Chapter 37
Acoustic Transduction
Chapter Outline
III. Mammalian Inner Ear Structure
IV. Cell Physiology of Endolymph Homeostasis
I Summary
The exquisite sensitivity of our sense of hearing results from the concerted action of many subsystems. The auditory peripheral organ, the cochlea, contains specialized cells for amplifying and sensing the mechanical auditory stimulus and other cells to create and maintain the unusual ionic environment in the cochlear lumen that powers and enables the transduction process. Function of these cells depends on specific gene expression patterns. A number of gene mutations are known to underlie specific clinical syndromic and non-syndromic hereditary hearing loss. It is perhaps surprising that most of these clinically important genes are located in the cells that engage in ion transport rather than the auditory sensory and neural cells. Major aspects of hearing and deafness can be understood as an integration of the many cellular processes that comprise the auditory organ.
II Introduction
The detection of sound by mammals depends on a series of biological systems beginning with the collection of sound pressure waves by the external ear, followed by the mechanical transmission through the middle ear ossicles to the cochlea of the inner ear (Fig. 37.1). The sound pressure waves in the cochlea induce motion of the basilar membrane to which the sensory organ, the organ of Corti, is attached. The organ of Corti is comprised of the sensory inner and outer hair cells (Fig. 37.2), which are surrounded by Deiters’ cells, pillar cells and Hensen’s cells. Motion of the organ of Corti with respect to another structure (tectorial membrane) causes movements of the sensory cilia (hairs) on the hair cells and modulation of the flow of current through these hair cells, leading to modulation of the rate of firing of the afferent auditory nerve fibers, which synapse to the base of the hair cells. The nerve fibers carry the auditory information to the brain where the signal undergoes central processing, leading to the perception of sound. Peripheral auditory processing is modulated by efferent signals originating from the brain. This chapter focuses on the cellular aspects of acoustic transduction in the auditory periphery. Much of what we know about the cellular physiology of the mammalian cochlea is derived from experiments performed on preparations from the vestibular labyrinth of mammals, birds and amphibians, and from the cochlea of birds. There are strong homologies between the cochlea and vestibular labyrinth which are reviewed elsewhere (Lee and Marcus, 2008; Marcus and Wangemann, 2009, 2010; Kim and Marcus, 2011).
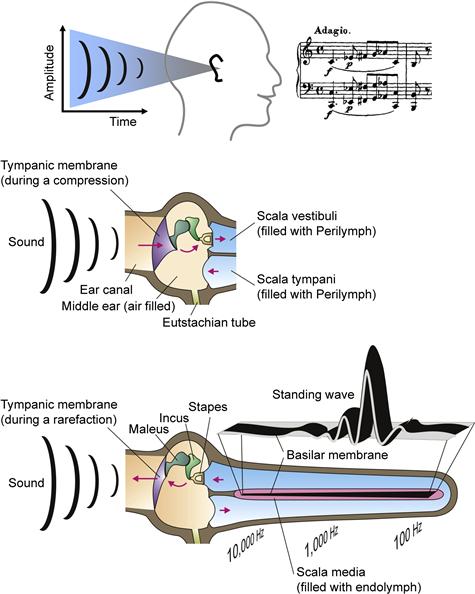
FIGURE 37.1 Diagrams of the physical pathway and effects of sounds from the environment to the cochlea. (Upper panel): Sound waves enter the outer ear and (middle panel) are transmitted by the tympanic membrane to the middle ear bones (ossicles: maleus, incus, stapes). The sound waves are then transmitted to scala vestibuli of the inner ear through the oval window. Acoustic energy enters the cochlea (lower panel; diagrammed uncoiled) where the frequency content of the sound waves is analyzed by the creation of standing waves along the basilar membrane and excites receptors and associated neurons at locations that code for the frequencies comprising the sound. (Reproduced with permission from Marcus and Wangemann, (2009). In F.J. Alvarez-Leefmans and E. Delpire, eds), Physiology and Pathology of Chloride Transporters and Channels in the Nervous System – From Molecules to Diseases, pp. 425–437. Elsevier, New York.)
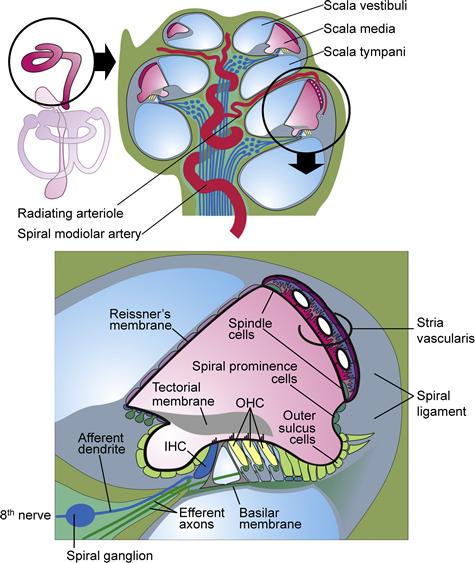
FIGURE 37.2 Diagram of a cross-section of a coiled cochlea. Upper panel shows the membranous structures of the inner ear (left) with the cochlea in the circle and a cross-section of the entire cochlea (right). Lower panel shows a cross-section of one turn of the cochlear lumen, scala media (pink), which is filled with endolymph, an unusual extracellular fluid that is high in K+ and low in Na+ and Ca2+ content. The composition of this fluid is maintained by the epithelial cells bounding the lumen that include the stria vascularis in the lateral wall, Reissner’s membrane and organ of Corti that contains the sensory inner hair cells and the outer hair cells that provide amplification of the sound-induced mechanical vibrations of the basilar membrane. (Reproduced with permission from Kim, H. M. and Wangemann, P. (2011) Epithelial cell stretching and luminal acidification lead to a retarded development of stria vascularis and deafness in mice lacking pendrin. PLoS.One. 6(3):e17949; Griffith, A. J. and Wangemann P. (2011) Hearing Loss Associated with Enlargement of the Vestibular Aqueduct: Mechanistic Insights from Clinical Phenotypes, Genotypes, and Mouse Models. Hear Res, doi:10.1016/j.heares.2011.05.009; Marcus and Wangemann, (2009). In (F.J. Alvarez-Leefmans and E. Delpire, eds), Physiology and Pathology of Chloride Transporters and Channels in the Nervous System–From Molecules to Diseases, pp. 425–337. Elsevier, New York.)
III Mammalian Inner Ear Structure
The transduction apparatus is part of an epithelium forming the cochlear duct and separating two distinct cochlear fluids, endolymph and perilymph. The composition and importance of these fluids is related later. A diagram of a cross-section of the mammalian cochlear duct is shown in Fig. 37.2. A mechanically stiff apical surface of the organ of Corti, the reticular lamina, is formed by cuticular plates just under the apical membrane of these cells. The organ of Corti sits on the basilar membrane, a fibrous sheet that transmits the acoustic stimulus.
In the medial direction from the organ of Corti, the duct is comprised of inner sulcus cells and interdental cells of the spiral limbus. The tectorial membrane is a gelatinous, acellular structure in the cochlear lumen apparently secreted by the interdental cells. Lateral from the organ of Corti, the cochlear duct consists of outer sulcus cells, spiral prominence cells and marginal cells of the stria vascularis. Reissner’s membrane forms the remaining wall of the triangular-shaped cochlear duct and is comprised of a thin, avascular sheet of epithelial cells.
The apical and basolateral membranes of all of these cells are separated by tight junction complexes near the endolymphatic surface, which serve to join each cell to its neighbor and to complete the barrier between endolymph and the fluid bathing the basolateral membranes. The basolateral fluid is perilymph for all cell types except strial marginal cells, as described later. The cochlear duct is closed at the apex of the cochlea and is joined at the base of the cochlea via a constriction in the epithelial lumen (ductus reuniens) to the vestibular system. Cellular physiologists have focused much of their attention on the strial marginal cells, which provide the energy source for the transduction process, and on the sensory hair cells themselves. In recent years, the contributions of other cell types bordering the cochlear lumen to endolymph ion homeostasis have been determined and are under continuing investigation.
IV Cell Physiology of Endolymph Homeostasis
IVA Composition
Even in the absence of an acoustic stimulus, the cochlea is highly active, maintaining the electrolyte composition of endolymph (Table 37.1) and a standing current analogous to the “dark current” of photoreceptors (see Chapter 38) (Marcus and Wangemann, 2010). The transduction current from endolymph through the hair cells is carried predominantly by K+ and so depends on the high concentration of that ion in endolymph. A low concentration of Ca2+ in endolymph is regulated by several cells types; this ion maintains the integrity of tip links between stereocilia of hair cells and enters the transduction channels of hair cells to modulate transduction processes. Gross changes in endolymph composition by a tear in Reissner’s membrane, by drug action or by genetic interference in K+ secretion led to degeneration of the hair cells with subsequent degeneration of the synapsing afferent auditory nerve (Vetter et al., 1996). The dimensions of the gelatinous tectorial membrane are sensitive to the levels of K+, Na+, H+ and Ca2+ as well as to unknown chemical factors (Shah et al., 1995). Swelling has been associated with substitution of Na+ for K+ and with decreases of Ca2+. Maintenance of the tectorial membrane structural properties is needed for normal hearing since it is one of the physical structures coupling the acoustic stimulus to hair cells.
TABLE 37.1. Approximate Ion Composition and Electrical Potential of Cochlear Endolymph and Perilympha
| Ion | Endolymph | Perilymph |
| Potassium (mM) | 157 | 4.2 |
| Sodium (mM) | 1.3 | 148 |
| Calcium (mM) | 0.02 | 1.3 |
| Chloride (mM) | 132 | 119 |
| Bicarbonate (mM) | 31 | 21 |
| Protein (mg/dL) | 38 | 178 |
| pH | 7.5 | 7.3 |
| Potential (mV) | +80 | 0 |
a Reproduced by permission from D.C. Marcus and P. Wangemann (2010). Inner ear fluid homeostasis. In P.A. Fuchs, ed.), The Oxford Handbook of Auditory Science: The Ear, pp. 213–230. Oxford University Press, Oxford.
IVB Cellular Basis of Endolymphatic Ion Homeostasis
The ion composition of fluid compartments in the body is maintained by epithelial ion transport. The known epithelial domains in the ear responsible for K+ and Na+ transport are shown in Fig. 37.3A. Homeostatic imbalance leads to swelling or collapse of the cochlear lumen (Fig. 37.3B), as observed in Pendred syndrome or Scheibe’s deformity, respectively.
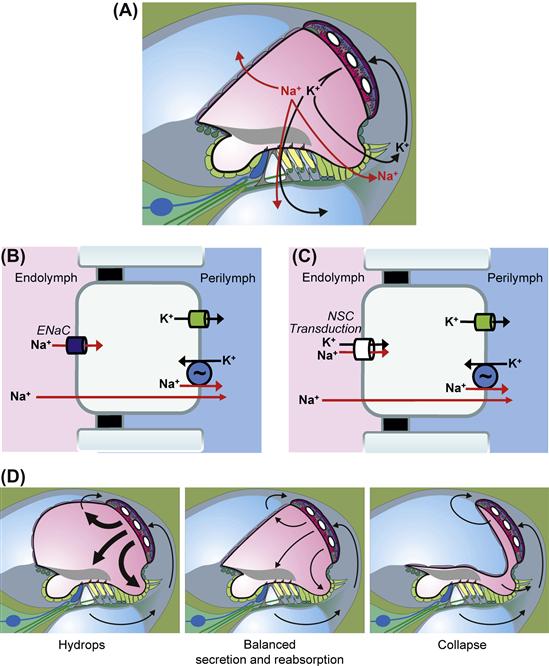
FIGURE 37.3 Schematic cross-sections through one cochlear turn illustrating inner ear ion and water transport. (A) K+ secretion by stria vascularis (SV) and absorption by sensory cells in the organ of Corti and outer sulcus cells; Na+ absorption by Reissner’s membrane, hair cells and outer sulcus cells. Cell models of transepithelial Na+ absorption via Na+-selective epithelial Na+ channels (ENaC) (B) or non-selective cation channels (C), including the non-selective transduction channels of hair cells. (D) Effects of hypoabsorption and/or hypersecretion (hydrops) of balanced transport (normal cross-section) and of hyperabsorption and/or hyposecretion (collapse). (C). (Reproduced with permission from Kim, S.H. and Marcus, D.C. (2011). Regulation of sodium transport in the inner ear. Hear Res. 280, 21–29, and Wangemann, P. (2002). Adrenergic and muscarinic control of cochlear endolymph production. Adv Otorhinolaryngol. 59, 42–50, and Marcus and Wangemann, (2009). In (F.J. Alvarez-Leefmans and E. Delpire, eds), Physiology and Pathology of Chloride Transporters and Channels in the Nervous System–From Molecules to Diseases, pp. 425–437. Elsevier, New York).
Transepithelial transport is accomplished through expression of a special constellation of specific ion channels, transporters and pumps in the apical and basolateral membranes. The influx and efflux pathways for Na+ and K+ in the cochlea are illustrated in Fig. 37.3A. K+ is taken up by the stria vascularis from the spiral ligament in the lateral wall and secreted into endolymph. K+ enters the hair cells through mechanosensitive transduction channels in the apical stereocilia and this entry flux is modulated by the acoustically-gated mechanosensitive channels. K+ then leaves the hair cells across their basolateral membranes via K+-selective ion channels. The accumulated K+ in the extracellular fluid surrounding the hair cells returns to the spiral ligament by diffusion. Three alternate pathways have been proposed and are described in Fig. 37.4. K+ reaching the spiral ligament is then returned to the endolymph by the stria vascularis. Additional descriptions of K+ transport by the stria vascularis and of the transduction process are given below.
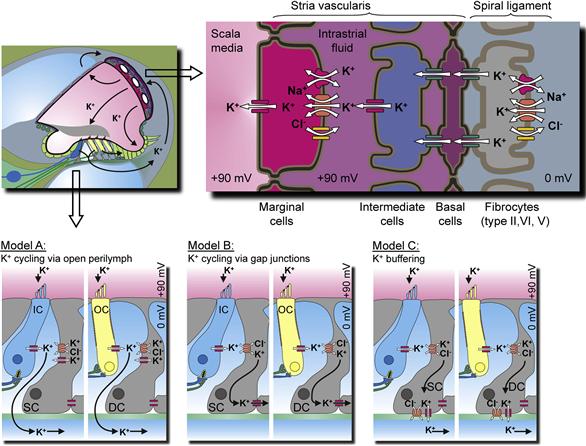
FIGURE 37.4 Overview of the stria vascularis and its K+ transport mechanisms and three models of K+ pathways that remove K+ from the hair cells. (Inset) Cochlear cross-section. (Top panel) The stria vascularis consists of three distinct cell types: marginal, intermediate and basal cells. Intermediate cells are connected via gap junctions to basal cells which, in turn, form gap junctions with underlying fibrocytes. Gap junctions among the fibrocytes are not shown. (Bottom panel) Model A: postulates that K+ released from hair cells cycles back to the stria vascularis through the open perilymph space; Model B: entails K+ recycling through inner phalangeal cells, marked as supporting cells (SC) by the inner hair cells (IC) or Deiters’ cells (DC) by the outer hair cells (OC); Model C: the supporting cells act as a K+ buffer. Note that these three models are not mutually exclusive. (Reproduced with permission from Zdebik, A.A., Wangemann, P. and Jentsch, T.J. (2009). Potassium ion movement in the inner ear: insights from genetic disease and mouse models. Physiology (Bethesda.) 24, 307–316, and Marcus and Wangemann, (2009). In (F.J. Alvarez-Leefmans and E. Delpire, eds), Physiology and Pathology of Chloride Transporters and Channels in the Nervous System–From Molecules to Diseases, pp. 425–437. Elsevier, New York.)
Endolymphatic [Na+] is maintained below electrochemical equilibrium with the perilymph; it “leaks” into the cochlear lumen and so must be actively removed. Two types of transport have been identified and described (reviewed in Kim and Marcus, 2011) (see Fig. 37.3B, C). The outer sulcus cells and the hair cells possess non-selective cation (NSC) channels in their apical membranes (small-conductance NSC channels and the large mechanosensitive transduction channels, respectively). Na+, as well as K+, enters the cells via these channels and are removed from the cells into perilymph by basolateral Na+, K+-ATPase (the Na+-pump) in parallel with K+ channels. The outer sulcus cells thereby act as a parasensory K+ pathway in addition to their function as a Na+ extrusion mechanism. This parasensory Na+ and K+ efflux is modulated by luminal ATP via P2X ionotropic receptors (Chapter 31).
A second, and perhaps more significant, Na+ efflux pathway is through Reissner`s membrane epithelium (reviewed in Kim and Marcus, 2011). These cells utilize apical epithelial Na+ channels (ENaC) to mediate Na+ absorption. ENaC activity can be under a variety of intracellular and extracellular control mechanisms. Na+ absorption by Reissner’s membrane is known to be stimulated by glucocorticoids via a genomic pathway and appears to be controlled by luminal ATP. ATP would increase the Na+ absorption via ligand-gated NSC channels (P2X receptors) and decrease Na+ absorption via signalling from P2Y4 receptors.
IVC Stria Vascularis
The stria vascularis has two primary known functions: secretion of K+ and generation of the lumen-positive endocochlear potential (EP; ca. +80 mV). The high endolymphatic K+ concentration provides the carrier of the transduction current. The driving force for that current consists almost exclusively of the voltage across the transduction channels in the stereocilia (see Section VI) and that voltage is the sum of the intracellular potential of the hair cells and the EP. The large EP therefore heightens the sensitivity of the cochlear transduction process compared to vestibular organs, which do not have this high transepithelial electrical polarization. The cellular transport model by which the stria vascularis secretes potassium and generates the EP is shown in Fig. 37.4 and described next. Recall that all epithelial cells that produce a vectorial transport of substances do so by virtue of different membrane properties of their apical and basolateral membranes.
IVC1 Division of Function between Marginal and Basal Cell Barriers
The stria vascularis consists of two barriers formed by the marginal cells and the basal cells. Each barrier consists of a continuous sheet of cells joined by tight junction complexes (see Fig. 37.4). Between these barriers is the intrastrial space with the capillary bed for which the tissue is named and a discontinuous layer of intermediate cells. The basal cells are joined via gap junctions (see Chapter 22) to the intermediate cells and to fibrocytes in the adjacent connective tissue, suggesting a level of cooperation among these three cell types (Kikuchi et al., 2000). Unlike most sheets of epithelial cells, strial marginal cells are not coupled to each other nor to other cells by gap junctions (Takeuchi and Ando, 1998; Kikuchi et al., 2000). The physiological significance of this functional independence of marginal cells is not known.
The basal cell/intermédiate cell syncytium produces and supports the endocochlear potential and the strial marginal cells secrete K+. In spite of the distinct cellular functions, the two processes are closely tied together through the composition of the intrastrial space. For example, inhibition of K+ secretion by the marginal cells leads to a decline of the endocochlear potential. This would occur by a rise in intrastrial K+ concentration upon cessation of K+ uptake by the marginal cells and the consequent depolarization of the K+-selective intermediate cell membrane (see below). Several lines of evidence, including histochemical, biochemical, electrophysiological and flux studies, have strongly suggested that the stria vascularis is responsible for secretion of K+ into the cochlear lumen and for production of the EP (reviewed in Marcus and Wangemann, 2010).
IVC2 K+ Secretion by Strial Marginal Cell Epithelium
Active K+ secretion in the cochlear duct has been demonstrated by flux measurements of radiolabeled K+ introduced in either the perilymphatic or vascular space and its appearance in endolymph (Konishi et al., 1978; Sterkers et al., 1982). These fluxes were inhibited by transport blockers and anoxia. The stria was assumed to be the site of secretion since the flux was nearly the same when the radiotracer was added to either scala vestibuli or scala tympani; possible contributions by the spiral limbus were disregarded.
More recently, the stria was isolated from the cochlear duct, a K+-selective self-referencing probe was placed near the tissue in vitro and a K+ gradient was found directed away from the luminal surface (Wangemann et al., 1995) (Fig. 37.5A). The marginal cell layer of the stria vascularis produces this K+ flux using the constellation of transport processes shown in Fig. 37.4. K+ is taken up across the basolateral membrane from the intrastrial space fluid by two transporters: the Na+,K+-ATPase (Na+ pump) and the NKCC1 Na+-K+-Cl− co-transporter. The first is a primary active process, which uses the energy from cytosolic adenosine triphosphate (ATP) and the second is secondary active and uses the large Na+ concentration gradient between extracellular and intracellular compartments created by the Na+,K+-ATPase. Na+ and Cl− taken up by the co-transporter are removed from the cytosol by the Na+,K+-ATPase and basolateral ClC-K/barttin (/BSND) Cl− channels, respectively (Marcus and Wangemann, 2010).
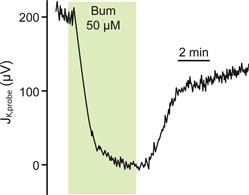
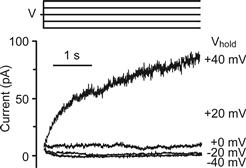
FIGURE 37.5 Transepithelial K+ flux and apical membrane K+ currents via KCNQ1/KCNE1 K channels. (A) K+ secretory flux from stria vascularis measured with a K+-selective self-referencing probe. The ordinate is the flux expressed as the difference in microvolts measured at the two positions of the probe. Flux was decreased by basolateral perfusion of the inhibitor of Na+-K+-Cl− co-transport, bumetanide (Bum). (Adapted from Wangemann, P., Liu, J. and Marcus, D.C. (1995). Ion transport mechanisms responsible for K+ secretion and the transepithelial voltage across marginal cells of stria vascularis in vitro. Hear Res. 84, 19–29, Copyright 1995 with kind permission of Elsevier Science-NL, Sara Burgerhartstraat 25, 1055 KV Amsterdam, The Netherlands.). (B) Patch-clamp currents from the apical membrane of a strial marginal cell, on-cell configuration. Sustained depolarizations activate the current slowly over several seconds, characteristic of KCNQ1/KCNE1 K+ channels. (Reproduced with permission from Shen, Z., Marcus, D.C., Sunose, H., Chiba, T. and Wangemann, P. (1997). IsK channel in strial marginal cells: voltage-dependence, ion-selectivity, inhibition by 293B and sensitivity to clofilium. Auditory Neurosci. 3, 215–230.)
K+ secretion occurs passively via channels in the apical membrane (see Figs. 37.4 and 37.5). These channels are products of the genes KCNQ1 and KCNE1, which code for the pore-forming α subunit and the regulatory β subunit of the channel, respectively. The KCNQ1/KCNE1 channels are characterized by slow activation (over seconds) in response to depolarization of the cell membrane and have a single-channel conductance of about 14 pS under in vivo-like conditions (see Fig. 37.5B) (Marcus and Shen, 1994; Shen et al., 1997; Marcus and Wangemann, 2010). The essential contributions to K+ secretion of the apical K+ channel and of the basolateral co-transporter have been demonstrated with gene knockout mice. Individual deletion of KCNQ1, KCNE1 or NKCC1 (Slc12a2) resulted in a collapsed lumen in knockout mice.
IVC3 Production of Endocochlear Potential by Strial Intermediate and Basal Cells
Two key genes expressed in the stria vascularis are at the heart of EP generation: those that code for the tight junction protein claudin-11 and the KCNJ10 K+ channel. Tight junctions are formed from an assemblage of a number of proteins and the family of claudin isoforms provides definition to the permeability characteristics of each junction. The basal cell barrier contains claudin-11 and the connections among these cells are the only location for claudin-11 in the inner ear. An important demonstration of the importance of claudin-11 to EP generation was the observation that the EP collapsed in claudin-11 knockout mice, while K+ secretion continued since marginal cell function was unimpaired (Gow et al., 2004).
The KCNJ10 K+ channel (also known as Kir4.1) is expressed in the membrane of intermediate cells (see Fig. 37.4). Intracellular K+ is maintained high by uptake from perilymph by fibrocytes in the spiral ligament and diffusion via gap junctions into basal cells and intermediate cells (see Fig. 37.4) (Kikuchi et al., 2000). Intrastrial K+ is maintained at an unusually low level in the intrastrial space by the marginal cells. The membrane potential of the intermediate cells is dominated by the KCNJ10 conductance. Normally, one would then expect the intermediate cells to be highly negative, as observed in symmetrical cells that are dominated by a membrane K+ conductance. However, the basal cells are highly depolarized, perhaps by non-selective cation channels, and the high density of gap junctions between basal cells and intermediate cells effectively “grounds” the cytosol of the intermediate cells. With the intermediate cells near zero with respect to the extracellular fluid of the spiral ligament (perilymph), the intrastrial space (electrically isolated from the perilymph by the claudin-11-containing tight junctions of the basal cells) is highly positive. That high positive potential appears in the endolymph as the EP. The marginal cell layer generates very little contribution to the EP, similar to the K+-secreting vestibular dark cell epithelium which is a monolayer without the intermediate cell/basal cell layer. The importance of the KCNJ10 channel to EP generation is supported by the observation that KCNJ10 knockout mice have no EP, but maintain an elevated endolymphatic [K+].
The general scheme of strial function shown in Fig. 37.4 was first supported by observations of the electrical and K+ profile of the stria obtained with double-barrel electrodes (Salt et al., 1987). In the spiral ligament, the potential was taken as zero and the K+ concentration was a few millimolar (Fig. 37.6). As the electrode was advanced, a region was found where the K+ concentration was also low but the potential had risen to about +80 mV. This was interpreted as being located in the intrastrial space. Further penetration led to a jump of the K+ concentration to that commonly found in endolymph with no appreciable change in the potential. The voltage gradient was therefore largest across the basal cell layer and the K+ gradient largest across the marginal cell layer.
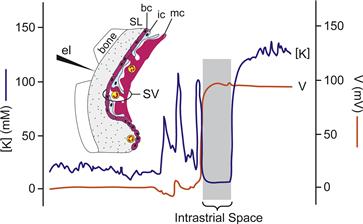
FIGURE 37.6 Recording of profiles for voltage (orange line; right axis) and K+ concentration (blue line; left axis) during penetration of the stria vascularis (SV). A region was found where the voltage with respect to perilymph was highly positive but the K+ concentration was low; this region was interpreted to be the intrastrial space. SL, spiral ligament; bc, basal cell; ic, intermediate cell; mc, marginal cell; el, penetrating electrode advanced from left to right. Horizontal axis is time/distance of electrode advancement. (Adapted with permission from Salt, A.N., Melichar, I. and Thalmann, R. (1987). Mechanisms of endocochlear potential generation by stria vascularis. Laryngoscope. 97, 984-991, and (inset) from Dallos, P. (1996). Overview: Cochlear neurobiology. In (P. Dallos, A.N. Popper, and R.R. Fay, eds), The Cochlea, pp. 1–43. Copyright 1996 by Springer-Verlag.)
IVC4 Regulation of Ion Transport in Strial Marginal Cells
Every cell type encounters perturbations and signals by systemic variables, such as by hormones and/or neurotransmitters, osmotic strength and extracellular [K+] and pH (pHo), and by cytosolic variables, such as intracellular Ca2+ and pH (pHi). K+ secretion in the inner ear is altered in response to many such stimuli.
Basolateral [K+] and Apical pH
The increase in flow of K+ through hair cells in response to acoustical stimulation is known to increase the concentration of K+ in the perilymph surrounding the basolateral membranes of the hair cells and the nerve endings. The rate of basolateral uptake of K+ in strial marginal cells and of its secretion through the apical K+ channel has been found to be exquisitely sensitive to the concentration of perilymphatic K+ (Fig. 37.7). This K+ sensitivity is believed to play a significant role in regulating the recirculation of K+ back into endolymph (Wangemann et al., 1996) (see Fig. 37.7). Extracellular pH and [Ca2+] of the lumen is under cellular control in the cochlea (see below). The luminal pH can control key ion transporters in the stria vascularis and other cells. Of particular importance is the stimulation of KCNQ1/KCNE1 K channel activity and the inhibition of TRPV5/6 Ca2+ channel activity by extracellular pH (see below).
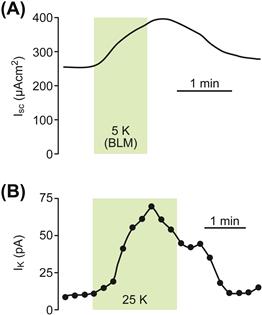
FIGURE 37.7 Stimulation of (A) short circuit current (Isc) and (B) K+ current through the apical KCNQ1/KCNE1 K+ channels (IK) on K+ concentration in vestibular dark cells, the homologs to strial marginal cells. Basolateral [K+] was increased from 3.6 mM to 5 mM (A) or 25 mM (B). (Adapted from Wangemann, P., Shen, Z., and Liu, J. (1996). K+ induced stimulation of K+ secretion involves activation of the IKs channel in vestibular dark cells, Hear Res. 100, 201–210. Copyright 1996 with kind permission of Elsevier Science-NL, Sara Burgerhartstraat 25, 1055 KV Amsterdam, The Netherlands.)
Hormones
There is no innervation of the stria vascularis, but these cells contain receptors coupled to ion transport for several local or systemic hormones, including catecholamines, ATP and adrenocorticosteroid hormones (Table 37.2). Vasopressin (antidiuretic hormone) affects the EP and longitudinal K+ concentration gradient (Mori et al., 1989; Julien et al., 1994), but the cells with vasopressin receptors have not yet been identified. Basolateral perfusion in vitro of the β-adrenergic agonist isoproterenol caused maximal increases in Isc of 40–75% in strial marginal cells (Wangemann, 2002).
TABLE 37.2. Hormones and Signal Pathways Regulating KCNQ1/KCNE1 Current
| Hormone/Signal | Stimulate | Inhibit |
| β-Adrenergic agonist | ||
| Basolateral | X | |
| Purinergic agonist | ||
| Apical | Transient | X |
| Basolateral | X | |
| cAMP | X | |
| Phospholipase C | X | |
| Protein kinase C | X | |
| Cytosolic Ca2+ | X | |
| Cytosolic pH | Transient | X |
| Cell swelling | X | |
β-Adrenergic receptors
β-Adrenergic receptors are commonly coupled via G proteins to adenylate cyclase (see Chapter 6). Indeed, an increase of cytosolic cAMP in strial marginal cells by direct stimulation of adenylate cyclase, by perfusion of a membrane-permeable cAMP analog, or by inhibition of phosphodiesterases that catalyze the breakdown of cAMP, all lead to an increase of Isc. The stimulation of K+ secretion by adrenergic agonists was found to be mediated by β-adrenergic receptors through determination of the potency order of specific antagonists and by demonstration of the presence of transcripts for this subtype in cochlear tissue.
In isolated strial marginal cell epithelium, both apical and basolateral perfusion of micromolar ATP significantly alter Isc (Fig. 37.8) (Lee and Marcus, 2008). Apical ATP and analogs monotonically downregulate Isc, whereas basolateral ATP and analogs transiently increase Isc followed by downregulation. The data are consistent with the presence of purinergic receptors of the P2Y4 subtype on the apical membrane and P2Y2 subtypes on the basolateral membrane. Additional purinergic receptors may also play a role.
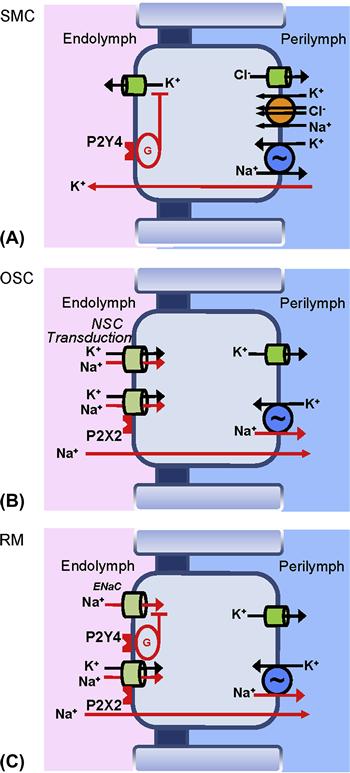
FIGURE 37.8 Purinergic signaling in the cochlear epithelium. (A) stria vascularis marginal cells; activation of P2Y4 receptors in the apical membrane reduce secretory K+ flux via a G-protein (G) signal cascade (Lee and Marcus, 2008). There are additional purinergic receptors on the basolateral side (Lee and Marcus, 2008). (B) Outer sulcus cells; activation of apical P2X2 receptors open the associated non-selective cation channels (Lee and Marcus, 2008). (C) Reissner’s membrane epithelial cells; activation of P2Y4 receptors (membrane location unknown) reduces ENaC-mediated Na+ absorption, and activation of apical P2X2 receptors open the associated non-selective cation channels. (Reproduced by permission from Kim, S.H. and Marcus, D.C. (2011). Regulation of sodium transport in the inner ear. Hear Res. 280, 21–29, and Lee, J.H., and Marcus, D.C. (2008). Purinergic signaling in the inner ear. Hear Res 235, 1–7.)
P2Y receptor subtypes are known in other cells to be coupled to G proteins that stimulate phospholipase C (PLC). PLC catalyzes the breakdown of membrane phospholipid to produce both inositol trisphosphate (IP3) and diacylglycerol (DAG) (see Chapter 6). It is most common that the IP3 branch of the PLC pathway regulates ion channel activity through the cytosolic level of free Ca2+. However, it was shown that the apical P2Y4 receptor downregulates K+ secretion primarily via the DAG-protein kinase C (PKC) branch (Lee and Marcus, 2008) even though inositol phosphate production is increased in the cochlear lateral wall in response to P2Y agonists (Ogawa and Schacht, 1995).
Basolateral muscarinic receptors (M3 and/or M4) were observed to inhibit K secretion by strial marginal cells, but the lack of direct innervations led to the speculation that circulating hormone might conceivably provide the agonist (Wangemann, 2002).
Steroid Hormones
Binding sites for both glucocorticosteroid (ten Cate et al., 1993) and mineralocorticosteroid (Yao and Rarey, 1996) have been demonstrated in the stria vascularis. Both corticoids control the activity of Na+, K+-ATPase in the stria vascularis (adrenalectomy reduced activity 60% and systemic administration of either the glucocorticosteroid dexamethasone or the mineralocorticosteroid aldosterone restored activity) (Curtis et al., 1993) and the increase in Na+, K+-ATPase with aldosterone was not dependent on major changes in blood plasma cation concentration (ten Cate et al., 1994). However, in spite of the strong dependence of the EP on strial Na+, K+-ATPase, a reduction of adrenocorticosteroids by adrenalectomy did not significantly reduce the EP in the presence or absence of strong acoustic stimulation (Ma et al., 1995). In addition, glucocorticoid hormones alone apparently do not stimulate the formation of Na+, K+-ATPase in the inner ear since no difference in the level of antibody binding to Na+, K+-ATPase was observed in glucocorticoid receptor knockout mice (Erichsen et al., 1998). Acute, non-genomic actions of steroid hormones were observed on currents from stria vascularis (Lee and Marcus, 2001, 2002). Corticosteroids stimulated currents only at therapeutic levels and not at normal circulating levels and estrogen inhibited currents at concentrations only found during the end of pregnancy.
A clear demonstration of steroid regulation of ion transport in the cochlea is on Reissner`s membrane. Both Na+ absorption and the expression of the α subunit of ENaC are markedly increased after 24 h exposure to the synthetic glucocorticoid dexamethasone (Fig. 37.9). This action of dexamethasone was via the glucocorticoid receptor (GR) and not via the mineralocorticoid receptor (MR) since both of the stimulatory actions were blocked by a specific GR inhibitor and not by an MR inhibitor.
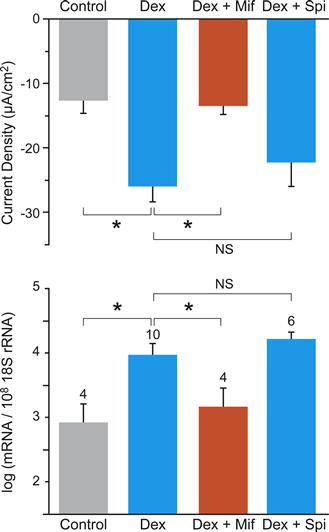
FIGURE 37.9 Stimulation of transepithelial sodium absorption by glucocorticoid, mediated by glucocorticoid receptor. (A) Changes of current density from Reissner’s membrane under control conditions (gray bar), dexamethasone (blue bar; 100 nM, 24 h), dexamethasone + the glucocorticoid receptor antagonist mifepristone (orange bar; Mif, 100 nM), and dexamethasone + the mineralocorticoid receptor antagonist spironolactone (2nd blue bar; Spi, 100 nM). (B) qRT-PCR evaluation of transcript expression of the α subunit of ENaC under the same conditions as in (A). ∗P < 0.05; NS, not significant. (Reproduced with permission from Kim, S.H., Kim, K.X., Raveendran, N.N., Wu, T., Pondugula, S.R. and Marcus, D.C. (2009). Regulation of ENaC-mediated sodium transport by glucocorticoids in Reissner’s membrane epithelium. Am J Physiol Cell Physiol. 296, C544–C557.)
IVD Calcium and Acid/Base Transport
Endolymphatic [Ca2+] is unusually low for an extracellular fluid (see Table 37.1) and its level is apparently maintained at its set point by a “push-pull” system. Ca2+ is secreted (“pushed”) into endolymph by the plasma membrane Ca2+-ATPase PMCA2, which is located in the stereocilia of hair cells and likely also in Reissner’s membrane (Yamoah et al., 1998; Kim et al., 2009; Marcus et al., 2011). Gene deletion/mutation of PMCA2 leads to a reduced level of endolymphatic [Ca2+] (Wood et al., 2004). An acid-sensitive Ca2+ absorptive system (“pull”) is expressed in the cochlea and its gate-keeper apical channels, TRPV5 and TRPV6, are located in the inner and outer sulcus cells and have also been observed in marginal cells (Yamauchi et al., 2010).
Endolymphatic acid/base balance is apparently also a “push-pull” system. H+ secretion from stria vascularis has been observed with self-referencing pH electrodes (Miyazaki, Wangemann and Marcus, unpublished observations). In addition, HCO3− secretion occurs via the Cl−/HCO3− exchanger pendrin (Slc26a4), which is expressed in the apical membrane of strial spindle cells, spiral prominence and outer sulcus epithelial cells (Wangemann et al., 2004, 2007; Griffith and Wangemann, 2011). Pendrin, and likely other HCO3− transporters, maintain endolymph alkaline with respect to perilymph (see Table 37.1). Deletion of Slc26a4 leads to an acidification of endolymph (Wangemann et al., 2007).
V Genetic Basis of Deafness
Nearly 50% of profound hearing loss results from hereditary factors (Nance, 2003; Bayazit and Yilmaz, 2006; Morton and Giersch, 2010). Genetic causes of hearing loss are classified and identified in several ways. Clinical manifestations that are solely hearing-related are “non-syndromic” and those with pathologies in the ear plus one or more other organs are “syndromic”. The chromosomal loci of non-syndromic hereditary Dea FNess are designated with DFN numbers, such as DFNA1 or DFNB 1. The A and B refer to autosomal dominant and autosomal recessive transmission of hearing loss and cases without A or B are X-linked; 80% of cases are of DFNB type (Bayazit and Yilmaz, 2006). Additional forms of deafness occur from mutations of the mitochondrial genome.
Several on-line sources of information on hereditary hearing loss include:
• the American Hearing Loss Foundation (http://www.american-hearing.org/disorders/congenital-deafness/)
• Dr Timothy C. Hain (http://www.dizziness-and-balance.com/disorders/hearing/cong_hearing.html)
• Hereditary Hearing Loss by Dr Guy Van Camp and Dr Richard Smith (http://hereditaryhearingloss.org/)
• Laboratory website of Dr Karen Avraham (http://www.tau.ac.il/~karena/overview-genetics.html).
DFNB1 is the most common DFNB and results from mutations of the gap junction gene coding for connexin-26, GJB2. Gap junctions between epithelial cells and between fibrocytes of the lateral cochlear wall and among fibrocytes, strial basal cells and strial intermediate cells (see Fig. 37.4) are crucial to acoustic transduction. The physical properties of junctions composed of heteromeric connexin-26 and connexin-30 as occur in the cochlea continue to be investigated (Change et al., 2008; Hoang et al., 2009).
Two of the important syndromic hearing losses are Jervell and Lange-Nielsen (JLN) syndrome and Pendred syndrome. JLN results from mutations of the KCNQ1/KCNE1 K channel in either of the subunits. It is “syndromic” because this K channel plays a prominent role in cardiac function as well as providing the sole route of K efflux from strial marginal cells (see above). Pendred syndrome results from mutations of the Cl−/HCO3−-exchanger coded by Slc26a4. As mentioned above, deletion of this gene in mice results in an acidic shift of endolymphatic pH, as expected from a HCO3−-secretory transporter. The effects of pendrin mutation, however, go far beyond this relatively obvious consequence. A series of recent studies (reviewed in Griffith and Wangemann, 2011) has found that hearing loss results from a constellation of events.
Without normal levels of expression of functional pendrin during late embryonic and early postnatal development, there is tremendous expansion of the developing cochlea (10-fold increase in luminal cross-sectional area) that leads to enlargement and acidification of cochlear endolymph, which spread the effect from pendrin-expressing cells to many other cell types. The result is a delayed development of the stria vascularis and a fluctuating local oxidative stress in the stria. This oxidative stress results in downregulation of expression of the KCNJ10 K channel in the intermediate cells (Fig. 37.10). KCNJ10 expression is known to be especially sensitive to oxidative stress and its downregulation compromises the EP, which was proposed to be a factor leading to fluctuating hearing loss (Griffith and Wangemann, 2011).
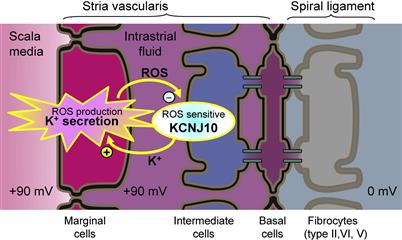
FIGURE 37.10 Proposed mechanism for fluctuating hearing loss. Diagram of the stria vascularis illustrating a feedback mechanism that leads to fluctuating loss of the K+ channel that generates the endocochlear potential, KCNJ10. An increased rate of K+ secretion related to the enlarged scala media generates increased levels of reactive oxygen species (ROS) in marginal cells. ROS diffuses to intermediate cells, where they downregulate expression of KCNJ10 channels (−). Reduced KCNJ10 decreases the EP and hearing and also reduces K+ flux to the intrastrial space, thus limiting K+ secretion by marginal cells; metabolic activity of marginal cells is reduced and ROS production drops. KCNJ10 expression is restored and the cycle repeats. Fluctuating loss of the endocochlear potential can thereby be expected to lead to fluctuating loss of hearing. (Reproduced with permission from Griffith, A.J. and Wangemann, P. (2011). Hearing loss associated with enlargement of the vestibular aqueduct: mechanistic insights from clinical phenotypes, genotypes, and mouse models. Hear Res. doi:10.1016/j.hedres.2011.05.009.)
VI Cell Physiology of Acoustic Transduction
The mammalian cochlea has an exquisite sensitivity, being able to detect sound pressure fluctuations of less than one millionth atmospheric pressure. At the threshold of hearing, the organ of Corti vibrates less than 1 nm. This sensitivity includes a gain of 100–1000 due to active mechanical amplification by the outer hair cells of the organ of Corti (but, see Ashmore et al., 2010). In spite of the delicacy of this process, the dynamic range for the amplitude detected by the cochlea is about one million to one due to compression of the response (Patuzzi, 1996).
The organization of the inner and outer hair cells and their innervations is depicted in Fig. 37.11. Afferent synapses to the inner hair cells account for most (95%) of the neural transmission from the cochlea to the brain. Each inner hair cell is innervated by 5–30 dendrites (Meyer and Moser, 2010). The remaining afferent fibers originate from the three rows of outer hair cells. Feedback occurs through an efferent neural supply that synapses with inner hair cell afferent fibers and directly with the body of outer hair cells (Elgoyhen and Fuchs, 2010). A local GABAergic innervation among outer hair cells has also been described (Thiers et al., 2008).
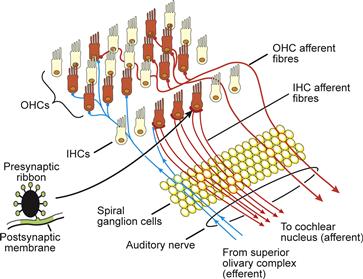
FIGURE 37.11 The organization of the cochlear inner and outer hair cells (IHCs and OHCs) and their (afferent and efferent) innervations. Each inner hair cell is innervated by 5–30 dendrites. The inset shows the schematic profile of the ribbon found at each afferent contact between the hair cells and the postsynaptic afferent terminal. (Adapted with permission from Hackney, C. (2002). From cochlea to cortex. In D. Roberts, (ed.), Signals and Perception–the Fundamental Human Sensation, p. 31. Palgrave Macmillan Press, New York.)
VIA Transduction Channels
The mechanosensitive organelle of the sensory cells is the hair bundle (Fig. 37.12), which consists of 30–300 stereocilia arranged in rows of increasing height. Motion of the hair bundle modulates the fractional open time (open probability Po) of the mechanosensitive transduction channels near the tips of the stereocilia. Modulation of Po leads to corresponding fluctuations of the current through the apical membrane and to changes in the membrane potential of the hair cell. These changes in the membrane voltage are referred to as the receptor potential, the primary event to modulate synaptic transmission.
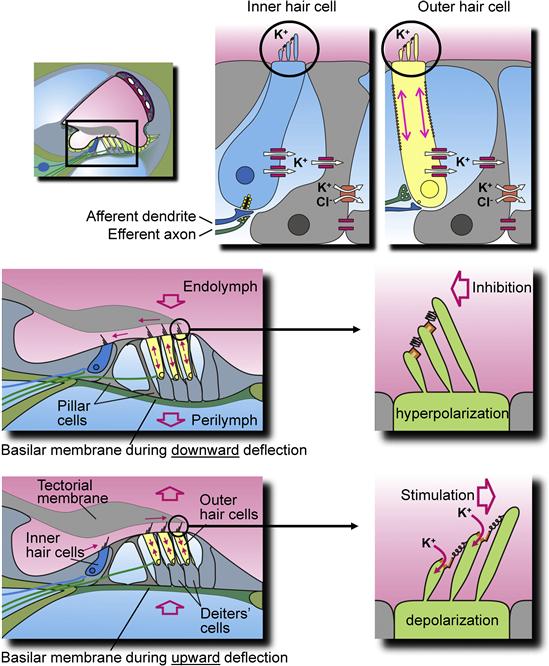
FIGURE 37.12 Diagram of the cochlear sensory structure, the organ of Corti and its inner and outer hair cells. (Top panel) The location of the organ of Corti (rectangle; left) and partial detail of an inner hair cell with supporting cell (middle) and an outer hair cell with supporting Deiters’ cell (right). Stereocilia at the apical membrane of the hair cells tip links and transduction channels are circled; channels and transporters involved in putative K+ buffering are described in the text and Fig. 37.4. (Middle panel) Downward deflection of basilar membrane (left) in response to sound is shown with medial movement of the outer hair cell stereocilia (right), leading to hyperpolarization of the hair cells (reduced afferent nerve transmission and elongation of the outer hair cell). (Bottom panel) Upward deflection of basilar membrane (left) in response to sound is shown with movement of the outer hair cell stereocilia (right), leading to depolarization. Arrows indicate movements of tectorial membrane, subtectorial fluid, basilar membrane (open arrow), K+ flux through transduction channels at the tips of stereocilia and length changes in outer hair cells. (Reproduced with permission from Marcus, D.C. and Wangemann, P. (2009). In (F.J. Alvarez-Leefmans and E. Delpire, eds), Physiology and Pathology of Chloride Transporters and Channels in the Nervous System – From Molecules to Diseases, pp. 425–437. Elsevier, New York.)
Bundle movement is believed to be the result of direct interaction of the outer hair cell stereocilia with the tectorial membrane and of the inner hair cell stereocilia with flowing endolymph pumped back and forth in the channel under the tectorial membrane (see Fig. 37.12). The cytoskeleton of the stereocilia is formed by a rigid matrix of actin filaments cross-linked by fimbrin. Deflection of the hair bundle results in a rotation of the individual cilia about a pivotal region at their base, which causes a shearing motion between the ciliary tips. Shear resulting from movement of the hair bundle in the direction of the tallest stereocilia increases Po of the transduction channels from the resting activity of about 5–15%, whereas movement of the hair bundle in the opposite direction decreases Po (see Figs. 37.12 and 37.13) (Hudspeth and Gillespie, 1994). Motion in the stimulatory direction is caused by the rarefaction phase of the external acoustic pressure wave. The asymmetric position on the receptor potential-stimulus transfer function of the stereocilia under unstimulated conditions (zero displacement in Fig. 37.13) leads to larger changes in the receptor potential with a given stimulatory displacement than for the same size inhibitory displacement. This asymmetry is a primary cause of distortion, which generates many of the non-linear properties of the auditory system, such as distortion products and otoacoustic emissions (see later section on reverse transduction).
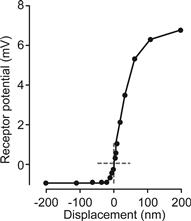
FIGURE 37.13 Response function of the receptor potential of outer hair cells to mechanical displacement of the stereocilia. The asymmetrical position of the quiescent point is indicated by dashed cross-hairs. (Adapted with permission from Russell, I.J., Koessi, M. and Richardson, G.P. (1992). Nonlinear mechanical responses of mouse cochlear hair bundles. Proc R Soc London Ser B. 250, 217–227.)
The shear between stereocilia is posited in the gating-spring model of hair cell function to transmit a mechanical force through an elastic element (the gating spring) to the mechanosensitive gate of each transduction channel (Hackney and Furness, 2010). Increased tension on the spring increases Po and decreased tension reduces Po. The most widely accepted hypothesis is that the fine extracellular links (called tip links) that run between the tips of shorter stereocilia and the sides of taller ones constitute the elastic element of the model. The location of the transduction channels with respect to the attachment of the tip links remains somewhat equivocal, but the channels are apparently near the tips of sterocilia (Hackney and Furness, 2010).
Tip links are thought to be maintained under tension (see next section on adaptation), which is either increased or decreased by motion toward or away from the stimulatory direction. The time constant for response of the receptor current to a step stimulus has been found in saccular hair cells of the bullfrog to be in the range of 100–500 μs at 4°C and to become faster at higher temperatures (Corey and Hudspeth, 1983). This time course is substantially faster than can be accounted for by typical enzymatic or second messenger pathways and points to a direct mechanical coupling between the stimulus and the gate of the transduction channel.
The conductance of the transduction channel has been estimated from patch-clamp records to be on the order of 300 pS under in vivo conditions (Kros, 1996). The channel is permeable to cations including monovalent, divalent and small organic cations such as tetraethylammonium while selecting against anions (Corey and Hudspeth, 1979). Because potassium and calcium in endolymph are above electrochemical equilibrium with respect to the hair cell cytosol, it is primarily these two cations that flow through the transduction channel. Potassium carries the bulk of the current due to its high concentration, whereas entry of Ca2+ through the transduction channel is thought to control adaptation (see below). It was found that Ca2+ carries a surprisingly high fraction of the current through the transduction channel (Ricci and Fettiplace, 1998) – about 20% (whereas the concentration of Ca2+ in endolymph is only about 0.01% that of K+; see Table 37.1). This apparent anomaly may be due to the behavior of the transduction channel like a multi-ion pore in which the different ions interact. Sufficient Ca2+ enters the stereocilia to drive adaptation despite its low concentration. The channel is known to be blocked by aminoglycoside antibiotics (multivalent cations) and amiloride (a blocker of several Na+ transport processes).
The non-selective cation permeability of the transduction channel, in concert with the basolateral K conductance and negative basolateral membrane potential, in hair cells is similar to the situation in outer sulcus cells. Therefore, an efflux of Na+ from endolymph can be expected through hair cells, especially outer hair cells which are greater in number and have a greater basolateral membrane potential than inner hair cells. The molecular identity of the channels is different among the cell types, but the constellation is favorable for Na+ absorption (Kim and Marcus, 2011).
VIB Adaptation
When a sustained displacement is applied to hair cells, the transduction current is first stimulated but then relaxes in the continued presence of the stimulus with a complex characteristic time course, processes referred to as fast- and slow-adaptation (Hackney and Furness, 2010). The mechanism underlying slow adaptation is thought to be an active process in which myosin motors in the insertional plaque maintain tension within the tip link through a balance of climbing and slipping (Fig. 37.14). The tip link slackens when there is a deflection in the inhibitory direction but tension is soon re-established as the motor climbs along actin filaments within the taller stereocilium of each pair. Deflection in the stimulatory direction increases tension in the tip links, opening the mechanosensitive transduction channel. Ca2+ coming in through the channel is thought to bind to calmodulin, a myosin regulatory protein found to be concentrated in the tips of stereocilia which, in turn, promotes the dissociation of myosin from the actin filaments, allowing the plaque with the motors to slip. Fast adaptation is thought to be a direct, calcium-dependent effect on the transduction channel.
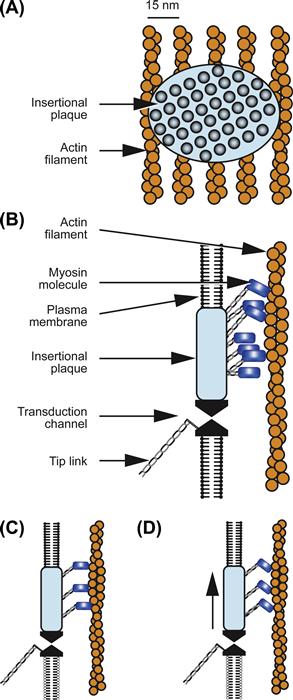
FIGURE 37.14 Proposed mechanism of adaptation: (A) frontal view of insertional plaque on actin filaments; (B) side view of insertional plaque with associated mechanosensitive transduction channel in cell membrane; and (C and D) power stroke of actin–myosin complex. (Reproduced with permission from Hudspeth and Gillespie, 1994. Copyright 1994 by Cell Press.)
The physiologic significance of adaptation is clear for vestibular hair cells where prolonged static displacements naturally occur during which sensitivity to transient stimuli must be maintained. However, the function of adaptation in the mammalian cochlea is less obvious (Kros, 1996). The adaptation process has been estimated to have an upper frequency limit of about 160 Hz, limiting its potential usefulness as a response to normal acoustic stimuli. Physiological significance in the cochlea may lie more with homeostasis of the system in the face of small readjustments of the position and/or size of cells during normal changes in systemic osmolarity and local metabolic processes or during development. There may also be a significant importance in controlling the response to infrasound, which gains attention with regard to insults by machinery such as wind turbine generators (Salt and Hullar, 2010).
VIC Basolateral Membrane Channels
Although there are differences among hair cell types, they all contain a major basolateral K+ conductance, comprised of voltage-dependent and Ca2+-activated K+ channels and voltage-gated Ca2+ channels (Fuchs, 1992; Kros, 1996). The K+ channels serve to shape the receptor potential initiated by the acoustically-gated transduction channels and the Ca2+ channels to initiate transmitter release at the presynaptic sites, respectively. The presence of voltage-gated Ca2+ channels alone would be expected to lead to regenerative depolarization during acoustic stimulation, however, the basolateral voltage-gated K+ channels provide an active repolarizing influence in the mature cochlea. Interestingly, immature inner hair cells have a different complement of channels including excitable Na+ channels and a relatively slow K+ channel, which lead to spontaneous and evoked action potentials. During development (see Chapter 25), a large, fast K+ channel is expressed that greatly speeds up the membrane time constant, preventing action potentials (Kros et al., 1998).
The Ca2+ channels in hair cells appear to be of the L type, which are characterized by activation at depolarized membrane potentials, little or no inactivation, and block by dihydropyridines such as nifedipine. In inner hair cells, the Ca2+ currents are activated above −60 mV and peak near −20 to 0 mV, a range that correlates well with that of receptor potentials found in recordings made in vivo and with predictions from in vitro experiments (Kros, 1996). These Ca2+ channels are therefore well poised to participate in the graded release of neurotransmitter at the basolateral synapses to type I afferent fibers in the auditory nerve. The kinetics of channel activation have been estimated to be sufficiently fast so as not to limit accurate following of nervous discharge rates to the acoustic stimulus (Kros, 1996). By contrast, the Ca2+ currents in outer hair cells are activated only above −30 mV, a range that is far removed from the resting membrane potential near −80 mV found in vivo and never reached by receptor potentials, which apparently saturate at 15 mV (Kros, 1996). There is therefore no evidence at this time for a function of these voltage-gated Ca2+ channels in outer hair cells; there are also no measurements of neural activity in the type II afferent fibers in the auditory nerve that synapse exclusively to the outer hair cells.
In inner hair cells, the K+ currents are activated by depolarization in the range of −60 to −20 mV, a range that correlates well with that of the resting and receptor potentials found in recordings made in vivo and with that of the voltage-gated Ca2+ channels described earlier. The resting potential of inner hair cells in vivo is about −40 mV, which reflects a compromise of the highly negative EMF from the basolateral K+ channels by the depolarized EMF of the non-selective cation conductance in the apical membrane (Dallos, 1986).
VID Synaptic Release of Vesicles
A comprehensive description of the afferent synapse is given in Ashmore (2010) and a diagram is in Fig. 37.11. Depolarization by acoustic stimulation leads to Ca2+-dependent release of neurotransmitter at the 30–40 synapses with the afferent fibers at the base of each inner hair cell. This process can be monitored in vitro as changes in membrane capacitance. Opening of Ca2+ channels during depolarization raises the local free Ca2+ concentration to tens or hundreds of micromolar (Roberts, 1994), which is thought to trigger synaptic exocytosis of the neurotransmitter glutamate (Ashmore, 2010) (see Chapter 32). Depolarization led to increases in membrane capacitance (up to 5% of the initial value) that continued for up to 2 s. It was estimated that this was sufficient to exhaust more than five times the number of vesicles initially in close apposition to the plasma membrane at active zones, suggesting that hair cells are capable of rapidly replenishing vesicles at release sites (Parsons et al., 1994). Capacitance returned during repolarization toward its prestimulus level with a time constant of about 14 s, but only in perforated-patch recordings (see Chapter 20), suggesting that membrane retrieval depends on a diffusible intracellular factor.
VIE Reverse Transduction: Cochlear Amplifier
Discrimination of frequency information in acoustic signals is performed broadly at an initial level by mechanical tuning of the basilar membrane as a function of position along the cochlea. In lower vertebrates, frequency discrimination is accomplished by tuning the electrical properties of the hair cells. The exquisite sensitivity and fine frequency discrimination of mammalian hearing depends on amplification of the incoming sound within the organ of Corti (Ashmore, 2010; Iwasa, 2010). It is widely held that the outer hair cells sense displacements caused by sound and feedback forces that enhance the basilar membrane motion by reducing the inherent damping of the cochlear partition. In support of this hypothesis, outer hair cells show membrane potential-induced length changes at acoustic rates. This process has been termed reverse transduction by the cochlear amplifier. This property of the organ of Corti leads to an epiphenomenon called otoacoustic emission in which mechanical fluctuations are generated by the inner ear, resulting in sounds emanating from the ear (Kemp, 2010).
Reverse transduction occurs by somatic and/or ciliary motility, the former due to a high concentration of the motor molecule prestin (Slc26a5) (Iwasa, 2010) (current opinions: Ashmore et al., 2010). Outer hair cells shorten and lengthen by up to 5% when depolarized and hyperpolarized, respectively. Longitudinal motions of the outer hair cells (see Fig. 37.12) are assumed to be transmitted in vivo to the whole organ of Corti via the tectorial membrane by the rigid reticular lamina at the top and by the Deiters’ cell bodies at the base, mechanically coupled to the basilar membrane. Shortening in vivo would cause an upward pull on the basilar membrane and/or a downward pull on the reticular lamina (see Fig. 37.12). The latency is less than 0.1 ms and can be driven to frequencies in excess of 22 kHz (measurement of upper frequency limited by instrumentation) (Dallos and Evans, 1995). This response is driven by membrane potential and is associated with an apparent non-linear capacitance. The presence of non-linear capacitance suggests that the motors act by conformational changes of the protein rather than by electrostatic repulsion between separate elements within the plane of the lipid bilayer.
Myosin in the stereocilia is estimated to be sufficiently plentiful and powerful to account for ciliary-driven amplification by the organ of Corti. However, if the stereociliary motion were modulated by Ca2+ rather than membrane potential, activation of stereociliar myosin by Ca2+ would not be restricted by the membrane’s time constant. It remains to be determined whether these motors can operate at the highest frequencies detected by the mammalian cochlea (Ashmore, 2010; Iwasa, 2010).
Deiters’ cells were previously thought to offer only a passive support to the outer hair cells and a mechanical connection to the acoustic stimulus at the basilar membrane. However, evidence suggests that these cells have a Ca2+-dependent motile response. Increases in cytoplasmic Ca2+ caused an extension of the head of the phalangeal process away from the body by 0.5 to 1 μm within a few hundred milliseconds and the stiffness of the phalangeal process increased by 28 to 51% (Dulon, 1995).
VIF Receptors and Neurotransmitters
A number of receptors for neurotransmitters and neuromodulators have been identified on cochlear hair cells (Ashmore, 2010; Elgoyhen and Fuchs, 2010). Some are known to mediate synaptic transmission while the physiological significance of others has not yet been identified.
VIF1 Outer Hair Cells
Receptors for several neurotransmitters and neuromodulators (Table 37.3) have been identified on outer hair cells by histochemical and electrophysiologic observations (Elgoyhen and Fuchs, 2010). The efferent synapses on the outer hair cells release acetylcholine (ACh) and γ-aminobutyric acid (GABA) as neurotransmitters from two populations of fibers and may co-release ATP with ACh. There is evidence for the presence of both ionotropic and metabotropic receptors for ATP and ACh and for the ionotropic GABAA receptor (Housley et al., 1995). Ionotropic receptors are coupled directly to ion channels, whereas metabotropic receptors act via G-protein pathways that ultimately regulate ion channel activity and/or other cellular processes (see Chapter 6). Cochlear Ach receptors contain the unusual α9-receptor isoform subunit. Activation of ACh receptors causes membrane hyperpolarization apparently through an ion channel permeable to cations including Ca2+. The entry of Ca2+ activates Ca2+-dependent K+ channels, likely the SK type (Elgoyhen and Fuchs, 2010), leading to hyperpolarization. Ionotropic ATP receptors depolarize the hair cell by opening associated non-selective cation channels permeable to Ca2+ as well as monovalent cations. The channels associated with the ionotropic ATP and ACh receptors admit Ca2+ into the cell, leading to a relatively slow elongation (a process referred to as slow motility or adaptation; see above). In addition to its effects on slow motility, ACh has been found to control the gain and magnitude of fast motility in outer hair cells (Sziklai et al., 1996). ACh evokes an increase in magnitude and gain of electromotility, which is sensitive to the muscarinic blocker atropine.
TABLE 37.3. Effects of Outer Hair Cell Receptors on Fast and Slow Motility, Membrane Potential, and Calcium Concentration
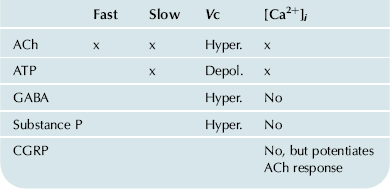
Receptors have also been found on outer hair cells for the peptides substance P and calcitonin gene-related peptide (CGRP). Substance P hyperpolarizes these cells by downregulating the non-selective cation conductance in the lateral wall via a pertussis toxin-insensitive G protein (Kakehata et al., 1993). CGRP had no effect on the resting cytoplasmic Ca2+ concentration but potentiated by about threefold the increase in Ca2+ caused by ACh stimulation in chick hair cells (Shigemoto and Ohmori, 1990).
VIF2 Inner Hair Cells
Inner hair cells have been found to have both metabotropic and ionotropic receptors for extracellular ATP (Sugasawa et al., 1996). At submicromolar ATP concentrations, the metabotropic receptors raise intracellular Ca2+ concentration, which hyperpolarizes inner hair cells via Ca2+-sensitive K+ channels. The ionotropic receptors are non-selective cation channels activated at higher ATP concentrations and which mainly have a depolarizing effect on the inner hair cells. Although the source of agonist for these receptors has not yet been identified, Ca2+-dependent release of ATP from the organ of Corti has been observed (Wangemann, 1996) and ATP release from cochlear cells via connexin/pannexin hemichannels has been reported to reduce outer hair cell motility via purinergic receptors (Zhao et al., 2005).
VIG Echolocation
Bats can capture flying prey by echolocation (biosonar) even among vegetation and other bats. Sounds are vocalized by the bat at frequencies near 60 kHz (the upper range of human hearing is about 20 kHz) and typically consist of a constant-frequency tone pulse for 10–100 ms followed by a shorter period of dropping frequency. The pulse is reflected from surfaces in front of the bat and is returned and heard before the end of the emitted pulse. Several characteristics of the returned sound pulse are interpreted by the bat as an acoustic image. A Doppler shift of the frequency of the returned sound from that of the emitted sound carries velocity information (the rate of closure on a target). For example, a Doppler shift from 61 to 62 kHz corresponds to 6.3 miles per hour. The delay of the echo gives the distance to the target such that a 1-ms echo delay corresponds to a target distance of 17 cm. Temporal delay acuity has been reported to be as fine as 10 ns (Simmons et al., 1990), a surprisingly short time span for recognition by a biological system. It has been suggested, however, that bats may not respond directly to processing of the time delay but rather to spectral processing, which would require only a resolution of about a few kilohertz (Neuweiler and Schmidt, 1993; Simmons, 1993; Simmons et al., 1996; Fuzessery et al., 2011).
The fine tuning of hearing used in echolocation is mostly achieved by mechanical specializations of the cochlea, which spread out the physical mapping of the biosonar frequencies over a full half turn of the cochlea. There is also an amplifying reverberation of the acoustic wave traveling on the basilar membrane, which is due either to reflections at a discontinuity of the basilar membrane thickness or to different radial oscillation modes of the basilar membrane (Russell and Koessl, 1995). As in other mammalian cochleae, the tuning (and the bat’s special reverberation) is thought to depend on active processes in the outer hair cells. Tuning of auditory nerve fibers, and therefore likely tuning of basilar membrane motion, to acoustic stimuli is amazingly high in the bat with Q10 values (center frequency/bandwidth 10 dB from the tip) as high as 610 in comparison to typical values of 1 to 10 for non-echolocating animals (Russell and Koessl, 1995). Processing by central neural pathways of the detected echoes has been reviewed by Fuzessery et al. (2011). An interesting specialization of the bats’ prey has been found in the dogbane tiger moth, which apparently emits clicks when a bat approaches in order to interfere with the sonar echoes, thereby jamming the signal and averting becoming a meal (Fullard et al., 1994)!
VII Concluding Remarks
The exquisite sensitivity of our sense of hearing results from the concerted action of many subsystems. Sound is conducted from our environment through the outer ear canal at the end of which it is conducted through the middle ear by the three ossicles to the oval window of the inner ear. Sounds can also reach the inner ear by bone conduction. This auditory peripheral organ transduces the mechanical stimulus into nerve impulses that travel to central auditory pathways in the brain where we interpret the impulses as sounds.
This chapter has focused on the cellular processes in the cochlea that support auditory transduction. In particular, several cell types contribute specific ion transport modalities to the creation and maintenance of the ionic composition of the cochlear luminal fluid, endolymph. The potassium, sodium, calcium and pH levels that comprise this unique extracellular environment all serve to power and support the transduction process performed by the sensory inner hair cells and the cochlear amplifier embodied in several cell types in the organ of Corti and especially the outer hair cells.
All of these cellular processes depend on specific gene expression patterns; a number of gene mutations are known to underlie specific clinical syndromic and non-syndromic hereditary hearing loss. The only “surprise” has been that most of these clinically important genes are located in the cells that engage in ion transport rather than the auditory sensory and neural cells. Current and future areas of investigation include extending our knowledge of ion transport function to early developmental times, development of gene therapy and drug delivery techniques and discovery of cochlear “repair” through new extrinsic nerve stimulation paradigms and/or cochlear cell replacements.
BIBLIOGRAPHY
1. Ashmore J. The afferent synapse. In: Fuchs PA, ed. The Oxford Handbook of Auditory Science: The Ear. Oxford: Oxford University Press; 2010;:259–282.
2. Ashmore J, Avan P, Brownell WE, et al. The remarkable cochlear amplifier. Hear Res. 2010;266:1–17.
3. Bayazit YA, Yilmaz M. An overview of hereditary hearing loss. Otorhinolaryngol Relat Spec. 2006;68:57–63.
4. Chang Q, Tang W, Ahmad S, Zhou B, Lin X. Gap junction mediated intercellular metabolite transfer in the cochlea is compromised in connexin30 null mice. PLoS One. 2008;3:e4088.
5. Corey DP, Hudspeth AJ. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979;281:675–677.
6. Corey DP, Hudspeth AJ. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci. 1983;3:962–976.
7. Curtis LM, ten Cate WJ, Rarey KE. Dynamics of Na, K-ATPase sites in lateral cochlear wall tissues of the rat. Eur Arch Otorhinolaryngol. 1993;250:265–270.
8. Dallos P. Neurobiology of cochlear inner and outer hair cells: intracellular recordings. Hear Res. 1986;22:185–198.
9. Dallos P, Evans BN. High-frequency motility of outer hair cells and the cochlear amplifier. Science. 1995;267:2006–2009.
10. Dulon D. Ca2+ signaling in Deiter’s cells of the guinea-pig cochlea: active process in supporting cells?. In: Flock A, Ottoson D, Ulfendahl M, eds. Active Hearing. New York: Elsevier Science; 1995;:195–208.
11. Elgoyhen AB, Fuchs PA. Efferent innervation and function. In: Fuchs PA, ed. The Oxford Handbook of Auditory Science: The Ear. Oxford: Oxford University Press; 2010;:283–306.
12. Erichsen S, Stierna P, Bagger-Sjoback D, et al. Distribution of Na, K-ATPase is normal in the inner ear of a mouse with a null mutation of the glucocorticoid receptor. Hear Res. 1998;124:146–154.
13. Fuchs PA. Ionic currents in cochlear hair cells. Prog Neurobiol. 1992;39:493–505.
14. Fullard JH, Simmons JA, Saillant PA. Jamming bat echolocation: the dogbane tiger moth Cycnia tenera times its clicks to the terminal attack calls of the big brown bat Eptesicus fuscus. J Exp Biol. 1994;194:285–298.
15. Fuzessery ZM, Razak KA, Williams AJ. Multiple mechanisms shape selectivity for FM sweep rate and direction in the pallid bat inferior colliculus and auditory cortex. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2011;197:615–623.
16. Gow A, Davies C, Southwood CM, et al. Deafness in Claudin 11-null mice reveals the critical contribution of basal cell tight junctions to stria vascularis function. J Neurosci. 2004;24:7051–7062.
17. Griffith AJ, Wangemann P. Hearing loss associated with enlargement of the vestibular aqueduct: Mechanistic insights from clinical phenotypes, genotypes, and mouse models. Hear Res 2011; doi:10.1016/J.heares.2011.05.009.
18. Hackney CM, Furness DN. Hair bundle structure and mechanotransduction. In: Fuchs PA, ed. The Oxford Handbook of Auditory Science: The Ear. Oxford: Oxford University Press; 2010;:231–258.
19. Hoang DE, Ahmad S, Chang Q, Tang W, Stong B, Lin X. Diverse deafness mechanisms of connexin mutations revealed by studies using in vitro approaches and mouse models. Brain Res. 2009;1277:52–69.
20. Housley GD, Connor BJ, Raybould NP. Purinergic modulation of outer hair cell electromotility. In: Flock A, Ottoson D, Ulfendahl M, eds. Active Hearing. New York: Elsevier Science; 1995;:221–238.
21. Hudspeth AJ, Gillespie PG. Pulling springs to tune transduction: adaptation by hair cells. Neuron. 1994;12:1–9.
22. Iwasa KH. Electromotility of outer hair cells. In: Fuchs PA, ed. The Oxford Handbook of Auditory Science: The Ear. Oxford: Oxford University Press; 2010;:179–212.
23. Julien N, Loiseau A, Sterkers O, Amiel C, Ferrary E. Antidiuretic hormone restores the endolymphatic longitudinal K+ gradient in the Brattleboro rat cochlea. Pflügers Arch. 1994;426:446–452.
24. Kakehata S, Akaike N, Takasaka T. Substance P decreases the non-selective cation channel conductance in dissociated outer hair cells of guinea pig cochlea. Ann NY Acad Sci. 1993;707:476–479.
25. Kemp DT. Otoacoustic emissions and evoked potentials. In: Fuchs PA, ed. The Oxford Handbook of Auditory Science: The Ear. Oxford: Oxford University Press; 2010;:93–137.
26. Kikuchi T, Kimura RS, Paul DL, Takasaka T, Adams JC. Gap junction systems in the mammalian cochlea. Brain Res Brain Res Rev. 2000;32:163–166.
27. Kim SH, Marcus DC. Regulation of sodium transport in the inner ear. Hear Res. 2011;280:21–29.
28. Kim SH, Kim KX, Raveendran NN, Wu T, Pondugula SR, Marcus DC. Regulation of ENaC-mediated sodium transport by glucocorticoids in Reissner’s membrane epithelium. Am J Physiol Cell Physiol. 2009;296:C544–C557.
29. Konishi T, Hamrick PE, Walsh PJ. Ion transport in guinea pig cochlea I Potassium and sodium transport. Acta Otolaryngol. 1978;86:22–34.
30. Kros CJ. Physiology of mammalian cochlear hair cells. In: Dallos P, Popper AN, Fay RR, eds. The Cochlea. New York: Springer-Verlag; 1996;:318–385.
31. Kros CJ, Ruppersberg JP, Rusch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394:281–284.
32. Lee JH, Marcus DC. Estrogen acutely inhibits ion transport by isolated stria vascularis. Hear Res. 2001;158:123–130.
33. Lee JH, Marcus DC. Nongenomic effects of corticosteroids on ion transport by stria vascularis. Audiol Neurootol. 2002;7:100–106.
34. Lee JH, Marcus DC. Purinergic signaling in the inner ear. Hear Res. 2008;235:1–7.
35. Ma YL, Gerhardt KJ, Curtis LM, Rybak LP, Whitworth C, Rarey KE. Combined effects of adrenalectomy and noise exposure on compound action potentials, endocochlear potentials and endolymphatic potassium concentrations. Hear Res. 1995;91:79–86.
36. Marcus DC, Shen Z. Slowly activating, voltage-dependent K+ conductance is apical pathway for K+ secretion in vestibular dark cells. Am J Physiol. 1994;267:C857–C864.
37. Marcus DC, Wangemann P. Cochlear and vestibular function and dysfunction. In: Alvarez-Leefmans FJ, Delpire E, eds. Physiology and Pathology of Chloride Transporters and Channels in the Nervous System – From Molecules to Diseases. New York: Elsevier; 2009;:425–437.
38. Marcus DC, Wangemann P. Inner ear fluid homeostasis. In: Fuchs PA, ed. The Oxford Handbook of Auditory Science: The Ear. Oxford: Oxford University Press; 2010;:213–230.
39. Marcus DC, Raveendran NN, Wu T. Reissner’s membrane, mouse. GEO 2011;:GSE6196.
40. Meyer AC, Moser T. Structure and function of cochlear afferent innervation. Curr Opin Otolaryngol Head Neck Surg. 2010;18:441–446.
41. Mori N, Shugyo A, Asai H. The effect of arginine-vasopressin and its analogues upon the endocochlear potential in the guinea pig. Acta Otolaryngol. 1989;107:80–84.
42. Morton CC, Giersch AB. Genetics of hearing loss. In: Fuchs PA, ed. The Oxford Handbook of Auditory Science: The Ear. Oxford: Oxford University Press; 2010;:377–408.
43. Nance WE. The genetics of deafness. Ment Retard Dev Disabil Res Rev. 2003;9:109–119.
44. Neuweiler G, Schmidt S. Audition in echolocating bats. Curr Opin Neurobiol. 1993;3:563–569.
45. Ogawa K, Schacht J. P2y purinergic receptors coupled to phosphoinositide hydrolysis in tissues of the cochlear lateral wall. Neuroreport. 1995;6:1538–1540.
46. Parsons TD, Lenzi D, Almers W, Roberts WM. Calcium-triggered exocytosis and endocytosis in an isolated presynaptic cell: capacitance measurements in saccular hair cells. Neuron. 1994;13:875–883.
47. Patuzzi R. Cochlear micromechanics and macromechanics. In: Dallos P, Popper AN, Fay RR, eds. The Cochlea. New York: Springer-Verlag; 1996;:186–257.
48. Ricci AJ, Fettiplace R. Calcium permeation of the turtle hair cell mechanotransducer channel and its relation to the composition of endolymph. J Physiol (Lond.). 1998;506:159–173.
49. Roberts WM. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J Neurosci. 1994;14:3246–3262.
50. Russell IJ, Koessl M. Measurements of the basilar membrane resonance in the cochlea of the mustached bat. In: Flock A, Ottoson D, Ulfendahl M, eds. Active Hearing. New York: Elsevier Science; 1995;:295–306.
51. Salt AN, Hullar TE. Responses of the ear to low frequency sounds, infrasound and wind turbines. Hear Res. 2010;268:12–21.
52. Salt AN, Melichar I, Thalmann R. Mechanisms of endocochlear potential generation by stria vascularis. Laryngoscope. 1987;97:984–991.
53. Shah DM, Freeman DM, Weiss TF. The osmotic response of the isolated, unfixed mouse tectorial membrane to isosmotic solutions: effect of Na+, K+, and Ca2+ concentration. Hear Res. 1995;87:187–207.
54. Shen Z, Marcus DC, Sunose H, Chiba T, Wangemann P. IsK channel in strial marginal cells: voltage-dependence, ion-selectivity, inhibition by 293B and sensitivity to clofilium. Auditory Neurosci. 1997;3:215–230.
55. Shigemoto T, Ohmori H. Muscarinic agonists and ATP increase the intracellular Ca2+ concentration in chick cochlear hair cells. J Physiol (Lond.). 1990;420:127–148.
56. Simmons JA. Evidence for perception of fine echo delay and phase by the FM bat, Eptesicus fuscus. J Comp Physiol A. 1993;172:533–547.
57. Simmons JA, Dear SP, Ferragamo MJ, Haresign T, Fritz J. Representation of perceptual dimensions of insect prey during terminal pursuit by echolocating bats. Biol Bull. 1996;191:109–121.
58. Simmons JA, Ferragamo M, Moss CF, Stevenson SB, Altes RA. Discrimination of jittered sonar echoes by the echolocating bat, Eptesicus fuscus: the shape of target images in echolocation. J Comp Physiol A. 1990;167:589–616.
59. Sterkers O, Saumon G, Tran Ba HP, Amiel C. K, Cl, and H2O entry in endolymph, perilymph, and cerebrospinal fluid of the rat. Am J Physiol. 1982;243:F173–F180.
60. Sugasawa M, Erostegui C, Blanchet C, Dulon D. ATP activates non-selective cation channels and calcium release in inner hair cells of the guinea-pig cochlea. J Physiol (Lond.). 1996;491:707–718.
61. Sziklai I, He DZ, Dallos P. Effect of acetylcholine and GABA on the transfer function of electromotility in isolated outer hair cells. Hear Res. 1996;95:87–99.
62. Takeuchi S, Ando M. Dye-coupling of melanocytes with endothelial cells and pericytes in the cochlea of gerbils. Cell Tissue Res. 1998;293:271–275.
63. ten Cate WJ, Curtis LM, Rarey KE. Effects of low-sodium, high-potassium dietary intake on cochlear lateral wall Na+, K+-ATPase. Eur Arch Otorhinolaryngol. 1994;251:6–11.
64. ten Cate WJ, Curtis LM, Small GM, Rarey KE. Localization of glucocorticoid receptors and glucocorticoid receptor mRNAs in the rat cochlea. Laryngoscope. 1993;103:865–871.
65. Thiers FA, Nadol Jr JB, Liberman MC. Reciprocal synapses between outer hair cells and their afferent terminals: evidence for a local neural network in the mammalian cochlea. J Assoc Res Otolaryngol. 2008;9:477–489.
66. Vetter DE, Mann JR, Wangemann P, et al. Inner ear defects induced by null mutation of the isk gene. Neuron. 1996;17:1251–1264.
67. Wangemann P. Ca2+-dependent release of ATP from the organ of Corti measured with a luciferin-luciferase bioluminescence assay. Auditory Neurosci. 1996;2:187–192.
68. Wangemann P. Adrenergic and muscarinic control of cochlear endolymph production. Adv Otorhinolaryngol. 2002;59:42–50.
69. Wangemann P, Itza EM, Albrecht B, et al. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Med. 2004;2:30.
70. Wangemann P, Liu J, Marcus DC. Ion transport mechanisms responsible for K+ secretion and the transepithelial voltage across marginal cells of stria vascularis in vitro. Hear Res. 1995;84:19–29.
71. Wangemann P, Nakaya K, Wu T, et al. Loss of cochlear HCO3− secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am J Physiol Renal Physiol. 2007;292:F1345–F1353.
72. Wangemann P, Shen Z, Liu J. K(+)-induced stimulation of K+ secretion involves activation of the IsK channel in vestibular dark cells. Hear Res. 1996;100:201–210.
73. Wood JD, Muchinsky SJ, Filoteo AG, Penniston JT, Tempel BL. Low endolymph calcium concentrations in deafwaddler2J mice suggest that PMCA2 contributes to endolymph calcium maintenance. J Assoc Res Otolaryngol. 2004;5:99–110.
74. Yamauchi D, Nakaya K, Raveendran NN, et al. Expression of epithelial calcium transport system in rat cochlea and vestibular labyrinth. BMC Physiol. 2010;10:1.
75. Yamoah EN, Lumpkin EA, Dumont RA, Smith PJ, Hudspeth AJ, Gillespie PG. Plasma membrane Ca2+-ATPase extrudes Ca2+ from hair cell stereocilia. J Neurosci. 1998;18:610–624.
76. Yao XF, Rarey KE. Localization of the mineralocorticoid receptor in rat cochlear tissue. Acta Otolaryngol (Stockh). 1996;116:493–496.
77. Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci USA. 2005;102:18724–18729.