9 |
Examination of the Special Senses |
And there I stood, a man grown, shaking in the sunshine with that old boyish emotion brought back to me by an odour! … Often and often have I known this strange rekindling of dead fires. And I have thought how, if our senses were really perfect, we might lose nothing out of our lives: neither sights, nor sounds, nor emotions….
I. THE SENSES
A. Sensation and subjectivity
The possibility for sensation begins when a chemical or physical change stimulates the receptor endings of sensory neurons and alters the flow of impulses in the sensory pathways. The impulses in the sensory pathways then lead to an experience that we call a sensation, such as pain, touch, or sight. Nothing is more real to the patient (Pt) than the experience of the sensation, such as pain, nor less real to the observer. Although the Pt can judge the degrees of a sensation, even on scale of 0 to 10 for pain, no one else can verify the sensation or measure it objectively in grams, centimeters, or seconds, the classic units of the physics. Nevertheless, by carefully eliciting the Pt’s history, the examiner (Ex) can recognize and diagnose various sensory syndromes, such as migraine or nerve root compression, with about the same degree of certainty as motor syndromes.
B. Classification of sensation
1. Aristotle recognized five primary senses:
a. Sight
b. Sound
c. Smell
d. Taste
e. Touch
2. Tradition also recognizes special and general senses. The special senses are sight, sound, taste, smell, and equilibrium/verticality. The general sensations are the rest. Sensation also can be classified as somatic or visceral.
3. Charles Sherrington (1857–1952) classified sensation as exteroception, proprioception, and interoception, depending on the origin of the stimulus and the location of the receptor tips of the axons.
a. Exteroceptor axonal tips are located near the external body surfaces. They respond to stimuli that impinge on the body’s external surfaces. These stimuli produce sight, sound, smell, taste, and superficial cutaneous sensation. Superficial skin sensations include:
i. Touch
ii. Superficial pain
iii. Temperature
iv. Itching, tickling, and wetness
b. Proprioceptor axonal tips are located beneath body surfaces. They respond to stimuli that originate from receptors deep in the dermis, in muscles, tendons, ligaments, and the vestibular labyrinth. In large part, they record the actions of the body on itself and orientation to the pull of gravity. (See page 395 for a fuller definition of proprioception.) Proprioceptive sensations include:
i. Position
ii. Movement
iii. Vibration
iv. Pressure, weight, or tension
v. Deep pain (sometimes included in proprioception)
vi. Equilibrium and verticality (via vestibular pathways and dorsal columns)
c. Interoceptor axonal tips located in the viscera and vessels respond to stimuli that act on the internal surfaces of the viscera or originate in the visceral walls:
i. Visceral and vascular pain
ii. Sense of fullness or distention of the viscera
4. Obviously the various sensory classifications overlap and are inconsistent. To obviate memorizing sensory classifications, think systematically and simply sort through your own senses.
C. The concept of sensory modalities
1. No normal person confuses the stench of carrion with a flash of light or a pinprick with a sound. Each unique sensation not resolvable into a more elementary sensation is called a primary sensory modality. Ay, but there is the rub: How does one define unique and elementary? (Synesthesia refers to the condition where one type of stimulation evokes the sensation of another; hearing a sound evokes the visualization of a color.)
2. A sensation felt in one part of the body as a result of stimulus applied to another, as in referred pain.
3. Operations to disclose primary sensory modalities: Stick yourself with a pin: There, that is pain, one modality. Stroke yourself with a piece of cotton: There, that is touch, another modality.
4. Operation to disclose multimodal sensations: Close your eyes and grasp any object, say a quarter, from your pocket or purse. You will recognize it as a metal disc by a combination of touch, texture, weight, size, circularity, and even its slightly cold feel. Thus we can resolve object recognition as a combination of several exteroceptive and proprioceptive modalities. Multimodal sensations include:
a. Form, size, shape, texture, and weight
b. Itching, tickling, and wetness
D. Implications of the theory of modality specificity
1. The neurologist constructs the entire sensory examination on modalities because the pathways of the nervous system provide for modality separation. In fact, we might even define a modality as any sensation that the nervous system represents by a unique pathway, but that requires negative definitions. What is sight? It is that sensation lost after cutting both optic nerves. Each of us has to rely on our private experience to distinguish different modalities.
2. Because unique receptors serve each of the special senses, investigators have sought unique receptors for all modalities. Carried to its extreme, the theory of modality specificity requires unique receptors, unique peripheral axons, unique pathways through the cord, brainstem, and thalamus, and unique cortical receptive areas. This theory of modality-specific pathways enables clinicians to localize lesions, but does not reflect the fact that most stimuli tend to excite all afferents and cortical neurons receive convergent input from multiple afferent fibers (Saal and Bensmaia, 2014; Vriens et al, 2014). For example, the sensations of shape, texture, wetness or vibration represent the integration of cutaneous sensory information that begins at the spinal cord and extends to subcortical and cortical levels (Abraira and Ginty, 2013; Filingeri et al, 2014).
3. By testing all sensations, the Ex tests the integrity of a large volume of neural tissue. Add the volume of tissue assayed by testing motor pathways, and the Ex has tested the integrity of the spinal cord, the brainstem, the cerebellum, and much of the diencephalon and cerebral hemispheres. The more pathways that function normally, the more the Ex can exclude neurologic disease. The more pathways that function abnormally, the more the Ex can predict the size, location, and type of the lesion.
E. Basic principles of sensory physiology
These principles are summarized from the doctrine of specific nerve energies of Johannes Müller (1801–1858):
1. Sensation is an awareness of the state of nerve impulses in the sensory neural pathways. We only know the external world by the changes that occur in the state of impulses in our receptor pathways.
2. Any stimulation of a sensory nerve by any means, electrical, mechanical, or chemical, causes only the type of sensation ordinarily mediated by the nerve. A blow on the eye causes a sensation of light, not taste.
3. The same stimulus applied to different sensory organs causes only the sensation appropriate to the organ. Put a stimulating electrode on the cochlea and you hear. Put the same electrode on the skin and you feel.
II. SMELL (OLFACTION): CRANIAL NERVE I
A. Olfactory receptor and nerve
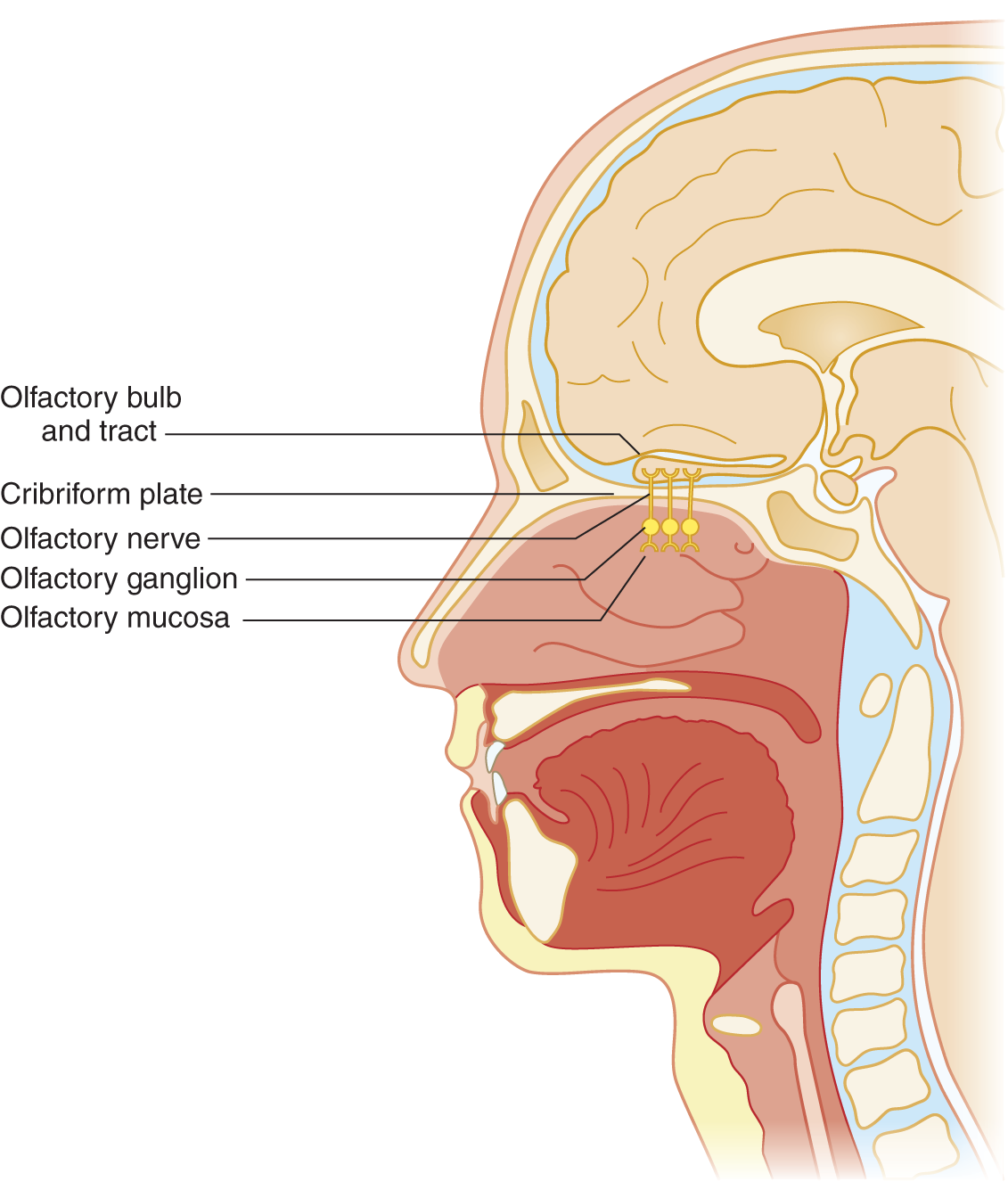
FIGURE 9-1. Sagittal section of head to show olfactory nerve, bulb, and tract.
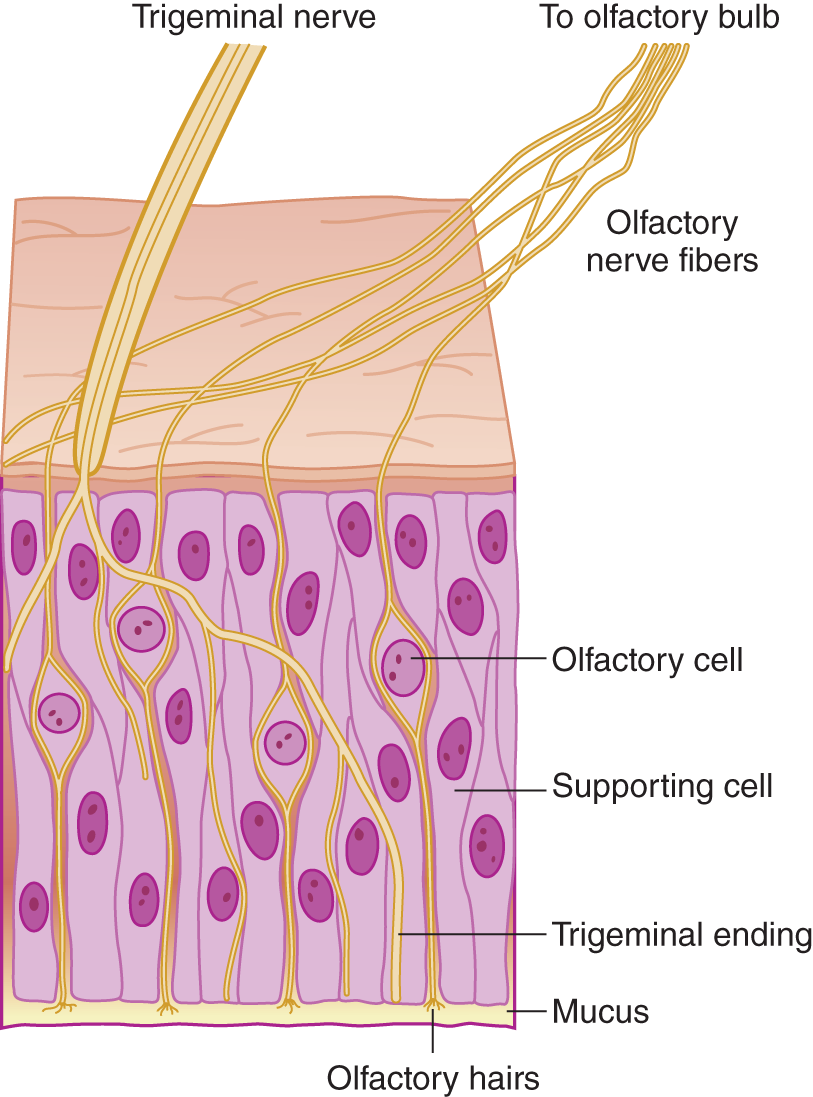
FIGURE 9-2. Microscopic section of olfactory mucosa, showing innervation by cranial nerves I and V. (Reproduced with permission from Amoore JE, Johnston JW, Rubin M. The stereochemical theory of odor. Sci Am. 1964;210:42–49.)
2. Mucus covers the olfactory nerve endings. Any odiferous agent must first dissolve in the mucus, which acts as the first censor for smell. Colds or allergic rhinitis impair olfaction by mechanical reduction of airflow and by excessive mucus secretion, a response triggered in part by these olfactory receptor neurons (Tizzano and Finger, 2013). Hyposmia means partial loss of the sense of smell, and anosmia means complete loss. These olfactory neurons are unique as they are not only the first-order neuron for olfaction, but can regenerate, possess receptors that bind odorants (respond to more than one odorant) and are directly exposed to the external environment.
3. Olfactory impulses travel centrally past the perikarya of the ganglion cells in the nasal mucosa. The ganglion cells are  external to/
external to/ within/
within/ internal to the cribriform plate. (
internal to the cribriform plate. ( external to (Fig. 9-1))
external to (Fig. 9-1))
4. Axons from the olfactory ganglion cells form olfactory nerve filaments. The filaments perforate the cribriform plate and attached dura. The olfactory axons then cross the subarachnoid space to synapse on their second order projection neurons (mitral and tufted cells) within specialized structures (glomerulus) in the olfactory bulbs (Crespo et al, 2013; Lucero, 2013). These second order neurons are the primary efferent projection neurons of the olfactory bulb. Organisms may gain access to the subarachnoid space or brain via the olfactory nerve filaments and cause encephalitis.
B. The olfactory stimulus
1. The two cranial nerves (CrNs) that supply sensory fibers to the olfactory epithelium are _____ and _____. Of these, only CrN _____ serves olfaction. (I; V (Fig. 9-2); I)
2. As a general law in testing any sensation, the Ex isolates the chosen modality from all other modalities. Otherwise, the Ex does not know which sensory pathway caused the response. To test only the sense of smell, should the Ex use an irritating substance such as  ammonia or an aromatic substance such as
ammonia or an aromatic substance such as  coffee? (
coffee? ( coffee)
coffee)
3. Ammonia irritates all receptors of a mucous membrane. Even the conjunctiva reacts to (smells, as it were) ammonia. To test smell, use a vial of coffee grounds. Should the vial be  opaque/
opaque/ transparent? Why? (
transparent? Why? ( opaque)
opaque)
_________
_________
4. Other readily available aromatic substances are oil of lemon, orange peel or apple skin, and soap.
5. Although not used in the routine neurologic examination (NE), there are commercially available smell identification testing kits that incorporate a battery of different odorants to test olfaction (Allis and Leopold, 2012).
C. Technique for testing olfaction
1. Successful sensory testing depends on communication between the Pt and Ex. Say to the Pt, “Close your eyes, sniff, and try to identify this odor.”
2. Compress one of the Pt’s nostrils. Hold the vial in front of the open nostril and ask the Pt to sniff. Wait a moment for the Pt to perceive the odor and then identify it.
3. For the second trial, compress the opposite nostril and this time do not present the stimulus. Withholding the stimulus tests the Pt’s suggestibility and attentiveness. Incorporate such safeguards in all sensory testing.
4. The third time, present the stimulus to the untested nostril.
D. Central olfactory pathways and the concept of a rhinencephalon
1. After receiving the synapses from the primary olfactory axons, the olfactory bulbs send secondary pathways to the adjacent basal frontotemporal junction (basal forebrain). Tertiary pathways then disperse through an array of circuits in the basal forebrain that are not directly accessible to clinical testing but can be imaged (Benarroch, 2010; Demaria and Ngai, 2010; Bekkers and Suzuki, 2013; Lepousez et al, 2013).
2. Taken together, the olfactory bulbs and tracts and their immediate central connections constitute the rhinencephalon. At one evolutionary stage, the cerebrum consisted mostly of rhinencephalon. Ontogenetically and phylogenetically, our own brain retains the primitive rhinencephalic ground plan (Fig. 9-3).
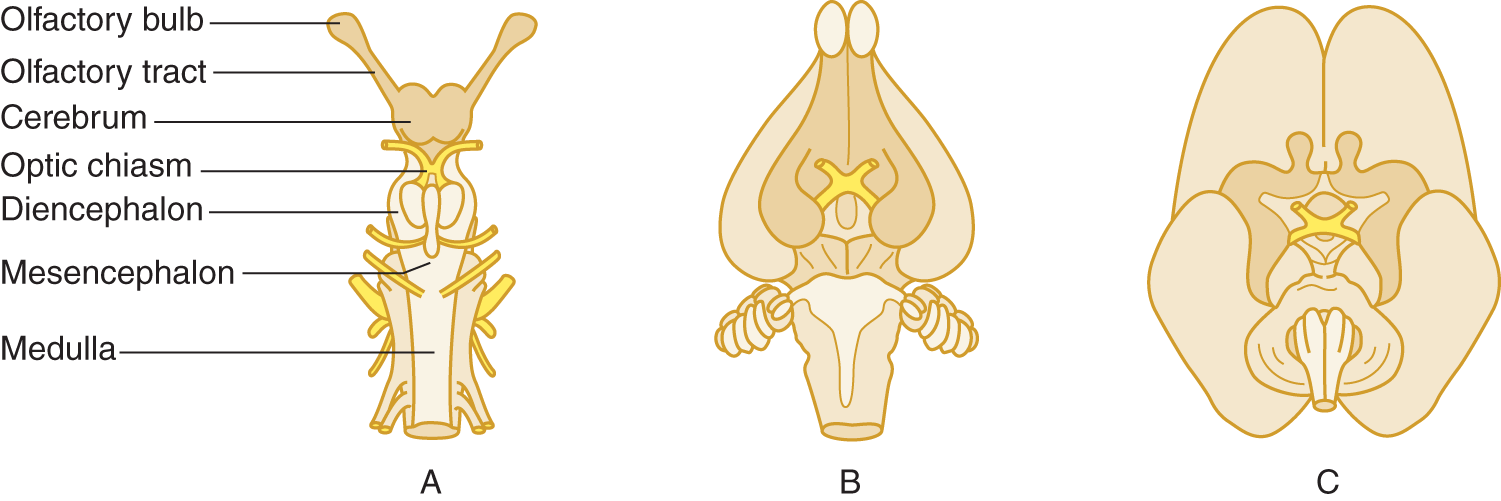
FIGURE 9-3. Ventral views of shark (A), rabbit (B), and fetal human (C) brains. The rhinencephalon (darker tan) comprises most of the shark brain. Notice in the rabbit and human brains that the nonrhinencephalic cortex (unshaded), which began as patches on the cerebral wall of primitive animals, has overgrown to dwarf the rhinencephalon. Nevertheless, the rhinencephalon set its imprint forever on the form and function of the human brain.
3. The sense of smell originally served the two fundamental functions of feeding and mating. These two visceral drives and their attendant visceral emotions were originally localized in the rhinencephalon before extending to those parts of the forebrain, essentially the limbic lobe that evolved most directly from the olfactory ground plan. These forebrain derivatives remain the “seats” of emotion and affective experience. Humans are not believed to exude pheromones which serve to activate neural circuits within the limbic system that result in specific behaviors or regulate hormonal levels (Liberles, 2014), but we assiduously replace them with perfumes and colognes so, perhaps they still play a role (Semin and de Groot, 2013). In any event, smell remains as the most evocative of sensations.
4. Déjà vu and déjà pensée: The uncus, the medial-most gyrus of the temporal lobe, contains a cortical area for smell. Uncal lesions cause olfactory hallucinations, usually of very disagreeable odors. One of my Pts tore down his bedroom walls because of the conviction that he smelled a dead animal entrapped within them. Each time the odor came powerfully to him, he also experienced a peculiar feeling of familiarity, of something happening that had happened before (just as Ray Shannard Baker described). Autopsy showed a metastatic bronchogenic carcinoma in his uncus. The feeling of familiarity, as if something had happened before, is called déjà vu (previously or already seen) or déjà pensée (previously or already thought). Although we each experience this sense of undue familiarity from time to time, when a Pt reports it in association with an olfactory hallucination, suspect a medial temporal lobe lesion. Get a magnetic resonance imaging (MRI) scan.
E. Olfactory-related consequences of head injuries
1. Head injuries may shear off the delicate olfactory nerve filaments, resulting in anosmia (Reiter et al, 2004) and compared to impaired olfaction following upper respiratory tract infections, recovery is infrequent and no clear medical interventions currently exist (Reden et al, 2006, 2012). If the wafer-thin cribriform plate fractures, the meninges may rupture initially or later when the Pt coughs, causing a fistula that allows cerebrospinal fluid (CSF) to gush into the nose. During physiologic fluctuations in intracranial pressure, fluid then refluxes back through the fistula into the subarachnoid space, introducing nasal organisms and causing meningitis or encephalitis. Therefore, consider a CSF fistula in the differential diagnosis of a runny nose (rhinorrhea). Rhinorrhea may occur intermittently and often increases upon bending forward or following the Valsalva maneuver. Suspect such a fistula whenever a Pt, usually one with a history of head injury, has a runny nose and anosmia but does not have a cold or allergic rhinitis. Persistent CSF fistulas at any level of the neuraxis require surgical closure (Prosser et al, 2011; Ziu et al, 2012).
2. To differentiate a CSF leak from nasal mucus or allergic rhinorrhea, a sample of the fluid is tested for β2-transferrin (B2Tr) produced in the brain by neuraminidase activity which is specific for CSF. While results of glucose (higher in CSF then in nasal secretions) were once used as a diagnostic test, current assays are too sensitive and hence often false positive; total protein content, and chloride are not specific for CSF. Technical expertise and delay in obtaining results for B2Tr led to the development of an assay for β-trace protein (present in high concentrations within the CSF) that is as sensitive as B2Tr, but for various reasons, it has yet replaced B2Tr as a diagnostic test. To localize the fistula the nasal cavity is inspected carefully with a speculum and endoscopy, but further diagnostic imaging studies (eg, high-resolution thin section computed tomography, MRI, MR cisternogram, or radionuclide cisternography) are necessary (Ziu et al, 2012); Fig. 9-4 provides a diagnostic algorithm.
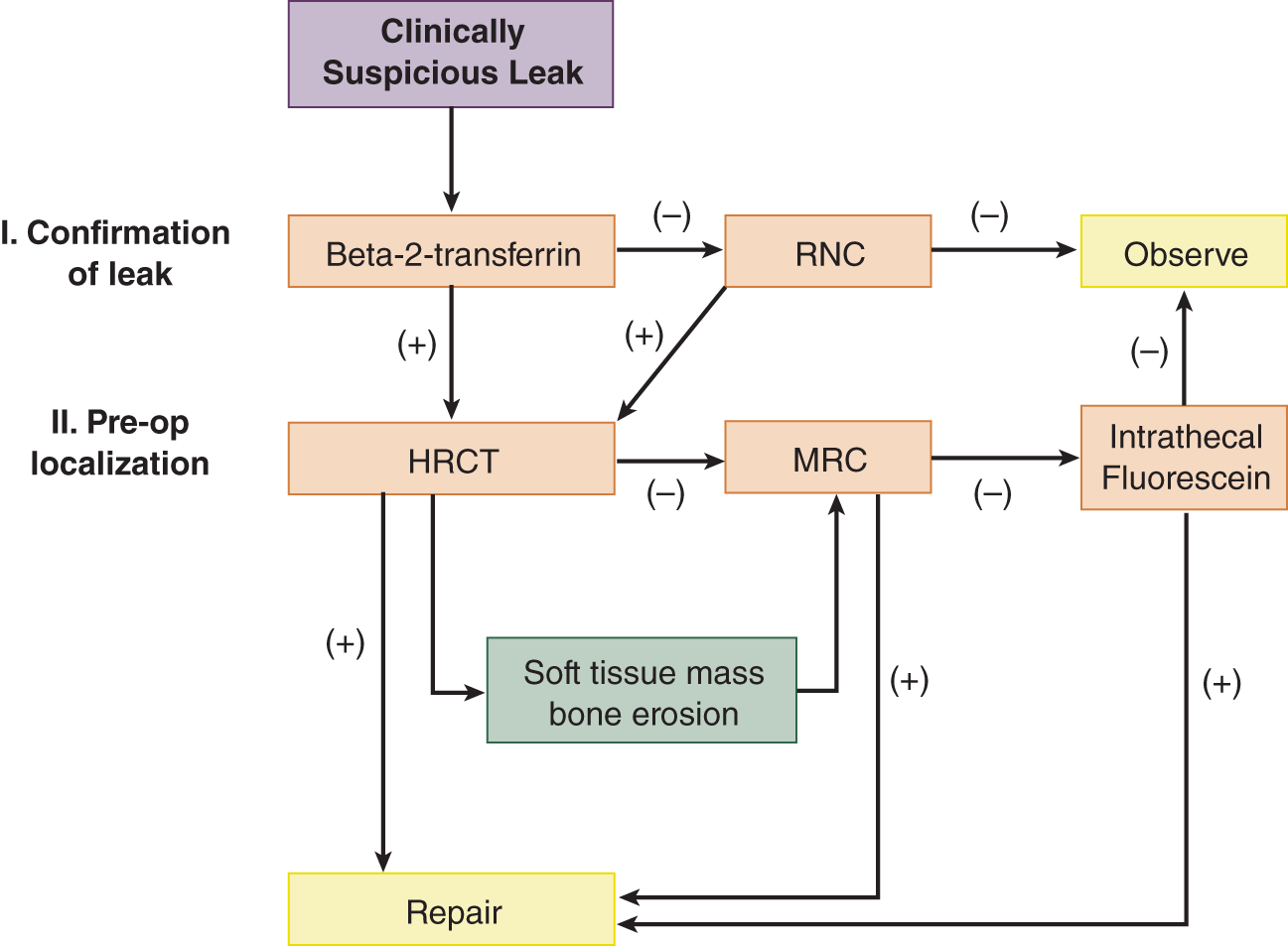
FIGURE 9-4. Diagnostic algorithm for diagnosis and management of suspected skull base cerebrospinal fluid fistulas. HRCT = high-resolution computed tomography; MRC = magnetic resonance cisternography; RNC = radionuclide cisternography. (Reproduced with permission from Zapalac JS, Marple BF, Schwade ND. Skull base cerebrospinal fluid fistulas: a comprehensive diagnostic algorithm. Otolaryngol Head Neck Surg. 2002;676:669–676.)
3. Some nasal complications of a head injury are
a. Loss of smell, a condition called _________
b. The formation of a fistula between the nasal cavity and the _________
c. A potentially lethal complication of such a fistula is _________
F. Differential diagnosis of anosmia
1. To analyze anosmia systematically, start at the receptor. What initial barrier must any aromatic agent in the inspired air pass through before it stimulates olfactory receptors? _________
a. The most frequent causes of anosmia are the common cold, allergic rhinitis, smoking, and head trauma (Allis and Leopold, 2012).
b. Sadly, aging diminishes the sensitivity of all sensations—sight, hearing, vibration sense, and so on. However, the origin of the age-related impairment of olfaction remains unclear and may have as much of a cortical as a peripheral origin (Mobley et al, 2014). Hyposmia has many etiologies and can accompany various endocrine disorders (eg, hypothyroidism, pseudohypoparathyroidism), meningitis, subarachnoid hemorrhage, local mechanical injury to the olfactory epithelium, medications, psychiatric disorders, Alzheimer and Parkinson disease (Greebe et al, 2009; Moman et al, 2009, Doty, 2012; Schecklmann et al, 2013; Schofield et al, 2014) and therefore requires a careful history to limit the differential diagnosis.
2. Next, consider lesions of the olfactory bulbs and tracts. Although rare, the most significant are meningeal neoplasms—classically, olfactory groove meningiomas—that compress the olfactory bulbs and tracts (Adappa et al, 2011; Jang et al, 2013). The olfactory bulbs and tracts may fail to evaginate (arhinencephaly), resulting in congenital lifelong anosmia (Assouline et al, 1998; DeMyer, 1987) or part of a clinical syndrome where the individual may never remember being able to smell (Karstensen and Tommercup, 2012).
3. Figure 9-5 reviews the differential diagnosis of anosmia.
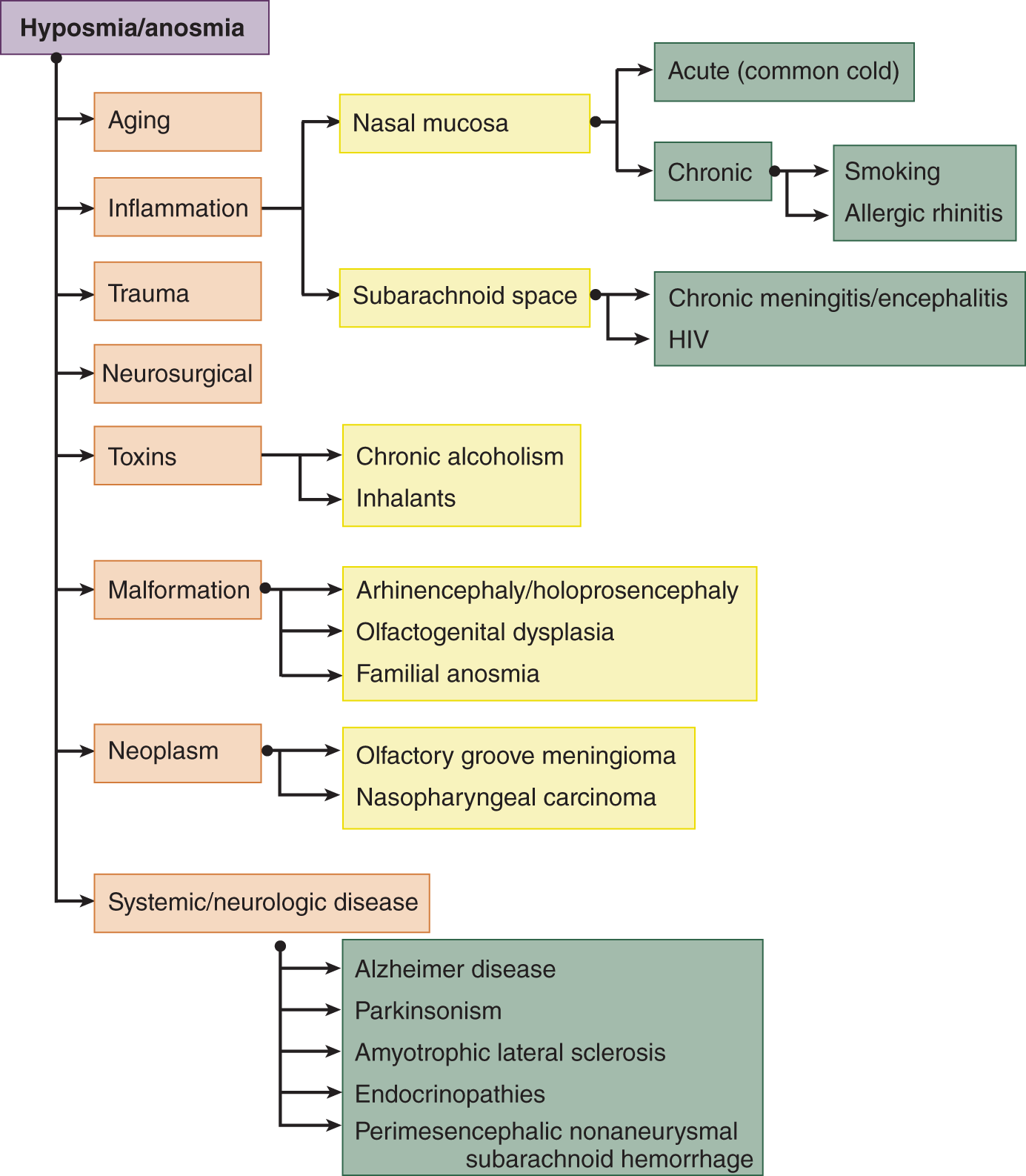
FIGURE 9-5. Dendrogram for the differential diagnosis of hyposmia and anosmia (reference).
4. What is the explanation for nasal drip caused by sneezing or coughing after a head injury?
_________
_________
5. The Pt with anosmia may complain mainly of loss of taste, because taste and smell are so intimately linked and quality of life often suffers with their impairment (Croy et al, 2014).
BIBLIOGRAPHY · Sensation
Abraira VE, Ginty DD. The Sensory Neurons of Touch. Neuron. 2013;79:618–639.
Filingeri D, Fournet D, Hodder S, Havenith G. Why wet feels wet? A neurophysiological model of human cutaneous wetness sensitivity. J Neurophysiol. 2014;112:1457–1469.
Saal HP, Bensmaia SJ. Touch is a team effort: interplay of submodalities in cutaneous sensibility. Trends Neurosci. 2014;37:689–697.
Vriens J, Nilius B, Voets T. Peripheral thermosensation in mammals. Nat Rev Neurosci. 2014;15:573–589.
Smell
Adappa ND, Lee JYK, Chiu AG, Palmer JN. Olfactory Groove Meningioma. Otolaryngol Clin N Am. 2011;44:965–980.
Allis TJ, Leopold DA. Smell and taste disorders. Facial Plast Surg Clin N Am. 2012;20:93–111.
Assouline S, Shevell MI, Zatorre RJ, et al. Children who can’t smell the coffee: isolated congenital anosmia. J Child Neurol. 1998;13:168–172.
Baker RS (David Grayson, pseudonym). Adventures in Contentment. New York, NY: Grossett; 1907.
Bekkers JM, Suzuki N. Neurons and circuits for odor processing in the piriform cortex. Trends Neurosci. 2013;36:429–438.
Benarroch EE. Olfactory system; functional organization and involvement in neurodegenerative disease. Neurology. 2010;75:1104–1109.
Crespo C, Liberia T, Blasco-Ibáñez JM, et al. The circuits of the olfactory bulb. The exception as a rule. Anat Rec. 2013;296:1401–1412.
Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life—an updated review. Chem Senses. 2014;39:185–194.
DeMaria S, Ngai J. The cell biology of smell. J Cell Biol. 2010;191:443–452.
DeMyer W. Holoprosencephaly (cyclopia-arhinencephaly). In: Vinken PJ, Bruyn GW, Klawans HL, eds. Malformations. Handbook of Clinical Neurology. Vol 6. Amsterdam: Elsevier Science; 1987, Chapter 13, pp. 225–244.
Doty RL. Olfaction in Parkinson’s disease and related disorders. Neurobiol Dis. 2012;46:527–552.
Greebe P, Rinkel GJE, Algra A. Anosmia after perimesencephalic nonaneursmal hemorrhage. Stroke. 2009;40:2885–2886.
Jang WY, Jung S, Jung TY, et al. Preservation of olfaction in surgery of olfactory groove meningiomas. Clin Neur Neurosurg. 2013;115:1288–1292.
Karstensen HG, Tommerup N. Isolated and syndromic forms of congenital anosmia. Clin Genet. 2012;81:210–215.
Lepousez G, Valley MT, Lledo PM. The impact of adult neurogeneses on olfactory bulb circuits and computations. Annu Rev Physiol. 2013;75:339–363.
Liberles SD. Mammalian pheromones. Annu Rev Physiol. 2014;76:151–175.
Lucero MT. Peripheral modulation of smell: fact of fiction? Sem Cell Dev Biol. 2013;24:58–70.
Mobley AS, Rodriguez-Gill DJ, Imamura F, Greer CA. Aging in the olfactory system. Trends Neurosci. 2014;37:77–84.
Moman MR, Verweij BH, Buwalda J, Rinkel GJL. Anosmia after endovascular and open surgical treatment of intracranial aneurysms. J Neurosurg. 2009;110(3):482–486.
Prosser JD, Vender JR, Solares CA. Traumatic Cerebrospinal Fluid Leaks. Otolaryngol Clin N Am. 2011;44:857–873.
Reden J, Mueller A, Mueller C, et al. Recovery of olfactory function following closed head injury or infections of the upper respiratory tract. Arch Otolaryngol Head Neck Surg. 2006;132:265–269.
Reiter ER, DiNardo LJ, Costanzo RM. Effects of head injury on olfaction and taste. Otolaryngol Clin N Am. 2004;37:1167–1184.
Schecklmann M, Schwenck C, Taurines R, et al. A systematic review on olfaction in child and adolescent psychiatric disorders. J Neural Transm. 2013;120:121–130.
Schofield PW, Finnie S, Yong YM. The role of the olfactory challenge tests in incipient dementia and clinical trial design. Curr Neurol Neurosurg Rep. 2014;14:479.
Semin GR, de Groot JHB. The chemical bases of human sociality. Trends Cogn Sci. 2013;17:427–429.
Tizzano M, Finger TE. Chemosensors in the nose: Guardians of the airways. Physiology. 2013;28:51–60.
Warnecke A, Averbeck T, Wurster U, et al. Diagnostic relevance of beta-2 transferrin for the detection of cerebrospinal fluid fistulas. Arch Otolaryngol Head Neck Surg. 2004;130:1178–1184.
Zapalac JS, Marple BF, Schwade ND. Skull base cerebrospinal fluid fistulas: a comprehensive diagnostic algorithm. Otolaryngol Head Neck Surg. 2002;126:660–676.
Ziu M, Savage JG, Jimenez DF. Diagnosis and treatment of cerebrospinal fluid rhinorrhea following accidental traumatic anterior skull base fractures. Neurosurg Focus. 2012;32:E3.
III. TASTE (GUSTATION) AND LOSS OF TASTE (AGEUSIA)
A. Receptors
The epithelium of the tongue and tonsillar pillars contains taste buds (fungiform and circumvallate papillae; Roper, 2013) where specific receptors detect different taste modalities, but these same receptors have been identified on nontaste tissues where they may exert metabolic roles (Li, 2013; Laffitte et al, 2014). As in olfaction, the chemical agents that stimulate taste must first dissolve in a liquid, the saliva. Loss of taste is called ageusia. Often the Pt who complains of ageusia actually has anosmia, because taste and smell complement each other in producing flavor and full gustatory sensation (Allis and Leopold, 2012). Hypogeusia may be present in up to 5% of the population, complete ageusia seems to be rare, but misinterpretations or categorization of tastes common (Welge-Lüssen et al, 2011). When pathologic changes in taste buds were found in patients with the syndrome of idiopathic hypogeusia with dysgeusia, hyposmia, and dysosmia (Henkin et al, 1971) it was later attributed to reduced total serum zinc levels. As hypogeusia was linked to zinc deficiency in humans, it was the rationale for related tests (and supplementation), but currently none seem to be sensitive and specific with regards to detecting marginal zinc levels (Gruner and Arthur, 2012).
B. Innervation of taste receptors
1. The taste buds of the anterior two-thirds of the tongue are innervated by … which CrN was it? Well, if you have forgotten, start at CrN I and sort through them:
a. CrN _______ smells, and _______ sees. (I; II)
b. CrNs _______, _______, and _______ rotate the eyeball. (III; IV; VI)
c. CrN _______ chews and feels the front of the head. (V)
d. CrN _______ moves the facial muscles, tears, snots, salivates, and _________
2. To test taste, use the anterior two-thirds of the tongue, the area innervated by CrN VII, because of the inconvenience of reaching the taste buds on the posterior third of the tongue and tonsillar pillars. Review Figs. 6-5 and 6-6. (VII; tastes (Should you review the brief description of the CrNs in Tables 2-5 and 2-6?))
3. In contrast to earlier maps, the tongue does not show clinically significant regional differences to salty, sweet, sour, and bitter tastes and there exists a great deal of overlap between each taste modality (Breslin, 2013). (There is also a visual aspect to eating and the color of foods can also inform, but not always indicate their nutritional value; Barnes et al, 2013).
C. Review of cranial nerve VII
1. CrN VII attaches to the brainstem at the _________
2. In ventrodorsal order, the CrNs attached to the pontomedullary sulcus are _________
3. CrN VII enters the internal auditory meatus in company with CrN _________
4. The primary neurons for taste occupy the only ganglion on CrN VII, the _________
5. The name geniculate ganglion comes from the knee-like downward bend of CrN VII after it clears the ganglion and heads for the stylomastoid foramen (Figs. 6-5 and 6-6).
D. Central pathways for taste
Lesions of the central taste pathways rarely cause isolated loss of taste, but often hypogeusia. The brainstem pathway ascends ipsilaterally within the central tegmental tract in the lateral part of the medulla and tegmentum of the pons arising from the nucleus of the tractus solitarius where the taste fibers from CrNs VII, IX, and X converge. (There is evidence that distinct taste modalities are transmitted via segregated pathways to the brain; Carleton et al, 2010). It appears these fibers cross (all or some?) at the midbrain so, lesions inferiorly will usually cause an ipsilateral deficit (Combarros et al, 2000), those superior contralateral, but a bilateral deficit assumed to reflect involvement of both crossed and uncrossed fibers. These fibers terminate in the most medial portion of the ventromedial nucleus of the thalamus and project to the insular cortex (island of Reil) and adjacent parietal operculum (Landis et al, 2006; Nakajima et al, 2010; Tsivgoulis et al, 2011; Maffei et al, 2012). Since an initial description of irritative lesions in this cortical region causing gustatory hallucinations (Penfield and Jasper, 1954), while cortical lesions typically cause bilateral hypogeusia or hemiageusia of which the Pt is unaware (Landis et al, 2006).
E. Technique of testing for loss of taste (ageusia)
1. Stimulus: The stimulus is a salty, sweet, sour, or bitter substance. Table salt, sugar, or quinine is suitable. Conceal the salt or sugar. The Pt who sees a white crystalline substance will almost automatically guess salt or sugar.
2. Communication with the patient
a. Tell the Pt, “I want to place something on your tongue for you to taste. Stick out your tongue and keep it out. When you recognize the taste, hold up your hand.”
b. Place a few crystals of your test material on the right or left half of the tongue and massage these around with the well-moistened cotton tip of an applicator stick. Take care to confine the stimulus to one-half of the tongue. Do not allow the Pt to return the tongue to the mouth because the saliva will diffuse the taste stimulus beyond the area selected for testing. If the tongue is dry, moisten it slightly. Allow 15 to 20 seconds for the substance to dissolve and for the Pt to respond. Some normal subjects will not perceive sugar. Test again with salt.
c. After the Pt rinses his mouth, test the opposite side of the tongue with the same or a different substance. For routine clinical purposes you need try only one substance to test for ageusia. Although not part of the routine NE, taste can be measured by impregnated paper discs and by galvanic currents.
d. Test your own sense of taste as described.
e. The main indications for testing taste are a complaint of loss of appetite, smell, or taste or the presence of CrN VII palsy. Taste is the only clinically testable sensation mediated by CrN VII. Patients may lose taste or suffer a perversion of taste (dysgeusia) because of various medications, systemic illness, cancer, and endocrinopathies (Allis and Leopold, 2012; Mennella et al, 2013).
F. Clinical value of testing taste in facial palsy
1. Patient protocol: A 26-year-old woman awoke one morning with her face “drawn to one side.” Examination disclosed that on the left side she could not wrinkle her forehead, close her eye, pull back the corner of her mouth, or wrinkle the skin of her neck. She moved the right side of her face normally. Her complaint of “drawing” of her face was due to the unopposed pull of the intact right-side facial muscles, which pulled her lips to the right when she spoke or smiled (see the Pt in Fig. 6-7). The remainder of the examination was completely normal, including taste sensation and hearing.
2. Analysis of the clinical data will lead to a conclusion as to where and what the CrN VII lesion is.
a. In analyzing a motor deficit, consider first its distribution. Does it match a central or a pyramidal tract (upper motoneuron) distribution? Does it match a root or peripheral nerve or myopathic distribution?
b. Which distribution does the motor deficit of the present Pt match?  upper motoneuron/
upper motoneuron/ peripheral nerve/
peripheral nerve/ myopathic. (
myopathic. ( peripheral nerve)
peripheral nerve)
c. The paralysis involves the muscles of one nerve, CrN _________
d. The distribution of the paralysis in the field of one nerve excludes a neuromyal junction disorder or myopathy; these are widespread disorders and not limited to a single nerve.
e. Because interruption of a single nerve explains the paralysis, the disorder consists of a  mononeuropathy/
mononeuropathy/ polyneuropathy/
polyneuropathy/ myopathy. (
myopathy. ( mononeuropathy)
mononeuropathy)
3. Having identified a mononeuropathy of CrN VII, we have to specify the location of the lesion along the course of the nerve. In analyzing a sensory disturbance or a reflex arc, we invoked the principle of starting at the _________
4. Where should you start to trace along the course of the impulses in a motor nerve? _________
5. The CrN VII nucleus occupies the  tectum/
tectum/ tegmentum/
tegmentum/ basis of the
basis of the  midbrain/
midbrain/ pons/
pons/ medulla. (
medulla. ( tegmentum;
tegmentum;  pons)
pons)
6. Because of the close packing of tracts and nuclei, a brainstem lesion would rarely affect just one CrN nucleus. It most likely would involve the neighboring lemniscal, cerebellar, or CrN VIII pathways or neighboring CrNs. In addition to VII, the CrN motor nuclei in the pons are _________
7. The Pt had no signs implicating structures in the vicinity of the CrN VII nucleus in the central nervous system (CNS); therefore, the lesion most likely interrupted the nerve  inside/
inside/ outside the brainstem. (
outside the brainstem. ( outside)
outside)
8. After leaving the pontomedullary sulcus of the brainstem and before entering the internal auditory meatus, CrN VII must cross the _________
9. The subarachnoid space between the cerebellum and the brainstem is called the cerebellopontine angle. A lesion here, such as a neoplasm, would interrupt not only CrN VII, but also CrN ______. (VIII)
10. As the neoplasm enlarged, in addition to CrNs VII and VIII, it would affect CrNs _________
11. Go to Fig. 2-20 to appreciate how a relatively common tumor, an acoustic neuroma, in the cerebellopontine angle can affect additional nerves. Start with your pencil on CrN VIII as the center and shade in a circle about 1 to 1.5 cm in diameter to see how the lesion would encroach on adjacent nerves and the brainstem as it grows.
12. If the lesion occupied the internal auditory meatus or canal, other than CrN VII, which CrN would it affect? _________
13. If the lesion interrupted the trunk of CrN VII somewhere between its point of exit from the brainstem and the geniculate ganglion, the Pt should have lost _________
14. CrN VII innervates one muscle in the middle ear, the _________
15. Because the Pt retained taste and had no hyperacusis, the CrN VII lesion must be / outside distal to/
outside distal to/ outside within the middle ear. (
outside within the middle ear. ( distal to)
distal to)
16. If distal to the middle ear, the lesion might be in the facial canal, but a lesion deep within the canal in the temporal bone bars direct clinical examination. If a lesion interrupted CrN VII after its exit from the stylomastoid foramen, the Ex should find pain or swelling in the parotid region, as from an inflammatory or neoplastic mass, but the Pt had no mass or pain in the parotid region.
17. The next link in a motor nerve comes at the terminal tips of its axons, where the axons synapse on the muscles, a region called the _________
18. Explain why the Pt’s lesion is not at the neuromyal junction or in the muscle itself._________
_________
19. Make a line across Fig. 6-5 at the most likely site of the Pt’s lesion.
20. The Pt had idiopathic facial paralysis (Bell palsy), the most common etiology for this common mononeuropathy of CrN VII. The lesion is usually an inflammation, frequently caused by viruses (particularly herpes simplex virus 1) and resulting in either demyelination along the course of the nerve or as the facial nerve swells, compression within the facial canal (de Almeida et al, 2014; Zandian et al, 2014). The Pt recovered good facial function by 6 weeks after onset and a typical outcome for 60% to 90% of Pts, but aberrant regeneration of CrN VII can lead to synkinetic movements (innervation of inappropriate muscles resulting in involuntary movements) or reinnervation of lacrimal rather than salivary glands (resulting in the phenomena of “Crocodile tears” manifested as tearing when eating). EMG can offer prognostic information early in the course and also contribute to clinical management decisions (Mancini et al, 2014; Schwartz et al, 2014). (Your line should cross the facial canal distal to the chorda tympani nerve but proximal to the stylomastoid foramen.)
21. This Pt shows how testing taste helps to localize a lesion along the course of CrN VII. Unless the Pt’s symptoms and signs implicate taste, smell, or CrN VII, you may omit taste testing; but make the omission by discretion, not carelessness.
BIBLIOGRAPHY · Taste Testing and Bell Palsy
Allis TJ, Leopold DA. Smell and taste disorders. Facial Plast Surg Clin N Am. 2012;20:93–111.
Barnes S, Prasain J, Kim H. In nutrition, can we “see” what is good for us? Adv Nutr. 2013;4:327S–334S.
Breslin PAS. An evolutionary perspective on food and human taste. Curr Biol. 2013;23:R409–R418.
Carleton A, Accolla R, Simon SA. Coding in the mammalian gustatory system. Trends Neurosci. 2010;33:326–334.
Combarros O, Sanchez-Huan P, Berciano J, et al. Hemiageusia from an ipsilateral multiple sclerosis plaque at the midpontine tegmentum. J Neurol Neurosurg Psychiatry. 2000;68:795–802.
de Almeida JR, Guyatt GH, Sud S, et al. Management of Bell palsy: clinical practice guidelines. CMAJ. 2014;186:917–922.
Gruner T, Arthur R. The Accuracy of the zinc taste test method. J Altern Complement Med. 2012;18:541–550.
Henkin RL, Schechter PJ, Hoye R, Mattern CFT. Idiopathic hypogeusia with dysgeusia, hyposmia and dysosmia. JAMA. 1971;217:434–440.
Laffitte A, Neiers F, Briand L. Functional roles of the sweet receptor in oral and extraoral tissues. Curr Opin Clin Nutr Metab Care. 2014;17:379–385.
Landis BN, Leuchter I, Millán Ruíz DS, et al. Transient hemiageusia in cerebrovascular lateral pontine lesions. J Neurol Neurosurg Psychiatry. 2006;77:680–683.
Li F. Taste perception: from the tongue to the testis. Mol Hum Reprod. 2013;19:349–360.
Maffei A, Haley M, Fontanini A. Neural processing of gustatory information in insular circuits. Curr Opin Neurobiol. 2012;22:709–716.
Mancini P, De Seta D, Prosperini L, et al. Prognostic factors of Bell’s palsy: multivariate analysis of electrophysiological findings. Laryngoscope. 2014;124:2598–2605.
Mennella JA, Spector AC, Reed DR, Coldwell SE. The bad taste of medications: overview of basic research on butter taste. Clin Ther. 2013;35:1225–1246.
Nakajima M, Ohtsuki T, Minematsu K. Bilateral hypogeusia in a patient with a unilateral paramedian thalamic infarction. J Neurol Neurosurg Psychiatry. 2010;81:700–701.
Penfield W, Jasper HH. Epilepsy and the Functional Anatomy of the Human Brain. Boston, MA: Little, Brown & Co; 1954.
Roper SD. Taste buds as peripheral chemosensory processors. Semin Cell Dev Biol. 2013;24:71–79.
Schwartz SR, Jones SI, Getchius TSD, Gronseth GS. Reconciling the clinical practice guidelines on Bell Palsy from the AAO-HNSF and the AAN. Neurology. 2014;82:1927–1929.
Tsivgoulis G, Ioannis H, Vadikolias K, et al. Bilateral ageusia caused by a unilateral midbrain and thalamic infarction. J Neuroimaging. 2011;21:263–265.
Welge-Lüssen A, Dörig P, Wolfensberger M, et al. A study about the frequency of taste disorders. J Neurol. 2011;258:386–392.
Zandian A, Osiro S, Hudson R, et al. The neurologist’s dilemma: a comprehensive clinical review of Bell’s palsy, with emphasis on current management trends. Med Sci Monit. 2014;20:83–90.
IV. HEARING
The specialist told him: “Fine let’s leave it at that.
The treatment is done: you’re deaf. That’s how
It is you have quite lost your hearing.”
And he understood only too well, not having heard.
A. Cranial nerve VIII
CrN VIII consists of cochlear (auditory) and vestibular divisions. Each division has its own specialized receptors, its own bundle within the trunk of VIII, and its own brainstem nuclei and central pathways. The cochlear division mediates hearing only. It detects sound vibrations between 20 and 20,000 cps. By its design, the ear is the most sensitive “vibration” detector in the human body and not only detects, but expends energy to enhance its mechanical input (Kazmierczak and Müller, 2012; Hudespeth, 2014).
B. Anatomy of the cochlear division of VIII
1. Receptor for hearing: The cochlea contains the receptor (the organ of Corti) and the cochlear (spiral) ganglion that originates the cochlear division of VIII (Fig. 9-6).
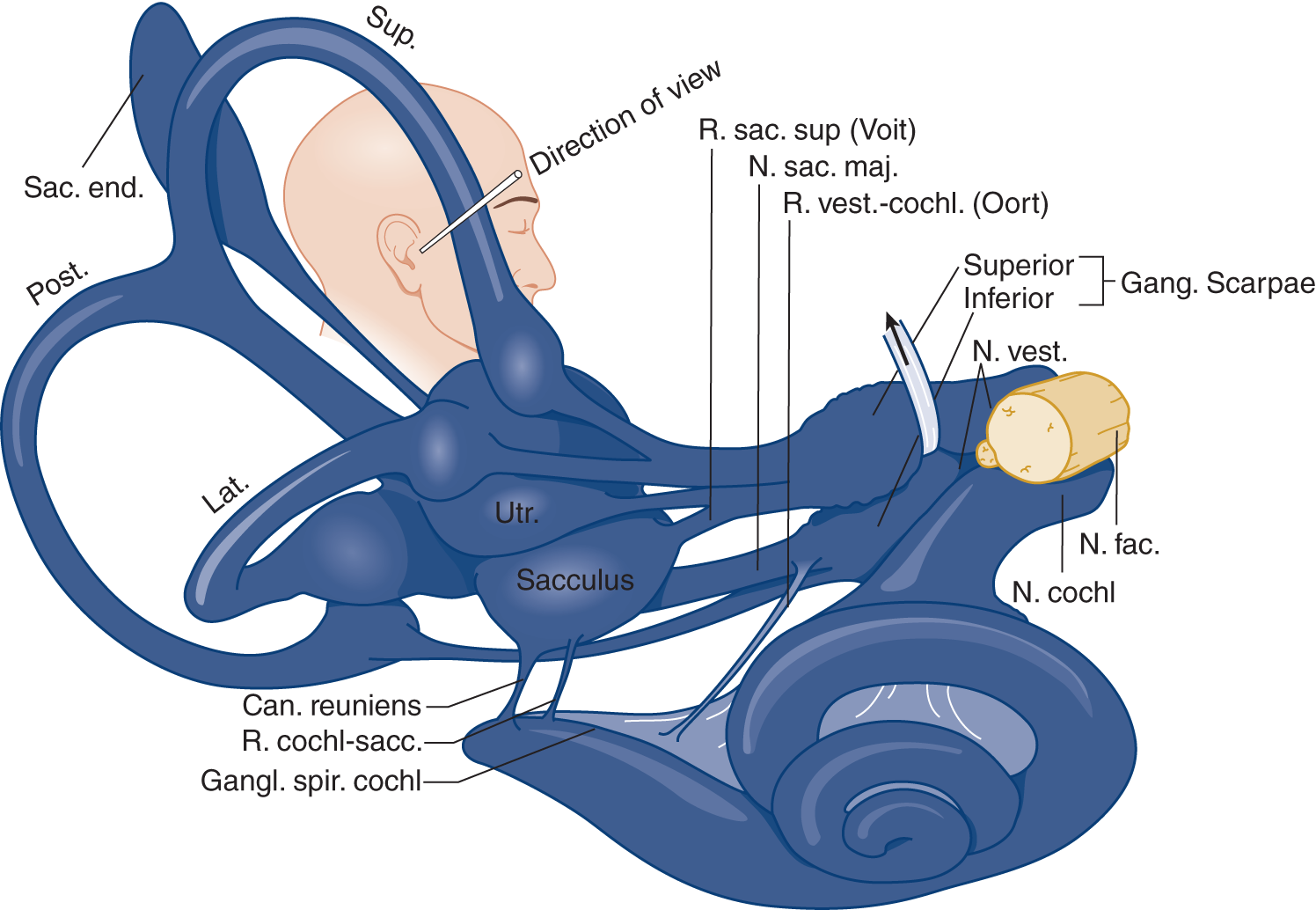
FIGURE 9-6. Drawing of the labyrinth, showing its nerve supply. Notice the intimate relation of the facial nerve (N. fac.) with the vestibular (N. vest.) and cochlear (N. cochl.) divisions of cranial nerve VIII. Lat = lateral semicircular canal; Post = posterior semicircular canal; N = nerve; R = ramus; Sup = superior semicircular canal; Utr = utriculus. (Reproduced with permission from Hardy M. Observations on the innervation of the macula sacculi in man. Anat Rec. 1934;59:403–418.)
2. The cochlear ganglion contains the  outside primary/
outside primary/ outside secondary/
outside secondary/ outside tertiary neurons for hearing. (
outside tertiary neurons for hearing. ( outside primary)
outside primary)
3. Peripheral course of the cochlear nerve: The cochlear and vestibular divisions of VIII run through the internal auditory canal, accompanied by CrN _________
4. CrNs VII and VIII attach to the brainstem at the _________
5. Central connections of the cochlear nerve. Learn Fig. 9-7.
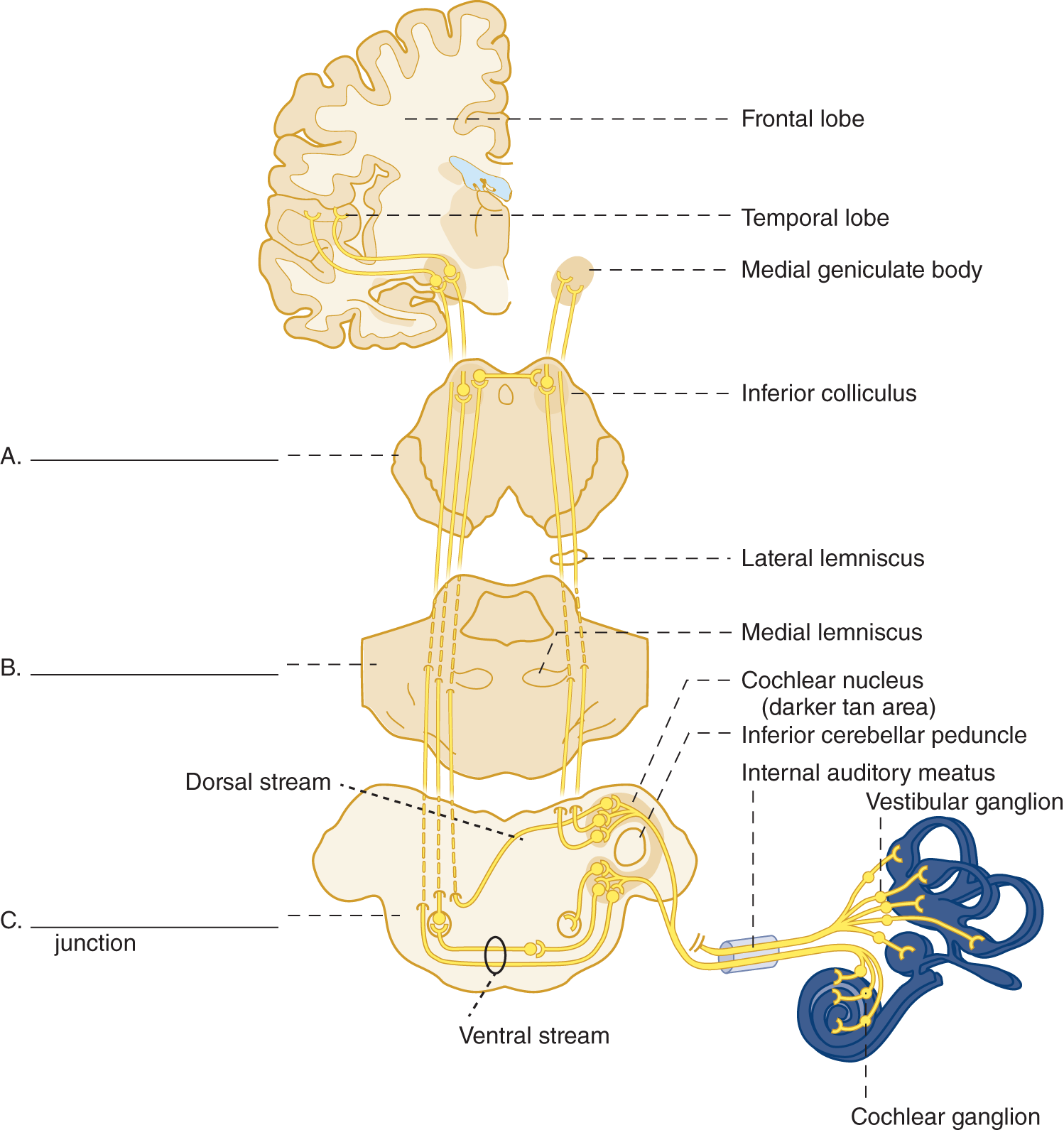
FIGURE 9-7. Diagram of the cochlear (auditory) pathway. On A, B, and C, label the subdivisions of the brainstem.
(A. Mesencephalon
B. Pons
C. Pontomedullary junction (and superior olivary nucleus))
a. Upon penetrating the brainstem, the cochlear axons synapse at the _________
b. These nuclei drape around the _________
c. In the auditory pathway, the cochlear nuclei contain the  primary/
primary/ secondary/
secondary/ tertiary neurons and preserve the tonotopic organization of the cochlea. (
tertiary neurons and preserve the tonotopic organization of the cochlea. ( secondary)
secondary)
d. The projections from the cochlear nuclei form two major bundles a dorsal stream (role in sound identification, multimodality integration and reflexes) and a ventral stream (binaural sound localization), both ascend through the brainstem and the auditory pathway disperses about equally ipsilaterally and bilaterally (Pickles, 2015). Therefore, if a Pt has a profound unilateral hearing loss, the lesion most likely would affect  a central pathway/
a central pathway/ an auditory nerve/
an auditory nerve/ the auditory receptive cortex. (
the auditory receptive cortex. ( an auditory nerve)
an auditory nerve)
e. The name of the auditory pathway that ascends through the brainstem is the lateral _________
f. From the inferior colliculus, the pathway runs to the _________
g. Neurons of the medial geniculate body relay to the superior surface of the _________
h. Would a unilateral temporal lobe lesion cause complete deafness in either ear?  Yes/
Yes/ No. (
No. ( No)
No)
Explain.
_________
i. Whenever you test a Pt’s hearing, “think through” the auditory pathway, naming the structures en route.
C. Symptoms and causes of cochlear nerve lesions
The most common symptoms are deafness and tinnitus. Tinnitus represents the perception of sound without a corresponding stimulus (see Section IV H; Langguth et al, 2013). The most common causes of impaired hearing and tinnitus are aging (presbyacusis), ototoxic medications such as aspirin, certain aminoglycoside antibiotics, chemotherapeutic agents such as cisplatin or carboplatin (Campo et al, 2013), viral infections, recurrent otitis media, hereditary cochlear degenerations (Alford et al, 2014), trauma, and chronic exposure to loud sound (Videos 9-1 to 9-3).

Video 9-1. Bilateral hearing loss in a patient with Susac syndrome.

Video 9-2. Bilateral hearing loss due to vestibular Schwannomas in a patient with NF-2.

Video 9-3. Bilateral peripheral facial paralysis and hearing loss.
D. The concept of threshold or sensitivity in testing
In testing olfaction and taste, the Ex merely seeks a yes or no answer and does not fractionate the strength of the stimulus or test the threshold. For the best test of muscle strength, the Ex requires the Pt to exert the maximum strength. For the best test of a sensory system, the Ex determines not the maximum stimulus it can withstand but the minimum stimulus it can detect; in other words, its sensitivity.
E. Technique for screening hearing
1. Ask the Pt about hearing deficits: Can the Pt hear the telephone, normal conversational voice, and whispering? Does the Pt have tinnitus?
2. Do otoscopy to ensure that the external auditory canals are open and that the eardrums are normal. In analyzing hearing deficits, the Ex has to decide whether the Pt has a mechanical impediment to conduction of sound or a nerve lesion. Otoscopy discloses some obvious mechanical impediments such as damaged eardrums, cerumen, or foreign bodies in the external auditory canal, but it does not disclose others, such as immobility of the ossicles (otosclerosis). The otologist measures the mechanical conductive ability of the eardrum and ossicles with impedance audiometry and tympanometry and more sophisticated testing evaluates central nervous system integrity (Musiek and Chermak, 2015).
3. Rub your fingers together beside one of the Pt’s ears and then the other.
4. Present a vibrating tuning fork to each ear and ask the Pt to compare the loudness.
a. To present the most uniform sound, hold the fork prongs perpendicular, not parallel, to the Pt’s ear. Forks with a frequency of 512 to 2000 cps match the frequencies most important for speech perception, but they do not vibrate very long. Neurologists compromise by using a fork of 126 or 256 cps (middle C) that also serves to test vibration sense in the digits.
b. To semiquantitate the test, move the fork from one ear to the other and ask the Pt to compare the loudness of the sound in the two ears. Also compare the distance from the ear at which you hear the sound with the distance at which the Pt hears it.
5. Masking the opposite ear: When the Ex tests one ear, the sound vibrations may travel through air or the skull bone, resulting in detection by the opposite ear, even though the ear directly tested is impaired. To improve test reliability, mask the opposite ear by rubbing the edge of a card along the helix of the ear or rustling your fingers beside it as you present the tuning fork to the other ear.
6. The air-bone conduction test of Rinne compares the efficiency of the conduction of sound vibrations by bone and by air.
a. Hold a faintly vibrating tuning fork on your mastoid process. Just after the sound disappears, hold the fork beside your ear. Can you hear the sound now?  Yes/
Yes/ No. Normally air conduction is
No. Normally air conduction is  more/
more/ less efficient than bone. (
less efficient than bone. ( Yes;
Yes;  more) (Sound normally reaches the ear by air conduction.)
more) (Sound normally reaches the ear by air conduction.)
b. If you press your fingertip in your ear while holding a tuning fork beside it, you know that that will block the sound. Try this: Place the fork against your mastoid and, as you listen to the sound, press your finger hard into your ear to completely occlude the canal. What happens to the sound? _________
c. This test shows that, although a mechanical obstruction of the auditory canal causes a/an  increase/
increase/ decrease in sound by air conduction, it also causes an apparent
decrease in sound by air conduction, it also causes an apparent  increase/
increase/ decrease in bone conduction. (
decrease in bone conduction. ( decrease;
decrease;  increase)
increase)
d. If anything impedes the conduction of sound vibrations through the external auditory canal or ossicles of the middle ear, the Pt has a conduction hearing loss. Conversely, reduction of hearing by a lesion of the organ of Corti or of the auditory nerve is called a neurosensory loss. Notice that a conduction loss of hearing refers to mechanical conduction of sound vibrations through the external and middle ear, not to conduction of nerve impulses through CrN VIII. Loss of hearing from a CrN VIII lesion is called a  conduction/
conduction/ neurosensory loss. (
neurosensory loss. ( neurosensory)
neurosensory)
e. After application of the tuning fork to the mastoid process, the bone mechanically transmits vibration to the inner ear, bypassing the conduction channels of the external and the middle ear. Bone conduction tests the integrity of the nerve, even though something blocks mechanical conduction of sound vibration through the external auditory canal. Characteristically, neurosensory hearing loss impairs the hearing of high frequencies via air conduction and decreases hearing via bone conduction. Thus, nerve lesions block hearing by air and bone, whereas mechanical lesions block the sounds transmitted through  air/
air/ bone. (
bone. ( air)
air)
f. Clinical analysis of the air-bone conduction test of Rinne
i. If a Pt hears better with the fork applied to his mastoid process than with it in the air beside his ear, the best inference is
 (a) The Pt is normal.
(a) The Pt is normal.
 (b) The Pt must have a lesion of the organ of Corti.
(b) The Pt must have a lesion of the organ of Corti.
 (c) The Pt has a conduction lesion, a mechanical impediment such as wax in the auditory canal, a damaged drum, or immobility of the ossicles.
(c) The Pt has a conduction lesion, a mechanical impediment such as wax in the auditory canal, a damaged drum, or immobility of the ossicles.
 (d) The Pt has a neurosensory lesion in the organ of Corti or in the auditory nerve. (
(d) The Pt has a neurosensory lesion in the organ of Corti or in the auditory nerve. ( (c))
(c))
ii. If the test had shown reduced hearing for air and for bone conduction of sound, the best inference is:  (a)/
(a)/ (b)/
(b)/ (c)/
(c)/ (d). (
(d). ( (d))
(d))
7. The sound-lateralizing test of Weber
a. For this test, the Ex places a vibrating tuning fork on the middle of the Pt’s forehead or the vertex of the skull. Try this test yourself. The sound seems to come from  the right/
the right/ the left/
the left/ the center. (
the center. ( the center (if you are normal))
the center (if you are normal))
b. Is the sound equally loud in both ears?  Yes/
Yes/ No (
No ( Yes (if you are normal))
Yes (if you are normal))
c. With the vibrating fork in place on the vertex of your head, press your fingertip in one ear and then the other. What happens to the sound?
_________
d. The normal person hears the vertex vibration equally in both ears. If a mechanical impediment blocks sound conduction in one ear, the vertex sound localizes to  the same/
the same/ the opposite/
the opposite/ neither side. (
neither side. ( the same)
the same)
e. If the Pt has an auditory nerve lesion on one side, the vertex vibration sounds loudest on  the same/
the same/ the opposite/
the opposite/ neither side. (
neither side. ( the opposite)
the opposite)
f. Only a consistent lateralization to one side after several trials is considered significant.
F. Analysis of patients for conduction versus neurosensory hearing loss
1. This Pt showed an increased auditory threshold to finger rustling on the left; the vertex test lateralized to the left, and bone transmission was better than air transmission. These findings most likely indicate:
 (a) A normal Pt.
(a) A normal Pt.
 (b) A mechanical impediment, a conduction lesion.
(b) A mechanical impediment, a conduction lesion.
 (c) An auditory nerve or cochlear lesion, a neurosensory lesion.
(c) An auditory nerve or cochlear lesion, a neurosensory lesion.
 (d) Temporal lobe lesion.
(d) Temporal lobe lesion.
 (e) Insufficient data to reach a conclusion. (
(e) Insufficient data to reach a conclusion. ( (b))
(b))
2. This next Pt showed an increased auditory threshold to the tuning fork on the left; bone and air transmissions of sound were reduced on the left, and the vertex test lateralized to the right. Which of the previous inferences applies to this Pt?  (a)/
(a)/ (b)/
(b)/ (c)/
(c)/ (d)/
(d)/ (e). (
(e). ( (c))
(c))
G. Testing the auditopalpebral reflex or startle response to sound
While standing just behind the Pt’s line of sight, make a loud, unexpected sound such as a hand clap. Observe the Pt for blinking or a startle response. A response indicates an intact auditory pathway. No response means deafness, or that the Pt ignored the stimulus. Use the auditopalpebral reflex to test auditory function in noncooperative, hysterical, or malingering Pts, infants, and unconscious Pts. Always ask a mother whether her infant shows an alerting response to sound.
H. Auditory tests for cerebral dysfunction
1. In testing visual fields, we found that some Pts could not detect visual stimuli from both sides. This test is called _________
2. Similarly, the Ex can present simultaneous auditory stimuli, if the previous tests have demonstrated intact auditory pathways. Stand behind the Pt and hold one of your hands beside each of the Pt’s ears. Gently rub your fingers together, first on one side and then the other, and have the Pt point to the side from which the stimulus comes. Then rub the fingers of both hands to see whether the Pt identifies the simultaneous stimuli from both sides. Consider only a consistent inattention to sound from one side significant. Repeat the test several times, randomly alternating single and simultaneous stimuli. Patients with large right cerebral hemisphere lesions tend to suppress sound from the left side (see also Chapter 11); disturbances of sound lateralization also can be related to unilateral visuospatial neglect (Tanaka, et al, 1999).
3. The Ex can test sound localization by presenting rustling fingers in the anterior or posterior quadrants of the right and left sides, with the Pt’s eyes closed. Chapter 11 describes how to test Pts for auditory aphasia, the inability to understand the symbolic significance of words.
I. Tinnitus
1. Tinnitus means an abnormal sound perceived in the ear, unrelated to an outside source. It usually has a buzzing, ringing, roaring, or clicking quality (Langguth et al, 2013). Tinnitus may be continuous or fluctuating and may be perceived in one or both ears.
2. There are two types of tinnitus: subjective and objective.
a. Subjective tinnitus arises not from real sounds but from some auditory system disorder. It may arise from a disease of the ear such as presbyacusis, cochlear disease, lesions along CrN VIII or central pathways, the auditory cortex, or from drugs such as salicylates, loop diuretics, “mycin” antibiotics, quinine or derivatives, and chemotherapeutic agents such as cisplatin. Tinnitus may be associated with palatal myoclonus (palatal tremor; Zadikoff et al, 2006), and with stapedius myoclonus characterized by rapid rhythmic movements of the tympanic membrane.
b. Objective tinnitus is caused by a real sound, audible to the Ex, usually a bruit from an arteriovenous malformation, fistula, or states of high blood flow as in anemia or hyperthyroidism. In these cases, the sound is pulsatile (Hofmann et al, 2013). Pulsatile tinnitus can also be associated with idiopathic intracranial hypertension.
3. The Pt with tinnitus requires a thorough head and neck examination, detailed auscultation with gentle pressure on the jugular vein and performance of the Valsalva maneuver to exclude a venous hum (Chapter 1), neuroimaging when appropriate, and laboratory testing for hearing loss, which often accompanies tinnitus. MRA and MRV, or CT angiographic studies, may be needed to look for arterial tortuosity, carotid dissections, fibromuscular dysplasia, ectopic internal carotid artery, carotid-cochlear dehiscence, persistent stapedial artery, aberrancies of the jugular bulb or jugular vein, glomus tumor or other highly vascularized skull-based tumors, carotid aneurysms, or dural arteriovenous fistulas (Hofmann et al, 2013)
J. Laboratory tests for auditory dysfunction
1. If the foregoing screening tests suggest loss of hearing, refer the Pt for diagnostic testing. These may include pure tone audiometry, speech discrimination batteries, recruitment, and the von Bekesy loudness discrimination test. Brainstem auditory evoked responses (BAERs) do not require conscious responses, so you can objectively test the integrity of the auditory pathways in conscious or unconscious Pts, as discussed under Electroneural Diagnosis in Chapter 13.
2. Common causes of delayed speech are deafness and intellectual disability. If the child has delayed speech or does not appear to hear, always test thoroughly with bedside and electronic hearing tests.
K. Rehearsal time
Do all of the foregoing hearing tests as outlined in Section V C 3 of the Standard NE.
BIBLIOGRAPHY · Auditory Testing and Physiology
Alford RL, Arnos KS, Fox M, et al. American College of Medical Genetics and Genomics guideline for the clinical evaluation and etiologic diagnosis of hearing loss. Genet Med. 2014;16:347–355.
Bizley JK, Cohen YE. The what, where and how of auditory-object perception. Nat Rev Neurosci. 2013;14:693–707.
Campo P, Morata TC, Hong O. Chemical exposure and hearing loss. Dis Mon. 2013;59:119–138.
Hofmann E, Behr R, Neumann-Haefelin T, Schwager K. Pulsatile tinnitus: imaging and differential diagnosis. Dtsch Arztebl Int. 2013;110:451–458.
Hudspeth AJ. Integrating the active process of hair cells with cochlear function. Nat Rev Neurosci. 2014;15:600–614.
Kazmierczak P, Müller U. Sensing sound: molecules that orchestrate mechanotransduction by hair cells. Trend Neurosci. 2012;35:220–229.
Langguth B, Kreuzer PM, Kleinjung T, De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12:920–930.
Levine RA. Typewriter tinnitus: a carbamazepine-responsive syndrome related to auditory nerve vascular compression. ORL J Othorhinolaryngol Relat Spec. 2006;68(1):43–46.
Musiek FE, Chermak GD. Psychophysical and behavioral peripheral and central auditory tests. Handb Clin Neurol. 2015;129:313–332.
Pickles JO. Auditory pathways: anatomy and physiology. Handb Clin Neurol. 2015;129:3–25.
Tanaka H, Hachisuka K, Ogata H. Sound lateralisation in patients with left or right cerebral hemispheric lesions: relation with unilateral visuospatial neglect. J Neurol Neurosurg Psychiatry. 1999;67:481–486.
Theunissen FE, Elie JE. Neural processing of natural sounds. Nat Rev Neurosci. 2014;15:355–366.
Wasserthal C, Brechmann A, Stadler J, et al. Localizing the human primary auditory cortex in vivo using structural MRI. Neuroimage. 2014;93:237–251.
Zadikoff C, Lang AE, Klein C. The ‘essentials’ of essential palatal tremor: a reappraisal of the nosology. Brain. 2006;129:832–840.
V. THE VESTIBULAR SYSTEM: VERTIGO AND ITS POSTURAL COMPENSATIONS
A. Dizziness and vertigo
1. Among the most common symptoms that plague humankind are headaches, backaches, dizziness, fatigability, and blackout spells.
2. In the Pt’s lexicon, dizziness may mean giddiness, light-headedness, unsteadiness, vertigo, spinning, and so on (van Leeuwen and Bruintjes, 2014). When the Pt complains of dizziness, ask for a more specific description (“Describe how you feel different when you have the dizziness”). Then echo the Pt’s own words to avoid prejudicing the answer. Later ask the Pt to compare the sensation with that produced by a merry-go-round. If strictly defined, vertigo excludes the multitude of less specific symptoms encompassed by dizziness. Vertigo means a specific sense of disequilibrium as though the person or the world were spinning around or undergoing a swerving or tilting movement (Molnar and McGee, 2014). It is an illusion of movement of self or environment. True vertigo implies a disorder of the vestibular receptors, their nerves, or central connections. The most frequent causes of peripheral vertigo are benign paroxysmal positioning vertigo (BPPV), Ménière disease, and vestibular neuritis. Central forms of vertigo are due to lesions involving the neuronal circuitry between the vestibular nuclei and the vestibulo-cerebellum, as well as those involving the vestibular nuclei, the vestibular and ocular motor structures of the brainstem, cerebellum, thalamus, and vestibular cortex (eg, Wallenberg syndrome, vestibular migraine, cerebellar atrophies).
3. Several afferent avenues contribute to a sense of equilibrium, balance, and verticality: vision, proprioception (vestibular and dorsal column), and cutaneous touch and pressure. Vertigo occurs whenever the major senses provide conflicting information. The vestibular system sends impulses to the brain and it accustoms itself to the balanced input. With rotation, the input from one side increases and that from the other decreases, thus magnifying the discrepancy between the two sides. The brain interprets the information as movement. If disease causes an imbalance in the vestibular input, the brain interprets it as movement in conflict with other senses that signal no movement (Seemungal, 2014). A person standing at the top of the Empire State Building or the Grand Canyon who suddenly glances down will feel vertiginous because of the loss of the visual stereoscopy. Even diplopia may cause vertigo or loss of the sense of balance, because of the mismatch of images from the two eyes. Which image is the person to believe and orient to? When you walk in the dark and put out your hand to touch a wall, you instinctively confirm the role of cutaneous sensation in the sense of balance.
4. Self-induction of vertigo: To appreciate how vertigo affects the Pt, try this experiment. Because you may fall, follow the instructions carefully.
a. Place a penny on the floor about 40 to 50 cm from a bed or a fully cushioned easy chair (to catch you in case you fall).
b. Stand directly over the penny, with it between your feet and with the receptacle to your right.
c. Flex your neck to stare down at the penny, and while staring at the penny, turn around to your right fairly rapidly for six complete turns. At the end of the six turns, stop with your right side toward the receptacle. Then try to stand erect and still and hold your arms straight out. Record these observations.
i. In which direction do you experience an illusion of movement?  to the right/
to the right/ to the left (
to the left ( to the left) (Most persons feel as if they were spinning to the left, but some may experience another type of movement.)
to the left) (Most persons feel as if they were spinning to the left, but some may experience another type of movement.)
ii. Which way do you tend to fall?  to the right/
to the right/ to the left (
to the left ( to the right)
to the right)
iii. Which way do your outstretched arms tend to deviate?  to the right/
to the right/ to the left (
to the left ( to the right)
to the right)
d. Repeat the rotation experiment, but this time rotate to the left. The direction of vertigo is to the  right/
right/ left, and the direction of falling and arm deviation is to the
left, and the direction of falling and arm deviation is to the  right/
right/ left. (
left. ( right; left)
right; left)
e. We can now generalize that, when the person experiences vertigo in one direction, the falling and arm deviation are in the  same/
same/ opposite direction. (
opposite direction. ( opposite)
opposite)
f. The vertigo comes from a conflict or mismatch of sensory information. When you stop rotating, momentum causes the fluid in the semicircular canals to continue to rotate briefly. The current, deflecting the hair cell receptors of the cristae, signals movement, but the other proprioceptors and vision signal no movement. What you have experienced is motion sickness.
g. During vestibular vertigo, eyelid closure increases postural instability by removing the compensatory information from vision, just as when the Pt has dorsal column disease (Romberg test, pp. 400–401). Thus, darkness or deprivation of vision makes Pts with vestibular or dorsal column disease worse, but has little effect on normal persons or Pts with cerebellar ataxia, an important point when taking the history.
B. Symptoms and signs of acute vestibular dysfunction
1. Acute transection of a vestibular nerve causes the full syndrome of vestibular dysfunction.
a. The symptoms consist of intense constant vertigo to the opposite side of the lesion, nausea, oscillopsia (when objects in the visual filed appear to oscillate), and anxiety. The Pt refuses to stand or walk and resists any change in position because it aggravates the symptoms. Nystagmus causes the oscillopsia.
b. The signs consist of falling and past-pointing to the side of the lesion, jerk nystagmus with the slow phase to the side of the lesion, and autonomic dysfunction: vomiting, pallor, sweating, and hypotension. The hypotension does not lead to syncope.
2. Bilateral, symmetrical vestibular disease may produce only unsteadiness of the gait and balance, without the vertigo or autonomic symptoms. Vision and dorsal columns compensate for the loss of vestibular function.
3. CNS or peripheral lesions, such as infarcts, involving the vestibular pathways may cause an ocular tilt reaction with skew deviation of the eyes and tilts of the perceived visual vertical or present with acute vertigo that may suggest a peripheral origin (Tehrani et al, 2014).
C. Physiology of the peripheral vestibular system
1. Vestibular receptors: The semicircular canals, utricle and saccule of the vestibular labyrinth of the inner ear, contain the hair cell receptors that initiate vestibular impulses (Fig. 9-7).
2. Each of the three semicircular canals contains an ampullary crista that has hair cells sensitive to current flow. The utricle and saccule each contain a macula that has otoliths resting on hair cells that are sensitive to inertia and the action of gravity. Head movements and the pull of gravity stimulate the vestibular receptors.
a. The cristae of the semicircular canals detect fast angular accelerations or rotations of the head in the plane of the canal. Figures 9-8 and 9-9 show the planes of the semicircular canals.
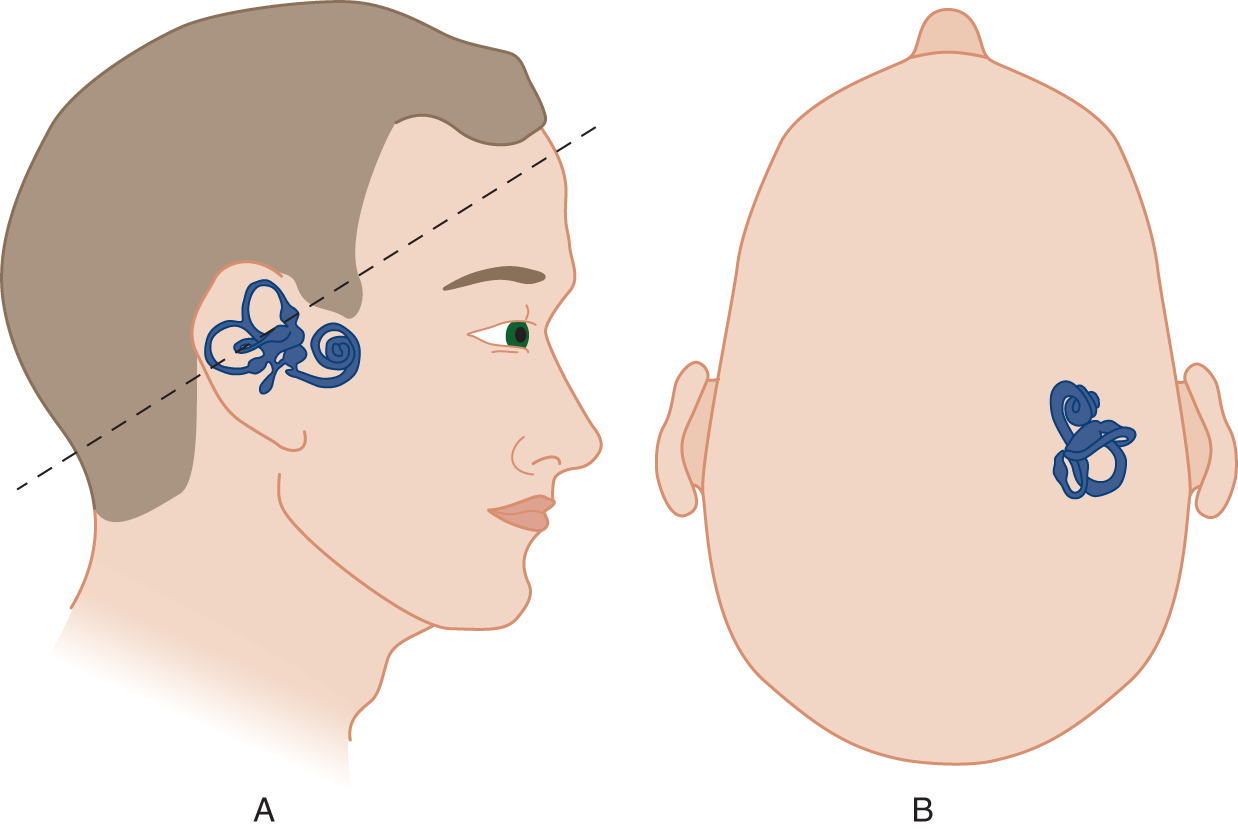
FIGURE 9-8. Orientation of the labyrinth. The size of the labyrinth is exaggerated for clarity of the drawing. (A) Lateral view of the right labyrinth. Notice that the plane (dotted line) of the “horizontal” semicircular canal angles 30° upward from the true horizontal. (B) Superior view of the labyrinth.
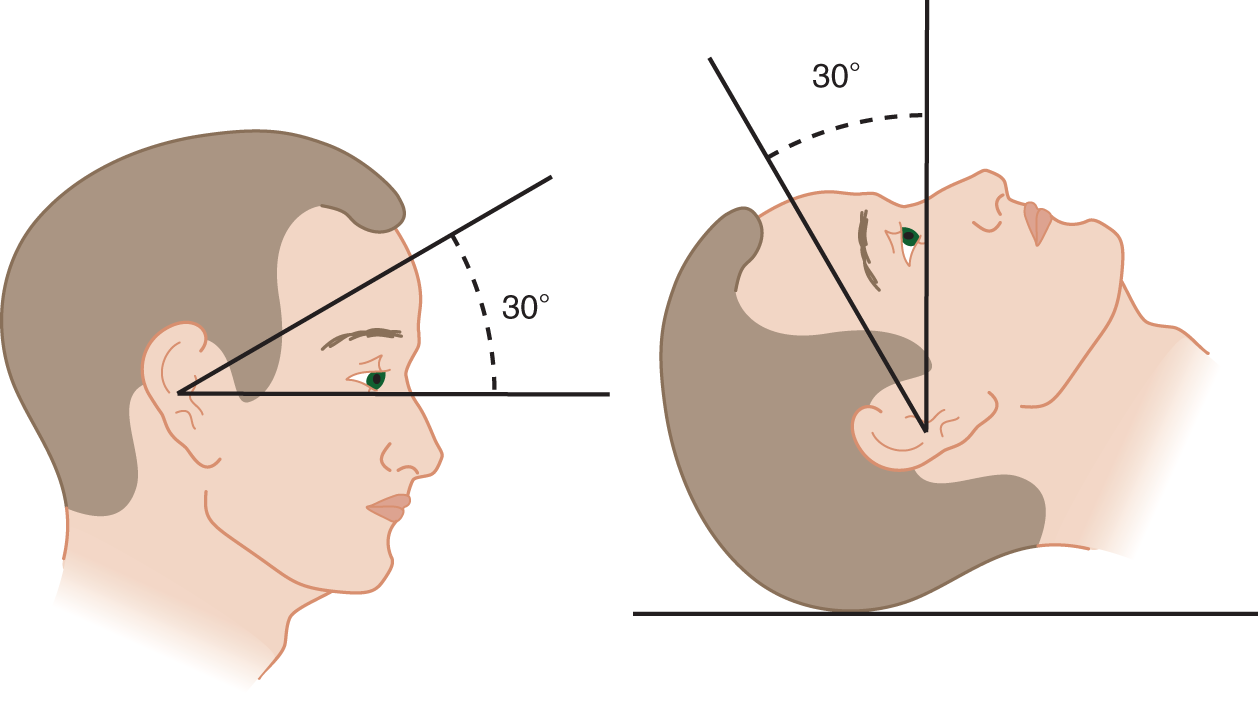
FIGURE 9-9. Inclination of the horizontal canal with the patient erect and supine.
b. In tilting your head downward while spinning around the penny, you placed the horizontal canal more truly  horizontal/
horizontal/ vertical and therefore in the plane of rotation. (
vertical and therefore in the plane of rotation. ( horizontal)
horizontal)
3. The otoliths of the maculae respond to linear acceleration in the lateral and vertical planes and to slow tilting. They act as “out-of-position” or tilt receptors in response to gravity and contribute to postural reflexes and a sense of balance and verticality.
4. In summary, the vestibular receptors initiate three functions:
a. They counter-roll the eyes against the direction of head rotation, thus keeping the eyes on the visual target when the head moves; for example, if the head turns to the right, the vestibular system counter-rolls the eyes to the left.
b. They detect angular and linear head movements and the inclination of the head with respect to the pull of gravity, which leads to a vertical posture and a sense of balance when the person sits, stands, and walks.
c. They alter the tone in the antigravity muscles. Through the pathways described next, the vestibular system mediates major postural reflexes, namely the symmetric and asymmetric tonic neck reflexes (Fig. 5-4), head- and trunk-righting reflexes, positive supporting reflexes, Moro reflex, and decerebrate rigidity (Fig. 12-13).
D. Vestibular pathways
1. The vestibular ganglion occupies the distal end of the internal auditory canal. Hence, the primary vestibular neurons, like the primary cochlear neurons, sit very close to their place of duty (Fig. 9-7). So does the sensory ganglion for CrN _________
2. The primary vestibular neurons synapse on the vestibular nuclei at the  cervicomedullary/
cervicomedullary/ medullopontine/
medullopontine/ pontomesencephalic region of the brainstem. (
pontomesencephalic region of the brainstem. ( medullopontine (review Fig. 2-21, if necessary))
medullopontine (review Fig. 2-21, if necessary))
3. The vestibular nuclei contain the  primary/
primary/ secondary/
secondary/ tertiary neurons in the vestibular pathway. (
tertiary neurons in the vestibular pathway. ( secondary)
secondary)
4. The central pathways for the signs of vestibular stimulation
a. Pathways from the vestibular nuclei run to the nuclei of CrNs III, IV, and VI. The pathway linking the vestibular system with CrN III, IV, and VI is the _________
b. These pathways mediate the counter-rolling effect of the vestibular system on eye movement and also mediate nystagmus of vestibular origin.
c. Extensive connections between the vestibular system and the dorsal motor nucleus of the vagus and the pontomedullary reticular formation mediate the autonomic signs of vestibular dysfunction.
d. The vestibular nuclei send strong descending pathways to the spinal cord via the MLF and vestibulospinal tracts (Cullen, 2012). These pathways and reticulospinal pathways coordinate postural reflexes involving the eyes, head, trunk, and limbs, listed in frame V C 4 c. Should you recite these reflexes?
5. The pathways for the symptoms of vestibular stimulation
a. The vestibular nuclei connect to the thalamus by two pathways:
i. A ventral pathway, ventral to the medial lemniscus, passes lateral to the red nucleus and dorsal to the subthalamic nucleus, terminating in the nucleus ventralis posterolateralis, pars oralis (nucleus ventralis intermedius).
ii. A dorsal pathway runs through the lateral lemniscus and brachium of the inferior colliculus to the medial geniculate body, thus paralleling the auditory pathway (Fig. 9-7).
b. The vestibular cortex consists of adjacent areas in the inferior parietal lobe, insula, and superior temporal region. The vestibular cortex is not confined to a discrete strip of striate cortex like other somatic senses. The vestibular cortical regions integrate auditory, visual somatosensory, and vestibular proprioceptive impulses that provide a sense of verticality, balance, and orientation of the body in space (Cullen, 2012; Mazzola et al, 2014).
6. Summary: Vestibular impulses that arrive at the vestibular nuclei can disperse to every major subdivision of the CNS: the cerebrum, diencephalon, brainstem, cerebellum, and spinal cord.
a. If they go caudally to the spinal cord, they descend via the MLF and the _________
b. If they go dorsally to the flocculonodular lobe of the cerebellum, they travel via the _________
c. If they go rostrally to nuclei for the ocular muscles, they travel via the _________
d. If they go into the reticular formation, the impulses travel through many short circuits of bewildering complexity.
e. If they go to the cortex, they follow a dual pathway to the thalamus and then to areas near the posterior end of the Sylvian fissure, close to the striate cortex of the auditory area.
E. Review of clinical features of acute vestibular dysfunction
1. Recall that the response to vestibular stimulation is subjective and objective. List the subjective responses, the symptoms, by recalling motion sickness.
_________
_________
2. List the objective responses—the signs including somatic and autonomic actions.
_________
_________
_________
F. Physiology of nystagmus from caloric irrigation of the external auditory canals
1. The inertia or flow of fluid within the semicircular canals due to head rotation provides the normal stimulus. Syringing the external auditory canal with warm or cold water induces convection currents in the fluid in the horizontal semicircular canal, the closest one. Cooled fluid descends and heated fluid rises. Because of the weak effects of convection, the Ex places the horizontal canal in the vertical plane to add the effect of gravity to the convection currents. Refer to Fig. 9-9 to answer these questions:
a. For a reclining Pt, tilt the head ____ degrees forward to get the horizontal canal vertical. (30)
b. For a sitting Pt, tilt the head ___ degrees  forward/
forward/ backward to get the horizontal canal vertical. (60;
backward to get the horizontal canal vertical. (60;  backward)
backward)
2. Characteristics of vestibular nystagmus
a. The nystagmus has fast and slow phases. It is therefore a  jerk/
jerk/ pendular nystagmus. (
pendular nystagmus. ( jerk)
jerk)
b. While observing the nystagmus, have the Pt’s eyes pursue your finger as you move it from side to side. The nystagmus increases in amplitude when the Pt looks in the direction of the fast component, because the volitional saccade adds to the kickback saccade.
3. Describing the direction of jerk nystagmus: Describing the direction of vestibular nystagmus presents a problem. The slow deviation of the eyes is the specific vestibulo-ocular action that initiates the nystagmus. A compensatory saccade then jerks the eyes back toward the midline, centering them for another deviation by the vestibular stimulus. Coma or anatomic interruption of the fronto-tegmental pathway for saccades may abolish the kickback saccade, even though the deviation phase remains (Molnar and McGee, 2014). By tradition, the direction of nystagmus is named for the kick-back phase, not for the deviation phase. Understanding that, you can use the well-known COWS mnemonic.
4. The term vestibulo-ocular reflex (oculocephalic reflex or doll’s eye test) means the slow deviation or counter-rolling of the eyes induced by caloric irrigation or by head rotation.
G. Indications for caloric irrigation
Although not part of the Standard NE, consider caloric irrigation if the history or examination indicates dizziness, vertigo, auditory dysfunction, or the recent onset of nystagmus (Baloh, 2002; Zapala et al, 2008). See Chapter 12 for its use in coma and brain death. Chapter 13 discusses objective recording of nystagmus by electronystagmography (Curthoys, 2012). Caloric irrigation becomes reliable in infants after 6 to 8 months of age (Fife et al, 2000).
H. Technique for caloric irrigation
1. Forewarn and position the Pt: Because of discomfort, the Ex should warn the Pt about the test, but mentioning the expected symptoms voids the objectivity and validity of the test. Therefore, say this: “I will be rinsing your ear. It is somewhat uncomfortable, but I want you to pay attention to what you feel.” Because vertigo may cause the Pt to fall, place the Pt in a sitting or reclining position.
2. Do otoscopy: Exclude a mechanical impediment such as cerumen, otitis, or a perforated eardrum that might allow water into the middle ear, causing pain and infection. Remove excessive cerumen that may preclude adequate heat conduction.
3. Place spectacles on the Pt that have strong positive lenses (+10 to 30 diopters, Frenzel lenses). (You can buy them cheaply in chain stores.) The lenses serve two purposes. They magnify the Pt’s eyes, making any nystagmus easier to see, and they impair fixation by blurring vision. Fixation inhibits vestibular-induced nystagmus. Thus, the glasses increase the likelihood of eliciting nystagmus, and make it easier to study if it occurs.
4. Instruct the Pt to gaze ahead: Place an emesis basin or a towel next to the Pt’s ear to prevent wetting the Pt (and also for emergency service should the Pt vomit).
5. Irrigate the ear with warm or cold water: Fill a 50-mL syringe with water at a temperature of 7°C above or below the normal 37°C of body temperature (30°C or 44°C). Gently instill the 50 mL of water through a short rubber tube into the external auditory canal over a timed period of 40 seconds (Baloh, 2000; Zapala et al, 2008). Test both ears because a consistent difference is required for significance. Wait about 5 minutes between each test.
6. Observe the Pt’s responses: At the end of irrigation, ask the Pt to direct the gaze more or less ahead and hold the arms straight out. Inspect the Pt for the following:
a. Nystagmus: Record the duration and direction.
b. Postural deviation and past-pointing: To test for past-pointing, have the Ex and Pt sit facing each other. Each extends the arms out from the shoulder so that the index fingers of Pt and Ex touch. The Ex instructs the Pt to close the eyes and extend the arm straight up and then bring it back down to touch the Ex’s finger in the original position. Look for the Pt’s finger to miss by constantly deviating to one side.
7. Ask about symptoms: Ask whether the caloric irrigation reproduced the Pt’s usual sensation of movement, and ask about the direction of any vertigo. Patients with vertigo and postural deviations sometimes report confusing directionality, depending on whether they are attending to their vertigo or their body tilt. Normal individuals also respond somewhat variably to caloric irrigation. Some Pts show little or no response from either ear. Determine whether irrigation of the two ears produces any consistent difference. Thus, a strong normal response from the right ear with little or no response from the left indicates a lesion of the vestibular end organ, nerve, or immediate central connections on the left (Curthoys, 2012).
8. To gain an enduring sympathy for your vertiginous Pts, submit to caloric irrigation yourself. Get a partner and follow the foregoing instructions. To make all the observations, you may have to irrigate more than once. Use the right ear and ice water and compare your observations with the answers to frames V E 1 and 2.
I. Summary of the results of caloric irrigation
1. If the vertigo goes in one direction, to the right or to the left, the slow deviation phase of the nystagmus, the truncal tilt, arm deviation, and past-pointing go to the opposite side.
2. Because the vertigo causes the Pt to feel movement or rotation in one direction, all of the postural deviations can be regarded as reflex compensation for the erroneous information coming from the artificially stimulated horizontal canal. Thus, the Pt compensates for the feeling of moving to the left by leaning and past-pointing to the right. In relation to the vertigo, the Pt compensates by postural deviations that go in the  same/
same/ opposite direction. (
opposite direction. ( opposite)
opposite)
J. Summary of vestibular testing
1. The symptoms of vestibular disease are_________
_________
_________
2. The signs of vestibular disease are_________
_________
_________
3. What precautions does the Ex take before doing the caloric irrigation test?
_________
_________
_________
4. Describe the nystagmus after irrigating the left ear of a normal person with cold water.
_________
_________
5. In general, the Pt experiences the vestibular-induced vertigo as rotation toward the direction of the  fast/
fast/ slow component. (
slow component. ( fast)
fast)
6. State the law that relates the postural deviations to the direction of the vertigo.
_________
_________
_________
7. Now can you put it all together? Figure 9-10 shows a person at the height of a strong vestibular response. Reason out which ear was irrigated with cold water:  right/
right/ left. (
left. ( right)
right)
FIGURE 9-10. Postural deviation after one ear was irrigated with cold water. The patient was sitting upright with his head tilted 60 degree backward during irrigation.
K. Positional vertigo and nystagmus
1. Introduction: Whenever a Pt complains of dizziness, the Ex should ask about the effect of changes in posture. Dizziness when first standing up, a phenomenon you have all experienced, suggests orthostatic hypotension. However, disease of the labyrinth or its central connections may cause true vertigo after changes of position, such as turning over in bed. Positional nystagmus provides an objective sign of organic disease of the labyrinthine system. Free-floating otolithic particles in the posterior semicircular canal of the vestibular labyrinth may cause benign paroxysmal positioning vertigo (BPPV; Kim and Zee, 2014). While classic BPPV involves the posterior semicircular canal, it is believed that horizontal involvement may occur more frequently then assumed, but spontaneously resolve. Anterior canal involvement is infrequent in BPPV as otolithic particles are unlikely to be entrapped. The episodes of vertigo in BPPV are typically sudden, last only 10 to 30 seconds and follow turning in bed or changes in position. In contrast, the vertigo of Ménière disease comes on spontaneously and lasts minutes to hours. Bedside maneuvers can move these particles to another location and alleviate the symptoms (Viirre et al, 2005; Fyrmpas et al, 2009).
Acoustically induced vertigo and oscillopsia (Tullio phenomenon), often accompanied by tinnitus, hearing loss, and ear pressure sensitivity that is induced by acoustic stimuli. This has been attributed to a perilymphatic fistula or the superior canal dehiscence syndrome (absence of bone over the superior semicircular canal) (Kaski, 2012).
2. Technique to test for positional nystagmus and vertigo (Dix-Hallpike maneuver and Epley canal repositioning maneuver)
a. Occlude visual fixation by Frenzel lenses if available, but are not necessary. Start with the Pt sitting upright near the end of an examining table. Instruct the Pt to look straight ahead during the procedure and to keep their eyes open so, they can be observed for nystagmus. If the Pt states that rolling to one side induces vertigo, and if they are anxious, test the other side first so, they are accustomed to the test procedure. The Ex assists the Pt so they drop back quickly, with the head in the midline and end with their head hanging below the level of the examination table (not necessary for their head to be over the table edge, but accomplished by elevating their shoulders with a pillow so, their head rests on the table). After erecting the Pt, then drop the Pt back with the head turned 45 degree to 60 degree to the right or the left (if they experienced vertigo when rolling to the right, then the head is turned to that same side). After laying the Pt’s head back, there is a latency for the nystagmus so, wait for 1 minute before considering the test negative (Fig. 9-11); if nystagmus occurs it resolves within a minute (Molnar and McGee, 2014).
FIGURE 9-11. Method for eliciting positional nystagmus.
b. After returning the Pt to the erect position, with their head still turned, inspect the eyes again for nystagmus. Classic posterior canal BPPV produces geotropic rotatory nystagmus (quick component moves the eyes up and a torsional rotation down) with the involved ear down.
c. Repeat the procedure with the head turned to the opposite direction.
d. At the end of any such positional tests, inquire whether the maneuver reproduced the Pt’s sensation of dizziness, but do not suggest that it should have. Once the involved side is determined, a canalith repositioning maneuver can be performed (Viirre et al, 2005).
e. Pts with a neurologic disorder such as multiple sclerosis, but not benign paroxysmal positional vertigo, may experience vertigo by these maneuvers (Frohman et al, 2000). Hence, the Ex should always consider and test for this possibility, but typically there is no lag period for their nystagmus to occur and accompanying findings suggest an etiology other than BPPV.
L. Hyperventilation and dizziness
1. Indications for hyperventilation: Hyperventilation causes dizziness and may occur in panic attacks. It may provoke fainting (hyperventilation syncope) and epileptic seizures, in particular petit mal attacks. Hyperventilate the Pt if the history suggests any of these possibilities.
2. Technique for hyperventilation: Because the Pt may faint, the Pt sits or semireclines during the test. Ask the Pt to breathe as deeply and quickly as possible for a full 3 minutes by the clock. Throughout the test, keep encouraging the Pt to breathe hard: “Come on now. We are racing up a mountain.” At the end, ask how the test made the Pt feel and whether it matched the original symptoms. Be sure to try this test on yourself.
3. Normal results of hyperventilation: The Pt feels lightheaded and somewhat faint and may experience tingling in the perioral region and extremities. Carpopedal spasm may also occur.
M. Summary of clinical tests for workup of the dizzy patient
1. See Table 9-1. See also Fife et al (2000) and Curthoys (2012).
TABLE 9-1 • Clinical Workup of a Patient with Dizziness or Vertigo
Tests for vestibular dysfunction |
Inquire about the circumstances at onset of vertiginous attacks and the necessity of remaining still to avoid vertigo |
Inspect the gait and posture for tilting and unsteadiness |
Inspect the eyes for nystagmus |
Caloric irrigation of the ear |
Whirling |
Direct whirling of infant |
Rotating (Barany) chair |
Tilt tests for postural vertigo and nystagmus |
Doll’s eye maneuver for counter-rolling of the eyes (Chapter 12) |
Nonvestibular tests |
Romberg test (increased swaying with vestibular or dorsal column disease, but not with cerebellar disease) |
Reclining/standing blood pressure for orthostatic hypotension |
Valsalva maneuver |
Hyperventilation for 3 minutes |
Carotid sinus stimulation |
2. Frequently the Ex should also order an electroencephalogram and MRI in Pts with disequilibrium or dizziness (Kerber et al, 1998).
BIBLIOGRAPHY · Vestibular System, Dizziness, and Vertigo
Baloh RW. Charles Skinner Hallpike and the beginnings of neurotology. Neurology. 2000;54:2138–2145.
Blanke O, Perrig S, Thut G, et al. Simple and complex vestibular responses induced by electrical cortical stimulation of the parietal cortex in humans. J Neurol Neurosurg Psychiatry. 2000;69:553–556.
Cullen KE. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trend Neurosci. 2012;35:185–196.
Curthoys IS. The interpretation of clinical test of peripheral vestibular function. Laryngoscope. 2012;122:1342–1352.
Fife TD, Tusa RJ, Furman JM, et al. Assessment: vestibular testing techniques in adults and children: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2000;55:1431–1441.
Frohman EM, Zhang H, Dewey RB, et al. Vertigo in MS: utility of positional and particle repositioning maneuvers. Neurology. 2000;55:1566–1568.
Fyrmas G, Rachovitsas D, Haidich AB, et al. Are postural restrictions after an Epley maneuver unnecessary? First results of a controlled study and review of the literature. Auris Nasus Larynx. 2009;36:637–643.
Kaski D, Davies R, Luxon L, et al. The Tullio phenomenon: a neurologically neglected presentation. J Neurol. 2012;259:4–21.
Kim JS, Zee DS. Benign paroxysmal positional vertigo. N Engl J Med. 2014;370:1138–1147.
Mazzola L, Lopez C, Faillenot I, et al. Vestibular response to direct stimulation of the human insular cortex. Ann Neurol. 2014;76:609–619.
Molnar A, McGee S. Diagnosing and treating dizziness. Med Clin N Am. 2014;98:583–596.
Seemungal BM. The cognitive neurology of the vestibular system. Curr Opin Neurol. 2014;27:125–132.
Tehrani AS, Kattah JC, Mantokoudis G, et al. Small strokes causing severe vertigo: frequency of false-negative MRIS and nonlacunar mechanisms. Neurology. 2014;83:169–173.
Valoh RW. Robert Bárány and the controversy surrounding his discovery of the caloric reaction. Neurology. 2002;58:1094–1099.
van Leeuwen RB, Bruintjes TD. Dizziness in the elderly: diagnosing its causes in a multidisciplinary dizziness unit. Ear Nose Throat J. 2014;93:162–167.
Viirre E, Purcell I, Baloh RW. The Dix-Hallpike test and the canalith repositioning maneuver. Laryngoscope. 2005;115:184–187.
Zapala DA, Olsholt KF, Lundy LB. A comparison of water and air caloric responses and their ability to distinguish between patients with normal and impaired ears. Ear Hear. 2008;29:585–600.
 Learning Objectives for Chapter 9
Learning Objectives for Chapter 9