Microscopic Studies and Associated Testing
Reasons for Performing Microscopic Studies
Procedural Care for Microscopic Studies
Overview
Reasons for Performing Microscopic Studies
Microscopic examinations are essential for the diagnosis and treatment of numerous diseases and infectious processes. Included in this chapter are microbiologic studies and studies that require a microscopic review of tissue. Microbiologic specimens can be collected from many sources, such as tissue and organ biopsies, blood, urine, wound drainage, cervical secretions, and sputum. This testing usually takes place in the microbiology or bacteriology section of the laboratory. Microscopic examination is used in a wide variety of clinical situations, some of which include the following:
1. To evaluate hematologic disorders (bone marrow biopsy, blood smear)
2. To detect sexually transmitted diseases (sexually transmitted disease culture, smear, and wet mount)
3. To evaluate dysfunctional uterine bleeding (endometrial biopsy)
4. To determine liver pathologic conditions (liver biopsy)
5. To detect lung cancer (lung biopsy)
6. To screen for cancer of the vagina, cervix, and uterus (Papanicolaou test)
7. To determine the sensitivity of breast cancer to hormonal therapy (estrogen and progesterone receptor assays)
8. To detect renal disease, such as malignancy, glomerulonephritis, and transplant rejection (renal biopsy)
9. To detect tuberculosis (tuberculosis culture, AFB stain)
10. To evaluate and treat infections (body fluids, wound and soft-tissue culture and sensitivity)
11. To evaluate the urologic tract (see Urinalysis, p. 956)
Microscopic examinations are used to evaluate histologic and cytologic specimens and to identify bacteria (and other infecting organisms). Determination of hormone receptor assay results along with chromatin identification also requires microscopic examination of various types.
Included with microscopic studies are culture and sensitivity testing. Microscopic examination is an important part of identifying an infecting organism. A Gram stain is just one of the microscopic examinations performed with microbiology testing. A Gram stain is a method by which all bacteria are classified. All forms of bacteria are grossly classified as gram-positive (blue staining) or gram-negative (red staining). Furthermore, knowledge of the shape of the organism (e.g., spherical, rod shaped) also may be very helpful in the tentative identification of the infecting organism. For example, if the Gram stain indicates gram-negative rods, the infection may be caused by Escherichia coli. With knowledge of the Gram stain results, the physician can institute reasonable antibiotic treatment based on past experiences regarding the organism's possible identity and the source of the specimen. The Gram stain can be reported in less than 10 minutes after smearing the specimen on a microscopic slide. Treatment can then be altered based on the final results of culture and sensitivity testing.
The usual culture is obtained from a smear of an infected area (e.g., a wound culture). However, body fluids or tissue can be sent for culture techniques. When plated on the appropriate culture medium, an infecting organism can be expected to grow. Frequently several different kinds of culture media are used in the culture process to maximize the chances of growing the infecting organism. Some bacteria or fungi grow better in one medium than another.
Most of the time the infecting organism is identified from a culture plate on which the organism is growing. In other, rarer situations the infecting organism is found by microscopic review of a tissue specimen. In still other situations the only evidence of infection is derived from serologic testing (e.g., antistreptolysin O titer; see Chapter 2).
Although there are no potential complications associated with culture testing, the risks involved in obtaining tissue for microscopic examination may be considerable. They are well outlined in the discussion of each specific study.
Procedural Care for Microscopic Studies
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Inform the patient of any special preprocedure requirements. For example, patients should not douche or tub bathe before cervical cultures for herpes. Men should not void for 1 hour before collection of urethral specimens.
Inform the patient of any special preprocedure requirements. For example, patients should not douche or tub bathe before cervical cultures for herpes. Men should not void for 1 hour before collection of urethral specimens.
![]() Inform the patient about the collection technique. These techniques vary from being noninvasive (throat culture) to lightly invasive (liver biopsy).
Inform the patient about the collection technique. These techniques vary from being noninvasive (throat culture) to lightly invasive (liver biopsy).
• Invasive studies require an informed consent. Coagulation profiles are often done before invasive studies because of the risk of bleeding.
• If an invasive procedure is to be performed to obtain tissue, the patient should be prepared as if surgery were a possibility because if bleeding or organ injury occurs, the patient must be ready to go to surgery.
During
• Follow universal precautions in handling all specimens because of the risk of transmitting infection or contaminating the specimen.
• Specific protocols for collection are described with each test in this chapter.
• Instruct the patient to remain still during specimen collection. The quality of the specimen depends in part on the cooperation of the patient.
After
• The specimen should be carefully labeled with the patient's name, the source, and any other pertinent information, such as antibiotic therapy.
• The specimen should be promptly transported to the laboratory or pathology department.
• Antibiotic therapy should be initiated after the specimen is collected.
• Vital signs should be carefully evaluated to detect bleeding, infection, or other potential complications of an invasive procedure.
• If the results indicate a sexually transmitted disease (STD), sexual partners should be notified, evaluated, and treated.
Laboratory Handling of Specimens
The laboratory has protocols to minimize factors that may interfere with testing results. Certain specimens must be processed immediately (such as cerebrospinal fluid [CSF] cultures). Some specimens (such as urine) may be refrigerated if there will be a delay before testing. Stained smears of medically urgent specimens should be evaluated and reported immediately. Cultures should be plated immediately. This is essential to avoid bacterial deterioration. All efforts must be made to avoid the contamination of a culture to decrease the likelihood of incorrect results.
In addition to protocols concerning timeliness in handling of the specimen, the laboratory must also have strict guidelines for rejecting a specimen. An improperly identified specimen is the main reason for rejection. It is obvious that labeling the wrong patient with a diagnosis such as gonorrhea or some other STD can have devastating consequences. Desiccated, poorly preserved specimens, or specimens placed in the wrong container would be considered unsatisfactory.
Reporting of Results
Most microbiologic examinations require several days before results are available. The specimens often must go through a staining process that takes at least 24 hours. Some tissue for microscopic examination needs to be sent to reference laboratories for evaluation. Results take much longer to obtain in these cases. Preliminary culture reports and Gram stain results, however, are available much sooner. The quality of microscopic study results depends on the quality of the personnel obtaining, transporting, and handling the specimens. The experience of the physician and technologist reporting the results is also a key component in the process. Microscopic studies are invaluable in making a diagnosis. Not only is communication among the various health care providers imperative, but also reporting must be timely, concise, and accurate.
Acid-Fast Bacilli Smear (AFB Smear)
Indications
This smear (usually of sputum) is used to support the diagnosis of tuberculosis (TB). The diagnosis of TB cannot be made with positive results of an AFB smear by itself. TB cultures are required. AFB smears are also used to monitor treatment of TB. The test is indicated in any patient with a persistent productive cough, night sweats, anorexia, weight loss, fever, hemoptysis, or abnormal chest x-ray. This smear should especially be considered in high-risk patients, such as those who are immunocompromised, are alcoholic, or have had a recent exposure to TB.
Test Explanation
The most clinically significant AFB is Mycobacterium tuberculosis. This is the causative agent in TB. After taking up the fuchsin dye, M. tuberculosis is not decolorized by acid alcohol (i.e., it is acid-fast). It is seen under the microscope as a red or pink, rod-shaped organism. If this bacillus is seen, the patient may have active TB. However, other species of microbes such as Mycobacterium, Nocardia, and some fungi are also acid-fast. The AFB smear is most commonly performed on sputum. At least 5000 organisms must be present in each milliliter of specimen to be seen on a microscope smear. Other specimens, such as CSF, tissue, and synovial fluid, may be used. Smears may be negative as much as 50% of the time even with positive cultures. One cannot make the diagnosis of TB based only on a positive smear for AFB. Cultures (p. 768) must be positive for a definitive diagnosis. Also, cultures are the only way to determine drug sensitivities for treatment.
AFB is also used to monitor treatment for TB. If after adequate therapy (2 months), the sputum still contains AFB (even though the culture may be negative because of anti-TB drugs), treatment failure should be considered. Cavitary disease may cause this same picture (positive smear, negative cultures).
Interfering Factors
• False-negative results can occur because of faulty laboratory techniques.
• False-positive results can occur when the water used to suspend the smear on the slide contains a non-TB organism.
Procedure and Patient Care
Before
![]() Explain the procedure for sputum collection.
Explain the procedure for sputum collection.
![]() Remind the patient that the sputum must be coughed up from the lungs and that saliva is not sputum. The first morning specimen is usually best.
Remind the patient that the sputum must be coughed up from the lungs and that saliva is not sputum. The first morning specimen is usually best.
• Hold antibiotics until after the sputum has been collected.
• Give the patient a sterile sputum container the night before the sputum is to be collected so that the morning specimen may be obtained when the patient awakens.
![]() Instruct the patient to rinse out his or her mouth with water before the sputum collection to decrease contamination by particles in the oropharynx. Remind the patient not to use antiseptic mouthwash.
Instruct the patient to rinse out his or her mouth with water before the sputum collection to decrease contamination by particles in the oropharynx. Remind the patient not to use antiseptic mouthwash.
During
• For best results, obtain sputum collection when the patient awakens in the morning.
• Collect at least 1 teaspoon of sputum in a sterile sputum container.
• Obtain sputum by having the patient cough after taking several deep breaths.
• If the patient is unable to produce a sputum specimen, stimulate coughing by lowering the head of the patient's bed or by giving the patient an aerosol administration of a warm hypertonic solution.
• Note that other methods to collect sputum, such as endotracheal aspiration, fiberoptic bronchoscopy, and transtracheal aspiration, may be used if necessary.
• For AFB determinations, collect sputum on three separate occasions.
Related Tests
Tuberculin Culture (p. 768). This is the only manner in which the diagnosis of TB can be made with certainty. When TB is grown from the culture of a specimen, the diagnosis of TB can be made and treatment started based on drug sensitivities.
Tuberculin Skin Testing (p. 1126). Purified protein derivative is administered intradermally to test for prior exposure to TB. Skin testing cannot indicate active or dormant TB. Positive results indicate nothing more than previous exposure.
Chest X-Ray (p. 1014). Because TB is usually infective to the lungs from inhalation of airborne infectious material, the chest x-ray examination often demonstrates the results (Ghon complex) of the acute granulomatous infection.
Blood Culture and Sensitivity (Blood C&S)
Test Explanation
Bacteremia (the presence of bacteria in the blood) can be intermittent and transient, except in endocarditis or suppurative thrombophlebitis. The episode of bacteremia is usually accompanied by chills and fever; thus the blood culture should be drawn when the patient manifests these signs to increase the chances of growing bacteria on the cultures. It is important that at least two culture specimens be obtained from two different sites. If one produces bacteria and the other does not, it is safe to assume that the bacteria in the first culture may be a contaminant and not the infecting agent. When both cultures grow the infecting agent, bacteremia exists and is a result of the organism that is growing in the culture. If the patient is receiving antibiotics during the time that the cultures are drawn, the laboratory should be notified. Resin can be added to the culture medium to negate the antibiotic effect in inhibiting growth of the offending bacteria in the culture. If cultures are to be performed while the patient is receiving antibiotics, the blood culture specimen should be taken shortly before the next dose of the antibiotic is administered. All cultures preferably should be performed before antibiotic therapy is initiated.
Culture specimens drawn through an intravenous (IV) catheter are frequently contaminated, and tests using them should not be performed unless catheter sepsis is suspected. In these situations, blood culture specimens drawn through the catheter help to identify the causative agent more accurately than a culture specimen from the catheter tip.
Most organisms require approximately 24 hours to grow in the laboratory, and a preliminary report can be given at that time. Often 48 to 72 hours are required for growth and identification of the organism. Anaerobic organisms may take longer to grow. Cultures may be repeated after antibiotic therapy to assess resolution of the infection. (See Figure 7-1.)

Figure 7-1 Bactec automated blood culture instrument. Note that each blood culture is separately cultured and incubated. CO2 is monitored in each culture bottle. Positive results are automatically indentified by rising CO2 levels. If positive, bacteria is isolated and identified, Automated antibiotic sensitivities are then performed.
Procedure and Patient Care
During
• Carefully prepare the proposed venipuncture site with an antiseptic solution. Allow the skin to dry.
• Clean the tops of the Vacutainer tubes or culture bottles with an antiseptic solution (such as chlorhexidine, 70% isopropyl alcohol, or Betadine). Allow the area to dry.
• Venous blood by venipuncture from each site is collected into a vacuum blood culture container containing culture media. One is for aerobic, and a second is for anaerobic cultures. A different vacuum container can be used if the amount of blood is less than 3 mL (pediatrics).
• Label the specimen with the patient's name, date, time, and tentative diagnosis.
• Indicate on the laboratory slip the collection site (e.g., left arm or IV line) and any medications that may affect test results.
Test Results and Clinical Significance
Bacteremia: The bacteria growing in the blood can often be grown in the culture medium within the microbiology laboratory. When bacteremia exists, the patient must be considered gravely ill and antibiotics should be started immediately after blood cultures are obtained.
Blood Smear (Peripheral Blood Smear, Red Blood Cell Morphology, RBC Smear, WBC Differential)
Indications
Examination of the peripheral blood smear can provide a significant amount of information concerning drugs and diseases that affect the RBCs, WBCs, or platelets. Furthermore, other congenital and acquired diseases can be diagnosed by an examination of the peripheral blood smear. When special stains are applied to the blood smear, infection, infestation, leukemia, and other diseases can be identified.
Test Explanation
When adequately prepared and examined microscopically by an experienced technologist or pathologist, a smear of peripheral blood is the most informative of all hematologic tests. All three hematologic cell lines—erythrocytes (RBCs), platelets, and leukocytes (WBCs)—can be examined. In the peripheral blood, five different types of leukocytes can routinely be identified—neutrophils, eosinophils, basophils, lymphocytes, and monocytes. The first three are also referred to as granulocytes. (See discussion of bone marrow biopsy on p. 712 for more information concerning the various elements of blood.)
Microscopic examination of the RBCs can reveal variations in RBC size (anisocytosis), shape (poikilocytosis), color, or intracellular content. Classification of RBCs according to these variables is most helpful in identifying the causes of anemia and the presence of other diseases.
RBC Intracellular Structure
Mature RBCs are round with a small central pallor without any intracellular structures. They do not have a nucleus. Immature RBCs (reticulocytes) do contain intracellular RNA. Immature nucleated cells are not normally found in the peripheral blood and indicate increased RBC synthesis.
Basophilic stippling (refers to bodies enclosed or included in the cytoplasm of the RBCs)
Howell-Jolly bodies (small, round remnants of nuclear material remaining within the RBC)
WBC Examination
The WBCs are examined for total quantity, percentage of each type of WBC, and degree of maturity. An increased number of immature WBCs can indicate leukemia or infection. A decreased WBC count indicates a failure of marrow to produce WBCs (drugs, chronic disease, neoplasia, or fibrosis), peripheral destruction, or sequestration.
Procedure and Patient Care
During
• Collect a drop of blood from a finger stick or heel stick (in an infant) and place it on a slide for smearing. The single drop of blood is spread across the slide with a second slide at a 25-degree angle to form a feathered edge.
• If necessary, perform a venipuncture and collect the blood in a lavender-top tube.
• Note that a blood smear is first studied with an automated calculator programmed to recognize abnormal blood cell shapes and other irregularities. A more accurate smear is performed by a technologist. Low counts may be “hand counted” to ensure accuracy. The most accurate smear requires review by a pathologist.
Related Tests
Complete Blood Cell Count and Differential Count (p. 174). This is a battery of tests performed on the peripheral blood to measure the quantity of each blood component.
Bone Marrow Biopsy (see following test). This is a test on the bone marrow, which forms the components of the peripheral blood.
Bone Marrow Biopsy (Bone Marrow Examination, Bone Marrow Aspiration)
Normal Findings
Active erythroid cell line, myeloid and lymphoid cell lines, and megakaryocyte (platelet) production:
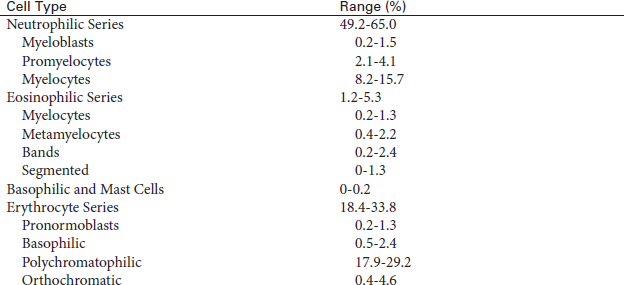
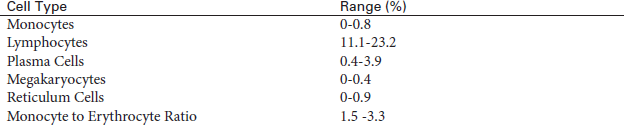
Normal iron content demonstrated by staining with Prussian blue
 Critical Values
Critical Values
A physician should be notified when there is a new diagnosis of leukemia, lymphoma, metastatic malignancy, infection, or hemolytic anemia. This notification is usually performed by the interpreting pathologist.
Indications
Bone marrow examination is an important part of the evaluation of patients with hematologic diseases. Indications for bone marrow examination include the following:
Test Explanation
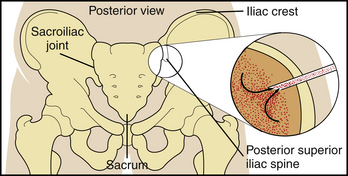
All of the cells circulating through the bloodstream—leukocytes (white blood cells) that fight infections, erythrocytes (red blood cells) that carry oxygen to the tissues, and platelets (clotting cells) that prevent bleeding—are made by precursor cells in a fatty matrix inside the hollow of our bones called bone marrow. At birth there is bone marrow in the hollow space inside all of the bones of the body as well as some bone marrow cells in the liver, spleen, and bloodstream. As the human body grows, the bone marrow cells are confined to the hollow space of the flat bones of the body, specifically, the skull, the sternum, the ribs, or the bones of the pelvis (the iliac bones). When a patient has unexplained abnormal blood counts, has abnormal cells circulating in the blood, or is diagnosed with a disease that can involve the bone marrow (lymphoma) or metastasize to the bone marrow (some carcinomas), a bone marrow biopsy is performed to examine the cells in the marrow space. Because the marrow in adults is in the flat bones, the standard location for sampling bone marrow is the iliac bones of the pelvis. Accepted practice is that the safest location to use for entering the iliac bone to obtain a sample is the posterior superior iliac spine of the pelvis (see Figure 7-2, p. 716). There, the blood-forming cells in the marrow produce the blood cells and release them into the circulation.
By examination of a bone marrow specimen the hematologist can fully evaluate hematopoiesis. Examination of the bone marrow reveals the number, size, and shape of the RBCs, WBCs, and megakaryocytes (platelet precursors) as these cells evolve through their various stages of development in the bone marrow. Samples of the bone marrow can be obtained by either aspiration or bone marrow biopsy. An aspiration provides a small quantity of different cell types and provides a sample for bone marrow cell morphology immunophenotyping, cytogenetics, or microbiology cultures. An aspiration is usually performed at the same time as the bone marrow biopsy.
Bone marrow biopsy includes estimation of cellularity, determination of the presence of infiltrative diseases (fibrosis or neoplasms, both primary and metastatic), and estimation of iron storage. The bone marrow biopsy is more accurate than the bone marrow aspiration because aspiration removes only a small amount of marrow and may not be truly representative of the entire marrow. Immunophenotyping by flow cytometry is able to identify cell specific antibodies on the surface of the cells examined. Cell percentages are more accurately determined and abnormal cell patterns can be identified. Ploidy status and S-phase analysis are also provided. Florescent hybridization (FISH) analysis is also performed with various DNA probes chosen based on the indication for bone marrow biopsy (e.g., lymphoma). These probes can identify genetic translocations and rearrangements that may impact disease prognosis and treatment.
For the estimation of cellularity, the specimen is examined and the relative quantity of each cell type determined. Leukemias or leukemoid drug reactions are suspected when increased numbers of leukocyte precursors are present. Physiologic marrow leukemoid compensation is also seen with infection. Decreased numbers of marrow leukocyte precursors occur in patients with myelofibrosis, metastatic neoplasia, or agranulocytosis; in elderly patients; and following radiation therapy or chemotherapy. Some drugs can diminish leukocyte production.
Increased numbers of marrow RBC precursors occur with polycythemia vera or as physiologic compensation to blood loss (hemorrhage or hemolysis). Decreased numbers of marrow RBC precursors occur with erythroid hypoplasia following chemotherapy, radiation therapy, administration of other toxic drugs, iron administration, or marrow replacement by fibrotic tissue or neoplasms.
Increased numbers of platelet precursors (megakaryocytes) can be the result of compensation to platelet loss from a recent hemorrhage. They are also seen in some forms of acute and chronic myeloid leukemias. This increase also may be compensatory in patients with platelet sequestration (secondary hypersplenism associated with portal hypertension). Platelet counts decrease, and the marrow compensates by increasing production. Decreased numbers of megakaryocytes occur in patients who have had radiation therapy, chemotherapy, or other drug therapy and in patients with neoplastic or fibrotic marrow infiltrative diseases. Patients with aplastic anemia also have decreased numbers of megakaryocytes.
Increased numbers of lymphocyte precursors occur in chronic, viral, or mycoplasma infections, lymphocytic leukemia, and lymphoma. Plasma cells and lymphocytes are increased in patients with multiple myelomas, lymphomas, hypersensitivity states, rheumatic fever, and other chronic inflammatory diseases.
Estimation of cellularity also can be expressed as a ratio of myeloid (WBC) to erythroid (RBC) cells (M/E ratio). The normal M/E ratio is approximately 3:1. The M/E ratio is greater than normal in those diseases in which leukocyte precursors are increased or erythroid precursors are decreased. The M/E ratio is below normal when either leukocyte precursors are decreased or erythroid precursors are increased.
Drug-induced or idiopathic myelofibrosis can be detected by examination of the bone marrow. Using special stains, one can estimate iron stores with a marrow biopsy.
Bone marrow aspiration/biopsy are performed by a physician. The duration of these procedures is approximately 20 minutes. The patient may have some apprehension when pressure is applied to puncture the outer table of the bone during biopsy-specimen removal or aspiration. The patient probably will feel pain during lidocaine infiltration and pressure when the syringe plunger is withdrawn for aspiration.
Contraindications
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Obtain a written informed consent for this procedure.
![]() Encourage the patient to verbalize fears because many patients are anxious concerning this study.
Encourage the patient to verbalize fears because many patients are anxious concerning this study.
• Assess the results of the coagulation studies. Report any evidence of coagulopathy to the physician.
• Obtain an order for sedatives if the patient appears extremely apprehensive.
![]() Remind the patient to remain very still throughout the procedure.
Remind the patient to remain very still throughout the procedure.
During
• Note the following procedural steps for bone marrow aspiration, which is performed on the sternum, iliac crest, anterior or posterior iliac spines, and proximal tibia (in children):
1. The procedure is usually performed at the patient's bedside using local anesthesia.
2. A preferred site is the posterior iliac spine, with the patient placed prone or on the side.
3. The area overlying the bone is prepared with an antiseptic solution and draped in a sterile manner.
4. The overlying skin and soft tissue, along with the periosteum, is infiltrated with lidocaine.
5. A small (<1 mm) incision is made in the skin directly over the posterior superior iliac spine.
6. A large-bore needle containing a stylus is slowly advanced through the soft tissue and into the outer table of the bone (Figure 7-2).
7. Once inside the marrow, the stylus is removed and a syringe is attached.
8. A 0.5- to 2-mL sample of bone marrow is aspirated, smeared on slides, and allowed to dry.
• Note the following procedural steps for bone marrow biopsy:
1. The skin and soft tissues overlying the bone are incised.
2. A core biopsy instrument is “screwed” into the bone.
3. The biopsy specimen is obtained and sent to the pathology laboratory for analysis.
• Note that aspiration is performed by a trained nurse or physician. Bone marrow biopsy is usually performed by a physician. The duration of these studies is approximately 20 minutes.
![]() Inform the patient that he or she may have some apprehension when pressure is applied to puncture the outer table of the bone during biopsy specimen removal or aspiration.
Inform the patient that he or she may have some apprehension when pressure is applied to puncture the outer table of the bone during biopsy specimen removal or aspiration.
![]() Tell the patient that he or she probably will feel pain during lidocaine infiltration and discomfort when the syringe plunger is withdrawn for aspiration.
Tell the patient that he or she probably will feel pain during lidocaine infiltration and discomfort when the syringe plunger is withdrawn for aspiration.
After
• Apply pressure to the puncture site to arrest minimal bleeding. Apply an adhesive bandage.
• Observe the puncture site for bleeding. Ice packs may be used to help control bleeding.
• Assess for tenderness and erythema, which may indicate infection. Report this to the physician.
• Evaluate the patient for signs of shock (increased pulse rate, decreased blood pressure).
• Normally, place the patient on bed rest for 30 to 60 minutes after the test.
• Note that some patients complain of tenderness at the puncture site for several days after this study. Mild analgesics may be ordered.
Test Results and Clinical Significance
Infiltrative diseases are evident histologically regarding the specific cause and by demonstrating hypocellularity within the marrow.
Infection—viral, bacterial, fungal: Bacterial infection is usually associated with increased neutrophilic elements. However, in overwhelming sepsis, these elements may be depressed. Viral and some fungal infections are characterized by increases in monocytic elements.
Agranulocytosis: The marrow has no myeloblast cells.
Polycythemia vera: There is an abundance of erythroid cellular elements.
Waldenström's macroglobinemia:
These cancers may be associated with overwhelming presence of mononuclear elements within the marrow.
Leukemia: The myeloid precursor cells are significantly increased and crowd the marrow.
Hypersensitivity states: This may be evident as an increase in eosinophilic and basophilic myeloblast elements.
Acute hemorrhagic marrow hyperplasia: Following an acute hemorrhage, the erythroid (and to some extent, the myeloid) elements are greatly increased to compensate for the loss of cells in the peripheral blood system.
Anemia: If the anemia is because of marrow failure, erythroid precursors will be deficient in number. Specifics about the appearance of the marrow cells (e.g., megaloblasts in B12 deficiency) may indicate the cause of the marrow failure. Special stains may reveal deficient iron storage, etc.
Mononuclear precursor elements may be increased.
Acquired immunodeficiency syndrome (AIDS): Decreased leukocytic elements may be noted, especially as the disease progresses.
Related Test
Blood Smear (p. 710). These test results are usually reported in the bone marrow biopsy report so as to corroborate the findings and support the indications for the procedure.
Breast Cancer Tumor Analysis (Breast Cancer Predictors, DNA Ploidy Status, S-Phase Fraction, Cathepsin D, HER 2 [c erbB2, neu] Protein, Ki67 Protein, p53 Protein)
Indications
This testing is performed on the breast cancer tissue and is used to predict the possibility of breast cancer relapse after curative primary surgery.
Test Explanation
The most important predictor of recurrent breast cancer is stage of disease, including lymph node status. Patients with positive lymph node metastasis are more likely to develop recurrence. However, nearly 30% of the patients whose tumor has been completely removed and who have no evidence of lymph node metastasis will also develop recurrence. It would be helpful to predict the patients who are destined for recurrence so that they can be selected for systemic therapy, while patients who will not have a recurrence can be spared the morbidity of a treatment that is not needed. Conventional predictors of tumor recurrence such as tumor size, grade, histologic type, and hormone receptors provide some information and are used alongside of the predictors that we mention in this discussion of breast cancer tumor analysis. Although estrogen and progesterone receptors are also breast cancer prognostic indicators, they are discussed separately on pp. 728 and 750. Furthermore, in addition to HER-2/neu testing, there are more accurate prognosticators for breast cancer (e.g., breast cancer genomic testing), discussed on p. 1086.
Ploidy (DNA Index) and S-Phase Fraction
Measurement of the rapidity with which the cells in a breast cancer grow includes ploidy status and S-phase analysis. Normally, cells are diploid (one set of paired chromosomes) and have a small number of cells in the S-phase of cell division. During the mitotic phase of cell division, the amount of DNA doubles (two sets of paired chromosomes) in preparation for cell division. Because the more aggressive cancer cells divide more rapidly, many cells are in various stages of the mitotic phase. These cells may have a variable number of chromosome sets (aneuploid).
It has been noted that the more aggressive cancer cells are more often in S-phase (a time of DNA replication in which the amount of DNA in the cell doubles while the ploidy remains the same). This is usually reported as S-phase fraction (SPF), that is, the number of cells in S-phase divided by the total number of cancer cells in the particular specimen. The laboratory methods have been automated by flow cytometry with laser-stimulated DNA fluorescence.
Cathepsin D
This protein catabolic enzyme was found to be absent in resting breast tissue but markedly elevated in malignant tissue. This presence of this protein on the cellular membrane of the malignant cells correlates with higher risk of recurrent breast cancer. The exact cutoff point between favorable prognosis and unfavorable prognosis has yet to be standardized. Like most cell surface protein markers, monoclonal antibody immunohistochemical techniques are used to identify this protein.
HER-2 (c erbB2, neu) Protein
HER-2/neu, which stands for “human epidermal growth factor receptor 2,” is a protein associated with a higher aggressiveness in breast cancers. The HER-2/neu oncogene encodes a transmembrane tyrosine kinase receptor with extensive similarity to other epidermal growth factor receptors. It is normally involved in the pathways leading to cell growth and survival. Approximately 15% to 20% of breast cancers have an amplification of the HER-2/neu gene or overexpression of its protein product. Overexpression of this receptor in breast cancer is associated with increased disease recurrence and worse prognosis.
There are two commonly used methods to measure HER-2/neu protein. Immunohistochemistry (IHC), although the easier, can be less accurate. This test measures the production of the HER-2 protein by the tumor. The test results are ranked as 0, 1+, 2+, or 3+. If the results are 3+, the cancer is HER-2–positive. Fluorescence in situ hybridization (FISH) has become the “standard criterion” method to measure HER-2/neu protein in tumor tissue. This test method uses fluorescent probes to look at the number of HER-2 gene copies in a tumor cell. If there are more than two copies of the HER-2 gene, the cancer is HER-2 positive. RT-PCR methods are more accurate, but more cumbersome and more costly.
HER-2 testing is also helpful in making treatment decisions. Because tumors that overexpress HER-2/neu are more aggressive, more aggressive adjuvant chemotherapy is recommended to women with these tumors. It has been found that the HER-2 gene can act as a target for an antineoplastic monoclonal antibody drugs (e.g., trastuzumab, Herceptin). Trastuzumab is effective only in breast cancer in which the HER-2/neu receptor is overexpressed. One of the mechanisms of trastuzumab after it binds to HER-2 is to halt cell proliferation.
p53 Protein
The p53 gene is a tumor suppressor gene that is overexpressed in more aggressive breast cancer cells. Mutation of the gene causes overexpression and a buildup of mutant proteins on the surface of the cancer cells. Overexpression of p53 can be identified by studying the gene or by immunohistochemical staining of paraffin-embedded breast cancer tissue for the mutant proteins.
Procedure and Patient Care
Related Tests
Estrogen Receptor Assay (p. 728) and Progesterone Receptor Assay (p. 750). These are other prognostic markers for breast cancer.
Breast Cancer Genomics (p. 1086). This test is used to predict the possibility of cancer susceptibility to chemotherapy. It is also a powerful indicator of the likelihood of breast cancer recurrence after primary breast cancer surgery.
Cervical Biopsy (Punch Biopsy, Endocervical Biopsy, LEEP Cervical Biopsy, Cone Biopsy, Conization)
Indications
A biopsy of the cervix is performed to more accurately identify and treat premalignant and superficial malignant lesions of the cervix.
Test Explanation
When a Papanicolaou (Pap) test reveals an “epithelial cell abnormality” or when a pelvic examination reveals a possible neoplastic abnormality in the cervix, a biopsy of that structure is indicated. There are several different methods to perform the biopsy, all of which obtain an increasing amount of tissue. Cervical biopsy procedures include:
• A simple cervical biopsy, sometimes called a punch biopsy, removes a small piece of tissue from the surface of the cervix. This is often performed during colposcopy, see p. 595.
• An endocervical biopsy (endocervical curettage) removes tissue from high in the cervical canal by scraping with a sharp instrument.
• Loop electrosurgical excision procedure (LEEP) uses a thin, low-voltage electrified wire loop to cut out abnormal tissue on the cervix and high in the endocervical canal (sometimes called a large loop excision of the transformation zone [LLETZ]).
• A cone biopsy (conization) is a more extensive form of a cervical biopsy. It is called a cone biopsy because a cone-shaped wedge of tissue is removed from the cervix. Both normal and abnormal cervical tissues are removed. This can be performed by LEEP, surgical knife (scalpel), or a carbon dioxide laser.
After colposcopy or a cervical biopsy, LEEP may be used to treat abnormal, precancerous cells found on biopsy. It can also be used to assess the extent and sometimes to treat noninvasive superficial cervical cancers.
Procedure and Patient Care
During
• Note the following procedural steps:
1. The patient is placed in the lithotomy position, and a vaginal speculum is used to expose the vagina and cervix.
2. The cervix is cleansed with a 3% acetic acid solution or iodine to remove excess mucus and cellular debris and to accentuate the difference between normal and abnormal epithelial tissues.
3. Medication may be injected to numb the cervix (cervical block).
4. With the instrument chosen by the doctor a punch biopsy, endocervical biopsy, LEEP, or cone biopsy is performed.
• Note that a physician performs the procedure in approximately 5 to 10 minutes.
• Although cone biopsy is done in the operating room, the other procedures can be performed in the doctor's office.
![]() Tell the patient that some women complain of pressure pains from the vaginal speculum and that discomfort may be felt if biopsy specimens are obtained.
Tell the patient that some women complain of pressure pains from the vaginal speculum and that discomfort may be felt if biopsy specimens are obtained.
• Most women can return to normal activities immediately after a simple cervical biopsy or an endocervical biopsy.
• Most women will be able to return to normal activities within 2 to 4 days after LEEP or cone biopsies. This can vary depending on the amount of tissue removed.
After
![]() Inform the patient that it is normal to experience the following:
Inform the patient that it is normal to experience the following:
• Vaginal bleeding if biopsy specimens were taken; suggest that she wear a sanitary pad
• Mild cramping for several hours after the procedure
![]() Instruct the patient that sanitary napkins should be used instead of tampons for 1 to 3 weeks.
Instruct the patient that sanitary napkins should be used instead of tampons for 1 to 3 weeks.
![]() Inform the patient when and how to obtain the results of this study.
Inform the patient when and how to obtain the results of this study.
Chlamydia
Indications
Chlamydia testing is performed on patients with symptoms compatible with the wide variety of diseases this organism can cause. In the United States, its most common form is pelvic inflammatory disease. This form of sexually transmitted disease (STD) commonly presents as pelvic pain and/or vaginal discharge. Cervical cultures or smears are performed on patients who have these complaints. See also Sexually Transmitted Disease Testing (p. 756).
Test Explanation
There are many Chlamydia species that cause various diseases within the human body. Chlamydia psittaci causes respiratory tract infections and occurs with close contact with infected birds. C. pneumoniae, another species, causes pneumonia. C. trachomatis infection is the most frequently occurring STD in developed countries and is also discussed in STD testing (p. 756). Infections of the genitalia are most common, followed by those of the conjunctiva, pharynx, urethra, and rectum. Lymphogranuloma venereum was the first form of venereal disease recognized as a C. trachomatis infection; this infection is very common in central Africa. The second serotype of C. trachomatis causes the eye disease trachoma, which is the most common form of preventable blindness. A third serotype produces genital and urethral infections different from lymphogranuloma. This third type is transmitted by direct contact of the infant with the mother's cervix during vaginal delivery or by direct contact during sexual activity.
Chlamydia infection is thought to be the most widespread STD in the United States. This disease is most prevalent in those younger than 20 years, in nulliparas, and in users of nonbarrier contraceptive methods. Also, in those with multiple or recent, new sexual partners, Chlamydia is frequently associated with gonorrhea.
Most women colonized with Chlamydia are asymptomatic. Chlamydia may be associated with pelvic inflammatory disease, particularly in adolescents.
The Chlamydia organism can be detected in many different ways. It seems to be most accurately demonstrated by tissue culture. Although these cultures require a special cell culture line, which takes several days, they are used as the standard criterion against which other methods of detection of Chlamydia are measured. It is now possible to detect the Chlamydia antigen by utilizing deoxyribonucleic acid (DNA) probes.
With the increasing use of ThinPrep Cervical cytology (see Papanicolaou Test, p. 743), Chlamydia infections are being increasingly diagnosed. Transcription mediated amplification can be used to amplify Chlamydial ribosomal RNA or using DNA probes on a ThinPrep sample. This same technology can detect Chlamydia from a urine specimen.
Procedure and Patient Care
During
• Collect a venous blood sample in a red-top tube.
• Acute and convalescent serum should be drawn 2 to 3 weeks apart.
• A conjunctival smear is obtained by swabbing the eye lesion with a cotton-tipped applicator or scraping with a sterile ophthalmic spatula and smearing on a clean glass slide.
• Sputum cultures (p. 761) are used to check for C. psittaci respiratory infections.
• Refer to p. 757 for cervical culture and p. 758 for urethral cultures.
• Note these procedures are performed by a nurse or physician in several minutes.
Related Test
Sexually Transmitted Disease (STD) Testing (p. 756). Discussed here are other STDs and their causative agents.
Colon Cancer Tumor Analysis (Microsatellite Instability [MSI] Testing, DNA Mismatch Repair [MMR] Genetic Testing, BRAF Mutation Analysis, Oncotype DX Colon Cancer Assay)
Indications
This test is used to indicate the prognosis of a patient recently surgically treated for colon cancer to determine if additional chemotherapy will improve survival. Furthermore this test can be used to indicate the possibility that the colon cancer was hereditary, thereby encouraging other members of the patient's family to undergo testing.
Test Explanation
Patients with stage 1 colon cancer have a high cure rate with surgery alone. Patients with stage 3 colon cancer benefit from the use of adjuvant chemotherapy. However, patients with stage 2 colon cancer may or may not benefit from adjuvant chemotherapy. Colon cancer tumor analysis can help differentiate stage 2 patients who may benefit from adjuvant chemotherapy. This test is used to indicate the risk of recurrence colon cancer in the years succeeding surgical treatment.
Deficiencies in DNA mismatch repair (MMR) gene function, either because of decreased gene expression or mutation, result in the accumulation of DNA alterations that can manifest as abnormal shortening or lengthening of microsatellite DNA sequences in the colon cancer cell. This causes microsatellite instability (MSI). Patients with MMR deficient (MMR-D) colon tumors have high MSI and have been shown to have significantly lower colon cancer recurrence risk. Therefore testing the colon tumor for MMR and MSI can assist in determining the likelihood of recurrence after surgery and quantify any benefit from adjuvant chemotherapy.
Furthermore hereditary colon cancers frequently are positive for MSI as compared with sporadic colon cancers. Lynch syndrome (a hereditary form of colon cancer) can be suspected if the tumor is MSI positive. MSI is performed by immunohistochemical identification of specific nucleic acid. MMR genetic testing is most frequently performed by PCR testing.
BRAF is another important gene that is used to indicate the likelihood that a colon tumor is hereditary. BRAF is a kinase-encoding gene in the RAS/RAF/MAPK pathway. The presence of a BRAF V600E mutation in a microsatellite unstable tumor indicates that the tumor is probably sporadic and not associated with hereditary non-polyposis colorectal cancer (HNPCC). The lack of this mutation indicates that a tumor may either be sporadic or HNPCC associated.
The Oncotype DX Colon Cancer Assay evaluates 12 genes and provides an individualized score reflective of the risk of colon cancer recurrence for individual patients with stage 2 colon cancer. The assay uses a RT-PCR platform to quantitate the level of expression of each of the 12 genes in the panel using the patient's colon tumor. For each patient, the assay produces a recurrence score that is closely associated with the patient's risk of recurrent colon cancer 3 years after surgery (the peak time of recurrence). MMR and MSI testing can complement the information provided by the Oncotype DX Colon Cancer Assay.
Test Results and Clinical Significance
Colon cancer with unfavorable prognosis: This helps determine patients, who by the genomic make up of their colon cancer and other prognostic factors will benefit from the use of adjuvant chemotherapy. Patients whose prognosis is very good by genomic testing will not benefit from the addition of preventive chemotherapy.
Hereditary colon cancer: Patients with a BRAF genetic mutation or whose colon cancer has microsatellite instability have an increased risk that their cancer was hereditary. After genetic counseling, other family members may want to consider being tested for genetic predisposition to colon cancer and have early screening testing such as colonoscopy (p. 591) if they are positive.
Related Test
Genetic Testing (p. 1093). This discussion included genetic testing for other forms of familial colon cancer, including Lynch syndrome and familial polyposis.
Endometrial Biopsy
Indications
Endometrial biopsy had been used to determine if the patient has adequate ovarian estrogen and progesterone levels. This is indicated in women with suspected ovarian dysfunction (such as women who are nearing menopause, are not menstruating, or are infertile). It is most often used to diagnose and evaluate women who have dysfunctional uterine bleeding and uterine cancer.
Test Explanation
An endometrial biopsy can determine whether ovulation has occurred. A biopsy specimen taken 3 to 5 days before normal menses should demonstrate a “secretory-type” endometrium on histologic examination if ovulation and corpus luteum formation have occurred. If not, only a preovulatory “proliferative-type” endometrium will be seen. This test can determine if a woman has adequate ovarian estrogen and progesterone levels.
Another major use of endometrial biopsy is to diagnose endometrial cancer, polyps, or inflammatory conditions and to evaluate dysfunctional uterine bleeding.
This procedure is performed by an obstetrician/gynecologist in approximately 10 to 15 minutes. Minor discomfort (menstrual-type cramping) may be felt. It is important to recognize that an endometrial biopsy is not a substitute for a dilation and curettage (D&C). The D&C is much more extensive and tests all surfaces of the endometrium.
Contraindications
• Patients with infections (e.g., trichomonal, candidal, suspected gonococcal) of the cervix or vagina, because the infection may spread to the uterus
• Patients in whom the cervix cannot be visualized (e.g., because of abnormal position or previous surgery), because the cervix is the access to the uterus
• Patients who are pregnant, because the procedure may induce labor/abortion
Procedure and Patient Care
During
• Note the following procedural steps:
1. The patient is placed in the lithotomy position, and a pelvic examination is performed to determine the position of the uterus.
2. The cervix is exposed and cleansed.
3. A biopsy instrument is inserted into the uterus, and specimens are obtained from the anterior, posterior, and lateral walls. The biopsy can be done with a curette, forceps, or suction device. Suction endometrial biopsy is most commonly performed because it is the least painful and can be performed in the office.
4. The specimens are placed in a solution containing 10% formalin and sent to the pathologist for histologic or cytologic examination.
After
• Any temperature elevation should be reported to the physician because this procedure may activate pelvic inflammatory disease.
![]() Advise the patient to wear a pad because some vaginal bleeding is to be expected.
Advise the patient to wear a pad because some vaginal bleeding is to be expected.
![]() Instruct the patient to call her physician if excessive bleeding (requiring more than one pad per hour) occurs.
Instruct the patient to call her physician if excessive bleeding (requiring more than one pad per hour) occurs.
![]() Inform the patient that douching and intercourse are not permitted for 72 hours after the biopsy.
Inform the patient that douching and intercourse are not permitted for 72 hours after the biopsy.
![]() Instruct the patient to rest during the next 24 hours and to avoid heavy lifting to prevent increased intraabdominal pressure and uterine hemorrhage.
Instruct the patient to rest during the next 24 hours and to avoid heavy lifting to prevent increased intraabdominal pressure and uterine hemorrhage.
Test Results and Clinical Significance
Anovulation: Without ovulation the endometrium is persistently in the proliferative stage. No secretory changes are noted.
Endometrial adenocarcinoma with or without squamous carcinoma components is the most common uterine cancer. Hyperplastic proliferative polyps are a common cause of dysfunctional uterine bleeding.
Inflammatory condition: Endometrial infections are rare but do occur. Ascending sexually transmitted diseases (STDs) are the most common type of primary infection not associated with prior surgical instrumentation.
Estrogen Receptor Assay (ER Assay, ERA, Estradiol Receptor)
Indications
Estrogen receptor assay is performed on breast cancer tissue to indicate sensitivity to hormonal manipulative therapy and to indicate prognosis of breast cancer.
Test Explanation
The ER assay is useful in determining the prognosis and treatment of breast cancer. The assay is used to determine whether a tumor is likely to respond to endocrine therapy. Hormone receptor assays should be performed on all breast cancers. Breast tumors in postmenopausal women tend to be positive more often than in premenopausal women.
Slightly more than half of patients with breast carcinoma who are ER positive respond to endocrine therapy (e.g., tamoxifen, estrogens, aromatase inhibitors, oophorectomy). The response is greater when the progesterone receptors (see p. 750) are also positive. Patients whose breast cancers lack these hormone receptors (i.e., are ER negative) have a much lower chance of tumor response to hormone therapy and may not be candidates for this form of treatment.
Specimens are obtained from surgical specimens by a pathologist. ER assays are performed most commonly using immunohistochemical methods on fixed, paraffin-embedded tissue. Positive reactivity by immunohistochemistry is observed in the nuclei of the tumor cells. This method of measuring ER receptors is considered very accurate. Results are usually available in less than 1 week.
Related Tests
Progesterone Receptor Assay (p. 750). Like estrogen receptor assay, this test predicts the likelihood of tumor response to endocrine manipulative therapy.
Breast Cancer Genomics (p. 1086). This test is used as a prognosticator indicating the risk of recurrent breast cancer. It is a powerful predictor of benefit from hormone therapy or chemotherapy.
Breast Cancer Tumor Analysis (p. 717). These tests are prognostic as they indicate the risk of tumor recurrence. They also can be predictive of tumor response to some anti–breast cancer therapies.
Fungal Antibody (Antifungal Antibodies; Beta-D-glucan (1→3)-β-D-glucan, Fungitell)
Test Explanation
Fungal infections can be superficial, subcutaneous, or systemic (deep). The systemic fungal infections (mycoses) are the most important, for which serologic antibody testing is performed. Generally mycoses are caused by the inhalation of airborne fungal spores. In the United States, the most serious fungal infections are coccidioidomycosis, blastomycosis, histoplasmosis, and paracoccidioidomycosis. These infections start out as primary pulmonary infections. Aspergillus, Candida, and Cryptococcus systemic infections usually affect only those with compromised immunity (Table 7-1).
TABLE 7-1
Diseases Resulting from Fungal Infections
| FUNGUS | SYSTEMIC DISEASE | ENDEMIC AREA |
| Candida albicans | Candidiasis, thrush, yeast of mouth/esophagus | Ubiquitous |
| Cryptococcus neoformans | Infection of the lung, bloodstream; meningitis | Ubiquitous |
| Histoplasma capsulatum | Pulmonary infection | Caribbean, Central and South America |
| Coccidioides immitis | Pulmonary infection | Southwestern United States, Mexico, Central America |
| Aspergillus | Pulmonary infection | Ubiquitous |
Fungal antibody testing is not highly reliable. Antibodies are present in only about 70% to 80% of infected patients. When positive, they merely indicate that the person has an active or has had a recent fungal infection. These antibodies can be identified in the blood or cerebrospinal fluid. In general, more specific antibodies are tested only after screening antibody testing (e.g., complement fixation studies) are performed. Immunodiffusion is most commonly used to detect immunoglobulin (Ig)G antibodies in the blood. IgA and IgM antibodies can also be identified by enzyme immunoassay (EIA). These antibodies can be tested singularly or as a fungal panel. Cross-reactions can occur (e.g., antibodies to blastomycosis can cross-react with histoplasmosis antigens).
(1→3)-β-D-glucan is an enzyme immunoassay used to support the diagnosis of invasive fungal disease (IFD) in at-risk patients. Normally serum contains low levels of (1→3)-β-D-glucan, presumably from yeasts present in the alimentary and gastrointestinal tract. (1→3)-β-D-glucan is produced by most invasive fungal organisms. D-glucan becomes elevated well in advance of conventional clinical signs and symptoms of IFD. As opportunistic infections, IFDs are common among hematologic malignancy and AIDS patients. They account for a growing number of nosocomial infections, particularly among organ transplant recipients and other patients receiving immunosuppressive treatments.
Fungal antigen assays are available to detect a portion of the infecting fungus such as Aspergillus galactomannan. This uses ELISA technology. In general, the antigen assays are best used on blood, but CSF may also be studied in some instances. DNA sequencing or PCR can also identify fungal organisms from the specimen or culture tissue. However, negative molecular studies do not rule out infection.
Fungal organisms can be identified by culture growth and macroscopy/microscopy. Fungi components can occasionally be seen on Gram stain. For optimal recovery of organisms, a sufficient specimen should be transported within 24 hours of collection. Fungi can be pathogens, colonizers, or contaminants. Correlation of the patient's clinical condition with culture results is necessary. Nucleic Acid Probe/16S rDNA Sequencing/Real-Time Polymerase Chain Reaction is used for the identification of some fungi. Other fungi such as Coccidioides are identified by real-time PCR. Fungi can be cultured from blood; body fluids; CSF; fresh tissue; bronchopulmonary secretions; a swab of the ear, nose, and throat; or urine. An accurate fungal culture is labor intensive and requires a highly experience laboratory. Results are not available quickly.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Acute fungal infection: Fungal antibodies develop only with systemic or deep infections. Negative results do not rule out a fungal disease. Fungal molecular tests are more accurate, but the definitive diagnosis of fungal disease requires a positive culture and identification to be associated with the clinical picture.
Related Tests
Cultures of the Blood (p. 708), CSF (p. 651), Sputum (p. 761), Throat (p. 765), Urine (p. 973). When infection is suspected, bacterial infection should be ruled out at the same time appropriate fungal testing is being performed. These cultures predominantly discuss bacterial infection. However, the methodology of specimen accrual is the same.
Herpes Simplex (Herpesvirus Types 1 and 2, Herpes Simplex Virus Type 1 and 2 [HSV 1 and 2], Herpes Genitalis)
Indications
Herpes testing is performed to diagnose acute initial herpes infections. It is used on patients with suspected initial genital infection. It is also used in immunocompromised patients who have aggressive oral mucosal or genital eruptions compatible with the infection. Furthermore, it is used on patients (especially immunocompromised patients) who have a fever of unknown origin.
Herpes cultures are used to identify active genital herpes infection in women who are expecting to vaginally deliver a baby in the next 6 to 8 weeks.
Test Explanation
HSV can be classified as either type 1 or type 2. Type 1 is primarily responsible for oral lesions (blisters on the lips— “cold sores”) or even corneal lesions. About half of the patients with HSV 1 develop recurrent infections. HSV 2 is a sexually transmitted viral infection of the urogenital tract. Vesicular lesions may occur on the penis, scrotum, vulva, perineum, perianal region, vagina, or cervix. Initial infections are often associated with generalized symptoms of fever and malaise.
Because most infants become infected if they pass through a birth canal containing HSV, determining its presence at delivery is necessary. Congenital infections may result in problems such as microcephaly, chorioretinitis, and mental retardation in the newborn. Disseminated neonatal herpes virus infections carry a high incidence of infant mortality. A vaginal delivery is possible if no virus is present, but birth by cesarean section is necessary if HSV is present. Viral testing can be performed on males or females to determine the risk for sexual transmission.
Culture is still the standard criterion for HSV detection and can identify HSV in 90% of infected patients. Culture can be performed only during an outbreak. Serologic tests are more easily and conveniently available for detection of HSV 1 and HSV 2 antibodies. Serologic tests for herpes simplex are useful to supplement cultures or molecular detection for acute infection. Only about 85% of patients who are culture positive have positive serologies. The advantage of serology tests is that results can be available in a day. Serologic tests for IgG antibodies are available to help differentiate type 1 from type 2 infection. IgG antibodies indicate a previous exposure. IgM antibodies indicate an acute infection, but do not differentiate well between types 1 and 2. Perhaps more than 50% of people in the United States have positive herpes antibodies. Serologic tests for antibodies require repeated blood tests during the acute and convalescent phases of an acute viral outbreak (about 2 weeks apart). A fourfold rise in titer is expected to diagnose acute initial herpes infection. Recurrent infections are far less likely to demonstrate titer elevations.
The antibody tests use immunofluorescent immunoassay or enzyme-linked immunosorbent assay (ELISA) methods. Antibody testing cannot diagnose whether there is active recurrent genital herpes. Culture testing is required.
Fresh tissue is the definitive specimen for detection of HSV, particularly with suspected CNS disease. However, because brain biopsy is an invasive procedure, it is infrequently performed for laboratory diagnosis. Similarly, it is difficult to recover HSV from cerebrospinal fluid (CSF) specimens in culture systems, and the serologic diagnosis of HSV CNS disease has not been informative during early-onset disease. HSV PCR molecular detection of HSV DNA from CSF (as well as oral, genital, ocular, and other sites) is a sensitive and specific alternative for detection. PCR is a qualitative assay and results are reported as negative, positive, or indeterminate. The lower limit of detection for PCR is 10 DNA target copies/microliter.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Tell the female patient to refrain from douching and tub bathing for 24 hours before the cervical culture is performed.
Tell the female patient to refrain from douching and tub bathing for 24 hours before the cervical culture is performed.
• Obtain the urethral specimen from the male patient before he voids.
• Note that blood study results can be diagnostic in both males and females.
During
Urethral Culture
1. A culture is taken by inserting a sterile swab gently into the anterior urethra or genital skin lesion of the male patient (see Figure 7-8, p. 758).
2. It is advisable to place the male patient in the supine position to prevent falling if vasovagal syncope occurs during introduction of the cotton swab or wire loop into the urethra.
3. The patient is observed for hypotension, bradycardia, pallor, sweating, nausea, and weakness.
Cervical Culture
1. The female patient is placed in the lithotomy position, and a vaginal speculum is inserted.
2. Cervical mucus is removed with a cotton ball.
3. A sterile cotton-tipped swab is inserted into the endocervical canal and moved from side to side to obtain the culture. If a genital lesion is present, swabs from that area will be more sensitive in indicating infection.
• For pregnant women with herpes genitalis, note that the cervix is cultured weekly for the herpes virus beginning 4 to 6 weeks before the due date. Vaginal delivery is possible if the following criteria are met:
Molecular PCR Tissue and Other Fluids
• Obtain CSF (p. 651) or other fluids by sterile technique as described elsewhere in this book.
• Obtain tissue by appropriate biopsy techniques.
• Place specimen in an appropriate container designated by the reference laboratory.
Test Results and Clinical Significance
Herpes virus infection: Like other sexually transmitted disease (STDs), this disease can significantly affect patients, their children, and their sexual partners. If herpes or other STDs have been diagnosed, treatment should begin immediately and active sexual partners should be evaluated. Although acute outbreaks of herpes genitalis are treatable, the disease is not curable.
Liver Biopsy
Indications
Liver biopsy is a safe, simple, and valuable method of diagnosing pathologic liver conditions.
Test Explanation
For this study a specially designed needle is inserted through the abdominal wall and into the liver (Figure 7-3). A piece of liver tissue is removed for microscopic examination. Percutaneous liver biopsy is used in the diagnosis of various liver disorders, such as cirrhosis, hepatitis, drug reaction, granuloma, and tumor. Biopsy is indicated for patients with the following conditions that cannot be identified by other tests:
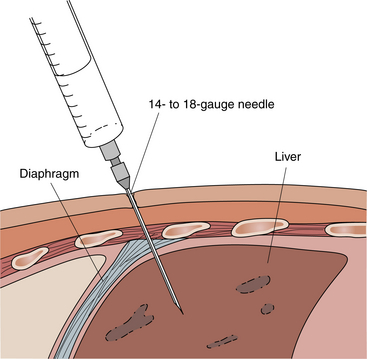
Figure 7-3 Liver biopsy. Percutaneous liver biopsy requires the patient's cooperation. The patient must be able to lie quietly and hold his or her breath after exhaling.
2. Persistently elevated liver enzyme levels
3. Suspected primary or metastatic tumor, as determined by other studies
6. Suspected infiltrative diseases (e.g., sarcoidosis, amyloidosis)
7. Hemochromatosis and Wilson's disease
8. Diseases in which biopsy is the only way to determine severity of disease
The biopsy may be performed by a “blind” stick or may be directed with the use of a computed tomography (CT) or magnetic resonance imaging (MRI) scan, ultrasound, or laparoscopy. Directed biopsy is used if there is a specific focal area of the liver that is suspect and from which tissue must be obtained (e.g., a metastatic tumor). The “blind” stick is used if the liver is diffusely involved.
This test is performed by a physician in approximately 15 minutes. Minor discomfort may be experienced during injection of the local anesthetic and during needle insertion and biopsy. In the past, blind biopsies were performed with small aspiration or small tissue-sampling needles. With guided biopsies, larger-core needles can obtain a significant amount of tissue for histologic review. This has reduced sampling errors both in placing the needle in the suspicious area and in obtaining enough tissue for histologic study.
Contraindications
• Uncooperative patients who cannot remain still and hold their breath during sustained exhalation
• Patients with impaired hemostasis
• Patients with anemia who could not tolerate major blood loss associated with inadvertent puncture of an intrahepatic blood vessel
• Patients with infections in the right pleural space or right upper quadrant, because the biopsy may spread the infection
• Patients with obstructive jaundice: In these patients, bile within the ducts is under pressure and may subsequently leak into the abdominal cavity after needle penetration.
• Patients with a hemangioma: This is a very vascular tumor, and bleeding after a biopsy may be severe.
• Patients with ascites, because persistent leak of fluid may occur: Further bile leaks will not seal off.
Potential Complications
• Hemorrhage caused by inadvertent puncture of a blood vessel within the liver
• Chemical peritonitis caused by inadvertent puncture of a bile duct, with subsequent leakage of bile into the abdominal cavity
• Pneumothorax (collapsed lung) caused by improper placement of the biopsy needle into the adjacent chest cavity
Procedure and Patient Care
Before
![]() Explain the procedure to the patient. Many patients are apprehensive about this procedure.
Explain the procedure to the patient. Many patients are apprehensive about this procedure.
• Obtain a medication history to be certain the patient is not taking medication that could affect coagulation.
• Ensure that all coagulation test results are normal.
![]() Instruct the patient to keep on nothing by mouth (NPO) status after midnight on the day of the test. Surgery may be necessary if a complication occurs. The patient must be prepared for the possibility of surgery.
Instruct the patient to keep on nothing by mouth (NPO) status after midnight on the day of the test. Surgery may be necessary if a complication occurs. The patient must be prepared for the possibility of surgery.
During
• Note the following procedural steps:
1. The patient is placed in the supine or left lateral position.
2. The skin area used for puncture is locally anesthetized.
3. The patient is asked to exhale and hold the exhalation. This causes the liver to descend and reduces the possibility of a pneumothorax. Frequently the patient practices exhalation two or three times before insertion of the needle.
4. During the patient's sustained exhalation the physician rapidly introduces the biopsy needle into the liver and obtains liver tissue.
• If laparoscopy is used to obtain the biopsy, follow the procedure outlined for laparoscopy (p. 617).
After
• Place the tissue sample into a specimen bottle containing formalin and send it to the pathology department.
• Apply a small dressing over the needle insertion site.
• Place the patient on his or her right side for approximately 1 to 2 hours. In this position the liver capsule is compressed against the chest wall, thereby decreasing the risk for hemorrhage or bile leak.
• Assess the patient's vital signs frequently for evidence of hemorrhage (increased pulse rate, decreased blood pressure) and peritonitis (increased temperature).
• If laparoscopy was performed, provide routine postoperative care.
• Evaluate the rate, rhythm, and depth of respirations. Assess breath sounds. Report chest pain and signs of dyspnea, cyanosis, and restlessness, which may be indicative of pneumothorax.
Test Results and Clinical Significance
Primary (hepatoma, cholangiocarcinoma),
Metastatic (bowel, breast, lung, etc.):
Biopsies of these focal lesions can be performed and specimens obtained for histologic study. Usually these biopsies are guided by imaging studies.
Infiltrative diseases (e.g., amyloidosis, hemochromatosis, cirrhosis, fat):
Related Tests
Computed Tomography (CT) Scan of the Abdomen (p. 1020). This is an x-ray study that provides excellent visualization of the liver for guided biopsy.
Magnetic Resonance Imaging (MRI) Scan of the Liver (p. 1106). This study uses variations in electromagnetic characteristics to provide an accurate image of the liver. Recently technology has advanced to allow the guidance of a biopsy needle to the suspect area within the liver.
Lung Biopsy
Indications
Lung biopsy is indicated to determine the nature of a pulmonary parenchymal nodule that has been identified on plain chest x-ray film or chest computed tomography (CT) scan. Carcinomas, granulomas, infections, and sarcoidosis can be diagnosed with this procedure. This procedure is also useful in detecting environmental exposures, infections, or familial disease, which may lead to better prevention and treatment.
Test Explanation
This invasive procedure is used to obtain a specimen of pulmonary tissue for a histologic examination by using either an open or a closed technique. The open method involves a limited thoracotomy. The closed technique includes methods such as transbronchial lung biopsy, transbronchial needle aspiration biopsy, transcatheter bronchial brushing, percutaneous needle aspiration biopsy, and video-assisted thoracostomy surgery (VATS).
Note that this procedure is performed by a radiologist, surgeon, or pulmonologist in 30 to 60 minutes. Most patients describe the percutaneous biopsy procedure as painful. Postoperative incisional pain can be expected if the open technique or VATS is used.
Contraindications
• Patients with bullae or cysts of the lung, because they have a greater risk of pneumothorax with needle lung biopsy
• Patients with suspected vascular anomalies of the lung, because bleeding may occur
• Patients with bleeding abnormalities, because bleeding may occur
• Patients with pulmonary hypertension, because bleeding is more likely to occur
• Patients with respiratory insufficiency, because they are not likely to survive a pneumothorax if it occurs
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Ensure that informed signed consent is obtained.
![]() Explain to the patient that fasting is usually ordered. The patient may be kept on NPO status after midnight on the day of the test.
Explain to the patient that fasting is usually ordered. The patient may be kept on NPO status after midnight on the day of the test.
• Administer the preprocedural medications 30 to 60 minutes before the test as ordered. Atropine is usually given to decrease bronchial secretions. Meperidine (Demerol) may be used to sedate anxious patients.
![]() Instruct the patient to remain still during the lung biopsy. Any movement or coughing could cause laceration of the lung by the biopsy needle.
Instruct the patient to remain still during the lung biopsy. Any movement or coughing could cause laceration of the lung by the biopsy needle.
During
• Note that the patient's position depends on the method used and that the histologic lung specimen may be obtained by several different methods:
Transbronchial Needle Aspiration
See discussion of bronchoscopy on p. 587.
1. The specimen is obtained via a fiberoptic bronchoscope using a needle (Figure 7-4).
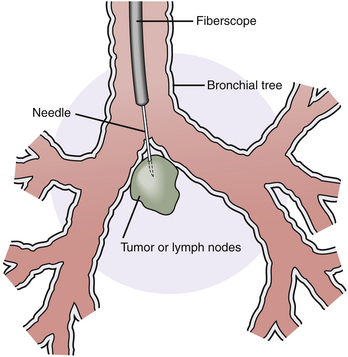
Figure 7-4 Transbronchial needle biopsy. The diagram shows a transbronchial needle penetrating the bronchial wall and entering a mass of subcarinal lymph nodes or tumor.
2. The bronchoscope is inserted, and the target site is identified using fluoroscopy.
3. The needle is inserted through the bronchoscope and into the tumor or desired area, where aspiration is performed with the attached syringe.
4. The needle is retracted within its sheath, and the entire catheter is withdrawn from the fiberoptic scope.
Transcatheter Bronchial Brushing
1. This is also performed via a fiberoptic bronchoscope (see discussion of bronchoscopy on p. 587).
2. During bronchoscopy a small brush is moved back and forth over the suspicious area in the bronchus or its branches.
3. The cells adhere to the brush, which is then removed and used to make microscopic slides.
Percutaneous Needle Biopsy
1. In this method for obtaining a closed specimen, the biopsy is obtained after using fluoroscopic radiograph or CT scan for localization of the desired site.
2. The procedure is carried out with a cutting needle or by aspiration with a spinal type of needle to obtain a specimen.
3. The main problem with this procedure is potential damage to major blood vessels.
4. During the lung biopsy procedure, assess the patient carefully for signs of respiratory distress (e.g., shortness of breath, rapid pulse rate, cyanosis).
Open Lung Biopsy
1. The patient is taken to the operating room, and general anesthesia is provided.
2. The patient is placed in the supine or lateral position, and an incision is made into the chest wall.
3. After a piece of lung tissue is removed, the lung is sutured.
4. Chest tube drainage is used for approximately 24 hours after an open lung biopsy.
5. This procedure can be performed by thoracoscopy as described in the following section.
Thoracoscopic Biopsy
1. The lung is collapsed with a double-lumen endotracheal tube placed during induction of general anesthesia.
2. With the use of a thoracoscope (similar to a laparoscope [p. 617]), the lung is grasped and a piece is cut off using a cutting/stapling device. Large wedge lung resections can be obtained.
3. The scope and trocars are removed, and a small chest tube is left in place.
4. The tiny incisions are closed, and the procedure is completed.
After
• Place biopsy specimens in appropriate containers for histologic and microbiologic examination.
• Observe the patient's vital signs frequently for signs of bleeding (increased pulse rate, decreased blood pressure) and for shortness of breath.
• Assess the patient's breath sounds and report any decrease on the biopsy side.
• A chest x-ray film is ordered to check for complications (e.g., pneumothorax).
• Observe the patient for signs of pneumothorax (e.g., dyspnea, tachypnea, decrease in breath sounds, anxiety, restlessness).
Test Results and Clinical Significance
Carcinoma: Biopsies of both primary and metastatic lesions can be performed using this technique. The lesion must be peripheral enough to ensure that one of the great vessels will not be punctured.
Granuloma: If the lesion is observed to contain calcium, it can be considered to be an old granuloma from previous granulomatous infection. If calcification is not observed, a biopsy of the lesion must be performed to rule out cancer, active fungal infection, or tuberculosis.
Exposure lung diseases (e.g., black lung, asbestosis): It is important to recognize and document the presence of exposure lung disease for medical (prognosis and treatment) and legal (workers' compensation or disability) reasons.
Sarcoidosis: Sarcoidosis of the lung is an interstitial disease highlighted by chronic inflammation and fibrosis. This is obvious on lung biopsy.
Infection: Unusual infections (such as that caused by Pneumocystis jiroveci) and unusual fungal diseases require tissue for culture and microscopic identification.