Evolution and Development: Organisms
Paul M. Brakefield
OUTLINE
1. Evolution of form and function
2. The rise of evolutionary developmental biology
3. Evolutionary constraints and patterns of allometry
4. Patterns of parallel evolution
5. The paradox of morphological stasis
6. Opportunities for future research
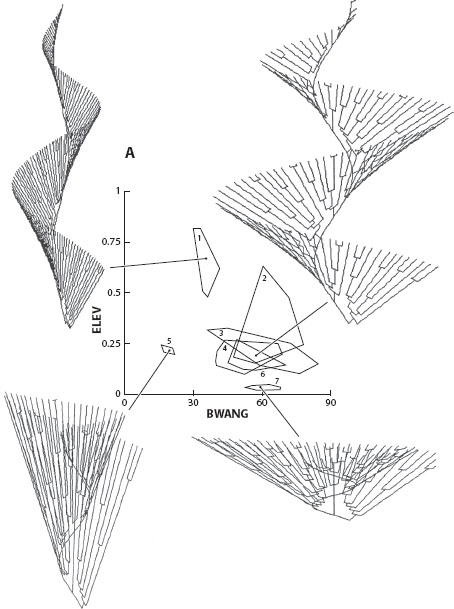
The essence of evolution by natural selection is uncomplicated: phenotypic variation among individuals is generated from genetic variation via the processes of development, and this “fuel” is then screened for performance in the ecological and reproductive arena. This chapter examines the extent to which both the generation of fuel and its performance influence the paths of evolution. Paleontologist David Raup showed in the 1960s that the set of all theoretical shapes of snail shells fit a cube, with each axis reflecting a simple mathematical description of one component of shell growth (figure 1). He then observed that only a small proportion of this theoretical morphospace had been occupied in the evolutionary history of gastropod snails. Such findings of substantial areas of potential phenotypic space that have not been explored in evolution are characteristic of this type of study of clades of related species. Furthermore, variation is observed in the density of taxa in the area of morphospace that has been occupied. It is then important to examine the extent to which such patterns reflect how natural selection screens the performance of individuals and the production of phenotypic variation. In other words, to what extent is the evolution of optimal phenotypes in response to adaptive landscapes compromised by the numerous potential constraints involved in the generation of phenotypic variation?
Figure 1. Evolution in morphospace in bryozoa: occupancy of theoretical morphospace and forms that have not evolved. Simulations of helical colony morphologies for living and fossil bryozoa are shown in panel A, while simulations of helical colony morphologies that have never been found are shown in panel B. The graphs are the same in panels A and B, and the polygons illustrate measurements of two geometric parameters of the helical colony model (BWANG and ELAV) taken from living and fossil bryozoans. (George McGhee 2007.)
GLOSSARY
Allometry. The change in proportion of various parts of an organism as a consequence of growth. Variation in the scaling relationships of parts or organs of organisms typically contributes substantially to evolutionary diversification within lineages.
Artificial Selection. Intentional breeding by humans for certain traits or combination of traits. This contrasts with natural selection, in which the environment acts as a sieve to screen variation among individuals for reproductive success.
Constraint. A limit on evolutionary change that slows the rate of adaptive evolution or prevents a population from evolving the optimal value of a trait. Constraints can be considered intrinsic to the organism (e.g., genetic or developmental) and involve the generation of form, or extrinsic and associated with the power of natural selection and the spatial-temporal matching between population structure and the environment.
Developmental Bias. A bias in the production of various phenotypes caused by the structure, composition, or dynamics of the developmental system.
Developmental Drive. Results from developmental processes that facilitate the production of phenotypic variants along certain axes of form and which, therefore, favor evolution in certain directions.
Evolvability. Capacity of a trait, set of traits, or a lineage to adapt to changing conditions.
Genetic Covariance. A summary statistic describing the genetic association or correlation among traits. It quantifies the extent to which the evolutionary response of one trait will be influenced by selection of another. Genetic covariance among traits can yield “axes of least resistance” along which species tend to evolve.
Modularity. Ability of individual parts or modules of an organism, such as repeated limbs or segments, to develop or evolve independently from one another. Different elements within a single module will lack individuality and the ability of independent evolution but show independence from other modules.
Morphospace. A theoretical morphospace represents an N-dimensional geometric hyperspace for the form of organisms produced by systematically varying the parameter values of a geometric or mathematical model for the generation of form. One can then compare observed patterns in the occupancy of morphospace by a group of organisms, or by different groups.
Parallel Evolution. Evolution of similar or identical features independently in related lineages, usually considered to be based on similar modifications of the same developmental pathways.
Stasis. The pronounced morphological stability displayed by many fossil species, often for millions of years. It contrasts sharply with the rapid, often-adaptive, evolutionary change documented in many extant species.
1. EVOLUTION OF FORM AND FUNCTION
Evolution of a trait by natural selection requires three components in any population, namely, phenotypic variation, a consistent relationship between phenotype and fitness, and genetic variation, a resemblance in the trait among related individuals. Thus, if phenotypic variation among individuals is not generated and is not based, at least in part, on some underlying genetic variation, adaptive evolution of the trait will not happen. Variation in the phenotype must exist among individuals so that selection can screen for the performance of individuals with differing phenotypes for some component of viability, survival, and reproductive success. Some genetic basis for the variation among individuals is then necessary for the selection at this phenotypic level to result in an evolutionary change, usually in the mean phenotype, from one generation to the next.
The null hypothesis for natural selection acting in a fully unconstrained manner would be that the observed patterns for the evolution of diversity are the result solely of natural selection at the phenotypic level; however, genetic constraints, such as patterns of genetic variation and especially genetic covariances among traits, can in theory retard, channel, or bias the extent or directions of evolutionary change. Developmental constraints can be considered in a similar framework for the evolution of form. John Maynard Smith made an early effort to integrate studies of evolution and development by bringing together a group of evolutionary biologists and developmental biologists. They established the crucial idea of developmental bias: do the properties of the developmental system, and the ways in which it generates variation, bias the course of the evolution of form? The terminology and semantics associated with descriptions of such constraints are extensive and arguably of limited value, but the issue of the mechanisms by which genetic or developmental constraints can influence the trajectories and patterns of evolution alongside natural selection remains a challenge to unravel; however, so-called intrinsic or generative constraints involving the processes by which phenotypic variation is generated are opening up to experimental dissection, and this is in turn leading to a more rounded assessment of the extent of the power of natural selection in the evolution of diversity.
2. THE RISE OF EVOLUTIONARY DEVELOPMENTAL BIOLOGY
Evolutionary developmental biology, hereafter referred to as evo-devo, grew out of the rich history of comparative embryology in the nineteenth and early twentieth centuries. It examines how developmental processes evolve, and how knowledge of development can contribute to understanding of evolutionary change. The genotype-to-phenotype map is a major focus of the research program, including an understanding of the concept of evolvability, or the potential for morphological traits to exhibit adaptive evolution (see chapter II.6). Although evo-devo is essentially concerned with morphogenesis, other areas of biology such as behavioral biology are embracing the attempt to more fully map phenotypes of functional significance to genotypes.
The field of evo-devo is providing evolutionary biologists with the tools to address in detail the ways in which variation in form is generated through development, and thus to explore the ways in which the developmental processes that build animal or plant forms can be reflected in observed patterns of diversity, whether between widely separate phylogenetic lineages or among closely related species. The mechanisms underlying the evolvability of form are currently being investigated, in the contexts of both fundamental changes in body plan, and adaptive radiations as a lineage of species encounters novel ecological opportunities and explores new morphological and phenotypic spaces. Different modes of key innovations or evolutionary novelties are being explored by research in a wide variety of lineages, as are the processes of elaboration and evolutionary tinkering involved in the exploitation of such novelties.
3. EVOLUTIONARY CONSTRAINTS AND PATTERNS OF ALLOMETRY
When observing many groups of related species of animals or plants, one often sees striking diversity in their shapes. Static allometry refers to the proportional scaling relationship among individuals of a species between one organ and total body size, or between two organs, after growth has ceased or at a single developmental stage. The evolution of static allometry underlies much of the diversity in shape and is therefore a fundamental component of an understanding of pattern and process in evolutionary diversification. The evolution of the scaling proportions of various body parts provides an interesting example to discuss in the light of intrinsic versus extrinsic constraints.
Numerous descriptive studies have revealed that patterns of allometry of specific parts of the body within a species are typically remarkably invariant. Thus, each male stalk-eyed fly is characterized by a particular scaling relationship between the width of its eye stalks and its body length. Both these traits can be highly variable, but the relationship between the two is comparatively invariant. While a tight phenotypic correlation is typically observed between traits in individuals of a species, different species of stalk-eyed flies can show remarkably divergent static allometry.
Patterns such as this raise the issue of the mechanisms by which striking differences among species can evolve if the phenotypic variation within species is very limited. This is perhaps especially pertinent where the morphological traits concerned share developmental processes and show high genetic correlations. An example is the regulation of development of both forewings and hindwings in a butterfly by essentially the same sets of developmental genes and physiological processes. How during evolution do these two sets of wings evolve to gain individuality and become different from each other in divergent patterns of size, shape, and scaling among species? Until recently, there was little knowledge of the developmental mechanisms that regulate such scaling relationships, but this is beginning to change, especially through work on organ development in Drosophila melanogaster. Feedback processes among growing organs can modulate their final sizes in relation to one another and to overall body size. In Drosophila, organ autonomous changes in expression of a gene called FOXO are one component that can alter the extent to which individual organs adjust their size, for example, in response to nutrition during growth.
One way to examine the role of generative constraints is to employ artificial selection. Recent experiments of this type with butterflies have explored the more general issue of the contribution to patterns of diversity in static allometry made by processes intrinsic or extrinsic to the organism; can the mechanisms involved in generating phenotypic variation act as a brake on the evolution in allometry, or is natural selection sufficiently powerful to drive the independent evolution of body parts when this is favored?
Tony Frankino applied artificial selection in lines established from an outbred laboratory stock of the tropical butterfly Bicyclus anynana for the scaling of the size of the forewing relative to the hindwing. These two traits show a positive genetic covariance with a genetic correlation not significantly different from unity, and yet selection was able to produce short-term evolution of the scaling relationship and novel phenotypes in each direction (figure 2). Additional experiments with free-flying butterflies in a spacious greenhouse used hybrids obtained from crosses of pairs of opposing selection lines to examine the fitness consequences of the change in phenotype. The results showed that the mating success of male butterflies with a divergent forewing to hindwing allometry was substantially lower than that of those with an intermediate wing scaling. This indicates a strongly stabilizing pattern of selection in favor of the original wild type and against either of the divergent morphologies (figure 2). Thus, the evolution of allometries in this insect is not limited by any developmental constraint, and the scaling relationships are shaped by strong natural selection. Comparable experiments have been performed with B. anynana with closely similar results for scaling of the forewing relative to body size.
Figure 2. Forewing:hindwing allometry in butterflies: positive responses to artificial selection versus stabilizing sexual selection in favor of wild-type butterflies. (Frankino et al. 2007.)
Artificial selection experiments have also been used to examine the flexibility of evolution in different directions of morphospace for two wing eyespots in this species of butterfly. Each eyespot pattern element consists of concentric rings of epithelial cells containing different-colored pigments. They are often arranged in an anterior to posterior column along the wing margins, and may then function in misdirecting the attacks of predators hunting by sight, away from the vulnerable body. A series of eyespots on the same wing surface behave as a module in which each eyespot is based on a central signal-diffusion system (or “organizer”) during development, and they all express the same set of genes during pattern determination. Thus, the eyespots represent a set of repeated elements exhibiting strongly positive genetic correlations for both eyespot size and color composition such that selection targeting either trait for a specific eyespot in B. anynana yields corresponding responses in the other eyespots (note that the two eyespot traits show little, if any, genetic covariance). A series of artificial selection experiments performed by Patrícia Beldade examined whether repeated eyespots can evolve independently. By simultaneously targeting two different eyespots on the same wing, the experiments explored the extent to which the pattern of eyespots, in terms of relative size or color composition, is flexible in evolutionary terms; for example, with respect to size, can two different eyespots be selected to both either increase or decrease in size, and also for one to become larger and the other smaller? A high flexibility in the evolution of extreme, novel forms toward all four corners of theoretical morphospace for this pattern was observed for the pattern of relative eyespot size over 25 generations of selection. This result is consistent with neither intrinsic constraints nor any strong bias in potential responses to natural selection. This flexibility of response in this experimental system appears to be reflected in a full exploration of morphospace for the same pattern when variation in the species-rich tribe of butterflies is considered. In contrast, experiments on eyespot color yielded very little response and no novel phenotypes when two eyespots were selected in opposing directions. The different responses of the two traits may be traceable to mechanisms of developmental regulation; whereas the size of each eyespot depends on the strength of the organizing “signaling” source at its center, the regulation of eyespot color involves threshold responses to the signals and occurs at the level of the whole wing epidermis.
4. PATTERNS OF PARALLEL EVOLUTION
Parallel evolution describes the development of similar traits or forms in related but distinct species descending from the same ancestor. Such patterns of occupancy in morphospace are of particular interest in considering how evolvability and constraints may influence the trajectories of adaptive evolution. Related clades that have colonized similar environments may show parallel patterns of diversification because the environments provide similar unexploited ecological niches and opportunities for adaptation. This could occur in a largely unconstrained way and be driven by natural selection and ecological performance. On the other hand, related clades may diversify in similar ways in part because they share developmental and genetic systems, and therefore have similar propensities to evolve along certain trajectories or axes of change; in other words, the patterns of parallel evolution may reflect a comparable process of developmental drive. It will become more feasible to examine the role of the evolvability of traits in shaping the ways in which organisms evolve through phenotypic space as research on model species within particular groups of related organisms more effectively maps patterns of phenotypic variation to genetic variation via developmental and physiological processes (see chapter V.12).
One of the most striking examples of parallel evolution is provided by the species flocks of cichlid fish in the Rift Valley lakes of Africa. Each lake was colonized independently by haplochromine cichlids, presumably via rivers entering the lakes. Although the lakes differ in age, topology, and history, each has harbored spectacular radiations of cichlid taxa established over remarkably short periods of time. In particular, the dramatic increases in morphological diversity have been associated with the evolution of clusters of taxa with readily identifiable ecomorphs or trophic morphologies. The ecomorph morphologies are remarkably similar across lakes, even though the taxa within each lake are substantially more similar to each other at a genetic level than they are to any taxon from one of the other lakes. Each ecomorph is associated with suites of life history and behavioral traits associated with exploitation of particular ecological environments within the lake. Such ecomorphs are also characteristic of the island faunas of Anoles in the Caribbean, and feeding morphologies are a major component of radiations in other animal groups, including Darwin’s finches (see chapter VI.10). The patterns of radiation that have evolved in these cichlid faunas may be driven primarily by natural selection, but shared genetic and developmental systems inherited from the haplochromine ancestors could also have facilitated evolution in certain directions of change, and not in others. Thus, although natural selection may, at least superficially, appear all-powerful, the properties in terms of the evolvability of traits and the generation of phenotypic variation could have biased observed patterns in occupancy of morphospace and contributed to the parallel nature of evolution in independent radiations.
Work by Peter and Rosemary Grant on Darwin’s finches of the Galápagos Island archipelago has yielded one of the most complete accounts of how natural selection can yield an adaptive radiation. They have demonstrated very clearly the ways in which bill morphology is a crucial target of natural selection. The diversity in bill morphology can then be compared in a 2-D morphospace by plotting an index of bill depth and width against length. Evo-devo research is now uncovering two developmental genetic pathways that have contributed to the phenotypic diversification in the finch bills. This work includes functional tests of the role of the pathways by employing the chick model for genetic manipulations in embryonic development. This provides promise that future evo-devo work will be able to compare the mechanisms by which diversity has evolved in independent radiations, whether of birds or of cichlid fishes, thus revealing more about the contributions of the properties of developmental systems to patterns of evolution (see chapter V.11).
5. THE PARADOX OF MORPHOLOGICAL STASIS
One of the most striking contrasts observed in patterns of evolution is apparent when studies of microevolutionary change over short periods of time are compared with those of rates of change in morphology over periods of geologic time. Estimates of natural selection obtained from the numerous studies of local populations conducted in recent decades collectively demonstrate a high intensity of natural selection (see chapter III.7). Such observations in nature are also matched by the generality of rapid responses to artificial selection, although there are important differences between the way selection can be made to target phenotypic variation by the human experimenter and the way natural selection interacts with population dynamics in the wild (see chapter III.6). In contrast to these short-term patterns of dynamic evolutionary change, paleontologists studying fossil remains frequently record very little change in the form of animals over geologic time (see chapter II.9). A dramatic disparity can be seen between the observed rates of evolution over short periods of time and those over long periods.
One of the most detailed descriptions of variation in animal form over geologic time is that of cheilostome bryozoans. Cheilostomes are small, clonal marine organisms that form colonies. They are abundant in recent seas and in the fossil record. The results of studies of their biology in present-day populations enable a high degree of confidence in interpretation of the fossil record, for example, with respect to the relationships between variability in morphology and the species-level taxonomy, ecology, and life history evolution of the organisms. A study of two tropical American genera of 11 species found that with both genera, all species tended to remain essentially unchanged in morphology over geologic time. In contrast, new species can appear comparatively abruptly, that is, within the limits of stratographic resolution of sampling of some 150,000 years. Observations from studies of extant species suggest that this pattern extends to stasis in life history traits.
Morphological stasis seems a dominant pattern in the fossil record, thus demanding an explanation in terms of the dynamic behavior of local populations in contemporary analyses. Numerous works have discussed several ways in which this apparent discrepancy could be reconciled. These are not mutually exclusive, and it is indeed likely that there is no single explanation. First, natural selection may tend to oscillate in direction over time on a scale that ensures that patterns of progressive change are seldom observed at the scale of sampling typical analyses of the fossil record. Examples of evolutionary reversals have been observed in studies at a more intermediate timescale, for example, in Darwin’s finches and in stickleback fish. A second class of explanations involves considering patterns of genetic pleiotropy and genetic covariances, and the dimensionality of combinations of traits that are the targets of natural selection. Such interactions among traits, both genetic and developmental, can theoretically constrain or restrain evolutionary change, as outlined earlier in this chapter. Third, and on a more spatial scale, several evolutionary biologists have emphasized the difference between observing dynamic evolutionary change in one, or a group, of local populations, and observing the spread of such change throughout the range of a (widely distributed) species. This has been referred to as the ephemeral divergence hypothesis, pointing out the likelihood of interactions with speciation processes. A later acquisition of reproductive isolation by a group of populations will enable the evolution of a phenotypic shift within them to be retained but not be detected as a change within a species in the fossil record. In contrast, stasis is likely to be observed unless speciation intervenes to capture such a pattern of phenotypic change within a subgroup of populations.
6. OPPORTUNITIES FOR FUTURE RESEARCH
There remain many challenges to expanding the analysis of ways in which intrinsic or generational constraints can restrain, bias, or limit evolutionary change; however, there are also increasing opportunities for using comparative and experimental approaches to exploring the extent to which such constraints can contribute alongside the power of natural selection to evolutionary diversification. Research in evo-devo and evolutionary genetics will increasingly refine our understanding of the evolvability of single traits and more complex character sets, and thus describe the potential pathways of evolutionary change within a lineage. This endeavor should increasingly reveal whether the way in which development works can indeed both facilitate certain trajectories of evolution in morphology and make others less likely to occur. We will then be in a better position to disentangle the contributions of different kinds of intrinsic and extrinsic constraints (including selection and gene flow) to the ways in which animal and plant life explore morphospace in evolution.
FURTHER READING
Allen, C., P. Beldade, B. J. Zwaan, and P. M. Brakefield. 2008. Differences in the selection response of serially repeated color pattern characters: Standing variation, development, and evolution. BMC Evolutionary Biology 8: 94. A description of an experimental analysis of the potential for developmental bias to become reflected in patterns of evolutionary divergence in the eyespots on butterfly wings.
Brakefield, P. M. 2006. Evo-devo and constraints on selection. Trends in Ecology & Evolution 21: 362–368. A review of evolutionary constraints that emphasizes how studies of evo-devo are beginning to open up the analysis of intrinsic constraints.
Eldridge, N., J. N. Thompson, P. M. Brakefield, S. Gavrilets, D. Jablonski, J.B.C. Jackson, R. E. Lenski, et al. 2005. The dynamics of evolutionary stasis. Paleobiology 31: 133–145. A review of ideas about the evolutionary dynamics of morphological stasis.
Frankino, W. A., D. S. Stern, and P. M. Brakefield. 2007. Internal and external constraints in the evolution of a forewing-hindwing allometry. Evolution 61: 2958–2970. A description of experiments to explore how constraints may influence the evolution of scaling of forewing:hind wing size in butterflies.
Futuyma, D. J. 2010. Evolutionary constraints and ecological consequences. Evolution 64: 1865–1884. A detailed review of current thinking about evolutionary constraints, especially in an ecological context.
Jackson, J.B.C., and A. H. Cheetham. 1994. Phylogeny reconstruction and the tempo of speciation in cheilostome bryozoa. Paleobiology 20: 407–423. This paper is part of one of the most thorough analyses of morphological stasis in the fossil record.
Kirkpatrick, M. 2010. Rates of adaptation: Why is Darwin’s machine so slow? In M. A. Bell et al., eds., Evolution since Darwin: The First 150 Years. Sunderland, MA: Sinauer. A discussion of the contrast between rates of selection observed in extant populations and those apparent from studies of the fossil record.
Maynard Smith J., R. Burian, S. Kauffman, P. Alberch, J. Campbell, B. Goodwin, R. Lande, D. Raup, and L. Wolpert. 1985. Developmental constraints and evolution. Quarterly Review of Biology 60: 265–287. An early attempt to facilitate a constructive dialogue between evolutionary biologists and developmental biologists. It yielded a key definition of developmental bias and a discussion of its potential relevance to evolution.
McGhee, G. 2007. The Geometry of Evolution: Adaptive Landscapes and Theoretical Morphospaces. Cambridge: Cambridge University Press. A recent book about analyses of theoretical morphospace in the context of adaptive landscapes and ecological performance. Several key examples of analyses are taken from the fossil record.
Raup, D. M. 1966. Geometric analysis of shell coiling: General problems. Journal of Paleontology. 40:1178–1190. The classic early study of the occupancy of a theoretical morphospace.
Shingleton, A. 2011. The regulation and evolution of growth and body size. In T. Flatt and A. Heyland, eds., Mechanisms of Life History Evolution. Oxford: Oxford University Press. A review about allometry that includes a discussion of recent work on the regulation of growth and body size in Drosophila melanogaster.
Wagner, G. P., and V. J. Lynch. 2010. Evolutionary novelties. Current Biology 20: R48–R52. This is a comprehensive primer on current views of evolutionary novelties.