Abdominal wall, peritoneal cavity, and the peritoneum
Histology of the gastrointestinal tract
Function of the gastrointestinal tract
Salivary and pancreatic secretion
Indigestion, peptic and duodenal ulcers
Digestion and absorption of nutrients
Fluid and electrolyte movement
Inflammatory diseases in the gut
Protein synthesis by the liver
Detoxification of xenobiotics 582
The structures of the abdominal wall are of obvious significance for surgical approaches to the abdomen. The abdominal wall consists of several distinct layers which are mostly consistent throughout. Skin is the outermost layer and overlies superficial fascia. Beneath this is a tough muscular layer superficial to another layer of fascia and extraperitoneal fat above the peritoneum. These layers protect and contain the abdominal viscera, thereby preventing herniation.
The subdermal superficial fascia comprises two distinct layers:
• Camper’s fascia is a superficial fatty layer containing variable amounts of fat. It covers the anterior abdomen and blends inferiorly with the superficial fascia of the thigh
• Scarpa’s fascia is fibrous tissue that forms the deeper of the two fascial layers. It is not present in the superior and lateral areas of the abdominal wall, but inferiorly merges with the fibrous fascial layer of the upper thigh (fascia lata, below the inguinal ligament) and with Colles’ fascia, which extends into the genital area and perineum.
The deep fascia of the abdomen is indistinct and is tightly bound to the superficial muscles.
The muscles of the anterior abdominal wall are arranged in layers. All the muscular layers thin to become fibrous, tendon-like sheets (aponeuroses) across the medial part of the anterior abdominal wall. The aponeuroses fuse in the midline to form the linea alba (white line).
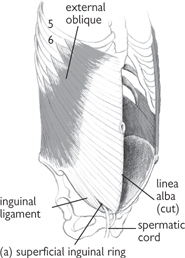
• External oblique is the most superficial muscular layer with fibres running inferiorly from lateral to medial. It attaches laterally to the anterior surface of the 5th–12th ribs, the xiphoid process, linea alba, anterior surface of iliac crest, and pubis. It forms the inguinal ligament inferiorly as the aponeurosis folds back on itself, between the anterior superior iliac spine and the pubic tubercle. An opening in external oblique just medial to the pubic tubercle forms the superficial inguinal ring
• Internal oblique lies beneath external oblique, with muscular fibres running at 90° to external oblique fibres. It arises from the anterior two-thirds of the iliac crest and the lateral half of the inguinal ligament, in front of the deep ring, and attaches to the linea alba, pubic crest, as well as the lumbar fascia. This fascial layer splits medially to ensheath rectus abdominis as two aponeuroses
• Transversus abdominis is the deepest muscular layer with muscular fibres running transversely. It attaches to the 7th–12th costal cartilages, the lumbar fascia, the anterior two-thirds of the iliac crest, the inguinal ligament, linea alba, and pubis. Inferiorly, transversus abdominis forms part of the roof of the inguinal canal as it arches over to become the conjoint tendon with the aponeurosis of internal oblique.


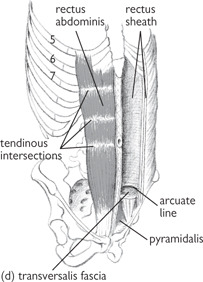
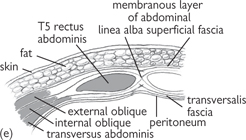
Fig. 8.1 Muscles of the anterior abdominal wall: (a) external oblique; (b) internal oblique; (c) transversus abdominis; (d) rectus abdominis; (e) section through anterior abdominal wall. The aponeuroses of the abdominal wall muscles which form the rectus sheath interdigitate to form the midline linea alba.
Reproduced from Mackinnon, Pamela and Morris, John, Oxford Textbook of Functional Anatomy, vol 2, p114 (Oxford, 2005). With permission of OUP.
• Rectus abdominis is a wide band of muscle, enveloped by the rectus sheath (aponeurotic layers) and divided into two bands of muscle by the fusion of the aponeurotic layers of the abdomen at the linea alba. Rectus abdominis is attached superiorly to the costal cartilages of the 5th–7th ribs and inferiorly to the pubic bone. It is marked by three tendinous intersections which form the ‘six pack’ seen in muscular, thin people. These adhere to the anterior (but not the posterior) part of the rectus sheath, thereby allowing rectus abdominis to lie free posteriorly. The intersections have constant positions and occur at:
• The tip of the xiphoid process
• A point halfway between the two intersections already described.
• The coverings of the rectus sheath vary, depending on abdominal level
• Above the costal margin, external oblique forms the anterior sheath and no posterior sheath is present. From the costal margin, down to a point halfway between the umbilicus and the pubis, the anterior sheath is formed by external oblique and the anterior aponeurotic slip of internal oblique, with the posterior part being formed by the other aponeurotic slip of internal oblique and the aponeurosis of transversus abdominis
• Below this halfway point, the sheath is formed by all three aponeurotic expansions lying anterior to the rectus muscle with only the transversalis fascia and the peritoneum lying between rectus abdominis and the viscera
• The arcuate line represents the inferior limit of the posterior rectus sheath. The inferior epigastric vessels pass upwards from the external iliac vessels and pierce the rectus sheath at the level of the arcuate line where they anastomose with the superior epigastric vessels.
• The nerve supply to the abdominal wall is segmental and follows dermatomal distribution of skin and muscular innervation
• T7–L1 supply the abdomen, with the umbilicus supplied by T10, and the groin and scrotal area supplied by L1
• Above the arcuate line, a neurovascular plane runs between internal oblique and transversus abdominis, and contains nerves and arteries supplying the abdominal wall.
• The inferior epigastric artery and deep circumflex iliac arteries are branches of the external iliac artery and supply the inferior part of the anterior abdominal wall
• The superior part of the anterior abdominal wall is supplied by the superior epigastric artery, a terminal branch of the internal thoracic artery, and branches from the posterior intercostal arteries of the 10th and 11th ribs and the subcostal arteries. These anastomose with the blood vessels supplying the inferior abdominal wall
• The inferior part of the abdominal wall is drained by three superficial inguinal veins into the great saphenous vein of the lower limb
• The superior part of the abdominal wall is drained by the superficial epigastric vein and the lateral thoracic vein
• The para-umbilical veins form a porto-systemic anastomosis between the superficial veins and the deep (portal) venous system.
• Peritoneum is a thin, single-celled layer of mesothelium that covers the internal surfaces of the abdominal wall (parietal peritoneum) and envelops abdominal viscera (visceral peritoneum)
• It originates from the endothelial lining of the primitive coelomic cavity of the embryo and forms a cavity (peritoneal cavity) which is punctured only by the uterine tubes in females
• The peritoneal cavity is filled with peritoneal fluid which lubricates parietal and visceral peritoneum, thereby permitting movement of abdominal viscera
• Peritoneum has several important functions, including support of viscera within the abdomen, fat storage, and sealing off infected bowel segments
• Peritoneal ligaments are formed from double layers of the peritoneal membrane connecting an organ with another organ or the abdominal wall. These include:
• The falciform ligament (which extends from the anterior abdominal wall to the liver)
• The gastrophrenic ligament (which extends from the stomach to the inferior surface of the diaphragm)
• The gastrosplenic ligament (which extends from the stomach to the hilum of the spleen)
• The gastrocolic ligament (which forms part of the greater omentum and extends from the stomach to the transverse colon).
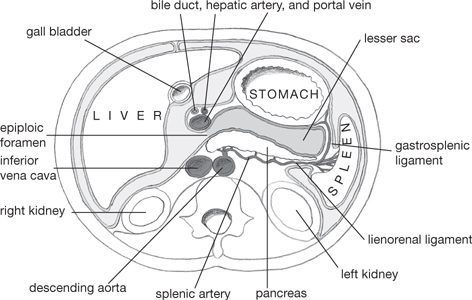
Fig. 8.2 Arrangement of the peritoneum of the greater and lesser sacs in a horizontal section of the abdomen through the stomach, viewed from below.
• Certain bowel segments and abdominal organs, in particular the small intestine, are attached to the posterior abdominal wall by a mesentery—a double-layered flap of peritoneum which is reflected from the abdominal wall and contains blood vessels, nerves and lymphatics, and fat stores. Certain abdominal viscera (e.g. the ascending colon) have no mesentery, are only covered by peritoneum anteriorly, and are retroperitoneal
• The omentum is a double-layered segment of peritoneum that connects the stomach to other organs (in contrast to a mesentery, which connects viscera to the abdominal wall)
• The lesser omentum runs between the liver and the lesser curvature of the stomach and proximal part of the duodenum as two peritoneal ligaments—the hepatogastric ligament and the hepatoduodenal ligament
• The greater omentum starts from the greater curvature of the stomach and proximal part of the duodenum as a large flap of tissue that hangs like an apron and passes back up to the transverse colon, where it merges with the transverse mesocolon. At the inferior fold of the greater omentum, its four layers are fused
• Two continuous sacs are partially separated within the abdomen by the greater and lesser omentum
• The lesser sac lies posterior to the lesser omentum and the stomach
• The greater sac forms the anterior cavity of the abdomen
• The foramen of Winslow (or epiploic foramen) connects these two cavities and is bounded anteriorly by the free border of the lesser omentum (which contains the common bile duct, hepatic artery, and the portal vein), posteriorly by the inferior vena cava, inferiorly by the first part of the duodenum, and superiorly by the caudate process of the liver.
• Where peritoneum folds, pouch-like peritoneal recesses are formed
• These are potential spaces that may become filled with pus or blood They include:
• The right and left subphrenic spaces (which lie between the liver and the diaphragm and are divided by the falciform ligament)
• The right subhepatic space (which lies between the liver and the posterior abdominal wall)
• The left subhepatic space (which is the lesser sac)
• The right extraperitoneal space (which lies between the diaphragm and the bare area of the liver).
 OHCM8 pp.616–7)
OHCM8 pp.616–7)• The inguinal canal connects the abdomen to the scrotum
• It is a weakness in the abdominal wall and is clinically important as a site prone to herniation of abdominal contents
• The inguinal canal is approximately 6cm long and passes obliquely between the deep and superficial inguinal rings. It carries the spermatic cord in men or the round ligament in women, as well as the ilio-inguinal nerve
• The spermatic cord contains several important structures including the vas deferens, the artery to the vas, the testicular artery, the cremasteric artery, the genital branch of the genitofemoral nerve supplying the cremasteric muscle, the pampiniform plexus of veins, autonomic nerve fibres, and lymphatics which drain to the aortic nodes
• The spermatic cord is covered in three layers of fascia:
• The outermost external spermatic (continuous with external oblique)
• The cremasteric muscle and fascia (continuous with internal oblique)
• The innermost internal spermatic fascia (continuous with transversalis fascia)
• The relations of the inguinal canal are important when considering surgical approaches to repairing inguinal hernias
• The base of the canal is formed by the inguinal ligament and the lacunar ligament (which forms the medial part of the floor as a continuation of the inguinal ligament)
• The roof of the canal is formed by transversus abdominis, internal oblique, and the pectineal line of the pubic bone
• The anterior wall of the canal is formed by the external oblique medially and the internal oblique laterally, whilst the posterior wall is formed by the transversalis fascia and the conjoint tendon.
• The deep inguinal ring is an opening in the transversalis fascia at the midpoint of the inguinal ligament (halfway between the anterior superior iliac spine and the pubic tubercle), 1.3cm above the ligament
• It is just lateral to the mid-inguinal point (halfway between the anterior superior iliac spine and the pubic symphysis)
• It is bounded by the transversalis fascia medially, the inferior epigastric vessels posteriorly, and, laterally, by the angle formed by the inguinal ligament and transversus abdominis.
• The superficial inguinal ring is an opening in the external oblique aponeurosis and lies above and medial to the pubic tubercle
• The medial crus of external oblique attaches to the pubic crest and the lateral crus attaches to the pubic tubercle. The pubic crest forms the base of the superficial inguinal ring
• The inferior epigastric artery runs around the medial edge of the superficial inguinal ring
• A direct hernia passes through a weakness in the transversalis fascia (i.e. the floor of the inguinal canal) and lies medial to the inferior epigastric vessels. An indirect hernia runs through the deep ring, the canal, and the superficial ring and lies lateral to the inferior epigastric vessels.
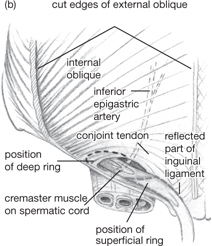
Fig. 8.3 (a) Position of superficial inguinal ring relative to the femoral sheath and its contents. Note the sharp edge of the lacuna ligament in relation to the femoral canal, which is continuous with the abdominal (extraperitoneal) cavity. (b) External oblique has been largely removed to show the floor and roof of the inguinal canal and the spermatic cord.
Reproduced from Mackinnon, Pamela and Morris, John, Oxford Textbook of Functional Anatomy, vol 2, p115 (Oxford, 2005). With permission of OUP.
• The primary function of the gastrointestinal tract is to digest and absorb food for energy, growth, and maintenance of the body. It is also an important immune organ for protecting against bacterial invasion
• Certain generalizations can be made about the histological structure of the digestive tract, specifically with regard to the layers of tissue surrounding the bowel lumen
• Differences in microstructure between different regions of the gastrointestinal tract reflect location and functional specializations
• There are four main layers of the gastrointestinal tract, moving from the lumen outwards:
• The mucosa consists of an epithelial layer immediately adjacent to the lumen, underneath which is a connective tissue layer (lamina propria) comprising a small amount of smooth muscle, blood vessels, and sometimes glands and lymphoid tissue. The muscularis mucosa is part of the mucosal layer and the innermost layer of smooth muscle. The mucosa contains gut-associated lymphoid tissue (GALT) in the lamina propria
• The submucosa is a layer of dense connective tissue containing a nervous plexus (Meissner’s plexus) as well as the main blood and lymph vessels
• Muscularis comprises two layers of smooth muscle—an inner circular layer (muscularis interna) and an outer longitudinal layer (muscularis externa)—sandwiching a myenteric (Auerbach’s) plexus
• The serosa comprises loose connective tissue, adipose tissue, and blood and lymph vessels, surrounded by the mesothelium (simple squamous epithelial layer).
• Transmits and propels food from mouth to stomach
• Lubricates food to aid propulsion
• Lined with non-keratinized, stratified, squamous epithelium, which becomes columnar epithelium at the junction with the stomach
• Within the lamina propria, certain glands (e.g. cardiac glands) secrete mucus into the lumen
• The muscularis mucosae are only found in the lower section
• Mucous glands in the submucosa secrete mucus into the lumen of the oesophagus, via ducts, aiding the lubrication of food boluses and protecting the lining from abrasions
• The muscularis externa in the upper third is made of striated muscle; the middle third is mixed (striated and smooth muscle); the lower third, near the junction with the stomach, consists of smooth muscle only. These muscles are responsible for propelling swallowed food to the stomach
• The adventitia forms the outer layer in the non-abdominal sections. This is a connective tissue layer which is only loosely restraining. In the abdominal sections, this layer is replaced by serosa.
• The mucosa of the stomach is highly specialized for secretory functions. The single-layered, columnar epithelium has many invaginations (folds) that extend into the lamina propria, forming branched specialized glands (gastric pits) for the secretion of acid, mucus, and digestive enzymes
• The lamina propria is thin and contains large numbers of capillaries, nerve fibres, and lymphatics
• The muscularis is made up of three smooth muscle layers with fibres running in different directions—longitudinal, circular, and oblique when running from the external to the internal layer. This promotes mixing of the stomach contents and propulsion towards the pyloric sphincter to enable emptying into the duodenum. The stomach wall is arranged into numerous folds or rugae that allow the stomach to distend and increase in size
• Different regions of the stomach have different functions and, thus, different lining specializations:
• The cardia: short simple or branched glands with mucus-secreting cells and a few parietal cells
• The fundus and body: branched tubular gastric glands with mucous neck cells (secrete mucus) and oxyntic (parietal) cells (secrete hydrochloric acid and intrinsic factor) are found at the neck of the glands. Chief cells (secrete pepsin and lipase) and endocrine cells (secrete 5-HT) are found at the base of these glands
• The pylorus: shorter branched glands (pyloric glands), which secrete mucus, gastrin (stimulates the production of acid secretion) and enteroendocrine cells, which secrete somatostatin (an inhibitor of gastrin).
• Several structural adaptations increase its surface area, enhancing absorptive and secretory processes:
• Plicae circulares are permanent folds in the mucosa and submucosa which are particularly prominent in the jejunum
• Villi are formed by outgrowths of the mucosa (epithelia and lamina propria), with simple tubular glands (glands of Lieberkühn) found at their base
• Absorptive cells are specialized epithelial cells with an absorptive surface of densely packed microvilli extending into the intestinal lumen as an apical brush border.
Aggregates of lymphoid material (in the lamina propria and submucosa) along the small intestine, covered by specialized epithelial cells (M (micro-fold) cells). M cells have pits on their basal membrane which contain antigen-presenting cells (APCs). M cells endocytose antigens and transport them to the underlying APCs which are able to trigger appropriate immune responses to foreign antigens.
Exocrine cells in the basal section of intestinal glands. They secrete lysozyme which digests bacterial walls of some types of bacteria and is, therefore, antimicrobial.
Deep coiled glands extending into the muscularis mucosa, with extensive branching. These glands secrete alkaline mucus and distinguish the duodenum from the rest of the small intestine.
• Production of mucus (lubricates lining of intestine)
• Formation of faecal material
• The large intestine has no mucosal folds (except the rectum) or villi. It has deep glands, lined by specialized columnar epithelium, including extensive goblet and absorptive cells, and few enteroendocrine cells. The absorptive cells are columnar with irregular, short microvilli
• Large amounts of bacteria are present in the lumen (this aids in the breakdown of cellulose material) and there is extensive lymphoid tissue in the lamina propria and submucosa to prevent bacterial invasion
• The outer layer of the muscularis differs from the small intestine, with the longitudinal layer forming three longitudinal bands of muscle: the taeniae coli. Appendices epiploicae are small outpouchings of adipose tissue in the serous layer. This helps distinguish the large from the small bowel during surgery
• Epithelial cell turnover is high, as cells are sloughed off the walls by passing matter. Stem cells at the base of the glands (the proliferative zone) are constantly replacing epithelial cells by mitosis.
• Excretion of faecal material at socially acceptable times
• The rectum is lined with columnar epithelium, with lots of goblet and absorptive cells
• The mucus membrane has vertical folds (rectal columns of Morgagni) forming valve-like folds (valves of Ball). The upper anal canal is also lined by a columnar epithelium, which becomes stratified squamous epithelium at the dentate line. The mucocutaneous junction is the area between the two types, where the epithelium is transitional
• The pectineal line defines the boundary of embryological development, with mucosa above endodermal in origin, and that below ectoderm in origin. Blood, lymphatic, and nervous supply, and histology reflect this boundary.
• The oesophagus is a long tube (~25cm), responsible for transporting food from the mouth to the stomach
• It is divided into three sections, named according to the vertebral level. This division is particularly useful when considering blood, nerve, and lymphatic supply
• The cervical oesophagus starts at the level of the cricoid cartilage and runs posterior to the trachea and anterior to the cervical vertebrae and prevertebral fascia. The common carotid arteries and recurrent laryngeal nerves run on either side of the cervical oesophagus
• The thoracic oesophagus lies within the superior and posterior mediastinum, posterior to the trachea, the left main bronchus, and the pericardial cavity which encloses the heart. The thoracic vertebrae, thoracic duct, the azygous vein, and descending aorta run posterior to this section of the oesophagus. It becomes the abdominal oesophagus after it pierces the right crus of the diaphragm at the T10 level
• The abdominal oesophagus is very short and terminates at the cardia of the stomach. It lies in the oesophageal groove of the posterior surface of the left liver lobe. Its anterior surface is covered with parietal peritoneum and, in common with the rest of the gastrointestinal tract, the abdominal oesophagus lies outside the peritoneal cavity
• Narrowings of the oesophagus are clinically important ( OHCM8 pp.240–1) since they are likely points for foreign bodies to become lodged if swallowed and are also likely sites for stricture and carcinoma development. They delay the passage of food and liquid after it has been swallowed. Four such sites are of greatest importance:
OHCM8 pp.240–1) since they are likely points for foreign bodies to become lodged if swallowed and are also likely sites for stricture and carcinoma development. They delay the passage of food and liquid after it has been swallowed. Four such sites are of greatest importance:
• The cricopharyngeal sphincter
• Where the aortic arch crosses the oesophagus
• Where the left main bronchus indents the oesophagus
• The point at which the oesophagus pierces the diaphragm.
• Cervical: inferior thyroid artery
• Thoracic: oesophageal branches from aorta and bronchial arteries
• Abdominal: branches from the left inferior phrenic and left gastric artery.
• Cervical: vertebral, brachiocephalic, and inferior thyroid veins
• Thoracic: azygous and hemiazygos veins
• Abdominal: left gastric vein, which drains to the portal system
• The oesophageal veins form an anastomosis between the left gastric (portal) and azygous (systemic) venous drainage systems. During portal hypertension, distension of the veins draining the oesophagus can occur, leading to oesophageal varices. These varices are at risk of rupture and severe haemorrhage.
• Cervical: deep cervical nodes
• Thoracic: tracheobronchial and posterior mediastinal nodes
• Abdominal: left gastric and coeliac nodes.
• Cervical: recurrent laryngeal nerve and middle cervical ganglion (sympathetic)
• Thoracic: vagus, sympathetic trunk, and greater splanchnic nerve
• Abdominal: vagus-forming plexuses (anterior and posterior).
• The stomach is a J-shaped tube that receives food from the oesophagus at the cardia. It is responsible for initiating many digestive processes and for mixing and breaking up swallowed food before presenting it to the duodenum in a more digestible form (called chyme)
• Food is mixed with a number of secretions, including digestive enzymes (e.g. pepsin), hydrochloric acid (which aids with digestion and kills bacteria), and intrinsic factor (essential for vitamin B12 absorption)
• The stomach is also a reservoir for food, and regulates its release into the duodenum.
• The stomach has two curves: the greater (which forms the left border of the stomach) and the lesser (which forms its right border)
• The cardia is the narrow neck of the stomach and, although it is not anatomically distinct, this whole region functions as a ‘physiological’ sphincter to prevent ‘reflux’ of stomach contents into the oesophagus (GORD,  OHCM8 p.244). This is dependent on several separate factors:
OHCM8 p.244). This is dependent on several separate factors:
• The oesophagus is narrowed as it pierces the right crus of the diaphragm
• A valve is formed at the lower oesophagus by circular muscle fibres
• Pressure differences between the thorax and the abdomen compress the walls of the intra-abdominal oesophagus
• The acute angle of entry of the abdominal oesophagus has a valve-like effect and the muscularis mucosa forms mucosal flaps, which also act as valves
• The fundus is the upper section of the stomach, seen as the gastric bubble on X-rays
• The body is the main section and the pyloric antrum is the widest part of the pylorus. The pylorus forms the terminal section before it joins with the first part of the duodenum
• The pyloric sphincter (which regulates the flow of stomach contents into the duodenum) is a circular muscle around the lumen of the pylorus. Its position is marked by an external constriction of the alimentary tract at the junction of the duodenum and pylorus. This is also the point at which the constant vein of Mayo crosses it vertically. The different regions of the stomach have specialized functions which are described
• The omenta are two-ply regions of peritoneum which are created where viscera impress upon the peritoneal cavity
• The greater omentum is attached to the greater curve of the stomach and extends downwards like an apron before folding inwards and upwards towards the transverse colon
• The lesser omentum is attached to the lesser curve of the stomach and extends to the liver
• The gastrosplenic omentum (ligament) runs from the stomach to the spleen.
The omenta contain the blood vessels and nerves supplying the stomach.
Fig. 8.4 Arterial supply to the stomach.
Reproduced from Mackinnon, Pamela and Morris, John, Oxford Textbook of Functional Anatomy, vol 2, p138 (Oxford, 2005). With permission of OUP.
• Anteriorly: the anterior abdominal wall, the diaphragm, and the left lobe of liver
• Posteriorly: the stomach is divided by the lesser peritoneal sac from the pancreas, left kidney and adrenal gland, spleen, aorta, coeliac trunk, and the transverse mesocolon
• Lesser curvature: left (from coeliac axis) and right (from hepatic artery) gastric arteries anastomose with each other and run in the lesser omentum
• Upper part of greater curvature: the short gastric arteries and the left gastroepiploic artery supply the upper part of the greater curvature of the stomach. Both arteries arise from the splenic artery and run in the gastrosplenic ligament
• Lower part of greater curvature: the right gastroepiploic artery (from the gastroduodenal branch of the hepatic artery) runs in the greater omentum. It sometimes anastomoses with the left gastroepiploic artery.
The course of the gastric veins largely mirrors that of the arteries and, ultimately, they drain into the portal veins.
• The left gastroepiploic and short gastric veins drain into the splenic vein
• The right gastroepiploic vein drains into the superior mesenteric vein
• The splenic and superior mesenteric veins merge, forming the portal vein. This receives drainage from the right and left gastric veins directly
• There is no gastroduodenal vein.
• Lesser curvature: left and right gastric nodes
• Upper left side of greater curvature: splenic and pancreatic nodes
• Lower greater curvature of the stomach: gastroepiploic and pyloric nodes.
All lymph from the stomach ultimately drains into the coeliac nodes.
• The coeliac plexus (T6–T9) supplies the stomach with sympathetic fibres which run with blood vessels
• The vagus supplies parasympathetic fibres that innervate motor and secretory targets.
• The plexus has two trunks, anterior and posterior
• The anterior trunk (mainly from the left vagus) supplies the gastric branches, large hepatic branches, and branches to the pylorus
• The posterior trunk (mainly from the right vagus) supplies the branches to the posterior stomach and a large coeliac branch, which forms the coeliac plexus.
• The duodenum is the first part of the small intestine, which also comprises the jejunum and ileum. The stomach passes chyme into the duodenum, which then continues the digestive process receiving bile from the gallbladder and pancreatic juices from the pancreas. It is protected against acidic contents from the stomach by alkaline secretions
• The duodenum is a C-shaped tube curving around the head of the pancreas. It is a retroperitoneal organ, with only its first part being covered in peritoneum: the rest is immobile and covered in a serous membrane
• It is commonly divided into four parts:
• 1st part (5cm): starts at the gastroduodenal junction and is known radiologically as the duodenal cap. Posterior to it are the portal vein, common bile duct, gastroduodenal artery, and, moving further posteriorly, the inferior vena cava. Anterior to this section of the duodenum are the liver and gallbladder
• 2nd part (7.5cm): this curves downwards, around the pancreatic head, anterior to the hilum of the right kidney and right ureter. It is crossed by the transverse colon anteriorly. The common bile duct and main pancreatic duct join, forming the hepatopancreatic ampulla, which opens into the duodenum at the sphincter of Oddi. This structure opens into the posteromedial wall of this section of the duodenum at the duodenal papilla. The accessory pancreatic duct opens just superior to the sphincter of Oddi in the duodenum
• 3rd part (10cm): the duodenum runs horizontally, with its superior margin running around the head of the pancreas. The root of the mesentery and the superior mesenteric vessels cross anteriorly to this part of the duodenum
• 4th part (2.5cm): runs superiorly and to the left. Termination of the duodenum is defined by a fibromuscular peritoneal fold from the right crus of the diaphragm called the suspensory ligament of Treitz. It attaches to the terminal part of the duodenum at the duodenal–jejunal junction. Contraction of this muscle (the ligament of Treitz) widens the flexural angle, aiding the movement of the contents of the duodenum. Running on the left side of this junction is the inferior mesenteric vein as it descends from behind the pancreas.
• Blood supply to the duodenum is regional and defined by its embryological development
• The foregut is supplied by the coeliac axis, and the midgut is supplied by the superior mesenteric axis. In the duodenum and pancreas, these two blood supplies anastomose
• The inferior pancreatico-duodenal artery (from the superior mesenteric artery) anastomoses with the superior pancreatico-duodenal artery (from the coeliac axis) at the level of the duodenal papilla, where the common bile duct enters the duodenum. Thus, the duodenal papilla is the dividing line between the foregut and the midgut
• Duodenal ulcers ( OHCM8 p.242) most commonly occur in the duodenal cap and, as a consequence of its rich blood supply, are devastating should they bleed.
OHCM8 p.242) most commonly occur in the duodenal cap and, as a consequence of its rich blood supply, are devastating should they bleed.
• The superior pancreatico-duodenal vein drains directly into the hepatic portal vein
• The inferior pancreatico-duodenal vein drains into the superior mesenteric vein.
Duodenal lymph drains to the superior mesenteric and coeliac nodes.
All nerves reach the duodenum with blood vessels.
• Sympathetic supply: coeliac and superior mesenteric plexus
• Parasympathetic supply: vagus nerve.
• The remainder of the small intestine is divided into two parts: the jejunum (upper two-fifths) and the ileum (lower three-fifths). It is a long section of bowel 3–10m long and is responsible for terminal food digestion and nutrient absorption
• There is no obvious junction between the two halves of the small intestine: the bowel characteristics gradually change. The mucosa of the jejunum is thicker, with a smaller diameter, than the ileum
• The two parts lie in different areas of the abdomen: the jejunum in the umbilical region and the ileum in the hypogastrium and pelvis
• The mesentery carries blood vessels to the small intestine, nerve fibres (autonomic), and lymphatic vessels.
• Starts at the duodenal–jejunal junction and attaches most of the small intestine to the posterior wall of the abdomen
• Originates from the posterior abdominal wall at the same level and to the left of L2
• It runs diagonally downwards from L2 towards the right sacroiliac joint along the left side of the second lumbar vertebrae
• The root of the mesentery crosses the abdominal aorta, inferior vena cava, the third part of the duodenum, right psoas major muscle, right ureter, and right testicular/ovarian vessels
• The ileum terminates at the ileocaecal junction.
• The superior mesenteric artery sends many branches to the intestine, which anastomose and form arterial arcades
• Vasa recta are straight arteries that arise from the arcades and supply individual sections of the small intestine wall. These do not form anastomoses within the mesentery, but have extensive anastomoses among the blood vessels of the wall of the intestine
• The jejunum is more vascular than the ileum and the arterial arcades are more complex and shorter. This (along with the higher levels of fat found in the mesentery of the ileum) helps surgeons distinguish and localize parts of the small intestine when operating.
Drained by the superior mesenteric vein, which forms the portal vein as it joins the splenic vein, posterior to the neck of the pancreas, anterior to the superior mesenteric artery.
The majority of the intestine drains into the mesenteric lymph nodes, which drain into the superior mesenteric lymph nodes. The terminal ileum drains into the ileocolic lymph nodes.
Parasympathetic and sympathetic nerves form the myenteric and submucosal plexuses in the intestinal walls.
• Sympathetic: coeliac plexus via the sympathetic trunks and greater splanchnic nerves.
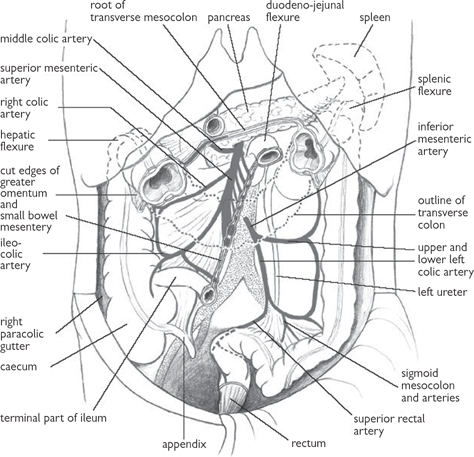
Fig. 8.5 Arterial supply to the small and large bowel derived from the superior and inferior mesenteric arteries.
Reproduced from Mackinnon, Pamela and Morris, John, Oxford Textbook of Functional Anatomy, vol 2, p147 (Oxford, 2005). With permission of OUP.
• The large intestine is the most distal part of the gastrointestinal tract. It is usually considered to comprise four sections:
• The large bowel is functionally important for the absorption of water and storage of faeces prior to defecation
• Most of the large intestine can be distinguished from the small intestine by certain distinguishing features, including:
• Taeniae coli (three flattened, thick, muscular bands which run across the entire wall of the caecum and colon)
• Haustrae (pouches of mucosa between the taeniae coli)
• Epiploic appendices (fat-filled pouches attached to the outer surfaces of the colon)
• These characteristics are useful landmarks during abdominal surgery.
• The caecum (Fig. 8.6) lies in the right iliac fossa distal to the ileocaecal junction, over iliacus and psoas
• It projects downwards as a pouch at the start of the ascending colon, with which it is continuous
• It is covered in peritoneum but has no mesentery.
• The appendix is devoid of taeniae coli, a feature which distinguishes it from the caecum and colon. It is a blind-ending tube on the postero- medial aspect of the caecum, although its position is highly variable, with 75% of cases lying behind the caecum
• The mesoappendix is a short, triangular-shaped mesentery from the mesentery of the terminal ileum, and contains the appendicular branch of the ileocolic artery and the ileocolic vein (a branch of the superior mesenteric vein). Lymph from the appendix (and caecum) drains to lymph nodes in the mesoappendix, and from there to ileocolic nodes. The superior mesenteric plexus supplies sympathetic and parasympathetic nerves to the appendix and caecum. The appendix is rich in lymphoid follicles
• Inflammation of the appendix (appendicitis,  OHCM8 p.610) is common, and usually caused by obstruction of the outlet of the appendix. The secretions cannot escape, causing swelling and stretching of the visceral peritoneum. Clinical features include acute, dull, generalized abdominal pain, which then localizes to McBurney’s point (one-third of the way along a diagonal line from the right anterior superior iliac spine to the umbilicus). This localization and tenderness occurs when the parietal peritoneum around the appendix becomes inflamed.
OHCM8 p.610) is common, and usually caused by obstruction of the outlet of the appendix. The secretions cannot escape, causing swelling and stretching of the visceral peritoneum. Clinical features include acute, dull, generalized abdominal pain, which then localizes to McBurney’s point (one-third of the way along a diagonal line from the right anterior superior iliac spine to the umbilicus). This localization and tenderness occurs when the parietal peritoneum around the appendix becomes inflamed.
The colon comprises four sections.
• Lies retroperitoneally on the posterior wall of the abdomen and is covered by peritoneum anteriorly and on its sides
• Paracolic gutters exist on either side of the ascending colon
• Travels upwards on the right side of the abdomen inferior to the liver, where it turns medially, forming the right colic flexure (hepatic flexure), and continues as the transverse colon.
• Completely covered in peritoneum
• Mesentery starts at the inferior border of the pancreas, where it is continuous with the posterior wall parietal peritoneum
• Runs from the right colic flexure to the left colic flexure at the spleen, at which point it becomes the descending colon. The diaphragm is attached to the left colic flexure by the phrenicocolic ligament.
• A retroperitoneal organ, covered in peritoneum over its lateral and anterior surfaces
• Descends to the sigmoid colon in the left lower quadrant of the abdomen
• Paracolic gutters run along the medial and lateral aspects of the descending colon. The lateral gutter is separated from the spleen by the phrenicocolic ligament. Pus in this gutter (e.g. as a result of diverticular disease ( OHCM8 p.630) causing bowel perforation) will drain into the pelvis.
OHCM8 p.630) causing bowel perforation) will drain into the pelvis.
• Connects the descending colon to the rectum
• The pelvic brim marks the beginning of the sigmoid colon and the rectum starts where the taeniae coli terminate, at the level of the third sacral segment
• Possesses a long mesentery (sigmoid mesocolon) and is therefore intraperitoneal. The attachment of the mesentery is V-shaped, running from the anterior aspect of the sacrum upwards to the bifurcation of the common iliac vessels, then turning laterally and crossing the external iliac vessels along the pelvic brim. Behind the apex of this mesentery lies the left ureter at the point at which it crosses the left common iliac artery—an important surgical landmark.
• The blood supply to the large bowel is a result of its embryological origins
• The midgut (distal duodenum, appendix, caecum, ascending colon, proximal part of the transverse colon) is supplied by the superior mesenteric artery
• The hindgut (distal part of the transverse colon, descending colon, sigmoid colon, rectum) is supplied by the inferior mesenteric artery
• In the rectum, there are anastomoses between branches of the inferior mesenteric and pudendal vessels, and an anastomosis also occurs between the superior and inferior mesenteric vessels via the marginal artery. These are important during bowel resection.
• All blood drains into the portal venous system via the superior mesenteric vein and the inferior mesenteric vein (corresponding to arterial supply)
• The inferior mesenteric vein joins the splenic vein, which merges with the superior mesenteric vein to form the portal vein.
• The right side of the large bowel drains into the inferior mesenteric nodes, and from there to the superior mesenteric nodes and para-aortic nodes
• The left side of the large bowel drains directly into the superior mesenteric nodes.
• Parasympathetic supply to the large bowel is partly via the vagus nerve, which supplies the entire bowel up to the distal transverse colon. The distal large bowel receives parasympathetic fibres from sacral segments via pelvic splanchnic nerves
• Sympathetic nervous supply to the large bowel is from spinal cord segments T10–L2. This is via the lumbar sympathetic chain and the superior hypogastric plexus (presacral nerves)
• Autonomic innervation of the large bowel is important for the regulation of vascular tone and bowel motility. Autonomic efferents supply the bowel via myenteric and submucosal plexuses in the bowel wall
• Visceral sensory fibres run with the lesser splanchnic nerve and can give rise to referred pain of abdominal organs. This pain is poorly localized and corresponds with the skin supplied by the spinal cord segment where visceral pain afferents first synapse.
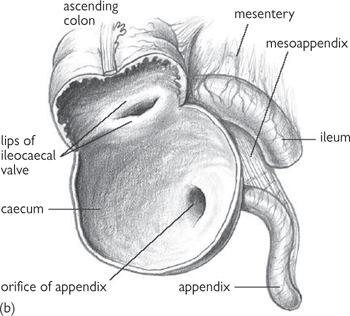
Fig. 8.6 (a) Ileo-caecal junction, caecum, and appendix with its mesentery and arterial supply. (b) Internal aspect of caecum, showing the ileo-caecal valve and appendicular orifice.
Reproduced from Mackinnon, Pamela and Morris, John, Oxford Textbook of Functional Anatomy, vol 2, p148 (Oxford, 2005). With permission of OUP.
• The rectum is the terminal, retroperitoneal section of the intestine which leads into the anal canal
• The rectum starts anterior to the sacrum (S3) and terminates as the dilated rectal ampulla, in front of the coccyx, having followed the curves of the sacrum and coccyx
• The rectal ampulla is extremely distensible and stores faeces prior to defecation
• The anterior and lateral sides of the upper third, and anterior part of the middle third of the rectum, are covered in peritoneum, whereas the inferior third has no such covering.
• The anatomical relations of the rectum are important and rectal examination is a common clinical procedure. In this way, local tumour invasion can be detected. Such growths usually originate from the rectum or prostate.
• Pouch of Douglas (between the rectum and bladder) and posterior wall of the vagina and distal part of the uterus (in females)
• Rectovesical pouch, seminal vesicles, and prostate (in males)
• The anorectal junction is at the level of the pelvic floor, where the taeniae coli merge to form a continuous layer of muscle and the sphincters of the anal canal start. Puborectalis forms a muscular sling around the anorectal junction to create a 90° angle with the pelvic floor. Puborectalis is continuous with the external anal sphincter
• The anal canal is the termination of the alimentary tract and forms the sphincter control for the excretion of waste products. The involuntary internal anal sphincter (smooth muscle) and voluntary external anal sphincter (skeletal muscle) are the two circular muscle layers which make up the wall of the anus. The intersphincteric groove is seen as an indentation on the wall of the anal canal. It marks the area between the internal and external sphincters
• Anal columns are longitudinal ridges formed in the upper anal canal and contain the terminal branches of the superior rectal arteries. Horizontal anal valves join the distal ends of the columns together and are formed by folding of the mucous membrane lining the walls of the canal. Above these valves, mucus is secreted by the submucosal anal glands. These glands can become infected, forming abscesses and fistulae
• The pectineal (or dentate) line is the junction of the two embryological origins of the anal canal, where the epithelium changes from a columnar to a stratified squamous morphology. This occurs just above the anal valves and the intersphincteric groove. Between the intersphincteric groove and the dentate line is a region of transitional epithelium called the pecten. Embryologically, mucosa above the dentate line originates from the endoderm, while below, it originates from the ectoderm. This divide is important when considering the blood, lymphatic, and nervous supplies, and the histology of the epithelial lining.
• The superior rectal artery (terminal branch of the inferior mesenteric artery) supplies the rectum and upper half of anal canal (above the dentate line)
• The median sacral artery (from the internal iliac artery) supplies the rectum
• The middle rectal artery (from the internal iliac artery) supplies the middle and inferior rectum
• The inferior rectal artery (a branch from the internal pudendal branch of the internal iliac artery) supplies the lower half of the anal canal (below the dentate line)
• Significant anastomoses exist between these blood vessels.
• The rectal venous plexus of rectal veins comprises an internal rectal plexus deep to the epithelial layer and an external rectal plexus external to the muscularis layer. There is free communication between these two plexuses, forming an anastomosis between the portal and systemic systems
• The superior rectal vein drains the superior part of the internal rectal plexus (which drains the anal canal above the dentate line) and the superior part of the external rectal plexus. The superior rectal vein is the first part of the inferior mesenteric vein
• The internal pudendal vein, via the inferior rectal veins, drains the inferior part of the external rectal plexus and the inferior part of the anal canal via the inferior part of the internal rectal plexus found below the dentate line
• The internal iliac vein, via the middle rectal vein, drains the middle part of the external rectal plexus
• The internal pudendal and internal iliac veins drain into the systemic circulation. The middle rectal veins anastomose with the superior and inferior rectal veins
• In the anus, venous anastomotic cushions are found in the upper third of the anal canal. They are found at the 3, 7, and 11 o'clock positions and, when dilated (e.g. during portal hypertension), can become haemorrhoids. They help to maintain sphincter control.
• Lymphatic drainage from the rectum largely follows the blood vessels supplying the rectum and anal canal
• Lymph vessels from the upper rectum follow the superior rectal vein to drain into the abdominal lymph nodes (para-aortic) and, from the lower rectum, into the inguinal nodes
• Lymphatic drainage of the anal canal is separated by the dentate line. Above the line, lymph drains to the internal iliac nodes, whereas below it drains to the superficial inguinal nodes.
• Parasympathetic: pelvic splanchnic nerves (S2, 3, and 4)
• Sympathetic: Plexuses of coeliac and hypogastric nerves.
• Parasympathetic: pelvic splanchnic plexus (relaxes internal sphincter)
• Sympathetic: pelvic plexus (contracts internal sphincter)
• Somatic: pudendal nerve (S2) via the inferior rectal branch (external sphincter and sensation to the distal anal canal—below the dentate line).
 OHCM8 p.367)
OHCM8 p.367)• The spleen forms part of the reticuloendothelial system. It is roughly the shape of a fist and is ~12cm long and 7cm wide in the adult, fitting neatly under the left side of the diaphragm
• It is protected by the rib cage and lies inferior and posterior to the stomach
• The spleen is contained within a fibrous capsule which is continuous with a meshwork of trabeculae which carry nerves and blood vessels (trabecular arteries and veins) into the parenchyma of the organ. The fibrous capsule of the spleen is covered with visceral peritoneum, except at the hilum, where the blood vessels emerge
• The main substance of the spleen is referred to as splenic pulp, which is further divided into white pulp and red pulp
• White pulp is lymphoid tissue containing large numbers of immune system cells and is concentrated in periarticular lymphoid sheaths which surround smaller arterioles as well as splenic nodules. Different areas of lymphoid tissue in the spleen have specific immunological functions
• Red pulp is so called because of the large amount of blood it contains. It consists of sinusoids (large diameter, thin-walled blood vessels) and splenic cords (composed of reticular cells, reticular fibres, and certain immune and blood cells e.g. macrophages, platelets, and red blood cells). Certain blood cells, including red blood cells, can pass between the sinusoids and the splenic cords
• The exact functions of the spleen vary between species, but in the human it has several well-defined roles:
• In the newborn, it produces lymphocytes
• Splenic phagocytes act as a filter removing foreign material and worn out red blood cells from the circulation
• In the fetus, it provides a source of red blood cells
• Gastrosplenic ligament (omentum)—attaches spleen to the greater curvature of the stomach
• Splenorenal ligament—attaches spleen to the left kidney.
• Posteriorly: spleen is divided from the 9th to 11th ribs by the diaphragm
• Anteriorly: stomach and tail of pancreas (at hilum of spleen)
• Laterally: splenic flexure of the descending colon and diaphragm.
The splenic artery (a branch of the coeliac trunk) divides into several branching arteries that supply the spleen at the hilum. The splenic artery runs in the splenorenal ligament behind the omental bursa.
Several venous branches drain the spleen and merge to form the splenic vein. The splenic vein receives the inferior mesenteric vein behind the pancreas. Here, the splenic vein merges with the superior mesenteric vein to form the portal vein draining into the liver.
The spleen drains to nodes at the hilum, which drain into pancreaticosplenic lymph nodes on the posterior surface and superior border of the pancreas.
The spleen is supplied with vasomotor fibres by the coeliac plexus. Generally, they follow the same route as branches of the splenic artery.
• The liver is the largest internal organ, situated in the right upper quadrant of the abdomen. Superiorly, the liver apposes the diaphragm and, inferiorly, it reaches the costal margin
• The liver is divided into right and left lobes by the falciform ligament. Anterosuperiorly lies the umbilical fissure, within which is the round ligament (an embryological remnant of the umbilical vein). The gallbladder is attached anteroinferiorly
• Between the umbilical fissure and gallbladder is the quadrate lobe. The caudate lobe lies posterior to the quadrate lobe, separated by the portal vein, hepatic artery, and hepatic duct. These structures enter the liver at the porta hepatis. The inferior vena cava sits at the posterior surface of the liver.
• The liver receives two main sources of blood:
• The hepatic artery (about 20% of flow and 60% of oxygen supply)
• The portal vein (about 80% of flow and 40% of oxygen supply)
• Blood enters the sinusoids within the liver, and drains via tributaries into the hepatic vein, which joins the inferior vena cava
• Sinusoids are arranged between layers of hepatocytes. They are lined by endothelial cells and Kupffer cells, which have a phagocytic function. Between the layers there is the space of Disse, which is composed of basement membrane and stellate cells
• When blood flow is obstructed, porto-systemic anastomoses open. This results in the formation of varices at a number of sites, which include the oesophagus and rectum. This is clinically important as they can be the cause of major gastrointestinal bleeds ( OHCM8 p.254).
OHCM8 p.254).
Lymph is formed in the perisinusoidal spaces of the liver and drains into hepatic ducts via smaller lymphatic vessels.
 OHCM8 pp.636, 637)
OHCM8 pp.636, 637)• Bile is formed in the liver and secreted via bile ductules into right and left hepatic ducts. The hepatic ducts join at the porta hepatis forming the common hepatic duct
• The gallbladder, which stores and concentrates bile, is connected to the common hepatic duct by the cystic duct, together forming the common bile duct
• The pancreatic duct and common bile duct join the second part of the duodenum at the ampulla of Vater.
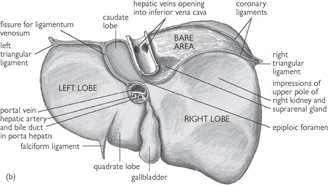
Fig. 8.7 (a) Anterior and (b) posterior views of the liver and its peritoneal reflections.
Reproduced from Mackinnon, Pamela and Morris, John, Oxford Textbook of Functional Anatomy, vol 2, p161 (Oxford, 2005). With permission of OUP.
• The pancreas is a retroperitoneal organ, located in the epigastrium. It can be anatomically divided into three parts: head, body, and tail (Fig. 8.8). The head is apposed to the duodenum and the tail contacts the spleen
• Microscopically, the pancreas is divided into exocrine and endocrine cells. The containing exocrine pancreas is made up of acinar glands, which produce digestive enzymes that drains an alkaline secretion into the pancreatic duct
• The endocrine pancreas is made up of Islets of Langerhans. These contain three main cell types that produce, store, and secrete different enzymes: α-cells (secrete glucagon); β-cells (secrete insulin); δ-cells (secrete somatostatin)
• The head of the pancreas receives arterial blood from the superior and inferior pancreatico-duodenal arteries, which are derived from the coeliac and superior mesenteric arteries. The remainder of the pancreas is supplied by the splenic artery, a branch of the coeliac artery. Venous blood drains into the portal vein behind the pancreatic neck. Lymphatic drainage follows blood vessels to lymph nodes around the coeliac axis
• The pancreas is innervated by sympathetic and parasympathetic fibres from the coeliac plexus. These regulate blood flow (sympathetic) and exocrine and endocrine secretions (parasympathetic)
• Pancreatitis is the inflammation of the pancreas. This can be acute or chronic and there are a number of causes ( OHCM8 p.280 (chronic), 636 (acute)).
OHCM8 p.280 (chronic), 636 (acute)).
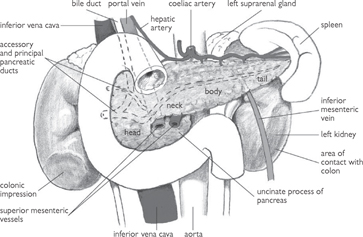
Fig. 8.8 Pancreas and its immediate relationships.
Reproduced from Mackinnon, Pamela and Morris, John, Oxford Textbook of Functional Anatomy, vol 2, p164 (Oxford, 2005). With permission of OUP.
• Gastrointestinal motility churns the contents of the tract to promote digestion and absorption; propels the contents along the tract; and stages the progression of the contents
• Most motility derives from circular and longitudinal smooth muscle layers in the walls of the gastrointestinal tract and sphincters that separate different segments. The first (upper oesophageal) and last (external anal) sphincters comprise striated muscle
• While striated muscle is under voluntary control, smooth muscle possesses an enteric nervous system (see Chapter 4, non-adrenergic, non-cholinergic nerves in the PNS); it can function without extrinsic innervation. The enteric nervous system coordinates muscular activity using reflex arcs:
• The mucosal layer possesses cells that detect chemicals (H+, protein digestion products) or mechanical forces (tension, stretch)
• The receptor cell bodies are in the submucosal (Meissner’s) plexus
• Interneurones transmit signals between the submucosal plexus and the myenteric (Auerbach’s) plexus
• Effector neurones in the myenteric plexus modulate motor activity in smooth muscle layers in response to sensory input
• Enteric neurones release a variety of neurotransmitters and neuromodulators, including ACh, substance P, vasoactive intestinal peptide (VIP), nitric oxide, cholecystokinin (CCK), serotonin, somatostatin. Neurones often release more than one transmitter. Enteric reflex arcs can be modulated by inputs from the autonomic nervous system
• Parasympathetic preganglionic fibres make cholinergic synapses with myenteric excitatory motor neurones (= post-ganglionic neurones, which release ACh, substance P) to increase motility
• Sympathetic post-ganglionic fibres form noradrenergic synapses with myenteric inhibitory motor neurones (which release VIP, nitric oxide) to reduce motility
• Autonomic afferent fibres relay information from mucosal receptors to the brain.
Swallowing is controlled by the swallowing centre in the medulla. Outputs pass through cranial nerves (V, IX, X). It has three phases:
• Oral phase: material moved to rear of mouth by tongue
• Soft palate moves up and back to close nasal passages
• Voluntary pharyngeal muscles contract—bolus propelled towards oesophagus
• Epiglottis closes over larynx, upper oesophageal sphincter relaxes
• Food enters oesophagus, sphincter constricts
• Oesophageal phase: peristaltic wave spreads along oesophagus, propelling bolus towards stomach. Secondary waves clear residual material. The lower oesophageal (cardiac) sphincter and proximal stomach relax and bolus enters the stomach. Sphincter constricts to prevent reflux ( OHCM8 p.244).
OHCM8 p.244).
Peristalsis and receptive relaxation of sphincter and stomach can fail in achalasia ( OHCM8 p.240).
OHCM8 p.240).
• Activity differs in fed and fasting states. In the fed state, motility patterns comprise:
• Propulsion: food is gradually propelled by peristalsis towards the antrum of stomach. Peristalsis initiates in pacemaker cells in the middle of greater curvature. Slow waves of depolarization spread and may excite circular muscle if the threshold is reached
• Mixing by smooth muscle in the antrum: contents are forced towards the pylorus, and small particles (<2mm) pass through the pyloric sphincter
• Retropulsion: contraction of pylorus and antrum closes the sphincter, forces larger material back toward antrum
• Repeated propulsion, mixing, retropulsion cycles ensue. Contents become increasingly fluid over time: the presence of the semi-liquid mass of partially digested food (chyme) induces the sphincter to remain open for longer periods
• Emptying depends on intragastric pressure, on volume and osmolarity for fluids, and on particle size and calorific content for foodstuffs. Duodenal content feeds back to modulate gastric emptying
• In the fasting (or interdigestive) state, there are migrating motor complexes (MMCs). These spread from stomach into small intestine
• The MMC comprises: long periods of inactivity, punctuated by periods when action potential frequency progressively increases until a sustained peak is achieved after which the frequency declines
• Contractility increases in parallel with action potential frequency. The MMC sweeps stomach contents (including acid) into and through the small intestine
• Motilin (28-amino acid peptide released from endocrine cells in duodenum) initiates MMC in the stomach
• Feeding terminates MMC, possibly through neurotensin released from endocrine cells throughout the tract.
• Exhibits slow waves of depolarization, which initiate contraction of circular smooth muscle
• Waves are result of transient relief of enteric inhibition. Frequency decreases along small intestine
• In the fed state, isolated contraction of circular muscle causes segmentation or churning; peristalsis causes propulsion. Peristaltic waves propagate only short distances, generally no more than 10cm
• In the fasting state, MMC pulses propel contents
• The ileocaecal sphincter separates small and large intestines: distension of the ileum initiates relaxation, distension of colon inhibits
• Vomiting ( OHCM8 pp.56, 240) is initiated by the vomiting centre in the medulla. There is reverse peristalsis of small intestine, relaxation of pyloric sphincter and stomach. Intestinal contents are swept into stomach
OHCM8 pp.56, 240) is initiated by the vomiting centre in the medulla. There is reverse peristalsis of small intestine, relaxation of pyloric sphincter and stomach. Intestinal contents are swept into stomach
• Abdominal contraction then forces the contents into the oesophagus, and there is reflex relaxation of oesophageal sphincters. Vomiting is induced by diverse stimuli that include emotion, pain, rotation, chemical composition of food, distension, obstruction, alcohol.
• In the large intestine, longitudinal smooth muscle forms three bundles (taeniae coli) that define folds or haustra
• Slow wave depolarization induces contraction of circular smooth muscle to produce segmentation. Waves increase in frequency along the large intestine
• Segmental contraction of haustra produces pendular movements of contents. Concerted contraction of haustra propels contents over short distances
• Large peristaltic propulsions (mass movements, around 20cm) occur infrequently (up to three times a day). Distension of stomach (gastrocolic reflex) and standing (orthocolic reflex) initiate mass movements. The distal colon demonstrates non-propulsive segmentation: this retards flow.
• Intermittently receives material and undergoes segmental contraction
• Distension of the rectum initiates rectosphincteric reflex: the internal anal sphincter transiently relaxes
• If the timing is not good, contraction of the external sphincter overrides the reflex. The urge passes for the moment. If appropriate, voluntary relaxation of the external anal sphincter leads to evacuation
• The rectum propels faeces into and through anal canal, assisted by voluntary contraction of the diaphragm and abdominal wall, which increases intra-abdominal pressure.
• There are a number of similarities between the salivary glands and the exocrine pancreas
• Both comprise a branching ductal arrangement into which epithelial cell secretions are released. Secretions are composed of water, electrolytes, and some digestive enzymes
• Both secretions aid digestion. Saliva lubricates ingested food, forms a protective buffer, and initiates digestion of starch. Alkaline pancreatic juice neutralizes stomach acid and completes digestion of ingested foodstuffs
• Glands comprise secretory units (lobules) made of an acinus: up to 100 cells lining an intercalated duct. Intercalated ducts drain into intralobular ducts, then into interlobular ducts, and, finally, into the main salivary or pancreatic duct. Post-ganglionic autonomic fibres innervate the cells.
• There are three types of salivary glands:
• Parotid: produces watery (serous) secretion amounting to 25% of total
• Submandibular: produces both serous and mucous secretions—70% of total
• Sublingual: produces mucous secretion—5% of total
• Serous secretions are supplemented with the enzyme, α amylase; mucous secretions with mucin. Average daily production = 1.5L day–1. Basal flow rate = 0.5mL min–1 rising to 5mL min–1 after stimulation
• Acinar cells secrete isotonic NaCl
• Basolateral Na+-K+-2Cl– co-transporter accumulates Cl– ions inside the cell
• Cl– ions diffuse across the apical membrane through channels. Na+ is pumped out at the basolateral membrane, K+ diffuses out through basolateral channels
• Na+ ions diffuse between cells through tight junctions, along electrical gradient established by Cl– movement
• Duct cells modify the primary secretion. Na+ and Cl– are reabsorbed and K+ and  secreted to a lesser degree. Permeability to water is low: water reabsorption is minimal and luminal fluid becomes hypotonic. At higher rates of secretion, the time available for modification is reduced: fluid is more similar in composition to plasma
secreted to a lesser degree. Permeability to water is low: water reabsorption is minimal and luminal fluid becomes hypotonic. At higher rates of secretion, the time available for modification is reduced: fluid is more similar in composition to plasma
• Different acinar and duct cells synthesize, store, and release proteins:
• Enzymes: α-amylase digests starch to maltose; lingual lipase starts fat digestion
• Kallikrein: cleaves proteins to yield vasodilator peptides (e.g. bradykinin)
• Lysozymes, lactoferrin, lactoperoxidase, proline-rich proteins, IgA: antimicrobial
• Salivary secretion is regulated by the autonomic nervous system (see Chapter 4, synaptic transmission):
• Parasympathetic outflow in cranial nerves V and VII is most important. ACh stimulates primary secretion, reduces secondary modification: produces large volumes of watery saliva. ACh binds M3 receptors, induces IP3 generation, Ca2+ mobilization in acinar and duct cells. In acinar cells, Ca2+ stimulates protein kinases which activate apical Cl– channels and basolateral K+ channels. Phosphorylation of cytoskeletal elements also induces export of protein-containing vesicles
• Sympathetic actions are less pronounced, although noradrenaline binding to α receptors also mobilizes Ca2+ through IP3. Binding to β receptors raises cAMP, activates protein kinase A, stimulates amylase secretion from vesicles: produces viscous saliva
• Substance P also raises Ca2+, initiates secretion.
• 90% of the pancreas is made up of exocrine cells; the remainder is made up of endocrine cells. The pancreas secretes 1.5L day–1 of alkaline, protein-rich fluid. The alkalinity helps neutralize stomach acid ( OHCM6 p.485)
OHCM6 p.485)
• Acinar cells produce a primary, plasma-like secretion. The mechanism of primary secretion is similar to that in salivary glands. The primary secretion hydrates digestive proteins released from the acinar cells
• Proteins secreted can be precursors (zymogens, activated in small intestine) or active enzymes:
• Proteases—for protein digestion (trypsinogens, chymotrypsinogens, proproteases, procarboxypeptidases)
• Amylases—for carbohydrate digestion
• Nucleases—for RNA, DNA digestion
• Constitutive secretion occurs but can be increased 10-fold by ACh M3 and CCK receptor activation, IP3 generation, Ca2+ mobilization
• Pancreatic duct cells perform secondary modification: there is secretion of isotonic NaHCO3
•  is generated in the cell from hydration of CO2 and secreted by apical Cl–-
is generated in the cell from hydration of CO2 and secreted by apical Cl–- exchange. Na+ and H2O follow through the paracellular pathway
exchange. Na+ and H2O follow through the paracellular pathway
• Cl– ions that enter the cell recycle across the apical membrane through the Cl– channel CFTR (cystic fibrosis transmembrane conductance regulator). In cystic fibrosis, mutations in CFTR compromise secondary secretion. Secretions are more viscous and clog the duct: The consequent deficiency in digestive enzymes can be treated with oral administration of enzymes (coated to prevent gastric digestion) with every meal
• As for gastric acid secretion, pancreatic secretion regulated by three phases of digestion:
• Cephalic phase: mediated primarily by ACh
• Gastric phase: mediated by CCK, gastrin
• Intestinal phase: mediated by secretin
• Goblet cells in ducts secrete mucus to facilitate lubrication, offer mechanical protection, bind pathogens.
• Secretions act in concert to facilitate digestion (and absorption) of ingested foodstuffs. Digestion is commenced by salivary secretions, continues in the stomach and is later assisted by secretions from the liver and pancreas
• The environment of the stomach is acidic: pH can be as low as 1. This acidity assists protein digestion by denaturing proteins and activating pepsin enzymes. A mucous lining on the epithelial surface of the stomach and the presence of  ions prevent autodigestion of the stomach
ions prevent autodigestion of the stomach
• The gastric epithelial lining is characterized by gastric glands, which increase the surface area. Glands comprise pits which open into a neck which leads to a base. A variety of cell types make up the lining:
• Parietal (oxyntic) cells in the base and neck secrete HCl and intrinsic factor (necessary for vitamin B12 absorption in the ileum)
• Chief (peptic) cells in the base and neck cells secrete pepsinogen
• Endocrine cells in the base secrete regulators such as gastrin and somatostatin via the bloodstream
• Mucous neck cells secrete mucus; superficial epithelial cells in the pit and on the surface lining secrete mucus, along with  ions.
ions.
• Parietal cells possess deep invaginations of apical membrane. In an unstimulated cell, there are large numbers of tubulovesicles in the subapical cytoplasm. Tubulovesicle membranes contain H+-K+ ATPase proteins
• Tubulovesicle fusion with the apical membrane leads to acid secretion:
• Intracellular carbonic anhydrase catalyses hydration of CO2 to yield H+ and 
• The ATPase pumps H+ into the lumen in exchange for K+. K+ recycles into the cell through apical K+ channels
•  exits across the basolateral membrane into interstitial fluid, then blood, on Cl–-
exits across the basolateral membrane into interstitial fluid, then blood, on Cl–- exchange
exchange
• Cl– diffuses through apical channels, joins H+ in the lumen
• Net result: secretion of HCl, alkalinization of blood (‘alkaline tide’)
• Tubulovesicle insertion is initiated by:
• Acetylcholine (neurocrine): from vagus nerve endings. Binds M3 receptors, triggers IP3 cascade to raise [Ca2+], activate kinases
• Gastrin (endocrine): from endocrine G cells in response to gastrin-releasing peptide (GRP) from peptidergic vagus nerve endings or protein digestion products in lumen. Binds CCKB receptors, triggers IP3 cascade to raise [Ca2+], activate kinases
• Histamine (paracrine): from enterochromaffin-like (ECL) cells. Binds H2 receptors, triggers cAMP generation, activates protein kinase A. ACh and gastrin bind M3 and CCKB receptors on ECL cells, induce histamine release. This ‘common mediator’ action explains effectiveness of H2 antagonists (e.g. ranitidine) as inhibitors of gastric acid secretion.
• There are three phases of gastric secretion (Fig. 8.9):
• Cephalic: thought, sight, smell, taste initiate vagal stimulation, ACh, GRP release. Responsible for 30% of secretion
• Gastric: distension initiates vagovagal reflex, protein digestion products promote gastrin release. Responsible for 60% of secretion
• Intestinal: protein digestion products stimulate duodenal G cells. Responsible for 10% of secretion
• Omeprazole inhibits the gastric H+-K+ ATPase.
• Release somatostatin, which inhibits adenylyl cyclase and reduces gastric acid secretion. ACh inhibits, and low luminal pH stimulates, somatostatin release
• Acid-induced release of secretin from duodenal S cells stimulates somatostatin release (and inhibits gastrin release)
• Gastric Helicobacter pylori infection inhibits somatostatin release and is an important cause of ulcers.
• Secrete inactive pepsin precursor, pepsinogen, which is activated by N-terminal truncation. Spontaneous activation occurs in acidic lumen. Low pH is also required for optimal activity of pepsin
• Once activated, pepsins catalyse pepsinogen truncation. Pepsin digests one-fifth of ingested protein. Pepsin release involves fusion of secretory granules with apical membrane and is stimulated by:
• ACh, via M3 receptors, Ca2+ signalling
• Gastrin and CCK, via CCKB receptor, Ca2+ signalling
• Secretin, via adenylyl cyclase-coupled receptors, cAMP signalling.
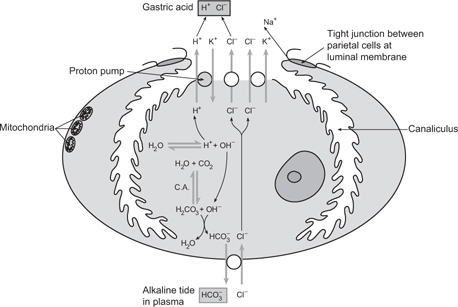
Fig. 8.9 Steps involved in the secretion of gastric acid by a parietal (oxyntic) cell.
Reproduced with permission from Pocock G, Richards CD (2006). Human Physiology: The Basis of Medicine, 3rd edn, p389. Oxford: Oxford University Press.
• Secrete mucin, a large glycoprotein with high viscosity. This, together with ions, water, and phospholipids forms a mucus gel up to 200µm thick that acts as a barrier to H+ ion diffusion from acidic bulk solution in the lumen
• Surface epithelial cells secrete  ions across the apical membrane, making the pH of the gel around 7 and neutralizing any H+ ions that reach it
ions across the apical membrane, making the pH of the gel around 7 and neutralizing any H+ ions that reach it
• Secreted acid bores through the mucus without lateral spread—stream of H+ is termed a ‘viscous finger’
• Secretion of mucous components is stimulated by ACh-induced Ca2+ signalling. Mucosa can be disturbed by aspirin, alcohol, anti-inflammatory drugs.
Dysfunction of one or more of the processes described can lead to dyspepsia (indigestion) and ulceration. Infection (H. pylori >80% of cases) ( see p.555) NSAIDs (
see p.555) NSAIDs ( see p.850) and smoking are risk factors. Duodenal ulcers are more common than gastric ulcers.
see p.850) and smoking are risk factors. Duodenal ulcers are more common than gastric ulcers.
Inhibition of prostaglandins that are thought to provide endogenous protection against acid-mediated damage of the gastric and duodenal mucosa through increased blood flow and mucus secretion is a major factor in induction of NSAID-mediated ‘gastric toxicity’ characterized by ulceration
Box 8.1 summarizes the treatments of ulcers.
• Bile is secreted by the liver. It promotes lipid digestion, absorption, and elimination of endogenous (cholesterol) and exogenous (heavy metals) components. There is constitutive secretion, which is up-regulated in the fed state
• Bile components are dissolved in an alkaline solution, with similar composition to pancreatic juice. Bile comprises:
• Hepatocytes line bile canaliculi, which drain into bile ductules, then into a series of ducts that unite to form the common hepatic duct. Blood-to-lumen trafficking occurs across the cell, with compounds bound to proteins or contained within vesicles
• Hepatocytes synthesize primary bile acids cholic acid and chenodeoxycholic acid from cholesterol
• Acids are conjugated to glycine or choline. They ionize and exist as Na+ or K+ bile salts
• Bile salts are actively secreted into the canalicular lumen by an ATPase in the hepatocyte apical membrane
• Secondary bile acids result from bacterial deconjugation, dehydroxylation of primary bile salts in the intestine. Some bile acids are reabsorbed by ileum, bound to albumin, and returned to the liver in the blood. After dissociation from albumin, they are taken up into hepatocytes by transporters in the basolateral membrane. This is an example of enterohepatic circulation. After reconjugation, they are secreted again
• Bile acids may recirculate up to three times before digestion of a meal is complete
• Conjugated bile acids are amphipathic: they possess water-soluble and lipid-soluble domains. Combined with phospholipid and cholesterol, they form mixed micelles in aqueous solution. They provide a vehicle for transport of lipid soluble substances in the aqueous environment of the small intestine
• The secretion of organic anions (e.g. thromboxanes), organic cations (e.g. choline, antibiotics), and the haemoglobin breakdown product, bilirubin, occurs by carrier-mediated transport in series, first across the basolateral and then across the apical membrane
• There is also secretion of inorganic ions (Na+, K+, Ca2+, Cl–,  ) through channels and carriers in hepatocyte membranes, and by movement through the paracellular pathway. Secretion of bile salts and organic compounds establishes an osmotic gradient for water movement into the canaliculi. Most bile flow is dependent on bile acid secretion
) through channels and carriers in hepatocyte membranes, and by movement through the paracellular pathway. Secretion of bile salts and organic compounds establishes an osmotic gradient for water movement into the canaliculi. Most bile flow is dependent on bile acid secretion
• Cells lining bile ducts (cholangiocytes) secrete a watery NaHCO3 solution using a mechanism similar to that in pancreatic duct cells. Secretion is stimulated by secretin, glucagon, VIP, which increase cAMP levels and activate CFTR. The solution secreted is water-rich and bile salt-poor. Somatostatin lowers cAMP, inhibits secretion
• The gallbladder stores secreted bile, especially in the fasting state.
• Gallbladder epithelial cells reabsorb an isotonic NaCl solution, concentrating bile acids
• CCK regulates release of gallbladder bile. It stimulates gallbladder smooth muscle contraction and relaxes the sphincter of Oddi, which allows bile to flow from the ducts into the duodenum.
• Carbohydrates, proteins, fats, and vitamins and minerals enter the body through the small intestine
• The small intestine surface area is enhanced by folds of mucosa and tiny, finger-like projections (called villi). Villi are lined by epithelial cells that absorb nutrients, and possess ruffled apical (‘brush border’) membranes ( p.54)
p.54)
• Some substances can be absorbed directly from the lumen by the cell, without prior digestion (e.g. glucose). Alternatively, uptake can involve:
• Hydrolysis in the lumen, absorption across the cell (e.g. proteins)
• Hydrolysis on the brush border membrane, absorption across the cell (e.g. disaccharides)
• Absorption into, and hydrolysis within, the cell (e.g. di- and tripeptides)
• Luminal hydrolysis, uptake, and resynthesis within the cell (e.g. triglycerides).
Carbohydrates are provided predominantly in the form of starch.
• Salivary amylase initiates digestion
• Pancreatic amylase renders most starch in the form of disaccharides, trisaccharides, and α-limit dextrins
• Brush border disaccharidases (sucrase, lactase) release glucose, fructose, galactose
• Glucose and galactose are reabsorbed across the apical membrane by a Na+-dependent carrier, SGLT-1; fructose is reabsorbed by the facilitated diffusion carrier GLUT5
• Monosaccharides exit the cell at the basolateral membrane on GLUT2
• Reabsorption of monosaccharides from interstitial fluid to blood completes the process. Absorption complete by mid-jejunum.
Digestion of proteins begins in the acidic environment of the stomach.
• Pepsin secreted and activated in the stomach initiates digestion
• Pancreatic peptidases (trypsin, chymotrypsin, elastase, carboxy-peptidases) responsible for the bulk of digestion of proteins to oligopeptides, amino acids
• Further digestion of oligopeptides to amino acids by brush border peptidases can occur
• Amino acids are absorbed by apical carriers, mostly Na+-dependent. Different carrier proteins exhibit different specificities for different classes of amino acids (neutral, cationic, basic)
• Di- and tripeptides can be directly absorbed by a H+-dependent apical carrier protein, PepT-1. Cytosolic peptidases then convert peptides to amino acids
• Amino acids exit the cell across basolateral membrane on Na+-independent carriers
• Reabsorption of amino acids from interstitial fluid into the blood completes the process. Absorption is mostly complete by end of jejunum.
Triglycerides are the most common dietary lipid. The absorption process is more complex, since fats are water insoluble.
• Muscular movements of the stomach emulsify fats (transformation into emulsion of oil droplets in water), aided by lingual lipases
• Most digestion occurs in small intestine. Pancreatic lipase digests triglycerides to monoglycerides and free fatty acids. Colipase, from the pancreas, coordinates binding of lipase to emulsion
• Monoglycerides—free fatty acids incorporated into bile micelles (spherical structures with the polar group facing outwards, the hydrophobic group facing towards the interior)—diffuse into an unstirred layer adjacent to the brush border
• Micelle components dissociate at the cell surface
• Digestion products enter cell by dissolving in lipid membrane—non-ionic diffusion
• Within the cell, the components are reassembled in smooth ER; they combine with apoproteins made in the rough ER to form microscopic particles of triglyceride called chylomicrons
• Chylomicrons are exported by the Golgi apparatus into interstitial fluid and pass into lymphatic capillaries. Lymph drains back into left subclavian vein via thoracic duct. In the fasting state, VLDLs, containing endogenous lipids, are secreted into lymphatic system.
Vitamins are organic substances that act as co-enzymes and/or regulators of metabolic processes. They can be water-soluble (e.g. thiamine B1, riboflavin B2, B12, vitamin C, folic acid) or fat-soluble (e.g. vitamins A, D, E, K). Water-soluble vitamins can be reabsorbed by:
• Specific carrier proteins (B1, C, folic acid)
• Binding to specific brush border receptors: B12 binds to intrinsic factor secreted by parietal cells of stomach. The complex formed binds an apical receptor which is then internalized. B12 exits across the basolateral membrane bound to a second protein, transcobalamin.
Fat-soluble vitamins are presented for absorption dissolved in bile micelles. In most cases, they exit the epithelial cell, unmodified, in chylomicrons.
Minerals are essential ions that need to be absorbed and include Ca2+, Mg2+, and Fe2+.
• Ca2+ is passively reabsorbed via the paracellular route throughout the intestine
• In addition, in the duodenum there is active transcellular absorption. Ca2+ enters across apical membrane through channels and is buffered in the cell by calbindin, exits at basolateral membrane on Ca2+ ATPase or Na+-Ca2+ exchange. Processes are stimulated by parathryroid hormone (PTH).
Absorption occurs through passive paracellular diffusion along the small intestine. Active absorption occurs in ileum.
• Absorption of non-haem iron occurs in the duodenum
• Fe2+ is the absorbed species
• Non-haem Fe3+ is reduced to Fe2+ by an apical membrane reductase
• Fe2+ is taken up using the apical H+-driven transporter DCT
• Within the cell, Fe 2+ binds mobilferrin
• The complex is translocated to the basolateral membrane, where a carrier, IREG1, mediates efflux
• An oxidase, hephaestin, oxidizes Fe2+ to Fe3+
• Fe3+ binds transferrin in plasma
• Haem iron is also absorbed in the duodenum. Haem is endocytosed at the apical membrane and split enzymatically. An intracellular reductase reduces Fe3+ to Fe2+, which is then processed in the same way as non-haem Fe2+
• The intestine is sole site for regulation of the body’s iron levels. If iron levels are adequate, iron absorption can ‘stall’ within the intestinal cells. Cells turn over every 5 days, and any surplus iron stored in the cell is excreted (the ‘iron curtain hypothesis’).
• Around 9L of fluid enters the gastrointestinal tract each day—2L ingested, 7L secreted. Yet only 100mL is excreted in the stool each day
• The small intestine absorbs the majority of the fluid—up to 8.5L. Daily absorption by the colon is only 500mL—approximately 10% of its maximum absorptive capacity
• Faeces are hypertonic. Water absorbed by the gastrointestinal tract replaces that lost from urination, perspiration, respiration, and can be used for subsequent secretions
• The small intestine behaves as a leaky epithelium ( p.56) with paracellular transport of ions and water playing a significant role. NaCl transport is not regulated directly. The colon behaves as a tight epithelium, with transport through the cells dominating transepithelial transport. Colonic transport is regulated
p.56) with paracellular transport of ions and water playing a significant role. NaCl transport is not regulated directly. The colon behaves as a tight epithelium, with transport through the cells dominating transepithelial transport. Colonic transport is regulated
• Water reabsorption occurs through osmosis and is the secondary consequence of solute (electrolyte or non-electrolyte) reabsorption. The movement of Na+ is central to this process. It, along with co-transported osmolytes, establishes a hypertonic interstitial fluid that draws water, by osmosis, through the cell and tight junctions. The water then moves from the interstitial space to the blood. Water may move through tight junctions or transcellularly through membrane aquaporins.
• There are a number of pathways for Na+ reabsorption:
• Passive diffusion through channels (ENaC)
• In exchange for H+ on Na+-H+ exchange (NHE)
• Co-transported with glucose (SGLT), amino acids (e.g. system B)
• Co-transport with Cl– ions (Na+-Cl– co-transport, NCC, or by parallel operation of NHE and Cl–- exchange (AE)
exchange (AE)
• Different processes play the dominant role in Na+ absorption at different points along the gastrointestinal tract:
• Duodenum, jejunum: mediated by NHE, SGLT
• Ileum: SGLT, NCC, or NHE//AE
• The Na+-K+ ATPase in the basolateral membrane transports Na+ ions into interstitial fluid. Other solutes exit the cell through channels and carriers. Aldosterone stimulates Na+ absorption.
• In addition to absorption by NCC and NHE//AE, Cl– can be absorbed passively:
• In the jejunum and ileum: through Cl– channels in apical, basolateral membranes, and by paracellular route
• Crypt cells at the base of villi can secrete Cl–, through a mechanism that resembles that of salivary or pancreatic acinar secretion. There is a basal level of secretion, masked by reabsorption. Secretagogues (e.g. cholera toxin, VIP, histamine) stimulate apical Cl– efflux mediated by CFTR. Cl– secretion increases: Na+, H2O follow through paracellular route. Signalling is through kinase activation following cAMP generation or mobilization of Ca2+ by IP3. Cholera activation is irreversible: it leads to massive Cl–-rich watery diarrhoea.
• Both reabsorption and secretion of K+ occurs:
• Passive absorption through paracellular route in jejunum, ileum
• Active absorption by H+-K+ ATPase in colon
• Passive secretion through paracellular route in colon
• Active secretion through apical K+ channels in colon
• The relative importance of these pathways depends on K+ balance:
 Aldosterone stimulates K+ secretion (by stimulating the Na+-K+ ATPase)
Aldosterone stimulates K+ secretion (by stimulating the Na+-K+ ATPase)
 Hypokalaemia stimulates absorption.
Hypokalaemia stimulates absorption.
 OHCM8 p.280)
OHCM8 p.280)Insufficient absorption of nutrients for the body’s needs, despite an adequate intake.
• Loss of epithelium in the gut so that there is an inadequate surface area for absorption
• Lack of digestive enzymes to break the food down into absorbable molecules
• Lack of specific transport proteins to bind to some molecules and to facilitate their transcellular transport.
• Gluten-sensitive enteropathy (coeliac disease) —allergy to gluten component of wheat produces a lymphocytic response which damages small bowel epithelium leading to villous atrophy and loss of absorptive surface area
• Post irradiation and chemotherapy—damage to epithelium leads to loss of absorptive surface area (e.g. after conditioning therapy before bone marrow transplant)
• Short bowel syndrome—loss of absorptive surface area due to resection of substantial amounts of small bowel or gross damage by inflammatory processes, most commonly Crohn’s disease
• Pernicious anaemia—autoimmune atrophic gastritis destroys the cells in the stomach which produce intrinsic factor, so vitamin B12 is not absorbed in the terminal ileum
• Pancreatic insufficiency—if the exocrine glands of the pancreas are destroyed (e.g. in cystic fibrosis or chronic alcoholism), there is a deficiency of lipases, etc., to digest fats and, so, malabsorption of these and fat-soluble vitamins
• Enzyme deficiencies—e.g. disaccharidase deficiency producing lactose intolerance.
Deficiencies of whatever nutrients are not being absorbed, which may be specific or general, depending on the cause.
 OHCM8 p.612)
OHCM8 p.612)Obstruction of the intestine by any process, at any level, producing obstruction to passage of food or faeces at that point.
• Obstruction within the lumen
• Dysfunction of the gastrointestinal muscle wall
• Obstruction due to pressure from elements outside the gastrointestinal tract.
• Obstruction within bowel lumen
• Polypoid tumour, e.g. adenomatous polyp/carcinoma in colorectum
• Foreign body, e.g. hair ball mass in stomach
• Fibrous diaphragms, e.g. with chronic use of NSAIDs or pancreatic enzyme supplements in cystic fibrosis
• Intussusception, when the bowel herniates into itself, e.g. at the point of a small tumour or with gross lymphoid hyperplasia in the small bowel
• Dysfunction of the gastrointestinal muscle wall
• Diffusely infiltrating tumour, e.g. diffusely infiltrating (‘signet ring’ type) adenocarcinoma in the stomach producing a rigid organ (well seen on barium meal—‘leather bottle’ stomach = linitus plastica)
• Congenital abnormality of the bowel innervation, e.g. Hirschsprung’s disease, where there is an absence of ganglion cells in the distal part of the colorectum with loss of motility and neonatal intestinal obstruction
• Obstruction by elements outside the gastrointestinal tract
• Intra-thoracic tumours obstructing the oesophagus, e.g. bronchial carcinoma
• Intra-abdominal tumours obstructing the intestine, e.g. disseminated ovarian carcinoma.
• Perforation due to increased pressure and ischaemic necrosis in the bowel proximal to the obstruction, e.g. caecal perforation in obstructing colorectal cancer—but only if the ileocaecal valve is competent (otherwise pressure is decompressed in the small bowel)
• Water/electrolyte imbalance due to gross pooling in the obstructed bowel
• Dysphagia if the obstruction is in the oesophagus
• Vomiting if the obstruction is in the stomach or lower, and obstruction is prolonged.
 OHCM8 p.246)
OHCM8 p.246)Rather vague. Usually means an increased frequency of more liquid stool.
• Toxin—which causes increased secretion of fluid by gut epithelial cells into the gut lumen e.g. the toxin of Vibrio cholera
• Damage to the gut epithelium—so that water is lost into the gut lumen and/or not resorbed
• Viral infection, e.g. enterovirus
• Bacterial invasion, e.g. salmonella
• Radiation damage, e.g. following conditioning for bone marrow transplantation
• Toxic damage, e.g. during systemic chemotherapy
• Immune damage, e.g. gluten-sensitive enteropathy
• Idiopathic inflammatory damage, e.g. Crohn’s disease
• Masking of the gut epithelium by organisms
• Protozoal infection, e.g. giardiasis
• Osmotic—passive movement into gut lumen
• Disaccharidase deficiency—undigested sugars left in gut lumen and thus greater osmolality
• Laxative abuse—usually osmotically acting laxatives such as lactulose.
• Anaemia—if blood is also being lost due to damaged epithelium
• Complications of specific cause e.g. systemic infection.
Box 8.2 summarizes the treatments for diarrhoea,
 OHCM8 p.276)
OHCM8 p.276)• Thought to affect ~15% of the population to some degree
• Symptoms vary from mild and occasional abdominal discomfort, to frequent severe pain and irregular bowel motility leading to either constipation (IBS-C), or diarrhoea (IBS-D), or both (IBS-A)
• Stress, depression, and infections are thought to trigger the disease while specific constituents of the diet and some drugs (NSAIDs) can exacerbate the symptoms. Although a genetic predisposition to IBS has been suggested, there is little clear evidence to support this notion at present
• IBS is associated with enhanced inflammation and there is some evidence that it is mediated by chronic infections that are poorly detected by current best practice (e.g. blastocystis, Dientamoeba fragilis or simply intestinal flora overgrowth)
For treatments for IBS, see Box 8.3.
Not to be confused with IBS, inflammatory bowel disease (IBD) includes a number of inflammatory conditions, of which Crohn’s disease and ulcerative colitis are the most common.
 OHCM8 p.274)
OHCM8 p.274)• Affects 25–50 people per 100 000 in industrialized countries
• Differential geographical and ethnic distribution, together with strong heritability suggests that there is a genetic component to the disease, However, there are also clear environmental factors involved, e.g. smoking is a recognized risk factor for the disease
• Widely regarded to be an autoimmune disease (i.e. driven by the damaging effects of the body’s own inflammatory cells)
• Characterized by deep lesions in the ileum, the ileo-colonic junction (most common) and/or the colon
• Symptoms include severe abdominal pain, diarrhoea, which might contain blood. Obstruction of the intestine can also occur (intestinal stenoses), particularly following surgery. The severe inflammation can also spill over into systemic effects, including, for example, depressed growth in children and adolescents.
Box 8.4 summarizes the treatments for IBD.
 OHCM8 p.272)
OHCM8 p.272)• Ulcerative colitis is a severe form of colitis (inflammation of the colon) that affects ~0.1% of the population
• The cause is complex: no single gene defect has been identified, but a combination of gene defects are almost certainly involved in predisposing individuals to ulcerative colitis
• However, environmental factors are also closely associated with incidence of the disease; dietary constituents have received particular attention
• Ulcerative colitis has similar symptoms to Crohn’s and IBS (abdominal pain or cramps, often associated with diarrhoea, which might contain blood). There might also be associated weight loss in the patient. Some patients complain of arthritic pain: on occasion this can pre-empt onset of the intestinal symptoms
• Diagnosis is best confirmed with endoscopy, although the most definitive diagnosis comes from microscopic analysis of biopsies: ulcerative colitis is characterized by shallow lesions and inflammation and crypt abscesses, while Crohn’s disease features deep inflammation
• There is an increased risk of colon cancer in patients with ulcerative colitis.
For treatments for ulcerative colitis, see Box 8.5.
Food always contain a certain number of microbial organisms however carefully prepared and in the times of early humans, would have contained many more. These organisms include viruses and bacterial and eukaryote parasitic organisms. Thus, there has evolved an immune system in the gut which largely prevents the invasion or colonization of disadvantageous organisms. In the stomach, the acidic environment is the major non-immune mechanism of antimicrobial defence but, distal to this, an immune defence predominates.
Although the organization of the mucosal immune cells in the gut does not, at first, appear to be as precise as in lymph nodes, there is a very compartmentalized distribution of these cells (Fig. 8.10):
• Intraepithelial lymphocytes—are found in varying numbers throughout the small and large intestine. They are of cytotoxic/suppressor phenotype (CD8+)
• Lymphoid aggregates—are found in the mucosa of the small and large intestine and sometimes extend into the submucosa. They may be visible endoscopically as Peyer’s patches or are sometimes mistaken for epithelial polyps. The epithelial cells overlying mucosal lymphoid aggregates contain membranous (M) cells as well as the usual absorptive cells. These M cells transcytose intact macromolecules from the lumen to be presented to the T-lymphocytes
• B-cells—in the gut tend to produce the dimeric IgA subclass of immunoglobulin rather than the more common IgG. This protein has a secretory chain which facilitates its secretion into the gut lumen where it will bind to specific antigens and which also protects the protein against proteolysis.
The immune tissue in the gut is part of the mucosa-associated lymphoid tissue (MALT) system ( OHCM8 p.356), which is found in other mucosal sites such as the bronchus and salivary glands.
OHCM8 p.356), which is found in other mucosal sites such as the bronchus and salivary glands.
• Neoplasia—lymphomas may develop in the lymphoid tissue of the gut, usually of B-cell origin and usually retaining some characteristics of MALT lymphoid cells, e.g. homing to MALT tissue in other sites in the body
• IgA deficiency—specific deficiency of this class of immunoglobulin may be associated with increased frequency of gastrointestinal infections
• Generalized immunodeficiencies (e.g. post-chemoradiotherapy,  OHCM8 p.370)—will lead to an increased rate of gastrointestinal infections.
OHCM8 p.370)—will lead to an increased rate of gastrointestinal infections.
Fig. 8.10 Organization of the immune system in the gut.
The liver is the metabolic workhouse of the body, playing a role in numerous biosynthetic and metabolic processes (see Chapter 2), including:
• The liver stores glycogen for use for sustaining other tissues during times of fasting
• In addition, the liver is the major site of gluconeogenesis during starvation
• The synthesis of fatty acids takes place in the liver (for storage as triacylglycerides in adipose tissue), as does cholesterol synthesis
• Ketogenesis is also performed by the liver during starvation
• Amino acid metabolism and urea synthesis
• Amino acids are metabolized in the liver and their carbon skeletons converted into glucose by the gluconeogenesis pathway
• In order to maintain nitrogen balance, the body has to excrete excess nitrogen
 The liver makes urea—the non-toxic, water-soluble nitrogenous compound excreted by renal filtration
The liver makes urea—the non-toxic, water-soluble nitrogenous compound excreted by renal filtration
 The ammonia groups incorporated into urea come from the breakdown of amino acids, especially glutamine.
The ammonia groups incorporated into urea come from the breakdown of amino acids, especially glutamine.
More minor but still important roles of the liver include:
• Synthesis of plasma proteins, such as albumin and the blood-clotting factors
• Alcohol metabolism ( see p.577)
see p.577)
• Vitamin/co-factor metabolism
Anything that affects these processes can have major detrimental effects on liver metabolism ( OHCM8 p.258).
OHCM8 p.258).
• Alcohol detoxification takes place primarily in the liver, converting ethanol → acetaldehyde → acetate
• Both reactions reduce NAD+ → NADH, and even moderate alcohol ingestion produces more NADH than can be oxidized by the ETC
• The high NADH:NAD+ ratio is an allosteric inhibitor of a number of pathways (e.g. gluconeogenesis, fatty acid breakdown).
 Consequences include hypoglycaemia and fatty acid accumulation in the liver
Consequences include hypoglycaemia and fatty acid accumulation in the liver
• In addition, acetaldehyde is a reactive compound that covalently binds to biologically important functional groups of proteins in liver and blood
• Hepatitis is an inflammatory infection ( OHCM8 pp.268, 406) of the liver which interferes with plasma protein synthesis, causing clotting disorders.
OHCM8 pp.268, 406) of the liver which interferes with plasma protein synthesis, causing clotting disorders.
 OHCM8 p.258)
OHCM8 p.258)• As the liver is the only organ capable of urea synthesis, serious liver damage will result in the rate of ammonia production exceeding the rate that it can be converted into urea for excretion
• Ammonia is toxic, and its build-up affects the functioning of the CNS
• In addition, there is a reduction in the amino acid metabolism capacity, and an accumulation of aromatic amino acids also affects CNS function
• There is also a reduction in capacity for gluconeogenesis, and if blood glucose levels drop, then hypoglycaemic coma and even death can occur in liver failure
• Finally, liver failure patients have muscle wasting due to a lack of insulin-like growth factor (IGF-1; made in the liver in response to growth hormone) compounding the problems.
Two of the most important proteins synthesized by the liver are albumin and the blood clotting factors.
• Makes up ~50% of the protein found in plasma (concentration of ~40g l–1)
• Rest are globulins (α, β, γ) and fibrinogen
• All except the γ-globulins are synthesized in the liver
• Albumin is a single molecular species of a 66KDa protein.
• The high concentration of albumin results in it making a large contribution to the osmolarity of the plasma, preventing oedema
• Oedema and ascites (accumulation of fluid in the peritoneal cavity causing swelling) are signs of chronic liver disease ( OHCM8 p.260) reducing the capacity for albumin synthesis
OHCM8 p.260) reducing the capacity for albumin synthesis
• Protein malnutrition (kwashiorkor) can lead to depleted levels of albumin and subsequent oedema
• Kidney disease (nephritis) can also deplete albumin through excessive excretion, with similar results
• Binding of small molecules—albumin is a major carrier of other molecules in the plasma, especially hydrophobic molecules and metal ions (trace elements)
• Albumin has several sites capable of binding hydrophobic molecules
 The binding is non-specific and relatively weak, but this is still important quantitatively due to the high concentration of albumin in plasma
The binding is non-specific and relatively weak, but this is still important quantitatively due to the high concentration of albumin in plasma
 Important molecules carried by albumin include: free fatty acids; steroid hormones; bilirubin (breakdown product of haemoglobin); hydrophobic drug molecules
Important molecules carried by albumin include: free fatty acids; steroid hormones; bilirubin (breakdown product of haemoglobin); hydrophobic drug molecules
• Trace elements such as Cu2+ and Zn2+ bind to the N-terminus region of the albumin protein
• Another example of a plasma protein synthesized by the liver with a role in metal transport is transferrin ( p.580).
p.580).
• Blood clotting factors are proteases that act upon one another in a cascade when activated, ultimately converting fibrinogen to fibrin which is insoluble and forms the blood clot
• Prevents further blood loss, maintaining haemostasis ( p.464)
p.464)
• Almost all of the blood clotting factors are synthesized by the liver
• Liver diseases ( OHCM8 pp.260–9) can depress the clotting system enough to cause severe tendency to bleed
OHCM8 pp.260–9) can depress the clotting system enough to cause severe tendency to bleed
• Liver synthesis of four of the most important clotting factors (prothrombin, factors VII, IX, and X) is vitamin K dependent
• Lack of the fat-soluble vitamin K can also lead to a serious tendency to bleed
• Normally, vitamin K is made by intestinal bacteria and absorbed with dietary fat
• Liver disease is one of the main causes of vitamin K deficiency
 Lack of bile secretion causes poor fat absorption and, thus, poor vitamin K uptake
Lack of bile secretion causes poor fat absorption and, thus, poor vitamin K uptake
 Compounds the reduced synthetic capacity of diseased liver for producing clotting factors
Compounds the reduced synthetic capacity of diseased liver for producing clotting factors
• It is obviously important that blood clotting does not occur when not required
• Antithrombin III and α-macroglobulin are the major anticoagulation plasma proteins, with heparin co-factor II and α-1-antitrypsin
 Protease inhibitors prevent blood clotting factors acting inappropriately
Protease inhibitors prevent blood clotting factors acting inappropriately
 The anticoagulation drug, heparin, strongly activates antithrombin
The anticoagulation drug, heparin, strongly activates antithrombin
• Many of the proteins involved in blood clotting are modified, post-translationally, to have γ-carboxylglutamyl (Gla) residues
• The proteins with GIa residues are effective at binding Ca2+ ions. Ca2+ is important for clotting factor interactions with other proteins and for enhancing their catalytic activity
• The post-translational modification is vitamin K dependent
 Vitamin K is an essential co-factor for the carboxylase enzyme responsible, located on the luminal side of the rough ER
Vitamin K is an essential co-factor for the carboxylase enzyme responsible, located on the luminal side of the rough ER
 Coumarin anticoagulants (e.g. warfarin) inhibit the carboxylase reaction by interfering with the vitamin K co-factor.
Coumarin anticoagulants (e.g. warfarin) inhibit the carboxylase reaction by interfering with the vitamin K co-factor.
 OHCM8 pp.318, 320)
OHCM8 pp.318, 320)Iron is important in the body, being an essential constituent of haemoglobin, myoglobin, and cytochromes. It is ingested in the diet in two major forms—haem and non-haem iron—absorbed by separate pathways.
• Haem is absorbed across the enterocyte apical membrane by a specific haem transporter. Once in the cell, the iron is released by haem oxidase
• Non-haem (or free iron) is reduced from Fe3+ to Fe2+ by ferrireductase, present on the luminal surface of enterocytes
• The reduction of Fe3+ to Fe2+ is also favoured by ascorbic acid (vitamin C) in the diet (vitamin C is used in iron supplements to aid absorption)
• Fe2+ is absorbed by the proton-coupled divalent metal transporter DMT1 (which can transport a variety of divalent cations).
Once inside the enterocyte, the iron can either be stored or transported across the basolateral membrane to enter the plasma.
• Storage is as a complex of Fe3+ with the protein ferritin
• Basolateral transport is by another transporter, regulatory protein 1 (REG1), and hephaestin. Hephaestin oxidizes Fe2+ back to Fe3+, which is the form that binds the iron-transporting plasma protein, transferrin.
Absorption of iron by the intestine is tightly regulated, as there is no mechanism for excreting iron once it has been taken up into the body.
• Healthy individuals are in iron balance, with daily loss equalling uptake at about 1mg/day
• Most of iron (~60% or 2 500mg) in the male body is in haemoglobin, with about 25% stored and the remainder in myoglobin and enzymes
• Females tend to have smaller stores (around 40% of that in males) and larger daily losses (averaging up to 2mg/day) due to blood loss during menstruation
• A poorly understood mechanism limits absorption of excess iron at the level of the enterocytes (‘mucosal block’).
Transferrin is a 76KDa β-globulin glycoprotein synthesized by the liver which is central to the control of iron metabolism by the body.
• 1 mole of transferrin binds 2 moles of Fe3+
• To enter cells, transferrin binds to a cell surface transferrin receptor, which is then internalized by receptor-mediated endocytosis
• The low pH in the lysosome causes the release of the iron, and it enters the cytoplasm via DMT1
• The transferrin receptor, with the transferrin still bound, is recycled, intact, to the plasma membrane. The transferrin is released and can bind more iron.
Synthesis of the intracellular storage protein, ferritin, and the transferrin receptor are reciprocally linked.
• Genes for both proteins have iron response elements in their promoter regions
• When iron levels are high, ferritin mRNA synthesis is increased to promote iron storage in cells, and that of transferrin receptor is reduced
• When iron levels are low, transferrin mRNA levels rise, while ferritin mRNA is kept in an inactive form.
In addition to iron deficiency, iron excess can also cause disease.
• Hereditary haemochromatosis is prevalent in Scotland, Ireland, USA ( OHCM8 p.262)
OHCM8 p.262)
• Total body iron is >15g (compared with 2.5–3.5g normally)
• Tissues damaged include liver, pancreatic islets (→ diabetes), heart
 Melanin and iron accumulate in skin, giving slate-grey colour
Melanin and iron accumulate in skin, giving slate-grey colour
• Usually linked to a mutation in the gene HFE, which codes for a protein related to major histocompatibility complex (MHC) class I antigens
• It is thought that this affects mucosal block by enterocytes, leading to excessive absorption of iron by the intestine.
The liver is the major site for metabolism of xenobiotics (foreign compounds, xenos = Greek for stranger), which can be both exogenous (e.g. drugs, carcinogens, pollutants) or endogenous (e.g. steroids, eicosanoids, fatty acids).
The main aim of the metabolism is to convert the mainly lipophilic xenobiotics into more water-soluble derivatives for excretion.
Xenobiotic metabolism can be divided into two phases:
• Phase 1 mainly involves hydroxylation, carried out by enzymes called mono-oxygenase or cytochrome P450
• Most tissues contain a number of different isoforms of cytochrome P450, especially the small intestine and liver. Present mainly in the membranes of smooth ER

• Phase 2 involves the conjugation of the hydroxylated xenobiotics to various other compounds to further increase their hydrophilicity and enable excretion in bile or urine
• The most common conjugation is the addition of glucuronic acid. Examples of conjugated compounds include phenol, steroids, meprobamate (a tranquillizer)
• Other conjugation reactions include sulphation, acetylation, methylation, and conjugation with glutathione.
Although xenobiotic hydroxylation and conjugation are referred to as detoxification, this is not always the outcome.
• Phase 1 reactions may make the xenobiotics more biologically active. This may be desirable if the xenobiotic is a prodrug which is being converted into its active form, or undesirable if it creates a carcinogen
• Phase 2 reactions can also theoretically increase activity, but this is rare.
Cytochrome P450 enzymes are often induced by the presence of their substrates.
• This can be the cause of drug interactions. For example, phenobarbital (for epilepsy) induces the cytochrome P450 that metabolizes warfarin (used to prevent blood clotting). Thus, the starting/discontinuing of phenobarbital will affect the dose of warfarin necessary to maintain the same level of effect
• Alcohol induces a cytochrome P450 isoform which metabolizes widely used solvents and components present in tobacco smoke. Alcohol consumption may therefore increase the risk of carcinogenicity.
Alcohol (ethanol) metabolism is catalysed by ethanol dehydrogenase.
• Ethanol + NAD+ → acetaldehyde + NADH + H+
• Subsequently, acetaldehyde is dehydrogenated to acetate (also NAD+-dependent)
• The acetate can be conjugated to CoA to form acetyl-CoA. Acetyl-CoA is a substrate for fat synthesis in the liver, leading to obesity and/or a fatty liver in nutrient-replete alcoholics
• Large amounts of alcohol can deplete the liver of NAD+ (i.e. the NADH: NAD+ ratio increases)
• This pushes the equilibrium of lactate dehydrogenase to lactate, reducing the amount of pyruvate available for gluconeogenesis
• Similarly, malate dehydrogenase will be pushed in the direction of malate (not oxaloacetate), which is also a gluconeogenic substrate
• If this coincides with a falling blood glucose level, hypoglycaemia can occur
 One of the most sensitive parts of the brain to hypoglycaemia controls body temperature, which will drop, resulting in hypothermia (
One of the most sensitive parts of the brain to hypoglycaemia controls body temperature, which will drop, resulting in hypothermia ( OHCM8 p.860).
OHCM8 p.860).
Haem catabolism takes place in the liver, the spleen, and bone marrow (Fig. 8.11).
• An average person metabolizes 6g of haemoglobin per day
• The globin protein is degraded and the amino acids recycled
• The iron from the haem group is released after it has been reduced with NADPH, and returned to the body iron pool for reuse
• The porphyrin ring is opened to give biliverdin (green)
• Biliverdin is reduced with NADPH to bilirubin (yellow).
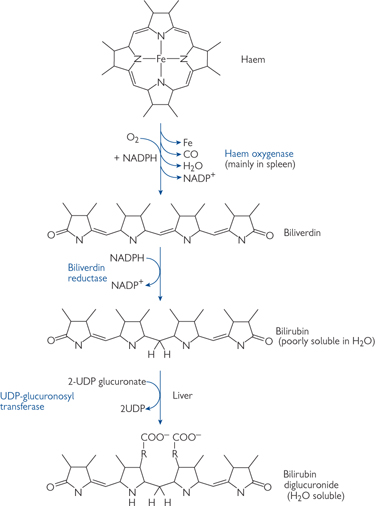
Fig. 8.11 Diagrammatic representation of the haem oxygenase reaction and the reduction of biliverdin.
 OHCM8 p.260)
OHCM8 p.260)Formation of bands of fibrous tissue between portal tracts in the liver which surround regenerative nodules of liver cells.
Continued damage to the liver while repair/regeneration is occurring—so is generally caused by chronic factors (e.g. decades of alcohol abuse, see Table 8.1) rather than a single acutely hepatotoxic episode (e.g. an overdose of paracetamol).
Table 8.1 Causes of hepatic cirrhosis
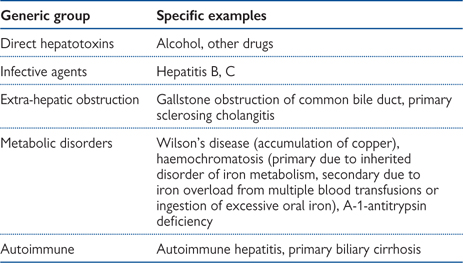
• Portal hypertension—due to obstruction of portal venous blood flow by the bands of fibrous tissue
• Oesophageal varices—may produce sudden and massive haematemesis
• Rectal varices ( OHCM8 p.254)—less recognized than oesophageal varices but can produce massive rectal bleeding. Distinguish from haemorrhoids (rectal varices are completely within the rectum)
OHCM8 p.254)—less recognized than oesophageal varices but can produce massive rectal bleeding. Distinguish from haemorrhoids (rectal varices are completely within the rectum)
• Hepatic failure ( OHCM8 p.258)—when the majority of the parenchymal cells are dysfunctioning, often due to some precipitating event (decompensated hepatic failure) such as large bleed from oesophageal varices. A cirrhotic liver can provide adequate function for many years.
OHCM8 p.258)—when the majority of the parenchymal cells are dysfunctioning, often due to some precipitating event (decompensated hepatic failure) such as large bleed from oesophageal varices. A cirrhotic liver can provide adequate function for many years.
 OHCM8 p.258)
OHCM8 p.258)Destruction or non-function of a sufficient amount of the liver cells so that it no longer performs its physiological functions.
The liver has a large functional reserve, so about 90% of its mass has to be non-functioning before hepatic failure ensues.
 Gallstones in common bile duct
Gallstones in common bile duct
 Primary sclerosing cholangitis
Primary sclerosing cholangitis
 Primary—hepatocellular carcinoma
Primary—hepatocellular carcinoma
 Metastatic—commonly, colorectal cancer
Metastatic—commonly, colorectal cancer
• Failure of production of proteins
• Albumin → oedema due to ↓ osmotic pressure