4. Invasive species and climate change
Human activities are profoundly altering the biodiversity of the earth. The principal drivers of change thus far have been the transformation of lands for human use, accompanying fragmentation of remaining natural habitats, hunting, and modification of native disturbance regimes. These forces have resulted in the extinction of numerous species and radically altered the abundances of countless others. Empirical and theoretical studies imply that many more of the world’s species will experience the same fate as humanity’s collective impacts on the planet further expand and intensify. Long-lived plants and animals and animal species in which individuals range widely in space appear to be particularly vulnerable because of the strong dependence of their populations on the dwindling number of regions that are free of significant human influence. In numerical terms, the impacts of humans on terrestrial biodiversity are generally larger in the tropics because of the restricted spatial distributions of many tropical species and the high species diversity of many tropical ecosystems compared to their high-latitude counterparts. Two additional, and increasingly important, modifiers of terrestrial ecosystem biodiversity are the introduction of exotic species into ecosystems and human-induced changes in climate. These more recently recognized agents of change may act independently of, or synergistically with, land cover change, habitat fragmentation, hunting, and altered disturbance regimes to yet further modify the composition of the world’s ecosystems over the coming century and beyond.
early successional species. Species that appear in an ecosystem following a disturbance event, such as a fire, landslide, or logging. Early successional species typically possess r-selected traits, such as high dispersal ability, short generation time, and rapid growth, but at the expense of having a short lifespan and poor competitive ability. As a result, their population sizes usually increase immediately after disturbances, and then decline later as conditions become more crowded and they are competitively replaced by late successional species.
endemics. Species that have a relatively narrow geographic range, such as species found only on a particular island, or in a particular habitat or region.
fire return interval. The number of years between two successive fire events at a particular location.
invasive species. Introduced or nonindigenous species that are rapidly expanding outside of their native range.
late successional species. Species found in an ecosystem that has not experienced a disturbance for a long period of time. Late successional species typically have K-selected traits, such as long generation time, slow rates of growth, but long lifespan and strong competitive ability. As a result, late successional species come to dominate an ecosystem when no further disturbances occur.
species-area curve. A graph showing the number of species found in an area as a function of the area’s size.
Human population growth and economic development have led to a radical transformation of the earth’s land surface. As plate 8 illustrates, humanity’s footprint on the planet is now pervasive, with 83% of the earth’s surface being significantly affected by one or more of human land transformation, population density, power infrastructure, and transportation networks. In this chapter, I review how these and other human activities are affecting the biodiversity of terrestrial ecosystems. The focus of this chapter is on ecosystem biodiversity; however, as discussed in more detail in chapters IV.2, IV.6, and IV.8, an ecosystem’s species composition has important consequences for its biophysical and biogeochemical functioning.
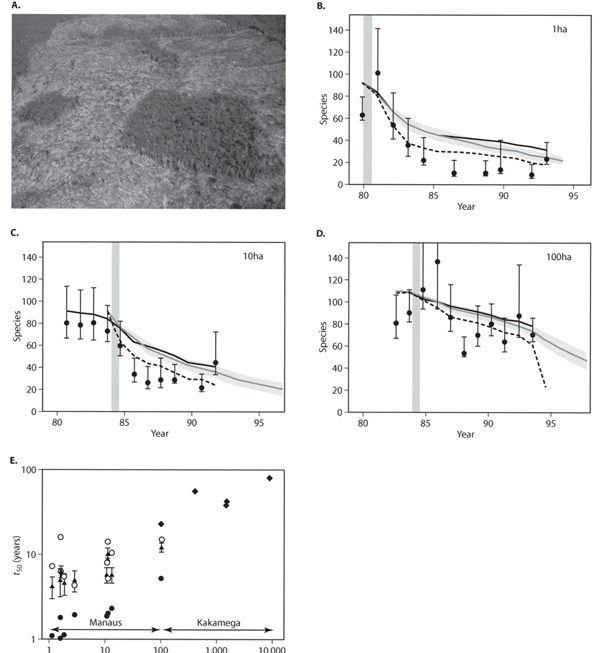
One of the most significant ways in which humans affect terrestrial biodiversity is the loss of species that accompanies the destruction of natural habitats. A particularly compelling study of this phenomenon is the Biological Dynamics of Forest Fragments Project (BDFFP) initiated by James Lovejoy and others in the late 1970s (Bierregaard et al., 2001), which is examining the consequences of habitat destruction in the tropical forests of the Amazon (figure 1A). Figure 1B-D shows the number of understory bird species in three forest fragments of different sizes experimentally created as part of the BDFFP. The initial species diversity of the understory bird community in the forest fragments was high, with the 3-, 11-, and 100-ha fragments, respectively, containing 90, 92, and 111 bird species (Ferray et al., 2003). However, following their creation, species diversity in the fragments declined rapidly: in a 13-year period, the number of species remaining in the 3-ha fragment decreased to approximately 30, and the diversity in the 11- and 100-ha fragments decreased to approximately 50 and 70 species, respectively (figure 1B-D). Figure 1E shows calculations of the time taken for the understory bird diversity to decrease to half its original value (t50) in a series of forest fragments created as part of the BDFFP, along with similar estimates for several larger (100 to 10,000 ha) forest fragments created in a tropical forest in Kenya (see Ferraz et al., 2003). As the figure illustrates, the rate at which species diversity decreases is strongly influenced by the size of the remaining forest fragment: small 1- to 10-ha fragments lost half their species in under 10 years, whereas the diversity of the larger 100- to 10,000-ha fragments declined more slowly, taking between 10 and 100 years to lose half of their species.
Ecologists have sought to predict the magnitude of species loss when habitat is removed. One simple approach for doing so utilizes so-called species-area curves, which, as implied by the name, describe how the number of species within a region increases as a function of its area. figure 2 shows species-area curves for reptiles on islands in the West Indies (figure 2A) and birds in the Sunda Islands (figure 2B) (MacArthur and Wilson, 1963, 1967). In both of these animal groups, the relationship between the number of species (S) and island area (A) can be reasonably described by a mathematical function of the form S = S0Az, where S0 and z are constants. When plotted on logarithmic axes, a straight-line relationship between area and species diversity results (figure 2), where the values of S0 and z, respectively, reflect the line’s intercept and its slope.
Knowing the relationship between species number and area yields a simple prediction for the biodiversity consequences of habitat loss. For example, figure 2C shows the predicted number of species lost following 10-fold reduction in habitat area for two different groups of organisms, one with a species-area exponent of 0.2 and the other with a species-area exponent of 0.35 (most species-area exponents lie in this general range; for example, the values for reptiles and birds plotted in figure 2A, B are 0.301 and 0.303, respectively). As figure 2C shows, following a 90% reduction in habitat, the number of species predicted to be lost when the species-area exponent is 0.2 is 37%, whereas for a species-area exponent of 0.35 the predicted loss is 55%, illustrating that, for a given amount of habitat loss, the fraction of species lost is higher for groups of organisms with higher species-area exponents.
Species-area curves have been widely applied to predict the rate at which habitat destruction is causing biodiversity loss. Two important cases to which this method has been applied are losses of bird species in eastern North America and in the Atlantic coastal forests of Brazil, areas that have both experienced extensive habitat loss. Eastern North American forests were extensively cleared in the centuries following the colonization of the North American continent, with forest cover declining to approximately 50% of its original extent during the mid-1800s. Given a species-area exponent between 0.2 and 0.3, species-area curves imply that between 13% and 19% of species diversity should have been lost as a result of this land clearing. Habitat loss in the Atlantic coastal forests of Brazil has been even more dramatic: approximately 90% of the original forest has been eliminated as a result of human agriculture and development. Species-area curves imply that this should have resulted in 37 to 50% of the original Brazilian Atlantic coastal forest bird species being eliminated (see figure 2C).
The actual rates at which species have been lost from these two regions have been significantly lower than the above species-area curve estimates. Only 2% of eastern North American bird species have become extinct, and less than 1% of Brazilian Atlantic coastal forest bird species are now extinct. Several factors account for this mismatch. In the case of eastern North American birds, of the 200 species found in the region, only 28 are endemic (i.e., are species found only within the region and not elsewhere). Considering only endemics, because these are species at risk of being lost as a result of habitat loss, 4 of 28 or 14% of the species were lost, a number that is consistent with the species-area curve predictions for the region (Pimm and Askins, 1995). In the case of Brazilian Atlantic coastal forest, there are 214 endemic bird species, and thus one would expect on the basis of typical species-area curve exponents that 79–108 bird species would be lost from the region (Brooks and Balmford, 1996). In reality, only one species has been lost thus far. However, because a significant amount of the coastal forest deforestation has been relatively recent, and the time scale for species extinctions is relatively long, it has been argued that it is more reasonable to consider threatened as well as extinct species because (in the absence of successful conservation intervention) it is only a matter of time before the threatened species also will become extinct. The number of endemic bird species in the Brazilian Atlantic coastal forest currently listed as threatened is 60, a number that is reasonably close to the 79–108 range predicted by species-area curve relationships.
Figure 1. (A) Photograph showing examples of the 1-ha and 10-ha forest fragments created as part of the BDFFP study of Amazonian deforestation. (From Bierregaard et al., 2001) (B–D) Plots of species loss for fragments according to four different estimation methods. The gray bars indicate the timing of isolation. (From Ferraz et al., 2003) (E) Estimated time to lose 50% of the species from the BDFFP forest fragments (open circles, closed circles, and triangles) and Kakamega (diamonds). The different symbols for the BDFFP fragements are estimates obtained from three different statistical models. (From Ferraz et al., 2003)
Figure 2. (A and B) Dots indicate the empirical species–area relationships for reptiles in the West Indies and birds in the Sunda Islands. Lines are fitted relations of the form S = S0Az, where A is area, S0 the number of species in a unit area of land, and z the species–area exponent. (C) Hypothetical species–area curves plotted for the case where z = 0.2 and 0.35, and S0 is 10. The shaded areas shows how a 90% decrease in area results in predicted species loss of 37% when z is 0.2 and 55% when z is 0.35. Steeper species–area relationships (higher values of z) imply greater proportional species loss for a given amount of habitat loss.
A further implication of the above focus on endemic species is that habitat loss will have a greater impact on biodiversity in areas with large numbers of endemics than in areas with few endemics. figure 3 shows a map of areas of high endemism—so-called “biodiversity hot spots”—around the globe. As the figure indicates, the majority of these areas are found in tropical regions, implying that the biodiversity consequences of habitat loss in tropical ecosystems will typically be considerably larger than in temperate ecosystems.
Human activities are rarely confined to small areas and instead are dispersed across landscapes. The resulting fragmentation of remaining natural habitats that accompany human land transformation has important consequences for an ecosystem’s flora and fauna over and above those caused by the reductions in the total area of natural habitat. The reason for this is various forms of “edge effects” caused by small patches of natural habitats having higher perimeter-to-area ratios compared to larger areas of natural habitat of equivalent total area (see chapter IV.5).
Species that range widely are particularly vulnerable to the deleterious effects of the edge effects caused by fragmentation. Clear evidence of this comes from a study by Woodroffe and Ginsberg (1998), who analyzed the occurrence of 10 large carnivore species as a function of reserve size (figure 4). As the figure shows, for each species there is a strong correlation between a reserve’s size and its probability of being occupied. These edge effects arise because, in small reserves, individuals of large carnivore species are more likely to stray outside of reserve boundaries, where they are much more likely to be killed by humans. Further support for this conclusion comes from that fact that the critical reserve size for each species, the reserve size at which there is a 50% chance of the species being present (point A50 on each panel of the figure), is positively correlated with the average home range size of the different species.
Figure 3. Twenty-five regions that contain areas of high endemism that are critical for preserving global biodiversity. Within each region, biodiversity “hot spots” comprise between 3% and 30% of the shaded area. (From Myers et al., 2000)
Similar evidence for the impacts of fragmentation has come from a number of studies. A high-profile case in the United States has been the case of the northern spotted owl (Strix occidentalis caurina), which lives in old-growth forests of the Pacific Northwest. Studies indicate that dispersing juvenile northern spotted owls suffer greatly increased rates of predation by great horned owls and goshawks when they fly over cleared areas of forest than in intact forest, making them vulnerable to the landscape fragmentation caused by clear-cut logging operations.
The loss of biodiversity arising from habitat fragmentation can have cascading effects on ecosystem composition, structure, and function. Dramatic evidence of this phenomenon has come from a study by John Terborgh and colleagues, who studied the effects of fragmentation of tropical forests on a series of islands that were created in 1986 by the rising waters that followed the construction of the Lago Guri hydroelectric dam in Venezuela. Terborgh and colleagues found that whereas large (150 ha) islands retained nearly all their original species diversity, medium (4-12 ha) and small (0.25-0.9 ha) islands lost more than 75% of their vertebrate species. Ecological guilds that were virtually absent on the small and medium-sized islands included frugivores, which are the principal seed dispersers in tropical forests, small mammal predators (felids, mustelids, snakes, and large raptors), and, in the case of small islands, armadillos, which prey on leaf-cutter ants (Terborgh et al., 2001).
The absence of the above ecological guilds following fragmentation had ramifying impacts on the remainder of the forest ecosystem. Leaf-cutter ant densities on the small islands were 100 times greater, and rodent densities on the small and medium-sized islands 35 times greater, than those found on the large islands and on the mainland. The subsequent increase in the intensity of herbivory, in turn, affected the forest canopy: densities of tree stems less than 1 m tall on small islands were, on average, 50% lower than those found on the large islands and on the mainland. The recruitment of canopy trees appears to have been particularly affected, with the average density stems of canopy species on the small islands being only ~20% of the average density found on the large islands and mainland. In addition to providing strong evidence for the biodiversity impacts of fragmentation on tropical forests, the Lago Guri study provides strong empirical support for the occurrence of ecological cascades within ecosystems and for the “green world hypothesis” (Hairston et al., 1960), which argues that in most ecosystems there is strong top-down regulation of herbivore abundance by predator species.
Figure 4. Proportion of reserves of various sizes in which 10 species of large carnivores have persisted. Population persistence is related to reserve area for all species. Curves show the probability of persistence predicted by a simple statistical model fitted to the binary data; filled circles show the critical reserve sizes (±SE) for which the models predict a 50% probability of population persistence (A50). Species: (A) black bear; (B) jaguar; (C) snow leopard; (D) tiger; (E) spotted hyena; (F) lion; (G) dhole; (H) gray wolf; (I) African wild dog; (J) grizzly bear. (From Woodroffe and Ginsberg, 1998)
Similar, albeit less well-documented, ecological cascades arising from habitat fragmentation appear to be occurring in many of the world’s tropical forests. In particular, in both the Amazon and in Southeast Asia, evidence suggests that the abundance and distribution of many species of wild pigs have been severely disrupted by habitat fragmentation. Like the frugivorous primates in the Lago Guri study, pigs are an important group of seed dispersers in many tropical forests. As a result, changes in their abundance and distribution are affecting forest canopy biodiversity in many tropical areas.
Another important edge effect occurring in fragmented tropical forest landscapes arises when surface fires started in surrounding agricultural areas spread into remaining areas of forest. Analysis of satellite imagery in Amazonia has shown that fire return intervals are reduced to less than 20 years within 500m of a forest edge, compared to more than 100 years in the forest interior (figure 5A). Evidence from the BDFFP described earlier suggests that the increased frequency of burning in forest areas that adjoin pastures is exacerbated by changes in microclimate at forest-pasture interfaces. These changes in microclimate extend over 100m into surrounding forest, causing significant declines in canopy tree biomass and shifting the composition of the canopy toward early successional and fire-adapted tree species (figure 5B). Analyses of satellite measurements of cloud cover and atmospheric modeling studies also suggest that, in heavily fragmented forest landscapes, changes in the biophysical properties of the land surface arising from the conversion from forest to pasture may be altering local scale atmospheric circulation patterns and resulting spatial and temporal patterns of precipitation.
Figure 5. (A) Fire frequency as a function of proximity to forest edge in Amazonia measured by Cochrane (2003). (B) Forest dynamics measured by Laurance et al. (1998) as a function of proximity to forest edge. Panel shows annual rates of mortality, tree damage, and turnover (turnover = (mortality + recruitment)/2).
The abundant evidence regarding the detrimental effects of habitat fragmentation has led to increasing calls by a number of conservation biologists for the establishment of “megareserves”—large areas of undisturbed natural habitat that are 10,000 km2 or larger in size. The logic for this is that only such extensive areas of natural habitats will avoid the various deleterious forms of edge effects described above and thus sustain the full complement of species within an ecosystem. Megareserves also have the additional advantage that concentrating areas of natural habitat in one area allows for easier and cheaper enforcement of boundaries than a widely scattered set of reserves of equivalent total size. For example, on a per-unit basis, the maintenance costs per unit area of Brazil’s approximately 38,670km2 Serra do Tumucumaque National Park are about 18,000 times lower than those for the 1.1km2 Sauim-Castanheira Ecological Reserve.
From the perspective of biodiversity preservation, the reductions in edge effects and the cost savings associated with megareserves must, however, be tempered against another ecologically important consideration: preserving regional species diversity by maintaining reserves across the range of habitat types found within a region. In most cases, this naturally implies a network of reserves rather than one single large reserve. Consideration then has to be given to the spatial arrangement of the reserves and the desirability of providing corridors for migration and dispersal of species among the different areas. Questions about the optimal spatial configuration of habitat reserves were historically argued in qualitative terms, with ecologists debating, often heatedly, about whether a single reserve would preserve a greater diversity of species than an equivalently sized collection of smaller reserves. This so-called single large or several small (SLOSS) debate has largely been superseded in modern analyses of habitat preservation, which instead focus on developing specific recommendations for particular species and habitats. In addition to ecological concerns regarding edge effects and how species composition varies among habitat types, modern analyses often also take into account pragmatic issues such as land costs and the potential for multiple-use areas designed to meet other human land needs such as recreation.
Simple approaches for predicting the impacts of human activities on ecosystem biodiversity, such as the species-area curve approach described earlier, do not differentiate among species. In reality, a number of ecological factors result in certain species being more vulnerable to human activities than others. One of the strongest predictors of a species’ risk is its body size. figure 6 shows the percentage of mammalian genera that have become extinct in the past 130,000 years. As can be seen in the figure, the extinction rate has been far higher in larger mammals than in smaller ones. Although only a few of these extinctions can be categorically attributed to human activities, the timing of the losses in different regions correlates closely with patterns of human migration, and thus, it is generally thought that the vast majority of the losses were, either directly or indirectly, caused by human activities.
The increased vulnerability of larger species is a natural consequence of their accompanying life-history characteristics. Larger species tend to have longer generation times and slower rates of reproduction (so-called K-selected species) compared to smaller species that have shorter generation times and higher rates of reproduction (so-called r-selected species). As a result, larger species have slower intrinsic rates of population growth, making them more vulnerable to increases in mortality and/or declines in recruitment caused by human activities. Larger-sized species also tend to have larger home range sizes, making them more vulnerable to habitat fragmentation and loss (see habitat fragmentation section).
Figure 6. The effects of body size on the extinctions of mammalian herbivore genera in North America, South America, Europe, and Australia. (Figure redrawn from Owen-Smith, 1987)
In the past, the principal mechanism of human-induced declines in species abundance and eventual species loss was almost certainly hunting, an explanation sometimes referred to as the “overkill hypothesis.” Hunting continues to be a major cause of population decline in some regions. So-called bushmeat continues to be a key source of protein for human populations in a number of developing countries. For example, the bushmeat trade in Ghana is estimated conservatively to be 400,000 tons/year and is a major contributor to the declines of many of its carnivore, primate, and large herbivore species. A recent study found that from 1970 to 1988, the biomass of 41 species of mammals in nature reserves in Ghana had deceased by 76%, with many species becoming locally extinct in many of the reserves (Brashares et al., 2004).
Many modern-day species population declines are, however, the result of ecological forces other than hunting. In addition to habitat loss and habitat fragmentation discussed above, another major driver of biodiversity modification and loss is arising from human modification of disturbance regimes. Most ecosystems are subject to one or more natural forms of episodic disturbance, such as hurricanes, flooding, fire, landslides, or pathogen outbreaks. These disturbances are significant from a biodiversity perspective because they maintain so-called successional diversity within ecosystems: species that in the absence of disturbance would be excluded from an area by competitively superior species are able to persist by continually exploiting newly disturbed areas. This connection between the species composition of an ecosystem and its disturbance regime means that any shift in the intensity or frequency of disturbance will almost inevitably alter its species composition.
From a global perspective, the most significant human modification of natural disturbance regimes has been through changes in fire frequency. Figure 7A, B shows the changes in fire regimes that have occurred in South America and Southeast Asia over the past 100 years. As the figure illustrates, over the past century, tropical forests in both the New World and Old World have experienced major increases in fire frequency. A similar, though less pronounced, trend is also seen in tropical Africa. As described earlier (see habitat fragmentation section), fire profoundly alters the structure and composition of tropical forests. In particular, when the mean return time between successive fire disturbances is reduced to under 100 years, late successional old-growth tree species, such as Mahogany species in South American forests and Dipterocarp species in Southeast Asian forests, are unable to persist because of their slow growth rates and long generation times. They are replaced by early successional pioneer species, such as Cecropia and Vismia in South America and Macaranga species in Southeast Asia, whose fast growth rates and short generation times enable them to flourish when the time between successive disturbances is on the time scale of decades rather than centuries.
Another major factor altering the disturbance regimes of tropical forest ecosystems is timber harvesting. Figure 7E shows rates of timber harvesting around the globe, showing the marked increases in the extent of forest logging operations that have occurred over the past 300 years in tropical forests. As with body size discussed earlier, the increases in fire frequency and rates of timber harvesting in tropical forests are yet another manifestation of human activities increasingly favoring r-selected species over K-selected species.
Unlike the tropics, temperate ecosystems in the United States have experienced significant declines in fire frequency over the past 100 years (figure 7C, D). The primary cause of this has been human fire-suppression activities, which, until recently, have been a significant feature of ecosystem management in both the eastern and western United States. As would be expected from ecological theory, the decrease in fire frequency in the temperate United States that occurred over the last century has favored late successional species at the expense of early successional species. In the arid savanna and woodland ecosystems of the southwestern United States, this has led to a widespread phenomenon of “woody encroachment” in which early successional grasses and forbs are competitively replaced by woody shrubs and trees. This phenomenon has become a major management issue in the Southwestern states of Arizona, Utah, New Mexico, and Texas.
In forested regions of the United States, the declines in fire frequency arising from fire-suppression activities have been accompanied by increases in rates of timber extraction (figure 7E). In the West, logging has primarily been in the form of clear-cuts in which essentially all of the merchantable timber is removed from an area. In the East, there was large-scale clearing of the eastern forests for agriculture during the eighteenth century, followed by large-scale agricultural abandonment in the late nineteenth and early twentieth centuries. This widespread agricultural abandonment resulted in a large-scale increase in forest area compared to 150 years ago. However, there has been continual selective logging of the eastern forests, which has kept the mean time between disturbances low and thus prevented any large-scale shift back toward late successional tree species that were present before European colonization.
Figure 7. Temporal trends in area burned per year over the past century in (A) South America, (B) Southeast Asia, (C) the western United States, and (D) the eastern United States. (From Mouillot and Field, 2005) (E) Magnitude of timber harvesting on different continents over the past 300 years. The global integrated wood harvest for the 1700–2000 period is 86 Pg C (FSU = Former Soviet Union). (From Hurtt et al., 2006)
A complicating factor in studying the changes in disturbance regimes is the continuing debate about past human activities. For example, analyses of pollen in lake sediments suggest that in the United States Native Americans maintained elevated fire disturbance regimes in many regions that significantly impacted their composition before European colonization (Delcourt et al., 1998).
Thus far, this chapter has focused on the impacts of human activities on terrestrial ecosystem biodiversity arising from habitat loss, hunting, and changing disturbance regimes. Two additional, and increasingly important, modifiers of terrestrial ecosystem biodiversity are invasive species and human-induced climate change.
The colonial era heralded a vast increase in the rate of exchange of organisms between regions, islands, and continents that were formerly isolated from each other. Continuing increases in the extent of global trade mean that this rate of biotic exchange is increasing still further. Not surprisingly, this large-scale reorganization of the global biota is having major impacts on the diversity of many terrestrial ecosystems, with certain countries, such as New Zealand, now containing as many alien species as they do native ones.
From a biodiversity perspective, the most profound consequence of an invasive species is extinction of native flora and fauna. Examples of this include the infamous introduction of the brown tree snake (Boiga irregularis) to Guam in the 1950s, which, in just a few decades, caused extinction of more than 10 bird species, including some endemic only to Guam (Rodda et al., 1997). Similarly, the introduction of the rosy wolf snail (Euglandina rosea) to the Society Islands in order to control the giant African snail (an agricultural pest) resulted in the extinction of numerous species of endemic Partula land snails (Strong et al., 2000; see Mooney and Cleland, 2001). To date, extinctions have arisen through the predatory and pathogenic effects of invasive species rather than as a result of competitive interactions between invasive and native species. Some ecologists have argued that it is simply a matter of time before such competition-induced extinctions occur, but others maintain that competing native and invasive species will in many cases coexist, thereby resulting in long-term increases rather than decreases in the biodiversity of many ecosystems.
Regardless of whether the long-term outcome of their competitive interactions is coexistence or eventual competitive exclusion, what is not in debate is that invasive species are having marked effects on the abundance of the native flora and fauna of many ecosystems. Sometimes these are the result of direct interactions, such as the competitive displacement of a native Californian ant species by the invasive Argentine ant (Linepthaema humile) or the predatory effects of the brown tree snake in Guam described earlier. Some of the most dramatic impacts of invasive species occur, however, when invasive species alter the disturbance regime of an ecosystem. A classic example of this is cheatgrass (Bromus tectorum), an invasive annual grass species in the United States that has resulted in a 10-fold increase in the fire frequency of over 40 million ha of land in the western states. This modified disturbance regime is, in turn, favoring yet further spread of this fire-adapted species at the expense of native vegetation. Similarly, several species of flammable grasses introduced into Hawaii for agriculture have spread into native woodlands, causing a 300-fold increase in fire frequency that is threatening to eliminate many species of woody native vegetation (D’Antonio and Vitousek, 1992).
One other biodiversity consequence of invasive species can be the production of novel species through hybridization. A classic case of this is Spartina alterniflora, a native coastal grass species of the eastern United States that was introduced to Great Britain around 1870. Following its introduction, S. alterniflora hybridized with the native S. maritima, producing S. anglica, an aggressive hybrid species that subsequently has spread widely along the British coastline (Thompson, 1991; Mooney and Cleland, 2001; Gray, 1986).
An important challenge for invasive species management has been diagnosing what traits make a species a successful invasive. An analysis of invasive and noninvasive pines in the United States found that the invasive species characteristically had r-selected traits, such as low seed mass, faster growth, and more frequent seeding compared to the noninvasive species. Another observation has been that a disproportionate number of thistle species in the Cirsium family and grass species in Poa and Bromus families are invasive. Analyses conducted for numerous other plant and animal groups have, however, failed to identify any key distinguishing traits of invasive species compared to either native species or noninvasive species. A related challenge is identifying what characteristics make an ecosystem vulnerable to invasion. A study of invasives in the United Kingdom found that plant communities could be ranked in terms of invasibility based on the proportion of bare ground and on the frequency and intensity of soil disturbance. Similar patterns have been found in other studies, indicating that invasions generally occur more readily into disturbed habitats compared to undisturbed ones.
Human-induced climate change is increasingly being recognized as another major driver of future biodiversity change. The two primary underlying causes of human-induced change are the increasing concentration of carbon dioxide in the atmosphere arising from the burning of fossil fuels and the changing biophysical properties of the land surface arising from human land-use transformation. A simple method for calculating the impacts of climate change on ecosystem diversity uses a “climate envelope” approach, in which the relationship between a species’ current distribution and climatic variables, such as temperature, precipitation, and rainfall, is used in conjunction with climate projections to predict the expected change in its spatial distribution that will occur as a result of climate change. This approach makes a number of simplifying assumptions, ignoring interactions between species, and assuming that species are in equilibrium with climate, both now and in the future. However, climate envelope approach predictions are nonetheless sobering.
Figure 8. (left) Frequency distribution showing the predicted magnitude of range shifts for 179 South African animals following a doubling of atmospheric CO2 concentrations calculated using climate envelope relationships. (right) Spatial pattern of species richness for the 179 South African animals under (A) current climate and (B) projected future climate. (From Erasmus et al., 2002)
Figure 8A shows a histogram of the predicted magnitudes of range shifts for 179 South African animal species (including mammals, birds, reptiles, and insects) following a doubling of atmospheric carbon dioxide concentrations. As the histogram illustrates, more than 25% of the species are predicted to undergo range shifts in excess of 90%. Climate-induced range shifts of this magnitude would be potentially catastrophic for many species, especially for species that have limited dispersal abilities or that are already endangered as a result of other human activities. Figure 8B and 8C shows the resulting changes in spatial patterns of species richness per unit area, calculated by overlaying the climate envelopes of the 179 species under the current climate (figure 8B) and under the projected future climate scenario (figure 8C). The projections imply that marked losses in species diversity will occur in the western and, to a lesser extent, the southern parts of the country. A particularly sobering statistic from this analysis is that Kruger National Park, the flagship national park in South Africa, is predicted to lose up to two-thirds of its species. A recent climate envelope-based estimate for the global extent of species loss implies, controversially, that 15–37% of the world’s species will be threatened by extinction by 2050 as a result of human-induced climate change.
Bierregaard, R. O., C. Gascon, T. E. Lovejoy, and R. Mesquita. eds. 2001. Lessons from Amazonia: The Ecology and Conservation of a Fragmented Forest. New Haven, CT: Yale University Press.
Brashares, J. S., P. Arcese, M. K. Sam, P. B. Coppolillo, A.R.E. Sinclair, and A. Balmford. 2004. Bushmeat hunting, wildlife declines, and fish supply in West Africa. Science 306: 1180–1183.
Brooks, T., and A. Balmford. 1996. Atlantic forest extinctions. Nature 380: 115.
Chapin, F. S., E. S. Zavaleta, V. T. Eviner, R. L. Naylor, P. M. Vitousek, H. L. Reynolds, D. U. Hooper, S. Lavorel, O. E. Sala, S. E. Hobbie, M. C. Mack, and S. Diaz. 2000. Consequences of changing biodiversity. Nature 405: 234 -242.
Cochrane, M. A. 2003. Fire science for rainforests. Nature 421: 913–919.
D’Antonio, C. M., and P. M. Vitousek. 1992. Biological invasions by exotic grasses, the grass-fire cycle, and global change. Annual Review of Ecology and Systematics 23: 63–87.
Davis, M. 2003. Biotic globalization: Does competition from introduced species threaten biodiversity? Bioscience 53: 481–489.
Delcourt, P. A., H. R. Delcourt, C. R. Ison, W. E. Sharp, and K. J. Gremillion. 1998. Prehistoric human use of fire, the eastern agricultural complex, and Appalachian oakchestnut forests: Paleoecology of Cliff Palace Pond, Kentucky. American Antiquity 63: 263–278.
Erasmus, B.F.N., A. S. Van-Jaarsveld, S. L. Chown, M. Kshatriya, and K. J. Wessel. 2002. Vulnerability of South African animal taxa to climate change. Global Change Biology 8: 679–693.
Ferraz, G., G. J. Russell, P. C. Stouffer, R. O. Bierregaard, Jr., S. L. Pimm, and T. E. Lovejoy. 2003. Rates of species loss from Amazonian forest fragments. Proceedings of the National Academy of Sciences, U.S.A. 24: 14069–14073.
Gray, A. J., R. N. Mack, J. L. Harper, M. B. Usher, K. Joysey, and H. Kornberg. 1986. Do invading species have definable genetic characteristics? Philosophical Transactions of the Royal Society of London B 314: 655–674.
Hairston, N. G., F. E. Smith, and L. B. Slobodkin. 1960. Community structure, population control, and competition. American Naturalist 94: 421–424.
Hurtt, G. C., S. Frolking, M. G. Fearon, B. Moore III, E. Shevliakova, S. Malyshev, S. Pacala, and R. A. Houghton. 2006. The underpinnings of land-use history: Three centuries of global gridded land-use transitions, wood harvest activity, and resulting secondary lands. Global Change Biology 12: 1–22.
Laurance, W. F., L. V. Ferreira, J. M. Rankin-de-Merona, and S. G. Laurance. 1998. Rainforest fragmentation and the dynamics of Amazonian rainforest communities. Ecology 79: 2032–2040.
MacArthur, R. H., and E. O. Wilson. 1963. An equilibrium theory of insular zoogeography. Evolution 17: 373–387.
MacArthur, R. H., and E. O. Wilson. 1967. The Theory of Island Biogeography. Princeton, NJ: Princeton University Press.
Manchester, S. J., and J. M. Bullock. 2000. The impacts of non-native species on UK biodiversity and the effectiveness of control. Journal of Applied Ecology 37: 845–864.
Mooney, H. A., and E. E. Cleland. 2001. The evolutionary impact of invasive species. Proceedings of the National Academy of Sciences, U.S.A. 10: 5446–5451.
Mouillot, F., and C. B. Field. 2005. Fire history and the global carbon budget: A 1° × 1° fire history reconstruction for the 20th century. Global Change Biology 11: 398–420.
Myers, N., R. A. Mittermeier, C. G. Mittermeier, G.A.B. da Fonseca, and J. Kent. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858.
Owen-Smith, N. 1987. Pleistocene extinctions: The pivotal role of megaherbivores. Paleobiology 13: 351–362.
Peres, C. A. 2005. Why we need megareserves in Amazonia. Conservation Biology 19: 728–733.
Pimm, S. L., and R. A. Askins. 1995. Forest losses predict bird extinctions in eastern North America. Proceedings of the National Academy of Sciences, U.S.A. 92: 9343–9347.
Pimm, S., P. Raven, A. Peterson, C. H. Sekercioglu, and P. R. Ehrlich, 2006. Human impacts on the rates of recent, present, and future bird extinctions. Proceedings of the National Academy of Sciences, U.S.A. 103: 10941–10946.
Rodda, G. H., T. H. Fritts, and D. Chiszar. 1997. The disappearance of Guam’s wildlife. BioScience 47: 565–574.
Sanderson, E. W., M. Jaiteh, M. A. Levy, K. H. Redford, A. V. Wannebo, and G. Woolmer. 2002. The human footprint and the last of the wild. BioScience 52: 891–904.
Terborgh, J., K. Freeley, M. Silman, P. Nunez, and B. Balukjian. 2006. Vegetation dynamics of predator-free land bridge islands. Journal of Ecology 94: 253–263.
Terbough, J., L. Lopez, P. Nunez, V. M. Rao, G. Shahabuddin, G. Orihuela, M. Riveros, R. Ascanio, G. H. Adler, T. D. Lambert, and L. Balbas. 2001. Ecological meltdown in predator-free forest fragments. Science 5548: 1923–1926.
Thomas, C. D., A. Cameron, R. E. Green, M. Bakkenes, L. J. Beaumont, Y. C. Collingham, B.F.N. Erasmus, M. F. de Siqueira, A. Grainger, L. Hannah, L. Hughes, B. Huntley, A. S. van Jaarsveld, G. F. Midgley, L. Miles, M. A. Ortega-Huerta, A. T. Peterson, O. L. Phillips, and S. E. Williams. 2004. Extinction risk from climate change. Nature 427: 145–148.
Thompson, J. D. 1991. The biology of an invasive plant. BioScience 41: 393–401.
Woodroffe, R., and J. R. Ginsberg. 1998. Edge effects and the extinction of populations inside protected areas. Science 280: 2126–2128.