Introduction: Biogeochemical Cycles as Fundamental Constructs for Studying Earth System Science and Global Change
1.1 Introduction
The latter part of the 20th century has seen remarkable advances in science and technology. Accomplishments in biochemistry and medicine, computer technology, and telecommunications have benefited nearly everyone on Earth to one degree or another. Along with these advances that have improved our quality of life, scientific research into the study of the Earth has revealed a planetary system that is more complex and dynamic than anyone would have imagined even 50 years ago. The Earth and the environment have become one of society’s greatest concerns, perhaps as the result of these discoveries combined with the quick dissemination of information that is now possible with modern telecommunications.
The basis of most environmental issues is pollution. But what is pollution? Keep in mind that with very minor exceptions, virtually all of the atoms in the solid, liquid, and gaseous parts of the Earth have been a part of the planet for all of its approximately 4.5 billion years of existence. Very few of these atoms have changed (i.e., by radioactive decay) or departed to space. This includes all of the atoms in your own body and in all other living things, which have also been permanent residents of the Earth through the eons. This means that the Earth is an essentially closed system with respect to atomic matter, and is therefore governed by the law of conservation of mass. This law dictates that all of the Earth’s molecules must be made of the same aggregation of atoms even though molecular forms may vary, evolve, and be transported within and around the planetary system. Pollution, therefore, is a human-induced change in the distribution of atoms from one place on Earth to another.
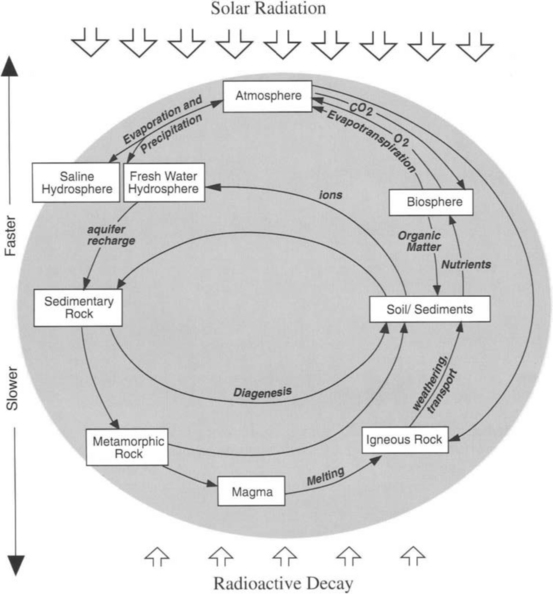
In order to understand the impact of pollution on Earth, we must realize that the planet itself is not stagnant, but continually moving material around the system naturally. Any human (anthropogenic) redistribution in the elements is superimposed on these continuous natural events. Energy from the sun and radioactive decay from the Earth’s interior drive these processes, which are often cyclic in nature. As a result, almost all of the rocks composing the continents have been processed at least once through a chemical and physical cycle involving weathering, formation of sediments, and subduction, being subjected to great heat and pressure to produce new igneous rocks. The water in the oceans has been evaporated, rained out, and returned via rivers and groundwater flow many tens of thousands of times. The main gases in the atmosphere (nitrogen and oxygen) are cycled frequently through living organisms. The combined effect of these dynamic transports and transformations is a planet that is in a state of continual physical, chemical and biological evolution. A bird’s eye, cartoon view of the dynamic Earth system is shown in Plate 1. This book is about putting together all of the different dynamic parts of this figure into an understandable, coordinated picture. In the last chapter of the book, we will revisit the topic of human modification of the system in detail.
1.1.1 Biogeochemical Cycles and Geospheres
Aside from the cyclic systems listed above, there is a complementary set of chemical cycles that we can describe for each of the most important biological elements (carbon, nitrogen, oxygen, sulfur, and the trace metals). These biogeochemical cycles are descriptions of the transport and transformation of the elements through various segments of the Earth system, called geospheres. We use these constructs to compartmentalize the larger Earth system into more manageable, chemically definable parts.
What are the geospheres? One of them is easily definable and requires no special introduction: the atmosphere is the gas-phase envelope surrounding the globe of the Earth. Another geosphere is the hydrosphere, which includes all of the oceans, and freshwater bodies of water on the planet. The lithosphere is the entirety of rocks on Earth, including rocks exposed to the atmosphere, under the waters of the hydrosphere, and the entire interior parts of the planet. The pedosphere (literally that upon which we walk) comprises the soils of the Earth. The geospheres listed thus far are more or less geographically definable, but there is a geosphere that can exist within all of the other geospheres: the biosphere, which is the collection of the biota (all living things) on the planet. The interfaces between the geospheres are often fuzzy and difficult to define. For example, ocean sediments contain water as well as rock and organic material; it is difficult to say exactly where the hydrosphere ends and the lithosphere starts. Part of the hydrosphere exists in the atmosphere as rain and cloud droplets.
The constant transport of material within and through the geospheres is powered by the sun and by the heat of the Earth’s interior. A simple diagram of these geospheric concepts and the energy that moves material within them is presented in Fig. 1-1. The result of the interactions shown in Plate 1 and Fig. 1-1 is an Earth system that is complex, coupled, and evolving.
In addition to the natural evolution of the interacting geospheres, human activities have brought about an entirely new set of perturbations to the system. Because many political and social issues surround the problem of human induced global change, there are both basic and applied scientific motivations to study biogeochemical cycles and their roles in the Earth system. The need for development and application of basic science to the broad policy issues of dealing with global change have inspired the formation of a new integrative scientific discipline, Earth system science (NASA, 1986).
The subject offers a number of challenges that are important for the scientific community to address. Probably the largest challenge is integrating knowledge and material from many disciplines. This is a major theme of this chapter and of this book, as will be seen in the sections that follow. If the scientific community is not able to integrate the science necessary to describe biogeochemical systems, it seems unlikely that it will be easy for society to derive solutions for the problems raised by global change.
The principal obstacles facing us as scientists studying Earth system science are the finite resources of most educational institutions. Development of this subject requires that we think of novel ways to do interdisciplinary work in a setting dominated by traditional disciplines. Although we can draw heavily on work being done in recently formed disciplines such as chemical oceanography, stable isotope geochemistry, and atmospheric chemistry, we may be able to glean some clues in how to accomplish our goal of integrating the disciplines by examining the history of Earth system study. Indeed, the recognition that biogeochemical issues may be significant for mankind goes back several hundred years.
1.2 History
Some of the earliest work in the study of biogeochemical cycles and their role in the physical functioning of the planet was by James Hutton (1788), who viewed the Earth as a “superorganism, and that its proper study should be by physiology.” More than 100 years later, a classic paper by Svante Arrhenius (1896) appeared, called “On the influence of carbonic acid in the air on the temperature of the ground.” This work provided a paradigm for quantitatively connecting the greenhouse effect of carbon dioxide to climate, as well as to the global biogeochemical cycle of carbon. A truly meaningful study of these issues clearly requires input from nearly all of the natural sciences (chemistry, physics, biology, geology, meteorology, etc.). These scientific disciplines, which were still in their early formative stages when Arrhenius’ pioneering work emerged, have since evolved over the past century into highly refined and useful, but largely separated entities. Accordingly, most scientists have adopted a reductionist approach to biogeochemistry (i.e., simplifying large scientific problems into smaller parts to be examined by an individual discipline). In contrast, as we stated above, one of the goals of Earth system science (referred to early on as natural philosophy) is to integrate the natural sciences to strive towards understanding the entire system.
In addition to the work of Arrhenius, several other scientists have made notable contributions that have helped to mold the approach and content of Earth system science (and of this book). The term biosphere was originally coined by the Austrian geologist Eduard Suess as a way of defining the parts of the Earth’s surface that support life (Suess, 1875). The global view portrayed by Arrhenius’ lengthy quote of the paper on the carbon cycle by Arvid Högbom set the tone for coupling the biosphere to geochemistry (Arrhenius, 1896). This coupling was strongly emphasized and promoted by the Russian mineralogist Vladimir Ivanovich Vernadsky, who published a series of lectures on the subject, first in Russian (1926), then in French (1929), and recently translated into English (1997). In these writings, Vernadsky developed an integrative definition of the biosphere as all living things and everything connected to them. He strongly believed that the Earth is a set of connected parts that can only be studied in an indivisible, holistic way. Vernadsky went so far as to suggest that geologic phenomena at the Earth’s surface are inherently caused by life itself, so his definition of the biosphere includes all of the Earth’s geologic features. Vernadsky’s teachings remained relatively obscure until Hutchinson (1970) popularized them in a famous article in Scientific American. Vernadsky came to be called the father of modern biogeochemistry by James Lovelock (1972), who went on to suggest that feedbacks in the biosphere-climatic system lead to homeostasis of basic Earth processes. Lovelock called this new feedback-based integrative science geophysiology and the system itself Gaia, which is a Greek word meaning Mother Earth.
This view of the coupled nature of the Earth system has not dominated the historical development of the key disciplines of the natural sciences. Many evolutionary biologists have viewed the changing physical climate of the Earth as an externally imposed factor to which the biosphere must adapt. Likewise, many geologists and geophysicists have viewed the evolution of the planet as being governed primarily or exclusively by chemical and physical processes. Under this perspective, free oxygen in the atmosphere is viewed as a constant factor. The disparate views of the biological and geologic communities have coexisted for nearly a century, and (with few exceptions) their merging has been controversial (see, e.g., Dawkins, 1976).
The Gaia hypothesis was put forward by Lovelock together with Lynn Margulis (Lovelock and Margulis, 1974) to provide a single scientific basis for integrating all components of the Earth system. This theory suggests that Earth’s biota as well as the planet itself are parts of a quasi-living entity that “has a capacity for homeostasis” (or self-regulation) (Lovelock, 1986). The earliest version of Lovelock’s Gaia hypothesis contained phrases like “by and for the biosphere,” which implied a sense of purposefulness on the part of the biota to evolve in ways that would suit its own continued existence. As the Gaia hypothesis has evolved, the interdependence of the evolution of biota and geophysical/geochemical systems is described in non-teleological terms. Lovelock (1991) himself recently stated what seems obvious: “In no way do organisms simply ’adapt’ to a dead world determined by physics and chemistry alone. They live in a world that is the breath and bones of their ancestors and that they are now sustaining.”
1.3 Evidence for the Coupled Nature of the Earth System
The biosphere is ultimately what ties the major systems of the Earth together. Studying the fundamental differences between a planet full of life (the Earth) versus one that lacks life (e.g., Mars) evidences this. Dead planets are in or near a state of perpetual thermodynamic equilibrium, but as pointed out by Lovelock (1972), the spheres of the Earth and the chemical constituents within them are far out of equilibrium in a thermodynamic sense, as evidenced by the following points:
• O2, N2, and H2O are the main molecular forms coexisting in the atmosphere, but the condition of thermodynamic equilibrium would require that HNO3 be formed from these gases and subsequently dissolve in the oceans.
• O2 coexists with combustible biomass in plants.
• Acidic materials in the atmosphere (e.g. CO2 and H2CO3, SO2 and H2SO3, HNO3 and so on) coexist with basic materials in both igneous and sedimentary rocks (e.g. FeS2 and CaCO3).
We cover each of these types of examples in separate chapters of this book, but there is a clear connection as well. In all of these examples, the main factor that maintains thermodynamic disequilibrium is the living biosphere. Without the biosphere, some abiotic photochemical reactions would proceed, as would reactions associated with volcanism. But without the continuous production of oxygen in photosynthesis, various oxidation processes (e.g., with reduced organic matter at the Earth’s surface, reduced sulfur or iron compounds in rocks and sediments) would consume free O2 and move the atmosphere towards thermodynamic equilibrium. The present-day chemical functioning of the planet is thus intimately tied to the biosphere.
All living organisms require at least one mobile phase (gas or liquid) in order to exist. Life on Earth as we know it would be impossible without the involvement of the liquid phase of water. The gas phase is necessary for life forms that consume gaseous substances or that produce gaseous waste products. Hence, the very functioning of the biosphere implicitly depends on the existence of the mobile atmosphere and hydrosphere, both of which are in intimate contact with the solid phases of the planet and exchange substances with them. Since the atmosphere and hydrosphere are distributed globally and because they each are mixed on large, often global spatial scales, the chemical influences of the biosphere are evident everywhere on Earth.
A rich base of empirical evidence convincingly illustrates the complexity of the couplings within the Earth system. One of the most important pieces of evidence of this interconnectedness is the chronology provided by chemical and isotopic analysis of ice cores from the deepest, oldest ice on the planet. The Vostok ice core (Lorius et al., 1985; Saltzman, pers. comm., 1998) currently yields information covering more than 400 000 years, from the current interglacial time, through the most recent ice age, through the previous interglacial time and back to an earlier ice age. Figure 1-2 shows plots of several chemical variables against time. The data are derived from detailed analyses of the chemical composition of the ice. These data will be discussed in more detail in Chapter 17, but even a cursory examination reveals features that indicate regulation of the system by the biosphere:
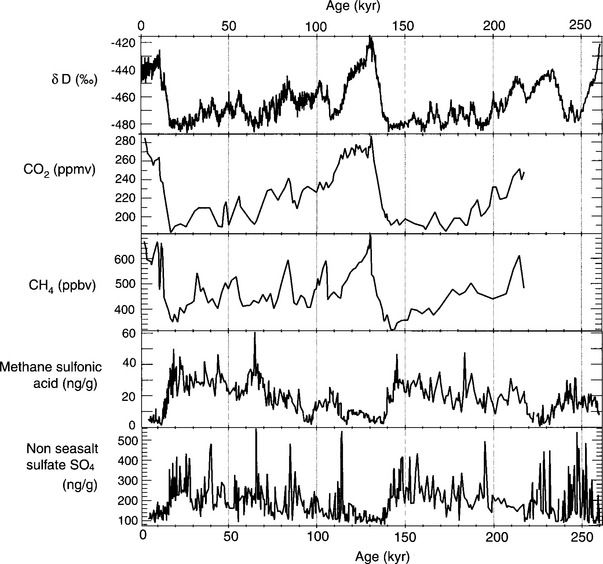
Fig. 1-2 Chemical data from the Vostok ice core. The graph of δD can be taken as a proxy for temperature changes, as described in Chapter 18. CO2 and CH4 are greenhouse gases and vary in the same direction as temperature. Non-seasalt sulfate and methane sulfonic acid are both sulfur species existing in the particle phase, and are positively correlated with each other, but negatively with T. Major variations for all of these variables seem to correlate either positively or negatively with each other, indicating a coupled system. δD, non-seasalt sulfate, and methane sulfonic acid data kindly provided by Dr Eric Saltzman. CO2 data are from Barnolaet al. (1987) and Jouzel et al. (1993). CH4 data are from Chappellaz et al. (1990) and Jouzel et al. (1993). (ppmv = parts per million by volume; ppbv = parts per billion by volime)
• The isotopically inferred temperature shows two relatively stable climatic states: ice ages and interglacial periods. The temperature during the current interglacial is about the same as during the past one. Likewise, the temperature during the coldest part of the last ice age was about the same as the minimum temperature during the previous glacial period.
• CO2 is higher in interglacial times and lower in ice ages, indicating significant differences in photosynthesis between these two climatic states.
• Sulfate and methane sulfonic acid (MSA) (both of which are produced primarily by photosynthetic algae in the ocean) are low during interglacials and high in ice ages, opposite to the CO2 trend in Antarctica (although not in Greenland).
These chemical constituents all vary in synchrony and two climatic states as defined by temperature coincide with the “climatic states” as defined by variation in the chemical variables. Although these correlations and anti-correlations do not prove that the Earth functions as an integrated biogeochemical system, it does strongly suggest that the many subsystems involved are coupled. Furthermore, the presence of the quasi “set points” of the temperature and chemical variables suggests the possible existence of feedbacks that steer the Earth system into one or another preferred climatic and biogeochemical state. Currently, no viable alternative hypothesis has been advanced to explain these correlations. To unravel the detailed functioning of the entire Earth system, we must first establish a basis for understanding the individual “spheres,” and the individual biogeochemical cycles that are involved.
1.4 Philosophy of Using the Cycle Approach to Describe Natural Systems on Earth
Viewing the Earth system as a set of coupled biogeochemical cycles that both depend on and influence the climate allows us to conceptually simplify the movement of material on Earth and its couplings to climate. Much of this book is presented using this approach, giving budgets for the flux of material into and out of various reservoirs. Take for instance the circulation of water between the oceans, atmosphere, and continents. In this example, the reservoirs would be “the oceans,” “the water in the atmosphere,” “the ground water,” etc. Using the most basic description of the cyclic processes that take place, we can mathematically and quantitatively model these cycles to describe and predict the distribution of the important chemical constituents of the planet. The fundamental concepts that govern modeling of biogeochemical cycles are covered in Chapter 4, but we introduce some of the main ideas here, since these concepts and definitions are useful for the entire text.
A basic goal of the cycle approach is to determine how the fluxes between the reservoirs depend on the content of the reservoirs and on other external factors. In many cases the details of the distribution of an element within each reservoir are disregarded, and for the most simplified calculations, the amounts of material in each reservoir are assumed to remain constant (i.e., there is a condition of steady state). This allows a chemical budget to be defined for the entire cycle.
A steady-state, flux-based approach to describing the physical-chemical environment on Earth has advantages as well as disadvantages. Some advantages are that:
• It provides an overview of fluxes and reservoir contents.
• It gives a basis for quantitative modeling.
• It helps to estimate the relative magnitudes of anthropogenic and natural fluxes.
• It stimulates questions such as, “Where is the material coming from? Where is it going next?”
There are, however, some disadvantages:
• The analysis is by necessity superficial. It provides little or no insight into what goes on inside the reservoirs or into the nature of the fluxes between them.
• It gives a false impression of certainty. Typically at least one of the fluxes in a cycle is calculated by the imposed mathematical necessity of balancing a steady-state budget. Such estimates may erroneously be taken to represent solid knowledge.
• The analysis is based on averaged quantities that cannot always be easily measured because of spatial variation and other complicating factors.
Also, many important geophysical problems cannot be studied using a simplified cycle approach. Weather forecasting, for example, requires a detailed knowledge about the distribution of winds, temperature, etc. within the atmosphere. Weather cannot be forecast using a reservoir model with the atmosphere as one of the reservoirs. It would not be much better even if the atmosphere were divided into several reservoirs. A forecast model requires a resolution fine enough to resolve explicitly the structure of the most important weather phenomena such as cyclones, anticyclones, and wave patterns on spatial scales as small as a few hundred kilometers. Spatially explicit models are based either on a division of the physical space into a large but finite number of grid cubes (grid point models) or on a separation of the variables into different wave numbers (spectral models).
1.5 Reservoir Models and Cycles – Some Definitions
The models used to study biogeochemical cycles are described by a set of terms whose definitions must be clearly understood at the outset. We define them here as they are used throughout the book.
Reservoir (box, compartment). An amount of material defined by certain physical, chemical or biological characteristics that, for the purposes of analysis we consider to be reasonably homogeneous. Examples:
• Carbon monoxide in the southern hemisphere.
• Carbon in living organic matter in the ocean surface layer.
If the reservoir is defined by its physical boundaries, the content of the specific element is called its burden. We will denote the content of a reservoir by M. The dimension of M would normally be mass, although it could also be, e.g., moles.
Flux. The amount of material transferred from one reservoir to another per unit time, in general denoted by F (mass per time). Examples:
• The rate of evaporation of water from the ocean surface to the atmosphere.
• The rate of oxidation of N2O in the stratosphere (i.e., flux from the atmospheric N2O-nitrogen reservoir to the stratospheric NOx-nitrogen reservoir).
In more specific studies of transport processes, flux is normally defined as the amount of material transferred per unit area per unit time. To distinguish between these two conflicting definitions, we refer to the latter as “flux density.”
Source. A flux (Q) of material into a reservoir.
Sink. A flux (S) of material out of a reservoir – very often this flux is assumed to be proportional to the content of the reservoir (S = kM). In such cases the sink flux is referred to as a first-order process. If the sink flux is constant, independent on the reservoir content, the process is of zero order. Higher-order fluxes, i.e. S=kMx with α > 1, also occur.
Budget. A balance sheet of all sources and sinks of a reservoir. If sources and sinks balance and do not change with time, the reservoir is in steady state, i.e., M does not change with time. Usually some fluxes are better known than others. If steady state prevails, a flux that is unknown a priori can be estimated by difference from the other fluxes. If this is done, it should be made very clear in the presentation of the budget which of the fluxes is estimated as a difference.
Turnover time. The turnover time of a reservoir is the ratio of the content M of the reservoir to the sum of its sinks S or the ratio of M to the sources Q. The turnover time is the time it will take to empty the reservoir in the absence of sources if the sinks remain constant. It is also a measure of the average time spent by individual molecules or atoms in the reservoir (more about this will be presented in Chapter 4).
Cycle. A system consisting of two or more connected reservoirs, where a large part of the material is transferred through the system in a cyclic fashion. If all material cycles within the system, the system is closed. Many systems of connected reservoirs are not cyclic, but instead material flows unidirectionally. In such systems some reservoirs (at the end of the chain) may be accumulative, whereas others remain balanced (nonaccumulative); cf. Holland (1978).
Biogeochemical cycle. As discussed early in the chapter, this term describes the global or regional cycles of the “life elements” C, N, S, and P with reservoirs including the whole or part of the atmosphere, the ocean, the sediments, and the living organisms. The term can be applied to the corresponding cycles of other elements or compounds.
Budgets and cycles can be considered on very different spatial scales. In this book we concentrate on global, hemispheric and regional scales. The choice of a suitable scale (i.e. the size of the reservoirs), is determined by the goals of the analysis as well as by the homogeneity of the spatial distribution. For example, in carbon cycle models it is reasonable to consider the atmosphere as one reservoir (the concentration of CO2 in the atmosphere is fairly uniform). On the other hand, oceanic carbon content and carbon exchange processes exhibit large spatial variations and it is reasonable to separate the surface layer from the deeper layers, the Atlantic from the Pacific, etc. Many sulfur and nitrogen compounds in the atmosphere occur in very different concentrations in different regions of the world. For these compounds regional budgets tell us more about local dynamics than do global budgets.
1.6 The Philosophy of Integration as a Basis for Understanding the Earth System
1.6.1 Recognizing the Interconnected Nature of the Earth System
Although it is clearly established that it is necessary to study all of the geospheres and all of the biogeochemical cycles in order to understand and predict the workings of the entire Earth system, it is not sufficient to stop there. The functioning of the biosphere and each of the individual physical spheres of the planet involves continuous and strong interactions, making all parts of the Earth system dependent to some degree on all the other parts. Earth system science attempts to understand current conditions by extending analyses backwards and forward in time – to include the earliest stages of the evolution of life on Earth, as well as projections of human-induced global change in the future.
The task of integrating the spheres and the biogeochemical cycles emerges as a necessary if daunting challenge. Disciplinary science has provided little in the way of precedent for us, although substantial guidance is provided by the pioneers like Arrhenius, Vernadsky, and Lovelock who presaged these global developments. Another major contributor to this kind of thinking is the field of systems analysis or systems engineering, and the field of cybernetics, though these lack any degree of focus on the Earth system.
1.6.2 Examples of Integration of Global Systems
In the chapters that follow, we cannot provide a complete and unified description for the integration of the entire system; however, we can provide a set of examples of integrated subsystems that will illustrate the nature of the interactions that constitute major pieces of the whole. Key features of the process of integrating natural-science fundamentals are (1) some degree of globality or global applicability, (2) interaction of two or more biogeochemical cycles, and (3) coupling of the interacting geospheres. While there are many such subsystems, we will mention a few of them to which we will return in the closing chapters. These examples of integrated subsystems also introduce and maintain a three-way focus on one or more geosphere(s), the biogeochemical cycles, and on systems integration. While these topics might appear to be non-parallel, they share the key feature of an integrative global view.
1.6.2.1 The hydrologic cycle
Far from being just the processing of water on Earth, this cycle is the basis for a wide range of meteorologic, geochemical, and biological systems. Water is the transport medium for all nutrients in the biosphere. Water vapor condensed into clouds is the chief control on planetary albedo. The cycling of water is also one of the major mechanisms for the transportation of sensible heat (e.g. in oceanic circulation) and latent heat that is released when water falls from the air.
1.6.2.2 Acid–base and oxidation-reduction systems
It is often taken for granted that the oxygen content of the air is nearly constant at ca. 20% of the atmospheric volume, that most of the liquid water on the planet is aerobic (i.e. contains O2), and that most water has pH values relatively close to “neutral” (close to 7). However, these circumstances are not mere coincidences but are in fact consequences of the interaction of key global biogeochemical cycles. For instance, the pH of rainwater is often determined by the relative amounts of ammonia and sulfuric acid cycled through the atmosphere, a clear example of interaction between the nitrogen and sulfur cycles.
1.6.2.3 Ice-age/interglacial climatic flip-flops
Although these are often viewed as being merely changes in physical climate as indexed by temperature, they really represent changes in a large range of biogeochemical phenomena. The rich and rapidly growing body of data from ice cores and sediments strongly supports the notion that the Earth functions as a coupled system.
1.6.2.4 The climate system with biogeochemical feedbacks
Climate is often viewed as the aggregate of all of the elements of weather, with quantitative definitions being purely physical. However, because of couplings of carbon dioxide and many other atmospheric species to both physical climate and to the biosphere, the stability of the climate system depends in principle on the nature of feedbacks involving the biosphere. For example, the notion that sulfate particles originating from the oxidation of dimethylsulfide emitted by marine phytoplankton can affect the albedo (reflectivity) of clouds (Charlson et al., 1987). At this point these feedbacks are mostly unidentified, and poorly quantified.
1.6.2.5 Anthropogenic modification of the Earth system
Again, the myriad influences of human activity are usually viewed as separate effects (global warming, acid rain, ozone loss, urban pollution, etc.) However, these individual symptoms clearly have major interdependencies that must be understood if humans are to learn how to coexist with a stable Earth system.
1.7 The Limitations and Challenges of Understanding Earth Systems
Earth system science is a young science with great potential, but we must exercise caution in not overlooking important details of traditional, disciplinary science in our attempt to develop this new and integrative science. The foundation upon which we will proceed in this book is to provide the basic disciplinary components, starting with fundamental concepts of modeling, Earth science, biology, and chemistry. Having reviewed these basic scientific building blocks we move on to a survey the biogeochemical cycles of key elements. Following this, we present a set of integrative topics.
The three user groups of this book (students, teachers, and researchers) will all discover that the challenge of understanding Earth system science is at least as much of a problem of integration of well developed fundamental fields into a global context as it is to refine the disciplinary pieces themselves. Users who are practiced in a traditional field utilizing reductionism will need to expand their thinking into other disciplines as well as into the problem of how to combine their own field into the larger picture. In doing so, they will find an opportunity to extend the scope of their own discipline towards and into the global context.
Arrhenius, S. On the influence of carbonic acid in the air upon the temperature of the ground. Philosophical Magazine and Journal of Science S. 5. 1896; Vol 4(No 251):237ff.
Barnola, J. M., Raynaud, D., Korotkevich, Y. S., Lorius, C. Vostok ice core provides 160 000 year record of atmospheric CO2. Nature. 1987; 329:408–413.
Chappellaz, J., Barnola, J. M., Raynaud, D., Korotkevich, Y. S., Lorius, C. Atmospheric methane record over the last climatic cycle revealed by the Vostok ice core. Nature. 1990; 345:127–131.
Charlson, R. J., Lovelock, J. E., Andreae, M. O., Warren, S. G. Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature. 1987; 326:655–661.
Dawkins, R. The Selfish Gene. Oxford University Press; 1976.
Holland, H. D., The Chemistry of the Atmosphere and Oceans. Wiley-Interscience, New York, 1978. 351.
September Hutchinson, G. E. The Biosphere. Scient. Am. 1970; * 45–53.
Hutton, J. Theory of the Earth; or an investigation of the laws observable in the composition, dissolution and restoration of land upon the globe. R. Soc. Edin. Trans. 1788; 1:209–304.
Jouzel, J., Lorius, J. R., Petit, C., et al. Vostok ice-core — a continuous isotope temperature record over the last climatic cycle (160 000 years). Nature. 1993; 329:403–408.
Lovelock, J. E. Gaia as seen through the atmosphere. Atmos. Environ. 1972; 6:452–453.
Lovelock, J. E., Margulis, M. Atmospheric homeostasis by and for the biosphere. Tellus. 1974; 26:1–10.
July 17 Lovelock, J. E. The Biosphere. New Scient. 1986; * 51.
Lovelock, J. E. Geophysiology — the science of Gaia. In: Schneider S. A., Boston P. J., eds. Scientists on Gaia. New York: MIT Press; 1991:3–10.
Lorius, C., Jouzel, J., Ritz, C., Merlivat, L., Barkov, N. I., Korotkevich, Y. S., Kotlyakov, V. M. A 150 000-year climatic record from Antarctic ice. Nature. 1985; 316:591–596.
NASA. Earth System Science — Overview — A Program for Global Change. Cambridge, MA: Earth System Sciences Committee, NASA Advisory Council; 1986.
(The Origin of the Alps) Suess, E. Die Enstehung der Alpen. Washington, DC: W. Braunmüller; 1875.
(D. B. Langmuir, transl. ; revised and annotated by M. A. S. McMenamin) Vernadsky, V. I. The Biosphere. Vienna: Copernicus Books; 1997.