6 |
Examination of the Motor Cranial Nerves V, VII, IX, X, XI, and XII |
To those I address, it is unnecessary to go farther, than to indicate that the nerves treated of in these papers are the instruments of expression, from the smile upon the infant’s cheek to the last agony of life.
I. V CRANIAL NERVE MOTOR FUNCTION: CHEWING
A. Functional anatomy of chewing
1. Motor-wise, cranial nerve (CrN) V only chews. Its motor axons innervate all and, for clinical purposes, only the chewing muscles: masseter, temporal, and lateral and medial pterygoids. CrN V conveys no efferents to glands or smooth muscle and no special sensory afferents (Table 2-7).
2. Jaw closure
a. Place your fingertips about 2 cm above and in front of the angle of your mandible. Bite hard and relax several times. The muscle felt, the masseter, is the easiest of the chewing muscle to palpate.
b. The other jaw-closing muscles are the temporalis muscles that originate in the temporal fossa and insert on the mandible and the medial pterygoid muscle.
3. Lateral jaw movement: Move your jaw from side to side. Chewing requires not only jaw closure but also a lateral, grinding action and jaw opening, caused by the lateral pterygoid muscles.
a. In Fig. 6-1 notice that the lateral pterygoid muscle originates from the skull base and inserts near the mandibular condyle.
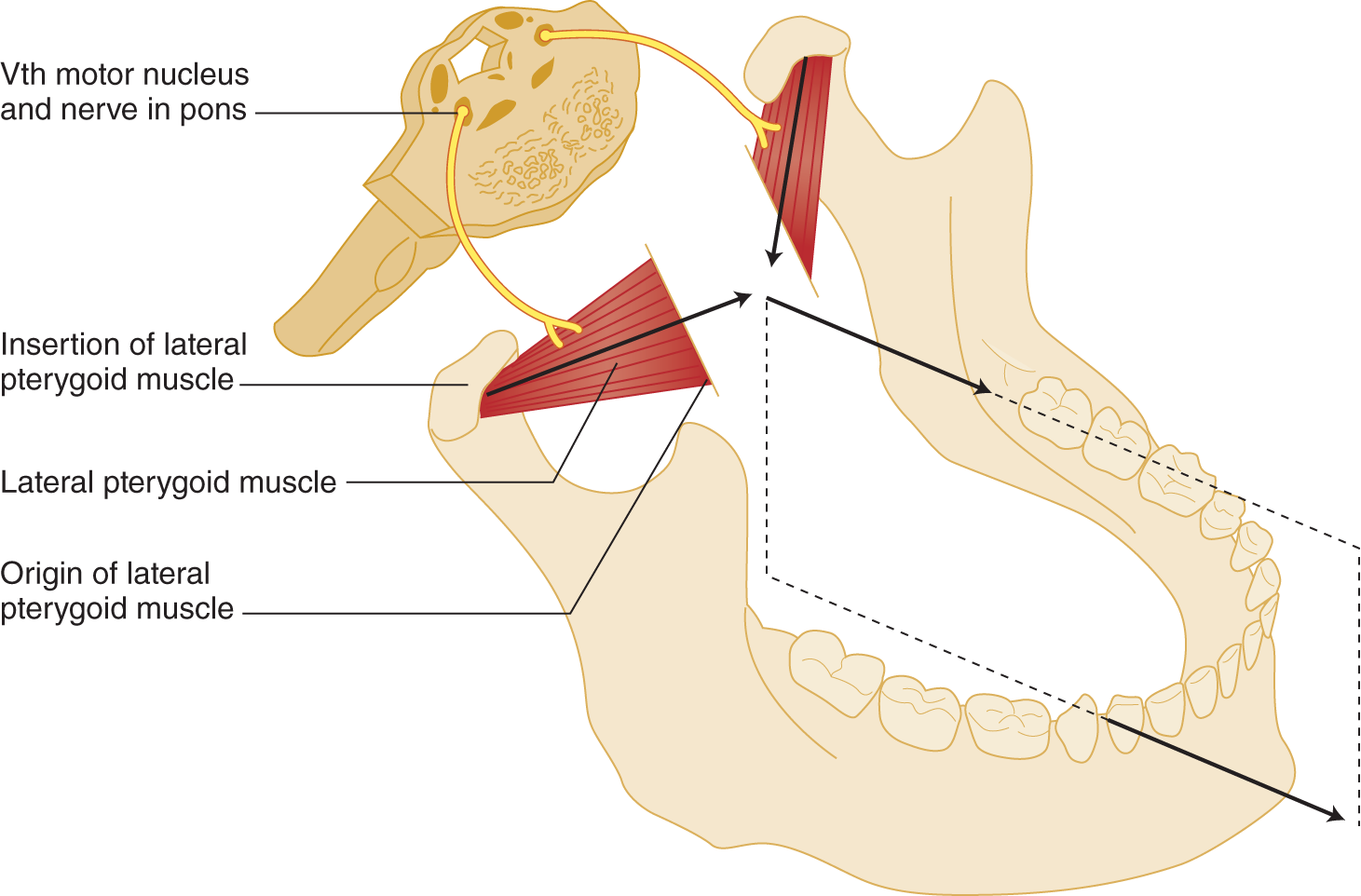
FIGURE 6-1. Innervation and action of the lateral pterygoid muscles. If both muscles contract equally, the tip of the mandible moves straight forward. If one muscle contracts, the tip moves forward and to the opposite side. Study the vector diagram (arrows between the muscles).
b. Because the skull base is fixed, only the mandible moves when the pterygoids contract. Equal contractions of right and left pterygoids pull the mandible straightforward (Fig. 6-1).
c. If only the right lateral pterygoid muscle contracts, the mandibular tip moves to the  right/
right/ left. (
left. ( left)
left)
d. If the patient (Pt) can move the jaw to the right but not to the left, the lateral pterygoid muscle on the  right/
right/ left is paralyzed. (
left is paralyzed. ( right)
right)
e. As a second action, the lateral pterygoid muscles act to open the jaw because they insert on the neck of the mandible (Fig. 6-2).
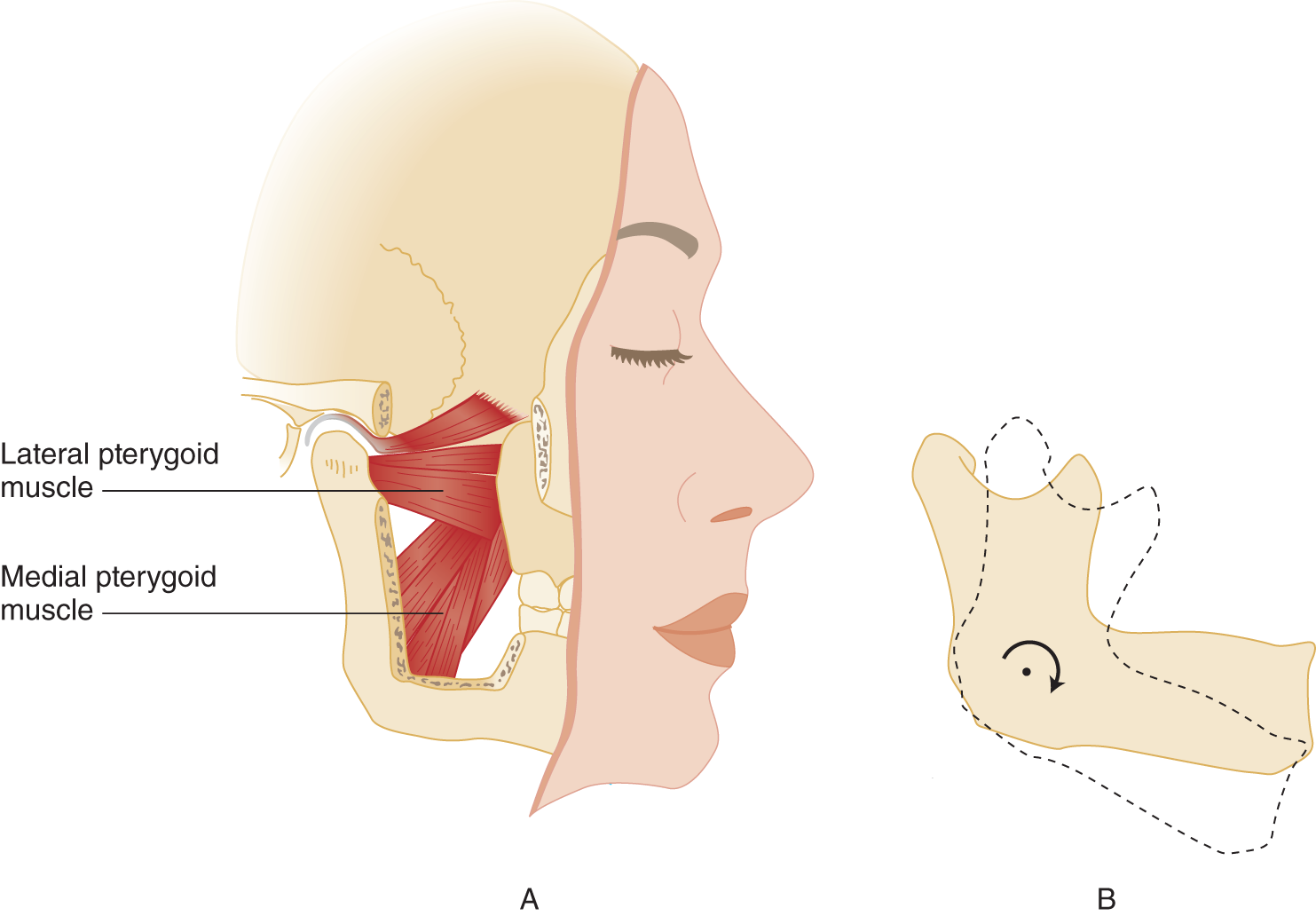
FIGURE 6-2. (A) Action of lateral pterygoid muscles to depress the tip of the mandible when the patient forcefully opens the jaw. The forward pull of the lateral pterygoid muscles also opens the jaw because the jaw is suspended to rotate around a lateral axis. (B) Arrow indicates the rotation.
f. If the Pt’s mandible moves forward and down in the midline on jaw opening, both _________
g. If the left lateral pterygoid muscle contracts, the tip of the mandible moves not only to the  right/
right/ left but also
left but also  up/
up/ down. (
down. ( right;
right;  down)
down)
4. Name the two major actions of the lateral pterygoid muscles.
_________
5. The remaining mandibular muscles innervated by CrN V all close the jaw. Name these muscles.
_________
B. Lower motoneuron lesions of cranial nerve V
1. Unilateral destruction of the perikarya or axons of CrN V causes complete paralysis of all ipsilateral chewing muscles.
2. The denervated muscle undergoes atrophy. Atrophy and paralysis are the two outstanding signs of lower motoneuron (LMN) lesions (Video 6-1). Which chewing muscle can you most readily palpate to check for atrophy? _________

Video 6-1. Atrophy of masticatory muscles in a patient with a remote history of nasopharyngeal carcinoma treated with chemotherapy and radiation.
C. Upper motor neuron innervation of cranial nerve V
1. Pursuant to general principles, one cerebral hemisphere sends upper motoneuron (UMN) axons to the contralateral V nerve nucleus. However, the contralateral innervation law requires some qualification. Compare your inability to contract half of your anal sphincter or half of your throat with the ease with which you flex a finger. These proximal (axial) muscles, such as those for trunk extension, chewing, and swallowing, that customarily act symmetrically much of or all the time usually receive about the same number of crossed and uncrossed UMN axons. The law of contralateral innervation holds best for independent unilateral movements, such as those of the hand. Hence, we find that:
a. Many proximal (axial) muscles that ordinarily contract symmetrically have  mostly ipsilateral/
mostly ipsilateral/ bilateral/
bilateral/ mostly contralateral UMN innervation. (
mostly contralateral UMN innervation. ( bilateral)
bilateral)
b. The distal (appendicular) muscles that ordinarily contract unilaterally have mainly  ipsilateral/
ipsilateral/ bilateral/
bilateral/ contralateral UMN innervation. (
contralateral UMN innervation. ( contralateral)
contralateral)
2. Because of bilateral UMN innervation, unilateral UMN lesions do not cause a severe or enduring unilateral paralysis of the chewing muscles (Willoughby and Anderson, l984). UMN lesions, as a rule, do not selectively paralyze individual muscles or sets of muscles innervated by one peripheral nerve.
D. Clinical tests of cranial nerve V motor function
1. Inspection: Inspect the temples and cheeks for atrophy of the temporalis and masseter muscles. The temporal muscle fills out the temple. Even when the Pt bites, the muscle is difficult to palpate, but after temporalis muscle atrophy, the temple sinks in. In myotonic dystrophy, the chewing muscles and sternocleidomastoid muscles atrophy. Review Fig. 1-13I. The masseters of some individuals undergo hypertrophy and stand out strongly.
2. Palpation: To test for masseter atrophy, ask the Pt to clench the teeth together strongly and unclench several times, while you simultaneously palpate the muscles of the two sides as they mound up and relax under your fingertips.
3. Testing for weakness of jaw closure.
a. Ask the Pt to clench the teeth strongly.
b. Place the heel of one palm on the tip of the Pt’s mandible and the other hand on the Pt’s forehead. Press hard on the tip of the mandible. You must brace the Pt’s head with your opposite hand because jaw closure is a very strong movement and you do not want to test the strength of the neck muscles and jaw closure at the same time. The principle is to test the strength of one muscle or one limited set of muscles at one time.
c. If the Pt complains of fatigability when chewing, as in myasthenia gravis, have the Pt chew for a period before testing.
4. Testing for weakness of the lateral pterygoid muscles.
a. Ask the Pt to forcefully open the jaw. Note whether its tip aligns with the crevice between the upper, medial incisor teeth. Weakness of one lateral pterygoid muscle would cause the jaw to deviate to the  ipsilateral/
ipsilateral/ contralateral side. (
contralateral side. ( ipsilateral (Fig. 6-2))
ipsilateral (Fig. 6-2))
b. Then ask the Pt to move the jaw from side to side.
c. Ask the Pt to hold the jaw forcefully to the side as you try to push it back to the center with the heel of your palm. Brace the Pt’s head by pressing your other hand against the opposite cheekbone.
5. A word of caution: Do not jerk or apply sudden force in testing jaw muscles, particularly in elderly or edentulous Pts. The temporomandibular joint may dislocate.
6. To ensure that you can do it, test V nerve motor function on yourself and on a partner. Do not just sit there: get up and do it! The text cannot substitute for the proprioceptive experience provided by using your own hands.
E. Analyze this 46-year-old man’s difficulty in chewing
Examination disclosed atrophy and paralysis of the left temporal and masseter muscles. When he opened his jaw, it deviated to the left. He could not move it forcefully to the right. No other muscles were weak. The evidence points to  an LMN/
an LMN/ a UMN lesion on the
a UMN lesion on the  right/
right/ left/
left/ both sides. Explain. (
both sides. Explain. ( an LMN;
an LMN;  left.)
left.)
_________
_________
_________
The paralysis affects the muscles of one nerve, CrN V. The paralysis is complete, and the muscles are atrophic. Because the atrophic and paralyzed muscles are on the left, the lesion interrupts the left V nerve.
II. VII CRANIAL NERVE MOTOR FUNCTIONS
A. Functional anatomy of facial movements
1. Except for the mandible and eyelid elevation, CrN VII innervates every other movement that the face can make. (For now study only the LMNs of VII nerve in Fig. 6-3.)
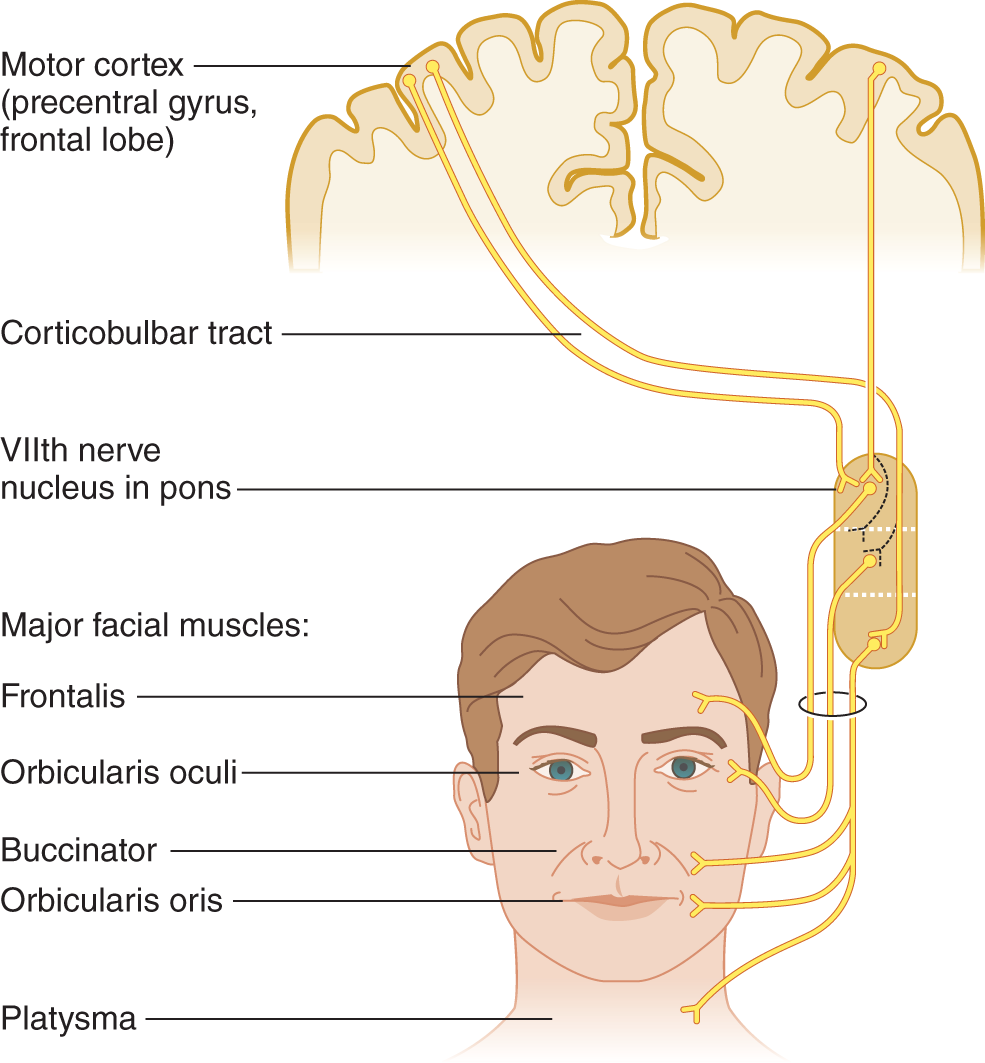
FIGURE 6-3. Upper and lower motoneuron innervations of the facial muscles. The dotted lines indicate that the number of crossed and uncrossed axons to the orbicularis oculi muscles vary from person to person. Therefore, the degree of weakness of the muscle differs after upper motoneuron lesions.
2. Look into a mirror and make every possible facial movement, including wiggling your nose and ears. (Which CrN do you suppose your dog uses to perk up his ears?)
3. Work through Table 6-1. Look into the mirror as you follow the commands the examiner (Ex) uses to test the facial muscles.
TABLE 6-1 • Summary of Tests of the Facial Muscles Innervated by Cranial Nerve VII
Examiner’s command |
Observation |
Muscle tested |
“Wrinkle up your forehead” or “Look up at the ceiling” |
Inspect for asymmetry |
Frontalis |
“Close your eyes tight and do not let me open them” |
Inspect for asymmetry of wrinkles; try to pull eyelids apart |
Orbicularis oculi |
“Pull back the corners of your mouth, as in smiling” |
Inspect for asymmetry of nasolabial fold |
Buccinator |
“Wrinkle up the skin on your neck” or “Pull down hard on the corners of your mouth” |
Inspect for asymmetry |
Platysma |
4. Functions of the facial muscles
a. Expression of emotions, such as when frowning and smiling.
b. Compression of lips for whistling, blowing, and spitting; labial sounds of speech; swallowing and other feeding actions.
c. Controlling and protecting the facial apertures: the palpebral fissures, oral fissure, nares, lips, and external auditory canals.
d. Dampening excessive movement of the ossicles by stapedius muscle contraction during loud sounds. After stapedius paralysis, ordinary sounds may seem uncomfortably loud, a symptom called hyperacusis.
B. Intra- and extra-axial anatomy of cranial nerve VII
1. Learn Fig. 6-4 and compare it with the generalized brainstem section in Fig. 2-14.
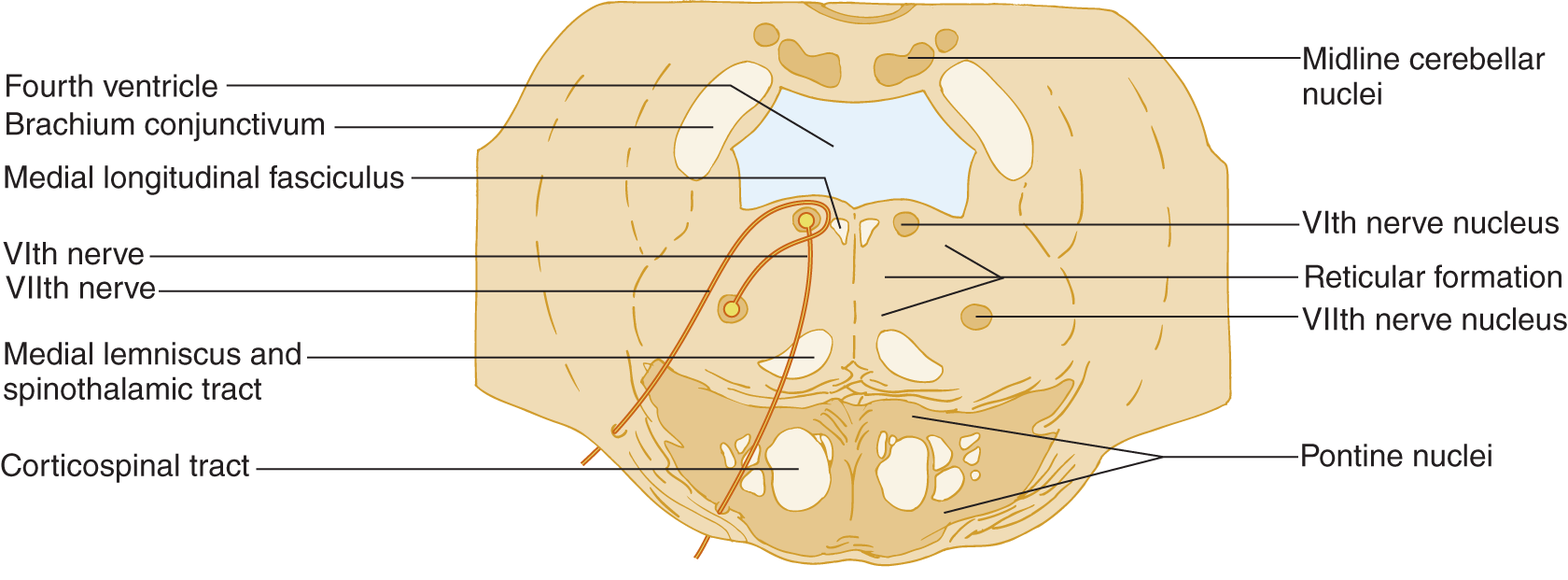
FIGURE 6-4. Transverse section at the caudal level of the pons that includes cranial nerve VI and VII nuclei.
2. Notice the peculiar internal loop of CrN VII around the VI nerve nucleus. Use a colored pencil to draw in the course of CrNs VI and VII on the opposite side of Fig. 6-4.
3. The pontine tegmentum contains motor nuclei for three CrNs: ____, ____, and ____. (V; VI; VII (Fig. 2-21))
4. Through the basis of the pons run the _________
5. Before exiting from the pons, the VII nerve fibers loop around the nucleus of the ____th CrN. (VI)
6. Three CrNs exit at the pontomedullary sulcus. In ventrodorsal order, these nerves are ____, ____, and ____. (VI; VII; VIII (review Fig. 2-20 if you erred))
7. As typifies peripheral nerves, the VII nerves do not cross the midline.
8. If a lesion destroys the VII nerve nucleus, the intra-axial course of the axons, or the peripheral nerve trunk, the result is paralysis of all facial muscles  ipsilaterally/
ipsilaterally/ contralaterally. (ipsilaterally)
contralaterally. (ipsilaterally)
9. The only sensory function of CrN VII tested clinically is taste (Chapter 8).
10. Mnemonics for remembering CrN VII functions: At first glance, mastery of CrN VII seems hopeless. See Fig. 6-5.
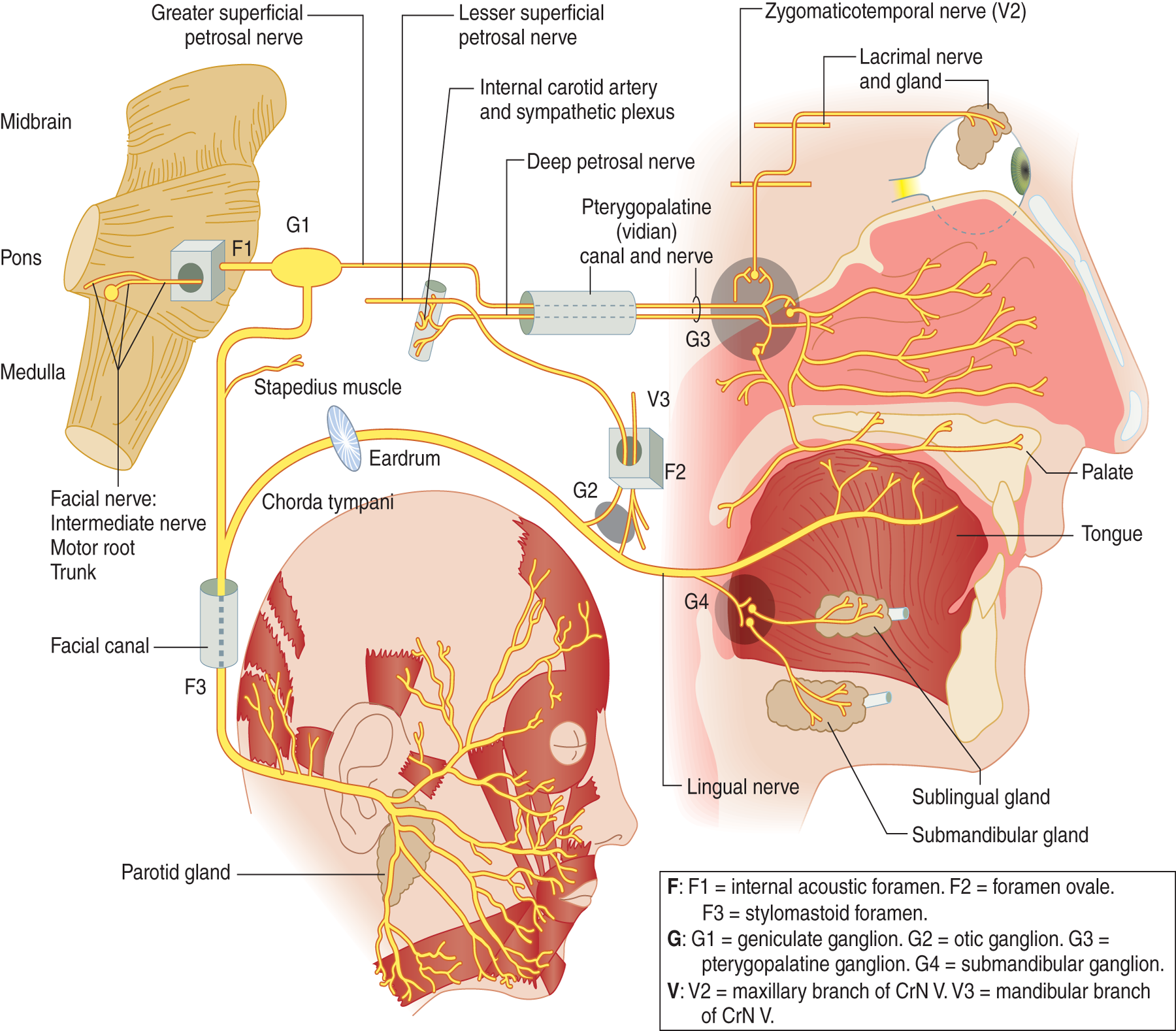
FIGURE 6-5. Diagram of the complete distribution of cranial nerve VII. (Reproduced with permission from DeMyer W. Neuroanatomy. 2nd ed. Baltimore MD: Williams & Wilkins, 1998.)
a. Salvation comes from considering CrN VII as three nerves, a branchiomotor nerve (Fig. 6-6A), a secretomotor nerve (Fig. 6-6B), and a taste nerve (Fig. 6-6C).
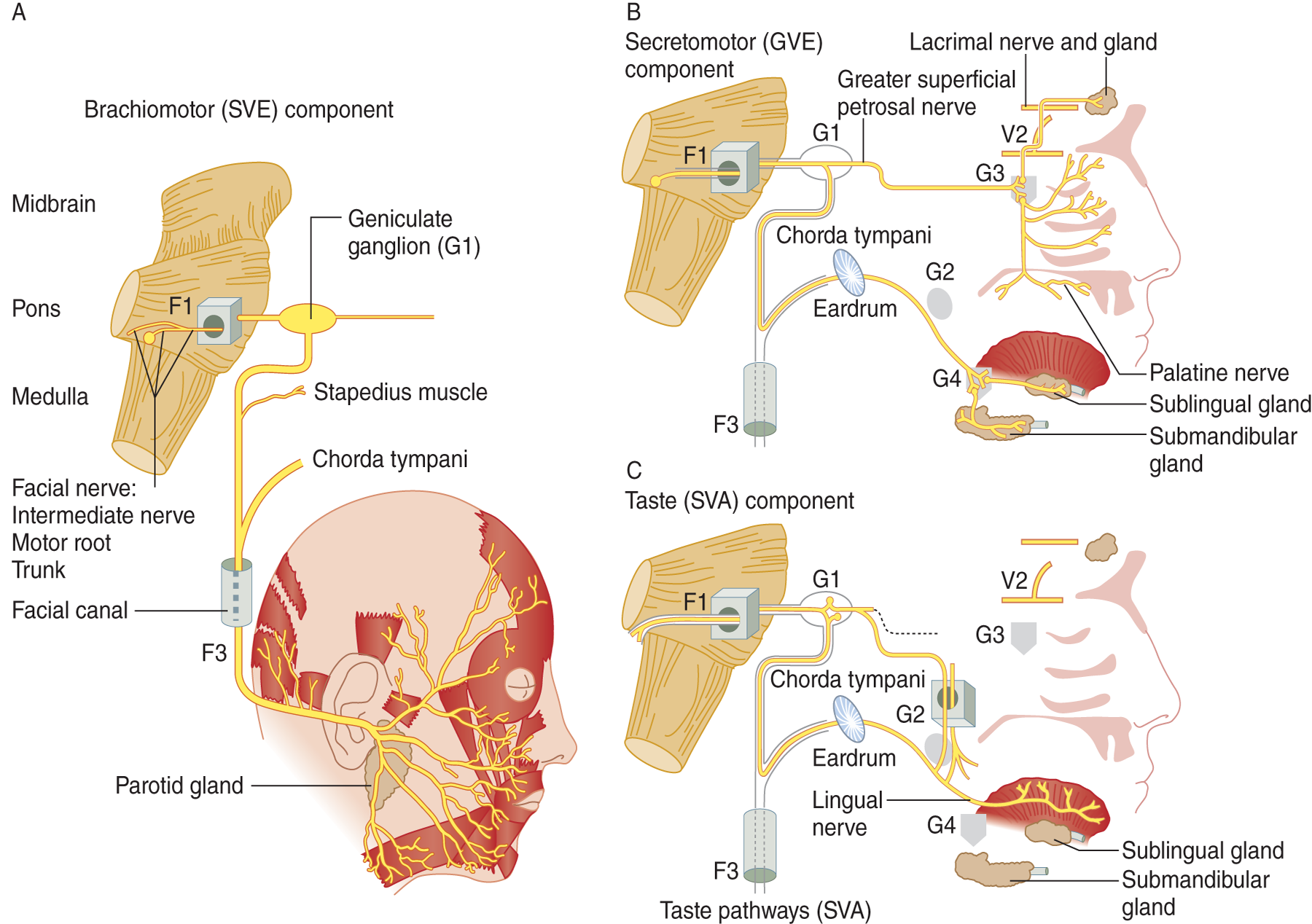
FIGURE 6-6. Diagram of cranial nerve VII as composed of three nerves: (A) a branchiomotor nerve, (B) a secretomotor nerve, and (C) a taste nerve. (Reproduced with permission from DeMyer W. Neuroanatomy. 2nd ed. Baltimore, MD: Williams & Wilkins, 1998.)
b. Remember that, in addition to moving the face, CrN VII innervates:
i. Tasting: taste from the anterior two-thirds of the tongue via the geniculate ganglion.
ii. Snotting: parasympathetic axons to the nasal mucosa via the pterygopalatine ganglion.
iii. Tearing: parasympathetic axons to the lacrimal gland via the pterygopalatine ganglion.
iv. Salivating: parasympathetic axons via the submandibular ganglion.
c. Complete interruption of CrN VII proximally causes ipsilateral facial paralysis, xerophthalmia, loss of nasal secretions, loss of taste, xerostomia, and hyperacusis.
d. Lesions can affect various sites distally along the course of CrN VII, causing the clinical signs to differ. The Ex can reason out the lesion site by “thinking along” the course of the VIIth nerve. For example, interruption of CrN VII at the facial canal (see facial canal in Fig. 6-5) would cause ipsilateral facial paralysis, but not the other clinical signs of a proximal lesion. An acute monosymptomatic LMN paralysis of the facial muscles, independent of etiology is called Bell palsy in many Anglo American countries (Peitersen, 2002) (Videos 6-2 and 6-3).

Video 6-2. Recurrent peripheral facial paralysis in a patient with a sixth nerve (CN VI).

Video 6-3. Bilateral sequential Bell’s palsies in a patient with aberrant regeneration and excessive involuntary facial movements.
e. In the Guillain–Barré syndrome (GBS), a bilateral LMN facial palsy may occur (facial diplegia). Sarcoidosis and Lyme disease also may cause bifacial palsy of the LMN type (May and Schaitkin, 1999).
C. Upper motoneuron innervation of cranial nerve VII and upper motoneuron paralysis of the face
1. By knowing the degree of unilaterality of various facial movements, you can unravel the pattern of UMN paralysis and fix it in your memory forever (Fig. 6-3).
2. As you watch in your mirror, make the movements listed in Table 6-2.
TABLE 6-2 • Tests for Unilaterality of Facial Movements
Movement |
Result |
Retract one corner of your mouth at a time. |
Every normal person can do it; the movement is unilateral. |
Wink one eye at a time; watch in your mirror for simultaneous contraction of the opposite orbicularis oculi muscle. |
Most can do it, but some cannot wink one eye without the other; when one eye winks, the opposite orbicularis oculi contracts to some degree. |
Elevate one eyebrow at a time. |
Few can do it unilaterally, but everyone can elevate them together; the movement is essentially bilateral. |
a. The freest unilateral facial movement normally is  forehead elevation/
forehead elevation/ eyelid closure/
eyelid closure/ lip retraction. (
lip retraction. ( lip retraction)
lip retraction)
b. The least free unilateral facial movement normally is  forehead elevation/
forehead elevation/ eyelid closure/
eyelid closure/ lip retraction. (
lip retraction. ( forehead elevation)
forehead elevation)
c. The utility of the various facial movements explains the gradient of unilaterality. Notice when eating that you make unilateral movements to manipulate food and clear it from your cheeks (buccinator muscle). Indeed a major discomfort of a facial palsy is that food lodges in the cheek. Unilateral forehead movements offer no such utility, and we usually activate both sides of the forehead equally. Although the eyes usually blink together, sometimes you do need to close only one. Thus, the utility of unilateral eyelid action falls between that of mouth retraction and forehead wrinkling.
3. Body parts such as the hand and lip that have the freest, most independent unilateral movements receive their UMN innervation  equally from each hemisphere/
equally from each hemisphere/ mainly from the contralateral hemisphere/
mainly from the contralateral hemisphere/ mainly from the ipsilateral hemisphere. (
mainly from the ipsilateral hemisphere. ( mainly from the contralateral hemisphere)
mainly from the contralateral hemisphere)
4. Proximal, customarily symmetrical movements, such as chewing and swallowing, receive about the same number of UMN axons from each hemisphere, let us say 50/50.
5. The freest, independent unilateral movements are innervated by crossed and uncrossed UMN axons in a ratio of, let us say, 90/10. For movements with an intermediate degree of unilateral independence, the ratio might be 60:40, and so on.
6. Now predict the pattern of facial muscle weakness after unilateral destruction of one corticobulbar pathway. The Pt would show most paralysis of  forehead wrinkling/
forehead wrinkling/ eyelid closure/
eyelid closure/ lip retraction and least paralysis of
lip retraction and least paralysis of  forehead wrinkling/
forehead wrinkling/ eyelid closure/
eyelid closure/ lip retraction. (
lip retraction. ( lip retraction
lip retraction  forehead wrinkling)
forehead wrinkling)
7. Facial weakness after acute, severe interruption of UMNs
a. After a large, acute UMN lesion, such as a massive cerebral infarct, eyelid closure is usually paretic (incomplete paralysis), along with paralysis of lip retraction. Rarely, even the frontalis muscle is somewhat paretic. Because such a Pt has weakness of eyelid closure, the Ex who does not understand the gradient of unilaterality of facial movement may erroneously diagnose a lesion of the ipsilateral facial nerve rather than the contralateral corticobulbar tract.
b. In the acute phase, shortly after a severe UMN lesion, lip retraction contralateral to the lesion will be paralyzed during volitional movement and during emotional expression, such as smiling (Videos 6-4 and 6-5).

Video 6-4. Left upper motor neuron facial paresis associated with a left pronator drift.

Video 6-5. A child with left spastic hemiparesis secondary to a right internal carotid artery dissection. Note characteristic features of upper motor neuron (UMN) pattern of facial paralysis.
c. In the chronic phase of the UMN lesion, especially if the Pt has bilateral UMN lesions, lip retraction may remain weak during volitional action but may be prominent or even exaggerated during emotional expression (Monrad-Krohn phenomenon). See pseudobulbar palsy in Section VIII of this chapter.
D. Clinical testing of cranial nerve VII motor function
1. Facial inspection begins upon meeting the Pt and continues while taking the history.
a. Notice the overall play of facial muscles during speech and emotional expression. The face may move too much or too little. Many disorders, such as muscular dystrophy (Fig. 1-13I), parkinsonism, and depression, reduce all facial movements, a condition called masked facies, as if the Pt wore an immobile mask.
b. Excessive or involuntary movements seen on inspection of the face include blepharospasm, which may close the eyelids so tightly that the Pt cannot see; hemifacial spasm, in which all the muscles innervated by one of the VII nerves twitch; mass movements or synkinesis, whereby involuntary movements accompany a voluntary one (Peitersen, 2002); tics, chorea, and athetosis, as described in Chapter 7.
c. Next, search for asymmetry of facial movements, asymmetry of blinking, and asymmetry of the movement and depth of the nasolabial skin creases. The nasolabial creases begin just lateral to the lips and bow upward to the nares. To detect mild unilateral weakness of lip retraction after partial UMN lesions, watch for asymmetry of the depth and movement of these creases. Using your mirror, observe these two creases when your lips are at rest, when you smile, and when you speak (say EEE loudly). Slight congenital asymmetries are common.
2. Work through Table 6-1 with a partner. Often it is quicker to demonstrate the desired movements and give the commands. In working through Table 6-1, pay particular attention to the strength of eyelid closure. Can you open your partner’s eyelids against a maximum effort at closure?
_________
E. Analysis of patients with facial weakness
1. The Pts shown in Figs. 6-7 and 6-8 were asked to pull back the corners of their mouths, as in smiling, and simultaneously to close their eyes tightly. Make sure that you understand that the side that shows the action, that “draws to one side,” is the normal one. The side that fails to move is the abnormal one. The Pt with a facial palsy may state in the history that the face “draws to one side,” as if that were the abnormality. Especially compare both sides of each face for asymmetry of facial movement and for differences in the bulk of the chewing muscles.
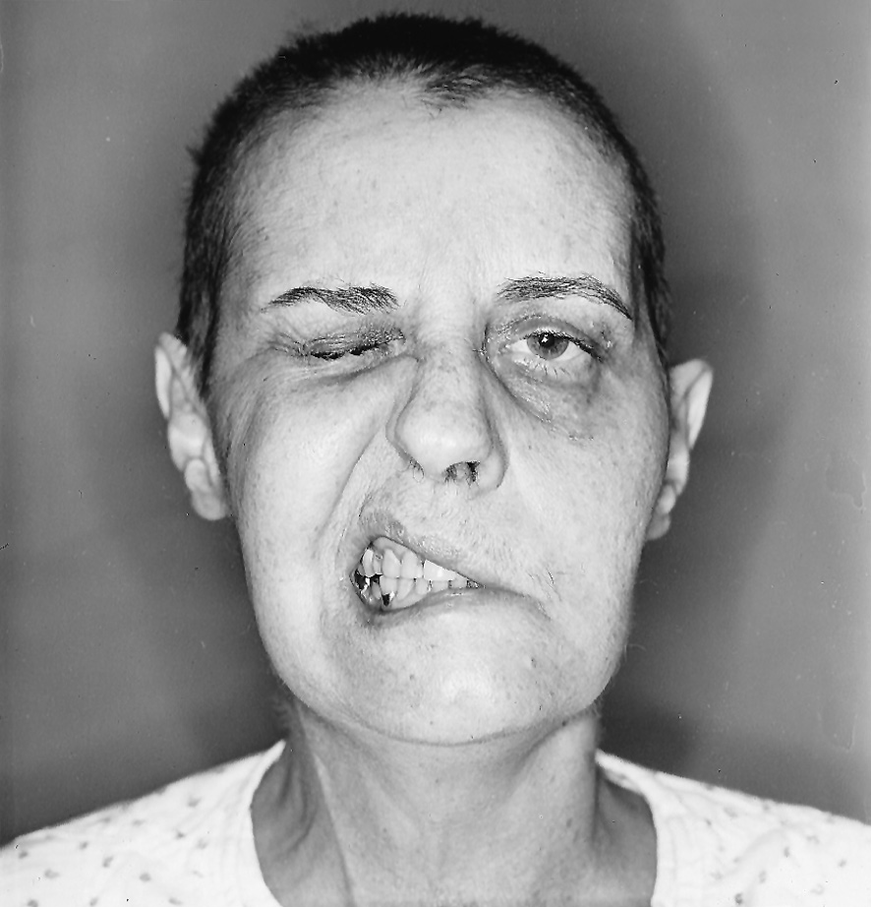
FIGURE 6-7. Patient 1 with facial palsy. She was asked to close her eyes tightly and to pull back the corners of her mouth, as in smiling.
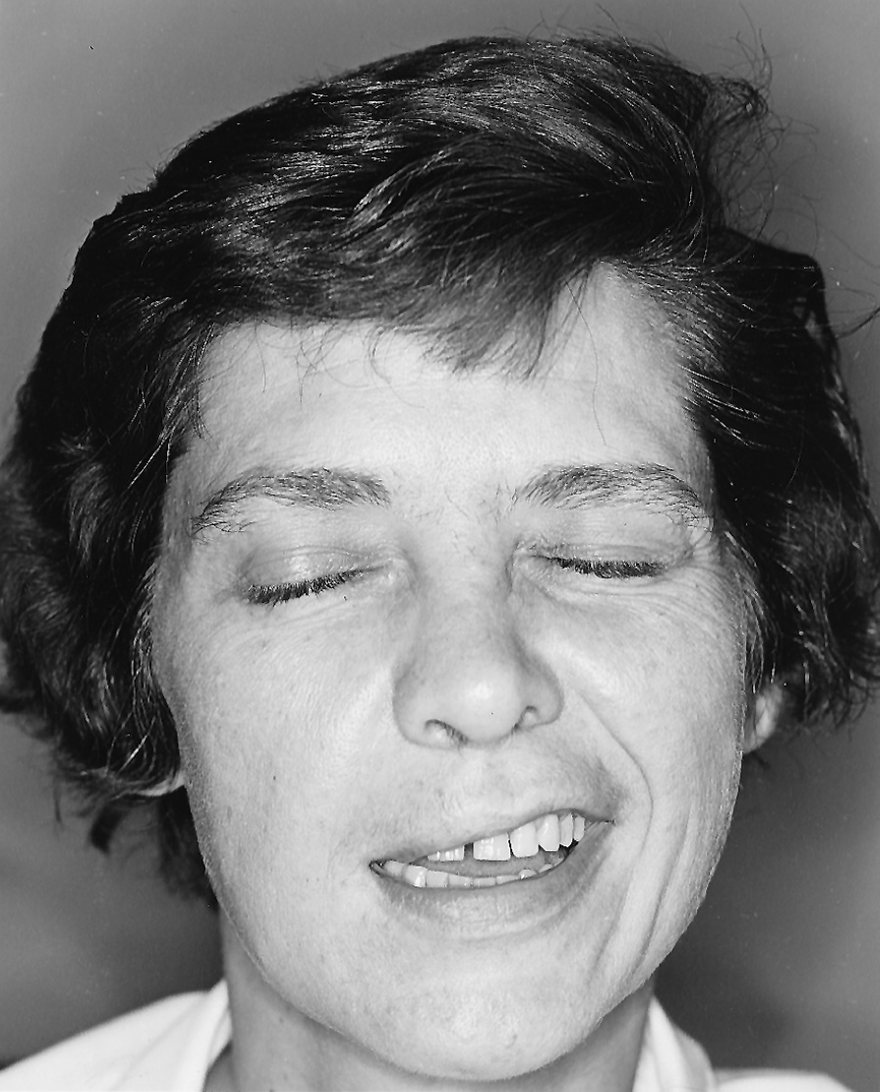
FIGURE 6-8. Patient 2 with facial palsy. She was asked to close her eyes tightly and to pull back the corners of her mouth, as in smiling.
2. Describe the abnormalities of the Pt in Fig. 6-7. Be sure that you start at the hair and systematically examine every facial contour and feature.
_________
_________
a. In Fig. 6-7 the Pt has weakness of eyelid closure on the left, as shown by lack of contraction of the orbicularis oculi muscle and absence of any “crow’s feet” wrinkles around the eye. The mouth retractors on the left are paralyzed. When this Pt looked up, the left half of her forehead failed to wrinkle. This pattern of total paralysis of all facial muscles on the left side implicates a lesion of the  right/
right/ left VII nerve or the
left VII nerve or the  right/
right/ left corticobulbar tract. (
left corticobulbar tract. ( left VII nerve)
left VII nerve)
b. The cognoscente of physical diagnosis will also have detected the hollowing of the Pt’s left temporal fossa and the concavity over the left masseter muscle, indicating atrophy of the chewing muscles and thus a lesion of CrN _____. (V)
c. If you missed this finding, remember that you must compare both halves of the body, specifically looking for just such asymmetries. The patient in Fig. 6-7 had undergone surgery to remove an acoustic nerve tumor, a neurinoma. The tumor, expanding in the cerebellopontine angle, had already destroyed CrNs V and VII, but full abduction of her left eye indicated sparing of CrN ________. (VI)
d. Did you also wonder about her very short hair? It was just growing back after having been shaved off for the operation.
e. This Pt was one of a minority who fail to show Bell phenomenon when attempting to close the eyelids. If Bell phenomenon had occurred, the eye would have turned _________
3. Describe the abnormalities of the Pt shown in Fig. 6-8.
_________
_________
a. In Fig. 6-8, the right side of the Pt’s mouth failed to retract, and eyelid closure on the right was weak, as shown by the wider exposure of the upper eyelid and lack of wrinkling around the right eye. This “crow’s feet” wrinkling on the normal side resulted from the purse-string, sphincter action of the orbicularis oculi muscle. When the Pt in Fig. 6-8 looked up, her forehead acted equally on both sides. Thus, on the right side of her face, the Pt had active forehead movements, weak eyelid closure, and paralysis of mouth retraction. This gradient of facial weakness on the right side of the Pt’s face indicates a lesion of the  right/
right/ left facial nerve or the
left facial nerve or the  right/
right/ left corticobulbar tract. (
left corticobulbar tract. ( left corticobulbar tract)
left corticobulbar tract)
b. The Pt shown in Fig. 6-8 had a UMN facial palsy after an occlusion of her left middle cerebral artery had caused infarction of her left cerebral hemisphere.
BIBLIOGRAPHY · Cranial Nerve VII
May M, Schaitkin BM. The Facial Nerve. 2nd ed. New York, NY: Thieme Medical Publishers; 1999.
Peitersen E. Bell’s palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol. 2002;549(Suppl.):4–30.
III. CRANIAL NERVE IX AND X MOTOR FUNCTIONS
A. Peripheral distribution of cranial nerves IX and X
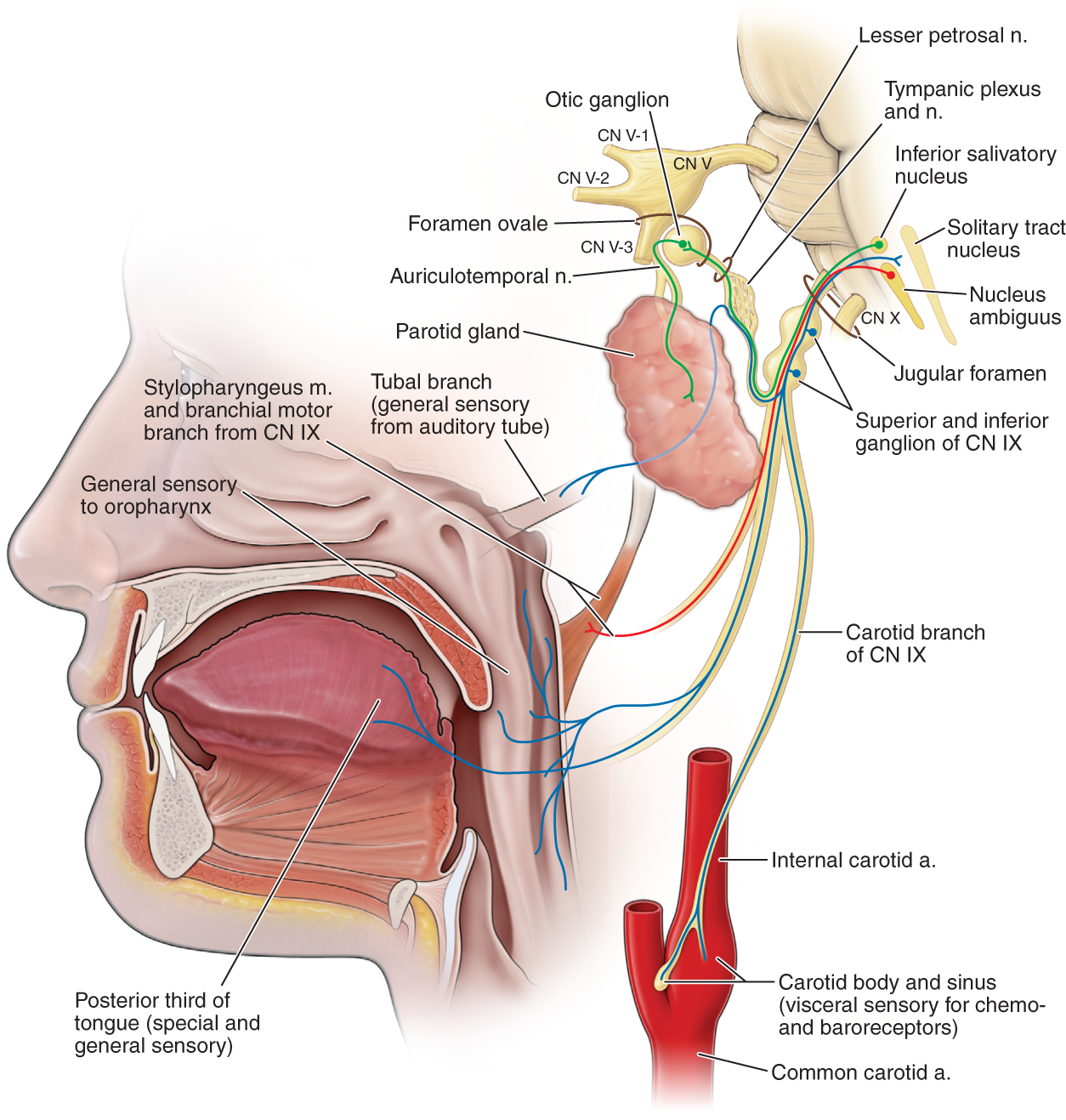
FIGURE 6-9. Innervation of the palate, tongue, and pharynx by cranial nerve IX. Motor axons innervate the stylopharyngeus and middle pharyngeal constrictor muscles. Sensory axons mediate taste from the tongue, the gag reflex, and the vasomotor, cardioinhibitory, and respiratory reflexes of the carotid body and sinus. (Reproduced with permission from Morton DA, Foreman K, Albertine KH. Chapter 17. Cranial Nerves. In: Morton DA, Foreman K, Albertine KH. eds. The Big Picture: Gross Anatomy. New York, NY: McGraw-Hill; 2011.)
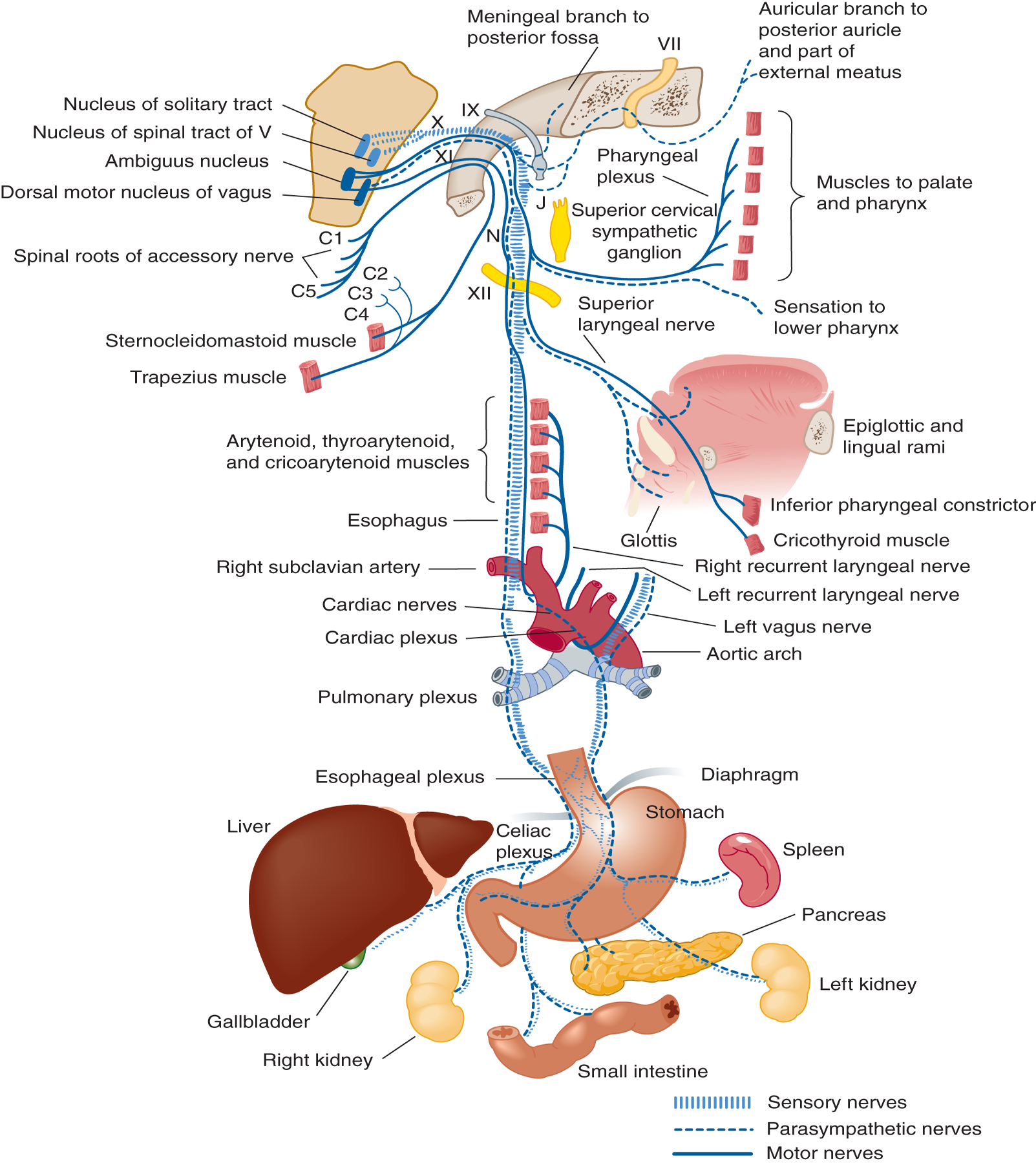
FIGURE 6-10. Innervation of the palate, pharynx, larynx, and thoracicoabdominal viscera by cranial nerve X. The palatal branch innervates the levator veli palatini muscle. The pharyngeal and laryngeal branches mediate sensory and motor functions of these structures. (Reproduced with permission from Waxman SG. Chapter 8. Cranial Nerves and Pathways. In: Waxman SG. eds. Clinical Neuroanatomy, 27e. New York, NY: McGraw-Hill; 2013.)
B. LMN innervation of the pharynx and larynx by cranial nerves IX and X
1. The skeletal muscles supplied by CrNs IX and X originally came from branchial arches. The branchial efferent nucleus for IX and X, the nucleus ambiguus, is in the  mesencephalon/
mesencephalon/ pons/
pons/ medulla. (
medulla. ( medulla (Fig. 2-14))
medulla (Fig. 2-14))
2. CrN IX supplies only one muscle exclusively (stylopharyngeus). Because this muscle aids in swallowing, its isolated function cannot be tested clinically. The remaining branchial efferent fibers of CrNs IX and X supply the pharyngeal constrictors. Because they act as a unit in swallowing, the isolated function of the individual constrictors cannot be tested at the bedside.
4. Because even complete interruption of CrN V has little clinical effect on palatal function, we can say that the motor functions of the palate, pharynx, and larynx are innervated by CrNs _______ and _______. (IX; X)
5. CrNs IX and X mediate sensation from the palate and pharynx and CrN X alone from the larynx. Hence, CrNs IX and X are the motor and sensory sentinels of the palatal orifice and pharynx, but CrN ___ alone is the sensorimotor nerve of the larynx. (X)
6. CrN IX also carries afferents from the carotid sinus that mediate baroceptive reflexes and from the carotid body that mediates baroceptive and chemoceptive reflexes.
C. Normal swallowing
1. Swallowing requires precise coordination of bulbar and respiratory muscles by a distributed network of neural connections involving the posterior inferior frontal gyrus, anterior insular region, basal motor nuclei, diencephalon, reticular formation, and cerebellum (Newton et al, 1994; Zald and Pardo, 1999). Lesions at various sites in this network may cause dysphagia (Groher, 1997). Unilateral lesions in the lowest part of the precentral gyrus and posterior part of the inferior frontal gyrus may impair swallowing without causing dysarthria (Wiles, 1991), although the two frequently coexist.
2. The tongue initiates the act of swallowing by voluntarily throwing a bolus of food back into the palatal archway. Tongue movements are innervated exclusively by CrN _____. (If you do not know, sort through CrNs I to XII to find the right one.) (XII)
3. Afferents from the palate via CrN IX then complete the act reflexly (Perlman and Schultze-Delrieu, 1997). The bolus stimulates the palate to elevate and deflect the bolus from the nasopharynx into the oropharynx. The pharyngeal constrictors contract, the larynx elevates, and the vocal cords close.
4. Swallowing requires afferent information via CrNs V, IX, and X, and motor actions are mediated by CrNs V, VII, IX, X, and XII. Connections in the region of the nucleus of the tractus solitarius in the medulla, in proximity to the respiratory center, act as a swallowing center. It coordinates the actions of swallowing and breathing to avoid aspiration (Newton et al, 1994; Smith Hammond, 2006); Section IV G describes clinical testing for disordered swallowing, ie, dysphagia).
5. Hold your jaw open and try to swallow with your lips open. You may succeed after a struggle, but normal swallowing requires sealing of the lips and closure of the jaw. CrN ___ innervates jaw closure and CrN ___ innervates lip closure. (V; VII)
D. Clinical physiology of the soft palate
1. The levator veli palatini muscle, innervated via the pharyngeal plexus by CrN X, swings the soft palate upward and backward to contact the posterior wall of the pharynx. This action seals off the naso pharynx from the oro pharynx. See Figs. 6-11A and 6-11B.
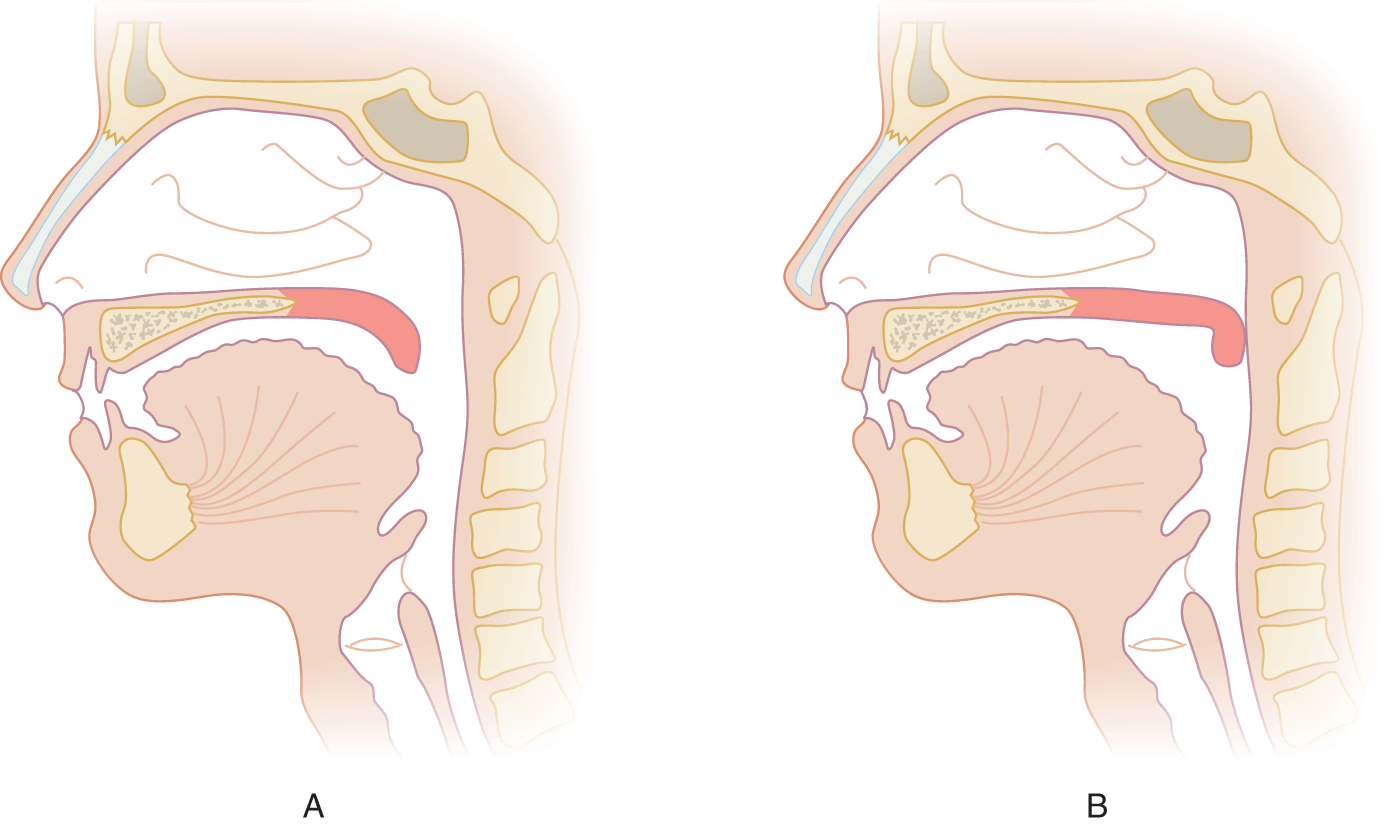
FIGURE 6-11. Sagittal section of the head. (A) Position of the soft palate when relaxed. (B) Position of the soft palate when elevated, occluding the nasopharynx from the oropharynx.
2. Unless the soft palate elevates properly, liquid will escape into your nose when you drink, and air will escape into your nose when you speak. The results are “nasal” swallowing and “nasal” speech. Because liquids and gases are fluids, we can agree that the soft palate and other branchial muscles of the throat control the fluid traffic at the oropharyngeal, nasopharyngeal, esophageal, and laryngotracheal openings. CrNs IX and X innervate, guard, and control the internal apertures of the head much as CrNs ____ (sensory) and ____ (motor) innervate the external apertures (eyes, mouth, ears, and nares). (V; VII)
3. The nasopharyngeal airway remains open until the palate elevates. The palate elevates as required to block anything in your oropharynx from entering your nasopharynx. Thus, the palate elevates when you:
a. Swallow
b. Whistle or trumpet
c. Make certain speech sounds
E. Upper motor neuron innervation of cranial nerves IX and X
1. The palate, pharynx, and vocal cords act with bilateral synchrony. By knowing this fact, you can predict that the number of crossed and uncrossed UMN fibers from each cerebral hemisphere would be about _________
2. Because of the usual bilateral UMN innervation, unilateral UMN lesions that cause hemiplegia only rarely cause unilateral weakness of the palate (Willoughby and Anderson, 1984), but Pts with acute hemiplegia frequently show mild dysarthria (about 60%).
BIBLIOGRAPHY · Cranial Nerves IX and X, Swallowing, and Dysphagia
Groher ME. Dysphagia: Diagnosis and Management. 3rd ed. Boston, MA: Butterworth Heinemann; 1997.
Newton HB, Newton C, Pearl D, et al. Swallowing assessment in primary brain tumor patients with dysarthria. Neurology. 1994;44:1927–1932.
Perlman AL, Schultze-Delrieu KS. Deglutition and Its Disorders: Anatomy, Physiology, Clinical Diagnosis, and Management. San Diego, CA: Singular Publishing Group; 1997.
Smith Hammond GA, Goldstein LB. Cough and aspiration of food and liquids due to oral-pharyngeal dysphagia ACCP evidence based guidelines. Chest. 2006;129(Suppl.):154S-N168S.
Wiles CM. Neurogenic dysphagia. J Neurol Neurosurg Psychiatry. 1991;57:1037–1039.
Willoughby EW, Anderson NE. Lower cranial nerve motor function in unilateral vascular lesions of the cerebral hemisphere. Br Med J. 1984;289:791–794.
Zald DH, Pardo JV. The functional neuroanatomy of swallowing. Ann Neurol. 1999;46:281–286.
IV. ROLE OF CRANIAL NERVES IN SPEECH
A. The basic physiology of speech
1. Speech requires a bellows, ergo lungs and respiratory muscles, that express a stream of air through the larynx and upper airway.
2. Speech requires a vibrator for phonation, ergo the vocal cords.
3. Speech requires articulation, ergo palate, tongue, lips, and mandible.
4. Speech requires resonance, ergo pharyngeal, oral, and nasal passages and cavities. The nasal passages resonate only three sounds of the English language: m, n, and ng.
5. Speech requires neural control, ergo UMNs to LMNs. Chapter 11 discusses cerebral circuits and cerebral disorders of speech.
B. Phonation: role of cranial nerve X
Galen (A.D. 130–200) of Pergamum was troubled by the squealing of piglets during surgical experiments. But his curiosity drove him to continue his investigations. Because he had no anesthetic, he eliminated the squealing and satisfied his curiosity with one stroke. After verifying the origin of the voice in the larynx, he found that he could silence it by cutting the recurrent laryngeal nerves, and that is how we learned what these nerves do.
C. Articulation of speech
1. Phonation versus articulation: After the larynx phonates, articulation shapes the sounds into speech. By whispering this sentence, you can clearly separate phonation from articulation. When whispering, you do not phonate at all, but you still articulate every word with perfect clarity. Articulate speech consists of a mixture of voiced and non-voiced sounds (Aronson, 1990).
2. Labial sounds: While watching your lips in a mirror, recite loudly each letter of the alphabet, but then try to repeat each letter without any lip movement. In Table 6-3, check the sounds that require strong labial action.
TABLE 6-3 • Letter Sounds Requiring Strong Lip Action (Labials)
Strong labial |
Strong labial |
A |
N |
B |
O |
C |
P |
D |
Q |
E |
R |
F |
S |
G |
T |
H |
U |
I |
V |
J |
W |
K |
X |
L |
Y |
M |
Z |
3. Lingual sounds: Complete Table 6-4 by reciting the alphabet loudly and checking the sounds that require strong lingual actions.
TABLE 6-4 • Letter Sounds Requiring Strong Tongue-Tip Elevation (Linguals)
Strong lingual |
Strong lingual |
A |
N |
B |
O |
C |
P |
D |
Q |
E |
R |
F |
S |
G |
T |
H |
U |
I |
V |
J |
W |
K |
X |
L |
Y |
M |
Z |
4. Vowel sounds
a. Compare the tongue actions when making D, G, and J sounds, strong linguals, with those needed to make the vowel sounds A, E, I, O, and U. To understand tongue action during vowel sounds, press down on your tongue with a tongue blade as you recite the vowels.
b. Vowels require palatal elevation. The palate does not completely seal off the nasopharynx during most speech sounds. Instead, it reduces the nasopharyngeal aperture, thereby detouring most of the air through the mouth, the path of least resistance. Only a few sounds require complete palatal closure:
i. Plosives: K or hard G, as in good.
ii. Vowels: sustained EEEEE… or Ah…
c. Clinicians traditionally test for palatal elevation by asking the Pt to say, Ahh. The vowel E requires tighter palatal closure, but the Pt can say Ah more easily with the mouth open to permit palatal inspection. As a test for palatal function, ask the Pt to repeat: “We see three gray geese.”
5. Plosive sounds
a. Plosives require momentary impounding of air and sudden release. Try this experiment: cup your palm and hold it about just in front of your lips. Loudly say, Puh, puh, puh; M, M, M; and kuh, kuh, kuh.
b. With two of these sounds, you felt a strong puff of air in your palm. The sound not requiring forceful air expulsion and therefore not a plosive was _________
c. To divert all air through your mouth, for plosives, the palate must effectively seal the nasopharynx off from the oropharynx. Therefore, if the Pt articulates vowels and plosives well, the velopharyngeal (palatal) valve closes well.
6. Sibilants and fricatives
a. Sibilants are hissing or whistling sounds. Cup your palm over your mouth and sustain a forceful SSS… or a prolonged Hisssss… Do you feel a stream of air against your palm?  Yes/
Yes/ No. (Try it yourself.)
No. (Try it yourself.)
b. Fricatives are high-frequency frictional or rustling sounds. Cup your hand to your mouth and forcefully pronounce V, V, V…; Z, Z, Z…; and F, F, F…
c. Try the sibilants and fricatives again, without using tongue or lips at all. Can you say them?
d. To produce sibilants and fricatives, you must force a strong stream of air through a small aperture formed by lips, tongue, and teeth. To divert air from the nose into the mouth, the velopharyngeal valve must close more or less completely.
7. Voiceless consonants: Many speech sounds, such as sibilants and plosives, require no phonation. As examples of such voiceless consonants, say Shhhh, P, T, and K. Hence, all speech sounds require  articulation/
articulation/ phonation, but not all speech sounds require
phonation, but not all speech sounds require  articulation/
articulation/ phonation. (
phonation. ( articulation;
articulation;  phonation)
phonation)
D. Nomenclature for disorders of speech
1. Mutism means the inability or refusal to speak. Neuromuscular disorders may cause mutism, but commonly it implicates a block at the level of the cerebrum or mind, as in dementia or hysterical mutism, or an afferent block, as in deaf mutism. In elective mutism a child, for example, may refuse to speak at all in public or at school but talks readily at home. Acute injury to the mid portion of the cerebellum, with or without nuclear involvement, can cause a spectrum of speech disturbances, including transient mutism as observed in certain cases following posterior fossa surgery (mostly tumors) in children (Jones et al, 1996).
2. Dysphonia means a disorder of phonation (in the sounds of speech produced by the larynx), such as hoarseness or spasmodic dysphonia.
3. Dysarthria means only faulty articulation of speech sounds. It does not refer to the content of speech, the words, syntax, rhythm, and the vocabulary. It may result from central lesions of the brain or cerebellum, intoxications (such as alcohol), or neuromuscular disorders. It commonly occurs with lesions along the course of the pyramidal tract, in which case the Pt usually has other pyramidal tract signs. The extent of associated pyramidal signs in stroke Pts varies from dysarthria and UMN facial palsy, to dysarthria and clumsy hand syndrome, dysarthria and ataxic hemiparesis, or dysarthria and hemiparesis, and most lesions are on the left side of the cerebrum or brainstem (Okuda and Tachibana, 2000; Urban et al, 2001). Chapter 11 discusses dysprosody and aphasia.
E. Hyponasal and hypernasal speech
1. In hyponasal speech, too little air escapes through the nose.
a. Pinch your nostrils together and say “Good morning.”
b. Lack of nasal escape of air transforms “Good morning” into “Good bordig,” as when a cold causes swelling of the nasal mucous membranes. This would be  hypernasal/
hypernasal/ hyponasal speech. (
hyponasal speech. ( hyponasal)
hyponasal)
2. In hypernasal speech, too much air escapes through the nose (Pollack and Shprintzen, 1979). Because of muscular weakness or mechanical defects, such as a cleft palate, the palate fails to seal off the oropharynx, a condition called velopharyngeal incompetence (velum curtain soft palate). A Pt with hypernasal speech would have  velopharyngeal incompetence/
velopharyngeal incompetence/ nasal obstruction, whereas a Pt with hyponasal speech would have
nasal obstruction, whereas a Pt with hyponasal speech would have  velopharyngeal incompetence/
velopharyngeal incompetence/ nasal obstruction. (
nasal obstruction. ( velopharyngeal incompetence;
velopharyngeal incompetence;  nasal obstruction)
nasal obstruction)
3. An unfortunate, common, but preventable error
a. Failure to distinguish between hyper- and hyponasal speech leads to serious errors in treatment. When the palate elevates, it contacts the dorsal pharyngeal wall (Fig. 6-11). In children, the adenoid tissue, which occupies the posterior pharyngeal wall, may hypertrophy and then bulge forward into the pharyngeal lumen. Hypertrophy of the adenoid would cause  hyponasal/
hyponasal/ hypernasal speech. (
hypernasal speech. ( hyponasal)
hyponasal)
b. Some Pts with neurogenic palatal weakness or submucous palatal clefts will have hypernasality, but the Ex may conclude that enlarged adenoids are at fault. Actually, the adenoids reduce the need for velopharyngeal closure. Removal of the adenoids of a Pt with hypernasal speech will make it  better/
better/ worse. Explain. _________
worse. Explain. _________ worse)
worse)
Removal of the adenoid tissue increases the distance the palate has to close to shut off the nasopharynx. A weak palate is now even less capable of preventing nasal escape of air.
F. Stuttering: involuntary pauses and repetitions interrupt the smooth flow of words and sentences
1. All of us have brief pauses and repetitions when we speak, the toddler more than the adult, but the stutterer’s pauses and repetitions impede communication. The Pt usually falters and repeats the first syllable of words, sometimes the middle or last syllables.
2. Although emotional stress worsens stuttering, the cause of stuttering is still unknown. The standard bedside neurologic examination (NE) of stutterers discloses no evidence of neurologic dysfunction per se.
G. Testing the motor function of cranial nerves IX and X
1. Speech
a. During the history, the Ex appraises the Pt’s speech almost automatically. Perfectly normal articulation requires no formal testing. Otherwise, test the articulation mechanisms individually.
b. Test articulation by the soft tissues, the soft palate, tongue, and lips, with the KLM test. Kuh, Kuh, Kuh tests the function of the _________
c. Most infants or young children with delayed speech or dysarthria require a thorough audiologic examination.
2. Difficulty swallowing (dysphagia)
a. To test for mild to moderate dysphagia, give the Pt a glass containing 150 mL of tap water. The Pt should swallow it at a rate exceeding 10 mL/s (Nathadwarawala, 1992).
b. Any Pt with dysphagia may aspirate food or fluids into the lung, causing aspiration pneumonitis. Avoid the timed swallowing test if the Pt gives a history of aspiration or obvious difficulty swallowing saliva. Deficits in coughing maybe as important as the impaired swallowing in determining aspiration risk (Smith Hammond et al, 2006).
3. Neurologic examination of the palate and larynx
a. As the Pt says Ahh, inspect the tonsillar pillars for asymmetry as they arch upward and medially to form the palate. Look at the arch, the arch above, not the uvula. Study your own palatal action in the mirror.
b. Students commonly mistake an asymmetrically hung uvula for palatal palsy. Let the uvula hang as it will. Look for asymmetry of the palatal arch. See Figs. 6-12A and 6-12B.
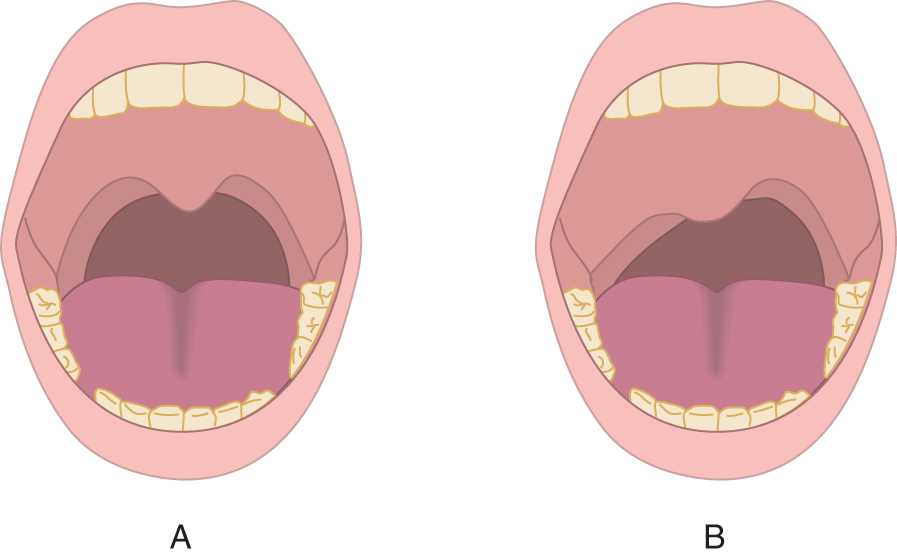
FIGURE 6-12. Palatal arch. (A) Symmetrical elevation of the palatal arch when a normal subject says Ah. … (B) Asymmetrical elevation of the palatal arch when a Pt with unilateral palatal weakness says Ah.…, indicating a weak levator veli palatini muscle.
c. Fig. 6-12A shows a normal person saying Ahh …, and Fig. 6-12B shows a palatal palsy. Which side is paralyzed in B?  right/
right/ left. (
left. ( right (the right side has failed to elevate))
right (the right side has failed to elevate))
d. The gag reflex is an additional test for palatal elevation. Touch one tonsillar pillar and then the other with a tongue blade. The afferent arc of the gag reflex is primarily CrN ___. The efferent arc is CrN ___. (IX; X)
e. If the palate fails to elevate when the Pt says Ah but does elevate during the gag reflex, the Pt would have a lesion of the  UMNs/
UMNs/ LMNs/
LMNs/ muscles. (
muscles. ( UMNs)
UMNs)
f. If the history and examination disclose no evidence of dysarthria or dysphagia, omit the gag reflex. It is uncomfortable and unnecessary. Furthermore, the gag reflex may be absent in a large number of healthy elderly people.
g. Causes of incompetent palatal elevation include UMN or LMN lesions, congenital malformations such as cleft palate, myopathies, or local lesions in the soft tissue, and palatopharyngeal discrepancy in size. Palatal incompetence is not one thing or another. Like every sign, it poses a puzzle to be solved.
4. Hoarseness and unilateral vagal lesions
a. Many mechanical or neurogenic disorders of the larynx can cause hoarseness. Vocal cord paralysis may cause hoarseness but not in all Pts. Therefore, in addition to listening to the voice and looking at the palate, vocal cords may need to be inspected by laryngoscopy.
b. Unilateral vocal cord paralysis usually causes a breathy, raspy voice or a voice flutter, because of vibration of the flaccid cord, and may cause stridor and anoxia.
i. In addition, paralysis of the cricothyroid muscles, the pitch adjustor muscles (innervated by the superior laryngeal nerve), results in a monotone sound without changes in pitch.
ii. Interruption of the entire vagus nerve results in dysphagia and dysphonia, because of paralysis of the pharyngeal constrictors.
5. After listening to many Pts, you will come to recognize disorders in rhythm and force (dysprosody) and timber of the voice. The speech affliction is often characteristic and even pathognomonic of the underlying disease.
a. In cerebral palsy, the cry or the speech has a characteristic slow, ratchet-like onset and a harsh, tight, and irritating dysphonic quality that is diagnosable without even seeing the Pt.
b. In the cat’s cry syndrome (cri du chat), the infant’s cry is high-pitched and sounds like the chromosome 5p deletion syndrome, (also called 5p minus syndrome). The Pt’s karyotype confirms the diagnosis: the fifth chromosome lacks a short arm.
c. In hypothyroidism, the voice is deep and raspy.
d. In parkinsonism, the muscular rigidity dampens the normal inflections and modulations of the Pt’s voice: The sound is all on one plane, that is, “plateau speech.” Cerebellar disease causes the opposite: The Pt overaccentuates some sounds and underaccentuates others; the voice scans from one peak of volume to another, a so-called scanning speech.
e. The most common dysarthria of all is slurring due to intoxication with alcohol and other drugs.
f. Table 6-5 presents a classification of speech disorders.
TABLE 6-5 • Classification of Speech Disorders
Dysphonia: disorder in the pitch, quality, or volume of the voice Spasmodic dysphonia Hoarseness Pubertal “squeaks” Vocal tics (Tourette syndrome) Dysarthria: disorder in the articulation of sounds Palatals Labials Linguals Fricatives and sibilants Plosives Dysprosody: disorder in the melody, stress, rhythms, and inflections that give shades of meaning to speech Aphasia: inability to express or receive words as symbols for communication Broca Wernicke Conduction Global Transcortical motor Transcortical sensory Mixed transcortical Anomic Striatocapsular Thalamic Mutism Selective Conversion disorder Deaf Echolalia: stereotyped, noncommunicative repetition of words or sentences heard Developmental disorders Pervasive developmental disorders (lack of communicative speech): mental retardation and autism Stammering: blocking or involuntary pauses in speech Stuttering: repetitions, prolongations, and interjections of syllables or whole words Cluttering: rapid speech characterized by dropping of syllables Speech disorders caused by lesions of specific motor system Ataxic speech (drunk-man speech): cerebellar lesions Spastic dysarthria (pseudobulbar speech): bilateral corticobulbar tract lesions Plateau speech: parkinsonism/substantia nigra lesion Bradylalia, choreiform, dystonic and athetoid speech: extrapyramidal lesions |
H. Ancillary diagnostic procedures for mutism, dysphonia, dysarthria, and dysphagia
Whenever the Pt, in particular infants or children, presents with disorders in speaking or swallowing, consider these tests:
1. Audiologic investigation: The Pt who does not hear correctly does not speak correctly.
2. Direct laryngoscopy.
3. Plain radiographs of the oropharyngeal airway.
4. Radiographic cinematography, often with a “barium swallow,” to visualize the palate and pharynx in action.
BIBLIOGRAPHY · Speech, Dysphonia, and Dysarthria
Aronson AE. Clinical Voice Disorders. 3rd ed. New York, NY: Thieme Medical Publishers; 1990.
Jones S, Kirollos RW, Van Hille PT. Cerebellar mutism following posterior fossa tumor surgery. Br J Neurosurg. 1996;10(2):221–224.
Nathadwarawala KM, Nicklin J, Wiles CM. A timed test of swallowing capacity for neurological patients. J Neurol Neurosurg Psychiatry. 1992;55:822–825.
Okuda B, Tachibana H. Isolated dysarthria. J Neurol Neurosurg Psychiatry. 2000;68:119–120.
Pollack MA, Shprintzen RF, Zimmerman-Manchester KL. Velopharyngeal insufficiency. The neurological perspective. A report of 32 cases. Dev Med Child Neurol. 1979;21:194–201.
Urban PP, Wicht S, Vukurevic G, et al. Dysarthria in acute ischemic stroke. Lesion topography, clinicoradiologic correlation, and etiology. Neurology. 2001;56:1021–1027.
V. CRANIAL NERVE XI MOTOR FUNCTIONS
A. Functional anatomy of cranial nerve XI
1. CrN XI has two parts, spinal and accessory. Hence, its full name is the _________
2. The spinal part supplies the sternocleidomastoid (SCM) and rostral portions of the trapezius muscles.
3. The accessory part is accessory to the vagus. The accessory fibers arise in the nucleus ambiguus of the medulla and merely hitchhike along the proximal part of CrN XI before joining CrN X for distribution to the pharynx and larynx. The NE tests the spinal part.
B. The sternocleidomastoid muscle
1. Study Fig. 6-13.
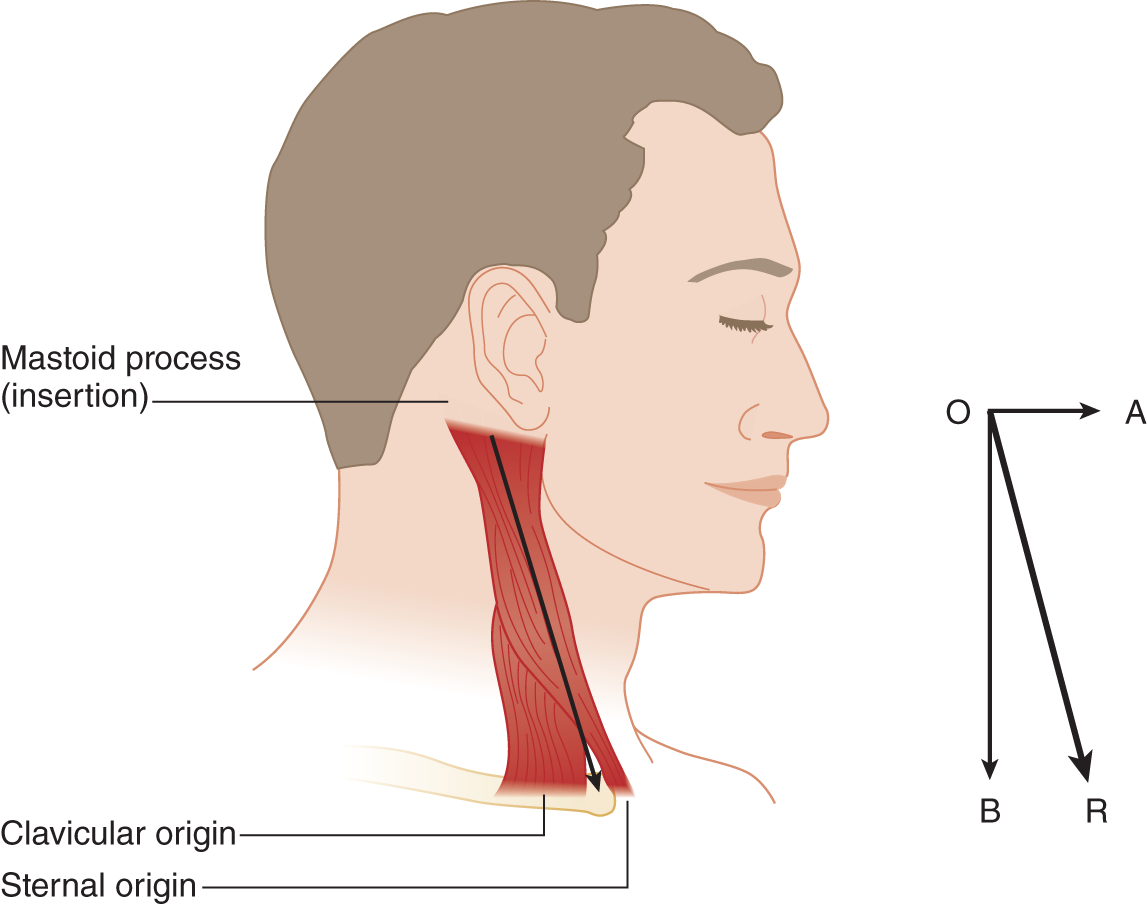
FIGURE 6-13. Origin and insertion of the sternocleidomastoid muscle. The oblique arrow in the vector diagram (O-R) is the actual line of pull. It resolves into a horizontal vector (O-A) that thrusts the head forward and turns it to the opposite side and into a vertical vector (O-B) that tilts the head to the same side as the muscle.
2. The SCM originates from the _________
3. Mnemonic: To remember once and for all the actions of the SCM muscle, make a C with your thumb and forefinger and place it on your SCM muscles (Fig. 6-14A).
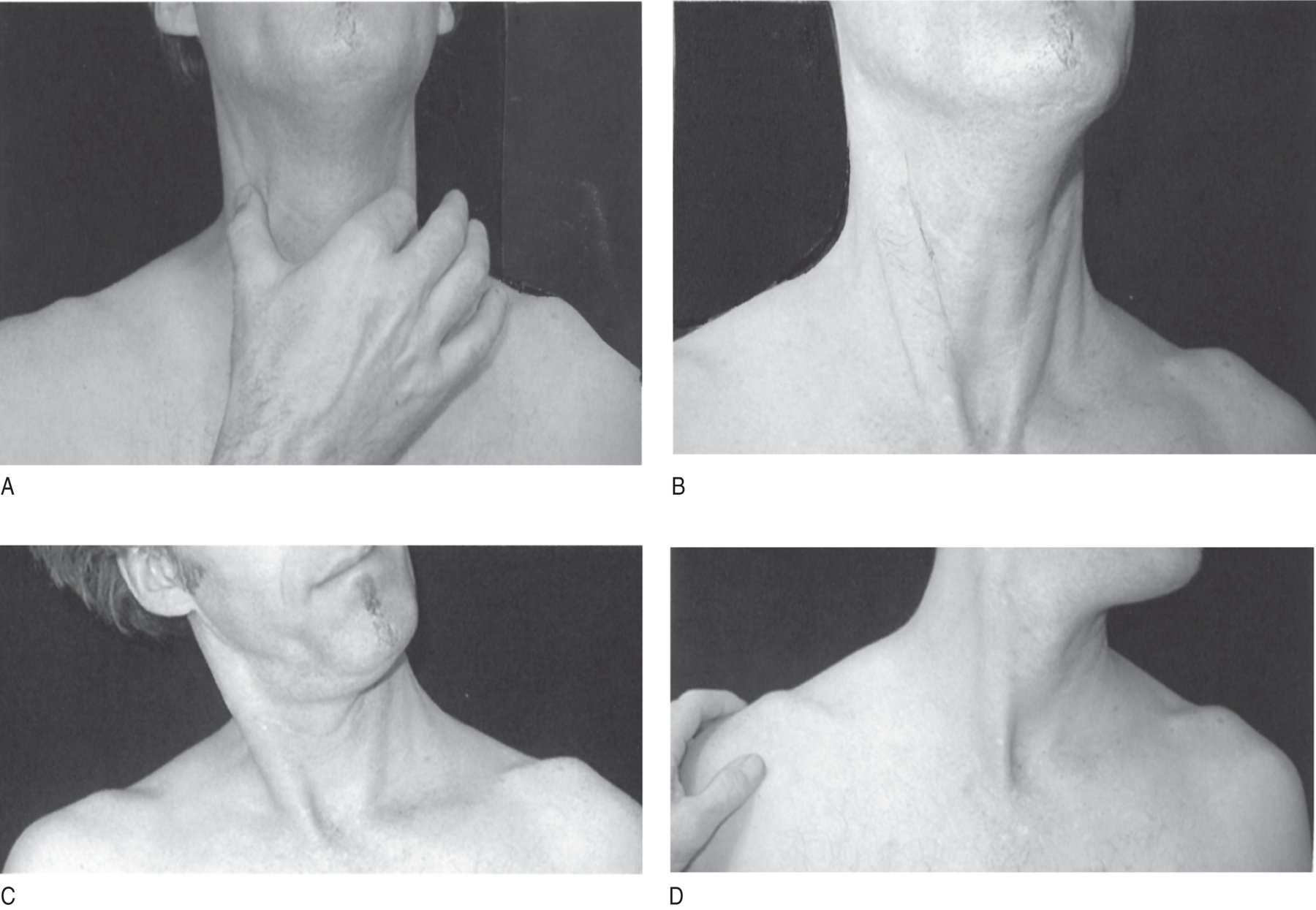
FIGURE 6-14. Mnemonic for remembering the actions of the SCM muscles. (A) Make a C of the thumb and forefinger and place it on the SCMs. (B) Press the head forward against the opposite hand, and the C fingers will feel the SCMs contract bilaterally. The hand is removed in Figs. 6-14B to 6-14D to expose the muscular contractions, but keep your hand in place as in step (A) to feel your own muscles act. (C) Tilt the head to the right (tilt, not turn) and feel the right SCM contract. (D) Turn the head to the left (turn, not tilt) and feel the right SCM contract. Thus the SCM acts to thrust the head forward, tilt it ipsilaterally, and turn it contralaterally. SCM = sternocleidomastoid.
a. Now start with your head in the neutral position and, while palpating your own SCM, place your other hand on your forehead and press back on it while you press your head forward. You will feel both SCM muscles contracting strongly (Fig. 6-14B).
b. Return your head to the neutral position and then tilt (tilt, not turn) your head fully to the right. You will feel your right SCM spring into action (Fig. 6-14C).
c. Next try turning (turning, not tilting) your head strongly to the left. You will feel your right SCM contract strongly (Fig. 6-14D).
d. SCM thrusts the head  forward/
forward/ backward. (
backward. ( forward)
forward)
e. SCM turns the head to the  same/
same/ opposite side. (
opposite side. ( opposite)
opposite)
f. SCM tilts the head to the  same/
same/ opposite side. (
opposite side. ( same)
same)
g. The action of one SCM in turning the head, like the action of one lateral pterygoid muscle in turning the mandible, results in turning  toward/
toward/ away from the side of the muscle that is contracting. (
away from the side of the muscle that is contracting. ( away from)
away from)
C. The trapezius muscle
1. The trapezius muscle originates in the midline from the occiput and the spinous processes of all cervical and thoracic vertebrae. It inserts into the clavicle and scapula.
2. CrN XI innervates only the rostral part of the trapezius muscle (Donner and Kline, 1993), the part that lifts the shoulders (Fig. 6-15). The cervical plexus innervates the rest of the muscle.
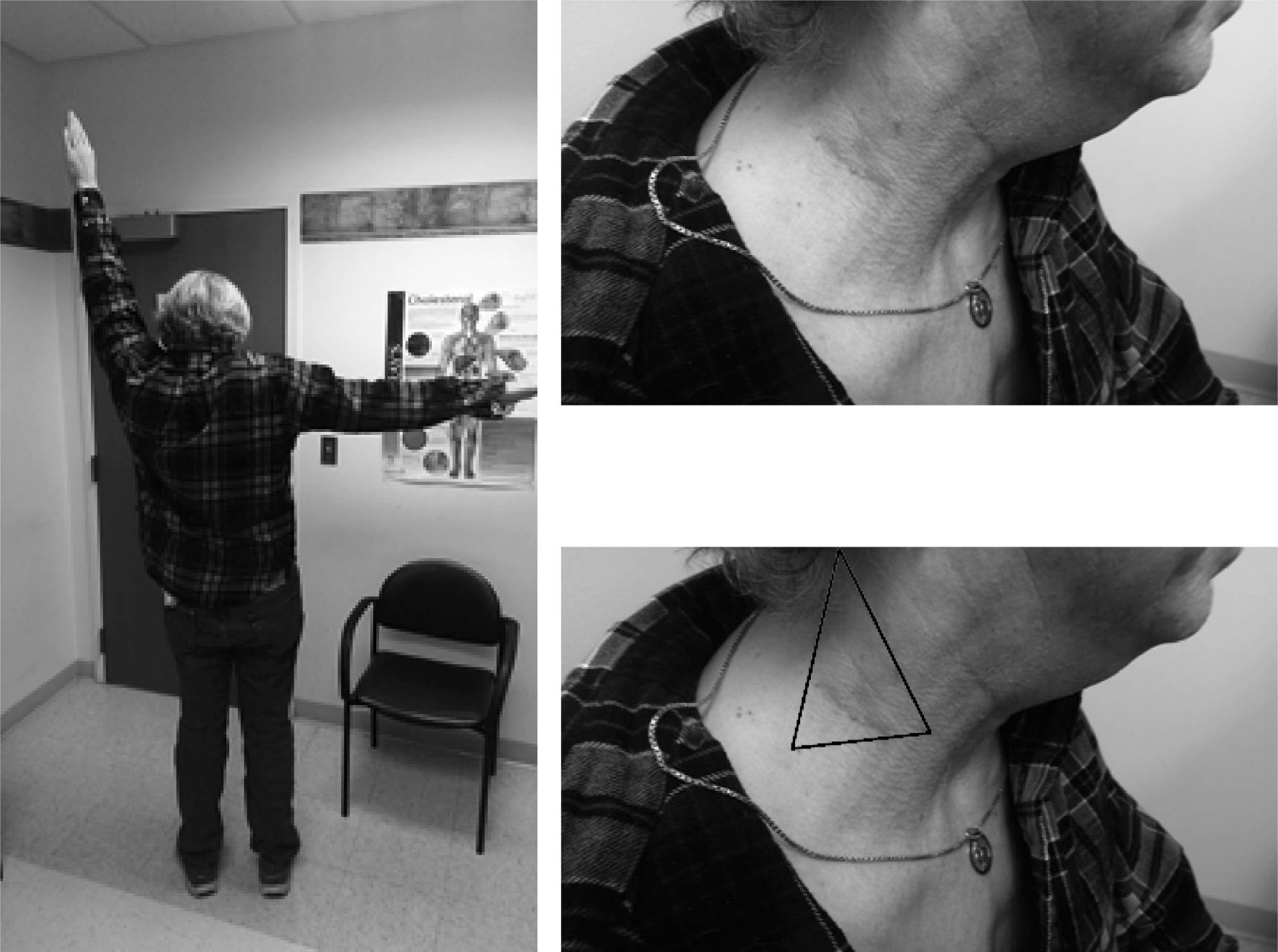
FIGURE 6-15. Isolated spinal accessory nerve palsy following lymph node biopsy in the posterior cervical triangle distal to the sternocleidomastoid muscle. Trapezius weakness occurred in isolation.
D. Clinical testing of muscles supplied by cranial nerve XI
1. Inspect the SCM and trapezius muscles for size and asymmetry.
2. Next palpate the muscles at rest and as they exert their actions.
3. To test the strength of SCM and trapezius muscles, try the maneuvers listed in Table 6-6 on another person.
TABLE 6-6 • Clinical Tests of the Motor Function of Cranial Nerve XI
Command to patient |
Examiner’s maneuver |
1. “Turn your head to the left. Do not let me push it back.” |
Place your right hand on the left cheek of the patient, your left hand on his right shoulder to brace him, and try to force his head to the midline. Repeat with the patient’s head turned to the right. With the patient’s head turned to the left, you test the |
2. “Push your head forward as hard as possible.” |
Place one hand on the patient’s forehead and push backward. What do you do with your other hand to brace the patient? In this maneuver you test the action of the |
3. “Try to touch your ears with the tips of your shoulders. Hold them there and don’t let me push them down.” |
Place your hands on both of the patient’s shoulders and press down. Observe from the front and back and watch for scapular winging that may occur with trapezius or serratus anterior weakness. |
ABBREVIATION: SCM = sternocleidomastoid. |
|
4. In step 1, why should you press on the Pt’s cheek, rather than on the mandible?
_________
_________
E. Upper motoneuron innervation of cranial nerve XI
1. An acute severe hemispheric lesion that interrupts the UMNs (the pyramidal tract) will cause contralateral hemiplegia. The Pt’s head turns to the side of the lesion because of assumed weakness of the SCM ipsilateral to the lesion. However, muscles which are ipsilateral may play a greater note in head turning than the contralateral SCM (Fitzgerald, 2001). Caudal to the brainstem, on the hemiplegic side, the SCM (and perhaps other cervical muscles that turn the head) are the only muscles that retain their strength (Willoughby and Anderson, 1984). In contrast, the trapezius muscle is paralyzed like the remaining muscles on the hemiplegic side. Thus these paralyzed muscles receive the classic contralateral UMN innervation (Brazis et al. 2016; Thompson and Thickbroom, 1997).
2. The course of the UMN axons to the SCM is still unclear. The clinical evidence suggests that the UMN fibers to one SCM derive mainly from the ipsilateral hemisphere and that they run directly without decussating to the LMNs for the SCM or that the fibers undergo a double decussation to end up ipsilateral to the hemisphere that activates them (Gandevia and Applegate, 1988; Marcus, 1989). However, transcranial magnetic stimulation studies suggest that innervation is predominantly contralateral, and there is minimal ipsilateral innervation.
BIBLIOGRAPHY · Cranial Nerve XI
Brazis PW, Masdeu JC, and Biller J. Localization in Clinical Neurology. 7th ed. Philadelphia, PA: Lippincott, Williams, and Wilkins, Wolters Kluwer Health. In Press, 2016.
Fitzgerald T. Sternocleidomastoid Paradox. Clin Anat. 2001;14:330–331.
Donner TR, Kline DG. Extracranial spinal accessory nerve injury. Neurosurgery. 1993;32:907–910.
Gandevia SC, Applegate C. Activation of neck muscles from the human motor cortex. Brain. 1988;111:801–813.
Marcus JC. The spinal accessory nerve in childhood hemiplegia. Arch Neurol. 1989;46:60–61.
Thompson ML, Thickbroom GW, Mastaglia FL. Corticomotor representation of the sternocleidomastoid muscle. Brain. 1997;120:245–255.
VI. XII CRANIAL NERVE MOTOR FUNCTIONS
A. Functional anatomy of the tongue
1. CrN XII controls tongue movements. Because XII runs under the tongue, it is named the _________
2. Action of the genioglossus muscle
a. To understand how XII nerve lesions affect tongue movements, learn the action of the genioglossus muscles. Notice in Fig. 6-16 the triangular shape of each genioglossus muscle.
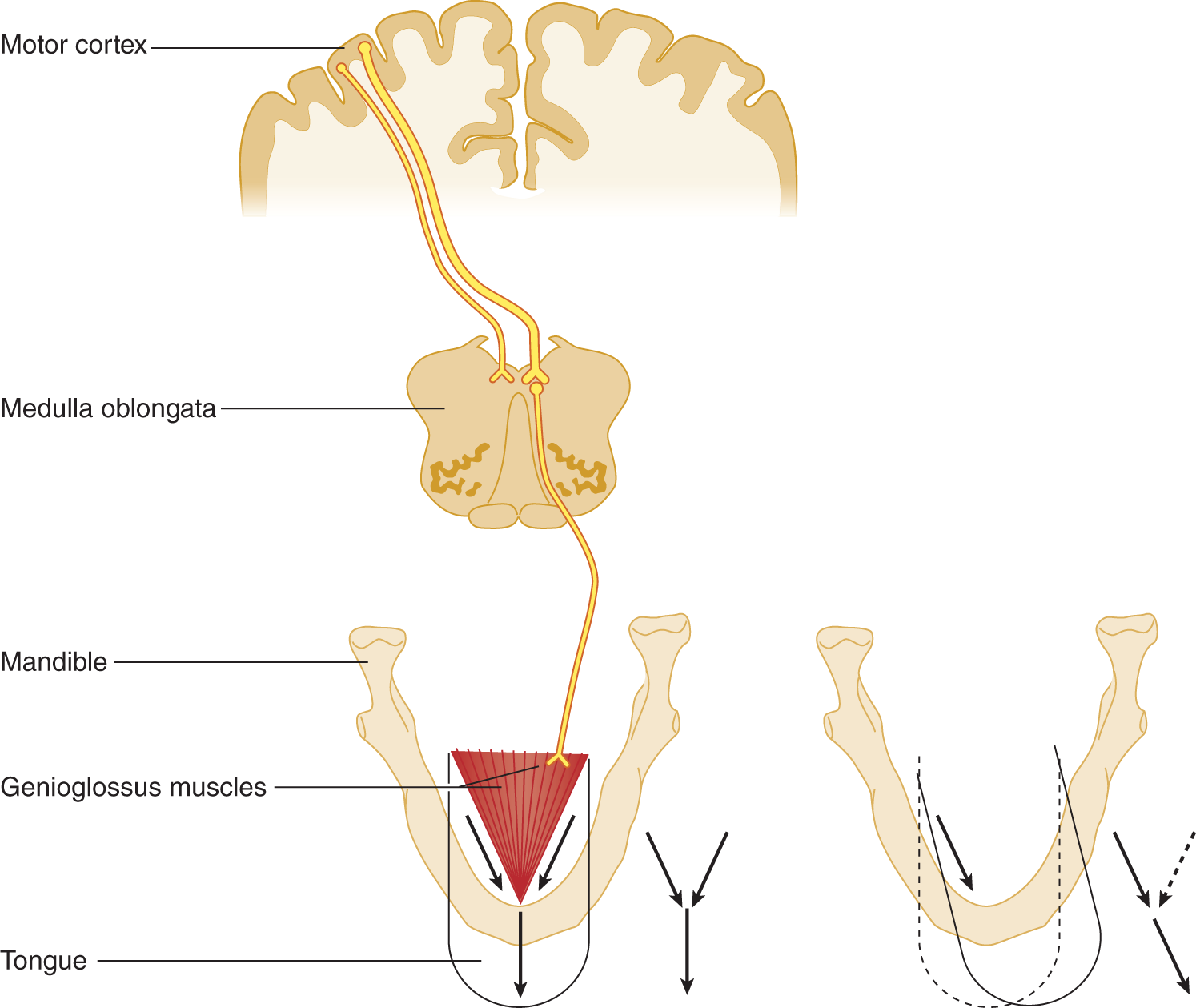
FIGURE 6-16. Innervation and action of the genioglossus muscle. The diagram to the right shows that, when only the right genioglossus muscle contracts, it pulls the right-sided base of the tongue forward, thereby protruding and deviating the tip of the tongue to the left.
i. Its apex originates from the apex of the mandible, which is hard, un-yielding, and immobile.
ii. Its base fans out to insert into the base of the tongue, which is soft, fleshy, and mobile. Symmetrical genioglossus contraction, therefore, must pull the base of the tongue  forward/
forward/ backward. (
backward. ( forward (Fig. 6-16))
forward (Fig. 6-16))
b. If the tongue protrudes in the midline, right and left _________
c. If the Pt attempts to protrude the tongue in the midline, and it deviates to the right, the weakness affects the  right/
right/ left genioglossus muscle. (
left genioglossus muscle. ( right)
right)
3. Compare the action of genioglossus in Fig. 6-16 with that of the lateral pterygoid muscle in Fig. 6-1. Clearly the mechanics of tongue and jaw protrusion are identical. Midline protrusion results from symmetrical muscular action. Compare the action of these two muscles with the SCM. All three muscles, when contracting unilaterally, turn the part they operate to the  same/
same/ opposite side. (
opposite side. ( opposite)
opposite)
4. The muscle that turns the tongue to the right is the  right/
right/ left _________
left _________ left; genioglossus)
left; genioglossus)
5. The muscle that turns the mandible to the left is the  right/
right/ left _________
left _________ right; lateral pterygoid)
right; lateral pterygoid)
6. One visible muscle that turns the head to the right is the  right/
right/ left _________
left _________ left; SCM)
left; SCM)
7. With the tongue inside the mouth, the Pt can deviate the tip of the tongue to the non-paralyzed side but not to the paralyzed side, in contrast to its deviation to the paralyzed side when outside the mouth. The genioglossus, an extrinsic muscle, acts on the protruded tongue, whereas intrinsic muscles cause lateral movement of the tip of the non-protruded tongue (Riggs, 1984).
B. Lower motoneuron lesions of cranial nerve XII
Notice in Fig. 6-16 that each XII nerve innervates one-half of the tongue. Although we have mentioned only the genioglossus, the bulk of the tongue is muscle. After interruption of the XII nerve, the muscle fibers on the ipsilateral half of the tongue undergo atrophy. Therefore, the signs of XII nerve interruption are ipsilateral atrophy and ipsilateral deviation of the tongue on attempted midline protrusion.
C. Upper motoneuron innervation of cranial nerve XII
1. Figure 6-16 shows that one hemisphere sends crossed and uncrossed axons to the hypoglossal nucleus. Using a colored pencil, draw in the UMN and LMN innervations from the left motor cortex. Notice the somewhat thicker line representing crossed fibers, indicating a slightly greater percentage of decussated corticobulbar fibers. In this respect, the UMN innervation of the tongue most closely resembles the innervation of  forehead elevation/
forehead elevation/ eyelid closure/
eyelid closure/ lip retraction. (
lip retraction. ( eyelid closure)
eyelid closure)
2. In 10% to 15% of Pts, the tongue deviates after a unilateral corticobulbar lesion. Thus, if the tongue deviates after a right hemisphere lesion, the tongue would deviate to the  right/
right/ left when protruded; use Fig. 6-16 to reason out the answer. (
left when protruded; use Fig. 6-16 to reason out the answer. ( left)
left)
3. In hysterical hemiplegia, if the tongue deviates, it typically deviates to the side opposite to the putatively paralyzed extremities (Keane, 1986).
4. Because the corticobulbar fibers for branchial nuclei and the corticospinal fibers run more or less together through the brainstem, isolated interruption of just the corticobulbar fibers to the tongue is unlikely. Thus, if a UMN lesion affects tongue movements, the Pt usually shows a UMN type of facial palsy and frank hemiplegia.
D. Clinical testing of cranial nerve XII
1. Inspection of the tongue at rest
a. Inspect the tongue for the most reliable sign of a XII nerve lesion, hemiatrophy. However, diseases that affect LMNs bilaterally, such as amyotrophic lateral sclerosis, result in bilateral tongue atrophy.
b. Palpation may help resolve questionable hemiatrophy. While wearing a rubber glove, palpate each half of the tongue between your thumb and index finger.
2. Testing tongue motility and deviation
a. Say to the Pt, “Stick your tongue straight out as far as possible and hold it there.” Check for alignment of the median raphe of the tongue with the crevice between the medial incisor teeth. Check the alignment of your own tongue in a mirror.
b. Then, if the history or findings suggest a bulbar problem, ask the Pt to move the tongue alternately to the right and to the left and to try to touch the tip of the tongue to the tip of the nose and then to the tip of the chin. On protrusion the tongue tip should extend well beyond the teeth. Many disorders may impair tongue motility:
i. Weakness due to UMN or LMN interruption.
ii. Myopathy.
iii. Rigidity, as in parkinsonism.
iv. Apraxia (Chapter 11), intellectual developmental disorder, dementia, or a major mental illness such as depressive disorders or schizophrenia.
3. Tongue strength: Tongue strength per se is hard to evaluate. Have the Pt press the tongue against the cheek while you press your finger against the cheek. Keep your tongue in your cheek while you evaluate this rather unreliable test.
4. Involuntary movements of the tongue:
a. Rippling of a normal tongue frequently indicates incomplete relaxation. Ask the Pt to make some tongue movements and then inspect it again after the Pt relaxes it. Rippling of one half of the tongue, if that half is weak and atrophic, suggests fasciculations (Chapter 7), which supports the diagnosis of an LMN lesion, but pathologic fasciculations and normal rippling are difficult to distinguish by clinical inspection.
b. Patients with involuntary movements such as chorea or athetosis (Chapter 7) cannot keep a protruded tongue still. Ask the Pt to hold the tongue protruded and still for 30 seconds.
c. Infants with intellectual disability or cerebral palsy often display an action called tongue thrusting. Whenever the mother puts food into the infant’s mouth, the tongue thrusts it back out, impairing nutritional input.
5. Dysarthria: Interruption of the UMN pathway to the tongue, the corticolingual pathway, causes dysarthria. Usually weakness of the lower part of the face on one side or frank hemiparesis accompanies the dysarthria. Occasionally an isolated lacune (a small infarct) will interrupt the corticolingual fibers from the motor cortex, causing dysarthria, but with no other signs of hemiparesis (Urban et al, 2001).
E. Distinguishing unilateral upper from lower motoneuron weakness of the tongue
1. If the tip of the protruded tongue deviates, the Pt has a UMN or an LMN lesion. Suppose the tongue deviates to the left. If the lesion is UMN, it involves the  right/
right/ left hemisphere. The weakness is usually
left hemisphere. The weakness is usually  severe/
severe/ moderate. (
moderate. ( right;
right;  moderate)
moderate)
2. The clinical distinction between unilateral UMN and LMN weakness of the tongue rests on supporting evidence of UMN signs in other movements or on positive evidence of LMN involvement. What would be the best evidence of a unilateral, LMN XII nerve lesion?
_________
F. Patient analysis
1. The Pt shown in Fig. 6-17 was asked to stick out his tongue straight out. Describe any abnormalities.
_________
_________

FIGURE 6-17. This patient was asked to stick his tongue straight out.
2. These clinical findings indicate interruption of the  right/
right/ left
left  XII nerve/
XII nerve/ corticobulbar tract. (
corticobulbar tract. ( right;
right;  XII nerve)
XII nerve)
3. The Pt shown in Fig. 6-17 had a glioma of the medulla oblongata that destroyed the fibers of CrN XII in their intra-axial course.
BIBLIOGRAPHY · Cranial Nerve XII
Brazis PW, Masdeu JC, and Biller J. Localization in Clinical Neurology. 7th ed. Philadelphia, PA: Lippincott, Williams, and Wilkins, in preparation.
Keane JR. Wrong-way deviation of the tongue with hysterical hemiparesis. Neurology. 1986;36:1406–1407.
Riggs JE. Distinguishing between extrinsic and intrinsic tongue muscle weakness in unilateral hypoglossal palsy. Neurology. 1984;34:1367–1368.
Urban PP, Wicht S, Vukurevic G, et al. Dysarthria in acute ischemic stroke. Lesion topography, clinicoradiologic correlation, and etiology. Neurology. 2001;56:1021–1027.
VII. MULTIPLE CRANIAL NERVE PALSIES, PATHOLOGIC FATIGABILITY, AND MYASTHENIA GRAVIS
A. Signs and symptoms of multiple cranial nerve palsies
If a Pt has diplopia, dysphagia, dysphonia, or dysarthria, particularly if these complaints are intermittent, or if the Ex finds an unexplained ocular, facial, or bulbar palsy, such as ptosis, strabismus, or mild hypernasal speech, suspect myasthenia gravis. Myasthenics may have little or no deficit when rested, as when first arising in the morning, but as the day wears on, or as they use their CrN muscles to look, talk, swallow, or chew, the weakness gets worse. Symptoms are often worsened by a rise in body temperature and often improved by cold. This pathologic fatigability of muscles, particularly of CrN muscles, is virtually pathognomonic of myasthenia gravis. Myasthenic Pts have a deficit in cholinergic transmission at the motor end plates of skeletal muscles. The diagnosis depends on clinical demonstration of the pathologic fatigability, repetitive nerve stimulation that shows a decrementing response, restoration of strength by giving a cholinergic drug, and demonstration of antibodies that block acetylcholine receptors at motor end plates of the skeletal muscles or IgG antibodies against the muscle-specific kinase (MuSK) in cases of seronegative myasthenia gravis.
B. Bedside tests for pathologic fatigability of cranial nerve muscles
1. The Ex chooses for testing the particular muscles implicated by the Pt’s history or that have displayed weakness during the NE. If the Pt complains of double vision or ptosis, select the eye muscles; if the complaint involves dysphagia, dysarthria, or dyspnea, select the oropharyngeal and breathing muscles. To bring out latent weakness of such muscles or of a muscle not overtly weak, require the Pt to make repetitive or prolonged contractions. Make sure that you enlist the Pt’s full cooperation and effort.
2. To test for ptosis or diplopia, carefully measure the height of the palpebral fissure and record the range of eye movements. Pay particular attention to the range of upward eye movement, the ocular movement that has the least range. Ask the Pt to follow your finger up and down through a full range of movement. Then measure the height of the palpebral fissure and again record the range of eye movements. The ice pack test may also be useful in cases of eyelid ptosis due to myasthenia gravis (Chatzistefanou et al, 2009). Test lateral eye movements by noting the distance between the limbus and lateral or medial canthi before and after repetitive exercise, or have the Pt hold the eyes in a deviated position for a timed period. Test oropharyngeal function by actually timing how long the Pt can read or count aloud without weakness of the voice, and then have the Pt try to swallow a glass of water (Section IV G 3). Test fatigability of the tongue by having the Pt waggle it from side to side. For masseter weakness, request the Pt to chew gum or paraffin a given number of times. Test palatal function by timing how long the Pt can sustain an EEEE°. Test breathing by measuring vital capacity before and after a timed period of hyperventilation. As a quick, quantitative, apparatus-less test for breathing insufficiency, useful in myasthenia or other neurologic disorders in lieu of spirometry, ask the Pt to take a full, deep breath and to count aloud softly from 1 up. Control the rate of counting by tapping your finger at the rate of one per second. The average adult Pt should reach at least 25. Try this test yourself. The point of the foregoing tests is to select some quantifiable or measurable end point to prove that repetitive use of the muscle causes pathologic fatigability or that cholinergic medication restores strength.
C. Electrical tests for pathologic fatigability in myasthenia gravis
Electrical testing for myasthenia includes repetitive stimulation of a peripheral nerve while recording the amplitude of the action potentials generated in the muscle fibers. Myasthenics show a decrement in the amplitude of muscular contraction after repetitive electrical stimulation of the nerve. The repetitive nerve stimulation test (Jolly test) provides entirely objective data. It eliminates the need for the Pt’s active participation, as required by the repetitive exercise tests. Single muscle fiber analysis also aids in establishing the diagnosis.
BIBLIOGRAPHY · Myasthenia Gravis
Chatzistefanou KI, Kounis T, Iliakis E, et al. The ice pack test in the differential diagnosis of myasthenic diplopia. Opthalmology. 2009;116:2236–2243.
D. Summary of common causes for multiple cranial nerve palsies or weakness of multiple ocular and faciobulbar muscles
1. Myasthenia gravis
2. Guillain–Barré syndrome (including the C. Miller Fisher variant)
3. Chronic basilar meningitis
4. Diabetes mellitus
5. Neoplasms along the base of the skull and nasopharynx
6. Botulism
7. Myotonic dystrophy (diffuse weakness of all CrN muscles with predilection for temporal, masseter, and SCM muscles and relative sparing of extraocular muscles; Fig. 1-13I)
VIII. PSEUDOBULBAR AND BULBAR PALSY: UPPER VERSUS LOWER MOTONEURON PALSIES OF THE CRANIAL NERVES
A. Bulbar paralysis
Clinicians use many different terms for UMN and LMN paralysis, terms that bear the imprint of medical history. The older anatomists visualized the medulla as a bulb-like expansion of the spinal cord. Thus, they called the medulla “the bulb.” They called LMN paralysis from a lesion of the bulb or its nerves, IX, X, and XII, “bulbar paralysis.” They called paralysis of speech and swallowing after UMN lesions “pseudobulbar,” or “false bulbar” paralysis, because the lesion was not truly in the bulb or its nerves. The UMNs to the bulb were called “corticobulbar fibers.” By usage, the term corticobulbar fibers has expanded to include all cortical efferent fibers to the brainstem, in particular to the LMNs of CrNs III through XII. But we limit the term bulbar paralysis to LMN paralysis of bulbar CrNs IX, X, and XII. Well, “A foolish consistency is the hobgoblin of little minds” (Ralph Waldo Emerson, 1803–1882). Thus, bulbar paralysis refers to an  LMN/
LMN/ UMN lesion of CrNs
UMN lesion of CrNs  IX to XII/
IX to XII/ III to XII. (
III to XII. ( LMN;
LMN;  IX to XII)
IX to XII)
B. The syndrome of pseudobulbar palsy
1. After acute, bilateral interruption of the corticobulbar fibers, as after bilateral cerebral infarction, the Pt becomes obtunded or comatose, mute, or severely demented, and loses all ability to speak or swallow. In the recovery phase or with gradual lesions of the corticobulbar tracts, the Pt shows a characteristic, virtually pathognomonic syndrome termed pseudobulbar palsy.
a. The Pt initiates speaking or swallowing very slowly, if at all, and has severe dysarthria or near mutism.
b. The voice has a peculiar strained pitch and quality.
c. The Pt may swallow or chew reflexly but cannot initiate these acts volitionally and may yawn automatically but cannot open the mouth volitionally.
d. The Pt exhibits extreme emotional lability by crying one moment and laughing the next, like turning on and off a faucet. The face appears immobile most of the time, as though it were a wooden mask, but when the Pt cries or laughs, the facial movements expressing the emotion become exaggerated; strangely, when queried, the Pt may not feel the emotion that the behavior of crying or laughing expresses (Lieberman and Benson, 1977). Sometimes the syndrome expresses itself by uncontrollable burst of inappropriate laughter, called fou rire prodromique (Parvizi et al, 2009). The most common causes in adults are bilateral strokes, amyotrophic lateral sclerosis (ALS), or other degenerative diseases (Wortzel et al, 2008) (Video 6-6). Gelastic seizures are another cause, and most commonly result from hypothalamic hamartomas.

Video 6-6. Pseudobulbar affect in a patient with progressive multifocal leukoencephalopathy (PML).
e. The pseudobulbar syndrome occurs in children who have bilateral perisylvian opercular and insular lesions from congenital malformations, hypoxia-ischemia, or encephalitis (Christen et al, 2000; Grattan-Smith et al, 1989).
2. Thus, the UMN pathways that activate the muscles for emotional expression differ from those for volitional movement, but we do not know their exact course. The motor pathways for behavioral expression of emotion can thus operate separately from the limbic pathways that produce the experience of emotion.
a. LMN lesions of CrNs would cause loss of  emotional movements only/
emotional movements only/ volitional movements only/
volitional movements only/ volitional and emotional movements. (
volitional and emotional movements. ( volitional and emotional movements)
volitional and emotional movements)
b. Acute bilateral UMN lesions would cause loss of volitional and emotional facial movements initially, but in the chronic stage emotional expression may be _________
c. Summarize the clinical features of pseudobulbar palsy and state the pathways affected.
_________
3. Table 6-7 lists the many different terms for UMN and LMN paralysis. Clearly, the terms UMN and LMN convey the concept concisely and informatively.
TABLE 6-7 • Synonyms or Near Synonyms for Muscular Paralysis from UMN and LMN Lesions
UMN paresis or paralysis |
LMN paresis or paralysis |
Central |
Peripheral |
Pseudobulbar |
Bulbar |
Suprasegmental |
Segmental/nuclear |
Supranuclear |
Infranuclear |
ABBREVIATIONS: LMN = lower motoneuron; UMN = upper motoneuron. |
|
BIBLIOGRAPHY · Pseudobulbar Palsy
Christen H-J, Hanefeld F, Kruse E, et al. Foix-Chavany-Marie (anterior operculum) syndrome in childhood: a reappraisal of Worster-Drought syndrome. Dev Med Child Neurol. 2000;42:122–123.
Grattan-Smith PJ, Hopkins IJ, Shield LK, et al. Acute pseudobulbar palsy due to bilateral focal cortical damage: the opercular syndrome of Foix-Chavany-Marie. J Child Neurol. 1989;4:131–136.
Lieberman A, Benson DF. Control of emotional expression in pseudobulbar palsy. Arch Neurol. 1977;34:717–719.
Parvizi J, Coburn KL, Shillcutt SD, et al. Neuroanatomy of pathological laughing and crying: a report of the American Neuropsychiatric Association Committee on Research. J Neuropsychiatry Clinc Neurosci. 2009;21:75–87.
Wortzel HS, Oster TJ, Anderson CA, et al. Pathological laughing and crying. Epidemiology, pathophysiology and treatment. CNS Drugs. 2008;22(7):531–545.
IX. THE NEUROLOGY OF BREATHING
A. Four functions of the breathing apparatus
1. Gas exchange for respiration
a. Homeostatic control of blood gases and pH
b. Clearing the airway by coughing, sniffing, and sneezing
2. Sucking and blowing
3. Emotional expression: sighs, laughter, crying, exclamations, hissing, breath-holding, hyperventilation
4. Speech
B. The neuroanatomy of breathing
1. Origin of the drive to breathe: The drive to breathe arises from two sources: the forebrain and the brainstem reticular formation. The isolated spinal cord by itself cannot produce any drive to breathe. That drive must descend from the brain. Thus separation of the spinal cord from the brain by transection at the medullocervical junction causes complete and irreversible apnea.
2. The forebrain and the control of volitional and emotional breathing
a. The forebrain mediates the volitional control of breathing via the pyramidal tracts, particularly for speech.
b. The forebrain also mediates the emotional control of breathing, in particular for laughing and crying, through descending pyramidal and nonpyramidal pathways. Other common examples of emotional expression through control of breathing are hyperventilation attacks in anxiety, breath-holding spells in infants, and sighing.
3. The reticular formation and the control of automatic breathing
a. Reticulospinal tracts, arising in the medullary reticular formation, control automatic, essentially homeostatic, breathing for the control of blood gases and pH.
b. The caudal half of the reticular formation, from the mid-pons to the medullocervical junction, generates the rhythmic drive to breath and controls the related reflexes served by the caudal CrNs (swallowing, chewing, rooting, sucking, coughing, and hiccoughing) and mediates the control of blood pressure and pulse. Bilateral permanent lesions of the caudal reticular formation permanently impair or abolish breathing and the related reflexes.
c. The medullary reticular formation provides the single most important pathway for survival, the reticulospinal pathway. It activates the LMNs of the muscles of respiration, essentially the diaphragm and intercostal muscles. The reticulospinal tracts decussate at the medullocervical junction, just ventral to the obex, and descend in the ventrolateral quadrants of the spinal cord to activate the phrenic and intercostal motoneurons (Fig. 6-18).
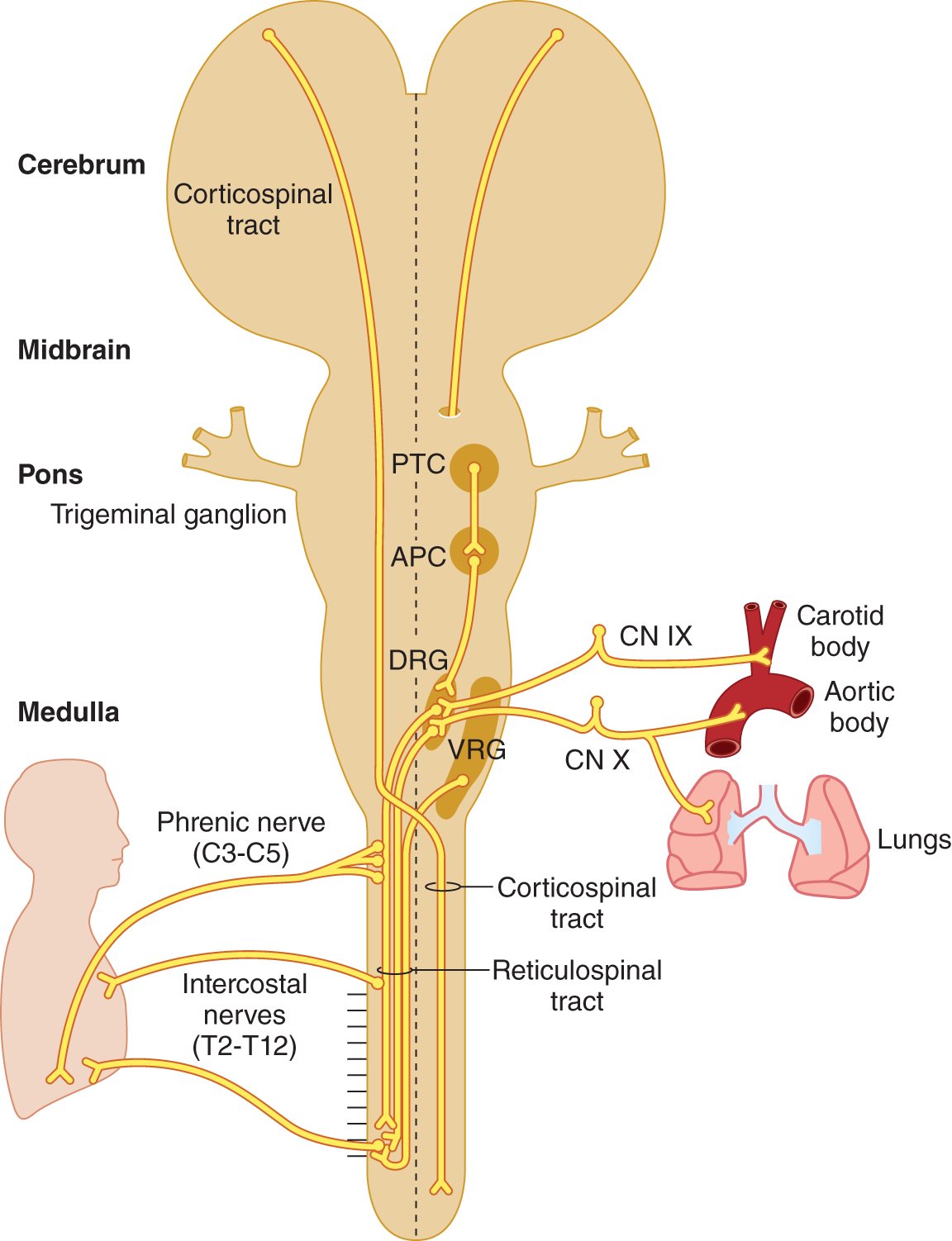
FIGURE 6-18. Neuroanatomy of breathing. By stimulating motoneurons in the spinal cord from C3 to T12, the corticospinal tracts control volitional breathing, and the reticulospinal tracts control automatic breathing. APC = apneustic center; CN = cranial nerve; DRG = dorsal respiratory group of neurons, mainly inspiratory, associated with the dorsal motor nucleus of the vagus nerve; PTC = pneumotaxic center; VRG = ventral respiratory group of mixed inspiratory and expiratory neurons associated with the nuclei ambiguus and retroambigualis. (Reproduced with permission from DeMyer W. Neuroanatomy. 2nd ed. Baltimore, MD: Williams & Wilkins; 1998.)
d. The phrenic nerves are the most important nerves in the body. With intact phrenic nerves and the drive to breathe descending through the reticulospinal tracts, the Pt can maintain life by diaphragmatic action alone, even after spinal cord transection caudal to the phrenic level (C3 to C5) paralyzes all the intercostal muscles. Transcutaneous stimulation of the phrenic nerves and electromyographic recording of the diaphragm can directly access the LMNs for breathing (Markand et al, 1984).
e. Figure 6-19 shows an infant who suffered spinal cord transection during a breech delivery. The infant shows dorsiflexed wrists secondary to intact extensor muscles and paralyzed flexors. The spared extensor muscle motoneurons are slightly rostral to the level of the flexors. The lesion has spared the phrenic nerve, which arises from C3 to C5, but has interrupted all ascending and descending spinal pathways at the C7 level, thus depriving the intercostal muscles of pyramidal and reticulospinal activation. Because of the descent of the diaphragm without intercostal action, the chest sucks in and the abdomen protrudes when the infant inspires, the so-called abdominal breathing (Fig. 6-19). The legs show the characteristic flexed posture that follows chronic spinal cord transection (paraplegia in flexion; Figs. 6-19A and 6-19B).
FIGURE 6-19. Eight-week-old infant showing the characteristic posture and breathing action after spinal cord transection at level C7 due to a breech delivery. (Reproduced with permission from DeMyer W. Anatomy and clinical neurology of the spinal cord. In Joynt RJ, ed. Clinical Neurology. rev. ed. Philadelphia, PA: Lippincott; 1990.)
4. Coughing
Another breathing-related function of the caudal reticular formation is coughing, which depends on the abdominal muscles for the explosive expulsion of air. Coughing is an important protective reaction to clear the airway in response to irritant gases, vomitus, or foreign bodies. Many diseases of the central and peripheral nervous systems impair coughing by interrupting the afferent or efferent arc or causing paralysis, thus leading to pneumonia. The Ex should record whether any Pt with respiratory difficulty can cough. Quantitative measurement of air flow is the most reliable way to assess the neuromuscular sufficiency for coughing (Smith Hammond, 2001).
C. Ondine curse and the dichotomy between volitional and automatic breathing
1. Consider a Pt with intact pyramidal tracts but interruption of the reticulospinal pathways. Such a condition may arise from any of the following conditions:
a. Bilateral destruction of the medullary reticular formation.
b. Interruption of the reticulospinal tracts at the obex (Fig. 6-18).
c. Interruption of the reticulospinal tracts as they descend in the ventro-lateral quadrants of the spinal cord white matter (Fig. 2-12C).
2. The Pt without a reticulospinal pathway lacks automatic breathing but continues to breathe while awake because of the respiratory drive generated in the waking forebrain, transmitted through the pyramidal tracts. Sleep removes the respiratory drive arising in the forebrain. Thus with no automatic breathing to take over, the Pt becomes apneic when going to sleep and may die. This condition, Ondine curse, condemns the Pt to death if he goes to sleep. To breathe and, hence, to live, the Pt must remain perpetually awake, never to enjoy Keats’ “sleep full of sweet dreams and quiet breathing.”
3. By contrast, interruption of the pyramidal tracts, with the reticulospinal tracts intact, abolishes volitional control of breathing, but automatic breathing, via the reticulospinal tracts, keeps the Pt alive, whether awake or asleep. Such Pts with bilateral pyramidal tract destruction also show the pseudo-bulbar palsy syndrome described in Section VIII.
D. Hypoventilation in hemiplegia
Hemiplegia causes paresis of respiratory actions. Because some Pts have a larger number of decussating axons in the pyramidal tract than others, the effects of UMN lesions may vary somewhat from Pt to Pt. Interruption of the pyramidal tract reduces diaphragmatic and intercostal actions contralaterally during voluntary breathing but not during automatic breathing (Polkey et al, 1999).
BIBLIOGRAPHY · Breathing
Markand O, Kincaid JC, Pourmand R, et al. Electrophysiologic evaluation of diaphragm by transcutaneous phrenic nerve stimulation. Neurology. 1984;34:604–614.
Polkey MI, Lyall RA, Moxhan J, et al. Respiratory aspects of neurological disease. J Neurol Neurosurg Psychiatry. 1999;66:5–15.
Smith Hammond CA, Goldstein LB, Zajac DJ, et al. Assessment of aspiration risk in stroke with quantification of voluntary cough. Neurology. 2001;56:502–506.
X. LOCALIZING DIAGNOSTICON FOR BRAINSTEM SYMPTOMS AND SIGNS
Figure 6-20 summarizes the clinical effects of brainstem lesions. One important, classic localizing combination is paralysis of a CrN in the midbrain, pons, and medulla ipsilateral to the lesion and motor or sensory signs contralateral to it due to interruption of a decussating tract, that is, right VI nerve palsy and left-sided pyramidal signs caused by a lesion of the basis pontis.
FIGURE 6-20. Localizing diagnosticon for brainstem lesions (exclusive of central ocular pathways). CDRTCT = cerebello-dentato-rubro-thalamo-cortical tract; CN = cranial nerve; ML = medial lemniscus; PT = pyramidal tract; TL = trigeminal lemniscus; RF = reticular formation; SN = substantia nigra; VP = vestibular pathways. (Reproduced with permission from DeMyer W. Neuroanatomy. 2nd ed. Baltimore, MD: Williams & Wilkins; 1998.)
XI. SEQUENTIAL SCREENING EXAMINATION OF THE MOTOR FUNCTIONS OF ALL CRANIAL NERVES
A. Preliminary observations
And now the crucial test: Can you actually and systematically examine CrN motor function? If you did your job when taking the history, you have already observed the Pt’s eye movements and blinking; noted the degree and symmetry of other facial movements; inspected the relation of the eyelids to palpebral fissures; looked for enor exophthalmos; listened to phonation and for the articulation of labials, linguals, and palatals; and noted the spontaneous swallowing of saliva. If these are all normal, the Pt cannot have too much wrong with CrN motor function, but you must do a minimum formal examination anyhow (Brazis et al, 2016; DeMyer, 1998; Samaii and Jannetta, 1981; Wilson-Pauwels et al, 2002).
B. Motor examination of all of the cranial nerves in 45 seconds
The formal examination of CrN motor function begins with the eyes. The NE outline at the beginning of the text lists motility last in the ocular sequence for a reason. The Ex can then flow smoothly through the entire CrN motor examination, yes, III to XII, in just 45 seconds, with a normal cooperative Pt. No, the 45 seconds is not a misprint. Get a partner and rehearse the commands and observations listed in Table 6-8 until you meet the 45-seconds criterion.
TABLE 6-8 • Rapid Sequential Screening of the Motor Function of the Cranial
|
BIBLIOGRAPHY · The Cranial Nerves
Brazis PW, Masdeu JC, and Biller J. Localization in Clinical Neurology. 7th ed. Philadelphia, PA: Lippincott, Williams, and Wilkins. Wolters Kluwer Health. In Press. 2016.
DeMyer W. Neuroanatomy. Baltimore, MD: Williams & Wilkins; 1998.
Samii M, Jannetta PJ, eds. The Cranial Nerves. New York, NY: Springer-Verlag; 1981.
Wilson-Pauwels L, Akesson EJ, Stewart PA, et al. Cranial Nerves in Health and Disease. Philadelphia, PA: BC Decker; 2002.
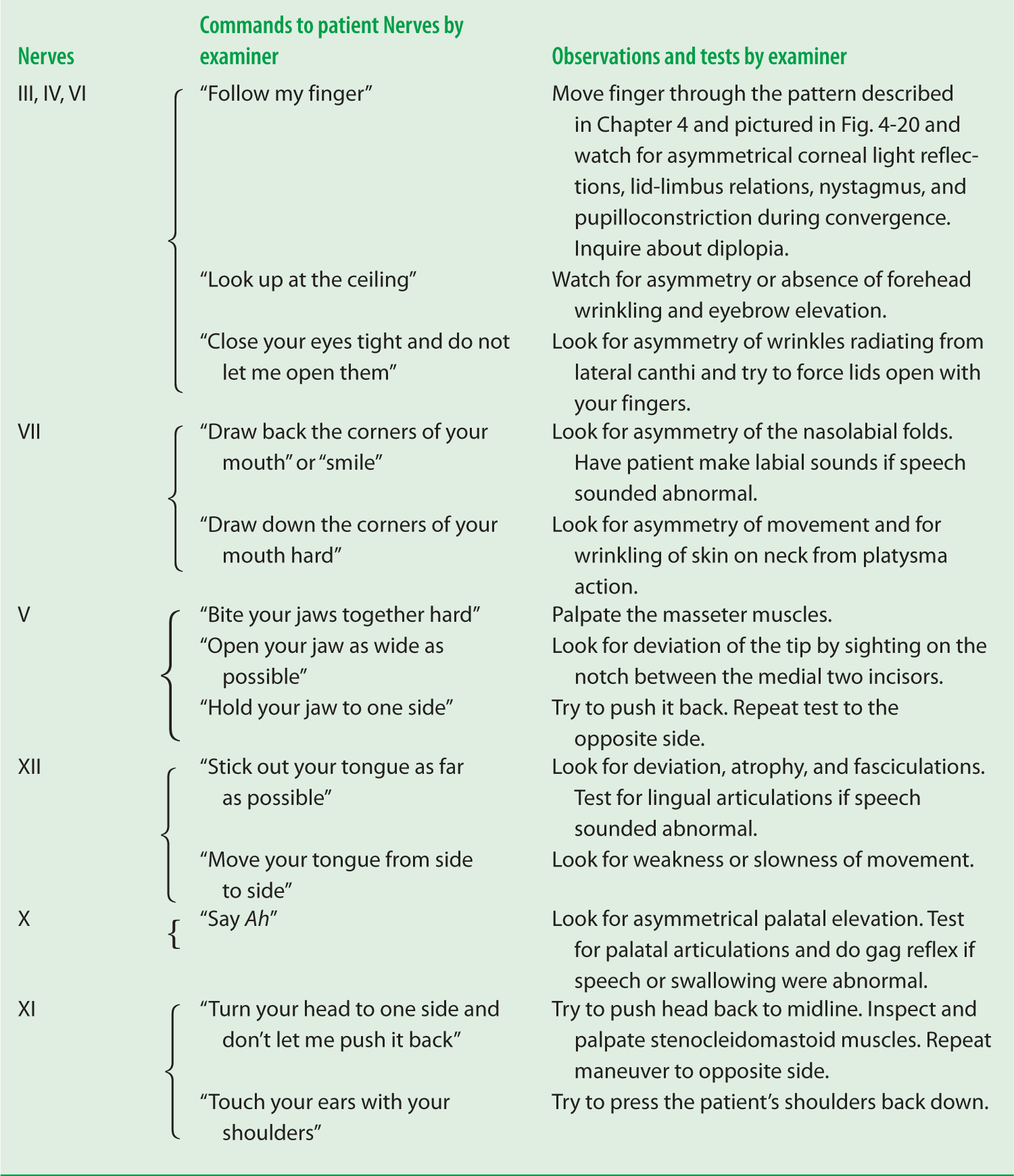
 Learning Objectives for Chapter 6
Learning Objectives for Chapter 6