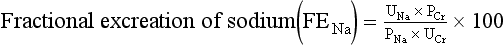Potassium, Urine (K)
Indications
This test measures the amount of potassium in a spot or 24-hour urine collection to aid in determining electrolyte balance.
Test Explanation
Potassium is the major cation within the cell. The electrolyte balance of potassium can be measured in a spot or a 24-hour urine collection. A 24-hour collection is essential to evaluate electrolyte (especially hypokalemia) balance, acid-base balance, and renal and adrenal diseases.
The serum potassium concentration depends on many factors. Aldosterone, and to a lesser extent glucocorticosteroids, tends to increase renal losses of potassium. If sodium blood levels are diminished, the renal tubules can reabsorb sodium in exchange for potassium, which is then excreted at increased rates. Acid-base balance depends to a small degree on potassium excretion. In alkalotic states, hydrogen can be reabsorbed in exchange for potassium. The kidneys cannot reabsorb potassium. Therefore potassium intake is balanced by kidney excretion through the urine.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Chronic renal failure: Sodium loss is increased in some forms of renal failure because of loss of reabsorptive capabilities of the kidneys. Potassium follows sodium loss.
Renal tubular acidosis: Reduced excretion of hydrogen increases excretion of potassium.
Starvation: To provide energy, protein- and fat-containing tissues are broken down. The cells in those tissues expel potassium into the bloodstream. The potassium is then excreted, at increased levels, into the urine.
Aldosterone increases potassium urinary excretion. Because glucocorticosteroids have an aldosterone-like effect, potassium excretion is also increased in Cushing syndrome.
Excessive intake of licorice: Licorice has an aldosterone-like effect, as described above.
Alkalosis: Hydrogen is reabsorbed in the renal tubules in exchange for potassium excretion.
Diuretic therapy: Most diuretics are potassium wasting and increase potassium urinary excretion.
 Decreased Levels
Decreased Levels
Dehydration: Decreased renal blood flow associated with dehydration diminishes urinary excretion of potassium.
Addison disease: This disease is associated with diminished aldosterone effect on the kidneys. Because aldosterone increases urinary excretion of potassium, reduced levels of aldosterone are associated with reduced urinary potassium levels.
Acute renal failure: Urinary excretion of potassium is diminished. This is the most common cause of hyperkalemia.
Pregnanediol
Indications
This test measures pregnanediol, a metabolite of progesterone. It is used in the evaluation and decision making in women who are having difficulty becoming pregnant or maintaining a pregnancy. It is also used to monitor “high-risk” pregnancies.
Test Explanation
Urinary pregnanediol is measured to evaluate progesterone production by the ovaries and placenta. The main effect of progesterone is on the endometrium. It initiates the secretory phase of the endometrium in anticipation of implantation of a fertilized ovum. Normally, progesterone is secreted by the ovarian corpus luteum after ovulation. Both serum progesterone levels and urine concentration of progesterone metabolites (pregnanediol and others) are significantly increased during the second half of an ovulatory cycle. Pregnanediol is the most easily measured metabolite of progesterone.
Because pregnanediol levels rise rapidly after ovulation, this study is useful in documenting whether ovulation has occurred and, if so, exactly when. During pregnancy, pregnanediol levels normally rise because of placental production of progesterone. Repeated assays can be used to monitor the status of the placenta in women who have difficulty becoming pregnant or maintaining a pregnancy. Repeated assays can also be used to monitor the status of the placenta in high-risk pregnancy.
Hormone assays for urinary pregnanediol are primarily used to monitor progesterone supplementation in patients with an inadequate luteal phase to maintain an early pregnancy. Urinary assays may be supplemented by plasma assays (progesterone assay, p. 750), which are quicker and more accurate.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Ovulation: Ovulation occurs with development of a corpus luteum, which makes progesterone. Pregnanediol is a metabolite of progesterone.
Pregnancy: A healthy placenta produces progesterone. Pregnanediol is a metabolite of progesterone.
Molar pregnancy: Hydatidiform mole can produce progesterone, although at lower levels than during pregnancy.
Luteal cysts of ovary: The corpus luteum produces progesterone in the nonpregnant woman and in the early stages of pregnancy. Cysts can also produce progesterone for prolonged periods of time. Pregnanediol is a metabolite of progesterone.
Arrhenoblastoma of ovary: This tumor can secrete sex hormones or their metabolites (usually testosterone). 17-Hydroxyprogesterone is a precursor of sex hormones. Pregnanediol is a metabolite of progesterone.
 Decreased Levels
Decreased Levels
These obstetrical emergencies are associated with decreased placental viability. Progesterone is made by the placenta during pregnancy. Pregnanediol is a metabolite of progesterone, which is decreased when placental viability is threatened.
Ovarian neoplasm: Ovarian epithelial cancers can destroy functional ovarian tissue. Progesterone levels may decrease.
Sodium, Urine (Na)
Indications
This test is used to evaluate fluid and electrolyte abnormalities, especially sodium. It can also be used to monitor therapy for these abnormalities.
Test Explanation
Many factors regulate sodium balance. Aldosterone causes conservation of sodium by stimulating the kidneys to reabsorb sodium, thus decreasing renal losses. Natriuretic hormone, or third factor, is stimulated by increased sodium levels. This hormone decreases renal absorption and increases renal losses of sodium. Antidiuretic hormone (ADH), which controls the reabsorption of water at the distal tubules of the kidney, affects sodium urine levels by dilution or concentration.
This test evaluates sodium balance in the body by determining the amount of sodium excreted in urine over 24 hours. Sodium is the major cation in the extracellular space. Measuring the amount of sodium in the urine is useful for evaluating patients with volume depletion, acute renal failure, adrenal disturbances, and acid-base imbalances. In the setting of acute renal failure, an increased value will indicate acute tubular necrosis, while a low value would be typical of prerenal azotemia.
This test is also useful when the serum sodium concentration is low. For example, in patients with hyponatremia caused by inadequate sodium intake, urine sodium will be low. However, in patients with hyponatremia caused by chronic renal failure, urine sodium concentration will be high.
Urine sodium excretions are helpful when the urine output is low (<500 mL/24 hr). However, a more accurate test to determine the cause of reduced urine output is the fractional excretion of sodium (FENa). This is the fraction of sodium actually excreted relative to the amount filtered by the kidney. FENa is a calculation based on the concentrations of sodium (Na) and creatinine (Cr) in the plasma and the urine as follows:
FENa is usually greater than 3% with acute tubular necrosis and severe obstruction of the urinary drainage of both kidneys. It is generally less than 1% in patients with acute glomerulonephritis, hepatorenal syndrome, and states of prerenal azotemia (such as congestive heart failure and dehydration). FENa can also be less than 1% with acute partial urinary tract obstruction.
Interfering Factors
• Dietary salt intake may increase sodium levels.
• Altered kidney function may affect levels.
![]() Drugs that may cause increased urine sodium levels include antibiotics, diuretics, and prostaglandins.
Drugs that may cause increased urine sodium levels include antibiotics, diuretics, and prostaglandins.
![]() Drugs that may cause decreased urine levels of sodium includes nonsteroidal antiinflammatory drugs (NSAIDs) and steroids.
Drugs that may cause decreased urine levels of sodium includes nonsteroidal antiinflammatory drugs (NSAIDs) and steroids.
Procedure and Patient Care
During
• See Box 11-2, Guidelines for a 24-Hour Urine Collection, p. 907.
• A spot urine specimen can be obtained and sent to the laboratory if information about urine sodium is needed sooner than 24 hours. In this situation, ask the patient to void in a nonsterile container, and transport the entire volume to the laboratory. The more urine available, the more accurately the spot urine specimen will reflect the 24-hour urine results.
• If FENa is ordered, venous blood is drawn in a gold-top tube for serum creatinine and sodium measurement.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Dehydration: Free water is maximally reabsorbed by the kidney, and urine sodium is more concentrated.
Adrenocortical insufficiency: Aldosterone and corticosteroids stimulate sodium reabsorption in the distal renal tubules. With inadequate levels of these hormones, sodium will not be reabsorbed, and large amounts are wasted into the urine.
Diuretic therapy: Most diuretics work by diminishing sodium reabsorption and increasing sodium loss in the kidney.
Syndrome of inappropriate antidiuretic hormone secretion (SIADH): ADH stimulates free water reabsorption in the kidney. With inappropriately high secretion of ADH, free water in the urine is diminished and sodium is more concentrated.
Diabetic ketoacidosis: The osmotic diuresis due to hyperglycemia tends to diminish sodium reabsorption in the kidney. Further, sodium salts combine with some ketotic products to further increase sodium losses into the urine.
Chronic renal failure: Renal reabsorption of sodium and many other products is diminished in a diseased, nonfunctioning kidney. Urine sodium levels increase.
 Decreased Levels
Decreased Levels
Congestive heart failure: Renal blood flow is diminished with reduced cardiac output. The renin-angiotensin system is activated (see pp. 447 to 448), and aldosterone production is stimulated. Aldosterone stimulates renal reabsorption of sodium, and urine levels diminish.
Intestinal absorption of sodium is reduced. The physiologic response is to reduce sodium excretion in the urine.
Cushing disease: Corticosteroids have an aldosterone-like effect on the kidney, which tends to stimulate renal reabsorption of sodium, and urine levels diminish.
Aldosteronism: Aldosterone stimulates renal reabsorption of sodium, and urine levels diminish.
Inadequate sodium intake: Intestinal absorption of sodium is very efficient. Therefore it is rare for a nutritional deficiency to occur as sodium insufficiency severe enough to significantly diminish renal excretion. However, with sodium deficit or ongoing sodium losses treated with inadequate sodium replacement, serum sodium levels significantly diminish and the kidneys are maximally stimulated to reabsorb sodium. Urine sodium levels diminish.
Related Tests
Sodium, Blood (p. 466). This is a direct measurement of sodium levels in blood.
Aldosterone (p. 43). More than any other hormone, aldosterone has a significant effect on blood sodium levels.
Antidiuretic Hormone (p. 73). By affecting free body water excretion, ADH alters sodium levels by virtue of dilution or concentration.
Substance Abuse Testing (Urine Drug Testing, Drug Screening)
Indications
Substance abuse testing is used to identify metabolites of illegal drugs used by the person being tested.
Test Explanation
Drug testing is mostly used by employers and by law enforcement agencies. Employers primarily use drug testing to promote and protect the safety, health, and well-being of their employees. Because many industrial fatalities are attributable to substance abuse, drug-testing programs are common in the workplace. Furthermore, drug use is responsible for decreased productivity and increased absenteeism. Industrial testing is used at the time of preemployment, prepromotion, annual physical, postaccident, or when there is reasonable suspicion. Other times include random testing or for follow-up surveillance of treatment.
Most commonly, a drug screen is performed to detect small amounts of any number of metabolites of commonly used drugs. If the screen result is positive, a more accurate and quantitative test is performed on the same specimen. Drug screens are available for a variety of substances. The most common are amphetamines, barbiturates, benzodiazepines, carisoprodol, cocaine, meprobamate, methamphetamine, opiates (morphine and heroin), cannabinoids (marijuana [tetrahydrocannabinol {THC}]), phencyclidine (PCP), and propoxyphene (Table 11-1). Alcohol testing is most commonly used by law enforcement (see p. 229). Not only is drug testing helpful in identifying users, but it also acts as a deterrent. Athletes are tested for anabolic hormones, stimulants, diuretics, beta blockers, street drugs, anti-estrogens, erythropoietin, and beta-2 agonists that may unfairly improve their performance. Health and life insurance companies routinely test for illicit drugs.
TABLE 11-1
Typical Multipanel Drug Screen
| Drugs/Drug Classes | Screen | Confirmation∗ |
| Marijuana | 20 ng/mL | 5 ng/mL |
| Cocaine | 150 ng/mL | 50 ng/mL |
| Opiates | 300 ng/mL | 5 ng/mL |
| Oxycodone | 100 ng/mL | 5 ng/mL |
| Phencyclidine | 25 ng/mL | 10 ng/mL |
| Amphetamines | 300 ng/mL | 200 ng/mL |
| MDMA (Ecstasy) | 500 ng/mL | 200 ng/mL |
| Barbiturates | 200 ng/mL | 50 ng/mL |
| Benzodiazepines | 200 ng/mL | 20 ng/mL |
| Methadone | 150 ng/mL | 10 ng/mL |
| Propoxyphene | 300 ng/mL | 10 g/mL |
∗Confirmatory tests are more sensitive and can detect metabolites at lower levels.
Substance abuse testing, up until recently, has used urine exclusively as the sample of choice. Urine is easily obtained and plentiful, and it contains a large amount of drug and metabolites. More importantly, urine can identify drug usage for several days after the last usage. THC can be identified in the urine for several weeks in chronic users. Blood testing reflects drug usage only during the past few hours. Saliva, breath, hair, and sweat are becoming increasingly important and accurate samples for specific drug testing. These testing methods are very expensive, however. Hair samples detect the presence of drugs used during the past 3 months. In addition, hair and nail samples may be used to detect or document exposure to arsenic and mercury. Nevertheless, urine testing remains the mainstay for drug testing (Figure 11-1).
The absence of an expected drug(s) and/or drug metabolite(s) may indicate compliance or a difficulty in identifying the substance because of inappropriate timing of specimen collection relative to drug administration, poor drug absorption, diluted/adulterated urine, or limitations of testing. The concentration at which the screening test can detect a drug or metabolite varies within a drug class.
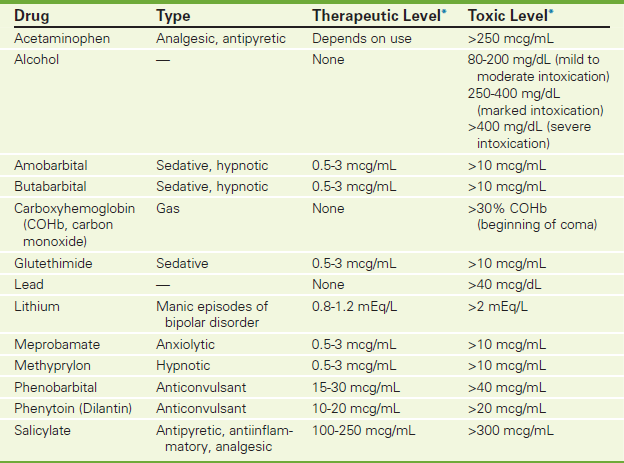
Toxicology screening tests for drug overdose (see Table 2-19 on p. 213) and poisoning (e.g., lead and carbon monoxide, see Table 11-2 on p. 952) are best performed on blood. Results indicate current drug levels, which are used to determine or alter therapy. Toxicology studies are used to incriminate drugs as a cause or factor in the death of a person. They are also used to assess patients when poisoning contributes to an illness.
Substance abuse testing can be ordered as the “drug abuse survey” that is an immunoassay to identify drugs of abuse by class (e.g., amphetamines, barbiturates, benzodiazepines). This testing is directed toward the patient's symptoms or medication history. The results are considered presumptive only. There is high cross-reactivity to over-the-counter medications.
Confirmed drug abuse survey is usually performed by immunoassay as described in the preceding paragraph. However, the results are confirmed by more definitive analytic techniques such as gas chromatography or liquid chromatography/tandem mass spectrometry. This testing method is especially useful for patients who are inclined to deny the results of the drug abuse survey immunoassay. When positive, a specific drug and its quantification are reported.
Because a positive result can have a profound effect on a person's life, job, and accountability, it is not uncommon for a drug abuser to attempt to alter the urine specimen (specimen adulteration). Therefore the urine sample is tested for odor, color, temperature, creatinine, pH, and specific gravity to ensure that it is a proper specimen. If the specimen does not meet these assessment standards, it is rejected and a second specimen is requested.
Interfering Factors
• Poppy seeds can cause positive opiate results.
• Second-hand marijuana smoke can cause positive results.
![]() Ibuprofen can cause a false-positive THC result in some assay systems.
Ibuprofen can cause a false-positive THC result in some assay systems.
![]() Cold remedies can cause false-positive amphetamine results in some assay systems, but not with the monoclonal antibody test.
Cold remedies can cause false-positive amphetamine results in some assay systems, but not with the monoclonal antibody test.
![]() Antibiotics (e.g., amoxicillin) can cause false-positive results for heroin and/or cocaine.
Antibiotics (e.g., amoxicillin) can cause false-positive results for heroin and/or cocaine.
![]() The aggressive use of diuretics can decrease drug levels in the urine.
The aggressive use of diuretics can decrease drug levels in the urine.
Procedure and Patient Care
During
• Collect blood and urine specimens as designated by the laboratory.
• Ensure that patients provide their own urine. Usually, the collection is supervised by a trained health care professional.
• Be sure that the patient does not alter the urine specimen.
• For hair testing, cut 50 strands of hair from the scalp.
• A second confirmatory specimen may be obtained (and is used if the results are positive).
After
• Follow the chain of custody for the specimen as provided by standard guidelines of the institution.
• Place the specimen in the required container for delivery.
• Check the temperature of urine specimens within 3 minutes after voiding. Temperature should be between 97° and 99° F.
• The specimen may be sent to a nationally certified laboratory for federal workers or workplace testing. Local hospital laboratories are often able to test for many drugs.
Related Test
Ethanol (p. 229). This is another common form of drug testing used in industry and by law enforcement agencies.
Toxicology
Normal Findings
See Tables 11-2 and 11-3 for blood toxicology and urine toxicology, respectively.
TABLE 11-3
Urine Toxicology Screening for Amphetamines
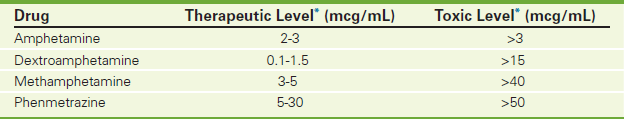
∗Varies according to institution performing the test.
Test Explanation
Detection of the most commonly abused nonprescription mood-altering drugs is discussed. These drugs are most commonly used in suicide attempts and chemical poisonings.
Testing for drug overdose and poisoning is best performed on blood. Results indicate immediate drug levels, which can indicate or alter therapy. Screening for use or abuse of nonprescription drugs is usually done on urine. Urine specimens are easily obtained without any invasive procedure. Often the specimen is obtained several hours or days after the drug administration. In this case, blood levels are low but urine levels are high. Further, drug metabolic products exist in the urine for longer periods, allowing detection of drug use in the past few hours or days. The disadvantages of urine drug tests are that they cannot indicate with any degree of accuracy when the drug was used and if the drug had any effect on the person's actions at any time. Also, the urine can be altered easily by changing the concentration (by drinking a large volume of water or adding water to the specimen), changing the pH, or adding foreign substances. Urine temperature, specific gravity, and creatinine concentration are often determined in urine specimens to ensure the specimen has not been altered. It is important to define the appropriate chain of transfer of the specimen from the moment it is obtained to the point of testing to prevent tampering.
Because of the impact on a person's life (socially, financially, and legally), positive results must be substantiated by another equally accurate test method. A popular combination is to screen with thin-layer or gas chromatography to separate out the constituents in the specimen, followed by mass spectrometry to identify those constituents.
Toxicology studies are used to incriminate drugs as a cause of or factor in a death. They are also used to assess patients when drug abuse or poisoning is contributing to an illness. Drug abuse is important to recognize in the workplace because of safety issues and in prisons because of disciplinary concerns.
Commonly Abused Drugs
Marijuana (Cannabis)
Marijuana is usually detected by identifying one of its metabolites (tetrahydrocannabinol [THC]) in the urine. Most laboratories detect carboxy-THC and use 100 ng/mL as a cutoff. Lower levels from passive inhalation of marijuana may be detected, but are not prosecutable in court. These metabolites can exist in the urine 1 hour after use and for 1 to 3 days afterward.
Cocaine (Including Crack)
Benzoylecgonine is a metabolite of cocaine. It is easily detectable in urine 1 to 4 hours after use and for 2 or 3 days. To indicate the timing of cocaine use, serum levels of cocaine must be determined.
Phencyclidine (PCP)
PCP or one of its metabolites is detectable in urine about 6 to 18 hours after use and for as long as 3 days.
Amphetamines (Especially Methamphetamine)
Amphetamines are identifiable in urine about 3 hours after use and for about 1 or 2 days. One must be careful in assuming abuse with detection of amphetamines, because many over-the-counter (OTC) cold medicines and weight loss medicines contain amphetamine analogs.
Morphine and Other Narcotic Alkaloids
Heroin, morphine, and codeine can be identified in the urine glucuronide conjugated forms 2 hours after use and for 2 to 3 days. Like amphetamines, one must be careful in assuming abuse with detection of codeine, because many OTC pain relievers and cough-suppressive medicines contain codeine.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient or significant others.
Explain the procedure to the patient or significant others.
• If the specimen is obtained for medicolegal testing, ensure that the patient or family member has signed a consent form.
• Obtain as much information as possible about the drug type, amount, and ingestion time.
• Carefully assess the patient for respiratory distress, a common adverse reaction to drug overdose.
During
• Collect blood or urine specimens as indicated. Urine specimens are collected in the presence of a trained health care professional.
• Collect gastric contents for analysis if indicated.
• Note that hair and nail samples may be used to detect or document exposure to arsenic and mercury.
• Immediately identify the sample and mark the patient's name on the specimen.
After
• Apply pressure or a pressure dressing to the venipuncture site.
• Assess the venipuncture site for bleeding.
• Assess the patient for respiratory distress, a common adverse reaction to drug overdose.
![]() Refer the patient for appropriate drug and psychiatric counseling.
Refer the patient for appropriate drug and psychiatric counseling.
• Follow the predetermined chain of transfer of the specimen to the laboratory for testing. Each person involved in handling the specimen must document his or her place in its handling.
![]() Remind the patient that all positive screening results must be confirmed.
Remind the patient that all positive screening results must be confirmed.
Test Results and Clinical Significance
Abuse or use of nonprescription drugs: Urine is most often used for this testing.
Heavy metal and lead poisoning: Blood, urine, cerebrospinal fluid, and tissue specimens may all be used to identify these poisons.
Suicide attempts: Determination of toxic levels of drugs is much more accurately determined with blood tests, although urine may also be used.
Related Tests
Ethanol (p. 229). This is a direct measurement of alcohol level in the blood.
Carboxyhemoglobin (p. 143). This test is used to detect carbon monoxide poisoning.
Delta-Aminolevulinic Acid (p. 922). This test is used to identify lead poisoning.
Drug Monitoring (p. 211). These tests are used to help identify toxic levels of therapeutic drugs such as aspirin and acetaminophen, commonly involved in suicide attempts.
Substance Abuse Testing (p. 948). This test is used to detect illegal drug use.
Uric Acid, Urine
Indications
Uric acid levels can be measured in both blood and urine. Urine levels of uric acid are helpful in evaluating uric acid metabolism in gout and for assessing hyperuricosuria in renal calculus formation. This test also helps to identify persons at risk for stone formation.
Test Explanation
Uric acid is a nitrogenous compound that is the final breakdown product of purine (a deoxyribonucleic acid [DNA] building block) catabolism. (See p. 514 for blood uric acid level.) Seventy-five percent of uric acid is excreted via the kidneys, and 25% by way of the intestinal tract. Elevated uric acid levels (hyperuricemia) may be indicative of gout, a form of arthritis caused by deposition of uric acid crystals in periarticular tissue. An elevated uric acid level in the urine is called uricosuria. Uric acid can become supersaturated in the urine and crystallize to form kidney stones, which can block the renal system.
Uric acid is produced primarily in the liver. Urinary excretion of uric acid depends on uric acid levels in the blood, along with glomerular filtration and tubular secretion of uric acid into the urine. Elevated uric acid levels can cause nephrolithiasis and ureterolithiasis. Uric acid is less well saturated in alkaline urine. As the urine pH rises, more uric acid can exist without crystallization and stone formation. Therefore urine known to have a high uric acid level can be alkalinized by ingestion of a strong base to prevent stone formation.
Procedure and Patient Care
During
• See Box 11-2, Guidelines for a 24-Hour Urine Collection, p. 907.
• A preservative may be used. Check with the laboratory.
![]() Show the patient where to store the urine container.
Show the patient where to store the urine container.
• Keep the specimen on ice or refrigerated during the entire 24 hours. (Note that some laboratories do not require that the specimen be kept cool.)
Test Results and Clinical Significance
 Increased Levels (Uricosuria)
Increased Levels (Uricosuria)
Gout: Uric acid levels are high in blood. With normal glomerular filtration, levels are high in urine.
Rapid cell destruction associated with rapidly growing cancers (with high cell turnover), and especially after chemotherapy for those rapidly growing tumors, causes the cells to lyse and spill their nucleic acids into the bloodstream. In the liver, these free nucleic acids are converted to uric acid. Blood and urine levels of uric acid increase.
High-purine diet: With increased uric acid production caused by a diet high in purines, uric acid levels in the urine will be increased.
Uricosuric drugs (e.g., ascorbic acid, calcitonin, citrate, dicumarol, estrogens, steroids, iodinated dyes, glyceryl guaiacolate, phenolsulfonphthalein, probenecid, salicylates, and outdated tetracycline): These drugs increase uric acid excretion into the urine.
Lead toxicity: Heavy-metal poisoning is associated with increased uric acid tubular secretion.
 Decreased Levels
Decreased Levels
Kidney disease: With decreased glomerular filtration rate and decreased tubular secretion of uric acid, urine levels fall.
Eclampsia: The pathophysiology of this observation is not well known.
Chronic alcohol ingestion: Chronic acidosis from excessive alcohol ingestion decreases renal tubular secretion of uric acid into the urine.
Acidosis (ketotic [diabetic or starvation], lactic): Renal tubular secretion of uric acid into the urine is decreased. Ketoacids, as occur in diabetic or alcoholic ketoacidosis, may compete with uric acid for tubular excretion, which is another cause of decreased uric acid excretion.
Urinalysis (UA)
Normal Findings
Urobilinogen: 0.01-1 Ehrlich unit/mL
Glucose (see Urine Glucose, p. 924)
Indications
Urinalysis (UA) is part of routine diagnostic and screening evaluations. It can reveal a significant amount of preliminary information about the kidneys and other metabolic processes. For example, it can detect urinary tract diseases (e.g., infection, glomerulonephritis, loss of concentrating capacity), and extrarenal disease processes (e.g., glucosuria in diabetes, proteinuria in monoclonal gammopathies, bilirubinuria in liver disease). It is done diagnostically in patients with abdominal or back pain, dysuria, hematuria, or urinary frequency. It is part of routine monitoring in patients with chronic renal disease and some metabolic diseases.
Test Explanation
Total UA involves multiple routine tests on a urine specimen. This specimen is not necessarily a clean-catch specimen. However, if urinary tract infection (UTI) is suspected, often a midstream, clean-catch specimen is obtained. This urine is then divided into two portions. One is sent for UA, and the other is held in the laboratory refrigerator for culture (see p. 973) if results of UA indicate infection. Routinely, UA includes remarks regarding the color, appearance, and odor; pH; and presence of proteins, glucose, ketones, blood, and leukocyte esterase. In addition, the urine is examined microscopically for RBCs, WBCs, casts, crystals, and bacteria. Because this is a spot urine test, volume is not measured. Volume of urine may be important in many clinical situations; in these cases, a full 24-hour specimen is required.(See Figure 11-2 for automated testing,)
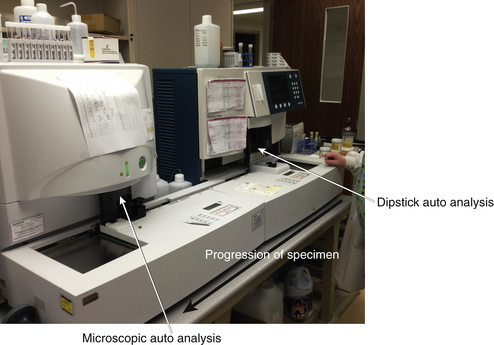
Figure 11-2 Siemens automated urinalysis analyzer. The urine specimen enters the machine on the far right. First, automated dipstick analysis is carried out. The specimen is then transferred to the left of the machine where microscopic automated analysis occurs. The machine will notify the technologist if any significant abnormality is noted. The findings are then individually corroborated. Many specimens can be processed in a short period of time.
Laboratory Examination
Appearance and Color
Urine appearance and color are noted as part of routine urinalysis. A normal urine specimen should be clear. Cloudy urine may be caused by the presence of pus (necrotic WBCs), RBCs, or bacteria; however, normal urine also may be cloudy because of ingestion of certain foods (e.g., large amounts of fat, urates, phosphates). Urine ranges from pale yellow to amber because of the pigment urochrome (product of bilirubin metabolism). The color indicates the concentration of the urine and varies with specific gravity. Dilute urine is straw colored, and concentrated urine is deep amber.
Abnormally colored urine may result from a pathologic condition or the ingestion of certain foods or medicines. For example, bleeding from the kidney produces dark red urine, whereas bleeding from the lower urinary tract produces bright red urine. Dark yellow urine may indicate the presence of urobilinogen or bilirubin. Pseudomonas infection may produce green urine. Eating beets may cause red urine, and rhubarb can color the urine brown. Many frequently used drugs also may affect urine color (Table 11-4).
TABLE 11-4
Frequently Used Drugs That May Affect Urine Color
| Generic and Brand Names | Drug Class | Urine Color |
| Cascara sagrada | Stimulant laxative | Red in alkaline urine; yellow-brown in acid urine |
| Chloroquine (Aralen) | Antimalarial | Rusty yellow or brown |
| Chlorzoxazone (Paraflex) | Skeletal muscle relaxant | Orange or purple-red |
| Docusate calcium (Doxidan, Surfak) | Laxative | Pink to red to red-brown |
| Doxorubicin (Adriamycin) | Antineoplastic | Red-orange |
| Iron preparations (Ferotran, Imferon) | Hematinic | Dark brown or black on standing |
| Levodopa | Antiparkinsonian agent | Dark brown on standing |
| Metronidazole (Flagyl) | Antiinfective | Darkening, reddish-brown |
| Nitrofurantoin (Macrodantin, Nitrodan) | Antibacterial | Brown-yellow |
| Phenazopyridine (Pyridium) | Urinary tract analgesic | Orange to red |
| Phenolphthalein (Ex-Lax) | Contact laxative | Red or purplish pink in alkaline urine |
| Phenothiazines (e.g., prochlorperazine [Compazine]) | Antipsychotic, neuroleptic, antiemetic | Red-brown |
| Phenytoin (Dilantin) | Anticonvulsant | Pink, red, red-brown |
| Riboflavin (vitamin B) | Vitamin | Intense yellow |
| Rifampin | Antibiotic | Red-orange |
| Sulfasalazine (Azulfidine) | Antibacterial | Orange-yellow in alkaline urine |
| Triamterene (Dyrenium) | Diuretic | Pale blue fluorescence |
Odor
Determination of urine odor is part of routine urinalysis. The aromatic odor of fresh, normal urine is caused by the presence of volatile acids. Urine of patients with diabetic ketoacidosis has the strong, sweet smell of acetone. In patients with a UTI, the urine may have a very foul odor. Urine with a fecal odor may indicate an enterobladder fistula.
pH
Analysis of the pH of a freshly voided urine specimen indicates the acid-base balance. The urine reflects the work of the kidneys to maintain normal pH homeostasis. Just as the lungs (respiratory component) help compensate for acid-base imbalance, so do the kidneys (metabolic component). The kidneys assist in acid-base balance by reabsorbing sodium and excreting hydrogen.
An alkaline pH is observed in a patient with alkalemia. Also, bacteria, UTI, or a diet high in citrus fruits or vegetables may cause increased urine pH. An alkaline urine is common after eating. Certain medications (e.g., streptomycin, neomycin, kanamycin) are effective in treating UTIs when the urine is alkaline. It is more common for the urine to be acidic. However, acidic urine is also observed in patients with acidemia, which can result from metabolic or respiratory acidosis, starvation, dehydration, or a diet high in meat products or cranberries. In patients with renal tubular acidosis, however, the blood is acidic and the urine is alkaline.
The urine pH is useful in identifying crystals in the urine and determining the predisposition to form a given type of stone. Acidic urine is associated with xanthine, cystine, uric acid, and calcium oxalate stones. To treat or prevent these urinary calculi, urine should be kept alkaline. Alkaline urine is associated with calcium carbonate, calcium phosphate, and magnesium phosphate stones. To treat or prevent these urinary calculi, urine should be kept acidic. See Urinary Stone Analysis, p. 971.
Protein
Protein is a sensitive indicator of kidney function. Normally, protein is not present in the urine because the spaces in the normal glomerular filtrate membrane are too small to allow its passage. If the glomerular membrane is injured, as in glomerulonephritis, the spaces become much larger, and protein (usually albumin, because it is a smaller molecule than the globulins) seeps into the filtrate and then into the urine. If this persists at a significant rate, hypoproteinemia can develop as a result of severe protein loss through the kidneys. This decreases the normal capillary oncotic pressure that holds fluid within the vasculature and causes severe interstitial edema. The combination of proteinuria and edema is known as nephrotic syndrome.
Proteinuria (usually albumin because it is a relatively small protein) is probably the most important indicator of renal disease. The urine of all pregnant women is routinely checked for proteinuria, which can be an indicator of preeclampsia. Urinary protein is used to screen for nephrotic syndrome and for complications of diabetes mellitus, glomerulonephritis, amyloidosis, and multiple myeloma (see test for Bence-Jones protein, p. 911).
If significant protein is noted at urinalysis, a 24-hour urine specimen should be collected so that the quantity of protein can be measured. This test can be repeated as a method of monitoring renal disease and its treatment. Usually, protein loss of more than 3000 mg/24 hr leads to the signs and symptoms of nephrotic syndrome. If proteinuria is identified, a random urine can be analyzed for protein quantification. This estimate of 24-hour protein excretion is usually performed with a urine creatinine, since hydration status and other factors may influence urine concentration. The normal protein/creatinine ratio is less than 0.15.
Specific Gravity
Specific gravity is a measure of the concentration of particles (including wastes and electrolytes) in the urine. High specific gravity indicates concentrated urine; low specific gravity indicates dilute urine. Specific gravity refers to the weight of the urine compared with that of distilled water (which has a specific gravity of 1.000). Particles in the urine give it weight, or specific gravity.
Specific gravity is used to evaluate the concentrating and excretory power of the kidneys. Renal disease tends to diminish concentrating capability. As a result, chronic renal diseases are associated with low specific gravity of the urine. Specific gravity must be interpreted in light of the presence or absence of glycosuria and proteinuria. Specific gravity is also a measurement of hydration status. With overhydration, the urine will be more dilute, with lower specific gravity, whereas with dehydration, specific gravity can be expected to be abnormally high. Nephrotoxic diabetes insipidus is associated with very little variation in specific gravity of the urine because the kidney cannot respond to variables such as hydration and solute load.
Measurement of urine specific gravity is easier and more convenient than measurement of osmolality (see p. 938). Specific gravity correlates roughly with osmolality. Knowledge of specific gravity is needed to interpret the results of most parts of the urinalysis. Specific gravity is usually evaluated with a refractometer (which measures the amount of light that can pass through a drop of urine) or a dipstick.
Leukocyte Esterase (WBC Esterase)
Leukocyte (WBC) esterase is a screening test used to detect leukocytes in the urine. Positive results indicate UTI. For this examination, chemical testing is performed with a leukocyte esterase dipstick; a shade of purple is considered a positive result. Some laboratories have established screening protocols in which a microscopic examination (see later discussion) is performed only if results of a leukocyte esterase test are positive.
Nitrites
Like the leukocyte esterase screen, the nitrite test is a screening test for identification of UTIs. This test is based on the principle that many (but not all) bacteria produce an enzyme called reductase, which can reduce urinary nitrates to nitrites. Chemical testing is done with a dipstick containing a reagent that reacts with nitrites to produce a pink color, thus indirectly suggesting the presence of bacteria. A positive test result indicates the need for a urine culture. Nitrite screening enhances the sensitivity of the leukocyte esterase test to detect UTIs.
Ketones
Normally, no ketones are present in the urine; however, a patient with poorly controlled diabetes and hyperglycemia may have massive fatty acid catabolism. The purpose of this catabolism is to provide an energy source when glucose cannot be transferred into the cell because of insulin insufficiency. Ketones (beta-hydroxybutyric acid, acetoacetic acid, and acetone) are the end products of this fatty acid breakdown. As with glucose, ketones (predominantly acetoacetic acid) spill over into the urine when blood levels in diabetic patients are elevated. Ketonuria is usually associated with poorly controlled diabetes. This test for ketonuria is also important in evaluating ketoacidosis associated with alcoholism, fasting, starvation, high-protein diets, and isopropanol ingestion. Ketonuria may occur with acute febrile illnesses, especially in infants and children.
Bilirubin and Urobilinogen
Bilirubin is a major constituent of bile. If bilirubin excretion is inhibited, conjugated (direct) hyperbilirubinemia will result (see p. 121). Obstruction of the bile duct by a gallstone is the classic example of obstructed bilirubin excretion causing conjugated hyperbilirubinemia. Unlike the unconjugated form, conjugated bilirubin is water soluble and can be excreted into the urine. Therefore, bilirubin in urine suggests disease affecting bilirubin metabolism after conjugation or defects in excretion (e.g., gallstones). Unconjugated bilirubin caused by prehepatic jaundice will not be excreted in the urine because it is not water soluble.
Bilirubin is excreted by way of the bile ducts into the bowel. There some of the bilirubin is transformed into urobilinogen by the action of bacteria in the bowel. Most of the urobilinogen is excreted from the liver back into the bowel, but some is excreted by the kidneys.
Microscopic Examination of Urine Sediment
Microscopic examination of the sediment from a centrifuged urine specimen provides substantial information about the urinary system. Because many different methods can be used to prepare the sediment for microscopic review, normal values may vary significantly among laboratories. Reference ranges are provided here to recognize marked abnormalities.
Crystals
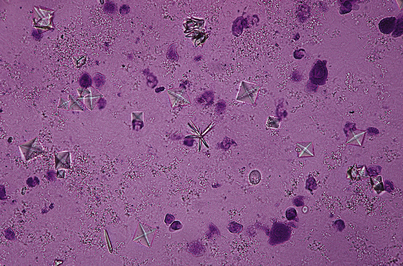
Crystals found in the urinary sediment on microscopic examination indicate that renal stone formation is imminent, if not already present. By themselves, crystals cause no symptoms until they form stones. Even then, stones produce symptoms only when they obstruct the urinary tract. Uric acid crystals occur in patients with high serum uric acid levels (e.g., gout). Phosphate and calcium oxalate crystals (Figure 11-3) occur in the urine of patients with parathyroid abnormalities or malabsorption states. The type of crystal found varies with the disease and the pH of the urine (see previous discussion on urinary pH). Small amounts of crystalline material and even casts (see next page) can be observed when the specific gravity of the urine is high.
Casts
Casts are rectangular clumps of materials or cells that form in the renal distal and collecting tubules, where the material is maximally concentrated. These amorphous clumps of material and cells are shaped like tubules, thus the term cast. Casts are usually associated with some degree of proteinuria and stasis within the renal tubules. There are two kinds of casts: hyaline and cellular. Casts are best seen on low power of the light microscope. Some casts are nearly clear (hyaline), and the condenser lamp must be dimmed to see them well.
Hyaline Casts
Hyaline casts are conglomerations of protein and are indicative of proteinuria. A few hyaline casts are normally present, especially after strenuous exercise.
Cellular Casts
Cellular casts are conglomerations of degenerated cells. Various types are described in the following paragraphs:
Granular casts result from the disintegration of cellular material into granular particles within a WBC or epithelial cell cast. Granular casts are found after exercise and in various renal diseases.
In some diseases, the epithelial cells desquamate into the renal tubule. As the cell degenerates, fatty deposits within the cell coalesce and become incorporated with protein into fatty casts. These are associated with glomerular disease or nephrotic syndrome/nephrosis. Free oval fat bodies may also be associated with fatty emboli, which occur in patients with bone fractures.
Waxy casts may be cell casts, hyaline casts, or renal failure casts. Waxy casts probably represent further degeneration of granular casts. They occur when urine flow through the renal tubule is diminished, giving time for granular casts to degenerate. Waxy casts are associated with chronic renal diseases and chronic renal failure. They also occur in diabetic nephropathy, malignant hypertension, and glomerulonephritis.
Epithelial cells and casts (renal tubular casts)
Epithelial cells can enter the urine at any point during the process of urinary excretion. These cells can be shed from the bladder as a result of tumor, infection, or polyps. They can result from cellular contamination of the urine by vaginal or urethral secretions. They can also result from desquamation of renal tubule cells into the lumen of the tubules and collecting system. These cells can form epithelial casts. The material in these cells can disintegrate first into coarse granules and then into fine granules, making granular casts. The presence of occasional epithelial cells is not remarkable; large numbers, however, are abnormal. Tubular (epithelial) casts are most suggestive of renal tubular disease or toxicity.
Normally, few WBCs are found in the urine sediment on microscopic examination. The presence of five or more WBCs in the urine indicates a UTI involving the bladder or kidneys, or both. A clean-catch urine culture should be done for further evaluation. WBC casts are most frequently found in infections of the kidney (e.g., acute pyelonephritis or interstitial nephritis).
Any disruption in the blood-urine barrier, whether at the glomerular, tubular, or bladder level, will cause RBCs to enter the urine. Hematuria can be microscopic or gross. Patients with more than three RBCs per high-power field in two out of three properly collected urine specimens should be considered to have microhematuria, and hence be evaluated for possible pathologic causes. Bladder, ureteral, and urethral diseases are the most common causes of RBCs in the urine. Pathologic conditions (e.g., tumors, trauma, stones, infection) that involve the mucous membrane in the collecting system can also cause hematuria. RBC casts suggest glomerulonephritis (which may be present in patients with acute bacterial endocarditis, renal infarct, Goodpasture syndrome, vasculitis, sickle cell disease, or malignant hypertension), interstitial nephritis, acute tubular necrosis, pyelonephritis, renal trauma, or renal tumor.
Interfering Factors
Appearance and Color
• Sperm remaining in the urethra after recent or retrograde ejaculation can cause the urine to appear cloudy.
• Urine that has been refrigerated for longer than 1 hour can become cloudy.
• Certain foods affect urine color. Eating carrots may cause dark yellow urine; beets may cause red urine; rhubarb may cause reddish or brownish urine.
• Urine darkens with prolonged standing because of oxidation of bilirubin metabolites.
![]() Many drugs, given the right environment, can alter the color of urine. See Table 11-4, p. 959.
Many drugs, given the right environment, can alter the color of urine. See Table 11-4, p. 959.
pH
• Urine pH becomes alkaline on standing, because of the action of urea-splitting bacteria, which produce ammonia.
• The urine pH of an uncovered specimen will become alkaline because carbon dioxide vaporizes from the urine.
• Dietary factors affect urine pH. Ingestion of large quantities of citrus fruits, dairy products, and vegetables produces alkaline urine, whereas a diet high in meat and certain foods (e.g., cranberries) produces acidic urine.
![]() Drugs that increase urine pH include acetazolamide, bicarbonate antacids, and carbonic anhydrase inhibitors.
Drugs that increase urine pH include acetazolamide, bicarbonate antacids, and carbonic anhydrase inhibitors.
![]() Drugs that decrease urine pH include ammonium chloride, chlorothiazide, and mandelic acid.
Drugs that decrease urine pH include ammonium chloride, chlorothiazide, and mandelic acid.
Protein
• Transient proteinuria may be associated with severe emotional stress, excessive exercise, and cold baths.
• Radiopaque contrast media administered within 3 days may cause false-positive results for proteinuria when turbidity is used as a measure of protein in the urine.
• Urine contaminated with prostate or vaginal secretions commonly causes proteinuria.
• Diets high in protein can cause proteinuria.
• Highly concentrated urine may have a greater concentration of protein than more dilute urine.
• Hemoglobin may cause a positive result with the dipstick method.
• Bence-Jones protein may not appear with the dipstick method.
![]() Drugs that may cause increased protein levels include acetazolamide, aminoglycosides, amphotericin B, cephalosporins, colistin, griseofulvin, lithium, methicillin, nafcillin, nephrotoxic drugs (e.g., arsenicals, gold salts), oxacillin, penicillamine, penicillin G, phenazopyridine, polymyxin B, salicylates, sulfonamides, tolbutamide, and vancomycin.
Drugs that may cause increased protein levels include acetazolamide, aminoglycosides, amphotericin B, cephalosporins, colistin, griseofulvin, lithium, methicillin, nafcillin, nephrotoxic drugs (e.g., arsenicals, gold salts), oxacillin, penicillamine, penicillin G, phenazopyridine, polymyxin B, salicylates, sulfonamides, tolbutamide, and vancomycin.
Bilirubin and Urobilinogen
• Bilirubin is not stable in urine, especially when exposed to light.
• pH can affect urobilinogen levels. Alkaline urine indicates higher levels; acidic urine may show lower levels.
![]() Phenazopyridine colors the urine orange. This may give the false impression that the patient has jaundice.
Phenazopyridine colors the urine orange. This may give the false impression that the patient has jaundice.
![]() Cholestatic drugs may reduce urobilinogen levels.
Cholestatic drugs may reduce urobilinogen levels.
![]() Antibiotics reduce intestinal flora, which in turn decreases urobilinogen levels.
Antibiotics reduce intestinal flora, which in turn decreases urobilinogen levels.
Procedure and Patient Care
During
• Collect a fresh urine specimen in a urine container.
• If the urine specimen contains vaginal discharge or bleeding, a clean-catch or midstream specimen will be needed. This requires meticulous cleaning of the urinary meatus with an antiseptic preparation to reduce contamination of the specimen by external organisms. The cleansing agent must then be completely removed so as not to contaminate the specimen. The midstream collection is obtained as follows:
1. Have the patient begin to urinate into a bedpan, urinal, or toilet, then stop urinating. This washes urine out of the distal part of the urethra.
2. Correctly position a sterile urine container and have the patient void 3 to 4 ounces of urine.
• Testing for ketones can be performed immediately after urine collection with a dipstick.
• For testing urine specific gravity, a first-voided specimen is best.
• Specific gravity can be measured with a refractometer. Light passes through the specimen, and the refractive index (difference between the velocity of light passing through air and the specimen) is determined.
• An easier method of measuring specific gravity is the dipstick method. A dipstick is placed in the specimen, and the resulting color is compared with a color chart.
• To test for protein, a first-voided specimen is best. Occasionally, however, a 24-hour urine collection is preferred. See Box 11-2, Guidelines for a 24-Hour Urine Collection, p. 907.
• Most laboratories use the dipstick method to determine protein in the urine.
• Nitrite, leukocyte esterase, pH, and ketones are measured with the dipstick method and the results determined by comparison with a color chart.
• Urine sediment is obtained by centrifuging a small volume (10 mL) of urine and discarding the supernatant. The remaining urine is a concentrated sediment that can be microscopically examined.
After
• Transport the urine specimen to the laboratory promptly.
• If the specimen cannot be processed immediately, refrigerate it. If the urine cannot be tested within 2 hours, a preservative may be required.
• If a 24-hour urine collection is requested, the specimen should be refrigerated and may need a preservative during the collection time.
• Casts will break up as urine is allowed to sit. Urine examinations for casts should be performed with fresh specimens.
Test Results and Clinical Significance
Appearance and Color
Infection: Infection may cause turbid, foul-smelling urine. Pseudomonas infection can give a green tint to urine.
Gross hematuria: RBCs in the urine cause the urine to be red. This is always a pathologic sign unless the blood is found to be from a source other than the urinary tract. Tumors, trauma, stones, and infection anywhere in the urinary tract can cause RBCs in the urine. Glomerulonephritis, interstitial nephritis, acute necrosis, and pyelonephritis are also associated with hematuria.
Drug therapy: See Table 11-4, p. 959.
Odor
Ketonuria: Associated with poor glucose tolerance, ketones in the urine cause a fruity smell.
Urinary tract infection: Most infections cause foul smelling urine.
Enterovesical fistula: This condition causes urine to smell like stool.
Maple sugar urine: This congenital defect in protein metabolism causes the urine to smell like burnt sugar.
Phenylketonuria: This disease causes the urine to smell musty.
pH
 Increased Levels
Increased Levels
Protein
 Increased Levels
Increased Levels
Trauma: Protein can spill into the urine as a result of traumatic destruction of the blood-urine barrier.
Macroglobulinemia: With increased globulin within the blood, albumin is excreted in an attempt to maintain oncotic homeostasis.
Multiple myelomas: Classically, multiple myelomas produce large amounts of proteins (e.g., Bence-Jones protein) in the urine.
Congestive heart failure (CHF):
The pathophysiologic factors of these observations are many. Suffice it to say that albumin leaks from the glomeruli, which are temporarily damaged by these illnesses.
Orthostatic proteinuria: As many as 20% of normal male patients have small amounts of protein in the urine when specimens are obtained from patients in the upright position. The pathophysiology is not known with certainty. It may be associated with passive congestion of the kidney in the upright position. This phenomenon can be diagnosed by obtaining a urine specimen before arising, and another after the patient has been up for 2 hours. The first has no protein, the latter does.
Severe muscle exertion: Prolonged muscular exertion can be associated with small amounts of protein in the urine.
Renal vein thrombosis: Congestion of the kidney is associated with proteinuria.
Bladder tumor: Tumors of the bladder secrete protein into the lumen of the bladder.
Urethritis or prostatitis: Inflammation in the periurethral glands or urethra can cause proteinuria.
Amyloidosis: Often associated with proteinuria, it may be so severe as to cause nephrotic syndrome. Usually, amyloidosis of the kidney is due to other severe, ongoing disease.
Specific Gravity
 Increased Levels
Increased Levels
Dehydration: The kidneys reabsorb all available free water; thus excreted urine is concentrated.
Pituitary tumor or trauma: Syndrome of inappropriate antidiuretic hormone (SIADH) results in excessive water reabsorption and concentrated urine.
Decreased renal blood flow (as in heart failure, renal artery stenosis, or hypotension): Urine is concentrated through secretion of antidiuretic hormone (ADH) and the renin-angiotensin system.
Glycosuria and proteinuria: These particles of glucose and protein increase specific gravity.
 Decreased Levels
Decreased Levels
Overhydration: Excess water is excreted, causing dilute urine with low specific gravity.
Diabetes insipidus: Inadequate ADH secretion causes decreased water reabsorption. Excess water is excreted, causing dilute urine with low specific gravity.
Renal failure: In chronic renal failure the kidney loses its ability to concentrate urine through water reabsorption. Excess water is excreted, causing dilute urine with low specific gravity.
Diuresis: Diuretics tend to cause dilute, voluminous urine flow.
Ketones
Poorly controlled diabetes mellitus,
Impaired glucose metabolism causes catabolism of fat for production of energy. Ketones (betahydroxybutyric acid, acetoacetic acid, and acetone) are formed and spill into the urine.
Febrile illnesses in infants and children,
Hypermetabolic states cause excessive utilization of glucose. Fats are then broken down. Ketones form and spill over into the urine.
Excessive aspirin ingestion: Aspirin toxicity is associated with reduced glucose production. Ketones form and spill over into the urine.
Anesthesia: The pathophysiology of this observation is probably multiple. Drug effect, starvation, and severe illness can all affect ketone formation.
Bilirubin
Extrahepatic duct obstruction (e.g., tumor, inflammation, gallstone, scarring, or surgical trauma),
Direct physical obstruction of flow of bile from the biliary tree causes elevated serum levels of conjugated (direct) bilirubin, which leads to elevated urine levels of bilirubin.
Cholestasis because of drugs: Some drugs affect bilirubin metabolism and excretion after glucuronide conjugation. Elevated serum levels of conjugated (direct) bilirubin lead to elevated urine levels of bilirubin.
Urobilinogen
 Increased Levels
Increased Levels
Crystals
Renal stone formation: Stones form with crystals as a nidus for production. Crystals in small quantities are not pathologic. Crystals do give some insight into metabolic diseases (e.g., gout, hyperparathyroidism) and other congenital defects of protein metabolism.
Urinary tract infection: Proteus infection, in particular, is associated with crystal formation, especially if chronic.
Granular Casts and Waxy Casts
Coarse and fine granular casts represent further degeneration of cellular casts. They appear when urine flow through the collecting system is diminished, allowing time for further degeneration. As a result, nearly any renal disease or toxicity can be associated with granular cast formation. Waxy casts represent further deterioration of granular casts over time.
Fatty Casts
Diabetic nephropathy (Kimmelstiel-Wilson syndrome),
Glomerulonephritis associated with streptococcal infection,
Chronic renal disease (glomerulonephritis),
Classically, fatty casts are associated with nephrotic syndrome. Any disease or poison that affects the tubular cells causes them to degenerate and desquamate into the lumen. The fatty deposits within those cells coalesce to mix with protein, to make fatty casts or oval fat bodies.
Fat embolism: About 50% of fat embolisms are associated with urinary fat.
Hyaline Casts
RBCs and Casts
 Increased RBC Levels
Increased RBC Levels
Primary renal diseases (e.g., glomerulonephritis, interstitial nephritis, acute tubular necrosis, pyelonephritis): These diseases are associated with the deterioration of the blood-urine barrier.
Renal tumor: Renal neoplasms are friable and hypervascular. RBCs are common with cancers of the kidney.
Renal trauma: Lacerations, contusions, and hematomas ultimately lead to blood in the urine.
Tumors of the ureters and bladder,
WBCs and Casts
 Increased WBC Levels
Increased WBC Levels
Bacterial infection in the urinary tract: WBCs in response to bacterial infections anywhere in the urinary tract can cause leukouria. It may be difficult to differentiate cystitis from urethritis, but it can be done with the two-specimen technique. Ask the patient to void about 20 mL of urine into one container and the rest into another container. More WBCs in the first container indicates urethritis; more in the second container indicates cystitis.
Urinary Stone Analysis (Renal Calculus Analysis)
Indications
Urinary stone analysis is performed to identify the chemicals that make up the kidney stone and to treat any underlying disease that may have caused the stone formation. This information is also used to determine the most effective methods to diminish the chance of another stone.
Test Explanation
About 5% of American women and 12% of men will develop a kidney stone at some time in their lives. Approximately 80% of stones are composed of calcium oxalate (CaOx) and calcium phosphate (CaP); 10% of struvite (magnesium ammonium phosphate produced during infection with bacteria that possess the enzyme urease); 9% of uric acid (UA); and the remaining 1% are composed of cystine or ammonium acid urate or are diagnosed as drug-related stones. Stones ultimately occur because of a supersaturated phase of these substances from liquid to solid state.
A kidney stone can be as small as a grain of sand or as big as 1 inch (2.5 cm) or larger in diameter. Sometimes a stone can leave the kidney and move down a ureter into the bladder. From the bladder, the stone passes through the urethra and out of the body in urine. Stone passage produces renal colic that usually begins as a mild discomfort and progresses to a plateau of extreme severity over 30 to 60 minutes. If the stone obstructs the ureteropelvic junction, pain localizes to the flank; as the stone moves down the ureter, pain moves downward and anterior. Colic is independent of body position or motion and is described as a boring or burning sensation.
Stones less than 5 mm in diameter have a high chance of passage; those of 5 to 7 mm have a modest chance (50%) of passage, and those greater than 7 mm almost always require urologic intervention. Renal stone burden is best gauged using computed tomography (CT) radiographs (see p. 1020) taken with 5-mm cuts, without infusion of contrast agents. The radiographic appearance and density of stones as measured by CT is a guide to their composition. About 90% of kidney stones can be seen on a KUB abdominal x-ray (see p. 1040).
Analysis is done on a kidney stone to determine its chemical makeup. The test, done on a stone that has been passed in the urine or removed from the urinary tract during surgery, shows the type of stone, which can guide treatment and give information that may prevent more stones from forming. People who have had a kidney stone have a risk for having another one. Therefore prevention measures are important.
Diagnosing a kidney stone includes an initial evaluation based on family history, associated medical conditions, medications, and diet; biochemical blood studies; urinalysis; x-rays; and analysis of the stone itself, if obtained. It also typically includes 24-hour urine collection to analyze volume, pH, calcium, magnesium, phosphate, oxalate, urate, creatinine, sodium, citrate, and cystine. If the stone is caused by a urinary tract infection (struvite or carbonate apatite), treatment of the infection will eliminate recurrence. Treating noninfectious stones will invariably involve some form of dietary manipulation, in particular increasing water intake.
Urinary stones can be partially prevented by altering the composition of the urine. In a simplified format, the following type of stones is often treated as follows:
• Hyperuricuria, predominantly uric acid stones, and cystine stones: Alkalinize urine to increase uric acid solubility with potassium alkali two or three times daily.
• Hypercalciuria and predominantly hydroxyapatite stones: Acidify urine to increase calcium solubility. However, treatment also depends on urine pH and urine phosphate, sulfate, oxalate, and citrate concentrations. Thiazide diuretics reduce urinary calcium and increase urinary volume.
• Hyperoxaluria and calcium oxalate stones: Increase daily fluid intake and consider reduction of daily calcium.
• Magnesium, ammonium, and phosphate stones (struvite): Investigate and treat urinary tract infection.
Related Tests
Computed Tomography (CT) Scan (p. 1020). A CT scan of the ureters and kidneys (also called a CT urogram) is the most common way to find kidney stones.
Abdominal Ultrasonography (p. 866). This is a quick method that may also be used to find kidney stones.
Intravenous Pyelogram (IVP) (p. 1057). An IVP can monitor the excretion of dye through the urologic tract. An obstruction could indicate a urinary stone.
Urine Culture and Sensitivity (Urine C&S)
Indications
This test is used to diagnose urinary tract infection (UTI) in patients with dysuria, frequency, or urgency. It is also indicated when patients have fever of unknown origin or when urinalysis (UA) suggests infection.
Test Explanation
Urine culture and sensitivity tests are performed to determine the presence of pathogenic bacteria in patients with suspected UTIs. Most often, urinary tract infections are limited to the bladder, although the kidneys, ureters, bladder, or urethra can be the source of infection. All cultures should be performed before antibiotic therapy is initiated, because the antibiotic may interrupt the growth of the organism in the laboratory. Most organisms require approximately 24 hours to grow in the laboratory, and a preliminary report can be given at that time. Usually, 48 to 72 hours are required for growth and identification of an organism. Cultures may be repeated after appropriate antibiotic therapy to assess for complete resolution of the infection, especially UTIs.
To save money, a urine sample is collected and divided. Half is sent for urinalysis, and the other half is held in the laboratory refrigerator and cultured only if results of urinalysis indicate a possible infection (e.g., increased number of WBCs, bacteria, high pH, leukocyte esterase).
An important part of any routine culture is assessment of the sensitivity to various antibiotics of any bacteria that are growing in the urine. The health care provider can then prescribe the safest, least expensive, and most effective antibiotic therapy for the specific bacteria.
Procedure and Patient Care
During
• Note that a clean-catch or midstream urine collection is required for culture and sensitivity testing. This requires meticulous cleansing of the urinary meatus with an antiseptic preparation to reduce contamination of the specimen by external organisms. The foreskin must be retracted in male patients. The cleansing agent must be completely removed so it will not contaminate the urine specimen. The midstream collection is obtained as follows:
1. Have the patient begin to urinate into a bedpan, urinal, or toilet, and then stop urinating. This washes urine from the distal portion of the urethra.
2. Correctly position a sterile urine container so the patient can void 3 to 4 ounces of urine.
• Note that urinary catheterization may be needed for patients unable to void. This procedure is not usually performed, however, because of the risk of introducing organisms into the bladder and because of patient discomfort.
• For inpatients with an indwelling urinary catheter, obtain a specimen by attaching a syringe at a built-in sampling port. Aspirate the urine and place it in a sterile urine container. Usually the catheter tubing distal to the puncture site needs to be clamped for 15 to 30 minutes before aspiration of urine to allow urine to fill the tubing. After the specimen is withdrawn, remove the clamp.
• Collect specimens from infants and young children in a disposable pouch called a U bag. This bag has adhesive backing around the opening to attach to the child's pubic skin. Clean the urinary meatus before applying the bag.
• Note that suprapubic aspiration of urine is a safe method of obtaining urine in neonates and infants. The abdomen is prepared with an antiseptic, and a 25-gauge needle is inserted into the suprapubic area 1 inch above the symphysis pubis. Urine is aspirated into the syringe, then transferred to a sterile urine container.
• Note that in patients with a urinary diversion (e.g., ileal conduit), catheterization should be performed through the stoma. Urine should not be collected from the ostomy pouch.
• Urine for culture and sensitivity testing should not be taken from a bedpan or brought from home, because it will be contaminated.
After
• Transport the specimen to the laboratory immediately (within 30 minutes). If this is not possible, the specimen may be refrigerated for up to 2 hours. Urine for cytomegalovirus culture, however, will be rendered useless by refrigeration.
• Notify the health care provider of any positive results so that appropriate antibiotic therapy can be initiated.
Related Test
Urinalysis (UA) (p. 956). This test is often performed before culture and sensitivity testing. The presence of WBCs, leukocyte esterase, nitrites, or bacteria indicates a UTI.
Vanillylmandelic Acid (VMA), Homovanillic Acid (HVA), and Catecholamines (Epinephrine, Norepinephrine, Metanephrine, Normetanephrine, Dopamine)
Indications
This 24-hour urine test for VMA, HVA, and catecholamines is a screening test for the diagnosis of catecholamine-producing tumors, such as neuroblastoma, pheochromocytoma, and other rare adrenal/neural crest tumors.
Test Explanation
A pheochromocytoma is a tumor of the chromaffin cells within the adrenal medulla that frequently secretes abnormally high levels of epinephrine and norepinephrine. Likewise, neural crest tumors such as neuroblastoma can also hypersecrete catecholamines. These hormones cause episodic or persistent severe hypertension by producing peripheral arterial vasoconstriction. Dopamine is the precursor of epinephrine and norepinephrine. HVA is a metabolite of dopamine. Metanephrine and normetanephrine are catabolic products of epinephrine and norepinephrine, respectively. VMA (3-methoxy-4-hydroxymandelic acid) is the product of catabolism of both metanephrine and normetanephrine. In pheochromocytoma, one or all of these substances will be present in excessive quantities in a 24-hour urine collection. These hormones may be measured singularly in the urine, but the collective metabolic end products, HVA and VMA, are more easily detected because their concentrations are much higher than any one catecholamine component.
VMA and HVA are primarily used as a screening test for neural crest tumors. These urinary tests can also be used to monitor tumor activity. HVA levels may also be altered in disorders of catecholamine metabolism. For example, monoamine oxidase (MAO) deficiency can cause decreased urinary HVA values, whereas a deficiency of dopamine beta-hydrolase (the enzyme that converts dopamine to norepinephrine) can cause elevated urinary HVA values.
A 24-hour urine test is preferable to a blood test because catecholamine secretion from the tumor may be episodic and could be missed at a random time during the day. A 24-hour urine reflects catecholamine production over an entire day. It is best to perform testing when symptoms (hypertension) of the potential adrenal tumor are significant. At that time, catecholamine production is greatest and can be more assuredly identified. That being said, VMA is not the analyte of choice to rule out a diagnosis of pheochromocytoma. Metanephrines measured in the plasma or urine may be more accurate.
In the past, these urinary tests have been performed by spectrophotometric assays. Now, high-performance liquid chromatography (HPLC)–tandem mass spectrometry has improved the accuracy of this testing. Nevertheless, urine testing is cumbersome and time consuming. With HPLC, measurement of plasma-free metanephrines (see p. 357) has nearly replaced urine testing for pheochromocytoma.
Interfering Factors
• Increased levels of VMA may be caused by certain foods (e.g., tea, coffee, cocoa, vanilla, chocolate, cider vinegar, soda, licorice).
• Vigorous exercise, stress, and starvation may cause increased VMA levels.
• Falsely decreased levels of VMA may be caused by uremia, alkaline urine, and radiographic iodine contrast agents.
![]() Drugs that may cause increased VMA levels include caffeine, epinephrine, levodopa, lithium, and nitroglycerin. Patients receiving L-dopa should stop taking it for 24 hours before the specimen is obtained.
Drugs that may cause increased VMA levels include caffeine, epinephrine, levodopa, lithium, and nitroglycerin. Patients receiving L-dopa should stop taking it for 24 hours before the specimen is obtained.
![]() Drugs that may cause decreased VMA levels include disulfiram (Antabuse), guanethidine, imipramine, monoamine oxidase (MAO) inhibitors, phenothiazines, and reserpine.
Drugs that may cause decreased VMA levels include disulfiram (Antabuse), guanethidine, imipramine, monoamine oxidase (MAO) inhibitors, phenothiazines, and reserpine.
![]() Drugs that may cause increased catecholamine levels include alcohol (ethyl), aminophylline, caffeine, chloral hydrate, clonidine (prolonged therapy), contrast media (containing iodine), disulfiram, epinephrine, erythromycin, insulin, methenamine, methyldopa, nicotinic acid (large doses), nitroglycerin, quinidine, riboflavin, and tetracyclines.
Drugs that may cause increased catecholamine levels include alcohol (ethyl), aminophylline, caffeine, chloral hydrate, clonidine (prolonged therapy), contrast media (containing iodine), disulfiram, epinephrine, erythromycin, insulin, methenamine, methyldopa, nicotinic acid (large doses), nitroglycerin, quinidine, riboflavin, and tetracyclines.
![]() Drugs that may cause decreased catecholamine levels include guanethidine, reserpine, and salicylates.
Drugs that may cause decreased catecholamine levels include guanethidine, reserpine, and salicylates.
Procedure and Patient Care
Before
![]() Explain the dietary restrictions and the 24-hour urine collection procedure to the patient.
Explain the dietary restrictions and the 24-hour urine collection procedure to the patient.
![]() For 2 or 3 days before and throughout the 24-hour collection for VMA, place the patient on a VMA-restricted diet. Instruct the patient to avoid coffee, tea, bananas, chocolate, cocoa, licorice, citrus fruit, all foods and fluids containing vanilla, and aspirin. Obtain specific restrictions from the laboratory.
For 2 or 3 days before and throughout the 24-hour collection for VMA, place the patient on a VMA-restricted diet. Instruct the patient to avoid coffee, tea, bananas, chocolate, cocoa, licorice, citrus fruit, all foods and fluids containing vanilla, and aspirin. Obtain specific restrictions from the laboratory.
![]() Instruct the patient to avoid taking antihypertensive medications, and sometimes all medications, during this period and possibly longer.
Instruct the patient to avoid taking antihypertensive medications, and sometimes all medications, during this period and possibly longer.
During
• See Box 11-2, Guidelines for a 24-Hour Urine Collection, p. 907.
• Use a preservative. Refrigerate the specimen or keep it on ice over the 24 hours.
• Identify and minimize factors contributing to patient stress and anxiety. Excessive physical exercise and emotion may alter catecholamine test results by causing increased secretion of epinephrine and norepinephrine.
Related Test
Pheochromocytoma Suppression and Provocative Testing (p. 389). This is used to identify pheochromocytoma when catecholamine levels are not assuredly diagnostic.
Water Deprivation (Antidiuretic Hormone [ADH] Stimulation)
Indications
This test is used to aid in the differential diagnosis of polyuria. Polyuria can occur as a result of neurogenic diabetes insipidus (DI), nephrogenic DI, or psychogenic polydipsia.
Test Explanation
In this test, the patient is deprived of fluids. Patients who have DI will dehydrate quickly as indicated by a rise in urine and serum osmolality. Patients with primary psychogenic polydipsia take a longer time to dehydrate. Next, ADH is administered. Patients with neurogenic DI have no endogenous ADH but can concentrate their urine and raise urine osmolality if ADH is provided exogenously. Patients with nephrogenic DI have kidneys that are insensitive to ADH. They will experience little or no increase in urine osmolality. Patients who have psychogenic polydipsia will experience a less than 9% increase in urine osmolality.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Explain the recommended fluid restriction.
Explain the recommended fluid restriction.
1. Patients with a urine output of less than 4000/24 hr undergo fluid restriction after midnight before the test.
2. Patients with a urine output of greater than 4000/24 hr begin fluid restriction at the time the test starts, because they may get dangerously dehydrated if asked to restrict water from midnight.
During
• Obtain and record the patient's body weight hourly for the duration of the procedure.
• Obtain urine osmolality hourly from 6 AM to noon or until three consecutive hourly determinations show a urine osmolality increase of less than 30 mOsm/kg.
• At that point obtain a serum osmolality. It must be greater than 288 mOsm/kg for the patient to be considered adequately dehydrated and water deprived.
• If the body weight drops more than 2 kg, discontinue the test and rehydrate the patient.
• Administer the prescribed dose of vasopressin (or desmopressin, an analog of ADH) subcutaneously.
• Obtain urine osmolality 30 to 60 minutes after the injection.
Test Results and Clinical Significance
Little or No Increase in Urine Osmolality During Deprivation Portion of Test or After Injection
Nephrogenic DI caused by primary renal diseases: Patients with nephrogenic DI because of chronic kidney diseases will experience little or no rise in urine osmolality during the dehydration phase of the test, because the kidney has lost its concentrating abilities. Furthermore, their kidneys are insensitive to the urine-concentrating effect of ADH.
Hypokalemia: These patients have the same lack of response as do patients with nephrogenic DI.
Psychogenic polydipsia: These patients frequently take longer than usual to dehydrate to a serum osmolality of 288, and the urine osmolality rises less than 9% after vasopressin injection.
Related Tests
Antidiuretic Hormone (ADH) (p. 73). This is a serum assay for direct measurement of ADH. This test is used in the differential diagnosis of neurogenic DI, nephrogenic DI, or psychogenic polydipsia.
Osmolality, Serum (p. 378). This test is a measurement of solute load in the serum.
Osmolality, Urine (p. 938). This test is a measurement of solute load in the urine.
Sodium, Blood (p. 466). This is a direct measurement of sodium level in the blood.
Sodium, Urine (p. 946). This is a direct measurement of sodium level in the urine.
∗BCE = bone collagen equivalents.