Causes and Consequences of Extinction
Michael J. Benton
OUTLINE
1. Species extinction
2. Some definitions: Extinction styles and magnitudes
3. Mass extinctions
4. Declining extinction risk and resetting the clock
5. Extinction and the drivers of macroevolution
Species extinction is a normal part of evolution, but there have been many times in the earth’s history when higher-than-expected numbers of extinctions have occurred. During sudden extinction events, and especially during mass extinctions, major physical environmental crises have wiped out large portions of life. The fact that selectivity during extinction events differs from natural selection suggests that higher-level macroevolutionary processes have continually affected the evolution of life.
GLOSSARY
Court Jester. The model of macroevolution that concentrates on changes in the physical environment as the main drivers (cf. Red Queen).
Ecospace. A combination of habitat and ecological activity at any scale.
Macroevolution. Evolution above the species level.
Mass Extinction. The sudden, worldwide loss of many species of diverse ecologies.
Morphospecies. A species defined on the assumption that all members share the same morphology, and other species show different external form.
Pseudoextinction. “Extinction” of a species when it evolves into another species.
Red Queen. The model of macroevolution that concentrates on biotic interactions as the main drivers (cf. Court Jester).
Taxon. A species or larger division of the tree of life.
1. SPECIES EXTINCTION
Extinction is the disappearance of a species or larger taxon. The geographic scale can be local or global. The concern here is with the latter, corresponding to the complete disappearance of a genetic lineage worldwide, not the local disappearance of a species by emigration or environmental change. In the global case, as has often been said, extinction is forever.
The extinction of species is inevitable. Each species has a duration, which is not predetermined but may be characteristic of the wider taxon. A common assertion, developed by George Gaylord Simpson and Steven Stanley, is that mammals evolve at 10 times the rate of clams, which means they originate and go extinct at rates differing by an order of magnitude. This declaration could be an artifact of how human taxonomists identify morphospecies (see chapter VI.1)—perhaps they subdivide mammalian species 10 times as finely as they do those of bivalves, possibly responding to the evident visual differences among mammalian species while missing the less visible species-specific cues in mollusk shells. Nonetheless, assuming that species of mammals and mollusks are somehow equivalent sections of the tree of life, then there are broad differences in mean species durations through geologic time, and therefore also in species extinction rates (table 1).
Table 1. Estimated mean durations of fossil species, taken from various sources
Group |
Mean duration (My) |
Reef corals |
25 |
Bivalves |
23 |
Benthic foraminifera |
21 |
Bryozoans |
12 |
Gastropods |
10 |
Planktonic foraminifera |
10 |
Echinoids |
7 |
Crinoids |
6.7 |
Monocot plants |
4 |
Horses |
4 |
Dicot plants |
3 |
Freshwater fish |
3 |
Birds |
2.5 |
Mammals |
1.7 |
Primates |
1 |
Insects |
1.5 |
Source: Summarized by McKinney 1997.
Note: Marine groups show longer durations (6.7–25 My) than terrestrial groups (1–4 My).
Such wide differences in species extinction rates and macroevolutionary rates have clear implications for the interpretation of times of intense extinction, such as extinction events and mass extinctions in the past, and the current biodiversity crisis (see chapter VIII.6): one would expect the fast-evolving species to be more liable to extinction and, indeed, more likely to recover following the crisis than the slowly evolving species. For example, the ammonoids, a long-lived group of mollusks, typically had short species durations but suffered near-complete wipeout during four mass extinction events, yet recovered rather rapidly in comparison with other marine invertebrates.
The prevalence of extinction—its inevitability—for all species is obvious to evolutionary biologists and paleontologists, but perhaps less so to nonscientists, and is germane to wider discussions about the current biodiversity crisis. Clarity is needed on three issues in this context: lineage extinction at some point is the norm, species differ innately in their extinction risk by wider clade membership, and these points are distinct from the immediate risk of extinction of any named species according to current ecological threats.
The aim here is not to discuss extinction as it manifests itself in the context of natural selection or phylogeography (see chapters II.5, III.1, III.6, III.7, and VI.4), nor in terms of its role in rates of evolution and species selection (see chapters VI.11, VI.12, and VI.14) and in the current biodiversity crisis (see chapter VIII.6) but at the macroevolutionary level and in two broad contexts: first, as a part of the debate about biotic and abiotic drivers of evolution, and second, in terms of the role of extinction events in punctuating the history of life.
2. SOME DEFINITIONS: EXTINCTION STYLES AND MAGNITUDES
The extinction of a species may occur according to one of two patterns in phylogenetic terms: the species terminates without leaving any issue, or it evolves sufficiently to be called a new species. In most molecular phylogenetic approaches, species terminate at the present day, and the issue of extinction does not arise. When fossil taxa are incorporated into phylogenies, they are generally treated as discrete entities that terminate with a definitive extinction. In densely sampled fossil records, however, some species apparently evolve directly into others, and the extinction of the older parts of the lineage is termed a pseudoextinction because the gene pool of the populations that constitute the original lineage continues into the replacing species. The relative prevalence of such pseudoextinctions is hard to determine: it could be argued that they are in fact rare, and quoted examples are based on nonobjective interpretations of sequences of rather simple fossils through numerous sampling horizons. Conversely, critics of cladistics have claimed that such transitional successions of species are relatively common and represent a challenge to the cladistic method because it can identify only species that arise by splitting.
If species extinction is the end of a lineage, the term extinction is also more widely used by evolutionists and paleontologists to denote the end of a clade or paraphyletic group. For example, the “extinction of the dinosaurs” means the end of all nonavian Dinosauria, in other words, the set of clades that includes all animals popularly called dinosaurs but not including the dinosaurian subclade Aves (Avialae), the birds. In this case, the extinction of the dinosaurs does mean the termination of a large number of clades, such as Ornithischia and Sauropodomorpha, and among the theropods, Ceratosauria, Carnosauria, Troodontidae, and Dromaeosauridae. In other cases, however, the term extinction is applied to even less cohesive groups that may share some general ecological characteristics, such as body size or geographic region. An example is the end-Pleistocene extinction of large mammals in the Northern Hemisphere, sometimes termed the “extinction of megafauna,” meaning some, but not all, large animals, in some, but not all, parts of the world.
Paleontologists divide extinctions into three categories: background extinctions, extinction events, and mass extinctions, each of which is a useful concept in particular contexts, but between which there are no sharp divisions.
Background extinction is the sum of all normal species terminations during a defined time interval (time bin). The termination of any particular lineage is not predictable, but the mean rate across a large clade, across a region, or worldwide for all life is predictable. Hence, all things being equal, global extinction is a stochastic process, and its rate should be predictable, dependent on the standing crop of species and their distribution through major clades (each of which has a characteristic mean extinction rate).
Extinction events are times when many species go extinct for a shared reason. Extinction events can be of all magnitudes, but the term is usually reserved for those smaller events that do not qualify as mass extinctions. Under this assumption, extinction events may be regional in scale or may apply to only certain clades or certain ecological guilds. The best-known example is the end-Pleistocene extinction of large mammals in the Northern Hemisphere, but there have been many others over the past 600 million years, such as the early Toarcian ocean anoxic event, 183 million years ago, that killed much of marine life in Europe, or the series of small extinctions in the late Cambrian, about 490 million years ago, each of which marked a major turnover in the trilobite faunas. Causes of these extinction events were varied, but they were generally associated with dramatic changes in the environment that affected many species at least in one or more world regions, such as the retreat of the northern ice 11,000 years ago, the spread of humans and their voracious hunting, an oceanic anoxic event, or major topographical change.
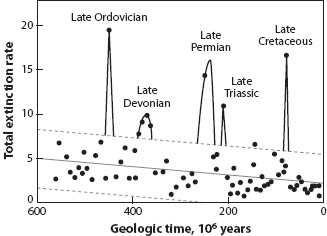
Mass extinctions are the most notable of all, the times of global disappearance of much of life, when many species of wide ecological range died out worldwide, and geologically speaking at least, did so rapidly. Paleontologists have struggled to constrain the terms “much of life” and “rapidly,” but without success, because the distribution of extinction event magnitudes is apparently continuous, with no qualitative distinction between small- and large-scale crises. David Raup and Jack Sepkoski famously identified a statistical distinction in which mean familial extinction rates were assessed for 100 time bins through the Phanerozoic, each 5–6 million years in duration, and they found that five of the points stood out as statistical outliers, beyond the 95 percent confidence envelope (figure 1). This result was broadly reasonable, as the five unusually high global extinction rates corresponded to the “big five” mass extinctions, but the method was statistically unreasonable because the error bars included negative extinction rates, which cannot occur.

Figure 1. Plot of total extinction rate through time for animal life in the sea. The timescale spans the late Proterozoic and Phanerozoic, the time of relatively abundant large animal life. The total extinction rate is assessed as the mean number of families becoming extinct per million years, in each geological stage (mean duration, 5–6 My). The solid lines indicate the best-fitting regression, and the dashed lines the 95 percent confidence envelope. The plot was interpreted to show declining mean extinction rate through the past 600 million years and to identify six times of unusually high extinction, the named positive outliers. (From Raup and Sepkoski 1982.)
3. MASS EXTINCTIONS
The identification of what is and is not a mass extinction is variously impossible (because there is a continuum of extinction events of all magnitudes, and so the dividing line between small extinction events and mass extinctions is a matter of choice) and trivial (because there is no category of unique entities called mass extinctions, there is no need to determine which event at the margin is or is not a mass extinction, nor to seek common rules or laws that apply to all). There is, however, a need for paleontologists to engage with the issue, because the subject has achieved wide popular interest and feeds through to concerns about the current biodiversity crisis: Are we living through the sixth mass extinction, as Richard Leakey and Roger Lewin termed it, or not?
The standard list of the big five mass extinctions comprises the end-Ordovician, Late Devonian, end-Permian, end-Triassic, and end-Cretaceous events (table 2). If these are the five, then the current biodiversity crisis can be said to scale with those events of the past, at least in terms of the rate of species loss in the past 500 years, and so it can be termed the “sixth mass extinction.” Annoyingly for the headline writers, however, there were earlier extinction events that might merit the term mass extinction, including the end of the Ediacaran faunas in the Neoproterozoic, 541 million years ago, and the assembled late Cambrian crises. So, is the present crisis the “eighth mass extinction”? Perhaps that designation is in doubt, as others, including Richard Bambach and colleagues, have argued quite reasonably that there are mass extinctions and mass extinctions: three of the big five were not rapid, single-cause events but summations of pulses of species losses, and perhaps only three of the large events count as mass extinctions: the end-Ordovician, end-Permian, and end-Cretaceous. These three stand out as times of unusually high rates of species loss compared with neighboring time intervals, and the catastrophic losses of biodiversity were caused primarily by high extinction rates. In contrast, during the Late Devonian and end-Triassic events, part of the depletion in biodiversity was caused by unusually low origination rates, and so these appear to have been complex episodes of turnover crisis, rather than simply mass killing.
Table 2. The “big five” mass extinctions, with principal victims and possible causes
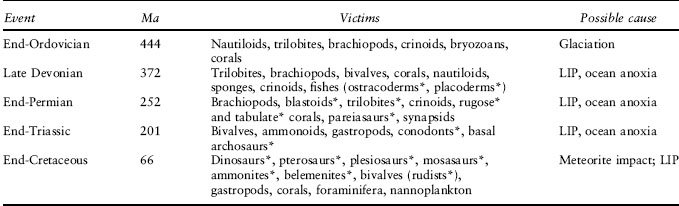
Note: LIP, large igneous province—basaltic eruptions
*Groups that entirely died out
However they may be defined and counted, there has been much study of the big five mass extinctions (table 2). Today, with thousands of publications each year, it might seem surprising that geologists and paleontologists hardly considered these events until the 1970s—indeed, somehow the “death of the dinosaurs” and earlier crises were ignored or trivialized. It seems that geologists were afraid of being labeled as crazy “catastrophists” at a time, even in the 1950s and 1960s, when it was considered dangerous to admit that the earth had ever been hit by large meteorites. Everything changed after 1980.
The tipping point for geologists occurred with the publication of Luis Alvarez’s proposal that the earth had been hit by a 10 km meteorite at the end of the Cretaceous period, that the impact threw dust high into the atmosphere, blacked out the sun, and caused global darkness and freezing for long enough to kill off much of life. This proposal was based on seemingly limited evidence—two locations, in Italy and Denmark, where there was a relatively high concentration of the platinum-group element iridium (the iridium spike) exactly at the Cretaceous-Tertiary (KT) boundary. This, Alvarez reasoned, indicated the arrival on earth of a massive amount of extraterrestrially derived material, transported through the medium of a meteorite or comet, because iridium does not generally occur naturally on the earth’s surface. Through a simple calculation, Alvarez and colleagues estimated the volume of dust needed to black out the sun, then the size of the crater required to generate such a dust volume (150 km diameter), and then the size of the colliding rock (10 km diameter).
These proposals were variously met with massive enthusiasm and angry denunciation, but the criticisms diminished as substantial amounts of confirming evidence were identified during the 1980s: the iridium spike was found everywhere at the KT boundary in both marine and terrestrial rocks; additional evidence for impact was identified (high-pressure minerals such as shocked quartz, coesite, and stishovite); and indeed, the crater itself was found, at Chicxulub in Mexico.
The Alvarez hypothesis led to a second consequence, the suggestion that all major extinctions, not just the big five, were triggered by impacts: Raup and Sepkoski presented evidence for periodicity of extinctions during the past 250 million years, noting a statistically prominent 26-million-year period between such events. Only three of the big five mass extinctions occurred within the past 250 million years, but Raup and Sepkoski identified many other medium-sized species extinctions during the Triassic, Jurassic, and Cretaceous. Indeed, the last of their events, in the Miocene, occurred 11 million years ago. The consequences of the periodicity theory were profound: all mass extinctions had a single cause, that cause was almost certainly extraterrestrial and involved impacts, and the next event will occur in 15 million years. The proposals led to massive interest from scientists across many disciplines, with contributions coming from astronomers, mathematicians, geologists, and biologists. The analyses were sophisticated, and some paleontologists and mathematicians are still intrigued by the proposal, yet the raw data are far from convincing: the fossil databases are patchy, revision of geologic timescales casts doubt on the periodic signal and the period length, and most devastatingly, many of the intermediate “smaller” extinctions disappear when inspected closely, as argued by Mike Benton and others.
Key questions about mass extinctions concern the causes, the victims, and the recovery. Here is not the place to present too much detail on the causes of mass extinctions—the literature on each event is huge, and the postulated causes, especially if older literature is included, are manifold. Recent work has concentrated on identifying plausible models, especially models that might explain more than one event. Some would still identify a single astronomical model as a driver and so explain all mass extinctions as the result of impact and perhaps a killing model akin to that for the KT event. Most paleontologists, however, are content to accept impact as the sole or major reason for the KT mass extinction, but they seek other explanations for the earlier mass extinctions. The most ubiquitous model appears to be volcanic eruption and its consequences, most notably, massive basaltic eruptions that span several hundred thousand years and form large igneous provinces (LIPs), and that appear to have coincided with at least three of the big five events (table 2), as well as the KT (the Deccan Traps in India). The model for extinction associated with such massive eruptions, as summarized by Paul Wignall, focuses on the huge volumes of carbon dioxide spewed out during the eruptions. This is a greenhouse gas and so causes global warming. In normal circumstances, excess carbon dioxide would be consumed by green plants through photosynthesis, but repeated and continuing large-scale eruptions perhaps swamped the normal feedback processes and caused increasingly severe atmospheric warming. On land, plants and animals succumbed if they could not move to the poles, and in the seas, warming of surface waters caused stagnation as the normal circulation of deep cold waters to the surface was slowed, and so oxygen could not reach the seabed, and life there died.
The victims of mass extinctions seem to be a random selection of life of the time. Raup famously contrasted the two assumptions about victims of extinction: they suffer from either “bad genes or bad luck.” In normal, Darwinian, evolution the focus is on bad genes; a species dies out because of some aspect of natural selection, perhaps competition with another species, or inability to adapt to changing conditions. However, during mass extinctions, environmental stresses are severe and unpredictable, and so species cannot be selected for their ability to survive such rare events, and those that succumb may be simply unlucky. Nonetheless, there might be biological characteristics that by chance enable species to survive the shock of the extinction crisis or the tough conditions that follow. Among such general characteristics the most important appears to be wide geographic range at the clade level, regardless of the geographic range of individual species. Other useful characters that seem to improve a species’ chances of survival are adaptation to a broad diet and broad physiological requirements, and modest body size.
The recovery of life after a mass extinction has clear significance for modern conservation concerns. Certainly, it seems that the rapidity of recovery is proportional to the scale of the extinction, but there may be nonscalar components: if a mass extinction removes certain species from ecosystems, the scaffold of the ecosystem may be available after the crisis for new species to slot in. If, however, most components of an ecosystem are removed by a larger extinction event, recovery may involve the construction of entirely new ecosystems, and so perhaps takes longer. Species recover according to their normal evolutionary dynamics, so it is notable, for example, that ammonoids recovered quickly after the end-Permian mass extinction, whereas other groups such as bivalves and echinoderms seem to have taken longer. Further, there may be a major difference between initial and subsequent recovery, meaning the initial rapid filling of ecospace versus the construction of longer-term, more stable ecosystems. So, for example, after the end-Permian mass extinction, species numbers within faunas—and globally—seemed to bounce back within 1–2 million years, but these consisted largely of disaster taxa, short-lived lineages that did not contribute to the eventual major clades or to the longer-term structure of the ecosystems. For example, on land, after the end-Permian event, the initial Lystrosaurus fauna was unusual in that it was dominated by one species; was associated with many amphibians, but no larger herbivores or carnivores; and was cosmopolitan. It took perhaps 10–15 million years for ecosystems to stabilize with a full range of body sizes and trophic levels—with a balance of the major clades that were to be significant for some time thereafter—and for continent-scale endemicity to become reestablished.
4. DECLINING EXTINCTION RISK AND RESETTING THE CLOCK
One of the key points of Raup and Sepkoski’s review of extinction rates (figure 1) was to demonstrate that these rates showed an apparently statistically significant decline through time, which these authors interpreted as a general improvement in the ability of organisms to resist extinction—presumably, as global mean extinction rates fell, mean duration of families of marine invertebrates increased. If this interpretation is correct, it would represent cogent evidence for progress in evolution, a notoriously tricky concept to define and prove.
The evidence has been disputed, for more or less geometric reasons. The fact that the analysis was carried out on families, not genera or species, immediately gives pause for thought: What if the families are largely human constructs, and we simply interpret families differently in older rocks? Further, all other things being equal, species are less likely to be preserved in older rocks than in younger ones, and so “families” in the Cambrian might well include far fewer species than families from younger rocks: with a constant species extinction rate, familial extinction rate will decline as the number of species per family increases. Third, even with a perfect fossil record, it is likely in any case that the number of species per family will increase through time, simply because the geometry of evolution demands lineage splitting and expansion of clades through time. Again, with a constant rate of species extinction, familial extinction rates must decline through time. In the end, then, there is no evidence that the mean of all family-level or genus-level extinction rates at any time can be compared with mean rates in neighboring time bins. Together, this means that there is no evidence for declining rates or improving competitive ability through time.
It has often been said that mass extinctions, or extinction events in general, reset the clock of evolution, cutting across all the existing arms races, coevolutionary species pairs, food webs, and ecosystems, and kick-start an entirely new phase in the history of life. Leigh Van Valen, for example, suggested that the history of life in the sea followed two major evolutionary cycles, one beginning with the origin of animals in the late Neoproterozoic and Cambrian, after which mean per-taxon extinction rates (probabilities of extinction) declined rather steadily to the end of the Permian. The huge end-Permian mass extinction then killed off all but 10 percent of species, which subsequently gave rise in the Triassic to new lineages that at first showed very high mean extinction probabilities, which in turn began a second long-term declining trend toward the present. In this case, he argued that the other mass extinctions had negligible effect on the broad patterns.
It would be wrong to assert that mass extinctions literally “reset” evolution, in the sense of wiping out the preexisting interactions and lineages and opening the world for something entirely unexpected and new. Indeed, many lineages survived even the most severe of mass extinctions, and they became reestablished in similar ecological roles after the crisis, occupying the same positions within ecosystems. These chance survivors may indeed have helped retain the frame of postextinction ecosystems, into which new taxa inserted themselves during the recovery process.
Nonetheless, mass extinctions do reset the pattern of macroevolution in enabling the radiation of clades that might otherwise not have been able to radiate, or not at the same time. For example, in the Early and Middle Triassic seas following the end-Permian mass extinction, several groups of marine reptiles—ichthyosaurs, thalattosaurs, and sauropterygians—became established as entirely new top predators. Likewise, on land the first dinosaurs emerged at this time and, after a further extinction event, took their important role in terrestrial ecosystems. Even better known perhaps are the ascents of modern mammals and birds after the extinction of the dinosaurs 65 million years ago. Had the extinctions not happened, these major groups might not have had their chance to rise to importance.
5. EXTINCTION AND THE DRIVERS OF MACROEVOLUTION
What, then, has been the significance of extinction events in driving evolution? The answer addresses the wider question of the relative roles of biotic and abiotic drivers of macroevolution, characterized as the Red Queen and Court Jester models. The Red Queen model, developed by Van Valen, stems from Charles Darwin’s work—in which he viewed evolution as primarily a balance of biotic pressures, most notably competition—and was characterized by the Red Queen’s statement to Alice in Through the Looking-Glass that “it takes all the running you can do, to keep in the same place.” In contrast, the Court Jester model, presented by Tony Barnosky, is that evolution, speciation, and extinction rarely happen except in response to unpredictable changes in the physical environment, recalling the capricious behavior of the licensed fool of medieval times. Note that neither model was meant to be exclusive, and both Darwin and Van Valen allowed for extrinsic influences on evolution in their primarily biotic, Red Queen views.
Species diversity in a Red Queen world depends primarily on intrinsic factors, such as body size, breadth of physiological tolerance, or adaptability to unusually harsh environmental conditions. In a Court Jester world, species diversity depends on fluctuations in climate, landscape, and food supply. In reality, of course, both worldviews can prevail in different ways and at different times. Traditionally, biologists have tended to think in a Red Queen, Darwinian, intrinsic, biotic factors way, and geologists in a Court Jester, extrinsic, physical factors way.
Much of the divergence between the Red Queen and Court Jester worldviews may depend on the scale of observation. It is evident that biotic interactions drive much of the local-scale success or failure of individuals, populations, and species (Red Queen), but natural selection and the Red Queen also accommodate constantly changing climate and topography at the scale of intergenerationally differing selection pressures. However, perhaps these processes are overwhelmed by large-scale tectonic and climatic processes at timescales above 100,000 years (Court Jester), which may be too drastic for most species lineages to adapt, and they go extinct locally or globally.
The Red Queen and the Court Jester are in opposition in that their consequences differ. Further, the two models could be said to emanate from two different starting points: the Red Queen from considerations and observations of natural selection experiments and evolutionary ecology, the Court Jester from paleobiological and geological studies of global change over longer time spans. The divergence between the two could be interpreted as epistemological, a result of differing methodologies, or ontological, meaning it is real. Evolutionary biologists and paleobiologists are often warned not to scale processes between levels, for example, to assume that large clades act like species in competition and predator-prey interactions, or to assume that geologically instantaneous processes can be ecologically instantaneous also. In this regard, macroevolution is likely pluralistic, with intense biotic interactions shaping ecosystems and species evolution on a daily and yearly basis, and abiotic drivers acting over all timescales, but especially on timescales of centuries to millions of years.
Importantly, no matter whether either the Red Queen or the Court Jester model actually prevails in evolution and how they interact, extinction has a key role in marking the tempo of evolution within clades, and in punctuating the larger-scale, long-term patterns of the history of life.
See also chapter II.9.
FURTHER READING
Alvarez, L. W., W. Alvarez, F. Asaro, and H. V. Michel. 1980. Extraterrestrial cause for the Cretaceous-Tertiary extinction. Science 208: 1095–1108. The classic presentation of impact as a cause of extinction.
Bambach, R. K., A. R. Knoll, and S. C. Wang. 2004. Origination, extinction, and mass depletions of marine diversity. Paleobiology 30: 522–542. A thoughtful consideration of ecological aspects of mass extinctions.
Barnosky, A. D. 2001. Distinguishing the effects of the Red Queen and Court Jester on Miocene mammal evolution in the northern Rocky Mountains. Journal of Vertebrate Paleontology 21: 172–185.
Benton, M. J. 1995. Diversification and extinction in the history of life. Science 268: 52–58.
Benton, M. J. 2009. The Red Queen and the Court Jester: Species diversity and the role of biotic and abiotic factors through time. Science 323: 728–732. A review of the relative roles of biotic and abiotic factors on macroevolution.
Chen, Z. Q., and M. J. Benton. 2012. The timing and pattern of biotic recovery following the end-Permian mass extinction. Nature Geoscience 5: 375–383. A review of the recovery of life from the most profound mass extinction of all.
Leakey, R., and R. Lewin. 1996. The Sixth Extinction: Patterns of Life and the Future of Humankind. London: Weidenfield and Nicolson.
McKinney, M. L. 1997. Extinction vulnerability and selectivity: Combining ecological and paleontological views. Annual Reviews of Ecology and Systematics 26: 495–516. An exploration of biological correlates of vulnerability to extinction.
Raup, D. M. 1992. Extinction: Bad Genes or Bad Luck? New York: W. W. Norton. A lively presentation by the master of numerical paleobiology, giving a slant to extraterrestrial causation.
Raup, D. M., and J. J. Sepkoski Jr. 1982. Mass extinctions in the marine fossil record. Science 215: 1501–1503. The classic attempt to define mass extinctions statistically.
Raup, D. M., and J. J. Sepkoski Jr. 1984. Periodicity of extinctions in the geologic past. Proceedings of the National Academy of Sciences USA 81: 801–805. One of the most cited papers in paleobiology, in which evidence for periodicity of mass extinctions is presented.
Simpson, G. G. 1953. The Major Features of Evolution. New York: Columbia University Press. One of several books by George Simpson, founder of numerical paleobiology.
Stanley, S. M. 1979. Macroevolution: Pattern and Process. New York: W. H. Freeman.
Van Valen, L. 1973. A new evolutionary law. Evolutionary Theory 1: 1–30.
Van Valen, L. 1984. A resetting of Phanerozoic community evolution. Nature 307: 50–52.
Wignall, P. B. 2001. Large igneous provinces and mass extinctions. Earth-Science Reviews 53: 1–33. An excellent overview of an earthbound model for mass extinction arising from massive volcanic eruption.