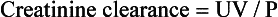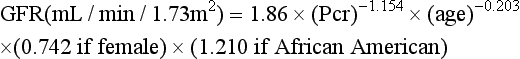Cholinesterase (CHS, Pseudocholinesterase [PChE], Cholinesterase RBC, Red Blood Cell Cholinesterase, Acetylcholinesterase)
Indications
This test is done to identify patients with PChE deficiency before anesthesia or to identify those who may have been exposed to phosphate poisoning.
Test Explanation
Cholinesterases hydrolyze acetylcholine and other choline esters and thereby regulate nerve impulse transmission at the nerve synapse and neuromuscular junction. There are two types of cholinesterases: acetylcholinesterase, also known as true cholinesterase, and PChE. True cholinesterase exists primarily in the red blood cells and nerve tissue. It is not in the serum. PChE, on the other hand, exists in the serum. Deficiencies in either of these enzymes can be acquired or congenital.
Because succinylcholine (the most commonly used muscle relaxant during anesthesia induction) is inactivated by PChE, people with an inherited PChE enzyme deficiency exhibit increased and/or prolonged effects of succinylcholine. Patients with a genetic variant of PChE may have a nonfunctioning form of PChE and will also experience prolonged effects of succinylcholine administration. Prolonged muscle paralysis and apnea will occur after anesthesia in these patients. This situation can be avoided by measuring serum cholinesterase (PChE) in all patients with a family history of prolonged apnea after surgery.
Because patients with a nonfunctioning variant of PChE will have normal total quantitative PChE levels yet still have prolonged paralytic effects of succinylcholine, a second test (dibucaine inhibition) usually is also performed. Dibucaine is a known local anesthetic that inhibits the function of normal PChE. The dibucaine inhibition number (DN) is the percent of PChE activity that is inhibited when dibucaine is added to the patient's serum sample. If total PChE is normal and DNs are low, the presence of a nonfunctioning PChE variant is suspected and the patient will be at risk for succinylcholine-induced prolonged paralysis. Decreased PChE enzyme activity in conjunction with a DN less than 30 suggests high risk for prolonged paralysis. Normal to decreased PChE enzyme activity in conjunction with a DN 30-79 suggests variable risk. Phenotype interpretation (homozygote or various types of heterozygosity) is based on the total PChE activity and the percent of inhibition caused by dibucaine.
A common form of acquired cholinesterase deficiency, either true or PChE, is caused by overexposure to pesticides, organophosphates, or nerve gas. The half-life of the pseudoenzyme in serum is about 8 days, and the “true” cholinesterase (AChE) of red cells is more than 3 months (determined by erythropoietic activity). Recent exposure (up to several weeks) is determined by assay of the pseudoenzyme and months after exposure by measurement of the red cell enzyme. Persons with jobs associated with chronic exposure to these chemicals are often monitored by the frequent testing of RBC cholinesterase activity. Other potential causes of reduced cholinesterase activity include chronic liver diseases, malnutrition, and hypoalbuminemia.
Increased cholinesterase activity, when found in the amniotic fluid, represents strong evidence for a neural tube defect (NTD). When an NTD is suspected based upon maternal serum alpha-fetoprotein (AFP) screening results or diagnosed via ultrasound, analysis of AFP and acetylcholinesterase (AChE) in amniotic fluid are useful diagnostic tools.
Interfering Factors
• Pregnancy decreases test values.
• It is important to recognize that pseudocholinesterase levels cannot be measured in postoperative patients in the recovery room if the patient is not regaining muscular function, because often one or more of the above drugs may be given during the surgery and could invalidate the results.
![]() Drugs that may cause decreased values include atropine, caffeine, codeine, estrogens, morphine sulfate, neostigmine, oral contraceptives, phenothiazines, quinidine, theophylline, steroids, and vitamin K.
Drugs that may cause decreased values include atropine, caffeine, codeine, estrogens, morphine sulfate, neostigmine, oral contraceptives, phenothiazines, quinidine, theophylline, steroids, and vitamin K.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Tell the patient that no fasting is required.
Tell the patient that no fasting is required.
• If the test is done to identify the presurgical patient who may be at risk for cholinesterase deficiency, be sure the test is done several days before the planned surgery.
• It may be recommended to withhold medications that could alter test results for 12 to 24 hours before the test.
Chromosome Karyotype (Blood Chromosome Analysis, Chromosome Studies, Cytogenetics, Karyotype)
Indications
This test is used to study an individual's chromosome makeup to determine chromosomal defects associated with disease or the risk for developing disease.
Test Explanation
The term “karyotyping” refers to the arrangement of cell chromosomes from the largest to the smallest to analyze their number and structure. Variations in either can produce numerous developmental abnormalities and diseases. A normal karyotype of chromosomes consists of a pattern of 22 pairs of autosomal chromosomes and a pair of sex chromosomes: XY for the male and XX for the female. Chromosomal karyotype abnormalities can be congenital or acquired. These karyotype abnormalities can occur because of duplication, deletion, translocation, reciprocation, or genetic rearrangement.
Chromosome karyotyping is useful in evaluating congenital anomalies, mental retardation, growth retardation, delayed puberty, infertility, hypogonadism, primary amenorrhea, ambiguous genitalia, chronic myelogenous leukemia, neoplasm, recurrent miscarriage, prenatal diagnosis of serious congenital diseases (especially when advanced maternal age is a factor), Turner syndrome, Klinefelter syndrome, Down syndrome, and other suspected genetic disorders. The products of conception also can be studied to determine the cause of stillbirth or miscarriage.
The most common form of karyotyping is performed by banding techniques. This technique provides a method of pairing similar chromosomes based on their size, location of the centromere (constriction that divides the chromosome into long and short arms), and other constrictions, ratio of long to short arms, satellite deoxyribonucleic acid (DNA), and banding patterns. With this method, a characteristic karyotype is determined. An extensive nomenclature system for the types has been developed.
Special chromosome studies can be performed on cells grown in special medium to identify certain chromosome abnormalities. DNA testing is now possible through the use of DNA probes and DNA linkage studies.
Procedure and Patient Care
During
• Specimens for chromosome analysis can be obtained from numerous sources. Leukocytes from a peripheral venipuncture site are the most easily obtained and most often used for this study.
• Bone marrow biopsies and surgical specimens also can sometimes be used as sources for analysis.
• During pregnancy, specimens can be collected by amniocentesis (see p. 632) and chorionic villus sampling (see p. 1088).
• Fetal tissue or products of conception can be studied as well to determine the reason for the loss of the fetus.
• Buccal mucosal cell specimens are less costly but not as accurate as other tissue for karyotyping.
After
• Aftercare depends on how the specimen was collected.
![]() Inform the patient that test results are generally not available for weeks to several months.
Inform the patient that test results are generally not available for weeks to several months.
• If an abnormality is identified, the entire family line may be tested. This can be exhaustive and expensive.
• If the test results show an abnormality, encourage the patient to verbalize his or her feelings. Provide emotional support.
Test Results and Clinical Significance
Abnormal Findings
Chromosome abnormalities can be a cause of congenital anomalies, mental retardation, growth retardation, delayed puberty, infertility, hypogonadism, primary amenorrhea, ambiguous genitalia, chronic myelogenous leukemia, neoplasm, recurrent miscarriage, Tay-Sachs disease, sickle cell anemia, Turner syndrome, Klinefelter syndrome, and Down syndrome. See Table 2-13 for some of the commonly known abnormalities.
TABLE 2-13
Common Chromosome Abnormalities
| Chromosome Abnormality | Clinical Manifestation or Syndrome |
| Trisomy 21 | Down |
| Single X | Turner |
| Extra X in male (XXY) | Klinefelter |
| 5 p deletion | Cat-cry |
| 15 q deletion | Prader-Willi |
| 3 q trisomy | Cornelia de Lange |
| Fragile X | Mental retardation |
| X centromere dislocation | Roberts |
| Philadelphia | Chronic myelogenous leukemia, acute myelogenous leukemia |
Related Tests
Barr Body Analysis. This is an inexpensive test for detecting chromatin material (X chromatin).
Genetic Testing (p. 1093). This is another method of DNA testing.
Coagulating Factor Concentration (Factor Assay, Coagulating Factors, Blood-Clotting Factors)
Indications
The coagulating factor concentration test measures the concentration of specific coagulating factors in the blood.
Test Explanation
These tests measure the quantity of each specific factor suspected to be responsible for suspected defects in hemostasis. Testing is available to measure the quantity of the factors listed in Table 2-14. When these factors exist in concentrations below their “minimal hemostatic level,” clotting will be impaired. These minimal hemostatic levels vary according to the factor involved.
TABLE 2-14
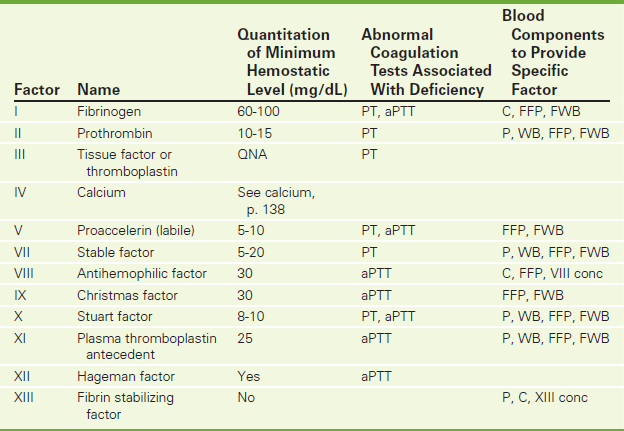
aPTT, Activated partial thromboplastin time; PT, prothrombin time; C, cryoprecipitate; FFP, fresh frozen plasma; FWB, fresh whole blood (less than 24 hours old); P, unfrozen banked plasma; WB, banked whole blood; VIII conc, factor VIII concentrate; XIII conc, factor XIII concentrate.
NOTE: Recombinant factors are now available for factor VII, VIII, IX, and XIII. Concentrates are also now available for II, VII, VIII, IX, and XIII.
Deficiencies of these factors may be a result of inherited genetic defects, acquired diseases, or drug therapy. Common medical conditions associated with abnormal factor concentrations are listed in Table 2-15). It is important to identify the exact factor or factors involved in the coagulating defect so that the appropriate blood component replacement can be administered (Table 2-14 and Figure 2-11).
TABLE 2-15
Conditions Associated With Abnormal Factor Concentrations
| Factor | Increased (excess) | Decreased (deficiency) |
| I (Fibrinogen) | Acute inflammatory reactions Trauma Coronary heart disease Cigarette smoking |
Liver disease (hepatitis or cirrhosis) DIC Congenital deficiency |
| II (Prothrombin) | ND | Vitamin K deficiency Liver disease Congenital deficiency Warfarin ingestion |
| V (Proaccelerin) | ND | Liver disease DIC Fibrinolysis |
| VII (Proconvertin [stable factor]) | ND | Congenital deficiency Vitamin K deficiency Liver disease Warfarin ingestion |
| VIII (Antihemophilic factor) | Acute inflammatory reactions Trauma/stress Pregnancy Birth control pills |
Congenital deficiency (e.g., Hemophilia A) DIC |
| von Willebrand factor | ND | Congenital deficiency (e.g., von Willebrand disease) Some myeloproliferative disorders |
| IX (Christmas factor) | ND | Congenital deficiency (e.g., Hemophilia B) Liver disease Nephrotic syndrome Warfarin ingestion DIC Vitamin K deficiency |
| X (Stuart factor) | ND | Congenital deficiency Liver disease Warfarin ingestion Vitamin K deficiency |
| XII (Hageman factor) | ND | Congenital deficiency Liver disease DIC |
DIC, Disseminated intravascular coagulation; ND, no common diseases states associated with excess of this factor.

Figure 2-11 Siemens automated hemostasis analyzer. This system can analyze multiple factors involved in hemostasis.
The hemostasis and coagulation system is a homeostatic balance between factors encouraging clotting and factors encouraging clot dissolution. The first reaction of the body to active bleeding is blood vessel constriction. In small-vessel injury this may be enough to stop bleeding. In large-vessel injury, hemostasis is required to form a clot that will durably plug the hole until healing can occur. The primary phase of the hemostatic mechanism involves platelet aggregation to the blood vessel (Figure 2-12). Secondary hemostasis then occurs. Secondary hemostasis can be broken down into a series of four reactions that culminate in the production of thrombin and fibrin. These act to create a blood clot at the site of vascular injury. In reaction one, sometimes called the intrinsic phase of coagulation, factor XII and other proteins form a complex on the subendothelial collagen in the injured blood vessel. Through a series of reactions, activated factor XI (XIa) is formed and activates factor IX (IXa). In a complex formed by factors VIII, IX, and X, activated X (Xa) is formed.

Figure 2-12 Secondary hemostasis (fibrin clot formation) and fibrinolysis (fibrin clot dissolution). Primary hemostasis involves platelet plugging of the injured blood vessel. Secondary hemostasis, as simply described here, takes place most rapidly on the platelet surface after attachment to the fractured endothelium. Four different reactions result in the formation of fibrin. As seen beneath the dark line in the figure, the fibrin clot supports the platelet clump so that the clot does not get swept away by the tremendous “shear forces” of the fast moving blood cells. Fibrinolysis follows formation of the fibrin clot in order to prevent complete occlusion of the injured blood vessel. Bold = Vitamin K–dependent coagulating factors.
At the same time, reaction two, the extrinsic pathway, is activated and a complex is formed between tissue factor and factor VII. Activated factor VII (VIIa) results. Factor VIIa can directly activate factor X. Alternatively, VIIa can activate factors IX and X together. In reaction three, factor X is activated by the proteases generated in the two previous reactions (VIIa and IXa in concert with VIII). As an alternative, VIIa can activate factors IX and X directly. In reaction four, sometimes referred to as the common pathway, Xa converts prothrombin in the presence of factor V, calcium, and phospholipid on the platelet surface. Thrombin, in turn, converts fibrinogen to fibrin, which is polymerized into a stable clot. Thrombin also activates factor VIII to stimulate further platelet aggregation and fibrin polymerization.
Almost immediately, three major activators of the fibrinolytic system act on plasminogen, which had previously been absorbed into the clot, to form plasmin. Plasmin degenerates the fibrin polymer into fragments, which are cleared by macrophages.
Roman numerals have been assigned by the order in which the factor had been identified, not by their order in the above-noted hemostatic mechanism. (See Table 2-14 for a list of factor names and for routine coagulation test abnormalities associated with factor deficiency.)
Fibrinogen, like many other of the coagulation proteins, is considered an acute-phase reactant protein and is elevated in many severe illnesses. It is also considered a risk factor for coronary heart disease (CHD) and stroke. Prothrombin is a vitamin K–dependent clotting factor. Its production in the liver requires vitamin K. This vitamin is fat soluble and is dependent on bile for absorption. Bile duct obstruction or malabsorption will cause a vitamin K deficiency and result in a reduced quantity of prothrombin and other vitamin K–dependent factors (VII, IX, X). It usually takes about 3 weeks before body stores of vitamin K are exhausted.
Factor VIII is actually a complex molecule with two components. The first component is related to hemophilia A and is involved in the hemostatic mechanism as described above. The second component is the von Willebrand factor and is related to von Willebrand disease. This second component is involved in platelet adhesion and aggregation. Factor XII deficiency is a common cause of prolonged activated partial thromboplastin time (aPPT) a nonbleeding patient. Patients with factor XII deficiency have been observed to have an increased risk for myocardial infarction (MI) and venous thrombosis.
Measurement of coagulation factors in relationship to other key coagulating proteins may be helpful in determining risks of hypercoagulation. A measure of the ratio of von Willebrand factor to ADAMTS13 (a factor-cleaving protease) is an accurate predictor of thromboembolic complication after liver surgery.
Coagulation factor inhibitors arise in patients who are congenitally deficient in a specific factor in response to factor replacement therapy. They can also occur spontaneously without known cause or in response to a variety of medical conditions, including the postpartum state, immunologic disorders, certain antibiotic therapies, some malignancies, and old age. Inhibitors of factor VIII coagulant activity are the most commonly occurring of the specific factor inhibitors. These can be identified and quantified.
Interfering Factors
• Many of these proteins are heat sensitive, and levels will be decreased if the specimen is kept at room temperature.
• Pregnancy or the use of contraceptive medication can increase levels of several of these factors, especially VIII and IX. A mild deficiency could be masked.
• Many of these protein coagulation factors are acute-phase reactant proteins. Acute illness, stress, exercise, or inflammation could raise levels.
Procedure and Patient Care
After
• Apply pressure or a pressure dressing to the venipuncture site.
• Assess the venipuncture site for bleeding, especially if the patient has had other episodes of clotting deficiency.
• Deliver the blood specimen to the laboratory as soon as possible.
• Freeze the specimen if testing is not going to be done immediately because these proteins are very labile.
Test Results and Clinical Significance
Related Tests
Partial Thromboplastin Time, Activated (aPTT) (p. 383). This test is used to evaluate the intrinsic system and the common pathway of clot formation.
Prothrombin Time (p. 434). This test is used to evaluate the adequacy of the extrinsic system and common pathway in the clotting mechanism.
Fibrinogen (p. 241). This is a separate discussion on this coagulating factor.
Cold Agglutinins
Indication
Cold agglutinins are used to identify and investigate cold agglutinin syndrome and unusual infections, such as Mycoplasma pneumoniae.
Test Explanation
Cold agglutinins are antibodies (usually IgM) to erythrocytes. All individuals have circulating antibodies directed against red blood cells, but their concentrations are often too low to trigger disease or symptoms (titers <1:64). In individuals with cold agglutinin syndrome, these antibodies are much higher (>1:512). At body temperatures of 28-31° C, such as those encountered during winter months, these antibodies can cause a variety of symptoms (from chronic anemia caused by intravascular hemolysis or extravascular sequestration of affected RBCs leading to acrocyanosis of the ears, fingers, or toes because of local blood stasis in the skin capillaries).
There are two forms of cold agglutinin disease, primary and secondary. The primary form has no precipitating cause. Secondary cold agglutinin disease is a result of an underlying condition, notably Mycoplasma pneumoniae. The Cold agglutinins test is not specific for Mycoplasma pneumoniae and is not recommended to diagnose the disease. It does provide supportive information, however. Mycoplasma pneumoniae serum antibodies (IgG and IgM) (p. 364) are also supportive of Mycoplasma infection.
Other possible conditions associated with cold agglutinins include influenza, mononucleosis, rheumatoid arthritis, lymphomas, HIV, Epstein-Barr virus, and cytomegalovirus. Temperature regulation is important for the performance of this test. Under no circumstances should the cold agglutinin specimen be refrigerated.
The cold agglutinin screen is performed on all specimens first to identify most of those with titer values in the normal range. If the screen is negative, no titration is required. If the screen is positive, a titer with serial saline dilutions is performed.
Test Results and Clinical Significance
Related Tests
Mycoplasma pneumonia Antibodies (p. 364). The serologic identification of IgG and IgM antibodies to Mycoplasma are used to support the clinical diagnosis of the infection.
Complement Assay (C2, C3, and C4 Complement)
Indications
Measurements of complement are used primarily to diagnose hereditary deficiencies of complement peptides and monitor the activity of infectious or autoimmune diseases (systemic lupus erythematosus nephritis, membranoproliferative nephritis, or poststreptococcal nephritis).
Test Explanation
Serum complement is a group of proteins that act as enzymes to instigate a cascade-like series of reactions that lead to the synthesis of a group of proteins that facilitate the immunologic and inflammatory responses. The total complement, sometimes labeled CH50, is made by the series of reactions involving proteins C1 through C9 (classic cascade reactions). Once activated, total complement (and some precursor proteins) acts to increase vascular permeability, allowing antibodies and white blood cells (WBCs) to be delivered to the area of the immune/antigen complex. Complement also acts to increase chemotaxis (attracting WBCs to the area), phagocytosis, and immune adherence of the antibody to antigen. The end result of the complement activation cascade is the formation of the lytic membrane attack complex (MAC).
These processes are vitally important in the normal inflammatory or immunologic response. The absence of early components (C1-C4) of the complement cascade results in the inability of immune complexes to activate the cascade. Patients with deficiencies of the early complement proteins are unable to clear immune complexes. Patients with deficiencies of the late complement proteins (C5, C6, C7, C8, and C9) are unable to form the MAC. Patients with deficient complement factors have increased susceptibility to infections with encapsulated microorganisms. They may also have symptoms that suggest autoimmune disease and complement deficiency may be an etiologic factor in the development of autoimmune disease. Besides the major complement components, there are some subcomponents and “inhibitor” components involved in the system that can affect complement function.
Reduced complement levels can be congenital or acquired. Although rare, C2 deficiency is the most common inherited complement deficiency. Acquired complement deficiencies are usually instigated by ongoing inflammatory/infectious diseases. As the complement system is activated, the complement components are “consumed” or used up. If the system is persistently or overly activated, serum levels decrease. The complement system is instigated by the presence of antibody/antigen complexes. As in hereditary angioedema, complement components are used up and serum levels fall. Diseases associated with these immune complexes include serum sickness, lupus erythematosus, infectious endocarditis, renal transplant rejection, vasculitis, some forms of glomerulonephritis, and infections. As these diseases are successfully treated, complement levels can be expected to return to normal. Complement components can be increased after the onset of various acute inflammatory diseases or acute tissue damage. This is very similar to an “acute reaction” protein.
The total complement assay should be used as a screen for suspected complement deficiencies before ordering individual complement component assays. Testing is usually automated using labeled liposomes. A deficiency of an individual component of the complement cascade may result in a reduced total complement level. Specific complement factor assay can be performed by radial immunodiffusion. Complement levels can be measured in body fluids, most commonly joint fluid. Low synovial fluid complement levels are characteristic of effusions from patients with rheumatoid arthritis, systemic lupus erythematosus, and bacterial infections.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
 Decreased Levels
Decreased Levels
Hereditary angioedema: Hereditary angioedema is a congenital lack of a C1 “inhibitor” (often called C1 esterase). The complement system is overly activated and the complement components are consumed or used up. Serum levels fall.
Severe liver diseases such as hepatitis or cirrhosis: The liver is the site of synthesis of many of the complement components. Synthesis is decreased in the presence of liver disease. Serum levels fall.
Autoimmune disease (SLE, glomerulonephritis, lupus nephritis, rheumatoid arthritis [severe and active], Sjögren syndrome),
Serum sickness (immune complex disease),
Renal transplant rejection (acute):
These diseases are associated with the increased presence of antibody/antigen complexes, which serve to act as complement activators. The complement system is overly activated and complement components are consumed. Serum levels fall.
These diseases are associated with protein depletion. Complement is a protein and its synthesis can be expected to be reduced in these illnesses.
Infection such as gram-negative sepsis or bacterial endocarditis,
Glomerulonephritis (specifically poststreptococcal and membranoproliferative):
Complete Blood Cell Count and Differential Count (CBC and Diff)
The CBC and differential count (diff) are a series of tests of the peripheral blood that provide a tremendous amount of information about the hematologic system and many other organ systems. They are inexpensively, easily, and rapidly performed as a screening test. The CBC and diff include automated multimeasurement of the following studies (Figure 2-13), which are discussed separately:

Figure 2-13 The Beckman Coulter automated CBC analyzer can perform many CBC tests in a few minutes. The automated system will notify the technologist if any significant abnormality is noted. Those findings will be corroborated by individual testing.
Red blood cell count (see p. 439)
Hemoglobin (see p. 281)
Hematocrit (see p. 277)
Red blood cell indices (see p. 442)
White blood cell count and differential count (see p. 526)
Blood smear (see p. 710)
Platelet count (see p. 401)
Mean platelet volume (MPV) (see p. 407)
Coombs Test, Direct (Direct Antiglobulin Test [DAT])
Indications
This test is performed to identify immune hemolysis (lysis of red blood cells [RBCs]) or to investigate hemolytic transfusion reactions (Box 2-7).
Test Explanation
Most of the antibodies to RBCs are directed against the ABO/Rh blood grouping antigens, such as those that occur in hemolytic anemia of the newborn or blood transfusion of incompatible blood. When a transfusion reaction occurs (Box 2-8), the Coombs test can detect the patient's antibodies or complement components coating the transfused RBCs. The Coombs test is therefore useful in evaluating suspected transfusion reactions.
Non–blood-grouping antigens can develop on the RBC membrane and stimulate the formation of antibodies. Drugs such as levodopa or penicillin can do this. Also, in some autoimmune diseases, antibodies not originally directed against the patient's RBCs can attach to the RBCs and cause hemolysis, which can be detected by the direct Coombs test. Examples of the latter would include:
• Antibodies developed in reaction to drugs such as penicillin
• Autoantibodies formed in various autoimmune diseases
• Antibodies developed in some patients with advanced cancer (e.g., lymphoma)
Frequently the production of these autoantibodies against RBCs is not associated with any identifiable disease, and the resulting hemolytic anemia is therefore called idiopathic.
The direct Coombs test demonstrates that RBCs have been attacked by antibodies in the patient's bloodstream. The RBCs of patients suspected of having antibodies against RBCs are washed to eliminate any excess free gamma globulins. Coombs serum is added to the RBCs. If the RBCs have antibodies on them, Coombs serum will cause agglutination. The greater the quantity of antibodies against RBCs, the more clumping occurs. This test is read as positive with clumping on a scale of micro-positive to 4+. If the RBCs are not coated with autoantibodies against RBCs (immunoglobulins), agglutination will not occur; this is a negative test.
Interfering Factors
• Antiphospholipid antibodies (see p. 68, Anticardiolipin Antibodies) can cause a false-positive DAT.
![]() Drugs that may cause false-positive results include ampicillin, captopril, cephalosporins, chlorpromazine (Thorazine), chlorpropamide, hydralazine, indomethacin (Indocin), insulin, isoniazid (INH), levodopa, methyldopa (Aldomet), penicillin, phenytoin (Dilantin), procainamide, quinidine, quinine, rifampin, streptomycin, sulfonamides, and tetracyclines.
Drugs that may cause false-positive results include ampicillin, captopril, cephalosporins, chlorpromazine (Thorazine), chlorpropamide, hydralazine, indomethacin (Indocin), insulin, isoniazid (INH), levodopa, methyldopa (Aldomet), penicillin, phenytoin (Dilantin), procainamide, quinidine, quinine, rifampin, streptomycin, sulfonamides, and tetracyclines.
Test Results and Clinical Significance
Hemolytic disease of the newborn,
Incompatible blood transfusion reaction:
Antibodies to the patient's RBCs have been created by mixing of incompatible blood grouping antigens.
Autoimmune hemolytic anemia (rheumatoid/collagen diseases, e.g., systemic lupus erythematosus [SLE], rheumatoid arthritis [RA]):
Hemolytic anemia after heart bypass: Autoantibodies formed during the use of the heart/lung bypass machine attach to RBCs.
Adult hemolytic anemia (idiopathic): Autoantibodies not otherwise associated with any other disease attach to RBCs.
Coombs Test, Indirect (Blood Antibody Screening, Indirect Antiglobulin Test [IAT])
Indications
This test is used to detect antibodies against red blood cells (RBCs) in the serum. This laboratory method is used most commonly for screening potential blood recipients.
Test Explanation
The indirect Coombs test detects circulating antibodies against RBCs. The major purpose of this test is to determine if the patient has minor serum antibodies (other than the major ABO/Rh system) to RBCs before receiving a blood transfusion. Therefore this test is the “screening” portion of the “type and screen” routinely performed for blood compatibility testing (crossmatching in the blood bank). This test is also used to detect other agglutinins, such as cold agglutinins that are associated with mycoplasmal infections.
In this test a small amount of the recipient's serum is added to donor RBCs containing known antigens on their surfaces. This is the first stage. In the second stage of the test, Coombs serum is added after the test RBCs have been washed of any free globulins. If antibodies exist in the patient's serum, agglutination occurs. In blood transfusion screening, visible agglutination indicates that the recipient has antibodies to the donor's RBCs. If the recipient has no antibodies against the donor's RBCs, agglutination will not occur; transfusion should then proceed safely without any transfusion reaction. Circulating antibodies against RBCs also may occur in an Rh-negative pregnant woman who is carrying an Rh-positive fetus.
Test Results and Clinical Significance
Incompatible crossmatched blood: ABO/Rh antigens in the donor blood cross-react with the patient's serum.
Hemolytic disease of the newborn:
Acquired immune hemolytic anemia,
Presence of specific cold agglutinin antibody: Drugs and other illnesses are associated with the development of antibodies detected in the patient's serum.
Related Test
Coombs Test, Direct (p. 175). This test is performed to identify hemolysis or to investigate hemolytic transfusion reactions.
Cortisol, Blood (Hydrocortisone, Serum Cortisol, Salivary Cortisol)
Indications
This test is a measure of serum cortisol. It is performed on patients who are suspected to have hyperfunctioning or hypofunctioning adrenal glands.
Test Explanation
An elaborate feedback mechanism for cortisol coordinates the function of the hypothalamus, pituitary gland, and adrenal glands. Corticotropin-releasing hormone (CRH) is made in the hypothalamus. This stimulates adrenocorticotropic hormone (ACTH) production in the anterior pituitary gland. ACTH stimulates the adrenal cortex to produce cortisol. The rising levels of cortisol act as a negative feedback to curtail further production of CRH and ACTH. Cortisol is a potent glucocorticoid released from the adrenal cortex. This hormone affects the metabolism of carbohydrates, proteins, and fats. It has a profound effect on glucose serum levels. Cortisol tends to increase glucose by stimulating gluconeogenesis from glucose stores. It also inhibits the effect of insulin and thereby inhibits glucose transport into the cells.
The best method of evaluating adrenal activity is by directly measuring plasma cortisol levels. Normally cortisol levels rise and fall during the day; this is called the diurnal variation. Cortisol levels are highest around 6 AM to 8 AM and gradually fall during the day, reaching their lowest point around midnight. Sometimes the earliest sign of adrenal hyperfunction is only the loss of this diurnal variation, even though the cortisol levels are not yet elevated. For example, individuals with Cushing syndrome often have upper normal plasma cortisol levels in the morning and do not exhibit a decline as the day proceeds. High levels of cortisol indicate Cushing syndrome, and low levels of plasma cortisol are suggestive of Addison disease.
For this test, blood is usually collected at 8 AM and again at around 4 PM. The 4 PM value is anticipated to be one third to two thirds of the 8 AM value. Normal values may be transposed in individuals who have worked during the night and slept during the day for long periods of time.
Serum cortisol assay is measured by an automated competitive binding immunoenzymatic assay. Cortisol can be measured in the urine (p. 920). The measurement of late-night salivary cortisol is another effective test for Cushing syndrome. It seems to be more convenient and superior to plasma and urine for detecting cortisol in patients with mild Cushing syndrome. Salivary cortisol assay cannot be used to diagnose hypocortisolism or Addison disease because liquid chromatography-tandem mass spectrometry laboratory methods are not sensitive enough at low levels. If late-night salivary cortisol levels are elevated, the results should be confirmed with a repeat salivary cortisol measurement, a midnight blood sampling for cortisol, or a 24-hour urinary collection of free cortisol. A dexamethasone suppression test (p. 204) is another confirmation test that can be used.
Interfering Factors
• Pregnancy is associated with increased levels.
• Physical and emotional stress can elevate cortisol levels. Stress stimulates the pituitary-cortical mechanism and thereby stimulates cortisol production.
![]() Drugs that may cause increased levels include amphetamines, cortisone, estrogen, oral contraceptives, and spironolactone (Aldactone).
Drugs that may cause increased levels include amphetamines, cortisone, estrogen, oral contraceptives, and spironolactone (Aldactone).
![]() Drugs that may cause decreased levels include androgens, aminoglutethimide, betamethasone and other exogenous steroid medications, danazol, lithium, levodopa, metyrapone, and phenytoin (Dilantin).
Drugs that may cause decreased levels include androgens, aminoglutethimide, betamethasone and other exogenous steroid medications, danazol, lithium, levodopa, metyrapone, and phenytoin (Dilantin).
Procedure and Patient Care
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Ectopic ACTH-producing tumors,
ACTH is overproduced as a result of neoplastic overproduction of ACTH in the pituitary or elsewhere in the body by an ACTH-producing cancer. Stress is a potent stimulus to ACTH production. Cortisol rises as a result.
Cushing syndrome (adrenal adenoma or carcinoma): The neoplasm produces cortisol without regard to the normal feedback mechanism.
Hyperthyroidism: The metabolic rate is increased and cortisol levels rise accordingly to maintain the elevated glucose needs.
Obesity: All sterols are increased in the obese, perhaps because fatty tissue may act as a depository or site of synthesis.
 Decreased Levels
Decreased Levels
Adrenal hyperplasia: The congenital absence of important enzymes in the synthesis of cortisol prevents adequate serum levels.
Addison disease: As a result of hypofunctioning of the adrenal gland, cortisol levels drop.
Hypopituitarism: ACTH is not produced by the pituitary gland, which is destroyed by disease, neoplasm, or ischemia. The adrenal gland is not stimulated to produce cortisol.
Hypothyroidism: Normal cortisol levels are not required to maintain the reduced metabolic rate of hypothyroid patients.
Related Tests
Adrenocorticotropic Hormone (ACTH) Stimulation (p. 34). This test is used for the differential diagnosis of Cushing syndrome or Addison disease.
Adrenocorticotropic (ACTH) Hormone (p. 31). The serum ACTH study is a test of anterior pituitary gland function that affords the greatest insight into the causes of either Cushing syndrome (overproduction of cortisol) or Addison disease (underproduction of cortisol).
Dexamethasone Suppression (p. 204). This test is important for diagnosing Cushing syndrome and distinguishing its cause.
Cortisol, Urine (p. 920). This test is a measure of urinary cortisol. It is performed on patients who are suspected to have hyperfunctioning or hypofunctioning adrenal glands.
C-Peptide (Connecting Peptide Insulin, Insulin C-Peptide, Proinsulin C-Peptide)
Indications
This test is used to evaluate diabetic patients and to identify patients who secretly self-administer insulin. C-peptide is also helpful in monitoring patients with insulinomas (tumors of the insulin-secreting cells of the islets of Langerhans).
Test Explanation
C-peptide (connecting peptide) is a protein that connects the beta and alpha chains of proinsulin. In the beta cells of the islet of Langerhans of the pancreas, the chains of proinsulin are separated during the conversion of proinsulin to insulin and C-peptide. C-peptide is released into the portal vein in nearly equal amounts. Because it has a longer half-life than insulin, more C-peptide exists in the peripheral circulation. In general, C-peptide levels correlate with insulin levels in the blood, except possibly in islet cell tumors and in obese patients. The capacity of the pancreatic beta cells to secrete insulin can be evaluated by directly measuring either insulin or C-peptide. In most cases, direct measurement of insulin is more accurate. However, in some instances, direct measurement of insulin does not accurately assess the patient's insulin-generating capability. C-peptide levels more accurately reflect islet cell function in the following situations:
1. Patients with diabetes who are treated with insulin and who have antiinsulin antibodies. This most often occurs in patients treated with old bovine or pork insulin. These antibodies falsely increase insulin levels.
2. Patients who secretly administer insulin to themselves (factitious hypoglycemia). Insulin levels will be elevated. Direct insulin measurement in these patients tends to be high, because the insulin measured is self-administered exogenous insulin. But C-peptide levels in that same specimen will be low, because exogenously administered insulin suppresses endogenous insulin (and C-peptide) production.
3. Diabetic patients who are taking insulin. The exogenously administered insulin suppresses endogenous insulin production. Insulin levels only measure the exogenously administered insulin and do not accurately reflect true islet cell function. C-peptide would be a more accurate test of islet cell function. This is performed to see if the diabetes is in remission and the patient may not need exogenous insulin.
4. Distinguishing type 1 from type 2 diabetes. This is particularly helpful in newly diagnosed diabetics. A person whose pancreas does not make any insulin (type 1 diabetes) has a low level of insulin and C-peptide. A person with type 2 diabetes has a normal or high level of C-peptide.
The C-peptide test is indicated for the clinical situations described above. Further, C-peptide is used in evaluating patients who are suspected to have an insulinoma. It can differentiate patients with insulinoma from patients with factitious hypoglycemia. In the latter patients, C-peptide levels are suppressed by exogenous insulin challenge. In patients with an autonomous secreting insulinoma, C-peptide levels are not suppressed. Furthermore, C-peptide can be used to monitor treated patients with insulinoma. A rise in C-peptide levels indicates a recurrence or progression of the insulinoma. Likewise, some clinicians use C-peptide testing as an indicator of the adequacy of therapeutic surgical pancreatectomy in patients with pancreatic tumors. C-peptide can also be used to diagnose “insulin resistance” syndrome.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Insulinoma: Insulin and C-peptide are made concomitantly by the neoplastic cells.
Pancreas transplant: Excess C-peptide is produced by the transplanted islet cells.
Renal failure: C-peptide is removed from the blood by the kidneys. Diminished kidney function will lead to elevated levels.
Administration of oral hypoglycemic agents: Oral hypoglycemic agents stimulate insulin and C-peptide synthesis.
Related Tests
Glucose, Blood (p. 253). This is a measurement of serum glucose.
Glucagon (p. 251). This is a direct measurement of glucagon, an islet cell hormone that acts to increase serum glucose levels.
Glycosylated Hemoglobin (p. 266). This is a test to measure the amount of glycosylated hemoglobin, which is an indirect measure of the chronic state of glucose levels.
Insulin Assay (p. 315). This is a direct measurement of insulin, an islet cell hormone that acts to decrease serum glucose levels.
C-Reactive Protein (CRP, High-Sensitivity C-Reactive Protein [hs-CRP])
Indications
C-reactive protein (CRP) is an acute-phase reactant protein used to indicate an inflammatory illness. It is believed to be of value in predicting coronary events.
Test Explanation
CRP is a nonspecific, acute-phase reactant protein used to diagnose bacterial infectious disease and inflammatory disorders, such as acute rheumatic fever and rheumatoid arthritis. It is also elevated when there is tissue necrosis. CRP levels do not consistently rise with viral infections. CRP is a protein produced primarily by the liver during an acute inflammatory process and other diseases. A positive test result indicates the presence, but not the cause, of the disease. The synthesis of CRP is initiated by antigen-immune complexes, bacteria, fungi, and trauma. CRP is functionally analogous to immunoglobulin G, except that it is not antigen specific. CRP interacts with the complement system.
The CRP test is a more sensitive and rapidly responding indicator than the erythrocyte sedimentation rate (ESR). In an acute inflammatory change, CRP shows an earlier and more intense increase than ESR; with recovery, the disappearance of CRP precedes the return of ESR to normal. The CRP also disappears when the inflammatory process is suppressed by antiinflammatory agents, salicylates, or steroids.
This test is also useful in evaluating patients with an acute myocardial infarction (AMI). The level of CRP correlates with peak levels of the MB isoenzyme of creatine kinase (see p. 186), but CRP peaks occur 18 to 72 hours later. Failure of CRP to normalize may indicate ongoing damage to the heart tissue. Levels are not elevated in patients with angina.
Atheromatous plaques in diseased arteries typically contain inflammatory cells. Multiple prospective studies have also demonstrated that baseline CRP is a good marker of future cardiovascular events. The CRP level may be a stronger predictor of cardiovascular events than the low-density lipoprotein (LDL) cholesterol level. When used together with the lipid profile (see Lipid Panel, Appendix C), it adds prognostic information to that conveyed by the Framingham risk score.
Recent development of a high sensitivity assay for CRP (hs-CRP) has enabled accurate assays at even low levels. Atheromatous plaques in diseased arteries typically contain inflammatory cells. Multiple prospective studies have also demonstrated that baseline CRP is a good marker of future cardiovascular events. The CRP level may be a stronger predictor of cardiovascular events than the low-density lipoprotein (LDL) cholesterol level. When used together with the lipid profile (see Lipid Profile, Appendix C), it adds prognostic information to that conveyed by the Framingham risk score. Because of the individual variability in hs-CRP, two separate measurements are required to classify a person's risk level. In patients with stable coronary disease or acute coronary syndromes, hs-CRP measurement may be useful as an independent marker for assessing likelihood of recurrent events, including death, myocardial infarction (MI), or restenosis after percutaneous coronary intervention (PCI). hs-CRP is most commonly used when other causes of systemic inflammation have been eliminated.
Another indicator of inflammation besides CRP that is instigating considerable attention as a cardiac risk factor is lipoprotein-associated phospholipase A2 (Lp-PLA2). Lp-PLA2 promotes vascular inflammation through the hydrolysis of oxidized LDL within the intima, contributing directly to the atherogenic process. When combined with CRP, testing for Lp-PLA2 markedly increases the predictive value in determining risk for a cardiac event, especially in patients whose cholesterol (see p. 154) is normal. The PLAC test is an enzyme-linked immunosorbent assay (ELISA) using two highly specific monoclonal antibodies to measure the level of Lp-PLA2 in the blood.
The CRP test also may be used postoperatively to detect wound infections. CRP levels increase within 4 to 6 hours after surgery and generally begin to decrease after the third postoperative day. Failure of the levels to fall is an indicator of complications, such as infection or pulmonary infarction.
Interfering Factors
• Elevated test results can occur in patients with hypertension, elevated body mass index, metabolic syndrome/diabetes mellitus, chronic infection (gingivitis, bronchitis), chronic inflammation (rheumatoid arthritis), and low high-density lipoprotein (HDL)/high triglycerides.
• Cigarette smoking can cause increased levels.
• Decreased test levels can result from moderate alcohol consumption, weight loss, and increased activity or endurance exercise.
![]() Medications that may increase test results include estrogens and progesterones.
Medications that may increase test results include estrogens and progesterones.
![]() Medications that may decrease test results include fibrates, niacin, and statins.
Medications that may decrease test results include fibrates, niacin, and statins.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Acute, noninfectious inflammatory reaction (e.g., arthritis, acute rheumatic fever, Reiter syndrome, Crohn disease),
Collagen-vascular diseases (e.g., vasculitis syndrome, lupus erythematosus),
Tissue infarction or damage (e.g., acute myocardial infarction [AMI], pulmonary infarction, kidney or bone marrow transplant rejection, soft-tissue trauma),
Bacterial infections such as postoperative wound infection, urinary tract infection, or tuberculosis,
Bacterial infection (e.g., tuberculosis, meningitis):
These diseases are all associated with an inflammatory reaction that instigates the synthesis of CRP.
Increased risk for cardiovascular ischemic events: Inflammation of the intimal lining of a blood vessel, and particularly the coronary vessels, is associated with an increased risk for intimal injury thereby leading to distal vessel plaque occlusions.
Related Tests
Erythrocyte Sedimentation Rate (p. 221). This is also an acute-phase reactant protein. It is a nonspecific test used to detect inflammatory, infectious, and necrotic processes.
Complement Assay (p. 172). Not only are some of the complement components acute-phase reactant proteins, but CRP also interacts with this complex immune system.
Fibrinogen (p. 241). This is an important part of the hemostatic mechanism. It is also an acute-phase reactant protein.
Lipoproteins (p. 342). This is an important risk factor for heart disease.
Homocysteine (p. 301). This is an important risk factor for heart disease.
Creatine Kinase (CK, Creatine Phosphokinase [CPK])
Indications
This test is used to support the diagnosis of myocardial muscle injury (infarction). It can also indicate neurologic or skeletal muscle diseases.
Test Explanation
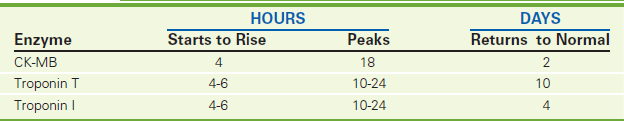
CK is found predominantly in the heart muscle, skeletal muscle, and brain. Serum CK levels are elevated when these muscle or nerve cells are injured. CK levels can rise within 6 hours after damage. If damage is not persistent, the levels peak at 18 hours after injury and return to normal in 2 to 3 days (Figure 2-14).
To test specifically for myocardial muscle injury, electrophoresis is performed to detect the three CK isoenzymes: CK-BB (CK1), CK-MB (CK2), and CK-MM (CK3). The CK-MB isoenzyme portion appears to be specific for myocardial cells. CK-MB levels rise 3 to 6 hours after infarction occurs. If there is no further myocardial damage, the level peaks at 12 to 24 hours and returns to normal 12 to 48 hours after infarction. CK-MB levels do not usually rise with transient chest pain caused by angina, pulmonary embolism, or congestive heart failure. One can expect to see a rise in CK-MB in patients with shock, malignant hyperthermia, myopathies, or myocarditis. Mild elevation of CK-MB (below the threshold of positive) can occur in patients with unstable angina and will signify an increased risk for an occlusive event. Very small amounts of CK-MB also exist in skeletal muscle. Severe injury to, or diseases of the skeletal muscle can also raise the CK-MB isoenzyme above normal.
The CK-MB isoenzyme level is helpful in both quantifying the degree of myocardial infarction (MI) and timing the onset of infarction. The CK-MB isoenzyme is often used to determine appropriateness of thrombolytic therapy, which is used for MI. High CK-MB levels would suggest that significant infarction has already occurred, thereby precluding the benefit of thrombolytic therapy.
Because the CK-BB isoenzyme is found predominantly in the brain and lung, injury to either of these organs (e.g., cerebrovascular accident, pulmonary infarction) will be associated with elevated levels of this isoenzyme.
The CK-MM isoenzyme normally makes up almost all of the circulatory total CK enzymes in healthy people. When the total CK level is elevated as a result of increases in CK-MM, injury to or disease of the skeletal muscle is present. Examples of this include myopathies, vigorous exercise, multiple intramuscular (IM) injections, electroconvulsive therapy, cardioversion, chronic alcoholism, or surgery. Because CK is made only in the skeletal muscle, the normal value of total CK (and therefore CK-MM) varies according to a person's muscle mass. Large muscular people may normally have a CK level in the high range of normal. Likewise, people of small stature or those with low muscle mass will be expected to have low CK levels. This is important because high normal CK levels in these patients can mask an MI.
Each isoenzyme has been found to have isoforms. The CK-MM isoforms MM1 and MM3 are most useful for cardiac disease. An MM3/MM1 ratio of greater than 1 suggests acute myocardial injury. A CK-MB ratio of MB2/MB1 greater than 1 also indicates acute myocardial injury.
CK is the main cardiac enzyme studied in patients with heart disease. Because its blood clearance and metabolism are well known, its frequent determination (on admission and at 12 hours and 24 hours) can accurately reflect timing, quantity, and resolution of an MI (see Figure 2-14). The clearance characteristics of commonly used cardiac enzymes are noted in Table 2-16.
New blood assays for cardiac markers have promised to rapidly and accurately detect acute MI (AMI) in the emergency room. One of these assays is troponin (see p. 508). A new assay is ischemia-modified albumin (see p. 326).
Interfering Factors
• Intramuscular (IM) injections can cause elevated CK levels.
• Strenuous exercise and recent surgery may cause increased levels.
• Early pregnancy may produce decreased levels.
• Muscle mass is directly related to a patient's normal CK level.
![]() Drugs that may cause increased levels include alcohol, amphotericin B, ampicillin, some anesthetics, anticoagulants, aspirin, captopril, clofibrate, colchicine, dexamethasone (Decadron), furosemide (Lasix), lithium, lidocaine, morphine, propranolol, statins, and succinylcholine.
Drugs that may cause increased levels include alcohol, amphotericin B, ampicillin, some anesthetics, anticoagulants, aspirin, captopril, clofibrate, colchicine, dexamethasone (Decadron), furosemide (Lasix), lithium, lidocaine, morphine, propranolol, statins, and succinylcholine.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Discuss with the patient the need and reason for frequent venipuncture in diagnosing MI.
Discuss with the patient the need and reason for frequent venipuncture in diagnosing MI.
• Avoid IM injections in patients with cardiac disease. These injections may falsely elevate the total CK level.
![]() Tell the patient that no food or fluid restrictions are necessary.
Tell the patient that no food or fluid restrictions are necessary.
During
• Collect a venous blood sample in a red-top tube. This is usually done initially and 12 hours later, followed by daily testing for 3 days and then at 1 week.
• Rotate the venipuncture sites.
• Record the time and date of any IM injection.
• Record the exact time and date of venipuncture on each laboratory slip. This aids in the interpretation of the temporal pattern of enzyme elevations.
Test Results and Clinical Significance
 Increased Levels of CK-BB Isoenzyme
Increased Levels of CK-BB Isoenzyme
Diseases that affect the central nervous system (CNS) (e.g., brain injury, brain cancer, cerebrovascular accident [stroke], subarachnoid hemorrhage, seizures, shock, Reye syndrome)
Adenocarcinoma (especially breast and lung): The pathophysiology of this observation is not known.
Pulmonary infarction: The lung tissue has small amounts of CK-BB. With cellular injury of this organ, the contents of the cell, including CK, spill out into the bloodstream, causing elevated CK-BB isoenzyme levels.
 Increased Levels of CK-MM Isoenzyme
Increased Levels of CK-MM Isoenzyme
Diseases affecting skeletal muscle cause CK-MM to spill out of the damaged cells and into the bloodstream, producing elevated CK-MM isoenzyme levels.
Injury affecting skeletal muscle causes CK-MM to spill out of the damaged cells and into the bloodstream, producing elevated CK-MM isoenzyme levels.
Related Tests
Aspartate Aminotransferase (AST) (p. 119). Elevated levels of this enzyme may indicate cardiac injury. It is not specific to the heart, however.
Lactic Dehydrogenase (LDH) (p. 329). This intracellular enzyme is used to support the diagnosis of injury or disease involving the heart, liver, red blood cells, kidneys, skeletal muscle, brain, and lungs.
Alanine Aminotransferase (ALT) (see p. 39). This enzyme is used similarly to AST and exists predominantly in the liver.
Leucine Aminopeptidase (LAP) (see p. 337). This enzyme is specific to the hepatobiliary system. Diseases affecting that system will cause elevation of this enzyme.
Gamma-Glutamyl Transpeptidase (GGTP) (p. 246). This is another enzyme existing predominantly in the liver.
Alkaline Phosphatase (p. 47). This is another enzyme existing predominantly in the liver.
5'-Nucleotidase (p. 376). This is another enzyme existing predominantly in the liver.
Troponins (p. 508). This is a biochemical marker used to assist in the evaluation of patients with chest pain.
Creatinine, Blood (Serum Creatinine)
Normal Findings
Less than 2 years: 0.1-0.4 mg/dL
2 years to <6 years: 0.2-0.5 mg/dL
6 years to <10 years: 0.3-0.6 mg/dL
10 years to <18 years: 0.4-1.0 mg/dL
18 years to <41 years: Female: 0.5-1.0 mg/dL
18 years to <41 years: Male: 0.6-1.2 mg/dL
41 years to <61 years: Female: 0.5-1.1 mg/dL
41 years to <61 years: Male: 0.6-1.3 mg/dL
Test Explanation
This test measures the amount of creatinine in the blood. Creatinine is a catabolic product of creatine phosphate, which is used in skeletal muscle contraction. The daily production of creatine, and subsequently creatinine, depends on muscle mass, which fluctuates very little. Creatinine, as blood urea nitrogen (BUN), is excreted entirely by the kidneys and therefore is directly proportional to renal excretory function. Thus, with normal renal excretory function, the serum creatinine level should remain constant and normal. Besides dehydration, only renal disorders, such as glomerulonephritis, pyelonephritis, acute tubular necrosis, and urinary obstruction, will cause an abnormal elevation in creatinine. There are slight increases in creatinine levels after meals, especially after ingestion of large quantities of meat. Furthermore, there may be some diurnal variation in creatinine (nadir at 7 AM and peak at 7 PM).
The serum creatinine test, as with the BUN, is used to diagnose impaired renal function. Unlike the BUN, however, the creatinine level is affected minimally by hepatic function. The creatinine is used as an approximation of the glomerular filtration rate (GFR). The serum creatinine level has much the same significance as the BUN level but tends to rise later. Therefore elevations in creatinine suggest chronicity of the disease process. In general, a doubling of creatinine suggests a 50% reduction in the glomerular filtration rate. The creatinine level is interpreted in conjunction with the BUN. These tests are referred to as renal function studies: The BUN/creatinine ratio is a good measurement of kidney and liver function. The normal range is 6 to 25, with 15.5 being the optimal adult value for this ratio.
While serum creatinine is the most commonly used biochemical parameter to estimate GFR in routine practice, there are some shortcomings to the use of this parameter. Factors such as muscle mass and protein intake can influence serum creatinine, leading to an inaccurate estimation of GFR. Moreover, in unstable, critically ill patients, acute changes in renal function can make real-time evaluation of GFR using serum creatinine difficult. On the other hand, cystatin C, a protein that is produced at a constant rate by all nucleated cells, is probably a better indicator of GFR. Because of its constant rate of production, its serum concentration is determined only by glomerular filtration. Its level is not influenced by those factors that affect creatinine and BUN.
Cystatin C might predict the risk for developing chronic kidney disease, thereby signaling a state of “preclinical” kidney dysfunction. Several studies have found that increased levels of cystatin C are associated with the risk for death, several types of cardiovascular disease (including myocardial infarction, stroke, heart failure, peripheral arterial disease, and metabolic syndrome). For women, the average reference interval is 0.52 to 0.90 mg/L with a mean of 0.71 mg/L. For men, the average reference interval is 0.56 to 0.98 mg/L with a mean of 0.77 mg/L.
Interfering Factors
• A diet high in meat content can cause transient elevations of serum creatinine.
![]() Drugs that may increase creatinine values include ACE inhibitors, aminoglycosides (e.g., gentamicin), cimetidine, heavy-metal chemotherapeutic agents (e.g., cisplatin), and other nephrotoxic drugs such as cephalosporins (e.g., cefoxitin).
Drugs that may increase creatinine values include ACE inhibitors, aminoglycosides (e.g., gentamicin), cimetidine, heavy-metal chemotherapeutic agents (e.g., cisplatin), and other nephrotoxic drugs such as cephalosporins (e.g., cefoxitin).
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Diseases affecting renal function, such as glomerulonephritis, pyelonephritis, acute tubular necrosis, urinary tract obstruction, reduced renal blood flow (e.g., shock, dehydration, congestive heart failure [CHF], atherosclerosis), diabetic nephropathy, nephritis: With these illnesses, renal function is impaired and creatinine levels rise.
Rhabdomyolysis: Injury of the skeletal muscle causes myoglobin to be released in the bloodstream. Large amounts are nephrotoxic. Creatinine levels rise.
Related Tests
Blood Urea Nitrogen (BUN) (p. 511). This is a test of renal function. Unlike creatinine, there are many nonrenal factors that can alter this test result.
Creatinine Clearance (see following test). This is a more accurate measurement of renal function. It is a direct measurement of glomerular filtration rate.
Creatinine Clearance (CrCl)
Test Explanation
Creatinine is a catabolic product of creatine phosphate, which is used in skeletal muscle contraction. The daily production of creatine, and subsequently creatinine, depends on muscle mass, which fluctuates very little. Creatinine is excreted entirely by the kidneys and therefore is directly proportional to the GFR (i.e., the number of milliliters of filtrate made by the kidneys per minute). CrCl is a measure of the GFR. Urine and serum creatinine levels are assessed and the clearance rate is calculated.
The amount of filtrate made in the kidney depends on the amount of blood to be filtered and on the ability of the nephron to act as a filter. The amount of blood present for filtration is decreased in renal artery atherosclerosis, dehydration, and shock. The ability of the nephron to act as a filter is decreased by diseases such as glomerulonephritis, acute tubular necrosis, and most other primary renal diseases. Significant bilateral obstruction to urinary outflow affects glomerular filtration (CrCl) only after it is long-standing.
When one kidney alone becomes diseased, the opposite kidney, if normal, has the ability to compensate by increasing its filtration rate. Therefore, with unilateral kidney disease or nephrectomy, a decrease in CrCl is not expected if the other kidney is normal.
Several nonrenal factors may influence CrCl. With each decade of age, the CrCl decreases 6.5 mL/min because of a decrease in the GFR. Because urine collections are timed, incomplete collections will falsely decrease CrCl. Muscle mass varies among people. Decreased muscle mass will give lower CrCl values. Likewise, ingestion of large amounts of meat will temporarily increase CrCl.
The CrCl test requires a 24-hour urine collection and a serum creatinine level. CrCl is then computed using the following formula:
where
U = number of milligrams per deciliter of creatinine excreted in the urine over 24 hours
Creatinine values are often used to assess the completeness of a 24-hour urine collection. In patients with normal creatinine, the CrCl should indicate whether all the urine has been collected for the full 24 hours.
The 24-hour urine collections used to measure CC are too time consuming and expensive for routine clinical use. The GFR can be estimated (estimated GFR [eGFR]) using the Modification of Diet in Renal Disease (MDRD) Study equation. This is an equation that uses the serum creatinine, age, and numbers that vary depending upon sex and ethnicity to calculate the GFR with very good accuracy. The prediction equation for GFR is as follows, with Pcr being serum or plasma creatinine in mg/dL:
The GFR is expressed in mL/min/1.73 m2
An increasing number of institutions across the country are beginning to report an eGFR on patients who are 18 years and older with every serum creatinine ordered. The eGFR calculation can be programmed into most laboratory information systems. As a result, chronic renal disease is being recognized more frequently in its early stages. Chronic kidney disease can be treated and progression to renal failure slowed or prevented. For example, if a patient with diabetes is found to have a reduced GFR of 49 at an annual examination, that patient's primary care physician can and should take steps to treat the early chronic kidney disease. This may include the use of ACE inhibitors, more aggressive treatment of high blood pressure, glycemic dietary control, and treatment of high cardiac risk factors. The eGFR can be used to calculate medication dosage in patients with decreased renal function.
Table 2-17 shows population estimates for mean (average) estimated glomerular filtration rate (eGFR) by age. There is no difference between races or sexes when eGFRs are expressed per square meter of body surface area. For diagnostic purposes, most laboratories report eGFR values above 60 as “>60 mL/min/1.73 m2,” not as an exact number.
TABLE 2-17
| Age (Years) | Mean eGFR |
| 20-29 | 116 mL/min/1.73 m2 |
| 30-39 | 107 mL/min/1.73 m2 |
| 40-49 | 99 mL/min/1.73 m2 |
| 50-59 | 93 mL/min/1.73 m2 |
| 60-69 | 85 mL/min/1.73 m2 |
| 70+ | 75 mL/min/1.73 m2 |
Cystatin C is a cysteine proteinase inhibitor that is produced by all nucleated cells and found in serum. Since it is formed at a constant rate and freely filtered by the kidneys, its serum concentration (like creatinine) is another accurate test that can estimate GFR.
Interfering Factors
• Exercise may cause increased creatinine values.
• Incomplete urine collection may give a falsely lowered value.
• Pregnancy increases CrCl. This is due in part to the increased load placed on the kidneys by the growing fetus.
• A diet high in meat can cause transient elevation of the serum creatinine and CrCl. When the creatinine is high, its clearance is increased. Therefore the CrCl overestimates the GFR.
• The eGFR may be inaccurate in extremes of age and in patients with severe malnutrition or obesity, paraplegia or quadriplegia, and in pregnant women.
![]() Drugs that may cause increased levels include aminoglycosides (e.g., gentamicin), cimetidine, heavy-metal chemotherapeutic agents (e.g., cisplatin), and nephrotoxic drugs such as cephalosporins (e.g., cefoxitin).
Drugs that may cause increased levels include aminoglycosides (e.g., gentamicin), cimetidine, heavy-metal chemotherapeutic agents (e.g., cisplatin), and nephrotoxic drugs such as cephalosporins (e.g., cefoxitin).
![]() Drugs that may cause a decrease in eGFR interfere with creatinine secretion (e.g., cimetidine or trimethoprim) or creatinine assay (cephalosporins). In these cases, a 24-hour creatinine clearance may be necessary to accurately estimate kidney function.
Drugs that may cause a decrease in eGFR interfere with creatinine secretion (e.g., cimetidine or trimethoprim) or creatinine assay (cephalosporins). In these cases, a 24-hour creatinine clearance may be necessary to accurately estimate kidney function.
Procedure and Patient Care
During
![]() See Box 11-2, Guidelines for 24-Hour Urine Collection, p. 907.
See Box 11-2, Guidelines for 24-Hour Urine Collection, p. 907.
![]() Encourage the patient to drink fluids during the 24-hour collection unless this is contraindicated for medical purposes.
Encourage the patient to drink fluids during the 24-hour collection unless this is contraindicated for medical purposes.
![]() Instruct the patient to avoid vigorous exercise during the 24 hours, because exercise may cause an increased CrCl.
Instruct the patient to avoid vigorous exercise during the 24 hours, because exercise may cause an increased CrCl.
• Make sure a venous blood sample is drawn in a red-top tube during the 24-hour collection.
• Mark the patient's age, weight, and height on the requisition sheet.
Cryoglobulin
Indications
This test is performed to identify cryoglobulins in patients with symptoms of purpura, arthralgia, or Raynaud phenomenon. Cryoglobulin testing is used to support the diagnosis of the diseases that are known to be associated with cryoglobulins.
Test Explanation
Cryoglobulins are abnormal immunoglobulin protein complexes that exist within the blood of patients with various diseases. These proteins will precipitate reversibly at low temperatures and will redissolve with rewarming. These cryoglobulins can precipitate within the blood vessels of the fingers when exposed to cold temperatures. This precipitation causes slugging of the blood within those blood vessels. These patients may have symptoms of purpura, arthralgia, or Raynaud phenomenon (pain, cyanosis, coldness of the fingers).
These proteins exist in varying quantities, depending on the disease entity with which they are associated. The cryoglobulins can be classified, which helps determine the underlying disease state. Type I (monoclonal) cryoglobulinemia is associated with monoclonal gammopathy of undetermined significance, macroglobulinemia, or multiple myeloma. Type II (mixed, two or more immunoglobulins of which one is monoclonal) cryoglobulinemia is associated with autoimmune disorders, such as vasculitis, glomerulonephritis, systemic lupus erythematosus, rheumatoid arthritis, and Sjögren syndrome. It may also be seen in such infections as hepatitis, infectious mononucleosis, cytomegalovirus, and toxoplasmosis. Type II cryoglobulinemia may also be essential (i.e., occurring in the absence of underlying disease). Type III (polyclonal) cryoglobulinemia is associated with the same disease spectrum as Type II cryoglobulinemia.
For this test, the blood sample is taken to the chemistry laboratory, where it is refrigerated for 72 hours. After that time the specimen is evaluated for precipitation. If precipitation is identified, it is measured and recorded. The tube is then rewarmed, and the specimen is reexamined for dissolution of that precipitation. If precipitation of the refrigerated specimen is identified and dissolved on rewarming, cryoglobulins are present. If cryoglobulin qualitative is positive, then immunofixation electrophoresis typing and quantitative IgA, IgG, and IgM is performed to classify the type of cryoglobulin that exists.
Test Results and Clinical Significance
The following is a list of diseases associated with the presence of cryoglobulins:
Cutaneous Immunofluorescence Antibodies (Indirect IFA Antibodies, Anti–Basement Zone Antibodies, Anti–Cell Surface Antibodies)
Indications
This test is used to diagnose and monitor autoimmune-mediated dermatitis and paraneoplastic dermatitis.
Test Explanation
Autoimmune-mediated skin lesions are often associated with the presence of elevated levels of antibodies in the serum (see Antiscleroderma Antibody, p. 93) and in the skin (see Skin Biopsy, p. 760). IgG anti-basement zone (BMZ) antibodies are produced by patients with pemphigoid, epidermolysis bullosa acquisita (EBA), and bullous eruption of lupus erythematosus (LE). The titer of anti-CS antibodies generally correlates with disease activity of pemphigus. This test is useful for confirming a diagnosis of these diseases and monitoring therapeutic response. Indirect immunofluorescence (IF) testing may be diagnostic when histologic or direct IF studies are only suggestive, nonspecific, or negative.
Anti–cell surface (CS) antibodies correlate with a diagnosis of pemphigus.
Anti–basement zone (BMZ) antibodies correlate with a diagnosis of bullous pemphigoid, cicatricial pemphigoid, epidermolysis bullosa acquisita (EBA), or bullous eruption of lupus erythematosus (LE).
Results should be interpreted in conjunction with clinical information, histologic pattern, and results of direct immunofluorescence (IF) study.
Related Test
Skin Biopsy (p. 760). Cutaneous immunofluorescence antibodies can also be detected directly on skin biopsy. This test is confirmatory for autoimmune dermatitis.
Cytokines
Indications
Cytokine assays are predominantly used for clinical research. Clinically, they may predominantly have the following uses:
• Measurement of acquired immunodeficiency syndrome (AIDS) progression
• Measurement of progression of inflammatory diseases, such as rheumatoid arthritis (RA) and other autoimmune diseases
• Tumor markers (e.g., breast cancer, lymphoma, and leukemia)
• Determination of risk for disease (e.g., risk for developing Kaposi sarcoma in AIDS patients)
• Determination of treatment of disease (e.g., which patients with RA may benefit from cytokine therapy)
• Determination of immune function and response
• Monitoring of patients receiving cytokine therapy or anticytokine therapy
Test Explanation
Cytokines are a group of proteins that have multiple functions but, in general, are produced by immune cells to communicate and orchestrate the immune response. The immune system has many different cells that must act together to effectively protect the body from infection, inflammation, or tumor. The cykotines are made by many different types of cells, including lymphocytes (T cells, B cells), monocytes, and eosinophils. Some cytokines stimulate each other, and some inhibit other cytokines to maintain balance. Originally, cytokines were named by their function (T cell growth factor, colony stimulating factor, etc.). As more was learned about this complex group of proteins, it became apparent that a single cytokine might act differently on different cells. Therefore naming the cytokine by function was confusing and misleading. As more cytokines have been identified, they were named interleukins and numbered by the sequence of discovery. Interleukins, in general, are made by leukocytes. Lymphokines and monokines are made by lymphocytes and monocytes, respectively. Other cytokines include interferon and growth factors.
Cytokines have receptors in other cells to which they attach and instigate a series of intracellular activity that may be associated with secretion, motion, or cell division. Cytokines are used therapeutically in stimulating bone marrow production of blood cells in patients with suppression (by chemotherapy) or disease of the bone marrow. They are used as potent antiinflammatory or antineoplastic agents. Some cytokines are produced at increased levels in particular disease states and are, thereby, markers for disease extent, progression, and response to therapy. For cancers that are associated with elevated cytokines, they act as “tumor markers.” Human Interferon Inducible protein 10 is a small cytokine belonging to the chemokine family that affects cellular chemotaxis, immune response, and bone marrow inhibition. This protein, when present in high quantities in an acutely ill patient, is an accurate predictor of multiple organ failure.
Any table designed to list all of the cytokines and their function quickly becomes inaccurate and imperfect. The discovery of new cytokines and new functions changes so frequently that any table is outdated in the delay to publication. Likewise, any listing of normal values will be just as quickly antiquated as methods of testing changes so frequently. It is suggested that reference to “normal values” be directed to the laboratory performing the assay.
Usually, cytokine testing is performed on serum. However, joint fluid is often tested in the evaluation of the patient with arthritis. Likewise, if inflammatory encephalitis or meningitis is considered, cerebrospinal fluid may be the specimen.
Test Results and Clinical Significance
Abnormal Findings
AIDS: The cytokine profile associated with the developing stages of AIDS or the susceptibility to AIDS related tumors has yet to be determined.
Various malignancies (breast cancer, lymphoma, and leukemia): Progression of these tumors may be the result or the instigator of elevated cytokines.
Impaired immune function: Cytokines are integral in the function of both cellular and humoral immune response. The exact cytokine profile for immune dysfunction has yet to be determined.
Rheumatoid arthritis: RA and other autoimmune diseases may be associated with increased cytokine levels compatible with a strong immune reaction. Measurement of certain cytokines may be important in monitoring more advanced anticytokine treatments for autoimmune diseases.
Cytomegalovirus (CMV)
Test Explanation
CMV belongs to the viral family that includes herpes simplex, Epstein-Barr, and varicella-zoster viruses. CMV infection is widespread. Infections usually occur in the fetus, during early childhood, and in the young adult. Certain populations are at increased risk. Male homosexuals, transplant patients, and acquired immunodeficiency syndrome (AIDS) patients are particularly susceptible. Infections are acquired by contact with body secretions or urine. Blood transfusions are commonly implicated in the spread of CMV. As many as 35% of patients receiving multiple transfusions become infected with CMV. Most patients with acute disease have no or very few (mononucleosis-like) symptoms. Others may have mononucleosis-like symptoms of fever, lethargy, and anorexia. After infection there is an asymptomatic incubation period of about 60 days. Acute symptoms then develop. This is followed by a latent phase. Reactivation can occur at any time.
CMV is the most common congenital infection. Pregnant mothers can get the disease during their pregnancy, or a previous CMV infection can become reactivated. Approximately 10% of infected newborns exhibit permanent damage, usually mental retardation and auditory damage. Fetal infection can cause microcephaly, hydrocephaly, cerebral palsy, mental retardation, or death.
The term TORCH (toxoplasmosis, other, rubella, CMV, herpes) has been applied to infections with recognized detrimental effects on the fetus. The effects on the fetus may be direct or indirect (e.g., precipitating abortion or premature labor). Included in the category of “other” are infections (e.g., syphilis). All of these tests are discussed separately.
Virus culture is the most definitive method of diagnosis. However, a culture cannot differentiate an acute infection from a chronic, inactive infection. Immunofluorescence, enzyme-linked immunosorbent assay (ELISA), and latex agglutination methods of identifying anti-CMV antibodies reveal much more information about the activity of the infection. CMV immunoglobulin (Ig) G antibody levels persist for years after infection. Identification of IgM antibodies, however, indicates a relatively recent infection. Three different CMV antigens can be detected immunologically. They are called early, intermediate-early, and late antigens and indicate onset of infection. CMV inclusion bodies can be identified in the renal cells sloughed into the urine and are seen during a routine urinalysis. PCR assays demonstrate sensitive and specific detection of CMV nucleic acid.
No specific therapy is known for this infection. If the diagnosis is established early by viral culture or serology, abortion may be an option. A fourfold increase in CMV titer in paired sera drawn 10 to 14 days apart is usually indicative of an acute infection.
Procedure and Patient Care
During
• For culture specimens, a urine, sputum, or mouth swab is the specimen of choice. Fresh specimens are essential.
• The specimens are cultured in a virus laboratory, which takes about 3 to 7 days.
• For an antibody or antigen titer, collect blood in a gold-top or red-top tube.
• Collect a specimen from the mother with suspected acute infection as early as possible.
D-Dimer (Fragment D-Dimer, Fibrin Degradation Product [FDP], Fibrin Split Products)
Test Explanation
The fragment D-dimer test assesses both thrombin and plasmin activity. D-dimer is a fibrin degradation fragment that is made through lysis of cross-linked (D-dimerized) fibrin. As plasmin acts on the fibrin polymer clot, fibrin degradation products and D-dimer are produced. The D-dimer assay provides a highly specific measurement of the amount of fibrin degradation that occurs. Normal plasma does not have detectable amounts of fragment D-dimer. For a discussion of other fibrin degradation products, see Thrombosis Indicators (p. 482).
This test provides a simple and confirmatory test for disseminated intravascular coagulation (DIC). Positive results of the D-dimer assay correlate with positive results of other thrombosis indicators. The D-dimer assay may be more specific than the FDP assay, but it is less sensitive. Therefore combining the FDP and the D-dimer provides a highly sensitive and specific test for recognizing DIC.
Levels of D-dimer can also increase when a fibrin clot is lysed by thrombolytic therapy. Thrombotic problems such as deep vein thrombosis (DVT), pulmonary embolism, sickle cell anemia, and thrombosis of malignancy are also associated with high D-dimer levels. D-Dimer is used as an effective screening test for DVT. It is able to accurately identify patients with DVT who are then sent for venous duplex scanning (p. 900). The d-dimer test, however, is often positive in patients who are already hospitalized. If the D-dimer test is negative, its high predictability indicates that the patient does not have PE/DVT, and further testing may not be necessary.
Finally, the D-dimer test can be used to determine the duration of anticoagulation therapy in patients with DVT. Patients with an abnormal D-dimer level 1 month after the discontinuation of anticoagulant therapy have a significant incidence of recurrent DVT. This incidence can be reduced by restarting anticoagulation therapy.
The D-dimer can be tested by immunoturbidimetric methods or latex quantitative/qualitative assay.
Interfering Factors
• The D-dimer level may be decreased in lipemic patients.
• The presence of rheumatoid factor at a level >50 IU/mL may lead to increased levels of D-dimer.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
DIC: This is a phenomenon of rapid intramicrovascular coagulation and synchronous fibrinolysis. D-dimer is produced by the action of plasmin on the fibrin polymer clot.
During thrombolytic or defibrination therapy:
Related Tests
The following are tests used to assist in the diagnosis of DIC:
Prothrombin Time (PT) (p. 434). The PT is used to evaluate the adequacy of the extrinsic system and common pathway in the clotting mechanism.
Coagulating Factor Concentration (p. 163). This is a quantitative measurement of specific coagulation factors.
Partial Thromboplastin Time, Activated (aPTT) (p. 383). This test is used to evaluate the intrinsic system and the common pathway of clot formation. It is most commonly used to monitor heparin therapy.
Dexamethasone Suppression (DS, Prolonged/Rapid DS, Cortisol Suppression, Adrenocorticotropic Hormone [ACTH] Suppression)
Indications
The DS test is important for diagnosing adrenal hyperfunction (Cushing syndrome) and distinguishing its cause.
Test Explanation
An elaborate feedback mechanism for cortisol exists to coordinate the function of the hypothalamus, pituitary gland, and the adrenal glands. Corticotropin-releasing hormone (CRH) is made in the hypothalamus. This stimulates ACTH production in the anterior pituitary gland. ACTH stimulates the adrenal cortex to produce cortisol. The rising levels of cortisol act as a negative feedback and curtail further production of CRH and ACTH. Cortisol is a potent glucocorticoid released from the adrenal cortex. This hormone affects the metabolism of carbohydrates, proteins, and fats. It especially has a profound effect on glucose serum levels.
The DS test is based on pituitary ACTH secretion being dependent on the plasma cortisol feedback mechanism. As plasma cortisol levels increase, ACTH secretion is suppressed; as cortisol levels decrease, ACTH secretion is stimulated. Dexamethasone is a synthetic steroid (similar to cortisol) that will suppress ACTH secretion. Under normal circumstances this results in reduced stimulation to the adrenal glands and ultimately a drop of 50% or more in plasma cortisol and 17-OCHS levels. This important feedback system does not function properly in patients with Cushing syndrome.
In Cushing syndrome caused by bilateral adrenal hyperplasia (Cushing disease), the pituitary gland is reset upward and responds only to high plasma levels of cortisone and steroids. In Cushing syndrome caused by adrenal adenoma or cancer (which acts autonomously), cortisol secretion will continue despite a decrease in ACTH. When Cushing syndrome is caused by an ectopic ACTH-producing tumor (as in lung cancer), that tumor is also considered autonomous and will continue to secrete ACTH despite high cortisol levels. Again, no decrease occurs in plasma cortisol. Knowledge of the following defects in the normal cortisol-ACTH feedback system is the basis for understanding the DST.
Cushing Syndrome Caused by Ectopic ACTH-Producing Tumor
The DS test also may identify depressed persons likely to respond to electroconvulsive therapy or antidepressants rather than to psychologic or social interventions. ACTH production will not be suppressed after administration of low-dose DS in these patients.
The prolonged DS test can be performed over a 6-day period on an outpatient basis. The rapid DS test is easily and quickly performed and is used primarily as a screening test to diagnose Cushing syndrome. It is less accurate and less informative than the prolonged DS test, but when its results are normal, the diagnosis of Cushing syndrome can safely be excluded.
Interfering Factors
• Physical and emotional stress can elevate ACTH release and obscure interpretation of test results. Stress is stimulatory to the pituitary, which thereby secretes ACTH.
![]() Drugs that can affect test results include barbiturates, estrogens, oral contraceptives, phenytoin (Dilantin), spironolactone (Aldactone), steroids, and tetracyclines.
Drugs that can affect test results include barbiturates, estrogens, oral contraceptives, phenytoin (Dilantin), spironolactone (Aldactone), steroids, and tetracyclines.
Procedure and Patient Care
During
• There are several documented methods of performing this test by varying the dose and duration of testing.
Prolonged Test
• Obtain a baseline 24-hour urine collection for corticosteroids (urine 17-OCHS [see p. 926] or urinary cortisol).
• Collect blood for determination of baseline plasma cortisol levels if indicated. Collect 24-hour urine specimens daily over a 6-day period. Because 6 continuous days of urine collections are needed, no urine specimens are discarded except for the first voided specimen on day 1, after which the collection begins.
• On day 3 administer a low dose of DS by mouth.
• On day 5 administer a high of DS by mouth.
• Administer the DS with milk or an antacid to prevent gastric irritation.
• The urine samples for cortisol and 17-OCHS do not need a preservative.
• Note that the creatinine content is measured in all the 24-hour urine collections to demonstrate their accuracy and adequacy.
• Keep the urine specimens refrigerated or on ice during the collection period.
Rapid Test
• Give the patient a dose of dexamethasone by mouth at 11 PM.
• Administer the DS with milk or an antacid to prevent gastric irritation.
• Attempt to ensure a good night's sleep. Sedative/hypnotics are used only if absolutely necessary.
• At 8 AM the next morning, draw blood for determination of the plasma cortisol level before the patient arises.
• If no cortisol suppression occurs after administration of the dose of dexamethasone, administer a higher dose to suppress ACTH production. This is referred to as the overnight 8-mg DS suppression test. Patients with adrenal hyperplasia will suppress. Patients with adrenal or ectopic tumors will not suppress.
Test Results and Clinical Significance
Adrenal Hyperfunction (Cushing Syndrome)
Ectopic ACTH-producing tumors:
In these illnesses, ACTH is produced without regard to the inhibitory feedback mechanism that normally exists. This is a result of neoplastic overproduction of ACTH in the pituitary or elsewhere in the body by an ACTH-producing cancer. ACTH is not suppressed. As a result, cortisol is not suppressed.
Adrenal adenoma or carcinoma: Neoplasms of the adrenal glands are not sensitive to the inhibitory feedback mechanism that normally exists. Therefore ACTH will be suppressed by the DS, but cortisol production (the end point of the test) is not.
Bilateral adrenal hyperplasia: The inhibitory feedback mechanism that normally exists in the pituitary-adrenal system is blunted. Therefore at low dexamethasone doses no change in cortisol production is seen. At high dexamethasone doses, however, the ACTH and subsequently cortisol are suppressed.
Mental depression: ACTH is not suppressed in individuals likely to require electroconvulsive or medicinal therapy for their depression.
Related Tests
Adrenocorticotropic Hormone (ACTH) Stimulation With Cosyntropin (p. 34). This test is used to evaluate the differential diagnosis of Cushing syndrome or Addison disease.
Adrenocorticotropic Hormone (ACTH) (p. 31). The serum ACTH study is a test of anterior pituitary gland function that affords the greatest insight into the causes of either Cushing syndrome (overproduction of cortisol) or Addison disease (underproduction of cortisol).
Cortisol, Blood (p. 179). This is a direct measurement of the cortisol blood level.
Cortisol, Urine (p. 920). This test is a measure of urinary cortisol. It is performed on patients who are suspected to have hyperfunctioning or hypofunctioning of the adrenal gland.
Diabetes Mellitus Autoantibody Panel (Insulin Autoantibody [IAA], Islet Cell Antibody [ICA], Glutamic Acid Decarboxylase Antibody [GAD Ab])
Indications
This test is used in the evaluation of insulin resistance. It is also used to identify type 1 diabetes and in patients with a suspected allergy to insulin. This antibody panel is also used in surveillance of patients who have received pancreatic islet cell transplantation.
Test Explanation
Type 1 diabetes mellitus (DM) is insulin-dependent diabetes (IDDM). It is becoming increasingly recognized that this disease is an “organ specific” form of autoimmune disease that results in destruction of the pancreatic islet cells and their products. These antibodies are used to differentiate type 1 DM from type 2 non-insulin-dependent DM. Nearly 90% of patients with type 1 diabetes have one or more of these autoantibodies at the time of their diagnosis. Patients with type 2 diabetes have low or negative titers.
These antibodies often appear years before the onset of symptoms. The panel is useful to screen relatives of IDDM patients who are at risk for developing the disease. Sixty percent to 80% of first-degree relatives with both ICA and IAA will develop IDDM within 10 years. GAD Ab provides confirmatory evidence. The presence of these antibodies identifies which gestational diabetic will eventually require insulin permanently. Once recognized, preventive diabetic treatment is instituted. This may include counseling plus antibody and glucose monitoring.
The most common type of anti-insulin antibody is immunoglobulin (Ig) G, but IgA, IgM, IgD, and IgE also have been reported. Most of these insulin antibodies do not cause clinical problems, but they may complicate most insulin assays. Anti-insulin antibodies act as insulin-transporting proteins and bind the free insulin. This can reduce the amount of insulin available for glucose metabolism. They may also contribute to insulin resistance (daily insulin requirements exceeding 200 units/day for 2 days). IgM, especially, may cause insulin resistance. Insulin allergy (most common with animal insulin) may result from IgE antibodies to insulin.
Although it was common in the past for diabetic patients to develop anti-insulin antibodies after prolonged treatment with exogenous insulin, the development and therapeutic use of recombinant DNA insulin has virtually eliminated that problem. Nevertheless the presence of insulin antibodies is diagnostic of factitious hypoglycemia from surreptitious administration of insulin. This antibody panel is also used in surveillance of patients who have received pancreatic islet cell transplantation. Finally these antibodies can be used to identify late onset type 1 diabetes in those patients previously thought to have type 2 diabetes.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Insulin resistance: The anti-insulin antibodies bind insulin and thereby diminish the amount of free insulin available for glucose metabolism.
Allergies to insulin: Although allergies occur most frequently with the use of animal-generated insulin, they can still occur with human insulin. A rash or lymphadenopathy may be the result of such an allergy.
Factitious hypoglycemia: Because most patients develop anti-insulin antibodies to exogenous insulin, the identification of these antibodies supports the secretive self-administration of insulin in a patient who denies the use of insulin.
Related Tests
C-Peptide (p. 182). This test is used to evaluate diabetic patients. It is also used to identify patients who secretly self-administer insulin.
Insulin Assay (p. 315). This test is used to diagnose insulinoma (tumor of the islets of Langerhans) and to evaluate abnormal lipid and carbohydrate metabolism. It is used in the evaluation of patients with fasting hypoglycemia.
2,3-Diphosphoglycerate (2,3-DPG in Erythrocytes)
Test Explanation
2,3-DPG is a by-product of the glycolytic respiratory pathway of the red blood cell (RBC). A congenital enzyme deficiency in this vital pathway alters the RBC shape and survival significantly. Nonspherocytic anemia is the result. Another result of the enzyme deficiency is reduced synthesis of 2,3-DPG. 2,3-DPG controls O2 transport from the RBCs to the tissues. Deficiencies of this enzyme result in alterations of the RBC O2 dissociation curve that controls release of O2 to the tissues. Many anemias not a result of 2,3-DPG deficiency are associated with increased levels of 2,3-DPG as a compensatory mechanism.
Usually, 2,3-DPG levels increase in response to anemia or hypoxic conditions (e.g., obstructive lung disease, congenital cyanotic heart disease, after vigorous exercise). Increases in 2,3-DPG decrease the O2 binding to hemoglobin so that O2 is more easily released to the tissues when needed (lower arterial PO2). Levels of 2,3-DPG are decreased as a result of inherited genetic defects. This genetic defect parallels sickle cell anemia and hemoglobin C diseases.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Anemia: Increased 2,3-DPG levels are compensatory to provide adequate O2 to the tissues.
Hypoxic heart and lung diseases (e.g., obstructive lung disease, cystic fibrosis, congenital cyanotic heart disease): Hypoxemia stimulates the production of 2,3-DPG.
Hyperthyroidism: Increased metabolic processes increase O2 requirements. This need is met by increased 2,3-DPG.
Chronic renal failure: Erythropoietin deficiency as a result of chronic renal failure causes anemia. Increased 2,3-DPG levels are compensatory to provide adequate O2 to the tissues.
Pyruvate kinase deficiency: This enzyme is important in the glycolytic respiratory pathway of the RBC. Its function is to metabolize 2,3-DPG by-products. In the absence of this enzyme, 2,3-DPG is not metabolized and increased levels result.
Compensation for higher altitudes: In compensation for the reduced oxygen availability, 2,3-DPG is increased in order to shift the oxygen dissemination curve to the right, making more oxygen available to the tissues.
 Decreased Levels
Decreased Levels
Polycythemia: 2,3-DPG is made in the RBC as a result of its glycolytic respiratory process. Increased numbers of RBCs will cause compensatory decreased 2,3-DPG.
Acidosis: Decreased 2,3-DPG is associated with metabolic or respiratory acidosis.
After massive blood transfusion: Banked RBCs lose their 2,3-DPG during storage.
2,3-DPG disease: The enzymes required for synthesis of 2,3-DPG are reduced. As a result, 2,3-DPG is reduced.
Respiratory distress syndrome: The pathophysiology of this observation is unknown.
Related Test
Complete Blood Cell Count (p. 174). This is a series of tests that provide information about the hematologic system and many other organ systems.
Disseminated Intravascular Coagulation Screening (DIC Screening)
Indications
This group of tests is indicated for patients who are suspected of having acute DIC (demonstrate a coagulopathy), for patients who have chronic DIC (chronic microembolic processes), and for patients who are at great risk for DIC (patients with sepsis or advanced cancer).
Test Explanation
This is a group of tests used to detect DIC. Many pathologic conditions can instigate or are associated with DIC. The more common ones include bacterial septicemia, amniotic fluid embolism, retention of a dead fetus, malignant neoplasia, liver cirrhosis, extensive surgery (especially on the prostate or liver), extracorporeal heart bypass, extensive trauma, severe burns, and transfusion reactions.
In DIC the entire clotting mechanism is triggered inappropriately. This results in significant systemic or localized intravascular formation of fibrin clots. Consequences of this futile clotting are small blood vessel occlusion and excessive bleeding caused by consumption of the platelets and clotting factors that have been used in intravascular clotting. The fibrinolytic system is also activated to break down the clot formation and the fibrin involved in the intravascular coagulation. This fibrinolysis results in the formation of fibrin degradation products (FDPs) (see Thrombosis Indicators [p. 482]) which, by themselves, act as anticoagulants; these FDPs only serve to enhance the bleeding tendency.
Organ injury can occur as a result of intravascular clots, which cause microvascular occlusion in various organs. This may cause serious anoxic injury in affected organs. Also, RBCs passing through partly plugged vessels are injured and subsequently hemolyzed. The result may be ongoing hemolytic anemia. Figure 2-15 summarizes DIC pathophysiology and effects. Heparin is sometimes used to treat DIC because it inhibits the ongoing futile thrombin formation. This decreases the use of clotting factors and platelets, and bleeding ceases.
Figure 2-15 Pathophysiology of DIC, which may result in bleeding tendency, organ ischemia, and hemolytic anemia. (FDP, Fibrin degradation products; RBCs, red blood cells.)
When a patient with a bleeding tendency is suspected of having DIC, a series of routinely performed laboratory tests are done (prothrombin time [PT], partial thromboplastin time [PTT], bleeding time, and platelet count). If results are abnormal, further testing should be performed (Table 2-18). With these tests, the hematologist can make the appropriate diagnosis confidently. All of these tests are discussed separately.
TABLE 2-18
Disseminated Intravascular Coagulation Screening Tests
| Test | Result |
| Platelet count (p. 401) | Decreased |
| Prothrombin time (p. 434) | Prolonged |
| Partial thromboplastin time (p. 383) | Prolonged |
| Coagulating factors (p. 163) | Decreased factors I, II, V, VIII, X, and XIII—more commonly used for diagnosis than screening |
| Fibrin degradation products (p. 202) | Increased |
| Fibrinogen (p. 241) | Decreased |
| D-Dimer (p. 202) | Increased |
| Fibrinopeptide A (p. 482) | Increased |
| Prothrombin fragment (p. 482) | Increased |
Related Test
Protein C, Protein S (p. 432). This test identifies patients who are deficient in protein C and/or S. This is part of an evaluation of hypercoagulation.
Drug Monitoring (Therapeutic Drug Monitoring [TDM])
Indications
TDM entails measuring blood drug levels to determine effective drug dosages and prevent toxicity. TDM is used to adjust the dosage of medications so as to maximize efficacy and minimize side effects.
Test Explanation
There are several factors that affect both efficacy and toxicity. They include patient compliance (TDM can be used to determine patient compliance), patient age and size, access to adequate care, optimal dosing, and drug pharmacology issues, including absorption, elimination, and drug interactions. Drug monitoring is helpful in patients who take other medicines that may affect drug levels or act in a synergistic or antagonistic manner with the drug to be tested. There are some medicines (e.g., antiarrhythmics, bronchodilators, antibiotics, anticonvulsants, cardiotonics) that have a very narrow therapeutic margin (i.e., the difference between therapeutic and toxic drug levels is small).
TDM is helpful if the desired therapeutic effect of the drug is not observed as expected. Dosages beyond normal may have to be prescribed. Likewise if toxic symptoms appear with standard doses, TDM can be used to determine reduced dosing.
Table 2-19 lists the therapeutic and toxic ranges for the average patient for commonly tested drug levels. This list is far from complete. These ranges may not apply to all patients because clinical response is influenced by many factors (Box 2-9). Also note that different laboratories use different units for reporting test results and normal ranges. It is important that sufficient time pass between the administration of the medication and the collection of the blood sample to allow for adequate absorption and therapeutic levels to occur.
Blood is routinely used for TDM because results indicate what is presently going on with the drug at any one particular time. Urine drug levels reflect the presence of the drug over the last several days. Therefore if data concerning drug levels at a particular time are necessary, blood testing is required.
Blood samples can be taken at the drug's peak level (highest concentration) or the trough level (lowest concentration). Peak levels are useful when testing for toxicity, and trough levels are useful for demonstrating a satisfactory therapeutic level. Trough levels are often referred to as residual levels. The time when the sample should be drawn after the last dose of the medication varies according to whether a peak or trough level is requested as well as the half-life (the time required for the body to decrease the drug blood level by 50%) of the drug. Table 2-20 lists the peak concentration times for some common drugs. If peak levels are higher than the therapeutic range, toxicity may be experienced. If trough levels are below the therapeutic range, drug therapy may not be successful.
Pharmacogenetics (Genetic Testing For Drug Monitoring)
All drugs undergo metabolism by enzymes systems to activate a bound (proactive) drug and/or to deactivate an active drug. The effectiveness of these enzymes' systems of metabolism are determined by the genetic makeup of the patient. With pharmacogenetics, four categories of drug metabolizers can be identified:
In general, PMs and, to a lesser extent, IMs are prone to exaggerated side effects from active drugs, whereas normal doses of the same drugs tend to be ineffectual for UMs. If a proactive drug is administered and must be hydrolyzed to its active form, PMs will not benefit from normal doses, whereas UMs will experience drug benefit from even small doses.
The cytochrome P (CYP) 450 system is a major family of drug-metabolizing enzymes. Several CYP450 enzymes are involved in the metabolism of a significant proportion of drugs (Table 2-21). Cytochrome P450 genotype testing using PCR amplification is a pharmacogenetic method of evaluating the metabolic effectiveness of the CYP450 system and provides data to categorize the patient's metabolizing ability as described in the preceding. This testing is performed on a buccal swab specimen.
TABLE 2-21
Enzymes Involved in Drug Metabolism
| Enzyme | Drugs |
| CYP2C9 | Warfarin, phenytoin, nonsteroidal anti-inflammatory drugs |
| CYP2C19 | Omeprazole, proguanil, amitriptyline, diazepam, propranolol |
| CYP2D6 | Codeine, antidepressants, haloperidol, amiodarone, tamoxifen, diltiazem, amphetamine, dextromethorphan, anticonvulsants, flecainide, disopyramide |
| Atypical pseudocholinesterase | Succinylcholine |
| NAT2 (slow acetylator) | Isoniazid, hydralazine |
| UGT1A1 | Irinotecan |
| GST | D-Penicillamine |
| TPMT | Azathioprine, mercaptopurine |
| CYP1A1 | Polycyclic aromatic hydrocarbons |
| CYP1A2 | Caffeine, theophylline, imipramine |
| CYP2 CYP2A6 | Nicotine |
| CYP2E1 | Ethanol |
| CYP3 CYP3A4 | Amitriptyline, clarithromycin, cyclosporine, erythromycin, tacrolimus, lidocaine, nifedipine, tamoxifen |
Thiopurine methyltransferase (TPMT) is another metabolic enzyme system used in the metabolism of thiopurine drugs (e.g., azathioprine, 6-mercaptopurine [6MP], and 6-thoguanine). Defects in the TPMT noted on TPMT gene mutation testing leads to decreased methylation and decreased inactivation of 6MP. This can lead to enhanced bone marrow toxicity, which may cause myelosuppression, anemia, bleeding tendency, leukopenia, and infection.
Pharmacogenetics allows physicians to consider genetic information from patients in selecting medications and dosages of medications for a wide variety of common conditions, such as cardiac disease, psychiatric disease, and cancer.
Gas chromatography and liquid chromatography are commonly used in TDM. Mass spectrometry can be combined with other methods to improve sensitivity of TDM. Enzyme-multiplied immunoassay technique offers an alternative to the traditional spectroscopic and chromatographic method for measuring blood concentrations of drugs.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Tell the patient that no food or fluid restrictions are needed.
Tell the patient that no food or fluid restrictions are needed.
• For patients suspected of having symptoms of drug toxicity, the best time to draw the blood specimen is when the symptoms are occurring.
• If there is a concern regarding whether an adequate dose of the drug is achieved, it is best to obtain trough levels.
During
• Collect a venous blood sample in a tube designated by the laboratory. Peak levels are usually obtained 1 to 2 hours after oral intake, approximately 1 hour after intramuscular (IM) administration, and approximately 30 minutes after intravenous (IV) administration. Residual (trough) levels are usually obtained shortly before (0 to 15 minutes) the next scheduled dose. Consult with the pharmacy for specific times.
After
• Apply pressure or a pressure dressing to the venipuncture site.
• Assess the venipuncture site for bleeding.
• Clearly mark all blood samples with the following information: patient's name, diagnosis, name of drug, time of last drug ingestion, time of sample, and any other medications the patient is currently taking.
Related Test
Toxicology Testing (p. 951). This is generally a urine test to determine the toxic effect of prescribed and nonprescribed drugs that are often used and abused in criminal behavior.
Drug Sensitivity Genotype Testing (AccuType)
Indications
This test is indicated if a patient is taking a medication with no therapeutic effect or is experiencing signs of toxicity at normal therapeutic doses.
Test Explanation
The efficacy of therapeutic drugs can vary considerably among different patients. Factors that influence these variations include genetic aberrations, patient age, race, body weight or surface area, sex, tobacco use, concomitant medications, and comorbid medical conditions. It is extremely important to identify differences in drug metabolism so as to preclude the possibility of overdosing or underdosing.
Drug sensitivity genotype testing identifies genetic aberrations that encode various proteins required for drug metabolism. If the gene is abnormal, the protein may be deficient in quantity or character to properly metabolize the medication given to the patient. Various laboratories have “trade marked” their testing methods. A common test is called AccuType Testing.
Drug sensitivity genotype testing is available for predicting a patient's response to warfarin, clopidogrel, interferon-ribavirin (and other retroviral medications), metformin, and anti-TB drugs (rifampin/isoniazid).
Test Results and Clinical Significance
Genetic aberrations that may alter drug metabolism: As a result of knowing genetic aberrations in drug metabolism, drug dosages can be modified to provide the therapeutic dose without risks of toxicity.
Related Test
Drug Monitoring (p. 211). This test includes a more general discussion of pharmacogenetics.
Epstein-Barr Virus Testing (EBV Antibody Titer)
Test Explanation
EBV infects 80% of the U.S. population. Once infection occurs, the virus becomes dormant but can be reactivated later. EBV infection can produce infectious mononucleosis. Mononucleosis is seen most often in children, adolescents, and young adults. Clinical features include acute fatigue, fever, sore throat, lymphadenopathy, and splenomegaly. Laboratory findings of lymphocytosis, atypical lymphocytes, and transient serum heterophil antibodies are seen in patients with acute EBV infection. Most patients with infectious mononucleosis recover uneventfully and return to normal activity within 4 to 6 weeks. In Africa, EBV has been associated with Burkitt lymphoma. In China, EBV infection has been associated with nasopharyngeal carcinoma.
After recovery from primary EBV infection, patients are life-long, latent EBV carriers. Specific immunologic tests to identify EBV activity indicate that latent EBV can reactivate and become associated with a constellation of chronic signs and symptoms resembling infectious mononucleosis. Clinical manifestations of chronic EBV are variable and include nonspecific symptoms, such as profound fatigue (chronic fatigue syndrome), pharyngitis, myalgia, arthralgia, low-grade fever, headache, paresthesia, and loss of abstract thinking.
The majority of EBV infections can be recognized, however, by testing the patient's serum for heterophile antibodies (rapid latex slide agglutination test; mononucleosis [mono] rapid test, see p. 363). Other more specific immunologic tests are recommended only when a mononucleosis screening procedure is negative and infectious mononucleosis or a complication of Epstein-Barr virus infection is suspected. Also they more precisely define the acuity of the infection (Table 2-22). In cases in which EBV is suspected but the heterophile antibody is not detected, an evaluation of the EBV-specific antibody profile (e.g., EBV viral capsid antigen [VCA] IgM, EBV VCA IgG, and EBV nuclear antigen [EBNA]) may be useful (Table 2-23). The viral capsid antigen-antibodies (VCAs) can be immunoglobulin (Ig) G or IgM. The EBV nuclear antigen (EBNA) is located in the nuclei of the infected lymphocyte. Another EBV antigen is called the early antigen (EA). There are two EA antigens. One is EA-D and is commonly associated with nasopharyngeal cancer. EA-R is commonly associated with Burkitt lymphoma.
TABLE 2-22
Serologic Studies and the Timing of Infections
| Serologic Study | Appears/Disappears | Clinical Significance |
| Mono spot heterophil | 5 days/2 wk | Acute or convalescent infection |
| VCA-IgM | 7 days/3 mo | Acute or convalescent infection |
| VCA-IgG | 7 days/exists for life | Acute, convalescent, or old infection |
| EBNA-IgG | 3 wk/exists for life | Old infection |
| EA-D | 7 days/2 wk | Acute or convalescent infection |
The interpretation of EBV antibody tests is based on the following assumptions:
1. Once the person becomes infected with EBV, the anti-VCA antibodies appear first.
2. Anti-EA (EA-D or EA-R) antibodies appear next or are present with anti-VCA antibodies early in the course of illness. An anti-EA antibody titer greater than 80 in a patient 2 years after acute infectious mononucleosis indicates chronic EBV syndrome.
3. As the patient recovers, anti-VCA and anti-EA antibodies decrease and anti-EBNA antibodies appear. Anti-EBNA antibody persists for life and reflects a past infection.
4. After the patient is well, anti-VCA and anti-EBNA antibodies are always present but at lower ranges. Occasionally anti-EA antibody also may be present after the patient recovers.
In an acute infection, heterophile antibodies usually appear on the mono spot within the first 3 weeks of illness, but then decline rapidly within a few weeks. The heterophile antibody, however, fails to develop in about 10% of adults, more frequently in children, and almost uniformly in infants with primary EBV infections. If EBV infection is suspected to have occurred more than a few weeks before testing, the mono spot test may be negative. Detecting anti-VCA IgG or EBNA will not be helpful because they indicate that an EBV infection has occurred sometime in the patient's life but not necessarily recently. But detecting anti-VCA IgM would indicate that the syndrome of complaints the patient experienced a few weeks prior was because of EBV.
In immunosuppressed patients (i.e., those with AIDS, transplantation, or long-term chemotherapy), EBV infection can be much more serious, instigating extranodal lymphoma and posttransplant lymphoproliferative disorders. These patients may have serologic negative tests because of their immunosuppression.
EBV specific antibodies are most frequently detected by using enzyme immunoassay or qualitative enzyme immunoassay. With qualitative/quantitative polymerase chain reaction laboratory methods, EBV viral nuclear particles can be identified and measured. This technique provides a much more direct indication of EBV infection.
Test Results and Clinical Significance
Related Test
Mononucleosis Rapid Test (p. 363). This test is used to detect heterophil antibodies that can support the diagnosis of EBV infection (infectious mononucleosis).
Erythrocyte Fragility (Osmotic Fragility [OF], Red Blood Cell Fragility)
Indications
This test is performed to detect hereditary spherocytosis and thalassemia when intravascular hemolysis is identified.
Test Explanation
Red blood cells (RBCs) are bound by a membrane that allows water to pass through while generally restricting the solutes. This process, called osmosis, causes RBCs to absorb water when in a hypotonic medium. This results in swelling and, ultimately, hemolysis as the cell bursts. The osmotic fragility test uses this fact to determine the concentration of solute inside the cell by subjecting it to salt solutions of different concentrations. The ability of the normal RBC to withstand hypotonicity results from its biconcave shape, which allows the cell to increase its volume by 70% before the surface membrane is stretched. Once this limit is reached, lysis occurs. When intravascular hemolysis is identified, OF is used to determine if the RBCs have increased fragility (tend to burst open when exposed to a higher-concentrated NaCl solution) or decreased fragility (tend to burst open in lower-concentrated, and thus more hypotonic, NaCl solution).
An osmotic fragility test primarily indicates the surface area–to-volume ratio (SAVR) of RBCs. The lower the ratio, the more fragile the RBC. OF of RBCs is defined as the ease with which the cells burst in hypotonic solutions. This is expressed in terms of the concentration of the saline solution in which the cells are hemolyzed. The numbers of cells that burst in varying concentrations of NaCl are plotted on a curve. That curve is compared to a normal curve. If the curve is shaped or shifted to the right, OF is abnormally increased (i.e., more cells lyse at higher concentrations NaCl). If the curve is abnormally shaped or shifted to the left, OF is decreased (i.e., fewer cells lyse at comparable NaCl concentrations). It is useful to record the concentration of sodium chloride solution causing 50% lysis (i.e., the median corpuscular fragility [MCF]). This value is normally 0.4% to 0.45% of NaCl. Other useful values include the concentration at which lysis begins (minimum resistance) and that at which lysis appears to be complete (maximum resistance). This test is performed by automated spectrophotometry.
Round cells (spherocytes) have increased OF compared to normal indented RBCs. In hereditary spherocytosis, there is abnormal morphology due to a lack of spectrin, a key RBC cytoskeletal membrane protein. This produces membrane instability, which forces the cell to the smallest volume—that of a sphere. This common disorder is associated with intravascular hemolysis. This is shown by increased osmotic fragility, which causes the entire curve to “shift to the right” or causes most of it to be within the normal range with a “tail” of fragile cells.
Thalassemia, on the other hand, is associated with thinner leptocytes whose OF is decreased. A single-tube osmotic fragility test has been proposed for thalassemia screening with a range of different saline concentrations. The sensitivity and specificity of a 0.36% buffered saline will provide a positive or equivocal result in nearly all patients with a thalassemia trait.
Erythrocyte Sedimentation Rate (ESR, Sed Rate Test)
Indications
The ESR is a nonspecific test used to detect illnesses associated with acute and chronic infection, inflammation (collagen-vascular diseases), advanced neoplasm, and tissue necrosis or infarction.
Test Explanation
ESR is a measurement of the rate at which the red blood cells (RBCs) settle in saline solution or plasma over a specified time period. It is nonspecific and therefore not diagnostic for any particular organ disease or injury. Because inflammatory, neoplastic, infectious, and necrotic diseases increase the protein (mainly fibrinogen) content of plasma, RBCs have a tendency to stack up on one another, increasing their weight and causing them to descend faster. Therefore in these diseases the ESR will be increased. ESR provides the same information as an acute-phase reactant protein. That is to say that it occurs as a reaction to acute illnesses as described above.
The test can be used to detect occult disease. Many physicians use the ESR test in this way for routine patient evaluation for vague symptoms. Other physicians regard this test as so nonspecific that it is useless as a routine study. The ESR test occasionally can be helpful in differentiating disease entities or complaints. For example, in a patient with chest pain the ESR will be increased with myocardial infarction (MI) but will be normal in a patient with musculoskeletal chest pain.
The ESR is a fairly reliable indicator of the course of disease and therefore can be used to monitor disease therapy, especially for inflammatory autoimmune diseases (e.g., temporal arteritis, polymyalgia rheumatica). In general, as the disease worsens, the ESR increases; as the disease improves, the ESR decreases. If the results of the ESR are equivocal or inconsistent with clinical impressions, the C-reactive protein test is often performed.
1. As mentioned above, it is nonspecific.
2. It is sometimes not elevated in the face of active disease.
3. Many other factors may influence the results (see following section).
ESR elevation may lag behind other indicators early in an infection. Likewise, in the convalescent stage of a disease or infection, the ESR may remain elevated longer than other disease indicators. ESR cannot be used as an indicator of tumor burden when it is associated with neoplastic diseases, such as myeloma or breast cancer.
Interfering Factors
• Artificially low results can occur when the collected specimen is allowed to stand longer than 3 hours before the test.
• Pregnancy (second and third trimester) can cause elevated levels.
• Menstruation can cause elevated levels.
• The sedimentation tube must be perfectly vertical. Any tilt can distort results.
• Some anemias can falsely increase the ESR. There are correction nomograms available for variations in RBC count.
• Polycythemia is associated with decreased ESR.
• Diseases associated with increased proteins (e.g., macroglobulinemia) can falsely increase the ESR.
![]() Drugs that may cause increased ESR levels include dextran, methyldopa (Aldomet), oral contraceptives, penicillamine, procainamide, theophylline, and vitamin A.
Drugs that may cause increased ESR levels include dextran, methyldopa (Aldomet), oral contraceptives, penicillamine, procainamide, theophylline, and vitamin A.
![]() Drugs that may cause decreased levels include aspirin, cortisone, and quinine.
Drugs that may cause decreased levels include aspirin, cortisone, and quinine.
Procedure and Patient Care
During
• Collect a venous blood sample in a yellow-top tube.
• In the laboratory, the blood is aspirated into a calibrated sedimentation tube and allowed to settle, usually for 60 minutes. The remaining clear area (plasma) is measured as the sedimentation rate.
• An alternate method is performed by measuring the distance (in millimeters) that RBCs descend (or settle) in normal saline solution in 1 hour. These processes are now automated (Figure 2-16).
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Chronic renal failure (e.g., nephritis, nephrosis): The pathophysiology of this observation is not well defined.
Malignant diseases (e.g., multiple myeloma, Hodgkin disease, advanced carcinomas): Malignant diseases are often associated with increased abnormal serum proteins. Diseases associated with increased serum proteins are associated with increased ESR.
Bacterial infection (e.g., abdominal infections, acute pelvic inflammatory disease, syphilis, pneumonia),
Inflammatory diseases (e.g., temporal arteritis, polymyalgia rheumatica, rheumatoid arthritis, rheumatic fever, systemic lupus erythematosus [SLE]),
Necrotic diseases (e.g., acute myocardial infarction, necrotic tumor, gangrene of an extremity):
Diseases associated with increased proteins (e.g., hyperfibrinogenemia, macroglobulinemia): Diseases associated with increased serum proteins are associated with increased ESR.
Severe anemias (e.g., iron deficiency or B12 deficiency): With lower RBC volumes, the RBCs settle faster than in blood containing normal RBC volume.
Related Tests
Complement Assay (p. 172). Some of the complement components are also acute-phase reactant proteins.
Fibrinogen (p. 241). This is an important protein involved in the hemostatic mechanism. It is also an acute-phase reactant protein.
C-Reactive Protein (p. 184). This is also an acute-phase reactant protein.