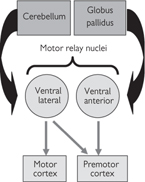
Fig. 11.47 Principal thalamic motor relay nuclei and their connections.
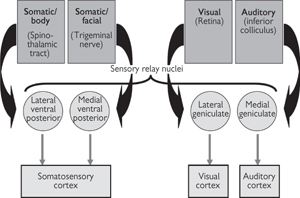
Fig. 11.48 Principal thalamic sensory relay nuclei and their connections.
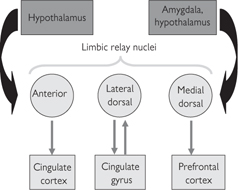
Fig. 11.49 Principal thalamic limbic relay nuclei and their connections.
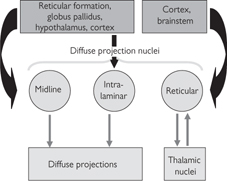
Fig. 11.50 Thalamic diffuse projection nuclei and their connections.
• In the waking state, thalamic neurones that project to the cortex (TC neurones) are tonically depolarized by cholinergic, aminergic (noradrenergic, serotonergic, and histaminergic), and peptidergic (orexins) inputs from the brainstem and hypothalamus. This allows TC neurones to respond faithfully to incoming sensory and motor signals and, thus, ensures accurate transfer of this information to higher cortical areas
• During non-rapid eye movement (NREM) sleep (slow-wave sleep (SWS)  p.769, Fig. 11.61), these brainstem and hypothalamic inputs diminish and TC cells first hyperpolarize, then switch to a pattern of slow membrane potential oscillations driven by intrinsic voltage-dependent ionic conductances in their neuronal membrane
p.769, Fig. 11.61), these brainstem and hypothalamic inputs diminish and TC cells first hyperpolarize, then switch to a pattern of slow membrane potential oscillations driven by intrinsic voltage-dependent ionic conductances in their neuronal membrane
• Under these conditions, processing of externally sourced sensory and motor information is depressed. Thus, cortical processing is regulated by intrinsic excitation within the cortex and, possibly to some extent, waves of activity known as ‘spindle’ waves (because of their relatively high frequency) generated in the thalamus as a result of disinhibition of inhibitory GABA neurones in the thalamic reticular nucleus
• During REM sleep, it is thought that an internal representation of the external world becomes the input to the thalamo-cortical circuitry. This change in thalamic and cortical activity is thought to be important in the iteration of information and essential for processes such as learning and memory.
• The hypothalamus (Fig. 11.51), is also located in the diencephalon. Observed in a frontal section, three divisions—lateral, medial, and periventricular (immediately bordering the third ventricle)—can be identified. In medial section it can be divided into anterior, middle, and posterior areas along the rostral–caudal axis
• The medial forebrain bundle acts as a pathway from the hypothalamus to the neocortex and other parts of the brain. However, it also carries fibres that are not of hypothalamic origin, such as aminergic fibres from brainstem nuclei
• The lateral region sends both long-distance connections to the cortex and spinal cord and shorter axons to ascending and descending pathways
• Defined nuclei in the medial region of the hypothalamus include:
 The preoptic and suprachiasmatic (SCN) nuclei in the anterior region (sleep and circadian regulation)
The preoptic and suprachiasmatic (SCN) nuclei in the anterior region (sleep and circadian regulation)
 The dorsomedial, ventromedial, and paraventricular (PVN) nuclei in the middle region
The dorsomedial, ventromedial, and paraventricular (PVN) nuclei in the middle region
 The posterior nucleus and mammillary bodies in the posterior region
The posterior nucleus and mammillary bodies in the posterior region
• Most nuclei have bidirectional fibre systems, sending and receiving signals via the medial forebrain bundle, mamillotegmental tract, and dorsal longitudinal fasciculus. There are two exceptions—supraoptic and paraventricular neurones project, via the hypothalamohypophyseal tract, to the posterior pituitary ( see p.737), while the SCN receives a unidirectional input from the retina (
see p.737), while the SCN receives a unidirectional input from the retina ( see p.738 and p.750).
see p.738 and p.750).
• The hypothalamus has a major role in maintaining homeostasis through its regulation of the autonomic nervous system, the endocrine system, and visceral function. These functions are carried out through both conventional, direct, synaptic contacts and indirect neurohormonal pathways that involve an intimate regulatory influence over the function of the pituitary
• By receiving information from, and acting directly on, the internal environment, the hypothalamus regulates body functions including temperature, heart rate, blood pressure, blood osmolarity, and water and food intake. In addition, extensive connections throughout the CNS provide routes for both direct and processed sensory information to modulate hypothalamic activity and influence behavioural processes
• Direct connections between the limbic system ( p.744) and the hypothalamus regulate autonomic and visceral responses associated with motivation and adaptive emotional behaviour. The limbic system also exerts control over the endocrine system through its regulation of the secretion of hypothalamic hormones.
p.744) and the hypothalamus regulate autonomic and visceral responses associated with motivation and adaptive emotional behaviour. The limbic system also exerts control over the endocrine system through its regulation of the secretion of hypothalamic hormones.
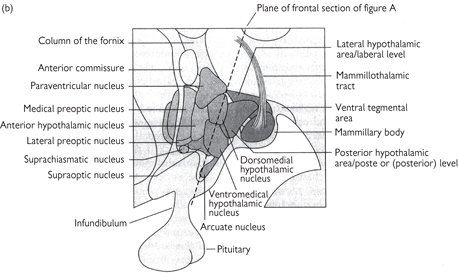
Fig. 11.51 The structure of the hypothalamus. (a) Frontal view of the hypothalamus (section along the plane shown in part b). (b) A medial view shows most of the main nuclei. The hypothalamus is often divided analytically into three areas in a rostrocaudal direction: the preoptic area, the tuberal level, and the posterior level.
Reproduced with permission from Kandel E, Schwartz JH, Jessell TM (2000). Principles of Neural Science, 4th edn. © The McGraw-Hill Companies Inc.
• The influence of the cortex on the hypothalamic expression of emotional behaviour is through a pathway involving the cingulate gyrus and hippocampus, while a reciprocal influence of the hypothalamus exists via the mammillary bodies, the anterior nucleus of the thalamus, and the cingulate gyrus
• Another component of the limbic system, the amygdala, also has direct projections to the hypothalamus and this region has been implicated in learning, particularly tasks that might involve associating stimuli with an emotional response.
• The hypothalamus exerts a major influence over the pituitary gland thereby regulating metabolism, feeding, water homeostasis, and the circadian timing of physiology and behaviour
• Connected to the hypothalamus by the infundibulum, the anterior pituitary is controlled by substances secreted primarily from small, peptide-releasing neurones found in the basal part of the middle region of the hypothalamus
• Parvocellular (small) neurones located primarily in the PVN, as well as the periventricular region and the preoptic nucleus, release peptides into the local plexus of blood vessels that drain into the vasculature of the anterior pituitary. These hormones then act to promote or inhibit the production of anterior pituitary hormones. For example, CRH promotes the release of ACTH from the anterior pituitary and this induces cortisol release by the adrenal cortex—an important mediator of the stress response. Other hypothalamic hormones that act on the pituitary in this way include TRH, leading to thyrotropin production and regulation of growth and metabolism, GnRH, stimulating FSH and LH release and, thus, controlling gametogenesis; GHRH; somatostatin; and dopamine
• In addition to this indirect pathway, magnocellular (large) neurones located in the PVN and supraoptic nucleus project to the posterior pituitary and directly release hormones into the circulation. In this way, water homeostasis is maintained by releasing the peptide, ADH (vasopressin), while the release of another peptide, oxytocin, controls uterine contraction and milk ejection
• The temporal organization of hormonal release and behavioural patterns is dictated by the SCN. This nucleus generates an endogenous circadian pattern that entrains many physiological processes to the light–dark cycle. Unidirectional input to the SCN from the visual system synchronizes the circadian clock to day length. The SCN itself has minimal projections, mainly to other parts of the hypothalamus. Thus, temporal synchronization of biological processes involves intra-hypothalamic interactions and output from other hypothalamic nuclei.
• Direct connections from the PVN to sympathetic pre-ganglionic neurones in the intermediolateral cell columns of the thoracic and lumbar spinal cord as well as projections to parasympathetic nuclei in the brainstem indicate direct neuronal control of the autonomic motor nervous system. This control is regulated by the sensitivity of the hypothalamus to stimuli in the blood such as glucose and insulin
• Visceral sensory information from the major organs is directed to the paraventricular and lateral hypothalamic nuclei via brainstem sensory nuclei, including the nucleus of the solitary tract
• Hypothalamic involvement in sleep appears to involve at least three nuclei. Histamine-releasing neurones of the tuberomammillary nuclei that project to the cortex are inhibited during sleep by ventrolateral preoptic neurones, thereby reducing histamine release, a neurotransmitter implicated in arousal. Other hypothalamic nuclei are involved in wider serotonergic and noradrenergic pathways that regulate arousal. In addition, the circadian timekeeping associated with the SCN is also implicated in the timing and modulation of sleep patterns
Sexual dimorphisms in the hypothalamus of animals implicate a role in sexual behaviour as well as gametogenesis. Although well established in animals, evidence for similar dimorphisms in the human hypothalamus is less secure. Nevertheless, structural differences have been identified between the male and female hypothalamus, particularly in the preoptic nucleus.
• The basal ganglia at the base of the brain receive nervous input from both the sensory and motor cortices. Nervous outflow from the basal ganglia is exclusively to the areas involved in higher processing of movement, namely the prefrontal, premotor, and supplementary motor cortices; there is no output to the spinal cord. The primary role of basal ganglia is thought to be related to the planning and control of complex motor behaviour by selectively activating some movements and suppressing others. These functions are highlighted in patients with Parkinson’s disease, where the cells of the basal ganglia degenerate and motor control is compromised ( p.775; OHCM8 p.498)
p.775; OHCM8 p.498)
• The cerebellum acts in concert with the motor cortex and basal ganglia to coordinate movement. In simple terms, the cerebellum can be seen to control fine motor tasks by constantly comparing ‘intention’ with ‘performance’. It receives input from a vast array of sensory nerves that allow it to compute the actual position of limbs and compare this information to the intended position. The net result is an error calculation, which is translated into an output signal via the Purkinje fibres to make fine adjustments to correct the error
• The role of the cerebellum in modulating motor output from the pyramidal and extrapyramidal systems, rather than instigating movement itself, is highlighted in patients with damage to the cerebellum. These individuals are not paralysed and still receive sensory information as normal, but they are unable to coordinate movements in complex or even quite simple tasks requiring motor control and timing. Lesions to the cerebellum give rise to a characteristic set of clinical signs ( OHCM8 p.503). It is now recognized that the cerebellum is also important in some aspects of speech recognition and learned responses, but not memory.
OHCM8 p.503). It is now recognized that the cerebellum is also important in some aspects of speech recognition and learned responses, but not memory.
• Walking may seem an automatic and straightforward task, but from a neuromuscular standpoint, it is highly complex and involves motor control of the majority of our skeletal muscles
• The key to the neuronal control of locomotion is so-called ‘pattern generation’, where a set of commands are relayed to all the relevant muscles so that they contract and relax in the correct order. On completion of one cycle of the task, the same set of commands is repeated so that a pattern of movement is instigated. The neural circuit must also include suitable facilities to modify the pattern to take into account any changes in the environment and to stop the pattern of movement.
• Neural control of locomotion stems from the spinal cord—it does not necessarily require instructions from the brain but does require sensory information from the limbs that are involved. Locomotion can be best described as a series of reflexes in response to incoming sensory information (e.g. from the Golgi tendon organs and the muscle spindles) about the position of the limb in motion
• Much of the experimental work relating to pattern generation has been conducted in primitive vertebrates, where neural circuits can be more easily identified and characterized. These studies clearly show that although basic pattern generation originates in the spinal cord in response to sensory information, higher centres in the brain, including the cerebellum, basal ganglia, sensory and motor cortices, are necessary to accommodate variation in locomotion and adaptability in response to the environment (e.g. response to a trip) (Fig. 11.52)
• The cerebellum sends modulating signals to motor neurones, via the descending pathways, to fine-tune the movements of the crude, ungainly gait that originates from pattern generators in the spinal cord. It also modulates activity to adjust the walking pattern to accommodate changes in the terrain (e.g. going up or down stairs)
• The basal ganglia are required to initiate movement and to help compute visual and sensory information from the parietal cortex, and generate appropriate spatially directed movement towards a given goal
• The parietal cortex collects visual and somatosensory information and collates it into a three-dimensional representation of position with respect to environment.
Fig. 11.52 Pattern generation for locomotion originates in the spinal cord; modulation is controlled by the cerebellum.
The somatosensory system comprises touch, nociception (pain), temperature, and proprioception ( pp.252, 748). Each relies on different types of receptor, pathway (Fig. 11.53), and processing.
pp.252, 748). Each relies on different types of receptor, pathway (Fig. 11.53), and processing.
• Touch is mediated by mechanoreceptors. In non-glabrous skin, there are four types of mechanoreceptor
• Pacinian corpuscles are found deep in the dermis, thus having large receptive fields, and are rapidly adapting
• Meissner’s corpuscles are also rapidly adapting, but are superficial and thus have small receptive fields
• Merkel’s discs are superficial, slowly adapting receptors
• Ruffini receptors are deep, slowly adapting receptors. No conscious sensation is associated with direct stimulation of Ruffini receptors and a role has been postulated for them in proprioception
• In glabrous skin, there are three types of follicle receptor (hair-guard, hair-tylotrich and hair-down receptors) and the same deep receptors as in non-glabrous skin
• Temperature is sensed by two types of thermoreceptor, one for heat (responding in the range of 30–45°C) and one for cold (responding in the range of 1–20°C). Cold receptors are also activated by extreme heat (at over 45°C), resulting in the sensation of ‘paradoxical cold’. Thermal sensitivity is punctate: hot and cold thermoreceptors are grouped in non-overlapping heat- or cold-sensitive zones. Heat nociceptors also respond to extreme heat
• Pain is mediated by nociceptors, which can be mechanical, thermal, or polymodal (mechanical nociceptors that can be activated or sensitized by inflammatory mediators and other indicators of tissue damage such as bradykinin, K+ ions, serotonin, prostaglandin, and histamine).
Transduction by mechanoreceptors relies on a simple mechanical deformation of the nerve ending, which opens mechanically gated cation channels, ion flow through which triggers an action potential that is transmitted along the nerve axon. Rapidly adapting mechanoreceptors have a large capsule around the nerve ending, formed by multiple foldings of a Schwann cell. These act to absorb a constant pressure, so that rapidly adapting mechanoreceptors are only activated by changes in pressure. This can be demonstrated by stripping the capsule off the nerve ending, which no longer adapts to constant pressure. Slowly adapting mechanoreceptors have a much smaller capsule around the nerve ending, so they respond with a more constant rate of firing to the absolute level of pressure. Nociceptors are all free nerve endings.
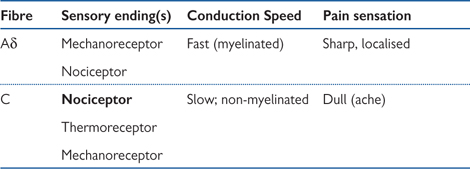
Fibre types are different for different modalities. All mechanoreceptors are carried by fast myelinated Aβ (type II) fibres. Mechanical nociceptors are carried by thinner, slower, myelinated Aδ (type III) fibres. Polymodal nociceptors are carried by thin, unmyelinated C (type IV) fibres, which are the slowest of all. Thus sharp, acute pain, mediated by mechanical nociceptors, is felt faster than dull inflammatory pain, which is mediated by polymodal nociceptors. The sensation of cold from thermal receptors is carried by Aδ fibres, while that of heat is carried by slower C fibres.
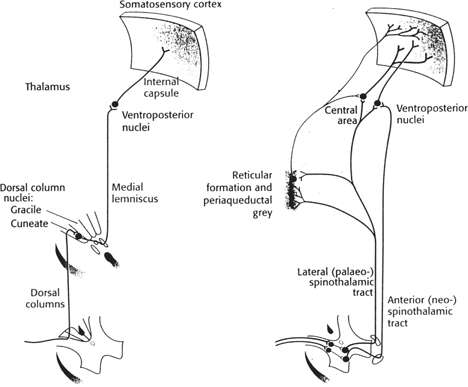
Fig. 11.53 The main ascending somatosensory pathways: (left) the lemniscal system; (right) the neo-spinothalamic and paleo-spinothalamic divisions of the anterolateral system.
Reproduced with permission from Carpenter R (2003). Neurophysiology, 4th edn, Edward Arnold.
• All sensory fibres enter the spinal cord through the dorsal roots, with their cell bodies lying just before the dorsal root in the dorsal root ganglia. Axons from mechanoreceptors and proprioceptors ascend the spinal cord in the dorsal columns. These carry fibres from the ipsilateral side of the body and continue to the dorsal column nuclei at the level of the medulla
• The dorsal columns are divided into the gracile and cuneate fascicles, which contain fibres representing the lower limb and trunk, and the upper limb and neck, respectively. The fascicles are divided by the dorsal intermediate septum. Similarly, the dorsal column nuclei are divided into the gracile and cuneate nuclei. At this level, the sensory axons synapse onto internal arcuate fibres, which decussate and continue to ascend on the contralateral side in the medial lemniscus. Fibres from the sensory trigeminal nucleus, representing touch sensation from the face and head, ascend from this level in the neighbouring trigeminal lemniscus. The medial lemniscus fibres synapse in the ventral posterior lateral nucleus of the thalamus, and trigeminal lemniscus fibres synapse in the ventral posterior medial nucleus, whence all tertiary fibres proceed to the primary somatosensory cortex on the postcentral gyrus. Through all levels of the pathways, fibres remain somatotopically arranged
• Nociception and temperature sensation are carried by the anterolateral system. Axons entering the spinal cord through the dorsal horns may ascend or descend one or two spinal segments in the tract of Lissauer, before synapsing onto secondary fibres and interneurones in the dorsal horn laminae. Aδ fibres synapse in laminae I (marginal zone) and V (part of the nucleus proprius), while C fibres synapse in lamina II (substantia gelatinosa). Referred pain is thought to be a result of cutaneous and visceral nociceptive afferents converging on the same secondary fibres through interneurones in the dorsal horn laminae
• The secondary fibres decussate at the same spinal segment as they arise, and ascend in one of three tracts that form the anterolateral system on the contralateral side
• The spinothalamic tract is the largest tract, arising from dorsal horn laminae I and V–VII (the nucleus proprius and Clarke’s nucleus), and ascends to the central lateral and the ventral posterior lateral nuclei of the thalamus
• The spinoreticular tract arises from laminae VII (Clarke’s nucleus) and VIII. Some fibres terminate in the reticular formation of the pons and medulla, while others continue to the central lateral nucleus of the thalamus. Fibres that terminate in the thalamus, from both spino-thalamic and spinoreticular tracts, synapse onto fibres that project to primary somatosensory cortex
• Fibres in the spinomesencephalic tract arise from laminae I and V, and project to the periaqueductal grey and the mesencephalic reticular formation. These projections provide inputs to the limbic system via the hypothalamus
Pain is a sensory function that deserves special attention because of the dramatic impact it can have on well-being, both in response to injury (acute) and chronic illness. Nociception is the term given to the perception of a noxious stimulus, but it is not necessarily painful: pain is much more subjective and is influenced by aspects other than the strength of the stimulus (e.g. emotion).
• The sensory endings that trigger pain sensation fall into the same categories as those described previously, but they have a higher threshold for activity, so are only stimulated by stimuli of sufficient (noxious) intensity to potentially cause damage
• Stimulation of the receptors triggers action potentials along afferent fibres to the dorsal horn of the spinal cord. The nerve type and speed of conduction is dependent on the type of receptor involved (Table 11.2)
Table 11.2 Nerve fibres transmitting pain sensations
• C fibres release neuropeptides (calcitonin gene-related peptide (CGRP) and substance P at both the peripheral and central nerve endings; the important role played by these peptides in pain has attracted attention as possible new targets for drugs to reduce pain
• Impulses from pain sensors are transmitted up the spinal cord via transmission neurones (ascending pathway) to the thalamus and on to the sensory cortex, resulting in pain sensation.
• As well as being subject to local interneurone modulation, nociception pathways can also be modulated by distal inhibition from the periaqueductal grey matter in the midbrain
• Noradrenergic and serotonergic efferent fibres from the raphe nucleus, driven by neurones from the periaqueductal grey matter, descend through the dorsolateral funiculus to inhibit nociceptive afferent fibres via enkephalinergic interneurones in laminae I and II of the dorsal horn.
• In another mechanism of pain modulation, called dorsal horn gating, Aβ afferent fibres inhibit secondary nociceptive afferents arising in lamina V via inhibitory interneurones.
Diseases that cause damage to neurones in the sensory pathway (e.g. stroke, multiple sclerosis, diabetes, shingles) are often associated with chronic and debilitating pain, most likely mediated via hyperactivity of damaged nerves
Box 11.1 summarizes current available therapies for management of pain.
Reflex movements are involuntary; they do not require a command from the brain to happen. Voluntary movements are entirely dependent on a signal from the motor cortex to be implemented. The reflex arc describes an integrated response to a stimulus that involves both somatosensory and motor neurones.
• Reflex responses are movements that are made very rapidly (~20ms; 0.02s) in response to a stimulus that is sensed by specific sensory receptors. These tend to be defensive reflexes that must be made quickly in order to avoid injury (e.g. hand withdrawal when placed on a hot surface)
• The speed of the response is facilitated by limiting the number of neurones (and hence synapses) involved. The fastest responses are mediated by monosynaptic reflexes (Fig. 11.54), whereby there is only one synapse, located in the spinal cord, between the sensory neurone and the motor neurone (e.g. the knee-jerk reflex)
• Polysynaptic reflex arcs include interneurones in the spinal cord (Fig. 11.55), which connect the sensory neurone to the motor neurone. These reflexes are marginally slower than monosynaptic reflexes but are still considerably faster than conscious movements that require input from the brain. The role of the interneurone is often to modulate the signal to the motor neurone; an excitatory signal from a sensory neurone can stimulate an inhibitory interneurone, resulting in inhibition of the motor neurone (e.g. Golgi tendon organs)
• Far more complex reflexes are constantly called upon to react to assimilated information from sensory organs such as the eyes and balance centre of the ear, just to stay upright.
Fig. 11.54 Monosynaptic reflex arc.
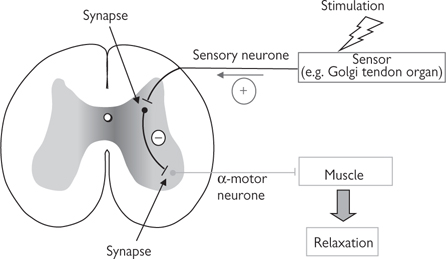
Fig. 11.55 Polysynaptic reflex. An interposed inhibitory interneurone results in reduced stimulation of the α-motor neurone, leading to muscle relaxation.
• The retina is composed of five layers (starting nearest the lens):
• The ganglion cell layer contains the cell bodies of the ganglion cells
• The inner plexiform layer contains the dendrites of amacrine, bipolar, and ganglion cells, and synapses between them
• The inner nuclear layer contains bipolar cell axons and the cell bodies of amacrine, bipolar, and horizontal cells
• The outer plexiform layer contains the dendrites of horizontal cells and synapses between rods, cones, horizontal cells, and bipolar cells
• The outer nuclear layer contains the rods and cones
• Cells in the retina are unmyelinated, in order to reduce visual distortion as light passes to the photoreceptors at the back of the retina. However, at the centre of the retina, in the fovea, maximal acuity is achieved by displacing to the side all but the photoreceptors themselves. Behind the retina, adjacent to the outer nuclear layer, is the pigment epithelium.
• The photoreceptive cells of the retina are the rods and cones. These cells contain photosensitive pigment made up of a light-absorbing molecule called retinal, bound to a membrane protein called opsin. Each cell type, rods, and the three types of cone, contains a different visual pigment, made up of retinal and a different opsin molecule, each of which has a different peak light absorption energy
• Colour vision relies on the three types of cone which are maximally excited by light with short (blue: 437nm), middle (green: 533nm), and long (red: 564nm) wavelengths. In contrast, the rod system is achromatic, since there is only one visual pigment, rhodopsin, with a peak absorbency at 498nm
• Rods and cones have an inner and an outer segment. The inner segment is where the nucleus, mitochondria, and other cellular apparatus are found. The outer segment comprises a comb-shaped series of folds of the cell’s membrane, which, in rods, often seal off to form free-floating discs within the rod. These folds provide a large surface area over which to collect photons with the photosensitive transmembrane proteins. The outer segments of rods are larger than those of cones, making the rods much more sensitive than cones in dim light.
• Transduction occurs via a cascade of events triggered by the absorption of a photon by pigment molecule. In rods the retinal usually exists as the 11-cis isomer. Excitation of the rhodopsin by a photon causes a series of conformational changes in the retinal, ending ultimately in the all-trans state. Rhodopsin is unable to bind all-trans retinal, so the retinal becomes detached. A semi-stable intermediate configuration called metarhodopsin II activates transducin, a G-protein, which in turn activates phosphodiesterase to convert cyclic GMP (cGMP) to 5′GMP. The lowered cGMP concentration decreases Na+ influx through a cGMP-gated channel. Thus in the dark, cGMP concentrations are raised, and so there is a constant Na+ current, known as the dark current. The resting membrane potential is therefore relatively depolarized, at around 40mV, and light causes a hyperpolarization to around 70mV. A similar cascade operates in cones
• Detached retinal is recycled in the pigment epithelium by converting the all-trans retinal to all-trans retinol (vitamin A). All-trans retinol is the precursor for 11-cis retinal and is not synthesized in the body. Dietary vitamin A deficiency can therefore cause night-blindness or, if severe, even total blindness
• The dynamic range of photosensitivity of cones is maintained through light adaptation. The transduction cascade also inactivates a cGMP-gated Ca2+ channel: Ca2+ inhibits guanylate cyclase (GTP→cGMP), thus a negative feedback loop operates to maintain a fairly constant cGMP concentration through a range of absolute levels of brightness, and so keep the cell maximally sensitive to changes in light level.
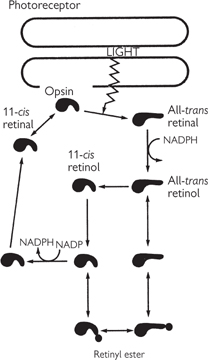
Fig. 11.56 Pigment epithelium. The cyclical sequence by which light leads to isomerization of retinal and its dissociation from opsin, followed by the relatively slow processes that lead to the final regeneration of rhodopsin.
Reproduced with permission from Carpenter R (2003). Neurophysiology, 4th edn, Edward Arnold.
• Cones synapse onto bipolar cells and horizontal cells. Bipolar cells synapse onto ganglion cells, which are the output cells of the retina. Horizontal cells and amacrine cells are interneurones that affect the information passed between photoreceptors and bipolar cells, and between bipolar cells and ganglion cells, respectively. Horizontal cells are inhibitory and synapse back onto the same cones that supply them. However, neighbouring horizontal cells are also all connected to each other through electrical synapses, forming one continuous sheet along which cone inputs are conducted electrotonically. Each cone is therefore inhibited by the activity of a number of neighbouring cones, producing the centre-surround antagonism that characterizes the receptive fields of cells in the very early stages of the visual system
• Cones release glutamate, which has one of two effects, depending on the type of bipolar cell
• In off-centre bipolar cells, glutamate opens Na+ channels, which causes depolarization
• In on-centre bipolar cells, glutamate closes Na+ channels and opens K+ channels, causing hyperpolarization
• Bipolar cells signal using electrotonic conduction, so there is a continuous, graded membrane potential rather than an all-or-nothing action potential. The ganglion cells onto which bipolar cells synapse are the first cells in the visual system to fire action potentials
• In photopic vision, rods synapse via gap junctions onto nearby cones. However, they are sufficiently sensitive that normal levels of brightness bleach them of visual pigment and their input is negligible
• For scotopic vision, there is a process called dark adaptation, during which the rod pathways change. The gap junctions onto nearby cones close and, instead, rods synapse onto rod bipolar cells, which in turn synapse onto AII amacrine cells. AII amacrine cells synapse both onto off-centre ganglion cells and on-centre bipolar cells, which synapse onto on-centre ganglion cells
• As discussed, colour vision is mediated by the cone system. The principle of univariance means that it is the ratio of activities between the three cone types that carries colour information. A single cone type (or rod) cannot alone convey colour information
• Colour blindness is a result of a defect in or absence of one or more cone pigments. Defects of a visual pigment are classified as protanomaly, deuteranomaly, or tritanomaly, whereas an absence of a pigment is classified as protanopia, deuteranopia, or tritanopia (with prot-, deuter-, and trit- denoting the long-, middle-, and short-wavelength pigments). An absence of two cone pigments is classified as atypical monochromatopsia, while an absence of all cone pigments is classified as typical monochromatopsia. All these are congenital defects of colour vision. However acquired, retinal disease can also damage colour vision. Tetrachromatopsia is sometimes found in women, who have an extra visual pigment
• The output of the retina is via the ganglion cells, which have circular, centre-surround antagonistic receptive fields. They can be either on-centre, off-surround, meaning that they are excited by light falling on the receptive field centre and inhibited by light falling on their receptive field surround, or off-centre, on-surround
• Ganglion cell axons converge at the optic disc and leave the retina as the optic nerve. The ganglion cells are closer to the lens than the rods and cones, so where these axons pass through the retina at the optic disc, there are no photoreceptors. The resulting small scotoma is known as the blind-spot
• The optic nerves from each eye meet at the optic chiasm. Fibres arising from the nasal retinae from each eye cross, so that when the fibres continue as the optic tract, each tract carries information about left and right hemifields, rather than from left and right retinae
• The optic tract continues to the lateral geniculate nucleus (LGN), when the ganglion cell fibres synapse. The LGN is divided into six layers, with layer 1 being the most ventral and layer 6 the most dorsal
• Layers 1, 4, and 6 contain fibres arising from the contralateral retina, while layers 2, 3, and 5 contain ipsilateral fibres. The six layers of the LGN are also split into magnocellular (‘M’—layers 1 and 2) and parvocellular (‘P’—layers 3–6) streams. M cells have larger receptive fields and have good temporal resolution, but have poor spatial resolution and are monochromatic. P cells have smaller receptive fields, carry colour information, and have good spatial resolution but poor temporal. M cells are thought to be the substrate for processing of motion in the visual cortices, while P cells are thought to contribute to fine feature processing and colour vision
• The visual pathway continues from the LGN as the optic radiation, which passes back to the primary visual cortex at the occipital pole. Some fibres initially travel forward around the front of the temporal horn of the lateral ventricle. The loop that is formed is known as Meyer’s loop. The LGN also projects to the pretectum, which controls pupillary reflexes via the IIIrd (oculomotor) cranial nerve, and to the superior colliculus, which controls saccadic eye movements via the IIIrd, IVth (trochlear), and VIth (abducens) cranial nerves
• The primary visual cortex, also known as striate cortex or area V1, is located at the occipital pole continuing along the calcarine sulcus on the medial surface of each cerebral hemisphere. The visual field is represented contralaterally in strict retinotopic order, with the central visual field represented most posteriorly, and the upper half of the visual field on the inferior banks of the calcarine sulcus and the lower half on the superior banks.
• As in all neocortex, area V1 is divided into six layers. Inputs from the LGN arrive in layer 4, which is further subdivided into layers 4A, 4B, 4C, and 4D. Fibres from the magnocellular layers of the LGN arrive in layer 4C, and fibres from the parvocellular layers of the LGN arrive in layer 4C.
Fig. 11.57 The functional destinations of fibres in the optic nerve. LGN (lateral geniculate nucleus); HThal (hypothalamus); PT (prectum and other visual proprioceptive areas); SC (superior colliculus); Par, Temp (parietal and temporal cortex).
Reproduced with permission from Carpenter R (2003). Neurophysiology, 4th edn. Edward Arnold.
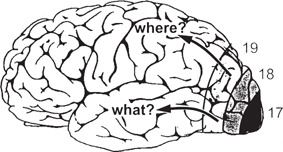
Fig. 11.58 Cerebral cortex, showing the general location of visual areas 17, 18, and 19.
Reproduced with permission from Carpenter R (2003). Neurophysiology, 4th edn. Edward Arnold.
• Receptive fields in area V1 have ‘on’ and ‘off’ bands running side-by-side, rather than the centre-surround structures of LGN cells. These cells are therefore selective for the orientation of visual features. Visual cortex is compartmentalized into columns of cells, through all six layers, that have similar receptive field structures. Neighbouring columns in area V1 will have selectivities for slightly different orientations and, thus, as one travels over a length of cortex there is a trend for a smooth rotation of orientation selectivity. Orientation columns are achromatic
• Primary visual cortex is the first stage in the visual pathway at which cells receive inputs from both eyes. Some cells have a preference for one eye over the other, and so area V1 is also compartmentalized by ocular dominance. Neighbouring columns will usually have a similar ocular dominance, and thus there is often a complete representation of all orientations within each region of a particular ocular dominance. A complete set of orientation columns for both left and right ocular dominance has been termed a hypercolumn
• Interspersed between orientation columns are columns in which all the cells are selective for particular wavelengths. These columns of colour-sensitive cells are known as blobs, after their appearance when visual cortex is stained for the enzyme cytochrome oxidase
• Another aspect of the processing of information from left and right eyes in area V1 is a sensitivity to retinal disparity—a slight mismatching of the relative positions of a visual feature on each retina. This difference in retinal position of the feature is a strong cue to the feature’s depth
• This early processing of visual information about feature orientation, colour, and depth is further refined in higher visual areas. Visual information from area V1 passes to the first extrastriate area, known as area V2, and thence to a multitude of other visual areas. These visual areas are broadly divisible into dorsal and ventral streams, deriving from the earlier magnocellular and parvocellular pathways
• The dorsal stream includes areas V5 and MST, where cells have strong selectivities for direction of motion and motion-in-depth, and has been labelled the ‘where’ pathway
• The ventral stream includes areas V4 and IT, where cells have strong selectivities for colour and depth contours, and has been labelled the ‘what’ pathway.
• A sound is a pressure wave transmitted through a medium, such as the air, consisting of alternate compression and rarefaction of the medium caused by the vibration of an object, such as vocal cords or the strings on a violin. The auditory system gathers information about the frequency composition and intensity of a sound and the direction of the source of the sound
• The auditory system is composed of the ear itself, in which the sound is transduced into neuronal impulses, and the central pathways in which the transduced signal is analysed
• The ear comprises three compartments:
• Outer ear—the pinna and external auditory meatus change the intensity of frequencies in the range of 2–7kHz (‘colouration’). This is important for zenith and front/back localization. It is separated from the middle ear by the tympanic membrane (ear drum)
• Middle ear—the ossicles (malleus, incus, and stapes—in order from outer to inner ear) are necessary for mechanical impedance matching between the tympanic membrane and the oval window, because the outer ear is filled with air, while the inner ear is filled with fluid. The tensor tympani and stapedius muscles alter the efficiency of impedance matching, and can thus protect the inner ear from extremely loud sounds. They are also active during speech. The middle ear is separated from the inner ear by the oval window, and joined to the nasopharynx by the eustachian tube
• Inner ear—the inner ear is divided into two portions: bony and membranous labyrinths. The sensory organs of the auditory system are in the auditory division of the membranous labyrinth (the cochlea). The cochlea consists of three scalae, in a spiral around the modiolus.
 The scala vestibuli begins at the oval window and joins the scala tympani at the helicotrema, at the head of the spiral, which runs back to another membrane between the middle and inner ears, just beside the oval window (the round window)
The scala vestibuli begins at the oval window and joins the scala tympani at the helicotrema, at the head of the spiral, which runs back to another membrane between the middle and inner ears, just beside the oval window (the round window)
 In between these runs the scala media, which is completely enclosed by Reissner’s membrane towards the scala vestibuli and by the basilar membrane towards the scala tympani. The scala media is filled with endolymph, which has a similar chemical constitution to intracellular fluid
In between these runs the scala media, which is completely enclosed by Reissner’s membrane towards the scala vestibuli and by the basilar membrane towards the scala tympani. The scala media is filled with endolymph, which has a similar chemical constitution to intracellular fluid
 The scalae vestibuli and tympani are filled with perilymph, which has a similar chemical constitution to CSF
The scalae vestibuli and tympani are filled with perilymph, which has a similar chemical constitution to CSF
• Transduction of sound waves into neuronal impulses occurs in the organ of Corti, which is found on the basilar membrane in the inner ear (Fig. 11.59). When the oval window is moved by the ossicles in the middle ear, a travelling wave is set off along the basilar membrane. The basilar membrane is thin and stiff at its base, at the oval window, and becomes increasingly wide and less stiff towards its apex, at the helicotrema. Thus, high-frequency travelling waves have a maximum amplitude near the oval window, while low-frequency travelling waves have a maximum amplitude near the helicotrema
• The organ of Corti contains rows of hair cells, which have stereocilia arranged either in linear rows (inner hairs cells) or in V or W formations (outer hair cells). There are three rows of outer hair cells and one row of inner hair cells along the length of the basilar membrane
• The stereocilia rest against the tectorial membrane, which lies above the organ of Corti. The tectorial membrane is stiff, so the stereocilia are bent sideways by a shearing force when the basilar membrane vibrates. The stereocilia on each hair cell are arranged in graded height order, with the tallest being thicker and known as the kinocilium. The tips of the stereocilia are linked, such that when the basilar membrane moves downwards, the stereocilia bend in one direction and the tip-links slacken, and when the basilar membrane moves upwards, the stereocilia bend the other way and the tip-links tighten
• Tightening of the tip-links opens stretch-activated non-selective cation channels, which allow K+ influx from the endolymph in the scala media. There is no chemical gradient, but there is an electrical gradient, resulting in an oscillatory membrane potential. At the basal end of the hair cells the oscillatory membrane potential causes oscillatory transmitter release.
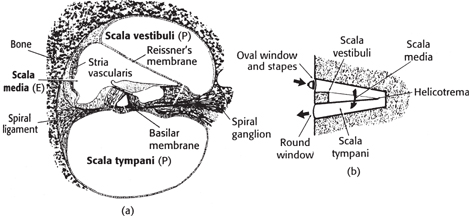

Fig. 11.59 (a) Section through the cochlea, showing the organ of Corti—shown in (c) in more detail; (b) a representation of the scala vestibuli, scala media, and scala tympani, and the path of sound through them. OHC, IHC (outer and inner hair cells); E (endolymph); P (perilymph).
Reproduced with permission from Carpenter R (2003). Neurophysiology, 4th edn, Edward Arnold.
• Innervation of the inner ear is by the VIIIth cranial (vestibulocochlear) nerve. It carries both afferent and efferent fibres
• ~90% of afferent fibres are from the inner hair cells, which synapse one-to-one. The rest innervate the outer hair cells, which are pooled ~20 outer hair cells per afferent fibre
• Efferent fibres are from the superior olive and innervate mostly the outer hair cells. They are thought to contribute to the sharpening of the frequency tuning along the basilar membrane
• Outer hair cells contribute to sharpening frequency tuning by changing the length of their stereocilia. Destruction of the outer hair cells (e.g. by drugs such as furosemide and gentamicin) can lead to hearing deficits
• Electrical resonance does not contribute to the sharpness of frequency tuning in mammals
• Afferent fibres in the auditory nerve have a characteristic frequency, determined by the hair cells they innervate. Other nearby frequencies will also stimulate the nerve fibre but require a greater sound intensity, resulting in a V-shaped frequency tuning curve. The afferent fibres carry the frequency of a sound in two ways, the combination of which is known as Duplex theory. With frequencies of up to ~10kHz, phaselocking is used. The firing of the nerve fibre coincides with the rising of the basilar membrane causing a depolarization of the hair cell. The firing of the fibre is thus at the same frequency as that of the sound whose signal it carries. This is important because the fibre may not fire on every cycle, especially at the higher frequencies. The position of the hair cell along the basilar membrane is the other way in which frequency is encoded in the afferent fibres (known as tonotopicity). Intensity is encoded simply in the number of action potentials. An increased amplitude of the sound wave causes an increased depolarization in the hair cells, and thus an increased amount of transmitter is released
• Sound localization, although contributed to by colouration in the outer ear, is primarily mediated by two binaural processes:
• The sound-shadow of the head causes intensity differences between the two ears. Intensity differences are measured by cells in the lateral superior olive that receive excitatory inputs from the contralateral cochlea and inhibitory inputs from the ipsilateral cochlea. Thus, the cells are activated if the sound source is on the contralateral side. Intensity differences are used for frequencies in the range of ~1.5–20kHz
• Phase differences occur because the distance to the sound source is different from each ear when the head is turned towards or away from the source. This cue is used for lower frequencies, in the range of ~40Hz–3kHz, and is measured by coincidence detectors in the medial superior olive ( OHCM8 p.468)
OHCM8 p.468)
• Primary auditory cortex (area A1) is located on the superior temporal gyrus. Area A1 contains several tonotopic maps, in a typical columnar organization. The columns are arranged in patterns of summation (binaural) and suppression (one ear dominant). Pitch is represented bilaterally, so that unilateral lesions have little effect on pitch discrimination, while sound source location is represented contralaterally. Higher auditory areas concerned with speech are:
• Wernicke’s area, which is located in the temporal lobe (usually left) and is involved in speech perception
• Broca’s area, which is located in the frontal lobe (usually left) and is involved in speech expression
• Lesions of these sites result in receptive and expressive dysphasias, respectively ( OHCM8 p.80). There is much feedback to earlier stages of the auditory pathways (to the medial geniculate nucleus; inferior collicular nucleus; dorsal and ventral cochlear nucleus, but not to the cochlea), most likely having a role in attention, giving rise to the ‘cocktail party effect’ of being able to single out a sole voice amid a noisy crowd.
OHCM8 p.80). There is much feedback to earlier stages of the auditory pathways (to the medial geniculate nucleus; inferior collicular nucleus; dorsal and ventral cochlear nucleus, but not to the cochlea), most likely having a role in attention, giving rise to the ‘cocktail party effect’ of being able to single out a sole voice amid a noisy crowd.
• The vestibular system measures linear and angular acceleration of the head. The sensory organs of the vestibular system are found in the inner ear, which is divided into two portions: bony and membranous labyrinths. There are two principal structures in the vestibular part of the membranous labyrinth:
• The otolith organs (utricle and saccule)
• Both the otolith organs and the semicircular ducts are filled with endolymph, which has a similar chemical constitution to intracellular fluid.
• Each semicircular duct arises and joins back to the utricle in a circular loop. Each duct is oriented at 90° to the other two. At one end of each duct there is a dilation of the duct (the ampulla) containing the hair cells. These are similar to those of the auditory system, each having a row of stereocilia arranged in order of height at their apex, of which the tallest is thicker and known as the kinocilium. The apices of the hair cells are embedded in the cupula, a gelatinous membrane stretching from the ampullary crest to the roof of the duct, thus completely occluding the lumen of the duct
• Angular acceleration of the head causes the cupula to move, due to the inertia of the endolymph in the ducts and, therefore, the stereocilia on the hairs cells are pushed to one side. The tips of the stereocilia on each hair cell are linked, so when the cupula moves and the stereocilia bend, the tip-links slacken or tighten, depending on which way the head is rotated. Tightening of the tip-links opens stretch-activated non-selective cation channels, which allow K+ influx from the endolymph down an electrical gradient (there is no chemical gradient)
• The utricle has a zone called the macula, which is covered with hair cells. The otolithic membrane—a layer of a gelatinous substance in which are embedded crystals of calcium carbonate (the otoliths)—lies over the stereocilia of these hair cells. The macula lies in the horizontal plane such that when the head is upright, the otoliths rest on it. When the head tilts, the otoliths deform the gelatinous membrane and therefore bend the stereocilia on the hairs cells. The utricle is thus sensitive to horizontal, linear forces. The axes of the stereocilia on the hair cells form an approximately radial pattern over the macula, in order to sense linear forces in any horizontal direction. The saccule has a similar structure, but is vertically orientated. Thus it is sensitive to any vertically directed linear force.
The vestibular organs are innervated by the VIIIth cranial (vestibulocochlear) nerve. These fibres terminate in the vestibular nuclei. The vestibular nuclear complex comprises four nuclei.
• The lateral vestibular nucleus is functionally related to the control of posture, with vestibular inputs primarily in the ventral portion of the nucleus and cerebellar and spinal inputs going to the dorsal portion
• The medial and superior vestibular nuclei control the vestibulo-ocular reflexes. These reflexes are important for maintaining a stable eye position when the head is moved
• The inferior vestibular nucleus (along with input from the cerebellum) mediates the vestibulospinal and vestibuloreticular pathways.
Disorders of vestibular system or its central connections results in vertigo, an illusion of rotatory movement ( OHCM8 p.466).
OHCM8 p.466).
• Taste is mediated by chemical receptor cells. The chemoreceptors are epithelial cells clustered in sensory organs called taste buds. Each taste bud contains around 50–150 taste receptors. Taste buds are found in the papillae (numerous projections) of the tongue. There are three types of papillae:
• Fungiform papillae contain between one and five taste buds and are found on the anterior two-thirds of the tongue
• Foliate papillae contain thousands of taste buds and are found on the posterior edge of the tongue
• Circumvallate papillae also contain thousands of taste buds and are found on the posterior third of the tongue
• Receptors have microvilli on their apical membrane that extend through an opening in the surface of the tongue, known as a taste pore, to reach the oral cavity
• There are four classes of taste receptor: bitter, sweet, sour, and salty. There is also mixed evidence for a fifth class for monosodium glutamate. Different receptor classes are distributed in broadly distinct regions around the tongue. There is a maximum density of each type in different locations, but each type can be found all over
• Taste receptors contain voltage-gated Na+, K+, and Ca2+ channels, similar to those in neurones, which are capable of generating action potentials. However, receptors appear to signal taste through graded subthreshold membrane potentials. The mechanism of transduction differs between the four submodalities:
• Bitter stimuli cause release of Ca2+ from intracellular stores, triggered by IP3 or cAMP second-messenger pathways. The raised Ca2+ concentration leads to transmitter release
• There are two putative mechanisms for transduction of sweet tastes
 In the first, the ‘sweet’ molecule directly activates a Na+-specific channel that depolarizes the cell. This hypothesis is supported by evidence that the depolarization has a reversal potential near the Na+ equilibrium potential, and that the depolarization is blocked by amiloride
In the first, the ‘sweet’ molecule directly activates a Na+-specific channel that depolarizes the cell. This hypothesis is supported by evidence that the depolarization has a reversal potential near the Na+ equilibrium potential, and that the depolarization is blocked by amiloride
 In the second, adenylate cyclase is activated via a G-protein, causing an increase in intracellular cAMP concentration. cAMP closes a voltage-dependent K+ channel that is open at the normal resting potential, causing depolarization. It is possible that both mechanisms act in ‘sweet’ receptors, or that there are two types of sweet receptor, one with each mechanism
In the second, adenylate cyclase is activated via a G-protein, causing an increase in intracellular cAMP concentration. cAMP closes a voltage-dependent K+ channel that is open at the normal resting potential, causing depolarization. It is possible that both mechanisms act in ‘sweet’ receptors, or that there are two types of sweet receptor, one with each mechanism
• Sourness is the taste associated with acids, which are thought to pass directly into the receptor cells through the cell membrane. The acids block voltage-dependent K+ channels in the apical membrane, but do not diffuse across the cell in sufficient quantities to block voltage-gated Na+ and Ca2+ channels in the basolateral membrane. This alteration in the balance of the resting ion flux results in depolarization of the cell
• Saltiness is thought to be mediated through a direct binding of the active chemical species to a voltage-independent, amiloride-sensitive, cation channel. Opening of this channel allows Na+ influx and thus depolarization.
• Receptor cells form chemical synapses with primary afferent fibres. Primary afferent fibres innervate several receptor cells within a number of taste buds in several papillae. Fibres thus have highly distributed innervations with complex and overlapping receptive fields
• A branch of the VIIth (facial) cranial nerve (the chorda tympani) innervates the anterior two-thirds of the tongue, while the lingual branch of the IXth (glossopharyngeal) nerve innervates the posterior third. Cell bodies of the chorda tympani are in the geniculate ganglion, and those of the glossopharyngeal nerve in the petrosal ganglion
• There are also some taste buds on the palate, innervated by the greater superficial petrosal branch of the VIIth cranial nerve, and on the epiglottis and oesophagus, innervated by the superior laryngeal branch of the Xth (vagal) cranial nerve, whose cell bodies lie in the nodose ganglion
• Gustatory afferents from the VIIth, IXth, and Xth cranial nerves enter the solitary tract in the medulla, synapse in the gustatory nucleus, in the rostral and lateral part of the solitary nuclear complex, and project, via the central tegmental tract, to the ventral posterior medial nucleus of thalamus, and thence to the area of primary somatosensory cortex that represents the tongue on the postcentral gyrus and to the insular cortex deep in the Sylvian sulcus
• Taste is represented in a specialized area of primary somatosensory cortex, next to the area that represents touch sensation on the tongue. Spatial segregation of receptor subtypes in the tongue is preserved in the gustatory nucleus, thalamus, and cortex.
• Smell is mediated by chemoreceptors that have a very high sensitivity to odorant molecules. They can detect molecules at concentrations as low as a few parts per trillion
• Receptors are located in a specialized region of olfactory epithelium, deep in the nasal cavity, which consists of receptor cells, supporting cells and basal cells
• Olfactory receptors are bipolar cells (Fig. 11.60). One end is short and extends to the mucosal surface where it expands into an olfactory knob. The other end is longer and forms an unmyelinated axon that projects through the cribriform plate to the ipsilateral olfactory bulb, on the undersurface of the frontal lobe. These axons join together in bundles of 10–100 surrounded by Schwann cells, to pass through the cribriform plate as olfactory nerves
• Olfactory receptors have a lifetime of ~60 days and so, unusually for neurones, are constantly replaced. However, the post-synaptic cells in the olfactory bulb do not regenerate, and must continually make fresh connections with each new generation of receptors.
• The olfactory knob has several cilia, which form a dense mat at the mucosal surface. Odorants in the mucus are bound by an olfactory binding protein to enable cilia to interact with odorant molecules in the layer of mucus that covers this surface
• Binding of odorants by membrane proteins causes an increase in intracellular cAMP concentration via a G-protein effect on adenylate cyclase. Depolarization arises through a cAMP-gated Na+ channel, in a process homologous to phototransduction in the retina. There is also evidence for depolarization mediated through an IP3 (inositol triphosphate) second-messenger system, without a change in the cAMP concentration
• There is a large family of olfactory receptors which are thought to act together, rather like the three cone types in colour vision, to give a very wide range of smells that can be discriminated.
• The olfactory bulb is divisible into four layers:
• In glomeruli, receptor axons synapse onto mitral cells, tufted cells, and periglomerular cells
• The external plexiform layer contains tufted cell bodies and the dendritic trees of granule cells
• The mitral body layer contains mitral cell bodies
• The granule layer contains the axons of mitral cells and granule cells, and granule cell bodies
• Mitral and tufted cells form the output from the olfactory bulbs, and their axons form the olfactory tract. Periglomerular cells and granule cells are inhibitory interneurones. Granule cells also receive efferent fibres from both olfactory nuclei
• Axons in the olfactory tract project to the anterior olfactory nucleus, the olfactory tubercle, the pyriform cortex, the cortical nucleus of the amygdala, and the entorhinal cortex
• The anterior olfactory nucleus projects to the contralateral olfactory bulb via the anterior commissure, and thence back to the contralateral olfactory bulb
• The olfactory tubercle and pyriform cortex project to other olfactory cortical regions and to the medial dorsal nucleus of the thalamus, and thence to orbitofrontal cortex, which is associated with conscious perception of smell
• The amygdala and entorhinal cortex are part of the limbic system, and are involved in the affective components of smell perception.
• There is no topographic central representation of olfaction as there is with other sensory modalities. However, glomeruli seem to have preferential sensitivities to certain odours over others, so there is clearly a distributed representation of odour.

Fig. 11.60 Simplified representation of the cell types of the olfactory bulb and their connections—p (periglomerular cells); g (granule cells).
Reproduced with permission from Carpenter R (2003). Neurophysiology, 4th edn. Edward Arnold.
The passive electroencephalogram reveals rhythms of activity at four different frequencies:
• Alpha rhythm (8–13Hz) characterizes the awake but resting EEG
• Faster beta waves are associated with mental activity (>13Hz)
• Higher frequency gamma rhythm (35–45Hz) may be a signature of the waking state
• Slower rhythms, theta (4–7Hz) and delta (<3.5Hz), are more common during reduced arousal in adults.
Two types of sleep can be defined using electrophysiological features detected with electroencephalography (EEG), electro-oculography (EOG), and electromyography (EMG)—REM (paradoxical) and non-REM (NREM, slow-wave sleep), see Fig. 11.61.
• NREM sleep occupies ~75% of sleep time and is predominantly associated with delta activity, while activity more like that seen during the waking state characterizes REM sleep. This is despite the individual being difficult to arouse at this point—hence the alternative term of paradoxical sleep
• In general, NREM sleep is characterized by synchronized activity in the brain, while activity during waking and REM sleep is desynchronized
• Four stages of NREM sleep have been defined related to the depth of sleep and the increasing dominance of low-frequency synchronized wave activity in the EEG:
• Stage 1 shows a slowing of the frequency of wave activity
• In stage 2, the EEG displays distinctive bursts of high-frequency activity (spindles)—thought to be of thalamic origin (p.734)
• In stages 3 and 4 (SWS), low-frequency, synchronized wave activity dominates the EEG.
REM sleep is characterized by ‘wake-like’ EEG activity (low-amplitude, high-frequency gamma waves, 30–80Hz), clusters of eye movements on the EOG, and low muscle tone (atonia) on the EMG.
• Neuronal networks in the pons, midbrain, thalamus, hypothalamus, and basal forebrain (all of which regulate the rostral cerebral hemispheres) control wakefulness, NREM, and REM sleep
• The areas of the brain implicated in arousal lie predominantly, but not exclusively, in the reticulum of the brainstem and midbrain (mesopontine junction—a region of the rostral brainstem containing the laterodorsal tegmental nucleus, pedunculopontine nucleus, dorsal raphe nucleus, and the locus coeruleus). An increase in activity in this ascending reticular activating system (ARAS) is suggested to underlie arousal
Fig. 11.61 Behavioural states in humans. States of waking, NREM sleep and REM sleep have behavioural, polygraphic and psychological manifestations. In the row labelled behaviour, changes in position (detectable by time-lapse photography or video) can occur during waking and in concert with phase changes of the sleep cycle. Two different mechanisms account for sleep immobility. The first is disfacilitation (during stages I–IV of NREM sleep). The second is inhibition (during REM sleep). During dreams, we imagine that we move, but we do not. Sample tracings of three variables used to distinguish the state are shown: an EMG, an EEG, and an EOG. The EMG tracings are highest during waking, intermediate during NREM sleep and lowest during REM sleep. The EEG and EOG are both activated during waking and inactivated during NREM sleep. Each sample shown is approximately 20s long. The three bottom rows describe other subjective and objective state variables.
Reprinted by permission from Macmillan Publishers Ltd: Nature 437: 7063, 1254–1256, Copyright (2005).
• The neurotransmitter systems that are prominently involved include the noradrenergic (locus coeruleus), serotonergic (5-HT, dorsal raphe) histaminergic (hypothalamus), cholinergic (pons/midbrain), and orexin/hypocretin (hypothalamus)
• In general, the release of these neurotransmitters is under the control of both circadian (light–dark cycle—SCN in the hypothalamus) and homeostatic (fatigue) influences
• The major projection of the ascending reticular activating system (ARAS) is the thalamus—a critical relay for sensory and intra-cerebral pathways (p.730). In the absence of activity in the ARAS, the thalamus and the cortex tend towards slow wave activity and unconsciousness
• The transfer into NREM sleep is associated with a reduction in activity of these networks
• The length of the NREM/REM cycle is also determined by signals from cholinergic and aminergic neurones. During NREM sleep, aminergic signalling dominates. However, the transition to REM sleep involves reduced activity of aminergic systems and an increase in cholinergic activity. During REM sleep, aminergic signalling is silent and cholinergic excitatory activity is dominant
• The termination of REM sleep is driven by increased noradrenergic and serotonergic system activity
• NREM and REM activity alternate in each of four or five ~90min cycles each night. NREM is deeper and longer early in the night while, in later cycles, REM sleep occupies progressively longer periods of the cycle (p.768)
• NREM sleep appears associated with conservation of energy and repair mechanisms, thereby suggesting a restorative function, although it has also been suggested to function in the iteration of information
• The brain activity associated with REM sleep may be important in brain development and plasticity. A widely supported idea is that memory consolidation is a major function of REM sleep
• The disruption of metabolic homeostasis that results from severe sleep deprivation can lead to death, emphasizing the crucial role of sleep.
• In healthy individuals, three main states of consciousness are recognized—wakefulness, NREM sleep, and REM sleep
• Awareness might also be regarded as an aspect of consciousness, as we are aware, whether awake or dreaming. Nevertheless, analysis is difficult because someone other than the ‘aware’ individual interprets awareness on the basis of objective parameters such as behavioural or neuronal activity
• The conscious state can be usefully assessed using objective criteria such as those in the Glasgow Coma Scale (GCS) (p.772)—though it may be limited if the patient is prevented from making the usual responses to consciousness (e.g. by paralysis). The GCS includes numerically graded assessment of three parameters—eye opening, motor function, and verbal responsivity
• Altered consciousness states, as a result of some pathology, range from coma to vegetative state to brainstem death:
• Coma is a state of continuous unconsciousness in the absence of a sleep–wake cycle. The degree to which an individual responds varies significantly, as does the level of cerebral metabolism. Coma may represent a transitional state between full recovery, re-establishment of the sleep–wake cycle with impaired awareness, or brainstem death
• Brainstem death is associated with irreversible loss of all brainstem functions
• The vegetative state illustrates distinctions between the underlying brain systems for wakefulness and awareness. In this state, several behaviours suggest wakefulness but any sense of purpose or attempts to communicate are absent. The vegetative state is often the result of severe brain injury (trauma/hypoxia/ischaemia) yet, while autopsies reveal damage to any or all of the cortical mantle, cerebral white matter, and thalamus, the brainstem networks associated with wakefulness are unaffected.
 OHCM8 p.802)
OHCM8 p.802)This gives a reliable, objective way of recording the conscious state of a person. It can be used by medical and nursing staff for initial and continuing assessment. It has value in predicting ultimate outcome. Three types of response are assessed.
Best motor response This has six grades:
6 Carrying out request (‘obeying command’): The patient does simple things you ask (beware of accepting a grasp reflex in this category)
5 Localizing response to pain: Put pressure on the patient’s fingernail bed with a pencil then try supraorbital and sternal pressure: purposeful movements towards changing painful stimuli is a ‘localizing’ response
4 Withdraws to pain: Pulls limb away from painful stimulus
3 Flexor response to pain: Pressure on the nail bed causes abnormal flexion of limbs—decorticate posture
2 Extensor posturing to pain: The stimulus causes limb extension (adduction, internal rotation of shoulder, pronation of forearm)—decerebrate posture
Note that it is the best response of any limb which should be recorded.
Best verbal response This has five grades:
5 Oriented: The patient knows who they are, where they are, and why, and the year, season, and month
4 Confused conversation: The patient responds to questions in a conversational manner but there is some disorientation and confusion
3 Inappropriate speech: Random or exclamatory articulated speech, but no conversational exchange
2 Incomprehensible speech: Moaning but no words
Record the level of best speech.
Eye opening This has four grades:
3 Eye opening in response to speech: Any speech, or shout, not necessarily request to open eyes
2 Eye opening in response to pain: Pain to limbs as above
An overall score is made by summing the score in the three areas assessed. For example, no response to pain + no verbalization + no eye opening =3. Severe injury, GCS ≤8; moderate injury, GCS 9–12; minor injury, GCS 13–15.
NB: An abbreviated coma scale, AVPU, is sometimes used in the initial assessment (‘primary survey’) of the critically ill:
• V = responds to vocal stimuli
Some centres score GCS out of 14, not 15, omitting ‘withdrawal to pain’.
NB: The GCS scoring is different in young children.
• The limbic system functions as an integrator of information from the external world and from within the body. Its proposed role is in the generation of emotional experience
• Anatomically, the limbic system consists of the limbic lobe, a ring of cortex around the brainstem comprising the parahippocampal gyrus, the cingulate gyrus, and the subcallosal gyrus (Fig. 11.62). In addition, the deeper lying hippocampal formation (including the hippocampus, dentate gyrus, and subiculum), the amygdala, parts of the hypothalamus, the septal area, the nucleus accumbens, and parts of the orbitofrontal cortex also contribute to the limbic system
• Pathways between the association cortex, entorhinal cortex, the hippocampus, and amygdala provide a link between the neocortex and the limbic system. Thus, connections between the limbic system and higher cortical areas enable the integration of emotional processing with cognitive functions such as attention, memory, and reasoning. In addition, it is well established that the hippocampus is involved in processes of memory storage, while the amygdala has also been implicated in learning, particularly tasks that might involve the association of stimulus and emotional response
• Both the amygdala and the hippocampus have direct, reciprocal connections with the hypothalamus. Pathways between the cortical cingulate gyrus, hippocampus, and hypothalamus extend the influence of the cortex over the hypothalamic expression of emotional behaviour
• The amygdala has two major projections:
• The stria terminalis that innervates the hypothalamus and nucleus accumbens
• The ventral amygdalofugal pathway that innervates the hypothalamus, dorsal medial nucleus of the thalamus, and the cingulate gyrus
• The connections between the limbic system and the hypothalamus regulate autonomic and visceral responses associated with motivational drives (food, water, sex) and emotional expression; they exert control over the endocrine system through its regulation of the secretion of hypothalamic hormones; and, through extensive connections with the sympathetic and parasympathetic nuclei of the brainstem and spinal cord, they exert influence over the autonomic motor system.
Fig. 11.62 The limbic system consists of the limbic lobe and deep-lying structures. This medial view of the brain shows the prefrontal limbic cortex and the limbic lobe. The limbic lobe consists of primitive cortical tissue that encircles the upper brain stem as well as underlying cortical structures (hippocampus and amygdala).
Reproduced with permission from Kandel E, Schwartz JH, Jessell TM (2000). Principles of Neural Science, 4th edn. © The McGraw-Hill Companies Inc.
Much of our understanding of memory systems and their neurological basis originates from observations of patient HM, who, after undergoing bilateral medial temporal lobe resection (including the hippocampal formation) in an attempt to cure intractable epilepsy, displayed an inability to learn or retain memories of events after the transection (anterograde amnesia) along with impaired recollection of memories in the few years preceding transection (retrograde amnesia). Nevertheless, longer-term memories could be retrieved and were intact. Interestingly, HM could learn some motor tasks and was able to retain some immediate and short-term information indicating a distinction between short- and long-term memory systems.
Memory has been categorized into two types—declarative and non-declarative.
The formation and retrieval of explicit memories of facts and events specific to an individual and involving the conscious recollection of past experiences.
• Episodic memory is the recollection of specific events occurring at a particular time and place. As seen in patient HM, the medial temporal lobe, including the hippocampus, perirhinal cortex, and parahippocampal cortex, is involved in both the formation and retrieval of this form of memory. Also involved is the prefrontal cortex. Damage in these regions can impair the ability to:
• Retrieve the time and place at which an event occurred
• Distinguish temporally, two or more events
• Recollect where or when a new task was learned
• Semantic memory involves the recollection of information not associated with time or place but relating more to meaning and function (e.g. of objects, words). It is also dependent on the medial temporal lobes, particularly the anterior and lateral regions of the left hemisphere—a localization that, despite its close relationship to episodic memory, clearly distinguishes semantic memory.
• Procedural memory relates to the acquisition of skills or habits. An intact cortico-striatal system, motor cortex, and cerebellum all appear important mediators of different types of procedural memory. Damage to the striatum, such as that seen in Huntington’s disease, impairs motor task learning without affecting declarative memory
• The perceptual representation system is important in the recognition of objects by their structure or form. This is distinct from the meaning or function that can be attributed to semantic memory. This form of memory also includes priming, or the ability to recognize a partial object as a result of prior exposure to the whole object. The posterior cortical regions that are activated during this form of memory are also distinct from those involved in semantic memory. Thus, visual object form involves the extrastriate occipital cortex, while global object structure involves areas at the interface between temporal and occipital cortices
• Working memory, a form of memory used for the short-term retention of information important for problem solving or reasoning, can be severely disrupted by damage to the orbital frontal and medial frontal cortex, suggesting involvement of these regions in memory processes. Two sub-systems appear to operate in the transfer of working memory into long-term memory:
• A phonological loop that allows rehearsal of speech-based information
• A visuo-spatial map, located in the visual association cortex, inferior parietal lobule, and prefrontal cortex of the right hemisphere, which retains the visual and spatial information.
• Associative learning describes the linking of two events, whereas non-associative learning involves the influence of a single event on the probability that it elicits a response. Associative learning is typified by the classical conditioning experiments of Pavlov. Repeated pairing of a neutral (unconditioned) stimulus (bell ring) preceding one that evokes a response (conditioned stimulus, food) eventually leads to salivation in response to the bell ring—a conditioned response
• The motor component of procedural associative memory, typified by the example of an eye-blink in response to a conditioned stimulus, is dependent on the cerebellum, even though the reflex eye-blink to an unconditioned stimulus is independent of cerebellar involvement. The hippocampus and amygdala, on the other hand, are both involved in storing the learning experience but not the acquisition of the motor response
• Associative learning involving an unpleasant conditioned stimulus invokes mechanisms associated with fear and links emotion with memory. The amygdala plays a major role in emotional associative memory, along with the hippocampus and medial prefrontal cortex (p.780)
• Non-associative learning, often expressed through reflex pathways, can involve a reduction in response (habituation), enhancement of a response (sensitization), or the removal of habituation by another, more powerful, stimulus (dishabituation).
Current ideas of the cellular basis of learning and memory revolve around mechanisms that lead to the selective strengthening or weakening of synaptic connections between neurones. Experimentally induced cellular mechanisms such as long-term potentiation (LTP) and long-term depression (LTD), involving the strengthening or weakening of synapses, respectively, have been proposed as cellular correlates for learning and memory. The changes in synaptic strength in LTP and LTD are mediated by alterations in transmitter release and/or the insertion or removal of post-synaptic receptors. Both types of molecular event have been obser-ved at synapses in pathways involved in the acquisition of particular tasks.
With normal ageing (senescence), most individuals experience some form of cognitive impairment that, while being short of dementia, can be problematic for quality of life. Mental capabilities most likely to show age-related impairment include aspects of memory, executive functions, and reasoning. It is significant that when one of these mental functions declines, so do the others. The impairment of these mental functions can be at least partially accounted for by a reduced speed of information processing that can be first detected in the third decade. In addition, sleep, motor, and sensory systems and brain circulation and metabolism may be affected.
Interconnectivity between temporal, prefrontal, and parietal cortical areas and the pathways between the entorhinal cortex, dentate gyrus (hippocampal formation), and the principal neurones of the hippocampus (all forming the hippocampal formation) play prominent roles in cognitive function (p.774).
As people grow older, there is a gradual decrease in brain volume. Traditionally, neuronal death spread across the cortex was proposed to underlie the reduced brain volume and, thus, explain cognitive impairment with ageing. This hypothesis was apparently supported by the large cortical cell loss seen in age-related disorders such as dementia and Alzheimer’s disease (AD,  OHCM8 p.492), which are also characterized by dramatic cognitive decline. More recent studies, however, indicate that the cognitive symptoms of normal ageing are not associated with significant neuronal death. Rather, the decrease in brain volume is thought to be due to shrinkage of neuronal cell bodies and dendrites and a progressive loss of myelin integrity.
OHCM8 p.492), which are also characterized by dramatic cognitive decline. More recent studies, however, indicate that the cognitive symptoms of normal ageing are not associated with significant neuronal death. Rather, the decrease in brain volume is thought to be due to shrinkage of neuronal cell bodies and dendrites and a progressive loss of myelin integrity.
A reduction in synapse number, without accompanying neuronal death, is suspected of underlying impaired cognitive function during senescence. The loss of synapses is associated with a reduction in dendritic spine number and density (the sites of synapses) on cortical neurones that may be as much as 50% in individuals over 50.
Also accompanying synaptic loss, degeneration of myelinated axons in the cortex and white matter has been reported. As white matter represents the major connective pathways within the CNS, it is likely that the damage to this cortical connectivity underlies the synaptic loss. This myelin damage is manifest as white matter lesions (small scars in the brain’s white matter) that can be detected using magnetic resonance brain imaging (MRI). They are found in healthy old people and accumulate with age.
Neuronal death with ageing may, however, be more significant in sub-cortical areas. Modulatory inputs to the hippocampus and cortex from cholinergic neurones in the basal forebrain and from brainstem monoaminergic neurones are reduced during senescence. Correlations exist between the degree of cholinergic cell loss and behavioural change, suggesting that the reduction of these neuromodulatory systems is likely to contribute to the impaired integrative function of the cortex with age.
Emotions of anger, fear, pleasure, and contentment are expressed as behavioural patterns that include facial expressions, bodily demeanour, and autonomic arousal. Conscious recognition of the physiological reactions to emotion-inducing stimuli can be described as ‘feelings’. In general, emotions influence all aspects of cognitive function (attention, memory, reasoning), yet we have restricted intentional control over them.
Key brain regions and their interactions that influence the processing of emotions include the prefrontal cortex, the amygdala, the hypothalamus, and the anterior cingulate cortex (Fig. 11.63). Connectivity between these and other brain areas enables the integration of emotional and cognitive processes.
The hypothalamus integrates and coordinates the motor and endocrine responses that constitute the behavioural expression of emotional states. Aggression is associated with neural activity in the medial hypothalamus. On the other hand, the lateral hypothalamus is active during expression of anger. Indeed, stimulating this region induces the behavioural profile associated with anger, though not the conscious experience of the emotion.
Expressions of anger are also associated with activity in other parts of the limbic system, particularly the cingulate cortex. Reciprocal connections between the cortex and the hypothalamus perform several functions:
• They allow the conscious experience of emotions in the form of feelings
• They activate appropriate bodily mechanisms in response to external stimuli
• They direct appropriate motor behaviour (e.g. avoidance) in response to the stimulus.
In addition, neocortical involvement may suppress emotional responses to minor stimuli.
The amygdala is critical for the processing of emotional signals that are expressed through changes in hypothalamic function. Fear, for example, is associated with autonomic (heart rate and blood pressure), endocrine (stress hormones), and motor behaviour changes, along with analgesia and potentiated somatic reflexes like the startle response. Fear conditioning describes the ability of a neutral stimulus to acquire fear-inducing properties when paired closely in time with a threatening event. Damage to the amygdala can impair the processing of signals of fear such as facial and vocal expressions. From a clinical point of view, such a mechanism may relate to the pathophysiology of phobic anxiety and post-traumatic stress disorders.
The amygdala also plays important roles in emotional conditioning, the consolidation of emotional memories, and associative learning involving reward and appetite. Modulation through β-adrenoceptors is essential for the enhancement of emotional memory.
The amygdala processes emotion-inducing stimuli through two pathways:
• Basic sensory input is received by the amygdala via a direct route from the thalamus and hippocampus. This elicits short-latency emotional responses, possibly without cortical (cognitive) involvement. This direct input may also prepare the amygdala for the reception of more complex information from cortical areas
• The prefrontal cortex receives processed sensory signals from the thalamus, as well as information concerning the output of the amygdala and homeostatic feedback concerning the rest of the body. A second pathway to the amygdala thus carries integrated cortical output, resulting in a slower response that also includes conscious awareness of the emotional experience.
The ventromedial prefrontal cortex is proposed to integrate signals originating within the body with other forms of cognitive information to modulate emotional intensity. This may enable decision-making in situations where the possible choices have subtly different emotional values. In this respect, earlier significant emotional events may be linked with patterns of physiological reactions. Alternatively, the role of the prefrontal cortex is suggested to modulate emotional decision making in favour of longer-term adaptive rather than immediate gains.
Homeostatic inputs from autonomic, visceral, and musculoskeletal sources via the brainstem, hypothalamus, and somatosensory and cingulate cortices are integrated into the generation of feelings. Likewise, the recall of feelings associated with emotional experience recruits similar brain areas including brainstem nuclei, hypothalamus, and somatosensory and orbitofrontal cortices. The anterior cingulate cortex is implicated in processing of emotional information with respect to the regulation of mood.
The distinction between emotion and its mental representation as feelings suggests separate systems exist for the perception of emotion and feeling states. The involvement of the amygdala in emotional perception, but not in the pathways associated with the expression of feelings, supports at least partial distinction in the mechanisms underlying these two aspects of emotion.

Fig. 11.63 Key structures in the human brain associated with emotion.
Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews: Neuroscience 5:7, 583–9, Copyright (2004).
 OHCS8 pp.336–43)
OHCS8 pp.336–43)Depression and mania are disorders of affect characterized by changes in mood. Two forms of depressive syndrome have been categorized:
• Bipolar depressive syndrome reflects oscillation between depression and mania, perhaps suggesting a biochemical imbalance. There is good evidence for a hereditary link to this form of depression
• Unipolar depressive syndrome or depression without associated mania. This is more common in older patients and can be associated with anxiety and agitation.
Symptoms associated with depression include:
• General feeling of apathy, misery, and pessimism
• Low self-esteem including guilt, inadequacy, ugliness
• Indecisiveness and loss of motivation
• Retardation of thought and actions
Mania is associated with symptoms of excessive exuberance, self-confidence, enthusiasm, physical activity, irritability, impatience, and anger.
Abnormal function of brain regions associated with emotion is implicated in mood disorders. The cognitive aspects of depression such as memory impairment, feelings of worthlessness, guilt, and suicidality may be mediated by the neocortex and hippocampus. The involvement of the amygdala, striatum, and nucleus accumbens in emotional memory indicates these regions may be involved in decreased drive for pleasurable activity, reduced motivation, and anxiety. The hypothalamus may also be involved in the disruption of sleep (circadian rhythm) as well as drives for food or sex.
Box 11.2 summarizes the treatments for depression.
Schizophrenia is a highly debilitating but common disorder with a lifetime risk in the general population of ~1%. A further 2–3% of the population has the related schizotypal personality disorder.
Schizophrenia is classified as a neurodevelopmental disorder that epidemiological studies suggest is predominantly, but not completely, attributable to genetic causes. Studies of identical (monozygotic) and non-identical (dizygotic) twins indicate ~50% and ~10% concordance, respectively—both considerably higher than the incidence in the general population.
Two types of symptoms characterize the disorder:
• Positive (type I) symptoms are manifest as psychotic episodes of the disorder and include auditory hallucinations, delusions, and incoherent or disordered cognitive processes
• Negative (type II or residual) symptoms characterize non-psychotic periods and represent the most unmanageable aspects of the disorder in that drug therapy is commonly ineffective. They include social isolation and withdrawal, decreased emotions, loss of drive, apathy, poverty of speech, and affective flattening.
The progression of the clinical signs of schizophrenia can be divided into four stages:
• During childhood, no obvious symptoms of the disorder are apparent (premorbid phase)
• During adolescence, non-specific behavioural changes appear, related to the development of negative symptoms (prodromal phase)
• The onset of the disorder, characterized by the first psychotic episode, occurs typically during the late teens to early twenties. Left untreated, the disorder will continue, with psychotic episodes interspersed by phases displaying negative symptoms, throughout adulthood (progressive phase). Also associated with disease progression are mood disorders and impaired cognitive function
• These symptoms persist during later life in the final residual stage of the disorder. Many sufferers never recover.
Based on the strong correlation between the potency with which neuroleptic drugs antagonize dopamine D2 receptors, and their effective therapeutic dose, the dominant hypothesis is that hyperactivity of brain dopaminergic pathways underlies the positive symptoms of schizophrenia.
Nevertheless, while neuroleptic drugs occupy D2 receptors very quickly after oral administration, a delay of 1–2 weeks is needed before an overall decrease in dopaminergic activity is obtained and therapeutic effects are seen. This suggests that while the dopaminergic system may be integral to the mechanism of neuroleptic action, indirect actions of these drugs are also likely to be involved.
There are four major dopaminergic pathways in the brain (Fig. 11.64):

Fig. 11.64 Dopamine pathways and schizophrenia.
• Dopaminergic neurones originating in the ventral tegmental area (VTA), and projecting to nuclei of the limbic system, form the mesolimbic dopamine pathway. Projections to the nucleus accumbens, amygdala, hippocampus, anterior cingulate, and entorhinal cortex—areas intimately involved in emotional function and memory—support the idea that hyperexcitability of this pathway mediates the positive symptoms of the disorder
• Dopaminergic neurones also originating in the VTA but projecting to the neocortex (in particular the prefrontal cortex) form the mesocortical dopamine pathway. These cortical areas are important in motivational planning, attention, and social behaviour. It is possible that abnormalities in these regions are important in the negative symptoms. Damage to cortical connections in these areas can result in similar symptoms. Thus, it is possible that reduced activity of this dopaminergic pathway is responsible for negative symptoms and this may explain their refractoriness to typical neuroleptic drugs
• Two additional dopaminergic pathways in the brain are of importance relative to unwanted effects of neuroleptic drugs:
• The nigrostriatal dopamine system is found in the basal ganglia and projects from the substantia nigra compacta to the striatum (Parkinson’s disease). Neuroleptic drugs acting on this pathway can induce unwanted extrapyramidal effects (movement disorders)
 Acute dystonias are reversible effects associated with Parkinsonism-type symptoms of muscle rigidity, tremor, and loss of mobility. Onset is dose-dependent and the severity often declines with progressive treatment
Acute dystonias are reversible effects associated with Parkinsonism-type symptoms of muscle rigidity, tremor, and loss of mobility. Onset is dose-dependent and the severity often declines with progressive treatment
 Tardive dyskinesia is a chronic, irreversible effect involving involuntary movement of particularly the face and tongue, but also the trunk and limbs. These unwanted effects arise in 20–40% of cases and have a delayed appearance after prolonged neuroleptic treatment for months to years. Their underlying cause is unknown but changes in D2 receptor sensitivity in the striatum and neuronal death in cortical motor areas may both contribute
Tardive dyskinesia is a chronic, irreversible effect involving involuntary movement of particularly the face and tongue, but also the trunk and limbs. These unwanted effects arise in 20–40% of cases and have a delayed appearance after prolonged neuroleptic treatment for months to years. Their underlying cause is unknown but changes in D2 receptor sensitivity in the striatum and neuronal death in cortical motor areas may both contribute
• The tuberoinfundibular dopamine system projects from the hypothalamus to the pituitary (p.734). Neuroleptic treatment can cause endocrine disturbances, notably lactation, due to increased prolactin production.
Box 11.3 summarizes the treatments for schizophrenia.
• A common disorder that affects up to 1% of the population
• Of unknown origin, but can develop after brain trauma (e.g. infection, tumour growth, neurological disease); only one rare type of epilepsy has been traced to a single gene defect, but there is about 30% heritability of epilepsy
• Characterized by seizures that range in intensity (loss of concentration through to convulsive fit), frequency and impact (specific functions affected (partial seizure) through to global effects throughout the body (generalized seizure))
• Associated with high frequency discharges from neurones in specific regions (partial seizure) or throughout the brain (generalized seizure)
• Generalized seizures are subcategorized into tonic–clonic type (grand mal) and absence seizure type (petit mal)
• Tonic–clonic seizures involve an initial global spasm (which can involve biting, cessation of respiration, salivation, and defecation), followed by a series of convulsions for a period of several minutes. The patient often remains unconscious for several minutes after the seizure and usually feels dazed and confused after regaining consciousness
• Absence seizures are less dramatic and occur in children: the child suddenly enters a trance-like state for several seconds, before returning to their activities, with no ill effects
• Seizures can be associated with enhanced release of excitatory neurotransmitters (most notably glutamate acting on NMDA receptors) and/or depressed release of inhibitory neurotransmitters (GABA), but there is no obvious reason for this in sufferers.
Box 11.4 summarizes the treatments for epilepsy.
• Addiction or dependence is a compulsion to perform an action (e.g. take a drug) with a loss of control in limiting that behaviour. This compulsion or craving is a form of psychological dependence
• Intimately associated with the concept of addiction is tolerance, a reduction in effect with repeated execution of the action, and withdrawal (also known as physical dependence) or the appearance of symptoms associated with the termination of chronic expression of the behaviour
• Anxiety, depression, and dysphoria are symptoms common to withdrawal from a wide range of addictive behaviours. It is possible that these symptoms may also be motivating factors for perpetuating addictive behaviour
• The development of addiction is associated with positive reinforcement (i.e. an increased probability of a response to a given stimulus). In the context of drug use, reinforcement could be through direct effects on the probability of self-administration or indirect effects such as a drug’s ability to enable reinforcing effects of associated but neutral stimuli
• While most frequently associated with drug use, other disorders such as impulse control disorders also display symptoms characteristic of addiction—namely, compulsion to perform the action, repeated performance, and withdrawal symptoms of dysphoria and depression. Such disorders are often associated with eating, exercise, gambling, sex, and shopping.
• The development of addiction has been associated with midbrain and forebrain systems that mediate motivated behaviour and natural reward mechanisms
• Ascending and descending pathways through the median forebrain bundle, particularly monoamine pathways, link several regions associated with motivated behaviour. Of particular importance are the VTA and the basal forebrain, including the nucleus accumbens, olfactory tubercle, frontal cortex, and amygdala
• Neurochemically, the mesolimbic dopamine pathway (p.785), linking the VTA and the basal forebrain, is seen as an integral component in linking dependence and natural reward systems
• In addition, opioid, GABA, and 5-HT systems modulate the function of the VTA and basal forebrain during natural and drug reward-related behaviours indicating the convergence of several neurochemical systems in positive reinforcement mechanisms
• During withdrawal, monoamine levels, particularly dopamine in the VTA and 5-HT, decrease dramatically, suggesting that addiction involves neuroadaptive mechanisms that contribute to both dependence and withdrawal symptoms.
Fig. 11.65 Neural circuitry implicated in addictive mechanisms. Dashed lines indicate limbic input to the nucleus accumbens (N.Acc); the wavy connection represents the mesolimbic dopaminergic pathway believed to be important in reward mechanisms, as is the pathway from nucleus accumbens to VTA, represented by the broken grey connection. Various neurotransmitter systems indicated in white modulate VTA dopaminergic activity.
Cocaine and amphetamine are psychomotor stimulants that induce euphoria and act as reinforcers for drug self-administration. Although they increase levels of all monoamines the reinforcing effects crucially depend on dopamine release.
Impairing the mesolimbic dopamine pathway, particularly in the region of the nucleus accumbens and central nucleus of the amygdala, abolishes the reinforcing effects of cocaine and amphetamine.
The reinforcing effects of opiates such as morphine and heroin involve actions at a specific receptor subtype, the µ-opioid receptor, found on neurones in the VTA and the nucleus accumbens. Blocking these receptors blocks positive reinforcement by opiates. This does not, however, involve the mesolimbic dopamine pathway as opiate addiction is unaffected by its pharmacological or anatomical disruption.
Weak, long-acting µ-opioid receptor agonists, such as methadone, are used to alleviate physical dependence during drug withdrawal.
Nicotine has a direct reinforcing effect that involves activation of both the mesolimbic dopamine and opioid pathways through its agonistic actions at nicotinic acetylcholine receptors. Nicotine replacement, to alleviate withdrawal symptoms and psychological dependence, in conjunction with counselling, is the primary treatment used to help smokers give up. The α2-adreoceptor agonist, clonidine, may also be used. It acts presynaptically to reduce neurotransmitter release that may reduce the efficacy of the reward pathways.
The pathways associated with alcohol dependence are more complex. Alcohol shares sedative and anxiolytic properties with benzodiazepines and these may contribute to the reinforcing properties of both drugs. Alcohol and benzodiazepines potentiate GABA systems and this effect, in the central nucleus of the amygdala, is considered important for the development of dependence.
Activity in the mesolimbic dopamine pathway may also contribute to alcohol reinforcement, though this is not essential. Glutamate, serotonin, and opioid systems have all been implicated in alcohol dependence.
Benzodiazepines are commonly used to alleviate withdrawal symptoms during the acute drying-out period, presumably because of their shared action on GABA systems. Disulfiram, an inhibitor of aldehyde dehydrogenase, promotes increased plasma acetaldehyde levels if alcohol is taken. This causes unpleasant symptoms (flushing, hyperventilation, tachycardia, panic) that can act as a form of aversion therapy. Acamprosate, a taurine analogue, reduces craving through an unknown mechanism, though it may be related to interactions with amino acid neurotransmission.
Neurones in the CNS are post-mitotic cells and the potential for replacement is, in general, limited. As such, pathological neuronal loss is likely to have serious consequences for brain function. Neuronal death is a common feature of a group of disorders termed neurodegenerative disorders.
Neuronal death in neurodegenerative disease can occur by one of two processes:
• Necrosis (usually induced by acute injury) involves cell swelling, vacuolization, and lysis and is often associated with an inflammatory response
• Apoptosis (or programmed cell death) can be triggered by extracellular signals that occur normally during development but can also be triggered pathologically during neurodegenerative disease
• Apoptosis is characterized by cell shrinkage, nuclear chromatin and DNA damage, and by the activation of caspases that break down certain intracellular proteins
• Macrophages remove dead cells without inducing inflammatory responses.
Two mechanisms known to induce necrosis as well as to trigger apoptosis, and hence implicated in neuronal death associated with neurodegenerative disease, are oxidative stress and excitotoxicity.
• Oxidative stress refers to the excessive production of reactive oxygen species (ROS) including O2–, hydroxyl free radicals, and H2O2 when mitochondrial oxidative phosphorylation, and hence ATP production, is compromised. ROS damage important intracellular components including enzymes, DNA, and membrane lipids
• Glutamate-induced excitotoxicity involves excessive stimulation of calcium-permeable NMDA receptors and a catastrophic increase in intracellular calcium that further promotes glutamate release, activates membrane-damaging proteases and lipases, and also induces the production of ROS
• The importance of efficient mitochondrial ATP production and endogenous safety mechanisms to protect against ROS—for example, enzymes such as SOD and catalase, and antioxidants such as glutathione, ascorbate, and α-tocopherol (vitamin E)—has led to the suggestion that compromising any of these mechanisms may underlie several neurodegenerative diseases.
In many neurodegenerative disorders, the causes of cell death can be attributed to inherited traits. However, many sporadic cases are idiopathic, suggesting non-genomic mechanisms may also be important. Thus, specific genetic mechanisms account for all cases of Huntington’s disease ( OHCM8 p.716). Genetic mutations also account for some cases of motor neurone disease (
OHCM8 p.716). Genetic mutations also account for some cases of motor neurone disease ( OHCM8 p.510), Parkinson’s disease (
OHCM8 p.510), Parkinson’s disease ( OHCM8 p.498), and Alzheimer’s disease (
OHCM8 p.498), and Alzheimer’s disease ( OHCM8 p.492). New variant Creutzfeldt–Jakob disease (nvCJD,
OHCM8 p.492). New variant Creutzfeldt–Jakob disease (nvCJD,  OHCM8 pp.710, 711), on the other hand, is attributable to a transmissible infective agent.
OHCM8 pp.710, 711), on the other hand, is attributable to a transmissible infective agent.
 OHCM8 p.492)
OHCM8 p.492)• In Alzheimer’s disease, neurones projecting from the entorhinal cortex to the dentate gyrus (hippocampal formation) and the principal neurones in the CA1 region of the hippocampus appear particularly vulnerable to death. This explains the usual course of the disease in which episodic memory is impaired early on (p.776)
• Pyramidal neurones in the cortical circuitry that link temporal, prefrontal, and parietal cortical areas also display vulnerability in Alzheimer’s disease and the involvement of these areas in cognitive function may explain the later decline in cognition with disease progression
• A characteristic of Alzheimer’s disease is a relatively selective loss of cholinergic neurones in the basal forebrain, leading to a reduction in the cholinergic innervation of the cortex and hippocampus. Attempts to alleviate this cholinergic loss using the cholinesterase inhibitors, tacrine or donepezil, has resulted in modest improvement in cognitive function in some patients
• Abnormal processing of amyloid precursor protein (APP) is regarded as a key step in pathogenesis of Alzheimer’s disease, leading to extracellular accumulation of β-amyloid protein in the form of amyloid plaques
• Another characteristic of Alzheimer’s disease is intraneuronal deposition of the phosp horylated microtubule associated protein, tau. When neurones die, phosphorylated tau filaments aggregate to form extracellular neurofibrillary tangles (NFT)
• Neurones die by apoptosis and necrosis in Alzheimer’s disease. However, it remains to be established whether amyloid plaques and NFTs mediate neurotoxicity.
 OHCM8 p.498)
OHCM8 p.498)• Parkinson’s disease is manifest primarily as a disease of motor control resulting from the degeneration of nigrostriatal dopaminergic neurones in the basal ganglia
• Both apoptotic cell death and death induced by ROS and mitochondrial dysfunction have been implicated in Parkinson’s disease
• Major treatment involves increasing brain dopamine levels by oral administration of the metabolic precursor, L-dopa. Dopamine itself cannot cross the blood–brain barrier. Brain levels of L-dopa are increased by the use of inhibitors of dopa decarboxylase (e.g. carbidopa) in the periphery
• Intraneuronal protein deposits, called Lewy bodies, are characteristic of Parkinson’s disease
• A central role for Lewy bodies in Parkinson’s disease neuropathology is suspected because mutations in genes encoding for their constituent proteins (e.g. α-synuclein) have been linked to some familial forms of the disease.
Mutations in the SOD-1 gene have been causally linked to amyotrophic lateral sclerosis, a form of motor neurone disease that results in the degeneration of motor neurones in the spinal cord, brainstem, and motor cortex.
 OHCM8 p.716)
OHCM8 p.716)• Huntington’s disease is characterized by involuntary jerky movements that result from GABAergic cell death in the striatum. It is thought this leads to hyperactivity of the nigrostriatal dopamine pathway. Treatment of the symptoms is through dopamine D2 antagonists (p.786) or GABA agonists such as baclofen
• Huntington’s disease is inherited and attributable to the presence of high repeats of the DNA nucleotide sequence, CAG, in the huntington gene (that encodes for a protein implicated in regulating apoptosis and other cell death mechanisms).
 OHCM8 pp.710, 711)
OHCM8 pp.710, 711)• NvCJD is a type of spongiform encephalopathy characterized by a vacuolated appearance of the postmortem brain and associated with dementia and loss of motor coordination
• It is postulated that an infective agent—an abnormal form of a protein called a prion—triggers the disease. The abnormal prion is thought to lead to accumulation of insoluble prion protein in the brain, triggering neurotoxic processes.