Chapter 16
Flowering and sexual reproduction
16.1 Introduction to Flowering
Charles Darwin called the sudden appearance and rapid spread of flowering plants during evolution an ‘abominable mystery’. No less mysterious is why the sexual mode of biological reproduction should have arisen in the first place, particularly in plants, where the property of cellular totipotency allows efficient asexual propagation. But sexual reproduction by means of flowers clearly confers fitness benefits and competitive advantages: the first flowering plants emerged 125 million years ago and within the geologically brief timespan of 60 million years angiosperms had established themselves as the dominant form of vegetation, accounting for more than 80% of the species in most ecosystems.
As with the principles determining body plan and morphogenesis (see Chapter 12), the reproductive strategies of seed plants and higher animals differ in fundamental aspects (Figure 16.1). During early embryogenesis in animals, somatic cells (destined to give rise to all body tissues other than those producing eggs and sperm) are differentiated from germ line cells (committed to becoming the gametes) (Figure 16.1A). The decision about whether a given cell takes the somatic or germ line route to maturity depends on intrinsic factors, such as cytoplasmic polarity, and on signaling from neighboring cells. Normally, once a cell is committed to the germ line or soma, neither it nor the cells derived from it can divert into the alternative developmental fate. As a consequence, the animal reproductive system is not exposed to the developmental signals that subsequently induce formation of the major somatic tissues and organs, and mutations can be inherited only if they affect germ line cells.
There is no such specification of germ line and soma cells in plants, however (Figure 16.1B). The shoot apex switches from the vegetative to reproductive mode of differentiation in response to developmental and environmental signals. The reproductive system of the flower is derived from cells with a history of exposure to internal and environmental influences experienced through many rounds of cell division during plant maturation. The alternation of generations between the diploid sporophyte and haploid gametophyte (see Chapter 1) is an efficient mechanism for selecting against potentially deleterious heritable somatic mutations occurring in meristem cells before gametogenesis (formation of eggs and sperm).
This chapter discusses the environmental and developmental factors determining the transition from the vegetative to the reproductive condition. The patterning of flower primordia and the differentiation of floral organs are considered and finally the formation of male and female gametes and their union during fertilization is discussed.
16.2 Induction of Flowering
To maximize reproductive success, flowering must take place at the right time. Most plants are cross-pollinated and will synchronize flower production with others of the same species and with the availability of pollinators. In latitudes where the annual cycle alternates between favorable and unfavorable seasons, flowering is usually subject to close environmental control so that it coincides with the best conditions for seed development and fruit dispersal. Flowering is a three-stage process. First, the plant must be competent to respond to the factors that invoke the inductive signal. Next, the signal must be received by the leaves (see Section 8.5.4) and a message sent to the shoot apex, which undergoes the vegetative to reproductive transition. Then the reprogrammed apex switches from the production of vegetative laterals to pathways of patterning and morphogenesis that lead to the differentiation of floral organs. The best understood factors stimulating the action of the inductive signal are light (photoperiodism) and temperature (vernalization).
16.2.1 Floral Induction Requires Both the Perceptive Organ and the Shoot Apex to Acquire Competence during Plant Maturation
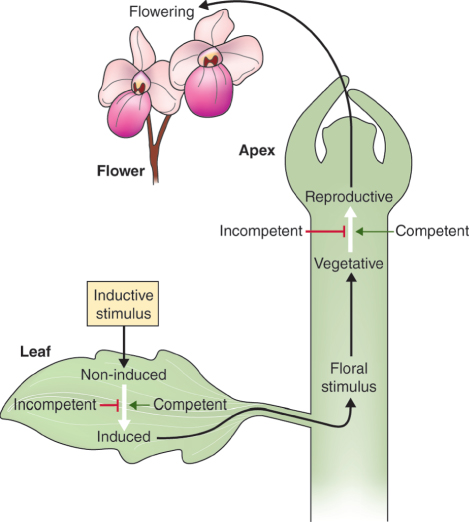
For flowering to occur, the plant must be competent. Competence resides in either the leaf, which perceives the inductive stimulus, or the shoot apex, or both. Figure 16.2 summarizes the possible combinations of foliar and apical competence and their outcomes in response to the inductive stimulus. Apical incompetence is characteristic of the shoots of young trees, which are usually unable to make the transition into flowering even when grafted onto mature plants. The reverse is the case in some species such as Kalanchoe (mother of thousands; see Figure 11.22A), in which the juvenile apex is competent and will form flowers when grafted onto a mature stock. The regulation of competence to flower is complex and varies across the taxonomic range, but an important part of the mechanism is the transition from the juvenile to mature phase of whole-plant development.
Distinct periods of juvenility and maturity are identifiable in the life spans of many species. The maturation process is referred to as heteroblasty or phase change. Four phases or maturation stages are recognized in the life cycle: embryonic; post-embryonic juvenile; adult vegetative; and adult reproductive. Each phase is associated with a characteristic package of morphological and physiological features. Ivy (Hedera helix) is a well-studied example of phase change. Table 16.1 compares the characteristics of juvenile ivy with those of the adult phase plant. As well as competence to flower, many aspects of morphology change with maturity in this species, including leaf form and phyllotaxy, growth habit, shoot determinacy and root development. If tissue taken from a juvenile ivy plant is cultured, plants regenerated from it have a stable juvenile phenotype. Similarly, mature tissue yields regenerants with mature characteristics. The difference between the phases has a strong epigenetic component (see Section 3.5).
Table 16.1 Distinguishing characteristics of juvenile and adult ivy.
|
|
|
| Three- or five-lobed palmate leaves |
Entire, ovate leaves |
| Alternate phyllotaxy |
Spiral phyllotaxy |
| Plastochron 4.2 days |
Plastochron 3.2 days |
| Shoot apex relatively narrow with large cells |
Wide apex with small cells |
| High rate of internode growth |
Low rate of internode growth |
| Anthocyanin pigmentation of young leaves and stems |
No anthocyanin pigmentation |
| Stems hairy |
Stems smooth |
| Climbing and spreading growth habit |
Upright or horizontal growth habit |
| Shoots show unlimited growth and lack terminal buds |
Shoots show limited growth terminated by buds with scales |
| Absence of flowering |
Presence of flowering |
| Rooting ability of cuttings good |
Cuttings root poorly |
| Adventitious roots present |
Adventitious roots absent |
Gibberellins (GAs) are important internal factors responsible for regulating phase change. Their effect is not the same in all species, however; for example, adult-phase ivy reverts to juvenility when sprayed with GA, whereas the juvenile–adult transition is delayed in Arabidopsis mutants with deficiencies in GA synthesis or perception. GA is also required for bolting, the process of stem elongation that precedes flowering in Arabidopsis and other rosette plants. Environmental variables, particularly light intensity and ambient temperature, significantly influence the timing of progress from juvenile to adult development. Figure 16.3 shows the phenotypes of phase-change mutants of maize (Zea mays), which produce shorter plants with more shoots than wild-type plants. The leaves of juvenile maize plants are short, hairless and covered in epicuticular wax. Adult-phase leaves are long and narrow with hairs but no wax. The analysis of mutants has identified the Corngrass (Cg) gene and genes of the Teopod (Tp) family as promoters of the adult condition and suppressors of the juvenile phase of vegetative development in this species. The signal that triggers phase transition is perceived directly in individual leaf primordia rather than by the shoot apical meristem. Mutations in Cg and Tp genes are associated with overexpression of the miR156 family of microRNAs (see Chapter 3). The miR156 locus has also been shown to be important in the architecture and maturation of rice, and variations in this locus are proposed to have been important in the evolution and domestication of cereal crops in general. Among the target genes silenced by miR156 are transcription factors of the extensive SPL (SQUAMOSA promoter-binding-like) family, which have a wide range of developmental functions including meristem identity, leaf shape and tissue patterning, and floral induction. The role of small RNAs in phase change, flowering and other morphogenetic processes is a fast-developing story which is expected to yield many new insights into the regulation of the development of plants and their parts.
16.2.2 Determinacy of the Reproductive Apex Affects Plant Morphology and Annual/Perennial Growth Habit
The shoot apex may be continuously meristematic (indeterminate) or may differentiate into a terminal organ and cease to make new structures (determinate). The early events of organogenesis at the shoot apex, whether the outcome is a leaf (see Chapter 12) or a floral structure, involve the formation of a determinate primordium. The pattern of apical and primordium determinacy is decisive not only for vegetative and floral morphology but also for plant habit and life cycle (see, for example, Figure 12.4). In annual species, all indeterminate vegetative shoot apices become determinate floral apices and the entire plant dies once the seeds have been dispersed. In perennial species, temporary determinacy of the vegetative apex results in the formation of a resting bud. Dormancy, the inactive condition adopted by plants to survive unfavorable seasons, is a state of arrested apical growth, and the position of resting buds in the plant is the basis of one of the widely used systems of classifying plant life forms (see Chapter 17).
Key Points
Of all the plants on Earth, only the angiosperms produce flowers. There are three requirements for flowering to occur. Firstly, the plant must have reached a state in which it is capable of responding to an inductive signal. Secondly, the signal must be perceived in the leaves and transmitted to the shoot apical meristem. Finally, the meristem must respond by transforming from vegetative proliferation to the production of floral parts. The best understood factors that trigger flowering are day length and temperature. Many species have distinct juvenile and adult phases, and only adult-phase plants are competent to flower. Gibberellins are important in regulating the phase change from juvenile to adult. The shoot apex may be indeterminate—that is, continuously meristematic—or it may differentiate into a terminal, determinate organ and cease to make new structures. In annual species, all indeterminate vegetative shoot apices become determinate floral apices. In perennial species, the vegetative apex becomes temporarily determinate in order to produce a resting bud. Determinacy of a floral apex is usually irreversible, but reversion sometimes occurs in response to environmental conditions.
Generally, determinacy of a terminal floral apex is irreversible, but there are numerous examples of reversion, in which the meristem is kick-started into further activity, leading to the abnormal production of vegetative or floral structures. Reversion may be an environmental response. For example, florally-induced soybean (Glycine max) that is transferred from inductive short days to non-inductive long days has been observed to revert to the vegetative condition, making compound leaves consisting of three leaflets instead of foliar bracts (modified simple leaves associated with floral parts) and initiating vegetative shoot growth after aborting further development of flower buds. In some species, such as garden balsam (Impatiens balsamina), the tendency to reversion (Figure 16.4) has a strong genetic component; the extent and nature of reversion behavior in parents and offspring varies between different varieties. Mutations in genes that determine and maintain the reproductive state often result in a form of floral reversion in Arabidopsis.
16.2.3 Different Inductive Pathways Lead to Flowering
Several environmental signals may be involved in the induction of flowering. These include photoperiod, light quality and temperature. Photocontrol of flowering is exerted through day length and light quality; the role of light in controlling flowering is discussed in Chapter 8 (see Figure 8.33). Exposure to low temperature is also critical for the acquisition of competence to respond to photoinductive conditions in many long-day (LD) winter annual, biennial and perennial species from temperate regions. The cold requirement is called vernalization and acts as a kind of time-computing mechanism that measures the passage of winter and ensures that flowering does not begin until the favorable conditions of spring arrive. The analysis of a class of delayed-flowering mutants that are insensitive to photoperiod has identified another regulatory pathway of floral induction in Arabidopsis. This so-called autonomous pathway is independent of day length and represents the route by which endogenous developmental cues feed into the network of flowering genes and their interactions.
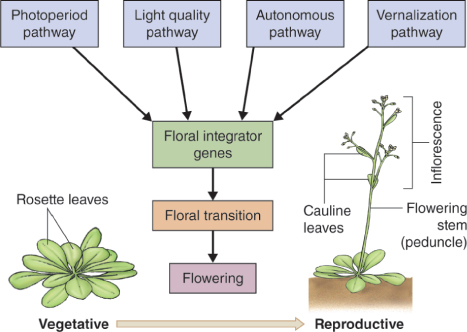
Figure 16.5 shows how the photoperiod pathway, light quality pathway, vernalization pathway and autonomous pathway form a regulatory network that converges on the activities of a set of genes that integrate the floral stimulus and trigger the vegetative to reproductive transition. Gibberellins play a number of roles in this transition. These include functions in competence (Section 16.2.1) and the development of floral organs (see Section 16.3) as well as in the promotion of bolting and flowering in Arabidopsis and many other LD and/or biennial species. Flowering in perennial species, on the other hand, tends to be insensitive to, or even inhibited by, GA. There is good evidence that GA is a mobile signal that transmits the photoperiodic floral stimulus in the LD grass Lolium temulentum; its action is independent from that of FT, the phloem-mobile protein that relays the flowering induction signal from leaf to shoot apex (see Section 8.5.4).
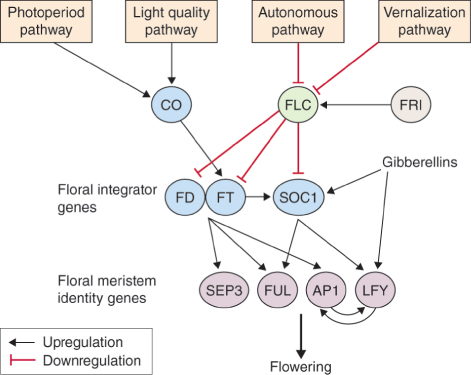
Expression of both SOC1 (SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1) and LFY in Arabidopsis is promoted by GA via the DELLA-mediated signaling mechanism (see Chapter 10). SOC1 is thus regulated in a multifactorial manner and integrates the autonomous, vernalization and GA pathways. The GA and photoinductive pathways converge on SOC1 and LFY (Figure 16.6).
Floral meristem identity genes stand at the point of conjunction between the different inductive pathways. The genes APETALA1 (AP1), LEAFY (LFY), SEPALLATA3 (SEP3) and FRUITFUL (FUL), are all named for the Arabidopsis mutants identified by their abnormal floral phenotypes (see Section 16.3). Activation of floral identity genes during the switch to reproductive development involves either derepression of the target gene (as in the case of AP1) or reorganization of sites of expression within the apex (LFY is an example) before floral primordia develop (Figure 16.7). As we shall see, during the vegetative to reproductive transition, floral identity genes have distinct but overlapping functions and cooperate to modulate each other's activity (Figure 16.6).
16.2.4 Regulation of Floral Induction by Vernalization is Epigenetic in Nature
Many strains of Arabidopsis, including those most commonly used for laboratory experimentation, are summer annuals and have no vernalization requirement. Studies of natural variation, however, have identified ecotypes (genotypes adapted to particular habitats) that, like winter annuals, delay flowering unless they are exposed to a period of chilling. Genetic analysis has established that this vernalization requirement is conferred by FLOWERING LOCUS C (FLC) and a second dominant gene, FRIGIDA (FRI). FLC is a MADS-box transcription factor that functions as a repressor of flowering, in part by blocking expression of FT. The FLC gene is expressed in all the vegetative parts of the plant, especially the vegetative shoot apex and the roots, but FLC mRNA is not detectable in young floral meristems, indicating that expression of FLC is downregulated in the apex after the transition to flowering. FLC is the point of convergence of the autonomous and vernalization pathways (see Figure 16.6). Mutations in the autonomous pathway delay flowering, an effect that is suppressed in flc null mutants. Genes in the autonomous pathway that block FLC expression have a range of functions in development, many of which are not directly concerned with flowering. FRI encodes a nuclear protein, unique to plants, that upregulates FLC expression, thereby repressing transcription of FT and other floral integrator genes (see Figure 16.6). Most of the natural variation in the vernalization requirement of Arabidopsis ecotypes is due to allelic variation at the FRI and FLC loci.
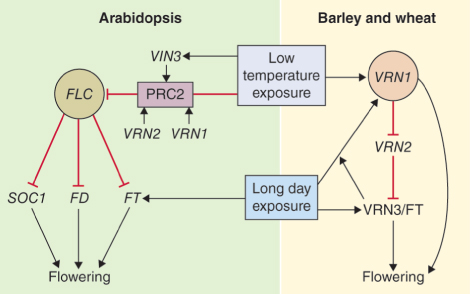
Vernalization is an example of an epigenetic switch. Even after chilling treatment has been discontinued, vernalization-mediated change in competence to flower is retained through a large number of mitotic divisions in the apical meristem. The switch takes the form of modifications to the chromatin of the FLC locus, resulting in mitotically-stable repression of FLC gene transcription. In Arabidopsis, low temperature induces the synthesis of VIN3, the product of the VERNALIZATION INSENSITIVE 3 gene. VIN3 is recruited to the so-called polycomb repressive complex 2 (PRC2) in association with other proteins including VRN2 and VRN1, encoded by VERNALIZATION (VRN) genes. Polycomb takes its name from the phenotype of a homeotic mutant of Drosophila. Proteins of the polycomb group silence gene expression in animals and plants by remodeling chromatin. The repressed state is perpetuated through cell divisions. PRC2 components increase during vernalization and modify histone methylation, which results in repression of FLC transcription and consequent derepression of flowering (Figure 16.8).
Winter cereals are planted in fall and have a vernalization requirement which is satisfied by exposure to cold temperatures before seedling growth resumes in the spring. Most temperate perennial grasses also require vernalization to become florally competent to respond to LDs. As their name suggests, spring cereals are planted after winter is over and will flower without vernalization. The vernalization pathways in Arabidopsis and winter cereals show points of similarity and difference (Figure 16.8). Flowering in Triticum spp. is regulated at three VRN loci. Confusingly, the genes designated VRN in grasses are not structurally related to the genes of the same name in Arabidopsis. VRN3 of wheat and barley (Hordeum) has high homology with FT. The VRN2 region of the cereal genome encodes two similar zinc finger proteins which act as repressors of VRN3/FT. The spring growth habit in wheat and barley is associated with loss of the VRN3/FT repressor VRN2, a genetic locus with no clear homolog in Arabidopsis. VRN1 is a MADS box gene with multiple functions. Plants with deletions at this locus remain permanently vegetative. VRN1 is thought to be the primary target for the vernalization signal in grasses. In contrast to FLC of Arabidopsis (a structurally unrelated MADS box gene), VRN1 in grasses is induced rather than repressed by low temperature. VRN1, VRN2 and VRN3/FT interact to regulate vernalization and flowering (Figure 16.8). VRN1, which is expressed at low levels during winter, becomes strongly upregulated by VRN3 in shoot apices as day length increases during spring and stimulates the shoot apical meristem to switch from the production of leaf primordia to flower primordia. Further development and elongation of the inflorescence generally require LDs. VRN1 is also a repressor of VRN2 transcription. As in Arabidopsis, chilling-induced floral competence in winter cereals is associated with alterations in histone methylation and chromatin structure and is stably transmitted during mitosis.
The epigenetic nature of vernalization control in winter cereals is of more than biological interest: it is also of major historical and political importance. In the 1930s, the influential Ukrainian agriculturalist Trofim Lysenko denounced the principles of Mendelian genetics, using vernalization as evidence for the inheritance of acquired characters. Lysenko's doctrine, enshrined in Soviet Communist Party orthodoxy (and propagated through its own journal, the Bulletin of Vernalization), dominated biology during the era of Josef Stalin and was responsible for the persecution of many prominent geneticists and agronomists, thereby contributing to the backwardness of Soviet agriculture and to frequent and widespread crop failure. It is no exaggeration to state that the genetic regulation of cereal vernalization was a significant influence on the course of 20th century history.
Key Points
Photoperiod and light quality are important in regulating the induction of flowering in many plants. Temperate species also often require a period of exposure to cold in order to become competent to flower; this cold requirement is called vernalization. Vernalization is an epigenetic switch, brought about by modification of the chromatin around the FLC gene. An autonomous pathway, which is independent of light regime and temperature, integrates endogenous development cues to ensure that flowering is not triggered inappropriately. The autonomous, photoperiod, light quality and vernalization pathways operate together to control expression of key genes that trigger the switch from vegetative to reproductive development.
16.3 Development of Floral Organs
The transition of shoot apex identity from the vegetative to the reproductive state described in Section 16.2 is only the first step in flowering. To make a functional flower, the identities of organ primordia in the apex must be established. Familiarity with the terminology used to describe floral morphology is necessary for understanding how flowers develop (see Section 1.7.1). The structure of the mature Arabidopsis flower is typical of that of many advanced angiosperms. It comprises four types of floral organs in an arrangement of four concentric whorls. The outermost whorl of four sepals (collectively called the calyx) and the next whorl of four petals (the corolla) enclose the male and female reproductive organs, comprising, respectively, a whorl of six stamens surrounding the central gynoecium that contains two carpels (see Figure 1.17). Collectively, the calyx and corolla comprise the perianth.
Once Arabidopsis has been induced to flower it bolts; that is, the rosette produces a shoot carrying the floral apex and cauline leaves—cauline means growing on a stem rather than in a rosette (see Figure 16.5). This main flowering stem (peduncle) is indeterminate and bears the primary inflorescence, the first inflorescence to form. An inflorescence is defined as a cluster or group of flowers on a stem. Successive flowers that make up an inflorescence are arranged at an angle of 130° to 150° to each other in a spiral around the center of the shoot apex, with the oldest flowers at the base and the youngest at the top of the meristem. Axillary buds of cauline leaves develop into additional secondary inflorescences, bearing flowers along their length. Flower initiation on the primary shoot meristem begins shortly before secondary inflorescences form. A flower primordium first appears as a bulge (buttress) on the shoot apical meristem, and organ primordia as bulges on a flower primordium. Under experimental conditions, the sequence of events giving rise to a normal Arabidopsis flower is highly predictable. Table 16.2 is a timetable of developmental landmarks and Figure 16.9 shows a corresponding scanning electron microscope sequence of the morphology of the floral apex and developing flower and floral organ primordia.
Table 16.2 Stages of flower development in Arabidopsis thaliana.
Arabidopsis has been the subject of detailed genetic and molecular dissection of floral morphogenesis. Analysis of flower development in this species is based on the availability of homeotic mutants. In a homeotic mutant an organ or tissue develops in an abnormal location, often replacing the structure that would normally occur in that position. Such mutants have been intensively studied in insects, notably Drosophila, where the body is metameric, that is, organized into segments bearing different organs. In the Drosophila mutant Antennapedia, for example, mis-expression of the corresponding homeotic gene (Antp) results in primordia on the head segment developing into legs instead of antennae (see Figure 3.30). The body plan of plants is similarly metameric and subject to homeotic mutation, as exemplified by genes of the KNOX family (see Chapters 3 and 12). Homeotic mutations affecting flowers were among the first recognized by geneticists. Familiar examples include the so-called double flowers of many commercial varieties of roses, camellias, carnations and other blooms, in which some or all of the stamens in a flower are replaced by petals. Genetic analysis of homeotic mutants has been the key to building a model of the regulation of floral organ identity in Arabidopsis.
The following discussion examines the picture of flower development that emerges from work on Arabidopsis mutants in which the normal course of floral morphogenesis is disrupted. We go on to consider how the model for the specification of flower structures established in Arabidopsis applies, with appropriate adjustments, across the range of angiosperms. Finally we describe how floral symmetry, inflorescence architecture and flower color are determined.
16.3.1 Specification of Floral Structures in Arabidopsis is Explained by the ABC Model of Gene Expression
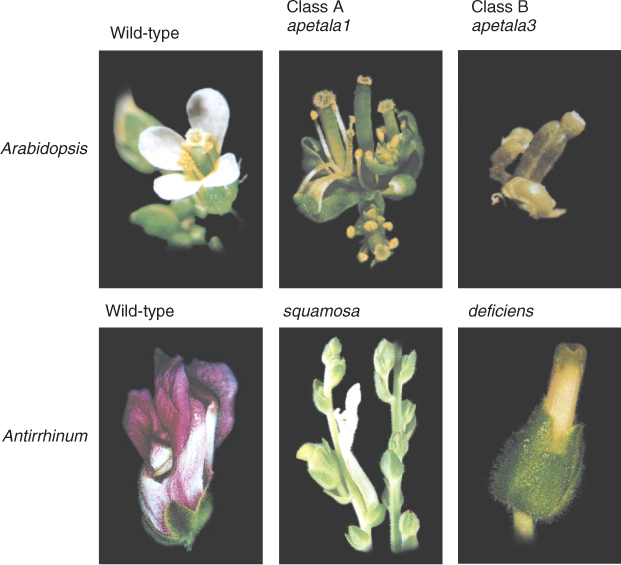
Analysis of Arabidopsis homeotic mutants that have abnormal identities of organs (sepals, petals, stamens, carpels) in their respective whorls supports a theory of flower development based on the interactions of a small number of regulatory genes. Some Arabidopsis mutants with mis-specified floral organs are shown in Figure 16.10. The flower of the apetala2 (ap2) mutant has stamens in the third whorl and carpels in the fourth whorl, like wild-type flowers. However, carpels replace sepals in the first whorl, and the second whorl may contain stamens or lack organs altogether. The flower of another mutant, pistillata (pi), has sepals in the first and second whorls and carpels in the third and fourth. An agamous (ag) flower consists of perianth parts only, with sepals in the first whorl, petals in the second and third whorls, and reiterations of this pattern in interior whorls. Observations of these and other homeotic mutants have been used to develop a model of floral organ determination that proposes three overlapping fields of gene activity, designated A, B and C, working in a combinatorial fashion to specify organ identity in each of the whorls of the flower primordium. The A field is conceived as covering whorls 1 and 2, the C field covers whorls 3 and 4, and B overlaps A and C in whorls 2 and 3, respectively. An important feature of the model is the antagonism between A and C, such that if expression of either is missing, the other will be expressed across the entire floral meristem (Figure 16.11). According to the model, in the wild-type flower production of a sepal in the normal position, whorl 1, requires class A gene expression. A combined with B specifies development of a petal in whorl 2. B and C together determine stamens in whorl 3, and class C gene expression alone establishes the identity of carpels in whorl 4 (Figure 16.11).
The ABC concept provides an explanation for the corresponding mutant phenotypes. The wild-type alleles of the genes shown in Figure 16.10 are classified according to the model thus: AP2 is an A-type gene, PI is a B-type and AG is a C-type gene. The combination of functional AP2, PI and AG specifies a normal flower with sepals, petals, stamens and carpels occupying whorls 1 to 4 sequentially. Figure 16.11 shows the consequence of mutations in AG (loss of C function), in AP2 (loss of A function) and in PI (loss of B function).
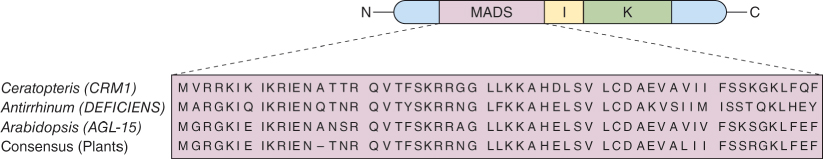
AG, AP2 and PI are classified as homeotic, but unlike KNOX and many other homeotic genes, they do not encode homeodomain proteins (see Chapter 3). AG and PI are members of the MADS-box family of transcriptional regulators. Figure 16.12 is a comparison of plant MADS-box sequences, showing a high degree of conservation. MADS-box genes in the fern Ceratopteris are expressed in gametophytes and sporophytes and show a high degree of homology with the MADS-box genes of the eudicots Antirrhinum and Arabidopsis. It is believed that MADS-box genes present in the last common ancestor of contemporary vascular plants underwent multiple duplications and diversifications and were recruited into novel developmental networks during the evolution of floral organs. AP2 does not encode a MADS-box protein but belongs to the ERF (ethylene responsive factor) family of transcription regulators.
16.3.2 The Original ABC Model has been Enhanced to Include E Class Factors
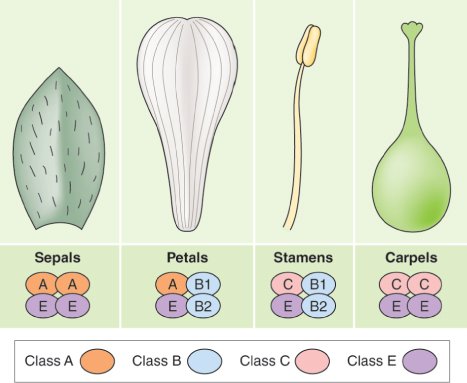
Since the ABC model of floral organ identity determination was originally proposed, further studies of homeotic mutants have led to its elaboration to include additional morphogenetic fields, designated D and E. Class D genes, which act in carpels to confer ovule identity, will be further discussed in Section 16.4.4. Here we consider Arabidopsis SEPALLATA (SEP), a set of four MADS-box genes required to specify petals, stamens and carpels. The pattern of expression of sep mutations has led to their corresponding wild-type loci being assigned to class E in the extended flower development model. Flowers of sep1-sep2-sep3 triple mutants consist of sepals only. A fourth SEP gene, SEP4, works with SEP1, SEP2 and SEP3 to specify sepal identity, and also contributes to the development of petals, stamens and carpels. In quadruple mutants (sep1-sep2-sep3-sep4) all floral organs are converted into leaf-like structures. Based on such observations the ABCE model has been proposed. In this model A + E function is needed for sepals, A + B + E function for petals, B + C + E function for stamens, and C + E function for carpels.
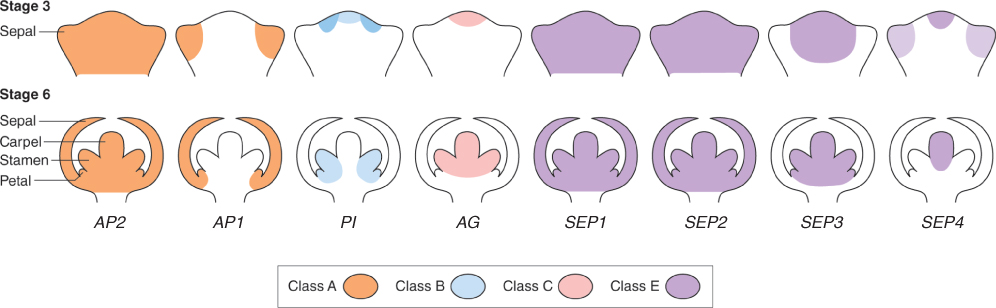
Genes of the ABCE model are expressed in the floral apex at characteristic locations that change as the flower primordium differentiates. Figure 16.13 shows how expression patterns become modified between early and later developmental stages (stages 3 and 6; see Table 16.2 and Figure 16.9). At stage 3, only sepal initials can be identified. Primordia of all four organ types are present at stage 6. AP2 (class A) is expressed in all four whorls of the flower. AP1 (also class A) is not detectable in the vegetative apex and is expressed specifically in the outer two whorls of the flower primordium (Figure 16.13). From an early stage, expression of the class B gene PI is strongly associated with cells destined to form petals and stamens. AG (class C) is expressed in the inner two floral whorls. Of the SEP genes (class E), SEP1 and SEP2 are expressed throughout the flower, SEP3 is localized to the inner three whorls and SEP4 is prominent in the fourth whorl and detectable at low levels in sepal primordia at stage 3.
The proteins that are encoded by floral identity MADS-box genes interact at their I, K and C-terminal domains (Figure 16.12) to form different tetramers which bind with so-called CArG DNA sequences in the regulatory regions of target genes. The name of these sequences comes from CC(A/T)rich6GG, meaning that each consists of two C nucleotides, followed by a region of six nucleotides which are all, or mostly, As and Ts, and finally two Gs. Figure 16.14 relates each organ type to the composition of the corresponding MADS-box tetramer that specifies it. Experimental studies of protein–protein and protein–DNA binding provide direct support for the ‘floral quartet’ mechanism underlying the ABCE model.
There is a certain degree of overlap between genes in the floral induction network (see Figure 16.6) and regulators of flower organ identity. For example, AP1 functions as a class A homeotic gene. Interaction with FT-FD and LFY activates AP1, whereas A–C antagonism integral to the ABC model means that AG is a suppressor of AP1 expression. The class E gene SEP3 is directly responsive to FT-FD.
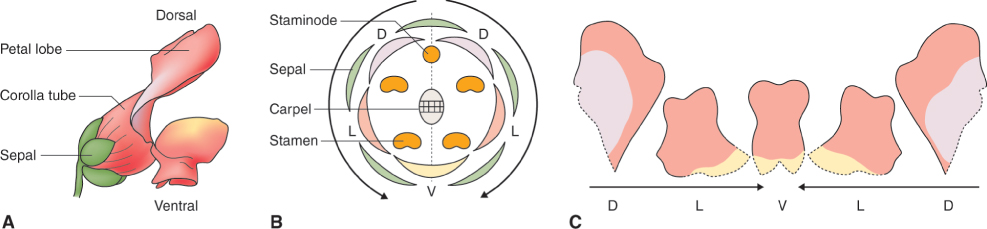
16.3.3 The ABCE Model, or Modifications of it, Applies to Floral Differentiation Across the Range of Angiosperms
The ABCE concept was largely based on genetic studies in Arabidopsis, but work using other species contributed significantly to aspects of the model and to its establishment as the general scheme that can be applied to floral development across the range of angiosperms. One such species is snapdragon, Antirrhinum majus, the mature flowers of which are morphologically very different from those of Arabidopsis (Figure 16.15). Antirrhinum flowers are bilaterally symmetrical (zygomorphic) whereas those of Arabidopsis are radially symmetrical (actinomorphic). The four petals of the Arabidopsis flower are identical and each is bilaterally symmetrical, whereas the Antirrhinum corolla consists of a single, bilaterally symmetrical dorsal (adaxial) petal, two asymmetrical lateral petals and two asymmetrical ventral (abaxial) petals which are fused at their bases to form a corolla tube (Figure 16.15). Note that the terms adaxial and dorsal are often used interchangeably in the literature on the orientation of plant organs, as are abaxial and ventral. Species with zygomorphic flowers are thought to have evolved from actinomorphic ancestors on several independent occasions during angiosperm diversification. Coevolution with specialized pollinators gave rise to bilateral floral symmetry, which facilitates efficient and specific pollen exchange. Many hypotheses seek to explain this trend. For example, it is suggested that floral characteristics such as the morphology and color of the corolla and the quantity and quality of the reward to the pollinator form phenotypic clusters that match perceptional or behavioral preferences in particular taxonomic groups of pollinators. The genetic regulation of floral symmetry is discussed in Section 16.3.5.
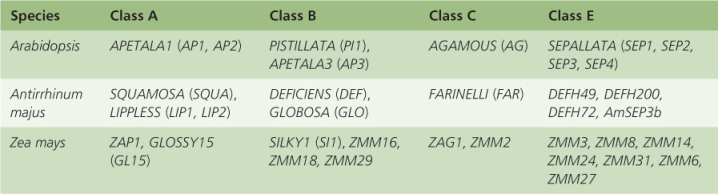
Despite the differences in floral structure, the organ-specifying genes and mutants on which the ABCE model is based are closely similar in Antirrhinum and Arabidopsis (Table 16.3, Figure 16.16). Indeed, some of the Arabidopsis genes for floral meristem identity were cloned using sequences first isolated from Antirrhinum. Comparative studies of ABCE-related DNA sequences has established that conservation of genes and mechanisms extends across the taxonomic range, including the so-called basal angiosperms, which represent survivors of the earliest lineages of flowering plants. They include plants such as magnolia (order Magnoliales), avocado (Persea americana; Laurales), black pepper (Piper nigrum; Piperales) and water lily (any of several members of the family Nymphaeaceae).
Table 16.3 Orthologs of ABCE floral genes in Arabidopsis, Antirrhinum and Zea.
Monocots are sometimes included among the basal angiosperms (see Figure 1.16). The principle of interacting morphogenetic fields applies to the specification of monocot floral organs, but the ABCE model has to be modified to account for some distinctive structural features in this group. Many non-grass monocot flowers, such as lily (Lilium spp.) and tulip (Tulipa spp.), have a variant of the four-whorl structure in which sepals and petals are replaced by petaloid organs called tepals, arranged in an outer and inner whorl of three each, surrounding six stamens and three fused carpels. The organ identity of tepals is explained by a model in which the B field extends to cover both outer whorls, so that A + B function specifies both outer and inner tepals, B + C specifies stamens and C alone specifies carpels (Figure 16.17).
Grasses are wind-pollinated and have correspondingly specialized flower structures in which sepals are highly modified and petals are absent (see Figure 6.6). A, B and C class genes have been isolated from a number of grass species (Table 16.3 lists orthologs from maize). Studies of homeotic mutants in maize and rice have confirmed that class B function is conserved in these species. The specification of carpels and the function of class C MADS-box genes are less clear. Although class E orthologs have been identified in many monocots (at least seven from maize for example; Table 16.3), their function is not yet confirmed.
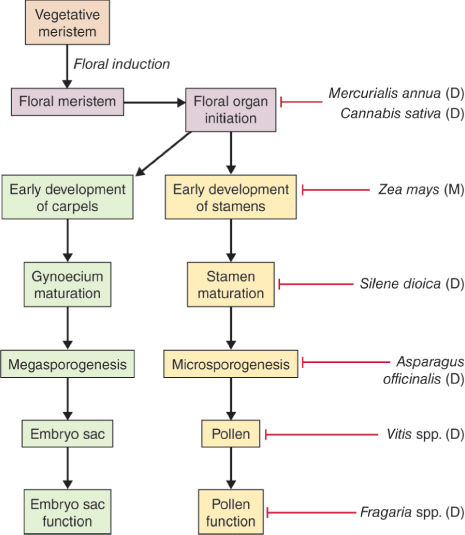
While most angiosperms have flowers with both stamens and carpels, a number of species have separate staminate (male) and pistillate (female) flowers. Maize is an example of a monoecious species bearing separate male and female flowers on the same plant. In dioecious species such as asparagus and holly (Ilex spp.), male and female flowers are carried on different plants. The sex of the flower in monoecious and dioecious plants is determined by mechanisms in which initiation, specification or differentiation of stamens or carpels is blocked at a point that varies from species to species (Figure 16.18). Maize flower development and the role of cell death in sex determination are described in Chapter 18.
16.3.4 Floral Symmetry is Determined by the Interplay between TCP- and MYB-type Transcription Factors
As we have seen, the flowers of Antirrhinum are zygomorphic. Studies of Antirrhinum mutants in which the zygomorphic symmetry is disturbed have identified four key genes regulating dorsal/ventral development. CYCLOIDEA (CYC) and DICHOTOMA (DICH) promote dorsal identity and encode proteins belonging to the TCP family. TCP factors, which are exclusive to flowering plants, are named after the genes TB1 (from maize), CYC (from Antirrhinum) and PCF (from rice), and are related to basic helix-loop-helix-type DNA-binding proteins. CYC and DICH interact with two members of the MYB gene family, RADIALIS (RAD), and DIVARICATA (DIV). Expression of the two TCP proteins in dorsal regions of the floral meristem leads to localized activation of RAD transcription. RAD and DIV act antagonistically so activation of RAD on the ventral side of the meristem leads to inactivation of DIV there. Figure 16.19 interprets the dorsoventral and lateral patterning of the Antirrhinum flower in terms of interplay between the pairs of TCP and MYB transcription factors.
Key Points
Flowers are made up of floral parts arranged in concentric whorls, with sepals and petals surrounding the inner, reproductive parts. Specification of different floral structures is best understood in Arabidopsis, where homeotic mutants that convert one type of floral part into another have been used to dissect the molecular control of flower structure. The ABC model of floral organ specification proposes overlapping fields of gene expression, so that, for example, class A gene expression is required to make a sepal in the normal position, whorl 1, while A combined with B specifies that a petal will develop in whorl 2. The original model has been expanded to include class E genes. A, B, C and E genes are expressed in distinct zones of the floral apex as the flower primordium develops. Variants of the ABCE model apply to the differentiation of flower parts across all angiosperms.
The decisive role of CYC homologs in the development of zygomorphy has been demonstrated for several species. In Lotus japonicus and Pisum sativum, for example, disruption of wild-type patterns of dorsoventral symmetry by ectopic (abnormally positioned) or reduced expression of CYC-like genes results in dorsalized or ventralized flower phenotypes, respectively. Evidence for involvement of CYC in the evolutionary transition from radially to bilaterally symmetrical flowers is seen in the zygomorphic candytuft (Iberis). In this genus, flowers have two dorsal petals that are smaller than the two ventral petals. In wild-type Iberis expression of a CYC-like gene in later stages of dorsal petal development is associated with a reduction in their size relative to that of ventral petals. This is in contrast to Antirrhinum, where the dorsal petals are larger than the ventral. In radially symmetrical mutants of Iberis, this pattern of CYC expression is absent. Expressing Iberis CYC-like genes in its actinomorphic close relative Arabidopsis has the effect of reducing petal size.
In addition to their role in floral symmetry, TCP genes also have a role in floral organ abortion. For example, the stamen in the adaxial position of the Antirrhinum flower is sterile (a staminode; see Figure 16.15B). CYC expression is necessary for dorsal stamen abortion. The desert ghost flower (Mohavea confertiflora), a relative of Antirrhinum, has both adaxial and lateral staminodes, and this correlates with expansion of CYC-like gene expression into the lateral domain. Genes of the TCP family have undergone extensive duplication, structural divergence and functional diversification during plant evolution and have been co-opted on multiple occasions into a variety of morphogenetic programs—and not only for floral organ patterning. TCP genes are known to be transiently expressed in a range of different developing tissues, including flower and shoot meristems and leaf and floral organ primordia. It is becoming clear that they are active in determining organ shape, size, number and curvature. For example the Antirrhinum gene CINCINNATA (CIN) encodes a TCP-type transcription factor responsible for regulating lobe formation both in petals and in leaves.
16.3.5 Inflorescence Architecture can be Modeled using Veg, a Meristem Identity Parameter
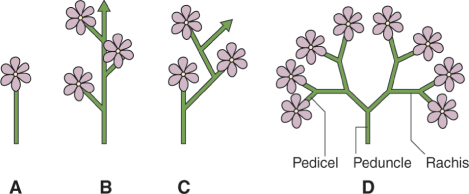
Inflorescence is the name given to the arrangement of flowers on the peduncle (floral axis). The terminology applied to the different forms of inflorescence architecture gives us some of the most beautiful words in the language (corymb, umbel, spadix, glomerule, thyrse are examples) but can be extremely complex and confusing. We can, however, group the diversity of forms into a small number of basic classes (Figure 16.20). The single flower is usually terminal. The raceme consists of a monopodial (that is, continuous) main stem bearing lateral flowers. The inflorescence of Arabidopsis is a raceme. The main stem of the cyme terminates in a flower, and growth is sympodial, that is, it continues from lateral shoots that also end in flowers, resulting in a flowering axis built of separate shoots. This gives the cyme a zigzag shape, though this is frequently evened out during development so that it comes to resemble the straight stem of the raceme. The panicle is a branching series of axes, each terminating in a flower. The stem within the inflorescence that bears the flowers or more branches within the inflorescence is called the rachis and the stalk of each single flower is the pedicel.
The inflorescence in species of the aster family (Asteraceae) is a highly modified raceme. Rachis and pedicels are so reduced that the minute individual flowers (florets) are compressed into a capitulum, a composite head resembling a single bloom. The individual ‘flower’ of Helianthus (sunflower), a typical example of a member of the Asteraceae, is in fact an entire inflorescence (Figure 16.21). Florets, each of which consists of perianth, stamens and carpels, are arranged on a cushion-like receptacle and are surrounded by a whorl of bracts. The inflorescence may, as in Helianthus, be differentiated into a central disk consisting of bisexual florets and the outer petal-like ray, in which the florets are usually female. In accordance with the fractal principle of plant architecture (see Chapter 12), groups of individual capitula are themselves clustered into second-order ‘inflorescences of inflorescences’ in many species. Since the Asteraceae is the most numerous of all plant families, the capitulum is evidently a highly successful floral configuration for promoting sexual propagation.
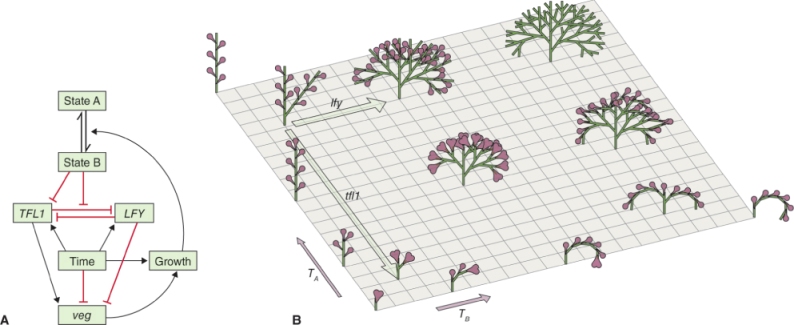
A scheme that accounts for different inflorescence architectures, called the transient model, has been used to simulate the development of racemes, cymes and panicles. It defines meristem identities in terms of ‘vegetativeness’ (veg) a quantifiable property that is influenced by factors such as plant age or the state of regulatory gene networks. Above a certain threshold value of veg, the meristem produces a vegetative phytomer consisting of an internode and lateral meristem subtended by a leaf. Below the threshold the meristem becomes floral. Below the vegetative/floral threshold, there is a second veg threshold that determines whether the floral meristem is determinate or indeterminate. If veg falls below the second threshold, the meristem is determinate, above it the meristem is indeterminate. After a period of branching, a simultaneous uniform decrease in veg in all meristems renders them determinate, resulting in the formation of a panicle. If veg reaches the threshold for floral determination in lateral meristems before apical meristems, a raceme is generated, whereas the reverse results in a cyme.
Inflorescence development has been simulated according to the transient model using two Arabidopsis architectural genes as determinants of veg. LFY, a floral identity gene that interacts directly with the class A gene AP1 (see Figure 16.6), acts to reduce veg. The effect of the gene TERMINALFLOWER 1 (TFL1) is to increase veg; as a result, tfl1 mutants produce inflorescences with short axes that terminate in flowers. A meristem with high expression of TFL1 and low LFY has a high veg score and is said to be in state A. High LFY and low TFL1 (low veg) corresponds to state B. Figure 16.22A combines these interactions into a model in which TFL1 and LFY are mutually antagonistic, are time-dependent and have opposite effects on veg. In state B, TFL1 is suppressed and growth promotes the state B to A transition. Running a computer simulation based on this model, varying time and also values for veg through the effects of introducing mutations in TFL1 and LFY, the branched raceme-type architecture of the wild-type Arabidopsis inflorescence is generated as well as mutant and overexpression phenotypes (Figure 16.22B). By altering the values of the various model parameters, cyme- and panicle-like inflorescence architectures can be created.
Grass inflorescences have distinctive structures. Each floral unit is called a spikelet, contains up to 40 individual florets and may be determinate or indeterminate depending on species. In rice, each spikelet meristem produces a single fertile floret and spikelets are arranged into panicles. Both male and female flowers of maize, the other grass species in which genetic regulation of floral architecture has been researched, have two florets per spikelet, only one of which is fertile. The functions of the Arabidopsis LFY gene are conserved in its maize homologs ZFL1 and ZFL2. There is a rice homolog, RFL, but its main roles seem to be in panicle branching and the control of tillering (branch growth). Homologs of CLAVATA (CLV), a gene that regulates patterning of vegetative meristem growth in Arabidopsis (see Chapter 12), play a distinctive part in the architecture of rice and maize inflorescence development by controlling the number of branches produced by the apical meristem of the inflorescence. Functional studies and modeling of factors determining inflorescence structure in monocots and dicots are revealing both common and phylogenetically-specific regulatory features.
16.3.6 Colors of Flower Parts are Due to Betalain, Anthocyanin or Carotenoid Pigments
Before the evolution of the angiosperms the color world of vegetation would have consisted largely of greens, yellows and browns, much as it does in modern conifer-dominated forest ecosystems. A rapid increase in the profusion of natural pigmentation accompanied the explosion in angiosperm evolution during the Cretaceous and was likely to have been an adaptive response to a combination of abiotic and biotic factors. For example, coevolution of angiosperms with insects and other animals was an important factor in the development of coloration in floral parts and in propagating structures such as seeds and fruits. Pigments in plant organs act as signaling molecules, advertising to pollinators, dispersers, or herbivores and other predators. Flower pigments are diverse in chemical structure, cellular localization and genetic regulation. Three major groups of pigments—betalains, carotenoids and anthocyanins—are responsible for the attractive colors of blooms (Figure 16.23). Carotenoids are tetraterpene products of the isoprenoid biosynthetic pathway described in Chapter 15 (see Figure 15.36). Anthocyanins are synthesized as part of the flavonoid pathway, also discussed in Chapter 15 (see Figure 15.13). Betalains are red, violet, yellow or orange water-soluble derivatives of the aromatic amino acid tyrosine, and the early stages of their biosynthesis are shared with those leading to alkaloids such as morphine (Figure 16.24; see also Figure 15.18). They are restricted to some, but not all, families within the order Caryophyllales. Curiously, betalains are absent from species that accumulate anthocyanins and vice versa, but why this should be is unclear.
Floral betalains are glycosylated at the level of both cyclo-DOPA (cyclo-dihydroxyphenylalanine) and betanidin (Figure 16.24) and are located in vacuoles of petal cells. Anthocyanin pigments are also water-soluble glycosides stored in the vacuole. The glycosylation enzyme of maize, anthocyanidin 3-O-glucosyltransferase, is encoded by the Bronze1 (Bz1) gene (see Figure 3.6). The carotenoids of colored flowers, like those of fruits, are hydrophobic and concentrated in chromoplasts, often in the form of globules, crystals or fibrils (see Chapter 18). The interaction between carotenoid-based flower coloration and the visual system of animals is an example of extreme convergence in evolution. The earliest prokaryotic photoautotrophs harvested light energy through carotenoid–protein receptor structures and metabolic systems. These show remarkable conservation of gene and protein sequence through to both the carotenoid structures and functions of angiosperm organs and the visual photoreceptors of the animals that interact with them. In contrast with the extremely ancient origins and functional organization of carotenoids, the vacuolar pigments of flowers, fruits and leaves are relatively late innovations of land plants, and their diversification was a key factor in angiosperm radiation.
Key Points
The symmetrical organization of flowers is controlled by interactions between genes in two families of transcription factor, the TCP family and MYB-type factors. TCP gene products also play a part in the developmental abortion of floral organs and in other aspects of plant development. The arrangement of flowers on the peduncle (floral axis) is called the inflorescence. Plants exhibit a very wide range of inflorescence architectures. Models of inflorescence development and structure are based on veg, a quantifiable parameter that specifies the state of the meristem and determines whether it will remain vegetative or become an indeterminate or determinate floral organ. Many floral parts are brightly colored and act as signals to potential pollinating and seed-dispersing insects, birds and other animals. Depending on species, this color is due to pigments in one or more of three major groups: betalains, anthocyanins and carotenoids.
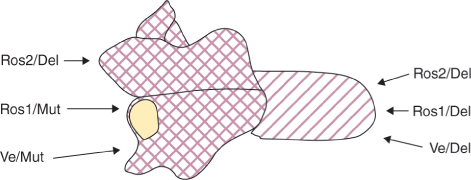
A number of genes determining the patterns and shades of flower colors have been identified in Antirrhinum. The intensity and regional distribution of the magenta-colored anthocyanin of Antirrhinum petals is under the control of DELILA (DEL), ROSEA (ROS), VENOSA (VEN) and MUTABILIS (MUT). Mutations in these regulatory genes do not abolish pigmentation but change the pattern of pigmentation within the flower. DEL determines corolla tube color. MUT affects pigmentation in the petal lobes. ROS regulates the pattern and intensity of color in both lobes and tube. VEN controls stripiness by specifying pigmentation of the epidermis overlying the veins in both lobes and tube. DEL encodes a basic helix-loop-helix (bHLH) protein and ROS and VEN encode MYBs. Each of the transcription factors activates expression of genes for anthocyanin biosynthesis with its own characteristic specificity. The pigmentation of different parts of the Antirrhinum flower is determined by interactions between pairs of genes: ROS1, ROS2 or VE with MUT or DEL, as shown in Figure 16.25. The range of anthocyanin pigmentation pattern across different Antirrhinum species can be attributed largely to variations in the activity of the ROS and VEN loci. This kind of region-specific activity in transcriptional regulators of pigment biosynthesis is one way of coloring flowers in sectors and stripes. Another common mechanism is transposon insertion and excision, as described in Chapter 3 for Petunia (see Figure 3.6).
16.4 Development of the Male and Female Gametophytes
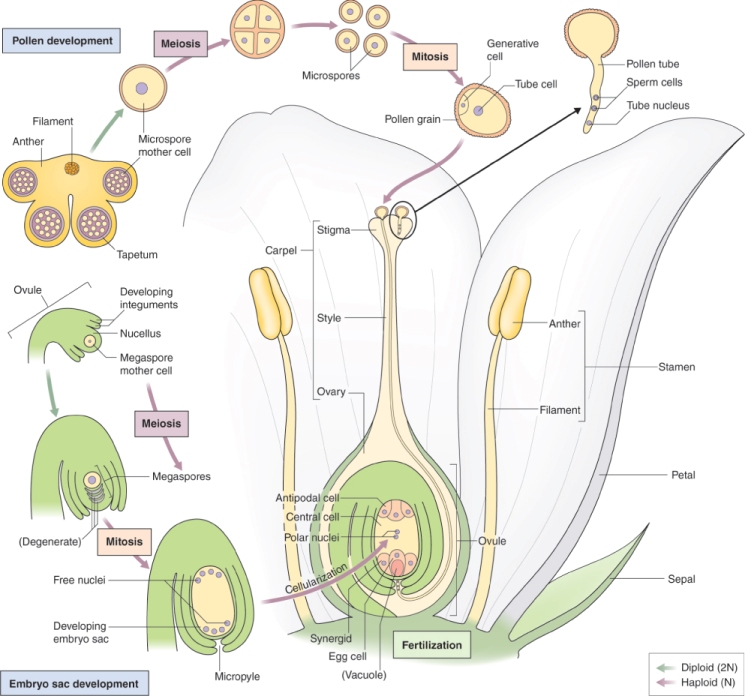
In order for the plant to complete its life cycle, it must form gametes: sperm and egg cells (Figure 16.26). In flowering plants, the male gametophyte—the structure that produces a male gamete—is the pollen grain, which is produced in the anther. The female gametophyte is the embryo sac produced within an ovule in the ovary of a carpel. When pollen is released from an anther, it is transferred to the stigma of the carpel by a process called pollination. As will be described in Section 16.5, when a pollen grain makes contact with stigmatic tissue, it extrudes a pollen tube which grows through the style until it reaches the embryo sac, where it releases its two sperm cells to carry out fertilization. At the molecular level, more is known about pollen grain formation than about the development of the embryo sac. This is, firstly, because a flower produces many more pollen grains than embryo sacs; for example a Zea mays plant can produce more than 25 million pollen grains, but only a few hundred embryo sacs, so that a typical maize plant bears one to two cobs with 200–400 kernels each. Secondly, because they are released from anthers, pollen grains are usually easy to isolate, whereas an embryo sac must be painstakingly dissected out from the ovule tissue that surrounds it in order to study the genes which are expressed exclusively in female gametes. In this and the subsequent section, we will describe the formation of male and female gametophytes of typical flowering plants.
16.4.1 The Male Gametophyte is the Pollen Grain, which Forms in the Anther
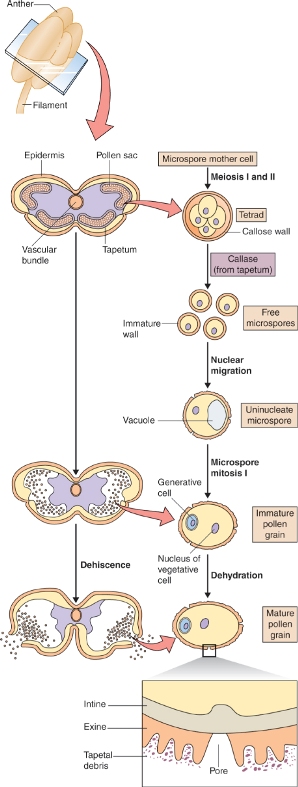
At both the cytological and the molecular level, the development of the plant's male reproductive tissue is highly conserved among all angiosperms. The sequence of events leading to the production of a male gametophyte, the pollen grain in the anther of the flower, is illustrated in Figure 16.27. The first stage in this process is the division of a diploid cell to give a tapetal initial cell and a microspore mother cell. These cells play very different roles in the production of pollen grains.
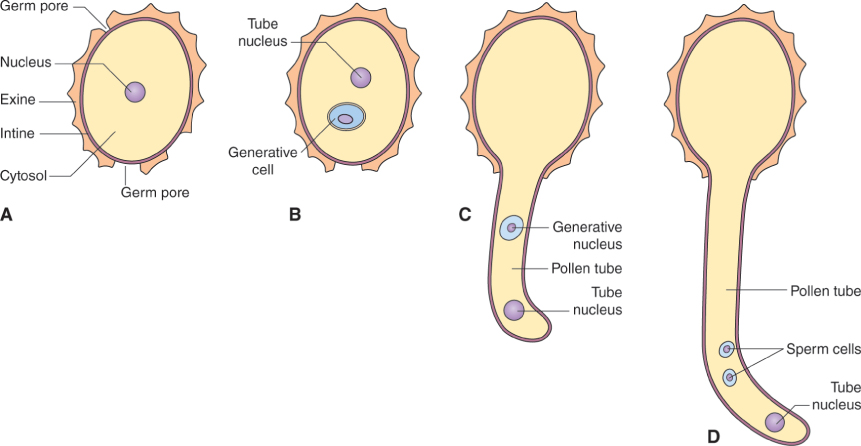
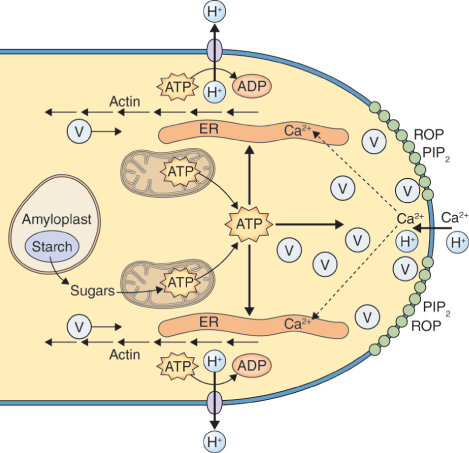
The microspore mother cell divides by meiosis (see Chapter 11) to produce four haploid cells, called microspores, which are initially surrounded by a wall that consists of the polysaccharide callose, β(1 → 3)-linked glucan. This group of four cells, known as a tetrad, is released from the wall by the enzyme callase which is secreted by the tapetum. Once released from the callose wall, each microspore undergoes an asymmetrical mitotic division to produce a pollen grain that consists of a large vegetative cell and a small generative cell. Subsequently, the generative cell will divide to yield two sperm cells. The timing of this division varies between plant species. In Arabidopsis, for example, it occurs while the pollen is still on the anther, but in the majority of angiosperms the pollen grain is shed from the anther while it is still at the two-cell (vegetative and generative) stage, and division of the generative cell takes place only after pollination when pollen tube growth begins (see Section 16.5). In either case, the final product is a pollen grain consisting of two small sperm cells surrounded by the cytoplasm of the vegetative cell (Figure 16.27). Nutrient reserves, proteins and transcripts required for pollen tube growth are accumulated in the vegetative cell during pollen maturation, so that once contact is made between pollen and the stigmatic surface, the pollen tube can grow rapidly.
The tapetal initial cells undergo mitotic divisions to form the tapetum, a tissue that lines the locule of the anther. The tapetum has two important functions. First, it produces the callase that is required to release the microspores from the wall of callose that surrounds them. Second, it manufactures many of the compounds, including structural polymers and pigments, that make up the outer wall of the pollen grain. Late in pollen development, the cells of the tapetum disintegrate and their cytoplasmic contents are deposited on the pollen coat. As we shall see in Section 16.5, the composition of the pollen coat is important for interactions between the pollen and the female tissue.
16.4.2 Many Genes are Expressed in the Anther and Nowhere else in the Plant
Certain genes are expressed only in anthers and not at any other stage in the plant's life cycle. In Arabidopsis, for example, there are over 700 of these anther-specific genes. Some of them are members of multigene families, such as those encoding the cytoskeletal proteins tubulin and actin, other members of which are expressed at different developmental stages. However, there are also genes that encode types of protein unique to pollen. Most pollen-specific genes are expressed only in the vegetative cell, not in the smaller generative cell that will give rise to the sperm. During the first, asymmetrical division of the microspore, the vegetative cell receives most of the cytoplasm and thus of the ribosomes and other components of the translational apparatus. The chromatin of the generative cell nucleus is highly condensed and seems to be largely transcriptionally inactive.
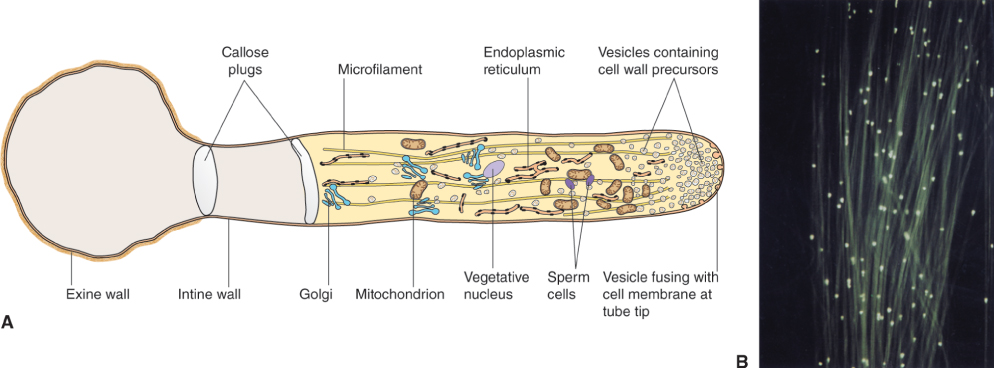
The cell wall that surrounds a mature pollen grain is a complex structure. It has an inner, or intine, wall which completely encloses the grain, and an outer, or exine, wall, which has apertures at intervals. Following pollination, a pollen grain germinates on the surface of the stigma and the pollen tube emerges at one of the apertures in the exine wall, growing by tip growth and extending the intine cell wall (Figure 16.28). The intine wall differs from other plant cell walls in that its main component is callose, although it also contains cellulose and arabinans like other cell walls.
The exine wall of the pollen grain in many species shows an elaborate pattern of spines, ridges and other decorations and is almost indestructible. Because the patterns are often unique to particular species (Figure 16.29), they can be useful in identifying the plants associated with a particular location. This attribute has been applied both in paleobotany, to determine which plant species occupied a habitat in the distant past, and in forensic science, to identify crime scenes. The extraordinary durability of pollen resides in the main structural component of the exine wall, sporopollenin, a highly decay-resistant phenolic polymer that allows the exine to retain its structure for millions of years and under harsh conditions.
The genetic control of exine patterns is interesting. If plants of two species that are very closely related but have different pollen grain morphologies are crossed, all the progeny plants have identical pollen grains, but their exine patterns can include characteristics from both parents (for example, the spine length from one parent and the spine density from the other). This suggests that different, and unlinked, genes control different elements of exine patterns, because the progeny plants inherit some from each parent. However, it also implies that pollen morphology is controlled not by the genetic makeup of the pollen grain itself (otherwise the pollen grains from a single parent plant would vary) but by the plant prior to the formation of the pollen grain. It is now known that the exine is made up of materials derived from three groups of cells: the microspore mother cells, the microspores themselves, and the tapetum. Genes that encode enzymes of the biosynthetic pathways for waxes and callose, as well as those needed for sporopollenin synthesis, have been shown to be important for normal development of the exine and for pollen fertility.
16.4.3 Mutations in Genes that are Active in the Sporophyte can Lead to Male Sterility
Several classes of mutations are known which lead to plants that are unable to act as male parents. Some male-sterile mutants have defects that affect an aspect of meiosis, and thus cannot form pollen. Others have abnormal tapetal cells, while still others appear to have normal tapetal tissues and pollen, but cannot release the pollen properly because of defects in anther structure. Although some genes underlying male sterility have been cloned, in most cases the functions of their products are poorly understood.
One class of male-sterile mutants deserves special mention because of their importance in plant breeding. They are the so-called cytoplasmic male-sterile (CMS) mutants. In such plants, which have been identified in a wide range of angiosperms including Zea mays, Oryza sativa and Helianthus and Phaseolus species, male sterility is transmitted through the female parent. In all the cases so far studied, it has been found that it is the mitochondria, which a plant inherits only from its female parent, that are responsible for the male sterility phenotype. In CMS plants, an abnormal protein is expressed in the mitochondria in all cells of the plant, but it is only during male gametophyte development that there is a phenotype, suggesting that mitochondria play a particularly important role in pollen formation. If the expression of the aberrant mitochondrially-encoded protein is reduced, the plant's fertility is restored. The nuclear genome in many plant species includes restorer genes, which can suppress expression of these toxic mitochondrial proteins.
Cytoplasmic male sterility in maize has been studied in detail. In one of the most intensively studied cases, the CMS-T line of maize has been shown to produce an abnormal mitochondrial protein called URF13 that causes complete male sterility. If, however, the CMS-T mutation is introduced into a genetic background in which the two restorer nuclear genes Rf1 and Rf2 are both expressed, male fertility is restored to normal. Rf1 expression greatly reduces the amount of URF13 produced, but Rf2 must also be expressed to confer fertility (Figure 16.30). The product of Rf2 is an aldehyde dehydrogenase, and it has been suggested that URF13 may cause buildup of toxic aldehydes in the mitochondria of developing tapetal cells. A further example of cytoplasmic male sterility with a different molecular basis, the CMS-S system, will be described in Chapter 18. Plant breeders use cytoplasmic male sterility to produce F1 hybrids in many crop plants: a CMS plant, which cannot self-fertilize, is used as the female parent and a plant with the nuclear restorer genes is used as the male. This restores fertility to the resulting F1 progeny, which contain the desirable characteristics of both parent plants.
16.4.4 The Female Gametophyte, or Embryo Sac, is Produced by One Meiotic Division followed by Several Mitotic Divisions
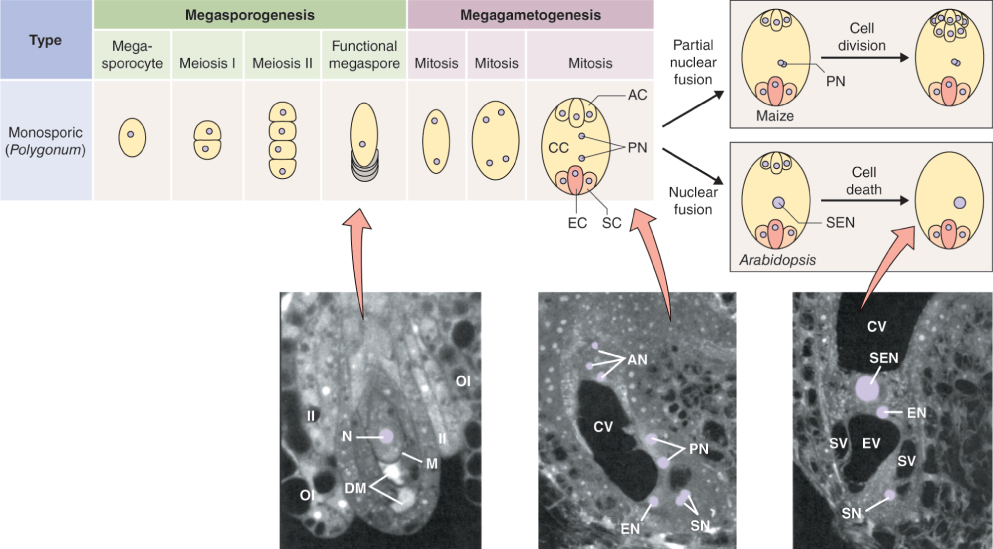
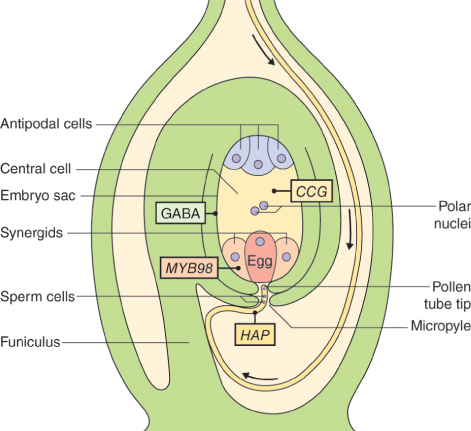
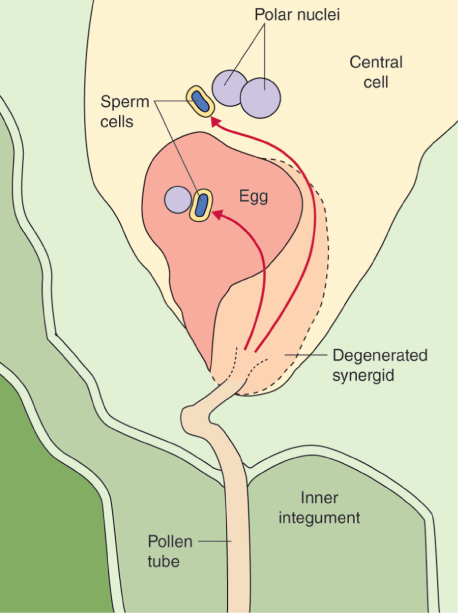
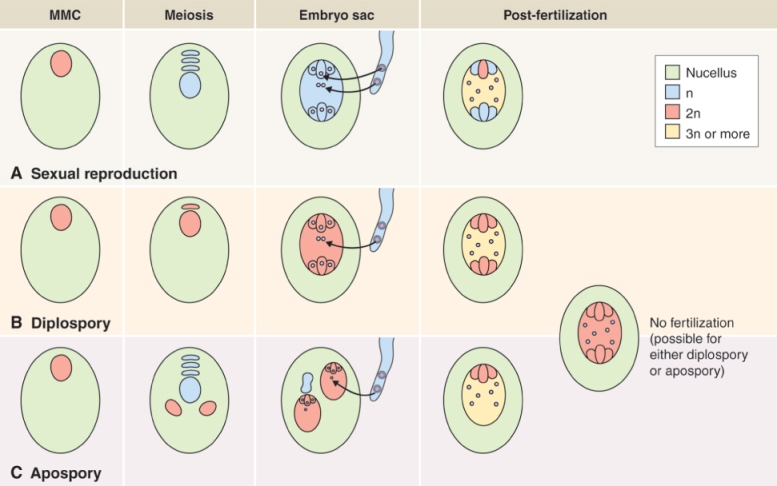
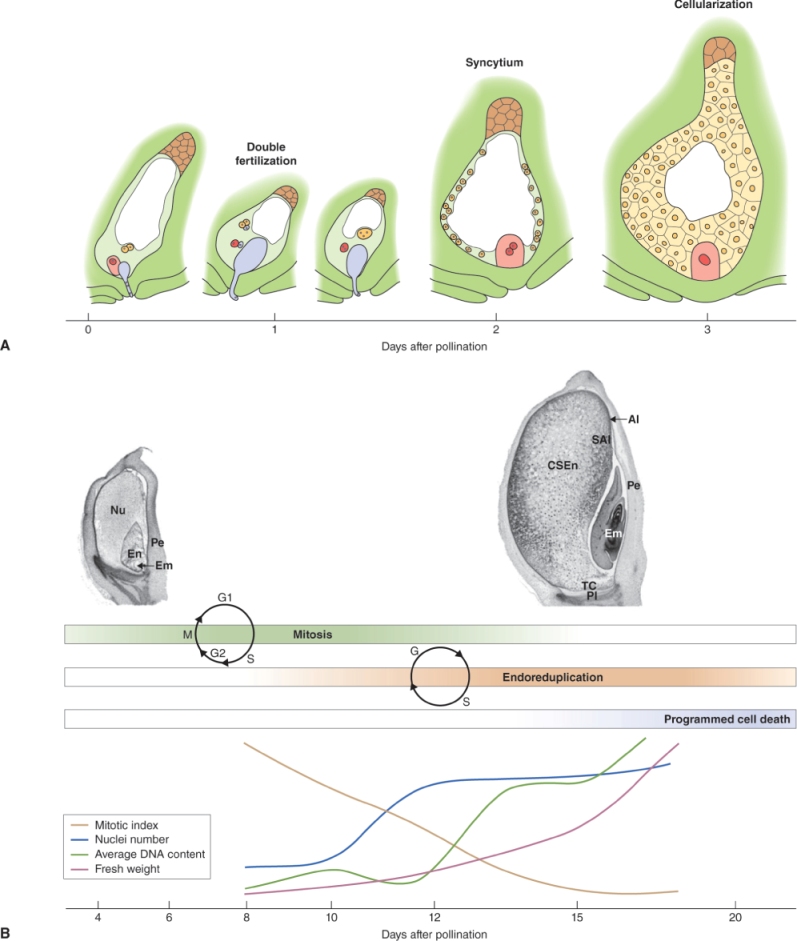
The female reproductive cells of seed plants are contained in the ovule, which consists of an outer layer (integument) enclosing the nucellus (megasporangium). Within the nucellus, meiotic division of the megaspore mother cell (MMC) gives rise to the female gametophyte (embryo sac), just as meiosis results in production of the male gametophyte in an anther (see Figure 16.26). The sequence of cell division events that give rise to the embryo sac varies between plant species, but more than 70% of flowering plants follow a pattern normally known as Polygonum-type development, named after the genus of dicots in which it was first identified. In these species, when an MMC divides by meiosis, three of the four haploid products die and the fourth, which is much larger than the other three, is the megaspore (Figure 16.31). The megaspore then undergoes three mitotic divisions, producing eight genetically identical daughter nuclei. Cytokinesis follows, producing a mature embryo sac which has seven cells: six uninucleate haploid cells (three antipodal cells, two synergids and the egg cell) and a central cell that contains two of the haploid nuclei. As will be discussed in Section 16.5.7, when the pollen tube delivers two sperm nuclei to the embryo sac, double fertilization occurs. The egg cell fuses with one sperm nucleus to form a diploid zygote which will give rise to an embryo progeny plant. The central cell fuses with the second sperm nucleus to produce the triploid primary endosperm cell which gives rise to the endosperm that surrounds the embryo in the seed and provides it with nutrients to support germination.
Key Points
A plant must form gametes (sperm and egg cells) in order to complete its life cycle. The male gametophyte—the structure that produces a male gamete—is the pollen grain, which is formed in the anther. The female gametophyte, which is formed within an ovule in the ovary of a carpel, is the embryo sac. Pollination is the process in which pollen grains are transferred to the stigma of the carpel. When a pollen grain makes contact with stigmatic tissue, unless a self-incompatibility reaction prevents it, a pollen tube extruded from the pollen grain grows through the style until it reaches the embryo sac. The two sperm cells carried by the pollen grain are then able to fertilize the embryo sac. Hundreds of genes are expressed only in anthers and not at any other stage in the plant's life cycle. Gene expression in pollen occurs mainly in the vegetative cell, not in the generative cell that will give rise to the sperm. A mature pollen grain is surrounded by a complex, two-layer cell wall. The outer layer (the exine) is almost indestructible and in many species it has a complex pattern of spines, ridges and other decorations which can be used for identification purposes. There are several classes of gene which, if mutated, lead to plants that cannot act as male parents; that is, they are male sterile. Some have aberrant meiosis and cannot form pollen, while others have abnormal tapetum or anther structure. In cytoplasmic male sterility, the mitochondria inherited by the plant from the female parent produce an aberrant protein that results in failure to form normal pollen. Cytoplasmic male sterility is important in plant breeding because it is useful for hybrid seed production.
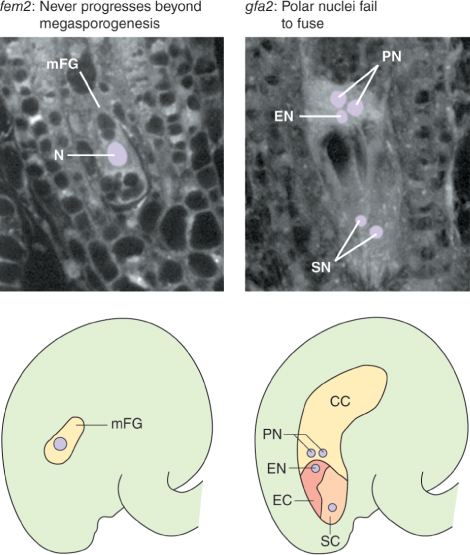
As with male gametophyte development, there are several genes that are expressed only during the steps that lead to the formation of the female gametophyte. Mutations in these genes cause aberrant development of the ovule and lead to female sterility. Some encode proteins that regulate the number of cell divisions, or the process of cell specialization in the embryo sac. Figure 16.32 shows the results of mutations in two such genes in Arabidopsis. In one mutant, development is arrested at the megaspore stage, while in the other the two polar nuclei do not fuse. Mutations in other genes can affect development of the ovule as a whole, causing ovules to be transformed into other structures and preventing normal embryo sac formation. For example, in Arabidopsis, four closely related genes of the MADS-box family of transcription factors, AG, SEEDSTICK (STK) and SHATTERPROOF 1 and 2 (SHP1 and SHP2), are required to confer the correct identity upon ovule tissues. In flowers of plants with the stk-shp1-shp2 triple mutation, ovules are converted into organs resembling carpels or leaves. These ovule identity genes are sometimes included in an extension of the ABCE model of floral organ patterning (see Section 16.3.3), under the name of class D function genes. Similar phenotypes result from mutations in genes of the SEP family of genes, which encode another group of MADS-box transcription factors (Figure 16.33).
Key Points
The sequence of events giving rise to an embryo sac varies between plant species, but in more than 70% of flowering plants, after a megaspore mother cell has undergone division by meiosis, three of the four haploid products die. The fourth, which is the largest, is the megaspore, and it undergoes three mitotic divisions to produce eight genetically identical daughter nuclei. These develop into a mature embryo sac which consists of seven cells. Six are haploid cells with single nuclei (three antipodal cells, two synergids and the egg cell) while the seventh, the central cell, contains two of the haploid nuclei.
16.5 Pollination and Fertilization
The goal of sexual reproduction is fertilization, the fusion of egg and sperm. Before fertilization can occur in flowering plants, a pollen grain must make contact with a receptive stigma. It must then germinate producing a pollen tube, which grows through the tissues of the style towards the embryo sac. Fertilization to produce a zygote, the beginning of a new diploid sporophyte generation, can then occur. Each point in the sequence of events from pollen touchdown on the stigmatic surface to the initiation of embryogenesis requires that the appropriate cell–cell signaling interaction occurs on cue. Following pollination the female partner must allow invasion of its tissues by the foreign cytoplasm of the male gametophyte; this step is mediated by highly specific molecular mechanisms for distinguishing self from non-self. Here we discuss the signals and regulatory networks that promote (and in some cases block) the union between male and female gametes which completes the cycle of alternation of generations.
16.5.1 Hydration and Germination of the Pollen Grain Require Specific Interaction between the Pollen Coat and Stigmatic Surface
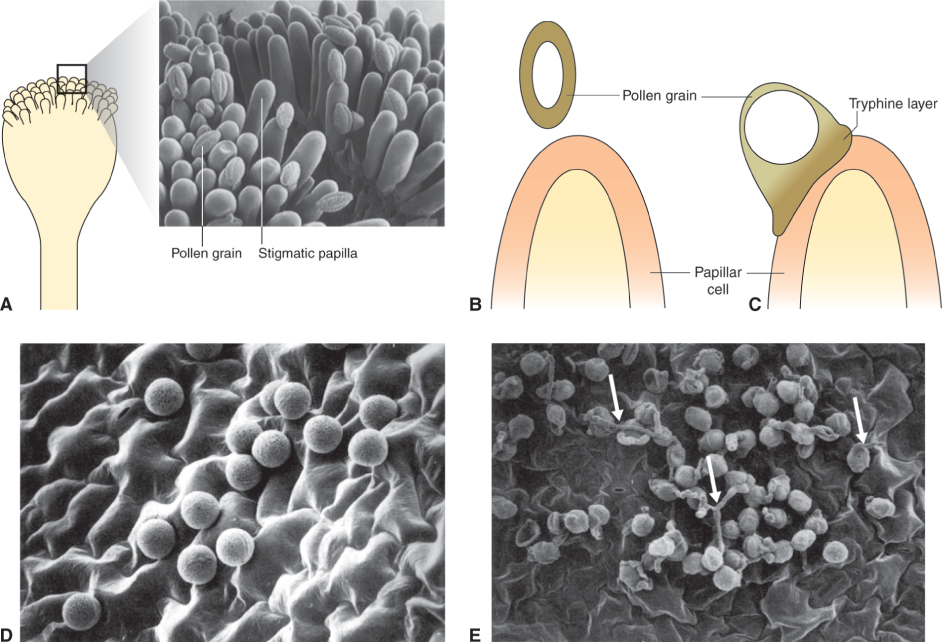
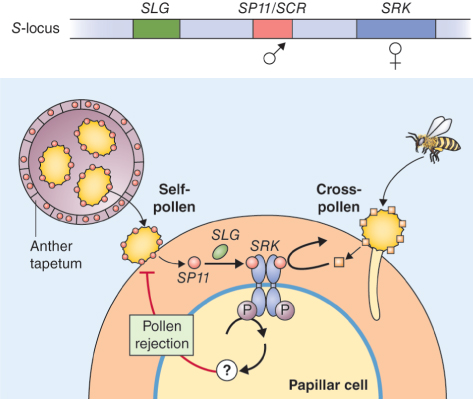
During pollen maturation the developing grain becomes dehydrated. The pollen coat protects the grain from excess desiccation while it is in transit from the anther to the stigma; it also is involved in the early stages of pollen interaction with the stigma by contributing to pollen–stigma adhesion and facilitating rehydration. The outer layer of the pollen coat, which is generated by the tapetum during gametogenesis, is a lipid–protein matrix that represents the point of interaction between pollen and stigma. When a grain makes contact with the stigma, rehydration is initiated as a prelude to growth of the pollen tube and delivery of sperm to an embryo sac within an ovule (Figure 16.34A–C). Rehydration is a highly regulated step, at which self-pollen in many self-incompatible species is rejected and interspecific pollination is blocked (see Section 16.5.3). Self-incompatible species are those in which pollen cannot fertilize eggs on the same plant in which it was produced. Depending on plant species and the self-incompatibility mechanism it uses, successful production of an embryo may be blocked at any of several stages, including pollen germination, growth of the pollen tube, fertilization and development of the embryo.
Rehydration of pollen grains is facilitated by release of water from the stigmatic surface. The mechanism of this water release has not been fully determined, but a number of pollen coat and stigmatic components have been implicated. These include aquaporin-like genes, which are relatively highly expressed in stigmatic papillae. Secondary metabolites such as flavonols can also facilitate the initiation of pollen tube growth in some plants. The interaction between pollen and stigma is abnormal in certain mutants defective in lipid metabolism. Pollen grains of the Arabidopsis mutant eceriferum (cer), for example, which are depleted in pollen coat lipids, fail to rehydrate unless exposed to high humidity or when triacylglycerides are added to the stigma. The pollen coats of Arabidopsis and related members of the family Brassicaceae also contain lipases and glycine-rich lipid-binding proteins called oleosins. Mutations in the gene that encodes the most abundant oleosin, GRP17, result in delayed pollen rehydration and reduce its ability to compete with wild-type pollen.
Further evidence that lipids are important in pollen grain germination comes from observations on fiddlehead (fdh), an Arabidopsis mutant with a modified epidermis that results in anomalous organ adhesion and fusion. Normally, pollen grains will not germinate on non-stigmatic tissues such as the epidermis of wild-type leaves (Figure 16.34D); but leaf surfaces of the fdh mutant can support pollen hydration and germination (Figure 16.34E). Plants with the fdh mutation retain the capacity for self- and non-self-recognition, however, since even the abnormal interaction with leaf epidermis occurs only with pollen from Arabidopsis or close relatives. The fdh mutant is deficient in β-ketoacyl-CoA synthase, an enzyme of long-chain fatty acid biosynthesis. As a consequence the high molecular mass lipids in fdh leaf cells differ from those in wild-type leaves, and epidermal cell permeability is altered. The range of genetic and biochemical studies of pollen rehydration in Arabidopsis, some of which we have discussed here, leads to the conclusion that plants normally restrict water flow across the cuticle in all tissues except the stigma, and that the stigmatic surface may be unique in its capacity interactively to change its water permeability on contact with pollen through alterations in long-chain fatty acid synthesis.
16.5.2 Pollen Allergens, the Cause of Hay Fever, have a Range of Functions in Fertilization
An unwelcome consequence of the complexity of pollen coat structure is that exposure to pollen can stimulate the immune systems of sensitive individuals. The result is allergic rhinitis, the debilitating condition commonly known as hay fever, which reportedly affects almost 500 million people worldwide. Pollen allergens cross-link with antibodies in the mucous membranes of susceptible humans, leading to the release of histamines, leukotrienes and other inflammatory mediators and consequently to the development of hay fever symptoms including widening of the blood vessels, redness, swelling, increased mucus secretion, itching and sneezing.
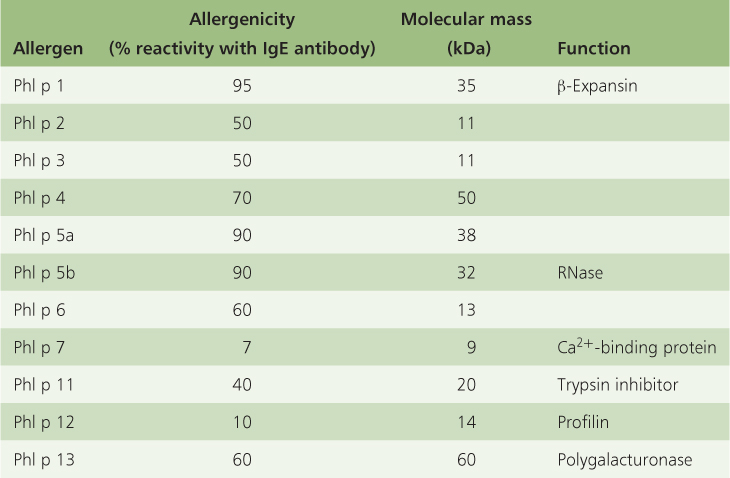
Of course, allergenic pollen proteins exist not to make people sneeze but to facilitate pollen germination and growth. The route from stigma through the style to the embryo sac (the transmitting tract) is lined with an extracellular matrix consisting of cell wall carbohydrates, pectins and glycoproteins, with which pollen allergens interact. Pollen allergens are structurally and functionally diverse. They include proteins such as pectin-degrading enzymes, disease-resistance proteins, Ca2+-binding proteins and unrelated structural proteins. Grass pollen is particularly allergenic: Table 16.4 lists the individual allergen classes in pollen from timothy grass (Phleum pratense), showing a typical range of sizes, immunological effects and protein functions. The major allergen, Phl p 1, is the P. pratense homolog of the Zea mays EXP B-1 allergen (see Figure 12.11). Allergens of this group are expressed at a low level prior to pollen mitosis and maximally in mature pollen. They function as β-expansins, relaxing the network of wall polysaccharides, thereby permitting turgor-driven cell enlargement (see Chapter 12). They are thought to facilitate invasion of the pollen tube into pistil tissues, by loosening the cell walls of the grass stigma and style.
Table 16.4 Immunological and biochemical characteristics of the Phleum pratense (timothy grass) pollen allergen groups.
Phl p 11 (Table 16.4) is a member of the OleI family, named for the major allergen of olive (Olea europaea) pollen, which is the commonest cause of hay fever in Mediterranean countries. Homologs have been described from Arabidopsis, tomato and maize. Allergens of this group accumulate from the early microspore stage onwards within the pollen wall and tapetum. The downregulation of Phl p 11 results in a gametophytic lethal phenotype in which pollen matures correctly, but fails to rehydrate properly or to grow an effective tube. Comparison of protein structural motifs suggests that members of this group of allergens are related to inhibitors of trypsin-type proteases, although the relevance of this to their function in pollination is unclear. Phl p 7 (Table 16.4) is a small, weakly allergenic Ca2+-binding protein that is highly expressed in the cytosol of mature pollen, in the wall of the grain during hydration and on the surface and tip of the pollen tube. Such proteins are thought to function as calcium-sensitive signal molecules (see Section 16.5.6).
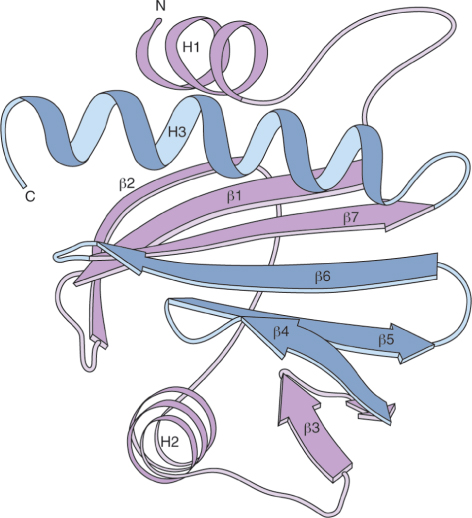
Phl p 12 is related to a family of small, highly conserved proteins called profilins. Profilin was first identified in the pollen of birch (Betula), the major cause of allergic responses in northern latitudes, where it is estimated to affect 15–20% of hay fever sufferers. The three-dimensional structure of the protein is shown in Figure 16.35. Profilins bind to the cytoskeleton component actin. Actin plays an important role in pollen tube growth, which suggests that profilin functions in pollination to support the process that delivers sperm cells to the embryo sac.
Phl p 13 (Table 16.4), a polygalacturonase, is one of the allergens that function in vivo in the enzymatic modification of pectins. In addition to polygalacturonases, pollen proteins with amino acid sequence similarity to pectate lyases have been identified as major allergens in a range of species, including tomato, common ragweed (Ambrosia artemisiifolia) and Japanese cedar (Cryptomeria japonica). Pectin is a major component of the middle lamella, which glues adjacent cells together (see Chapter 4). Pectin-modifying enzymes are employed by pathogenic microorganisms to soften plant tissues; it is likely that allergens with such activity play a role in the penetration of the style during pollen tube growth.
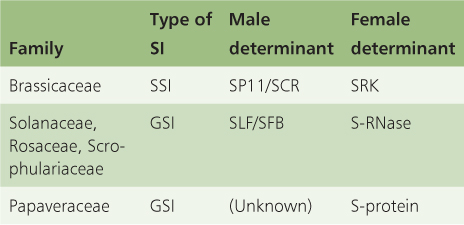
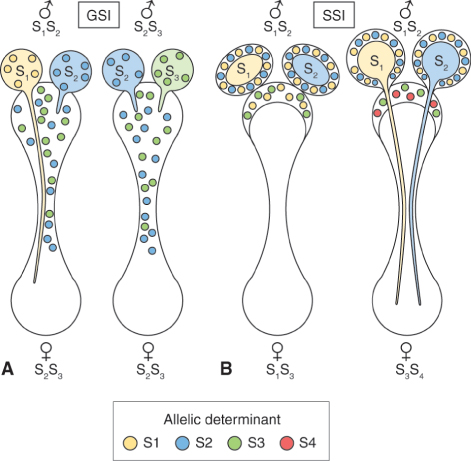
Key Points
Pollination is followed by fertilization, the union of egg and sperm. On contact with the stigma, the pollen grain is rehydrated in a sequence of regulated events that includes expression of aquaporins and changes in lipid metabolism. Pollen allergens are a diverse group of proteins with a variety of functions in fertilization. They include cell wall modifiers, disease-resistance factors and Ca2+-binding proteins. Profilin, the major allergen of birch pollen, interacts with the cytoskeleton during pollen tube growth and delivery of sperm cells to the embryo sac prior to fertilization.
16.5.3 Incompatibility Mechanisms Prevent Self-pollination and Promote Outbreeding
Over 85% of angiosperms are hermaphrodites (that is, they are bisexual, bearing flowers each of which has both male and female reproductive organs). A further 5% or so are plants that have unisexual flowers and are monoecious, like maize (see Figure 16.3), where separate male and female flowers occur on the same plant. Therefore, for the great majority of plants, it is highly likely that pollen will land on a stigma from the same individual, a process called self-pollination. Self-pollination has the disadvantage of promoting inbreeding, which may decrease the fitness of the progeny. Several mechanisms have evolved to minimize or prevent fertilization of the egg cell by self-pollen. For example, there may be asynchrony between maturation of the stamens and pistils within a given hermaphroditic flower, or between the male and female flowers in monoecious individuals. Pollen produced either too early or too late in relation to when the female tissue is receptive to pollen tube growth will fail to achieve fertilization.
Here we discuss genetic strategies for promoting outcrossing. Such self-incompatibility mechanisms ensure that pollen of an individual plant fails on the stigmas of the same plant, but pollen from other plants of the same species is successful. Self-incompatibility has arisen independently several times in evolution, and the molecular basis differs between different plant families. Self-incompatibility is usually controlled by an S locus, which consists of multiple genes (determinants) that are expressed either in the pollen grain (male) or in the pistil (female). Both the male and female determinants have many alleles and are inherited as a single segregating unit. The variants of this gene complex are called S haplotypes. Pollen is recognized as self (incompatible) or non-self (compatible) according to the proteins encoded by the different alleles of determinant genes. An incompatible response occurs if the partners carry determinants from the same S haplotype; but if the male and female carry male and female determinants from different S haplotypes, pollination and fertilization are permitted. There are two major types of self-incompatibility mechanisms, gametophytic and sporophytic, distinguished by the relationship between pollen and pistil tissue (Table 16.5). Gametophytic self-incompatibility (GSI) is determined by the haploid pollen genotype at the S locus. In contrast, sporophytic self-incompatibility (SSI) is determined by the diploid genotype of the male parent at the S locus. In a GSI interaction an incompatible pollen tube often initiates growth through the style before it arrests, while in SSI, the pollen grain fails to germinate (Figure 16.36).
Table 16.5 Types of self-incompatibility (SI) system. The SI system of Brassicaceae is of the sporophytic type (SSI). The system in Solanaceae, Rosaceae and Scrophulariaceae is gametophytic self-incompatibility (GSI). The identities and functions of male and female determinants are described in the text. The GSI system in Papaveraceae is based on a programmed cell death mechanism.
16.5.4 In Gametophytic Self-incompatibility Growth of the Pollen Tube is Arrested by Ribonucleases or Programmed Cell Death
Gametophytic self-incompatibility systems are the commonest, having been identified in more than 60 plant families. There are at least two distinct GSI systems. Incompatibility in members of the Solanaceae, Rosaceae and Scrophulariaceae is based on destruction of the RNA in the tube of self-pollen by cytotoxic ribonucleases (RNases). In species of the poppy family (Papaveraceae), the incompatible reaction is a programmed cell death process that is described in further detail in Chapter 18.
Studies of GSI in the Solanaceae established a correlation between specific, abundant glycoproteins of molecular mass about 30 kDa in stylar extracts and the presence of particular S alleles. Subsequent analysis showed that these female determinant proteins are RNases. S-RNase gene expression occurs exclusively in the pistil. The protein is localized mostly in the upper segment of the style, the site of inhibition of self-pollen tube growth. Mutations that eliminate stylar S-RNase activity result in a self-compatible plant. The S haplotype specificity determinant of S-RNases has been found to reside in their protein backbone and not in their glycan side-chains. In genetic engineering experiments in which the N-glycosylation site of Petunia S3-RNase was knocked out, plants retained the capacity to reject S3 pollen. The genes encoding S-RNases can be highly polymorphic; S-RNases encoded by different alleles share amino acid identities ranging from 38% to 98%. Figure 16.37 is a model of a typical S-RNase of the Rosaceae, the S3-RNase of Pyrus pyrifolia. A loop in the N-terminal half of the molecule, referred to as a hypervariable region, is the location of most of the allelic diversity in amino acid sequence between different female determinants in the S locus.
The nature and mode of action of male determinants in the GSI system of the Solanaceae, Rosaceae and Scrophulariaceae are not well understood. Genes encoding novel F-box proteins, components of one type of Cullin/RING E3 ubiquitin ligases (see Figure 5.36) are associated with the S locus in species from these families. Such proteins are referred to as SLF (S-locus F-box) or SLB (S-haplotype-specific F-box). The molecular mechanisms by which SLF/SLB and S-RNases interact and specifically inhibit self-pollen growth are not yet known. One simple model proposes that S-RNases pass non-specifically from the style to the pollen tube. Interaction between SLF/SLB and incompatible RNases protects the latter from degradation and they are therefore able to break down RNA in the pollen tube, halting its growth and preventing fertilization. RNases from a compatible source, however, are unprotected, or associate with an unidentified inhibitor, resulting in the survival of RNA in the growing tube. Other, more complex, schemes have also been suggested. Additional components of the system remain to be characterized and further research will be required before the full picture becomes clear.
16.5.5 Sporophytic self-incompatibility in the Brassicaceae is Mediated by Receptor Kinases in the Female and Peptide Ligands in the Pollen Coat
In sporophytic self-incompatibility, the diploid S genotype of the plant that produced the pollen grain determines whether the pollen tube is able to grow through the style (Figure 16.36B). The interaction in SSI systems is generally highly localized at the stigma surface and incompatibility takes the form of a blockage in pollen rehydration or pollen tube emergence. A single papillar cell can discriminate between genetically different pollen grains, inhibiting a self-type grain while allowing the development of non-self pollen. The response is typically rapid, occurring within minutes of pollen–stigma contact. Unlike GSI, SSI does not involve cytotoxic RNases or cell death. In this case incompatible pollen grains that have not formed pollen tubes remain viable for a time after landing on an incompatible stigma, and can develop pollen tubes when transferred to a compatible stigma.
The most fully understood SSI mechanism occurs in species of the family Brassicaceae; it is characterized by interaction between a stigma epidermal receptor kinase and the corresponding peptide ligand in the pollen coat. The S locus in Brassicaceae consists of three genes: two female determinants, SRK and SLG, and one male determinant, SP11 (Figure 16.38). SRK (S-locus Receptor Kinase) encodes a protein that spans the plasma membrane of a stigmatic papilla cell and has an extracellular domain, a transmembrane domain that anchors the protein in the plasma membrane, and a cytoplasmic domain with serine/threonine kinase activity. The male determinant, SP11 (S-locus protein 11), which is also designated SCR (S-locus cysteine-rich protein), is a small peptide that is expressed predominantly in the anther tapetum and accumulates in the exine layer during pollen maturation. Allelic variation gives rise to over 70% amino acid divergence among SRKs and 35% among SP11s. SLG (S-locus glycoprotein), which localizes to the papillar cell wall, is not essential for self/non-self recognition but enhances the incompatibility reaction in some S haplotypes.
When a self-pollen grain makes contact with the stigma, SP11 protein penetrates the papilla cell wall and binds to the extracellular domain of SRK in an S haplotype-specific manner (Figure 16.38). This induces autophosphorylation of SRK and triggers a signaling cascade that results in the rejection of the pollen. This mechanism is supported by the analysis of mutants and transgenic plants. For example, Arabidopsis thaliana, which is normally self-fertile, can be rendered self-incompatible by transformation with an SRK-SP11 gene pair from its SSI-type relative Arabidopsis lyrata. Knowledge of the signaling cascade downstream of SRK is incomplete (Figure 16.38).
Key Points
Self-incompatibility is the means by which a plant prevents fertilization of its egg by its own pollen and thereby promotes outbreeding. Genetic control of self-incompatibility generally resides in multiple genes, called determinants, that constitute the S locus. Gametophytic self-incompatibility (GSI) is specified by the haploid pollen genotype and involves either destruction of pollen tube RNA by cytotoxic ribonucleases or, in poppy species, a type of programmed cell death. Sporophytic self-incompatibility (SSI) is determined by the male parent's diploid genotype at the S locus. An incompatible reaction between pollen and stigma in SSI results in an interaction between a pollen coat peptide ligand specified by the male S locus determinant and a kinase encoded by the female S locus determinant. This triggers a signaling cascade that results in blockage of pollen rehydration or tube growth.
16.5.6 The Growing Pollen Tube is Actively Guided toward the Embryo Sac
Following touchdown, rehydration and germination, the vegetative cell of the compatible male gametophyte produces a pollen tube that grows through the transmitting tract of the pistil toward an ovule. It enters the ovule at the micropyle and continues on to the embryo sac. Elongation of the pollen tube by tip growth can achieve rates as fast as 3 μm s−1. Cytoplasm is concentrated near the tip as it grows (Figure 16.39A), and the part of the tube closest to the grain is blocked off by deposition of callose plugs (Figure 16.39A, B). Vesicles containing cell wall material are transported via the cytoskeleton to the tip of the pollen tube where they deliver their contents to the cell wall by exocytosis.
An important question is what guides the pollen tube on its journey to the waiting embryo sac. The route and rate of pollen tube growth are thought to be determined by signals from the embryo sac and the style, which also act as an energy source for the pollen tube. The chemical influences exuded by the style have been studied in Easter lily (Lilium longiflorum), which is experimentally convenient because, unusually, the style is hollow in this species, allowing access to pollen tubes as they grow. Gradients in Ca2+ concentration, which are mediated by Ca2+-binding allergen proteins (see Section 16.5.2), appear to play an important role in determining the path of pollen tube growth. The direction of growth can be changed if the Ca2+ gradient experienced by the tip is manipulated, for example by the application of chelators.
Fluxes of protons (H+ ions) have been implicated in growth. There is a pH gradient in the tip region of pollen tubes; the most apical region has a pH of 6.8, while the region at the base of the vesicular zone (Figure 16.39A) is alkaline (pH 7.5). Proton fluxes in the tip, probably driven by plasma membrane H+-ATPase activity, control a current loop in the apical dome, which in turn directly influences tube growth. Evidence of the link between protons and growth is provided by high-resolution studies that show regular oscillations in tube growth rate (between 100 and 500 nm s−1 in Lilium, with a periodicity of about 20–50 seconds) and an anticipatory oscillation in pH with the same cycle time. Similar rhythms in ATP supply have also been observed.
In addition to the roles of Ca2+ and H+, regulation of pollen tube growth is exerted through signaling pathways involving phosphoinositides and small G (guanosine triphosphate-binding) proteins. Phosphoinositides such as phosphatidyl inositol-4,5-bisphosphate (PIP2) are membrane lipids. PIP2 associates with profilin (see Figure 16.35) and other actin-binding proteins. G proteins include ROPs (Rho family GTPases of plants) which represent a subfamily of molecular switches, unique to plants, with functions in developmental and signaling events. ROP and PIP2 are localized to the plasma membrane of the pollen tube apex and contribute to cell polarity. Figure 16.40 is a model of the tip of the growing pollen tube showing ROP and PIP2 and the participation of Ca2+, H+ and OH− fluxes, respiratory ATP formation from starch stored in pollen amyloplasts, and interactions between vesicles, endoplasmic reticulum (ER) and actin.
As the pollen tube reaches the end of the transmitting tract, guidance is taken over by signals from the embryo sac (Figure 16.41). Pollen tubes are not attracted to embryo sacs that are developmentally aberrant, or have already been fertilized, or have been heat-killed. There is evidence that GABA (γ-aminobutyric acid) secreted by the embryo sac can act as a chemical attractant in pollen tube guidance. Analysis of Arabidopsis mutants has identified a number of gametophytic genes whose expression is required for the correct routing of the pollen tube to the embryo sac. For example, synergid cells, located on either side of the egg, are believed to have a critical role in attracting the pollen tube to and through the micropyle. A transcription factor, MYB98, is specifically expressed in synergids. In myb98 mutants, synergid ultrastructure is disrupted and ovules are unable to attract the pollen tube to the micropyle. The central cell may also play a role in pollen tube guidance; the gene CENTRAL CELL GUIDANCE (CCG) is thought to encode a transcriptional regulator, which must be functional in the central cell in order to ensure the pollen tube is properly guided through the micropyle. Pollen-borne genes are also important in the process. Pollen carrying mutations in genes of the HAPLESS (HAP) family produces tubes that meander aimlessly over the ovule surface, a behavior that suggests that a mechanism required for the recognition of guidance cues from the embryo sac has been disrupted. Figure 16.41 summarizes the components of the gametophytic guidance system.
16.5.7 Double Fertilization Completes the Alternation of Generations
When the pollen tube reaches the embryo sac, its tip bursts and releases its two sperm into one of the two synergid cells, which degenerates at the time of sperm release or shortly before it (Figure 16.42). In the Arabidopsis mutants feronia (fer) and sirene (sir), the pollen tube continues to grow, even though the synergid has degenerated, indicating that a signal from the embryo sac that normally halts pollen tube growth is missing in these genotypes. Generally, each ovule attracts only one pollen tube. Fertilization by extra pollen tubes is called polyspermy or heterofertilization. In Arabidopsis the normal rate of heterofertilization is less than 1%, but multiple pollen tubes are able to gain access to ovules in fer and sir mutants. This suggests that pollen tube reception is part of the mechanism that repulses additional pollen tubes and prevents polyspermy. FERONIA has been cloned and shown to encode a plasma membrane-localized, receptor-like serine-threonine kinase. A proposed signaling pathway suggests an interaction between FER and an (unidentified) ligand from the pollen tube in which activated FER causes the synergid to send another signal back to the pollen tube, inducing both growth arrest and bursting.
During double fertilization, one sperm fuses with the haploid egg cell to form a diploid zygote, thus initiating a new sporophyte generation. The second sperm fertilizes the central cell; its nucleus fuses with the two central cell nuclei, thus giving rise to a triploid primary endosperm cell (Figure 16.42). In some plants, the two sperm cells differ in size or shape or in the numbers and types of organelles they carry. An extreme example is white leadwort (Plumbago zeylanica). In this species the smaller sperm, which has more plastids, always fuses with the egg cell. Preferential fertilization related to unequal quantities of heritable organelles in the sperm is referred to as cytoplasmic heterospermy. Alternatively, differentiation between sperm cells can be the result of differences in nuclear content. Such a system, termed nuclear heterospermy, has been described for Zea mays. B chromosomes are extra chromosomes that are not homologous to the karyotype (the A chromosome set) and are found across the range of eukaryotes from plants to fungi to animals, although not all species have B chromosomes and not all genotypes within a species contain them. Unlike the A chromosomes, B chromosomes in some organisms frequently exhibit non-disjunction (‘not coming apart’), in which a pair of chromosomes fails to separate correctly during meiosis I, meiosis II or mitosis. In lines of maize carrying B chromosomes, non-disjunction at the second pollen mitosis results in one sperm cell receiving two or more B chromosomes while the other receives none. Sperm cells with B chromosomes fertilize egg cells more frequently than those without (in a ratio of up to 3:1). In both nuclear and cytoplasmic heterospermy, selectivity is believed to be the result of signaling between egg and sperm, but the molecular mechanisms remain to be discovered.
Fertilization is the point at which organelle genomes are transmitted to the next generation, a phenomenon called cytoplasmic inheritance. The predominant type of cytoplasmic inheritance is uniparental maternal, in which the genotypes of plastids and mitochondria in the progeny are derived exclusively from the female parent. Uniparental paternal inheritance of plastids is found in a significant minority of flowering plants. Inheritance of mitochondria via the paternal line has been described for alfalfa (Medicago sativa), Populus and Brassica napus. The plastids of the egg cell are usually of variable size and shape, contain some starch grains and lamella-like structures, and tend to cluster around the nucleus. Plastid number per cell ranges from more than 700 in Plumbago to as few as eight in Daucus (carrot) species.
16.5.8 Apomixis, Asexual Reproduction through Seeds, Occurs in a Large Number of Taxa and is a Target Trait for Crop Breeding
In some plant species, seeds can be produced asexually as well as by sexual reproduction. The processes by which seeds are produced in the absence of fertilization of an egg cell by a sperm are called apomixis (also termed agamospermy); a plant that reproduces by apomixis is called an apomict. Apomixis pathways are classified as gametophytic or sporophytic according to the source of the cells that develop into an embryo. The apomictic habit has evolved independently several times and occurs in more than 400 taxa. Sporophytic apomixis, also called adventitious embryony, is so-named because embryos arise spontaneously from cells of ovule integuments or from nucellar cells that are adjacent to a haploid embryo sac. It is common among citrus species. The maturation and survival of adventitious embryos is dependent on the development of endosperm derived from normal double fertilization. This kind of apomixis is essentially a form of somatic embryogenesis, equivalent to the developmental pathway undertaken by cells in tissue culture that have been induced to regenerate into plants.
Three families, the Asteraceae, Rosaceae and Poaceae, which collectively constitute only 10% of flowering plant species, account for about 75% of gametophytic apomicts. Often, but not always, apomixis is associated with self-incompatibility, perenniality and dehiscent fruits (i.e. those that split open at maturity). Gametophytic apomixis originates with a diploid embryo sac, which develops by mitosis of a diploid cell with apomictic potential rather than from a haploid megaspore (Figure 16.43). Endosperm development may be either spontaneous (autonomous) or induced by fertilization with a pollen sperm nucleus (pseudogamous).
Two pathways of gametophytic apomixis, diplospory and apospory, are recognized, based on the cell type that gives rise to the diploid embryo sac. In diplospory (Figure 16.43B), the megaspore mother cell may initiate meiosis but abort it at an early stage and switch to mitotic development of the embryo sac, a condition referred to as meiotic diplospory. Dandelion (Taraxacum) is an example of such a diplosporous apomict. An alternative mechanism is mitotic diplospory, where the MMC proceeds directly to form the unreduced embryo sac by mitosis without an initial meiotic phase. The second form of gametophytic apomixis is apospory, exemplified by members of the hawkweed genus (Hieracium). In apospory the embryo sac is derived from one or more somatic ovule cells, called aposporous initials. Diploid embryo sacs can differentiate from aposporous initials at various times during ovule development and may coexist with meiotically haploid embryo sacs in the same ovule or may continue to develop while the haploid sexual embryo sac degenerates (Figure 16.43C).
Apomixis has been called an evolutionary dead end or blind alley because of its postulated association with genetic uniformity, low adaptive potential and the accumulation of deleterious mutations. On the other hand apomixis, like other forms of asexual reproduction (discussed in detail in Section 11.4.2 and in Chapter 17), is an abundant and widely distributed attribute that clearly confers adaptive and evolutionary advantages in dynamic natural populations. This makes the genetic regulation of apomixis a subject of ecological interest.
Apomixis has also attracted agronomic and biotechnological attention because introducing the trait into major crop species has the prospect of revolutionizing crop breeding. Many of the most productive and stress-resistant agricultural and horticultural crop varieties are hybrids. The superior performance of the progeny of a cross between two inbred parents is called heterosis or hybrid vigor. Conversely, the consequence of self-pollinating hybrids over several generations is inbreeding depression, that is, progressive reduction in heterozygosity and vigor. An example of the exploitation of heterosis in a major crop is maize. The discoverer of heterosis in maize was Charles Darwin, who noted that the progeny of cross-pollinated maize were 25% taller than the progeny of inbred parents. Hybrid varieties were first developed by maize breeders early in the 20th century. Since that time their use has increased, together with crop yields, until today hybrids are planted in about 95% of maize acreage in the United States, and two-thirds worldwide. Figure 16.44 shows that the heterotic effect is apparent not only in grain yield at maturity but also from the earliest phase of seedling development.
The downside to F1 hybrid varieties is that they do not breed true, and suffer from inbreeding depression if propagated by conventional methods of seed multiplication. It is therefore necessary to recreate the hybrid each season by crossing inbred parents, an expensive and time-consuming business. This is where interest in apomixis comes in, because it offers the prospect of creating hybrids that propagate asexually via seeds and in which desirable traits are therefore transmitted faithfully to the progeny. The genus Tripsacum (gamagrass), which is related to Zea, includes apomictic species. Tripsacum dactyloides diploids are sexual, but tetraploid genotypes are pseudogamous diplosporous apomicts. Diplospory in Tripsacum is inherited as a single dominant locus. T. dactyloides will cross with maize and there have been numerous attempts to transfer the apomictic locus into maize by exploiting the interfertility between the two species. So far success has been limited, at least in part because of low rates of recombination between the genomes of the two species, but backcross programs to integrate the apomixis locus into the maize genetic background continue. Some authorities believe the organization of genes for apomixis is too complex for intergeneric transfer of the trait by conventional crossing ever to be possible.
An alternative strategy is to intervene in the process of meiosis in the MMC by genetic engineering. An example of this approach is a recent study that identified an Arabidopsis gene named OSD1 (OMISSION OF SECOND DIVISION 1). The products of meiosis in osd1 mutants are dyad rather than tetrad spores. In triple mutants consisting of osd1 with a gene that eliminates recombination and another that modifies chromatid segregation, meiosis is totally replaced by mitosis. Such so-called MiMe plants produce diploid male and female gametes that are genetically identical to their parent, and ploidy doubles at each generation. As more detailed molecular understanding of meiosis and apomixis is acquired, new tools and approaches such as MiMe plants are becoming available to help the biotechnologist and plant breeder reach the goal of being able to manipulate the reproductive systems of crops at will.
16.6 Seed and Fruit Development
Pollination and double fertilization are followed by the development of major structures of the seed: the embryo, endosperm and seed coat. The seed coat originates in ovule integuments, the maternal tissues that provide protection and facilitate nutrient transfer to the developing embryo they surround. A fruit (see Figure 1.22) is defined as a structure derived from an ovary and bearing or containing seeds. It may be simple (originating as a single ovary), aggregate (from several separate ovaries of a single flower) or multiple (derived from an inflorescence). The winged fruit of Acer spp. is an example of a simple type. Raspberries (Rubus idaeus) are aggregate fruits. Hop (Humulus) is a multiple fruit. The structures of some fruits include tissues other than those of the gynoecium, in which case they are called false fruits or pseudocarps. Strawberry (Fragaria), in which the red fleshy part is derived from the floral receptacle, is a false fruit (see Figure 6.5A).
Key Points
The pollen tube elongates by tip growth, and is actively guided by signals from the style and the embryo sac, mediated by Ca2+ gradients, Ca2+-binding allergens and proton fluxes. Pollen tube development is also regulated by phosphoinositide and G protein signaling pathways. The embryo sac produces chemical attractants that guide the pollen tube toward the point of fertilization. Transcription factors expressed in synergids, central cells and the pollen tube itself are essential for delivering the sperm cells to the embryo sac. Fertilization by more than one pollen tube is prevented by signals from the embryo sac that halt further tube growth. Double fertilization results in a 2n zygote and 3n endosperm. Fertilization transmits organelle genomes to the next generation, primarily from the maternal line, though some instances of paternal transmission are known. Asexual reproduction through seeds is called apomixis. In sporophytic apomixis, embryos are derived from diploid ovule cells adjacent to the haploid embryo sac. Gametophytic apomixis is the result of embryogenesis from a diploid embryo sac. Apomixis is of interest to crop breeders as a means of counteracting inbreeding depression and promoting hybrid vigor (heterosis), but there are formidable obstacles to be overcome before it can be a practical tool.
The fates of the different cell types destined to form embryo, endosperm, seed coat and maternally-derived fruit tissues are under the control of networks of gene activity. The regulation of embryogenesis is described in detail in Section 12.3. In the period immediately after fertilization, endosperm proliferates and nourishes the early embryo. In many plants the endosperm is absorbed by the developing embryo and is absent from the mature seed, as in, for example, garden pea (Pisum sativum) and soybean (Glycine max). In other species, notably the grasses, endosperm is persistent and represents the major repository of the stored reserves that support germination and seedling growth. Here we discuss seed development in a typical non-endospermous species, the legume soybean. Subsequently, endosperm development in the cereal grain is considered. Finally we look at some general aspects of fruit tissue differentiation and its control.
16.6.1 Genomics Analysis Reveals Tissue Specificities and Changes with Time in Gene Expression Patterns during Seed Development
A seed is an anatomically complex structure, comprising a number of tissues with a variety of developmental origins. One way of making sense of this complexity is to take a genomics approach, in which expression patterns of a global sample of genes from the species under examination are determined in different seed tissues over time. We can illustrate this approach with a study of soybean.
Figure 16.45 shows the major events in soybean seed development over the period from flowering and fertilization to maturity. The fully developed soybean seed is non-endospermous. Endosperm is present over the period of cell division and embryogenesis; but as cells expand, endosperm is absorbed and has disappeared by the time of reducing water content as the seed approaches maturity. During the period of desiccation the general level of transcription and translation declines, but some genes are upregulated, particularly those for late embryogenesis-abundant (LEA) and other proteins with protective functions in water-limited tissues (see Section 15.6.1). Protein, lipid and starch reserves are laid down in the cotyledons during the middle phase of seed development (see Section 17.3). DNA of the embryo at early maturation undergoes endoreduplication, a process of genome replication that increases the number of gene copies per cell and enables a high rate of transcription to be supported (see Section 11.4.3).
More than 30 000 unique sequences from the soybean genome, arrayed on a gene chip, were probed with DNA copies (cDNAs) of the mRNAs expressed in soybean seed tissues at different times during maturation. Measuring the degree to which each gene hybridizes with its complementary cDNA quantifies the abundance of each mRNA and allows the pattern of transcription of the corresponding genes to be profiled in time and space. Figure 16.46 is an example of the output from such a study. The micrograph (Figure 16.46A) identifies the different tissues of the seed at the globular stage of the embryo. Figure 16.46B is an example of the functional classification of the genes transcribed in a particular tissue—in this case, the suspensor. Notice that more than half the expressed genes have not yet been assigned a function or identity. This degree of ignorance is typical of the current state of knowledge of genomics and emphasizes that much work remains to be done before we have anything like a complete picture of development and the genes that control it. Of the classes of known genes expressed in the suspensor, those related to general metabolism, protein storage, transcription and signal transduction are predominant. It is estimated that to make a globular-stage soybean embryo requires the expression of genes corresponding to at least 20 000 different transcript sequences, of which 74 are found to be confined to the suspensor and no other tissue. An example of such a region-specific gene is that encoding a transcription factor of the NAM family (see Sections 12.5.4 and 18.3.5).
Genomics technology changes rapidly. Global transcription profiling using DNA microarrayed on gene chips is being replaced by high-throughput DNA sequencing methodology. The ever-increasing pace of data generation from such studies requires more bioinformatics, systems biology and computer capacity to turn the flood of information into understanding.
16.6.2 The Development of Nuclear Endosperm Comprises Phases of Syncytium Formation, Cellularization, Endoreduplication and Programmed Cell Death
The primary endosperm cell is the triploid fusion product of the two nuclei of the embryo sac central cell with the haploid nucleus of the second male gamete. Endosperm tissue development from the primary cell may take one of three forms. During cellular endosperm formation, cell walls are laid down at the same time as nuclei divide. Cellular endosperm formation occurs in about 25% of angiosperm families. In nuclear endosperm formation there is repeated free nuclear division without cell wall formation, resulting in a syncytium (a tissue consisting of cytoplasm containing many nuclei but not differentiated into separate cells); cell walls may form subsequently. Nuclear endosperm is the commonest type, occurring in 56% of angiosperms. Coconut (Cocos nucifera) is an example of a plant with nuclear endosperm formation (see Figure 1.21B). As the seed matures, the syncytial endosperm begins to cellularize, forming the coconut ‘meat’; the coconut ‘water’ remains a syncytium. Helobial endosperm is an intermediate type, in which the first mitotic division is followed by cytokinesis, resulting in the formation of two unequal cells. Subsequent divisions of these cells are of the free nuclear type; cell walls may form later in development, after completion of free nuclear division. Helobial endosperm formation is found in 19% of families, comprising mostly aquatic and other non-gramineous monocots. Here we discuss the formation of nuclear endosperm in cereals where it produces the storage tissue of grains, on which world food supply depends.
Early events in the formation of maize endosperm are shown in Figure 16.47A. After fertilization the primary endosperm nucleus rapidly enters a period of intense mitotic activity. Several rounds of more or less synchronized division without the formation of the cell plate and cytokinesis (see Section 11.1.2) produce a syncytium. Mitotic activity in early endosperm development is much greater than that in the embryo. As the proliferation rate of endosperm nuclei declines towards the level of embryo cell division, the syncytial phase is succeeded by a period of cellularization (Figure 16.47A). Microtubules radiating from the nuclear surface define nuclear–cytoplasmic domains. The pattern of cellularization takes the form of centripetal growth of cell files extending to the center of the endosperm cavity.
Endosperm cell fate is specified by positional signals. The mature endosperm comprises two types of storage tissue: starchy endosperm and aleurone (see Section 6.2.3). Some endosperm cells near vascular tissue and immediately surrounding the embryo have nutrient transfer functions. The cytoplasm of these transfer cells is typically dense, with many small, spherical mitochondria. A number of genes have been described with presumed functions in transfer cell specification and development. Mutation of EMPTY PERICARP4, a maize gene encoding a protein that regulates mitochondrial gene expression, results in a defective transfer cell layer and endosperm. Genes of the BETL (Basal Endosperm Transfer Layer) and EBE (Embryo-sac Basal-endosperm-layer Embryo-surrounding-region) families are preferentially expressed in maize transfer cells under the transcriptional control of the MYB-related protein ZmMRP-1. The basal layer of transfer cells in the maize mutant globby-1 is abnormal as a consequence of errors in the timing of patterning events. There is evidence that signals from maternal sporophytic tissue exert a controlling influence on the development of basal transfer cell layers.
The maize mutant dek1 (defective kernel1) lacks aleurone but has normal transfer cells, indicating that differentiation of each cell type is regulated independently of the other. Dek1 encodes a membrane-associated protease that is thought to be part of a mechanism for transmitting positional information during aleurone cell specification. As is evident from the phenotypes of the waxy, sugary and shrunken mutants of maize, the development of starchy endosperm is sensitive to modifications in the enzymology of starch synthesis and the structure of starch granules and amyloplasts (see Sections 6.3.1 and 17.3.1). Storage proteins, mainly prolamins in cereals (see Section 6.3.4), accumulate throughout the middle to late phases of endosperm development (Figure 16.47B) and their expression is regulated predominantly at the transcription level.
A distinctive feature of nuclear endosperm development is the changing nature of the cell cycle (Figure 16.47). During formation of the syncytium cytokinesis is suppressed. Most of the cells of the mature endosperm are the products of mitosis uncoupled from cell division. These cells exhibit a high degree of endopolyploidy (up to 96x) as a result of extensive endoreduplication, involving reiterated DNA replication without chromatin condensation, chromatid segregation or cytokinesis. The roles of a number of cell cycle regulators (see Chapter 11) have been examined during endosperm development. For example, it has been shown that downregulation of mitotic cyclin-dependent kinases (CDKs) and upregulation of S-phase CDKs occurs at the point where the mitotic cycle switches to the endoreduplication cell cycle.
Further characteristic attributes of endosperm development are discussed elsewhere. They include the phenomenon of imprinting and epigenetic control (see Section 3.5.4 and Figure 3.36) and the ultimate mummification of the starchy endosperm at maturity at the culmination of a phase of programmed cell death (see Section 18.4.4).
16.6.3 Differentiation of Fruit Tissues is Associated with the Activities of MADS-box Transcription Factors
The extensive molecular resources established for Arabidopsis have been exploited to analyze aspects of fruit development in this species. The fruits of Arabidopsis and other members of the family Brassicaceae are siliques, derived from two fused carpels (valves) separated by a septum bearing the seeds (Figure 16.48A). Siliques are dry fruit that shed their seed by dehiscence when ripe. Valve and valve margin development are under the control of FRUITFUL (FUL) and SHATTERPROOF (SHP), tissue-specific MADS-box proteins, and INDEHISCENT (IND), a bHLH transcription factor. DNA profiling has identified more than 15 transcription factors associated with development of the pericarp tissues of the valve. These regulatory components seem to be well conserved across the range of species with dry dehiscent pod-type fruits, but details of how they network to direct fruit development remain to be established.
The evolutionary origins of fruits stretch back to more than 125 million years ago, in the Cretaceous period, when angiosperms began to appear with seeds enclosed in carpels. It is believed that the earliest fruits were dry and were the evolutionary predecessors of fleshy types, which date from about 65 million years ago. There is evidence of conservation of regulatory genes during this evolutionary progression. Tomato is an example of a fleshy fruit (a berry), consisting of a skin (exocarp) enclosing seeds embedded in mesocarp tissue (Figure 16.48B). Together the exocarp and mesocarp constitute the pericarp, the homolog of the tissue of the silique valve wall. It is not clear how fleshiness arises in evolution or development, but the effects of overexpressing the gene TAG1 may give a clue. TAG1 is the tomato homolog of the homeotic MADS-box gene AGAMOUS (AG; see Section 16.3.1). Overexpressing TAG1 in tomato was observed to induce increased swelling and ripening of sepals. TDR4 (TOMATO DEFICIENS-RELATED4) is a MADS-box gene with close sequence similarity to FUL and is thought to have a role in tomato pericarp development. Differentiating the structure of fleshy fruits is tightly integrated with ripening processes, and their regulatory networks overlap. One common factor is RIPENING INHIBITOR (RIN), a MADS-box protein that functions in the control of mesocarp softening. Chapter 18 further develops the story of RIN and other regulators of ripening in fleshy fruit (see Section 18.5).
Key Points
Double fertilization gives rise to the embryo and endosperm of the seed, and the seed coat develops from ovule integuments. Seeds are borne in or on a fruit, which is derived from the ovary. In non-endospermous seeds, such as those of soybean, the endosperm is absorbed by the developing embryo and the cotyledons accumulate protein, lipid and starch reserves during maturation. Endoreduplication of DNA occurs early in embryogenesis. Desiccation as the seed approaches maturity is associated with the upregulation of genes with functions in adaptation to water stress. Genomics studies suggest that differentiation of the globular-stage soybean embryo requires expression of over 20 000 different transcript sequences. In cereals and other endospermous species, the endosperm persists as the major reserve tissue of the mature seed. Endosperm differentiation in maize is an example of nuclear endosperm formation (the other, less common, types are cellular and helobial endosperm). In the period after fertilization, mitosis without cell wall formation results in a syncytium. Subsequently there is a phase of cellularization and specification of different endosperm cell types—starchy endosperm, aleurone and transfer cells. Cell identity and tissue differentiation are determined by transcription factors, are sensitive to the operation of the pathways of starch synthesis and are subject to epigenetic control. Mature starchy endosperm cells are highly endopolyploid and are in a mummified state. Species with fleshy fruits evolved relatively recently from those with dry fruits, and regulatory mechanisms have been conserved. In both cases the activities of MADS-box transcription factors are implicated in the differentiation of fruit tissues.