KEY CONCEPTS
The first family studies of schizophrenia were conducted in the early twentieth century, but only recently, myriad genomic, bioinformatic, and analytic tools have made possible an incipient understanding of the molecular genetics of schizophrenia. We review here the status of the field and its main developments.
Schizophrenia is typically a chronic and severe psychotic disorder with a median lifetime prevalence of 4.0 per 1,000 and a morbid risk of 7.2 per 1,000 (McGrath et al., 2008). As there are no diagnostic laboratory tests, the diagnosis of schizophrenia relies on clinical observations and on information gathered from informants—relatives and various health care providers. Although ascertainment and assessment methods have been quite variable, the large majority of epidemiologic studies of the clinical phenotype have pointed consistently to the importance of genetic factors in schizophrenia.
From the very beginning, much effort focused on the characterization of schizophrenia as an individual diagnostic entity with a unified pathophysiology. Emil Kraepelin grouped periodic and circular insanity, simple mania, and melancholia under the term manic-depressive psychoses, which he thought did not result in deterioration, and defined dementia praecox (later called schizophrenia) as having a tendency toward poor prognosis (Kraepelin, 1899). However, schizoaffective disorder (Kasanin, 1933), considered intermediate between schizophrenia and bipolar disorder, is a relatively common clinical phenotype characterized by a mixture of psychotic and severe mood symptoms and episodes. The existence of schizoaffective disorder makes it difficult to reconcile the diagnostic categories with a pure dichotomous model, where schizophrenia and bipolar disorder would be at each end, with no clinical entities in between. Family studies show that schizoaffective disorder is present in excess in families ascertained from probands with schizophrenia and in families ascertained from probands with bipolar disorder (Gershon et al., 1982; Gershon et al., 1988; Kendler et al., 1993; Maier et al., 1993; Valles et al., 2000; Maier et al., 2002). However, the clinical separation of schizophrenia and schizoaffective disorder is reliable only after careful clinical training (Spitzer, Endicott, & Robins, 1978; Nurnberger et al., 1994; Suarez et al., 2006; Heckers, 2009). One continuing major complication is that the specific time criterion for affective symptoms relative to the schizophrenic symptoms is arbitrary (and sometimes ambiguous or simply unspecified), varying in different modern classifications. Also, clinical records frequently are insufficiently detailed with regard to temporal overlap of psychotic and affective symptoms. The possibility of shared family history of schizophrenia and other severe non-mood-related mental disorders has been less investigated, but the existing evidence suggests a relationship with autism is plausible (Stahlberg et al., 2004; Larsson et al., 2005; Daniels et al., 2008; Mouridsen et al., 2008; see the section “Pleiotropy and Overlap with Bipolar Disorder, Autism, and Other Disorders” in this chapter).
A Landscape of Extreme Complexity
The complexity of schizophrenia genetics is at this point unsurprising, because despite a century of biological research, our knowledge of the specific molecular mechanisms underlying schizophrenia remains at a very tentative level; some main reasons include the following. First, the absence of well-defined, specific, or focal neuropathology, and specific symptoms, diminishes the number of research approaches, in contrast with the relatively well-characterized brain abnormalities in Parkinson’s and Alzheimer’s disease that have enabled their respective fields to move faster. Second, the immense number of neuronal interconnections and permutations (approximately 2 × 1010 neocortical neurons and approximately 1014 synapses) (Drachman, 2005; Sporns, Tononi, & Kotter, 2005) and extreme redundancy (Morris, Nevet, & Bergman, 2003; Bastian, Chacron, & Maler, 2004; Chechik et al., 2006) make the brain disproportionately more complicated than any other human organ; consequently, our knowledge of the physiologic basis of higher brain functions (and of schizophrenia) is still very incomplete.
Schizophrenia is conceptualized as a complex disorder. Genomewide association studies (GWAS) (see the section on them later in this chapter) show that many genes are involved in complex disorders, with each gene conferring only a small effect on the phenotype (a polygenic model being the logical extreme). Individual genetic variants do not predict risk well. It has also been proposed that complex disorders are “system disorders” that result from dysfunction of entire molecular networks (as opposed to arising from abnormal function of individual genes); this is being tested (Schadt, 2009). Epistasis (i.e., nonadditive interactions between these genes or their protein products) and interactions between genes and environmental risk factors are also assumed. However, the study of genetic interactions remains largely unexplored because of the need to correct for a large number of statistical comparisons, although approaches continue to develop (Cornelis et al., 2010). GWAS data suggest a frequency spectrum with many common and rare mutations (Purcell et al., 2009; Shi et al., 2009; Stefansson et al., 2009).
Twin studies show incomplete concordance for schizophrenia in monozygotic twins, making conceivable an epigenetic mechanism whereby changes in phenotype not explained by DNA sequence may contribute to the transmission of schizophrenia (see chapter 9, this book). Few studies have tested this hypothesis, however, because of methodologic difficulties (Roth et al., 2009).
Evidence for Environmental Factors
As reviewed elsewhere in this book, multiple environmental risk factors increase risk for schizophrenia—namely, various obstetric complications (Cannon, Jones, & Murray, 2002; Byrne et al., 2007; Mittal, Ellman, & Cannon, 2008) (see chapter 3, this book), urban birth or residence, famines and micronutrient deficiencies (Susser & Lin, 1992; St Clair et al., 2005; Brown et al., 2007) (see chapter 2, this book), migrant status, seasonal effects (possibly by way of prenatal infections, including influenza) (McGrath et al., 2008; Brown & Derkits, 2010) (see chapters 1 and 10, this book), advanced paternal age (Malaspina et al., 2001; Torrey et al., 2009) (see chapter 5, this book), and maternal stress (see chapter 4, this book).
Evidence for Genetic Factors
Three epidemiologic sources have underscored the importance of genetic factors in schizophrenia and to the apportioning of environmental and genetic risks: family studies, twin studies, and adoption studies.
Family Studies
Family studies have consistently shown that the child of a parent with schizophrenia has a risk about tenfold over the general population and that the risk for schizophrenia to a relative decays much more rapidly than the proportion of shared genes, which is inconsistent with a simple Mendelian model. It is interesting that most cases of schizophrenia in the general population are sporadic (Kendler, 1987; Yang, Visscher, & Wray, 2009). Although surprising at first glance for such a familial disorder, assuming polygenic inheritance, many more sporadic (approximately 80–90%) than familial cases are expected via simulations (Yang, Visscher, & Wray, 2009) that match well with family study data (Lichtenstein et al., 2006; Lichtenstein et al., 2009).
Twin Studies
Differences between monozygotic (identical) twins are attributed to the environment, and differences between dizygotic (fraternal) twins are attributed to both hereditary and environmental factors. Contrasting findings for each twin type enables estimations of the proportion of variance explained by genetic factors, or heritability. The concordance (diagnostic agreement) rates of schizophrenia for monozygotic twins have been found to be about 40–50% both in older and in more recent population or hospital registry-based studies (Klaning, Mortensen, & Kyvik, 1996; Cannon et al., 1998; Franzek & Beckmann, 1998; Cardno et al., 1999), and heritability estimates are around 80% (Cardno & Gottesman, 2000; Sullivan, Kendler, & Neale, 2003). Notably, the risk of schizophrenia and schizophrenia-related disorders seems similar for the offspring of both the unaffected and the affected monozygotic twins (Gottesman & Bertelsen, 1989; Kringlen & Cramer, 1989), which suggests that the unaffected twins do carry a heritable genetic risk for schizophrenia without expressing the disease, supporting either or both epigenetic factors and nonshared environments (although the data are from only about 20 reproducing monozygotic twin pairs from each study). It has recently been proposed that DNA methylation differences might be the cause of monozygotic twin discordance (Mill et al., 2008).
Adoption Studies
Adoption studies allow further dissection of genetics, from environmental contributions to a disorder. Key findings from many studies (see the review by Ingraham & Kety, 2000) include the following: (1) the risk for schizophrenia is conferred by (i.e., travels with) the biological relationship, not the adoptive relationship; (2) the risk is conferred regardless of when a schizophrenic parent experiences the onset of illness; and (3) risk is conferred independently of rearing environment (foster parents or institutional).
Darwinian Paradox
Schizophrenia is associated with decreased fertility, particularly in males (Rüdin, 1916; Kallmann, 1938; Haukka, Suvisaari, & Lonnqvist, 2003; Svensson et al., 2007). Although natural selection should decrease the population frequencies of genes that diminish fertility, the prevalence of schizophrenia, which is highly heritable, has not markedly diminished in the global population. Multiple hypotheses have been proposed for the “Darwinian paradox” of how schizophrenia seemingly circumvents the effect of natural selection (see the review by Keller & Miller, 2006). Earlier evidence for balancing selection—that relatives of schizophrenia patients might have a compensatory increase in fertility (Fananas & Bertranpetit, 1995)—did not replicate in larger samples (Haukka, Suvisaari, & Lonnqvist, 2003; Svensson et al., 2007; MacCabe et al., 2009). Potential explanations for balancing selection include (1) heterozygote advantage, in which either homozygote shows reduced fitness compared to the heterozygote (such as in sickle cell anemia [Allison, 1954], where heterozygotes have less susceptibility to malaria than do major allele homozygotes, and less burden of sickle cell anemia than do minor allele homozygotes); and (2) antagonist pleiotropy, where an allele might reduce fitness for one trait while increasing fitness for a related trait. Both of these explanations are weakened by the lack of evidence for increased fertility in relatives of schizophrenia patients (Keller & Miller, 2006).
The clinical schizophrenia phenotype might also have poor correlation with its underlying genetic susceptibility (i.e., genotype). It has been suggested that endophenotypic variables (sometimes called intermediate phenotypes) such as structural and functional neuroimaging characteristics may constitute a better index of the underlying gene effects than does the clinical phenotype (Gottesman & Gould, 2003). However, a large body of genetic epidemiology is based on the clinical phenotype, and none of the proposed endophenotypes has been proved yet to be more heritable than the aggregate clinical phenotype (Greenwood et al., 2007; see also the recent review for other limitations of the endophenotype approach by Kendler & Neale, 2010).
A high mutation rate might maintain schizophrenia susceptibility alleles in the population even against negative selection (Book, 1953), although, as noted (Malaspina et al., 2001), this idea was largely abandoned because it was thought that such mutation rates would be unsustainably high (Huxley et al., 1964). However, more recently it was shown that humans (and other hominids) have substantial new deleterious mutation rates (e.g., 1.6 such mutations per diploid genome per generation [Eyre-Walker & Keightley, 1999], renewing interest in the area [Malaspina et al., 2001]). One should keep in mind, though, that the more mildly deleterious a mutation is the longer it will persist before elimination from the gene pool, so much of the mutational burden is not from new mutations (Keller & Miller, 2006). Nevertheless, as reviewed in chapter 5, advanced paternal age could be a putative risk factor as spermatogonia replicate many more times over life than oocytes and the age of fathers is greater than expected for some autosomal dominant diseases that are due to new mutations (Friedman, 1981). In a study on the Jerusalem Perinatal Cohort, an epidemiologic sample, the relative risk of schizophrenia increased continuously with the age of the fathers (adjusting for maternal age) to a maximum of 2.96 in offspring of fathers age 50 and 54 years, but there were no maternal age effects after adjusting for paternal age (Malaspina et al., 2001) (see chapter 5, this book). This finding has been replicated in larger samples from different populations, especially for older fathers (see the review by Torrey et al., 2009), and the effect was found to be stronger in sporadic (family history negative) cases (Sipos et al., 2004), as would be predicted for de novo mutations. As reviewed (Keller & Miller, 2006), paternal age effects are expected under a mutation-selection model (Crow, 2000). This is due to mutations accumulating much more rapidly in male than in female gametes because of the much greater number of cell divisions, leaving most other schizophrenia cases resulting from milder, older, and more numerous mutations (Keller & Miller, 2006). Polygenic mutation-selection balance (with many new rare alleles at many loci, which in the aggregate form a large pool) can preserve genetic variation in spite of stabilizing selection, and it is consistent with the prevalence and reproductive fitness costs of schizophrenia (Keller & Miller, 2006), as well as with recent GWAS findings (see the section “Genomewide Association Studies” in this chapter).
Linkage
During meiotic recombination, or crossing over between homologous chromosomes, adjacent loci are unlikely to be separated and are thus likely to be inherited together. The hypothesis that one or a few common major gene effects can explain schizophrenia in the general population was tested in genomewide linkage scans, but results mostly fell short of genomewide significance. However, similar (or overlapping) regions on chromosome 8p have been implicated (suggestive, i.e., expected to occur about once per genome scan just by chance) in previous linkage studies (e.g., Blouin et al., 1998; Stefansson et al., 2002; Suarez et al., 2006), in meta-analyses (Badner & Gershon, 2002; Lewis et al., 2003), and in a combined genomewide linkage scan, which consists of multiple clinical samples jointly genotyped rather than only a joint statistical analysis (Holmans et al., 2009). For example, the maximum evidence for linkage (multipoint Zlr of 3.25) in a sample of schizophrenia-affected sibling pairs from 409 pedigrees was in this chromosome 8p region (1-LOD 18.4–32.1 Mb) (Suarez et al., 2006). Note that there is overlap between some of the above samples—for example, with regard to earlier versus later meta-analyses and individual linkage studies included in the meta-analyses. The clearest and most inclusive study, in our view, is the most recent genomewide linkage study meta-analysis of 22 European ancestry (EA) samples (1,813 pedigrees, 4,094 genotyped cases, including samples from most of the other studies). In this study, the strongest linkage region, which was genomewide-significant, resided on chromosome 8p (15.7–32.7 Mb) (Ng et al., 2009), a region of continuing interest in schizophrenia genetics, given that it contains NRG1 (Stefansson et al., 2002) and PPP3CC (Gerber et al., 2003).
First Modern Association Studies
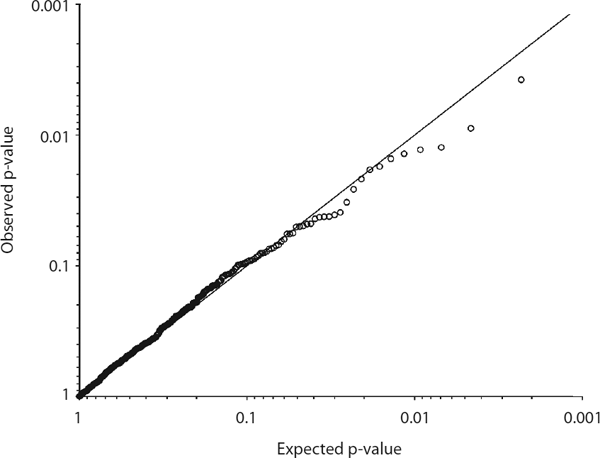
Association studies are based on linkage disequilibrium (LD), a nonrandom statistical association of alleles at two or more loci, characteristically correlated with short physical distance between genetic markers. The effect size can be conceptualized as the strength of the association between a marker and the disorder, and it can be expressed as the odds ratio (OR), which is the odds for an event (in this instance, the odds for possession of a risk allele) in cases, divided by the odds in controls. Before the availability of GWAS, most gene association studies consisted of tests of candidate gene involvement. Close to 800 genes have been tested for association (see www.schizophreniaforum.org/res/sczgene; Allen et al., 2008), making schizophrenia one of the most studied disorders through a candidate gene approach. Unfortunately, none of these candidate genes can be considered established. Given that samples in previous candidate gene association studies frequently lacked sufficient statistical power, the problem of nonreplication has been far from trivial. In a comprehensive study of some of the most cited candidate genes (e.g., DISC1, DTNBP1, NRG1, DRD, HTR2A, and COMT), 14 genes were each tested by genotyping an EA sample of 1,870 cases and 2,002 screened controls (Sanders et al., 2008). A total of 789 single nucleotide polymorphisms (SNPs), including tags for common variation in each gene (tag SNPs are SNPs that are correlated with many other nearby SNPs, for which they are proxies), SNPs previously reported as associated, and SNPs located in functional domains of genes were genotyped, but no association was found (figure 7.1). These findings contradict the relatively large ORs that would be predicted from association observed in small samples.

There are an abundance of positive and negative associations between candidate genes and schizophrenia. It is likely that the use of small sample sizes and inadequate or loose statistical thresholds accounts for many of the unreplicated observations. Other potential causes of false positive findings are multiple analyses and selective reporting (Ioannidis, 2008). It is possible that genetic heterogeneity in some specific cases would preclude a replication, but it would seem unlikely that this would be a robust general argument. (For a detailed discussion of heterogeneity, see McCarthy et al., 2008). Multiplicative epistasis (where the individual gene effects might not be detectable but the product of the individual effects might become detectable) is another largely unexplored possibility that could in principle explain nonreplication. Another source of heterogeneity is environmental variation. Furthermore, very provocative work (Richter, Garner, & Wurbel, 2009) suggests that increased standardization—such as in experiments designed to decrease heterogeneity—that allows and frequently requires smaller sample sizes can actually decrease reproducibility in animal behavioral experiments. This finding challenges long held ideas and might be important for the design of association studies. In the aggregate, recent schizophrenia GWAS results (in which each candidate gene is covered with more SNPs than in most candidate gene experiments) have not supported most associations to classical candidate genes (table 7.1; see supplementary data file 3 from Hindorff et al., 2009; Shi et al., 2009; this is a pattern consistent with the general results of GWAS in complex disorders, www.genome.gov/gwastudies). Most associations discovered from GWAS for complex disorders have been either in genes that were not previously suspected to be involved in the disease, or in regions of the genome with no obvious genes. Additional research and the analysis of cumulative data, with particular attention to both quality control and statistical rigor, will be required for definitive conclusions.
Table 7.1 Top Genes or Genomic Regions Identified in Recent Schizophrenia GWAS

a Combined analysis of ISC, SGENE-plus (GWAS set), and MGS.
b Combined analysis of ISC and MGS, along with SGENE-plus and follow-up samples.
c or (odds ratio) is for common allele of the associated SNP, which is different from that in ISC and MGS.
SOURCE: Reprinted from Gejman, Sanders, & Duan (2010) with permission from Psychiatric Clinics of North America (copyright Elsevier).
Genomewide Association Studies
Genomewide studies, in combination with other fields in systems biology, yield comprehensive information and have been demonstrated to be more useful in dealing with complex phenotypes. Systems biology is a biology-based interdisciplinary approach focusing on complex interactions in biological systems—i.e., the “-omics” strategies. The main assumption under GWAS is the common disease/ common variant hypothesis (Pritchard, 2001; Reich & Lander, 2001). Thus, GWAS interrogate common SNPs across the human genome, investigating all genes and the majority of the nongenic regions, whether they were previously implicated by pathophysiologic hypotheses or not. The Human Genome Project (see www.ornl.gov/sci/techresources/Human_Genome/home.shtml), to a large extent, made GWAS possible. Major improvements in SNP genotyping and DNA sequencing were spinoffs from the Human Genome Project, and microarrays made possible rapid and accurate genomewide genotyping that resulted in a map of common genetic variation in a reference set of individuals of European, Asian, and African descent (the HapMap project). The majority of the markers used for GWAS are tag SNPs; thus the most significant associated SNP in a GWAS may reflect an indirect association (i.e., be in LD with a causative variant). The Affymetrix 6.0 and Illumina 1M SNP arrays include approximately 1 million common SNPs and probes for analysis of copy number variants (CNVs), with their SNPs assaying about 80% of the common variation in the genome for EA samples (Li, Li, & Guan, 2008). However, the estimated number of common (minor allele frequency, MAF > 1%) SNPs is approximately 10 million, but our genotyping capabilities are not sufficiently developed yet to genotype every SNP in a very large clinical sample, although deep resequencing technology and new arrays may soon overcome this difficulty. In the meantime, imputation—the computational prediction of genotypes from nongenotyped SNPs—is used to extend GWAS map coverage (Halperin & Stephan, 2009; Nothnagel et al., 2009).
The large number of SNPs makes GWAS highly susceptible to false positives; therefore, the estimation of an appropriate genomewide significance threshold is fundamental. The genomewide significance threshold, for a value of 5% significance assuming tests for all common SNPs, has been estimated to be around p < 5 × 10−8 (Dudbridge & Gusnanto, 2008; Hoggart et al., 2008; Pe’er et al., 2008). GWAS have been more successful than any previous approach to find new susceptibility loci for complex disorders. According to www.genome.gov/gwastudies (as of September 20, 2009), 732 genes were reported to be associated to one or more complex disease phenotypes at genomewide significant levels (p < 5 × 10−8) (Hindorff et al., 2009; Manolio et al., 2009), and many of these associations have already been replicated. (Small individual ORs do not permit prediction of “caseness” from specific individual susceptibility loci.)
Recent GWAS of complex disorders show two main characteristics. First, common loci with small effects are typically reported (ORs = 1.1–1.5); these are an empirical confirmation that a large body of epidemiologic studies predicting multiple small common genetic effects for complex disorders was correct, including for schizophrenia (Gottesman & Shields, 1967; Risch, 1990), because loci with larger effects are eliminated rapidly from the population through selection. Second, most studies have tended to detect new susceptibility loci, and only very large samples obtained from combining studies are powered to show robust replication. This is because the power to detect one out of many possible risk loci is much larger than the power to detect specific disorder alleles (Kraft & Hunter, 2009). Furthermore, if only small effects are found, many genes would be predicted to underlie the pathophysiology of most complex genetic disorders. On the other hand, it is important to also emphasize some main GWAS limitations. The statistical power of GWAS to detect an association with rare alleles (MAF < 1%) is very limited. Resequencing is more useful than GWAS in the study of rare variants. The study of gene-gene interactions (epistasis), although widely expected to be a significant source of heritability, is strictly limited by the statistical power of currently existing samples due to the large number of such tests.
GWAS have already yielded genomewide significant results for schizophrenia, which we will now discuss in more detail. Seven GWAS for schizophrenia have been published (table 7.1). The sizes of the investigated discovery samples have ranged from 322 to more than 16,161, but even the largest studies did not yield a genomewide significant result before combined testing of independent samples. This was not unexpected. A typical susceptibility locus has an OR of 1.1–1.3, which often necessitates extremely large samples for detection. A sample with a total N of 5,334, such as the Molecular Genetics of Schizophrenia (MGS) EA sample (most investigated samples have been smaller), has adequate statistical power only to detect very common risk alleles (30–60% frequency, log additive effects) with genotypic relative risks of about 1.3 (Shi et al., 2009). To reach sufficient statistical power, the combined analysis of independent data sets is useful. Although the diagnostic spectrum of the final combined sample is naturally wider than for the component data sets, combining data sets has been remarkably successful for a variety of complex disorders including schizophrenia (Purcell et al., 2009; Shi et al., 2009; Stefansson et al., 2009) (see the section immediately following in this chapter). Different samples often were typed with different platforms, but imputation largely overcomes the limitation of many nonoverlapping SNPs. These results suggest that schizophrenia, despite its very high reported heritability, is among the most complex of human genetic disorders. An additional analysis of the International Schizophrenia Consortium (ISC) and MGS samples (Purcell et al., 2009) supported a polygenic model for schizophrenia susceptibility, involving a set of hundreds of genes, each with unquantified but very small individual effects (Gottesman & Shields, 1967). (See the section “Polygenic Contributions to Schizophrenia” in this chapter.) Finally, rapidly mounting evidence shows that cases have more rare (< 1%) and large (> 100 kb) CNVs than do controls (chapter 8, this book).
Meta-analysis of Genomewide Association Studies Data and the Major Histocompatibility Complex Locus
The initial attempts to map schizophrenia to the major histocompatibility complex (MHC) started in the 1970s (Worden et al., 1976), only a few years after the discovery of the human leukocyte antigen (HLA) system (Bach, Bach, & Poo, 1969). Many attempts have been made since then, and some yielded suggestive evidence (Wei & Hemmings, 2000), but compelling evidence of MHC involvement was only recently obtained from a combined analysis of GWAS data. Three GWAS studies published jointly in 2009 (ISC, MGS, and the Schizophrenia Genetics Consortium, or SGENE), reaching a total EA sample of 8,008 cases and 19,077 controls, performed a meta-analysis of schizophrenia GWAS for the first time (Purcell et al., 2009; Shi et al., 2009; Stefansson et al., 2009). The meta-analysis combined the p-values for all imputed and genotyped SNPs from the most significant regions of each study. This analysis generated a genomewide significant association at the MHC region on chromosome 6.
The MHC signal extends over much of the MHC region, from approximately 26 Mb to approximately 33 Mb (figure 7.2). The strongest evidence (rs13194053, p = 9.54 × 10−9) for association from the meta-analysis was near a cluster of histone genes and several immune-related genes, including butyrophilin subfamily 3 member A2 and A1 (BTN3A2 and BTN3A1) and protease serine 16 (PRSS16), but each individual data set has a different location for its strongest association. The MHC region has a very high gene density and long-range LD blocks (Traherne, 2008)—the human genome is structured in many “blocklike” islands of LD generated by a great variation of recombination rates. Blocks from regions of low recombination are long and interspersed with interblock regions of higher recombination (Daly et al., 2001; Gabriel et al., 2002). The location of the causative variation remains indeterminate, but it could be in one or more genes or a nongenic region within the MHC. In the MGS sample, about 50% of the 1,000 highest-ranking GWAS SNPs were intergenic, located outside the 10 kb region on either side of a gene, although many of these may represent a genic region signal because of LD. Even at the MHC locus, rs13194053—the SNP with the most significant association from the meta-analysis—is roughly 29 kb away from its closest gene, HIST1H2AH (histone cluster 1, H2ah) (Purcell et al., 2009; Shi et al., 2009; Stefansson et al., 2009). A functional role for many intergenic regions would not be surprising, as they might contain highly conserved sequences, which are believed to have a regulatory function (Kleinjan & van Heyningen, 2005). The associated variants, or variants in LD with them, in intergenic regions may then alter expression of upstream or downstream genes. Moreover, most of the human genome is transcribed, with some transcripts serving as regulatory RNAs, but the function of most human transcripts is still undefined (Birney et al., 2007).

The genes in the MHC region have many different biological functions, but genes with an immune function predominate. Histones regulate DNA transcription by chromatin modification through histone methylation or acetylation (Costa et al., 2007; Shi, 2007; Adegbola et al., 2008) (see chapter 9, this book), and have a role as antimicrobial agents by disrupting the bacterial cell membrane and interfering with microbial gene expression (Kawasaki & Iwamuro, 2008). In human placenta, histones (H2A and H2B) neutralize bacterial endotoxins (Kim et al., 2002). This raises the possibility that genetic variation in histones underlies variation in placental susceptibility to infections, and thus genetic variation may increase the susceptibility to schizophrenia. These findings are also consistent with previous studies indicating associations between in utero exposure to infection and risk of schizophrenia (see chapter 1, this book, as well as Brown & Derkits, 2010).
A Danish registry study reported an increased risk of autoimmune disorders (thyrotoxicosis, intestinal malabsorption, acquired hemolytic anemia, chronic active hepatitis, interstitial cystitis, alopecia areata, myositis, polymyalgia rheumatica, and Sjögren’s syndrome) for schizophrenia, and a history of any autoimmune disorder (of 29 evaluated) was associated with a 45% increase in risk for schizophrenia (Eaton et al., 2006). The MHC region has been implicated in many genetic disorders with immune-related abnormalities (Shiina, Inoko, & Kulski, 2004), including type 1 diabetes (T1D), multiple sclerosis (MS), Crohn’s disease, and rheumatoid arthritis, among many others (see www.genome.gov/gwastudies; Hindorff et al., 2009). It is noteworthy that rs3800307 (found on the DRB1*03-DQA1*0501-DQB1*0201 haplotype)—an SNP in complete LD (r2 = 1) with rs13194053 that reached genomewide significant association with schizophrenia in the combined GWAS meta-analysis (Purcell et al., 2009; Shi et al., 2009; Stefansson et al., 2009)—is associated with T1D (Viken et al., 2009). In addition, rs3131296 at NOTCH4 is in strong LD (r2 > 0.73) with the classical HLA allele DRB1*03 and other SNPs that are associated with several autoimmune disorders (T1D, celiac disease, systemic lupus erythematosus, and so forth), albeit with opposite alleles (Stefansson et al., 2009). Finally, the MGS GWAS showed some evidence, with p = 3.5 × 10−5 in the EA data set and p = 1.9 × 10−6 in the EA plus African American (AA) data set, for association with schizophrenia at the chromosome 1p22.1 FAM69A-EVI-RPL5 gene cluster (Shi et al., 2009), which has been implicated in MS (Oksenberg et al., 2008).
Other genes in the same region are involved in chromatin structure (high mobility group nucleosomal binding domain 4, HMGN4); transcriptional regulation (activator of basal transcription 1, ABT1; zinc finger protein 322A, ZNF322A; zinc finger protein 184, ZNF184); G-protein-coupled receptor signaling (FKSG83); and the nuclear pore complex (nuclear pore membrane protein 121-like 2, POM121L2). The SGENE-plus (their GWAS set) and follow-up samples (i.e., an extended SGENE data set that added a follow-up EA sample of 4,999 cases and 15,555 controls) analysis reported an independent association (i.e., in weak LD with rs13194053 at the histone gene cluster) at NOTCH4 (Notch homolog 4 [Drosophila], rs3131296, p = 2 × 10−8), located at 32.28 Mb on chromosome 6, and the combined meta-analysis of SGENE-plus and follow-up samples, along with MGS and ISC samples, gave a p = 2.3 × 10−10 there (Stefansson et al., 2009). For a list of genes mentioned in the text and their functions, see table 7.2.
Table 7.2 Genes Mentioned in Review, and Their Functions

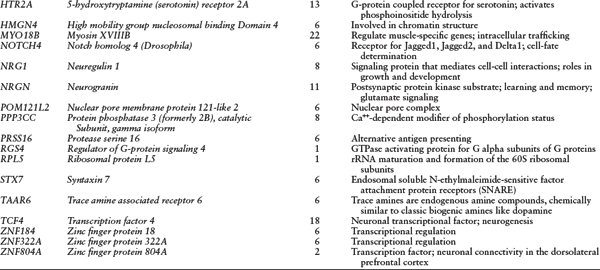
NOTES: Genes are listed alphabetically by symbol. All chromosome 6 genes are in the MHC except DTNBP1.
SOURCE: Reprinted from Gejman, Sanders, & Duan (2010) with permission from Psychiatric Clinics of North America (copyright Elsevier).
Non-Major Histocompatibility Complex Loci that Have Also Achieved Genomewide Significance
The combined analysis of SGENE-plus GWAS samples and replication samples uncovered associations with neurogranin (NRGN) and with transcription factor 4 (TCF4) that subsequently reached genomewide significance in the combined analysis of SGENE-plus and replication samples along with ISC and MGS samples (table 7.1) (see Stefansson et al., 2009). NRGN encodes a postsynaptic protein kinase substrate that binds calmodulin, mediating N-methyl-d-aspartate (NMDA) receptor signaling that is important for learning and memory and also relevant to the proposed glutamate pathophysiology of schizophrenia (Harrison & Weinberger, 2005; Wang et al., 2008). TCF4 is a neuronal transcriptional factor essential for brain development, specifically neurogenesis (Gulacsi & Anderson, 2008). Mutations in TCF4 cause Pitt-Hopkins syndrome, a neurodevelopmental disorder characterized by severe motor and mental retardation, including absence of language development, microcephaly, epilepsy, and facial dysmorphisms (Brockschmidt et al., 2007; Flora et al., 2007; Kalscheuer et al., 2008). It is also of interest that homozygous and compound-heterozygote deletions and mutations in CNTNAP2 and NRXN1 can symptomatically resemble Pitt-Hopkins syndrome along with autistic behavior (Zweier et al., 2009), and both NRXN1 (via the 2p16.3 CNV) and CNTNAP2 (a rarer CNV) (Friedman et al., 2008) have previously been implicated in schizophrenia (and also autism spectrum disorders and epilepsy, as reviewed in Zweier et al., 2009). Another new schizophrenia susceptibility gene from schizophrenia GWAS is zinc finger protein 804A (ZNF804A), which was identified by a two-stage study, with a GWAS discovery phase using 479 cases and 2,937 controls, followed with 6,829 cases and 9,897 controls for loci with a discovery p < 10-5 (O’Donovan et al., 2008). A combined p = 1.61 × 10−7 was obtained for SNP rs1344706 in the initial report, and the association evidence was supported in a later large GWAS of schizophrenia (Purcell et al., 2009; Shi et al., 2009; Stefansson et al., 2009). Subsequently, rs1344706 in ZNF804A was reported to be associated with altered neuronal connectivity in the dorsolateral prefrontal cortex in a functional magnetic resonance imaging study of healthy controls (Esslinger et al., 2009).
Polygenic Contributions to Schizophrenia
Many genetic variants with very small effects, combined together, can increase risk substantially under a polygenic model, first hypothesized for schizophrenia four decades ago (Gottesman & Shields, 1967). Simulations show that even a disorder with 1,000 risk loci with low mean relative risks (RR = 1.04), when evaluated on a large scale (10 K cases and 10 K controls), GWAS would still allow prediction of individual disorder risk with an accuracy greater than 0.75 by using 75 loci explaining approximately 50% of the risk variance (Wray, Goddard, & Visscher, 2007). ISC used their GWAS (discovery data set) to define a large set of very small effect common variants as “score” alleles with increasingly liberal association significance thresholds (Purcell et al., 2009). ISC then generated an aggregate risk score for each individual in independent target GWAS data sets, using the MGS EA and AA data sets as well as a U.K. sample (O’Donovan et al., 2008; Shi et al., 2009). Aggregate risk scores in cases were found to be higher than in controls in each of the GWAS data sets of schizophrenia, and also in GWAS data sets of bipolar disorder (WTCCC, 2007; Sklar et al., 2008), but not in control GWAS data sets of nonpsychiatric disorders (WTCCC, 2007; Purcell et al., 2009). They concluded that thousands of common polygenic variants with very small individual effects explain about one-third of the total variation in genetic liability to schizophrenia (Purcell et al., 2009). In an independent bioinformatics-based study (Sun et al., 2009), candidate genes selected from literature mining were found to be enriched in the list of genes with small p-values from schizophrenia GWAS data sets (the Clinical Antipsychotic Trials of Intervention Effectiveness [CATIE] study [Sullivan et al., 2008] and the Genetic Association Information Network [GAIN] portion of MGS [Shi et al., 2009]). A polygenic model with less common causal alleles (MAF < 5%) did not fit the data as well (Purcell et al., 2009). Simulated and empirical data indicate that the spectrum of risk alleles for common disorders includes both common and rare variants (Pritchard, 2001; Kathiresan et al., 2009). The problem of how to explain the substantial missing heritability remains fundamental. Missing heritability here refers to heritability that is unexplained after well-powered GWAS have been conducted. Although it has been argued that the heritability of some behavioral traits and disorders may have been overestimated (Kamin & Goldberger, 2002), this seems unlikely for schizophrenia given the large body of high-quality evidence that is available, and other reasons seem more plausible (see an excellent review on the topic by Herold et al., 2009).
Pleiotropy and Overlap with Bipolar Disorder, Autism, and Other Disorders
Pleiotropy refers to the common phenomenon of variation in a gene simultaneously affecting different phenotypes. It has been found in humans for genes for body weight and height (Weedon et al., 2008) and also for disorders such as type 2 diabetes (T2D) (Zeggini et al., 2008) and prostate cancer (Thomas et al., 2008). The molecular genetic overlaps between schizophrenia and bipolar disorder, and between schizophrenia and autism, are consistent with pleiotropy, but shared genetic loci may actually determine an aspect (somewhat in isolation from the overall phenotype) shared by two disorders such as psychosis in schizophrenia and in bipolar disorder (Schulze et al., 2006).
Overlap with Bipolar Disorder
Schizophrenia and bipolar disorder share several characteristics: peak onset in early adulthood, similar prevalence, psychotic symptoms in more than half of bipolar disorder type 1 subjects, response to antipsychotic medications, substance use comorbidity, increased suicide risk, and severe mood episodes (often present in schizophrenia). Family studies have shown that schizoaffective disorder is more common in families—as ascertained from probands with schizophrenia or with bipolar disorder type 1—than in the general population (Gershon et al., 1982; Gershon et al., 1988; Kendler et al., 1993; Maier et al., 1993; Valles et al., 2000; Maier et al., 2002). A recent meta-analysis of family studies found familial coaggregation of schizophrenia and bipolar disorder (Van Snellenberg & de Candia, 2009). The largest family study (Sweden; schizophrenia N approximately 36,000 and bipolar disorder N approximately 40,000 probands) found familial coaggregation between schizophrenia and bipolar disorder to be roughly 63% because of additive genetic effects common to both disorders (Lichtenstein et al., 2009).
Overlap of susceptibility genes has been postulated (Berrettini, 2000; Craddock & Owen, 2007; van Os & Kapur, 2009), including for more circumscribed aspects such as psychosis proneness (Schulze et al., 2006; Goes et al., 2007). An overlapping CNV for schizophrenia and bipolar disorder has been reported for the 16p11.2 duplication in a meta-analysis with an association p = 4.8 × 10−7 for schizophrenia and p = 0.017 for bipolar disorder (McCarthy et al., 2009). GWAS SNP data suggest a genetic overlap between schizophrenia and bipolar disorder, clinical entities shown to share polygenic common variants with very small effect sizes (Purcell et al., 2009). A genewide analysis found a significant excess of genes showing associations with both disorders (Moskvina et al., 2009). ZNF804A and CACNA1C (calcium channel, voltage-dependent, L type, alpha 1C subunit) are two such genes that are shared by both disorders: ZNF804A was identified initially as a schizophrenia susceptibility gene, and CACNA1C was identified initially as a bipolar disorder susceptibility gene, in respective GWAS (O’Donovan et al., 2008; Green et al., 2009).
Overlap with Autism
Schizophrenia and autism also share a few clinical features such as social interaction and communication impairments, and some negative or deficit symptoms (Konstantareas & Hewitt, 2001). This is more noticeable for childhood-onset schizophrenia, where developmental delays may be more marked, than in adult-onset schizophrenia, in which such findings are subtler (Rapoport et al., 2009). However, autism remains an exclusion criterion for schizophrenia in the DSM-IV unless prominent hallucinations and delusions are present for at least a month (APA, 1994). A study of 129 adults with autism spectrum disorders (ASD) found that 7% had psychotic bipolar disorder and 8% had schizophrenia or other psychotic disorders (Stahlberg et al., 2004). The current diagnostic hierarchy, which largely treats ASD and schizophrenia as mutually exclusive, could mask some ASD-schizophrenia comorbidity because an autism case might be less likely to be diagnosed with schizophrenia in adulthood (Rapoport et al., 2009), even in the presence of overt and chronic psychosis. A twofold increase of schizophrenia in the parents of individuals with autism (Daniels et al., 2008), a risk ratio of 3.44 for autism when prenatal parental history of a schizophrenia-like psychosis was present in a nationwide Danish study (Larsson et al., 2005), and an increased risk of schizophrenia in autism patients (Stahlberg et al., 2004; Mouridsen et al., 2008) suggest overlapping risk factors between schizophrenia and autism.
It is interesting that the strongest SNP association of p = 4.6 × 10−7 in the MGS GWAS EA data set was found with centaurin gamma 2 (CENTG2, also known as AGAP1), a gene that has been implicated in autism (Wassink et al., 2005). It is also noteworthy that our exploratory analysis of MGS GWAS data combining both EA and AA (3,967 cases and 3,626 controls) showed a p = 1.9 × 10−6 association with fragile X mental retardation, autosomal homolog 1 (FXR1; see Shi et al., 2009), and the association reached genomewide significance when the ISC and SGENE-plus GWAS data sets were included in the analysis along with MGS EA and AA (data not shown). FXR1 is a paralog of fragile X mental retardation 1 (FMR1), dysfunction of which causes the FMR syndrome that includes autism as a common feature (Bassell & Warren, 2008). CNV loci common to autism and schizophrenia have also been reported (see the next section and chapter 8).
Overlap with Other Conditions
The idea that there is genetic overlap between schizophrenia and autism (and other conditions) has recently received indirect support because a number of individuals with schizophrenia carrying rare and large CNVs have comorbidities such as learning disabilities, mental retardation, autism and autism spectrum disorders, and seizures or epilepsy, and because such disorders’ own CNV scans have implicated the same CNV loci (Sebat et al., 2007; Brunetti-Pierri et al., 2008; Christian et al., 2008; Kumar et al., 2008; Marshall et al., 2008; Mefford et al., 2008; Sharp et al., 2008; Weiss et al., 2008; de Kovel et al., 2009; Dibbens et al., 2009; Helbig et al., 2009; McCarthy et al., 2009). Seizures are also overrepresented in schizophrenia (Hyde & Weinberger, 1997; Cascella, Schretlan, & Sawa, 2009). Finally, evidence for pleiotropy from model organisms (Griswold & Whitlock, 2003) suggests that pleiotropic genes might not be selectively neutral: the “unintended” effect (e.g., conferring susceptibility to a common disorder) may be overshadowed by the “intended effect” (e.g., protecting against another disorder, such as one that is more lethal or that has similar lethality but earlier expression [see also Cheverud, 2007]). The recent observation that somatic mutations in PARK2, a gene with an established role in Parkinson’s disease, are associated with glioblastoma and other human malignancies also suggests a pleotropic effect (Veeriah et al., 2010). If a gene (pleiotropically) increases the risk for schizophrenia through two unrelated mechanisms, one independent (i.e., directly affecting the risk for schizophrenia) and another one through an association with a secondary associated phenotype (e.g., mood disorder or nicotine addiction, which are both associated with schizophrenia), it may induce bias in association analyses, depending on the specific ascertainment rules and their effect on recruitment of subjects with the secondary phenotype (Monsees, Tamimi, & Kraft, 2009).
The New Reality and Challenges for the Field of Schizophrenia Genetics
The GWAS experiments have defined multiple chromosomal regions of high interest, in addition to 6p21.3-p22.1, and several have already surpassed the genomewide significance threshold. Ongoing larger multisample combined GWAS (e.g., the Psychiatric GWAS Consortium [PGC)] for schizophrenia [Gejman, 2009], with a sample N > 21,000) and large-scale replication studies (e.g., the PGC combined schizophrenia replication with a sample N > 30,000) are very likely to expand the number of chromosomal regions that attain genomewide significance and the number of other regions of high interest that also merit further laboratory efforts. As additional large samples are studied, the number of regions that reach genomewide significance is expected to increase, a general trend for complex genetics (albeit the gene effects become progressively smaller because the larger-effect size loci are found first). Therefore it is reasonable to conclude that GWAS-enabled discoveries in the schizophrenia genetics field reflect incremental progress. However, it is also clear that we have not yet identified the DNA loci associated with the bulk of the transmission of schizophrenia in the general population, or the biological mechanisms for any of the confirmed associations. In GWAS, SNPs that constitute a genomewide genotyping array typically are selected because they are common and are informative of many other SNPs, not because of their functional properties. This method of SNP selection tends to yield results where the large majority of associated alleles are unlikely to be causative. Furthermore, the associated SNPs typically are not in coding sequences where a signal would be easier to interpret, but in intergenic and intronic regions for which the function of most associated variation remains unknown. The planning of follow-up resequencing experiments is not simple, either. The association signals frequently are from regions with an extended LD block that spans many genes, an extreme example being the MHC locus implicated in schizophrenia where it is challenging to disentangle which of the several hundred genes in the region is or are likely to be causal. The causative variants may be close to the associated locus but may also be farther removed, even on a different chromosome (as could happen with a trans-acting gene expression factor). The extent of rare variation involvement in schizophrenia in the general population remains unknown. This includes CNVs within risk loci already detected as being associated by GWAS, or in other regions of the genome for which there is no evidence of association, although genomewide resequencing will empirically address this question. In the context of our still incipient knowledge of the genome and of the genetics of complex disorders, it is important to avoid trying to fit our hypotheses to a Procrustean bed and instead keep an open mind to a variety of approaches. From the collective experience of model organisms (Mackay, 2009), it is unlikely that explanations relying only on single genes will capture the fundamental complexity of most human complex traits. All associated genetic variation needs to be pursued, and their effects integrated, to understand the pathophysiology of a complex disorder. There are a few issues of momentous importance, which we have selected for brief discussion.
Replication and Fine Mapping of Genomewide Association Studies Associations
This includes the location and boundaries of each locus and is best done by analyzing, in the aggregate and separately, a large combined sample. (Single samples lack the necessary size or power.) The follow-up experiments to replicate GWAS results need to be thorough and systematic. It is essential to predefine the statistical thresholds of loci that are to be included in replication experiments. These do not necessarily need to be only genomewide significant loci, because the combination of GWAS and new replication samples may establish new risk loci (e.g., among SNPs that were strongly associated but did not fulfill GWAS criteria for genomewide significance previously). Replication is one of the main aims of the PGC. The preferred approach is to combine GWAS data from independent samples, but for those samples that lack GWAS data, focused genotyping is still useful, although less informative. Non-EA populations might have some nonoverlapping susceptibility loci; thus, it is fundamental to investigate for these differences because they are potentially informative for both genetic and environmental risk factors. An important characteristic of African populations (e.g., AA) is reduced LD, which helps in narrowing the associated genomic intervals; existing limitations of CNV and SNP map coverage and imputation of AA data sets are currently being addressed (for examples, see Hao et al., 2009; McElroy et al., 2009). The new data-sharing policy (see gwas.nih.gov) has strongly stimulated collaboration, including follow-up replication studies.
Large-Scale Functional Approaches
The integration of GWAS data with measures of gene expression regulation is fundamental. The combined study of genomewide transcription and GWAS association data may generate mechanistic explanations for formerly purely statistical GWAS associations. It is important that such studies be sufficiently powered to allow for the detection of small effects. The approach has proved successful in asthma (Moffatt et al., 2007). It is interesting that within the MHC region implicated in schizophrenia, there are more than 10 cis-expression QTL (cis-eQTL, cis meaning nearby on the same chromosome) (figure 7.2) (see Dixon et al., 2007), and the SNP showing the most significant association with schizophrenia, rs13194053 with p = 9.54 × 10−9, is in LD (r2 = 0.43) with an SNP showing the strongest association with BTN3A2 expression (figure 7.2). It is noteworthy that, in the absence of buffering effects of entire multiorgan physiologic systems, simple in vitro models offer the advantage of allowing the observation of smaller effects, particularly when the system of interest can be stimulated (perturbed to further expose variation) in pharmacogenetic experiments.
Basic genomics research has produced major breakthroughs during recent years. Examples are the discovery of microRNAs, long-range promoters, epigenetic factors, and variable CNVs. Many more will probably be made as our knowledge of the genome rapidly increases. It should not be surprising if still unknown genetic mechanisms will, in the end, explain a substantial proportion of the heritability of schizophrenia. Nonetheless, the task of defining the spectrum of molecular genetic mechanisms in schizophrenia is now at the forefront of our field. Rapid progress in biotechnology (Mardis, 2009) is making the study of rare variants in many genes or in large genomic regions in large samples increasingly feasible (see chapter 8, this book). Proof of principle is provided by the 1,000 genomes project (see www.1000genomes.org), which is designed to build a deep catalog of human genetic variation. The design of experiments aimed at fine mapping of regions of association and the precision of imputation will both benefit from this project.
It is anticipated that as genetic discoveries accumulate, the application of myriad tools from systems biology (e.g., genomics, transcriptomics, proteomics, and so forth) will lead to a delineation of biological pathways involved in the pathophysiology of schizophrenia, and eventually to new therapies. Developments in treatment still lag behind discoveries of new genetic associations for complex disorders (see O’Rahilly, 2009), but this situation is expected to change as biological research makes inroads into still purely statistical associations. A long-term aim, and a task of utmost importance, is the integration of the spectrum of mutations found in schizophrenia into explanations that take into account constantly changing environments and evolutionary forces.
KEY AREAS FOR FUTURE RESEARCH
Acknowledgments
This review was supported by funding from National Institutes of Health grant U01MH79469 to Pablo V. Gejman and by the Paul Michael Donovan Charitable Foundation.
Selected Readings
Daly, M. J., Rioux, J. D., Schaffner, S. F., Hudson, T. J., & Lander, E. S. (2001). High-resolution haplotype structure in the human genome. Nature Genetics 29(2): 229–232.
Gottesman, I. I. & Shields, J. (1967). A polygenic theory of schizophrenia. Proceedings of the National Academy of Sciences U.S.A. 58(1): 199–205.
Ioannidis, J. P. (2008). Why most discovered true associations are inflated. Epidemiology 19(5): 640–648.
Keller, M. C. & Miller, G. (2006). Resolving the paradox of common, harmful, heritable mental disorders: Which evolutionary genetic models work best? Behavioral Brain Science 29(4): 385–404.
Ng, M. Y., Levinson, D. F., Faraone, S. V., Suarez, B. K., DeLisi, L. E., Arinami, T., Riley, B., Paunio, T., Pulver, A. E., Irmansyah, Holmans, P. A., et al. (2009). Meta-analysis of 32 genome-wide linkage studies of schizophrenia. Molecular Psychiatry 14(8): 774–785.
Purcell, S. M., Wray, N. R., Stone, J. L., Visscher, P. M., O’Donovan, M. C., Sullivan, P. F., & Sklar, P. (2009). Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460(7256): 748–752.
Sanders, A. R., Duan, J., Levinson, D. F., Shi, J., He, D., Hou, C., Burrell, G. J., Rice, J. P., Nertney, D. A., Olincy, A., et al. (2008). No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: Implications for psychiatric genetics. American Journal of Psychiatry 165(4): 497–506.
Shi, J., Levinson, D. F., Duan, J., Sanders, A. R., Zheng, Y., Pe’er, I., Dudbridge, F., Holmans, P. A., Whittemore, A. S., Mowry, B. J., et al. (2009). Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 460(7256): 753–757.
Suarez, B. K., Duan, J., Sanders, A. R., Hinrichs, A. L., Jin, C. H., Hou, C., Buccola, N. G., Hale, N., Weilbaecher, A. N., Nertney, D. A., et al. (2006). Genomewide linkage scan of 409 European-ancestry and African American families with schizophrenia: Suggestive evidence of linkage at 8p23.3-p21.2 and 11p13.1-q14.1 in the combined sample. American Journal of Human Genetics 78(2): 315–333.
Wray, N. R., Goddard, M. E., & Visscher, P. M. (2007). Prediction of individual genetic risk to disease from genome-wide association studies. Genome Research 17(10): 1520–1528.
References
Adegbola, A., Gao, H., Sommer, S., & Browning, M. (2008). A novel mutation in JARID1C/ SMCX in a patient with autism spectrum disorder (ASD). American Journal of Medical Genetics A 146A(4): 505–511.
Allen, N. C., Bagade, S., McQueen, M. B., Ioannidis, J. P., Kavvoura, F. K., Khoury, M. J., Tanzi, R. E., & Bertram, L. (2008). Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: The SzGene database. Nature Genetics 40(7): 827–834.
Allison, A. C. (1954). Notes on sickle-cell polymorphism. Annals of Human Genetics 19(1): 39–51.
APA. (1994). Diagnostic and Statistical Manual of Mental Disorders (4th ed.). Washington, DC: American Psychiatric Association.
Bach, M. L., Bach, F. H., & Joo, P. (1969). Leukemia-associated antigens in the mixed leukocyte culture test. Science 166(3912): 1520–1522.
Badner, J. A. & Gershon, E. S. (2002). Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Molecular Psychiatry 7(4): 405–411.
Bassell, G. J. & Warren, S. T. (2008). Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron 60(2): 201–214.
Bastian, J., Chacron, M. J., & Maler, L. (2004). Plastic and nonplastic pyramidal cells perform unique roles in a network capable of adaptive redundancy reduction. Neuron 41(5): 767–779.
Berrettini, W. H. (2000). Are schizophrenic and bipolar disorders related? A review of family and molecular studies. Biological Psychiatry 48(6): 531–538.
Birney, E., Stamatoyannopoulos, J. A., Dutta, A., Guigo, R., Gingeras, T. R., Margulies, E. H., Weng, Z., Snyder, M., Dermitzakis, E. T., Thurman, R. E., et al. (2007). Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447(7146): 799–816.
Blouin, J. L., Dombroski, B. A., Nath, S. K., Lasseter, V. K., Wolyniec, P. S., Nestadt, G., Thornquist, M., Ullrich, G., McGrath, J., Kasch, L., et al. (1998). Schizophrenia susceptibility loci on chromosomes 13q32 and 8p21. Nature Genetics 20(1): 70–73.
Book, J. A. (1953). Schizophrenia as a gene mutation. Acta Genetica et Statistica Medica 4(2–3): 133–139.
Brockschmidt, A., Todt, U., Ryu, S., Hoischen, A., Landwehr, C., Birnbaum, S., Frenck, W., Radlwimmer, B., Lichter, P., Engels, H., et al. (2007). Severe mental retardation with breathing abnormalities (Pitt-Hopkins syndrome) is caused by haploinsufficiency of the neuronal bHLH transcription factor TCF4. Human Molecular Genetics 16(12): 1488–1494.
Brown, A., Bottiglieri, T., Schaefer, C., Quesenberry, C., Liu, L., Bresnahan, M., & Susser, E. (2007). Elevated prenatal homocysteine levels as a risk factor for schizophrenia. Archives of General Psychiatry 64(1): 31–39.
Brown, A. S. & Derkits, E. J. (2010). Prenatal infection and schizophrenia: A review of epidemiologic and translational studies. American Journal of Psychiatry 167(3): 261–280.
Brunetti-Pierri, N., Berg, J. S., Scaglia, F., Belmont, J., Bacino, C. A., Sahoo, T., Lalani, S. R., Graham, B., Lee, B., Shinawi, M., et al. (2008). Recurrent reciprocal 1q21.1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nature Genetics 40(12): 1466–1471.
Byrne, M., Agerbo, E., Bennedsen, B., Eaton, W. W., & Mortensen, P. B. (2007). Obstetric conditions and risk of first admission with schizophrenia: A Danish national register based study. Schizophrenia Research 97(1–3): 51–59.
Cannon, M., Jones, P. B., & Murray, R. M. (2002). Obstetric complications and schizophrenia: Historical and meta-analytic review. American Journal of Psychiatry 159(7): 1080–1092.
Cannon, T. D., Kaprio, J., Lonnqvist, J., Huttunen, M., & Koskenvuo, M. (1998). The genetic epidemiology of schizophrenia in a Finnish twin cohort: A population-based modeling study. Archives of General Psychiatry 55(1): 67–74.
Cardno, A. G. & Gottesman, I. (2000). Twin studies of schizophrenia: From bow-and-arrow concordances to Star Wars Mx and functional genomics. American Journal of Medical Genetics 97(1): 12–17.
Cardno, A. G., Marshall, E. J., Coid, B., Macdonald, A. M., Ribchester, T. R., Davies, N. J., Venturi, P., Jones, L. A., Lewis, S. W., Sham, P. C., et al. (1999). Heritability estimates for psychotic disorders: The Maudsley twin psychosis series. Archives of General Psychiatry 56(2): 162–168.
Cascella, N. G., Schretlen, D. J., & Sawa, A. (2009). Schizophrenia and epilepsy: Is there a shared susceptibility? Neuroscience Research 63(4): 227–235.
Chechik, G., Anderson, M. J., Bar-Yosef, O., Young, E. D., Tishby, N., & Nelken, I. (2006). Reduction of information redundancy in the ascending auditory pathway. Neuron 51(3): 359–368.
Cheverud, J. M. (2007). The relationship between development and evolution through heritable variation. Novartis Foundation Symposium 284: 55–65.
Christian, S. L., Brune, C. W., Sudi, J., Kumar, R. A., Liu, S., Karamohamed, S., Badner, J. A., Matsui, S., Conroy, J., McQuaid, D., et al. (2008). Novel submicroscopic chromosomal abnormalities detected in autism spectrum disorder. Biological Psychiatry 63(12): 1111–1117.
Cornelis, M. C., Agrawal, A., Cole, J. W., Hansel, N. N., Barnes, K. C., Beaty, T. H., Bennett, S. N., Bierut, L. J., Boerwinkle, E., Doheny, K. F., et al. (2010). The Gene, Environment Association Studies consortium (GENEVA): Maximizing the knowledge obtained from GWAS by collaboration across studies of multiple conditions. Genetic Epidemiology 34(4): 364–372.
Costa, E., Dong, E., Grayson, D. R., Guidotti, A., Ruzicka, W., & Veldic, M. (2007). Reviewing the role of DNA (cytosine-5) methyltransferase overexpression in the cortical GABAergic dysfunction associated with psychosis vulnerability. Epigenetics 2(1): 29–36.
Craddock, N. & Owen, M. J. (2007). Rethinking psychosis: The disadvantages of a dichotomous classification now outweigh the advantages. World Psychiatry 6(2): 84–91.
Crow, J. F. (2000). The origins, patterns and implications of human spontaneous mutation. Nature Reviews Genetics 1(1): 40–47.
Daly, M. J., Rioux, J. D., Schaffner, S. F., Hudson, T. J., & Lander, E. S. (2001). High-resolution haplotype structure in the human genome. Nature Genetics 29(2): 229–232.
Daniels, J. L., Forssen, U., Hultman, C. M., Cnattingius, S., Savitz, D. A., Feychting, M., & Sparen, P. (2008). Parental psychiatric disorders associated with autism spectrum disorders in the offspring. Pediatrics 121(5):, e1357–e1362.
de Kovel, C. G., Trucks, H., Helbig, I., Mefford, H. C., Baker, C., Leu, C., Kluck, C., Muhle, H., von Spiczak, S., Ostertag, P., et al. (2009). Recurrent microdeletions at 15q11.2 and 16p13.11 predispose to idiopathic generalized epilepsies. Brain 133(Pt 1): 23–32.
Dibbens, L. M., Mullen, S., Helbig, I., Mefford, H. C., Bayly, M. A., Bellows, S., Leu, C., Trucks, H., Obermeier, T., Wittig, M., et al. (2009). Familial and sporadic 15q13.3 microdeletions in idiopathic generalized epilepsy: Precedent for disorders with complex inheritance. Human Molecular Genetics 18(19): 3626–3631.
Dixon, A. L., Liang, L., Moffatt, M. F., Chen, W., Heath, S., Wong, K. C., Taylor, J., Burnett, E., Gut, I., Farrall, M., et al. (2007). A genome-wide association study of global gene expression. Nature Genetics 39(10): 1202–1207.
Drachman, D. A. (2005). Do we have brain to spare? Neurology 64(12): 2004–2005.
Dudbridge, F. & Gusnanto, A. (2008). Estimation of significance thresholds for genomewide association scans. Genetic Epidemiology 32(3): 227–234.
Eaton, W. W., Byrne, M., Ewald, H., Mors, O., Chen, C. Y., Agerbo, E., & Mortensen, P. B. (2006). Association of schizophrenia and autoimmune diseases: Linkage of Danish national registers. American Journal of Psychiatry 163(3): 521–528.
Esslinger, C., Walter, H., Kirsch, P., Erk, S., Schnell, K., Arnold, C., Haddad, L., Mier, D., Opitz von Boberfeld, C., Raab, K., et al. (2009). Neural mechanisms of a genome-wide supported psychosis variant. Science 324(5927): 605.
Eyre-Walker, A. & Keightley, P. D. (1999). High genomic deleterious mutation rates in hominids. Nature 397(6717): 344–347.
Fananas, L. & Bertranpetit, J. (1995). Reproductive rates in families of schizophrenic patients in a case-control study. Acta Psychiatrica Scandinavica 91(3): 202–204.
Flora, A., Garcia, J. J., Thaller, C., & Zoghbi, H. Y. (2007). The E-protein Tcf4 interacts with Math1 to regulate differentiation of a specific subset of neuronal progenitors. Proceedings of the National Academy of Sciences U.S.A. 104(39): 15382–15387.
Franzek, E. & Beckmann, H. (1998). Different genetic background of schizophrenia spectrum psychoses: A twin study. American Journal of Psychiatry 155(1): 76–83.
Friedman, J. I., Vrijenhoek, T., Markx, S., Janssen, I. M., van der Vliet, W. A., Faas, B. H., Knoers, N. V., Cahn, W., Kahn, R. S., Edelmann, L., et al. (2008). CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Molecular Psychiatry 13(3): 261–266.
Friedman, J. M. (1981). Genetic disease in the offspring of older fathers. Obstetrics and Gynecology 57(6): 745–749.
Gabriel, S. B., Schaffner, S. F., Nguyen, H., Moore, J. M., Roy, J., Blumenstiel, B., Higgins, J., DeFelice, M., Lochner, A., Faggart, M., et al. (2002). The structure of haplotype blocks in the human genome. Science 296(5576): 2225–2229.
Gejman, P. V. (2009). Combined “Freeze 1” Schizophrenia-GWAS analysis. Plenary session: The psychiatric GWAS consortium. Paper presented at the World Congress of Psychiatric Genetics, San Diego.
Gejman, P. V., Sanders, A. R., & Duan, J. (2010) The role of genetics in the etiology of schizophrenia. Psychiatric Clinics of North America 33(1): 35–66.
Gerber, D. J., Hall, D., Miyakawa, T., Demars, S., Gogos, J. A., Karayiorgou, M., & Tonegawa, S. (2003). Evidence for association of schizophrenia with genetic variation in the 8p21.3 gene, PPP3CC, encoding the calcineurin gamma subunit. Proceedings of the National Academy of Sciences U.S.A. 100(15): 8993–8998.
Gershon, E. S., DeLisi, L. E., Hamovit, J., Nurnberger, J. I., Jr., Maxwell, M. E., Schreiber, J., Dauphinais, D., Dingman, C. W., & Guroff, J. J. (1988). A controlled family study of chronic psychoses: Schizophrenia and schizoaffective disorder. Archives of General Psychiatry 45(4): 328–336.
Gershon, E. S., Hamovit, J., Guroff, J. J., Dibble, E., Leckman, J. F., Sceery, W., Targum, S. D., Nurnberger, J. I., Jr., Goldin, L. R., & Bunney, W. E., Jr. (1982). A family study of schizoaffective, bipolar I, bipolar II, unipolar, and normal control probands. Archives of General Psychiatry 39(10): 1157–1167.
Goes, F. S., Zandi, P. P., Miao, K., McMahon, F. J., Steele, J., Willour, V. L., Mackinnon, D. F., Mondimore, F. M., Schweizer, B., Nurnberger, J. I., Jr., et al. (2007). Mood-incongruent psychotic features in bipolar disorder: Familial aggregation and suggestive linkage to 2p11–q14 and 13q21–33. American Journal of Psychiatry 164(2): 236–247.
Gottesman, I. I. & Bertelsen, A. (1989). Confirming unexpressed genotypes for schizophrenia: Risks in the offspring of Fischer’s Danish identical and fraternal discordant twins. Archives of General Psychiatry 46(10): 867–872.
Gottesman, I. I. & Gould, T. D. (2003). The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry 160(4): 636–645.
Gottesman, I. I. & Shields, J. (1967). A polygenic theory of schizophrenia. Proceedings of the National Academy of Sciences U.S.A. 58(1): 199–205.
Green, E. K., Grozeva, D., Jones, I., Jones, L., Kirov, G., Caesar, S., Gordon-Smith, K., Fraser, C., Forty, L., Russell, E., et al. (2009). The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Molecular Psychiatry 15(10): 1016–1022.
Greenwood, T. A., Braff, D. L., Light, G. A., Cadenhead, K. S., Calkins, M. E., Dobie, D. J., Freedman, R., Green, M. F., Gur, R. E., Gur, R. C., et al. (2007). Initial heritability analyses of endophenotypic measures for schizophrenia: The consortium on the genetics of schizophrenia. Archives of General Psychiatry 64(11): 1242–1250.
Griswold, C. K. & Whitlock, M. C. (2003). The genetics of adaptation: The roles of pleiotropy, stabilizing selection and drift in shaping the distribution of bidirectional fixed mutational effects. Genetics 165(4): 2181–2192.
Gulacsi, A. A. & Anderson, S. A. (2008). Beta-catenin-mediated Wnt signaling regulates neurogenesis in the ventral telencephalon. Nature Neuroscience 11(12): 1383–1391.
Halperin, E. & Stephan, D. A. (2009). SNP imputation in association studies. Nature Biotechnology 27(4): 349–351.
Hao, K., Chudin, E., McElwee, J., & Schadt, E. E. (2009). Accuracy of genome-wide imputation of untyped markers and impacts on statistical power for association studies. BMC Genetics 10: 27.
Harrison, P. J. & Weinberger, D. R. (2005). Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Molecular Psychiatry 10(1): 40–68.
Haukka, J., Suvisaari, J., & Lonnqvist, J. (2003). Fertility of patients with schizophrenia, their siblings, and the general population: A cohort study from 1950 to 1959 in Finland. American Journal of Psychiatry 160(3): 460–463.
Heckers, S. (2009). Is schizoaffective disorder a useful diagnosis? Current Psychiatry Reports 11(4): 332–337.
Helbig, I., Mefford, H. C., Sharp, A. J., Guipponi, M., Fichera, M., Franke, A., Muhle, H., de Kovel, C., Baker, C., von Spiczak, S., et al. (2009). 15q13.3 microdeletions increase risk of idiopathic generalized epilepsy. Nature Genetics 41(2): 160–162.
Herold, C., Steffens, M., Brockschmidt, F. F., Baur, M. P., & Becker, T. (2009). INTERSNP: Genome-wide interaction analysis guided by a priori information. Bioinformatics 25(24): 3275–3281.
Hindorff, L. A., Sethupathy, P., Junkins, H. A., Ramos, E. M., Mehta, J. P., Collins, F. S., & Manolio, T. A. (2009). Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proceedings of the National Academy of Sciences U.S.A. 106(23): 9362–9367.
Hoggart, C. J., Clark, T. G., De Iorio, M., Whittaker, J. C., & Balding, D. J. (2008). Genomewide significance for dense SNP and resequencing data. Genetic Epidemiology 32(2): 179–185.
Holmans, P. A., Riley, B., Pulver, A. E., Owen, M. J., Wildenauer, D. B., Gejman, P. V., Mowry, B. J., Laurent, C., Kendler, K. S., Nestadt, G., et al. (2009). Genomewide linkage scan of schizophrenia in a large multicenter pedigree sample using single nucleotide polymorphisms. Molecular Psychiatry 14(8): 786–795.
Huxley, J., Mayr, E., Osmond, H., & Hoffer, A. (1964). Schizophrenia as a genetic morphism. Nature 204: 220–221.
Hyde, T. M. & Weinberger, D. R. (1997). Seizures and schizophrenia. Schizophrenia Bulletin 23(4): 611–622.
Ingraham, L. J. & Kety, S. S. (2000). Adoption studies of schizophrenia. American Journal of Medical Genetics 97(1): 18–22.
Ioannidis, J. P. (2008). Why most discovered true associations are inflated. Epidemiology 19(5): 640–648.
Kallmann, F. J. (1938). The Genetics of Schizophrenia: A Study of Heredity and Reproduction in the Families of 1,087 Schizophrenics. New York: J. J. Augustin.
Kalscheuer, V. M., Feenstra, I., Van Ravenswaaij-Arts, C. M., Smeets, D. F., Menzel, C., Ullmann, R., Musante, L., & Ropers, H. H. (2008). Disruption of the TCF4 gene in a girl with mental retardation but without the classical Pitt-Hopkins syndrome. American Journal of Medical Genetics A 146A(16): 2053–2059.
Kamin, L. J. & Goldberger, A. S. (2002). Twin studies in behavioral research: A skeptical view. Theoretical Population Biology 61(1): 83–95.
Kasanin, J. (1933). The acute schizoaffective psychoses. American Journal of Psychiatry 90(1): 97–126.
Kathiresan, S., Willer, C. J., Peloso, G. M., Demissie, S., Musunuru, K., Schadt, E. E., Kaplan, L., Bennett, D., Li, Y., Tanaka, T., et al. (2009). Common variants at 30 loci contribute to polygenic dyslipidemia. Nature Genetics 41(1): 56–65.
Kawasaki, H. & Iwamuro, S. (2008). Potential roles of histones in host defense as antimicrobial agents. Infectious Disorders—Drug Targets 8(3): 195–205.
Keller, M. C. & Miller, G. (2006). Resolving the paradox of common, harmful, heritable mental disorders: Which evolutionary genetic models work best? Behavioral and Brain Sciences 29(4): 385–404.
Kendler, K. S. (1987). Sporadic vs familial classification given etiologic heterogeneity: I. Sensitivity, specificity, and positive and negative predictive value. Genetic Epidemiology 4(5): 313–330.
Kendler, K. S., McGuire, M., Gruenberg, A. M., O’Hare, A., Spellman, M., & Walsh, D. (1993). The Roscommon Family Study. I. Methods, diagnosis of probands, and risk of schizophrenia in relatives. Archives of General Psychiatry 50(7): 527–540.
Kendler, K. S. & Neale, M. C. (2010). Endophenotype: A conceptual analysis. Molecular Psychiatry 15(8): 789–797.
Kim, H. S., Cho, J. H., Park, H. W., Yoon, H., Kim, M. S., & Kim, S. C. (2002.). Endotoxinneutralizing antimicrobial proteins of the human placenta. Journal of Immunology 168(5): 2356–2364.
Klaning, U., Mortensen, P. B., & Kyvik, K. O. (1996). Increased occurrence of schizophrenia and other psychiatric illnesses among twins. British Journal of Psychiatry 168(6): 688–692.
Kleinjan, D. A. & van Heyningen, V. (2005). Long-range control of gene expression: Emerging mechanisms and disruption in disease. American Journal of Human Genetics 76(1): 8–32.
Konstantareas, M. M. & Hewitt, T. (2001). Autistic disorder and schizophrenia: Diagnostic overlaps. Journal of Autism and Developmental Disorders 31(1): 19–28.
Kraepelin, E. (1899). (English translation 1921). Manic-Depressive Insanity and Paranoia. Edinburgh: E. & S. Livingstone.
Kraft, P. & Hunter, D. J. (2009). Genetic risk prediction—are we there yet? New England Journal of Medicine 360(17): 1701–1703.
Kringlen, E. & Cramer, G. (1989). Offspring of monozygotic twins discordant for schizophrenia. Archives of General Psychiatry 46(10): 873–877.
Kumar, R. A., KaraMohamed, S., Sudi, J., Conrad, D. F., Brune, C., Badner, J. A., Gilliam, T. C., Nowak, N. J., Cook, E. H., Jr., Dobyns, W. B., et al. (2008). Recurrent 16p11.2 microdeletions in autism. Human Molecular Genetics 17(4): 628–638.
Larsson, H. J., Eaton, W. W., Madsen, K. M., Vestergaard, M., Olesen, A. V., Agerbo, E., Schendel, D., Thorsen, P., & Mortensen, P. B. (2005). Risk factors for autism: Perinatal factors, parental psychiatric history, and socioeconomic status. American Journal of Epidemiology 161(10): 916–928.
Lencz, T., Morgan, T. V., Athanasiou, M., Dain, B., Reed, C. R., Kane, J. M., Kucherlapati, R., & Malhotra, A. K. (2007). Converging evidence for a pseudoautosomal cytokine receptor gene locus in schizophrenia. Molecular Psychiatry 12(6): 572–580.
Lewis, C. M., Levinson, D. F., Wise, L. H., DeLisi, L. E., Straub, R. E., Hovatta, I., Williams, N. M., Schwab, S. G., Pulver, A. E., Faraone, S. V., et al. (2003). Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. American Journal of Human Genetics 73(1): 34–48.
Li, M., Li, C., & Guan, W. (2008). Evaluation of coverage variation of SNP chips for genomewide association studies. European Journal of Human Genetics 16(5): 635–643.
Lichtenstein, P., Bjork, C., Hultman, C. M., Scolnick, E., Sklar, P., & Sullivan, P. F. (2006). Recurrence risks for schizophrenia in a Swedish national cohort. Psychological Medicine 36(10): 1417–1425.
Lichtenstein, P., Yip, B. H., Bjork, C., Pawitan, Y., Cannon, T. D., Sullivan, P. F., & Hultman, C. M. (2009). Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: A population-based study. Lancet 373(9659): 234–239.
MacCabe, J. H., Koupil, I., & Leon, D. A. (2009). Lifetime reproductive output over two generations in patients with psychosis and their unaffected siblings: The Uppsala 1915–1929 Birth Cohort Multigenerational Study. Psychological Medicine 39(10): 1667–1676.
Mackay, T. F. (2009). The genetic architecture of complex behaviors: Lessons from Drosophila. Genetica 136(2): 295–302.
Maier, W., Lichtermann, D., Franke, P., Heun, R., Falkai, P., & Rietschel, M. (2002). The dichotomy of schizophrenia and affective disorders in extended pedigrees. Schizophrenia Research 57(2–3): 259–266.
Maier, W., Lichtermann, D., Minges, J., Hallmayer, J., Heun, R., Benkert, O., & Levinson, D. F. (1993). Continuity and discontinuity of affective disorders and schizophrenia: Results of a controlled family study. Archives of General Psychiatry 50(11): 871–883.
Malaspina, D., Harlap, S., Fennig, S., Heiman, D., Nahon, D., Feldman, D., & Susser, E. (2001). Advancing paternal age and the risk of schizophrenia. Archives of General Psychiatry 58(4): 361–367.
Manolio, T. A., Collins, F. S., Cox, N. J., Goldstein, D. B., Hindorff, L. A., Hunter, D. J., McCarthy, M. I., Ramos, E. M., Cardon, L. R., Chakravarti, A., et al. (2009). Finding the missing heritability of complex diseases. Nature 461(7265): 747–753.
Mardis, E. R. (2009). New strategies and emerging technologies for massively parallel sequencing: Applications in medical research. Genome Medicine 1(4): 40.
Marshall, C. R., Noor, A., Vincent, J. B., Lionel, A. C., Feuk, L., Skaug, J., Shago, M., Moessner, R., Pinto, D., Ren, Y., et al. (2008). Structural variation of chromosomes in autism spectrum disorder. American Journal of Human Genetics 82(2): 477–488.
McCarthy, M. I., Abecasis, G. R., Cardon, L. R., Goldstein, D. B., Little, J., Ioannidis, J. P., & Hirschhorn, J. N. (2008). Genome-wide association studies for complex traits: Consensus, uncertainty and challenges. Nature Reviews Genetics 9(5): 356–369.
McCarthy, S. E., Makarov, V., Kirov, G., Addington, A. M., McClellan, J., Yoon, S., Perkins, D. O., Dickel, D. E., Kusenda, M., Krastoshevsky, O., et al. (2009). Microduplications of 16p11.2 are associated with schizophrenia. Nature Genetic, 41(11): 1223–1227.
McElroy, J. P., Nelson, M. R., Caillier, S. J., & Oksenberg, J. R. (2009). Copy number variation in African Americans. BMC Genetics 10: 15.
McGrath, J., Saha, S., Chant, D., & Welham, J. (2008). Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiology Reviews 30: 67–76.
Mefford, H. C., Sharp, A. J., Baker, C., Itsara, A., Jiang, Z., Buysse, K., Huang, S., Maloney, V. K., Crolla, J. A., Baralle, D., et al. (2008). Recurrent rearrangements of chromosome 1q21.1 and variable pediatric phenotypes. New England Journal of Medicine 359(16): 1685–1699.
Mill, J., Tang, T., Kaminsky, Z., Khare, T., Yazdanpanah, S., Bouchard, L., Jia, P., Assadzadeh, A., Flanagan, J., Schumacher, A., et al. (2008). Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. American Journal of Human Genetics 82(3): 696–711.
Mittal, V. A., Ellman, L. M., & Cannon, T. D. (2008). Gene-environment interaction and covariation in schizophrenia: The role of obstetric complications. Schizophrenia Bulletin 34(6): 1083–1094.
Moffatt, M. F., Kabesch, M., Liang, L., Dixon, A. L., Strachan, D., Heath, S., Depner, M., von Berg, A., Bufe, A., Rietschel, E., et al. (2007). Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature 448(7152): 470–473.
Monsees, G. M., Tamimi, R. M., & Kraft, P. (2009). Genome-wide association scans for secondary traits using case-control samples. Genetic Epidemiology 33(8): 717–728.
Morris, G., Nevet, A., & Bergman, H. (2003). Anatomical funneling, sparse connectivity and redundancy reduction in the neural networks of the basal ganglia. Journal of Physiology (Paris) 97(4–6): 581–589.
Moskvina, V., Craddock, N., Holmans, P., Nikolov, I., Pahwa, J. S., Green, E., Owen, M. J., & O’Donovan, M. C. (2009). Gene-wide analyses of genome-wide association data sets: Evidence for multiple common risk alleles for schizophrenia and bipolar disorder and for overlap in genetic risk. Molecular Psychiatry 14(3): 252–260.
Mouridsen, S. E., Rich, B., Isager, T., & Nedergaard, N. J. (2008). Psychiatric disorders in individuals diagnosed with infantile autism as children: A case control study. Journal of Psychiatric Practice 14(1): 5–12.
Need, A. C., Ge, D., Weale, M. E., Maia, J., Feng, S., Heinzen, E. L., Shianna, K. V., Yoon, W., Kasperaviciute, D., Gennarelli, M., et al. (2009). A genome-wide investigation of SNPs and CNVs in schizophrenia. PLoS Genetics 5 (2): e1000373.
Ng, M. Y., Levinson, D. F., Faraone, S. V., Suarez, B. K., DeLisi, L. E., Arinami, T., Riley, B., Paunio, T., Pulver, A. E., Irmansyah, Holmans, P. A., et al. (2009). Meta-analysis of 32 genome-wide linkage studies of schizophrenia. Molecular Psychiatry 14(8): 774–785.
Nothnagel, M., Ellinghaus, D., Schreiber, S., Krawczak, M., & Franke, A. (2009). A comprehensive evaluation of SNP genotype imputation. Human Genetics 125(2): 163–171.
Nurnberger, J. I., Jr., Blehar, M. C., Kaufmann, C. A., York-Cooler, C., Simpson, S. G., Harkavy-Friedman, J., Severe, J. B., Malaspina, D., & Reich, T. (1994). Diagnostic interview for genetic studies: Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry 51(11): 849–859.
O’Donovan, M. C., Craddock, N., Norton, N., Williams, H., Peirce, T., Moskvina, V., Nikolov, I., Hamshere, M., Carroll, L., Georgieva, L., et al. (2008). Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nature Genetics 40(9): 1053–1055.
O’Rahilly, S. (2009). Human genetics illuminates the paths to metabolic disease. Nature 462(7271): 307–314.
Oksenberg, J. R., Baranzini, S. E., Sawcer, S., & Hauser, S. L. (2008). The genetics of multiple sclerosis: SNPs to pathways to pathogenesis. Nature Reviews Genetics 9(7): 516–526.
Pe’er, I., Yelensky, R., Altshuler, D., & Daly, M. J. (2008). Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genetic Epidemiology 32(4): 381–385.
Pritchard, J. K. (2001). Are rare variants responsible for susceptibility to complex diseases? American Journal of Human Genetics 69(1): 124–137.
Purcell, S. M., Wray, N. R., Stone, J. L., Visscher, P. M., O’Donovan, M. C., Sullivan, P. F., & Sklar, P. (2009). Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460(7256): 748–752.
Rapoport, J., Chavez, A., Greenstein, D., Addington, A., & Gogtay, N. (2009). Autism spectrum disorders and childhood-onset schizophrenia: Clinical and biological contributions to a relation revisited. Journal of the American Academy of Child and Adolescent Psychiatry 48(1): 10–18.
Reich, D. E. & Lander, E. S. (2001). On the allelic spectrum of human disease. Trends in Genetics 17(9): 502–510.
Richter, S. H., Garner, J. P., & Wurbel, H. (2009). Environmental standardization: Cure or cause of poor reproducibility in animal experiments? Nature Methods 6(4): 257–261.
Risch, N. (1990). Genetic linkage and complex diseases, with special reference to psychiatric disorders. Genetic Epidemiology 7(1): 3–16.
Roth, T. L., Lubin, F. D., Sodhi, M., & Kleinman, J. E. (2009). Epigenetic mechanisms in schizophrenia. Biochimica et Biophysica Acta 1790(9): 869–877.
Rüdin, E. (1916). Zur Vererbung und Neuenstehung der Dementia Praecox. Berlin: Springer.
Sanders, A. R., Duan, J., Levinson, D. F., Shi, J., He, D., Hou, C., Burrell, G. J., Rice, J. P., Nertney, D. A., Olincy, A., et al. (2008). No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: Implications for psychiatric genetics. American Journal of Psychiatry 165(4): 497–506.
Schadt, E. E. (2009). Molecular networks as sensors and drivers of common human diseases. Nature 46(7261): 218–223.
Schulze, T. G., Hedeker, D., Zandi, P., Rietschel, M., & McMahon, F. J. (2006). What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Archives of General Psychiatry 63(12): 1368–1376.
Sebat, J., Lakshmi, B., Malhotra, D., Troge, J., Lese-Martin, C., Walsh, T., Yamrom, B., Yoon, S., Krasnitz, A., Kendall, J., et al. (2007). Strong association of de novo copy number mutations with autism. Science 316(5823): 445–449.
Sharp, A. J., Mefford, H. C., Li, K., Baker, C., Skinner, C., Stevenson, R. E., Schroer, R. J., Novara, F., De Gregori, M., Ciccone, R., et al. (2008). A recurrent 15q13.3 microdeletion syndrome associated with mental retardation and seizures. Nature Genetics 40(3): 322–328.
Shi, J., Levinson, D. F., Duan, J., Sanders, A. R., Zheng, Y., Pe’er, I., Dudbridge, F., Holmans, P. A., Whittemore, A. S., Mowry, B. J., et al. (2009). Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 460(7256): 753–757.
Shi, Y. (2007). Histone lysine demethylases: Emerging roles in development, physiology and disease. Nature Reviews Genetics 8(11): 829–833.
Shiina, T., Inoko, H., & Kulski, J. K. (2004). An update of the HLA genomic region, locus information and disease associations: 2004. Tissue Antigens 64(6): 631–649.
Sipos, A., Rasmussen, F., Harrison, G., Tynelius, P., Lewis, G., Leon, D. A., & Gunnell, D. (2004). Paternal age and schizophrenia: A population based cohort study. British Medical Journal 329(7474): 1070.
Sklar, P., Smoller, J. W., Fan, J., Ferreira, M. A., Perlis, R. H., Chambert, K., Nimgaonkar, V. L., McQueen, M. B., Faraone, S. V., Kirby, A., et al. (2008). Whole-genome association study of bipolar disorder. Molecular Psychiatry 13(6): 558–569.
Spitzer, R. L., Endicott, J., & Robins, E. (1978). Research diagnostic criteria: Rationale and reliability. Archives of General Psychiatry 35(6): 773–782.
Sporns, O., Tononi, G., & Kotter, R. (2005). The human connectome: A structural description of the human brain. PLoS Computational Biology 1(4): e42.
St Clair, D., Xu, M., Wang, P., Yu, Y., Fang, Y., Zhang, F., Zheng, X., Gu, N., Feng, G., Sham, P., et al. (2005). Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. Journal of the American Medical Association 294(5): 557–562.
Stahlberg, O., Soderstrom, H., Rastam, M., & Gillberg, C. (2004). Bipolar disorder, schizophrenia, and other psychotic disorders in adults with childhood onset AD/HD and/or autism spectrum disorders. Journal of Neural Transmission 111(7): 891–902.
Stefansson, H., Ophoff, R. A., Steinberg, S., Andreassen, O. A., Cichon, S., Rujescu, D., Werge, T., Pietilainen, O. P., Mors, O., Mortensen, P. B., et al. (2009). Common variants conferring risk of schizophrenia. Nature 460(7256): 744–747.
Stefansson, H., Sigurdsson, E., Steinthorsdottir, V., Bjornsdottir, S., Sigmundsson, T., Ghosh, S., Brynjolfsson, J., Gunnarsdottir, S., Ivarsson, O., Chou, T. T., et al. (2002). Neuregulin 1 and susceptibility to schizophrenia. American Journal of Human Genetics 71(4): 877–892.
Suarez, B. K., Duan, J., Sanders, A. R., Hinrichs, A. L., Jin, C. H., Hou, C., Buccola, N. G., Hale, N., Weilbaecher, A. N., Nertney, D. A., et al. (2006). Genomewide linkage scan of 409 European-ancestry and African American families with schizophrenia: Suggestive evidence of linkage at 8p23.3–p21.2 and 11p13.1–q14.1 in the combined sample. American Journal of Human Genetics 78(2): 315–333.
Sullivan, P. F., Kendler, K. S., & Neale, M. C. (2003). Schizophrenia as a complex trait: Evidence from a meta-analysis of twin studies. Archives of General Psychiatry 60(12): 1187–1192.
Sullivan, P. F., Lin, D., Tzeng, J. Y., van den Oord, E., Perkins, D., Stroup, T. S., Wagner, M., Lee, S., Wright, F. A., Zou, F., et al. (2008). Genomewide association for schizophrenia in the CATIE study: Results of stage 1. Molecular Psychiatry 13(6): 570–584.
Sun, J., Jia, P., Fanous, A. H., Webb, B. T., van den Oord, E. J., Chen, X., Bukszar, J., Kendler, K. S., & Zhao, Z. (2009). A multi-dimensional evidence-based candidate gene prioritization approach for complex diseases-schizophrenia as a case. Bioinformatics 25(19): 2595–2602.
Susser, E. S. & Lin, S. P. (1992). Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944–1945. Archives of General Psychiatry 49(12): 983–988.
Svensson, A. C., Lichtenstein, P., Sandin, S., & Hultman, C. M. (2007). Fertility of first-degree relatives of patients with schizophrenia: A three generation perspective. Schizophrenia Research 91(1–3): 238–245.
Thomas, G., Jacobs, K. B., Yeager, M., Kraft, P., Wacholder, S., Orr, N., Yu, K., Chatterjee, N., Welch, R., Hutchinson, A., et al. (2008). Multiple loci identified in a genome-wide association study of prostate cancer. Nature Genetics 40(3): 310–315.
Torrey, E. F., Buka, S., Cannon, T. D., Goldstein, J. M., Seidman, L. J., Liu, T., Hadley, T., Rosso, I. M., Bearden, C., & Yolken, R. H. (2009). Paternal age as a risk factor for schizophrenia: How important is it? Schizophrenia Research 114(1–3): 1–5.
Traherne, J. A. (2008). Human MHC architecture and evolution: Implications for disease association studies. International Journal of Immunogenetics 35(3): 179–192.
Valles, V., van Os, J., Guillamat, R., Gutierrez, B., Campillo, M., Gento, P., & Fananas, L. (2000). Increased morbid risk for schizophrenia in families of in-patients with bipolar illness. Schizophrenia Research 42(2): 83–90.
van Os, J. & Kapur, S. (2009). Schizophrenia. Lancet 374(9690): 635–645.
Van Snellenberg, J. X. & de Candia, T. (2009). Meta-analytic evidence for familial coaggregation of schizophrenia and bipolar disorder. Archives of General Psychiatry 66(7): 748-755.
Veeriah, S., Taylor, B. S., Meng, S., Fang, F., Yilmaz, E., Vivanco, I., Janakiraman, M., Schultz, N., Hanrahan, A. J., Pao, W., et al. (2010). Somatic mutations of the Parkinson’s disease-associated gene PARK2 in glioblastoma and other human malignancies. Nature Genetics 42(1): 77–82.
Viken, M. K., Blomhoff, A., Olsson, M., Akselsen, H. E., Pociot, F., Nerup, J., Kockum, I., Cambon-Thomsen, A., Thorsby, E., Undlien, D. E., et al. (2009). Reproducible association with type 1 diabetes in the extended class I region of the major histocompatibility complex. Genes and Immunity 10(4): 323–333.
Wang, H., Feng, R., Phillip Wang, L., Li, F., Cao, X., & Tsien, J. Z. (2008). CaMKII activation state underlies synaptic labile phase of LTP and short-term memory formation. Current Biology 18(20): 1546–1554.
Wassink, T. H., Piven, J., Vieland, V. J., Jenkins, L., Frantz, R., Bartlett, C. W., Goedken, R., Childress, D., Spence, M. A., Smith, M., et al. (2005). Evaluation of the chromosome 2q37.3 gene CENTG2 as an autism susceptibility gene. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 136B(1): 36–44.
Weedon, M. N., Lango, H., Lindgren, C. M., Wallace, C., Evans, D. M., Mangino, M., Freathy, R. M., Perry, J. R., Stevens, S., Hall, A. S., et al. (2008). Genome-wide association analysis identifies 20 loci that influence adult height. Nature Genetics 40(5): 575–583.
Wei, J. & Hemmings, G. P. (2000). The NOTCH4 locus is associated with susceptibility to schizophrenia. Nature Genetics 25(4): 376–377.
Weiss, L. A., Shen, Y., Korn, J. M., Arking, D. E., Miller, D. T., Fossdal, R., Saemundsen, E., Stefansson, H., Ferreira, M. A., Green, T., et al. (2008). Association between microdeletion and microduplication at 16p11.2 and autism. New England Journal of Medicine 358(7): 667–675.
Worden, F. G., Childs, B., Matthysse, S., & Gershon, E. S. (1976). Frontiers of psychiatric genetics. Neuroscience Research Program Bulletin 14(1): 8–86.
Wray, N. R., Goddard, M. E., & Visscher, P. M. (2007). Prediction of individual genetic risk to disease from genome-wide association studies. Genome Research 17(10): 1520–1528.
WTCCC. (2007). Wellcome Trust Case Control Consortium: Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447(7145): 661–678.
Yang, J., Visscher, P. M., & Wray, N. R. (2009). Sporadic cases are the norm for complex disease. European Journal of Human Genetics 18: 1039–1043.
Zeggini, E., Scott, L. J., Saxena, R., Voight, B. F., Marchini, J. L., Hu, T., de Bakker, P. I., Abecasis, G. R., Almgren, P., Andersen, G., et al. (2008). Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nature Genetics 40(5): 638–645.
Zweier, C., de Jong, E. K., Zweier, M., Orrico, A., Ousager, L. B., Collins, A. L., Bijlsma, E. K., Oortveld, M. A., Ekici, A. B., Reis, A., et al. (2009). CNTNAP2 and NRXN1 are mutated in autosomal-recessive Pitt-Hopkins-like mental retardation and determine the level of a common synaptic protein in drosophila. American Journal of Human Genetics 85(5): 655–666.