
The peripheral nervous system: somatic nervous system
The peripheral nervous system: autonomic nervous system
Neuromuscular transmission: drug intervention
Ganglionic synaptic transmission in the autonomic nervous system
Synaptic transmission at the post-ganglionic parasympathetic nerve terminal
Clinical use of muscarinic agonists and antagonists
Synaptic transmission at the post-ganglionic sympathetic nerve terminal
Therapeutic uses of α and β agonists
Non-adrenergic, non-cholinergic nerves in the PNS
Together, the peripheral and central nervous systems comprise an integrated network of neurones, which allows rapid transmission and processing of information as nervous impulses. In conjunction with the endocrine system, this network is responsible for homeostasis of an organism, as well as for its behavioural patterns. In humans, this includes higher-level functions such as thinking and memory, as well as more basic activities.
Nervous tissue comprises two basic cell types:
• Neurones (excitable cells which transmit nervous impulses over long distances)
• Glial cells (non-neural support cells which are closely associated with neurones).
The CNS includes the brain and the spinal cord and is divided into grey and white matter. Grey matter contains the cell bodies, dendrites, parts of axons, and glial cells, and makes up the cerebral and cerebellar cortex, as well as the central sections of the spinal cord. The white matter contains the nerve fibres and glial cells, but not the cell bodies, except in grey matter nuclei (islands of aggregated cell bodies within the white matter) and includes those parts of the brain which are not grey matter as well as the outer regions of the spinal cord.
The PNS includes all nervous tissue located outside the CNS. It includes nerves, ganglia, and nerve endings, as well as associated supporting cell types. PNS nerves may be efferent, conducting signals away from the CNS to effector organs or tissues (e.g. skeletal muscle) or afferent, conducting nervous impulses towards the CNS (e.g. from sensory receptors).
All neurones (e.g. Fig. 4.1), whether central or peripheral, include three separate parts:
• Dendrites—multiple long processes which are specialized for receiving external stimuli from other cells
• Cell body (perikaryon)—contains the cell nucleus
• Axon—conducts a nervous impulse to other cells. Axons form synapses with other cells (neurones and non-nerve cells) via swellings called terminal boutons.
Dendrites are branching extensions of the nerve which, unlike axons, become thinner as they divide. They receive many synapses and are the signal reception and processing site of the nerve.
The cell body encompasses the nucleus surrounded by cytoplasm. Free ribosomes and RER can be seen as Nissl bodies (basophilic granular areas) in the cytoplasm, and are responsible for the production of proteins, including neurotransmitters. Abundant neurofilaments (intermediate filaments) can be found within nerve cell bodies. Mitochondria are present in large numbers in the cytoplasm (as well as in axon terminals).
Axons are cylindrical processes and are often very long. The axon hillock arises from the perikaryon as a pyramidal shaped region, from which the axon projects. The axoplasm is contained within the axon, which is surrounded by a plasma membrane (axolemma). The axons of nerves can be myelinated or unmyelinated.
There are three major axonal shapes. The cell body position determines the type of axonal shape by altering the number of cell processes formed by the dendrites and axons:
• Multipolar neurones: have more than two cell processes (e.g. motor neurones)
• Bipolar neurones: have one dendrite and one axon. Found only in specialized sensory areas such as the olfactory area and retina
• Pseudounipolar neurones: the cell body forms a T-shape, with the dendritic process and the axon ‘bypassing’ the cell body. Impulses avoid the perikaryon, e.g. spinal ganglia (sensory neurones).

Fig. 4.1 Schematic diagram showing arrangement of motor and sensory neurones.
Reproduced with permission from Ross MH, Kaye GI, Pawlina W (2003). Histology: A Text and Atlas, 4th edn. Baltimore: Lippincott Williams and Wilkins.
Bundles of nerve fibres surrounded by a sheath of connective tissue make up the peripheral nerves. Nerve fibres are ensheathed in three layers:
• The endoneurium—reticular fibres produced by Schwann cells surrounding axons
• The perineurium—a layer of flattened epithelial-like cells, which form a protective barrier surrounding a bundle of nerve fibres, preventing macromolecules from approaching the fibres
• The outermost epineurium—a dense fibrous external sheath which envelops several bundles and forms the outermost layer of the nerve.
There are 10 times more glial cells than neurones in the brain and they have many important functions. They insulate neurones, provide structural support and help maintain the surrounding environment, play a role in repair of neurones, and form part of the blood–brain barrier. Several different types of glial cell exist and these differ between the peripheral and central nervous systems.
Star-shaped cells with many processes. Their most important function is to form the blood–brain barrier. In so doing, they control metabolic exchange with the extracellular fluid and blood, thus controlling the extracellular environment of the brain.
Small cells with multiple processes and dense drawn-out nuclei. They are the mononuclear phagocytic arm of the immune system within the CNS. They are important in inflammation and repair. When activated, the morphology of the cell changes to resemble that of a macrophage, and they perform the same functions, namely phagocytosis and antigen presentation.
Cells found only in the CNS. They produce myelin to insulate neuronal axons and increase the speed of conduction. They can each insulate more than one neurone, unlike peripheral myelin-producing cells (Schwann cells), and wrap around their axons. Modified membrane layers with raised lipoprotein content make up the myelin which insulates the axons.
Myelinate axons in the PNS. The Schwann cell wraps its membrane around the axon. The membranes of the cell merge to form myelin (a lipoprotein). More than one cell is required to insulate each axon. There are gaps between the Schwann cells, called nodes of Ranvier. The internodal gap is 1–2mm (the length of the Schwann cells). Unmyelinated axons are of smaller diameter. Unmyelinated cells still have a covering of Schwann cells but each Schwann cell can cover many unmyelinated axons. The Schwann cells lie adjacent to one another to form a continuous sheath, hence there are no nodes of Ranvier. In the CNS, there are many unmyelinated axons which have no sheath whatsoever.
Neuronal cell bodies and glial cells supported by connective tissue make up a ganglion. It is where nerves synapse with each other as one nerve enters and another exits. The ganglion cells are supported by a connective tissue framework and capsule, surrounding the cell bodies and glial cells. The axons found in ganglia tend to be pseudounipolar. There are two types of ganglion:
• Sensory ganglia receive impulses that are relayed to the CNS. There are:
• Spinal ganglia (from the dorsal root of the spinal nerves)
• Autonomic ganglia of the parasympathetic nervous system tend to be located within the organ that they innervate such as the gastrointestinal tract, as intramural ganglia. They are supported by the surrounding stroma of the organ, so have minimal or no connective tissue or supporting glia. Sympathetic ganglia are located away from target organs, e.g. the cervical ganglion or the ganglia of the sympathetic trunk.
The nervous system is divided into the CNS (CNS; the brain and the spinal cord;  pp.710–715) and the PNS, consisting of the nerves that control skeletal muscle contraction (voluntary movements) as well as smooth muscle and organ function (involuntary activity).
pp.710–715) and the PNS, consisting of the nerves that control skeletal muscle contraction (voluntary movements) as well as smooth muscle and organ function (involuntary activity).
Nerves that stimulate contraction of skeletal muscle belong to the somatic efferent system, whilst those that modulate involuntary actions belong to the autonomic system. The different functions of the somatic and autonomic nervous systems are reflected in different neuronal arrangements, neurotransmitters, and receptors associated with each system.
There are two elements to the somatic nervous system:
• Somatic motor (efferent) neurones that stimulate contraction of skeletal muscle
• Somatosensory neurones that convey information about muscle and tendon stretch to the spinal cord.
A number of key features distinguish somatic neurones from their autonomic counterparts:
• A single neurone spans between the spinal cord and the muscle—there are no intervening synapses
• The neurotransmitter released at the neuromuscular junction (NMJ) is exclusively acetylcholine (ACh)
• The post-synaptic receptors at the NMJ are exclusively nicotinic ACh receptors.
The autonomic nervous system is divided into two main branches: the parasympathetic and the sympathetic nervous systems (Fig. 4.2). In some instances, these systems act in opposition to each other (e.g. increased parasympathetic stimulation slows the heart, increased sympathetic drive increases heart rate). However, some organs or tissues are only innervated by one of the systems (e.g. blood vessels usually only have sympathetic innervation).
There are a number of similarities between the sympathetic and parasympathetic nervous systems:
• Both generally feature a single synapse, found in ganglia positioned between the spinal cord and the target organ or tissue. The neurones that stretch between the spinal cord and the ganglia are called pre-ganglionic; those between the ganglia and the target tissue are post-ganglionic. The sympathetic nerves that supply the adrenal medulla are an exception to this rule, as they do not feature ganglia
• The cell bodies of the pre-ganglionic cells are found in the ganglia; those of the post-ganglionic cells are generally found in the tissue
• The neurotransmitter released by the pre-ganglionic neurone at the ganglionic synapse is exclusively ACh, which acts on nicotinic ACh receptors on the post-ganglionic neurone to propagate the signal
• A cocktail of neurotransmitters is often released at the synapse with the tissue. Co-transmitters often have different rates and durations of action.
There are, however, a number of very important differences between the sympathetic and parasympathetic nervous systems (Table 4.1).
A third division of the autonomic nervous system forms a complex net around the viscera, with cell bodies embedded in the intramural plexuses of the intestinal wall. Neurones from both the sympathetic and parasympathetic systems synapse onto enteric nerves, which release a number of neuropeptides and transmitters other than noradrenaline and ACh (e.g. 5-HT, nitric oxide, ATP).
Table 4.1 Differences between the parasympathetic and sympathetic nervous systems
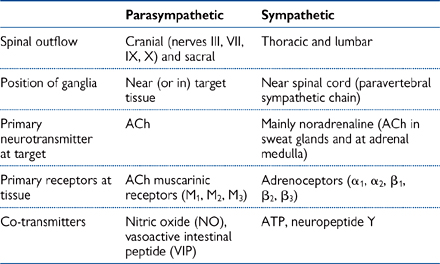

Fig. 4.2 Features of the sympathetic and parasympathetic branches of the autonomic nervous system.
All cells have a membrane potential (Em). Em is the voltage difference (in mV) between the extracellular and intracellular faces of the cell membrane. Em is usually referenced to the extracellular face. At rest, Em is negative, meaning that the inside of the membrane is negative with respect to the outside.
Em is established by the permeability properties of the cell membrane. At an equilibrium potential, there is not net movement of charge (no current). For a membrane which is selectively permeable to ion X, the equilibrium potential is described by the Nernst equation:
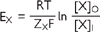
where EX = equilibrium potential
R = gas constant
T = absolute temperature
F = Faraday constant
ZX = the ionic charge of X
[X]I = intracellular concentration of X
[X]O = extracellular concentration of X
With appropriate values for the constants, and converting natural log to log10, the equation for a monovalent cation at 37°C becomes
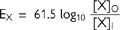
• The Na++K+ ATPase establishes high extracellular [Na+] and high intracellular [K+].
• For an idealized cell membrane which is permeable only to K+ ions, passive efflux of K+ down its concentration gradient will polarize the membrane: the inside face becomes negative with respect to the outside.
• The negative inside potential opposes further efflux. As more ions exit the cell, so the opposing force becomes greater.
• A point is reached at which chemical and electrical gradients are equal but opposing
• At this point, there is no net ion current, and equilibrium is achieved. The Nernst equation predicts EK = –90mV in mammalian nerves. Similar calculations for a membrane solely permeable to Na+ ions predict Na+ ion influx would cease at +60mV.
Since the resting cell membrane has high K+ permeability, K+ ion movements are most important for establishing Em. However, other ions (notably Na+, Cl–) are also permeable, but to lesser extents. Their movements also contribute to the potential difference across the membrane. Em is not the Nernst potential for any single ion, but rather an aggregate based on contributions weighted by membrane permeability. In a real cell, no ion is at equilibrium—although there are ionic currents, Em is at equilibrium. These currents therefore add up to zero.
The constant field equation describes this situation:

where P = permeability of the ion and [] signifies concentration.
In a mammalian nerve, Em = –70mV. Ion gradients are maintained by the Na++K+ ATPase. This pump transporter has a 3Na+:2K+ pumping ratio which makes it electrogenic. Its activity makes only a very small contribution to the inside negative Em. Note, however, that ion movements down gradients which the ATPase establishes are responsible for Em.
The number of ions moving is miniscule in respect of the total present in the extracellular and intracellular solutions, and ion numbers do not change in bulk solution, only at the faces of the membrane.
Changing ion gradients or membrane permeability can alter Em.
• Increasing [K+]O reduces the outward chemical gradient for K+: the contribution of K+ ion movement to Em is smaller, so Em is less negative or depolarized
• Likewise, increasing PNa will enhance the contribution of Na+ ion movements. Em again becomes less negative.
The action potential is a rapid (1–2ms) transient electrical impulse in which membrane potential (Em) is displaced by up to 100mV. It is exhibited by electrically excitable cells, and is the foundation for nervous conduction and muscle contraction.
Action potentials arise from changes in membrane permeability. Electrically excitable cells possess voltage-activated channels that open in response to depolarization. The action potential is an all-or-none response: once a threshold point is passed, the events progress in a time-dependent fashion. So long as the threshold can be attained, the magnitude of the depolarizing stimulus is irrelevant.
Key events in the nerve action potential (Fig. 4.3) are:
• Initial depolarization: a relatively gentle increase in Em. In nerve endings, this results from cation channel opening by chemical or physical stimuli and, within nerves, by the spread of current from neighbouring, already excited regions
• The upstroke: once threshold (generally +15mV above Em) is reached, voltage-activated Na+ channels open and Na+ ions enter the cell down their electrochemical gradient. The process is regenerative: once some open, the depolarization they initiate promotes the opening of others. The increase in Na+ permeability causes Em to move rapidly towards, but not reach, ENa. Em overshoots zero to approximately +35mV. The increase in permeability is short lived: the depolarization initiates fast opening of activation gates, followed by slower closing of inactivation gates
• Repolarization: this represents the inactivation of Na+ channels and opening of voltage-activated K+ channels. K+ ions efflux down their electrochemical gradient. Em recovers towards resting levels. K+ channels are activated at the same point as Na+ channels, but opening takes longer. Most K+ current flows after Na+ channels have closed
• After hyperpolarization: the closing of K+ channels by repolarization is slow. The transient raised permeability to K+ takes Em closer to EK. Repolarization also returns Na+ channels from the inactive to closed state.
The ion fluxes occurring in this sequence are very small in comparison with the number of ions present in the extracellular and intracellular solutions. Voltage-activated Na+ channels can be inhibited by tetrodotoxin (TTX), voltage-activated K+ channels by tetraethylammonium.
There is a refractory period during which the neurone is resistant to firing another action potential.
• The absolute refractory period is the time during which voltage-activated Na+ channels recover from inactivation, and during which there are insufficient channels which can be recruited to initiate an upstroke.
• The relative refractory period correlates with the after hyperpolarization, where larger stimuli are required to elicit a second action potential.
• Action potentials propagate by local current flow:
• At the region of excitation, the nerve potential is reversed
• The negative outside region acts as a current sink, drawing positive charges from areas behind and ahead of the excited region
• The polarity of the flanking membrane regions is reduced—this is termed electrotonic depolarization
• Na+ channels in the membrane behind the action potential remain inactivated, preventing firing. If depolarization in the region ahead reaches threshold, however, the action potential can fire
• This ‘one-way’ propagation is called orthodromic conduction
• Electrotonic spread of depolarization decays from the site of initiation.
As nerve diameter increases, cytoplasmic resistance decreases, local current flow is more rapid, and conduction velocity is faster. Nerves can be myelinated with concentric wrappings of Schwann cell membranes, to increase membrane resistance, minimize electrotonic current decay across the nerve cell membrane, and speed up conduction. Gaps in the myelination (called nodes of Ranvier) have high densities of voltage-activated channels. The current sink at one node triggers electrotonic depolarization in the next, so the action potential ‘jumps’ from node to node. This is termed saltatory conduction, and speeds up conduction by up to 50 times.
The fastest conducting fibres are therefore
• Aα (15–20µm)—myelinated: innervate skeletal muscle
• Aγ (2–5µm)—myelinated: sensory (pain, temperature)
• C (0.5–1µm)—unmyelinated: sensory.
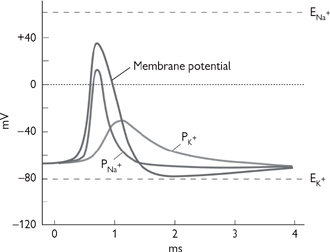
Fig. 4.3 The permeability changes that are responsible that are responsible for the action potential of a nerve.
Reproduced with permission from Pocock G, Richards CD (2006). Human Physiology: The Basis of Medicine, 3rd edn, p69. Oxford University Press.
 OHCM8 p.574)
OHCM8 p.574)Few developments have had a greater impact on medicinal practice than the discovery and development of anaesthetics from the early 1800s. General anaesthesia is routinely used to prevent patients feeling pain during surgery and have been instrumental in the development of the specialty of surgery. Increasingly, however, surgeons are turning to means of inducing local anaesthesia, in order to minimize the risk associated with anaesthetic practice.
It is perhaps surprising, in light of their widespread use, that the mechanism(s) by which general anaesthetics render patients unconscious and oblivious to pain are relatively poorly understood, particularly as, unlike drugs that act on specific receptors, the substances that are able to induce general anaesthesia do not have many molecular characteristics in common. Many general anaesthetics are volatile liquids or gases and are administered via inhalation. However, a number of intravenous anaesthetics are commonly used to induce rapid anaesthesia prior to prolonged administration of an inhaled anaesthetic during operations.
• Volatile liquids (e.g. those related to ether, including halothane, enflurane, and isoflurane) or gases (nitrous oxide, N2O—‘laughing gas’)
• Show a clear relationship of potency with lipid solubility: the more lipid-soluble anaesthetics are more potent. However, lipid solubility also carries the inherent danger of accumulating in body fat, leading to a slow recovery on cessation of anaesthesia
• Are thought to act either through altering the characteristics of the lipid bilayer of cells (e.g. increased fluidity or bilayer volume) or by interacting with hydrophobic regions of critical channels and receptors to modify their function. Both mechanisms are plausible and it is possible that a mixture of the two is responsible for anaesthetic activity
• Inhibit synaptic transmission, rather than axonal conduction (compare with local anaesthetics, see  pp.225–226)
pp.225–226)
• When administered at the appropriate levels for surgery, cause unconsciousness and a loss of response to painful stimuli (analgesia) and reflexes. Muscles also relax but neuromuscular blocking drugs are often administered alongside general anaesthetics to prevent muscle contraction during surgery ( p.230)
p.230)
• Generally, result in a depression in cardiovascular and respiratory function. This is an adverse effect that requires careful monitoring: overdose can result in death because of cardiovascular and/or respiratory collapse.
• Have a much quicker action than inhaled anaesthetics: typically an intravenous anaesthetic acts within 20s of administration (the time taken to reach the brain in the bloodstream). A single injection usually induces anaesthesia for about 10min
• Are very lipid-soluble to accelerate the onset of effect: they cross the blood–brain barrier rapidly
• Are centrally acting agents such as barbiturates (e.g. thiopental, propofol), benzodiazepines (e.g. diazepam), or N-methyl-D-aspartate (NMDA) receptor blockers (e.g. ketamine)
• Can be rapidly redistributed to tissues with high blood flow and, more slowly, to poorly perfused fat. This is a problem that affects thiopental in particular, for which blood concentrations fall very rapidly, but its slow redistribution to fat prior to metabolism means that it stays within the body long after anaesthesia, leading to a ‘hangover’. The effect is exacerbated with repeated injections—thiopental is only administered to induce anaesthesia prior to maintenance by inhaled anaesthetics ( p.224). However, propofol and ketamine have sufficiently long duration of action to facilitate their sole use for minor surgery.
p.224). However, propofol and ketamine have sufficiently long duration of action to facilitate their sole use for minor surgery.
Modern general anaesthetic practice, therefore, usually involves induction with an intravenous injection of an agent such as thiopental prior to prolonged administration of a combination of inhaled anaesthetics, such as isoflurane and nitrous oxide. A neuromuscular blocking drug such as atracurium is also administered to induce muscle paralysis.
 OHPDT2 p.768)
OHPDT2 p.768)• Generally conform to a common structure consisting of an aromatic region (containing a benzene ring) linked, via an ester or amide bond, to an amine side-chain that makes the molecule basic
• Have a pKa ~8–9, meaning that they are almost entirely ionized at physiological pH resulting in relatively slow penetration of nerves ( p.86). Quaternary compounds are entirely ionized and are not able to cross lipid membranes—instead, they enter nerves through open Na+ channels. Inflamed tissue can be resistant to local anaesthetics because it is often acidic, resulting in increased drug ionization and reduced cellular penetration (see Pharmacology–General Principles (Chapter 1) (absorption distribution and clearance of drugs))
p.86). Quaternary compounds are entirely ionized and are not able to cross lipid membranes—instead, they enter nerves through open Na+ channels. Inflamed tissue can be resistant to local anaesthetics because it is often acidic, resulting in increased drug ionization and reduced cellular penetration (see Pharmacology–General Principles (Chapter 1) (absorption distribution and clearance of drugs))
• Are rapidly hydrolysed by enzymes in the blood. Local anaesthetics that contain ester bonds are rapidly metabolized by esterase enzymes and typically have a plasma half-lives of <1h. Amides are more stable (half-life ~2h) and are, therefore, longer lasting
• Act to block Na+ channels essential to nerve conduction ( pp.222–223). Some local anaesthetics have a ‘use-dependent’ effect—their action increases the more the channel is open. This is because the ionized form of the drug is able to enter open channels to reach its binding site within (the only means of entry for completely ionized quaternary compounds). Partially ionized tertiary local anaesthetics can reach their site of action by this route (ionized) or through the influx of non-ionized drug into the lipid bilayer
pp.222–223). Some local anaesthetics have a ‘use-dependent’ effect—their action increases the more the channel is open. This is because the ionized form of the drug is able to enter open channels to reach its binding site within (the only means of entry for completely ionized quaternary compounds). Partially ionized tertiary local anaesthetics can reach their site of action by this route (ionized) or through the influx of non-ionized drug into the lipid bilayer
• Affect small nerve fibres more readily than large ones. This is of theoretical importance because the fibres that carry nociceptive impulses are the smallest (Aδ and C fibres), while those conducting impulses relating to other senses (e.g. touch) or motor control are much larger. In reality, however, the relative non-selectivity of local anaesthetics for Na+ channels in all axons results in some loss of sensation and motor control in the local area
• Can be applied to the skin (eutectic mixture of local anaesthetics—EMLA®) or injected directly into the target tissue, into the bloodstream distal to a pressure cuff to prevent systemic distribution of the drug, in the vicinity of a nerve trunk for the target region, or into the subarachnoid space to block transmission in the spinal cord
• Have side-effects due to their ultimate absorption into the systemic circulation. As with general anaesthetics, systemic side-effects include centrally induced respiratory depression, as well as hypotension due to reduced sympathetic nervous system activity and depressed cardiac contractility. The cardiac effects are used to advantage in the use of lidocaine as an antiarrhythmic drug ( p.431). Lower systemic doses of local anaesthetics have a stimulatory effect on the CNS, resulting in agitation and tremor.
p.431). Lower systemic doses of local anaesthetics have a stimulatory effect on the CNS, resulting in agitation and tremor.
Epidural is a specific type of local anaesthesia that involves infusion of local anaesthetic (e.g. lidocaine) into the epidural space surrounding the spinal cord. The target area is delineated by the level in the spine at which the drug is administered: infusion into the lumbar region anaesthetizes the lower abdomen and the legs; a thoracic epidural would have a more global effect but carries more risk because it might impact on nerves supplying the heart and muscles involved in breathing. Epidurals are used routinely to reduce the pain associated with childbirth, but their use is increasingly being advocated for abdominal surgery, and even cardiothoracic surgery.
When an action potential reaches the end of a nerve, the excitation can be transmitted to another nerve or to an effector by the release of a transmitter substance. The excitation of skeletal muscle by ACh release from a motor neurone exemplifies the processes underlying chemical transmission (Fig. 4.4).
Motor nerves send processes to individual muscle fibres, which collectively form a motor unit. Each nerve process terminates at the NMJ or end plate, where unmyelinated bulb-like nerve endings (boutons) interface with invaginations of the muscle fibre membrane. The 50nm space between the two membranes is termed the synaptic cleft. The membrane here is highly invaginated to increase the surface area.
The unmyelinated nerve membrane contains voltage-activated Ca2+ channels, which are opened by the arrival of the action potential. Ca2+ ions flow into the cell down their electrochemical gradient and trigger fusion of approximately 200 vesicles containing up to 10 000 molecules of ACh. Vesicles contain 150mM ACh, which is synthesized in the nerve ending from choline and acetylcoenzyme A, and concentrated within the vesicle by a H+-driven exchanger. Ca2+ ions are then extruded from the cell to terminate vesicle fusion.
The ACh released into the synaptic cleft diffuses to the post-junctional membrane of the muscle fibre where two molecules bind to each nicotinic ACh receptor. These receptors are doughnut-shaped heteropentamers and are ionotropic (ion channel) receptors. When ACh binds, a non-specific cation channel is opened, which leads to net influx of positive ions and depolarization.
This depolarization is approximately 40mV and is termed the end plate potential (epp). If sufficiently large, the threshold is reached for opening of voltage-activated Na+ channels in the muscle fibre membrane, and an action potential is initiated. The action potential involves the same sequence of events as that in the motor nerve. Small, spontaneous depolarizations (miniature epps) are observed in the absence of nerve stimulation. The magnitude of miniature epps is always a multiple of 0.4mV. The elemental depolarization of 0.4mV represents the release of ACh contained in one vesicle or quantum.
ACh is rapidly hydrolyzed in the synaptic cleft by the enzyme acetylcholinesterase, and re-uptake of choline into the nerve ending for synthesis of more ACh occurs on a Na+-dependent transporter.
Fig. 4.4 Cholinergic transmission.
 OHPDT2 pp.364, 670
OHPDT2 pp.364, 670The ability to prevent muscle contraction is clinically important as an adjunct to anaesthesia during surgery. The global paralysis caused by neuromuscular blocking drugs means that they prevent breathing and can only be used when artificial ventilation is available.
• Tubocurarine was the first ‘non-depolarizing’ blocking agent to be identified. Tubocurarine is a constituent of curare, the poison that has long been used by South American Indians on their arrows and darts
• It is a competitive antagonist at nicotinic ACh receptors, where it inhibits binding of ACh in a concentration-dependent manner
• Tubocurarine has been superseded by modern alternatives with shorter action and fewer side-effects (e.g. pancuronium, vecuronium, atracurium)
• The side-effects of these compounds are related to their non-selective action on other nicotinic ACh receptors, including those in the autonomic ganglia. Hypotension is sometimes experienced, as well as bronchospasm due to histamine release from mast cells ( p.844).
p.844).
The effect of these drugs is easily reversed by inhibitors of acetylcholine esterase (AChE), the enzyme that breaks down ACh. Inhibition of this enzyme ultimately leads to the persistence of ACh in the synaptic cleft, leading to an increase in its concentration and successful competition for ACh receptors.
Another class of drug that causes muscle paralysis consists of the depolarizing blocking drugs (e.g. suxamethonium).
• Unlike tubocurarine, these drugs do not act to compete with ACh for nicotinic ACh receptors; instead, they act to stimulate nicotinic ACh receptors and cause paradoxical depolarization of the motor end plate
• In the first instance, this depolarization leads to contraction—transient twitching is an early feature in depolarizing block
• Within a few seconds, this twitching (or fasciculation) gives way to paralysis as the electrical excitability of the end plate region is lost
• The major advantage of depolarizing block over non-depolarizing blocking agents is that there is less impact on ganglionic nicotinic ACh receptors. However, there are dangers, particularly bradycardia.
There are a number of other drugs that impact on the pre-synaptic synthesis and release mechanisms of ACh.
• Vesamicol inhibits uptake of ACh into pre-synaptic vesicles
• The antibiotics streptomycin and neomycin prevent Ca2+ entry and can occasionally cause paralysis
• Botulinum toxin (from the bacterium, Clostridium botulinum, responsible for the type of food poisoning called ‘botulism’ ( OHCM8 p.421) inhibits specific proteins involved in exocytosis, resulting in paralysis and inhibition of the parasympathetic nervous system. This agent is currently used ‘therapeutically’ to treat a number of conditions characterized by muscle spasm, including cervical dystonia, blepharospasm, hemi-facial spasm associated with stroke, focal spasticity and lower limb dysfunction associated with cerebral palsy. Injections into the skin of the armpits also relieves excessive sweating, and injections into facial skin can temporarily reduce the appearance of wrinkles (Botox®)
OHCM8 p.421) inhibits specific proteins involved in exocytosis, resulting in paralysis and inhibition of the parasympathetic nervous system. This agent is currently used ‘therapeutically’ to treat a number of conditions characterized by muscle spasm, including cervical dystonia, blepharospasm, hemi-facial spasm associated with stroke, focal spasticity and lower limb dysfunction associated with cerebral palsy. Injections into the skin of the armpits also relieves excessive sweating, and injections into facial skin can temporarily reduce the appearance of wrinkles (Botox®)
• β-bungarotoxin is found in cobra snake venom and has a similar action to botulinum toxin. α-bungarotoxin in the same venom inhibits the nicotinic ACh receptor
• A number of AChE inhibitors exist alongside neostigmine. Edrophonium is very short acting and is used in the diagnosis of myasthenia gravis ( OHCM8 p.516) (muscle weakness due to autoimmune destruction of ACh receptors), and physostigmine is used in the treatment of glaucoma
OHCM8 p.516) (muscle weakness due to autoimmune destruction of ACh receptors), and physostigmine is used in the treatment of glaucoma
• Hemicholinium and triethylcholine prevent the uptake of choline into the nerve terminal, but do not have any clinical application.
The nervous system is not ‘hard-wired’—there are spaces at connecting points called synapses. Synapses are highly complex structures that convert an electrical signal in one neurone into a chemical signal that acts, in turn, to generate (or inhibit) an electrical signal in adjacent neurones or other excitable cells (e.g. muscle cells). It is the existence of synapses and the chemical mediators that they employ that is central to much of our pharmacological interventional strategy.
Interneuronal synapses offer a means of exquisite control over the transmission of signals. The key features of synaptic transmission are summarized in Figure 4.5.
• Action potentials arrive at the synaptic terminal of the pre-synaptic neurone: the greater the frequency of action potentials, the more neurotransmitter that is ultimately released
• The membrane at the synaptic terminal depolarizes ( p.222)
p.222)
• Voltage-gated Ca2+ channels open to facilitate the influx of Ca2+ into the pre-synaptic terminal
• Neurotransmitter-containing synaptic vesicles migrate to, and fuse with, the pre-synaptic membrane in response to increased intracellular Ca2+
• Neurotransmitter molecules are released into the synaptic cleft
• The neurotransmitter molecules diffuse across the cleft where they stimulate ion channel-linked receptors on the post-synaptic membrane, resulting in the altered permeability to a specific ion that causes a change in membrane potential. For example, activation of nicotinic ACh receptors is excitatory because it causes an influx of Na+ and depolarization; activation of GABA receptors is inhibitory because it causes an influx of Cl– ions and hyperpolarization
• The change in potential of the post-synaptic membrane caused by neurotransmitter release from a single action potential in the pre-synaptic terminal is called an excitatory (or inhibitory) post-synaptic potential (epsp or ipsp; Fig. 4.6).
The ultimate effect of the synaptic transmission is dependent on a number of factors:
• The neurotransmitter released
• The receptor subtype on the post-synaptic neuronal membrane
• The ion channel linked to the receptor.
The simplest scenario for interneuronal synaptic transmission involves a single synapse between the pre- and post-synaptic neurones. In this situation, the only controlling factor that determines whether the action potential is propagated in the post-synaptic neurone is the frequency of signals arriving at the synaptic terminal of the pre-synaptic neurone: increased frequency causes summation of the depolarization caused by epsps until they exceed the necessary threshold for an action potential in the post-synaptic neurone (Fig. 4.6).
Fig. 4.5 Synaptic transmission.
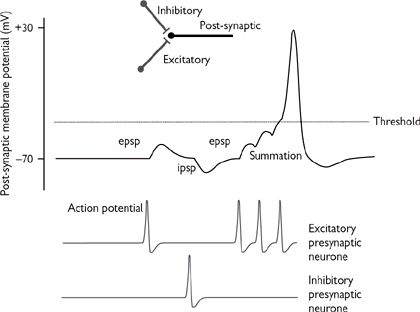
Fig. 4.6 Excitatory (epsp) and inhibitory (ipsp) post-synaptic potentials—summation.
It is more likely, however, that transmission at a synapse is susceptible to modulation by a number of other signals that impact on the pre- or post-synaptic neurones:
• Inhibitory neurones can synapse onto either the pre- or post-synaptic terminals. The neurotransmitters that are released by these neurones (e.g. GABA) act to hyperpolarize the neurone, reducing the likelihood of sufficient depolarization at the pre-synaptic terminal to facilitate transmitter release, or sufficient depolarization in the post-synaptic terminal to reach threshold for action potential propagation (Fig. 4.6). The balance of inhibitory and excitatory signals will ultimately determine whether an action potential is generated in the post-synaptic terminal
• Facilitatory neurones that synapse onto the pre-synaptic terminal act to increase the amount of neurotransmitter released by that neurone. This is generally through ‘sensitization’ of the pre-synaptic terminal to action potentials. For example, a facilitatory neurone might release 5-HT onto receptors on the pre-synaptic terminal. Activation of receptors stimulate G-protein-coupled phospholipase C and adenylate cyclase, leading to longer depolarization on the arrival of an action potential at the pre-synaptic terminal
• Other stimulatory neurones might synapse onto the post-synaptic neurone. Here, there may be ‘spatial summation’ of epsps from a number of excitatory neurones that trigger an action potential in the post-synaptic neurone.
The huge variety of synaptic connections present in the nervous system contributes to its enormous complexity and facilitates signal integration (Fig. 4.7).
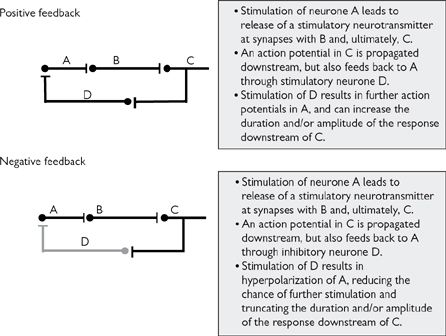
Fig. 4.7 Signal integration through synapses.
Many of the features already described for transmission at the NMJ (Fig. 4.7) are shared by synaptic transmission in the ganglia of both the parasympathetic and sympathetic nervous systems, on account of the fact that both processes involve the neurotransmitter ACh, and post-synaptic nicotinic ACh receptors (although there are minor structural differences between neuronal and muscular nicotinic receptors). Indeed, the events leading to transmitter release at the autonomic ganglia are indistinguishable from those already described, except that far less transmitter is required to stimulate an action potential in a neurone compared to a muscle. Equally, the events at the ACh receptor are identical to those at the NMJ, except that depolarization of the post-synaptic membrane results in propagation of an action potential in the post-ganglionic neurone, instead of a muscle.
The similarity between transmission at the NMJ and autonomic ganglia is responsible for some of the unwanted side-effects experienced with therapeutic intervention targeted at the NMJ. Nevertheless, there are some compounds with specificity for nicotinic ACh receptors at one or other of the muscular or ganglionic sites. In reality, specific ganglionic nicotinic ACh receptor stimulants or inhibitors have proved of little therapeutic use on account of their non-selective effects on both the sympathetic and parasympathetic branches of the autonomic nervous system. This lack of selectivity was responsible for the unwanted side-effects of the ganglion blocker, hexamethonium—the first drug used to treat hypertension: inhibition of the sympathetic pathway was responsible for reducing blood pressure (but also caused postural hypotension), but inhibition of parasympathetic pathways caused constipation, long-sightedness, impotence, urine retention, and dry mouth. This drug is no longer used in practice, although trimetaphan is a very short-acting ganglion blocking drug that is occasionally used in specific surgical operations.
ACh is also the neurotransmitter released by post-ganglionic parasympathetic nerves, but it acts upon ACh muscarinic receptors on the post-synaptic membrane of the relevant tissue. To date, five subclasses of muscarinic receptor have been identified, but only three have been well-characterized:
• M1 receptors are found in the CNS, autonomic, and enteric ganglia (neural M1), and on parietal cells that secrete gastric acid (gastric M1)
• M2 receptors (‘cardiac’) are found in the heart (primarily the atria) and on pre-synaptic neurones
• M3 (glandular) receptors are found on exocrine glands, smooth muscle, and vascular endothelium.
All muscarinic receptors are G-protein coupled ( pp.50–51)
pp.50–51)
• Activation of M1 and M3 receptors activates G-protein-coupled phospholipase C to generate IP3 and diacylglycerol, leading to cellular activation
• Activation of M2 receptors inhibits G-protein-coupled adenylate cyclase, leading to reduced rate and force of contraction in the heart.
Unlike drugs that act on ganglionic nicotinic receptors, those that act selectively on muscarinic receptors are selective for the parasympathetic nervous system. In addition, the existence of structurally distinct receptor subclasses raises the possibility of further selectivity.
In reality, however, only two muscarinic agonists are in regular clinical use:
• Pilocarpine can be used to reduce the intraocular pressure caused by glaucoma ( OHCM8 p.430) by causing activation of the constrictor pupillae muscle (the iris), increasing the size of the pupil and allowing aqueous humour to flow more easily out through the canal of Schlemm. This is a long-acting drug (effects last for a day) and it is administered directly onto the surface of the eye
OHCM8 p.430) by causing activation of the constrictor pupillae muscle (the iris), increasing the size of the pupil and allowing aqueous humour to flow more easily out through the canal of Schlemm. This is a long-acting drug (effects last for a day) and it is administered directly onto the surface of the eye
• Bethanechol is very occasionally used to aid bladder emptying or to ease constipation.
However, a number of muscarinic receptor antagonists are in clinical use:
• Atropine was the first muscarinic antagonist to be characterized as the active ingredient of deadly nightshade (Atropa belladonna). Locally delivered atropine used to be employed to dilate the pupils for ophthalmic inspections ( OHCM8 p.76), but was accompanied by long-sightedness caused by inhibition of contraction of the ciliary muscle. Cyclopentolate and tropicamide (
OHCM8 p.76), but was accompanied by long-sightedness caused by inhibition of contraction of the ciliary muscle. Cyclopentolate and tropicamide ( OHCM8 p.76) are preferred for this function nowadays. The antagonistic effects of systemically administered atropine are widespread, from the inhibition of secretions and sweating, to an increase in heart rate, bronchodilation, and constipation, reflecting the non-selective nature of this drug for different muscarinic receptor subtypes. These peripheral effects are accompanied by central effects that include agitation and disorientation. Hyoscine has very similar peripheral effects, but has sedative properties at low doses. It is sometimes used to treat motion sickness. Atropine methonitrate is an analogue of atropine that does not cross the blood–brain barrier, rendering it selective for peripheral parasympathetic neurones
OHCM8 p.76) are preferred for this function nowadays. The antagonistic effects of systemically administered atropine are widespread, from the inhibition of secretions and sweating, to an increase in heart rate, bronchodilation, and constipation, reflecting the non-selective nature of this drug for different muscarinic receptor subtypes. These peripheral effects are accompanied by central effects that include agitation and disorientation. Hyoscine has very similar peripheral effects, but has sedative properties at low doses. It is sometimes used to treat motion sickness. Atropine methonitrate is an analogue of atropine that does not cross the blood–brain barrier, rendering it selective for peripheral parasympathetic neurones
• Ipratropium ( OHCM8 p.177) is used as a bronchodilator in chronic obstructive airways disease—selectivity for bronchial receptors is imparted by delivery through an inhaler
OHCM8 p.177) is used as a bronchodilator in chronic obstructive airways disease—selectivity for bronchial receptors is imparted by delivery through an inhaler
• Pirenzepine is a relatively selective M1 receptor antagonist that is used to reduce gastric acid secretion and help prevent gastric ulcers.
With the exception of sweat glands and the adrenal medulla (Fig. 4.2) the main neurotransmitter in the sympathetic nervous system is noradrenaline. Like ACh, noradrenaline is synthesized and stored in the pre-synaptic nerve terminal of the post-ganglionic neurone and is released in response to action potentials. However, there are a number of differences between the two systems, not only in relation to the receptors that are activated but also in the re-uptake and metabolic processes.
• Synthesis from the amino acid, tyrosine, is mediated by a series of enzymes, the first of which catalyses the rate-limiting step of the process (see Fig. 4.8)
• Noradrenaline is actively transported into vesicles by the monoamine transporter, to protect it from the enzyme, monoamine oxidase (MAO), which catalyses its inactivation in the cytoplasm
• Exocytosis of noradrenaline is stimulated by an influx of Ca2+ through voltage-sensitive Ca2+ channels that open in response to action potential-mediated depolarization of the nerve terminal
• Noradrenaline diffuses across the synaptic cleft, where it stimulates post-synaptic adrenoceptors, resulting in an effect that is dependent on the adrenoceptor subtype and the tissue (see Table 4.2)
• Noradrenaline is reabsorbed into the pre-synaptic neurone via a transport protein, known as uptake 1. A similar process takes place in the surrounding tissue, primarily via a different protein—uptake 2. Uptake 1 is more selective for noradrenaline, whilst uptake 2 is less selective and also takes up adrenaline
• Noradrenaline reabsorbed into the pre-synaptic terminal is largely inactivated by MAO, although some is recycled into the vesicles. Noradrenaline and adrenaline taken into surrounding tissues via uptake 2 are inactivated by catechol-O-methyl transferase (COMT)
• As well as its action on post-synaptic receptors, noradrenaline can also activate receptors on the pre-synaptic terminal (α2 adrenoceptors) that act to inhibit further exocytosis of noradrenaline (negative feedback).
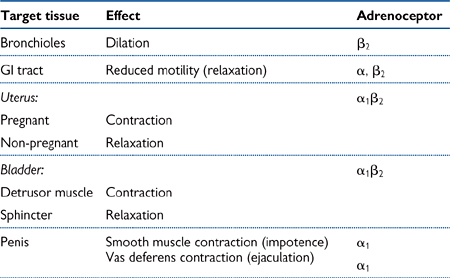
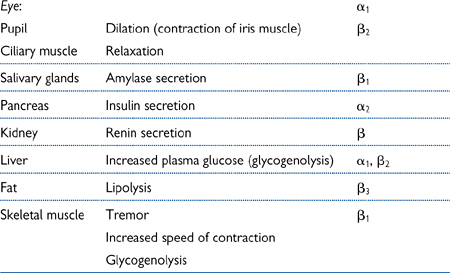
Tissue effects that are mediated by sympathetic nervous activity are extremely varied and often confusing (see Table 4.2). The following are examples that highlight some of the apparent contradictions:
• Noradrenaline can cause dilation of some blood vessels and constriction of others, depending on whether the vessels contain predominantly β2 or α1 adrenoceptors, respectively
• Stimulation of α1 receptors in most smooth muscle causes contraction through phospholipase C activation and an increased intracellular Ca2+, but in gastrointestinal muscle, it causes relaxation due to activation of K+ channels and hyperpolarization
• Stimulation of α2 adrenoceptors located on vascular smooth muscle cells causes constriction of blood vessels. However, the same receptors located on the endothelium of blood vessels can ultimately cause relaxation of the underlying vascular smooth muscle through release of endothelium-derived relaxing factors (nitric oxide, prostacyclin). Moreover, in the gastrointestinal tract, stimulation of α2 adrenoceptors causes relaxation of smooth muscle because the receptors are located on the pre-synaptic terminal and their stimulation encourages inhibition of further NA release by negative feedback.
To accurately predict the effect of NA on a given tissue, it is essential to know:
• The identity of the predominating adrenoceptor subtype
• The locality of the adrenoceptors (pre- or post-synaptic, endothelium or smooth muscle cell)
• The nature of the G-coupled 2nd messenger system.
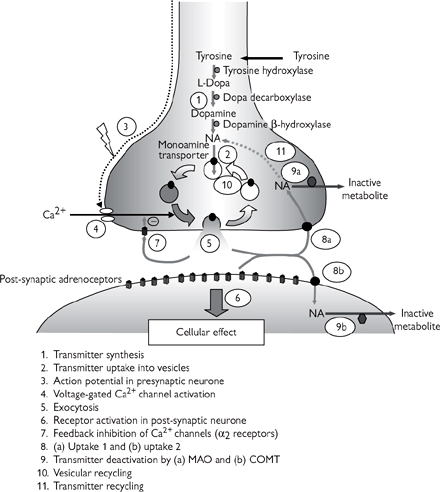
Fig. 4.8 Noradrenergic transmission.
Some of the most important actions of sympathetic stimulation relate to the cardiovascular system and it is in this field that much of the pharmacological interest has centred. The main cardiovascular actions of noradrenaline and adrenaline are:
• Increased heart rate and force of contraction (β1 receptors)
• Increased renin secretion in the kidney, leading to elevated angiotensin II synthesis, increased plasma volume, and vasoconstriction (β receptors)
• Redistribution of blood due to vasoconstriction of blood vessels in the splanchnic vascular beds (primarily α1 receptors) and dilatation of vessels supplying skeletal muscles in particular (primarily β2 receptors)
• Reduced compliance (or increased stiffness) of large arteries (α receptors).
The overall effect of these changes in the cardiovascular system is to increase blood pressure, making the sympathetic nervous system a legitimate target for reducing blood pressure in hypertension.
 OHPDT2 p.158
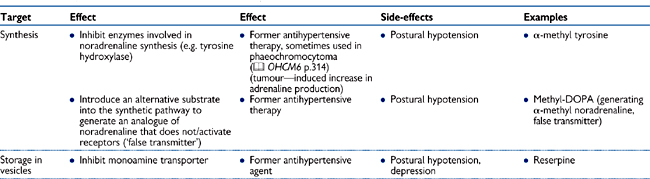
OHPDT2 p.158• α-adrenoceptor antagonists inhibit noradrenaline-mediated vascular constriction, but their effects are rapidly overcome by reflex increases in heart rate and force of contraction. Nevertheless, phenoxybenzamine (irreversible) and phentolamine (reversible) are non-selective α adrenoceptor antagonists, and prazosin is a selective α1 adrenoceptor antagonist. All have previously been used as antihypertensive drugs, but their use is limited by side-effects that include postural hypotension (a dramatic fall in blood pressure on standing up due to a loss of reflex α1-mediated vasoconstriction ( pp.240–241)) and nasal congestion
pp.240–241)) and nasal congestion
• β-adrenoceptor antagonists (β-blockers) ( OHCM8 p.108) are effective antihypertensive (
OHCM8 p.108) are effective antihypertensive ( OHCM8 p.134) drugs that are also used in heart failure (
OHCM8 p.134) drugs that are also used in heart failure ( OHCM8 p.130) and angina (
OHCM8 p.130) and angina ( OHCM8 p.110) (
OHCM8 p.110) ( p.438). The primary antihypertensive effect of β-blockers is now believed to be through inhibition of renin secretion in the kidney; renin is an enzyme involved in the synthesis of angiotensin II, which promotes salt and water retention together with vasoconstriction. The benefits of β-blockers in heart failure and angina, however, are primarily mediated by their inhibitory effects on β1 receptors in the heart, leading to reduced heart rate and force of contraction. As a result, the cardiac workload is reduced, together with the demand for oxygen. Side-effects of β-blockers include excessive slowing of the heart or even heart failure (due to inhibition of cardiac β1 adrenoceptors), bronchospasm in asthmatic people (due to inhibition of β2 adrenoceptors in the bronchioles), and worsening plasma lipid profile. The best-known non-selective β-blocker is propranolol; atenolol is a β1 selective β-blocker; and labetalol is a mixed α-and β adrenoceptor antagonist.
p.438). The primary antihypertensive effect of β-blockers is now believed to be through inhibition of renin secretion in the kidney; renin is an enzyme involved in the synthesis of angiotensin II, which promotes salt and water retention together with vasoconstriction. The benefits of β-blockers in heart failure and angina, however, are primarily mediated by their inhibitory effects on β1 receptors in the heart, leading to reduced heart rate and force of contraction. As a result, the cardiac workload is reduced, together with the demand for oxygen. Side-effects of β-blockers include excessive slowing of the heart or even heart failure (due to inhibition of cardiac β1 adrenoceptors), bronchospasm in asthmatic people (due to inhibition of β2 adrenoceptors in the bronchioles), and worsening plasma lipid profile. The best-known non-selective β-blocker is propranolol; atenolol is a β1 selective β-blocker; and labetalol is a mixed α-and β adrenoceptor antagonist.
Table 4.2 Non-cardiovascular effects mediated by adrenoceptors
Amongst the most widely used adrenoceptor agonists in clinical use is the endogenous catecholamine, adrenaline. Adrenaline is more β adrenoceptor selective than noradrenaline and it is used in the following crises:
• In cardiac arrest ( OHCM8 inside back cover): routinely administered during cardiopulmonary resuscitation (CPR) to help stimulate the heart (β1 adrenoceptors)
OHCM8 inside back cover): routinely administered during cardiopulmonary resuscitation (CPR) to help stimulate the heart (β1 adrenoceptors)
• In anaphylactic shock ( OHCM8 pp.806–7): alleviates bronchoconstriction through β2 adrenoceptor stimulation and helps prevent cardiovascular collapse, primarily through cardiac β1 adrenoceptor stimulation.
OHCM8 pp.806–7): alleviates bronchoconstriction through β2 adrenoceptor stimulation and helps prevent cardiovascular collapse, primarily through cardiac β1 adrenoceptor stimulation.
More specific adrenoceptor agonists include:
• α1 adrenoceptor agonists (e.g. phenylephrine) are primarily used in nasal decongestants, where they act to inhibit mucus secretion through α1 adrenoceptor activation. There is a theoretical risk of hypertension through vascular constriction
• α2 adrenoceptor agonists (e.g. clonidine—partial agonist) have been used in the past as antihypertensive drugs. Their primary action is through activation of the negative feedback inhibitory mechanism for noradrenaline release through pre-synaptic α2 adrenoceptors (see Fig. 4.7 and Table 4.3)
• β1 adrenoreceptor agonists (e.g. dobutamine) improve cardiac contractility and are used in cardiogenic shock ( OHCM8 p.814). They can promote cardiac arrhythmias by increasing the heart rate (
OHCM8 p.814). They can promote cardiac arrhythmias by increasing the heart rate ( p.426)
p.426)
• β2 adrenoceptor agonists (e.g. isoprenaline, salbutamol) are used in some inhalers to promote bronchodilation in people with asthma ( OHCM8 p.172). Isoprenaline (non-selective β agonist) has been largely superseded by salbutamol (β2 selective agonist)
OHCM8 p.172). Isoprenaline (non-selective β agonist) has been largely superseded by salbutamol (β2 selective agonist)
• β3 adrenoceptor agonists (e.g. BRL 37344) are being developed as treatments for obesity. They act to accelerate β3-mediated lipolysis (breakdown of fat; see Table 4.2).
Modulation of the synthesis, storage, release, or metabolism of noradrenaline is an alternative means of modifying the effects of sympathetic nervous stimulation. However, the obvious disadvantage of this approach is that it does not carry the specificity of modulation of post-synaptic receptors: the effects are global and impact on both α-and β-adrenoceptor-mediated effects. As a result, this type of modulation is often associated with a wide range of more severe side-effects. Nevertheless, drugs that inhibit synthesis storage and release of noradrenaline have been used in the past to treat hypertension, and some are still used in specific conditions (see Table 4.3).
Table 4.3 Drugs that affect the sympathetic nervous system

ACh and noradrenaline have long been recognized as the major neurotransmitters of the somatic and autonomic nervous systems. However, many of the neurones in the enteric nervous system that is associated with the gastrointestinal tract, the abdominal organs, and the genitalia, do not release either of these neurotransmitters and are known as non-adrenergic, non-cholinergic (NANC) nerves. The elusive NANC neurotransmitter has now been identified as the inorganic free radical, nitric oxide (NO), which also acts as a neuromodulator in the CNS and has important roles in the cardiovascular and immune systems ( pp.444, 846).
pp.444, 846).
• Chemically, No is very different from noradrenaline and ACh. Its free radical status means that it reacts very rapidly with a wide range of biological molecules and has a short biological half-life
• It cannot be stored like other neurotransmitters; instead it is synthesized on demand by the neuronal isoform of the enzyme, NO synthase (nNOS). Synthesis is highly regulated by the intracellular levels of Ca2+ in the neurone, which is ultimately dependent on depolarization of the pre-synaptic terminal in response to action potentials (Fig. 4.9)
• Unlike conventional neurotransmitters, NO does not activate a receptor on the post-synaptic membrane; instead, it diffuses readily through the membrane and stimulates soluble guanylate cyclase, an enzyme which catalyses the conversion of guanosine triphosphate (GTP) to the cyclic nucleotide, cyclic guanosine monophosphate (cGMP)
• There is no specific pathway for the degradation of NO because it is rapidly inactivated in biological media through oxidation to nitrite ( ) and nitrate (
) and nitrate ( )
)
• cAMP and cGMP, through protein kinase A and protein G respectively, can inhibit MLCK. Some of the effects of protein kinase G represent phosphorylation and activation of MLCP (a phosphatase, which reduces myosin light chain phosphorylation) and of K+ channels (which hyperpolarise the membrane and reduce Ca2+ influx through voltage-gated channels).
• Sildenafil (Viagra®) is a drug that is used to overcome impotence. It prevents the deactivation of cGMP to 5′GMP by an enzyme called phosphodiesterase V, which is abundant in the corpus cavernosum.
Fig. 4.9 Non-adrenergic, non-cholinergic neurotransmitter in the peripheral nervous system.
Skeletal muscle is striated (‘striped’).
• Skeletal muscle cells (or myofibres) are made up of a large number of cylindrical myofibrils
• Myofibrils are divided into sarcomeres, containing interdigitating myofilaments (Fig. 4.10)
• Sarcomeres are defined by Z lines
• Thick filaments are approximately 10nm wide and 1.5µm long and comprise two heavy chains of myosin
 Myosin chains have entwined α helix tails, which split open at a hinge region, and globular heads. Associated with the head is the regulatory light chain of myosin
Myosin chains have entwined α helix tails, which split open at a hinge region, and globular heads. Associated with the head is the regulatory light chain of myosin
• Thin filaments are approximately 5nm wide and 1µm long and made primarily of two intertwined α-helix strands of actin. Thin filaments also contain tropomyosin and troponin
 Two α helices of tropomyosin protein twist around the grooves formed by the actin. Tropomyosin obstructs myosin binding sites in the actin groove
Two α helices of tropomyosin protein twist around the grooves formed by the actin. Tropomyosin obstructs myosin binding sites in the actin groove
 Troponin is a trimer: troponin T binds to tropomyosin; troponin I binds to actin; and troponin C binds to Ca2+ ions (Fig. 4.11)
Troponin is a trimer: troponin T binds to tropomyosin; troponin I binds to actin; and troponin C binds to Ca2+ ions (Fig. 4.11)
• Z lines contain α-actinin, which anchors the thin filaments. Thick filaments lie between the thin filaments
• The partial interdigitation confers the appearance of light and dark banding on the sarcomere:
 Light I bands = portions of actin that do not overlap with myosin
Light I bands = portions of actin that do not overlap with myosin
 Dark A bands = overlap of actin and myosin filaments
Dark A bands = overlap of actin and myosin filaments
• ATP-energized movement of thin filaments over thick filaments underlies skeletal muscle contraction
• When sarcomeres contract, the I bands shorten but the A band length remains unchanged (Fig. 4.10).
• Skeletal muscle fibre Em is around –90mV. Action potentials are initiated by ACh release from motor nerves and last for 2–4ms. The initiation of an action potential in the muscle fibre membrane triggers muscle contraction, by elevating intracellular [Ca2+]
• Excitation spreads along the muscle membrane and into transverse (T) tubule invaginations at the boundary between the I and A bands which penetrate deep into the myofibre and closely abut pairs of cisternae of SR to form triads
• The muscle membrane contains voltage-activated L-type Ca2+ channels (also called dihydropyridine or DHP receptors) arranged in clusters of four
• These clusters are adjacent to Ca2+ channels in the SR membrane, called ryanodine receptors. Each DHP receptor interfaces with one of the four subunits of the ryanodine receptor
Fig. 4.10 The organization of skeletal muscle at various degrees of magnification.
Reproduced with permission from Pocock G, Richards CD (2006). Human Physiology: The Basis of Medicine, 3rd edn, p85. Oxford University Press.
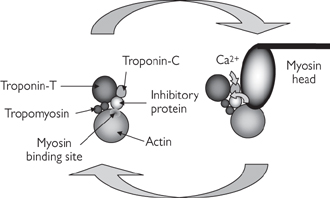
Fig. 4.11 Ca2+-mediated regulation of actin–myosin interaction.
• The arrival of the action potential in the T tubule membrane opens DHP receptors and Ca2+ ions enter the muscle fibre, although this is not essential for muscle contraction. The conformational change upon channel opening, however, initiates a conformational change in the ryanodine receptor, which opens it and releases Ca2+ ions from the cisternae
• Ca2+ ions bind to low affinity sites on troponin C, inducing a conformational change in the protein (Fig. 4.11). Troponin I moves away from actin and troponin T displaces tropomyosin. Binding sites for myosin on actin monomers are revealed, and actin–myosin cross bridges are formed.
The sliding of filaments requires cross-bridge cycling (Fig. 4.12):
• ATP binds to the head of the heavy chain, disengaging myosin from actin
• ATP is hydrolysed by the myosin head. The head pivots to attain a 90° orientation to the thin filaments
• The head forms a cross-bridge with an actin monomer two positions along the filament
• Inorganic phosphate is released from myosin and the head swivels through 45°, drawing the actin past the myosin filament
• ADP is released and the cycle is complete
• The cycle repeats for as long as [Ca2+] remains elevated and actin sites are accessible to myosin. [Ca2+] levels fall when stimulation of the muscle stops, with Ca2+ ions taken back up into the SR, or extruded from the cell.
In the short term, ATP is rapidly resynthesized by transfer of phosphate from phosphocreatine to ADP. Longer-term recovery of ATP levels can be achieved through production of pyruvate from glycogen stores. This anaerobic process yields some ATP, which explains why muscles can cope with short-term deprivation of oxygen. Pyruvate can also be used in oxidative metabolic pathways to generate greater amounts of ATP.
• The relation between length and active tension is biphasic in skeletal muscle
• Increasing or decreasing sarcomere length from normal reduces the number of cross-bridges which can be formed: myosin and actin filaments fail to overlap at increased lengths and opposing actins obstruct each other in shortened conditions. In isometric contraction, tension is generated by the fibre without any change in its length
• In isotonic contraction, the muscle contracts against a constant load and shortens.
Fig. 4.12 A schematic representation of the molecular events responsible for the relative movement of the thin and thick filaments of a strained muscle.
Reproduced with permission from Pocock G, Richards CD (2006). Human Physiology: The Basis of Medicine, 3rd edn, p87. Oxford University Press.
Force generated can be increased by a quick-fire sequence of twitches, where a second action potential fires before Ca4 levels have fallen to resting levels and the muscle fibre has relaxed (Fig. 4.13). This summation is frequency dependent: at very high frequencies a state of tetany will exist. The contraction of whole muscles is enhanced by recruitment of additional motor units.
The rate of force development can vary between fibres:
• Slow twitch (‘red’) fibres are prevalent in postural muscles. They have a profuse blood supply, are rich in myoglobin, and can maintain long contractions without fatigue.
• In contrast, fast twitch (‘white’) fibres in precision muscles are glycolytic, perform oxidative metabolism, and have short twitch durations.
Motor units are functional units of contraction, consisting of an α-motor neurone and all the fibres that it innervates. The fibres of a particular motor unit are of the same type.
Skeletal muscle contraction is controlled by α-motor neurone activity. When stimulated by a threshold action potential, fibres of a motor unit contract in an all-or-nothing fashion. Contraction of the muscle is graded in a number of ways, which include initial stretch of the muscle fibres (the length–tension relationship, Fig. 4.14, which shows an increase in developed tension with stretch, up to an optimal length); increased frequency of action potential stimulation; and recruitment of additional motor units.
Muscle spindles and Golgi tendon organs sense changes in length and tension of muscle fibres. Muscle fibres consist of extrafusal fibres (responsible for contraction) and intrafusal fibres (important for sensing muscle stretch). The intrafusal fibres are innervated by type Ia and type II sensory afferents and make up the muscle spindle.
Stretch of the muscle spindle results in reflex muscle contraction (Ia-α monosynaptic reflex arc). During volitional muscle contraction, resulting from somatic α-motor neurone firing, the spindle would shorten. This would result in counter-productive reflex relaxation of the muscle; to prevent this, there is concomitant γ-motor neurone activation, which contracts the spindle.
Golgi tendon organs are located within tendons of groups of muscle fibres. They are innervated by Ib sensory afferents. Increased muscle tension results in activation of Ib afferents which, via an interneurone, inhibits motor neurone activity.
Fig. 4.13 Contraction of skeletal muscle.

Fig. 4.14 Length–tension relationship for muscle contraction.
Cardiac muscle is another striated muscle, but is different from skeletal muscle in a number of ways. The most striking difference is that cardiac muscle initiates contractions itself: it is myogenic. It does not require nervous stimulation (hence the viability of heart transplantation), but its activity can be modulated by nervous impulses.
• In contrast to skeletal muscles, fibres are branched and interdigitate
• At Z lines, ruffled cell membranes abut each other to form an intercalated disk, which facilitates transmission of mechanical forces from one fibre to the next
• The membranes in intercalated disks contain gap junctions. These promote the spread of electrical excitation between neighbouring fibres
• A T-tubule system is again present, with infoldings of cell membrane present at the Z lines, although there are fewer, albeit more pronounced, tubules.
• Excitation is initiated in a cluster of cells in the right atrium—the sinoatrial node (SAN) or pacemaker region
• This rhythmically generates spontaneous action potentials which spread throughout the atria within 40ms
• The atria are separated from the ventricles by a non-conducting fibrous ring of tissue: excitation can spread into the ventricles only at the atrioventricular node (AVN)
• After a 100ms delay, the excitation passes through the bundle of His and Purkinje fibres (30ms) and then throughout the ventricular muscle (30ms). Slowing the wave of excitation at the AVN allows time for blood to move from atria to ventricles
• Em in cardiac myocytes during diastole is around –80mV
• The spread of depolarization through the cardiac muscle initiates action potentials
• Ventricular action potentials are far longer (200ms) and more complicated than those in skeletal muscle (Fig. 4.15). The events underlying the action potential in a ventricular cell can be summarized (Fig. 4.16):
• Phase 0: rapid upstroke and overshoot to around +20mV. Voltage-activated Na+ channels open at around –65mV and inward Na+ current depolarizes the cell
• Phase 1: an immediate incomplete repolarization towards 0mV. Na+ channels inactivate and, at positive voltages, the background K+ conductance of the membrane decreases (rectification)
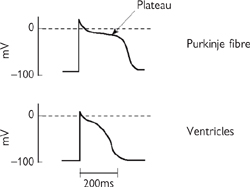
Fig. 4.15 Characteristic appearance of action potentials recorded from various types of myocardial cell.
Reproduced with the permission from Pocock G, Richards CD (2006). Human Physiology: The Basis of Medicine, 3rd edn, p267. Oxford University Press.
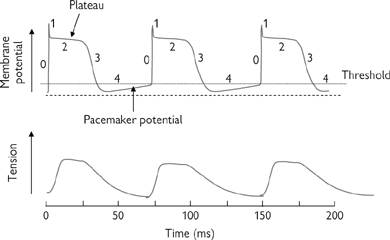
Fig. 4.16 Contraction of cardiac muscle and its association with cardiac action potentials. 0–4 (top panel) refers to phases 0–4 described in the text.
• Phase 2: a prolonged plateau phase due to slower opening of voltage-activated Ca2+ channels during the upstroke (at around –45mV) and inward Ca2+ current. The influx opposes the small hyperpolarizing effect of the (reduced) background K+ current. The plateau is also sustained by activation of electrogenic Na+ × Ca2+ exchange to extrude Ca2+ ions: the 3Na+:1Ca2+ ratio means that each transport cycle is associated with influx of one net positive charge which has an additional depolarizing effect
• Phase 3: repolarization. Ca2+ channels close after 100ms and a K+ current (through slowly activating voltage-activated K+ channels) dominates
• Phase 4: diastolic phase, with only background currents, where Em drifts towards the threshold for phase 0
• Action potentials in atrial cells last for only 8ms, and phase 2 is far less evident. This is the result of an additional transient K+ current, activated at potentials positive to –30mV, which contributes to the repolarizing currents from an early stage.
• SAN cells exhibit spontaneous depolarizations
• The diastolic potential in phase 4 is less negative than in ventricular cells, and is not steady: there is a progressive increase in Em from –65mV to –45mV, then an action potential is initiated
• SAN cells lack the background K+ conductance which stabilizes Em, making them susceptible to depolarization
• There are three contributions to the depolarization:
• Activation of the so-called ‘funny’ current (Na+ ion influx through a non-selective channel): hyperpolarization in phase 3 of the preceding action potential activates the current. This starts the depolarization but closes the channel
• Inactivation of the outward K+ current as phase 3 hyperpolarization ends
• Increasing inward Ca2+ current as depolarization opens Ca2+ channels.
• At threshold, a slow phase 0 upstroke occurs. This represents opening of activating voltage-activated Ca2+ channels: voltage-activated Na+ channels make little or no contribution to the upstroke
• There is no phase 2 plateau in the SAN cell action potential. Neurotransmitters modulate the currents determining the slope of phase 4 depolarisation, so altering heart rate.
AVN cells have similar properties to SAN cells. They have latent rhythmicity and can assume a pacemaker role in the absence of impulses from the SAN. Purkinje fibres exhibit action potentials broadly similar to ventricular cells. ‘Funny’ currents are also present in Purkinje fibres, which can initiate slow pacemaking when the AVN is blocked.
• Contraction initiated by the action potential lasts for around 300ms
• The Ca2+ -dependent sliding filament mechanism of contraction is identical to that in skeletal muscle
• In contrast to skeletal muscle, there is an absolute requirement for Ca2+ influx from the extracellular solution for excitation contraction coupling. Ca2+ ions which enter during the plateau phase of the action potential activate Ca2+ channels in the SR membrane to release further Ca2+: Ca2+ -induced Ca2+ release (CICR)
• Cross bridge formation is not optimized in cardiac muscle: increasing fibre length enhances tension generated: one explanation of Starling’s Law of the heart ( p.434). CICR can be exploited to regulate contraction: neurotransmitters alter the magnitude of Ca2+ entry and thereby modulate force of contraction
p.434). CICR can be exploited to regulate contraction: neurotransmitters alter the magnitude of Ca2+ entry and thereby modulate force of contraction
• Contraction is terminated by reuptake into the SR or extrusion across the cell membrane. The plateau phase of the action potential prevents tetanic contractions and ensures the heart pumps.
Smooth muscle (Figs 4.17, 4.18) is a non-striated muscle found within the walls of blood vessels, in the respiratory, gastrointestinal, urinary, and reproductive tracts, in the piloerector muscles associated with hairs in the skin, and in the ciliary muscle and iris of the eye.
It is different in its structure to striated muscles:
• Although actin and myosin are present they are not organized into parallel arrays
• Dense bodies (or attachment plaques) take the place of Z lines
• These contain α-actinin, which binds actin
• Tropomyosin is present but troponin is absent
• Intermediate cytoskeletal filaments assist in transmitting force between cells
• A SR Ca2+ store exists but is less highly developed.
Smooth muscle contractions are slow to establish but are sustained with low energy consumption for long periods. Innervation is derived from the autonomic nervous system. Smooth muscles have some degree of tension even at rest —contractions augment basal tone.
Smooth muscle can be divided into:
• Visceral or unitary smooth muscle
• Large sheets of cells with common innervation connected by gap junctions which function as low-resistance electrical connections and permit coordinated contraction
• Spontaneous contractions can occur; stretch increases tone
• Contractions primarily result from circulating hormones but can be modulated by nervous stimuli
• Found in walls of visceral organs (e.g. gastrointestinal tract, blood vessels, airways)
• Fibres receive individual innervation and act independently
• Spontaneous contractions do not occur. Contractions initiated primarily by nervous triggers, modulated by hormones
• Functions are more like those of skeletal muscle, with fine graded contractions (e.g. iris, piloerector muscles of skin).
• Em in smooth muscle is not steady: there is constant drift (a few mV). Minimum values are around –50mV
• Visceral smooth muscle can exhibit pacemaker activity, similar to the cardiac SAN. Pacemaker regions shift around within the muscle
• In some cases, spontaneous slow waves of graded depolarization are seen, with spike potentials superimposed at more positive potentials. Oscillating but opposing membrane currents for Ca2+ and K+ probably explain slow waves
• Action potentials have slower upstroke and last longer than skeletal muscle. They can be spiked or exhibit a plateau.
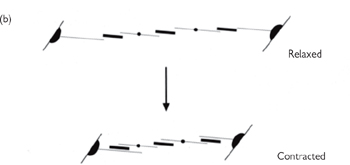
Fig. 4.17 The organization of the contractile elements of smooth muscle fibres. (a) Note the points of close contact for mechanical coupling (dense bands) and the gap junction for electrical signalling between cells. (b) A simple model of the contraction of smooth muscle. As the obliquely running contractile elements contract, the muscle shortens.
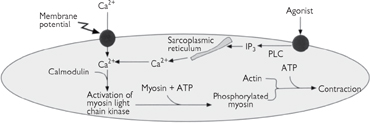
Fig. 4.18 Excitation–contraction coupling in smooth muscle. As in cardiac and skeletal muscle, the development of tension is regulated by calcium which can enter the sarcoplasm via either voltage-gated calcium channels in the plasma membrane or the intracellular calcium stores in the sarcoplasmic reticulum. Calcium binds to calmodulin, which regulates the interaction between actin and myosin via myosin light-chain kinase.
• Upstrokes represent opening of voltage-activated Ca2+ channels; repolarization is due to opening of voltage-activated and Ca2+-activated K+ channels
• Multi-unit smooth muscle does not usually exhibit action potentials.
Increases in [Ca2+] initiate contraction and can originate from:
• Influx of Ca2+ ions during slow waves or action potentials
• IP3-mediated Ca2+ mobilization from the SR in response to neurotransmitters, hormones, or Em changes
Note that changes in Em are not an absolute requirement for smooth muscle contraction.
Contraction is a slow sequence of events:
• Ca2+ ions bind to calmodulin (the smooth muscle functional homologue of troponin C)
• In the absence of Ca2+, actin–myosin binding is prevented by the myosin light chain (MLC), not by tropomyosin
• Ca2+-calmodulin activates MLC kinase. Phosphorylation of MLC relieves the inhibition; hydrolysis of ATP by the myosin head occurs; cross-bridge cycling begins
• A phosphatase dephosphorylates MLC when [Ca2+] falls.
Some smooth muscle can exist in a latch state. Tension is generated for long periods, yet [Ca2+] is lower (but still above resting levels), MLC phosphorylation is reduced, and energy consumption is minimal. The interaction of two Ca2+ binding proteins, caldesmon and calponin, with thin filaments is believed to alter the kinetics of actin–myosin binding.
Neurones are surrounded by glial cells which produce a myelin sheath around them. This sheath has two functions:
• It provides nutrition for the neurones
• It speeds the transmission of signals along the nerve since depolarization ‘jumps’ between the gaps between Schwann cells (the nodes of Ranvier) rather than all the way along the cell membrane.
Thus, if a neurone loses its myelin sheath, there will be severe malfunction and possible death of that neurone. There are several human diseases which are characterized by demyelination.
 OHCM8 pp.500, 501)
OHCM8 pp.500, 501)This is characterized by demyelination in any area of the brain and usually progresses with episodes of acute demyelination occurring at different sites at different times. The mechanism by which this demyelination occurs is not known but there are T-lymphocytes and macrophages around the demyelinated areas and, in animal models, demyelination can be induced by the transfer of T-lymphocytes from one animal to an unaffected animal.
This disease usually follows a viral infection and is characterized by a single widespread episode of demyelination affecting most of the white matter. It is possible that this represents an acute autoimmune reaction to myelin. There is a high fatality rate (about 20%), but the other cases usually recover completely.
 OHCM8 p.686)
OHCM8 p.686)A disease with demyelination in the distribution, as described by its name. It is associated with alcoholism, severe electrolyte imbalances, and liver transplantation. One possible mechanism is rapid correction of low blood sodium (hyponatraemia). It presents as a sudden quadriplegia.
Box 4.1 summarizes the treatments for demyelination diseases.
 OHCM8 p.716)
OHCM8 p.716)The alternative name of this disease, acute inflammatory demyelinating polyradiculoneuropathy, describes the pattern of demyelination. The disease often occurs after an acute viral illness and presents as an ascending neuropathy which can progress from loss of plantar reflexes through to paralysis of respiratory muscles. Patients may require many weeks of nursing in intensive care units when their breathing is affected, but go on to a complete recovery, as evidenced by some professional sports people returning to their sport after an episode of this illness. The pathogenesis of the disease is still uncertain but an autoimmune reaction precipitated by the viral infection is currently favoured.
Box 4.2 summarizes the management of patients with Guillain–Barré syndrome.
 OHCM8 p.710)
OHCM8 p.710)A rare inherited peripheral neuropathy that is characterized by recurrent demyelination and remyelination of peripheral nerves.
 OHCM8 p.516)
OHCM8 p.516)Myasthenia gravis is an autoimmune disease in which auto-antibodies inhibit neuromuscular transmission ( p.228).
p.228).
The auto-antibody is directed against the ACh receptor of the post-synaptic membrane of the neuromuscular junction. Binding of the antibody to this receptor causes an increased rate of degradation of this protein (rather than a simple blocking of the ACh binding site as originally thought) and damage to the post-synaptic membrane by complement binding.
• Drooping eyelids (ptosis) and double vision due to weakness of the extraocular muscles
• Generalized muscular weakness—characteristically fluctuates
• Sensory and autonomic nervous systems are normal.
Box 4.3 summarizes the treatments for myasthenia gravis.
 OHCM8 p.510)
OHCM8 p.510)This is a rare disease but one with a high public profile in the UK, mainly due to sufferers of the disease seeking to legally end their lives by euthanasia.
Motor neurone disease is characterized by a loss of neurones in both the anterior horns of the spinal cord and the corticospinal tracts, producing a mixture of upper and lower motor neurone signs.
• Early—weakness of fine hand muscles, cramping and spasticity of arms and legs
• Later—gross motor weakness, weakness of respiratory muscles
• Course of disease—progressive, with death within a year or two usually due to recurrent respiratory infections.
• Inherited—about 10% of cases are familial and, in some of these, there is a gain of function mutation in the copper-zinc SOD gene on chromosome 21
• Sporadic—there is no clear mechanism for the disease. The motor neurones appear to die individually by apoptotic processes but the factors which precipitate this have not been identified.
Box 4.4 summarizes the treatments for motor neurone disease.
Neurones in the central and peripheral nervous systems do not regenerate if the cell body dies. The axons of peripheral nerves do regrow if the surrounding myelin sheath is still intact (at a rate of about 1mm a day). This lack of regenerative capacity has profound consequences for people who experience injury to their spinal cord since such injuries usually cause disruption of the whole cord, so there is no regrowth of neurones to connect across the deficit. This leaves these people with complete paralysis of any muscles supplied by neurones below the level of the injury.
• Trauma ( OHCM8 pp.766–74)—is the most common cause and is usually sustained in car/motorbike accidents or in a sporting context, especially in the front row of the scrum in rugby union and falling from a height (horse riding, diving). There is usually transection of the cord by a displaced vertebra. The usual features of damage in the nervous system, such as initial inflammation followed by gliosis, are seen in the damaged cord
OHCM8 pp.766–74)—is the most common cause and is usually sustained in car/motorbike accidents or in a sporting context, especially in the front row of the scrum in rugby union and falling from a height (horse riding, diving). There is usually transection of the cord by a displaced vertebra. The usual features of damage in the nervous system, such as initial inflammation followed by gliosis, are seen in the damaged cord
• Syringomyelia ( OHCM8 p.520)—is characterized by development of a fluid-filled cavity in the centre of the spinal cord which causes pressure atrophy of neurones. Since the first neurones to be affected by this atrophy are the crossing anterior spinal commissural fibres, this produces the unusual neurological pattern of dissociated sensory loss of pain and temperature senses in the upper limbs. Syringomyelia may be associated with a congenital Arnold Chiari malformation but some occur after spinal cord trauma.
OHCM8 p.520)—is characterized by development of a fluid-filled cavity in the centre of the spinal cord which causes pressure atrophy of neurones. Since the first neurones to be affected by this atrophy are the crossing anterior spinal commissural fibres, this produces the unusual neurological pattern of dissociated sensory loss of pain and temperature senses in the upper limbs. Syringomyelia may be associated with a congenital Arnold Chiari malformation but some occur after spinal cord trauma.
Box 4.5 summarizes the management of patients with spinal cord damage.