Chapter 60 NURSING MANAGEMENT: peripheral nerve and spinal cord problems
1. Explain the aetiology, clinical manifestations, multidisciplinary care and nursing management of trigeminal neuralgia and Bell’s palsy.
2. Describe the aetiology, clinical manifestations, multidisciplinary care and nursing management of Guillain-Barré syndrome, botulism, tetanus and neurosyphilis.
3. Discuss the classification of spinal cord injuries and associated clinical manifestations.
4. Explore the clinical manifestations, multidisciplinary care and nursing management of spinal cord shock.
5. Correlate the clinical manifestations of spinal cord injury with the level of disruption and rehabilitation potential.
6. Apply knowledge of nursing management to the management of the major physical and psychological problems of the patient with a spinal cord injury.
7. Describe the effects of spinal cord injury on the older adult population.
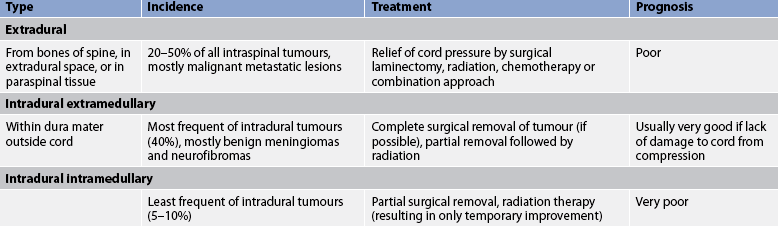
8. Explain the types, clinical manifestations, multidisciplinary care and nursing management of spinal cord tumours.
9. Describe the pathophysiology, clinical manifestations, and nursing and collaborative management of post-polio syndrome.
CRANIAL NERVE DISORDERS
Cranial nerve disorders are commonly classified as peripheral neuropathies. The 12 pairs of cranial nerves are considered the peripheral nerves of the brain. The disorders usually involve the motor or sensory (or both) branches of a single nerve (mononeuropathies). Causes of cranial nerve problems include tumours, trauma, infections, inflammatory processes and idiopathic (unknown) causes. Two cranial nerve disorders are trigeminal neuralgia (tic douloureux) and acute peripheral facial paralysis (Bell’s palsy).
Trigeminal neuralgia
AETIOLOGY AND PATHOPHYSIOLOGY
Trigeminal neuralgia (tic douloureux) is a relatively uncommon cranial nerve disorder; however, it is the most commonly diagnosed neuralgic condition. It is seen approximately twice as often in women as in men. The majority of cases (>90%) are diagnosed in individuals over the age of 40.1 The trigeminal nerve is the fifth cranial nerve (CN V) and has both motor and sensory branches. In trigeminal neuralgia the sensory or afferent branches, primarily the maxillary and mandibular branches, are involved (see Fig 60-1).
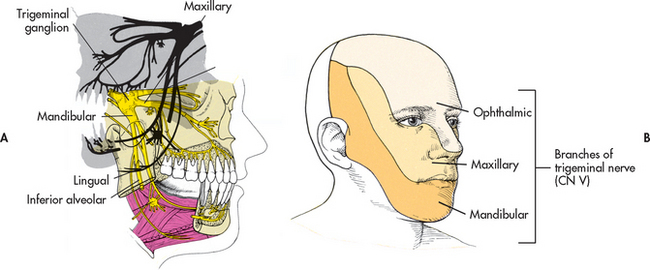
Figure 60-1 A, Trigeminal (fifth cranial) nerve and its three main divisions—the ophthalmic, maxillary and mandibular nerves. B, Cutaneous innervation of the head.
The pathophysiology of trigeminal neuralgia is not fully understood. One theory is that nerve compression by blood vessels, the superior cerebellar artery in particular, occurs, resulting in chronic irritation of the trigeminal nerve at the root entry zone. This irritation results in increased firing of the afferent or sensory fibre. Other factors that may result in neuralgia include herpes virus infection, infection of teeth and jaw, and a brainstem infarct. The effectiveness of antiepileptic drug therapy in reducing pain may be related to the ability of these drugs to stabilise the neuronal membrane and decrease paroxysmal afferent impulses of the nerve.1
CLINICAL MANIFESTATIONS
The classic feature of trigeminal neuralgia is an abrupt onset of paroxysms of excruciating pain described as a burning, knife-like or lightning-like shock in the lips, upper or lower gums, cheek, forehead or side of the nose. Intense pain, twitching, grimacing and frequent blinking and tearing of the eye occur during the acute attack (giving rise to the term tic). Some patients may experience facial sensory loss as well. The attacks are usually brief, lasting only seconds to 2–3 minutes, and are generally unilateral. Recurrences are unpredictable; they may occur several times a day, or weeks or months apart. After the refractory (pain-free) period, a phenomenon known as clustering can occur. Clustering is characterised by a cycle of pain and refractoriness that continues for hours.
The painful episodes are usually initiated by a triggering mechanism of light cutaneous stimulation at a specific point (trigger zone) along the distribution of the nerve branches. Precipitating stimuli include chewing, teeth brushing, a hot or cold blast of air on the face, washing the face, yawning or even talking. Touch and tickle seem to predominate as causative triggers rather than pain or changes in temperature. As a result, the patient may eat improperly, neglect hygiene practices, wear a cloth over the face and withdraw from interaction with other individuals. The patient may sleep excessively as a means of coping with the pain.
Although this condition is considered benign, the severity of the pain and the disruption of lifestyle can result in almost total physical and psychological dysfunction or even suicide.
DIAGNOSTIC STUDIES
It is important to rule out other problems with similar manifestations, such as other forms of facial and cephalic neuralgias and pain arising from the sinuses, teeth and jaws. In young adults with bilateral facial pain, a computed tomography (CT) scan is performed to rule out any lesions or vascular abnormalities, and a lumbar puncture and magnetic resonance imaging (MRI) are done to rule out multiple sclerosis. A complete neurological assessment is done, including audiological evaluation, although results are usually normal. Additional tests used to rule out other pathological conditions include electromyography (EMG), cerebrospinal fluid (CSF) analysis, arteriography and myelography. Once the diagnosis is made, the goal of treatment is relief of pain either medically or surgically (see Box 60-1 and Table 60-1).
MULTIDISCIPLINARY CARE
Collaborative therapy
Drug therapy (e.g. phenytoin, carbamazepine, sodium valproate, oxcarbazepine, gabapentin, lamotrigine, topiramate)
Surgical intervention (see Table 60-1)
CSF, cerebrospinal fluid; CT, computed tomography; EMG, electromyography; MRI, magnetic resonance imaging.
MULTIDISCIPLINARY CARE
Drug therapy
The majority of patients obtain adequate relief through antiepileptic drugs such as carbamazepine, phenytoin and sodium valproate. Carbamazepine is considered the first-line therapy for trigeminal neuralgia. By acting on sodium channels, carbamazepine and other antiepileptic drugs lengthen the time needed for neuron repolarisation, resulting in decreased neuron firing. Side effects of carbamazepine may include bone marrow suppression leading to blood abnormalities. Routine full blood counts (FBC) are required. Newer antiepileptic drugs used in the management of trigeminal neuralgia include oxcarbazepine, gabapentin, lamotrigine and topiramate. These drugs may prevent an acute attack or promote a remission of symptoms. Because drug therapy may not provide permanent pain relief, some patients may seek continued treatment from their general practitioner or from therapies such as acupuncture and megavitamins.
Conservative therapy
Nerve blocking with local anaesthetics is another treatment possibility. Local nerve block results in complete anaesthesia of the area supplied by the injected branches. Relief of pain is temporary, lasting from 6 to 18 months. This treatment is usually tolerated well by older adults.
Biofeedback is another strategy that may be helpful for some patients. In addition to controlling the pain, the patient may experience a strong sense of personal control by mastering the technique and altering certain body functions.
Surgical therapy
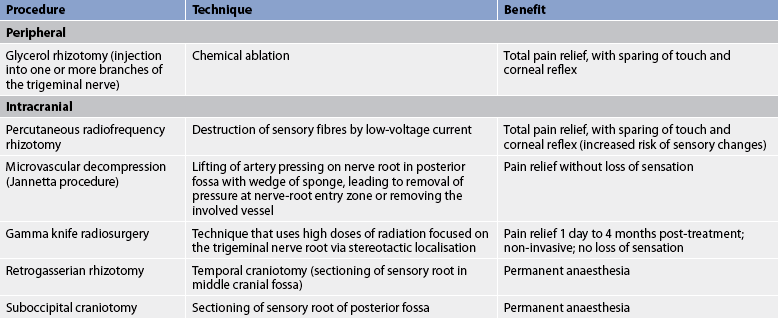
If a conservative approach including drug therapy is not effective, surgical therapy is available (see Box 60-1). Glycerol rhizotomy is a percutaneous procedure that consists of an injection of glycerol through the foramen ovale into the trigeminal cistern (see Fig 60-2). It is a more benign procedure with less sensory loss and fewer sensory aberrations than radiofrequency rhizotomy and with comparable or better pain relief. However, for some patients the pain will return over time.2,3

Figure 60-2 Glycerol rhizotomy. A, Patient with trigeminal neuralgia having needle placed. B, Doctor injecting glycerol.
Percutaneous radiofrequency rhizotomy (electrocoagulation) and microvascular decompression afford the greatest relief of pain. Percutaneous radiofrequency rhizotomy consists of placing a needle into the trigeminal rootlets that are adjacent to the pons and destroying the area by means of a radiofrequency current. This can result in facial numbness (although some degree of sensation may be retained), corneal anaesthesia and trigeminal motor weakness. This procedure is easily performed with minimal risk to the patient and is based on the exchange of pain for numbness. The procedure is usually performed on an outpatient basis with few complications. It is tolerated well by older adults and avoids a major operative procedure in the high-risk patient.2
Microvascular decompression of the trigeminal nerve is another commonly used procedure for neuralgia. It is accomplished by displacing and repositioning blood vessels that appear to be compressing the nerve at the root entry zone where it exits the pons. This procedure relieves pain without residual sensory loss but it is potentially dangerous, as is any surgery near the brainstem. Microvascular decompression has a long-term success rate equal to or superior to those of percutaneous procedures without the higher rate of permanent neurological outcomes, such as numbness. It is a safe procedure with an almost negligible mortality and low morbidity when performed in younger adults by a skilled surgeon.2
Gamma knife radiosurgery is another surgical treatment that is used for trigeminal neuralgia. Radiosurgery using the gamma knife provides precise radiation of the proximal trigeminal nerve, which is identified on high-resolution imaging. This image-guided approach has been useful both for patients with persistent pain after other surgical approaches have been tried and as a primary surgical option. Other intracranial procedures include retrogasserian rhizotomy and suboccipital craniotomy.
 NURSING MANAGEMENT: TRIGEMINAL NEURALGIA
NURSING MANAGEMENT: TRIGEMINAL NEURALGIA
 Nursing assessment
Nursing assessment
Assessment of the attacks, including the triggering factors, characteristics, frequency and pain management techniques, helps the nurse plan for patient care. The nursing assessment should include the patient’s nutritional status, hygiene (especially oral) and behaviour (including withdrawal). Evaluation of the degree of pain and its effects on the patient’s lifestyle, drug history, emotional state and suicidal tendencies are other important factors.
 Nursing diagnoses
Nursing diagnoses
Nursing diagnoses for the patient with trigeminal neuralgia include, but are not limited to, the following:
• acute pain related to inflammation or compression of the trigeminal nerve
• imbalanced nutrition: less than body requirements related to fear of triggering pain by eating or chewing
• anxiety related to uncertainty of timing and initiating event of pain and uncertainty regarding effectiveness of pain-relieving treatments
• impaired oral mucous membrane related to unwillingness to practise oral hygiene measures secondary to potential for initiating pain
• social isolation related to anxiety over pain attacks and desire to maintain non-stimulating environment.
 Planning
Planning
The overall goals are that the patient with trigeminal neuralgia will: (1) be free of pain; (2) maintain adequate nutritional and oral hygiene status; (3) have minimal to no anxiety; and (4) return to normal or previous socialisation and occupational activities.
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
The aetiology of trigeminal neuralgia remains unknown, so health promotion is directed at reducing recurrent episodes in those who have trigeminal neuralgia. Awareness and reduction of triggering events may be possible in some patients.
 Acute intervention
Acute intervention
Patients with trigeminal neuralgia are treated primarily on an outpatient basis. Pain relief is mainly obtained by the administration of the recommended drug therapy. The nurse monitors the patient’s response to therapy and notes any side effects. Opioids such as morphine may be prescribed for the patient who is not a surgical candidate and whose pain is not controlled by other therapeutic measures. Although patients often fear that they may become addicted they need to be reassured that the likelihood of addiction to opioids when used for pain relief is less than 1%. Careful assessment of pain, including history, pain relief and drug dependency, can assist in selecting appropriate interventions.
Environmental management is essential during an acute period to lessen triggering stimuli. The room should be kept at an even, moderate temperature and free of drafts. A private room is preferred during an acute period. The nurse must use care to avoid touching the patient’s face or jarring the bed. Many patients prefer to carry out their own care, fearing that someone else will inadvertently injure them.
The nurse must teach the patient about the importance of nutrition, hygiene and oral care, and convey understanding if previous oral neglect is apparent. The nurse should provide lukewarm water and soft cloths or cotton saturated with solutions not requiring rinsing for cleansing the face. A small, soft-bristled toothbrush or a warm mouthwash assists in promoting oral care. Hygiene activities are best carried out when analgesia is at its peak.
The patient will probably not engage in extensive conversation during the acute period. Alternative communication methods, such as paper and pencil, should be provided.
Food should be high in protein and kilojoules and easy to chew. It should be served lukewarm and offered frequently. The diet should be individualised according to personal, cultural and religious preferences. When oral intake is sharply reduced and the patient’s nutritional status is compromised, a nasogastric tube can be inserted for enteral feedings.
The nurse is responsible for instruction related to diagnostic studies to rule out other problems, such as multiple sclerosis, dental or sinus problems and neoplasms, and for preoperative teaching if surgery is planned. The nurse may also need to reinforce the surgeon’s instructions related to postoperative expectations; appropriate teaching related to postoperative activities depends on the type of procedure planned (e.g. percutaneous, intracranial). The patient needs to know that they will be awake during local anaesthetic procedures so that they can cooperate when corneal and ciliary reflexes and facial sensations are checked. Patients are informed about the potential risk of postoperative facial numbness. After the procedure, the patient’s pain is compared with the preoperative level. The corneal reflex, extraocular muscles, hearing, sensation and facial nerve function are evaluated frequently (see Ch 55). If there is impairment of the corneal reflex, special attention must be paid to eye protection. This includes the use of artificial tears or eye shields. General postoperative nursing care after a craniotomy is appropriate if intracranial surgery is performed. (Nursing care related to craniotomy is discussed in Ch 56.) Diet and ambulation should be increased according to the patient’s progress or specific orders.
After a percutaneous radiofrequency electrocoagulation procedure, an ice pack is applied to the jaw on the operative side for 3–5 hours. To avoid injuring the mouth, the patient should not chew on the operative side until sensation has returned.
 Ambulatory and home care
Ambulatory and home care
Regular follow-up care should be planned. The patient needs instruction regarding the dosage and side effects of medications. Although relief of pain may be complete, the patient should be encouraged to keep environmental stimuli to a moderate level and to use stress reduction methods. The patient may have developed protective practices to prevent pain and may need counselling or other assistance in the readjustment, especially in re-establishing personal relationships.
Herpes simplex virus (HSV) infection (cold sores) can occur from manipulation of the gasserian ganglion. Treatment consists of antiviral agents such as aciclovir.
Long-term management after surgical intervention depends on the residual effects of the type of procedure. If anaesthesia is present or the corneal reflex is altered, the patient should be taught to: (1) chew on the unaffected side; (2) avoid hot foods or beverages, which can burn the mucous membranes; (3) check the oral cavity after meals to remove food particles; (4) practise meticulous oral hygiene and continue with semi-annual dental visits; (5) protect the face against extremes of temperature; (6) use an electric razor; and (7) wear a protective eye shield.
Bell’s palsy
AETIOLOGY AND PATHOPHYSIOLOGY
Bell’s palsy (peripheral facial paralysis, acute benign cranial polyneuritis) is a disorder characterised by a disruption of the motor branches of the facial nerve (CN VII) on one side of the face in the absence of any other disease such as a stroke. Bell’s palsy is an acute, peripheral facial paresis of unknown cause. Each year approximately 20 people per 100,000 will be diagnosed with Bell’s palsy. It can affect any age group but it is more commonly seen in the 20–60-year-old age range. Despite its good prognosis, Bell’s palsy leaves more than 25% of sufferers with permanent, potentially disfiguring facial weakness.4
Although the exact aetiology is not known, there is evidence that reactivated HSV may be involved in some cases. The reactivation of HSV causes inflammation, oedema, ischaemia and eventual demyelination of the nerve, creating pain and alterations in motor and sensory function.
Bell’s palsy is considered benign, with full recovery after 6 months in about 85% of patients, especially if treatment is instituted immediately. The remaining 15% of patients continue to be bothered by asymmetrical movement of facial muscles.4
CLINICAL MANIFESTATIONS
The onset of Bell’s palsy is often accompanied by an outbreak of herpes vesicles in or around the ear. Patients may complain of pain around and behind the ear. In addition, manifestations may include fever, tinnitus and hearing deficit. The paralysis of the motor branches of the facial nerve typically results in a flaccidity of the affected side of the face, with drooping of the mouth accompanied by drooling (see Fig 60-3). An inability to close the eyelid, with an upward movement of the eyeball when closure is attempted, is also evident. Also common are a widened palpebral fissure (the opening between the eyelids), flattening of the nasolabial fold and inability to smile, frown or whistle. Unilateral loss of taste is common. Decreased muscle movement may alter chewing ability and although some patients may experience a loss of tearing, many patients complain of excessive tearing. The muscle weakness causes the lower lid to turn out, allowing overflow of normal tear production. Pain may be present behind the ear on the affected side, especially before the onset of paralysis.
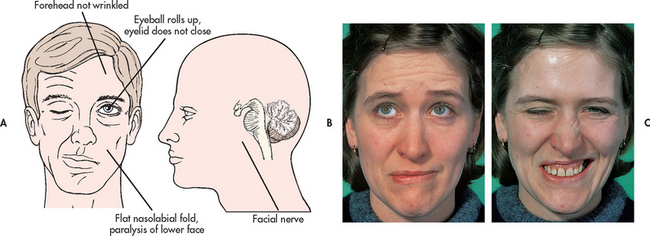
Figure 60-3 Facial characteristics of Bell’s palsy. A and B, At rest the face may look almost normal but the patient is not able to wrinkle their forehead on the affected side and the right corner of the mouth droops. C, When the patient is asked to close their eyes and show their teeth, the differences between the affected and unaffected sides become more obvious.
Source: Forbes CD, Jackson WF. Colour atlas and text of clinical medicine. 3rd edn. London: Mosby; 2003.
Complications can include psychological withdrawal because of changes in appearance, malnutrition, dehydration, mucous membrane trauma, corneal abrasions, muscle stretching, and facial spasms and contractures.
DIAGNOSTIC STUDIES
The diagnosis of Bell’s palsy is one of exclusion. There is no definitive test. The diagnosis and prognosis are indicated by observation of the typical pattern of onset and signs and the testing of percutaneous nerve excitability by EMG.
MULTIDISCIPLINARY CARE
Methods of treatment for Bell’s palsy include moist heat, gentle massage and electrical stimulation of the nerve, and prescribed exercises. Stimulation may maintain muscle tone and prevent atrophy. Care is primarily focused on relief of symptoms, prevention of complications and protection of the eye on the affected side.
Drug therapy
Corticosteroids, especially prednisolone, are started immediately and the best results are obtained if corticosteroids are initiated before paralysis is complete.4 When the patient improves to the point that the corticosteroids are no longer necessary, they should be tapered off over a 2-week period. Usually, the corticosteroid treatment decreases the oedema and pain but mild analgesics can be used if necessary. Because HSV is implicated in approximately 70% of cases of Bell’s palsy, treatment with aciclovir, alone or in conjunction with prednisolone, is used.4 Additional antiviral agents, including valaciclovir and famciclovir, have also been used in the management of Bell’s palsy.
 NURSING MANAGEMENT: BELL’S PALSY
NURSING MANAGEMENT: BELL’S PALSY
 Nursing assessment
Nursing assessment
Early recognition of the possibility of Bell’s palsy is important. Because HSV is a possible aetiological factor, any person who is prone to HSV infection should be alerted to seek healthcare if pain occurs in or around the ear. Assessment of facial muscles for any signs of weakness should also be done. Careful recording of assessment data provides information related to the progress of the syndrome.
 Nursing diagnoses
Nursing diagnoses
The nursing diagnoses for the patient with Bell’s palsy may include, but are not limited to, the following:
• acute pain related to the inflammation of CN VII (facial nerve)
• imbalanced nutrition: less than body requirements related to an inability to chew secondary to muscle weakness
• risk of injury (corneal abrasion) related to an inability to blink
• disturbed body image related to the change in facial appearance secondary to facial muscle weakness.
 Planning
Planning
The overall goals are that the patient with Bell’s palsy will: (1) be pain-free or have pain controlled; (2) maintain adequate nutritional status; (3) maintain appropriate oral hygiene; (4) not experience injury to the eye; (5) return to normal or previous perception of body image; and (6) be optimistic about disease outcome.
 Nursing implementation
Nursing implementation
The patient with Bell’s palsy is treated on an outpatient basis and the following interventions are used throughout the course of the disease. Mild analgesics can relieve pain. Hot wet packs can reduce the discomfort of herpetic lesions, aid circulation and relieve pain. The face should be protected from cold and drafts because trigeminal hyperaesthesia (extreme sensitivity to pain or touch) may accompany the syndrome. Maintenance of good nutrition is important. The patient should be taught to chew on the unaffected side of the mouth to avoid trapping food and to enjoy the taste of food. Thorough oral hygiene must be carried out after each meal to prevent the development of parotitis, caries and periodontal disease from accumulated residual food.
Dark glasses may be worn for protective and cosmetic reasons. Artificial tears (methylcellulose) should be instilled frequently during the day to prevent drying of the cornea. The eye should be inspected for the presence of eyelashes. Ointment and an impermeable eye shield can be used at night to retain moisture. In some patients, taping the lids closed at night may be necessary to provide protection. The patient is taught to report ocular pain, drainage or discharge.
A facial sling may be helpful to support affected muscles, improve lip alignment and facilitate eating. The facial sling is usually made and fitted by a physiotherapist or occupational therapist. Vigorous massage can break down tissues but gentle upward massage has psychological benefits even if physical effects other than the maintenance of circulation are questionable. When function begins to return, active facial exercises are performed several times a day.
The change in physical appearance as a result of Bell’s palsy can be devastating. The patient must be reassured that a stroke did not occur and that chances for a full recovery are good. The patient’s need for privacy should be respected, especially during meals, but the nurse’s assistance in the patient’s adjustment to the physical changes should not be delayed. Enlisting support from family and friends is important. It is important to tell the patient that most people recover within about 6 weeks of the onset of symptoms.
POLYNEUROPATHIES
Polyneuropathies are conditions in which many parts of the nervous system may be afflicted with a disorder.
Guillain-Barré syndrome
AETIOLOGY AND PATHOPHYSIOLOGY
Guillain-Barré syndrome (Landry-Guillain-Barré-Strohl syndrome, postinfectious polyneuropathy, ascending polyneuropathic paralysis) is an acute, rapidly progressing and potentially fatal form of polyneuritis. It affects the peripheral nervous system and results in loss of myelin (segmental demyelination) and oedema and inflammation of the affected nerves, causing a loss of neurotransmission to the periphery.5 The syndrome affects both sexes equally and is more commonly seen in adults, although it is observed in all age groups. Worldwide the incidence has varied from 10 to 20 million people each year. With adequate supportive care, 85% of patients recover completely from this disorder.5
The aetiology of this disorder is unknown but it is believed to be a cell-mediated immunological reaction directed at the peripheral nerves. The syndrome is often preceded by immune system stimulation from a viral infection, trauma, surgery, viral immunisations or human immunodeficiency virus (HIV).6 Campylobacter jejuni is the organism most commonly associated with Guillain-Barré syndrome.5,6 C. jejuni gastroenteritis is thought to precede Guillain-Barré syndrome in approximately 30% of cases. Other potential pathogens include Mycoplasma pneumoniae, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus and vaccines (rabies, swine influenza). These stimuli are thought to cause an alteration in the immune system, resulting in sensitisation of T lymphocytes to the patient’s myelin and, ultimately, myelin damage. Demyelination occurs and the transmission of nerve impulses is stopped or slowed down. The muscles innervated by the damaged peripheral nerves undergo denervation and atrophy. In the recovery phase, remyelination occurs slowly and neurological function returns in a proximal to distal pattern.
CLINICAL MANIFESTATIONS
Guillain-Barré syndrome is a heterogeneous condition with symptoms ranging from mild to severe. Symptoms usually develop 1–3 weeks after an upper respiratory or gastrointestinal (GI) tract infection. Weakness of the lower extremities (evolving more or less symmetrically) occurs over hours to days to weeks, usually peaking about the 14th day. Distal muscles are more severely affected. Paraesthesia (numbness and tingling) is frequent and paralysis usually follows in the extremities. Hypotonia (reduced muscle tone) and areflexia (lack of reflexes) are common, persistent symptoms. Objective sensory loss is variable, with deep sensitivity more affected than superficial sensations.
Miller Fisher syndrome is a clinical variant of Guillain-Barré syndrome, accounting for 5–10% of cases. It is characterised by a triad of symptoms: ataxia, areflexia and ophthalmoplegia (paralysis of motor nerves of the eye). Other subtypes include acute inflammatory demyelinating polyneuropathy, acute motor axonal neuropathy, and acute motor and sensory axonal neuropathy.
In Guillain-Barré syndrome, autonomic nervous system dysfunction results from alterations in both the sympathetic and the parasympathetic nervous systems. Autonomic disturbances are usually seen in patients with severe muscle involvement and respiratory muscle paralysis. The most dangerous autonomic dysfunctions include orthostatic hypotension, hypertension and abnormal vagal responses (bradycardia, heart block, asystole). Other autonomic dysfunctions include bowel and bladder dysfunction, facial flushing and diaphoresis. Patients may also have syndrome of inappropriate antidiuretic hormone (SIADH) secretion. (SIADH is discussed in Ch 49.) Progression of Guillain-Barré syndrome to include the lower brainstem involves the facial, abducens, oculomotor, hypoglossal, trigeminal and vagus nerves (CN VII, VI, III, XII, V and X, respectively). This involvement manifests itself through facial weakness, extraocular eye movement difficulties, dysphagia and paraesthesia of the face.
Pain is a common symptom in the patient with Guillain-Barré syndrome. The pain can be categorised as paraesthesias, muscular aches and cramps, and hyperaesthesias. Pain appears to be worse at night. Opioids may be indicated for those experiencing severe pain. Pain may lead to a decrease in appetite and may interfere with sleep.
COMPLICATIONS
The most serious complication of this syndrome is respiratory failure, which occurs as the paralysis progresses to the nerves that innervate the thoracic area. Constant monitoring of the respiratory system by checking respiratory rate, depth, forced vital capacity and negative inspiratory force provides information about the need for immediate intervention, including intubation and mechanical ventilation. Respiratory or urinary tract infections (UTIs) may occur. Fever is generally the first sign of infection and treatment is directed at the infecting organism. Immobility from the paralysis can cause problems such as paralytic ileus, muscle atrophy, deep vein thrombosis, pulmonary emboli, skin breakdown, orthostatic hypotension and nutritional deficiencies.
DIAGNOSTIC STUDIES
Diagnosis is based primarily on the patient’s history and clinical signs. CSF is normal or has a low protein content initially, but after 7–10 days it shows an elevated protein level of 7 g/L (normal protein is 0.15–0.45 g/L) with a normal white blood cell (WBC) count. Results of EMG and nerve conduction studies are markedly abnormal (reduced nerve conduction velocity) in the affected extremities.
MULTIDISCIPLINARY CARE
Management is aimed at supportive care, particularly ventilatory support, during the acute phase. Plasma exchange is used in the first 2 weeks of Guillain-Barré syndrome. In patients with severe disease who are treated within 2 weeks of onset, there is a distinct reduction in the length of hospital stay, the length of time on the ventilator and time required to resume walking. Intravenous (IV) administration of high-dose normal human immunoglobulin has also been shown to be as effective as plasma exchange and has the advantage of immediate availability and greater safety. However, patients receiving high-dose immunoglobulin need to be well hydrated and to have adequate renal function. (Plasmapheresis is discussed in Ch 14.) After 3 weeks of disease onset, plasma exchange and immunoglobulin therapies have little value. Corticosteroids appear to have little effect on the prognosis or duration of the disease.6
Nutritional therapy
Nutritional intake is compromised in the patient with Guillain-Barré syndrome. During the acute phase, the patient may experience difficulty swallowing because of cranial nerve involvement. Mild dysphagia can be managed by placing the patient in an upright position and flexing the head forwards during feeding. For more severe dysphagia, tube feedings may be required. Patients who experience paralytic ileus or intestinal obstruction may require total parenteral nutrition. Later in the course of the disease, motor paralysis or weakness continues to affect the ability to self-feed. The patient’s nutritional status, including body weight, serum albumin levels and energy intake, must be evaluated at regular intervals.
 NURSING MANAGEMENT: GUILLAIN-BARRÉ SYNDROME
NURSING MANAGEMENT: GUILLAIN-BARRÉ SYNDROME
 Nursing assessment
Nursing assessment
Assessment of the patient is the most important aspect of nursing care during the acute phase. The nurse must monitor the ascending paralysis; assess respiratory function; monitor arterial blood gases (ABGs); and assess the gag, corneal and swallowing reflexes during the routine assessment. Reflexes are usually decreased or absent.
Monitoring blood pressure and cardiac rate and rhythm is also important during the acute phase because transient cardiac arrhythmias have been reported. Autonomic dysfunction is common and usually takes the form of bradycardia and arrhythmias. Orthostatic hypotension secondary to muscle atony may occur in severe cases. Vasopressor agents and volume expanders may be needed to treat the low blood pressure. However, the presence of SIADH may require fluid restriction.
 Nursing diagnoses
Nursing diagnoses
Nursing diagnoses for the patient with Guillain-Barré syndrome may include, but are not limited to, the following:
• impaired spontaneous ventilation related to progression of the disease process resulting in respiratory muscle paralysis
• risk of aspiration related to dysphagia
• acute pain related to paraesthesias, muscle aches and cramps, and hyperaesthesias
• impaired verbal communication related to intubation or paralysis of the muscles of speech
• fear related to uncertain outcome and the seriousness of the disease
• self-care deficits related to inability to use muscles to accomplish activities of daily living (ADLs).
 Planning
Planning
The overall goals are that the patient with Guillain-Barré syndrome will: (1) maintain adequate ventilation; (2) be free from aspiration; (3) be pain-free or have pain controlled; (4) maintain an acceptable method of communication; (5) maintain adequate nutritional intake; and (6) return to usual physical functioning.
 Nursing implementation
Nursing implementation
The objective of therapy is to support body systems until the patient recovers. Respiratory failure and infection are serious threats. Monitoring the vital capacity and ABGs is essential. If the vital capacity drops to less than 800 mL (15 mL/kg or two-thirds of the patient’s normal vital capacity) or the ABGs deteriorate, endotracheal intubation or tracheostomy may be needed so that the patient can be mechanically ventilated (see Ch 67). Meticulous suctioning technique is needed to prevent infection whether the patient has an endotracheal tube or tracheostomy. Thorough bronchial hygiene and chest physiotherapy help clear secretions and prevent respiratory deterioration. If fever develops, sputum cultures should be obtained to identify the pathogen. Appropriate antibiotic therapy is then initiated.
A communication system must be established based on the patient’s abilities. This is extremely difficult if the disease progresses to involvement of the cranial nerves. At the peak of a severe episode, the patient may be incapable of communicating. The nurse must explain all procedures before doing them and reassure the patient that muscle function will return.
Urinary retention is common for a few days. Intermittent catheterisation is preferred to an indwelling catheter to avoid UTIs. However, for the acutely ill patient receiving a large volume of fluids (>2.5 L/day), indwelling catheterisation may be safer to reduce overdistension of a temporarily flaccid bladder and to prevent vesicoureteral reflux.
Physiotherapy is indicated early to help prevent problems related to immobility. Passive range-of-motion exercises and attention to body position help maintain function and prevent contractures. Patients who develop facial paralysis must receive meticulous eye care to avoid cornea irritation or damage (exposure keratitis). Artificial tears should be instilled frequently during the day to prevent drying of the cornea. The eyes should be inspected for the presence of eyelashes. Lubricating eye ointment and an impermeable eye shield can be used at night to retain moisture.
Nutritional needs must be met in spite of possible problems associated with delayed gastric emptying, paralytic ileus and potential for aspiration if the gag reflex is lost. In addition to checking for the gag reflex, the nurse should note drooling and other difficulties with secretions, which may be more indicative of an inadequate gag reflex. Initially, tube feedings or parenteral nutrition may be used to ensure adequate energy intake. Because of delayed gastric emptying, residual volumes of the feedings should be assessed at regular intervals or before feedings (see Ch 41). Fluid and electrolyte therapy must be monitored carefully to prevent electrolyte imbalances. A bowel program should be initiated because constipation is a common problem related to diet changes, immobility and decreased GI motility.
Throughout the course of the illness, the nurse needs to provide support and encouragement to the patient and family. Because residual problems and relapses are uncommon except in the chronic form of the disease, complete recovery can be anticipated, although it is generally a slow process that takes months or years if axonal degeneration occurs.
Botulism
AETIOLOGY AND PATHOPHYSIOLOGY
Botulism is the most serious type of food poisoning. It is caused by GI absorption of the neurotoxin produced by Clostridium botulinum. This organism is found in the soil and the spores are difficult to destroy. It can grow in any food contaminated with the spores. Improper home canning of foods is often the cause. Botulism is very rare: there was 1 reported case of botulism in Australia in 20077 and none since, and no reported cases in New Zealand. Botulism is a reportable disease in Australia; in New Zealand it is reported under the broad category of acute gastroenteritis, although this is currently under review as botulism is a serious condition and the implications of an outbreak can be severe.7,8 Botulism can be contracted through nasal inhalation, as well as oral ingestion. It is thought that the neurotoxin destroys or inhibits the neurotransmission of acetylcholine at the myoneural junction, resulting in disturbed muscle innervation. It has been highlighted as a potential bioterrorism agent, which is why it is included here.
CLINICAL MANIFESTATIONS
Symptoms are usually nausea, vomiting and abdominal cramps, generally within 6–48 hours after consumption of the contaminated food. Neurological manifestations develop rapidly over 2–4 days. They include difficulty in convergence of the eyes, photophobia, ptosis, paralysis of extraocular muscles, blurred vision, diplopia, dry mouth, sore throat and difficulty in swallowing. Other manifestations include paralytic ileus, mild muscle weakness, seizures and respiratory symptoms that can rapidly deteriorate to respiratory arrest and/or cardiac arrest. The course of the disease depends on the amount of toxin absorbed from the gut. If only a small amount is absorbed, symptoms are mild and recovery is complete. When large amounts are absorbed, death usually occurs in 4–8 days from circulatory failure, respiratory paralysis or the development of pulmonary complications.7
DIAGNOSTIC STUDIES AND MULTIDISCIPLINARY CARE
Blood and CSF are obtained for studies to rule out other diseases. In the patient with botulism, the blood and CSF results are normal.
The initial treatment of botulism is IV administration of botulinum antitoxin. Before administration of the antitoxin, an intradermal test dose is given for sensitivity to horse serum. If there are no reactions, the test dose is followed by daily doses of 50,000 units of botulinum antitoxin until improvement begins.
The GI tract is purged by laxatives, high colonic enemas and gastric lavage to decrease the absorption of the toxin. Activated charcoal is most effective if administered within 1 hour of ingestion.
 NURSING MANAGEMENT: BOTULISM
NURSING MANAGEMENT: BOTULISM
 Nursing implementation
Nursing implementation
Primary prevention is the goal of nursing management through educating consumers to be alert to situations that may result in botulism. Particular attention should be given to foods with a low acid content, which support germination and the production of botulin, a deadly poison. These foods include fish, vichyssoise and peppers. All varieties of spores are destroyed by boiling for 10 minutes or maintaining a temperature of 80°C for 30 minutes. Specific suggestions related to the preparation, storage and use of food include the following:
• In home canning, the equipment manufacturer’s directions should be followed. Only fresh fruits and vegetables (with all questionable spots removed) should be used. All containers and utensils must be cleansed and the seal on the can or jar must be airtight. Tinned foods should be stored properly in a cool, dry place.
• A can with a swollen end should never be used; the swelling may be caused by gases from C. botulinum.
• If the food is forcefully expelled when a container is opened, it should be discarded immediately and the contents should not be tasted.
• If the contents of a can look or smell bad after opening, the can should be discarded without tasting the contents. Materials may be flushed down the toilet or disposed of in the compost bin or garbage disposal if a large amount of water is used.
Nursing care during the acute illness is similar to that for Guillain-Barré syndrome. Supportive nursing interventions include rest, activities to maintain respiratory function, adequate nutrition and prevention of loss of muscle mass. Because the recovery process is slow, the patient may develop problems related to a feeling of helplessness, boredom and low morale.
Tetanus
AETIOLOGY AND PATHOPHYSIOLOGY
Tetanus (lockjaw) is an extremely severe polyradiculitis and polyneuritis affecting spinal and cranial nerves. It results from the effects of a potent neurotoxin released by the anaerobic bacillus Clostridium tetani. The toxin interferes with the function of the reflex arc by blocking inhibitory transmitters at the presynaptic sites in the spinal cord and brainstem. The spores of the bacillus are present in soil, garden mould and manure. Thus, C. tetani enters the body through a traumatic or suppurative wound that provides an appropriate low-oxygen environment for the organisms to mature and produce toxin. Other possible sources include dental infection, injections of heroin, human and animal bites, frostbite, compound fractures and gunshot wounds. The incubation period is usually 7 days but can range from 3 to 21 days, with symptoms frequently appearing after the original wound has healed.8 In general, the longer the incubation period, the milder the illness and the better the prognosis.
The number of cases worldwide per year is estimated to be 1 million, mainly in developing countries. In Australia, there were 120 hospitalisations in a 5-year period where tetanus was the principal diagnosis. In New Zealand, 8 cases of tetanus were reported in the same period. Of the reported cases, the majority of patients are over the age of 50 years. Mortality rates vary according to age, with infants and those over 50 most seriously affected. Overall mortality rates are declining and are at about 10% in both Australia and New Zealand.8
CLINICAL MANIFESTATIONS
Manifestations of generalised tetanus include a feeling of stiffness in the jaw (trismus) or neck, slight fever and other symptoms of general infection. Generalised tonic spasms occur because of the lack of reciprocal innervation. As the disease progresses, the neck muscles, back, abdomen and extremities become progressively rigid. In severe forms, continuous tonic convulsions may occur with opisthotonos (extreme arching of the back and retraction of the head). Laryngeal and respiratory spasms cause apnoea and anoxia. Additional effects are manifested by overstimulation of the sympathetic nervous system, including profuse diaphoresis, labile hypertension, episodic tachycardia, hyperthermia and arrhythmias. The slightest noise, jarring motion or bright light can set off the seizure. These seizures are agonisingly painful. Mortality is almost 100% in the severe form. Death is usually attributable to asphyxia or heart failure, the result of constantly recurring spasms. Residual injury, such as vertebral fracture, muscle contracture and brain damage secondary to hypoxia, may be long-term consequences.
MULTIDISCIPLINARY CARE
Serum electrolyte levels, FBC, albumin level, clotting factors, glucose level and ABGs are monitored. Cardiac function is monitored by electrocardiogram (ECG) and auscultation. As increasing numbers of nerve cells become involved, their inhibitory control over muscle activity decreases and symptoms develop.
Drug therapy
The management of tetanus includes administration of tetanus toxoid booster (Td) and tetanus immune globulin (TIG) before the onset of symptoms to neutralise circulating toxins (see Table 68-5). Control of spasms is essential and is managed by deep sedation, usually with diazepam, barbiturates or chlorpromazine. Chlorpromazine is also helpful in reducing hyperthermia. A 10-day course of penicillin is recommended to inhibit further growth of the organism.
Because of laryngospasm, a tracheostomy is usually performed early and the patient is maintained on mechanical ventilation. If sedation does not control seizures, skeletal muscle paralysing drugs, such as pancuronium, are used. Pain is relieved by means of codeine or pethidine, often with the addition of promethazine. Any recognised wound should be debrided or an abscess drained. Antibiotics may be given to prevent secondary infections.
Nutrition is maintained through parenteral nutrition or nasogastric feeding. The mortality rate associated with tetanus is declining. However, for those who recover there is a long convalescence, which includes extensive physiotherapy.
 NURSING MANAGEMENT: TETANUS
NURSING MANAGEMENT: TETANUS
 Nursing implementation
Nursing implementation
Health teaching is aimed at ensuring tetanus prophylaxis, which is the most important factor influencing the incidence of this disease. Tetanus prevention and immunisation protocols are summarised in Table 68-5. The patient should be taught that immediate, thorough cleansing of all wounds with soap and water is important in the prevention of tetanus. If an open wound occurs and the patient has not been immunised within 10 years, they are advised that they need to have a tetanus booster as soon as possible.
If equine tetanus antitoxin is to be used, the patient should be tested for sensitivity. Administration of equine antitoxin is not recommended if sensitivity occurs; anaphylactic shock is potentially life-threatening, and desensitisation is ineffective. The side effects of routine administration of the antitoxin are mild and include a sore arm, swelling at the site and itching. Serious side effects rarely occur. Routine administration of a booster shot to an adequately immunised patient can cause arm swelling and lymphadenopathy.
Every patient should receive a written record of immunisations and be encouraged to complete the active immunisation schedule. The patient’s immunisation history should be recorded accurately.
The acute nursing management of the patient with tetanus is aimed at supportive care based on the treatment of clinical manifestations. The patient should be placed in a quiet, darkened room that is insulated against noise. Judicious sedation should be given. Nursing care should be administered with the utmost caution to avoid triggering spasms. For example, the nurse should avoid unnecessary touching, use firm touching when necessary, avoid the use of linen to cover the patient and maintain a slightly higher than normal ambient temperature. Nursing care related to tracheostomy and mechanical ventilation is given as appropriate. An indwelling urinary catheter may be used to prevent bladder distension and urinary reflux in the presence of spasms in the muscles of the pelvic floor. Attention is also given to skin care. The patient needs emotional support during the acute phase because the fear of death is real. The family also needs support and education.
Neurosyphilis
Neurosyphilis (tertiary syphilis) is an infection of any part of the nervous system by the organism Treponema pallidum. It is the result of untreated or inadequately treated syphilis (see Ch 52). The organism can invade the central nervous system within a few months of the original infection. Except for causing some changes in the CSF, including increased WBCs and protein and positive serological reaction, the organism lies dormant for years. Untreated neurosyphilis, although not contagious, can be fatal.9 Penicillin therapy is effective for syphilitic meningitis but the neurological deficits remain.
Late neurosyphilis results from degenerative changes in the spinal cord (tabes dorsalis) and brainstem (general paresis). Tabes dorsalis (progressive locomotor ataxia) is characterised by vague sharp pains in the legs, ataxia, ‘slapping’ gait, loss of proprioception and deep tendon reflexes, and zones of hyperaesthesia. Charcot’s joints, which are characterised by enlargement, bone destruction and hypermobility, also occur as a result of joint effusion and oedema. Other manifestations include seizures and vision and hearing problems.
Neurological symptoms associated with neurosyphilis are numerous and in many cases non-specific.8 Neurosyphilis is a differential diagnosis for patients with neurological and psychiatric symptoms. Dementia paralytica is an ongoing spirochetal meningoencephalitis that causes a general dissolution of mental and physical capabilities. It may mimic a number of major or minor psychoses. Management includes treatment with penicillin, symptomatic care and protection from physical injury.
Spinal cord trauma
Before World War II, the life expectancy for the person with a spinal cord injury ranged from months to 10 years from the onset of injury. The leading causes of death were renal failure, respiratory failure, pressure sores or sepsis generated from these. Today, with improved treatment strategies even the very young patient with a spinal cord injury can anticipate a long life. The prognosis for life is generally only about 5 years less than for persons of the same age without spinal cord injury.10 The cause of premature death in the patient with tetraplegia (paralysis of both arms and legs), which was formerly called quadriplegia, is usually related to compromised respiratory function.
The potential for disruption of individual growth and development, altered family dynamics, economic loss in terms of absence from work, and the high cost of rehabilitation and long-term healthcare make spinal cord trauma a major consideration for prevention programs. More than 350 cases of traumatic spinal cord injury are reported in Australia each year,9 and around 75 per year are reported in New Zealand.11
The costs of spinal cord injury care can be high. Although many people with spinal cord injuries can care for themselves independently, those with the highest level of injury may require round-the-clock care at home or in a long-term care facility. Today, almost 90% of patients with spinal cord injury are discharged from the hospital to home or another non-institutionalised residence.9 The remaining 10% are discharged to nursing homes, chronic care facilities or group homes.
AETIOLOGY AND PATHOPHYSIOLOGY
The segment of the population with the greatest risk of spinal cord injury is young adult men between the ages of 16 and 30 years, with the majority of cases in all age groups being male (except those caused as a result of domestic injury and medical illness in New Zealand). The most common age at injury is 19 years.10
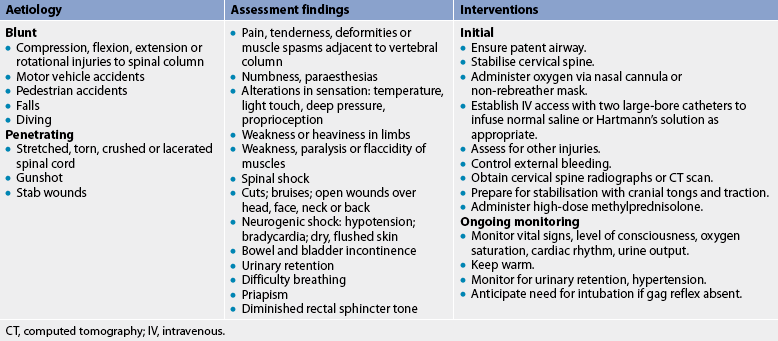
Causes of spinal cord injury include many types of trauma. In Australia, road trauma (which includes accidents involving motor vehicles, motor cycles, bicycles and pedestrians) accounts for 46% of spinal cord injuries; falls, 28%; diving and water-related incidents, 15%; sports injuries, 10%; and other miscellaneous causes, 9%.10 In New Zealand, the data are grouped slightly differently, although causes are similar, with 37% (female)/39% (male) of injuries related to motor vehicle accidents; 18% (female)/26% (male) related to sport and recreation; 10% (female)/5% (male) related to domestic causes; 5% (female)/17% (male) related to work-related injuries; 20% (female)/8% (male) related to medical illnesses; and 10% (female)/5% (male) related to ‘other’ causes.11
There has been an increase in the number of older adults with spinal cord injuries. The proportion of people who were at least 61 years of age when injured has increased from 4.7% of patients with spinal cord injury in the 1970s to 10% currently. Besides having greater mortality, older adults with traumatic injuries experience more complications than younger ones and are hospitalised longer. This trend towards older age at the time of injury explains the overall increase in the mean age of people with spinal cord injury from 28 years in the 1970s to 33 years in 2006.10
Spinal cord injury can be caused by cord compression by bone displacement, interruption to the blood supply to the cord or traction as a result of pulling on the cord. The spinal cord is wrapped in tough layers of dura and is rarely torn or transected by direct trauma. Penetrating trauma, such as gunshot and stab wounds, can result in tearing and transection. The initial mechanical disruption of axons as a result of stretch or laceration is referred to as the primary injury. Secondary injury refers to the ongoing, progressive damage that occurs after the initial injury.12
There are several theories about what causes this ongoing damage at the molecular and cellular levels. These include free radical formation, uncontrolled calcium influx, ischaemia and lipid peroxidation. At the molecular level, apoptosis (cell death) occurs and may continue sometimes for weeks or months after the initial injury. Thus the complete cord damage (previously thought to be transection) in severe trauma is related to autodestruction of the cord. This is confirmed by observations shortly after the injury, when petechial haemorrhages are noted in the central grey matter of the cord. Haemorrhagic areas in the centre of the spinal cord appear within 1 hour and by 4 hours there may be infarction in the grey matter.12 This ongoing, destructive process makes it critical that the initial care and management of the patient with a spinal cord injury limit further activation of these processes.
Figure 60-4 illustrates the cascade of events causing secondary injury following traumatic spinal cord injury. The resulting hypoxia reduces the oxygen tension below the level that meets the metabolic needs of the spinal cord. Lactate metabolites and an increase in vasoactive substances, including noradrenaline, serotonin and dopamine, are noted. At high levels, these vasoactive substances cause vasospasms and hypoxia, leading to subsequent necrosis. Unfortunately, the spinal cord has minimal ability to adapt to vasospasm.
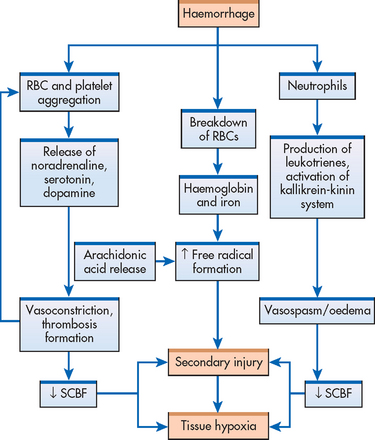
Figure 60-4 Cascade of metabolic and cellular events that leads to spinal cord ischaemia and hypoxia of secondary injury. RBC, red blood cell; SCBF, spinal cord blood flow.
By 24 hours or less, permanent damage may occur because of the development of oedema. Oedema, secondary to the inflammatory response, is particularly harmful because of the lack of space for tissue expansion. The resultant compression of the cord and the extension of oedema above and below the injury will increase the ischaemic damage.
The extent of the neurological damage caused by a spinal cord injury results from primary injury damage (actual physical disruption of axons) and secondary injury damage (ischaemia, hypoxia, microhaemorrhage and oedema). Because secondary injury processes occur over time, the extent of injury and prognosis for recovery are most accurately determined at 72 hours or more after injury.12
Spinal and neurogenic shock
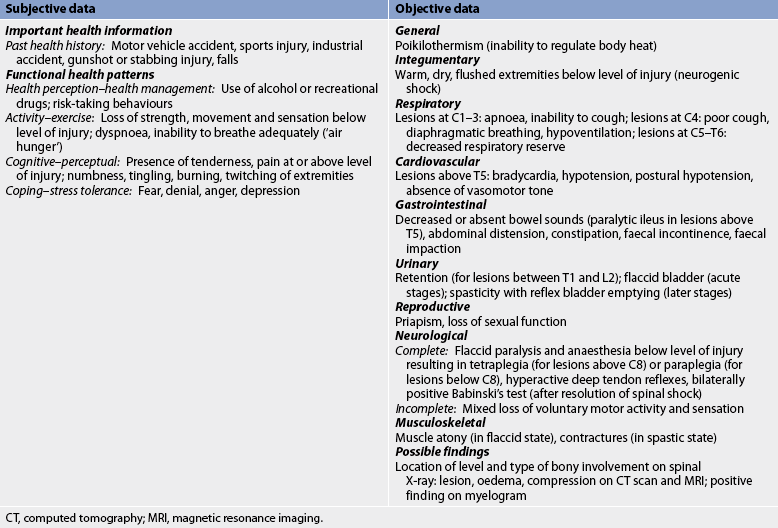
About 50% of people with acute spinal cord injury experience spinal shock, a temporary neurological syndrome that is characterised by decreased reflexes, loss of sensation and flaccid paralysis below the level of the injury.13 This syndrome lasts days to months and may mask post-injury neurological function. Active rehabilitation may begin in the presence of spinal shock.
Neurogenic shock, in contrast, is due to the loss of vasomotor tone caused by injury and is characterised by hypotension, bradycardia and warm, dry extremities. Loss of sympathetic innervation causes peripheral vasodilation, venous pooling and a decreased cardiac output. These effects are generally associated with a cervical or high thoracic injury.
Classification of spinal cord injury
Spinal cord injuries are classified by the mechanism of injury, skeletal and neurological level of injury, and completeness or degree of injury.
Mechanisms of injury
The major mechanisms of injury are flexion, hyperextension, rotation (usually combined with flexion injury and occasionally extension) and compression (see Fig 60-5). The flexion–rotation injury is the most unstable of all injuries because the ligamentous structures that stabilise the spine are torn.13 This injury is most often implicated in severe neurological deficits.
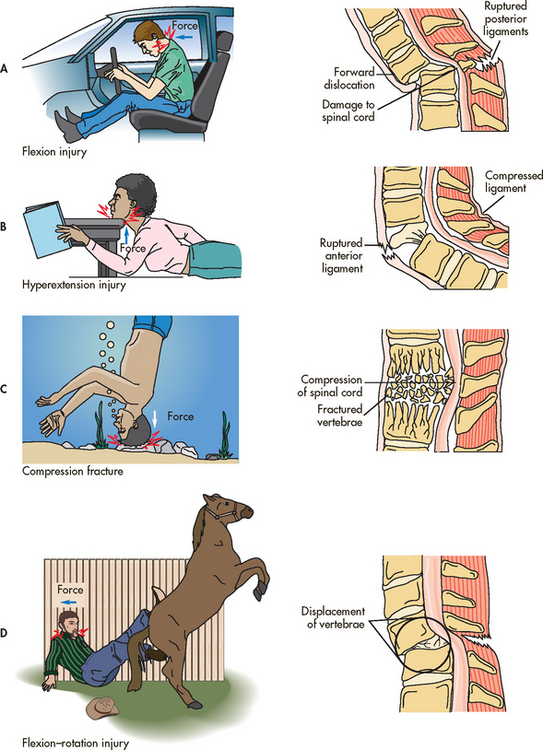
Figure 60-5 Mechanisms of spinal cord injury. Many situations may produce these injuries. This shows only some examples. A, Flexion injury of the cervical spine ruptures the posterior ligaments. B, Hyperextension injury of the cervical spine ruptures the anterior ligaments. C, Compression fractures crush the vertebrae and force bony fragments into the spinal canal. D, Flexion–rotation injury of the cervical spine often results in tearing of ligamentous structures that normally stabilise the spine.
Source: Copstead LC, Banasik JL. Pathophysiology. 3rd edn. Philadelphia: Saunders; 2005.
Level of injury
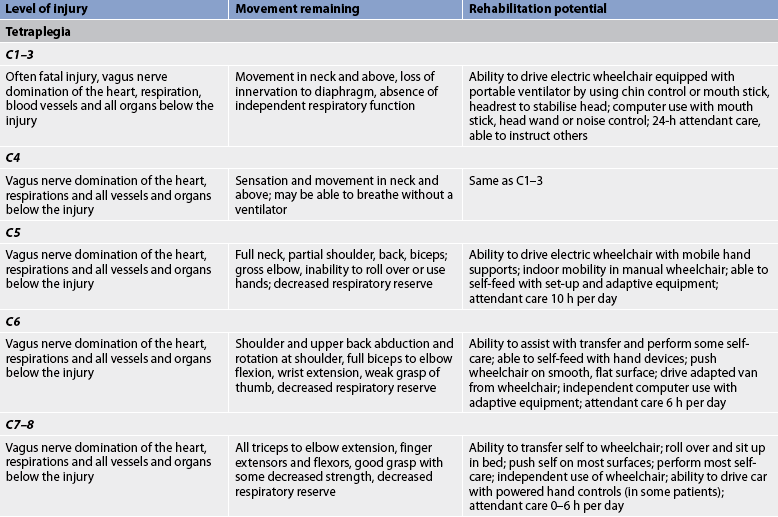
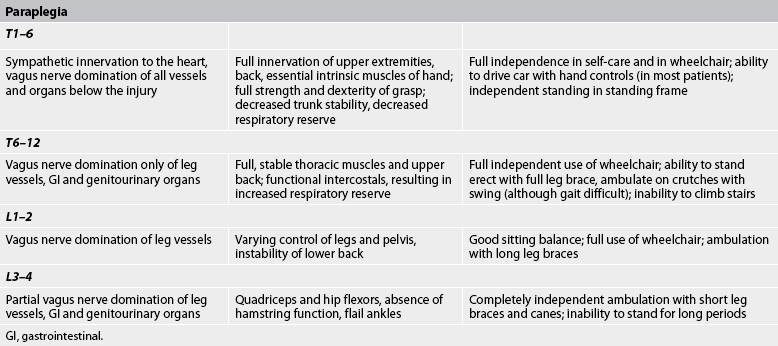
The skeletal level of injury is the vertebral level where there is the most damage to vertebral bones and ligaments. The neurological level is the lowest segment of the spinal cord with normal sensory and motor function on both sides of the body. The level of injury may be cervical, thoracic or lumbar. Cervical and lumbar injuries are the most common because these levels are associated with the greatest flexibility and movement. If the cervical cord is involved, paralysis of all four extremities occurs, resulting in what is called tetraplegia. However, even with a cervical injury the arms are rarely completely paralysed; the focus of functional deficit is in the hands. If the thoracic or lumbar cord is damaged, the result is paraplegia (paralysis and loss of sensation in the legs). Figure 60-6 shows affected structures and functions at different levels of cord injury.
Degree of injury
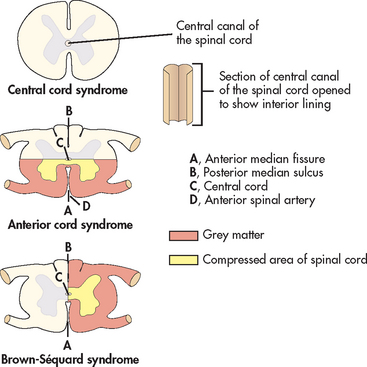
The degree of spinal cord involvement may be either complete or incomplete (partial). Complete cord involvement results in total loss of sensory and motor function below the level of the lesion (injury). Incomplete cord involvement results in a mixed loss of voluntary motor activity and sensation and leaves some tracts intact. The degree of sensory and motor loss varies depending on the level of the lesion and reflects the specific nerve tracts damaged and those spared. Six syndromes are associated with incomplete lesions: central cord syndrome, anterior cord syndrome, Brown-Séquard syndrome, posterior cord syndrome, cauda equina syndrome and conus medullaris syndrome.
• Central cord syndrome. Damage to the central spinal cord is termed central cord syndrome (see Fig 60-7). It occurs most commonly in the cervical cord region and is more common in older adults, as a result of a hyperextension injury. Motor weakness and sensory loss are more pronounced in the upper extremities with minimal effects in the trunk and lower limbs.
• Anterior cord syndrome. Anterior cord syndrome is caused by damage to the anterior spinal artery. It typically results from injury causing acute compression of the anterior portion of the spinal cord, often a flexion injury (see Fig 60-7), or disruption to the blood supply of the anterior spinal artery. Manifestations include motor paralysis and loss of pain and temperature sensation below the level of injury. Because the posterior cord tracts are not injured, sensations of light touch, position, vibration and proprioception remain intact.
• Brown-Séquard syndrome. Brown-Séquard syndrome is a result of damage to one half of the spinal cord (see Fig 60-7). This syndrome is characterised by a loss of motor function and position and vibratory sense, as well as vasomotor paralysis on the same side (ipsilateral) as the lesion. The opposite (contralateral) side has loss of pain and temperature sensation below the level of the lesion.
• Posterior cord syndrome. Posterior cord syndrome results from compression or damage to the posterior spinal artery; it is a very rare condition. Generally, the dorsal columns are damaged, resulting in loss of proprioception. However, pain, temperature sensation and motor function below the level of the lesion remain intact.
• Conus medullaris syndrome and cauda equina syndrome. Conus medullaris syndrome and cauda equina syndrome result from damage to the very lowest portion of the spinal cord (conus) and the lumbar and sacral nerve roots (cauda equina). Injury to these areas produces flaccid paralysis of the lower limbs and areflexic (flaccid) bladder and bowel.
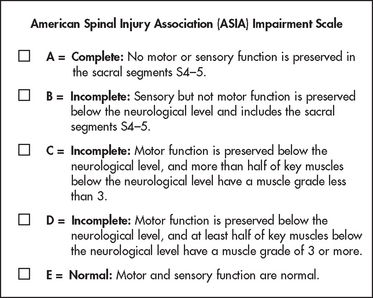
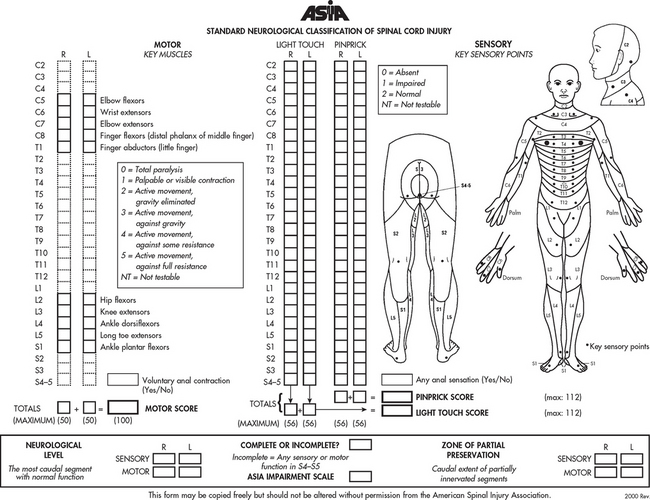
The American Spinal Injury Association (ASIA) impairment scale is commonly used in Australia and New Zealand for classifying the severity of impairment resulting from spinal cord injury. It combines assessments of motor and sensory function to determine neurological level and completeness of injury (see Figs 60-8 and 60-9). This scale is useful for recording changes in neurological status and identifying appropriate functional goals for rehabilitation.14
CLINICAL MANIFESTATIONS
The manifestations of spinal cord injury are generally the direct result of trauma that causes cord compression, ischaemia, oedema and possible cord transection. Manifestations of spinal cord injury are related to the level and degree of injury. The patient with an incomplete lesion may demonstrate a mixture of symptoms. The higher the injury, the more serious the sequelae because of the proximity of the cervical cord to the medulla and brainstem. Movement and rehabilitation potential related to specific locations of the spinal cord injury are described in Table 60-2. In general, sensory function closely parallels motor function at all levels.
Immediate post-injury problems include maintaining a patent airway, adequate ventilation and adequate circulating blood volume, and preventing extension of cord damage (secondary damage).
Respiratory system
Respiratory complications closely correspond to the level of the injury.14 Cervical injury above the level of C4 presents special problems because of the total loss of respiratory muscle function. Mechanical ventilation is required to keep the patient alive. At one time most of these patients died at the scene of the injury but with improved emergency medical services, more of these patients are surviving the initial events of their spinal cord injury. Injury or fracture below the level of C4 results in diaphragmatic breathing, with partial or full phrenic nerve functioning. Even if the injury is below C4, spinal cord oedema and haemorrhage can affect the function of the phrenic nerve and cause respiratory insufficiency.15 Hypoventilation almost always occurs with diaphragmatic respirations because of the decrease in vital capacity and tidal volume, which occurs as a result of impairment of the intercostal muscles.
Cervical and thoracic injuries cause a paralysis of abdominal musculature and often intercostal musculature. This means that the patient cannot cough effectively enough to remove secretions, leading to atelectasis and pneumonia. An artificial airway provides direct access for pathogens, making bronchial hygiene and chest physiotherapy extremely important to reduce infection. Neurogenic pulmonary oedema may occur, secondary to a dramatic increase in sympathetic nervous system activity at the time of injury, which shunts blood to the lungs. In addition, pulmonary oedema may occur in response to fluid overload.
Cardiovascular system
Any cord injury above the level of T6 greatly decreases the influence of the sympathetic nervous system and as a result bradycardia occurs. Peripheral vasodilation results in hypotension. A relative hypovolaemia exists because of the increase in venous capacitance. Cardiac monitoring is necessary. In marked bradycardia (heart rate <40 beats/min), appropriate drugs (atropine) are necessary to increase the heart rate and prevent hypoxaemia.13 The peripheral vasodilation reduces the venous return of blood to the heart and subsequently decreases cardiac output, resulting in hypotension. IV fluids or vasopressor drugs may be required to support blood pressure.
Urinary system
Urinary retention is a common development in acute spinal cord injuries and spinal shock. While the patient is in spinal shock, the bladder is atonic and becomes overdistended. An indwelling catheter is inserted to drain the bladder. In the post-acute phase, the bladder may become hyperirritable, with a loss of inhibition from the brain resulting in reflex emptying. Chronic indwelling catheterisation increases the risk of infection. Once the patient is medically stable and large quantities of IV fluids are no longer required, the indwelling catheter should be removed and intermittent catheterisation should begin as early as possible. This helps to maintain bladder tone and decrease risk of infection. (Intermittent catheterisation is discussed in Ch 45.) Alternatively, placement of a suprapubic catheter may be considered, particularly for females with limited options long term.
Gastrointestinal system
If the cord injury has occurred above the level of T5, the primary GI problems are related to hypomotility. Decreased GI motor activity contributes to the development of paralytic ileus and gastric distension. A nasogastric tube for intermittent suctioning may relieve the gastric distension. Metoclopramide may be used to treat delayed gastric emptying. The development of stress ulcers is common because of excessive release of hydrochloric acid in the stomach. Histamine H2-receptor antagonists, such as ranitidine and famotidine, and proton pump inhibitors (e.g. pantoprazole, omeprazole or lansoprazole) are frequently used to prevent the occurrence of stress ulceration during the initial phase. Intraabdominal bleeding may occur and is difficult to diagnose because no subjective signs, such as pain, tenderness and guarding, are observed. Continued hypotension in spite of vigorous treatment and decreased haemoglobin and haematocrit may be indications of bleeding. Expanding girth of the abdomen may also be noted.
Less voluntary neurological control over the bowel results in a neurogenic bowel. In the early period after injury when spinal shock is present, and for patients with an injury level of T12 or below, the bowel is areflexic and sphincter tone is decreased. As reflexes return, the bowel becomes reflexic, sphincter tone is enhanced and reflex emptying occurs. Both types of neurogenic bowel can be managed successfully with a regular bowel program coordinated with the gastrocolic reflex to minimise untimely accidents.
Integumentary system
A major consequence of lack of movement is the potential for skin breakdown over bony prominences in areas of decreased or absent sensation. Pressure ulcers can occur quickly and can lead to major infection or sepsis, an unnecessary complication and setback for the trauma patient.
Thermoregulation
Poikilothermism is inability to adjust the body temperature to the environmental temperature. This occurs because the injury interrupts the sympathetic nervous system, preventing peripheral temperature sensations from reaching the hypothalamus. Spinal cord disruption also decreases the ability to sweat or shiver below the level of the lesion, which also affects body temperature regulation. The degree of poikilothermism depends on the level of injury. Those with high cervical injuries have a greater loss of the ability to regulate temperature than do those with thoracic or lumbar injuries.
Metabolic needs
Nasogastric suctioning may lead to metabolic alkalosis, and decreased tissue perfusion may lead to acidosis. Electrolyte levels, including sodium and potassium, can be altered by gastric suctioning and must be monitored until suctioning is discontinued and a normal diet is resumed. Loss of body weight (10% or more) is common, with nitrogen excretion mirroring weight loss.13 Nutritional needs are much greater than would be expected for an immobilised person. A positive nitrogen balance and a high-protein diet help to prevent skin breakdown and infections and decrease the rate of muscle atrophy. (Nutrition is discussed in Ch 39.)
Peripheral vascular problems
Deep vein thrombosis (DVT) is a common problem accompanying spinal cord injury during the first 3 months. It is more difficult to detect DVT in a person with a spinal cord injury because the usual signs and symptoms, such as pain and tenderness, will not be present.15 Pulmonary embolism is one of the leading causes of death in patients with spinal cord injury. Techniques for assessment of DVT include Doppler examination, impedance plethysmography, and measurement of leg and thigh girth. Preventative measures, such as prophylactic anticoagulation therapy, combined with interventions such as calf muscle stimulators, antiembolic stockings and passive limb movements, are widely accepted as best practice.
DIAGNOSTIC STUDIES
Once the patient is immobilised, diagnostic studies can be done. Complete spine films are performed to assess for vertebral fracture. X-rays, including visualisation of C1–T1, are done to document the presence of vertebral injury. A CT scan may be used to assess the stability of the injury, the location and degree of bony injury, soft and neural tissue changes, and the degree of spinal canal compromise.13 MRI is used in cases where there is unexplained neurological deficit or worsening of neurological status. A comprehensive neurological examination is performed along with assessment of the head, chest and abdomen for additional injuries or trauma. Patients with cervical injuries who demonstrate altered mental status may also need vertebral angiography to rule out vertebral artery damage.
MULTIDISCIPLINARY CARE
The initial goals for the patient with a spinal cord injury are to sustain life and prevent further cord damage. Table 60-3 outlines the emergency management of the patient with a spinal cord injury. Systemic and neurogenic shock must be treated to maintain blood pressure. For injury at the cervical level, all body systems must be maintained until the full extent of the damage can be evaluated. Multidisciplinary care during the acute phase for a patient with a cervical cord injury is described in Box 60-2.
MULTIDISCIPLINARY CARE
Diagnostic studies
Collaborative therapy
Acute care
Immobilisation of vertebral column by skeletal traction
Maintenance of heart rate (e.g. atropine) and blood pressure (e.g. dopamine)
Methylprednisolone high-dose therapy
Insertion of nasogastric tube and attachment to suction
Intubation (if indicated by ABGs and PFT)
The systemic support required by the patient is less intense for spinal cord injuries of the lower thoracic and lumbar vertebrae. Respiratory compromise is not as severe and bradycardia is not a problem. Specific problems are treated symptomatically. After stabilisation at the accident scene, the person is transferred to a medical facility. A thorough assessment is done to evaluate specifically the degree of deficit and to establish the level and degree of injury. A history is obtained, with emphasis on how the accident occurred and the extent of injury as perceived by the patient immediately after the accident. Assessment involves testing muscle groups rather than individual muscles. Muscle groups should be tested with and against gravity, alone and against resistance, and on both sides of the body. Spontaneous movement should be noted. The patient should be asked to move the legs and then the hands, spread the fingers, extend the wrists and shrug the shoulders. After assessment of motor status, a sensory examination, including touch and pain as tested by pinprick, should be carried out, starting at the toes and working upwards. If time and conditions permit, position sense and vibration can also be assessed.
The types of accidents that cause spinal cord trauma may also result in brain injury. The patient should therefore be assessed for a history of unconsciousness, and signs of concussion and increased intracranial pressure (see Ch 56). In addition, a careful assessment for musculoskeletal injuries and trauma to internal organs should be performed. Because there are no muscle, bone or visceral sensations, the only clue to internal trauma with haemorrhage may be a rapidly falling haematocrit level. Urinary output is examined for haematuria, which is also indicative of internal injuries.
The patient must be moved in alignment as a unit or moved ‘as a log’ during transfers and when repositioning to prevent further injury. Respiratory, cardiac, urinary and GI functions should be monitored closely. The patient may go directly to surgery following initial immobilisation and stabilisation or to the intensive care unit (ICU) for monitoring and management.
Non-operative stabilisation
Non-operative treatments are focused on stabilisation of the injured spinal segment and decompression, either through traction or through realignment. Stabilisation methods eliminate damaging motion at the injury site. They are intended to prevent secondary spinal cord damage caused by repeated contusion or compression.15 However, these techniques are rarely used, unless consideration to rest in bed is necessary for other injuries such as a fractured pelvis.
Surgical therapy
The decision to perform surgery on a patient with a spinal cord injury often depends on the preference of the treating doctor. When cord compression is certain or the neurological disorder progresses, benefit may be seen following immediate surgery. Surgery stabilises the spinal column. There is some evidence to suggest that early cord decompression results in reduced secondary injury to the spinal cord and therefore improved outcomes.15 Other criteria used in the decision for early surgery include: (1) evidence of cord compression; (2) progressive neurological deficit; (3) compound fracture of the vertebrae; (4) bony fragments (may dislodge and penetrate the cord); and (5) penetrating wounds of the spinal cord or surrounding structures.
The more common surgical procedure is decompression laminectomy by anterior or posterior cervical and posterior thoracic approaches. Surgical instrumentation using acrylic wire mesh, insertion of stabilising rods, surgical plates and donor iliac crest bone grafts are also used to stabilise the vertebral fractures. (Specific surgical and nursing interventions for these techniques are discussed in Ch 62.)
Drug therapy
The National Acute Spinal Cord Injury Study II (NASCIS II, 1990) and NASCIS III (1997) showed that methylprednisolone, when administered early and in a large dose, resulted in greater recovery of neurological function.16,17 Based on these studies, methylprednisolone was adopted as a standard of care for acute spinal cord injury management. However, the use of methylprednisolone has been a hotly contested issue in the literature for more than 10 years. Many authors have criticised the NASCIS studies, specifically the study design and the way the statistical analyses was conducted. In addition to the problems of study design, the use of methylprednisolone in spinal cord injury has been debated due to its adverse effects.18,19 While patients enrolled in NASCIS II had no differences in wound infection and gastrointestinal bleeding, patients in NASCIS III had higher rates of pneumonia and more severe sepsis. Mortality rates were similar in both studies.16,17 Higher rates of respiratory tract infection, sepsis, urinary tract infection and wound site infection have also been observed with high-dose methylprednisolone. Moreover, blood glucose levels measured every 6 hours and more frequently in some studies were found to be higher among those receiving methylprednisolone.18 One study found that patients who received methylprednisolone had significantly longer intensive care unit stays and longer hospitalisation.19
NASCIS III also determined that tirilazad mesylate, a potent lipid peroxidation inhibitor, administered for 48 hours post-injury provided motor recovery rates equivalent to those with methylprednisolone. There was an indication of fewer adverse effects in comparison with 48-hour treatment with methylprednisolone.17 Other neuroprotective drugs are being tested and more treatment options may be available soon.19
Until then, methylprednisolone remains a treatment option.17,18 The treating doctor needs to consider whether the potential benefits outweigh the risks. If ordered, methylprednisolone is to be given within 8 hours of injury. When the loading dose of 30 mg/kg is given within 3 hours of injury, this is followed by 24 hours of 5.4 mg/kg IV methylprednisolone drip. If this loading dose is given between 3 and 8 hours post-injury, the IV drip is maintained for 48 hours. There is no reported benefit from methylprednisolone if it is given more than 8 hours post-injury.17 Methylprednisolone, a blocker of lipid peroxidation by-products, is thought to improve blood flow and reduce oedema in the spinal cord. Side effects of methylprednisolone include immunosuppression, increased frequency of upper GI bleeding and increased risk of infection.
Vasopressor agents such as dopamine are employed in the acute phase as adjuvants to treatment. These agents are used to maintain the mean arterial pressure at a level greater than 80–90 mmHg (11–12.5 kPa) so that perfusion to the spinal cord is improved.
Pharmacological properties and drug metabolism are altered in spinal cord injury and so drug interactions may occur. The differences in drug metabolism correlate with the level and completeness of injury, with greater change apparent in people with cervical cord injury than in those with injury of lower spinal levels.19
Pharmacological agents are used to treat specific autonomic dysfunctions, such as GI hypoactivity, bradycardia, orthostatic hypotension, inadequate emptying of the bladder and autonomic dysreflexia. The nurse must know the intended effects of such agents, observe responses and provide specific interventions when adverse reactions are seen.
 NURSING MANAGEMENT: SPINAL CORD TRAUMA
NURSING MANAGEMENT: SPINAL CORD TRAUMA
 Nursing assessment
Nursing assessment
Subjective and objective data that should be obtained from the patient with a recent spinal cord injury are presented in Table 60-4.
 Nursing diagnoses
Nursing diagnoses
Nursing diagnoses for the patient with a spinal cord injury depend on the severity of the injury and the level of dysfunction. The nursing diagnoses for a patient with a spinal cord injury may include, but are not limited to, those presented in NCP 60-1. The care plan presented is for a patient with a complete cervical cord injury.
 Planning
Planning
The overall goals are that the patient with a spinal cord injury will: (1) maintain an optimal level of neurological functioning; (2) have minimal or no complications of immobility; (3) learn new skills, gain new knowledge and acquire new behaviours to be able to care for self or successfully direct others to do so; and (4) return to home and the community at an optimal level of functioning.
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
Nursing interventions for injury prevention include identification of risk populations, counselling and education. Education about the use of seat belts in cars, the wearing of helmets by motorcyclists and cyclists, the use of child safety seats and not drink-driving is a professional responsibility.
Roles of nursing in health promotion as it applies to spinal cord injury include injury prevention, counselling and education of people with spinal cord injury regarding health behaviours (e.g. smoking, substance abuse, diet, exercise) and ensuring that ongoing healthcare after hospital discharge includes appropriate general health screening and health promotion, as well as spinal cord injury care.
 Acute intervention
Acute intervention
High cervical cord injury caused by flexion–rotation is the most complex spinal cord injury and is discussed in this section. Interventions for this type of injury can be modified for patients with less severe problems.
 Immobilisation
Immobilisation
Proper immobilisation of the neck involves the maintenance of a neutral or slight extension position.20 Sandbags, hard cervical collars and backboards can be used to stabilise the neck to prevent lateral rotation of the cervical spine. The body should always be correctly aligned and turning should be performed so that the patient is moved as a unit (e.g. logrolling) to prevent movement of the spine. For cervical cord injuries, skeletal traction is sometimes provided by Crutchfield (see Fig 60-10), Vinke or Gardner-Wells tongs or other types of skull tongs. Traction is provided by a rope that is extended from the centre of the tongs over a pulley and has weights attached at the end. Traction must be maintained at all times. One disadvantage of skull tongs is that the skull pins can be displaced. If this occurs, the head should be held in a neutral or extended position and help should be summoned. Sandbags can be positioned to stabilise the head while the doctor reinserts the tongs.
Infection at the sites of tong insertion is another potential problem. Preventative care includes cleansing the sites twice a day with normal saline solution and applying an antibiotic ointment, which acts as a mechanical barrier to the entrance of bacteria. The preventative care of insertion sites may vary depending on individual hospitals’ standards of care.
Special beds are often used for patients with spinal cord injuries. Kinetic therapy uses a continual side-to-side slow rotation 62° laterally with the patient in constant motion. The bed allows a frequency of turns greater than 200 times per day. The bed is used to decrease the likelihood of pressure sores and cardiopulmonary complications. Other turning beds place the patient in a side-lying position, which is useful in preventing pressure and urinary stasis, aids chest drainage and provides a different outlook for the rest-in-bed patient. However, in some patients the turning can induce motion sickness and fear of falling out of bed when turned to the extremes. (Motion sickness is unlikely with automatic rather than manual turning.)
Depending on the type of injury and therapeutic interventions, different methods of immobilisation and stabilisation of the vertebral column may be used. In a stable injury for which surgery is not done, halo traction may be applied. The application of a collar brace or halo traction device allows the patient to be more mobile and to begin active rehabilitation. The halo apparatus applies cervical traction by means of a jacket-like arrangement that allows greater mobility and wheelchair activity than other traction systems. After cervical fusion or other stabilisation surgery, a Philadelphia, Aspen collar or sternal–occipital–mandibular immobiliser (SOMI) brace is worn until the fusion becomes solid (see Fig 60-11).20 Patients with thoracic or lumbar spine injuries are immobilised with a custom-built thoracolumbar orthosis (‘body jacket’), which controls spinal flexion, extension and rotation, or with a Jewett brace, which restricts forward flexion.
Immobilisation of the neck of the patient with a spinal cord injury prevents further injury but the effects of immobility are profound. Meticulous skin care is critical because decreased sensation and circulation make the patient particularly susceptible to skin breakdown. Patients should be removed from backboards as soon as possible and cervical collars should be properly fitted or replaced with other forms of immobilisation to prevent coccygeal and occipital area skin breakdown. It is important that areas under the halo vest (see Fig 60-12) or jacket or under braces or orthoses be inspected regularly to assess skin condition.
 Respiratory dysfunction
Respiratory dysfunction
During the first 48 hours after injury, spinal cord oedema may increase the level of dysfunction and respiratory distress may occur. If the injury is at or above C3 level, or if the patient is exhausted from the effort of breathing or the ABGs deteriorate (indicating inadequate oxygenation or ventilation), endotracheal intubation or tracheostomy and mechanical ventilation should be initiated. Respiratory arrest is a possibility that requires careful monitoring of the respiratory system and prompt action, should it occur. Pneumonia and atelectasis are potential problems because of reduced vital capacity and the loss of intercostal and abdominal muscle function, resulting in diaphragmatic breathing, pooled secretions and an ineffective cough.13 The older adult has a more difficult time responding to hypoxia and hypercapnia and is extremely intolerant of hypoxia caused by lack of reserve. Therefore, aggressive chest physiotherapy, adequate oxygenation and proper pain management are essential to maximise respiratory function and gas exchange. Other problems include nasal stuffiness and bronchospasms.
The nurse needs to regularly assess: (1) breath sounds; (2) ABGs; (3) tidal volume; (4) vital capacity; (5) skin colour; (6) breathing patterns (especially the use of accessory muscles); (7) subjective comments about the ability to breathe; and (8) the amount and colour of sputum. A PaO2 (partial pressure of oxygen in arterial blood) above 60 mmHg (10 kPa) and a PaCO2 (partial pressure of carbon dioxide in arterial blood) below 45 mmHg (5.2 kPa) are acceptable values in a patient with uncomplicated tetraplegia. A patient who is unable to count to 10 out loud without taking a breath needs immediate attention.
In addition to monitoring, the nurse can intervene in maintaining ventilation. Oxygen is administered until ABGs stabilise. Chest physiotherapy and assisted coughing facilitate the raising of secretions. Assisted coughing simulates the action of the ineffective abdominal muscles during the expiratory phase of a cough. The nurse places the heels of both hands just below the xiphoid process and exerts firm upward pressure to the area timed with the patient’s efforts to cough (see Fig 67-6). Tracheal suctioning should be performed if crackles or rhonchi are present. Incentive spirometry is an additional technique that can be used to improve the patient’s respiratory status.
 Cardiovascular instability
Cardiovascular instability
Because of unopposed vagal response, the heart rate is slowed, often to below 60 beats per minute. Any increase in vagal stimulation, such as turning or suctioning, can result in cardiac arrest. Loss of sympathetic tone in peripheral vessels results in chronic low blood pressure with potential postural hypotension. Lack of muscle tone to aid venous return can result in sluggish blood flow and predispose the patient to DVT.
Vital signs should be assessed frequently. If bradycardia is symptomatic, an anticholinergic drug such as atropine is administered. A temporary pacemaker may be inserted in some extreme cases. Hypotension is managed with a vasopressor agent, such as dopamine or noradrenaline, and fluid replacement. In the older adult, the prevalence of cardiovascular disease must be considered. The cardiovascular system becomes less able to handle the stress of traumatic injury because heart contractions weaken and cardiac output is reduced. Maximum heart rate is also reduced.
CLINICAL PRACTICE
Situation
A 25-year-old man suffered a spinal cord injury to C7–8 following a motorcycle accident. He was diagnosed with anterior cord syndrome and has motor paralysis, which may prevent him from riding motorcycles again. He has become extremely depressed and no longer wishes to live. Due to his emotional state, he is now refusing to eat. Can he be forced to receive enteral nutrition (tube feeding)?
Important points for consideration
• Withholding treatment in a newly injured but otherwise healthy young adult may present an ethical dilemma for healthcare professionals.
• Approximately 20–30% of patients with a new spinal cord injury experience a major depressive disorder due to the sudden loss of bodily control and feelings of helplessness; a high (nearly 50%) spontaneous remission occurs within 1 week.
• A thorough mental health and psychological evaluation is warranted; treatment of depression is necessary before determining the capacity to make decisions.
• Most people (more than 90%) with a spinal cord injury who receive healthcare and have access to appropriate resources report a good quality of life. Therefore, requests to withhold treatment soon after spinal cord injury should be scrutinised carefully.
• If, after adequate treatment for pain, depression or other medical conditions, the patient persists in requests to withhold treatment, his ability to make an informed choice must be reassessed.
• A competent adult can decide to withhold treatment. If possible, action on the request should be delayed to ensure adequate informed consent and a determination whether an adequate quality of life may be possible.
Compression gradient stockings can be used to prevent thromboemboli and to promote venous return. The stockings must be removed every 8 hours for skin care. The use of pneumatic compression devices for the calves is advocated and they must be applied as soon as possible after admission and maintained throughout the hospitalisation. Venous duplex studies may be performed before applying compression devices. The nurse should also perform range-of-motion exercises and heel–cord stretching regularly. The thighs and calves of the legs should be assessed every shift for signs of DVT.
Prophylactic heparin or low-molecular-weight heparin (e.g. enoxaparin) therapy may be used to prevent DVT unless contraindicated. Contraindications include internal bleeding and recent surgery.
If blood loss has occurred from other injuries, haemoglobin and haematocrit levels should be monitored and blood should be administered according to protocol. The nurse also should monitor the patient for indications of hypovolaemic shock secondary to haemorrhage.
 Fluid and nutritional maintenance
Fluid and nutritional maintenance
During the first 48–72 hours after the injury the GI tract may stop functioning (paralytic ileus) and a nasogastric tube must be inserted. Because the patient cannot have oral intake, fluid and electrolyte needs must be monitored carefully. Specific solutions and additives are ordered based on individual requirements. Once bowel sounds are present or flatus is passed, fluids and then oral food can gradually be introduced. Because of severe catabolism, a high-protein, high-kilojoule diet is necessary for energy and tissue repair. In patients with high cervical cord injuries, swallowing must be evaluated before starting oral feedings. If the patient is unable to resume eating, total parenteral nutrition may be started to provide nutritional support.
Increased fibre should be included to promote bowel function. Some patients experience anorexia, which can be due to depression, boredom with institutional food or discomfort at being fed (often by a hurried nurse). Some patients normally have a small appetite. Occasionally, refusal to eat is used as a means of maintaining control over the environment because of diminished or absent body control. If the patient is not eating adequately, the cause should be assessed thoroughly. Based on this assessment, a contract may be made with the patient using mutual goal setting about the diet and eating. This gives the patient increased control of the situation and often results in improved nutritional intake. General measures, such as providing a pleasant eating environment, allowing adequate time to eat (including any self-feeding the patient can achieve), encouraging the family to bring in special foods and planning social rewards for eating, may be useful. A kilojoule count should be kept and the patient’s daily weight recorded as a means of evaluating progress. If feasible, the patient should participate in recording the kilojoule intake. The nurse should avoid allowing the patient’s nutritional intake to become a basis for a power struggle.
 Bladder and bowel management
Bladder and bowel management
Immediately after injury, urine is retained because of the loss of autonomic and reflex control of the bladder and sphincter. Because there is no sensation of fullness, overdistension of the bladder can result in reflux into the kidney with eventual renal failure. Bladder overdistension may even result in rupture of the bladder. Consequently, an indwelling catheter is usually inserted as soon as possible after injury. Its patency must be ensured by frequent inspection and irrigation if necessary. In some institutions, a doctor’s order is required for this procedure. Strict aseptic technique for catheter care is essential to avoid introducing infection.
After the patient is stabilised, the best means of managing long-term urinary function is assessed. Usually the patient is started on an intermittent catheterisation program. Intermittent catheterisation has been shown to reduce UTIs when compared to indwelling catheterisation, and it is the safest method of bladder management for protecting the kidneys.11,12 The patient is often maintained on a fluid restriction of 1800–2000 mL per day to facilitate a bladder training program. Urinary output is monitored closely.
UTIs are a common problem. The best method for preventing UTIs is regular and complete bladder drainage. During the period of indwelling catheterisation, a large fluid intake is required. The catheter should be checked frequently to prevent kinking and ensure free flow of urine. During intermittent catheterisation, fluid intake should be moderate and regular (200–300 mL every 2–3 hours). Catheterisation should be done at least 4 hourly to prevent bacterial overgrowth resulting from urinary stasis. Cranberry juice and/or cranberry extract tablets may be helpful for UTI prevention because there is some evidence that they may prevent bacteria from adhering to the bladder wall. Ascorbic acid and a urinary antiseptic, such as hexamine hippurate, are sometimes given, although their use in preventing UTIs remains controversial. If the appearance or odour of the urine is suspicious or if the patient develops symptoms of a UTI (chills, fever, malaise), a specimen is sent for culture.
Age-related changes in renal function should be considered. The older adult is more likely to develop renal calculi, and older men may have prostatic hyperplasia, which may interfere with urinary flow and complicate urinary management.
Constipation is generally a problem during spinal shock because no voluntary or involuntary (reflex) evacuation of the bowels occurs. A bowel program should be started during acute care. This consists of choosing a rectal stimulant (suppository or mini-enema) to be inserted daily at a regular time of day followed by gentle digital stimulation done by the nurse until evacuation is complete. Initially the program may be done in bed in the right side-lying position to facilitate gravity, but when the patient has resumed sitting, it should be done in the upright position on a padded commode chair in the privacy of a bathroom.
 Temperature control
Temperature control
Because there is no vasoconstriction, piloerection (erection of body hair) or heat loss through perspiration below the level of injury, temperature control is largely external to the patient. Therefore, the nurse must monitor the environment closely to maintain an appropriate temperature. Body temperature should be monitored regularly. The patient should not be overloaded with covers or unduly exposed (such as during bathing). If an infection with high fever develops, it may be necessary to use other means of temperature control, such as a cooling blanket.
 Stress ulcers
Stress ulcers
Stress ulcers are a problem for the patient with a spinal cord injury; these are due to the physiological response to severe trauma, psychological stress and high-dose corticosteroids. Peak incidence of stress ulcers is 6–14 days after injury. Stool and gastric contents are tested daily for blood and the haematocrit is observed for a slow drop. When corticosteroids are given, they should be accompanied by antacids or food. Histamine H2-receptor antagonists, such as ranitidine and famotidine, or proton pump inhibitors, such as omeprazole, may be given prophylactically to decrease the secretion of hydrochloric acid.
 Sensory deprivation
Sensory deprivation
The nurse must compensate for the patient’s absent sensations to prevent sensory deprivation. This is done by stimulating the patient above the level of injury. Conversation, music, strong aromas and interesting flavours should be a part of the nursing care plan. Prism glasses are provided so that the patient can read and watch television. Every effort should be made to prevent the patient from withdrawing from the environment.
Patients with spinal cord injury often report altered sensorium and vivid dreams during the acute phase of their treatment. Whether this is due to drugs used to manage pain and anxiety is not known. Patients may also experience disrupted sleep patterns as a result of the hospital environment or posttraumatic stress disorder.
 Reflexes
Reflexes
Once spinal cord shock is resolved, the return of reflexes may complicate rehabilitation. Lacking control from the higher brain centres, reflexes are often hyperactive and produce exaggerated responses. Penile erections can occur from a variety of stimuli, causing embarrassment and discomfort. Spasms ranging from mild twitches to convulsive movements below the level of the lesion may also occur.21 This reflex activity may be interpreted by the patient or family as a return of function, and the nurse must tactfully explain the reason for the activity. The patient may be informed of the positive use of these reflexes in sexual, bowel and bladder retraining. Spasms may be controlled with the use of antispasmodic drugs. Most commonly prescribed are baclofen and low-dose diazepam. Baclofen may be given orally or, in cases of severe spasm, it can be administered intrathecally via a pump implant. For protracted spasticity, which interferes with ADLs and rehabilitation, botulism toxin injections may be considered.21
 Autonomic dysreflexia
Autonomic dysreflexia
The return of reflexes after the resolution of spinal shock means that patients with an injury level at T6 or higher may develop autonomic dysreflexia. Autonomic dysreflexia or hyperreflexia (as it is sometimes referred to) is a massive uncompensated cardiovascular reaction mediated by the sympathetic nervous system.22 It occurs in response to visceral stimulation once spinal shock is resolved in patients with spinal cord lesions above T6. The condition is a life-threatening situation that requires immediate resolution. If resolution does not occur, it can lead to status epilepticus, stroke, myocardial infarction and even death.
The most common precipitating cause is a distended bladder or rectum, although any sensory stimulation may cause autonomic dysreflexia. Contraction of the bladder or rectum, stimulation of the skin or stimulation of the pain receptors may also cause autonomic dysreflexia. Manifestations include hypertension (up to 250 mmHg systolic), blurred vision, throbbing headache, marked diaphoresis above the level of the lesion, bradycardia (30–40 beats per minute), piloerection as a result of pilomotor spasm, flushing of the skin above the level of the lesion, blurred vision or spots in the visual fields, nasal congestion, anxiety and nausea. It is important to measure blood pressure when a patient with a spinal cord injury complains of a headache.23
The pathology of autonomic dysreflexia involves the stimulation of sensory receptors below the level of the cord lesion. The intact autonomic nervous system below the level of the lesion responds to the stimulation with a reflex arteriolar vasoconstriction that increases blood pressure. Baroreceptors in the carotid sinus and the aorta sense the hypertension and stimulate the parasympathetic system. This results in a decrease in heart rate but the visceral and peripheral vessels do not dilate because efferent impulses cannot pass through the cord lesion.
Nursing interventions in this serious emergency are elevation of the head of the bed to 45° or sitting the patient upright, notification of the doctor and assessment to determine the cause. The most common cause is bladder irritation. Immediate catheterisation to relieve bladder distension may be necessary.22 Lignocaine gel should be instilled in the urethra before catheterisation. If a catheter is already in place, it should be checked for kinks or if draining adequately. If blocked, small-volume irrigation (no more than 30 mL at a time) should be performed slowly and gently, or a new catheter may be inserted. The procedure of bladder irrigation remains controversial with many institutions and community agencies preferring to change the catheter, thus avoiding unnecessary bladder irrigations. Stool impaction can also result in autonomic dysreflexia. A digital rectal examination should be performed only after application of an anaesthetic ointment to decrease rectal stimulation and to prevent an increase of symptoms. The nurse should remove all skin stimuli, such as constrictive clothing and tight shoes. Blood pressure should be monitored frequently during the episode. If symptoms persist after the source has been relieved, an α-adrenergic blocker or an arteriolar vasodilator (e.g. anginine or nitrolingual spray) is administered. Careful monitoring must continue until the vital signs stabilise, and for the ensuing 24–72 hours post-episode as further episodes may occur due to the unstable autonomic nervous system.
The patient and family should be taught the causes and symptoms of autonomic dysreflexia (see Box 60-3). They must understand the life-threatening nature of this dysfunction and know how to relieve the cause. In the community setting, the person with a spinal cord injury should carry an emergency card with instructions for paramedics or carers on how to manage an episode of autonomic dysreflexia.
BOX 60-3 Autonomic dysreflexia
PATIENT & FAMILY TEACHING GUIDE
Patients and family members must know the signs and symptoms of autonomic dysreflexia so that timely intervention can occur. These include the following:
• sudden onset of acute headache
• elevation in blood pressure and/or reduction in pulse rate
• flushed face and upper chest (above the level of the lesion) and pale extremities (below the level of the lesion)
Immediate interventions include the following:
• raise the person to a sitting position
• remove the stimulus (faecal impaction, kinked urinary catheter)
• call the healthcare provider if the above actions do not relieve the signs and symptoms.
Efforts to decrease the likelihood of autonomic dysreflexia include the following:
 Rehabilitation and home care
Rehabilitation and home care
The physiological and psychological rehabilitation of the patient with a spinal cord injury is complex and involved. With physical and psychological care and intensive and specialised rehabilitation, the patient learns to function at the highest level of wellness. It is recommended that all patients with a new spinal cord injury receive comprehensive inpatient rehabilitation in a rehabilitation unit or centre that specialises in spinal cord rehabilitation.
Many of the problems identified in the acute period become chronic and continue throughout life. Rehabilitation focuses on refined retraining of physiological processes and extensive patient and family teaching about how to manage the physiological and life changes resulting from injury (see Fig 60-13).
Rehabilitation is a multidisciplinary endeavour carried out through a team approach. Team members include rehabilitation nurses, doctors, physiotherapists, occupational therapists, speech therapists, vocational counsellors, psychologists, prosthetists (denturists), podiatrists and dieticians. Rehabilitation care is organised around the individual patient’s goals and needs. During rehabilitation, patients are expected to be involved in therapies and learn self-care for several hours each day. Such intensive work at a time when the patient is dealing with the sudden change in health and functional status can be very stressful. Progress may be slow and frequent encouragement may be required. The rehabilitation nurse has a pivotal role in providing encouragement, specialised nursing care, patient and family teaching, and helping to coordinate the efforts of the rehabilitation team.
 Respiratory rehabilitation
Respiratory rehabilitation
The patient with high cervical spinal cord injury may have greatly increased mobility with phrenic nerve stimulators or electronic diaphragmatic pacemakers. These devices are not appropriate for all ventilator-dependent patients but may be helpful for those with an intact phrenic nerve. Today, ventilators are also reasonably portable and ventilator-dependent tetraplegic patients can be mobile and somewhat independent. Patients and family members should be taught all aspects of home ventilator care and referrals should be made to appropriate community agencies. Patients with cervical level injuries and high thoracic injuries who are not ventilator dependent should be taught assisted coughing and regular use of incentive spirometry or deep breathing exercises.
 Neurogenic bladder
Neurogenic bladder
A neurogenic bladder is any type of bladder dysfunction related to abnormal or absent bladder innervation.23 After spinal cord shock resolves, depending on the completeness of the spinal cord injury, patients usually have some degree of neurogenic bladder. Normal voiding requires nervous system coordination of urethral and pelvic floor relaxation with simultaneous contraction of the detrusor muscle.23 Depending on the lesion, a neurogenic bladder may have no reflex detrusor contractions (areflexic, flaccid), may have hyperactive reflex detrusor contractions (hyperreflexic, spastic) or may have lack of coordination between detrusor contraction and urethral relaxation (dyssynergia). Common problems with a neurogenic bladder include urgency, frequency, incontinence, inability to void and high bladder pressures resulting in reflux of urine into the kidneys.
Neurogenic bladder can be classified according to reflex detrusor activity, intravesical filling pressure and continence function.23 Types of neurogenic bladder are outlined in Table 60-5. Diagnostic and multidisciplinary care of neurogenic bladder is described in Box 60-4. The patient with a spinal cord injury and a neurogenic bladder requires a comprehensive program to manage bladder function.
MULTIDISCIPLINARY CARE
After the patient’s overall condition is stable and neurological reflexes are present, urodynamic testing, an IV pyelogram and cystourethrogram should be performed to form baseline observations for management and future reference of renal and bladder function. The method used for urinary drainage depends on the type of neurogenic bladder dysfunction, the lifestyle and preference of the patient with advice from the doctor. Numerous drainage methods are possible, including bladder reflex retraining if partial voiding control remains, an indwelling catheter, intermittent catheterisation and an external catheter (condom catheter). Surgical options include sphincterectomy, implantation of a functional electrical stimulation device and urinary diversion (e.g. an ileal conduit).
Many factors are considered when selecting a bladder management strategy. These include upper extremity function, carer burden and lifestyle choices. The type of bladder dysfunction also defines treatment goals and management options. Areflexic bladder with detrusor and sphincter dyssynergia requires intervention to provide low-pressure storage, low-pressure voiding and adequate emptying. Anticholinergic drugs (e.g. oxybutynin) may be used to suppress bladder contraction. α-adrenergic blockers (e.g. terazosin, doxazosin) may be used to decrease outflow resistance at the bladder neck, and antispasmodic drugs (e.g. baclofen) may be used to decrease spasticity of pelvic floor muscles.
Drainage options include intermittent catheterisation, an external catheter or an indwelling catheter. Areflexic bladder with detrusor hyperreflexia may be treated with anticholinergic drugs, intravesical capsaicin or botulinum A toxin. Drainage options include volitional voiding as well as the methods listed above. An areflexic bladder is usually managed with intermittent catheterisation or an indwelling catheter.
The long-term use of an indwelling catheter should be carefully evaluated because of the associated high incidence of UTIs, fistula formation and diverticula. However, there may be patients for whom this is the best option. Adequate fluid intake and patency of the catheter should be ensured. The frequency of routine catheter changes ranges from 1 week to 3 months, depending on the type of catheter used and agency policy.
Intermittent catheterisation is the most commonly recommended method of bladder management in the group of patients with spinal cord injury who have hand function (see Ch 45). Nursing assessment is important in selecting the time interval between catheterisations. Initially, catheterisation is done every 4 hours. Bladder volume can be assessed before catheterisation using the portable bladder ultrasound machine. If less than 200 mL of urine is measured, the time interval may be extended. If 500 mL or more of urine is measured, the time interval is shortened. An overdistended bladder can cause ischaemia of the bladder wall, which may predispose tissues to bacterial invasion and infection. Patients often experience diuresis at a regular time during a 24-hour period. The number of intermittent catheterisations per day is usually five or six.
Urinary diversion surgery may be necessary if the patient has repeated UTIs with renal involvement or repeated stones or if therapeutic intervention has been unsuccessful (see Table 45-8). Surgical treatment of neurogenic bladder includes bladder neck revision (sphincterotomy), bladder augmentation (augmentation cystoplasty), penile prosthesis, artificial sphincter, perineal ureterostomy, cystotomy, vesicotomy and anterior urethral transplantation.
No matter which bladder management strategy is selected, the nurse must teach the patient and family or carer about how to accomplish successful self-management. This includes teaching management techniques, how to obtain necessary supplies, care of supplies and equipment, and when to seek healthcare. Resources and referrals for supplies and ongoing care must also be arranged.
 Neurogenic bowel
Neurogenic bowel
Careful management of bowel evacuation is necessary in the patient with a spinal cord injury because voluntary control of this function may be lost as a result of a condition called neurogenic bowel.24 The usual measures for preventing constipation include a high-fibre diet and adequate fluid intake. Patient and family teaching guidelines related to bowel management are presented in Box 60-5. However, these measures by themselves may not be adequate to stimulate evacuation. In addition, suppositories (bisacodyl or glycerine) or small-volume enemas and digital stimulation by the nurse or patient may be necessary. In the patient with an upper motor neuron lesion, digital stimulation is necessary to relax the external sphincter to promote defecation.24 A stool softener such as sodium sulphosuccinate can be used to regulate stool consistency. Oral stimulant laxatives may be used to establish a regular routine, but consideration should be given to decreasing the dose and the ongoing use of such aperients. A healthy diet high in fibre (25 g per day) should be encouraged along with an adequate fluid intake (2–3 L per day) in order to avoid the ongoing use of laxatives.
BOX 60-5 Bowel management after spinal cord injury
PATIENT & FAMILY TEACHING GUIDE
The following are teaching guidelines for a patient with a spinal cord injury:
1. Optimal nutritional intake includes:
2. Fibre intake should be approximately 20–30 g per day. The amount of fibre eaten should be increased gradually over 1–2 weeks.
3. Three litres of fluid per day should be consumed unless contraindicated. Water or fruit juices should be used, and caffeinated beverages, such as coffee, tea and cola, should be avoided. Fluid softens hard stools; caffeine stimulates fluid loss through urination.
4. Foods that produce gas (e.g. beans) or upper GI upset (spicy foods) should be avoided.
5. Timing: A regular schedule for bowel evacuation should be established. A good time is 30 mins after the first meal of the day.
6. Position: If possible, an upright position with feet flat on the floor or on a step enhances bowel evacuation. Staying on the toilet, commode or bedpan for longer than 20–30 mins may cause skin breakdown. Based on stability, someone may need to stay with the patient.
7. Activity: Exercise is important for bowel function. In addition to improving muscle tone, it decreases GI transit time and increases appetite. Muscles should be exercised. This includes stretching, range-of-motion, position changing and functional movement.
8. Drug treatment: Suppositories may be necessary to stimulate a bowel movement. Manual stimulation of the rectum may also be helpful in initiating defecation. Stool softeners should be used as needed to regulate stool consistency. Oral laxatives should be used only if necessary.
In general, a bowel movement every other day is considered adequate. However, pre-injury patterns should be considered. Incontinence can result from too much stool softener or faecal impaction.
The Valsalva manoeuvre and manual evacuation are useful in patients with lower motor neuron lesions.24 The Valsalva manoeuvre requires intact abdominal muscles, so it is used in those patients with injuries below T12. The aim of bowel management in this group of patients is a daily routine, with firm, formed faeces to avoid stress incontinence in activities such as transfers.
Careful recording of bowel movements, including amount, time and consistency, is critical in trouble-shooting and important to the overall success of the program. Timing of evacuations may also be an important factor. If bowel evacuation is planned for 30–60 minutes following the first meal of the day, this may enhance success by taking advantage of the gastrocolic reflex induced by eating. Again, patient and family education is required to promote successful independent bowel management.
 Neurogenic skin
Neurogenic skin
Prevention of pressure ulcers and other types of injury to insensate skin are essential skills required by every patient with spinal cord injury. Nurses working in rehabilitation are responsible for teaching these skills and providing information about daily skin care. A comprehensive visual and tactile examination of the skin should be done twice daily with special attention given to areas over bony prominences. The areas most vulnerable to skin breakdown include the ischia, trochanters, heels and sacrum. Careful positioning and repositioning should be done initially every 2 hours with gradual increases in the times between turns if there is no redness over bony prominences at the time of turning. Pressure-relieving cushions must be used in wheelchairs and special mattresses may also be needed. Movement during turns and transfers should be done carefully to avoid stretching and folding of soft tissues (shear), as well as friction or abrasion. Nutritional status should be assessed regularly. Both weight loss and weight gain can contribute to skin breakdown. Adequate intake of protein is essential for skin health. Measurement of pre-albumin, total protein and albumin levels can help identify inadequate protein intake. The importance of nutrition to skin health should be stressed to the patient and family.
Protection of insensate skin also requires avoidance of thermal injury. Burns can be caused by hot food or liquids, bath or shower water that is too warm, radiators, heating pads and uninsulated plumbing. Thermal injury can also result from extreme cold (frostbite). Injuries may not be noticed until severe damage is done. Anticipatory guidance about potential risks is essential. Patient and family education related to skin care is provided in Boxes 60-6 and 60-7.
BOX 60-6 Skin care for the patient with spinal cord injury
PATIENT & FAMILY TEACHING GUIDE
Skin breakdown is a potential problem after spinal cord injury. The following measures are used to decrease this possibility.
PATIENT & FAMILY TEACHING GUIDE
The following are teaching guidelines for a patient with a halo vest:
1. Inspect the pins on the halo traction ring. Report to the healthcare provider if pins are loose or if there are signs of infection, including redness, tenderness, swelling or drainage at the insertion sites.
2. Clean around the pin sites carefully with hydrogen peroxide on a cotton swab. Repeat the procedure using water.
3. Use alcohol swabs to cleanse the pin sites of any drainage.
4. Apply antibiotic ointment as prescribed.
5. To provide skin care, have the patient lie down on a bed with their head resting on a pillow to reduce pressure on the brace. Loosen one side of the vest. Gently wash the skin under the vest with soap and water, rinse it and then dry it thoroughly. At the same time, check the skin for pressure points, redness, swelling, bruising or chafing. Close the open side and repeat the procedure on the opposite side.
6. If the vest becomes wet or damp, it can be dried carefully with a blow dryer.
7. When mobilising, an assistive device (e.g. cane, walker) may be used to provide greater balance. Flat shoes should be worn.
8. Turn the entire body, not just the head and neck, when trying to view sideways.
9. In case of an emergency, keep a set of wrenches close to the halo vest at all times.
10. Mark the vest strap such that consistent buckling and fit can be maintained.
11. Avoid grabbing the bars or vest to assist the patient.
12. Keep a sheepskin pad under the vest. Change and wash it at least weekly.
13. If perspiration or itching is a problem, a cotton T-shirt can be worn under the sheepskin. The T-shirt can be modified with Velcro seam closure on one side.
 Sexuality
Sexuality
Knowledge of the level and completeness of injury is needed to understand the male patient’s potential for orgasm, erection and fertility and the patient’s capacity for sexual satisfaction (see Table 60-6). Sexuality is an important issue regardless of the patient’s age or gender. To provide accurate and sensitive counselling and education about sexuality, the nurse must have awareness and an acceptance of personal sexuality, as well as knowledge of human sexual responses. When discussing sexual potential, the nurse should use scientific terminology rather than slang whenever possible.
Reflex sexual function capability is possible if the patient has an upper motor neuron lesion. The presence of tone in the external rectal sphincter indicates an upper motor neuron lesion. The absence of external rectal sphincter tone, bulbocavernosus reflex or both indicates that the patient has lower motor neuron involvement and may be capable of psychogenic erection but not reflex erection. If ejaculation occurs, it may be retrograde into the bladder.
The type of lesion determines the physical sexual response. Men with upper motor neuron lesions may have reflexogenic erections that are produced by reflex activity or external stimuli or that occur spontaneously. These spontaneous erections are often short-lived and uncontrolled and cannot be maintained or summoned at the time of coitus. Orgasm and ejaculation are usually not possible for men with a complete upper motor neuron lesion.
Most patients with a complete lower motor neuron lesion are unable to have either psychogenic or reflexogenic erections. Patients with incomplete lower motor neuron lesions have the highest possibility of successful psychogenic erection with ejaculation and up to 10% of these patients are fertile.
Treatments for erectile dysfunction include drugs, vacuum devices and surgical procedures. Sildenafil has become the treatment of choice since several studies have documented its effectiveness in men with spinal cord injury. Penile injection of vasoactive substances (papaverine, prostaglandin E) is another medical treatment. Risks include priapism (prolonged penile erection) and scarring, so these substances are often considered only after failure of sildenafil. Vacuum suction devices use negative pressure to encourage blood flow into the penis. Erection is maintained by a constriction band placed at the base of the penis. The main surgical option is implantation of a penile prosthesis. (Erectile dysfunction is discussed in Ch 54.)
Male fertility is affected by spinal cord injury causing poor sperm quality and ejaculatory dysfunction. Recent advances in methods of retrieving sperm (penile vibratory stimulation and electro-ejaculation) combined with ovulation induction and intrauterine insemination of the female partner have changed the prognosis for men with spinal cord injury to father children from unlikely to a reasonable possibility of successful outcomes.24
The effect of spinal cord injury on female sexual response is less clear. Vaginal lubrication is similar to erection in males, with reflex and psychogenic components. Women with upper motor neuron injuries may retain the capacity for reflex lubrication, whereas psychogenic lubrication depends on the completeness of injury. Orgasm is reported by about 50% of women with spinal cord injury.24
The woman of childbearing age with a spinal cord injury usually remains fertile. The injury does not affect the ability to become pregnant or to deliver normally through the vagina. Menses may cease for as long as 6 months. If sexual activity is resumed, protection against an unplanned pregnancy is necessary. A normal pregnancy may be complicated by UTIs, anaemia and autonomic dysreflexia. Because uterine contractions are not felt, a precipitous delivery is always a danger.
Sexual rehabilitation for both men and women should begin informally after the acute phase of the injury has passed. Questions such as, ‘Have you had an erection since your accident?’ and ‘Have your menstrual periods continued since the accident?’ are non-threatening ways to introduce the topic of sexual functioning. The male patient may pose a question such as, ‘Can I ever be a man again?’
Open discussion with the patient is essential. This important aspect of rehabilitation should be handled by someone specially trained in sexual counselling. A nurse or other rehabilitation professional with such expertise works with the patient and partner to provide support with the emphasis on open communication. The nurse’s educational role requires respect for every couple’s personal standards of religious and cultural beliefs. Alternative methods of obtaining sexual satisfaction, such as oral–genital sex (cunnilingus and fellatio), may be suggested. Explicit films may also be used. For example, the film Touching demonstrates the sexual activities of a patient with tetraplegia and a non-disabled partner. Graphics should be used cautiously because they may be too limiting or focus too much on the mechanics of sex rather than on the relationship.
Sexual activities may require more planning and be less spontaneous than before the injury. For example, an attendant may have to undress the patient and remove equipment. A relaxed atmosphere with music and perfume creates an attractive environment. Ample time for caressing, fondling and kissing is essential. The partners should be encouraged to explore each other’s erogenous areas, such as the lips, neck and ears, which can arouse psychogenic erection or orgasm. Few demands should be made initially.
Care should be taken not to dislodge an indwelling catheter during sexual activity. If an external catheter is used, it should be removed before sexual activity and the patient should refrain from fluids. The bowel program should include evacuation the morning of sexual activity. The partner should be informed that an accident is always possible. The woman may need a water-soluble lubricant to supplement diminished vaginal secretions and facilitate vaginal penetration.
 Grief and depression
Grief and depression
Patients with spinal cord injuries may feel an overwhelming sense of loss. They may temporarily lose control over everyday life activities and must depend on others for ADLs and for life-sustaining measures. Patients may believe that they are useless and a burden to their family. At a stage when independence is often of the greatest importance, they may be totally dependent on others.
The patient’s response and recovery differ in some important aspects from those experiencing loss from amputation or terminal illness. First, regression can and does occur at different stages. Working through grief is a difficult, lifelong process with which the patient needs support and encouragement. With recent advances in rehabilitation, it is usual for the patient to be independent physically and discharged from the rehabilitation centre before completion of the grief process. The goal of recovery is related more to adjustment than to acceptance. Adjustment implies the ability to go on with living with certain limitations. Although patients who are cooperative and accepting are easier to treat, the nurse should expect a wide fluctuation of emotions from patients with spinal cord injuries. Depression may not be a component of the recovery process. Societal norms allow depression after severe loss and almost impose it on those confronted with death or radical lifestyle changes. However, not every patient may experience depression.
The nurse’s role in grief work is to allow mourning as a component of the rehabilitation process. Table 60-7 summarises the mourning process and appropriate nursing interventions. Maintaining hope is an important strategy during the grieving process and should not be interpreted as denial.25 During the shock and denial stage the nurse reassures the patient and stresses the expertise of the entire healthcare team. During the anger stage, the nurse assists the patient in achieving control over the environment, particularly by allowing the patient’s input into the plan of care. The nurse should not respond to anger or manipulation or become involved in a power struggle with the patient. As self-care abilities increase, the patient’s independence increases.
The patient’s family also requires counselling to avoid promoting dependency in the patient through guilt or misplaced sympathy. The family is also experiencing an intense grieving process. A support group of family members and friends of patients with spinal cord injury can help increase family members’ knowledge and participation in the grieving process, physical difficulties, rehabilitation plan and the meaning of the disability in society.26
If the patient experiences a stage of depression, the nurse must be patient and persistent and maintain a sense of humour. Pity is not helpful. The patient should be treated in an adult manner and be involved in decision making about care, but the nurse must insist that the care be performed. A primary nurse relationship is helpful. Staff planning and sessions in which staff members can express their feelings are helpful in providing consistency of care. To achieve the stage of adjustment, the patient needs continual support throughout the rehabilitation process in the forms of acceptance, affection and caring. The nurse must be attentive when the patient needs to talk and sensitive to needs at the various stages of the grief process. Although the stage of depression during the grief process usually lasts days to weeks, some individuals may become clinically depressed and require treatment for depression. Evaluation by a mental health nurse or psychiatrist is recommended. Treatment may include drugs and psychotherapy.26
NEW DIRECTIONS IN SPINAL CORD INJURY
Up until recently, the care of those with spinal cord injury has been based solely on the deficit model. That is, the healthcare team has worked with the injured person to restore as much function as possible and to compensate for physical deficits by the use of wheelchairs, braces and other assistive devices. The underlying, unquestioned assumption has been that the central nervous system is hard-wired, non-malleable and incapable of repairing itself. However, there is now growing and convincing evidence that the nervous system has more neuroplasticity (capacity for renewal and change) than has been previously thought, especially in relation to recovery of the ability to walk.27 This evidence suggests that the neural network at the level of the spinal cord has the capacity to: (1) integrate incoming information; and (2) respond with motor output.
Research suggests that a comprehensive exercise program, which includes stimulation of paralysed limbs, can promote neural recovery, and as a result, innovative activity-based therapies are now being introduced into Australia based on work started in the US.27–32 The training method appears to be critical to progress and the goal is walking and standing.27,31–33 An investigation conducted in 2007 by an Australian physiotherapist into the effects of active rehabilitation programs found that ‘exercise is the only known intervention that can have lasting effects on function after spinal cord injury, both in promoting neural recovery and in reducing secondary complications’ and that ‘a paradigm shift has occurred in the way spinal cord injury rehabilitation is delivered in leading centres in the US, Canada and Europe. Best practice includes provision of a comprehensive exercise program that includes, but is not limited to, the use of functional electrical stimulation and body weight-supported treadmill training to stimulate the paralysed limbs and improve functional outcomes’.33 A randomised control trial reported in 2008 illustrated that multimodal intensive exercise can significantly improve motor function in participants with chronic spinal cord injury. The researchers further concluded that an organised program may provide greater motor benefits than a self-regulated program.32
This forms the rationale for the Walk On program now being offered by Spinal Cord Injuries Australia (SCIA; see the Resources on p 1733). Walk On is a private rehabilitation program based on the Project Walk® program from the US. The first Walk On facility was opened in Brisbane in 2008 in partnership with the Sporting Wheelies and Disabled Association of Queensland. The second Walk On facility was established in 2010 in partnership with the University of Sydney and operates out of the Clinical Exercise and Rehabilitation facility at the university. Since the commencement of the program in Australia, many clients have experienced significant practical and functional improvements that have had a major impact on their quality of life. The program involves intense, dynamic, weight-bearing exercises. All exercises are performed out of the wheelchair, on the floor, a table or rehabilitative exercise equipment, and at least one therapist works individually with each client. Exercises involve the entire body and are designed to reactivate the nervous system through a series of repetitive movements (see Fig 60-14). Each exercise program incorporates strengthening of the trunk and upper and lower limbs, whole body coordination, core balance (see Fig 60-15) and stability, as well as postural and gait training.

Figure 60-14 Dynamic weight-bearing exercises to develop whole-body coordination.
Walk On. Spinal Cord Injuries Australia. Used with permission.

Figure 60-15 Training includes core strengthening exercises.
Walk On. Spinal Cord Injuries Australia. Used with permission.
Interviews with clients in the Project Walk® program, which has been going for more than10 years, reveal unanimous improvements in clients’ sense of health and wellbeing.33 Many report improvements in bowel function and some have progressed to the stage of walking, using a gutter frame or forearm crutches. Others report substantial improvements in sitting balance. The Walk On program does not claim to be a cure for spinal cord injury; however, it is a different approach to rehabilitation and one that is showing promise.
Gerontological considerations: spinal cord injury
The demographics of patients living with spinal cord injuries are changing. The fact that people with spinal cord injuries now have longer life spans has contributed to the increasing number of older adults living with these injuries. Ageing is also associated with an increased likelihood of other chronic illnesses that may have a serious impact on the older adult with a spinal cord injury. As patients with spinal cord injuries age, both individual ageing changes and duration since injury influence functional ability. For example, bowel and bladder dysfunction can increase with duration and severity of spinal cord injury, and musculoskeletal repetitive trauma injuries are more common.
Health promotion and screening are important for the older patient with a spinal cord injury. Daily skin inspections, UTI prevention measures, monthly breast examinations for women and regular prostate cancer screening for men are recommended. Cardiovascular disease is the most common cause of morbidity and mortality among people with spinal cord injuries. The lack of sensation, including angina, in those with high-level injuries may mask acute myocardial ischaemia. Altered autonomic nervous system function and decreases in physical activity can place the patient at risk of cardiovascular problems, including hypertension.34
At the same time, because of the increased work and recreational activities of older adults, more older adults are experiencing spinal cord injuries. Health promotion to decrease injury risk includes fall prevention strategies (e.g. using a step stool or a grab bar to reach high shelves, using handrails on stairs). Rehabilitation for the older person who has undergone a spinal cord injury will probably take longer because of other pre-existing conditions and poorer health status at the time of the initial injury.
Spinal cord tumours
AETIOLOGY AND PATHOPHYSIOLOGY
Tumours that affect the spinal cord account for 0.5–1% of all neoplasms and are rare in both Australia and New Zealand. These tumours are classified as primary (arising from some component of cord, dura, nerves or vessels) or secondary (from primary growths in the breast, thyroid, lung, prostate, kidney and other sites). Spinal cord tumours are further classified as extradural (outside the spinal cord), intradural extramedullary (within the dura but outside the actual spinal cord) and intradural intramedullary (within the spinal cord itself). These latter tumours are usually astrocytomas or ependymomas (see Fig 60-16 and Table 60-8). Approximately 90% of all spinal tumours are extradural. Extradural tumours are usually metastatic and most often arise in the vertebral bodies. These metastatic lesions can invade intradurally and compress the spinal cord. Spinal intradural extramedullary tumours account for two-thirds of all intraspinal neoplasms and are mainly represented by meningiomas and schwannomas.
Because many of these tumours are slow growing, their symptoms stem from the mechanical effects of slow compression and irritation of nerve roots, displacement of the cord or gradual obstruction of the vascular supply. The slowness of growth does not cause autodestruction (secondary injury) as in traumatic lesions. Therefore, complete functional restoration may be possible when the tumour is removed, except with intradural intramedullary tumours.
CLINICAL MANIFESTATIONS
Both sensory and motor problems may result, with the location and extent of the tumour determining the severity and distribution of the problem. The most common early symptom of a spinal cord tumour outside the cord is pain in the back with radicular pain simulating intercostal neuralgia, angina or herpes zoster. The location of the pain depends on the level of compression. The pain worsens with activity, coughing, straining and lying down. Sensory disruption is later manifested by coldness, numbness and tingling in an extremity or in several extremities, slowly progressing upwards until it reaches the level of the lesion. Impaired sensation of pain, temperature and light touch precedes a deficit in vibration and position sense that may progress to complete anaesthesia. Motor weakness accompanies the sensory disturbances and consists of slowly increasing clumsiness, weakness and spasticity. The sensory and motor disturbances are ipsilateral to the lesion. Bladder disturbances are marked by urgency with difficulty in starting the flow, progressing to retention with overflow incontinence.
Manifestations of intradural spinal tumour develop as progressive damage to the long spinal tracts, producing paralysis, sensory loss and bladder dysfunction. Pain can be severe as a result of compression of spinal roots or vertebrae.
 NURSING AND COLLABORATIVE MANAGEMENT: SPINAL CORD TUMOURS
NURSING AND COLLABORATIVE MANAGEMENT: SPINAL CORD TUMOURS
Extradural tumours are seen early on routine spinal X-rays, whereas intradural and intramedullary tumours require MRI or CT scans for detection. CSF analysis may reveal tumour cells. The cord is decompressed after removal of the tumour by a laminectomy. More than 85% of primary tumours are benign and can be completely resected; 90% of patients recover without residual problems.
Compression of the spinal cord is an emergency. Relief of the ischaemia related to the compression is the goal of therapy. Corticosteroids are generally prescribed immediately to relieve tumour-related oedema. Dexamethasone is usually used, often in large doses.
Treatment for nearly all spinal cord tumours is surgical removal. The exception is a metastatic tumour that is sensitive to radiation and that has caused only minimal neurological deficits in the patient. In general, tumours of the extradural or intradural extramedullary group can be completely removed surgically. Intramedullary tumours offer a less favourable prognosis; however, exploration and removal are usually attempted.
Radiation therapy after the operation can be effective. Maximum permissible tissue dose is given over 6–8 weeks. Chemotherapy has also been used in conjunction with radiation therapy.
Relief of pain and return of function are the ultimate goals of treatment. The nurse must be aware of the neurological status of the patient before and after treatment. Ensuring that the patient receives pain medication as needed is an important nursing responsibility. Depending on the amount of neurological dysfunction exhibited, the patient may need to be cared for as though recovering from a spinal cord injury.35 Rehabilitation of patients with spinal cord tumours is similar to spinal cord injury rehabilitation.
Post-polio syndrome
Polio, also known as poliomyelitis, is an infectious viral disease transmitted through the oral route by ingestion of contaminated water or food, or contact with infected sources such as unwashed hands. The virus is shed in the faeces of infected individuals for as long as 6 weeks. The disease produces a range of presentations, from flu-like symptoms that resolve in 24–36 hours (also known as abortive poliomyelitis) to non-paralytic poliomyelitis (the majority of cases) to paralytic poliomyelitis that attacks the motor neurons in the anterior horn of the spinal cord and/or brainstem.
Polio was effectively eradicated in the developed world, including Australia and New Zealand, by the Salk injectable polio vaccine in 1955 and the Sabin oral polio vaccine in 1961. Currently, the replacement of oral polio vaccine (OPV) with inactivated polio vaccine (IPV) is recommended on the basis that the risk of vaccine-associated paralytic poliomyelitis (VAPP) from OPV now exceeds the risk of catching naturally occurring polio.36 Polio is still a threat in developing countries, such as India, Asia and Africa, due to lack of effective immunisation programs. Even though Australia and New Zealand are currently free of the wild polio virus, there is still a risk of the virus being imported from countries where it exists.37,38
Some polio survivors who recovered from the disease decades ago, notably those who had paralytic poliomyelitis, are now experiencing a recurrence of neuromuscular symptoms as they age. These late effects of polio are collectively referred to as post-polio syndrome. The World Health Organization estimates that approximately 40% of people who survived paralytic poliomyelitis may develop additional symptoms 15–40 years after the original illness.39 These symptoms include new progressive muscle weakness, severe fatigue and pain in the muscles and joints. Polio once ravaged Australian and New Zealand communities in post-war epidemics during the 1940s and 1950s. There is no central register of polio survivors, so no one is certain exactly how many are still living today. The accepted figure is that there are 20,000 survivors of paralytic poliomyelitis in Australia, but estimates have ranged from 20,000 to 70,000.37 In New Zealand, the last recorded case of polio was in 1999 and it is estimated that there are 3000–5000 survivors of paralytic poliomyelitis still living today.38 Many of these survivors are at risk of post-polio syndrome.
AETIOLOGY AND PATHOPHYSIOLOGY
The aetiology of post-polio syndrome is not completely clear. The most commonly accepted theory is that enlarged distal motor neurons that had recovered after polio degenerate and subsequently begin to fail.40 It appears that cellular damage caused by the effects of the polio virus may lead to exhaustion and premature failure of the motor neurons. The result is slowly progressive muscle weakness and fatigue. Factors thought to contribute to post-polio syndrome include age-related motor neuron loss, musculoskeletal overuse and disuse, weight gain, pain and other neuromuscular or systemic illnesses. There is no evidence to support that post-polio syndrome is caused by reactivation of the original polio virus.
CLINICAL MANIFESTATIONS AND DIAGNOSTIC STUDIES
Post-polio syndrome is manifested by a new onset of joint and muscle weakness, easy fatiguability, generalised fatigue and pain. Uncommonly, individuals may also exhibit speech, swallowing and respiratory difficulties. Post-polio syndrome is diagnosed by exclusion, as there is no definitive test. Patients should undergo thorough diagnostic testing to rule out other medical conditions that may produce similar symptoms. Criteria used to establish the diagnosis of post-polio syndrome include a history of polio in the abortive, non-paralytic or paralytic forms, recovery from polio, a lengthy period of stability of at least 10–20 years’ duration and clinical manifestations of post-polio syndrome that are not associated with other medical disorders. Although the disabilities caused by post-polio syndrome are not necessarily obvious, they can have a significant impact on the patient’s quality of life.
 NURSING AND COLLABORATIVE MANAGEMENT: POST-POLIO SYNDROME
NURSING AND COLLABORATIVE MANAGEMENT: POST-POLIO SYNDROME
There is no specific treatment for post-polio syndrome.37,38 Management approaches are targeted at controlling symptoms, particularly fatigue, weakness and pain. An interdisciplinary team approach is essential to manage the patient. The cornerstone of management is lifestyle modification to conserve energy and support performance of ADLs.
During polio epidemics, polio victims were subjected to rigorous therapy to regain muscle function. A particular challenge may be helping the patient to understand that aggressive or strenuous therapy to strengthen muscles is now considered counterproductive in treating post-polio symptoms. Overexertion can worsen fatigue and weakness. Instead, it is important to promote pacing of activities to avoid feelings of fatigue. Planning to include rest periods, as well as using assistive devices such as scooters, canes and wheelchairs, may be beneficial. Adaptive equipment can be helpful for patients who experience difficulty with self-care. Other strategies include arranging for a disabled licence plate or sticker to facilitate parking close to shops and public buildings, shopping on the internet to avoid walking, and enlisting the support of family and friends to perform necessary tasks. Physiotherapy can be undertaken to support mobility and fitness in the light of the patient’s limitations. Weight loss interventions should be considered for the individual who is overweight.
Effective pain management through both pharmacological and non-pharmacological approaches can enable the individual to remain active and achieve a greater sense of wellbeing. Non-pharmacological measures include massage, relaxation strategies and guided imagery. Protection from the cold can also aid in pain relief; the individual with post-polio syndrome may be especially sensitive to a cold environment. It is important to collaborate with the healthcare provider or pain management team to derive an effective plan that meets individual patient needs.
For the individual with speech, swallowing or respiratory difficulties, it is important to take measures to prevent aspiration, maintain airway patency and promote optimal nutrition. Nursing interventions for these problems are similar to those described for patients with Guillain-Barré syndrome and spinal cord injury.
Experiencing the re-emergence of symptoms related to polio can have a devastating impact on the patient’s psychosocial wellbeing. Memories of paralysis and the challenges of recovery can lead to fear in a patient diagnosed with post-polio syndrome. Anxiety and depression, as well as difficulties with coping, may occur. The nurse can assist the individual by actively listening to concerns, providing information about post-polio syndrome and available resources, and referring the patient to support groups or counselling when necessary. Gaining a sense of control through active participation in lifestyle modifications and therapy may improve the patient’s ability to cope with post-polio syndrome.
The patient with spinal cord injury
CASE STUDY
Patient profile
Samuel Li, a 25-year-old man, is admitted to the emergency department with the diagnosis of a cervical spinal cord injury. Samuel was swimming in a neighbour’s backyard pool. He dived into the shallow end, striking his head on the bottom of the pool. His friends noticed that he did not resurface. They rescued him and brought him to the side of the pool, keeping his neck immobilised until the ambulance arrived.
CRITICAL THINKING QUESTIONS
1. What nursing activities would be a priority on this patient’s arrival in the ICU?
2. What physiological problems are causing this patient to have hypotension and bradycardia?
3. What would the first line of treatment be for his hypotension and bradycardia?
4. What signs and symptoms would indicate respiratory distress and what physiological problem would cause respiratory distress in this patient’s injury state?
5. What can the nurse do to decrease this patient’s anxiety?
6. Based on the assessment data provided, write one or more nursing diagnoses. Are there any collaborative problems?
1. What is the best method of education in the prevention of spinal cord injuries?
2. What type of support or education is best for the families of patients with spinal cord injuries to help them to cope with their situation?
3. What nursing interventions enhance self-care in the patient with a spinal cord injury?
4. What is the best method of preventing urinary tract infections in the patient with a spinal cord injury?
5. What is the relationship between the functional ability of spinal cord injury and quality of life?
1. During assessment of the patient with trigeminal neuralgia, the nurse should:
2. During routine assessment of a patient with Guillain-Barré syndrome, the nurse finds the patient to be short of breath. The patient’s respiratory distress is caused by:
3. A patient is admitted to the intensive care unit with a C7 spinal cord injury and diagnosed with Brown-Séquard syndrome. On physical examination, the nurse would most likely find:
4. A patient is admitted to the hospital with a spinal cord injury following a motor vehicle accident. The nurse recognises that the pathophysiology of secondary spinal cord injury involves:
5. A rehabilitation goal for the patient with an injury at the C5 level includes:
6. A patient with a C7 spinal cord injury undergoing rehabilitation tells the nurse he must have the flu because he has a bad headache and nausea. The initial action of the nurse is to:
7. For a 65-year-old female patient who has lived with a T1 spinal cord injury for 20 years, the nurse would emphasise the following health teaching information:
8. The most common early symptom of a spinal cord tumour is:
9. Which of the following descriptions best describes post-polio syndrome?
1 Bennetto L, Patel NK, Fuller G. Trigeminal neuralgia and its management. Br Med J. 2007;334:201–205.
2 Gronseth G, Cruccu G, Alksne J, et al. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review). Neurology. 2008;71:1183–1190.
3 Ritter PM, Friedman WA, Bhasin RR. The surgical treatment of trigeminal neuralgia: overview and experience at the University of Florida. J Neuro Nurs. 2009;41(4):211–214.
4 Tiemstra J, Khatkhate N. Bell’s palsy: diagnosis and management. 2007;76(7):997–1002.
5 Kuwaba S. Guillain-Barré syndrome: epidemiology, pathology and management. Drugs. 2004;64(6):597–610.
6 Kinson SB, Lamb Carr R, Maybee P, Haynes D. The challenge of managing and treating Guillain-Barré syndrome during the acute phase. Dimen Crit Care Nurs. 2006;25(6):256–263.
7 NNDSS Annual Report Writing Group. Australia’s notifiable disease status, 2008. Comm Dis Intell. 2010;34:2.
8 Department of Health and Ageing. Tetanus fact sheet and notifiable diseases. Available at www.immunise.health.gov.au/internet/immunise/publishing.nsf/content/tetanus. accessed 24 January 2011.
9 LaFond RE, Lukehart SA. Biological basis for syphilis. Clin Microbio Rev. 2006;19(1):29–49.
10 Norton L. Spinal cord injury Australia, 2007–2008. Canberra: Australian Institute of Health & Welfare, 2010.
11 Fitch MT, Silver J. CNS injury, glial scars and inflammation. Exp Neurol. 2008;209(2):294–301.
12 The Cat Walk Spinal Injury Trust. Spinal cord injury statistics. Available at www.catwalk.org.nz/node/25, Updated April 2010. accessed February 2011.
13 Wuermser LA, Ho CH, Ciodo AE, et al. Spinal cord injury medicine 2: acute care management of traumatic and nontraumatic injury. Arch Phys Med Rehabil. 2007;88(suppl 1):S55–S81.
14 American Spinal Injury Association/International Medical Society of Paraplegic (ASIA/IMSOP). International standards for neurological functional classification of spinal cord injury patients (revised). Chicago: American Spinal Injury Association, 2002.
15 Fehlings M, Wilson J, Dvorak M, Vaccaro A, Fisher C. The challenges of managing spine and spinal cord injuries: an evolving consensus and opportunities for change. Spine. 2010;35(21S):S161–S165.
16 Bracken MB, Holford TR. Neurological and functional status 1 year after acute spinal cord injury: estimates of functional recovery in National Acute Spinal Cord Injury Study II from results modeled in National Acute Spinal Cord Injury Study III. J Neurosurg. 2002;96(3 suppl):259–266. (Seminal paper)
17 Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277(20):1597–1604. (Seminal paper)
18 Pandya KA, Weant KA, Cook AM. High dose methylprednisolone in acute spinal cord injuries: proceed with caution, Orthopaedics. 2010;33(5):237. Available at www.orthosupersite.com/view.aspx?rid=63709 accessed 24 January 2011.
19 Tsao YT, Chen WL, Tsai WC. Steroids for acute spinal cord injury: revealing silent pathology. Lancet. 2009;374:500.
20 Kwan I, Bunn F, Roberts IG. Spinal immobilisation for trauma patients (review). The Cochrane Library. (4):2009.
21 Hsieh J, Wolfe D, Connolly AF, et al. Spasticity after spinal cord injury: an evidence-based review of current interventions. Top Spinal Cord Inj Rehabil. 2007;13(1):81–97.
22 Krassioukov A, Warburton DE, Teasau R, Eng JJ, SCIRE Research Team. A systematic review of the management of autonomic dysreflexia after spinal cord damage. Arch Phys Med Rehabil. 2009;90:682–695.
23 Wyndaele JJ. Conservative treatment of patients with neurogenic bladder, Eur Urol Supplements. 2008;7:557–565. Available at www.eu-acme.org/europeanurology/upload_articles/Wyndaele.pdf accessed 24 January 2011.
24 Valles M, Mearin F. Pathophysiology of bowel dysfunction in patients with motor incomplete spinal cord injury: comparison with patients with motor complete spinal cord injury. Dis Colon Rectum. 2009;52:1589.
25 Angel S, Kirkevold M, Pedersen B. Getting on with life following a spinal cord injury: regaining meaning through six phases. Int J Wellbeing. 2009;4:39–50.
26 Sakakibara BM, Miller WC, Orenczuk SG, Wolfe SG. A systematic review of depression and anxiety measures used with individuals with spinal cord injury. Spinal Cord. 2009;47(12):841–851.
27 Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: an emerging paradigm shift in rehabilitation and walking recovery. Phys Ther. 2006;86:1406–1425.
28 Behrman AL, Lawless-Dixon AR, Davis SB, et al. Locomotor training progression and outcomes after incomplete spinal cord injury. Phys Ther. 2005;85:1356–1371.
29 Behrman AL, Harkema SJ. Locomotor training after human spinal cord injury: a series of case studies. Phys Ther. 2000;80:688–700.
30 Barbeau H. Locomotor training in neurorehabilitation: emerging rehabilitation concept. Neurorehabil Neural Repair. 2003;17:3–11.
31 Goldshmit Y, Lythgo N, Galea MP, Turnley A. Treadmill training after spinal cord hemisection in mice promotes axonal sprouting and synapse formation and improves motor recovery. J Neurotrauma. 2008;25(5):449–465.
32 Harness ET, Yozbatira N, Cramer SC. Effects of intense exercise in chronic spinal cord injury. Spinal Cord. 2008;46(11):733–777.
33 Galea MP. To investigate active rehabilitation programs for people with spinal cord injury. The Winston Churchill Memorial Trust of Australia; 2007. Available at http://scia.org.au/walk-on/research accessed February 2011.
34 Charlifue S, Jha A, Lammertse D. Ageing with spinal cord injury. Phy Med Rehabil Clin N Am. 2010;21(2):383–402.
35 Cole A. Rehabilitation in advanced cancer. Cancer Forum 2010; 34(2). Available at www.cancerforum.org.au/Issues/2010/July/Forum/Rehabilitation_in_advanced_cancer.htm. accessed 21 January 2011.
36 National Health and Medical Research Council (NHMRC). Australian immunisation handbook. 9th edn. Canberra: NHMRC; 2008. Available at www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/Handbook-home, Updated 2009. accessed 24 January 2011.
37 Post-Polio Network (NSW). The late effects of polio. Available at www.post-polionetwork.org.au/late.html. accessed 25 January 2011.
38 Post-Polio Support Society of NZ (Inc). NZ polio data. Available at www.postpolio.org.nz/polio_and_post_polio_/nz_polio_data. accessed 25 January 2011.
39 Polio Global Eradication Initiative. Available at www.polioeradication.org/Polioandprevention.aspx, September 2010. accessed July 2011.
40 National Institute of Neurological Diseases. Post-polio fact sheet. Available at www.ninds.nih.gov/disoders/post_polio/detail_post_polio.htm. accessed 25 January 2011.
Australian Quadriplegic Association Victoria. www.aqavic.org.au
Christopher Reeve Paralysis Foundation. www.christopherreeve.org
Guillain-Barré Syndrome of New South Wales. www.gbsnsw.org.au
Guillain-Barré Syndrome Support Group of New Zealand. www.gbsnz.org.nz
Neurological Foundation of New Zealand. www.neurological.org.nz
Paraplegic and Physically Disabled Association Auckland. www.parafedauckland.co.nz
ParaQuad Association South Australia. www.pqasa.asn.au
Post-Polio Network (NSW) Inc. www.post-polionetwork.org.au
Spinal Cord Injuries Australia. www.spinalcordinjuries.com.au
Spinal Injuries Trust. www.nzspinaltrust.org.nz/library_resources_info.asp