging from a globular leaf gall are produced by HEDGEHOG GALL WASP (Acaraspis erinacei)1 on white oak in eastern states. In California, leaf galls on valley oak covered with stout hairs are distinctive of WOOLLYBEAR GALL WASP (Atrusca trimaculosa). Philonix fulvicollis is a species commonly associated with bur oak that produces round leaf galls that are made of multiple sections with tiny spines. Leaf galls of cottony appearance, produced by a proliferation of plant hairs, are common forms produced by various Andricus species. Leaf galls covered with stout spines are typical of various “spined turban galls” produced by Antron species. SPINED TURBAN GALL WASP (Antron douglasii)1 is a common species on valley, blue, and California scrub oak in the western states.
A few Diplolepis species1 produce galls on leaves of rose; others develop on twigs. Typically the leaf galls are rounded, reddish, and spiny. D. nebulosus and ROSE BLISTERGALL WASP (D. rosaefolii) are examples of such species. SPINYROSE GALL WASP (D. bicolor) makes highly spined galls, usually in clusters, on leaves.
1 Hymenoptera: Cynipidae

B. Roly-poly type gall. DAVID SHETLAR

C. Roly-poly type gall cut away to show interior and an adult. DAVID SHETLAR

D. Translucent oak gall. DAVID SHETLAR

E. Translucent oak gall, cut away to show interior cell. DAVID SHETLAR

F. Jumping oak galls. DAVID SHETLAR

G. Galls produced by Neuroteras quercusverrucum on bur oak. WHITNEY CRANSHAW

H. Hedgehog type of gall. DAVID SHETLAR

I. Hairy gall on leaf midrib produced by gall wasp. WHITNEY CRANSHAW

J. Segmented ball-form gall produced by Philonix fulvicollis. WHITNEY CRANSHAW

K. Simple midrib swelling type of gall produced by Callirhytis flavipes. WHITNEY CRANSHAW

L. Adult of Callirhytis flavipes. WHITNEY CRANSHAW

M. Small, spiny leaf gall produced by a gall wasp on rose. WHITNEY CRANSHAW

N. Bud gall produced by gall wasp on rose. WHITNEY CRANSHAW
OTHER GALL-MAKING WASPS THAT DEVELOP ON LEAVES
Several dozen Pontania species1 make leaf galls on willow. WILLOW REDGALL SAWFLY (P. proxima) is typical, producing a reddish bean-shaped swelling on the leaves of willow. The insect spends winter in soil or under protective debris. Adults emerge in spring and lay eggs in young, expanding leaves in late spring and early summer. The developing larvae feed on the soft tissues in the gall, later moving into firmer tissues to feed but remaining in the gall. When full grown, they drop to the ground and spin a cocoon. The related species P. s-pomum creates prominent rounded yellow or red swellings on willow leaves that develop in small clusters.
Josephiella microcarpae is an introduced species present in Florida and southern California that produces galls on the leaves of Cuban laurel, Ficus microcarpa. The galls are in the form of small leaf blisters, often in groups, and can cause significant leaf distortion.
1 Hymenoptera: Tenthredinidae
2 Hymenoptera: Agaonidae
GALL-MAKING FLIES THAT DEVELOP ON LEAVES
The gall midge family Cecidomyiidae is one that contains many dozens of species that develop on leaves and needles. Adults are inconspicuous midgelike flies, and the maggot-form larvae are found in the galls. These are typically in the form of abnormal swellings, thickenings, and/or stunting of foliage. Other gall midges distort developing buds and flowers (e.g., rose midge, swede midge) or fruit, and these are discussed in chapter 7. Other gall-producing flies, in the families Agromyzidae (leafminer flies) and Tephritidae (fruit flies) develop in twigs and shoots (pages 418).
Larvae of the HONEYLOCUST PODGALL MIDGE (Dasineura gleditschiae)1 feed on the developing leaves of honeylocust, causing them to distort into thickened, podlike galls. The galls darken, dry, and drop a few weeks after adults emerge. This defoliation gives a thin appearance to the plant, particularly when most of the leaflets on a leaf are affected.
Honeylocust podgall midge spends the winter in the adult stage under protective cover around previously infested honeylocust plantings. The adults move to emerging honeylocust buds as they first start to break. Eggs are laid among the emerging leaves, and the larvae feed on the leaflets, causing them to curl and thicken into the characteristic pod gall. Larvae become full grown in about 3–4 weeks, and pupation occurs in the gall. As the adults emerge, the old pupal skin is often pulled partially out at the gall opening. There are typically three generations per year, with populations usually declining by early July. Later infestations may extend for additional generations where sprout growth continues to be produced or in highly fertile, irrigated sites such as nurseries.
ASH BULLETGALL MIDGE (Dasineura pellex) produces large globular galls on the larger veins of ash leaves. Dasineura pseudacaciae1 induces young leaves of black locust to thicken and fold. Thickened veins on boxelder are changes produced by the developing larvae of the GOUTY VEINGALL MIDGE (C. negundinis); similar vein thickening on red and sugar maples is produced by MAPLE GOUTY VEINGALL MIDGE (D. communis) in parts of the Midwest. More modest leaf distortions occur from feeding of the PEAR LEAFCURLING MIDGE (D. pyri) and APPLE LEAFGALL MIDGE (D. mali), which can cause a curling distortion along the leaf edge.

B. Cut away of gall by willow redgall sawfly. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

C. Cut away of gall by willow redgall sawfly. KEN GRAY COLLECTION, OREGON STATE UNIVERSITY

D. Gall produced by Pontania s-pomum. DAVID LEATHERMAN

E. Leaf galls produced by a Josephiella microcarpae on Cuban laurel. DOUG CALDWELL, UNIVERSITY OF FLORIDA

F. Honeylocust podgalls. WHITNEY CRANSHAW

G. Adult of the honeylocust podgall midge. WHITNEY CRANSHAW

H. Ash bullet galls. JIM KALISCH, UNIVERSITY OF NEBRASKA

I. Gall produced by grape tube gallmaker midge. DAVID SHETLAR
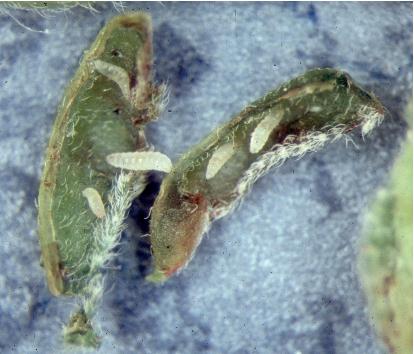
J. Larvae of the honeylocust podgall midge in gall. WHITNEY CRANSHAW

K. Pod galls produced by a Blaesodiplosis species in hawthorn. DAVID SHETLAR

L. Gouty veingall. WHITNEY CRANSHAW

M. Gall produced by Blaesodiplosis crataegifolia in hawthorn. WHITNEY CRANSHAW

N. Larvae of gouty veingall midge exposed within gall. WHITNEY CRANSHAW
GRAPE TUMID GALLMAKER (Janetiella brevicauda)1 develops on wild and cultivated grapes in the northeastern U.S. and southeastern Canada. Round, succulent galls are produced on leaves, petioles, and flower clusters. Three generations are produced annually, with most injury caused by the first, which occurs as flower buds are present. Elongate, pointed galls “tube galls” on grape are characteristic of Schizomyia viticola (GRAPE TUBE GALL MAKER).
Blaesodiplosis crataegifolia produces a ridged thickening between leaf veins of hawthorn. Another Blaesodiplosis species produces large bladderlike galls to form in leaves of hawthorn.
The genus Contarinia1 includes several species that produce thickened leaf foldings. GOUTY VEINGALL MIDGE (C. negundinis) makes a prominent thickening along the midrib of boxelder maple, in which numerous pale larvae may be found. ASH MIDRIBGALL MIDGE (C. canadensis) can produce a thickening along the midrib of ash leaves in late spring. LINDEN WARTGALL of basswood is produced by C. verrucicola. Leaves and developing seedpods of catalpa may be distorted by CATALPA MIDGE (C. catalpae). A Contarinia species that produces a modest folding along the leaf edges of pin oak has been associated indirectly with problems of a biting mite in Nebraska. Pyemotes herfsi,2 known as the OAK LEAF ITCH MITE, develops as a predator of gall midge larvae but may drop from the trees and bite humans.
OCELLATE GALL MIDGE (Acericesis ocellaris)1 produces distinctive “eyespot” galls on red maple leaves. The galls are yellow margined with red and form on the upper surface of the leaf. Eyespotting of tuliptree leaves is produced by EYESPOT GALL MIDGE (Thecodiplosis liriodendri).1
Distortion and discoloration of rhododendron foliage can result from damage by RHODODENDRON GALL MIDGE (Clinodiplosis rhododendri).1 Adults emerge from overwintering pupae and lay eggs on emerging leaves. Multiple generations are produced coinciding with the spring flush of growth, and peak damage is typically caused by the second and third generations. Twisting and distortion of new growth on chrysanthemum may be due to injury by CHRYSANTHEMUM GALL MIDGE (Rhopalomyia chrysanthemi).1 Larvae feeding on buds may result in formation of cone-shaped galls. Fleshy knobbed distortions of the shoot tips of coyote bush (Baccharis pilularis) are produced by BACCHARIS GALL FLY (R. californica). VIOLET GALL MIDGE (Phytophaga violicola) can produce a leaf edge curl that can develop into a bladder-form gall on African violet.
Close to two dozen Caryomyia species1 are associated with leaves of hickory. These “hickory gall midges” produce a wide range of gall forms, many rivaling the complexity of those produced by gall wasps.
In the Mountain States, STUBBY NEEDLEGALL MIDGE (Contarinia coloradensis)1 produces basal swellings and stunting of ponderosa pine needles. In the Pacific Northwest and Pennsylvania the DOUGLAS-FIR NEEDLE MIDGE (C. pseudotsugae) is an important pest of Christmas tree plantings. Adults lay eggs in the newly expanding buds in spring, and the larvae develop in the base of needles. Infested needles yellow and then brown and drop prematurely. Winter is spent in the soil as a full-grown larva that pupates the following March and April. One generation is produced annually. BALSAM NEEDLEGALL MIDGE (Paradiplosis tumifex) causes a similar injury to balsam fir. A common gall of pinyon grown in ornamental plantings is PINYON SPINDLEGALL MIDGE (Pinyonia edulicola).1 It produces a basal swelling of new needles, in which as many as a dozen or more pale orange larvae develop. Needles turn brown and drop the following year. Basal swelling combined with needle stunting is produced by PINYON STUNT NEEDLEGALL MIDGE (Janetiella spp.).1
CYPRESS TWIGGALL MIDGE (Taxodiomyia cupressiananassa) cause the buds of pond cypress and baldcypress to become grossly enlarged. One generation is produced in the northern areas, with larval stages surviving winter within the dropped galls. In parts of Florida two generations may occur.
1 Diptera: Cecidomyiidae
2 Trombidiformes: Pyemotidae

B. Leaf edge curl produced by a Contarinia species of gall midge of pin oak. JIM KALISCH, UNIVERSITY OF NEBRASKA

C. Larvae of a Contarinia species of gall midge that produces a leaf edge curl of pin oak. JIM KALISCH, UNIVERSITY OF NEBRASKA

D. Bull’s-eye type gall produced by ocellate gall midge. DAVID SHETLAR

E. Fleshy gall of coyote bush terminal produced by the baccharis gall fly. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

F. Violet gall midge. JACK KELLY CLARK, COURTESY OF UNIVERSITY OF CALIFORNIA STATEWIDE IPM PROGRAM

G. Gall produced by Caryomyia subulata on hickory. TOM MURRAY

H. Gall produced by Caryomyia tubicola on hickory. TOM MURRAY

I. Gall produced by Caryomyia persicoides on hickory. TOM MURRAY

J. Needle gall of pine produced by a Contarinia species of gall midge. DAVID SHETLAR

K. Douglas-fir needle gall. WARD STRONG, BC MINISTRY OF FORESTS, BUGWOOD.ORG

L. Balsam needle gall. DAVID SHETLAR

M. Balsam needle gall with midge larva exposed. DAVID SHETLAR

N. Cypress twiggalls. DAVID SHETLAR

O. Pinyon spindlegalls. WHITNEY CRANSHAW

P. Adult emergence of pinyon spindlegall midge. WHITNEY CRANSHAW