Figure 143. Comparison of aging patterns of total outlet in human female and male
Showing active median frequency of orgasm in total sexual outlet. Data estimated for pre-adolescence are shown by broken line. Data from Table 154 and our 1948:226.
We have seen that sexual responses depend upon a basic anatomy which is essentially the same in the female and the male (Chapter 14 ), and involve physiologic processes which, again, are essentially the same in the two sexes (Chapter 15 ). Throughout the present volume we have found, however, that there are differences in the sexual behavior of females and males, and we have presented data which suggest that some of these may depend upon differences in capacities to be affected by psychosexual stimuli.
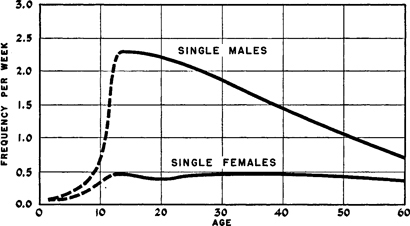
Some of the most striking differences between the sexual patterns of the human female and male are not, however, explainable by any of the data which we have yet presented. Throughout the present volume we have emphasized, for instance, the later development of sexual responsiveness in the female and its earlier development in the male. We have pointed out that the male’s capacity to be stimulated sexually shows a marked increase with the approach of adolescence, and that the incidences of responding males, and the frequencies of response to the point of orgasm, reach their peak within three or four years after the onset of adolescence (Figure 143 ). On the other hand, we have pointed out that the maximum incidences of sexually responding females are not approached until some time in the late twenties and in the thirties (Figures 99 , 150 ), although some individuals become fully responsive at an earlier age.

Figure 143. Comparison of aging patterns of total outlet in human female and male
Showing active median frequency of orgasm in total sexual outlet. Data estimated for pre-adolescence are shown by broken line. Data from Table 154 and our 1948:226.
We have pointed out that the frequencies of sexual response in the male begin to decline after the late teens or early twenties, and drop steadily into old age (Figure 143 ). On the other hand, we have shown that among females the median frequencies of those sexual activities which are not dependent upon the male’s initiation of socio-sexual contacts, remain more or less constant from the late teens into the fifties and sixties (Figures 143 –145 ). Nothing that we know about the anatomy or physiology of sexual response, or about the relative significance of psychologic stimuli in females and males, would account for these differences in the development of sexual responsiveness, and for these differences in the aging patterns of the two sexes.
In attempting to identify other factors which might affect sexual capacities, it should, again, be emphasized that sexual response is primarily a function of the nervous system. Muscles and blood vessels and other anatomic structures become involved only as a result of the stimulation of the nerves which control those organs. Factors which affect the level of an individual’s capacity to respond sexually must be factors which in some way determine the capacities of the nervous system, or some portion of it, to be affected by sexual stimuli.
There is usually considerable variation in an animal’s capacity to respond sexually at different periods in its life, and even on different occasions within a short span of time. The newly-born animal’s capacity to be sexually aroused may be less than the capacity of the somewhat older animal. Individuals who have reached old age are no longer as capable of responding as they were at an earlier age. The capacity of an animal to respond in a particular sexual situation may be considerably reduced or may totally disappear if the stimulation is continued without interruption for a protracted period of time. Individuals who are physically exhausted, starved, or in ill health are not easily aroused sexually; or if they are aroused, they may not be capable of effective action and may fail to reach orgasm. Such data suggest that anything that modifies the physiologic level at which an animal functions may, through its effect upon the nervous system, modify the general nature of its sexual behavior.
Among the internal factors which may affect the way in which the animal body functions, the best understood are the hormones. These are chemical substances which are produced chiefly in endocrine organs, from which they are ultimately carried by the blood stream to every part of the vertebrate body. Because of their accessibility to all parts of the body, hormones may have more effect on bodily functions than any other mechanism except the nervous system.
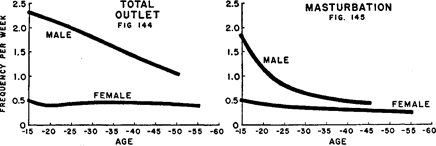
Figures 144–145. Comparison of aging patterns among single females and males
Showing contrasts in active median frequencies of orgasm in female activities which are not primarily dependent on the male. Data from Tables 154 and 23 , and our 1948:226, 240.
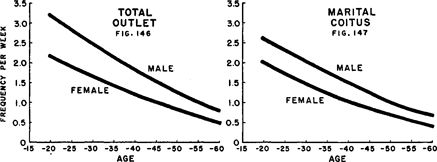
Figures 146–147. Comparison of aging patterns among married females and males
Showing how active median frequencies of orgasm attained in socio-sexual contacts in the female are affected by the patterns of male activity. Data from Tables 154 and 93 , and our 1948:226, 241.
Discovery of the Hormones . Among all of the hormones that are now known to exist in the vertebrate body, the so-called sex hormones were practically the first to be discovered.

Figures 148–150. Comparisons of Female and Male Experience and Orgasm
Accumulative incidences. For most outlets, male experience and orgasm curves are nearly identical. Female curves showing experience in heterosexual activities are closer to male curves, because the male determines the pattern. Female curves showing orgasm, rise more slowly and do not reach their peak until the mid-twenties or still later. Data from Tables 25 , 42 , 56 , 75 , 131 , and our 1948:500, 520, 534, 550, 624.
It is probable that most races of men, including even the most primitive, have recognized that the testes served two roles—one in connection with reproduction, and one in connection with the growth and function of the body as a whole. 1 Long before it was known that the ovaries and testes produce specific reproductive cells, the eggs and the sperm, it was known that some substance produced by the male had to be transferred to the female tract before she could reproduce; and the inability of a male to contribute to such reproduction after his testes had been removed provided early evidence that they were the source of an essential part of this fertilizing substance.
Figure 151. Multiple orgasm in female and male
Active incidences in coitus. For the male the curves show an aging effect, and for the female a plateau extending from the mid-teens into the late fifties. The differences between the two curves more or less parallel the differences between the curves for the total outlet of females and males (Figure 143 ). Data from Table 176 and our 1948:232.
Although there was, of course, no understanding that a chemical mechanism was involved, both primitive and ancient peoples also recognized that the early physical development of the male animal and its capacity to engage in sexual activity also depended upon its possession of testes. Human male castrates as well as castrates among farm animals were known to the earliest peoples in all parts of the world, and consequently the effects of castration were well understood at an early date. 2 Because the ovaries are within the body cavity, female castrations are more difficult to perform, and were rarely done before the days of modern medicine. Consequently the significance of the ovaries in regulating body functions was not understood at as early a date.
Table 177. Multiple Orgasm
Average Number of Orgasms in Each Coital Experience
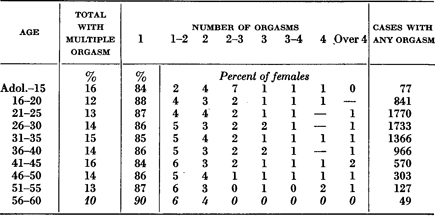
Table based on all females who had had coitus at least 25 times, irrespective of marital status.
Italic figures throughout the series of tables indicate that the calculations are based on less than 50 cases. The dash (—)indicates a percentage smaller than 0.5.
Table 178. Accumulative Incidence: Onset of Menopause
By Educational Level

The ancients knew that the effects of castration depended upon the age at which the human or other male was castrated. They knew that when the testes of the human male were removed before the onset of adolescence, the effects were more marked than they were when the castration was performed on a male who was a fully grown adult. 3 The basic biology of these matters did not find an explanation, however, until the middle of the nineteenth century.
In 1835, Graves recognized the relation between thyroid pathologies and the physiologic disturbances which accompany the disease which now bears his name. In 1849, Berthold, studying castrations and testicular implants in fowl, concluded that the testes secreted one or more blood-borne substances which were responsible for the modifications which his experimental castrations had produced. Within the next decade, Addison had noted the deterioration of the adrenal cortex in victims of the disease which bears his name. By the mid-seventies, Gull had identified the role of the thyroid in certain pathologic conditions; by 1887 Minkowski associated acromegaly with pituitary hyperfunction, and two years later (in 1889) von Mering and Minkowski had removed the pancreas and experimentally produced diabetes. The work of Brown-Sequard in 1889 revived interest in the utilization of testicular extracts in clinical practice, and since then there has been a tremendous development of experimental work on the significance of both testicular and ovarian hormones. 4
The list of endocrine glands which are now recognized in the vertebrate body include the ovaries and testes (or the gonads , as the two sets of organs are generically called), the pituitary gland which is located at the base of the brain, the thyroid and parathyroid glands which are located in the throat, and the adrenal glands which are located at the top of the kidneys near the small of the back. The thymus is a gland which reaches its maximum development in the early life of the animal but degenerates considerably after that. 5 The pineal gland, in the brain, may be an endocrine organ. 6 The liver and the pancreas, in addition to secreting substances which directly affect digestion, also produce important hormones which influence the development and the maintenance of activities of other organs of the body. 7
The testes are the chief source of the several hormones known as androgens (the so-called male hormones) in the body of the male. One of the best known androgens is testosterone. The ovaries are the chief source of the so-called female hormones in the female. The most prominent of the female hormones are the estrogens and progesterone. 8 As a group, hormones from the ovaries and testes may be referred to as gonadal hormones.
In recent years, the hormones produced by most of the endocrine glands have been isolated and identified as specific chemical substances. Many of them are closely related compounds of carbon, hydrogen, and oxygen. For instance, the androgens and estrogens, the 17-ketosteroids which are produced by the adrenal glands and by some other structures in the body, and the steroids which are chemicals characteristically found in all animal tissues, are all closely related chemical compounds, even though each may have a different and unique effect on the physiology of the body. Some of the other hormones, such as those produced by the thyroid and the pituitary glands, are totally different in their chemical composition.
Nature of the Hormones . A general knowledge of the hormones has become widespread in the population as a whole, but in regard to certain critical matters this knowledge is quite incorrect. Journalistic accounts of scientific research, over-enthusiastic advertising by some of the drug companies, over-optimistic reports from clinicians who have found a lucrative business in the administration of sex hormones, and some of the discussions among state legislators and public administrators who hope that hormone injections will provide one-package cure-ails for various social ills, have led the public to believe that endocrine organs are the glands of personality, and that there is such an exact knowledge of the way in which they control human behavior that properly qualified technicians should, at least in the near future, be able to control any and all aspects of human sexual behavior. It is, therefore, important that the general reader understand the nature of the hormones, and understand some of the difficulties that are involved in the accumulation and interpretation of data in the field of endocrinology.
Hormones are products of the physiologic processes that go on in certain of the gland cells that are to be found in the plant or animal body. Any cell which secretes a liquid content which becomes a significant part of the total volume of the cell, or which secretes materials which work their way out of the cell through a permeable cell wall or through some rupture of the wall, may be identified as a gland cell. Many of the cells of the body, and particularly those that line various body cavities, may be considered gland cells even though they are not part of a specific organ which is identifiable as a gland. Consequently, it is not always possible to identify all of the sources of the hormones in an animal’s body, even including the androgens and estrogens and the 17-ketosteroids, for part of the hormone may come from cells or groups of cells which lie outside of the specific organs which are known to be the chief sources of these hormones. For instance, the removal of the ovaries or testes (as in a complete castration) may not eliminate all the sources of the sex hormones, and this is one reason that it is difficult to interpret some of the experimental data. 9
In more complex glands, the secreting cells may pour their products into internal cavities from which ducts may carry them away. This is true, for instance, of the salivary glands. On the other hand, the glandular structures which give rise to the best known of the hormones do not have either internal cavities or ducts. Their secretions are picked up by the blood vessels which enter or surround the glands, and are thus carried away by the blood stream to other parts of the body. The structures are therefore known as ductless glands, or glands of internal secretion, or endocrine (meaning internally secreting ) organs. There are, however, hormones produced by gland cells in other types of structures, such as the placenta and the duodenal mucosa; and there is some reason for believing that most of the organs in the mammalian body may produce, in actuality, substances which, when circulated through the blood stream, may influence the activities of at least some of the other organs. 10
The hormones produced by any endocrine gland may affect other endocrine glands as well as organs which are not glandular. For instance, the secretions of the testes and ovaries have a direct effect on the anterior lobe of the pituitary and on the adrenal glands, and each of these has a direct effect on the testes and ovaries. Consequently an increase or decrease in the secretory capacity of any one of these glands may be reflected in the activities of the other glands. 11 Some of the other endocrine organs, such as the thyroid, may similarly affect the secretory capacities of the ovaries and testes, and of the pituitary and adrenal glands.
Although the effectiveness of any hormone is usually proportionate to the amount which is available, there is usually a point of optimum effectiveness, and an increase in the amount of hormone beyond that point may have negative effects which, in certain respects, may be as extreme as those obtained when there is an under-supply or complete removal of the source of supply of the hormone. 12
Usually the amount of hormone produced in an endocrine organ such as a testis or ovary is very small, and the amount that is to be found in the blood or the urine or at any other point in the body is so exceedingly minute that its recovery and chemical identification may be very difficult. Consequently, most of the reports of female and male hormone levels do not rest upon physical or chemical measurements, but upon such indirect evidence as can be obtained by injecting urine or blood extracts into experimental animals, and upon measurements of the changes which are thus effected in the growth or degeneration of some structure (like a rooster’s comb) in the experimental animal. Difficulties in measurement have been the source of considerable error in much of the reported work, including the studies which have attempted to analyze the relationships of the sex hormones and sexual behavior. 13
Moreover, when the amount of hormone in an animal’s body is determined by measuring the hormone in its urine, it is questionable what relation the amount of excreted material may have to the amount of the hormone that the body is actually utilizing. The hormone in the urine may merely represent that portion which the body has been unable to utilize. Recent endocrinologic research indicates that this latter may be the correct interpretation, especially when an animal is receiving an over-supply of hormones. In an effort to allow for this, hormonal measures are often made on materials recovered from the blood; but it is not clear that this eliminates the difficulty, for it is still not certain how much of the hormone carried in the blood stream may ultimately be utilized by an animal. Consequently reports on hormonal levels in females and males, or in heterosexual and homosexual males, are acceptable only when allowances are made for possible errors in interpreting the measurements, when a sufficient allowance is made for variation in the same individual on different occasions, when the series of reported cases is of some size, and when the report is based on a statistically adequate experimental group which can be compared with an adequate control group.
Development of Physical Characters in Young Mammals . The most certain effects of the gonadal hormones are their effects on the development of physical characters in the young animal. This includes the development in size and function of many parts of the body, including some of the characters (the secondary sexual characters) which most clearly differentiate adult females from males. Many of these characters do not fully differentiate until the onset of adolescence. The development of these secondary sexual characters, as well as the development of the adult anatomy as a whole, depends upon the animal’s possession of intact gonads. This is true of the human female and male, and of the females and males of the lower species of mammals.
If the gonads fail to develop normally-as not infrequently happens when testes of the human male, for instance, are retained in the body cavity and fail to descend into the scrotum-or if the gonads of the pre-adolescent human female or male become diseased, or if they are removed by castration any time before the onset of adolescence, the normal development of adult characters is usually slowed up or completely stopped. And when a castrated animal does reach its full size, its body proportions are not typical of those usually found in the normal adult, i.e. , the animal becomes a typical capon, a gelding, or a eunuch in form and structure. 14
In the normal human adolescent, female or male, the genitalia are among the first structures to acquire adult size. 15 When the gonads have been damaged before adolescence, the genitalia may develop even more slowly than the rest of the body and may remain more or less infantile even into later years.
In the human female and male, hair normally begins to develop in the armpits during adolescence. In normal development, pubic hair appears in both sexes, although the pubic hair of the female is ordinarily confined to a more limited triangle while it may spread over a more extensive area in the male. Ultimately, but often not until late in the twenties or thirties, the pubic hair of the male may develop upward along a midline (the linea alba ) on the abdomen. The normal male also develops hair on his face, on his chest, on his legs, and elsewhere on his body, while such hair is usually absent or scant in the female. But if the testes of the pre-adolescent male are damaged or eliminated, the hair in these several parts of the body may fail to appear at the usual age. If it does subsequently develop in the castrated male, it may appear in a pattern which is in many respects more typical of the very young adolescent. The face and chest and other parts of the body of such a male may remain more or less hairless. 16 While the early castration of a female does not have as marked an effect on the development of her body hair, it may contribute to the appearance of facial hair and other hair developments which are not typical of her sex. 17
The Adam’s apple is characteristic of the adult human male. Associated with this, his voice is rougher and usually at a pitch which is lower than that characteristic of the female. The Adam’s apple is ordinarily not developed in the female. The male who is castrated before adolescence fails to develop an Adam’s apple and retains a high voice.
In the course of adolescent development the shoulders of a normal male widen more than they do in the normal female. In adult females the hips become characteristically larger and wider than they are in the male. The buttocks in an adult female usually become larger and more elongated, while the buttocks of the adult male remain smaller and are more often rounded. The male who is castrated before adolescence retains body proportions which are closer to those of the juvenile.
The breast of the female normally enlarges in size, and the colored, corrugated (areolar) area surrounding the nipple becomes considerably expanded. The female who has diseased or damaged ovaries does not show such a normal breast development, her voice may become lower in pitch, and she may fail to acquire an adult female body form.
In general, castrations performed on young females and young males of the infra-human species of mammals affect their physical development in ways which are comparable to those just noted for human females and males.
Some of the effects of castration may be partially or largely corrected by the administration of hormones from an outside source. This is true for both females and males, and for both the human and other mammalian species. But injections of hormones have their maximum effect if they are made at an early age. They cannot fully correct the damage done by a castration if they are not administered until some time after adolescence has begun, but they may still have some value even when the therapy is not started until the individual is essentially adult. But the corrective effects of hormonal administrations to young castrates can be maintained only if the treatment is continued throughout the growth period. Otherwise the individual may lapse into its castrated state. If hormonal treatments are continued until the individual has become completely adult-which in the human species means into the middle twenties or sometimes later-then the continued administration of hormones is not so necessary. 18
Maintenance of Physical Characters in Adults . Damage to the gonads, or a complete castration of a human female or male after physical maturity has been acquired, prevents reproduction, but there are usually minimum effects on other physical characters. Some individuals (particularly some females) who have been castrated as adults may go for years and may even reach old age before they show any marked physical changes. Some females and males, on the contrary, may show more physical deterioration in the course of time. Clinicians commonly report characteristic aging effects on the genitalia of females who have had their ovaries removed. 19 It is generally believed that the deteriorations of old age come on sooner, although the specific data are inadequate on this point.
Castrations may, however, have marked effects on the physiologic well-being of an adult. 20 Since gonadal secretions affect the levels of secretion of the pituitary, adrenal, and thyroid glands, all of which are important in the regulation of the general physiology of an animal, it is inevitable that castrations, even of adults, should have some effect; but this effect is usually minor among human females, and it is not clear that most human males have their physiologic well-being particularly modified by castration if the operation is performed after complete physical maturity has been acquired.
There seem to be more marked effects on the physical characters and on the physiologic well-being of males of lower mammalian species which are castrated as adults. 21
The effects of castration on an adult animal, such as they are, may be more or less completely corrected by the administration of a sufficient supply of gonadal hormones. This has been demonstrated for laboratory animals, and hormones are often administered in clinical practice to middle-aged and older women who have had their ovaries removed. Usually testosterone is given to a male who is castrated as an adult, and estrogens to a female, but sometimes both hormones are given to individuals of both sexes. It is significant that the corrective administrations of hormones do not need to be kept up indefinitely in an adult, at least in an adult female. In some way the adult human body can adjust in a matter of months to a lack of gonadal hormones, and then it appears to be capable of more or less normal function, even though an important link in the endocrine chain has been eliminated. The capacity of the adult male body to adjust may not be as complete as that of the female. Some adult male castrates appear to adjust to a lack of male hormones for long periods of years; but others show some physical degeneration within a shorter period of years. Until there are further studies of long-time adult male castrates, we are uncertain how to interpret these contradictions in the reported data. 22
Much of the confusion concerning the function of the hormones which originate in the ovaries and the testes is a consequence of the unwarranted opinion that anything associated with reproduction must, ipso facto , be associated with an animal’s sexual behavior and, contrariwise, that all sexual behavior is designed to serve a reproductive function. Since the glands which produce eggs and sperm also produce hormones, scientists and philosophers alike have considered it logical to believe that these must be the hormones which control sexual behavior. Reasoning thus, men throughout history have castrated criminals as punishment for sexual activity which their gonads were supposed to have inspired, and with the intention of controlling their further sexual activity. 23 In recent years, courts and state legislatures are again considering gonadal operations as a means of controlling sex offenders. 24 There has been some experimentation with hormone injections in an attempt to achieve that end. Castrations and the administration of sex hormones have been carried out under court order and under the direction of physicians and psychiatrists in various parts of the United States, in Denmark, in Holland, and still elsewhere in mental and penal institutions. 25 On the even more amazing assumption that anatomic defects in the genitalia may explain the social misuse of those organs, some of the medical and psychiatric officers in police courts and in penal and mental institutions routinely examine the genitalia of persons committed on sex charges.
But the fact that hormones are produced in the gonads is, without further evidence, no reason for believing that they are the primary agents controlling those capacities of the nervous system on which sexual response depends. It is unfortunate, as we shall see, that these hormones were ever identified as sex hormones, and especially unfortunate that they were identified as male and female sex hormones, for the terminology inevitably prejudices any interpretation of the function of these hormones.
Estrogen Levels at Younger Ages . It should be borne in mind that estrogens (the female hormones), are to be found in the bodies of both females and males. The ovaries of the female are a chief source of her estrogens. The origins of the estrogens in the male are not so well established, but they seem to be produced, at least in part, by the testes. 26
Estrogens are reported to occur in about equal amounts in the preadolescent human female and pre-adolescent human male until they reach the age of ten (Figure 152 ). But at about the time of adolescence, the estrogens increase abruptly in the female. There is only a slight increase in estrogens in the male at adolescence. 27 In the adult female there is, in consequence, a much higher estrogen level than in the adult male. There is, of course, wide individual variation in this matter. 28
There is nothing, however, in the development of sexual responsiveness and activity, either in the female or in the male, which parallels these reported levels of estrogens in the human female or male. At the onset of adolescence there is no upsurge of sexual responsiveness and sexual activity in the female which parallels the dramatic rise in the levels of her estrogens (Figure 152 ). It is the male who suddenly becomes sexually active at adolescence, but his estrogens stay near their pre-adolescent levels.
Androgen Levels at Younger Ages . Androgens are also found in both females and males. The testes of the male are the chief source of his androgens; but the ovaries of the female apparently produce androgens as well as estrogens, and it is probable that the adrenal glands and still other structures in her body also produce androgens. 29
In the human species, from about age seven or eight until the middle teens, the androgen levels in the female and the male are about equal (Figure 153 ). Then the androgen levels begin to rise more markedly in the male, and less so in the female, and it is generally considered that older females have androgen levels that are about two-thirds as high as those of the males.
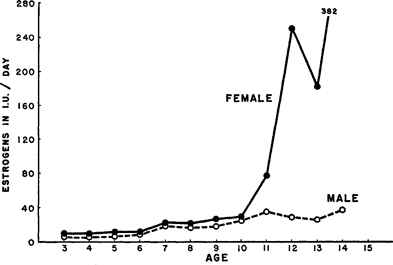
Figure 152. Estrogen levels in pre-adolescent and adolescent female and male
Averages from urinary assays reported by Nathanson et al. 1941.
Since we found a sudden upsurge of sexual responsiveness and overt sexual activity among human males at the beginning of adolescence, there may seem to be some correlation with the androgen picture; but the upsurge of sexual responsiveness in the male is much more abrupt than the steady rise in the levels of his androgens (compare Figures 143 and 153 ).
As for the female, there seems to be no correlation at all between the levels of her androgens and her slow and gradual development of sexual responsiveness and overt sexual activity (Figures 143 and 153 ). Although she has nearly as much androgenic hormone as the male in her pre-adolescent and early adolescent years, her levels of sexual response and overt sexual activity at that period are much lower than the levels in the average male. The near identity of the androgen levels in the female and male at the very age at which the two sexes develop such strikingly different patterns of behavior, makes it very doubtful whether there is any simple and direct relationship between androgens and patterns of pre-adolescent and adolescent sexual behavior in either sex.
Levels of Gonadal Hormones in Older Adults . Unfortunately, levels of gonadal hormones seem not to have been established for any adequate series of older human adults, either female or male. There is some reason for assuming that the levels of male hormones drop in the male at advanced ages, and if this were proved to be so it would parallel the drop in the levels of sexual response and overt activity which we have found in the male. On the other hand, there is as much reason for assuming that the levels of male hormone similarly drop in the older female, but such a drop would not correlate with the fact that the frequencies of female response and sexual activity stay on a level from the teens into the fifties or sixties (Figure 143 ), 30
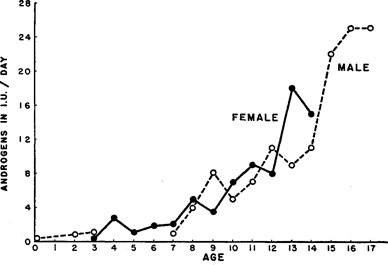
Figure 153. Androgen levels in pre-adolescent and adolescent female and male
Averages from urinary assays reported by Dorfman 1948.
Although the importance of the gonadal hormones in respect to the physical growth and development of the young mammal is clearly established by castration experiments, it is more difficult to measure the effects of castration on the capacity of an animal to respond sexually. In any case, it is difficult to know how many of the observed modifications of behavior represent the direct consequences of hormonal action, and how many are a product of the fact that the gonads influence other endocrine organs such as the pituitary and the thyroid which may affect the general metabolic level of all physiologic functions, including the functions of the nervous system. Finally, in the case of the human species, it should be noted that there may be pronounced psychologic effects from a castration. This is particularly true of the male because of the great importance which our culture attaches to his genital integrity and sexual potency. Many of the reported effects of castrations on sexual behavior are undoubtedly the product of the social maladjustments in which castrates often become involved. In those cultures where castration is observed as a religious duty, and in religious cults where the priests are regularly castrated, no social opprobrium is attached to such an operation, and the effects of castration do not seem as apparent as they are in our culture. 31
Castration and Sexual Response in Young Females and Males . The behavioral effects of gonadal insufficiencies, whether they are the product of undeveloped or diseased ovaries or testes, or the product of complete castrations, are most evident in young animals. In many of the lower mammalian species, early castration more or less completely stops the development of all sexual responsiveness in both the female and male.
In the human male, pre-adolescent gonadal insufficiencies regularly delay the development of sexual responsiveness. The responses of a twenty-year-old male whose testes have degenerated because they have failed to descend into the scrotum, or of a male who has been castrated in pre-adolescence, may be on a level with those of the average pre-adolescent boy of eight or fen years of age. Erections and other signs of response occur less frequently in an adult who was an early castrate, arousal is not effected by as large a number of stimuli as in the normal male, and arousal by psychologic stimuli in particular may occur less frequently than in the normal male. Some degree of sexual responsiveness may develop in later years; but the levels of response in the few cases on which we have original data, and in the well known histories of eunuchs who were castrated at an early age, usually do not reach the levels which are typical of the average male. 32 There is great need, however, for the accumulation of more data, for there appears to be considerable individual variation in such cases.
Early castrations of males of lower mammalian species similarly may prevent the development of any sexual responsiveness or reduce the levels of response. 33 But in some instances, castrations may have little effect on the development of sexual responsiveness. For instance, there are data on two male chimpanzees who were castrated at a very young age, and these castrations have not prevented the subsequent development of sexual responses comparable to those of normal pre-adolescent or adult chimpanzees. 34
In lower mammalian females, early castrations have somewhat similar effects on the development of sexual responsiveness. 35 There are, however, practically no data on the effects of such early castrations on the sexual behavior of the human female.
The importance of the gonadal hormones in the development of sexual responses is further confirmed by the fact that when testosterone is administered to an early castrate, whether it is a human or a mammal of some lower species, and whether it is a female or male, the levels of sexual response may be raised to something approaching the normal. The administration of estrogens to a female who was castrated in preadolescence has a less marked effect on her behavior, and a still lesser effect (as far as the data are yet available) when administered to males who were castrated at an early age. 36
Nevertheless, these demonstrations of the importance of the gonadal hormones in the development of sexual responsiveness in the young female and male, do not seem to warrant the conclusion that androgens and estrogens have more specific effects upon the development and functioning of the nervous system than they have upon the development and functioning of various other physical structures in the animal body. Quite to the contrary, the retardation of sexual development in a castrate is exactly what might be expected if the gonadal hormones provide, as they appear to provide, simply one of the conditions necessary for the normal growth and development of the body as a whole. Similar damage done to the pituitary, to the thyroid, or to some of the other endocrine organs of a developing animal, may have similarly disastrous effects on the normal course of its development and on the development of its capacity to respond. 37 All of these endocrine organs, as well as many of the other organs in the body, seem necessary for the development of sexual responsiveness in the young animal.
Castration and Sexual Response in Adult Females . There are some contradictions in the reported effects of the castration of a human adult. There appear to be differences in the effects on different individuals; Some of the recorded effects may depend upon the fact that the general level of all physiologic activities may be lowered by a gonadal insufficiency. Again, it is to be noted that the psychologic effects of a castration on an adult, and especially on an adult male, may be more severe than the psychologic effects on a pre-adolescent.
The effects of castration on the sexual behavior of a fully mature female have generally been reported to be minor, or none at all. We have the histories of 123 females who had had ovaries removed, and our examination of these cases confirms the general opinion that there is no modification of sexual responsiveness or capacity for orgasm, following an ovarian operation, which can be clearly identified as the result of such an operation. 38 Some of the sexually most active females in our sample were women in their fifties and sixties who were well past the age of menopause. Some of them had had their ovaries removed ten to fifteen years before.
Of our total sample of 123 castrated (ovariectomized) females (Table 179 ), twenty-three had not experienced orgasm for a year or two before the operation, and did not experience orgasm after the operation. Of the remaining one hundred cases, forty-one appraised their own record as follows: 54 per cent had not recognized that their loss of ovaries had had any effect on their sexual responses or overt behavior. Some 19 per cent believed that their sexual responses had been increased by their operations, and 27 per cent believed that their sexual responses had been decreased. The record of specific activities on the full hundred cases showed that 42 per cent had not changed in their overt behavior, 15 per cent had increased their activity, and 43 per cent had decreased their activity. However, the median frequencies of orgasm calculated for the whole sample both before and after operation, indicate that over a ten-year period the drop had paralleled the drop in frequencies of total outlet in approximately that same age period, among the females in our total sample. It is to be recalled that the declining frequencies of socio-sexual activities among females are not primarily dependent on an aging process in the female, but upon an aging process in the male which reduces his interest in having frequent coitus (see pp. 353–354). Our cases, therefore, do not provide evidence that females deprived of their normal supplies of gonadal hormones have their levels of sexual responsiveness or their frequencies of overt activity lowered by ovarian operations.
The increases in sexual activity shown in some of our histories may have depended on the fact that some women who have gone through a natural or induced menopause feel more free to engage in sexual activity as soon as they are relieved of the possibility of becoming pregnant.
We have detailed data on 173 cases of females who had gone through natural menopause (Table 179 ). It is ordinarily considered that there is a considerable reduction in the amount of estrogen secreted by the ovaries after menopause. However, in our sample it would be difficult to identify any reduction of sexual response or activities which could be considered the consequence of any change at menopause. Out of the 173 cases, forty-six had not experienced orgasm for a year or two before menopause, and there was no change in their status following menopause. In the other 127 cases, thirty-one appraised their own record as follows: 39 per cent believed that their sexual responses and activities had not been affected by the menopause, 13 per cent believed that their responses had increased, and 48 per cent believed that their responses had decreased. The detailed record of the activities of the full 127 women, confirmed this distribution of cases (Table 179 ). Again, however, the decrease in median frequencies in this sample had merely paralleled the decrease in median frequencies in our total sample of females of approximately the same age. Note again that this decrease is primarily dependent upon the male’s declining interest in socio-sexual activities.
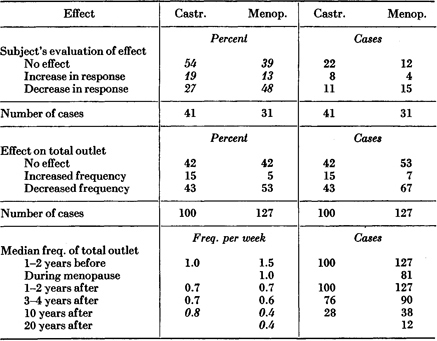
Table 179. Effect of Castration and of Menopause on Sexual Response and Outlet
Table based on all females available in sample, whose marital status remained constant before and after castration or menopause and who had experienced orgasm within 1 to 2 years before and/or after castration or menopause.
The median age at castration was 38.6 years, the range was 17 to _53 years. The median age at the onset of menopause was 46.3 years, the range was 33 to 56 years.
Some of the decreased frequencies also depended upon the fact that some of these women had seized upon menopause or their ovarian operations as an excuse for discontinuing sexual relationships in which they were never particularly interested. Some of the cases of increased activity were, again, a product of the fact that some of these women had been relieved of their fear of pregnancy after going through menopause.
Castration of Adult Females of Lower Mammalian Species . This lack of effect of castration on an adult human female is not in accord with the reported effects of castrations on adult females of rats, guinea pigs, and some other species of mammals. It is reported that castrations in those species eliminate all evidence of sexual response. 39 Examination of the literature, however, indicates that the chief bases for these reports, persistent as they are, is the fact that the castration of a lower mammalian female puts an end to her periods of estrus-the period during which she will accept coitus from the male. It has always been assumed that she accepts coitus during estrus because she becomes sexually more responsive at that time. But we question whether the submission to a male is, in itself, sufficient evidence of erotic arousal. Moreover, we find considerable evidence that lower mammalian females who are not in estrus are frequently aroused erotically.
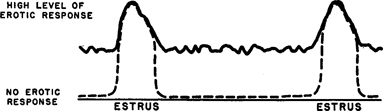
Figure 154. Relation of erotic response to estrus in infra-human females
Theoretic diagram. Lighter line shows previous interpretation, darker line shows present interpretation. In both instances, response is at a maximum during estrus; the present data indicate that there is also a considerable capacity for response in periods between estrus.
We have noted that a bull ordinarily gives evidence of his sexual arousal before he ever mounts a cow by showing a development of neuromuscular tensions throughout his body; the muscles on the sides of his abdomen become tensed in a corrugated design, his tail is arched as a result of tensions in that part of his body, he may show a partial erection, and his Cowper’s secretions may start flowing before he has touched the cow. Usually a cow which is in estrus does not show similar evidences of sexual arousal until after she is mounted. Then the tensed muscles on the sides of her abdomen, her arched tail, her tumescent genital labia, and her vaginal mucous secretions provide evidence of her arousal and response. We have also noted (page 450) that cows quite regularly and frequently mount other cows, and that when this occurs, the cow that does the mounting is the one that first shows evidence of erotic arousal, although the cow which is mounted may not give such evidence until after she has been mounted. But the cows that do the mounting are usually not in estrus, while the cows that are mounted are almost always in estrus.
There is, of course, no question that an animal that is in estrus is capable of being aroused erotically, and it is common knowledge that some animals, like female dogs, may become more responsive and actively search for males when they are in estrus; but the data indicate that sexual arousal among infra-human females that are not in estrus may also occur with some frequency (Figure 154 ). There are also records of castrated adult female rats and dogs that will sometimes mount other females after castration, just as they did before castration; and this seems clear-cut evidence of sexual responsiveness after castration. 40
Since reports on the effects of castrations in lower mammalian species usually do not describe those physiologic phenomena which are the best measures of sexual response (Chapter 15 ), and since there is so much evidence of sexual arousal outside of estrus, we doubt whether the presence or absence of estrus provides a sufficient measure of a lower mammalian female’s capacity to respond sexually.
It has been said that the gonadal hormones are more important in controlling the sexual responses of the lower mammalian female, while cerebral controls are more important in the human female. 41 But this, again, seems to be based primarily on the fact that estrus stops after the castration of the lower mammalian female, while responsiveness after castration is retained in the adult human female. If reexamination of the experimental data or further experimental work shows that the lower mammalian female does not actually lose sexual responsiveness as a result of castration, it would mean that the role of the gonadal hormones in the lower mammalian female is, to this extent, about the same as in the human female.
Castration and Sexual Response in Adult Males . Reports on the sexual behavior of human males who have been castrated as fully grown adults have usually been very brief, and the various reports are contradictory in spite of the fact that such operations have been performed with some frequency throughout history. Castrations of adult males were performed with religious objectives in various ancient groups, and have been performed for that purpose within the present century in certain groups. 31 In various cultures, castrations of adults were performed for the sake of obtaining eunuchs who could be used as household servants, or servants in harems, without the danger of their fathering offspring. 42 Castrations have been most frequently performed as indignities which were inflicted upon enemies captured or killed in battle, and as punishments for certain classes of criminals. Ancient Egyptian drawings, and the derived art of Northern Africa today, depict mounds of severed genitalia gathered from enemies destroyed in battle 43 ; and during warfare in probably every part of the world, such mutilation has been considered the supreme subjugation which the conqueror could bestow upon the conquered. There is no doubt that the recurring interest in castration as a legal punishment today is, at least in part, a product of the same sadistic eroticism which has inspired genital mutilation throughout human history.
In more recent decades, both in Europe and in this country, castrations have been rationalized as attempts to modify some aspect of the individual’s sexual behavior: to stop masturbation, 44 to transform homosexual into heterosexual patterns of behavior, to control exhibitionists and, in particular, to control adults who sexually “molest” children. Castrations have been used both in Europe and in this country to prevent feeble-minded, criminal, or irresponsible individuals from becoming parents; but simple sterilizations would satisfy that end if there were no other objective in a castration. 25
Castrations have, of course, been necessarily performed when testes were diseased; and recently castrations of older males have become fairly frequent as a means of reducing their androgen levels, because these may influence cancerous growths or other hypertrophies of the prostate gland. In addition there are a fair number of males who have had to be castrated as a result of war injuries. There has, in consequence, been no shortage of cases for studying the effects of castration on adult males, but there have been very few detailed reports on the sexual behavior of such castrates.
A considerable proportion of the studies have reported, without specific data, that there was an improvement in health as a result of a castration, or an increase or decrease in sex drive, or a generally beneficial effect. Unfortunately some of the reports on which state legislators have recently relied have been in these same general terms, without specific data on the frequencies of response, the intensities of response, the number of items to which the castrated individual responded, the frequencies of erection, the frequencies of masturbatory and overt socio-sexual activities, or the frequencies of total activity to the point of orgasm.
Many of the reports have concerned castrations of older males past the age of fifty, and in many cases between sixty and eighty years of age. Males of such advanced ages normally have their rates so reduced that it would be difficult to determine how much of their inactivity should be credited to a castration. Even at fifty years of age there are 7 per cent of the males who are already impotent and unresponsive sexually, whether they are castrated or not.
The studies that do report frequencies of activity after castration, fail to allow for the fact that most males have their frequencies of sexual activity steadily reduced with advancing age. It means nothing to find that castrated males gradually, over some period of years, show diminished sexual interests and capacities, unless it is shown that the diminution of their activity occurs more rapidly than that which occurs in the population as a whole. It is to be recalled that in our total male sample we found (1948:226) that the average twenty-year-old, married male experienced orgasm with a median frequency of 3.2 per week, but that these frequencies dropped steadily through the years until they had reached 0.8 per week at sixty years of age among males who were not castrated.
Because of the general misunderstanding of the reliability of the evidence on the effects of adult castrations, and because some courts and state legislators have uncritically accepted the published records as justification for their consideration of castration as a means of controlling certain types of sex offenders, it seems appropriate to summarize briefly the data in the published studies. They are here arranged in chronologic order.
1. Barr 1920. A study of 6 male castrates, most of them with records of low intelligence. Ages from eleven to twenty. Results cannot be evaluated because they are reported in nothing but general terms. They note “an improvement in general behavior” in most instances.
2. Commins and Stone 1932. An extensive review of the literature on the effects of castration on basal metabolism, on the nervous system, on reflex action, on voluntary activity, on sexual behavior in general, and on learning. Deals primarily with lower mammals.
3. Lange 1934. The most extensive and most specific study of the effects of male castration, based on 310 cases which included 242 complete castrates and 68 partial castrates. The data are drawn from a longtime study of 247 cases originating in war injuries, and 63 cases in which there had been a surgical removal of testes following tuberculosis. The following summary applies only to those cases in which there had been complete castrations.
At the time of castration, 10 per cent of the men were under twenty, 50 per cent under twenty-five, and 15 per cent over thirty-five years of age. All but 6 of the cases were observed for fifteen years or longer following castration. The physical changes, which were reported in detail, included regressive effects which appeared chiefly in the earliest and the latest years; but wide individual differences were noted, probably because of the wide age range of the subjects. Data on potency following castration are given on 99 complete castrates: 52 per cent lost potency immediately, 22 per cent lost potency gradually, and 26 per cent still retained their potency at the end of the period of observation. This loss was related to their ages at the time of castration (and hence to their ages at the final report). Some 73 per cent of those retaining potency were under the age of twenty-five at the time of castration. The author points out that the reports of defects due to injury or operation tended to be exaggerated in order to support claims for government compensation.
The sexual desire of many of the subjects had exceeded their potency. The author discusses the fallacy of castration as a cure for sex criminals, since such criminal violence is often the result of the conflict of weakened potency and strong sexual impulses (pp. 44, 101), and he questions the favorable results reported in Switzerland and Denmark on the basis of the selectivity of the groups and the short period of observation of those cases.
4. McCullagh and Renshaw 1934. A study of 12 subjects, 4 castrated between twenty-three and forty, 6 between forty and sixty, and 2 after sixty; observed from six months to twenty-seven years after castration. Partial responses remained in 3 cases, but were entirely lost in 9. There was a shrinking of the penis in 5 cases, a reduction of body and pubic hair in 11 cases, a decrease of energy and endurance in 10 cases, and changes in weight and general appearance. The specific data are, however, still insufficient for final analyses.
5. Wolf 1934. A German study summarizing 162 castrations of human males, many on the basis of fragmentary records from the older literature. Many of the subjects were feeble-minded or mentally deficient. There were 50 cases from the author’s own data. The information on the sexual responses of the subjects after operation was quite incomplete. For 72 cases, the effects on responsiveness were minor in 35 per cent, the responses were much reduced in 28 per cent, the responses were completely gone in 37 per cent. However, the data are uninterpretable because there are no correlations with the ages at castration and no exact data on the subsequent frequencies of sexual activity.
6. Kopp 1938. A survey of the status of castration and sterilization of criminals in the United States and Europe; but the material is in very general terms, and chiefly historical in interest.
7. Feinier and Rothman 1939. A single male, castrated at twenty-three for tuberculosis of the testes, reported a normal married life and potency which had increased after castration and after recovery from the tuberculosis. At fifty-three (thirty years after the castration) he was having weekly coitus with his wife. The wife corroborated the story. The authors conclude that the only indispensable function of the testes in a fully grown adult is that of procreation, and that sexual responses and potency “are functions of and controlled by the prepituitary and psychic centers.”
8. Tauber 1940. A good review of the literature, without original data. Reports studies on religious castrations; on castrations of criminals in Germany and Switzerland (where the author feels the data are not reliable); on castrations due to injuries; and on medical castrations. Feels the psychologic aspect of a castration is very important. Concludes that the sexual behavior of male castrates is highly variable, and that the range includes behavior which would be considered normal in non-castrates.
9. Huggins, Stevens, and Hodges 1941. A report on 21 patients castrated to control cancer of the prostate; 3 were between fifty-four and sixty years of age; the others ranged between sixty and eighty-four. Reports sexual drive and potency absent in all cases after operation; but all of the patients were so old that it is impossible to make any critical analysis of the reports on sexual behavior.
10. Engle in Cowdry 1942:489–491. Cites Rössie’s study on 125 men castrated under German law for criminal sex offenses, in half of whom sexual responses were “weakened.” Ages not given and no records of the specific frequencies of sexual activity. Cites a British report by Hammond of 7 males castrated at ages ranging from thirty to fifty-one, in whom coitus continued after castration, in one case for seventeen years. Concludes that “The evidence suggests then that in men in whom the psychic and neuromotor behavior patterns of sexual activity have been established, complete loss of the testes does not necessarily prevent participation in sexual activity.”
11. Hamilton 1943. One male castrated because of cancer at twenty-five years demonstrated normal erections at age forty-three. One male castrated at twenty-six reported marital coitus two to three times a week, at age thirty-nine. Wife confirmed report. Androgen and estrogen levels low, so writer concludes that capacity for erection does not depend upon a supply of androgens from extra-gonadal sources.
12. Stürup 1946a. A psychiatrist’s report on 123 males voluntarily castrated in the Danish asylum for psychopathic criminals at Herstedvester. Only general statements on sexual behavior after castration. “Some degree of sexuality is retained in certain cases, at any rate for a number of years, and some cases have been able to achieve a coitus satisfactory to their wives at intervals of about a month. To the majority of cases, however, this does not apply.” Insufficient data on ages and on sexual responses and frequencies of activity before and after castration.
13. Stürup 1946b. A discussion of the psychiatric treatment of criminal psychopaths in Denmark. Only a general statement of “good results,” without specific data which would allow critical analyses of the results. States that “The detainee must show hyper-sexuality beyond doubt or a stable sexually conditioned criminality, before we use this irreversible treatment.”
14. Beach 1948:23–27. Surveys the studies on male castration. Concludes: “Despite the frequency and possibly the accuracy of generalizations regarding the depressing consequences of human castration, the literature is replete with references to complete retention of sexual function in individuals who have been castrated for many years.” Then adds: “The frequency of accounts describing the survival of normal sexuality following castration need not obscure the fact that in many, if not the majority of cases, the human male exhibits a gradual loss of mating ability as a result of testicular removal.” This last statement, however, ignores the fact that non-castrated males similarly show a gradual loss of mating ability with advancing age.
15, Fuller 1950. No original data. Points out that the medical profession is not agreed on the use of castration as a means of reducing sexual responses. States that there is no assurance that “in man the sexual urge may not persist for years after the castration.”
16. Hawke 1950. In a paper delivered before the Illinois Academy of Criminology, Hawke discusses the program of castration employed, under his direction, at the State Training School at Winfield, Kansas, where 330 male castrates furnished material for a nine-year research program. These cases were also the source of physiologic data on castrates in Hamilton 1948:257–322, who gives ages at castration in 57 of these cases. These ranged from eight to twenty-two years, and included 11 boys who were 12 years of age or younger. The cases were largely drawn from a defective delinquent group. The sweeping generalizations as to psychologic improvement, lack of inferiority feelings, increased stability, and lessening of the “social menace” in these cases are not substantiated in the paper by any sufficient data. Only three case histories of individuals, castrated at sixteen, eighteen, and twenty-four, are described in detail, and the only evidence given as to the satisfactory result of these castrations was the fact that they later adjusted to life outside the institution. Hamilton 1948:286–288 presents detailed data, however, showing that a group of these same subjects had difficulty in carrying out their motor activities. Hawke, nonetheless, states that the castrate is “physically a better organism.” There do not seem to be any data on the sexual behavior of these subjects which allow reliable analyses of the sexual effects of the castrations.
On the basis of the more reliable of these published studies, and on the basis of the few cases we ourselves have seen, we may generalize as follows: Human males who are castrated as adults are, in many but not in all cases, still capable of being aroused by tactile or psychologic stimuli. They may still be capable of showing essentially all of the physiologic concomitants of sexual response, including the tumescence of all parts of the body and the specifically genital tumescence which may effect normal erection. They may still be capable of developing neuromuscular reactions which include rhythmic pelvic thrusts of the sort necessary for coitus, and they may still be capable of attaining orgasm. The frequency and intensity of response may or may not be reduced by the castration. The psychologic effects of such an operation may make it difficult for some of the males to make socio-sexual adjustments.
Ejaculation may or may not follow orgasm in a castrated human male. In the lower mammals, removal of the testes may cause degeneration of the prostate and seminal vesicles, which are the chief sources of the ejaculate, but there are some recorded instances of a reduced and modified ejaculate in some castrated human males. In most cases there is no ejaculation. The individual variation probably depends on the length of time which has elapsed since the castration and the stage of degeneration of the secreting glands. None of this, however, makes it impossible for a castrated male to have orgasm. We have the history of a male engaging in sexual activity with normal frequencies and with orgasm fifteen years after the castration, and it will be noted that in the literature cited above there are instances of the retention of sexual capacity for similarly long periods, including one case of a male who was normally active thirty years after castration.
Castrations of adult males of lower mammalian species produce, as we have already noted, a more general physical deterioration than is recorded for adult human male castrates. In general, male animals castrated as adults show diminished sexual responses, but the data are insufficient to allow critical analyses, 45 We have already noted that in chimpanzees there are records of adult male castrates who were as active sexually as males who had not been castrated.
In any event, the laboratory experiments on animals, and the data which are at present available on human male castrates, do not justify the opinion that the public may be protected from socially dangerous types of sex offenders by castration laws.
The administration of an extra supply of male hormone to an animal, female or male, which has intact gonads, may increase its sexual responsiveness. This may appear to contradict the data on the effects of a castration, but the two bodies of data are not actually in conflict.
Excessive Gonadal Hormones in Young Animals . The administration of androgenic hormones to a young, non-castrated male, whether infrahuman or human, ordinarily speeds up its physical development and the development of its sexual responsiveness and overt sexual activity. 46 There are similar results when estrogens are given to young females. 47 There is considerable work on laboratory animals which establishes this fact. In the case of the human male, the clinical administration of testosterone to a pre-adolescent is ordinarily avoided because of the probability that it will start precocious development. But when adolescent development seems to be delayed, and particularly when there seems to be an under-development of the gonads, some physicians do administer testosterone. There may be complications, however, if more than the optimum dose is given, for an excessive supply of gonadal hormones may inhibit the secretory activity of the anterior lobe of the pituitary.
Precocious adolescent development sometimes, although rarely, may occur in children at five or six, or even at two or three years of age. In some of these cases there may be an endocrine imbalance which sometimes involves an androgen disturbance; but there are other cases in which clinical studies fail to show any sort of endocrine disturbance. Such children may show physical developments equal to those of a normal thirteen- or fourteen-year-old. However, the sexual responses and overt activities of such precocious children are ordinarily typical of those among normal pre-adolescent children of the same age. Investigators are inclined to emphasize that such a child masturbates, shows sexual curiosity, or engages in some form of socio-sexual play, and they are likely to conclude that these activities are a product of the precociousness. But it should not be forgotten that such activities are ordinarily found in the histories of normal pre-adolescent children (Chapter 4 ).
In the several cases which we have of precocious adolescent development, we have rarely found sexual activities which exceeded those ordinarily found among normal children of the same age. 48 We have the history of a five-year-old with the physical development of a fourteen-year-old, but with sexual responses which were normal or even lower than normal for a five-year-old. We have one group of four related cases in which hereditary factors seem to have been involved, for precociousness had appeared among the males of at least two generations in separated branches of the family. We have the history of another boy who turned adolescent at seven, and he was highly responsive and exceedingly active in socio-sexual contacts. Since cases of very early adolescent development are relatively rare, it is highly important that more extensive data be accumulated on the responses and overt sexual activity of such children.
The administration of androgens to a pre-adolescent human female may do considerable damage because of their over-stimulation of physical development. 49 There seem to be no data on the effects of such an early administration of androgens on the sexual responsiveness and the overt sexual activity of the human female.
Excessive Androgens in Adults . When an extra supply of androgens is given an adult animal that has not been castrated, there may be an increase in the general level of its physical activity, its aggressiveness, and its frequency of sexual response and overt sexual performance. 50 This is true of laboratory and farm animals, and it is equally true of the human male. It is also true when androgens are given females, whether they are lower mammalian or human females.
When testosterone, for instance, is given the normal human male, there may be an increase in the frequency of his morning erections, the frequency of his erotic response to various stimuli, the frequency of his masturbation, and the frequency of his socio-sexual contacts. 51 This is ordinarily true of adult males of ages ranging at least from the twenties into the fifties or sixties. Testosterone has also been used clinically to increase the levels of sexual response in cases in which a failure to have offspring appears to depend on low rates of coitus. We have several histories of males who had had their coital frequencies increased by such clinical treatment. Sperm counts may also be increased by the administration of testosterone, and this may contribute to the relief of the sterility. 52 The indiscriminate use of testosterone, however, may involve some danger, for if the dose exceeds the amount necessary for optimum effectiveness, pituitary functions may be inhibited, and this may do damage to various structures, including the gonads themselves, and there may be negative effects on sexual activities. 53 In laboratory animals, excessive doses of testosterone may reduce the gonads to more or less vestigial structures.
There is some clinical experience in administering testosterone to normal human females, and the results obtained are quite similar to those obtained in males. 54 Once again, the levels of physical activity may be increased, and the general level of aggressiveness may be increased. In the case of the lower mammals, this increased aggressiveness increases the frequency with which the female mounts other animals, either females or males. Because males are normally more aggressive than females, normal females usually find few opportunities to mount males; but females who have been given testosterone may become so aggressive that they succeed in mounting a larger number of males. 55
The increased responsiveness of a normal female or male who has received an increased supply of testosterone has ordinarily been taken as evidence that the hormone plays a prime part in controlling sexual behavior. Such an interpretation, however, ignores the evidence of the castration experiments on adult females and males. It seems more correct to conclude that androgens, at every level which does not exceed the point of optimum effectiveness, are among the physiologic agents which step up the general level of metabolic activity in an animal’s body, including the level of its nervous function and therefore of its sexual activity. For instance, as an example of the effect of testosterone on other physiologic activities, it may be noted that dairy breeders sometimes administer it to cattle in order to increase their food utilization. Thereby the breeder may increase the amount of meat which he secures when he gives the animal a given quantity of food.
There are so many other factors which affect the levels of metabolic activity in a fully mature animal that the loss of the usual supply of androgens, as in a castration, does not make it impossible for an animal to hold its metabolism at something approaching a normal level. This, however, does not preclude the possibility that supplies of male hormones in excess of those normally provided by the gonads, may raise the metabolic levels and consequently the levels of sexual response and performance. But male hormones are not the only agents that step up the levels of activity, including the levels of sexual activity, for the administration of pituitary extracts, of thyroid, and of some other substances may have similar effects. In fact, good health, sufficient exercise, and plenty of sleep still remain the most effective of the aphrodisiacs known to man.
Excessive Estrogens in Adults . What effect the administration of an extra supply of estrogens may have on the sexual behavior of the human or lower mammalian female or male, is a matter which may not be asserted with assurance in our present state of knowledge. Some clinicians assert that they have raised the levels of sexual responsiveness in female patients by administering estrogens, while other clinicians make just as positive statements that they have never secured such a result. 56 Animal breeders and students experimenting with laboratory animals, female and male, give similarly contradictory reports. 57 The contradictions may mean that there is no simple and direct relationship between estrogens and sexual behavior, or they may mean that the effectiveness of an increased supply of estrogens depends upon the concomitance of a variety of factors, including such things as the general metabolic level, the general physical health, the levels of the other hormones in the body, the age, the point in the estrus cycle at which the estrogens are administered, and probably still other factors.
There is a theory that estrogens counteract the effectiveness of androgens. The theory is as yet unsubstantiated by adequate experiment, 58 and is confused by the fact that both androgens and estrogens occur simultaneously in both the female and male bodies. However, some clinicians in this country, in Denmark, and in Holland are using estrogens in an effort to reduce the levels of sexual responsiveness of males convicted as sex offenders. 59 It is possible that estrogens do reduce the amount of androgen which is secreted, but the attempt to control sexual behavior by lowering androgens depends, of course, on a misinterpretation of the role of the androgens in the sexual activities of the male. There are optimistic reports of “good results” from the estrogen injections, but, as usual, the reports are not supported by adequate details of what the “good results” are supposed to be. Since an excessive supply of estrogens may affect many body functions besides sexual behavior, and since an excessive supply may do irreparable damage to other glandular structures, several research endocrinologists assert that they consider the use of estrogens to lower the sexual responsiveness of a male nothing less than medical malpractice.
Because of the effects which secretions from the anterior lobe of the pituitary gland may have on all of the other endocrine organs, and particularly on the gonads and adrenal glands, the pituitary has often been described as a master gland dominating all of the other endocrine organs in the body. It seems, however, more correct to think of the gonads and the pituitary and adrenal glands as a chain of organs whose secretions have interlocking effects. The effect of the pituitary on the gonads, for instance, seems hardly more significant than the effect of the gonads on the pituitary.
Relation of Pituitary to Physical Characters . Damage to the anterior lobe of the pituitary in a young animal, or in the human female or male before the onset of adolescence, may affect physical development more seriously than a gonadal castration. 60 In the case of the human female or male, the individual remains immature or develops slowly and at a late age, and is abnormal in his or her physical proportions and functions. However, the effects of a pituitary insufficiency in a young animal may be relieved by the administration of pituitary hormones. Because of the general, regulatory function of the pituitary gland, some laboratory students and some clinicians are inclined to depend upon pituitary hormones to correct a gonadal insufficiency.
It is reported that the administration of pituitary hormones to a normal pre-adolescent in whom there is neither a pituitary nor a gonadal insufficiency, may induce a precocious adolescent development. 61
Relation of Pituitary to Sexual Behavior . A pre-adolescent pituitary deficiency affects the sexual behavior of an animal in much the same way that an early castration affects its sexual behavior. Responsiveness develops slowly if at all, and it is probable that the levels of response which are ultimately reached by such an individual are below those which are normal. 62 Since the gonads are among the structures which do not develop normally when there is an early pituitary insufficiency, the effects of the pituitary hormones on sexual behavior may depend upon their regulation of the supply of gonadal hormones, and this interpretation is favored by the fact that the administration of androgens to an animal which has a pituitary deficiency may induce normal sexual behavior. On the other hand, it is also possible that the pituitary hormones directly affect the development and the physiologic function of the nervous system on which sexual behavior depends. 63
Because the pituitary glands are not located near the organs of reproduction, there are only scattered references to the effects of pituitary deficiencies on sexual behavior. The opinion is generally held that such deficiencies in adults, or the administration of pituitary hormones in cases of deficiencies, or the administration of pituitary hormones to normal adults, does not have as pronounced an effect on sexual behavior as the administration of gonadal hormones.
Pituitary Secretions and Levels of Sexual Response . There are indications that the levels of pituitary secretion may correlate with the fundamental patterns of sexual behavior which we have found in the human female and male. Recently published studies on fowl show that the cells of the anterior lobe of the pituitary in a very young male contain a quite clear cytoplasm, which, however, begins to accumulate granular materials, the so-called mitochondria, soon after the animal begins to grow. 64 These mitochondria are associated with the normal physiologic processes that go on in these cells, but as the male animal ages, the mitochondria steadily increase in quantity until the cells of the glands of the older male become more or less filled with the granular inclusions.
The secretory capacity of the cell is inversely proportional to the volume of the granular inclusions in the cell and therefore to the age of the animal, and in the older male bird the cells may, in consequence, lose most of their secretory capacity. The functional significance of the pituitary is therefore steadily lowered as the male becomes more advanced in age.
On the other hand, the cells of the anterior lobe of the pituitary of the female do not accumulate such a quantity of granular material, and cases are reported of ten- and twelve-year-old female birds in which the cells of the anterior lobe of the pituitary are practically as clear of mitochondria as they are in very young females. This means that the secretory capacities of the pituitaries of these females are maintained at a more or less constant level throughout the life of the bird.
This gradual loss of function in the cells of the pituitary of the males, among fowls, parallels the steady decline which we have found in the sexual activities of the human male. The maintenance of pituitary function on a more or less constant level in the females, among fowls, parallels the maintenance of sexual capacity which we have found in the human female. Among all of the biologic phenomena which we are aware of, these differences between female and male pituitary secretion, and the differences which we shall note below in the levels of the 17-ketosteroids among females and males, are the only phenomena that seem to parallel the differences which we find between the sexual activities of the human female and male.
Since cytologic and experimental studies of these differences in female and male pituitary functions are of very recent date, we have no idea whether such a differentiation applies to the human or to any other species of mammal. If the differentiation does hold in the human species, it does not necessarily imply that there is any simple, causal relationship between pituitary secretions and sexual behavior. The relationship may depend upon a whole chain of physiologic phenomena which are initiated by the pituitary hormones; or the pituitary picture may reflect some other physiologic condition of the female on which both pituitary and sexual function depend. In any event there is a parallel between these reported pituitary functions in the bird, and the data which we have on the sexual behavior of the human female and male. We did not find such a correlation between the levels of gonadal hormones and the patterns of human sexual behavior.
Thyroid secretions have well known effects upon the general physiologic level of an animal’s activities, including its nervous responses. Low levels of thyroid secretion lower the rates of most activities, and high levels raise the rates of activity; but there is no invariable relationship for, once again, the effects depend upon the concomitance of various other factors which may also influence metabolic activities. It is reported that male hormones tend to stimulate thyroid function, but female hormones are said to inhibit their function, thereby reducing metabolic levels. 65
It is well known that early insufficiencies of thyroid secretions may delay or modify adolescent physical developments and lead to an infantilism of genital structures in both females and males. 66 Cases of such adolescent deficiencies which we have in our histories, show delayed or low levels of sexual response and activity.
There are few published data, but there is a rather widespread clinical understanding that thyroid deficiencies in an older individual may be associated with some lack of responsiveness, and an excess may be associated with rates of sexual activity which are above the average. Not a few physicians administer preparations of thyroid in order to increase the responsiveness of some of their patients. We have some histories of females and a fair number of histories of males to whom thyroid extracts were administered with that express purpose. In some cases, increased frequencies of coitus seem to have been the direct consequence of this therapy, in other cases the record did not seem to indicate that there was any modification of sexual activity, and in some cases a decrease in sexual activity was reported after the thyroid administration. It is, of course, difficult to know how much of the reported change in any particular case was the direct result of the administration of the hormone, how much was the consequence of a change in other physiologic functions, and how much depended on social situations.
Just as some physicians have used testosterone to increase the frequencies of coitus in cases of sterility in which the sterility appeared to be a consequence of low rates of coitus, so there are some who use thyroid to accomplish the same end. Just as with the administration of gonadal hormones, there are reports that such thyroid therapy increases the sperm count and the motility and viability of the sperm, thereby increasing the chances for conception to occur. 67 There is, however, a considerable need for the accumulation of more specific data on these matters, and more carefully controlled experimentation. Since the administration of thyroid extracts may affect a number of other body functions, there is need for caution in the use of these hormones in any attempt to control sexual responses.
The adrenal glands are paired organs lying above the kidneys, which places them in the small of the back of the human animal. They are known to produce a considerable number—perhaps twenty or thirty or more—chemical compounds (steroids) which function as hormones. The possibility that some of these are related to sexual behavior has been considered for some time, but there are still few specific data to establish such a relationship.
Adrenaline . The best known of the secretions of the adrenal glands is adrenaline. This is a product of the central core, the medulla, of the adrenal organ. The role of adrenaline in various types of emotional response, and particularly in the case of fear and fright, was one of the first things known about the glands of internal secretion (p. 692).
Because many of the gross physiologic changes which occur during sexual response are similar to those which may be induced in an animal by injecting it with adrenaline, or by stimulating it so its adrenal glands secrete an extra supply of adrenaline, it has frequently been suggested that adrenaline is responsible for the initiation of sexual response or for some portion of it.
On the other hand, there is a longstanding theory that adrenaline interferes with sexual responses. 68 The theory may have originated in the observation that persons who are frightened during sexual activity may have their activity interrupted. There are, however, no experimental data to establish this inhibitory effect of adrenaline on sexual response.
We have already emphasized (Chapter 17 ) that sexual reactions may so quickly involve the whole animal body that it is difficult to believe that a blood-circulated agent such as adrenaline could be responsible for the major body of physiologic changes which constitute sexual response. Nevertheless, many of the physiologic phenomena involved in sexual response, and particularly the activity of the autonomic nervous system during sexual response, may lead to the secretion of adrenaline, and it is quite possible that some of the phenomena seen in the later stages of sexual response may be products of adrenaline secretion (p. 693).
Cortical Hormones . The outer portion of the adrenal organ, its cortex, is the source of a variety of hormones, including androgens, estrogens, and the various compounds known as the 17-ketosteroids. It is understandable that the adrenal cortex should have some of the same functions as the gonads, for the testes, the ovaries, and the cortex of the adrenals originate from a common embryonic cell mass, even though the organs into which they develop may move apart in the adult anatomy. It has generally been considered that the effects of a gonadal castration are not more severe than they are because the adrenal cortex shares some of the responsibility for the production of the same hormones. 69 The importance of the adrenals in this regard, however, may have been overemphasized in the earlier literature.
The 17-ketosteroids are so called because they have a ketone group at the 17 position on the molecule. Some, but not all, of the 17-ketosteroids are androgens. The 17-ketosteroids are supposed to originate in various organs, including the gonads and the adrenal cortex. It has been estimated that the adrenal cortex may produce as much as 60 or 70 per cent of the 17-ketosteroids found in the mammalian body; but theremoval of both the testes and the adrenal cortex in a laboratory animal does not wholly deprive it of its 17-ketosteroids, and this indicates that other organs also produce these compunds. 70
There are suggestions in the literature that the 17-ketosteroids are in some fashion related to levels of sexual responsiveness, and to frequencies of overt sexual activity in a mammal. The suggestions, however, have been theoretic, and there seems to have been no demonstration of such a relationship in either experimental or clinical data. We do find, however, that the reported levels of the 17-ketosteroids in the human male differ from the reported levels of the 17-ketosteroids in the human female in a manner which more or less parallels the differences which we have found between the median frequencies of orgasm at various ages in the two sexes (compare Figures 143 and 155 ). 71
It is reported that the 17-ketosteroids increase sharply in the urine of the developing human male at the approach of adolescence and during early adolescence (Figure 155 ). They reach a peak in the late teens or early twenties in the male, but from that point they drop steadily into old age. Consequently, the levels of sexual activity in the human male more closely match the levels of the 17-ketosteroids than the levels of gonadal hormones. We have already noted that there is also a parallel to the human male behavior curves, in the secretory capacity of the anterior lobe of the pituitary in one species of laboratory animal.
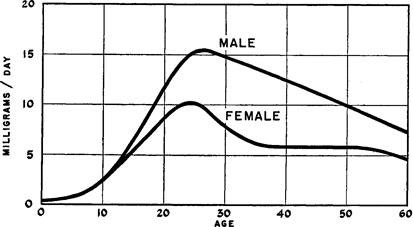
Figure 155. Levels of 17-Ketosteroids in Human Female and Male
Showing aging effect in male, and a prolonged plateau in the female, somewhat similar to average median frequencies of total outlet among females and males shown in Figure 143 . Data from Hamburger 1948.
The levels of the 17-ketosteroids which are reported for the human female are quite different from those reported for the male (Figure 155 ). During adolescence and the later teens, there is a sharp rise in the ketosteroid levels of the female. The curve reaches its peak somewhere in the middle twenties, shows a rather sharp drop in the next ten years, and then lies on a plateau for some years. It continues on this level through menopause and into the middle fifties, and does not show a further drop until the late fifties or sixties. Another study shows a more or less continuous plateau from ages seventeen to sixty-four, without the teen-age rise which the first-mentioned study showed. This plateau parallels the plateau which we have found in the sexual activities of the female (Figure 143 ). The chief difference lies in the rise in the 17-ketosteroids reported in one study during the late teens and early twenties, and this rise is not found in the female’s sexual performance. This may raise a question as to the significance of the correlation; but it is also clear that a part of the lack of sexual response in the younger American female is a product of cultural restraing on her sexual performance. We have presented evidence for this throughout the present volume, particularly in connection with the data showing the effect of the religious tradition on her behavior.
These correlations between ketosteroid levels and the sexual activities of the human female and male may represent a simple causal relationship, or each of these phenomena may be a product of some other basic physiologic phenomenon, or of a whole complex of physiologic phenomena which are responsible for adrenal function, for pituitary function, and for the capacities of the nervous system to be affected by what we call sexual stimuli.
While the data which we have presented show that hormones may affect the capacity of the central nervous system to be stimulated sexually, and therefore may affect the levels of sexual response, there are no data which warrant the conclusion that an individual’s choice of a sexual partner or of a particular type of sexual activity may be directly influenced by any of the hormones. As far as sexual performance is concerned, it is incorrect to think of endocrine organs as the glands of personality.
We have already pointed out that the sexual capacity of an animal depends upon its inherited anatomy and particularly upon its nervous structures and the physiologic capacities of those structures. The capacity of an animal to be aroused by tactile stimuli or psychologic stimuli, and to respond with a development of neuromuscular tensions which produce, among other things, the rhythmic pelvic thrusts which are typical of mammalian coitus, is a capacity which is inherent at birth (Chapter 15 ).
We have also emphasized (Chapter 16 ) that all organisms, and particularly such highly evolved organisms as the human animal, are conditioned by their experience, and consequently in the course of time come to respond more readily to particular types of stimuli. Thus, they develop preferences for the repetition of those types of sexual experience which have been most satisfactory, and become conditioned against the repetition of those types of sexual activity which have been unsatisfactory. In addition to being affected by firsthand experience, the individual may be conditioned by the reported experience of his or her friends, and by the social tradition (Chapter 16 ). Such conditioning seems a sufficient explanation of a preference for a sexual partner who is a blonde or a brunette, or a preference for a sexual partner of the same or of the opposite sex, or for the utilization of one type of technique rather than another in the course of a sexual relationship. It is curious that psychologists and psychiatrists, who are the ones who most often emphasize the importance of psychologic conditioning, so often look for hormonal explanations of any type of sexual behavior which departs from the Hittite and Talmudic codes.
On the unwarranted assumption that homosexuality represents some sort of femininity in an anatomic male, and masculinity in an anatomic female, there have been clinical attempts to redirect the sexual behavior of homosexual males, and occasionally of homosexual females, by giving them gonadal hormones. Recent suggestions concerning the importance of the 17-ketosteroids have led to investigations of the possibilities of using those substances to control behavior. Certain clinicians have developed a lucrative business supplying hormones to patients with homosexual histories, and androgens have been given males who have been discovered in homosexual activity in penal institutions, in the Armed Forces, and elsewhere. Certain of the drug companies have encouraged the use of male hormones for this purpose.
The possibility that hormones might modify homosexual patterns of behavior found some encouragement in early reports on the hormonal injections of laboratory animals. These reports, unfortunately, involved an erroneous use of the term homosexual . 72 Among most of the mammals a female in heterosexual coitus typically assumes a crouched position, often with her head drawn down and her haunches raised in a manner which allows the male to mount in coitus. The female rat arches her back convexly (in a lordosis ) and her ears go into a tremor. The male, on the other hand, typically sits or rises on his hind legs while he effects coitus. The assumption of the crouched position by a male during a sexual relationship, or the assumption of an upright position by a female, was recorded in the earlier literature on animal behavior as a sex reversal or homosexual activity. But this use of the term had no relation to its use in human psychology, where homosexual refers to the choice of a partner of the same sex.
We have already noted (p. 747) that rats which are injected with testosterone become more aggressive in all of their behavior, including their sexual behavior, and that females who are given testosterone mount other females and males more frequently than they did before they were given the hormones. But they do this because their aggressiveness has been increased; and when they mount males their behavior is still heterosexual and not homosexual, even though the positions assumed in the coitus have been reversed.
In connection with the clinical treatment of homosexuality there are, of course, reports of “good results,” but no adequate data to show that the behavioral patterns of such subjects have ever been modified by hormonal treatments. 73 We have the histories of an appreciable number of males who had received hormonal treatments, but we have never seen an instance in which a homosexual pattern had been eliminated by such therapy. Not infrequently, however, the levels of sexual responsiveness had been raised by the treatment with male hormones, and this, in many instances, had complicated the situation by increasing the capacity of the homosexual individual to be aroused by homosexual stimuli. Such failures to attain the desired results in therapy had led to social tragedies in a number of the cases.
Our present understanding of the relations between hormones and the sexual behavior of the human female and male may, then, be summarized as follows:
1. The early development of sexual responsiveness in the human male and its later development in the female, the location of the period of maximum responsiveness for the male in the late teens and early twenties and for the female in the late twenties, the subsequent decline of the male’s sexual capacities from that peak into old age, and the maintenance of female responsiveness on something of a level throughout most of her life, are patterns which are not explained by the known anatomy or physiology of sexual response. Neither are they explained by the known differences in the capacities of females and males to be aroused by psychosexual stimuli.
2. Various hormones may affect the levels of sexual responsiveness in the human female and male because of their effects on the levels of all physiologic activities, including those capacities of the nervous system on which sexual behavior depends.
3. Estrogens, the so-called female hormones, originate primarily in the ovaries and the adrenal cortex. Androgens, the so-called male hormones, originate in the testes, the adrenal cortex, and still other organs. Both estrogens and androgens are present in both females and males. They occur in about equal abundance in human females and males in the pre-adolescent years, but during adolescence the androgens rise to a somewhat higher level in the male, and the estrogens rise to a much higher level in the female. In neither sex, however, does this rise correlate with the levels of sexual responsiveness in the adolescent or older female or male.
4. Gonadal hormones are among the elements necessary for normal physical development in young animals, both female and male. Consequently they are necessary for the development of the capacities of the nervous system and therefore may, indirectly, affect the development of sexual responsiveness in the female and male.
5. In human adults, gonadal hormones may be withdrawn (as in a castration) with minimum effects on physical characters, with a minimum or no effect on the sexual responsiveness of the female, and with little or no effect on the sexual responsiveness of half or more of the males. In a smaller number of cases there may be a slow physical degeneration in the male which may be accompanied by a reduction in his sexual responsiveness.
6. Estrogen and androgen levels, as far as they are known in the normal human female and male, do not correlate with the frequencies of sexual activity in the two sexes. There seem to be no correlations with the aging patterns of the male in his sexual performance, and with the female’s maintenance of a level of response throughout most of her life.
7. Increased supplies of androgens given to adult human females or males do raise their levels of sexual response. There is serious danger, however, in the administration of such hormones except under the direction of an experienced clinician.
8. Pituitary hormones are also among the elements necessary for the normal physical development of the female and male, and for the development of many physiologic capacities, including those capacities of the nervous system upon which sexual behavior depends.
9. The levels of pituitary secretion are known to drop steadily among the males of one species of laboratory animal, while the levels of pituitary secretion in the female of the same species may remain constant for some years. If this is also true of the human species, this would parallel the differences which we find in levels of sexual responsiveness among human females and males. The correlations might represent a causal relationship, or both phenomena might be independent products of more basic physiologic factors.
10. The levels of thyroid secretion sometimes show a direct correlation with the levels of sexual responsiveness. Higher thyroid levels sometimes correlate with higher levels of response, and lower thyroid levels sometimes correlate with lower levels of response; but the correlations are not invariable, for there appear to be other factors which determine the effectiveness of the hormone from the thyroid.
11. Adrenaline, from the medulla of the adrenal glands, does not seem to be involved in the initiation of sexual response. However, as sexual activities progress, the adrenal glands may be stimulated into secretion, and this secretion may be responsible for certain of the phenomena which appear in the later stages of sexual activity. There is no evidence that adrenaline actually inhibits sexual response.
12. The levels of the 17-ketosteroids, which originate largely in the adrenal cortex but also develop in the testes and ovaries and apparently in other organs of the body, show rather striking correlations with the levels of sexual response in the human female and male. In the human male, the 17-ketosteroids drop steadily from a peak in the late teens or twenties into old age, while they remain on a plateau for some period of years in the female. Again, this may represent a direct causal relationship, or (what is more likely) both the 17-ketosteroids and the levels of sexual responsiveness may reflect a more basic and probably more complex physiologic situation.
13. While hormonal levels may affect the levels of sexual response—the intensity of response, the frequency of response, the frequency of overt sexual activity—there is no demonstrated relationship between any of the hormones and an individual’s response to particular sorts of psychologic stimuli, an individual’s interest in partners of a particular sex, or an individual’s utilization of particular techniques in his or her sexual activity. Within limits, the levels of sexual response may be modified by reducing or increasing the amount of available hormone, but there seems to be no reason for believing that the patterns of sexual behavior may be modified by hormonal therapy.
1 The Trobriand Islanders and certain Australian tribes are said not to be aware of the relationship between coitus and pregnancy, but even they consider that coitus paves the way for subsequent supernatural impregnation. The Trobrianders castrate boars. “to improve their condition” and note that such boars cease copulation. This renders suspect Malinowski’s statement that the testes are considered to be only ornamental appendages. See: Malinowski 1929:168, 180–181, 190–191. Ashley Montagu 1937:199–202.
2 The understanding and practice of castration may have begun as early as 7000 B.C. in the early Neolithic when animals were first domesticated. See: Steinach 1940:3, 25. Turner 1948:324. Many primitives who were at a Neolithic cultural level when first visited by Europeans practiced castration. See, for instance: Malinowski 1929:191 (Trobriand Islanders). Evans-Pritchard 1940:33 (Nuer of Africa). It is interesting to note that in cases of self-castration, as on the islands of Ponape and Tonga (Westermarck 1922(1):561) and among the Hottentot of Africa (Bryk 1934:123), the males were careful to remove only one of the testes. In addition, there are references to castration in some of the oldest myths of Europe and Egypt. See: Möbius 1903:12. Pritchard 1950:181 (Middle Assyrian laws, 15th century B.C. , tablet A, laws 15 and 20, provide castration as a punishment).
3 Aristotle (384–322 B.C. ), in the Historia Animalium, Bk. IX:631b–632b, has a lengthy discussion of the effects of castration in man and in other male animals, differentiates between pre-adolescent and adult castration, and refers to the removal of the ovaries of sows to lessen their sexual responsiveness.
4 For brief histories of endocrinology, see: Corner 1942:228–233. C. D. Turner 1948:5–9.
5 The removal of the thymus and/or injection of thymus extracts are usually without effect; but the development of the gonads at the onset of adolescence is associated with an atrophy of the thymus, and castrates retain the large thymus of childhood. See: Hoffman 1944:275. C. D. Turner 1948:21–22. Selye 1949:679, 683.
6 The functions of the pineal gland are poorly known and the data are conflicting, but see: Hoffman 1944:282–284. C. D. Turner 1948:19–21. Selye 1949:595.
7 It is not fully demonstrated that the liver secretes a hormone, but secretions of the liver inactivate gonadal and probably other hormones. See: C. D. Turner 1948:25. Williams 1950:7.
8 The estrogenic hormones found in human tissues and fluids are estradiol, estrone, and estriol. Estradiol is thought to be the true hormone and the others to be products derived from it, according to: Smith in Williams 1950:351. Talbot et al. 1952:296.
9 For instance, after removal of the testes, the cortex of the adrenal gland enlarges and secretes an additional amount of androgenic (male) hormone in mice and guinea pigs. See: Hartman and Brownell 1949:331. Selye 1949:130–131.
10 The placenta secretes estrogen, progesterone, and a gonadotropin. The duodenal mucosa secretes several hormones concerned with digestion. See: C. D. Turner 1948:13–16,
11 For the interrelationships between the secretions of the anterior lobe of the pituitary, the ovaries, testes, and adrenal cortex, see: Heller and Nelson 1948: 229–243. C. D. Turner 1948:241–244, 462–468. Hartman and Brownell 1949: ch. 26. Selye 1949:22–23. Burrows 1949:329–343. Williams 1950:21–32. Talbot et al. 1952:385–392.
12 For instance, both a thyroid deficiency and an excess of thyroid retard sexual maturation. See: Lisser 1942:29–32. Hoffman 1944:239–240. Williams 1950: 122, 175. Talbot et al. 1952:21, 31. See footnotes 37 , 66 .
13 As an example of the difficulties encountered in analyses: testicular androgen is metabolized and excreted partly in the form of ketosteroids in the urine, and hence the injection of an androgen such as testosterone propionate may be followed by an increase in urinary ketosteroids. But another androgen, methyl testosterone, may have no effect on the levels of the urinary ketosteroids, or even decrease them. See: Selye 1949:628. Howard and Scott in Williams 1950:337.
14 The failure of pre-adolescent castrates, either surgical or functional, to develop the secondary sexual characters typical of the adult, is described in such standard works as: Laughlin 1922:435. Lipschutz 1924:6 ff. Pratt in Allen et al. 1939:1267–1268, 1282. Hamilton 1941:1904. Greenblatt 1947:182, 254–257. Mazer and Israel 1951:231. Ford and Beach 1951:170.
15 For data on the growth of the human male genitalia before the rest of the body is fully grown, see: Schonfeld and Beebe 1942:771.
16 Absence of pubic hair and abnormal hair distribution in males with gonadal insufficiencies are well illustrated in Selye 1949:651–660.
17 A diminution of axillary and pubic hair, and sometimes hirsutism in females with congenitally absent or undeveloped ovaries are noted in Selye 1949:399–400.
18 In the abundant literature on the correction of a gonadal insufficiency through the administration of hormones, see, for instance: Kenyon 1938:121–134. Vest and Howard 1938:177–182. McCullagh 1939. Hamilton and Hubert 1940:372. Escamilla and Lisser 1941. Biskind et al. 1941. Keams 1941. Moore 1942:39. Heller, Nelson, and Roth 1943. Hurxthal 1943. Heller and Maddock 1947:414–418. Beach 1948:38–41,45. Selye 1949:645–666. Howard and Scott in Williams 1950:328–333. H. H. Turner 1950:32–48.
19 Menopausal-like involutional changes in the genitalia of castrated adult females, and their control by estrogen therapy, are noted, for example, in: Hoffman 1944:33–35. The lack of regressive changes in some hypogonadal males is discussed in: Heller and Maddock 1947:395.
20 The general physiologic effects of castration are discussed, for instance, in: Möbius 1903:28 ff. Laughlin 1922:435–436. Lipschütz 1924:12–14. Wolf 1934:257–268, 279. Lange 1934. Greenblatt 1947:254–257. Heller and Maddock 1947:393 ff. Ford and Beach 1951:221–225, 229–232.
21 In animals, adult male castrates show a genital atrophy, an accumulation of fat, and a decrease in metabolism, sexual drive, and aggressiveness. See: Tandler and Grosz 1913:25–41. Rice and Andrews 1951:115–116.
22 For a brief resumé of the studies on human male castrates, see pages 740–744 of this chapter.
23 For example, in the Middle Assyrian laws, which may date back to the 15th century B.C. , tablet A, laws 15 and 20, provide castration as a punishment for certain sexual offenses. See: Pritchard 1950:181.
24 There are statutes in some ten states providing for involuntary asexualization, or sterilization as a eugenic or therapeutic measure for criminal conduct. In several of these states (e.g. , Kansas and Oregon) the practice has been to allow castration as one of the permissible operations. Since the United States Supreme Court decision in Skinner v. Oklahoma 1941:316 U.S. 535, all operations have been “voluntary.” In order to circumvent constitutional objections, the California Legislature at its Third Extraordinary Session in 1951 passed a bill, A.B.2367, providing for mandatory life imprisonment for persons convicted of certain types of criminal conduct who did not “consent” to being castrated. This bill was vetoed by Governor Warren on July 18, 1951. A milder version of the same bill, S.B.19, failed to pass in the 1952 regular session of the Legislature. We are informed that a similar proposal was introduced in the 1953 session of the Oregon Legislature. To our knowledge castration proposals were summarily rejected by the commissions on sex offenders in New Jersey and Illinois. See also, Michigan, Governor’s Study Commission 1951:4, 6.
25 For such a use of castration or the administration of hormones, see: Hirschfeld 1928:54. Lange 1934:44, 101. Wolf 1934:16–23. Bohme 1935:10–34. Sand and Okkels 1938:374. Kopp 1938:698–704. Hawke 1950. Tappan 1951:242–246 (Denmark). Bowman 1952:70 (cites Judge Turrentine of San Diego court as stating that “behavior disorders have been well controlled by castration in about 70 men,” and that these men failed to become involved again with the law after castration. Since sex offenders are always among the lowest in their rate of recidivism, the Judge’s criterion is inadequate.). Bowman 1952:79–80 gives a report from the Swedish authorities on 166 legal castrations between 1944 and 1950. Bowman and Engle 1953:10.
26 For data on the two estrogens, estradiol and estrone, found in the testes, see: Selye 1949:54, 84. For data on estrogenic substances from the adrenal cortex, see: Hartman and Brownell 1949:105. Selye 1949:80–81. Kepler and Locke in Williams 1950:203. Thorn and Forsham in Williams 1950:261.
27 For levels of gonadal hormones in pre-adolescence and adolescence, see Nathanson et al. 1941.
28 For androgen and estrogen levels in normal adult males and females, see: Gallagher et at. 1937:695–703. Koch 1938:228–230. Heller and Maddock 1947: 395–398. Dorfman in Pincus and Thimann 1948:496–508.
29 The production of androgenic substances by the ovary is noted in: Hoffman 1944:47. C. D. Turner 1948:337. Burrows 1949:123–124. Selye 1949:627. Parkes 1950: 108. The adrenal cortex as another source of androgens is noted in: Koch 1938:218. Nathanson et al. 1941:862. C. D. Turner 1948:337. Burrows 1949:120. Selye 1949:80, 126–127. Kepler and Locke in Williams 1950:203. Parkes 1950:102. Perloff in Mazer and Israel 1951:124.
30 Dorfman in Pincus and Thimann 1948:502–504 differentiates the androgens from the 17-ketosteroid data and shows a decline in androgens with advancing age in both female and male. Ford and Beach 1951:227 state that it is generally agreed that testicular androgens decrease in later life.
31 For the social status of castrates in societies in which castration is socially approved, see: Möbius 1903:14, 16, 84.
32 The effect of pre-adolescent castrations or gonadal under-development in lowering the sexual responsiveness of the human male is also recorded in: Lipschütz 1924:12–13. Commins and Stone 1932:497–499. Pratt in Allen et al. 1939: 1268. Hoffman 1944:623, 625. Beach 1948:23–28. Selye 1949:646, 661. Ford and Beach 1951:231.
33 That an early castration depresses the sex drive of a male animal, although it does not abolish all indications of sexual responsiveness, is noted in: Stone in Allen et al. 1939:1219. Beach 1947a:34–35. Beach 1948:20–23. Rice and Andrews 1951:115. Ford and Beach 1951:229.
34 At the Yerkes Laboratories at Orange Park, Florida, the male chimpanzee named Don, who is now almost 19 years old, was castrated at approximately 2 years of age (ace. Nissen, verbal communic.). Physically he lies within the norms for adult males. His frequency of erection and sexual interest in females are equal to those of an intact male, but he does not usually reach orgasm and ejaculate. Owing to this inability, he can copulate more frequently than an intact male. Under androgen therapy Don is capable of orgasm and ejaculation, and his behavior and coital frequency then are identical with those of an intact male. The case is also noted in: Clark 1945. Ford and Beach 1951: 231. Beach in Blake and Ramsey 1951:78.
Another male chimpanzee named Dag, now about 8 years old, was castrated at about 2 months of age. Physically Dag falls within the norms, and his frequency of erection and his incidental masturbation are the same as that of intact males of comparable age.
35 When the females of mammalian species below the primates are castrated at an early age, they never develop sexual receptiveness, according to Beach 1947a: 35.
36 According to Wilson and Young 1941:781–783, estrogens administered to preadolescent guinea pig castrates can produce estrus. Estrogens administered to castrated pre-adolescent male rats show less specific effects; see Ball 1939: 282.
37 Delayed sexual development consequent on insulin deficiencies, on thyroid over- and under-development, on pituitary disturbances, and on adrenal malfunction, are noted, for instance, in: Hoffman 1944:255, 268. Hartman and Brownell 1949:353. Selye 1949:124, 126–127, 524, 737. Williams 1950:42, 122, 173–175. See also footnotes 12 , 66 .
38 The lack of any consistent effect of castration of an adult female on her sex drive is also noted in: Hegar 1878:71–72, 74. Canu 1897:ch. 1. Möbius 1903: 87 (sex drive remains after late castration). Glävecke ace. Kisch 1907: 187–188 (drive reported lessened in two-thirds of 27 castrated women). Commins and Stone 1932:499–501. Havelock Ellis 1936(I,2):11–14. Hoskins 1941:234 (desire often not lessened). Filler and Drezner 1944:123–124 (41 female castrates, 88 per cent reported no change in sex drive, majority under 35 years of age). Huffman 1950:915–917 (68 adult female castrations, ages 26–43, loss of responsiveness reported in 2 cases). Ford and Beach 1951:222–224. Masters in Cowdry 1952: 668 (atrophy of uterus, breasts, and vagina; more rapid in younger females; usually decreased sex drive, although exceptions are noted).
39 In the earlier work, distinctions were made between the receptivity of estrus and sexual responsiveness, as in: Young and Rundlett 1939:449. But more recent scientific publications have been inclined to consider sexual responsiveness and estrus synonymous, just as most farmers and animal breeders do. See: Williams 1943:125. Ford and Beach 1951:221. Beach in Blake and Ramsey 1951: 75–76 (“Removal of the ovaries in lower mammals is followed by total and permanent loss of female sexual responses … the animal’s tendency to become sexually aroused and to execute coital reactions is heavily dependent upon hormones from the ovaries.” And again, “Removal of the ovaries promptly and permanently abolishes all sexual behavior”). See also Chapter 15, footnote 18 .
40 That castrated female rats and dogs will sometimes mount other females is reported by: Beach and Rasquin 1942. Ford and Beach 1951:142.
41 This “phylogenetic interpretation” of hormonal control is suggested in: Beach 1947b:293–294. Beach 1948:9–10. Beach 1950:261–269.
42 For historical accounts of castrations, see such references as: Möbius 1903:12–25. Tandler and Grosz 1913:45–46. Hirschfeld 1948:65–66.
43 For a description of such mutilations in battle see: Möbius 1903:12–13. We have seen the ancient Egyptian and modem Ethiopian drawings.
44 For an example of the use of castration to cure masturbation see: Flood [?1901] who reports the castration of 24 males, half of them under 14 years of age, for persistent masturbation and epilepsy, apparently in the Hospital for Epileptics at Palmer, Mass. (See Laughlin 1922:433). Flood concludes: “persistent masturbators … unpleasant for a refined woman to see … it seemed an absolute necessity to try something which we had not yet tried.” See also the account of Dr. Pilcher’s castrations of “confirmed masturbators” at the Kansas State Training School at Winfield, Kansas, in: Flood [?1901]:16. Cave 1911:123. Hawke 1950:1 (“Our castrations first started during the administration of Dr. Pilcher who conceived the idea that castration might help control excessive masturbation and pervert sexual acts”). Bowman 1952: 69–70.
45 Data on the reduced sexual responses of castrated adult males of lower mammalian species may be found, for example, in: Stone 1927:369 (rats). Commins and Stone 1932:497 (rabbits and rats). Stone in Allen et al. 1939:1246 (rats). Beach 1944c:255 (rats). Beach 1948:21 (summarizes earlier studies). Rice and Andrews 1951:115–116 (farm animals).
46 Experimental and clinical data on the administration of male hormones to young, non-castrated males are cited in: Beach 1942d. Hoffman 1944:157–159. Heller and Maddock 1947:417. Beach 1948:206–207. Burrows 1949:169. Howard and Scott in Williams 1950:339.
47 Experimental data on the administration of female hormones to young, lower mammalian females, are cited in: Beach 1948:196, 203–204. Corner 1951: 57–58.
48 Summaries of published cases of precocious adolescent development in which there are data on sexual behavior, may be found in: Doe-Kulmann and Stone 1927:319. (adult sexuality not present in most precocious cases). Singer 1940: 19–22 (summary of published reports on 59 females, 48 males). Stotijn 1946:56 (a Dutch study). Dennis in Carmichael 1946:656–658. Sandblom 1948:110 (one case of a precocious three-year-old male, aggressive sexually).
49 Discussion of dosages of androgens affecting precocious physical development in young females is found in: Selye 1949:614. Mazer and Israel 1951:126. Talbot et al. 1952:381, 440–441, 484.
50 Effects of androgens on non-castrate adult male animals is described in: Beach 1942b:181–182, 193–194. Beach 1942c:227–247. Beach 1948:34, 36. Cheng et al. 1950:452 (increased sex activity in male rabbit).
51 While there is a general use of androgen therapy on intact, normal males in clinical practice, there are few published accounts; but see: Miller, Hubert and Hamilton 1938:538–540. Kenyon et al. 1940:35. Barahal 1940:319 (increased drive in homosexual males). Heller and Maddock 1947:419 (says “normal” men fail to have an increase in sex drive, but on p. 422 agrees that such treatment seems to increase “the power of the sex drive in both normal and homosexual males…”). Burrows 1949:169 (excessive masculine urges in “normal” male adults). Perloff 1949:133 ff. H. H. Turner 1950: 38–43 (a survey; indicates contradictions in data).
52 Surveys and studies on the effect of testosterone on sperm count include: Weisman 1941:240–242 (concludes small doses stimulate sperm production, large doses inhibit it). Heller and Maddock 1947:419 (temporary reduction of sperm). C. D. Turner 1948:344 (considers evidence for increase in sperm inconclusive; lack of sperm if dosage is excessive). H. H. Turner 1950:24–25, 53–54 (no change in sperm production, although recognizes that other reports are contradictory). Cheng et al. 1950:447–452 (increases volume of ejaculate in rabbit, but total number of sperm unchanged). Heckel and McDonald 1952:725–733 (reduces sperm production in 72 men; a return to a higher level after treatment stopped in twenty-three out of forty-five men).
53 The harmful effects of various doses of testosterone are pointed out in: Moore 1942:40–41. Heller and Maddock 1947:415–416. Ludwig 1950:453, 465 (small doses more injurious than large doses in male rats).
54 For clinical use of androgens to increase sex drive in human females, see: Geist 1941. Salmon 1941 (survey of animal and clinical experiments). Salmon and Geist 1943. Greenblatt 1947:177–178. Hyman 1946(3):2406. Carter, Cohen and Shorr 1947:361–364 (a comprehensive survey). Beach 1948:62. Selye 1949:619.
55 Increased aggressiveness among females of lower mammalian species after treatment with testosterone dosage are reported in: Stone 1939. Ball 1940:151–165. Young 1941:135. Birch and Clark 1946 (chimpanzee).
56 Various effects of estrogen therapy are reported in: Frank 1940:1509. Soule and Bortnick 1941. Mazer and Israel 1946:34. Greenblatt 1947:177–178. Emmens and Parkes 1947:240–241. Kimbrough and Israel 1948:1217–1219. Perloff 1949:135. Ford and Beach 1951:225–226. Mazer and Israel 1951:33. Masters in Cowdry 1952:669.
57 For estrogen effects on the behavior and physical structures of mammalian males, see: Clark and Birch 1945:328 (loss of dominance in chimpanzee). Emmens and Parkes 1947:236 ff. (atrophy of testis, prostate, Cowper’s glands, spermatogenesis). Beach 1948:61–62. Selye 1949:360 (decreases sexual responsiveness). Goldzieher and Goldzieher 1949: 1156 (male impotence). Howard and Scott in Williams 1950:342 (depresses spermatogenesis). Paschkis and Rakoff 1950:137–138 (testicular degeneration). Howard et al. 1950:134. Lynch 1952:734–741 (atrophy of rat testis, depresses spermatogenesis).
58 The lack of any antagonistic effect between androgens and estrogens is noted in: Koch 1937:206–208. Hartman 1940:449–471 (female monkey). Hoffman 1944:42–43. Burrows 1949:122. Selye 1949:64–65.
59 The use of estrogens on male sex offenders and others to reduce sex drive is discussed or reported in: Dunn 1940:2263–2264 (a single case, 96 day treatment, resulting in loss of sex drive and degeneration of genitalia). Dunn 1941: 643 (later report on same case. Some effects disappeared after stopping therapy, but sexual levels still reduced). Rosenzweig and Hoskins 1941:87–88 (massive doses, no effect on one homosexual male). Greenblatt 1947:279–280. Golla and Hodge 1949 (13 males, uncontrollable sex urge, treated with estrone successfully). Perloff 1949:135. Brown 1950:52–53 (cites 300 male homosexuals, unsuccessful treatment with androgens). Tappan 1951:247–248 (in Holland).
60 The physical effect of pituitary damage in young animals and human subjects, and subsequent hormonal therapy are discussed in: Pratt in Allen et al. 1939: 1294. Corner 1942:141 (females), 222 (males). Greenblatt 1947:183. Beach 1948:12–17, 22, 208. Selye 1949:262–317. Williams 1950:ch.2. Howard and Scott in Williams 1950:318–319. Ford and Beach 1951:167–169. Dempsey in Stevens 1951:221.
61 Precocious adolescent development resulting from the pituitary treatment of young animals is described in: Smith and Engle 1927 (ablation of thyroid made no difference, showing it was not involved; mice, rats, cats, rabbits, guinea pigs). Young 1941:322. Beach 1948:12–16. Burrows 1949:22–23, 39. Ford and Beach 1951:168–170.
62 That a lack of pituitary hormone depresses sex drive, is reported by: Burrows 1949:169. Selye 1949:271–282. Williams 1950:43, 46.
63 Conversely, the effect of the nervous system on the secretory capacity of the pituitary is well-known. Examples of such neural control are in: Hoffman 1944:309–310 (suppression of menses). Hartman 1945:27 (rabbit ovulation). C. D. Turner 1948:364–365 (pseudopregnancy). Durand-Wever 1952:209–211 (gonads deteriorate as a result of fear). Talbot et al. 1952:302–303 (puberty depends on neural mechanisms operating via pituitary).
64 For the relation of age to the mitochondria in the cells of the anterior lobe of the pituitary, see: Payne 1949:197–198. Payne in Cowdry 1952:385.
65 Effects of male and female hormones on thyroid function are noted in: Hoffman 1944:240–241 (reciprocal relationship). Williams 1950:115 (estrogen antagonizes thyroid function; testosterone stimulates thyroid and increases metabolic rate).
66 Delayed adolescence resulting from lack of thyroid is described in: Lisser 1942. Hoffman 1944:239. Williams 1950:173–175. Talbot et al 1952:21, 31.
67 The administration of thyroid in sterility cases is noted in: Siegler 1944:311–313. Smith in Williams 1950:430. Mazer and Israel 1951:461, 488 (to increase sperm count).
68 The opinion that adrenaline interferes with sexual response, or inhibits erection, is implied or specifically stated in: Cannon 1920:270–271. Kuntz 1945:311.
69 The compensatory function of the adrenal cortex in castrates is described in: Burrows 1949:189. Dempsey in Stevens 1951:216 (potency of male depressed more severely by destroying adrenals than by castration).
70 For the relative importance of the cortex in supplying 17-ketosteroids in the body see: Fraser et al. 1941:255 (two-thirds adrenal in male, all from adrenal in female). Dorfman et al. 1947:487 (17-ketosteroids still significant in male and female monkeys 40 days after removal of both gonads and adrenals). Hamburger 1948:31–32. Dempsey in Stevens 1951:215 (one-third gonadal, remainder from adrenal cortex). Engle in Cowdry 1952:712 (two-thirds from adrenal). Maddock et al. 1952: 668 (both testes and cortex are important).
71 Data on levels of 17-ketosteroids are found in the following sources: Fraser et al. 1941:237–255 (shows decrease in older ages, both male and female). Talbot et al. 1943:364. Hamburger 1948:34. Hamilton and Hamilton 1948: 438. Howard et al. 1950:127 ff. Salter, Humm, and Oesterling 1948:30.2–303, fig. 3 (decrease with age in male after twenties). Kenigsberg, Pearson, and McGavack 1949:426–427 (in males increase up to thirties, decrease thereafter. In contrast to other studies, notes no changes in twenty females aged 17 to 64).
72 The concept of a “reversal” of “masculinity” or “femininity” in sexual behavior among lower mammals is found, for instance, in: Ball 1937, 1939, 1940. Beach 1938, 1941, 1942b, 1945, 1947b. Young and Rundlett 1939:449 (labelling such a reversal homosexual). Beach corrects this interpretation in: Beach 1948:68. Ford and Beach 1951:41–42.
73 For the hormonal treatment of homosexual cases, see: Barahal1940:329 (7 cases, increased the sex drive of homosexuals, failed to change its direction). Glass, Deuel and Wright 1940. Kinsey 1941 (a critical appraisal of earlier data). Glass and Johnson 1944 (some benefit in 3 cases; no benefit in 8). T. V. Moore 1945:80. Heller and Maddock 1947:419–422 (a well balanced discussion). Perloff 1949:136–139. Howard and Scott in Williams 1950:333. Brown 1950: 52–53 (300 male homosexuals, unsuccessful treatment with androgens). See also footnote 59 .