8 |
Examination for Cerebellar Dysfunction |
But how great was his apprehension, when he farther understood, that [the force of parturition] acting upon the very vertex of the head, not only injured the brain itself, or cerebrum—but that it necessarily squeezed and propelled the cerebrum towards the cerebellum, which was the immediate seat of the understanding!—Angels and ministers of grace defend us! Cried my father—can any soul withstand this shock?—No wonder the intellectual web is so rent and tattered as we see it; and that so many of our best heads are no better than a puzzled skein of silk,—all perplexity—all confusion within-side.
I. FUNCTIONS OF THE CEREBELLUM
A. What the cerebellum does not do
1. Laurence Sterne correctly satirized the speculative neurophysiology of his time, which localized “the immediate seat of the understanding” to the cerebellum. Nevertheless, the cerebellum apparently participates in the regulation of cognition, emotion, and autonomic functions more than generally appreciated (Buckner, 2013). Cerebellar lesions can result in the cerebellar cognitive affective syndrome (CCAS) comprising a constellation of executive behavioral dysfunction, visual-spatial deficits, linguistic impairments, and behavioral-affective disturbances and a posterior fossa syndrome, presenting with similar symptoms but more often described in children after cerebellar tumor surgery. There is no general consensus with regard to assigning parts of the cerebellum to specific cognitive dysfunction, but it has been proposed that the anterior lobe and parts of medial lobule VI and lobule VIII of the posterior lobe contain the representation of the sensorimotor cerebellum; parts of lobule VI and lobule VII of the posterior lobe comprise the cognitive cerebellum; and the posterior vermis and the fastigial nucleus are the anatomical substrate for the limbic cerebellum (Buckner, 2013; De Smet et al, 2013).
2. The cerebellum has no clinically evident role in consciousness per se.
3. The cerebellum has no clinically evident role in the conscious appreciation of sensation, despite massive sensory connections. Holmes (Holmes, 1939; van Gijn, 2007) repeatedly stated that standard clinical tests did not reveal sensory deficits in cerebellar patients (Pts).
B. What the cerebellum does do
1. The most explicit function of the cerebellum for clinical testing is its role in coordinating willed muscular contractions. To coordinate means to adjust the rate, range, force, and sequence of willed muscular contractions. In so acting, the cerebellum belongs to a distributed sensorimotor network for coordination that includes the cerebral cortex, basal motor nuclei, thalamus, and reticular formation (De Smet et al, 2013).
2. As Hughling’s Jackson (1834–1911) stated, “It will not suffice to speak of coordination as a separate ‘faculty.’ Coordination is the function of the whole and every part of the nervous system.” Not the least are the sensory systems. Visual, tactile, and auditory systems send afferents to the cerebellum, but coordination pre-eminently requires proprioceptive input from joints, muscles, and vestibular system.
3. To make a movement, the brain must know where the body part starts from to orchestrate the sequence, rate, and force of muscular contractions required to get the part from point A to point B. Musculoskeletal proprioceptors and other senses inform the cerebellum about extremity position and movement, joint angles, and the length of and tension on muscles and joints, that is, the state of the muscles and skeletomuscular levers at any given instant to generate an internal model that assists with error detection and correction (Ebner et al, 2011; Manto et al, 2012). From this information, the cerebellum coordinates muscular contractions, through its feedback to the cerebral cortex as well as directly through the reticular formation and via their reticulospinal tracts, to produce steady volitional movements and steady volitional postures (Mottolese et al, 2013; Thach, 2014). Thus, the crucial clinical tests for cerebellar dysfunction expose unsteadiness of volitional movements and unsteadiness of volitionally sustained postures.
4. Now, if you understand the role of the cerebellum, you can answer this question: Could you test a paralyzed or comatose Pt for cerebellar dysfunction?  Yes/
Yes/ No. Explain. (
No. Explain. ( No)
No)
_________
_________
A comatose or paralyzed Pt makes no willed movements and maintains no willed postures.
5. Patients with cerebellar lesions make lower scores on neuropsychological tests and tend to have personality changes, with apathy or disinhibited, socially inappropriate behavior (De Smet et al, 2013). As yet no specific pattern of cognitive or affective dysfunction clearly localizes a lesion to the cerebellum, as do the standard motor tests. At one time control of movement was believed to be the main function of the cerebellum (“The cerebellar doctrine”) and Purkinje cells the only neuron involved in and the site of motor learning (Galliano and De Zeeuw, 2014). The rich bidirectional communication between widespread cortical areas and limbic system with the cerebellum and further insights into the cytoarchitecture and physiological characteristics of the microcircuits of the cerebellum provides further support for its major role in motor activity and some “explanations” for the observed role it plays in language, cognition, and behavior (Cerminara and Apps, 2011; Chadderton et al, 2014; Llinás 2014; Cerminara et al, 2015).
BIBLIOGRAPHY · Cerebellum
Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80:807–815.
De Smet HJ, Paquier P, Verhoeven J, Marien P. The cerebellum: its role in language and related cognitive and affective functions. Brain Lang. 2013;127:334–342.
Cerminara NL, Apps R. Behavioral significance of cerebellar modules. Cerebellum. 2011;10:484–494.
Cerminara NL, Lang EJ, Sillitoe RV, Apps R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nature Rev Neurosci. 2015;16:79–93.
Chadderton P, Schaefer AT, Williams SR, Margrie TW. Sensory-evoked synaptic integration in cerebellar and cerebral cortical neurons. Nature Rev Neurosci. 2014;15:71–83.
Ebner TJ, Hewitt AI, Popa LS. What features of limb movements are encoded in the discharge of cerebellar neurons? Cerebellum. 2011;10:683–693.
Galliano E, De Zeeuw CI. Questioning the cerebellar doctrine. Prog Brain Res. 2014;210:59–77.
Llinás RR. The olivo-cerebellar system: A key to understanding the functional significance of intrinsic oscillatory brain properties. Front Neural Circuits. 2014;7:96.
Manto M, Bower JM, Comfort AB, et al. Consensus Paper: roles of the cerebellum in motor control—the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2012;11:457–487.
Mottolese C, Richard N, Harquel S, et al. Mapping motor representations in the human cerebellum. Brain. 2013;136:330–342.
Thach WT. Does the cerebellum initiate movement? Cerebellum. 2014;13:139–150.
Van Gijn J. From the Archives. Symptomatology of cerebellar tumours; a study of forty cases. by T. Grainger Stewart (Registrar) and Gordon Holmes (Resident Medical Officer, National Hospital, Queen Square, London). Brain 1904;27:522–591. With The symptoms of acute cerebellar injuries due to gunshot injuries. By Gordon Holmes. Brain 1917;40:461–535. With the cerebellum of man. By Gordon Holmes. Brain 1939;62:1–30. Brain 2007;130:4–7.
II. ANATOMY OF THE CEREBELLUM
A. The three cerebellar lobes
1. Larsell divided the cerebellum transversely into three lobes and longitudinally into three parts, one midline vermis uniting two hemispheres (Fig. 8-1).
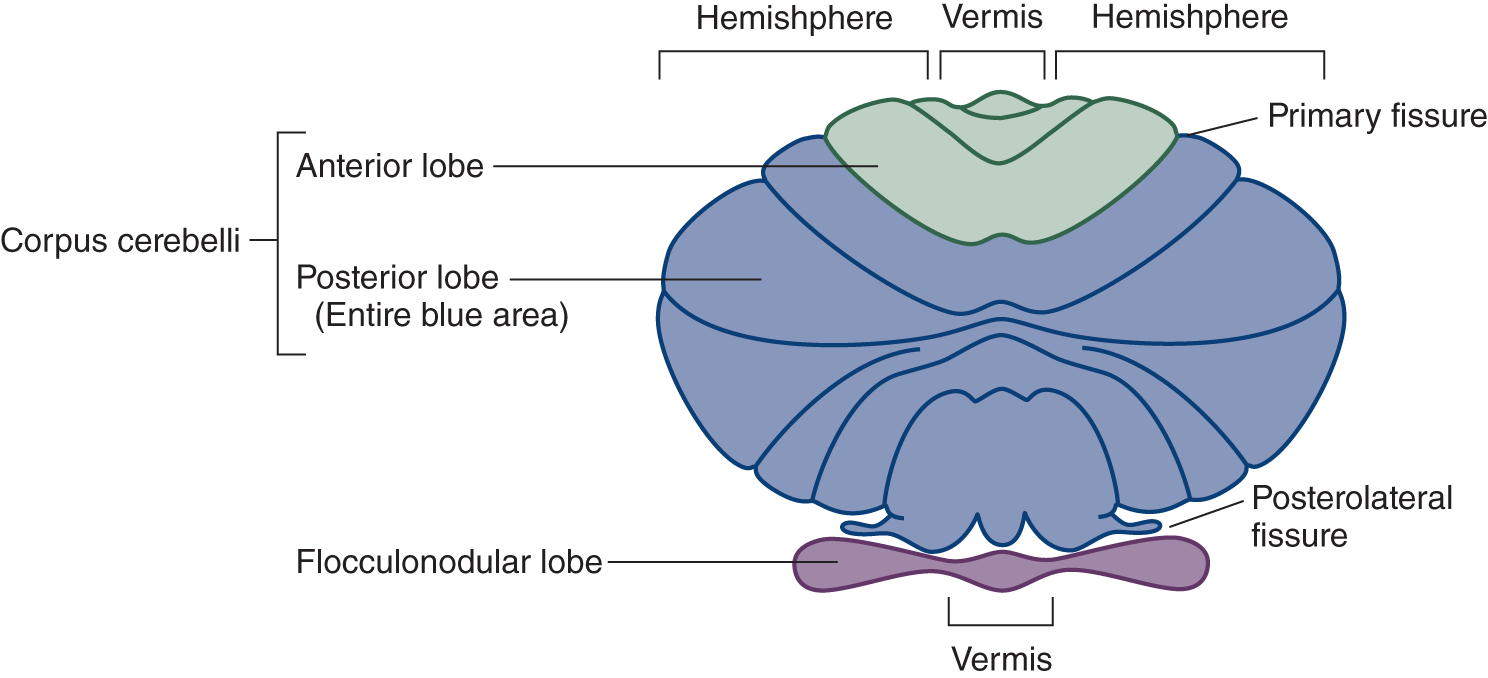
FIGURE 8-1. Schematic dorsal view of the cerebellum (Larsell nomenclature). In reality, the flocculonodular lobe is rolled under, out of sight, when the cerebellum is viewed dorsally (Fig. 8-2).
2. In contrast to the schematic depiction of lobes shown in Fig. 8-1, Fig. 8-2 shows the flocculonodular lobe rolled under in its true position. Label the lobes.
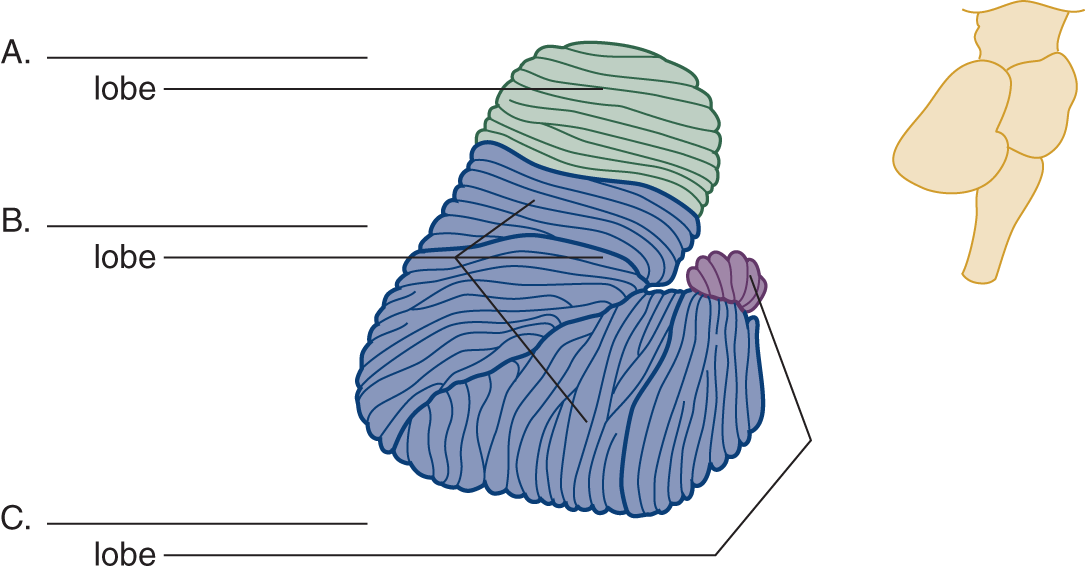
FIGURE 8-2. Right lateral view of the cerebellum. The inset at the right shows the relation of the cerebellum to the brainstem. Label the lobes A to C.
B. Cerebellar phylogenesis
1. Phylogeny provides the best understanding of the clinical syndromes of the cerebellum. The cerebellum evolved out of the vestibular nuclei. Its vestibular origin condemns it to straddle forever the vestibular nerves and nuclei, at the pontomedullary junction, and to retain forever its connections with the vestibular system—the law of retained original innervation.
2. Vestibular proprioceptors provide information about the movement of the head and its position in relation to the pull of gravity. Having no limbs, primitive animals require only a small nubbin of cerebellum to coordinate the axial muscles that position the eyes, head, and trunk. This nubbin is the flocculonodular lobe (as its major input is derived from the vestibular system it may be appropriate to use another frequently encountered name, vestibulocerebellum, or the one that has a teleological inspiration, archicerebellum).
3. All higher animals retain the vestibulocerebellar connections and their axial functions, but the budding limbs impress new roles on the cerebellum; it must now coordinate axial (trunk) and appendicular (limb) muscles. The emergence of the vertical bipedal from the quadrupedal posture places particular demands on gait coordination. The second portion of the cerebellum evolves to receive most of the proprioceptive input from the limbs and trunk, the anterior lobe (the term spinocerebellum is often used, but a misnomer as it implies that these are the only sources of input and again the term paleocerebellum is encountered implying an evolutionary origin).
4. The third cerebellar lobe expands in equal measure with the cerebrum, motor cortex, pyramidal tract, pontine basis, and inferior olivary nuclei. The corticopontocerebellar and olivocerebellar pathways send the major inputs to this newest part, the posterior lobe (the term pontocerebellum is occasionally used to indicate its input from the pons, but not entirely and hence confusing, and finally as a sign of its relative evolutionary “newness,” neocerebellum). The inferior olivary nuclei project topographically to all three cerebellar lobes.
5. To recapitulate: The cerebellum consists of three lobes: the anterior, posterior, and flocculonodular, based on phylogenesis and the major source of afferent connections (Baizer, 2014; Butts et al, 2014; Smaers, 2014). Complete Table 8-1.
TABLE 8-1 • Some Major Afferent Pathways to the Lobes of the Cerebellum
Cerebellar lobe |
Major afferent pathway |
Anterior lobe (spinocerebellum) |
_________ |
Posterior lobe (cerebrocerebellum) |
_________ |
Flocculonodular lobe (vestibulocerebellum) |
_________ |
All lobes |
_________ |
6. Lesions of each of the lobes cause different clinical syndromes. From the clinical findings, the examiner (Ex) can predict the location and often the type of lesion (Table 8-3).
C. The three pairs of cerebellar peduncles and their pathways
1. Three pairs of peduncles anchor the cerebellum to the pons: the superior (rostral), middle, and inferior (caudal). These thick stalks convey only and all afferent and efferent cerebellar connections (Roostaei et al, 2014). Transection of the peduncles allows the cerebellum to fall free from the brainstem (Fig. 8-3).
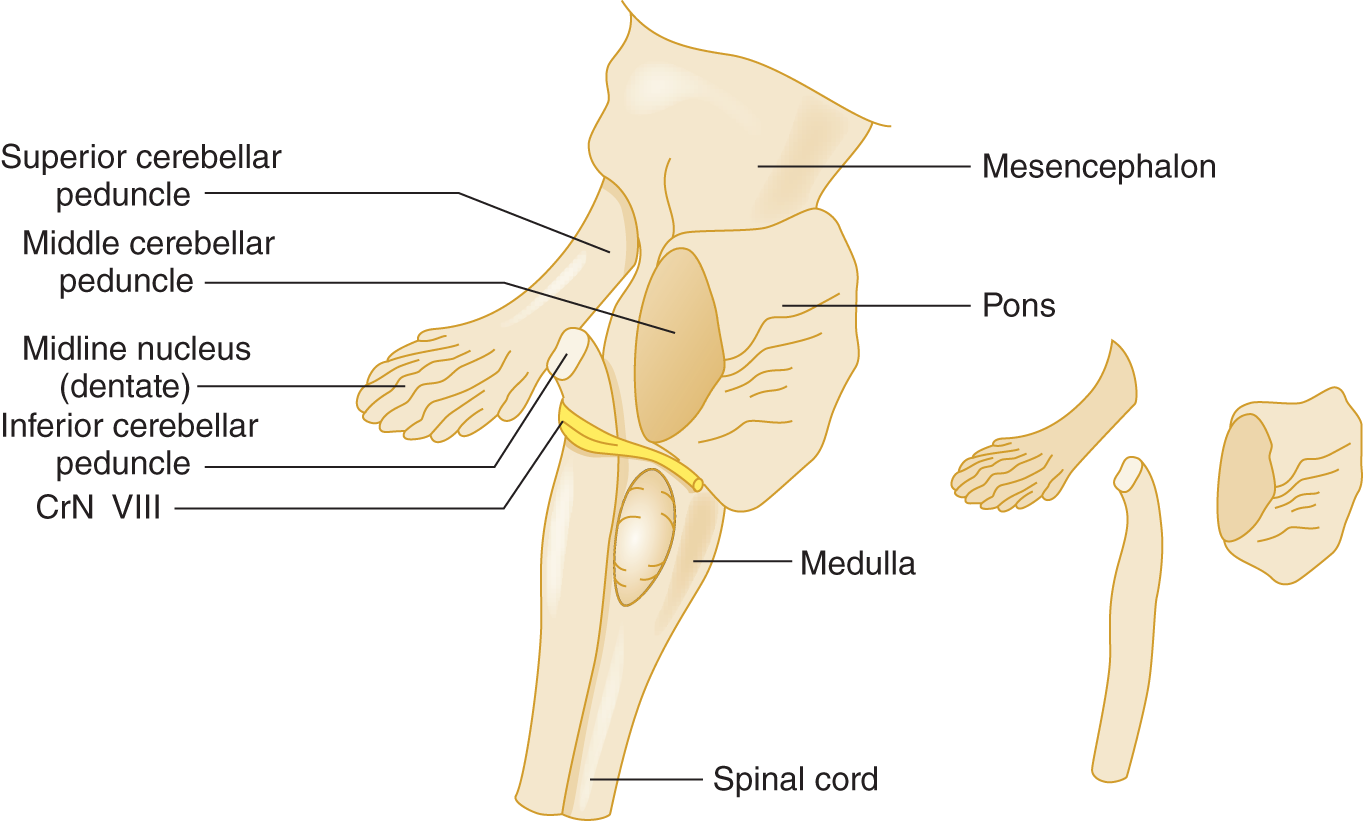
FIGURE 8-3. Right lateral view of the brainstem to show the cerebellar peduncles. The inset at the right is an exploded view of the peduncles.
2. The three pairs of peduncles anchor the cerebellum to only one part of the brainstem, the  mesencephalon/
mesencephalon/ pons/
pons/ medulla. Therefore, all cerebellar afferents and efferents must pass through the pons and through one of the peduncles. (
medulla. Therefore, all cerebellar afferents and efferents must pass through the pons and through one of the peduncles. ( pons)
pons)
3. The middle peduncle, the simplest peduncle in composition, conveys almost exclusively pontocerebellar fibers.
4. The connections of the inferior cerebellar peduncle are predicted by its neighbors: the spinocerebellar tracts, medulla, and vestibular nerve. Thus, the inferior peduncle transmits dorsal spinocerebellar, trigeminocerebellar, and olivocerebellar afferents and interchanges afferent and efferent connections with the medullary reticular formation and the vestibular nuclei.
5. The superior cerebellar peduncle (or brachium conjunctivum) angles forward and ventrally through the pons and into the midbrain (Fig. 8-3). It contains the major efferent cerebellar pathway that aims toward the contralateral red nucleus and thalamus. For the priggish neuroanatomist, we note that the dorsal spinocerebellar tract veers directly into the cerebellum via the inferior peduncle and that the ventral spinocerebellar tract ventures rostrally before entering via the superior peduncle—but the latter fact has no particular clinical value. More importantly, both spinocerebellar tracts end in the anterior lobe, especially in the vermis, making the anterior lobe the “spinocerebellum.” These tracts convey proprioceptive information to the anterior lobe from the muscles and joints of the neck, trunk, and extremities. Subpopulations of neurons that give rise to these tracts are also influenced by spinal cord interneurons of the spinal central pattern generators and descending tracts. Such a system allows proprioceptive and other information that is “expected” by a movement as well as unexpected to be provided to the cerebellum (Stecina et al, 2013).
D. Recapitulation of the cerebellar peduncles
1. In activating muscles for voluntary contractions, the cerebrum communicates with the cerebellum via the corticopontocerebellar pathway, which ends mainly in the _________
2. The corticopontocerebellar pathway runs in the _________
3. The major efferent peduncle is the _________
4. Afferents of the olivary, vestibular, and dorsal spinocerebellar systems and cerebellar efferents run through the _________
5. Recall that the cerebellar lobe that arose out of the vestibular system and retains strong vestibular connections throughout its phylogenetic history is the _________
6. Of the three cranial nerves (CrNs) attached along the pontomedullary sulcus, the most dorsal one, closest to the inferior peduncle, is CrN ________ and the most ventral one, farthest from the peduncle, is CrN _________
E. Circuits of the cerebellum
1. Major sources of afferents to the cerebellum arise from the following:
a. Spinocerebellar system, via inferior and superior peduncles, to the anterior lobe (“paleocerebellum,” “spinocerebellum”).
b. Corticopontocerebellar system, via middle peduncle, mainly to the posterior lobe (“neocerebellum,” “cerebrocerebellum”).
c. Vestibulocerebellar system, via inferior peduncle, mainly to the flocculonodular lobe (“archicerebellum,” “vestibulocerebellum”).
d. Olivocerebellar system, via inferior peduncle, to all cerebellar lobes.
e. The afferent fibers send collaterals to the deep cerebellar nuclei on their way to the cerebellar cortex.
2. Intrinsic cerebellar circuits: All afferent fibers ascend through the cerebellar white matter to influence ultimately the large Purkinje neurons found ubiquitously throughout the cerebellar cortex (Cerminara et al, 2015). The Purkinje axons afford the only way out of the cerebellar cortex. They descend through the cerebellar white matter, and the vast majority synapse on the deep nuclei of the cerebellum, and a small minority synapse directly on the vestibular nuclei. The deep cerebellar nuclei, the largest of which is the dentate nucleus, sit in the roof of the IV ventricle. Learn Fig. 8-4.
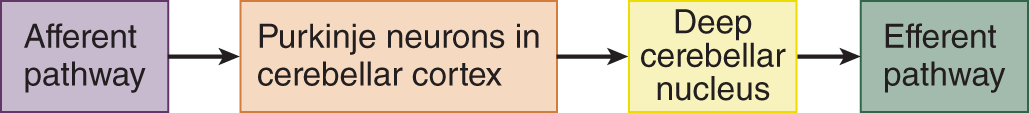
FIGURE 8-4. Diagram showing the flow of impulses through the cerebellum.
3. Extrinsic cerebellar circuits: The cortico-ponto-cerebello-thalamo-pyramidal circuit.
a. Well, yes, instead of inventing such a word, we could just introduce a law: Cerebral hemisphere lesions cause contralateral motor signs, whereas cerebellar hemisphere lesions cause ipsilateral signs, if that is all you want to know. It is more elegant, if more demanding, to learn the actual circuit that underlies the correlation between lesion site and clinical signs (Fig. 8-5). Learning it will cause no harm and has practical value which you can verify at your next opportunity.
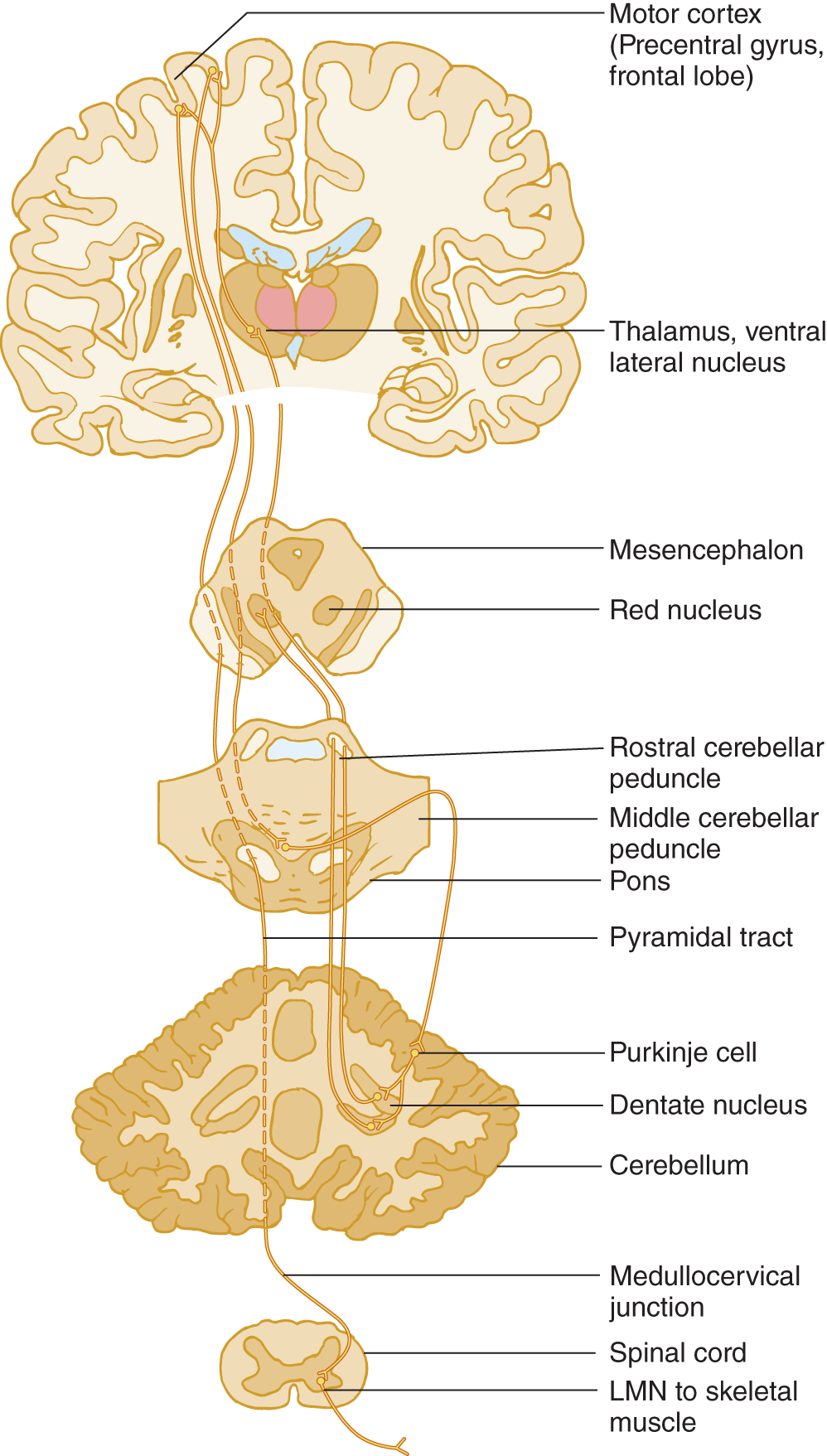
FIGURE 8-5. Diagram of the cerebro-cerebello-cerebral circuit. By this pathway, the cerebellum coordinates volitional movements by feeding back information to the cerebral motor cortex to influence its commands to the lower motoneurons via the pyramidal tract. Start at the motor cortex and trace through the circuit. Notice that the cerebro-cerebello-cerebral circuit double crosses the midline and that the pyramidal crossing brings the influence of one cerebellar hemisphere back to the same side.
b. Master Fig. 8-5 this way:
i. Learn the labeled structures.
ii. Start at the motor cortex in the precentral gyrus of the frontal lobe and trace a motor impulse from the motor cortex down to the cerebellar cortex, back to the motor cortex, and down the pyramidal tract.
iii. Using a colored pencil, draw the circuit on the opposite half of Fig. 8-5. Be sure to include the synapses, marked by Y.
c. By means of the single pyramidal decussation, cerebral hemisphere controls volitional movements on the  ipsilateral/
ipsilateral/ contralateral side of the body. (
contralateral side of the body. ( contralateral)
contralateral)
d. By means of double crossing pathways in conjunction with the pyramidal decussation, one cerebellar hemisphere ultimately coordinates muscular contractions on the  contralateral/
contralateral/ ipsilateral side of the body. (
ipsilateral side of the body. ( ipsilateral)
ipsilateral)
e. Hence, a lesion of one cerebellar hemisphere causes incoordinated muscular contractions on the  ipsilateral/
ipsilateral/ contralateral side. (
contralateral side. ( ipsilateral. Review the law in point 3-a.)
ipsilateral. Review the law in point 3-a.)
III. CLINICAL SIGNS OF CEREBELLAR DYSFUNCTION
A. The keys to detecting cerebellar dysfunction
Four cardinal cerebellar signs consist of ataxia (=dystaxia), tremor (intention tremor and postural), and, especially in acute lesions, hypotonia and asthenia. Taxis means “ordered,” as in taxonomy. Ataxia means “not ordered” or, as applied to the effect of cerebellar lesions, “uncoordinated” contractions of muscles during volitional movements or during volitionally sustained postures. Cerebellar signs derive not from weakness or loss of sensation but from loss of the movement coordination provided by the cerebellum.
Depressants such as alcohol preferentially affect vestibulocerebellar neurons. If you have ever been or seen an inebriated person, you will understand the syndrome immediately. A drunken person cannot coordinate any volitional muscular contractions. Thus, the person sways when standing (when maintaining a volitional posture), reels and missteps when walking, slurs words when talking, and his eyes oscillate when attempting to look at a target. The limbs hang loose and floppy. When a finger approaches a target, such as the nose, it may start to show a tremor as the movement progresses but will then definitely increase as the finger nears the target. If we apply technical terms to these signs, as is the habit of physicians, we can define the cerebellar syndrome as follows:
1. The overall incoordination of intentional movements is ataxia or dystaxia (Klockgether 2008; Pandolfo and Manto, 2013). Sensory ataxia also occurs with peripheral nerve or dorsal column lesions, as in the locomotor ataxia of tabes dorsalis due to syphilis, where proprioceptive input is impaired (Koike et al, 2010; Tong et al, 2013).
2. The tremor of intentionally maintained head or trunk posture or of a limb suspended in front of the body is called postural, positional, or static type of action tremor. (The “action” when holding a volitional posture is the active contraction of the muscles; see Fig. 7-38.) The unsteady oscillations of the head and trunk are also called titubation, occur a few times per second, and while it may be seen in cerebellar dysfunction is not pathognomonic or localizing.
3. The tremor as a limb approaches a target is called intention, end-point, or kinetic tremor. Classic cerebellar tremor occurs uni- or bilaterally depending on the underlying cerebellar disorder and usually has a frequency below 5 Hz.
4. The uncoordinated, slurred speech is called dysarthria, like any neurogenic disturbance of voice articulation.
5. The uncoordinated oscillations of the eyes are called nystagmus.
6. The loose floppy joints and muscles are called hypotonia, more frequently identified in affected children.
7. The person becomes silly, illogical, disinhibited, and socially inappropriate.
8. List the major clinical signs of cerebellar lesions (Hint: Start with the head and eyes and work caudally, visualizing a drunken person).
_________
_________
9. Another common neurogenic tremor, not caused by cerebellar lesions, is the tremor of Parkinson disease. This tremor appears when the part  rests/
rests/ undergoes volitional movement and disappears when the part
undergoes volitional movement and disappears when the part  rests/
rests/ undergoes volitional movement. (
undergoes volitional movement. ( rests;
rests;  undergoes volitional movement)
undergoes volitional movement)
B. The effect of cerebellar lesions on speech
Dysarthria in cerebellar Pts consists of slowness, slurring of words, and scanning speech. In scanning speech, the Pt’s voice varies from a low volume to a high volume as if scanning from peak to peak. The Pt fails to meter and modulate the strength of the muscular contractions that produce the speech sounds, thus accentuating the wrong syllables or words, or the Pt may speak too loudly and garrulously (Ogawa et al, 2010; Urban, 2013). A tremulousness, analogous to postural tremor, also may occur. Cerebellar lesions seem to affect speech less in children, but they may undergo a peculiar period of mutism after surgery for cerebellar neoplasms (Pitsika and Tsitouras, 2013).
C. The effects of cerebellar lesions on eye movement
Cerebellar lesions result in nystagmus, dysmetria of saccades, jerky rather than smooth pursuit, slowness in initiating eye movements, and skew deviation (Kheradmand and Zee, 2011; Eggenberger, 2014; Thurtell, 2014). Cerebellar nystagmus occurs pre-eminently during volitional use of the eyes and thus is gaze evoked; see the nystagmus dendrogram in Fig. 5-7.
To test for saccadic dysmetria, have the Pt look straight ahead and place your index fingers in the temporal fields. Ask the Pt to look first at one finger and then the other and then direct the Pt to look rapidly from one to the other several times and it will be noted that the eyes over or undershoot the target.
D. Clinical tests for dystaxia of station (stance) and gait
1. Symptomatic cerebellar lesions universally impair the gait and stance (the standing posture). Inspect the Pt for swaying when standing, which involves volitional posture, and for ataxia of gait, which involves volitional foot placement. The unsteady stance and reeling gait of the drunken person need no wordy description (lateropulsion is the tendency to move from side to side). To compensate for unsteadiness of stance and gait, the cerebellar Pt assumes a broad-based stance and a broad-based gait, just as a toddler does before gaining coordination, or an elderly Pt does after losing some. The signs witnessed not only consist of the motor and ocular disorders that accompany cerebellar dysfunction but also the safety strategies used by the Pt as well as their inaccurate adjustments undertaken to deal with their loss of balance (Ilg and Timmann, 2013).
2. To challenge the Pt’s coordination and overcome the compensatory broad-base, the Ex asks the Pt to stand with the feet together. Similarly, to expose gait incoordination, use a test known to every policeman: Ask the Pt to step along a straight line, placing the heel of one foot directly in front of the toe of the other, the so-called tandem walking, a sensitive test for gait ataxia. Now stand up and try tandem walking yourself. You will find that balancing on a narrow base when walking takes some ability.
3. To judge broad-based gaits, you must know where the heels fall in relation to the midline when a normal person walks. First, just for fun, guess where the medial margins of the heels fall in relation to the midline sagittal plane:  just on the midline/
just on the midline/ 2.5 cm off/
2.5 cm off/ 3 to 5 cm off/
3 to 5 cm off/ >5 cm off. (
>5 cm off. ( just on the midline)
just on the midline)
4. Unless the person has huge thighs, the medial margin of the heels falls exactly on the line. Verify this the next time you watch someone walk or, just as instructive, note the neat, precise tightrope placement of a dog’s hind feet.
5. Next stretch a string in a straight line or find a straight line on your floor and walk along it with your midline directly above it. Now walk straddling the string by deliberately placing each foot 2 to 3 in. to the side of the midline. Notice that even slight displacement of your heels from the midline will introduce a waddle in your gait. To the original signs of cerebellar dysfunction, we can add a swaying, broad-based stance and gait.
E. Clinical tests for arm dystaxia
1. Postural tremor and tremor of the arms during the finger-to-nose test
a. Ask the Pt to extend the arms straight out in front. Inspect the arms for wavering, indicating incoordination during this volitionally maintained posture, and for frank, rhythmic postural tremor. Having the Pt hold the fingers a little apart in front of the nose, with the arms elevated horizontally, in “the batswing” position, also demonstrates postural instability of the arms or postural tremor (Alusi et al, 2000).
b. After you have inspected the Pt with the arms held straight out, instruct the Pt to place his index finger on the tip of his nose.
i. To enlist the Pt’s best effort, say, “Move your finger in and place the tip of your finger exactly on the tip of your nose. Do not miss!”
ii. Inspect for dystaxia of the movement in progress or frank tremor that increases as the finger approaches the nose (intention type of kinetic tremor), and whether the Pt fails to precisely place the tip of the finger to the tip of the nose (dysmetria).
iii. Have the Pt perform this test (and later the heel-to-shin test) three times. If uncertain of the result, have the Pt alternately touch his nose, your finger, and his nose several times.
c. A tremor of the outstretched hands is called a _________
d. A tremor that increases as the finger approaches the nose or is reaching a target is called a _________
2. Cerebellar signs on one side implicate a lesion of the  ipsilateral/
ipsilateral/ contralateral cerebellar hemisphere because of
contralateral cerebellar hemisphere because of  one/
one/ two/
two/ three decussations. (
three decussations. ( ipsilateral;
ipsilateral;  three (Fig. 8-5))
three (Fig. 8-5))
3. Dysmetria: The dystaxic Pt, in seeking a specific endpoint, such as the nose on the finger-to-nose test, frequently undershoots or overshoots the target because of failure to control, or meter, the muscular contractions that set the distance.
4. The rapid alternating-movements tests for dystaxia and dysmetria (dysdiadokokinesia = dysdiadochokinesia)
a. The technical term for dystaxia-dysmetria of rapid alternating, movements, dysdiadochokinesia, is a lovely dactylic trimeter. This is the fórest priméval: dys di á dó ko ki né si a. This term describes nothing qualitatively different. It means only incoordination of muscular contractions during rapid alternating movements.
b. The Pt holds out the hands and pronates and supinates them as rapidly as possible. Test the hands separately and together. The dystaxic hand overshoots one time, undershoots the next, and is slower than normal (Fig. 8-6).
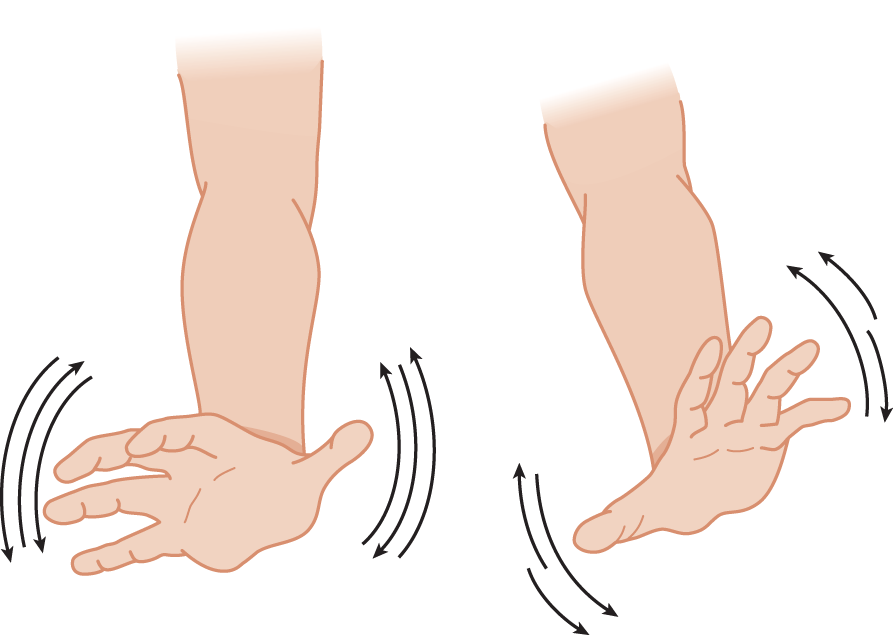
FIGURE 8-6. Pronation and supination test for dystaxia and dysmetria of the hands. Notice the even excursions of the normal right hand and the uneven excursions of the ataxic left hand.
c. A better method is the thigh-patting test. Test each hand separately and together. First demonstrate the action to the Pt by lightly slapping your own thigh, alternating first by slapping the palm and then the back of the hand, as rapidly and rhythmically as possible. Be sure to make an audible sound with each pat. Instruct the Pt to make actions that sound exactly like yours. The Ex sees and hears the slow rate and dysrhythmia of the ataxic hand (Fig. 8-7). You can detect the irregular rhythm of the alternating movements much better by sound than by sight.
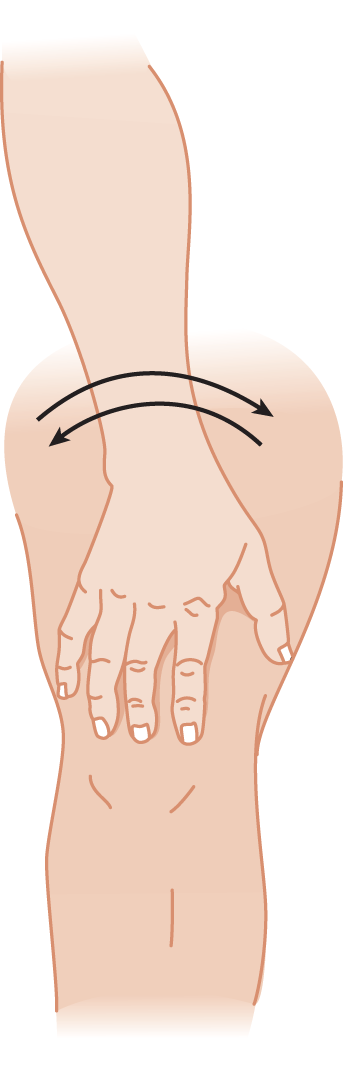
FIGURE 8-7. Thigh-patting test for dystaxia and dysmetria. The cerebellar patient slaps irregularly and turns the hand too much or too little in alternately slapping the front and the back of his hand on his thigh.
d. Here is a challenge to your mastery of this test: If you listen carefully, you will hear a slight but definite difference in the pitch of the sound from slapping the right or left thigh. We do not know the explanation, but the difference is real.
5. The finger-tapping test: Listen for dysrhythmia and slowness (Strauss, 2006). See Fig. 7-42.
F. Overshooting and checking tests of the arms
1. The cerebellar Pt has difficulty in maintaining a posture or position against a sudden, unexpected displacement. Have the Pt stand with eyes closed and arms outstretched.
2. Tell the Pt, “I am going to tap your arms. Hold them still. Do not let me budge them.” The Ex delivers to the back of the Pt’s wrist a quick push, strong enough to displace the arm. The normal subject’s arm returns quickly to its initial position. The cerebellar Pt’s arm oscillates back and forth: It overshoots several times (Fig. 8-8).
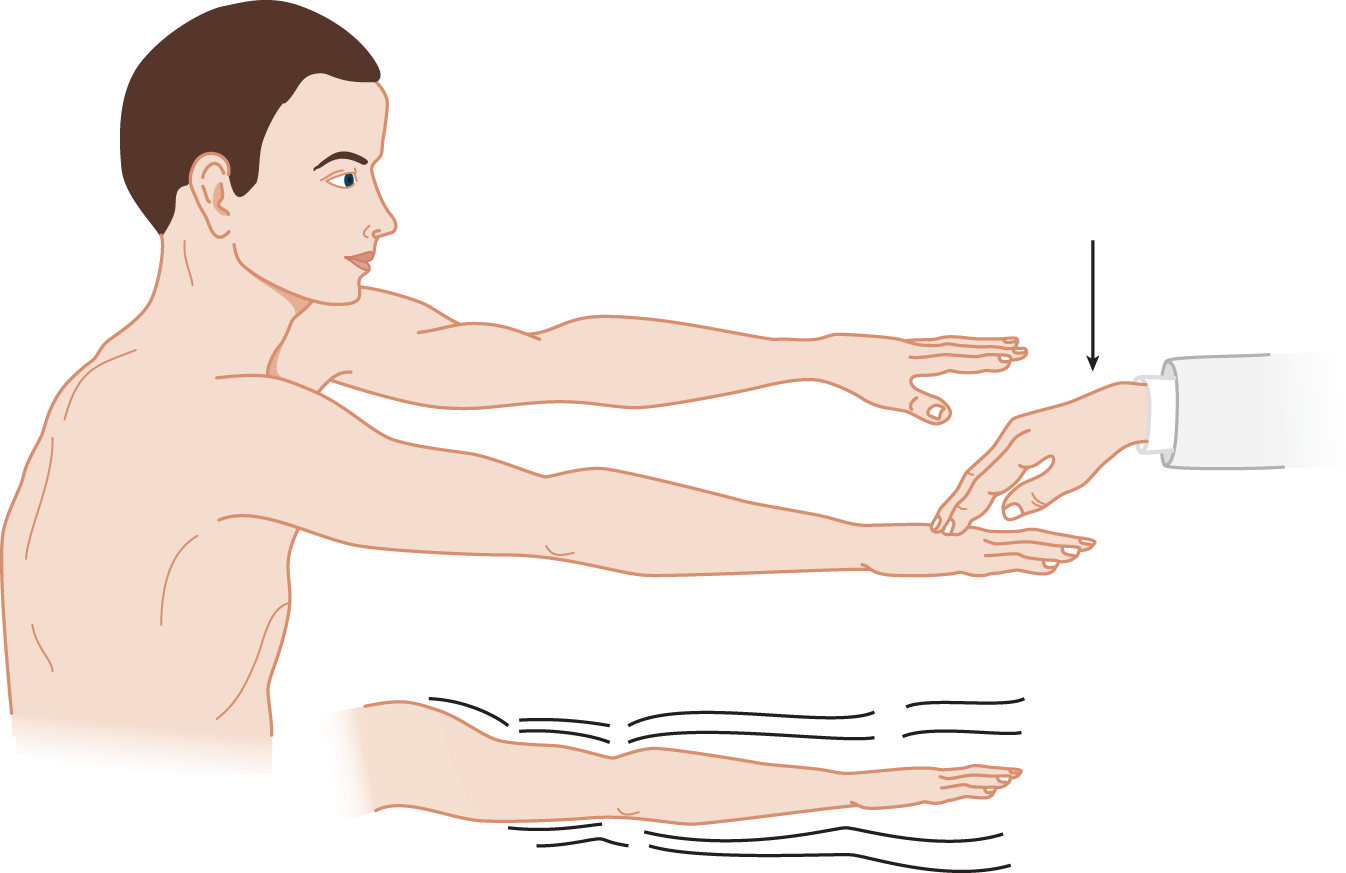
FIGURE 8-8. Wrist-tapping test for abnormal overshooting oscillation after sudden displacement of a part that is maintaining a volitional posture. The thin arrow shows the direction of the examiner’s push, which displaces the part.
3. Pts with cerebellar dysfunction can lack the normal rebound response.
a. Have the Pt holds the arms extended out front.
b. Grasp the wrists and ask the Pt to try to hold them in place as you pull down on them. Suddenly release your grip. The normal arm will fly upward a small distance, check and rebound, and quickly resume the resting position. The cerebellar Pt specifically overshoots by flying upward a greater distance and then overshoots the up and down position several times.
c. Angel (1977) pointed out that neurologists have erroneously called the overshooting phenomenon the “rebound sign of Holmes” (or Stewart-Holmes symptom; Pearce, 2004), when in reality the abnormality is failure to check before rebounding. Spastic limbs also rebound excessively.
4. The arm-pulling test also demonstrates overshooting
a. The Ex pulls hard against the Pt’s flexed arm. When the Ex suddenly releases the Pt’s arm, the cerebellar Pt fails to check the arm’s flight (Fig. 8-9).
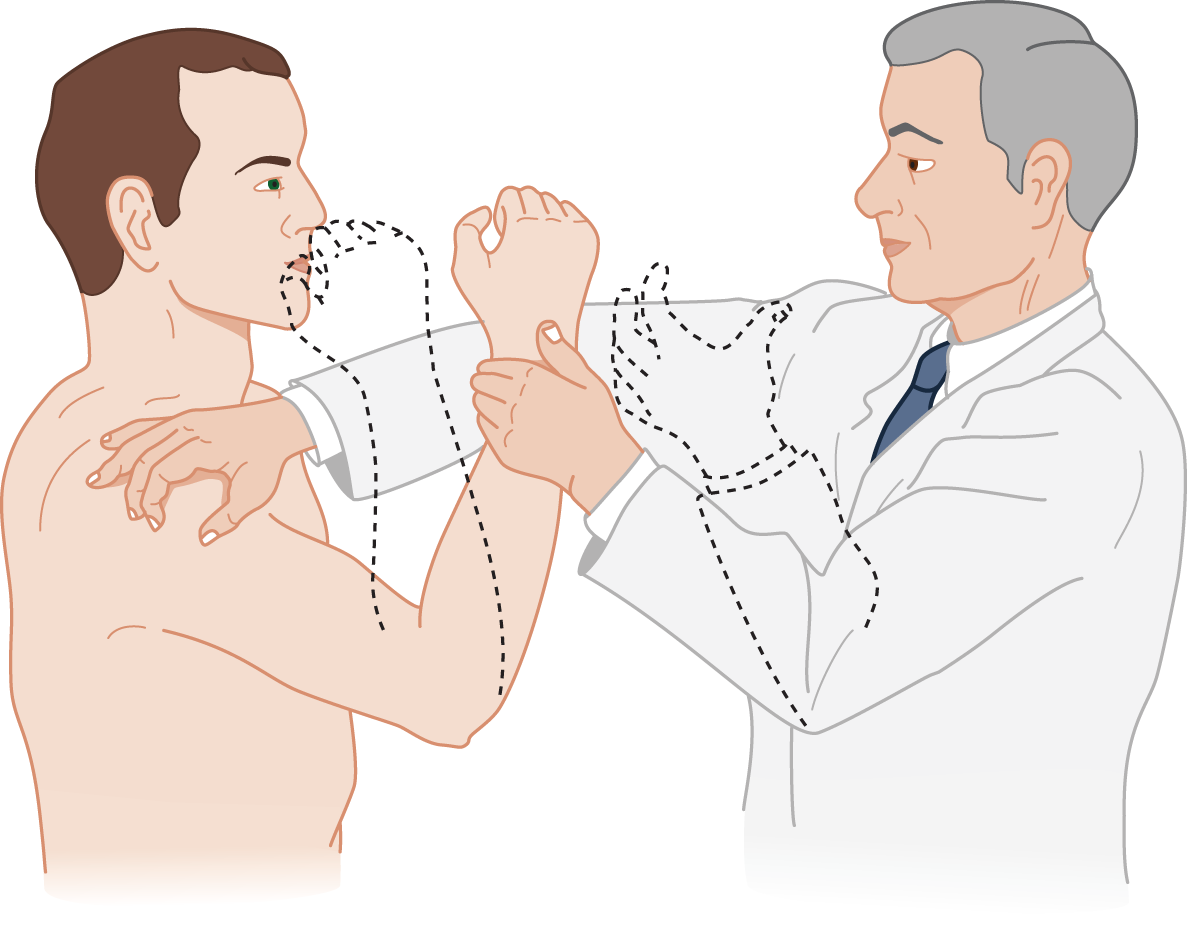
FIGURE 8-9. The arm-pulling test for overshooting. It tests how well the cerebellum functions to check movement and to maintain a given posture after a sudden release of tension on a muscle that is voluntarily contracting.
b. Precaution: Notice in Fig. 8-9 how the Ex places an arm to protect the Pt’s face in case the Pt’s arm fails to check and overshoots.
G. Decomposition of movement
1. In decomposition of movement, the Pt performs a movement as though it were decomposed “by the numbers.” The Pt moves the arm from his side in two stages to touch his nose:
a. Movement 1 consists of lifting the arm to the level of the nose.
b. Movement 2 then consists of bringing the fingertip to the nose; the two movements produce a long, inefficient trajectory.
2. The normal person performs both movements simultaneously to produce the optimal and most efficient movement trajectory. Gordon Holmes (1876–1965) summarized decomposition of movement among other cerebellar tests in classic articles that you might enjoy reading (1939). You will gain profound insight into the mind of a master clinician at work.
H. Clinical tests for leg dystaxia: the heel-to-shin and heel-tapping tests
1. The heel-to-shin test for dystaxia supplements gait testing. Have the Pt supine (or sitting). Instruct the Pt to place one heel precisely on the opposite knee. Have the Pt hold the heel to the knee for a few seconds and observe for a positional tremor. Then direct the Pt to run the heel in a straight line precisely down the shin. Again emphasize the importance of accuracy in the heel placement and movement.
2. For the heel-tapping test, ask the Pt to place one heel over the other shin and to tap the shin with the heel as rapidly as possible on one spot. The cerebellar Pt misses the spot (dysmetria) and taps dysrhythmically (dysdiadochokinesia).
3. Try these tests yourself. Again, such simple tasks offer a considerable challenge. Does your leg waver at all as you do the tests?
I. Clinical tests for hypotonia
1. Muscle tone is operationally defined as _________
2. Passive movement discloses the increased range of joint excursions.
3. Inspection for hypotonia
a. At rest, the hypotonic Pt assumes floppy postures and joint positions uncomfortable for a normal subject—rag-doll or dumped-in-a-heap postures. In a normal person, muscle tone helps to limit joint excursions.
b. When walking, the hypotonic Pt presents a floppy, sagging, loose-jointed appearance. The arms fail to swing properly, the knees may bend backward slightly (genu recurvatum), and the head and trunk bob—a rag-doll gait, as seen in drunkenness.
4. Pendular or hypotonic muscle stretch reflexes (MSRs) in cerebellar Pts: The MSRs, once elicited in the cerebellar Pt, fail to check normally, as best seen with the quadriceps femoris reflex. The Pt sits with the legs swinging freely over a table edge. After the quadriceps MSR is elicited, the leg normally stops swinging after one or two excursions. The cerebellar Pt’s leg swings to-and-fro several times, like a pendulum, without the normal checking of the excursions by muscle tone.
5. Describe how to detect hypotonia in the cerebellar Pt._________
J. Effect of cerebellar lesions on strength and endurance
The cerebellar Pt may experience mild asthenia, that is, weakness, fatigability, and a reluctance to move. Formal strength testing by dynamometry does show some decrease in the maximum force of contraction, particularly in the acute hypotonic phase (Pamdolfo and Manto, 2013). The feedback pathway that connects the cerebellar cortex with the cerebral motor cortex (Fig. 8-5) might explain the asthenia. Destruction of the pathway might alter the output of the cerebral motor cortex.
K. Summary of clinical tests for cerebellar dysfunction
1. Review Fig. 8-10, which summarizes the motor tests for cerebellar dysfunction. Act out each test in the dendrogram to ensure that you know how to do it.
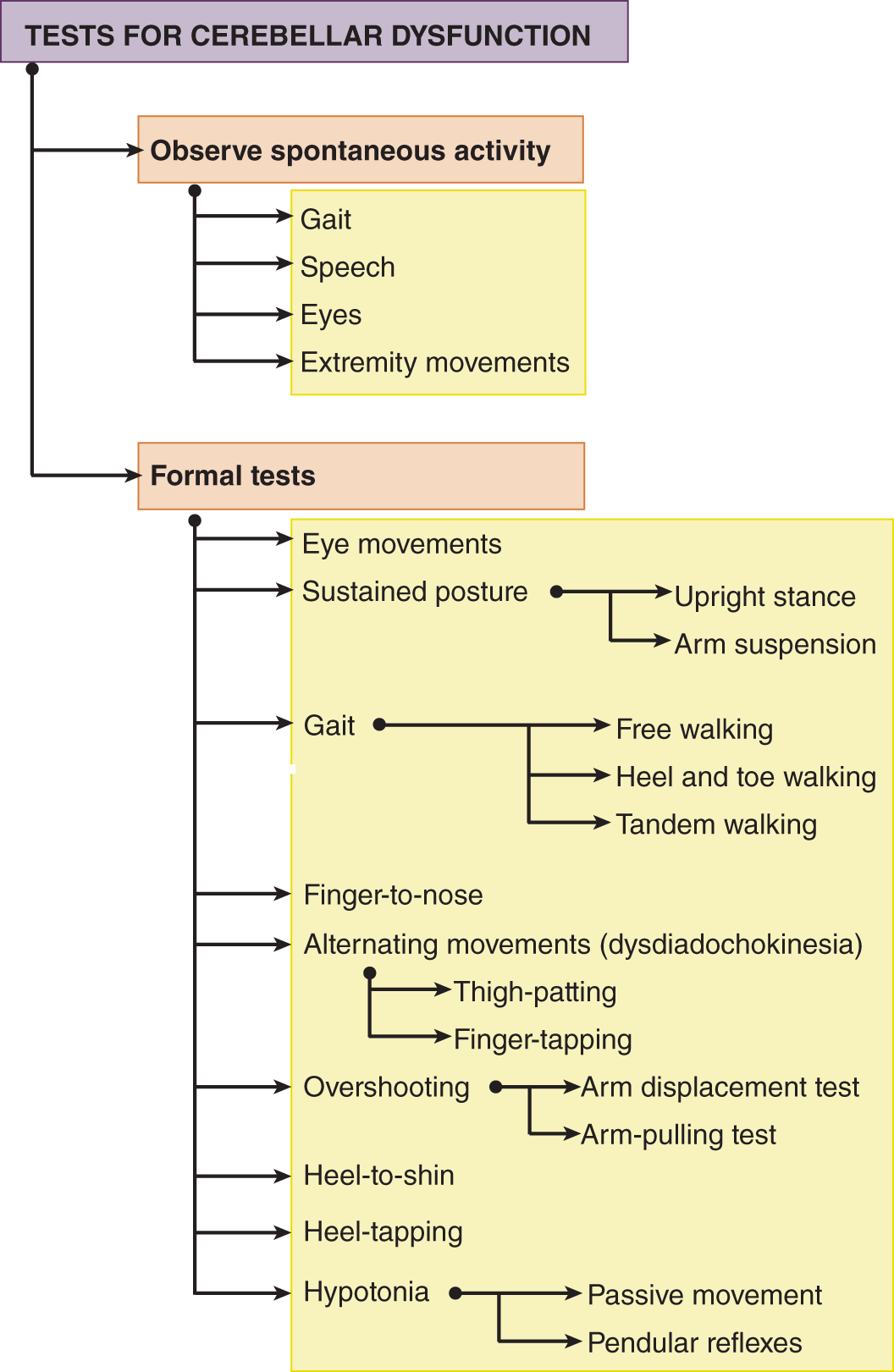
FIGURE 8-10. Dendrogram of motor tests for cerebellar dysfunction.
2. A quantitative battery standardizes the clinical tests for cerebellar dysfunction (Morton et al, 2010).
L. Review of circuitry for localization of cerebellar syndromes
Draw the cerebro-ponto-cerebello-dentato-thalamo-cortico-spinal circuit in Fig. 8-11. Compare your drawing with Fig. 8-5.
FIGURE 8-11. Blank for drawing the cerebro-cerebello-pyramidal pathway.
IV. ANALYSIS OF PATIENTS AND THE FOUR CEREBELLAR SYNDROMES
A. Patient 1
1. Medical history: This 62-year-old woman awakened and, on arising, fell to the left. She became dizzy, vomited, and struggled back into bed. When she called to her husband, he noticed slurred speech. Hypertensive for many years, she had suffered a myocardial infarct at the age of 60.
2. Physical findings: The Pt was conscious, cooperative, and intact mentally. She had mild dysarthria. She had a bidirectional nystagmus, with the slow movement toward a null point a little to the right of center. The nystagmus increased when looking to the left. She had slight ptosis of the left eyelid and miosis on the left. The corneal reflex was reduced on the left, and she had hypalgesia on the left side of her face. Pain and temperature discrimination were reduced on the right side of the body and in the right extremities, but the right side of the face was spared. The left side of the palate failed to elevate when she said Ahhh. Otherwise, the CrNs functioned normally. She could not walk unless supported. She had somewhat less strength on the left side and definitely less muscle tone. She had severe dystaxia on finger-to-nose and heel-to-knee testing on the left side only. She had left-sided dysdiadochokinesia. Her left arm overshot. Her left quadriceps femoris reflex was pendular. She had flexor plantar responses.
3. Lesion localization in Pt 1: Before continuing the text, try to diagnose the location of the lesion (Grimaldi and Manto, 2012). First review the brainstem cross sections in Figs. 2-15 to 2-18. In seeking a single lesion or at least a single pathologic process, first assemble the clinical data that require explanation (Table 8-2). The left-sided dystaxia and other cerebellar signs implicate a lesion of the  vermis/
vermis/ right cerebellar hemisphere/
right cerebellar hemisphere/ left cerebellar hemisphere. (
left cerebellar hemisphere. ( left cerebellar hemisphere)
left cerebellar hemisphere)
TABLE 8-2 • Localizing Findings in Patient 1 (Collate with Fig. 10-17)
Clinical sign |
Anatomic basis |
Left-side ataxia, hypotonia, and mild asthenia |
Lesion of the left cerebellar hemisph |
Pendular muscle stretch reflexes on the left |
Lesion of the left cerebellar hemisphere |
Bidirectional nystagmus, increased when looking left (Fig. 5-7) |
Lesion of the left cerebellar hemisphere |
Left-side ptosis and miosis (Horner syndrome) |
Interruption of descending sympathetic pathway on left |
Left-side palatal palsy |
Interruption of cranial nerve X intra-axially on the left |
Left-side hemifacial hypalgesia |
Interruption of the descending root of cranial nerve V on the left |
Right-side hypalgesia, sparing face |
Interruption of decussated spinothalamictract on the left |
4. Review the nystagmus dendrograms (Figs. 5-6 and 5-7) and state whether the Pt’s nystagmus helps to localize the lesion and, if so, to where.
_________
5. Noncerebellar findings in Pt 1: Because lesions limited to the cerebellum do not impair sensation or cause CrN palsies, damage at another site must have caused ptosis, miosis (Horner syndrome), reduced left corneal reflex palatal palsy, and right-side sensory loss.
6. Clinicopathologic correlation in Pt 1
a. Magnetic resonance imaging (MRI) showed infarction of the lateral aspect of the medulla on the left and of the left cerebellar hemisphere. The lateral wedge of medullary tissue that was infarcted conveys descending autonomic axons and the descending root of CrN V and ascending spinocerebellar tracts (Figs. 2-15 to 2-18, and especially see Fig. 10-23A).
b. Interruption of the autonomic fibers that descend from the hypothalamus through the medullary tegmentum on their way to the intermediolateral cell column of T1 and T2 accounts for Horner syndrome.
c. Interruption of the ipsilateral descending root of CrN V accounts for the reduced the corneal reflex and hemifacial loss of pain and temperature sensation (Fig. 10-2).
d. Interruption of the crossed, ascending spinothalamic tract that mediates pain and temperature accounts for the loss of pain and temperature on the body and extremities (Figs. 2-28 and 10-23B).
e. The vertigo and vomiting reflect interruption of the vestibular connections with the medullary reticular formation. With any presentation manifested by prominent vertigo, demonstration of sensory loss identifies a brainstem, and not a peripheral origin of the vertigo (Nozaki and Yamada, 2012).
f. To assign the cerebellar and medullary findings to one cause requires knowledge of the arterial supply of the posterior fossa. One artery, the posterior inferior cerebellar artery (PICA), irrigates the lateral medullary wedge and the overlying cerebellum. Because of the Pt’s hypertension, the probable diagnosis is a lateral medullary syndrome (Wallenberg syndrome or PICA syndrome), that is, ischemia or frank infarction in the distribution of PICA resulting from occlusion of the intracranial vertebral artery or its dissection (Kim, 2003; Fukuoka et al, 2012; Balami et al, 2013; Ogawa et al, 2015). Notice how the history, general physical findings, and knowledge of neuroanatomy, blood supply, and probable pathogenesis converge to make the diagnosis.
Medial medullary infarction (Dejerine syndrome) presents with paralysis of the tongue on the side of the lesion and impaired sensation and hemiplegia of the limbs and trunk (excluding the face) on the contralateral side. Opalski syndrome is a variant of the (lower) lateral medullary syndrome and Pts present with ipsilesional hemiparesis (its origin remains unclear and may reflect injury to the ipsilateral corticospinal tract after its decussation or possibly hypotonia from cerebellar involvement), hyperreflexia and Babinski sign, and contralesional hypothermalgesia (Kim, 2003; Herman et al, 2009; Chen et al, 2013). While syndromes abound in medicine, anatomy helps to clarify the “variants” we encounter so, for all of the syndromes mentioned, it is more likely they will be “incomplete” as it reflects the extent of the underlying lesion (Kim, 2003). The anatomy is what we need to learn, and eponyms only serve as a recognition of our history.
g. If we set aside the medullary signs, Pt 1 had the typical cerebellar hemisphere syndrome (essentially the posterior lobe syndrome), consisting of the full panoply of cerebellar signs (Table 8-3) ipsilateral to the lesion, including a particular type of bidirectional nystagmus. Figure 8-12A shows the unilateral distribution of signs in the cerebellar hemisphere syndrome. In Table 8-3, complete columns C and D.
TABLE 8-3 • Four Cerebellar Syndromes That Correlate Lesion Site with the Distribution of Clinical Signs
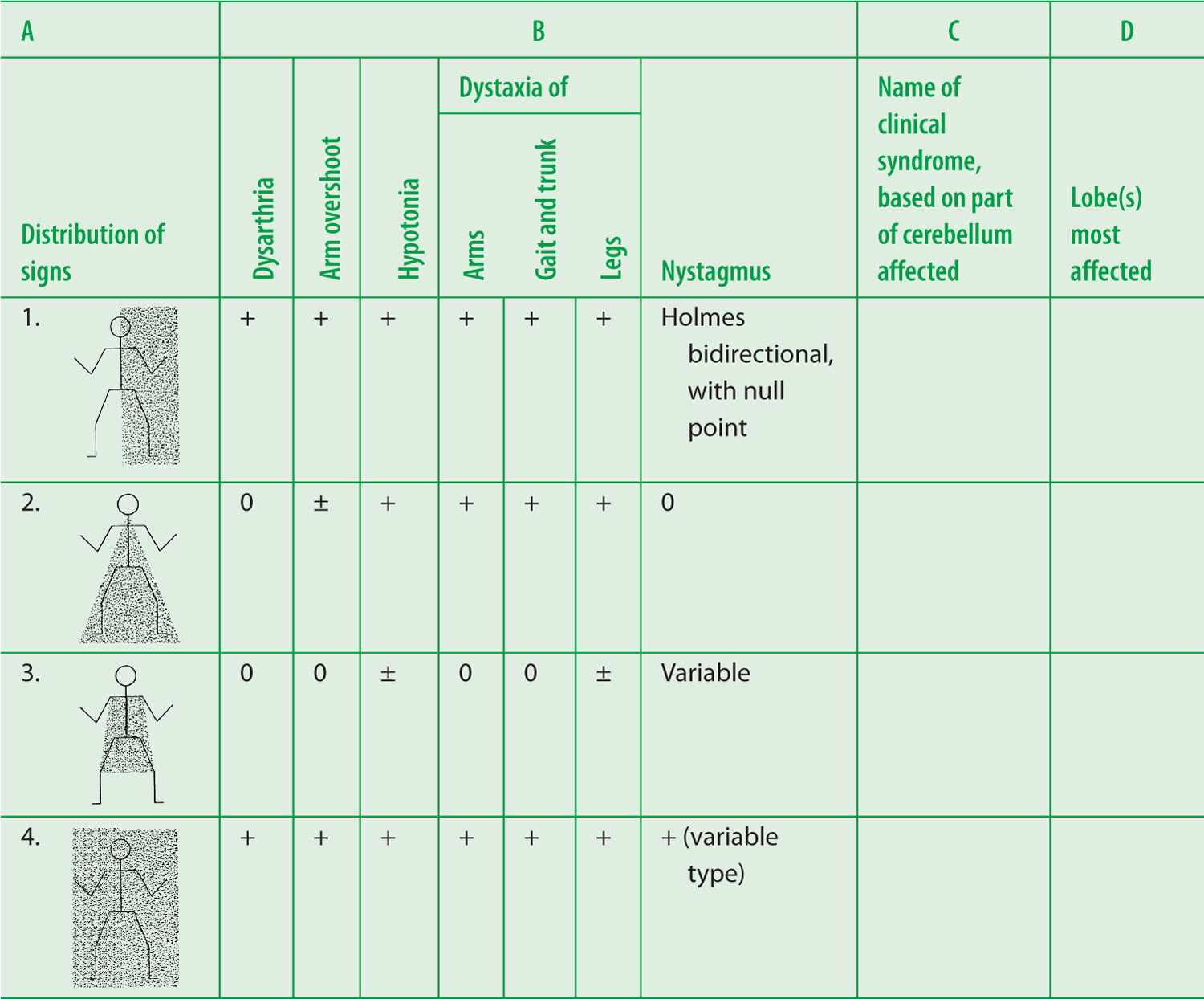
Answers—Table 8-3
C |
D |
1. Hemisphere syndrome |
1. Mainly posterior, variably anterior lobe |
2. Rostral vermis syndrome |
2. Anterior lobe |
3. Caudal vermis syndrome |
3. Flocculonodular and posterior lobe |
4. Pancerebellar syndrome |
4. All lobes |
FIGURE 8-12. Distribution of cerebellar signs. (A) Distribution of cerebellar signs in the cerebellar hemisphere syndrome (left hemisphere in this instance). (B) Distribution of signs in the rostral vermis syndrome. (C) Distribution of signs in the caudal vermis syndrome.
h. The cerebellar hemisphere syndrome follows any acute cerebellar hemisphere lesion: infarct, abscess, trauma, hemorrhage, neoplasm, or demyelinating disease.
i. Summarize the neurologic signs of a lateral medullary (PICA) syndrome. Ipsilaterally:_________
B. Patient 2
1. History: This 48-year-old man had drunk alcohol excessively for 13 years, resulting in numerous hospitalizations for drunkenness, convulsions, and delirium tremens. For 3 years his gait had become increasingly unsteady, to the degree that his family thought he was drunk even when he was sober. He stopped drinking 3 days before the examination.
2. Physical examination: The Pt was malnourished and unkempt but sober. He was somewhat disoriented as to time and date. The CrNs functioned normally. He had no nystagmus or dysarthria. Although generally somewhat tremulous, his finger-to-nose, alternating-movements, and overshoot tests of the arms were fairly normal. He had moderate truncal unsteadiness when sitting or standing. He had an unsteady, broad-based gait and could not tandem walk. His heel-to-shin movements were ataxic. His patellar reflexes were pendular. Notice in Fig. 8-13 that the triceps surae MSRs are recorded as ±. Chronic alcoholics often have some degree of peripheral neuropathy.
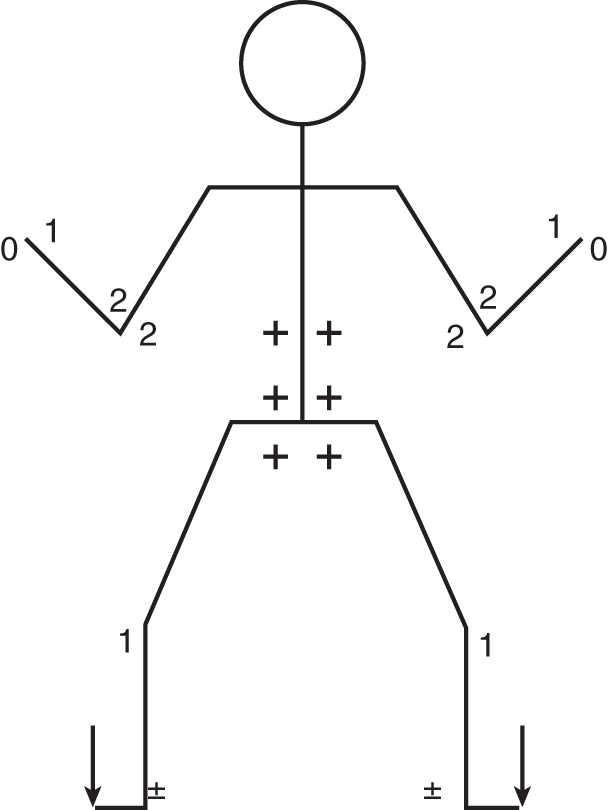
FIGURE 8-13. Reflex stick figure of patient 2.
3. Course of Pt 2: Five days after hospitalization, the Pt began to display severe delirium tremens with hyperthermia and convulsions. He died from irreversible hyperthermia. At autopsy, a sagittal cut through cerebellar vermis showed severe atrophy of all the folia of the rostral part. Microscopically the rostral part of the vermis and adjacent anterior lobe cortex showed severe depletion of neurons.
4. Clinicopathologic correlation in Pt 2
a. Which panel in Fig. 8-12 shows the distribution of cerebellar signs in Pt 2?  A/
A/ B/
B/ C. (
C. ( B)
B)
b. The rostral vermis and adjacent cortex belong to which lobe of the cerebellum?  anterior/
anterior/ posterior/
posterior/ flocculonodular. (
flocculonodular. ( anterior)
anterior)
c. The rostral part of the vermis receives proprioceptive information from the legs and trunk via the _________
d. The clinical picture of classical cerebellar signs in the legs, mild truncal ataxia, minimal or no arm ataxia, and absence of dysarthria or nystagmus predicts atrophy limited to the rostral part of the vermis, as shown by Pt 2 (Victor et al, 1959; Sullivan et al, 2010; Laureno, 2012; Pitel et al, 2012).
e. The gradient of cerebellar signs predicts the lesion location. The signs affect least the  CrN musculature/
CrN musculature/ arms/
arms/ trunk/
trunk/ legs and most the
legs and most the  CrN musculature/
CrN musculature/ arms/
arms/ trunk/
trunk/ legs. (
legs. ( CrN musculature;
CrN musculature;  legs)
legs)
C. Patient 3
1. History: This 6-year-old boy had trouble walking for 3 months, with increasing headaches and vomiting. Formerly very active, he no longer ran or played.
2. Physical examination
a. CrN examination showed questionable papilledema. He had very faint symmetrical jerk nystagmus in the extreme field of gaze to each side, with the quick component in the direction of gaze. When his eyes were returned slightly toward the midline, the nystagmus disappeared. He walked with a somewhat unsteady gait, but he could not tandem walk. At times he veered to the right, at others to the left. With the boy reclining in bed, formal cerebellar testing showed no definite abnormalities on finger-to-nose or heel-to-knee testing or on the rapid-alternating movements and overshoot tests. However, he had an unsteady trunk in any vertical position—thus when sitting, standing, or walking. In other words, he had predominately an axial or truncal ataxia, or titubation. Sensation was normal. Figure 8-14 shows the reflex pattern.
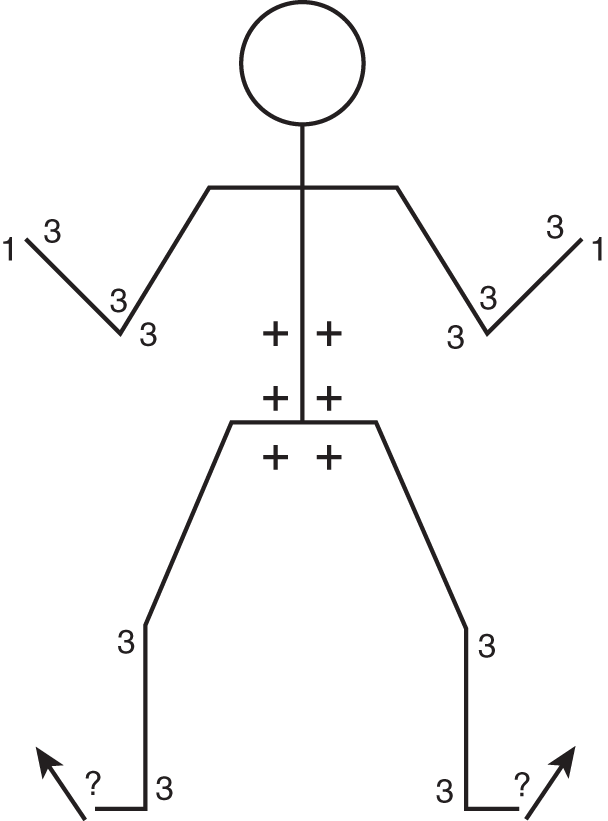
FIGURE 8-14. Reflex stick figure of patient 3.
b. MRI examination showed a posterior fossa tumor occluding the IV ventricle, with enlargement of the aqueduct, third, and lateral ventricles, indicating obstructive hydrocephalus (Figs. 1-25C and 1-25D). Posterior fossa craniotomy disclosed a medulloblastoma involving the flocculonodular lobe and posterior vermis.
3. Clinicopathologic correlation in Pt 3
a. From the nystagmus dendrograms, identify the type of nystagmus (Figs. 5-6 and 5-7): _________
b. Pseudonystagmus usually has no pathologic significance and was not clearly related to the neoplasm. The Ex always has to consider whether a finding is new or pre-existent and not relevant. The equivocal extensor toe signs and vomiting probably reflect compression of the medulla and the increased intracranial pressure. Because the skull sutures had split, the boy had no florid papilledema, as often occurs from an obstructed IV ventricle.
c. The localizing clinical features in this 6-year-old boy were a nearly pure syndrome of postural dysequilibrium or truncal ataxia whenever he had to voluntarily maintain an erect posture, either sitting or standing, and severe impairment of tandem walking. However, formal cerebellar tests with the Pt reclining were very nearly normal. It is tempting to suggest that disruption of vestibulocerebellar and cerebellovestibular connections caused the postural disturbance, that he had a flocculonodular lobe syndrome or caudal vermis syndrome of ataxia of the axial muscles and severe disturbance of tandem walking (the posterior vermal split syndrome was described in children whose posterior vermis was split for removal of fourth ventricular tumors, attributed to disruption of crossing cerebellar fibers that transmitted vestibular and visual inputs that coordinated legs and trunk across the midline, affecting tandem gait while self-paced gait, hopping on one leg and voluntary movements of fingers, arm, and leg were mildly affected (Bastian et al, 1998). Current surgical, often endoscopic approaches to the fourth ventricle now allow this syndrome to be avoided (Di Ivea et al, 2012). But we schematize too much. “The facile gates of hell are too slightly barred” (John Milton, 1608–1674). The large size of the neoplasm and distorted posterior fossa anatomy silence further speculation.
d. Which panel in Fig. 8-12 shows the distribution of cerebellar signs in Pt 3?  A/
A/ B/
B/ C. (
C. ( C)
C)
D. Summary of the four syndromes of the cerebellum
1. Because the nodule of the flocculonodular lobe is in the caudal part of the vermis, we can contrast the caudal vermis syndrome of Pt 3 with the rostral vermis syndrome of Pt 2 and the cerebellar hemisphere syndrome of Pt 1. Then if we recognize a pancerebellar syndrome caused by any agent affecting the entire cerebellum bilaterally, we see that the first step in diagnosing cerebellar lesions is to classify, if possible, the Pt’s deficits into one of the four cerebellar syndromes, as follows:
2. Ataxia predominantly in the legs, sparing the CrN musculature, is the _________
3. Ataxia or dysequilibrium of stance and gait (axial dystaxia) with little or no extremity dystaxia is the _________
4. Cerebellar signs lateralized to one half of the body are the cerebellar _________
5. Tabular summary of the four cerebellar syndromes: Study the clinical characteristics listed in Table 8-3, and your responses for the entries in columns C and D.
6. Run down each column in Table 8-3 to discover an important bonus from displaying the data in tabular form. The only column with a strong plus for each of the four cerebellar syndromes is the gait ataxia column. Gait incoordination is the one ubiquitous cerebellar sign (Bastian, 1998; Ilg and Timmann, 2013) common to all four cerebellar syndromes. The upright posture and gait demand integration of the entire sensory and motor systems by the cerebrum and cerebellum. Hence, gait testing, in particular tandem walking, is the most efficient clinical test for cerebellar dysfunction and many other neurologic deficits.
E. Etiologic implications of the four cerebellar syndromes
1. Earlier I stated that, by identifying the cerebellar syndrome, the Ex could predict the probable lesion. The rostral vermis syndrome can result from alcohol use disorder and nutritional deficiency. The caudal vermis syndrome implies a midline cerebellar neoplasm, usually a medulloblastoma, ependymoma, or astrocytoma. The cerebellar hemisphere syndrome comes from an acute destructive lesion, most likely an infarct, hemorrhage, neoplasm, abscess, or trauma. The pancerebellar syndrome requires a lesion that affects the entire cerebellum, and, therefore, usually results from vitamin deficiencies (eg, vitamin E), toxic or metabolic, demyelinating, immune mediated, paraneoplastic, or hereditary (autosomal recessive or autosomal dominant ataxias) or non-hereditary degenerative ataxias (eg, multiple system atrophy—cerebellar type) (Pandolfo and Manto, 2013). Thus, by identifying the cerebellar syndrome, the Ex defines or delimits the diagnostic probabilities, which in turn leads to the most effective diagnostic workup.
2. The pancerebellar syndrome presents the most difficulty in differential diagnosis because the causative agents span the ocean of possibilities from heredofamilial diseases to the toxic effects of drugs. Intermittent pancerebellar signs suggest a metabolic disorder with intermittent flare-ups, disclosed by measuring amino acids and organic acids, or a demyelinating disorder, such as multiple sclerosis. The fairly common vascular lesions that affect the distribution of only one cerebellar artery appear “relatively easy” to diagnose (Ye et al, 2010; Venti, 2012), but vertigo and headache are the most common symptoms at the onset of cerebellar infarction and Pts may initially (or solely) present with few neurologic deficits (Lee and Kim, 2013; Masuda et al, 2013).
3. A final warning: A mass lesion in the cerebellum or posterior fossa is always a danger. It may cause the posterior fossa contents to herniate upward through the tentorial notch or downward through the foramen magnum or directly compress the medullary respiratory center (Chapter 13).
V. SUMMARY OF THE CLINICAL EXAMINATION FOR CEREBELLAR DYSFUNCTION
Rehearsal time again! Can you give the commands, make the observations, and do the tests for cerebellar dysfunction? Work through Fig. 8-10. If you have followed the Standard Neurologic Examination outline, you have already tested muscle tone and elicited the MSRs, which would have disclosed pendular reflexes and hypotonia.
BIBLIOGRAPHY · Cerebellar Dysfunction
Alusi SH, Worthington J, Glickman S, et al. Evaluation of three different ways of assessing tremor in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;68:756–760.
Angel RW. The rebound phenomenon of Gordon Holmes. Arch Neurol. 1977;34:250.
Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nature Rev Neurosci. 2009;10:670–681.
Baizer JS. Unique features of the human brainstem and cerebellum. Front Hum Neurosci. 2014;8:202. doi:10.3389/fnhum. 2014. 00202
Balami JS, Chen RL, Buchan AM. Stroke syndromes and clinical management. Q J Med. 2013; 106:607–615.
Bastian AJ, Mink JW, Kaufman BA, et al. Posterior vermal split syndrome. Ann Neurol. 1998;44:601–610.
Butts T, Green MJ, Wingate RJT. Development of the cerebellum: simple steps to make a ‘little brain’. Development. 2014;141:4031–4041.
Cerminara NL, Lang EJ, Sillitoe RV, Apps R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat Rev Neurosci. 2015;16:79–93.
Di leva A, Komatsu M, Komatsu F, Tschabitscher M. Endoscopic telovelar approach to the fourth ventricle: anatomic study. Neurosurgical Review. 2012;35(3):341–349.
Eggenberger ER. Supranuclear eye movement abnormalities. Continuum (Minneap Minn). 2014;20:981–992.
Fukuoka T, Takeda H, Dembo T, et al. Clinical review of 37 patients with medullary infarction. J Stroke Cerebrovas Dis. 2012;21:594–599.
Glickstein M, Voogd J. Lodewijk Bolk and the comparative anatomy of the cerebellum. Trends Neurosci. 1995;18:206–210.
Grimaldi G, Manto M. Topography of cerebellar deficits in humans. Cerebellum. 2012;11:336–351.
Hermanna DM, Jung HH, Bassetti CL, Lateral medullary infarct with alternating and dissociated sensorimotor deficits: Opalski syndrome revisited. Eur J Neurosci. 2009;16:e72–e74.
Holmes G. The cerebellum of man. Brain. 1939;62:1–30.
Ilg W, Timmann D. Gait ataxia—Specific cerebellar influences and their rehabilitation. Movt Disord. 2013;28:1566–1575.
Karatekin C, Lazareff JA, Asarnow RF. Relevance of the cerebellar hemispheres for executive functions. Pediatr Neurol. 2000;22:106–112.
Kheradmand A, Zee DS. Cerebellum and ocular motor control. Front Neur. 2011;2:53.
Kim JS. Pure lateral medullary infarction: clinical–radiological correlation of 130 acute, consecutive patients. Brain. 2003;126:1864–1872.
Klockgether T. Ataxia, In: Hallet M and Poewe W, eds. Therapeutics of Parkinson’s Disease and Other Movement Disorders, Wiley-Blackwell; 2008, Chap. 27, 407–415.
Koike H, Atsuta N, Adachi H, et al. Clinicopathological features of acute autonomic and sensory neuropathy. Brain. 2010;133:2881–2896.
Larsell O, Jansen J. The Comparative Anatomy and Histology of the Cerebellum. The Human Cerebellum, Cerebellar Connections, and Cerebellar Cortex. Minneapolis: University of Minnesota Press; 1972.
Laureno R. Nutritional cerebellar degeneration, with comments on its relationship to Wernicke disease and alcoholism. Handb Clin Neurol. 2012;103:175–187.
Lee H, Kim H. Nystagmus in SCA territory cerebellar infarction: pattern and a possible mechanism. J Neurol Neurosurg Psychiatry. 2013;84:446–451.
Masuda Y, Tei H, Shimizu S, Uchiyama S. Factors associated with the misdiagnosis of cerebellar infarction. J Stroke Cerebrovas Dis. 2013;22:1125–1130.
Morton SM, Tseng Y, Zackowski KM, et al. Longitudinal tracking of gait and balance impairments in cerebellar disease. Mov Disord. 2010;25:1944–1952.
Nozaki A, Yamada M. An easy diagnostic tool distinguishing lateral medullary infarction from peripheral vertigo. Clin Neurol Neurosurg. 2012;114:68–69.
Ogawa K, Yoshihashi H, Suzuki Y, et al. Clinical study of the responsible lesion for dysarthria in the cerebellum. Inter Med. 2010;49:861–864.
Ogawa K, Suzuki Y, Oishi M, Kamei S. Study of 46 patients with lateral medullary infarction. J Stroke Cerebrovasc Dis. 2015; 5:1065–1074.
Pandolfo M, Manto M. Cerebellar and afferent ataxias. Continuum (Minneap Minn). 2013;19:1312–1343.
Pearce JMS. Historical note: Sir Gordon Holmes (1876-1965). J Neurol Neurosurg Psychiatry. 2004;75:1502–1503.
Pitsika M, Tsitouras V. Cerebellar mutism: a review. J Neurosurg Pediatrics. 2013;12:604–614.
Roostaei T, Nazeri A, Sahraian MA, Minager A. The human cerebellum: a review of physiologic anatomy. Neurol Clin. 2014;32:859–869.
Smaers JB. Modeling the evolution of the cerebellum: from macroevolution to function. Prog Brain Res. 2014;210:193–216.
Stecina K, Fedirchuk B, Hultborn H. Information to cerebellum on spinal networks mediated by the dorsal spinocerebellar tract. J Physiol. 2013;591:5433–5443.
Stolze H, Klebe S, Petersen G, et al. Typical features of cerebellar ataxic gait. J Neurol Neurosurg Psychiatry. 2002;73:310–312.
Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844.
Strauss E, Sherman, EMS, Spreen, O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. Oxford University Press; 2006.
Sullivan EV, Rose J, Pfefferbaum A. Physiological and focal cerebellar substrates of abnormal postural sway and tremor in alcoholic women. Biol Psychiatry. 2010;67:44–51.
Thurtell MJ. Diagnostic approach to abnormal spontaneous eye movements. Continuum (Minneap Minn). 2014;20:993–1007.
Tong M, Lin L, Zhang H, et al. Spectrum and characterization of movement disorders secondary to neurosyphilis. Parkinsonism Rel Disord. 2013;19:441–445.
Urban PP. Speech motor deficits in cerebellar infarctions. Brain Lang. 2013;127:321–326.
Venti M. Cerebellar infarcts and hemorrhages. Front Neurol Neurosci. 2012;30:171–175.
Victor M, Adams R, Mancall E. A restricted form of cerebellar degeneration occurring in alcoholic patients. AMA Arch Neurol. 1959;1:579–688.
Ye BS, Kim YD, Nam HS, et al. Clinical manifestations of cerebellar infarction according to specific lobular involvement. Cerebellum. 2010;9:571–579.
VI. AN ESSAY ON TESTING STATION (STANDING) AND GAIT (WALKING)
A. Importance of the station and gait examination
If we had just one chance to make the diagnosis, we would choose the most important single part of the neurologic examination: watching the Pt rise, stand, and walk. First notice how the Pt rises and the steadiness of the vertical posture. Then ask the Pt to walk freely back and forth. As the Pt walks, look for irregular strides, lack of a heel-to-toe foot action, unsteadiness, a wide-based gait, an overplay of involuntary movements, and lack of or excessive arm swinging. Notice whether the Pt turns by stepping around freely or rotates on the spot, en bloc, with tiny steps (see Parkinson gait and its marche à petit pas, later). Next, test triceps surae strength and balance by having the Pt walk on the balls of the feet and then on the heels. Next request tandem walking (heel-to-toe down a straight line). Finally, request a deep knee-bend. Ask a child to run and hop. Throughout, note how well the Pt comprehends and executes the commands. Pts with an intellectual developmental disorder, dementia, and mental disorder and those who are oppositional defiant disorder require constant coaxing.
If the Pt rises, stands, and walks completely normally, then, in all probability, the Pt’s motor system is completely intact. If the Pt’s motor system is completely intact, then, in all probability, the sensory system is completely intact. If the Pt follows all commands promptly and well, with no confusion or hesitancy, then in all probability the Pt’s mental state and sensorium are intact. With motor, sensory, and mental functions intact, then, in all probability, the Pt’s nervous system is intact. Of course, you must still complete the entire neurologic examination to confirm these initial inferences. A normal gait requires the integrity of vast circuits of the peripheral and central nervous systems: circuits that underlie the willing of movements and the antigravity, supporting, and righting reflexes; circuits that coordinate the rate, regularity, and force of the muscular contractions; circuits that generate reciprocal limb actions; and circuits that mediate touch, proprioception, and vision. Most disorders of the muscles, nerves, spinal cord, cerebellum, brainstem, basal ganglia, or cerebrum impair the gait and in a characteristic way. Thus, the features of the gait disorder suggest the lesion location and probable cause.
B. Developmental gaits
Gait examination begins with the genetically preprogrammed automatic or reflex stepping of the neonate. If the Ex holds the neonate vertically, with its feet contacting the bed surface, the infant reflexly lifts its legs alternately and steps. Voluntary trunk control and voluntary standing will later replace automatic stepping, leading to a cruising gait, in which the infant takes steps when holding onto a couch or when steadied by a parent. Then, at about 1 year the infant walks freely, with a toddler’s gait, featured by a broad-base, short, jerky, irregular steps, a semiflexed posture of the arms, and frequent falls (progression by three steps and a plop). After the toddler stage the child develops a normal mature gait, with a narrow-based, heel-toe stride, contra-body movement, and reciprocal swinging of the arms (Haywood and Getchell, 2014). The gait sequence merely reflects a general law of infant development, that inborn, so-called primitive reflexes or behaviors predate all voluntary actions. Thus, smiling, chewing, sucking, grasping, breathing, and walking occur reflexly before the brain and its pathways mature to control these actions voluntarily.
C. Neuromuscular gaits
Let us start with the neuromuscular system and work up to cerebral lesions. If you wish to learn the most from this essay, I strongly recommend that you get up and act out the gaits described. First get up and imitate the toddler gait. If an infant has a club-foot gait, the gait depends on which of a variety of valgus or varus deformities exists. With tibial torsion, the infant has an in-toed or pigeon-toed gait. Many clubfoot deformities can be corrected without surgical interventions (Mindler et al, 2014). Most myopathies (the muscular dystrophies and the polymyositises) weaken the proximal muscles of the shoulders, back, and hips. Because of weak paraspinal muscles, the myopathic Pt shows a characteristic, usually sway-backed, waddle, a lordotic waddling gait. Because of the weak proximal muscles, the myopathic Pt has trouble when getting up on or down from the examining table or when standing up from a sitting or especially a reclining position. In so arising, the myopathic Pt may display Gower sign, the bracing of the arms against the thighs to push the weak trunk erect (Chang and Murbarak, 2012).
This 4-year-old boy, striding on the balls of his feet, without a definite heel strike has a toe-walking gait that can be a normal finding up to the age of 3 years (Williams et al, 2014). Tight heel cords limit dorsiflexion of the foot to about 90°. Such a gait occurs in Duchenne muscular dystrophy, in spastic diplegia, and in children who are autistic or with an intellectual disability. But the next toe-walking child moves along jauntily, runs, skips, and hops normally and has none of these serious disorders, and may be idiosyncratic, sometimes familial, gait pattern, although a neurological rather than a musculoskeletal difficulty may still be the cause.
The next Pt’s toes do not clear the floor because of paralysis of foot dorsiflexion, causing a toe-drop or foot-drop gait. In compensation, the Pt jerks the knee high, flipping the foot up into dorsiflexion, and the foot slaps down. With unilateral or bilateral foot-drop gait, the sound of the slapping feet alone permits the Ex to suspect the diagnosis, without even seeing the Pt. Unilateral foot drop suggests a unilateral, perhaps mechanical or compressive, neuropathy of the common peroneal nerve, frequently from a crossed-knee palsy. A bilateral foot-drop or steppage gait suggests a symmetrical distal peripheral neuropathy of toxic, metabolic, or heredofamilial type, as in alcoholic neuropathy or Charcot-Marie-Tooth progressive peroneal atrophy.
A tibial nerve palsy, in contrast to a peroneal nerve palsy, causes a heel-drop gait. The Pt can dorsiflex the foot but not plantar flex it. A complete sciatic palsy causes a flail-foot gait in which the Pt can neither dorsiflex nor plantar flex the foot. Now you see why the Ex asks the Pt to walk on the toes and the heels. These actions test all the muscles innervated by the sciatic nerve, in addition to proprioception and balance.
D. Sensory gaits
A tabetic, dorsal column, or sensory ataxic gait resembles a double foot-drop or step-page gait but has its own unique signature. In tabes dorsalis, syphilitic infection causes degeneration of dorsal roots and dorsal columns of the spinal cord (Berger 2011; Pandey 2011; Zhang et al, 2013). Lacking position sense, the Pt lifts the knees too high and slaps the feet down, placing them irregularly and on a broad base, because of sensory ataxia. The Pt simply does not know where the legs are. When in bed, the Pt literally has to peek under the covers to locate the feet and legs. To compensate for the lack of position sense, the tabetic Pt must use visual cues to stand. Eye closure removes the visual cues that compensate for the absence of position sense. The Pt can sway and fall over, thus failing the Romberg test (Chapter 10), which the cerebellar Pt more or less passes. The steppage gait of dorsal column disease differs from the double foot-drop gait of peroneal palsy by the presence of normal dorsiflexor power, irregular foot placement due to the sensory ataxia, the absence of position and vibration sense in the legs, absence of MSRs, and abnormal Romberg test (Video 8-1). The presence of Argyll Robertson pupils and a positive serologic test for syphilis (Table 4-5) separates tabes dorsalis from other dorsal column diseases such as the subacute combined degeneration of pernicious anemia or the spinocerebellar degenerations. The experienced Ex will not confuse any of the gaits in this entire essay with the slow, deliberate, searching steps of the blind Pt, the blind person’s gait.

Video 8-1. High steppage gait due to polyneuropathy.
The next Pt has a painful sole or hyperesthetic gait. The Pt sets the foot down gingerly, bears as little weight on it as possible, and limps off the foot as soon as possible while wincing and hunching the shoulders. This feature, the limiting of weight bearing by pain, is the antalgic gait. If the pain is unilateral and on the bottom of the ball of the foot, suspect Morton metatarsalgia, a painful neuroma of an interdigital nerve; if it affects the large toe (podagra), consider gout. If the pain is bilateral, the Pt looks like a person walking barefoot on a hot pavement; suspect hyperesthesia of both soles, common in painful distal peripheral neuropathies, usually metabolic, toxic, or alcoholic or nutritional in origin. When the Pt complains of foot pain, always examine the Pt’s shoes—the wear pattern tells a tale in itself—and compare the size and shape of the shoe with the size and shape of the foot and note the heel height.
The next Pt has a radicular or back pain posture and an antalgic gait. The Pt complains of extreme pain radiating into the big toe, caused, in all probability, by a herniated intervertebral disc compressing the L5 nerve root. Coughing or straight-leg raising causes shooting pain into the foot. To rise from a chair, the Pt pushes up with the arms and has a stiff back, with a completely flat lumbar curve. When standing, the Pt does not put weight on the painful leg and gets off it as soon as possible (antalgic gait). The Achilles tendon feels soft to compression by the Ex’s thumb, as compared with the weight-bearing leg. When walking, the Pt places little weight on the painful leg and takes stiff, slow, short strides with no heel strike, to avoid painful jarring. Often the Pt’s trunk tilts slightly to the side opposite the pain.
Even upper extremity neuropathies may cause a characteristic gait disorder. If the Pt’s transverse carpal ligament compresses the median nerve, causing a carpal tunnel syndrome, excruciating pain in the hand typically awakens the Pt at night. Night after night, the Pt gets up and paces the bedroom flipping or shaking the hand in an effort to gain some relief, the nocturnal flipping-hand gait, and a nearly pathognomonic gait. Autistic and other children with an intellectual developmental disorder show a variety of flipping-hand gaits as repetitive, self-stimulating mannerisms.
E. Cerebellar ataxic gaits
Cerebellar lesions cause ataxia of voluntary movements and of voluntarily maintained postures, hence, a reeling stance and gait. A unilateral cerebellar lesion causes only ipsilateral cerebellar signs, most likely from neoplasm, infarct, or demyelinating disease. After an acute cerebellar lesion, the Pt frequently veers or falls in one direction (lateropulsion, anteropulsion, or retropulsion). Bilateral cerebellar signs, thus a pancerebellar syndrome, imply a toxic, metabolic, or heredofamilial disorder or multiple sclerosis, if combined with other exacerbating and remitting signs. Relatively pure ataxia of the legs and gait, with little or no dystaxia of the arms, and no dysarthria or nystagmus, suggests a rostral vermis syndrome, most commonly secondary to alcoholism. The painful peripheral neuropathy of such Pts also may cause an antalgic gait. Relatively pure truncal ataxia suggests a flocculonodular lobe or a caudal vermis lesion, generally a tumor (Bastain et al, 1998; Marquer et al, 2014; Table 8-3). Gait and balance may even be altered in those Pts with essential tremor despite their lack of subjective complaints (Hoskovcová et al, 2013).
F. Spastic gaits
With a hemiplegic gait, the Pt circumducts a leg, dragging the toe, placing the ball down without a heel strike, with the ipsilateral arm held in partial flexion or, more rarely, flaccidly at the side. Hemiplegia affects the hand and arm more than the leg and usually causes a closed fist because of flexor spasticity (Video 8-2). The lesion is most likely an infarct, tumor, or trauma. The next Pt walks with stiff legs, not clearing the floor with either foot, the exact opposite of the Pt with a high steppage gait. This Pt gives the appearance of wading through water because they must work against the spastic opposition of their own muscles, as if walking in a viscid environment of molasses instead of air. Their knees tend to rub together in a scissoring action. They have a spastic gait (Video 8-3). If the Pt has a spastic diplegic gait from cerebral palsy, they have small, short legs in contrast to normally developed chest, shoulders, and arms. In spastic diplegia, in direct contrast to double hemiplegia, the Pt has severe spasticity in their legs, minimal spasticity in her arms, and little or no deficit in speaking or swallowing (Chan and Miller, 2014), whereas the double hemiplegic has pseudobulbar palsy and the arm is weaker than the leg. The cerebral palsy Pt may adduct the legs strongly when walking, causing a scissors gait. The knees of some spastic diplegics may remain bent when walking, the spastic diplegic crouch gait (Chang et al, 2010). The Pt looks as if wading through water or molasses with the knees bent. A pure spastic or paraplegic gait, with no sensory deficits, coming on after infancy, suggests a pure corticospinal tract disorder, such as one of the familial spastic paraplegias. If, in addition to spasticity, the disease impairs the dorsal columns or cerebellum, the Pt will have a wider-based unsteady gait and take irregular steps—the spastic-ataxic gait, suggesting one of the spinocerebellar degeneration or multiple sclerosis.

Video 8-2. Hemiparetic gait following stroke.

Video 8-3. Spastic paraparetic gait in a patient who sustained a T11 end-plate fracture.
G. Basal motor nuclei gaits
When the Pt with a choreiform gait walks, the play of finger and arm movements increases or may even appear clearly for the first time. Random missteps mar the evenness of the strides as the choreiform twitches supervene. Station is characteristically broad-based. A family history of chorea and dementia suggests Huntington chorea (Video 8-4). A history of rheumatic fever, the acute onset of chorea, and a “fussy personality” signify Sydenham chorea. When the athetoid Pt walks, the slow writhing movements of fingers and arms tend to increase. A combination of athetosis with moderate spastic diplegia or double hemiplegia, a spastic-athetoid gait, usually signifies status marmoratus (état marbre) of the basal ganglia and thalamus, secondary to perinatal hypoxia. The Pt’s great toe may automatically extend when walking, a so-called striatal toe.

Video 8-4. Choreiform gait in a patient with Huntington chorea.
Dystonia may first manifest in a child, say of 9 years of age, by an intermittent in-turning of one foot that impedes walking, a dystonic equinovarus gait. In the later stages, dystonic truncal contortions and tortipelvis may cause the trunk to incline strongly forward. The Pt may take giant uneven strides, exhibiting flexions or rise and fall of the trunk, the so-called dromedary gait, hip bulges backward and sideways with the shoulders and neck turned opposite as if to compensate while the head is held vertically. While appearing psychogenic the Pt has dystonia musculorum deformans, a hereditary disorder. Genetic screening for the DYT gene abnormalities may be useful for patients with early onset dystonia or those with affected relatives. Patients with involuntary movement disorders can sometimes walk backward or dance better than they can walk forward.
This next Pt, with a parkinsonian gait, has a tremor at rest that disappears during voluntary movement, a flexed posture, rises and walks slowly with short steps, lacks any arm swing, and turns en bloc like a statue rotating on a pedestal. The Pt does not have a wide-based gait, as in cerebellar disease. If the Ex (after a warning and care to prevent the Pt from actually falling) were to push the parkinsonian Pt, the Pt will move forward or backward on tiny steps of increasing speed and decreasing length, as if chasing the center of gravity, and may fall over, a festinating gait. Patients with the marche à petit pas often also turn en bloc and festinate. Parkinson disease results from degeneration of the substantia nigra and parkinsonism can result from neuroleptic medication. Primary progressive freezing gait belongs to the parkinsonism category, but has multiple etiologies and does not respond to levodopa (Nutt et al, 2011; Perez-Lloret et al, 2014). The Pts freeze when starting to walk and when turning or in response to some stimulus. They show bradykinesia and a masked face. They get progressively worse, begin to fall, exhibit retropulsion, and soon become wheelchair dependent.
H. Higher-level gait disorders
Gait abnormalities often precede dementia of any type (Verghese et al, 2002). This elderly Pt with the shuffling, short steps, who does not lift the feet far from the floor, has the marche à petit pas (gait apraxia, lower body parkinsonism, or Bruns ataxia). When the Pt tries to speak, steps cease, leading to the somewhat pejorative, but expressive, colloquialism: “He can’t walk and chew gum [in this case walk and talk] at the same time.” Many of the elderly Pts in a nursing home display this type of gait and it may result from a primary degenerative major neurocognitive disorder due to Alzheimer disease or vascular cognitive impairment with periventricular lesions identified on MRI (Masdeu et al, 1989; Benson et al, 2002; Snijders et al, 2007).
A glance around a nursing home will disclose many Pts with forward flexion of the head, head drop or head ptosis. Many times the Pt has lost the sense of verticality: the entire trunk tilts in the chair, and the Pt goes to sleep in the head-dropped, body-tilted posture. The posture may result from neuromuscular disorders that cause weakness of the neck muscles, but it often coexists with dementia, parkinsonism, a forward flexed posture of the entire spine, called camptocormia (Jankovic, 2010) and usually a disorder of gait.
The next Pt has difficulty in initiating the sequence of movements to rise, stand, or walk. When lying down, they make fairly normal leg movements. When trying to rise from the chair, they rock up and down several times to rise. When commencing to walk, they make several efforts to move their feet. After these efforts, they appear somewhat puzzled, as if searching for lost motor engrams, or the right button to press to initiate walking. The effort to progress may result only in stepping on the same spot, as if trying to free the feet from thick, sticky mud, the dancing bear gait. If they do progress, their feet stick to the floor as if magnetized. Some observers have called the combination of short steps, wide base, and difficulty in picking up the feet (magnet sign or magnetic gait, cortical-subcortical gait, gait ignition failure, motor blocks) and many Pts previously described with such terms may have primary progressive freezing gait, but its neuropathologic basis is unknown (Nutt et al, 2011; Perez-Lloret et al, 2014). Overall, just the presence of a clinically evident gait disorder in the elderly and the rate that it worsens correlate with the risk of death (Wilson et al, 2002).
Some Pts have a syndrome of gait disorder, dementia, and urinary incontinence with ventriculomegaly on CT or MRI. They take small steps of reduced velocity and variable stride length and the constellation of symptoms is assumed to be caused by ventricular enlargement, but a finding that can arise from hydrocephalus or ex vacuo change. Called normal pressure hydrocephalus, Bret et al (2002) emphasized that it can also occur in children and prefer the name chronic hydrocephalus, but in adults attempts to lessen “pressure” by removal of CSF or shunting lead to inconsistent or short-term results (Stolze et al, 2001; Klassen and Ahlskog, 2011; Lenfeldt et al, 2012) so, the pathophysiology remains unclear.
I. Psychogenic gaits
To fully appreciate the astasia-abasia (astasia = not standing; abasia = not walking) of the Pt with a psychogenic gait disturbance, the neophyte physician will have to witness it. Often the Pt tilts, gyrates, and undulates all over the place, unwittingly proving by not falling during the marvelous demonstration of agility that strength, balance, coordination, and sensation have to be intact (also refer to Chapter 14, Video 14-2). However, do not mistake some of the bizarre involuntary movement disorders, in particular the dromedary gait of dystonia, or some focal seizures for functional neurological symptom disorder. Lempert et al (1991) listed what was felt to be six features of a psychogenic gait, but any type of movement disorder can have a psychogenic origin (not representative of a factitious disorder) (Thenganatt and Jankovic, 2015):
1. Moment-to-moment fluctuation
2. Excessive slowness or hesitation
3. Exaggerated sway on the Romberg test, improved by distraction
4. Postures that waste energy
5. Extremely cautious, restricted steps, like walking on ice
6. Sudden buckling of the knees without falling
To learn how other mental disorders affect the gait, just watch any group of Pts in a psychiatric hospital as they walk along the ward. Hardily a single Pt steps out with a perfectly normal gait. This Pt, hopelessly moving along, sighing, shoulders sagging, head down looking at the floor, may suffer from depression. The Pt wringing her hands and wrinkling her brow may have an agitated depression. That unkempt middle-aged person, walking with small irregular, wincing steps placed gingerly on a wide base has spindly arms and legs that contrast with a disproportionate fullness of the abdomen. Their alcohol use disorder has caused mild shoulder girdle weakness, ascites, a rostral vermis syndrome, and a painful sensory neuropathy with hyperesthetic soles. That young adult Pt with a mild parkinsonian gait is probably a schizophrenic taking large doses of neuroleptic medication. That young person, gesticulating as if conversing as they walk along, is a schizophrenic, attending to their hallucinations. They are underdosed or their medication has not yet taken hold. That next grim-faced Pt, walking cautiously and peering around suspiciously, suffers from severe paranoid schizophrenia (Lallart et al, 2012). The teenager who, of inner necessity, steps on every crack, pats every door, and suddenly halts their progression to whirl around and utter expletives suffers from the disabling compulsions of severe Tourette syndrome. That aged Pt with silvery white hair, confusion of purpose and direction, and marche à petit pas has a dementia and probably Alzheimer disease. The autistic children or those with an intellectual disability have their gait peculiarities, often characterized by behavioral stereotypies such as hand flapping. Thus, does the gait often disclose the mental and the neurologic status of the Pt?
Gentle reader, if we have overdone the gait examination a little, forgive us. If not quite the whole neurologic examination, nothing else discloses so much so quickly.
BIBLIOGRAPHY · Gait Analysis
Bastain AJ, Mink JW, Kaufman BA, et al. Posterior vermal split syndrome. Ann Neurol. 1998;44:601–610.
Benson RR, Guttmann CRG, Wei X, et al. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology. 2002;58:48–55.
Berger JR. Neurosyphilis and the spinal cord; then and now. J Nerv Ment Dis. 2011;199:912–913.
Bret P, Guyotat J, Chazal J. Is normal pressure hydrocephalus a valid concept in 2002? A reappraisal in five questions and proposal for a new designation of the syndrome as “chronic hydrocephalus.” J Neurol Neurosurg Psychiatry. 2002;73:9–12.
Chan G, Miller F. Assessment and treatment of children with cerebral palsy. Orthop Clin N Am. 2014;45:313–325.
Chang FM, Rhodes JT, Flynn KM, Carollo JJ. The role of gait analysis in treating gait abnormalities in cerebral palsy. Orthop Clin N Am. 2010;41:489–506.
Chang RF, Mubarak SJ. Pathomechanics of Gowers’ sign: a video analysis of a spectrum of Gowers’ maneuvers. Clin Orthop Relat Res. 2012;470:1987–1991.
Haywood K, Getchell N. Life Span Motor Development. 6th ed. Champaign: Human Kinetics; 2014.
Hoskovcová M, Ulmanová O, Sprdlík O, et al. Disorders of balance and gait in essential tremor are associated with midline tremor and age. Cerebellum. 2013;12:27–34.
Jankovic J. Camptocormia, head drop and other bent spine syndromes: heterogeneous etiology and pathogenesis of parkinsonian deformities. Mov Disord. 2010;25:527–528.
Klassen BT, Ahlskog JE. Normal pressure hydrocephalus. How often does the diagnosis hold water? Neurology. 2011;77:1119–1125.
Lallart E, Jouvent R, Hermann FR, et al. Gait and motor imagery of gait in early schizophrenia. Psychiatry Research 2012;198(3):366–370.
Lenfeldt N, Hansson W, Larsson A. Three-day CSF drainage barely reduces ventricular size in normal pressure hydrocephalus. Neurology 2012;79:237–242.
Marquer A, Barbieri G, Pérennou D. The assessment and treatment of postural disorders in cerebellar ataxia: a systematic review. Ann Phys Rehabil Med. 2014;57:67–78.
Mindler GT, Kranzl A, Lipkowski CAM, et al. Results of gait analysis including the Oxford Foot Model in children with clubfoot treated with the Ponseti Method. J Bone Joint Surg Am. 2014;96:1593–1599.
Snijders AH, van de Warrenburg BP, Giladi N, Bloem BR. Neurological gait disorders in elderly people: clinical approach and classification. Lancet Neurol. 2007;6:63–74.
Snijders AH, Haaxma CA, Hagen YJ, et al. Freezer or non-freezer: clinical assessment of freezing gait. Parkinsonism Relat Disord. 2012;18:149–154.
Stolze H, Kuhtz-Buschbeck JP, Drucke H, et al. Comparative analysis of the gait disorder of normal pressure hydrocephalus and Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2001;70:289–297.
Thenganatt MA, Jankovic J. Psychogenic movement disorders. Neurol Clin. 2015;33:205–224.
Verghese J, Lipton RB, Hall CB, et al. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347:1761–1768.
Williams CM, Tinley P, Curtin M, et al. Is idiopathic toe walking really idiopathic? The motor skills and sensory processing abilities associated with idiopathic toe walking gait. J Child Neur. 2014;29:71–78.
Wilson RS, Schneider JA, Beckett LA, et al. Progression of gait disorder and rigidity and risk of death in older persons. Neurology 2002;58:1815–1819.
Zhang HL, Lin LR, Liu GL, et al. Clinical spectrum of neurosyphilis among HIV-negative patients in the modern era. Dermatology 2013;226:148–156.
 Learning Objectives for Chapter 8
Learning Objectives for Chapter 8