CHAPTERS 5 through
9 presented a survey of the dinosaurs and many conclusions about their biology and behavior. This chapter summarizes some of that information and explores other aspects of dinosaur biology and behavior. The study of dinosaur soft-tissue (nonskeletal) anatomy and behavior is full of speculation, some of it sensational and unwarranted. Here, I shall take a cautious approach to these subjects, presenting reasonable speculation and avoiding science fiction.
Many aspects of dinosaur biology, especially skeletal anatomy, have already been reviewed in this book, and
chapter 14 discusses the complex subject of dinosaur metabolism. The focus of this chapter is on four topics not addressed in detail elsewhere in this book: dinosaur external appearance, weight, growth, and longevity.
Fossils of the integument (scales, feathers, scutes, and
skin impressions) are known for most of the major dinosaur taxa. They indicate that the skin of most dinosaurs was covered with scales similar to the scales of some living reptiles (
figure 13.1). However, recent discoveries also indicate that many theropods had feathers or a feather-like body covering (see
chapter 15).
Skin impressions of hadrosaurs are particularly well known and indicate a body covered in scales. A recent study of the skin impressions of
Saurolophus, a Late Cretaceous hadrosaur, known from Canada and Mongolia, distinguished the two species of the genus by differences in scale shape and pattern. This is an exciting result because today herpetologists distinguish many living species of reptiles (especially lizards) based on scale patterns. This can now be done with
Saurolophus, and a better record of skin impressions may allow such distinctions among other dinosaur species.
FIGURE 13.1
This close-up fossilized skin impression of a hadrosaurid shows reptilian scales only a few millimeters wide. Many dinosaurs were covered with such scales.
The color of dinosaurs is totally a matter of conjecture, because the pigment in their skin and scales did not fossilize. However, color patterns have been preserved in some dinosaurs, and some indicate a striped coloration, a common form of camouflage in living animals.
Classically, paleontologists and artists who create paintings or sculptures of dinosaurs used the color patterns of living reptiles, especially lizards, as a guide to the probable colors of dinosaurs. However, most artists now paint dinosaurs with very flamboyant, bright color patterns unlike those of most living reptiles and more like those of birds. These flashy dinosaurs are eye-catching, but no serious student of dinosaurs views the coloration attributed to any dinosaur as anything but speculation.
The posture and overall body shape of a dinosaur is determined by analyzing its skeleton. Skeletal anatomy is a guide to the size, shape, and configuration of the muscles and provides an understanding of how the dinosaur moved (
figure 13.2). Of course, this is old news to the readers of this book. What is not old news, and what is not so certain, is how fat or thin a given dinosaur was, whether it had flaps of skin on its neck or head as do some modern lizards and birds, and other features of dinosaur external anatomy that are difficult to predict from skeletal anatomy. Fortunately, some exceptionally preserved dinosaur fossils provide direct knowledge of some dinosaur soft tissues (
box 13.1).
FIGURE 13.2
The dinosaur skeleton provides the basis for reconstruction of the shape, size, and arrangement of muscles. (© Scott Hartman)
We now know a tremendous amount about the biology of the ceratopsian dinosaur Psittacosaurus, much more than we know about most dinosaurs. This is because of its exceptional fossil record, which includes some remarkable recent discoveries from the Lower Cretaceous of northeastern China. Some highlights include the following:
1. Studies of the growth of Psittacosaurus indicate that it grew faster than modern reptiles but slower than living mammals or large dinosaurs.
2. These studies also indicate maturity was reached at about nine years of age.
3. An adult skeleton found together with 34 juvenile skeletons has been interpreted as a nest with evidence of a high degree of parental care.
4. Analysis of skeletons of psittacosaurs killed in a volcanic mudflow suggests that groups of closely related dinosaurs lived together as a sort of “family.”
5. Soft-tissue preservation of one exceptional psittacosaur fossil shows a bushy tail with quill-like spines (
box figure 13.1). This dinosaur otherwise had scaly skin, and its fossil retains a color pattern that indicates dark and light bands (possibly for camouflage) and countershading (lighter coloring on the ventral side of the body).
BOX FIGURE 13.1
This exquisitely preserved skeleton of Psittacosaurus, from the Lower Cretaceous of northeastern China, retains skin impressions and shows quill-like projections around the tail. (Courtesy of Naturmuseum Senckenberg, Frankfurt)
It is certain that paleontologists’ and artists’ views of the external appearance of dinosaurs have evolved as new ideas about dinosaur biology and behavior have emerged (see
chapter 11). Older ideas of sluggish, cold-blooded dinosaurs produced restorations of flabby and lethargic creatures. New ideas of fast, warm-blooded dinosaurs produce renderings of sleek and agile animals. We can see that ideas about dinosaur external appearance not only involve careful inferences from dinosaur skeletal anatomy but also require speculation about
coloration and
soft-tissue anatomy that cannot be directly inferred from skeletal anatomy. Furthermore, paleontological conceptions of dinosaur biology and behavior have always shaped perceptions of dinosaur external appearance.
Many popular dinosaur books list weights of dinosaurs. These weight estimates are one way to state the size of a dinosaur. Indeed, most dinosaur weight estimates emphasize the very large size of the dinosaurs. How are dinosaur weights estimated?
Several methods are used. Many methods are mathematical in nature. One of these measures the
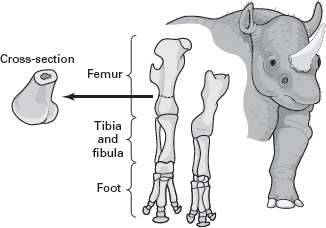
cross-sectional area of weight-bearing limb bone (
figure 13.3). The more weight a limb bone bears, the larger its cross-sectional area, and an equation that predicts weight borne from the cross-sectional area of a limb bone (usually the femur) can be calculated for living vertebrates. The cross-sectional area of the dinosaur limb bone can be plugged into such an equation.
What is determined, of course, is not the weight of the dinosaur but only the weight supported by the limb bone. A further adjustment upward of this value must be made in order to estimate the dinosaur’s body weight. This adjustment depends on the dinosaur’s posture and shape, leading to some uncertainty in the weight estimate. Also uncertain is just how applicable the cross-section-to-weight-borne equation is to extinct animals much larger, and presumably heavier, than the living animals from which the equation was originally determined. Because of these uncertainties, the cross-sectional area method is not the ideal method of estimating a dinosaur’s weight.
Scale models of dinosaurs have long been a common way to estimate weights. This is because estimating dinosaur weights from scale models is relatively straightforward once a model of known scale (say one-fiftieth the length of the dinosaur) is available. The volume of the model is calculated by displacement of water. That volume is then multiplied by the cube of the linear scale of the model so that it becomes the volume of a full-size dinosaur identical in shape to the model. This volume of the “real” dinosaur is then multiplied by 0.9 kilograms per liter, which is the mass of a liter of living crocodile, to arrive at a mass (weight) in kilograms (
box 13.2).
FIGURE 13.3
The cross-sectional area of a limb bone is related to the weight that bone must bear. (Drawing by Network Graphics)
Most paleontologists estimate the weights of dinosaurs by using scale models. The more accurate the scale model, the more accurate the weight estimate. You can test this method by using available scale models, such as the plastic dinosaurs you can purchase in a toy store. Here’s how to do it.
First, you need the following: solid, waterproof plastic scale models of dinosaurs; a water supply; a calculator; a ruler; and a measuring cup from the kitchen or a graduated cylinder from a chemistry lab. The more precisely calibrated the measuring cup or graduated cylinder, the more accurate will be your estimate.
Start by measuring the length of the dinosaur scale model. If your ruler is not metric, convert this measurement into centimeters (1 inch = 2.54 centimeters). Then, determine the actual length of the full-size dinosaur. Now, divide the length of the actual dinosaur by the length of the model. For a 6.7-meter-long Stegosaurus and a 10.2-centimeter-long model, this value is approximately 66, so the model is 1/66 the size of the dinosaur.
Now submerge the model in water to measure its volume (
box figure 13.2). Fill the measuring cup or graduated cylinder to a set level, say 5 ounces, or 100 milliliters if the cylinder is metric. Drop in the dinosaur toy. If it doesn’t sink, gently push it with your finger or a pen point until it is submerged. Read the new level of the water; the difference between it and the original water level is the volume of the scale model. If you must push the model to submerge it, make sure you do not put your finger or the pen point into the water any more than necessary, because that will artificially increase the estimate of the model’s volume.
BOX FIGURE 13.2
The volume of a dinosaur scale model can be determined by measuring the amount of water it displaces. This volume can then be used to estimate the weight of a full-size dinosaur.
If your estimate of the volume of the model is in fluid ounces, you need to convert it to milliliters (1 fluid ounce = 29.6 milliliters). The 10.2-centimeter-long toy Stegosaurus displaces 0.9 ounces, or about 27 milliliters.
Now, cube the scale of the model to obtain its cubic scale. The Stegosaurus model is 1/66 of the length of the actual dinosaur, so it is 1/66 × 66 × 66 (or 663) = 1/287,496 the volume of the actual dinosaur. If we multiply the inverse of this value by the volume of the model, we get the volume (in milliliters) of a life-size replica of the toy dinosaur.
So,
287,496 × 27 = 7,762,392 mL
Dividing this value by 1,000 converts the volume to liters:
7,762,392/1,000 = 7,762 L
A liter of water weighs 1 kilogram, and the average living crocodile weighs 0.9 kilograms per liter. If we apply this to our Stegosaurus, the weight of a dinosaur with a volume of 7.762 liters should be as follows:
7,762 L × 0.9 kg/L = 6,986 kg
A metric ton is 1,000 kilograms, so this is about 7 metric tons. One kilogram = 2.2 pounds, so this is 15,369 pounds, or about 7.7 tons.
This method is simple, and it forms the basis of many published dinosaur weight estimates. However, these estimates are only as accurate as the models themselves (
figure 13.4). Because we are often uncertain exactly how “fleshy” or “lean” dinosaurs were, dinosaur model-making is an imprecise art. Dinosaur weight estimates are full of uncertainty, which is why this book uses skeletal lengths, not weight estimates, to convey the size of a given dinosaur.
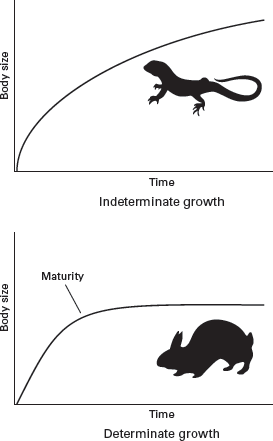
Living reptiles grow throughout their lives, although the rate at which they grow decreases as they age (
figure 13.5). This type of growth, called
indeterminate growth, contrasts with the
determinate growth of mammals and birds. Determinate growth means that after an animal grows to adulthood it stops growing. (In general, rates of determinate growth are faster than those of indeterminate growth.) Another factor affecting growth rate is metabolism; on average, warm-blooded vertebrates grow at least 10 times faster than do cold-blooded vertebrates.
FIGURE 13.4
Different scale models yield different weight estimates of the same type of dinosaur.
FIGURE 13.5
Living reptiles undergo indeterminate growth, whereas mammals and birds undergo determinate growth. (Drawing by Network Graphics)
FIGURE 13.6
Dinosaurs grew rapidly and underwent determinate growth. (Drawing by Network Graphics)
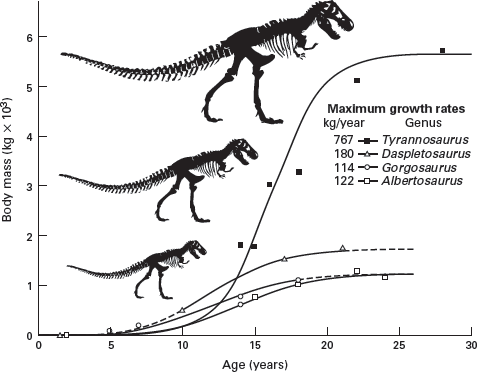
What type of growth did dinosaurs have, and how long did an individual dinosaur typically live? These questions were long difficult to answer directly from dinosaur fossils because fossils provide little direct evidence of the age, in years, of an individual dinosaur. However, recent studies of dinosaur growth based on new techniques that analyze bone microstructure and the spacing of growth lines in bone have given new insight. These studies suggest relatively rapid and determinate growth in dinosaurs and that no dinosaur had a lifespan of more than 100 years (
figure 13.6). Dinosaurs experienced growth rates faster than those of living reptiles and even as fast as some living birds. However, according to the new analyses, the dinosaurs thought of as ancestral to birds, the small theropods, actually had relatively slow growth rates, as was the case with
Archaeopteryx. The giant dinosaurs were able to grow so large because their growth rate accelerated through life. In other words, a late spurt of growth produced the giant dinosaurs.
Although
chapters 5 through
9 discussed many aspects of dinosaur behavior, it is useful to summarize them here and to discuss some subjects mentioned briefly at greater length. Important aspects of dinosaur behavior were feeding and locomotion, reproduction and parenting, attack and defense, and social (group) behavior.
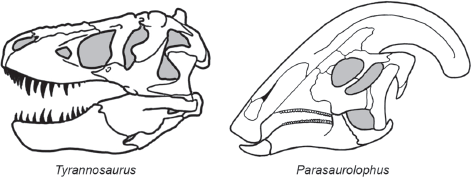
Tooth and jaw structure allow paleontologists to easily distinguish plant-eating from meat-eating dinosaurs (
figure 13.7). As we repeatedly saw in
chapters 4 and
9, meat-eating dinosaurs had numerous sharp, serrated, blade-like teeth set in powerful jaws. This was a feeding mechanism designed to stab, tear, and slice flesh.
Plant-eating dinosaurs, in contrast, had flatter, leaf-shaped teeth, sometimes arranged in dental batteries, set in massive jaws and skulls. This was a feeding mechanism designed to tear, slice, pulp, and/or grind vegetation. Herbivory evolved independently many times among the dinosaurs: three times in ornithopods, once in the sauropodomorphs, and several times in the theropods. Most herbivorous dinosaurs ate high-fiber plants. And, some dinosaurs, notably some theropods, were likely omnivorous.
It is extremely difficult to determine exactly what kinds of plants or animals a given kind of dinosaur ate. Stomach contents—conifer twigs and needles in hadrosaur mummies, a lizard in
Compsognathus, and so forth—provide some direct evidence. Coprolites even indicate that some meat-eating dinosaurs resorted to cannibalism (
box 13.3). Inferences of the
feeding range of plant-eating dinosaurs—how far above the ground the dinosaur could crop vegetation—suggest some specific types of plant food that may have been favored by different kinds of dinosaurs (
figure 13.8). But, preserved gut contents are not known for most dinosaurs, and inferences about feeding do little to narrow the range of possible plant foods. So it remains difficult to identify the specific food items most dinosaurs ate.
One of the principal reasons most animals, including dinosaurs, move (locomote) is to obtain food. Dinosaur locomotion has been discussed at various points in this book, and we can now draw some general conclusions.
Dinosaur skeletons indicate that most dinosaurs were ground-dwelling walkers and runners. However, there is now evidence that some small theropods were likely arboreal (lived in trees) or at least capable of climbing trees. Other than some hadrosaurs, no compelling argument can be presented that any dinosaur was aquatic (lived in the water). Indeed, as we saw in
chapter 6, earlier ideas that sauropods were aquatic do not stand up to a critical analysis of sauropod anatomy. Therefore, dinosaurs stand out as a remarkable group of almost wholly ground-dwelling animals.
FIGURE 13.7
The teeth, jaws, and skull structure of meat-eating Tyrannosaurus contrast with those of plant-eating Parasaurolophus. (© Scott Hartman)
Cannibalism (eating one’s own kind) is common among some living predators, such as sharks and crocodiles. Evidence of cannibalism exists for some predatory dinosaurs. Most impressive are skeletons of the small theropod
Coelophysis found at the Upper Triassic Ghost Ranch dinosaur quarry in northern New Mexico. One of these skeletons has coprolites in the pelvic area full of the bones of juvenile
Coelophysis (
box figure 13.3).
BOX FIGURE 13.3
These tiny juvenile bones of Coelophysis (scale bar in millimeters) came out of a coprolite of an adult Coelophysis.
Today, cannibalism occurs in many predatory reptiles and mammals. Most often, a reptile or mammal will eat younger individuals of its species when hungry, simply because they are easy to capture. This may seem cruel, but it may actually make sense in situations where the population density of young reptiles or mammals is very high and/or other food is difficult to catch.
The large adult individual of Coelophysis from Ghost Ranch with small juvenile Coelophysis in its coprolites fits the modern pattern of reptilian cannibalism. Clearly, cannibalism has a long antiquity, at least 210 million years, and may have been common among theropod dinosaurs.
FIGURE 13.8
Feeding range may provide some clues to the types of plants eaten by some dinosaurs. (© Scott Hartman)
Chapter 12 discussed the evidence suggesting that dinosaurs reproduced by laying eggs.
Clutches of eggs are particularly well known for several kinds of dinosaurs.
Very significant for interpretations of dinosaur reproductive and parenting behavior have been Late Cretaceous egg sites in Montana. There, at a place called “Egg Mountain,” a number of clutches of eggs attributed to two dinosaurs—the hadrosaur
Maiasaura and the primitive ornithopod
Orodromeus—have been discovered (
figures 13.9 and
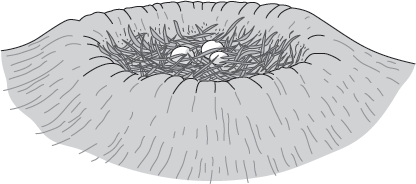
13.10). These clutches are in oval to subcircular, crater-like depressions with raised rims. It is almost certain the eggs were not exposed to the air after being laid, but were covered with a thin layer of soil or vegetation (
figure 13.11).
Numerous skeletons of hatchling dinosaurs have been found around these nests, as have some bones and footprints of adult dinosaurs of the same species as the hatchlings. This provides circumstantial (though not incontrovertible) evidence of parental care.
There is very suggestive evidence of parental care of young dinosaurs in the hadrosaurid
Maiasaura (
figure 13.12), but what of other dinosaurs? Certainly the
nests and hatchlings of some theropods could have been cared for, as were those of
Maiasaura. But evidence of such care is inferred, not actually fossilized, except in rare cases like the
Oviraptor that died brooding its nest (see
box 5.4). Other dinosaurs, such as the cannibalistic
Coelophysis, probably did not care for their young beyond the amount of care characteristic of living crocodilians (see
box 13.3). But new discoveries in the Lower Cretaceous of northeastern China of the ceratopsian
Psittacosaurus suggest not just parental care, but even “family groups” of dinosaurs living together (see
box 13.1). Thus, there appears to have been a spectrum of parental care among dinosaurs, ranging from no care at all to the feeding and protection of hatchlings to living in “family groups.”
FIGURE 13.9
Nests and dinosaur bones have been discovered at “Egg Mountain” in Montana. (Drawing by Network Graphics)
FIGURE 13.10
These three views (top, oblique, and side) are of an Orodromeus clutch from “Egg Mountain.” Each egg is about 20 centimeters long. (Drawing by Network Graphics)
FIGURE 13.11
This restoration of a Maiasaura nest includes a layer of vegetation that may have covered the eggs. (Drawing by Network Graphics)
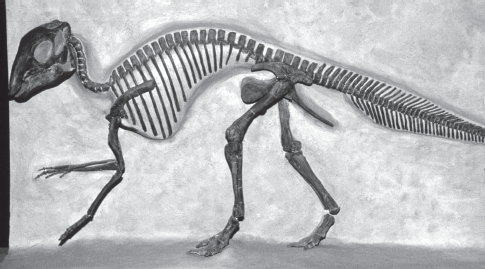
FIGURE 13.12
This skeleton of a very young Maiasaura is about 1 meter long and provides a remarkable record of the early growth of a hadrosaurid. (Courtesy Robert M. Sullivan)
How predatory dinosaurs hunted and how they and other dinosaurs defended themselves are subjects for which there is some good, hard evidence and much unfounded speculation.
How did theropods hunt? Here, we enter the realm of speculation, although some interesting suggestions have been made. One is that some theropods, such as Allosaurus, when hunting large prey, such as sauropods, hunted in packs, like wild dogs do today. An Allosaurus attack on a sauropod is well documented by a skeleton of Apatosaurus in which some of the caudal vertebrae were bitten off. The bite marks match the tooth spacing of Allosaurus, and broken teeth of Allosaurus were associated with the sauropod fossil. Pack hunting by smaller theropods when attacking large prey items seems necessary if such comparatively small predators were to take much larger prey.
A different, ambush style of predation has been suggested for some of the larger theropods. This style is analogous to that of the great white shark, which attacks its victim from behind and below, inflicting a terrible bite and then retreating and waiting until its victim either goes into shock or bleeds to death. Another, slightly different, view sees large theropods as the “big cats” (lions and tigers) of the Mesozoic. This view suggests that large carnosaurs either ran down or ambushed their victims, delivering a single (when possible) killing blow with their huge, tooth-filled jaws.
It is important to emphasize that these different ideas about how theropods hunted are speculation. In general, most paleontologists view the very large theropods as solitary hunters and many of the smaller theropods as pack hunters when taking big game, such as sauropods. However, many of the truly small theropods (less than three meters long) probably were solitary hunters of small animals, such as insects and lizards.
Defensive behavior varied greatly in dinosaurs. Among the predatory dinosaurs, perhaps the most that can be said is that they followed the maxim “the best defense is a good offense.” Their speed and agility, and their slashing teeth and claws, must have been used to defend against enemies, as with many predators today.
Among the plant-eating dinosaurs, three different defensive strategies appear to have evolved (
figure 13.13). In the sauropods, huge body size and powerful whip-like tails provided defense against enemies. Some sauropods, such as the titanosaurs, evolved body
armor. But defensive armor was the hallmark of most ornithischians. This armor ranged from the impervious plating of ankylosaurs to the spiked tails of stegosaurs.
Ornithopods were the exception. They lacked body armor and must have defended themselves from attackers in other ways—by speed, camouflage, fleeing to the water, or some sort of group defensive behavior. But it is not clear which one (or more) of these strategies ornithopods employed. Indeed, defensive behavior probably varied greatly among the ornithopods, from the small and speedy
Hypsilophodon to the large, spike-thumbed
Iguanodon.
FIGURE 13.13
At least three defensive strategies evolved in plant-eating dinosaurs. Ceratopsians (A) and ankylosaurs (B) had body armor. Sauropods (not shown) were gigantic. The defensive strategy of hadrosaurs is not easily determined. (© Mark Hallett. Reproduced with permission of Mark Hallett Paleoart)
The evidence for group behavior (gregariousness or sociality) among dinosaurs can be listed as follows:
1. Display structures—crests on allosaur skulls, tubes and crests on hadrosaur skulls, and so forth—suggest sociality among some groups of dinosaurs. These display structures presumably would have enabled the recognition of potential mates or opponents in a social group.
2. Sexual dimorphism (differences between males and females of the same species) of these display features and other structures (for example, tusks) in some dinosaurs also supports sociality. Often (though not always), sexual dimorphism among living social animals allows them to distinguish males from females and provides males with display and defensive structures that aid in the defense of territory and the acquisition of mates.
3. The change in shape during the growth of some dinosaur display structures could indicate the need to distinguish juveniles from adults in a social group.
4. Multiple dinosaur fossils (mass death assemblages) might also indicate group behavior. Dinosaurs that lived in groups would, occasionally, die in groups. However, many dinosaur mass death assemblages, such as the Late Jurassic fossils at Dinosaur National Monument in Utah, represent river-transported accumulations of carcasses. Not all dinosaur mass death assemblages actually represent groups of animals that lived and died together.
5. The evidence for parental care and nesting behavior discussed earlier also suggests some sort of group behavior among some dinosaurs.
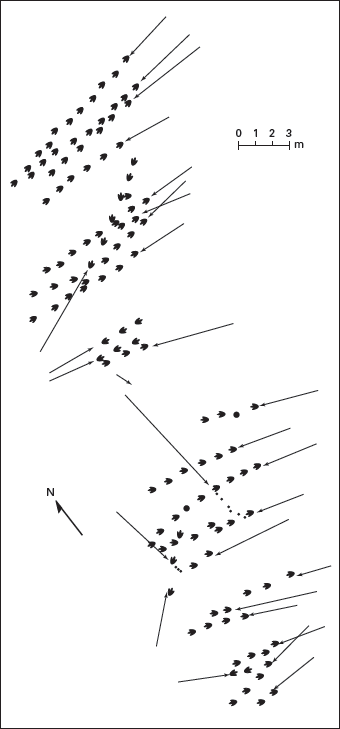
6. Finally, and regarded by many paleontologists as the strongest evidence, multiple trackways of dinosaurs that walked in the same direction suggest social behavior. Indeed, many dinosaur track sites preserve more than one trackway of the same type of dinosaur, all heading the same way and, in many cases, at the same estimated speed (
figure 13.14). Because so many tracksites document this pattern, an alternative interpretation—that at each site individual dinosaurs walked, at different times, to a common goal—seems unlikely.
These six points of evidence suggest some form of group behavior among dinosaurs, especially theropods, ornithopods, and sauropods. But they don’t allow us to infer the exact types of social structures of these dinosaurs. Some books talk of dinosaurs living in “herds.” But a herd is a complicated and specific kind of social group in which a dominant animal (usually a male) leads other animals. No unequivocal evidence for herd behavior exists among dinosaurs, although much evidence for social groupings does exist for many different types of dinosaurs.
1. Fossilized skin impressions suggest many kinds of dinosaurs had reptilian scales covering their bodies. Many (possibly all) theropods had feathers or feather-like body coverings.
2. Dinosaur coloration does not fossilize and is usually arrived at in renderings by analogy to the coloration of living reptiles or birds.
3. Restorations of the external appearance of dinosaurs have evolved with changing ideas about the biology and behavior of dinosaurs.
4. Dinosaur weight estimates are based mostly on scale models and are only as accurate as the models themselves.
5. Living reptiles experience indeterminate growth, whereas mammals and birds experience determinate growth.
FIGURE 13.14
Most of these Early Jurassic dinosaur trackways (from Massachusetts) are heading in the same direction and suggest the possibility of group behavior. Note that 20 trackways of the same kind of dinosaur are heading west. (Drawing by Network Graphics)
6. Dinosaurs grew quickly and experienced determinate growth. They achieved gigantic size by an acceleration of growth rate.
7. Although jaw and tooth structures allow paleontologists to distinguish meat-eating from plant-eating dinosaurs, it is seldom possible to determine exactly what food a dinosaur ate.
8. The skeletons of almost all dinosaurs identify them as ground-dwelling walkers and runners. Only a few lived in the trees or in water.
9. All dinosaurs probably laid eggs, some in nests that may have been protected and, after hatching, tended by adult dinosaurs.
10. Dinosaurs defended themselves in a variety of ways, from speedy escape to impervious body armor.
11. Several lines of evidence suggest group behavior, especially in some theropods, ornithopods, and sauropods. But the exact kinds of social structures of these dinosaurs cannot be determined, and there is no evidence of herd behavior among dinosaurs.
armor
cannibalism
clutch
Coelophysis
coloration
cross-sectional area
determinate growth
feeding range
group behavior
herd
indeterminate growth
locomotion
Maiasaura
mass death assemblage
nest
scale model
skin impression
social structure
soft-tissue anatomy
weight estimate
1. When a restoration of a dinosaur is examined, what features are inferred from sound skeletal evidence, and what features are based on speculation?
2. How have changing ideas about dinosaurs influenced dinosaur restorations?
3. How are dinosaur weights estimated?
4. What do we now know about dinosaur growth rates?
5. How long did dinosaurs live?
6. How do paleontologists determine what dinosaurs ate, and what are the limitations of these determinations?
7. What does the locomotion of dinosaurs tell us about where they lived?
8. What sort of parental care did hadrosaurids confer on their young? What evidence supports this?
9. List the defensive strategies employed by different types of dinosaurs. What is the evidence to indicate which dinosaurs employed a particular strategy?
10. What is the evidence for group behavior among dinosaurs?
Barrett, P. M. 2014. Paleobiology of herbivorous dinosaurs. Annual Review of Earth and Planetary Science 42:207–230. (Reviews the evolution of dinosaur herbivory)
Bell, P. R. 2012. Standardized terminology and potential taxonomic utility for hadrosaurid skin impressions: A case study for Saurolophus from Canada and Mongolia. PLoS ONE 7:e31295. (Detailed study of the skin impressions of the hadrosaur Saurolophus)
Brusatte, S. L. 2012.
Dinosaur Paleobiology. Chichester: Wiley-Blackwell. (Book-length review of all aspects of dinosaur biology)
Case, T. J. 1978. Speculations on the growth rate and reproduction of some dinosaurs. Paleobiology 4:320–328. (A classic technical analysis of dinosaur growth rates based on the growth rates of living reptiles)
Christian, A. 2002. Neck posture and overall body design in sauropods. Mitteilungen Museum für Naturkunde der Humboldt-Universität zu Berlin, Geowissenschaftliche Reihe 5:271–281. (Analyzes the neck postures and feeding ranges of sauropods)
Colbert, E. H. 1962. The weights of dinosaurs. American Museum Novitates 2076:1–16. (Weight estimates of dinosaurs using scale models)
Erickson, G. M. 2014. On dinosaur growth. Annual Review of Earth and Planetary Science 42:675–697. (Current knowledge of growth in dinosaurs)
Erickson, G. M., P. J. Makovicky, P. J. Currie, M. A. Norell, S. A. Yerby, and C. A. Brochu. 2004. Gigantism and comparative life-history parameters of tyrannosaurid dinosaurs. Nature 430:772–775. (Posits rapid growth rates for tyrannosaurids based on growth lines in bones)
García-Ortiz, E., and F. Pérez-Lorente. 2014. Palaeoecological inferences about dinosaur gregarious behavior based on the study of tracksites from La Rioja area in the Cameros basin (Lower Cretaceous, Spain). Journal of Iberian Geology 40:113–127. (Reviews the evidence for gregarious behavior in dinosaurs based on trackways)
Horner, J. R. 1984. The nesting behavior of dinosaurs. Scientific American 250:130–137. (A popular review of the dinosaur nests from Montana)
Lingham-Soliar, T., and G. Plodowski. 2010. The integument of Psittacosaurus from Liaoning Province, China: Taphonomy, epidermal patterns and color of a ceratopsian dinosaur. Naturwissenschaften 97:479–486. (Scales and color pattern of an exceptionally preserved fossil of Psittacosaurus)
Ostrom, J. H. 1972. Were some dinosaurs gregarious? Palaeogeography, Palaeoclimatology, Palaeoecology 11:287–301. (A now classic, technical review of the trackway evidence for social behavior in dinosaurs)
Sander, P. M., C. T. Gee, J. Hummel, and M. Clauss. 2010. Mesozoic plants and dinosaur herbivory, pp. 331–359, in C. T. Gee, ed., Plants in Mesozoic Time: Morphological Innovations, Phylogeny, Ecosystems. Bloomington: Indiana University Press. (Reviews dinosaur herbivory)
Schweizer, M. H. 2011. Soft tissue preservation in terrestrial Mesozoic vertebrates. Annual Review of Earth and Planetary Science 39:187–216. (Reviews soft tissues in Mesozoic vertebrates, including dinosaurs, and how they are identified)