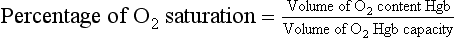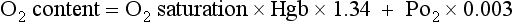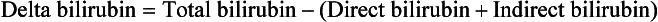Anti–Smooth Muscle Antibody (ASMA, F-Actin Smooth Muscle Antibody)
Indications
The ASMA is used primarily to aid in the diagnosis of autoimmune chronic active hepatitis (CAH), which has also been referred to as “lupoid” CAH.
Test Explanation
ASMA is an anticytoplasmic antibody directed against actin, a cytoskeletal protein. Normally the serum does not contain ASMA at a titer greater than 1:20. ASMA is the most commonly recognized autoantibody in the setting of CAH. It appears in 70% to 80% of patients with CAH. Patients with some types of CAH do not test positive for ASMA antibodies. This disease may be an autoimmune disease that occurs predominantly in adult women. The clinical presentation of CAH is similar to that of viral hepatitis. That clinical picture, along with serologic and pathologic criteria, must exist for more than 6 months to be classified as CAH.
ASMA is not specific for CAH and can be positive in patients with viral infections, malignancy, multiple sclerosis, primary biliary cirrhosis, and Mycoplasma infections. Usually the titer of ASMA is low in these diseases. With CAH, the titer is usually higher than 1:160. The titers are not helpful in predicting prognosis, nor do they indicate response to therapy. ASMA is also used to distinguish autoimmune hepatitis from lupus erythematosus. For the ASMA test, immunofluorescent assay (IFA) or enzyme-linked immunosorbent assay (ELISA) techniques are used.
Related Tests
Liver Enzymes, such as Alkaline Phosphatase (ALT) (p. 47) and Aspartate Aminotransferase (AST) (p. 119). These enzymes exist within the hepatocytes and are elevated in patients with hepatitis.
Antimitochrondrial Antibody (p. 84). This is an antibody that is most frequently associated with primary biliary cirrhosis. Positive titers of AMA are found in 30% of the patients with CAH.
Anti–Liver/Kidney Microsomal Type 1 Antibodies (p. 83). This antibody is helpful in the diagnosis of autoimmune hepatitis.
Antispermatozoal Antibody (Sperm Agglutination and Inhibition, Sperm Antibodies, Antisperm Antibodies, Infertility Screen)
Indications
The antispermatozoal antibody test is an infertility screening test used to detect the presence of sperm antibodies. Antibodies directed toward sperm antigens can result in diminished fertility.
Test Explanation
This test is used in the evaluation of an infertile couple usually after a postcoital test is positive. For fertilization to occur, the sperm head must first attach to the zona pellucida of the egg. Sperm antibodies interfere with this binding. Although there is consensus that these antibodies play a role in infertility, the percentage of sperm that must be bound by antibodies before fertility is adversely affected is less clear. The IgA antisperm antibodies to the sperm tail are associated with poor motility and poor penetration of cervical mucus. IgG antisperm antibodies are associated with blockage of sperm-ovum fusion. Semen and serum may contain sperm antibodies. Semen is the preferred specimen type for males. In cases in which semen production may present difficulties, a serum specimen can be tested instead. Serum is the preferred specimen type in females.
Positives are reported as percentage of sperm with positive bindings, the class of antibody involved (IgG, IgA, and IgM), and the site of binding (head, midpiece, tail, and/or tail tip). Greater than 50% binding is usually required to significantly lower a patient's fertility.
Not only is this test indicated for male infertility studies, but it is also used as a follow-up test when sperm agglutination is noted in the ejaculate. It is also used in men with a history of testicular trauma, biopsy, vasectomy reversal, genital tract infection, or obstructive lesions of the male ductal system. Antisperm antibodies may be found in the blood of men with blocked efferent ducts of the testes (a common cause of low sperm counts or poor sperm mobility) and in 30% to 70% of men who have had a vasectomy. Resorption of sperm from the blocked ducts results in the formation of autoantibodies to sperm as a result of sperm antigens interacting with the immune system. High titers of IgG autoantibodies are often associated with postvasectomy degeneration of the testes, which explains why 50% of men remain infertile after successful repair of a previous vasectomy.
Procedure and Patient Care
Before
Sperm Specimen
![]() Inform the man that a semen specimen should be collected after avoiding ejaculation for at least 3 days.
Inform the man that a semen specimen should be collected after avoiding ejaculation for at least 3 days.
• Give the male patient the proper container for the sperm collection.
![]() If the specimen is to be collected at home, be certain the patient is told that it must be taken to the laboratory for testing within 2 hours of collection.
If the specimen is to be collected at home, be certain the patient is told that it must be taken to the laboratory for testing within 2 hours of collection.
After
• Apply pressure or a pressure dressing to the venipuncture sites.
• Check the venipuncture sites for bleeding.
• For a sperm, blood, or cervical specimen, the specimen may be placed in a plastic vial, frozen, and sent to a reference laboratory on dry ice.
![]() Instruct the couple when and how to obtain the test results.
Instruct the couple when and how to obtain the test results.
Test Results and Clinical Significance
Infertility: Antispermatozoal antibodies may be present in the man or the woman and may inhibit the number or motility of sperm or the ability of the sperm to penetrate the ovum.
Blocked efferent ducts in the testes: This is considered to be a common cause of male infertility. Reabsorption of sperm from the blocked ducts results in the formation of autoantibodies to sperm as a result of sperm antigens interacting with the immune system.
Vasectomy: Reabsorption of sperm from the occluded vas deferens results in the formation of autoantibodies to sperm as a result of sperm antigens interacting with the immune system.
Related Tests
Sims-Huhner Test (p. 676). This test consists of a postcoital examination of the cervical mucus to measure the ability of the sperm to penetrate the mucus and maintain motility. It is used in the diagnostic workup of infertility. This analysis is also helpful in documenting cases of suspected rape by testing the vaginal and cervical secretions for sperm.
Luteinizing Hormone and Follicle-Stimulating Hormone Assay (p. 348). These hormones are useful for determining the pituitary effect on gonadal function and spermatogenesis.
Semen Analysis (p. 671). This test is used to evaluate the quality of sperm. Semen analysis is used to evaluate an infertile couple and to document the adequacy of operative vasectomy.
Estrogen and Progesterone (pp. 226 and 416). These tests are used to indicate ovarian function and reserve.
Anti–SS-A (Ro), Anti–SS–B (La), and Anti–SS-C Antibody (Anti-Ro, Anti-La, Sjögren Antibodies)
Indications
These three antinuclear antibodies are considered antiextractable nuclear antigens (see p. 79) and are used to diagnose Sjögren syndrome.
Test Explanation
Ro, La, and SS-C antibodies are subtypes of antinuclear antibodies (ANA) (see Table 2-3, p. 89) and react to nuclear antigens extracted from human B lymphocytes. Ro and La produce a speckled immunofluorescent pattern when seen under the ultraviolet microscope. They are strongly associated with Sjögren syndrome. This disease is an immunologic abnormality characterized by progressive destruction of the lacrimal and salivary exocrine glands leading to mucosal and conjunctival dryness. This disease can occur by itself (primary) or in association with other autoimmune diseases, such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and scleroderma. In the latter case it is referred to as secondary Sjögren syndrome.
Anti–SS-A antibodies may be found in approximately 60% to 70% of patients with primary Sjögren syndrome. Anti–SS-B antibodies may be found in approximately 50% to 60% of patients with primary Sjögren syndrome. When anti–SS-A and SS-B antibodies are positive, Sjögren syndrome can be diagnosed accurately. These antibodies are more rarely found when secondary Sjögren syndrome is associated with RA. In fact, SS-B is only found in primary Sjögren syndrome. However, anti–SS-C is positive in about 75% of patients with RA or patients with secondary Sjögren syndrome associated with RA. Yet anti–SS-A and anti–SS-B are almost never found in patients with Sjögren syndrome associated with RA. Therefore these antibodies are also useful in differentiating primary from secondary Sjögren syndrome.
SS-A can also be found in 25% of patients with SLE. This is particularly useful in “ANA-negative” patients with SLE, because these antibodies are present in the majority of such patients. Anti–SS-B is rarely found in patients with SLE. In general, the higher the titer of anti–SS antibodies, the more likely that Sjögren syndrome exists and the more active the disease is. As Sjögren syndrome becomes less active with therapy, the anti–SS antibodies titers can be expected to fall. These antibodies can be quantified by the Semi-quantitative Multi-analyte Fluorescent Detection methodology.
Test Results and Clinical Significance
Positive
Sjögren syndrome: When high titers of anti–SS-A or anti–SS-B are present, Sjögren syndrome can be diagnosed with confidence.
Rheumatoid arthritis (RA): When high titers of anti–SS-C are present, RA with or without Sjögren syndrome can be diagnosed with confidence.
ANA-negative SLE: Anti–SS-A will be positive in most of these patients.
Neonatal lupus: Anti–SS-A will be positive in 95% of these patients.
Related Tests
Antinuclear Antibody (ANA) (p. 88). This test is used to diagnose SLE and other autoimmine diseases. The ANA ELISA screen is designed to detect antibodies against dsDNA, histone, SS-A (Ro), SS-B (La), Smith, snRNP/Sm, Scl-70, Jo-1, and centromere.
Anticentromere Antibody (p. 69). This test is used to diagnose CREST syndrome. Anti-DNA Antibody (p. 78). This test is used to dignose SLE.
Antiextractable Nuclear Antigen (ENA) (p. 79). This test is used to diagnose SLE and mixed connective tissue disease. This is a first-line test for connective tissue disease screening.
Antiscleroderma Antibody (p. 93). This test is used to diagnose scleroderma.
Antithrombin Activity and Antigen Assay (Antithrombin III [AT-III] Activity/Assay, Functional Antithrombin III Assay, Heparin Cofactor, Immunologic Antithrombin III, Serine Protease Inhibitor)
Indications
This test is used to evaluate patients suspected of having hypercoagulable states. It is also used to help identify the cause of heparin resistance in patients receiving heparin therapy.
Test Explanation
AT-III is an alpha2-globulin produced in the liver. It inhibits the serine proteases involved in coagulation (II, X, IX, XI, XII). In normal homeostasis, coagulation results from a balance between AT-III and thrombin. ATT-III is the principal plasma anticoagulant mediating inactivation of serine protease procoagulant enzymes, chiefly thrombin and coagulation factors Xa and IXa. (A deficiency of AT-III increases coagulation or the tendency toward thrombosis.) A hereditary deficiency of AT-III is characterized by a predisposition toward thrombus formation. This is passed on as an autosomal-dominant abnormality. Individuals with hereditary AT-III deficiency typically develop thromboembolic events in their early twenties. These thrombotic events are usually venous.
Acquired AT-III deficiency may be seen in patients with cirrhosis, liver failure, advanced carcinoma, nephrotic syndrome, disseminated intravascular coagulation (DIC), protein-losing enteropathies, and acute thrombosis. AT-III is also decreased as much as 30% in pregnant women and those who take estrogens. Antithrombin activity testing is ordered, along with other tests for hypercoagulable disorders (such as protein C and protein S, and lupus anticoagulant), when a patient has been experiencing recurrent venous thrombosis. Antithrombin should be measured after a blood clot has been treated and resolved because both the presence of the clot, and the therapy used to treat it, will affect antithrombin results. AT-III provides most of the anticoagulant effect of heparin. Heparin increases antithrombin activity by 1000-fold. Patients who are deficient in AT-III may be heparin resistant and require unusually high doses for an anticoagulation effect. In general, patients respond to heparin if more than 60% of normal AT-III levels exist.
There are two tests for AT-III. The first is a “functional” assay and measures AT-III activity. The second quantifies the AT-III antigen. The antithrombin activity test is performed before the antigen test, to evaluate whether the total amount of functional antithrombin activity is normal. Antithrombin activity is the primary (screening) antithrombin assay. If antithrombin activity is normal, AT III is not the cause of the hypercoagulable state. If antithrombin activity is abnormal, antithrombin antigen should be quantified.
There are two types of inherited AT-III syndromes identified by using these tests. In type 1, the antithrombin activity and quantities of antithrombin antigen are decreased. In this case, the activity is decreased because there is less antithrombin available to participate in clotting regulation. In type 2 (very rare), there is reduced antithrombin activity and normal levels of antithrombin antigen, suggesting that there is sufficient antithrombin but it is not functioning as it should.
Asymptomatic individuals with an antithrombin deficiency should receive prophylactic anticoagulation to increase their antithrombin levels before any medical/surgical interventions in which inactivity increases the risk of thrombosis. Increased levels of AT-III are not usually considered a problem and may occur in patients with acute hepatitis, obstructive jaundice, vitamin K deficiency, and kidney transplantation.
Antithrombin studies are also used as an adjunct in the diagnosis and management of carbohydrate-deficient glycoprotein syndromes (CDGS) because defective glycosylation of this AT-III in individuals with CDGS will cause hypercoagulation. Deficient AT-III may also contribute to recurrent miscarriages.
Antithrombin activity testing is also used to monitor treatment of antithrombin deficiency disorders by infusion of antithrombin concentrates. Antithrombin antigen and activity is determined using automated latex immunoassay (LIA) methodology, commonly on a Beckman Coulter ACL TOP.
Test Results and Clinical Significance
 Decreased Levels
Decreased Levels
Related Tests
Coagulating Factor Concentration (p. 163). This test measures the concentration of specific coagulating factors in the blood.
Protein C, Protein S (p. 432). This is part of the evaluation of patients with coagulation disorders.
Lupus anticoagulant (p. 68 [anticardiolipin antibodies]). This test is done in many patients with thrombus.
Antithyroglobulin Antibody (Thyroid Autoantibody, Thyroid Antithyroglobulin Antibody, Thyroglobulin Antibody)
Test Explanation
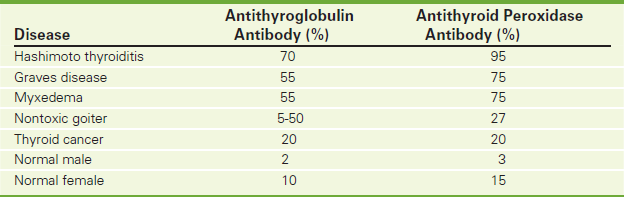
Thyroglobulin autoantibodies bind thyroglobulin (Tg), a major thyroid-specific protein that plays a crucial role in thyroid hormone synthesis, storage, and release. Tg remains in the thyroid follicles until hormone production is required. Tg is not secreted into the systemic circulation under normal circumstances. However, follicular destruction through inflammation (Hashimoto's thyroiditis or chronic lymphocytic thyroiditis and autoimmune hypothyroidism), hemorrhage (nodular goiter), or rapid disordered growth of thyroid tissue (as may be observed in Graves disease or follicular cell-derived thyroid neoplasms) can result in leakage of Tg into the bloodstream. This results in the formation of autoantibodies to Tg in some individuals. Of individuals with autoimmune hypothyroidism, 30% to 50% will have detectable anti-Tg autoantibodies (Table 2-7).
The antithyroglobulin test is usually performed in conjunction with the antithyroid peroxidase antibody test (p. 104). When this is done, the specificity and sensitivity are greatly increased. Antithyroglobulin assay is a Quantitative Chemiluminescent Immunoassay. Normal results vary based on the methodology used. A small percentage of the normal population has antithyroglobulin antibodies. Normally women tend to have higher levels than men.
Tg antibodies are also used when testing Tg as a marker for follicular cell thyroid cancer. If Tg antibodies are present, Tg is then considered an inaccurate marker for recurrent/metastatic cancer.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Chronic thyroiditis (Hashimoto thyroiditis): Antithyroglobulin antibodies attack the globulin in the thyroid cells. The immune complex creates an inflammatory and destructive process in the gland, which is mediated through the complement system.
The association with other autoimmune diseases is well known; however, the mechanism of this association has not been elucidated.
Pernicious anemia: Anti–parietal cell antibodies have been associated with the presence of antithyroglobulin antibodies.
Thyroglobulin, which leaks out of the thyroid as a result of these destructive diseases, stimulates the immune system to produce antithyroglobulin antibodies.
Myxedema: The antithyroid microsomal antibodies destroy the thyroid cell, resulting in hypofunction of the gland.
Related Tests
Antithyroid Peroxidase Antibody (p. 104). This test is primarily used in the differential diagnosis of thyroid diseases, such as Hashimoto thyroiditis and chronic lymphocytic thyroiditis (in children).
Thyroid-Stimulating Immunoglobulins (p. 491). These thyroid-stimulating immunoglobulins are used to support the diagnosis of Graves disease, especially when the diagnosis is complex.
Thyroid-Stimulating Hormone (p. 486). This test is used to diagnose primary hypothyroidism and to differentiate it from secondary (pituitary) and tertiary (hypothalamus) hypothyroidism.
Thyroxine, Total (p. 497). This is one of the first tests done to assess thyroid function. It is used to diagnose thyroid function and to monitor replacement and suppressive therapy.
Triiodothyronine (p. 506). A T3 test is used to evaluate thyroid function, primarily to diagnose hyperthyroidism. It is also used to monitor thyroid replacement and suppressive medical therapy.
Antithyroid Peroxidase Antibody (Anti-TPO, TPO-Ab, Antithyroid Microsomal Antibody, Thyroid Autoantibody, Thyroid Microsomal Antibody)
Indications
This test is primarily used in the differential diagnosis of thyroid diseases, such as Hashimoto thyroiditis and chronic lymphocytic thyroiditis (in children).
Test Explanation
Thyroid microsomal antibodies are commonly found in patients with various thyroid diseases. They are present in 70% to 90% of patients with Hashimoto thyroiditis. Microsomal antibodies are produced in response to microsomes escaping from the thyroid epithelial cells surrounding the thyroid follicle. These escaped microsomes then act as antigens and stimulate the production of antibodies. These immune complexes initiate inflammatory and cytotoxic effects on the thyroid follicle. This test is often performed in conjunction with the antithyroglobulin antibody test, which greatly increases the specificity and sensitivity.
Although many different thyroid diseases are associated with elevated antimicrosomal antibody levels, the most frequent is chronic thyroiditis (Hashimoto thyroiditis in the adult and lymphocytic thyroiditis in children and young adults) (Table 2-7, p. 103). Both of these chronic inflammatory diseases have been associated with other autoimmune (collagen-vascular) diseases. Twelve percent of normal females and 1% of normal males have positive antimicrosomal antibodies.
The most sensitive assay for antimicrosomal antibodies is for the antithyroid peroxidase (anti-TPO) antibody. This assay uses enzyme immunoassays. Anti-TPO is often performed in conjunction with the antithyroglobulin antibody test (see p. 102). When this is done, the specificity and sensitivity are greatly increased.
Anti-TPO is present in almost all patients with Hashimoto thyroiditis, in more than 70% of those with Graves disease, and, to a variable degree, in patients with nonthyroid autoimmune disease. Anti-TPO correlates with the degree of lymphocytic infiltrations (inflammation) in the thyroid. Among healthy people, 5% to 10% have elevated anti-TPO levels.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Chronic thyroiditis (Hashimoto thyroiditis): Antimicrosomal antibodies attack the microsome in the thyroid cells. The immune complex creates an inflammatory and destructive process in the gland, which is mediated through the complement system.
The association with other autoimmune diseases is well known. The mechanism of this association, however, is not well known.
Pernicious anemia: Anti–parietal cell antibodies have been associated with the presence of antimicrosomal antibodies.
Microsomes that leak out of the thyroid as a result of the presence of these destructive diseases stimulate the immune system to produce antimicrosomal antibodies.
Myxedema: Antithyroid microsomal antibodies destroy the thyroid cell, resulting in hypofunction of the gland.
Related Test
Antithyroglobulin Antibody (p. 102). This test is primarily used in the differential diagnosis of thyroid diseases such as Hashimoto thyroiditis.
Apolipoproteins (Apolipoprotein A-I [Apo A-I], Apolipoprotein B [Apo B], Lipoprotein [a] [Lp(a)], Apolipoprotein E [Apo E])
Indications
This test is used to evaluate the risk of atherogenic heart and peripheral vascular diseases. These levels may be better indicators of atherogenic risks than high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very-low-density lipoprotein (VLDL).
Test Explanation
Apolipoproteins are the protein part of lipoproteins (e.g., HDL, LDL). In general, apolipoproteins play an important role in lipid transport in the lymphatic and the circulatory system. They also act as enzyme cofactors in lipoprotein synthesis. Apolipoproteins also act as receptor ligands to improve transport of fat particles in the cell. Apolipoprotein synthesis in the liver is controlled by a host of factors, including dietary composition, hormones (insulin, glucagon, thyroxin, estrogens, androgens), alcohol intake, and various drugs (statins, niacin, and fibric acids).
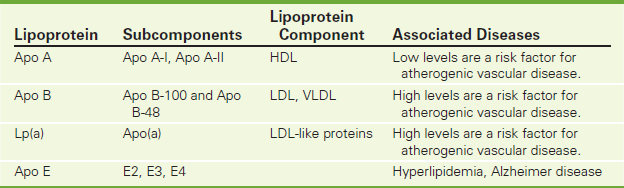
There are several types of apolipoproteins, including apo A-I, apo B, and apo E (Table 2-8). Apolipoprotein A (apo A) is the major polypeptide component of HDL. Low levels of apo A are associated with increased risk of coronary or peripheral artery disease (CPAD). Elevated levels may protect against CPAD.
Apo B is the major polypeptide component of LDL and chylomicrons. Apo B makes cholesterol soluble for deposition in the arterial wall. Forty percent of the protein portion of VLDL is composed of apo B. Familial hypercholesterolemia type B is caused by mutations in the Apo B gene.
Lp(a) (referred to as Lipoprotein little a) is a heterogenous group of lipoproteins consisting of an Apo A molecule attached to an Apo B molecule. An increased level of Lp(a) may be an independent risk factor for atherosclerosis and is particularly harmful to the endothelium. Serum concentrations of Lp(a) appear to be largely related to genetic factors; diet and lipid-lowering pharmaceuticals do not have a major impact on Lp(a) levels. Nevertheless, measurement of serum Lp(a) may contribute to a more comprehensive risk assessment in high-risk patients.
Apolipoprotein E (apo E) is involved in cholesterol transport. Through genotyping, three alleles for apo E have been identified: E2, E3, and E4. Each person gets an allele from each parent. E3/3 is the normal. E2/2 is found rarely and is associated with type III hyperlipidemia. E4/4 or E4/3 is associated with high LDL levels. The apo E4 gene has been proposed as a risk factor for Alzheimer disease. Apo E2 and E4 are associated with increased triglycerides.
Lp-PLA2 is a lipase enzyme located on the surface of circulating LDL. This protein is atherogenic.
Testing is performed by immunoturbidimetric assay or nephelometry.
Interfering Factors
Apo A-I
• Physical exercise may increase apo A-I levels.
• Smoking may decrease levels.
• Diets high in carbohydrates or polyunsaturated fats may decrease apo A-I levels.
![]() Drugs that may increase apo A-I levels include carbamazepine, estrogens, ethanol, lovastatin, niacin, oral contraceptives, phenobarbital, pravastatin, and simvastatin.
Drugs that may increase apo A-I levels include carbamazepine, estrogens, ethanol, lovastatin, niacin, oral contraceptives, phenobarbital, pravastatin, and simvastatin.
![]() Drugs that may decrease apo A-I levels include androgens, beta blockers, diuretics, and progestins.
Drugs that may decrease apo A-I levels include androgens, beta blockers, diuretics, and progestins.
Apo B
• Diets high in saturated fats and cholesterol may increase apo B levels.
![]() Drugs that may increase apo B levels include androgens, beta blockers, diuretics, ethanol, and progestins.
Drugs that may increase apo B levels include androgens, beta blockers, diuretics, ethanol, and progestins.
![]() Drugs that may decrease apo B levels include cholestyramine, estrogen (postmenopausal women), lovastatin, neomycin, niacin, simvastatin, and thyroxine.
Drugs that may decrease apo B levels include cholestyramine, estrogen (postmenopausal women), lovastatin, neomycin, niacin, simvastatin, and thyroxine.
Test Results and Clinical Significance∗
 Increased Apo A-I Increased Apo A-I |
 Decreased Apo A-I Decreased Apo A-I |
| Familial hyperalphalipoproteinemia | Coronary artery disease (CAD) |
| Pregnancy | Ischemic coronary disease |
| Weight reduction | Myocardial infarction (MI) |
| Familial hypoalphalipoproteinemia | |
| Fish eye disease | |
| Uncontrolled diabetes mellitus | |
| Tangier disease | |
| Nephrotic syndrome | |
| Chronic renal failure | |
| Cholestasis | |
| Hemodialysis | |
 Increased Apo B Increased Apo B |
 Decreased Apo B Decreased Apo B |
| Hyperlipoproteinemia (types IIa, IIb, IV, V) | Tangier disease |
| Nephrotic syndrome | Hyperthyroidism |
| Pregnancy | Inflammatory joint disease |
| Hemodialysis | Malnutrition |
| Biliary obstruction | Chronic pulmonary disease |
| Coronary artery disease (CAD) | Weight reduction |
| Diabetes | Chronic anemia |
| Hypothyroidism | Reye syndrome |
| Anorexia nervosa | |
| Renal failure | |
 Increased Lp(a) Increased Lp(a) |
 Decreased Lp(a) Decreased Lp(a) |
| Premature coronary artery disease (CAD) | Alcoholism |
| Stenosis of cerebral arteries | Malnutrition |
| Uncontrolled diabetes mellitus | Chronic hepatocellular disease |
| Severe hypothyroidism | |
| Familial hypercholesterolemis | |
| Chronic renal failure | |
| Estrogen depletion | |
| Apo E-4 Gene | |
| Alzheimer disease |
Related Test
Lipoprotein (p. 342). This is a test also used to assess the risk of atherogenic vascular disease.
Arterial Blood Gases (Blood Gases, ABG)
Indications
Measurement of arterial blood gasses (ABGs) provides valuable information in assessing and managing a patient's respiratory (ventilation) and metabolic (renal) acid-base and electrolyte homeostasis. It is also used to assess the adequacy of oxygenation.
Test Explanation
ABGs are used to monitor patients on ventilators, monitor critically ill nonventilator patients, establish preoperative baseline parameters, and regulate electrolyte therapy. Although O2 saturation monitors can accurately indicate O2, ABGs are still used to monitor O2 flow rates in the hospital and at home. ABG measurement is often performed in conjunction with pulmonary function studies.
pH
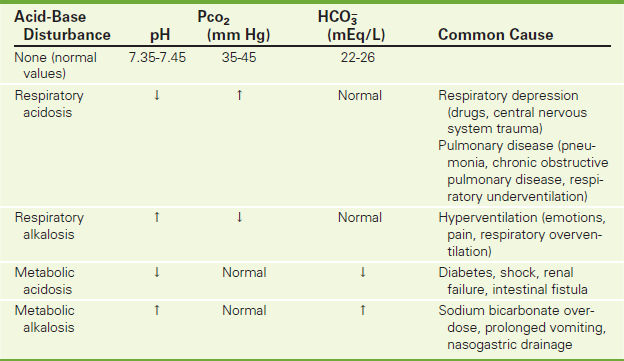
The pH is the negative logarithm of the hydrogen ion concentration in the blood. It is inversely proportional to the actual hydrogen ion concentration. Therefore, as the hydrogen ion concentration decreases, the pH increases, and vice versa. The acids normally found in the blood include carbonic acid (H2CO3), dietary acids, lactic acid, and ketoacids. The pH is a measure of alkalinity (pH >7.4) and acidity pH <7.35). In respiratory or metabolic alkalosis the pH is elevated; in respiratory or metabolic acidosis the pH is decreased. The pH is usually calculated by a machine that directly measures pH.
Pco2
The PCO2 is a measure of the partial pressure of CO2 in the blood. CO2 is carried in the blood as follows: 10% in the plasma and 90% in the red blood cells (RBCs). PCO2 is a measurement of ventilation. The faster and more deeply the patient breathes, the more CO2 is blown off, and Pco2 levels drop. Pco2 is therefore referred to as the respiratory component in acid-base determination, because this value is primarily controlled by the lungs. As the CO2 level increases, the pH decreases. The CO2 level and the pH are inversely proportional. The PCO2 in the blood and the cerebrospinal fluid is a major stimulant to the breathing center in the brain. As PCO2 levels rise, breathing is stimulated. If PCO2 levels rise too high, breathing cannot keep up with the demand to blow off or ventilate. As Pco2 levels rise further, the brain is depressed and ventilation decreases further, causing coma.
The Pco2 level is elevated in primary respiratory acidosis and decreased in primary respiratory alkalosis (Table 2-9). Because the lungs compensate for primary metabolic acid-base derangements, Pco2 levels are affected by metabolic disturbances as well. In metabolic acidosis the lungs attempt to compensate by blowing off CO2 to raise pH. In metabolic alkalosis the lungs attempt to compensate by retaining CO2 to lower pH (Table 2-10).
TABLE 2-10
Acid-Base Disturbances and Compensatory Mechanisms
| Acid-Base Disturbance | Mode of Compensation |
| Respiratory acidosis | Kidneys will retain increased amounts of HCO3– to increase pH |
| Respiratory alkalosis | Kidneys will excrete increased amounts of HCO3– to lower pH |
| Metabolic acidosis | Lungs “blow off” CO2 to raise pH |
| Metabolic alkalosis | Lungs retain CO2 to lower pH |
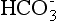 or CO2 Content
or CO2 Content
Most of the CO2 content in the blood is  . The bicarbonate ion (
. The bicarbonate ion ( ) is a measure of the metabolic (renal) component of the acid-base equilibrium. It is regulated by the kidney. This ion can be measured directly by the bicarbonate value or indirectly by the CO2 content (see p. 141). It is important not to confuse CO2 content with Pco2. CO2 content is an indirect measurement of
) is a measure of the metabolic (renal) component of the acid-base equilibrium. It is regulated by the kidney. This ion can be measured directly by the bicarbonate value or indirectly by the CO2 content (see p. 141). It is important not to confuse CO2 content with Pco2. CO2 content is an indirect measurement of  . PCO2 is a direct measurement of the tension of CO2 in the blood and is regulated by the lungs.
. PCO2 is a direct measurement of the tension of CO2 in the blood and is regulated by the lungs.
As the  level increases, the pH also increases; therefore the relationship of bicarbonate to pH is directly proportional.
level increases, the pH also increases; therefore the relationship of bicarbonate to pH is directly proportional.  is elevated in metabolic alkalosis and decreased in metabolic acidosis (see Table 2-9). The kidneys also are used to compensate for primary respiratory acid-base derangements. For example, in respiratory acidosis the kidneys attempt to compensate by reabsorbing increased amounts of
is elevated in metabolic alkalosis and decreased in metabolic acidosis (see Table 2-9). The kidneys also are used to compensate for primary respiratory acid-base derangements. For example, in respiratory acidosis the kidneys attempt to compensate by reabsorbing increased amounts of  . In respiratory alkalosis the kidneys excrete
. In respiratory alkalosis the kidneys excrete  in increased amounts in an attempt to lower pH through compensation (see Table 2-10).
in increased amounts in an attempt to lower pH through compensation (see Table 2-10).
PO2
This is an indirect measure of the O2 content of the arterial blood. PO2 is a measure of the tension (pressure) of O2 dissolved in the plasma. This pressure determines the force of O2 to diffuse across the pulmonary alveoli membrane. The PO2 level is decreased in:
1. Patients who are unable to oxygenate the arterial blood because of O2 diffusion difficulties (e.g., pneumonia, shock lung, congestive failure)
2. Patients in whom venous blood mixes prematurely with arterial blood (e.g., congenital heart disease)
3. Patients who have underventilated and overperfused pulmonary alveoli (pickwickian syndrome; i.e., obese patients who cannot breathe properly when in the supine position or in patients with significant atelectasis)
PO2 is one of the measures used to determine the effectiveness of O2 therapy.
O2 Saturation
O2 saturation is an indication of the percentage of hemoglobin saturated with O2. When 92% to 100% of the hemoglobin carries O2, the tissues are adequately provided with O2, assuming normal O2 dissociation. As the Po2 level decreases, the percentage of hemoglobin saturation also decreases. This decrease (see an oxyhemoglobin-dissociation curve) is linear to a certain value. However, when the Po2 level drops below 60 mm Hg, small decreases in the Po2 level will cause large decreases in the percentage of hemoglobin saturated with O2. At O2 saturation levels of 70% or lower the tissues are unable to extract enough O2 to carry out their vital functions.
O2 saturation is calculated by the blood gas machine using the following formula:
Pulse oximetry (see p. 1114) is a noninvasive method of determining O2 saturation. This can be done easily and continuously. This machine measures O2 saturation. It actually measures all forms of O2-saturated hemoglobin, including carboxyhemoglobin (which rises during smoke inhalation or after using some inhalants). Therefore, in cases of carbon monoxide poisoning when carboxyhemoglobin is high, oximetry will indicate an inaccurately high O2 saturation. During oximetry monitoring a small clip-like sensor is applied to the tip of the finger or earlobe. The oximeter transmits light from one side and records the amount of light on the other side, thus determining O2 saturation.
O2 Content
This is a calculated number that represents the amount of O2 in the blood. The formula for calculation is
Nearly all O2 in the blood is bound to hemoglobin. O2 content decreases with the same diseases that diminish PO2.
Base Excess/Deficit
This number is calculated by the blood gas machine using the pH, PCO2, and the hematocrit. It represents the amount of buffering anions in the blood.  is the largest of these. Others include hemoglobin, proteins, phosphates, and so on. Base excess is a way to take all of these anions into account when determining acid/base treatment based on the metabolic component. A negative-base excess (deficit) indicates metabolic acidosis (e.g., lactic acidosis). A positive-base excess indicates metabolic alkalosis or compensation to prolonged respiratory acidosis.
is the largest of these. Others include hemoglobin, proteins, phosphates, and so on. Base excess is a way to take all of these anions into account when determining acid/base treatment based on the metabolic component. A negative-base excess (deficit) indicates metabolic acidosis (e.g., lactic acidosis). A positive-base excess indicates metabolic alkalosis or compensation to prolonged respiratory acidosis.
Alveolar (A) to Arterial (a) O2 Difference (A-a Gradient)
This is a calculated number that indicates the difference between alveolar O2 and arterial O2. The normal value is less than 10 mm Hg (torr). If the A-a gradient is abnormally high, there is either a problem in diffusing O2 across the alveolar membrane (thickened edematous alveoli) or unoxygenated blood is mixing with the oxygenated blood. Thickened alveolar membranes can occur in patients with pulmonary edema, pulmonary fibrosis, and acute respiratory distress syndrome (ARDS). Mixing of unoxygenated blood occurs in patients with congenital cardiac septal defects, arteriovenous (AV) shunts, or underventilated alveoli that are still being perfused (atelectasis, mucus plug, etc.).
Interpretation of ABG levels can seem difficult but is really quite easy when one follows a system of evaluation (see Table 2-9). One such system is as follows:
A If the PCO2 is high in a patient who has been said to have acidosis (by step 1), the patient has respiratory acidosis.
B If the PCO2 is low in a patient who has been said to have acidosis (by step 1), the patient has metabolic acidosis (MA) and is compensating for that situation by blowing off CO2.
C If the PCO2 is low in a patient who has been said to have alkalosis (by step 1), the patient has respiratory alkalosis.
D If the PCO2 is high in a patient who has been said to have alkalosis (by step 1), the patient has metabolic alkalosis and is compensating for that situation by retaining CO2.
3. Next look at the bicarbonate ion ( ).
).
In patient A,  can be expected to be high in an attempt to compensate for the respiratory acidosis.
can be expected to be high in an attempt to compensate for the respiratory acidosis.
In patient B,  can be expected to be low as a reflection of the MA.
can be expected to be low as a reflection of the MA.
In patient C,  can be expected to be low to compensate for the respiratory alkalosis.
can be expected to be low to compensate for the respiratory alkalosis.
In patient D,  can be expected to be high as a reflection of the metabolic alkalosis.
can be expected to be high as a reflection of the metabolic alkalosis.
Contraindications
Arterial access should not be performed if:
• Cellulitis or open infection is present in the area considered for access.
• The Allen test is negative, indicating that there is no ulnar artery. If the radial artery is used for access, thrombosis may occur and jeopardize the viability of the hand.
• There is an AV fistula proximal to the site of proposed access.
Interfering Factors
• O2 saturation can be falsely increased by the inhalation of carbon monoxide, which increases the carboxyhemoglobin level.
• In patients with chronic obstructive pulmonary disease (COPD), the stimulus to breathe is not triggered by CO2 levels (as normal) but by O2 levels. If a large amount of O2 is provided to these patients, they will no longer be driven to breathe and will hypoventilate.
![]() Respiration can be inhibited by the use of sedative-hypnotics or narcotics. Overdosage of these drugs can cause hypoventilation in patients with normal lungs.
Respiration can be inhibited by the use of sedative-hypnotics or narcotics. Overdosage of these drugs can cause hypoventilation in patients with normal lungs.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Notify the laboratory before drawing ABGs so that the necessary equipment can be calibrated before the blood sample arrives.
• Perform the Allen test to assess collateral circulation before performing the arterial puncture on the radial artery (Figure 2-6). To perform the Allen test, make the patient's hand blanch by obliterating both the radial and the ulnar pulses and then release the pressure over the ulnar artery only. If flow through the ulnar artery is good, flushing can be seen immediately. The Allen test is then positive, and the radial artery can be used for puncture. If the Allen test is negative (no flushing), repeat it on the other arm. If both arms give a negative result, choose another artery (femoral) for puncture.
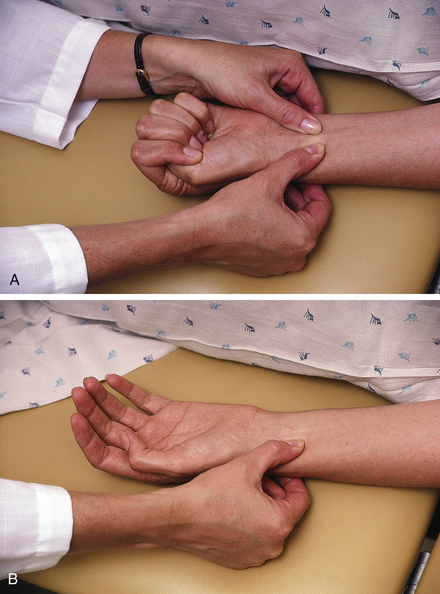
Figure 2-6 The Allen test for evaluating collateral circulation of the radial artery. A, Step 1, While the patient's fist is closed tightly, obliterate both the radial and ulnar arteries simultaneously. Instruct the patient to relax the hand, and watch for blanching of the palm and fingers. B, Step 2, Release the obstructing pressure from only the ulnar artery. Wait 15 seconds, observing the hand for flushing caused by capillary refilling. Flushing indicates a positive Allen test, verifying that the ulnar artery alone is capable of supplying the entire hand. If flushing does not occur within 15 seconds, the Allen test is negative and radial artery cannot be used.
• Note that the Allen test ensures collateral circulation to the hand if thrombosis of the radial artery should follow the puncture.
During
• Note that arterial blood can be obtained from any area of the body in which strong pulses are palpable, usually from the radial, brachial, or femoral artery. The artery chosen for access should have adequate collateral vessels, be easily accessible, and be surrounded by few other vital structures.
• Cleanse the arterial site carefully with an antiseptic (e.g., alcohol or povidoneiodine).
• Use a small-guage needle to collect the arterial blood in an air-free heparinzed syringe.
• After drawing the blood, remove the needle and apply pressure to the arterial site for 3 to 5 minutes.
After
• Place the arterial blood on ice and immediately take it to the chemistry or pulmonary laboratory for analysis.
• Hold pressure or apply pressure or a pressure dressing to the arterial puncture site for 3 to 5 minutes to avoid hematoma formation.
• Assess the puncture site for bleeding. Remember that an artery rather than a vein has been accessed.
• If the patient has an abnormal clotting time or is taking anticoagulants, apply pressure for a longer period (approximately 15 minutes).
Test Results and Clinical Significance (see Table 2-9)
 Increased pH (Alkalosis)
Increased pH (Alkalosis)
 Increased
Increased 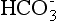
 Increased PO2 and O2 Content
Increased PO2 and O2 Content
 Decreased PO2 and O2 Content
Decreased PO2 and O2 Content
 Increased A-a O2 Gradient
Increased A-a O2 Gradient
Nonventilated lung tissue is still perfused. The perfused blood does not get oxygenated, however, because there is no ventilation in that area of the lung to bring O2 to the blood. The perfused yet unoxygenated blood mixes with the oxygenated blood in the pulmonary veins. By dilution, the O2 content of the mixed blood returning to the heart is lowered. The arterial blood is therefore lowered.
Related Tests
Pulmonary Function Tests (p. 1117). This is measurement of lung volume, which aids in the diagnosis and treatment of obstructive and restrictive lung diseases.
Fetal Scalp Blood pH (p. 239). Measurement of fetal scalp blood pH provides valuable information on fetal acid-base status. This screening test is useful clinically for diagnosing fetal distress.
Aspartate Aminotransferase (AST, Formerly Serum Glutamic Oxaloacetic Transaminase [SGOT])
Normal Findings
| Age | Normal Value (units/L) |
| 0-5 days | 35-140 |
| <3 yr | 15-60 |
| 3-6 yr | 15-50 |
| 6-12 yr | 10-50 |
| 12-18 yr | 10-40 |
| Adult | 0-35 units/L or 0-0.58 μkat/L (SI Units)(Females tend to have slightly lower levels than males) |
| Elderly | Slightly higher than adults |
Test Explanation
This enzyme is found in very high concentrations within highly metabolic tissue, such as the heart muscle, liver cells, skeletal muscle cells, and to a lesser degree in the kidneys, pancreas, and red blood cells (RBCs). When disease or injury affects the cells of these tissues, the cells lyse. The AST is released, picked up by the blood, and the serum level rises. The amount of AST elevation is directly related to the number of cells affected by the disease or injury. Furthermore, the elevation depends on the length of time that the blood is drawn after the injury. AST is cleared from the blood in a few days. Serum AST levels become elevated 8 hours after cell injury, peak at 24 to 36 hours, and return to normal in 3 to 7 days. If the cellular injury is chronic, levels will be persistently elevated.
Because AST exists within the liver cells, diseases that affect the hepatocyte will cause elevated levels of this enzyme. In acute hepatitis, AST levels can rise 20 times the normal value. In acute extrahepatic obstruction (e.g., gallstone), AST levels quickly rise to 10 times the norm and swiftly fall. In patients with cirrhosis, the level of AST depends on the amount of active inflammation.
Serum AST levels are often compared with alanine aminotransferase (ALT) levels. The AST/ALT ratio is usually greater than 1 in patients with alcoholic cirrhosis, liver congestion, and metastatic tumor of the liver. Ratios less than 1 may be seen in patients with acute hepatitis, viral hepatitis, or infectious mononucleosis. The ratio is less accurate if AST levels exceed 10 times normal.
Patients with acute pancreatitis, acute renal diseases, musculoskeletal diseases, or trauma may have a transient rise in serum AST. Patients with RBC abnormalities such as acute hemolytic anemia and severe burns also can have elevations of this enzyme. AST levels may be decreased in patients with beriberi or diabetic ketoacidosis and in patients who are pregnant.
Interfering Factors
• Pregnancy may cause decreased AST levels.
• Exercise may cause increased levels.
• Levels are falsely decreased in patients with pyridoxine deficiency (beriberi, pregnancy), severe long-standing liver disease, uremia, or diabetic ketoacidosis.
![]() Drugs that may cause increased levels include antihypertensives, cholinergic agents, coumarin-type anticoagulants, digitalis preparations, erythromycin, hepatotoxic medications, isoniazid, methyldopa, oral contraceptives, opiates, salicylates, stains, and verapamil.
Drugs that may cause increased levels include antihypertensives, cholinergic agents, coumarin-type anticoagulants, digitalis preparations, erythromycin, hepatotoxic medications, isoniazid, methyldopa, oral contraceptives, opiates, salicylates, stains, and verapamil.
Procedure and Patient Care
During
• Collect a venous sample of blood in a red-top tube. This is usually done daily for 3 days and then again in 1 week.
• Rotate the venipuncture site.
• Indicate on the laboratory slip any drugs that may cause false-positive results.
• Record the time and date of any intramuscular injection given.
• Record the exact time and date when the blood test is performed. This aids in the interpretation of the temporal pattern of enzyme elevations.
Test Results and Clinical Significance
Related Tests
Creatine Kinase (CK) (see p. 186). This enzyme is used similarly to AST and exists predominantly in heart and skeletal muscle.
Alanine Aminotransferase (ALT) (see p. 39). This enzyme is used similarly to AST and exists predominantly in the liver.
Lactic Dehydrogenase (LDH) (p. 329). This is an intracellular enzyme used to support the diagnosis of injury or disease involving the heart, liver, RBCs, kidneys, skeletal muscle, brain, and lungs.
Leucine Aminopeptidase (LAP) (see p. 337). This enzyme is specific to the hepatobiliary system. Diseases affecting that system will cause elevation of this enzyme.
Gamma-Glutamyl Transpeptidase (GGTP) (p. 246). This is another enzyme existing predominantly in the liver.
Alkaline Phosphatase (p. 47). This is another enzyme existing predominantly in the liver.
5'-Nucleotidase (p. 376). This is another enzyme existing predominantly in the liver.
Bilirubin
Indications
This test is used to evaluate liver function. It is a part of the evaluation of adult patients with hemolytic anemias and newborns with jaundice.
Test Explanation
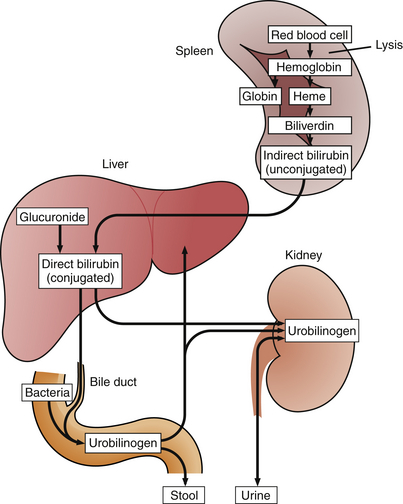
Bile, which is formed in the liver, has many constituents, including bile salts, phospholipids, cholesterol, bicarbonate, water, and bilirubin. Bilirubin metabolism begins with the breakdown of red blood cells (RBCs) in the reticuloendothelial system (mostly the spleen) (Figure 2-7). Hemoglobin is released from RBCs and broken down to heme and globin molecules. Heme is then catabolized to form biliverdin, which is transformed to bilirubin. This form of bilirubin is called unconjugated (indirect) bilirubin. In the liver, indirect bilirubin is conjugated with a glucuronide molecule, resulting in conjugated (direct) bilirubin. The conjugated bilirubin is then excreted from the liver cells and into the intrahepatic canaliculi, which eventually lead to the hepatic ducts, the common bile duct, and the bowel.
Jaundice is the discoloration of body tissues caused by abnormally high blood levels of bilirubin. This yellow discoloration is recognized when the total serum bilirubin exceeds 2.5 mg/dL. Jaundice results from a defect in the normal metabolism or excretion of bilirubin. This defect can occur at any stage of heme catabolism.
Physiologic jaundice of the newborn occurs if the newborn's liver is immature and does not have enough conjugating enzymes. This results in a high circulating blood level of unconjugated bilirubin, which can pass through the blood-brain barrier and deposit in the brain cells of the newborn, causing encephalopathy (kernicterus). In newborns, if bilirubin levels are greater than 15 mg/dL, immediate treatment is required to avoid mental retardation. This may include exchange transfusions. High levels of bilirubin in the newborn are often treated with light therapy.
If the defect in bilirubin metabolism occurs after addition of glucuronide, conjugated (direct) hyperbilirubinemia will result. Obstruction of the bile duct by a gallstone is the classic example of obstructed bilirubin excretion causing a direct hyperbilirubinemia.
Once the jaundice is recognized either clinically or chemically, it is important (for therapy) to differentiate whether it is predominantly caused by indirect (unconjugated) or direct (conjugated) bilirubin. This in turn will help differentiate the etiology of the defect. In general, jaundice caused by hepatocellular dysfunction (e.g., hepatitis) results in elevated levels of indirect bilirubin. This dysfunction usually cannot be repaired surgically. On the other hand, jaundice resulting from extrahepatic dysfunction (e.g., gallstones, tumor blocking the bile ducts) results in elevated levels of direct bilirubin; this type of jaundice usually can be resolved by open surgery or endoscopic surgery.
The total serum bilirubin level is the sum of the conjugated (direct) and unconjugated (indirect) bilirubin. These are separated out when “fractionation or differentiation” of the total bilirubin to its direct and indirect parts is requested from the laboratory (Figure 2-8). Normally the indirect (unconjugated) bilirubin makes up 70% to 85% of the total bilirubin. In patients with jaundice, when more than 50% of the bilirubin is direct (conjugated), it is considered a direct hyperbilirubinemia from gallstones, tumor, inflammation, scarring, or obstruction of the extrahepatic ducts. Indirect hyperbilirubinemia is diagnosed when less than 15% to 20% of the total bilirubin is direct bilirubin. Diseases that typically cause this form of jaundice include accelerated erythrocyte (RBC) hemolysis, hepatitis, or drugs.

Figure 2-8 Siemens multiple channel chemistry analyzer. This is one of six chemical analyzer machines that are assembled in series and in which specimens are directed by a computerized master distributor.
Delta bilirubin is a form of bilirubin that is covalently bound to albumin. It has a longer half-life than the other bilirubins; therefore it remains elevated during the convalescent phases of hepatic disorders when the conjugated bilirubin has typically returned to normal. It can be derived by the following calculation:
When the defect in bilirubin metabolism occurs after conjugation, elevated levels of direct (conjugated) bilirubin occur. Unlike the unconjugated form, direct bilirubin is water soluble and can be excreted into the urine. Therefore bilirubin in urine suggests disease affecting bilirubin metabolism after conjugation or defects in excretion (e.g., gallstones). There may be a small amount of bilirubin in the urine. Testing for bilirubin in the urine is a part of routine urine analysis (U/A).
Interfering Factors
• Blood hemolysis and lipemia can produce erroneous results.
![]() Drugs that may cause increased blood levels of total bilirubin include allopurinol, anabolic steroids, antibiotics, antimalarials, ascorbic acid, azathioprine, chlorpropamide (Diabinese), cholinergics, codeine, dextran, diuretics, epinephrine, meperidine, methotrexate, methyldopa, monoamine oxidase inhibitors, morphine, nicotinic acid (large doses), oral contraceptives, phenothiazines, quinidine, rifampin, salicylates, steroids, sulfonamides, theophylline, and vitamin A.
Drugs that may cause increased blood levels of total bilirubin include allopurinol, anabolic steroids, antibiotics, antimalarials, ascorbic acid, azathioprine, chlorpropamide (Diabinese), cholinergics, codeine, dextran, diuretics, epinephrine, meperidine, methotrexate, methyldopa, monoamine oxidase inhibitors, morphine, nicotinic acid (large doses), oral contraceptives, phenothiazines, quinidine, rifampin, salicylates, steroids, sulfonamides, theophylline, and vitamin A.
![]() Drugs that may cause increased urine bilirubin levels include allopurinol, antibiotics, barbiturates, chlorpromazine, diuretics, oral contraceptives, phenazopyridine (Pyridium), steroids, and sulfonamides.
Drugs that may cause increased urine bilirubin levels include allopurinol, antibiotics, barbiturates, chlorpromazine, diuretics, oral contraceptives, phenazopyridine (Pyridium), steroids, and sulfonamides.
![]() Drugs that may cause decreased blood levels of total bilirubin include barbiturates, caffeine, penicillin, and salicylates (high dose).
Drugs that may cause decreased blood levels of total bilirubin include barbiturates, caffeine, penicillin, and salicylates (high dose).
![]() Drugs that can cause false-negative results in urine levels include ascorbic acid (vitamin C) and indomethacin (Indocin).
Drugs that can cause false-negative results in urine levels include ascorbic acid (vitamin C) and indomethacin (Indocin).
![]() Drugs that can cause false-positive results in the urine level include “pyridium-like” drugs and urochromes. These drugs can color the urine yellow or orange and foil the color analysis tests. Bilirubin is not stable in urine, especially when exposed to light.
Drugs that can cause false-positive results in the urine level include “pyridium-like” drugs and urochromes. These drugs can color the urine yellow or orange and foil the color analysis tests. Bilirubin is not stable in urine, especially when exposed to light.
Procedure and Patient Care
During
Blood
• Collect a venous blood sample in a red-top tube.
• Use a heel puncture for blood collection in infants.
• Prevent hemolysis of blood during phlebotomy.
• Do not shake the tube, because inaccurate test results may occur.
• Protect the blood sample from bright light. Prolonged exposure (over 1 hour) to sunlight or artificial light can reduce bilirubin content.
Urine Testing With Multistix Reagent Strips
• Note that this is a firm, plastic strip with seven separate areas for testing pH, protein, glucose, ketones, bilirubin, blood, and urobilinogen.
• For testing bilirubin, obtain a fresh urine specimen and examine it as soon as possible.
• Immerse the dipstick in the well-mixed urine and then remove immediately to avoid dissolving other reagents.
• Tap the dipstick against the rim of the urine container to remove excess urine.
• Hold the strip horizontally and compare it with the color chart on the label of the bottle in the designated time period.
Test Results and Clinical Significance
 Increased Blood Levels of Conjugated (Direct) Bilirubin
Increased Blood Levels of Conjugated (Direct) Bilirubin
Extrahepatic duct obstruction (tumor, inflammation, gallstone, scarring, surgical trauma):
These diseases cause a blockage of the bile ducts. Bile, containing bilirubin, cannot be excreted. Blood levels rise.
Extensive liver metastasis: The intrahepatic ducts or hepatic ducts become obstructed because of tumor. Bile, containing bilirubin, cannot be excreted. Blood levels rise.
Cholestasis from drugs: Some drugs inhibit the excretion of bile from the hepatocyte into the bile canaliculi. Bile, containing bilirubin, cannot be excreted. Blood levels rise.
 Increased Blood Levels of Unconjugated (Indirect) Bilirubin
Increased Blood Levels of Unconjugated (Indirect) Bilirubin
Large-volume blood transfusion,
RBC destruction occurs. Large amounts of heme are available for catabolism into bilirubin. This quantity exceeds the liver's capability to conjugate bilirubin. Indirect (unconjugated) bilirubin levels rise.
Related Tests
Liver Enzymes such as Alkaline Phosphatase (ALP) (p. 47), Lactic Dehydrogenase (LDH) (p. 329), Aspartate Aminotransferase (AST) (p. 119), Alanine Aminotransferase (ALT) (p. 39), and 5'-Nucleotidase (p. 376). These tests are very helpful in the evaluation of the liver.
Complete Blood Cell Count (p. 174), Haptoglobin (p. 274), and other blood tests. These tests are helpful in the evaluation of hemolytic anemias.
Blood Typing (Blood Group Microarray Testing)
Indications
This test is used to determine the blood type of the patient before donating or receiving blood and to determine the blood type of expectant mothers to assess the risks of Rh incompatibility between mother and newborn.
Test Explanation
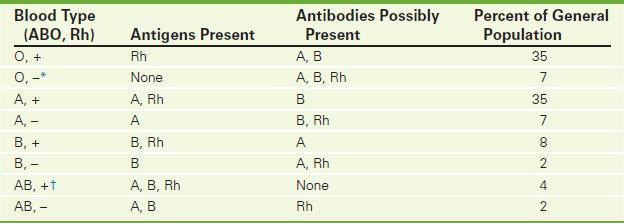
With blood typing, ABO and Rh antigens can be detected in the blood of prospective blood donors and potential blood recipients. This test is also used to determine the blood type of expectant mothers and newborns. A description of the ABO system, Rh factors, and blood crossmatching is reviewed here. The incidence of each blood type is noted in Table 2-11.
ABO System
Human blood is grouped according to the presence or absence of A or B antigens. The surface membranes of group A red blood cells (RBCs) contain A antigens (Figure 2-9); group B RBCs contain B antigens on their surface; group AB RBCs have both A and B antigens; and group O RBCs have neither A nor B antigens. In general, a person's serum does not contain antibodies to match the surface antigen on his or her RBCs. That is, persons with group A antigens (type A blood) will not have anti-A antibodies; however, they will have anti-B antibodies. The converse is true for persons with group B antigens. Group O blood will have both anti-A and anti-B antibodies. These antibodies against A and B blood group antigens are formed in the first 3 months of life after exposure to similar antigens on the surface of naturally occurring bacteria in the intestine.
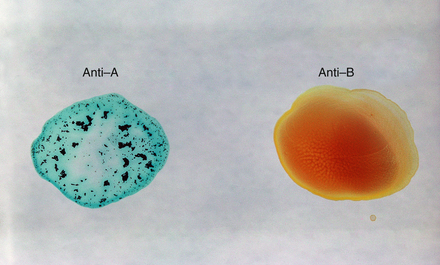
Figure 2-9 Blood typing by agglutination laboratory method. An “A” antigen that is present on the surface of the patient's red blood cell reacts with known anti-a antibody that has been added to a drop of the patient's blood. Agglutination (clumping) is visible on the left. No agglutination is noted on the right when anti-B antibody is added to a drop of the patient's blood. This patient has type A blood. In most laboratories, blood typing is automated by a machine that utilizes agglutination inhibition (see p. 2).
Blood transfusions are actually transplantations of tissue (blood) from one person to another. It is important that the recipient not have antibodies to the donor's RBCs. If this were to occur, there could be a hypersensitivity reaction, which can vary from mild fever to anaphylaxis with severe intravascular hemolysis. If donor ABO antibodies are present against the recipient antigens, usually only minimal reactions occur.
Persons with group O blood are considered universal donors because they do not have antigens on their RBCs. People with group AB blood are considered universal recipients because they have no antibodies to react to the transfused blood. Group O blood is usually transfused in emergent situations in which rapid, life-threatening blood loss occurs and immediate transfusion is required. The chance of a transfusion reaction is least when type O is used. Women of childbearing potential should receive group O negative blood, and men generally receive group O positive blood when emergency transfusion prior to type-specific or crossmatched blood is required.
ABO typing is not required for autotransfusions (blood donated by a patient several weeks prior to a major operation and then transfused postoperatively). However, in most hospitals, ABO typing is performed on those patients in the event that further blood transfusion of banked blood is required.
Rh Factors
The presence or absence of Rh antigens on the RBC's surface determines the classification of Rh positive or Rh negative. After ABO compatibility, Rh factor is the next most important antigen affecting the success of a blood transfusion. The major Rh factor is Rho(D). There are several minor Rh factors. If Rho(D) is absent, the minor Rh antigens are tested. If negative, the patient is considered Rh negative (Rh–).
Rh– persons may develop antibodies to Rh antigens if exposed to Rh positive (Rh+) blood by prior transfusions or fetal-maternal blood mixing. All women who are pregnant should have a blood typing and Rh factor determination. If the mother's blood is Rh–, the father's blood should also be typed. If his blood is Rh+, the woman's blood should be examined for the presence of Rh antibodies (by the indirect Coombs test). If the initial screening is negative (no antibodies to Rh found), the test is repeated at 28 to 30 weeks and 36 weeks of pregnancy. If these tests are also negative, the fetus is not at risk. However, if the test is positive, the fetus is at risk for hemolytic disease of the newborn (erythroblastosis fetalis). In this disease the mother is Rh– and the fetus is Rh+. Any fetal bleeding that occurs can sensitize the mother to form anti-Rh antibodies. These antibodies cross the placenta and hemolyze the fetal RBCs. Problems ranging from mild fetal anemia to in utero fetal death could occur. The severity of the hemolytic anemia can be evaluated by determining the quantity of bilirubin in the amniotic fluid (amniocentesis [p. 632]).
Hemolytic disease of the newborn can be prevented by Rh typing during pregnancy. If the mother is Rh–, she should be advised that she is a candidate for Rho-GAM (Rh immunoglobulin that “neutralizes” the Rh antigen) after the delivery. RhoGAM can reduce the chance of fetal hemolytic problems during subsequent pregnancies.
Other Blood Typing Systems
There are nine different gene codes for blood groups assayed. Most are minor and not clinically significant. However in certain clinical circumstances, these minor blood group antigens and acquired antigens can become significant. This may occur with frequent blood transfusions or in patients with leukemia or lymphoma. Multiplex PCR microarray analysis provides identification of the many variants involving these blood group systems and is particularly helpful in the described patients.
Blood Crossmatching
Although typing for the major ABO and Rh antigens is no guarantee that a reaction will not occur, it does greatly reduce the possibility of such a reaction. Many potential minor antigens are not routinely detected during blood typing. If allowed to go unrecognized, these minor antigens also can initiate a blood transfusion reaction. Therefore blood is not only typed but also crossmatched to identify a mismatch of blood caused by minor antigens. Crossmatching consists of the mixing of the recipient's serum with the donor's RBCs in saline solution followed by the addition of Coombs serum (indirect Coombs test). Only blood products containing RBCs need to be crossmatched. Plasma products do not need to be crossmatched but should be ABO compatible because other cells (WBCs and platelets) have ABO antigens (Figure 2-10).

Figure 2-10 Immunohematology section of the laboratory showing the area where units of blood are processed and labeled.
Homologous (donor and recipient are different people) and directed (recipient chooses the donor) blood for donation must be rigorously tested before transfusion (Box 2-4). Autologous (recipient and donor is the same person) blood for transfusions, however, is not subject to that same testing. It is important to note, however, that autologous blood transfusion is not 100% safe. As a result of the additives used for banking purposes, blood and hypersensitivity reactions can still occur.
Finally, one must be aware of graft-versus-host disease (GVHD) in which donor lymphocytes included in the blood transfusion may engraft and multiply in the recipient. These lymphocytes can react against the recipient's tissues. This is most common among immunocompromised patients. Pretransfusion radiation of the unit of blood to be transfused will avoid this problem.
CA 15-3 and CA 27-29 Tumor Marker (Cancer Antigen 15-3, Cancer Antigen 27-29)
Indications
The CA 15-3 and CA 27-29 antigens are tumor-associated serum markers available for breast cancer monitoring.
Test Explanation
Carcinoembryonic antigen (CEA), the most widely used tumor marker, is limited by poor sensitivity and specificity for patients with breast cancer. Monoclonal antibody technology has permitted the development of CA 15-3 and CA 27-29 antigens. These antigens are not as sensitive for the diagnosis of primary breast cancer as other tumor markers are for their respective tumors. That is, CA 15-3 and CA 27-29 levels are high in only 50% of patients who have a localized breast cancer or a small tumor burden. However, 80% of patients with metastatic breast cancer do have elevated CA 15-3 levels and 65% have elevated CA 27-29 levels; therefore the usefulness of these antigen tests as a screening technique in early breast cancers (the most common cancer of women) is quite limited.
Benign breast disease and nonbreast malignancies (e.g., lung, pancreas, ovary, prostate) also can cause elevation of these antigen levels. These antigens are useful in monitoring the patient's response to therapy of metastatic breast cancer. A partial or complete response to treatment will be confirmed by declining levels. Likewise, a persistent rise in these antigen levels despite therapy strongly suggests progressive disease.
CA 15-3 and CA 27-29 have a high sensitivity but a somewhat lower specificity. Many diseases, both benign and malignant, can cause elevations in these values. Therefore they cannot be used to diagnose recurrence. However, in the patient who has symptoms, signs, or other test results that indicate recurrence, an elevation in one of these tumor markers would corroborate the diagnosis of recurrent breast cancer. These tumor markers are better suited for indicating response of metastatic disease to treatment (when already elevated). CA 15-3 was the first breast tumor marker available. It is usually performed by a reference laboratory using competitive inhibition radioimmune assay. The CA 27-29 marker is done by immunoradiometric assay.
Related Test
Carcinoembryonic Antigen (CEA) (p. 145). This is another tumor marker used in the monitoring of breast cancer.
CA 19-9 Tumor Marker (Cancer Antigen 19-9)
Indications
CA 19-9 antigen is a tumor marker used for the diagnosis of patients with pancreatic or hepatobiliary cancer, evaluation of response to treatment, and surveillance.
Test Explanation
CA 19-9 is a carbohydrate cell-surface antigen. It exists on the surface of some cancer cells. Although initially thought to be specific for colorectal cancer, it is now used primarily in the evaluation of patients with pancreatic or hepatobiliary cancers. In the diagnosis of pancreatic carcinoma, for example, the presence of a pancreatic mass or biliary obstruction and greatly elevated CA 19-9 levels would support the diagnosis of pancreatic cancer over benign pancreatitis. Hepatobiliary cancer is suspected in patients whose presenting symptoms are ascites, jaundice, and elevated CA 19-9 levels. CA 19-9 levels may not be elevated in all patients with pancreatic carcinoma. Approximately 70% of patients with pancreatic carcinoma and 65% of patients with hepatobiliary cancer have elevated levels.
CA 19-9 levels areary cancers. In the few patients with pancreatic or biliary cancer who have a good response to surgery, chemotherapy, or radiation therapy, a decline in serum levels of CA 19-9 will confirm this response. A rapid rise in CA 19-9 levels may be associated with recurrent or progressive tumor growth. Mildly elevated levels may exist in patients with gastric cancer, colorectal cancer, hepatoma, and even in 6% to 7% of patients with non-gastrointestinal (GI) malignancies. Patients who have pancreatitis, gallstones, cirrhosis, inflammatory bowel disease (IBD), or cystic fibrosis (CF) also can have minimally elevated levels of CA 19-9.
Because of its lack of sensitivity and specificity, CA 19-9 is not effective in screening for pancreatobiliary tumors in the general population.
Related Test
Carcinoembryonic Antigen (CEA) (p. 145). This is another tumor marker that is elevated in patients with pancreatobiliary cancer.
CA-125 Tumor Marker (Cancer Antigen-125)
Indications
CA-125 is used in the detection of ovarian cancer. It is also used to determine the extent of disease and to monitor the response to treatment.
Test Explanation
This tumor marker has a high degree of sensitivity and specificity for ovarian cancer. Just as alpha-fetoprotein (AFP) and human chorionic gonadotropin (hCG) are accurate tumor markers for germ cell tumors of the ovary, CA-125 is an extremely accurate marker for nonmucinous epithelial tumors of the ovary. It is elevated in more than 80% of women with ovarian cancer.
The CA-125 marker can be used in many ways. By itself, it cannot be used to diagnose ovarian cancer, but it helps support the diagnosis of ovarian cancer. For example, a greatly elevated CA-125 level in women who have abdominal distention, ascites, and a palpable pelvic mass is strong confirmation that the underlying etiology is an epithelial ovarian malignancy.
The CA-125 serum tumor marker is also used to determine a patient's response to therapy. Comparative serial testing will show a progressive decline in CA-125 levels for patients responding to treatment. Also, CA-125 tumor markers can predict whether or not a second-look (repeat) diagnostic laparotomy will be positive. A second-look laparotomy will detect a residual tumor in 97% of patients whose CA-125 level is greater than 35 units/mL, whereas only 56% of patients with ovarian cancer whose CA-125 level is less than 35 units/mL will have a positive second-look laparotomy. A precipitous fall in CA-125 after two courses of chemotherapy is an accurate predictor of a complete response to chemotherapy and is interpreted as a good prognostic sign.
Finally, CA-125 determinations can be used in posttreatment surveillance of patients with ovarian cancer. In a patient who has had a complete response to radiation therapy, chemotherapy, or surgery, a delayed rise in the CA-125 level is an early predictor of a recurrent tumor in 93% of patients. Abnormal levels can antedate the appearance of obvious recurrent ovarian cancer by 2 to 7 months.
CA-125 is not an effective screening test for the asymptomatic general public because of its lack of specificity. It is used in “high-risk” women who have a strong family history of ovarian cancer or have a breast cancer antigen (BRCA) genetic defect (p. 1094). Elevated levels in the general population indicate that either benign or malignant disease is present in 95% of patients.
Other tumors and benign processes can cause elevated CA-125 levels as well. Diseases that affect the peritoneum, such as cirrhosis, pancreatitis, peritonitis, endometriosis, and pelvic inflammatory disease (PID), can cause elevated levels of CA-125. Other malignancies occurring in the female genital tract, pancreas, colon, lung, and breast can also be associated with elevated levels of this protein. A toftal of 1% to 2% of the normal population has CA-125 levels in excess of 3535 units/mL.
Interfering Factors
• The first trimester of pregnancy and normal menstruation may be associated with mild elevations of CA-125 levels.
• Patients with benign peritoneal diseases (e.g., cirrhosis, endometriosis) will have mildly increased levels.
• Smoking can falsely increase CA-125 levels.
• Patients who have had recent abdominal surgery may have elevated CA-125 levels for as long as 3 weeks after surgery.
Calcitonin (Human Calcitonin [HCT], Thyrocalcitonin)
Indications
This test is usually indicated to evaluate persons with suspected medullary carcinoma of the thyroid. Calcitonin is useful in monitoring response to therapy and predicting recurrences of medullary thyroid cancer. It is also useful as a screening test for those with a family history of medullary cancer.
Test Explanation
Calcitonin is a hormone secreted by the parafollicular or C cells of the thyroid gland. Secretion is stimulated by elevated serum calcium levels. Calcitonin contributes to calcium homeostasis. It decreases serum calcium levels by inhibiting bone resorption and increasing calcium excretion by the kidneys.
This test is usually used in the evaluation of patients who have confirmed or suspected medullary carcinoma of the thyroid. Seventy-five percent of these patients have hypersecretion of calcitonin despite normal serum calcium levels. Calcitonin is useful in monitoring response to therapy and predicting recurrences of medullary thyroid cancer. It is also useful as a screening test for those with a family history of medullary cancer and therefore at high risk (20%) for medullary cancer. This is a cancer of the thyroid with a familial tendency and if found late has a poor prognosis. This cancer is often associated with multiple endocrine neoplasia (MEN) syndromes. Routine screening for elevated calcitonin levels can detect medullary cancer early and improve chances for cure. Calcitonin can be used as a tumor marker in monitoring patients with medullary cancer of the thyroid. Increases in calcitonin levels herald progression of the cancer. Declining levels indicate tumor regression. C-cell hyperplasia, a benign calcitonin-producing disease that also has a familial tendency, is also associated with elevated calcitonin levels.
Equivocal elevations in calcitonin levels should be followed with further provocative testing using pentagastrin or calcium to stimulate calcitonin secretion. Pentagastrin stimulation involves an intravenous (IV) infusion with blood samples drawn before the injection and at 90 seconds, 2 minutes, and 5 minutes following the infusion. The calcium infusion test can be performed in a variety of ways but is most commonly administered with blood drawn to establish baseline and 5- and 10-minute postinfusion blood levels. With medullary cancer of the thyroid, the provocative tests can cause the calcitonin to rise significantly.
Elevated levels of calcitonin also may be seen in people with cancer of the lung, breast, and pancreas. This is probably a form of paraneoplastic syndrome in which there is an ectopic production of calcitonin by the nonthyroid cancer cells.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Medullary carcinoma of the thyroid,
Calcitonin is secreted by the thyroid in these diseases despite the calcium blood levels. These abnormalities are not responsive to the normal regulatory feedback mechanisms.
Secondary hyperparathyroidism as a result of chronic renal failure:
These states are associated with high serum calcium levels. High calcitonin levels may be compensatory.
Several endocrine familial and nonfamilial multiple endocrinopathies (Apudoma) may be associated with high calcitonin levels.
Alcoholic cirrhosis: The mechanism is not well defined. Perhaps the liver cannot metabolize hormones well and high levels of calcitonin result.
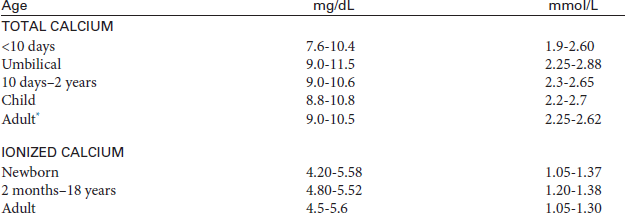
Calcium, Blood (Total/Ionized Calcium, Ca)
Indications
The serum calcium test is used to evaluate parathyroid function and calcium metabolism by directly measuring the total amount of calcium in the blood. Serum calcium levels are used to monitor patients with renal failure, renal transplantation, hyperparathyroidism, and various malignancies. They are also used to monitor calcium levels during and after large-volume blood transfusions.
Test Explanation
Serum calcium is necessary in many metabolic enzymatic pathways. It is vital for muscle contractility, cardiac function, neural transmission, and blood clotting. The serum calcium test is used to evaluate parathyroid function and calcium metabolism by directly measuring the total amount of calcium in the blood. The bone and the teeth act as a reservoir for calcium. When blood levels decrease, parathyroid hormone (PTH) release is stimulated. This hormone acts on the reservoirs to release calcium into the blood. About one half of the total calcium exists in the blood in its free (ionized) form, and about one half exists in its protein-bound form (mostly with albumin). The serum calcium level is a measure of both. As a result, when the serum albumin level is low (as in malnourished patients), the serum calcium level will also be low, and vice versa. As a rule of thumb, the total serum calcium level decreases by approximately 0.8 mg for every 1-g decrease in the serum albumin level. Serum albumin should be measured with serum calcium.
The ionized form of calcium also can be measured by ion-selective electrode techniques or can be calculated from several available formulas. An advantage of measuring the ionized form is that it is unaffected by changes in serum albumin levels. Many laboratories do not have the equipment to perform the ionized calcium assay. Certainly, when albumin levels are variable, measurement of ionized calcium can allow more accurate calcium replacement therapy if needed. This is especially true during open heart surgery, major organ transplantation, and renal dialysis.
When the serum calcium level is elevated on at least three separate determinations, the patient is said to have hypercalcemia. Symptoms of hypercalcemia may include anorexia, nausea, vomiting, somnolence, and coma. The most common cause of hypercalcemia is hyperparathyroidism. Parathormone causes elevated calcium levels by increasing gastrointestinal (GI) absorption, decreasing urinary excretion, and increasing bone resorption. Malignancy, the second most common cause of hypercalcemia, can cause elevated calcium levels in two main ways. First, tumor metastasis (myeloma, lung, breast, renal cell) to the bone can destroy the bone, causing resorption and pushing calcium into the blood. Second, the cancer (lung, breast, renal cell) can produce a PTH-like substance that drives the serum calcium up (ectopic PTH). Excess vitamin D ingestion can increase serum calcium by increasing renal and GI absorption. Granulomatous infections such as sarcoidosis and tuberculosis are associated with hypercalcemia.
In some instances a normal serum calcium does not preclude hypercalcemia. For example, if the serum calcium is normal in a patient with reduced serum albumin (the calcium should be reduced in these patients), hypercalcemia should be suspected. A similar situation exists in patients with chronic renal failure. These patients have high phosphate levels and other anions that tend to chronically lower serum calcium. As a result, PTH is persistently stimulated to increase calcium levels. The calcium levels return to normal in time, but that “normal” level actually represents a “high” level when one considers that it should be low in these individuals. This is the classic case of secondary hyperparathyroidism.
Hypocalcemia occurs in patients with hypoalbuminemia. The most common causes of hypoalbuminemia are malnutrition (especially in alcoholics) and large-volume intravenous infusions. Because one half of the calcium is bound to albumin, when albumin is low, calcium should be expected to be low. Large blood transfusions are associated with low serum calcium levels, because the citrate additives used in banked blood for anticoagulation bind the free calcium in the recipient's bloodstream. Intestinal malabsorption, renal failure, rhabdomyolysis, alkalosis, and acute pancreatitis (because of saponification of fat) are also known to be associated with low serum calcium levels. Hypomagnesemia can be associated with refractory hypocalcemia. Symptoms of hypocalcemia include nervousness, excitability, and tetany.
Interfering Factors
• Vitamin D intoxication may cause increased calcium levels.
• Excessive ingestion of milk may cause increased levels.
• Serum pH can affect calcium values. A decrease in pH causes increased calcium levels.
• Prolonged tourniquet time will lower pH and factitiously increase calcium levels.
• There is normally a small diurnal variation in calcium, with peak levels occurring around 9 PM.
• Hypoalbuminemia is associated with decreased levels of total calcium.
![]() Drugs that may cause increased levels include alkaline antacids, androgens, calcium salts, ergocalciferol, hydralazine, lithium, PTH, thiazide diuretics, thyroid hormone, and vitamin D.
Drugs that may cause increased levels include alkaline antacids, androgens, calcium salts, ergocalciferol, hydralazine, lithium, PTH, thiazide diuretics, thyroid hormone, and vitamin D.
![]() Drugs that may cause decreased levels include acetazolamide, albuterol, anticonvulsants, asparaginase, aspirin, calcitonin, cisplatin, corticosteroids, estrogens, heparin, laxatives, loop diuretics, magnesium salts, oral contraceptives, and thiazide diuretic.
Drugs that may cause decreased levels include acetazolamide, albuterol, anticonvulsants, asparaginase, aspirin, calcitonin, cisplatin, corticosteroids, estrogens, heparin, laxatives, loop diuretics, magnesium salts, oral contraceptives, and thiazide diuretic.
Test Results and Clinical Significance
 Increased Levels (Hypercalcemia)
Increased Levels (Hypercalcemia)
Nonparathyroid PTH-producing tumor (e.g., lung or renal carcinoma):
Milk-alkali syndrome: With increased ingestion of milk products or antacids (which contain calcium), the serum calcium level can be elevated.
Vitamin D intoxication: Vitamin D works synergistically with PTH to increase serum calcium.
Granulomatous infections such as sarcoidosis and tuberculosis:
These diseases are associated with enhanced levels of vitamin D, which works synergistically with PTH to increase serum calcium.
Addison disease: Glucocorticosteroids inhibit vitamin D activity. When steroid activity is decreased, vitamin D action is enhanced. Vitamin D works synergistically with PTH to increase serum calcium.
 Decreased Levels (Hypocalcemia)
Decreased Levels (Hypocalcemia)
Hypoparathyroidism: PTH acts to increase serum calcium. If PTH levels are reduced, serum calcium declines.
Hyperphosphatemia secondary to renal failure:
Vitamin D acts synergistically with PTH. PTH acts to increase serum calcium. Without that synergism, calcium levels decline.
Pancreatitis is associated with saponification (binding of calcium to fats) of the peripancreatic tissue. This reduces the calcium from the blood.
Alkalosis: High pH in the blood drives the calcium to intracellular spaces. Blood levels decline.
Related Tests
Parathyroid Hormone (p. 380). This is a measurement of PTH, which increases serum calcium levels.
Albumin (p. 424). This is a direct measurement of serum albumin. Albumin has a major effect on serum calcium metabolism.
Vitamin D (p. 520). Adequate levels of this vitamin are required for calcium absorption.
Carbon Dioxide Content (CO2 Content, CO2-Combining Power, Bicarbonate [HCO−3])
Indications
The CO2 content is a measure of CO2 in the blood. In the peripheral venous blood this is used to assist in evaluating the pH status of the patient and to assist in evaluation of electrolytes.
Test Explanation
The serum CO2 test is usually included with other electrolyte assessments. It is usually performed using a multiphasic testing machine that also measures sodium, potassium, chloride, blood urea nitrogen (BUN), and creatinine. It is important not to confuse this test with Pco2. This CO2 content measures H2CO3, dissolved CO2 and the bicarbonate ion ( ) that exists in the serum. Because the amounts of H2CO3 and dissolved CO2 in the blood are so small, CO2 content is an indirect measure of
) that exists in the serum. Because the amounts of H2CO3 and dissolved CO2 in the blood are so small, CO2 content is an indirect measure of  anion.
anion.  anion is second in importance to the chloride ion in electrical neutrality (negative charge) of extracellular and intracellular fluid; it plays a major role in acid-base balance.
anion is second in importance to the chloride ion in electrical neutrality (negative charge) of extracellular and intracellular fluid; it plays a major role in acid-base balance.
Levels of  are regulated by the kidneys. Increases occur with alkalosis, and decreases occur with acidosis. This test can be performed on arterial blood as discussed further on p. 109. When CO2 content is measured in the laboratory with other serum electrolytes, air affects the specimen and the CO2 partial pressure can be altered. Therefore venous blood specimens are not highly accurate for measuring true CO2 content or
are regulated by the kidneys. Increases occur with alkalosis, and decreases occur with acidosis. This test can be performed on arterial blood as discussed further on p. 109. When CO2 content is measured in the laboratory with other serum electrolytes, air affects the specimen and the CO2 partial pressure can be altered. Therefore venous blood specimens are not highly accurate for measuring true CO2 content or  . It is primarily used as a rough guide for acid-base balance.
. It is primarily used as a rough guide for acid-base balance.
Interfering Factors
• Underfilling the tube with blood allows CO2 to escape from the serum specimen and may significantly reduce  values.
values.
![]() Drugs that may cause increased serum CO2 and
Drugs that may cause increased serum CO2 and  levels include aldosterone, barbiturates, bicarbonates, ethacrynic acid, hydrocortisone, loop diuretics, mercurial diuretics, and steroids.
levels include aldosterone, barbiturates, bicarbonates, ethacrynic acid, hydrocortisone, loop diuretics, mercurial diuretics, and steroids.
![]() Drugs that may cause decreased levels include methicillin, nitrofurantoin (Furadantin), paraldehyde, phenformin, tetracycline, thiazide diuretics, and triamterene.
Drugs that may cause decreased levels include methicillin, nitrofurantoin (Furadantin), paraldehyde, phenformin, tetracycline, thiazide diuretics, and triamterene.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Chronic obstructive pulmonary disease (COPD): 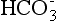 ions are increased to compensate for chronic hypoventilation (high Pco2). This is compensation for respiratory acidosis.
ions are increased to compensate for chronic hypoventilation (high Pco2). This is compensation for respiratory acidosis.
Metabolic alkalosis: Metabolic alkalosis is defined by an increased amount of 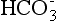 anions in the blood.
anions in the blood.
Related Test
Arterial Blood Gases (ABGs) (p. 109). This is a battery of arterial blood tests that are used to evaluate acid-base status. CO2 content and  are components of that test.
are components of that test.
Carboxyhemoglobin (COHb, Carbon Monoxide [CO])
Test Explanation
This test measures the amount of serum COHb, which is formed by the combination of CO and hemoglobin (Hgb). CO combines with Hgb 200 times more readily than O2 can combine with Hgb (oxyhemoglobin). This results in fewer Hgb bonds available to combine with O2. Furthermore, when CO occupies the O2-binding sites, the hemoglobin molecule is changed so as to bind the remaining O2 more tightly. This greater affinity of CO for Hgb and change in O2-binding strength does not allow the O2 to readily pass from the red blood cells (RBCs) to the tissue. Less O2 is therefore available for tissue cell respiration. This results in hypoxemia. CO poisoning is documented by Hgb analysis for COHb. A specimen should be drawn as soon as possible after exposure, because CO is rapidly cleared from the Hgb by breathing normal air. O2 saturation studies and oximetry are inaccurate in CO-exposed patients, because they measure all forms of O2-saturated hemoglobin, including COHb. In these circumstances the results will be normal, even though the patient is hypoxemic.
This test can also be used to evaluate patients with complaints of headache, irritability, nausea, vomiting, and vertigo, who may have been unknowingly exposed to CO. Its greatest use, however, is in patients exposed to smoke inhalation, exhaust fumes, and fires. Other sources of CO include tobacco smoke, petroleum and natural gas fuel fumes, automobile exhaust, unvented natural-gas heaters, and defective gas stoves. Symptoms of CO poisoning correlated with blood levels are shown in Table 2-12. CO toxicity is treated by administering high concentrations of O2 to displace the COHb.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient or the family.
Explain the procedure to the patient or the family.
• Obtain the patient history for possible source of CO inhalation.
• Assess the patient for signs and symptoms of mild CO toxicity (e.g., headache, weakness, dizziness, malaise, dyspnea) and moderate to severe CO toxicity (e.g., severe headache, bright-red mucous membranes, cherry-red blood). Maintain patient safety precautions if confusion is present.
After
• Apply pressure or a pressure dressing to the venipuncture site.
• Assess the venipuncture site for bleeding.
• Treat the patient as indicated by the physician. Usually the patient receives high concentrations of O2. Severe CO toxicity may be treated with hyperbaric oxygen.
• Encourage respirations to allow the patient to clear CO from the Hgb.
Carcinoembryonic Antigen (CEA)
Indications
This tumor marker is used for determining the extent of disease and prognosis in patients with cancer (especially gastrointestinal [GI] or breast). It is also used in monitoring the disease and its treatment.
Test Explanation
CEA is a protein that normally occurs in fetal gut tissue. By birth, detectable serum levels disappear. In the early 1960s, CEA was found to exist in the bloodstream of adults who had colorectal tumors. It was originally thought to be a specific indicator of the presence of colorectal cancer. Subsequently, however, this tumor marker has been found in patients who have a variety of carcinomas (e.g., breast, pancreatic, gastric, hepatobiliary), sarcomas, and even many benign diseases (e.g., ulcerative colitis, diverticulitis, cirrhosis). Chronic smokers also have elevated CEA levels.
Because the CEA level can be elevated in both benign and malignant diseases, it is not a specific test for colorectal cancer. Furthermore, not all colorectal cancers produce CEA. Therefore CEA is not a reliable screening test for the detection of colorectal cancer in the general population. Its use is limited to determining the prognosis and monitoring the response of tumor to antineoplastic therapy in a patient with cancer. This is especially helpful in patients with breast and GI cancers. The initial pretreatment CEA level is an indicator of tumor burden and therefore prognosis. Patients with smaller and early stage tumors are likely to have low, if not normal, CEA levels. Patients with more advanced or metastatic tumors are likely to have high CEA levels. A drastic reduction of the preoperative CEA to normal levels indicates complete eradication of the tumor. Therefore this test is used to determine the efficacy of treatment.
This test also is used in the surveillance of patients with cancer. A steadily rising CEA level is occasionally the first sign of tumor recurrence. This makes CEA testing very valuable in the follow-up of patients who have already had potentially curative therapy. It is important to reiterate that many (about 20%) patients with advanced breast or GI tumors may not have elevated CEA levels.
CEA can also be detected in body fluids other than blood. Its presence in those body fluids indicates metastasis. This test is commonly performed on peritoneal fluid or chest effusions. Elevated CEA levels in these fluids indicate metastasis to the peritoneum or pleura, respectively. Likewise, elevated CEA levels in the cerebrospinal fluid (CSF) indicate central nervous system (CNS) metastasis.
Interfering Factors
• Smokers tend to have higher CEA levels than nonsmokers.
• Benign diseases (e.g., cholecystitis, colitis, diverticulitis) and especially liver diseases (e.g., hepatitis, cirrhosis) are also associated with elevated CEA levels.
• Results may vary considerably depending on the method used for quantification. Because of this, results from different laboratories cannot be compared or interchangeably interpreted.
Test Results and Clinical Significance
Cell Surface Immunophenotyping (Flow Cytometry Cell Surface Immunophenotyping, Lymphocyte Immunophenotyping, AIDS T-Lymphocyte Cell Markers, CD4 Marker, CD4/CD8 Ratio, CD4 Percentage)
Indications
This test is used to detect the progressive depletion of CD4 T lymphocytes, which is associated with an increased likelihood of clinical complications from acquired immunodeficiency syndrome (AIDS). Test results can indicate if a patient with AIDS is at risk for developing opportunistic infections. It is also used to confirm the diagnosis of acute myelocytic leukemia (AML) and to differentiate AML from acute lymphocytic leukemia (ALL).
Test Explanation
All lymphocytes originate from reticulum cells in the bone marrow. Normal hematopoietic cells undergo changes in expression of cell surface markers as they mature from stem cells into cells of a committed lineage. Monoclonal antibodies have been developed that react with lymphoid and myeloid glycoprotein antigens on the cell surface of peripheral blood cells. One kind of lymphocyte that matures in the bone marrow is called a B lymphocyte. B lymphocytes provide humoral immunity (produce antibodies). A second type of lymphocyte matures in the thymus and is called a T lymphocyte. T lymphocytes are responsible for cellular immunity. Finally, there is a group of lymphocytes that has neither T nor B markers. These are called “natural killer cells” and will chemically attack foreign or cancer cells without prior sensitization. Monoclonal antibodies against cell-surface markers are used to identify the various forms of lymphocytes. The absolute numbers and percentages are then counted using flow cytometry. This can be performed on blood or on cell suspensions of tissue.
CD4 helper cells and CD8 cells are examples of T-lymphocytes. T-lymphocytes, and especially CD4 counts, when combined with HIV RNA viral load testing (p. 294), are used to determine the time to initiate antiviral therapy. They also can be used to monitor antiviral therapy. Successful antiviral therapy is associated with an increase in CD4 counts. Worsening of disease or unsuccessful therapy is associated with decreasing T-lymphocyte counts.
There are three related measurements of CD4 T lymphocytes. The first measurement is the total CD4-cell count. This is measured in whole blood and is the product of the WBC count, the lymphocyte differential count, and the percentage of lymphocytes that are CD4 T cells. The second measurement, the CD4 percentage, is a more accurate prognostic marker. It measures the percentage of CD4 lymphocytes in the whole blood sample by combining immunophenotyping with flow cytometry. This procedure relies on detecting specific antigenic determinants on the surface of the CD4 lymphocyte by antigen-specific monoclonal antibodies labeled with a fluorescent dye. The third prognostic marker, which is also more reliable than the total CD4 count, is the ratio of CD4 (T-helper) cells to CD8 (T-suppressor) cells.
Of the three T-cell measurements, the total CD4 count is the most variable. There is substantial diurnal variation in this count. Because it is a calculated measurement, the combination of possible laboratory error and personal fluctuation can result in wide variations in test results. With the CD4 percentage and CD4/CD8 ratios, very little diurnal variation and laboratory error exist. The Multicenter AIDS Cohort Study suggests that the latter two measurements are more accurate than the total CD4 count. However, because the total CD4-cell count was originally thought to be the best marker, this test was used in many of the studies that now form the basis for practice recommendations. It will take time before the more accurate measurements find clinical pertinence in practice recommendations.
The pathogenesis of AIDS is largely attributed to a decrease in the T lymphocyte that bears the CD4 receptor. Progressive depletion of CD4 T lymphocytes is associated with an increased likelihood of clinical complications from AIDS. Therefore CD4 measurement is a prognostic marker that can indicate whether a patient infected with human immunodeficiency virus (HIV) is at risk for developing opportunistic infections. The measurement of CD4-cell levels is used to decide whether to initiate Pneumocystis jiroveci pneumonia prophylaxis and antiviral therapy and for determining the prognosis of patients with HIV infection.
Both immunodeficiency and the dosage of immunosuppressive medications used after organ transplant are also monitored with the use of this cell surface immunophenotyping. Lymphomas and other lymphoproliferative diseases are now classified and treated according to the predominant lymphocyte type identified. In some instances, the prognosis of these diseases depends on this lymphocyte phenotyping.
The U.S. Public Health Service has recommended that CD4 prognostic markers be monitored every 3 to 6 months in all persons infected with HIV. Because the CD4 counts gradually fall in virtually all such patients, periodic review of the count can be emotionally stressful for both the patient and physician. The patient confronts his or her mortality as the health care provider confronts his or her ultimate powerlessness against the relentlessly advancing infections.
As the CD4-cell measurements decrease, the probability of developing AIDS increases. Forty-eight percent of patients can be expected to develop AIDS within 6 months when their CD4 count is less than 100 cells/mm3. It is recommended that antiviral therapy be started in patients whose CD4 count is less than 500 to 600 cells/mm3. P. jiroveci pneumonia prophylaxis should be started when the CD4 count is less than 200 to 300 cells/mm3.
CD4 prognostic markers also can be useful in guiding the approach to the patient's symptoms. Complaints such as cough and headache are common in most people; however, in patients infected with HIV, these symptoms often raise concerns about opportunistic infections. If the CD4 cell count exceeded 500 cells/mm3 in the past 6 months, there is a very low probability that these symptoms result from opportunistic infections. Knowing this, the patient and physician can feel comfortable with routine care.
Flow cytometry is able to analyze thousands of cells in less than a minute. The flow cytometer has three components in testing: an optical, a fluid, and an electronic system. The optical system consists of an argon laser that emits a single wavelength of light at 488 nm (blue region). Cells are labeled with one of several fluorochromes, including fluorescein (FITC), phycoerythrin (PE), and peridinin-chlorophyll protein (PCP), as a result of monoclonal antibody–fluorochrome conjugate binding to a specific blood cell. The fluorochromes are excited by the laser and emit green (FITC), orange (PE), and red (PCP) light that is measured through optical filters designed to capture their specific wavelength. The fluid system introduces the fluorochrome-bound cells in suspension into a pressurized sheath of fluid that travels through a clear cuvette. The laser light intersects a stream of cells that pass single file through the cuvette. The electronic system measures electronic signals from the detectors that provide measures of the magnitude of fluorescence intensity and the extent of light scatter associated with each cell as it passes through the laser. Most clinical flow cytometers measure five parameters on each cell: two nonfluorescence measures (magnitude of forward and side scatter) and three fluorescence measures (green, orange, and red light intensity). Multiple markers can be used simultaneously to identify different cell populations.
By using a combination of monoclonal antibodies recognizing B-cell, T-cell, and myeloid antigens, it is possible to confirm the diagnosis of acute myelocytic leukemia (AML) and to differentiate AML from acute lymphocytic leukemia (ALL) if morphology and traditional immunohistochemistry are inconclusive (<15% of cases). It is also helpful in identifying mixed patterns of leukemia that may affect prognosis and treatment. Furthermore, flow cytometric cell surface immunophenotyping is extremely helpful in differentiating various forms of immunodeficiency diseases.
Interfering Factors
• Although diurnal variation is usually of no significance, it may have some impact when counts are low. Higher counts can be expected in the late morning hours.
• A recent viral illness can decrease total T-lymphocyte counts.
• Nicotine and very strenuous exercise have been shown to decrease lymphocyte counts. However, such data are now being questioned.
Procedure and Patient Care
During
• Record the time of day when the blood specimen is obtained.
• Observe universal body and blood precautions. Wear gloves when handling blood products from all patients.
• Never recap needles. Dispose of needles and syringes in a puncture-proof container.
• Collect a venous blood sample in a large green-top tube (containing sodium heparin).
• Collect a venous blood sample in a small lavender-top tube (containing ethylene diamine tetraacetic acid).
After
• Keep the specimen at room temperature. Do not refrigerate.
• The specimen must be evaluated within 24 hours.
• Often specimens are sent to a central laboratory.
• Apply pressure or a pressure dressing to the venipuncture site.
• Assess the puncture site for bleeding.
![]() Instruct the patient to observe the venipuncture site for infection. Patients with AIDS or organ recipients are immunocompromised and susceptible to infection.
Instruct the patient to observe the venipuncture site for infection. Patients with AIDS or organ recipients are immunocompromised and susceptible to infection.
![]() Encourage the patient to discuss his or her concerns regarding the prognostic information that may be provided by these results.
Encourage the patient to discuss his or her concerns regarding the prognostic information that may be provided by these results.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
These patients can be expected to have increased B-lymphocyte counts in their tumor tissue or in their peripheral blood.
T-cell lymphoma: These patients can be expected to have increased T-lymphocyte counts in their tumor tissue or in their peripheral blood (if their bone marrow is heavily involved with tumor).
 Decreased Levels
Decreased Levels
Organ transplant patients: A decreased lymphocyte count is expected and desirable for immunosuppression of organ rejection.
HIV-positive patients: When CD4 counts are below 200/mm3, the patient is at increased risk for clinical symptoms from AIDS and the opportunistic infections that accompany this disease.
Congenital immunodeficiency: Children with DiGeorge syndrome and thymic hypoplasia will have decreased or no B lymphocytes.
Ceruloplasmin (Cp)
Indications
This test is an acute-phase reactant protein and can indicate an acute illness. However, its primary use is in the diagnosis of preclinical states of Wilson disease.
Test Explanation
Cp is an alpha2-globulin that binds copper for transport within the bloodstream after it is absorbed from the gastrointestinal (GI) tract. Levels are decreased in most instances of Wilson disease, which is an inherited disorder. Patients who are homozygous for this disease make very little Cp. High unbound copper blood levels result and are toxic to tissues. The copper is deposited in the eye, brain, liver, and kidney. Wilson disease is fatal unless early treatment is instituted. If this disease is identified before significant copper deposits affect major organs, the ravages of the disease can be avoided. Cp levels are obtained in children at high risk for the disease. Teenagers and young adults with hepatitis, cirrhosis, or recurrent neuromuscular incoordination (signs compatible with Wilson disease) should also have this test. Early detection is important, because therapy is effective in most cases.
Cp is also an acute-phase reactant protein that becomes elevated during stress, infection, and pregnancy. However, it rises more slowly than other acute-phase reactants, such as C-reactive protein and erythrocyte sedimentation rate.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Acute inflammatory reaction (e.g., infection, rheumatoid arthritis [RA]),
Copper intoxication: Copper elevation will stimulate Cp in unaffected individuals.
 Decreased Levels
Decreased Levels
Wilson disease: Patients with this disease have homozygous or heterozygous genes and are unable to make Cp. Homozygous patients have lower Cp levels than heterozygous patients.
Normal infants (6 months): Young infants normally are unable to make adequate amounts of acute-phase reactant proteins (alpha2-globulins) until they are 6 months of age.
These are protein-losing diseases. Cp is a protein that is lost in these diseases and therefore blood levels fall.
Menkes (kinky-hair) syndrome: This is an inherited disorder associated with defects in the production of alpha2-globulins such as Cp.
Chloride, Blood (Cl)
Indications
This test is performed as a part of multiphasic testing for what is usually called “electrolytes.” By itself, this test does not provide much information. However, with interpretation of the other electrolytes, chloride can give an indication of acid-base balance and hydration status.
Test Explanation
Chloride is the major extracellular anion. Its primary purpose is to maintain electrical neutrality, mostly as a salt with sodium. It follows sodium (cation) losses and accompanies sodium excesses in an attempt to maintain electrical neutrality. For example, when aldosterone encourages sodium reabsorption, chloride follows to maintain electrical neutrality. Because water moves with sodium and chloride, chloride also affects water balance. Finally, chloride also serves as a buffer to assist in acid-base balance. As carbon dioxide (and H cations) increases, bicarbonate must move from the intracellular space to the extracellular space. To maintain electrical neutrality, chloride will shift back into the cell.
Hypochloremia and hyperchloremia rarely occur alone and usually are part of parallel shifts in sodium or bicarbonate levels. Signs and symptoms of hypochloremia include hyperexcitability of the nervous system and muscles, shallow breathing, hypotension, and tetany. Signs and symptoms of hyperchloremia include lethargy, weakness, and deep breathing.
Interfering Factors
• Excessive infusions of saline solution can result in increased chloride levels.
![]() Drugs that may cause increased serum chloride levels include acetazolamide, ammonium chloride, androgens, chlorothiazide, cortisone preparations, estrogens, guanethidine, hydrochlorothiazide, methyldopa, and nonsteroidal antiinflammatory drugs.
Drugs that may cause increased serum chloride levels include acetazolamide, ammonium chloride, androgens, chlorothiazide, cortisone preparations, estrogens, guanethidine, hydrochlorothiazide, methyldopa, and nonsteroidal antiinflammatory drugs.
![]() Drugs that may cause decreased levels include aldosterone, bicarbonates, corticosteroids, cortisone, hydrocortisone, loop diuretics, thiazide diuretics, and triamterene.
Drugs that may cause decreased levels include aldosterone, bicarbonates, corticosteroids, cortisone, hydrocortisone, loop diuretics, thiazide diuretics, and triamterene.
Test Results and Clinical Significance
 Increased Levels (Hyperchloremia)
Increased Levels (Hyperchloremia)
 Decreased Levels (Hypochloremia)
Decreased Levels (Hypochloremia)
Syndrome of inappropriate secretion of antidiuretic hormone (SIADH):
Congestive heart failure: Chloride is retained with sodium retention but is diluted by excess total body water.
Vomiting or prolonged gastric suction,
Chronic diarrhea or high-output gastrointestinal (GI) fistula:
Chloride is driven into the cell to compensate for the 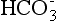 that leaves the cell to maintain pH neutrality.
that leaves the cell to maintain pH neutrality.
Burns: Sodium and chloride losses from the massive burn can be great.
Cholesterol
Indications
Cholesterol testing is used to determine the risk for coronary heart disease (CHD). It is also used for evaluation of hyperlipidemias.
Test Explanation
Cholesterol is the main lipid associated with arteriosclerotic vascular disease. Cholesterol, however, is required for the production of steroids, sex hormones, bile acids, and cellular membranes. Most of the cholesterol we eat comes from foods of animal origin. The liver metabolizes the cholesterol to its free form, and cholesterol is transported in the bloodstream by lipoproteins (see p. 342). Nearly 75% of the cholesterol is bound to low-density lipoproteins (LDL), and 25% is bound to high-density lipoproteins (HDLs). Cholesterol is the main component of LDL and only a minimal component of HDL and very-low-density lipoprotein (VLDL). It is the LDL that is most directly associated with increased risk for CHD.
The purpose of cholesterol testing is to identify patients at risk for arteriosclerotic heart disease. Cholesterol testing is usually done as a part of a lipid profile, which evaluates lipoproteins and triglycerides (see pp. 342 and 504), because, by itself, cholesterol is not a totally accurate predictor of heart disease. There is considerable overlap in what are considered “normal” and “high-risk” levels. “Normal” levels have been derived from a group of patients who have no obvious evidence of CHD. However, this may not be accurate because these patients may have preclinical CHD and may not truly reflect a “no-risk” population.
There is considerable variation in cholesterol levels. Day-to-day cholesterol values in the same individual can vary by 15%. An 8% difference can even be identified within the same day. Positional changes can affect these levels. Levels can decrease by as much as 15% in the recumbent position. As a result, hospitalized patients can be expected to have lower levels than outpatients. Because of these significant variabilities, elevated results should be corroborated by repeating the study. The two results should be averaged to obtain an accurate cholesterol level for risk assessment.
Because the liver is required to metabolize ingested cholesterol products, subnormal cholesterol levels are indicative of severe liver diseases. Furthermore, because our main source of cholesterol is our diet, malnutrition is also associated with low cholesterol levels. Certain illnesses can affect cholesterol levels. For example, patients with an acute myocardial infarction (AMI) may have as much as a 50% reduction in cholesterol level for as long as 6 to 8 weeks.
Total cholesterol is used most accurately as a predictor of the risk for CHD when studied as part of the updated Framingham Coronary Prediction algorithm. This prediction model is used to determine a person's risk for developing an ischemic event (angina, myocardial infarction, or myocardial death) over the course of the following decade. Besides cholesterol, other factors used to estimate risk for CHD include age, lipoproteins, blood pressure, cigarette smoking history, diabetes mellitus, and gender.
This risk model uses a system whereby points are given for each factor in the model (Boxes 2-5 and 2-6). The total number of points is used to provide the patient's CHD risk. By dividing the CHD risk by age-related data (comparative risk), a risk relative to peers can be calculated. The CHD risk can be used to determine whether or not medicinal cholesterol lowering intervention is indicated.
Familial hyperlipidemias and hyperlipoproteinemias are often associated with high cholesterol.
Interfering Factors
• Pregnancy is usually associated with elevated cholesterol levels.
• Oophorectomy and postmenopausal status are associated with increased levels.
• Recumbent position is associated with decreased levels.
![]() Drugs that may cause increased levels include adrenocorticotropic hormone, anabolic steroids, beta-adrenergic blocking agents, corticosteroids, cyclosporine, epinephrine, oral contraceptives, phenytoin (Dilantin), sulfonamides, thiazide diuretics, and vitamin D.
Drugs that may cause increased levels include adrenocorticotropic hormone, anabolic steroids, beta-adrenergic blocking agents, corticosteroids, cyclosporine, epinephrine, oral contraceptives, phenytoin (Dilantin), sulfonamides, thiazide diuretics, and vitamin D.
![]() Drugs that may cause decreased levels include allopurinol, androgens, bile salt-binding agents, captopril, chlorpropamide, clofibrate, colchicine, colestipol, erythromycin, isoniazid, liothyronine (Cytomel), monoamine oxidase inhibitors, niacin, nitrates, and statins.
Drugs that may cause decreased levels include allopurinol, androgens, bile salt-binding agents, captopril, chlorpropamide, clofibrate, colchicine, colestipol, erythromycin, isoniazid, liothyronine (Cytomel), monoamine oxidase inhibitors, niacin, nitrates, and statins.
Procedure and Patient Care
Before
![]() Instruct the patient to fast 12 to 14 hours after eating a low-fat meal before testing. Only water is permitted. Actually, cholesterol levels increase only minimally after a meal. However, early-morning fasting cholesterol levels are more easily compared when performed serially. Furthermore, lipoproteins, which are often ordered simultaneously, are affected by a previous meal. Therefore fasting is usually requested.
Instruct the patient to fast 12 to 14 hours after eating a low-fat meal before testing. Only water is permitted. Actually, cholesterol levels increase only minimally after a meal. However, early-morning fasting cholesterol levels are more easily compared when performed serially. Furthermore, lipoproteins, which are often ordered simultaneously, are affected by a previous meal. Therefore fasting is usually requested.
![]() Indicate to the patient that dietary intake for 2 weeks before testing will affect results. It is suggested that the patient eat a normal diet for at least 1 week before testing.
Indicate to the patient that dietary intake for 2 weeks before testing will affect results. It is suggested that the patient eat a normal diet for at least 1 week before testing.
![]() Tell the patient that no alcohol should be consumed within 24 hours before the test.
Tell the patient that no alcohol should be consumed within 24 hours before the test.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Familial hypercholesterolemia,
Increased cholesterol levels are associated with hypothyroidism, uncontrolled diabetes mellitus, nephrotic syndrome, pregnancy, high-cholesterol diet, xanthomatosis, hypertension, myocardial infarction (MI), atherosclerosis, biliary cirrhosis, and extrahepatic biliary occlusion, stress, and nephrotic syndrome: The pathophysiology of the association of cholesterol with these diseases is not well known. The association has been made by observation.
 Decreased Levels
Decreased Levels
Most of the cholesterol is synthesized from fat eaten in the diet. When dietary intake is decreased, fat levels and subsequently cholesterol levels fall.
Decreased levels of cholesterol are also associated with hyperthyroidism, cholesterol-lowering medication, pernicious anemia, hemolytic anemia, sepsis/stress, liver disease, and acute MI (AMI): The pathophysiology of the association of cholesterol with these diseases is not well known. The association has been made by observation.
Related TesTs
Apolipoproteins (p. 106). These polypeptides are associated with lipoproteins and have been used as accurate indicators of risk for CHD.
Lipoprotein (p. 342). HDL and LDL play an important role in the transport of lipids in the bloodstream. They, too, have been used in the assessment of risk for CHD.
Triglycerides (p. 504). This test is a measure of total triglycerides in the blood. It is a part of the lipid profile.