Chapter 48
Electrocytes of Electric Fish
Chapter Outline
III. Anatomy of Electrophorus and Mechanism of the Electrical Discharge
IV. Electrocyte Membrane Electrophysiology
V. Comparative Physiology of Electrophorus and Torpedo – Models for Mammalian Excitable Cells
I Summary
Both the freshwater electric eel and the saltwater electric ray produce extraordinarily powerful electrical discharges with membrane ion channels, receptors and pumps common to other excitable cells. These fish have separately evolved a specialized anatomy and cellular morphology designed for this function. Because of their specialized membrane asymmetry, action potentials and end-plate potentials generated on the innervated membrane are not reproduced on the non-innervated membrane, thereby setting up an asymmetrical flow of current across the cell. The arrangement allows transcellular potentials to be generated, which is essentially the basis for the generation of bioelectricity within the electric organs of these fish. Connective tissue septa that delineate columns of electrocytes prevent transcellular potentials from being short-circuited around the outside of individual electrocytes and also channel the resulting current along the electric organ.
The membrane potentials used by electrocytes to produce transcellular potentials are remarkably similar to those of other excitable cells, such as myocytes and neurons. The electrophysiology, therefore, can be explained by currents conducted through ligand-gated receptors and channels having known characteristics. Currents that give rise to electrocyte membrane potentials can even be represented by equivalent circuits similar to those of other excitable cells. However, two major differences exist between electrocytes and other excitable cells: (1) electrocytes express exaggerated amounts of key excitable membrane proteins, such as the Na+ channel of Electrophorus and the acetylcholine receptor (AChR) of Torpedo. These proteins that exist in high density tend to produce greater currents and peak potentials than what is customarily seen on other excitable cells. (2) Membrane proteins are polarized to particular sides of the cell to facilitate the production of transcellular potentials. In the past, however, researchers have taken advantage of these differences to utilize these fish as useful model systems.
Electrophorus electrocytes provide a general model system for excitable cells, such as neurons and myocytes, since they contain common membrane receptors, channels and ATPases. They are also large and easy to dissect in order to perform potential recording, voltage-clamp analysis and patch-clamp measurements. Since it expresses large quantities of proteins, such as the Na+ channel, the Na+,K+-ATPase, AChR and calmodulin, eel electric tissue has been used as a source for the purification of these proteins for molecular and functional analysis.
Torpedo electrocytes, on the other hand, are richly innervated with ACh-releasing electromotor neurons and are electrically inexcitable. Therefore, they provide a very specialized model for the motor end-plate. Because of the exaggerated cholinergic nature of Torpedo electric tissue, it has been used as a rich protein and mRNA source for the AChR and AChE. The expression of Cl− channels on the non-innervated membrane of these cells has led researchers to use this tissue as a source for ClC-0 protein and mRNA as well.
Investigations with electric tissue of both the electric eel and the electric ray have opened wide avenues of study in electrophysiology, protein biochemistry and clinical research. Electrophysiological techniques have been used, refined and, in some cases, developed while using electrocytes as model systems. Like the squid giant axon, these cells have been instrumental in defining and confirming the ionic currents responsible for excitable cell membrane potential changes. Biochemically, Electrophorus and Torpedo electric tissue has supplied abundant quantities of key excitable membrane proteins that exist in only trace amounts in mammalian tissues. Since electric tissue develops from skeletal muscle, the biochemical properties and three-dimensional structures of these proteins are similar, if not identical, to those of mammalian skeletal muscle and other excitable cells. These discoveries will continue to further our understanding of the mechanisms by which membrane potentials of excitable cells are generated and regulated, as well as the understanding and the treatment of disease.
II Introduction
Electric fish such as the marine electric ray (genus Torpedo) and the freshwater electric eel (Electrophorus electricus) are capable of generating powerful electrical discharges that can be measured in the water surrounding these animals. These fish use the production of bioelectricity as an effective mechanism to stun prey and ward off predators. Electrical discharges are generated by electric cells, called electroplax or electrocytes, that produce end-plate potentials and action potentials (APs) that are remarkably similar to the membrane potentials of neurons and myocytes. In fact, the membrane receptors, ion channels and ATPases responsible for electric tissue electrophysiology are biochemically and functionally identical to those of mammalian muscle and nerve. For this reason, electrocytes have been used extensively as a specialized and appropriate model system for the study of excitable cell membrane electrophysiology and biochemistry. Due to the specialized nature of electric tissue, it has also been used as an enriched source of membrane proteins for biochemical studies. Previous chapters have described in detail the generation of acetylcholine (ACh)-mediated muscle end-plate potentials and the propagation of action potentials of nerve and muscle (see Chapters 18, 19, 32, and 42). This chapter examines the anatomy and cellular morphology that electric fish have evolved in order to produce powerful electrical discharges. An electrophysiological and biochemical comparison is made between the electrocytes of the freshwater electric eel and the marine electric ray. The major contributions that electric tissue has made to the understanding of the electrophysiology and biochemistry of excitable membranes are also reviewed.
The shocking sensations produced by electric fish were undoubtedly experienced by mankind long before the recording of scientific phenomena. Some of the first recorded reports of unusual effects produced by electrical discharges of electric fish were of the Nile river catfish, Malapterus electricus. Nile river fishermen reported unpleasant sensations when handling live Malapterus, or even the water-soaked nets containing the fish. Godigno, a seventeenth century Jesuit father, noted that dead fish could be induced to move when a live Malapterus was thrown among them (Grundfest, 1957). At the time when Ben Franklin and other investigators were experimenting with static electricity of the Leyden jar, the electric eel provided insight into the basic conductive properties of electricity. In 1775, John Walsh conducted numerous experiments, one of which involved 10 people holding hands in a circle where the first and last “subjects” touched the opposite ends of a moderate-sized eel. All 10 people received a severe shock. The relative conductivities of various materials, including glass, wood, silk, brass chains and iron rods, were then determined by holding these materials between two of the investigators and noting the severity of the electrical discharge. Although these experiments were likely to be very convincing to Walsh and his assistants, others doubted the electrical nature of the discharge from Electrophorus and Torpedo. The bioelectric nature of the discharge had not gained widespread acceptance until Du Bois-Raymond demonstrated that nerve and muscle were electrogenic (Grundfest, 1957). Since that time, the usefulness of Electrophorus, Torpedo and other electric fish as models for excitable membranes has been realized.
III Anatomy of Electrophorus and Mechanism of the Electrical Discharge
Powerful electric fish possess a specialized anatomy and cellular morphology devoted to the production of electrical discharges. The electric eel is an excellent example of this specialization. It has been well characterized on the cellular and biochemical level and is used here to describe the production of bioelectricity. Figure 48.1A depicts the location of the electric organs within Electrophorus. The viscera are crowded into the rostral 20% of the animal; the remaining 80% is comprised predominantly of electric tissue and swimming muscles. The electric organs are confined to the ventral portion of this caudal region, whereas most of the swimming muscles, major blood vessels and spinal cord are situated in the dorsal one-third (Fig. 48.1B). The eel also possesses a tubular swim bladder that extends the length of the fish and is positioned dorsal to the main electric organ and ventral to the spinal cord. The central nervous system consists of a small brain typical of teleost fish and a spinal cord that extends down the length of the animal. The electrical discharge is coordinated in the central control nucleus in the medulla. Axons from these neurons of the brain project caudally and synapse on neurons of the electromotor nucleus of the spinal cord. Electromotor neurons radiate into the electric organ, innervating individual electrocytes (Bennett and Sandri, 1989). To generate the whole-animal electrical discharge, each electrocyte of the entire electric organ must be stimulated simultaneously. In other words, action potentials reaching proximal electrocytes of the electric organ must be delayed to varying degrees relative to more distal regions. Neurons innervating proximal electrocytes are smaller in diameter and conduct action potentials more slowly. Some of these neurons wind their way to these rostral electrocytes, thereby slowing stimulation of this part of the electric organ, aiding in synchronous activation (Bennett, 1971). The delay may also occur in the electromotor nucleus of the spinal cord where central neurons synapse on electromotor neurons. Presumably, synaptic transmission is slower to electromotor neurons innervating proximal portions of the electric organ, whereas faster signaling occurs between neurons that innervate electrocytes near the tail (Szabo, 1961).
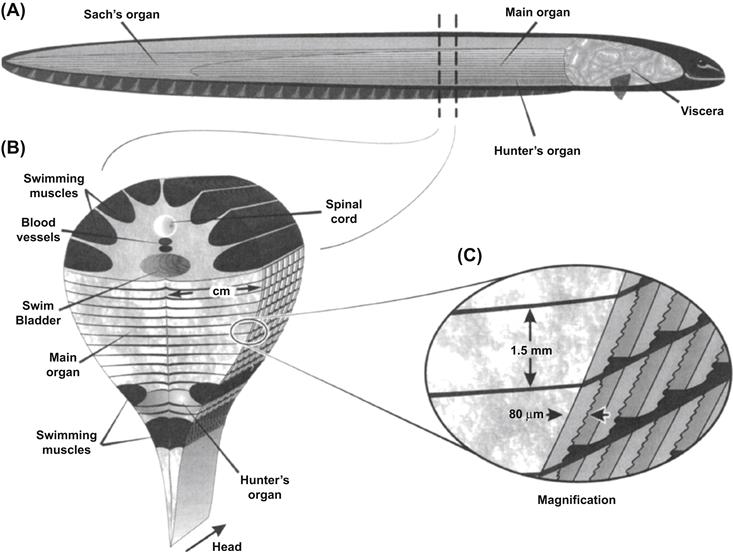
FIGURE 48.1 Anatomy of the electric eel. (A) Diagram illustrating the anatomical orientation of electric organs. (B) A section through the middle portion of the eel, drawn such that the anterior surface is nearest the reader. (C) Columns of electrocytes extend the length of the electric organ. In this panel, the flatter, caudal surface of each electrocyte would be innervated by numerous electromotor neurons (not shown).
As depicted in Fig. 48.1, Electrophorus has three well-defined electric organs. The main organ is the largest and is responsible for voluntarily generating powerful high-voltage discharges. The main organ extends from behind the peritoneal cavity of the viscera down the tail of the eel, where it eventually gives rise to Sach's organ. This organ, along with Hunter's organ, generates repetitive low-voltage discharges and is thought to be involved in electrolocation of objects in the eel's environment. In the cross-sectional view of Fig. 48.1B, Hunter's organ is seen to be partially delineated from the main organ by two columns of skeletal muscles. Electric tissue develops from these columns of skeletal muscle tissue in immature eels and is thought to arise from embryonic myocyte precursor cells (Keynes, 1961). As shown later, the membranes of electrocytes are biochemically and functionally very similar to skeletal muscle sarcolemma.
Electrocytes of both the main electric organ and Sach's organ are large ribbon-shaped cells. Each electrocyte extends laterally from the midline of the electric organ to the skin, a distance of up to 4 cm. They have a width of up to 1.5 mm and thickness of 80 μm. As seen in Fig. 48.1C, electrocytes are positioned one after another along their flat axis to give rise to long rectangular columns of cells running along the longitudinal axis of the eel. These columns are delineated and electrically insulated from one another by connective tissue septa, which help to maintain the physical structure of the electric organ. This stacked arrangement is a common feature of electric organs in electric fish and enables greater voltages to be produced (see later). When viewed under light microscopy, electrocytes are seen as multinucleated syncytia, similar to the skeletal muscle myocytes from which they are derived. Electrocytes are seen in cross-section to have one relatively flat posterior membrane relative to the other more undulated anterior membrane. The flat caudal membrane is innervated by ACh-releasing electromotor neurons that form synapses that are morphologically similar to motor end plates of skeletal muscle cells (Chapter 32).
Immunofluorescent localization microscopy and electrophysiological experiments have led to the understanding of how the electrical discharge is generated at the cellular level. Electrocytes use membrane receptors and ion channels polarized to either the innervated or non-innervated membrane in order to produce transcellular potentials that give rise to the discharge of the electric organ (Fig. 48.2A). The caudal innervated membrane contains acetylcholine receptors (AChRs), inward and outward rectifying K+ channels, a high density of voltage-gated Na+ channels and only trace amounts of Na+, K+-ATPase. This membrane is both chemically and electrically excitable. That is, this surface of the electrocyte produces APs in response to artificial stimulation with AChR agonists or direct electrical stimulation. The non-innervated membrane, on the other hand, does not respond to these manipulations, since it has no AChRs or voltage-gated Na+ channels. Instead, this membrane contains a high concentration of Na+, K+-ATPase and ion channels responsible for maintaining the resting potential of −70 to −85 mV. Evidence suggests that the resting current of Electrophorus electrocytes is carried predominantly by K+ channels (Lester, 1978), but the contribution of a Cl− conductance cannot be excluded (Nakamura et al., 1965). In Torpedo, the resting current has been shown to be carried at least partially by Cl− (Miller and White, 1980). In skeletal muscle myocytes, 30–70% of the resting current is carried by this anion (Chapter 42). Since Electrophorus electric tissue is derived from skeletal muscle, it is possible that the resting current of these cells is also carried by Cl−.
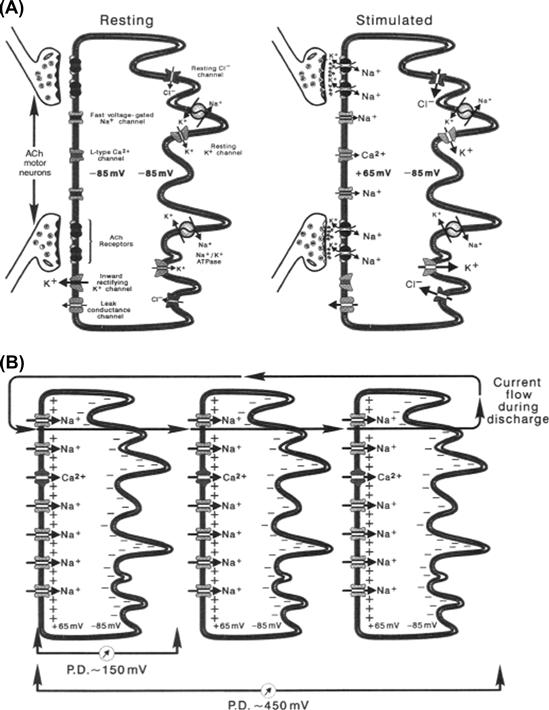
FIGURE 48.2 Diagrammatic representation of electrocytes. The left surface of each cell represents the posterior innervated membrane. (A) At rest, both the innervated and non-innervated membranes exhibit a potential of −85 mV. When stimulated, activated AChRs generate EPPs, triggering Na+ channel-mediated APs peaking at +55 mV on the innervated membrane. The non-innervated membrane contains no voltage-gated Na+ channels and maintains the −85-mV resting potential. The result is a transcellular potential difference of approximately 150 mV. (B) Because each cell is stimulated simultaneously, electrocyte transcellular potentials summate. The potentials of three electrocytes culminate to produce 450 mV. Currents generated by stimulated electrocytes flow down electrocyte columns in the posterior-to-anterior direction. The circuit is closed by current flowing out the head of the eel, through the water, and back into the tail region.
APs arriving at the nerve termini of electromotor neurons cause ACh to be released onto the innervated membrane of electrocytes (see Fig. 48.2A). End-plate potentials (EPPs) produced by AChRs surpass the threshold for Na+ channel activation and trigger the production of APs that propagate very short distances between electromotor junctions and have overshoots of +40 to +65 mV. Meanwhile, the potential of the non-innervated membrane remains at the resting value of up to −85 mV, due to the abundance of resting current channels and the absence of voltage-gated Na+ channels. When the AP peaks on the innervated membrane, a net transcellular potential of up to 150 mV results across the electrocyte (+65 mV of the innervated membrane minus −85 mV of the non-innervated membrane). This transcellular potential difference is accompanied by a net flow of positive current moving in the innervated membrane-to-non-innervated membrane direction (left to right as shown in Fig. 48.2). Insulating septa that form electrocyte columns effectively insulate the extracellular regions on either side of the electrocyte. These connective tissue structures prevent current from flowing around the outside of the electrocyte, which would short-circuit the transcellular potential difference (see Fig. 48.2B). This arrangement allows each electrocyte to act as a simple battery having an electrical potential of up to 150 mV. Since each electrocyte of a column is stimulated simultaneously, the potentials of each electrocyte battery within a column summate to generate a large voltage, as predicted by Ohm's law. In Fig. 48.2B, the potentials of three electrocytes summate to give a potential of 450 mV. In large eels, where the potentials of many thousands of electrocytes summate, the net electric discharge can reach 700 V.1 Connective tissue septa channel the current down the longitudinal axis of the electric organ toward the head of the eel. The current leaves the eel through low-resistance regions of the skin and is conducted through the water, producing an electric field, the magnitude of which diminishes with the square of the distance from the animal. Objects, like other fish or the human hand, experience a potential difference in this electric field and currents sufficient to excite muscles, nerves and sensory endings flow through them, producing a shocking sensation. The circuit of the discharge is closed by current flowing through the skin of the tail region of the eel back into electrocyte columns from which the electromotive force (EMF) originated (Bennett, 1971).
IV Electrocyte Membrane Electrophysiology
IVA Membrane and Extracellular Potentials
Electrocyte membranes contain many of the same protein elements found in myocytes and neurons. In fact, the individual APs of the innervated membrane of Electrophorus electrocytes are quite similar to those of other excitable cells. However, electric cells are different in that potential changes are polarized to a particular membrane, resulting in the generation of transcellular potentials and an asymmetric flow of current. Also, to produce whole-animal electrical discharges having the maximum possible voltage or current output, electrocytes have evolved to express exaggerated amounts of key excitable membrane proteins.
In electric tissue preparations where the flat innervated electrically excitable surface of electrocytes is exposed, APs can be triggered with extracellular stimulating electrodes situated close to the membrane surface. Membrane potentials and transcellular potentials can then be measured through recording electrodes lowered across the innervated membrane or through the entire cell, respectively (Fig. 48.3). As the recording electrode approaches the innervated membrane and a stimulus is applied, a negative deflection is recorded (Fig. 48.3A). Because the recording electrode measures the potential difference between the region just outside the membrane compared to the reference electrode placed in the bath, the AP here is recorded as a negative deflection. After the electrode is advanced through the innervated membrane, an AP that has propagated from the site of stimulation to the recording electrode is detected. The characteristics of electrocyte membrane potentials vary considerably from cell to cell, but are typically similar to those measured on the myocyte sarcolemma or neuronal axolemma. As seen in Fig. 48.3B, a typical resting potential is about −75 mV and ranges from −65 to −85 mV. The electrocyte AP seen in Fig. 48.3B is typical in its 3.5-ms duration and +50-mV overshoot. Generally, the duration ranges from 2 to 4 ms and the overshoot between +35 and +65 mV. These values for the overshoot are considerably larger than that of other excitable cells and are due to an extraordinarily high density of voltage-gated Na+ current and a relatively low level of outward rectifying K+ current (see discussion later). When the recording electrode is lowered even further until it completely penetrates the electrocyte, transcellular APs are recorded. Because the recording electrode is once again in the extracellular space, the resting potential here is measured as 0 mV. However, the interstitium where the recording electrode is positioned is electrically insulated by connective tissue septa from the reference electrode located in the bath solution. When the electrocyte is stimulated, an AP is recorded that is identical to the intracellular AP, except that it initiates at 0 mV. It has a peak equal to the total amplitude of the intracellular AP, in this case about 125 mV. This transcellular AP arises because the non-innervated non-excitable membrane does not fire an AP that would cancel out the spike of the innervated membrane. Insulating connective tissue septa prevent the potential difference from being short-circuited around the outside of the electrocyte. If the stimulus is increased such that the electrocyte beneath the recording electrode is also stimulated, a negative deflection in the extracellular potential is recorded just behind the 125-mV transcellular AP (see Fig. 48.3C, right). This negative deflection corresponds to the resulting AP of the lower electrocyte, which is stimulated later than the electrocyte on the surface. In the intact electric organ, these electrocytes would be stimulated simultaneously by the eel's nervous system such that these APs would be occurring at the same time. In this way, the transcellular potentials summate to yield a powerful electrical discharge.
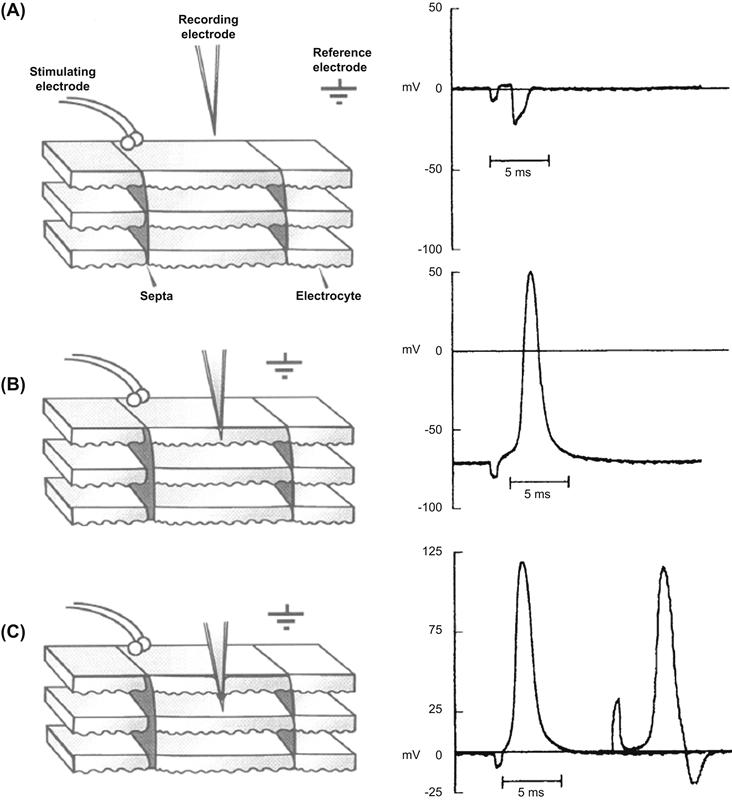
FIGURE 48.3 Extracellular potentials, APs and transcellular potentials of Electrophorus electrocytes in an electric tissue slice preparation. Diagrams to the left depict a slice of electric tissue in cross-section where electrocytes are oriented such that the innervated membrane is uppermost. Columns of electrocytes run in the vertical direction and are delineated by insulating connective tissue septa. Potentials recorded by the recording electrode in the indicated positions are shown on the right. (A) The recording electrode near the innervated membrane records a negative deflection in the extracellular potential. (B) The recording electrode penetrating the innervated membrane records an intracellular AP. (C) Transcellular potentials measured after the recording electrode has penetrated the entire electrocyte. The second potential recording on the right shows the negative deflection of the electrocyte beneath the recording electrode when a higher intensity stimulus is applied.
Ionic currents responsible for Electrophorus electrocyte resting potentials, as well as EPPs and APs are similar to other excitable cells and the reader is referred to previous chapters where their mechanisms have been described in detail. However, some differences between electrocyte membranes and those of neurons and myocytes are worthy of mention. The non-innervated membrane of electrocytes has a very low resistance of about 0.1 Ω/cm2, which is one to two orders of magnitude less than that typically found for nerve or muscle (Nakamura et al., 1965). Physiologically, the eel needs this high K+ and Cl− current to clamp the non-innervated membrane at the resting potential in order to set up transcellular potentials like those observed in Fig. 48.3C. On the other hand, the innervated membrane at rest has a resistance of 3–6 Ω/cm2 (Nakamura et al., 1965). On stimulation, this resistance decreases due to a large voltage-activated Na+ current. In fact, Shenkel and Sigworth (1991) were able to measure macroscopic Na+ currents in excised patches of the innervated membrane corresponding to a density of as much as 1300 channels/μm2. This high density of Na+ channels is accompanied by relatively few outwardly rectifying K+ channels. Because of this distribution of channels in the innervated membrane, the electrocyte AP has a large overshoot that nearly reaches the Na+ equilibrium potential, ENa. The repolarization phase is therefore due primarily to the inactivation of Na+ channels and secondarily to delayed rectifier K+ channels and the resting current of the innervated membrane.
Skeletal muscle-like EPPs that trigger APs on the surface of electrocytes are generated by AChR-mediated currents. Basically, macroscopic and single-channel currents conducted by the eel AChR are very similar to those seen on skeletal muscle sarcolemma. The permeability of the eel receptor to both Na+ and K+ is nearly equal since the reversal potential is the midpoint between ENa and EK (Sheridan and Lester, 1977; Pasquale et al., 1986). In other words, the peak of the EPP moves toward a value of approximately −10 mV in order to activate Na+ channels for an AP. Eel AChRs elicit single-channel opening events that are similar to the receptor from mammalian sources in that their mean open time is dependent on membrane potential, temperature and the AChR agonist used. Single-channel conductances through individual receptors do not depend on the ligand used. However, these preparations of the eel AChR are different from other excitable cells in that single-channel open times can be fitted to a single exponential compared to the more complex distributions found for other sources of the receptor. This indicates that the eel expresses only one isoform of each of the receptor subunits, yielding a receptor with a single unique conductance.
IVB Equivalent Circuits
From what is known of electrocyte electrophysiology, equivalent circuits can be derived that explain the production of transcellular potentials that arise during the AP. Because electrocytes are large flat cells comprised of essentially two parallel membranes having a uniform potential across their entire surfaces, whole-cell potentials can be described with two equivalent circuits, pertaining to the innervated and non-innervated membranes, connected by a resistor representing the resistance of the cytoplasm (Rt). At rest, the permeability of both membranes to K+ and Cl− is high, so that their equilibrium potentials are expressed more than that of Na+, resulting in an Em of about −85 mV (for a detailed description, see Chapter 9). The equivalent circuit for the electrocyte at rest can then be reduced to that seen in Fig. 48.4A (right), where both membranes have composite Em values that drive an outward flow of positive current. Notice that the equivalent circuits for both membranes are mirror images of one another, both having a symmetrical outward movement of current that cancels to give a transcellular potential of 0 mV. At the peak of the AP, however, the permeability of the innervated membrane to Na+ increases dramatically, such that the membrane potential is influenced primarily by ENa. The composite Em of the innervated membrane has now reversed its polarity, so that current across this membrane moves inward (Fig. 48.4B, right). Because the non-innervated membrane has no Na+ conductance, the polarity of the potential here is the same as at rest and a net outward current continues to flow. Now, the total driving force for both membranes is in the same direction, so that current flows from the innervated membrane to and through the non-innervated membrane. If one considers that Rcyt and Rm(non) are negligible compared to Rm(inn), the equivalent circuit for the electrocyte can be reduced to a resistor and battery in series, where the resistance is equal to Rm(inn) and the battery represents the composite potentials of Em(inn) + Em(non). During stimulation, the potential across this unit, then, equals the transcellular potential and has a magnitude equal to that of the amplitude of the innervated membrane's AP, or approximately 150 mV.
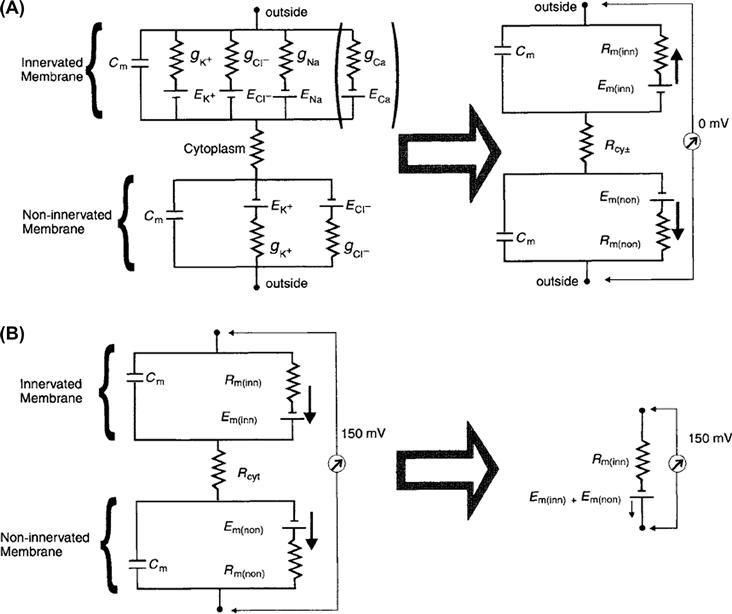
FIGURE 48.4 Electrical equivalent circuit diagrams for both the innervated and non-innervated membranes of an electrocyte. The innervated membrane is uppermost in both A and B. Both membranes are represented by parallel resistance–capacitance circuits and are connected by the cytoplasmic resistance, Rcyt. Cm is the membrane's capacitance. The conductances for K+, Cl−, Na+ and Ca2+ across the membranes are represented by gK, gCl, gNa and gCa, respectively and are inversely proportional to the resistance of the membrane for these ions. Nernst potentials for each of these ions across the membranes are EK, ECl, ENa and ECa. The Ca2+ leg of the circuit is in brackets, since the existence of a selective Ca2+ conductance in the electrocyte has not yet been demonstrated. (A) At rest, the innervated membrane reduces to a circuit where Rm(inn) represents the total membrane resistance of this face of the cell and is a composite of the resistances of the membrane to each of the ions listed to the left. The Em(inn) and Em(non) are the resting membrane potentials for the innervated membrane and non-innervated membrane, respectively. At rest, EK and ECl are expressed the most, since the conductance of the membrane to both these ions is greatest. In this state, the circuit diagrams are mirror images of one another, no net current flows across the cell and the transcellular potential is 0 mV. (B) At the peak of the AP, the conductance of the membrane to Na+ (and possibly Ca2+) increases dramatically and ENa and ECa is expressed more than EK and ECl. The polarity of the Em(inn) battery is now reversed, such that there is a net flow of positive current in the direction of the non-innervated membrane and a transcellular 150-mV potential results. Given that Rcyt and Rm(non) are negligible compared to Rm(inn) , the equivalent circuit can be reduced to the “electrocyte unit” on the right.
In the electric organ of Electrophorus, electrocytes are stacked one after another in very long columns. Electrically, this arrangement is represented by many electrocyte resistor-battery units connected in series, as shown in Fig. 48.5A. Each unit contributes an additional 150 mV to the overall electrical discharge. However, Kirchhoff's first law dictates that the current measured at every point along an unbranching leg of a circuit, such as a series of batteries, is constant.2 In other words, the value of the overall current output of a column of electrocytes does not depend on the number of cells in series, but on the electrocyte that has the greatest resistance to the flow of current. (For a comprehensive description of Ohm's law and Kirchoff's laws applied to biological equivalent circuits, see Sperelakis, 1979.) In order for an electric organ to increase the current of an electrical discharge, it must have additional electrocytes arranged in parallel. This amplification is accomplished in electric fish by having numerous electrocyte columns situated alongside one another. The electric eel, being a long slender animal, has fewer electrocyte columns arranged in parallel relative to some other electric fish. Because of this anatomical arrangement, the eel produces discharges of very high voltage with less current compared to other electric fish, such as Torpedo, the electric ray.
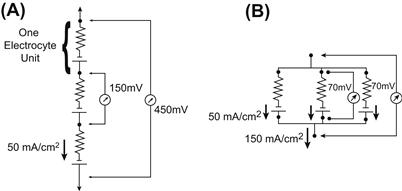
FIGURE 48.5 Electrocyte units in series and in parallel. (A) When connected in series, the potentials of each 150-mV electrocyte unit summate, while the current remains constant. In this case, three electrocyte units are shown to summate their potentials to yield a total of 450 mV. This tends to be the case in the electric organ of Electrophorus, where transcellular potentials of 150 mV and currents of 50 mA/cm2 are measured. (B) The currents of electrocytes in parallel summate while the potential remains constant. Three electrocyte units, each having a current of 50 mA/cm2, are shown to produce a 150 mA/cm2 current when arranged in parallel. This is the case in Torpedo, where many electrocytes are situated next to one another in parallel. The transcellular potential produced by Torpedo electrocytes is 70 mV.
The electric ray is a marine elasmobranch with two large electric organs positioned laterally on either side of its flattened head (Fig. 48.6). Hexagonal-shaped columns of electrocytes run in the vertical direction and conduct current up away from the ocean floor, around the edge of the lateral fin and back into the bottom of the electric organ. Torpedo has a short but wide electric organ accommodating large numbers of electrocyte columns situated next to one another. As shown in Fig. 48.5B, Kirchoff's law predicts that electrocyte resistor-battery units in parallel will produce electrical discharges of low voltage and high current, proportional to the total number of electrocyte units in parallel. This is indeed the case with the electric ray, in which discharges of up to 16 A and 60 V, totaling up to 1 kW, have been measured (Grundfest, 1960).
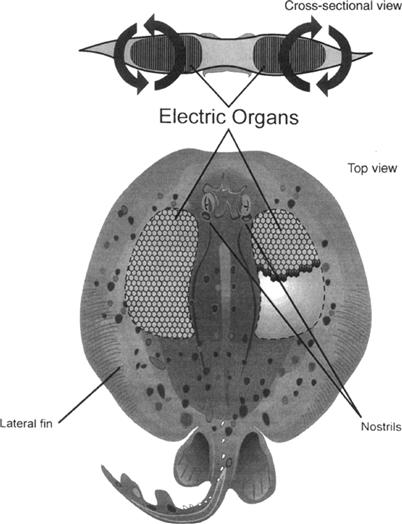
FIGURE 48.6 Drawing of an electric ray (genus Torpedo), depicting the location of its two lateral electric organs. The inset shows the direction of the flow of current around the fish while producing an electrical discharge.
V Comparative Physiology of Electrophorus and Torpedo – Models for Mammalian Excitable Cells
VA Electrophorus
VA1 Na+ Channel
Because electric tissue is specialized for membrane excitability and carries out its function with membrane proteins common to mammalian tissues, electrocytes of electric fish provide excellent models for mammalian excitable cell membranes. Compared to electrocytes of Torpedo and other electric fish, Electrophorus electrocytes express more of the membrane proteins common to mammalian excitable tissues and therefore provide a more general model for excitable membranes. For example, both voltage-gated Na+ and AChR-mediated currents have been measured from the innervated membranes of these cells. Early preparations took advantage of the large size of these cells by sealing single electrocytes over windows in Lucite chambers (Schoffeniels, 1961). Because the resistance of the non-innervated membrane is negligible relative to the innervated membrane, electrocytes in this configuration are treated as a single membrane without having to thread a space-clamping electrode down the middle of the cell. With this method, Nakamura et al. (1965) were able to measure Na+ currents responsible for the rising phase of eel electrocyte APs and to construct a current–voltage (I-V) relation for the Electrophorus Na+ channel. These researchers also described an abundant inward rectifying K+ current that has an I-V relation similar to those seen in myocytes. However, since delayed K+ currents are not seen in these preparations, the repolarization of the eel electrocyte AP is apparently due to Na+ channel inactivation. Much of what is known about the electrogenesis of the AP of electrocytes and other excitable cells has come from studies such as this. The innervated membrane of Electrophorus electrocytes was also patch-clamped to examine macroscopic Na+ currents in order to determine minute charge movements associated with channel opening, as well as to determine the permeability of the channel to K+ relative to Na+. Upon membrane depolarization, the Na+ channel undergoes a conformational change that is associated with the movement of positively charged amino acids within the protein. This movement of positive charge is thought to be a consequence of the opening of the channel gate and can be measured in a population of Na+ channels as a small outward current. Opening of the eel Na+ channel is associated with the movement of approximately 1.5 charges upon channel opening, a value similar to nerve and muscle preparations (Shenkel and Bezanilla, 1991; for a comprehensive description of gating mechanisms, see Hille, 1992). On examining the selective permeability of the eel Na+ channel for this ion relative to K+, Shenkel and Sigworth (1991) found PNa /PK ratios of 8 to 43. This range represents a substantial variation that was even seen in membrane patches taken from the same cell. Given that Electrophorus electrocytes are known to express just one isoform of the channel protein, these results suggested that this variability might arise from post-translational modifications such as glycosylation or even phosphorylation. This possibility was substantiated by experiments that showed the eel Na+ channel to be modulated by exogenously applied protein kinase A (Emerick et al., 1993; also see Chapter 23 of this book). These studies contributed to our basic understanding of the electrophysiological function of the Na+ channel protein.
The first Na+ channel ever purified, and later sequenced, came from electric tissue of Electrophorus. The purified 260-kDa protein is heavily glycosylated and consists of a single functional α subunit (Agnew et al., 1978; Miller et al., 1983). Na+ channels from mammalian brain and muscle express additional β subunits. Since it has been suggested that these auxiliary subunits may regulate channel gating, preparations of the eel Na+ channel are advantageous in that they eliminate the possibly complicating influence of β subunits. An Electrophorus electric tissue cDNA library was used to clone and sequence the channel for the first time (Noda et al., 1984). Its structure includes four homologous repeats that each contain six membrane-spanning helices. The fourth transmembrane segment contains a cluster of positively charged amino acids thought to be involved in gating of the channel. Movement of these positive amino acids during opening of the channel is thought to be responsible for the minute currents measured in patch-clamp experiments such as those discussed earlier. Compared to sequences of Na+ channels of mammalian muscle and brain, the eel Na+ channel shows the greatest homology with the muscle protein, as expected since electrocytes develop ontogenetically from myocytes. Both of these channels, however, lack a 202-amino-acid segment located between the first and second homologous repeat domains of the brain Na+ channel (for complete discussions of Na+ channel purification, structure and diversity, see Chapter 21 in this book and Hille, 1992).
VA2 Acetylcholine Receptor
As mentioned in a previous section, the large size of eel electrocytes has facilitated their dissection in order to measure single-channel AChR conductances. Other excitable cells express numerous isoforms of the subunits that make up the receptor and produce single-channel recordings that show complex mean open-time distributions and variable conductance values. The eel AChR yields more homogeneous values owing to its simplified subunit composition (Pasquale et al., 1986). The simple mean open-time distributions for the eel AChR were found for both the main electric organ as well as for Sach's organ, suggesting that AChR diversity evolved in order to meet varying physiological needs of myocytes and neurons. The eel receptor also desensitizes to a lesser extent in the presence of sustained concentrations of agonists (Pallotta and Webb, 1980). Simple subunit composition and lack of agonist-induced desensitization make the eel AchR advantageous for electrophysiological and biochemical studies.
Recognizing the cholinergic nature and the specialization of Electrophorus electrocytes, biochemists utilized electric tissue as a source for some of the first purifications of AChRs. Using various separation techniques, including differential centrifugation to separate membrane fractions and affinity chromatography to purify selectively the AChR from other membrane proteins, a 260-kDa macromolecule was isolated (Olsen et al., 1972; Biesecker, 1973). Not knowing a priori that the receptor is a pentameric protein made up of α, β, γ and δ subunits (in a respective ratio of 2:1:1:1), the initial isolation and identification of the peptides that make up the whole receptor were arduous. After some debate, the 44-kDa α subunit was established as the ligand-binding portion of the protein. The 50- to 65-kDa β, γ and δ subunits, along with the α subunit, are arranged symmetrically around a central axis to make up the ion channel pore of the protein (Karlin and Cowburn, 1973; Chang, 1974).
VA3 Na+, K+-ATPase
As we have seen, electrocytes express massive quantities of membrane receptors and ion channels in order to carry out their specialized function. To maintain resting potentials in the face of currents associated with EPPs and APs that dissipate Na+ and K+ gradients, electrocytes need to express large amounts of Na+, K+-ATPase. For this reason, Electrophorus electric tissue has been used as a source for the purification of this enzyme for structure–function studies. The eel protein, like that from other tissues, consists of a 94-kDa α subunit and a glycosylated 47-kDa β subunit. During purification, the ATPase is solubilized from electrocyte membranes with various detergents. To analyze its functional characteristics, the protein was reconstituted into liposomes where its ATP-driven translocation of radiolabeled Na+ and K+ has been found to be similar to preparations of native electrocyte membranes containing the ATPase (Yoda et al., 1984). These preparations of the eel protein have been invaluable in determining the reaction mechanisms of the ATPase involved in its function. Drugs that target and inhibit different partial reactions of the protein's translocation mechanism have also been investigated and used to examine the pump's function in cell physiology. Drugs, such as the cardiac glycoside digoxin, have also been used clinically specifically to inhibit Na+, K+-ATPase function in the heart. This treatment dissipates the membrane Na+ gradient, which indirectly augments intracellular Ca2+ within cardiac myocytes. This results in an increase in the heart's force of contraction, which can alleviate some forms of heart disease (for literature review, see Lingrel and Kuntzweiler, 1994.)
VA4 Calmodulin
Electrophorus electric tissue also expresses large quantities of the calcium-binding protein calmodulin. (For a detailed discussion of Ca2+-binding protein function, see Chapter 7.) In fact, calmodulin makes up roughly 2%, by weight, of electrocyte protein (Munjaal et al., 1986). Once again, electric tissue was used as a source for the purification of this 17-kDa soluble protein (Childers and Siegel, 1975). Unlike the membrane proteins discussed earlier, the function of calmodulin within electrocytes remains elusive, even though some of the functions of this Ca2+-mediator protein in intracellular signaling mechanisms is well documented in other electrically excitable cells. Figure 48.7 shows the intracellular location of calmodulin within electrocytes. Calmodulin is present throughout the cytoplasm of electrocytes, but is particularly concentrated near both the innervated and non-innervated membranes. In light of this membrane localization, along with the fact that calmodulin is so abundant in this tissue specialized for membrane excitability, a role for this protein in membrane function is likely. Determining the role of calmodulin in electrocyte function will undoubtedly lend insight into the role of this protein in membrane function of other excitable cells.
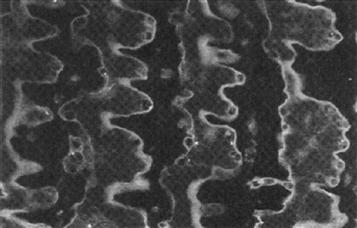
FIGURE 48.7 Immunofluorescent localization of calmodulin within main organ electrocytes. Paraffin-embedded 4-μm sections were probed with anti-calmodulin sheep antibodies. The location of primary antibodies was visualized with fluorescein-labeled rabbit anti-sheep secondary antibodies and photographed using epifluorescence microscopy.
VB Torpedo
VB1 Comparative Electrophysiology
The marine electric ray (genus Torpedo and various species: marmorata, californica, nobilianae, occidentalis) can produce high-amperage electrical discharges by virtue of numerous electrocyte columns arranged in parallel, as discussed briefly earlier. In this way, it differs from Electrophorus which produces high-voltage discharges owing to numerous electrocytes arranged in series. The basic arrangement of Torpedo electrocytes within electric organ columns is remarkably similar to that of Electrophorus, considering that these two fish belong to different orders and the existence of electric tissue in both orders of fish represents convergent evolution. Although Torpedo electrocytes are smaller and pancake shaped (10–30 μm × 5 mm in diameter) relative to wafer-shaped Electrophorus electrocytes, they are still stacked one after another in columns delineated by electrically insulating connective tissue septa. Torpedo electrocytes also display membrane polarity similar to that depicted in the diagrams of Fig. 48.2.
Some basic differences in the membrane biochemistry and electrophysiology exist between electrocytes of these two fishes, however. Like the eel, Torpedo electrocytes can be stimulated to produce EPPs in response to nervous stimulation. That is, these electrocytes are chemically excitable. Torpedo electrocytes, however, do not fire APs in response to EPPs or artificially applied electrical stimuli and are therefore electrically non-excitable. This is due to a lack of voltage-dependent Na+ channels on the innervated membrane that would generate and propagate APs. Instead, these cells are richly innervated by ACh-releasing electromotor neurons and have an abundance of postsynaptic AChRs. These ligand-gated channels conduct EPPs of 5-ms duration that peak just below 0 mV, halfway between ENa and EK. Like Electrophorus electrocytes, the non-innervated membrane of these cells has a large resting current, partially carried by Cl−. Transcellular potentials measured across Torpedo electrocytes then, have an amplitude equal to that of the EPP, or 70 to 85 mV. The equivalent circuits diagrammed in Figs. 48.4A and B also apply to these electrocytes, except that the potential produced by each electrocyte unit equals 70 to 85 mV, instead of 150 mV for Electrophorus electrocytes.
VB2 Acetylcholine Receptor
Without voltage-gated Na+ channels to propagate an AP, EPPs decay exponentially with the distance traveled from the electromotor end-plate. However, very little EPP decay is actually measured on the innervated membrane of Torpedo electrocytes, because the density of end-plates is so great. In fact, one might describe the innervated membrane of these cells as one large electromotor end-plate. These cells, therefore, provide a very specialized model for the motor end-plate. Like Electrophorus electric tissue, Torpedo electric tissue has been used for the purification of the AChR, which has supplied a wealth of knowledge about the biochemical properties of the receptor, as described in the previous section. The first sequences ever to be determined for each of the subunits of the receptor were obtained by screening Torpedo electric tissue cDNA libraries (Noda et al., 1982, 1983a,b; Claudio et al., 1983). The mRNAs encoding each of the receptor subunits were together injected into Xenopus oocytes in order for the functional protein to be expressed on the membrane of these cells. Interestingly, an increase in the ACh-induced conductance could be measured after microinjection (Mishina et al., 1984). By knowing the sequence of the receptor's subunits, a three-dimensional model of the receptor has been constructed and continually amended in light of ongoing biochemical research. (A model of the AChR appears in Chapter 32.) These studies established the basic protein structure of the nicotinic AChR, the findings of which have only been slightly modified to apply to the receptor of mammalian muscle and nerve.
VB3 Acetylcholinesterase
AChE is an important enzyme found in the postsynaptic membrane of cholinergic synapses of neurons, motor end-plates of myocytes and the electromotor end-plates of electrocytes. It catalyzes the hydrolysis of ACh to choline and acetate, thereby terminating ligand-gated activation of the AChR. Some pesticides and chemical warfare agents contain anti-AChE agents that cause acute and chronic alterations in central nervous system and neuromuscular function. Anti-AChE drugs have also been developed to alleviate symptoms of glaucoma, Alzheimer's dementia and myesthenia gravis – diseases marked by attenuated postsynaptic AChR density or compromised ACh release (for review, see Millard and Broomfield, 1995). Obviously, great care is needed when administering these drugs because overmedication can cause side effects similar to exposure to harmful anti-AChE agents.
Since the Torpedo electrocyte represents an exaggerated cholinergic system, it was used as a source for the purification and subsequent structural analysis of AChE. AChE is an 80-kDa protein that self-associates into tetramers, octamers and dodecamers and is anchored in the postsynaptic membrane through a phospholipid linkage (Parker et al., 1978; Ratman et al., 1986). Torpedo electric tissue provided massive enough quantities of AChE for the protein to be crystallized for subsequent x-ray diffraction studies. The resulting diffraction pattern obtained from x-rays shot through these crystals was analyzed to construct a three-dimensional structure of the protein, localizing atoms within the enzyme to within 2.8 Å (Sussman et al., 1991). These experiments with the Torpedo enzyme will continue to be invaluable to the development of new drugs aimed at the treatment of cholinergic diseases and for therapies for individuals exposed to toxic anti-AChE agents.
VB4 Cl− Channel
Perhaps the most dramatic contribution that electric tissue has made to recent membrane biochemistry and physiology has been toward elucidating the structure and function of Cl− channels. Recall that, in order for electrocytes to produce large transcellular potentials, the non-innervated membrane must have a tremendous resting current. This current clamps the non-innervated membrane potential at highly negative resting potentials even while the innervated membrane depolarizes dramatically. In Torpedo electrocytes, the resting current is carried at least partially by Cl−. Electric tissue of the electric ray, therefore, has been used to isolate the channel protein for physiological studies and as a source for mRNA used in cloning and sequencing of the channel.
In the course of developing the planar lipid bilayer method for measuring ion channel conductances, Miller and White (1980) found that vesicles derived from the non-innervated membrane of Torpedo electrocytes contained Cl− channels having novel “double-barreled” gating kinetics. With depolarization, individual Cl− channel complexes acted as two channels with two separate but equal conductances. When one channel of the complex was open, the other was more likely to be subsequently activated as well. Single-channel recordings showed periods of inactivity until one channel of the complex was opened, after which a second equal conductance would superimpose on the first. Purification of the Torpedo Cl− channel confirmed that the protein was a homodimer consisting of two 90-kDa polypeptides. When the purified protein was incorporated into planar lipid bilayers for single-channel recording, the same double-barreled gating kinetics were observed (Middleton et al., 1994).
The first Cl− channel ever to be sequenced came from Torpedo electric tissue and has greatly expanded the field of Cl− channel molecular biology. Using the expression cloning technique, the mRNA responsible for the Torpedo Cl− conductance was identified and its corresponding cDNA sequenced. The encoded protein was predicted to consist of 805 amino acids and to have a molecular weight similar to that of the purified protein (Jentsch et al., 1990). When mRNA for this channel, termed ClC-0, was injected into Xenopus oocytes, Cl− conductances having double-barreled gating kinetics were expressed (Bauer et al., 1991). Recognizing that electric tissue is a model for skeletal muscle membranes, Steinmeyer et al. (1991b) screened a rat muscle cDNA library with oligonucleotide sequences derived from the Torpedo Cl− channel. In this way, the sequence for the major Cl− channel of mammalian skeletal muscle (called ClC-1) was obtained. It was later found that genetic aberrations in the mammalian ClC-1 gene result in symptoms of skeletal muscle myotonia (Steinmeyer et al., 1991a). These findings confirmed the results of Bryant and Morales-Aguilera (1971) that showed this disease to be associated with compromised Cl− conductance. Once the sequences were known for both ClC-0 and ClC-1, investigators began screening libraries derived from virtually every mammalian tissue. Numerous Cl− channel sequences have now been determined and have been implicated in various physiological functions from neuronal membrane excitability to epithelial solute transport (for a review, see Fong and Jentsch, 1995). More Cl− channels having even greater diversity and function will undoubtedly be uncovered in the future.
BIBLIOGRAPHY
1. Agnew WS, Levinson SR, Brabson JS, Raftery MA. Purification of the tetrodotoxin-binding component associated with the voltage-sensitive sodium channel from Electrophorus electricus electroplax membranes. Proc Natl Acad Sci USA. 1978;75:2606–2610.
2. Bauer CK, Steinmeyer K, Schwartz JR, Jentsch TJ. Completely functional double-barreled chloride channel expressed from a single Torpedo cDNA. Proc Natl Acad Sci USA. 1991;88:11052–11056.
3. Bennett MVL. Electric organs. In: Hoar WS, Randall DJ, eds. Fish Physiology. New York: Academic Press; 1971;:347–491.
4. Bennett MVL, Sandri C. The electromotor system of the electric eel investigated with horseradish peroxidase as a retrograde tracer. Brain Res. 1989;488:22–30.
5. Biesecker G. Molecular properties of the cholinergic receptor purified from Electrophorus electricus. Biochemistry. 1973;12:4403–4409.
6. Bryant SH, Morales-Aguilera A. Chloride conductance of normal and myotonic goat fibres and the action of monocarboxylic aromatic acids. J Physiol (London). 1971;219:367–382.
7. Chang HW. Purification and characterization of acetylcho-line receptor-I from Electrophorus electricus. Proc Natl Acad Sci USA. 1974;71:2113–2117.
8. Childers SR, Siegel FL. Isolation and purification of a calcium-binding protein from electroplax of Electrophorus electricus. Biochim Biophys Acta. 1975;455:99–108.
9. Claudio T, Ballivet M, Patrick J, Heinemann S. Nucleotide and deduced amino acid sequences of Torpedo californica acetylcholine receptor γ subunit. Proc Natl Acad Sci USA. 1983;80:1111–1115.
10. Emerick MC, Shenkel S, Agnew WS. Regulation of the eel electroplax Na channel and phosphorylation of residues on amino- and carboxyl-terminal domains by cAMP-dependent protein kinase. Biochemistry. 1993;32:9435–9444.
11. Fong P, Jentsch TJ. Molecular basis of epithelial Cl channels. J Memb Biol. 1995;144:189–197.
12. Grundfest H. The mechanisms of discharge of the electric organs in relation to general and comparative electrophysiology. Prog Biophys. 1957;7:3–74.
13. Grundfest H. Electric organ. McGraw-Hill Encycl Sci Technol. 1960;8:427–433.
14. Hille B, ed. Ionic Channels of Excitable Membranes. Sunderland: Sinauer Associates; 1992.
15. Jentsch TJ, Steinmeyer K, Schwarz G. Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature. 1990;348:510–514.
16. Karlin A, Cowburn D. The affinity-labeling of partially purified acetylcholine receptor from electric tissue of Electrophorus. Proc Natl Acad Sci USA. 1973;70:3636–3640.
17. Keynes RD. The development of the electric organ in Electrophorus electricus. In: Chagas C, Paes De Carvalho A, eds. Bioelectrogenesis. New York: Elsevier; 1961;:14–19.
18. Lester H. Analysis of sodium and potassium redistribution during sustained permeability increases at the innervated face of Electrophorus electroplaques. J Gen Physiol. 1978;72:847–862.
19. Lingrel JB, Kuntzweiler T. Na+, K+-ATPase. J Biol Chem. 1994;269:19659–19662.
20. Middleton RE, Pheasant DJ, Miller C. Purification, reconstitution, and subunit composition of a voltage-gated chloride channel from Torpedo electroplax. Biochemistry. 1994;33:13189–13198.
21. Millard CB, Broomfield CA. Anticholinesterases: medical applications of neurochemical principles. J Neurochem. 1995;64:1909–1918.
22. Miller C, White MM. A voltage-dependent chloride conductance channel from Torpedo electroplax membrane. Ann NY Acad Sci. 1980;80:534–551.
23. Miller JA, Agnew WS, Levinson SR. Principal glycopeptide of the tetrodotoxin/saxitoxin binding protein from Electrophorus electricus: isolation and partial chemical and physical characterization. Biochemistry. 1983;22:462–470.
24. Mishina M, Kurosaki T, Tobimatsu T, et al. Expression of functional acetylcholine receptor from cloned cDNAs. Nature. 1984;307:604–608.
25. Munjaal RP, Conner CG, Turner R, Dedman JR. Eel electric organ: hyperexpressing calmodulin system. Molec Cell Biol. 1986;6:950–954.
26. Nakamura Y, Nakajima S, Grundfest H. Analysis of spike electrogenesis and depolarizing K inactivation in electroplaques of Electrophorus electricus. L J Gen Physiol. 1965;49:321–349.
27. Noda M, Shimizu S, Tanabe T, et al. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984;312:121–127.
28. Noda M, Takahashi H, Tanabe T, et al. Primary structure of α-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982;299:793–797.
29. Noda M, Takahashi H, Tanabe T, et al. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983a;302:528–532.
30. Noda M, Takahashi H, Tanabe T, et al. Primary structures of β- and δ-subunit precursors of Torpedo californica acetylcholine receptor deduced from cDNA sequences. Nature. 1983b;301:251–255.
31. Olsen RW, Meunier J-C, Changeux J-P. Progress in the purification of the cholinergic receptor protein from Electrophorus electricus by affinity chromatography. FEBS Lett. 1972;28:96–100.
32. Pallotta BS, Webb GD. The effects of external Ca++ and Mg++ on the voltage sensitivity of desensitization in Electrophorus electroplaques. J Gen Physiol. 1980;75:693–708.
33. Parker KK, Chan SL, Trevor AJ. Purification of native forms of eel acetylcholinesterase: active site determination. Arch Biochem Biophys. 1978;187:322–327.
34. Pasquale EB, Udgaonkar JB, Hess GP. Single-channel current recording of acetylcholine receptors in electroplax isolated from the Electrophorus electricus main and Sachs' electric organs. J Memb Biol. 1986;93:195–204.
35. Ratman M, Sargent PB, Sarin V, et al. Location of antigenic determinants on primary sequences of subunits of nicotinic acetylcholine receptor by peptide mapping. Biochemistry. 1986;25:2621–2632.
36. Schoffeniels E. The flux of cations in the single isolated electroplax of Electrophorus electricus (L.). In: Chagas C, Paes De Carvalho A, eds. Bioelectrogenesis. New York: Elsevier; 1961;:147–165.
37. Shenkel S, Bezanilla F. Patch recordings from the electrocytes of Electrophorus Na channel gating currents. J Gen Physiol. 1991;98:465–478.
38. Shenkel S, Sigworth FJ. Patch recordings from the electrocytes of Electrophorus electricus Na currents and PNa/PK variability. J Gen Physiol. 1991;97:1013–1041.
39. Sheridan RE, Lester HA. Rates and equilibria at the acetylcholine receptor of Electrophorus electroplaques A study of neurally evoked postsynaptic currents and of voltage-jump relaxations. J Gen Physiol. 1977;70:187–219.
40. Sperelakis N. Origin of the cardiac resting potential. In: Berne RM, Sperelakis N, eds. Handbook of Physiology, Vol 1, The Cardiovascular System. Bethesda: American Physiological Society; 1979;:187–267.
41. Steinmeyer K, Klocke R, Ortland C, et al. Inactivation of muscle chloride channel by transposon insertion in myotonic mice. Nature. 1991a;454:304–308.
42. Steinmeyer K, Ortland C, Jentsch TJ. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 1991b;354:301–304.
43. Sussman. JL, Harel M, Frolow F, et al. Atomic structure of acetylcholinesteras, from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991;253:872–879.
44. Szabo TH. Anatomo-physiologie des centres nerveux specifiques de quelques organes electriques. In: Chagas C, Paes De Carvalho A, eds. Bioelectrogenesis. New York: Elsevier; 1961;:185–201.
45. Walsh J. Experiments and observations on the Gymnotus electricus, or electric eel. Philos Trans. 1775;65:94–101.
46. Yoda. A, Clark AW, Yoda S. Reconstitution of (Na+ + K+)-ATPase proteoliposomes having the same turnover rate as the membraneous enzyme. Biochim Biophys Acta. 1984;778:332–340.
1 A discharge of this magnitude requires that at least 4700 electrocytes be stimulated simultaneously. That is, 4700 electrocytes × 0.15 V per electrocyte = 705 V. This situation is analogous to a flashlight, where more batteries aligned in series produce a brighter light source.
2 Specifically, Kirchoff's first law states that the current entering a point along a circuit is equal to the sum of the currents of all the branches leaving that point. Therefore, if the circuit is unbranching, then the current at every point is constant.