Blood Studies
Acetylcholine Receptor Antibody Panel
Adrenocorticotropic Hormone Stimulation
Adrenocorticotropic Hormone Stimulation With Metyrapone
Age-Related Macular Degeneration Risk Analysis
Anticyclic-Citrullinated Peptide Antibody
Antidiuretic Hormone Suppression
Antiextractable Nuclear Antigen
Antiglomerular Basement Membrane Antibody
Anti-Liver/Kidney Microsomal Type 1 Antibodies
Antineutrophil Cytoplasmic Antibody
Anti–SS-A, Anti–SS-B, and, Anti–SS-C Antibody
Antithrombin Activity and Antigen Assay,
Antithyroid Peroxidase Antibody
CA 15-3 and CA 27-29 Tumor Marker
Cell Surface Immunophenotyping
Coagulating Factor Concentration
Complete Blood Cell Count and Differential Count
Cutaneous Immunofluorescence Antibodies
Cytochrome P450 Genotype Testing
Diabetes Mellitus Autoantibody Panel
Disseminated Intravascular Coagulation Screening
Drug Sensitivity Genotype Testing
Erythrocyte Sedimentation Rate
Estimated Glomerular Filtration Rate
Glucose-6-Phosphate Dehydrogenase
HIV Serologic and Virologic Testing
Human T-Cell Lymphotrophic Virus
Iron Level, Total Iron-Binding Capacity, Transferrin, Transferrin Saturation
Lipoprotein-Associated Phospholipase A2
Luteinizing Hormone and Follicle-Stimulating Hormone Assay
Mycoplasma pneumoniae Antibodies, IgG and IgM
Neutrophil Gelatinase–Associated Lipocalin
Partial Thromboplastin Time, Activated
Pheochromocytoma Suppression and Provocative Testing
Plasminogen Activator Inhibitor 1 Antigen/Activity
Pregnancy-Associated Plasma Protein-A,
Septin 9 DNA Methylation Assay
Squamous Cell Carcinoma Antigen
Streptococcus Serologic Testing
Thyroid-Stimulating Hormone Stimulation
Thyroid-Stimulating Immunoglobulins
Thyrotropin-Releasing Hormone Stimulation Test
Vitamin B12 and Methylmalonic Acid
Overview
Reasons for Obtaining Blood studies
Blood is the body fluid most frequently used for analytic purposes. Blood studies are used to assess a multitude of body processes and disorders. Common studies assess the quantity of red and white blood cells, and the levels of enzymes, lipids, clotting factors, and hormones. Most blood studies are performed for one of the following reasons:
1. To establish a diagnosis (e.g., high blood urea nitrogen [BUN] and creatinine levels are indicative of renal failure).
2. To rule out a clinical problem (e.g., hypokalemia is ruled out with a normal potassium level).
3. To monitor therapy (e.g., glucose levels are used to monitor treatment of diabetic patients, and partial thromboplastin time [PTT] values are used to regulate heparin therapy).
4. To establish a prognosis (e.g., declining CD4 counts reflect a poor clinical prognosis for the acquired immunodeficiency syndrome [AIDS] patient).
5. To screen for disease (e.g., prostate-specific antigen levels are used to detect prostate cancer).
6. To determine effective drug dosage and to prevent toxicity. (Peak and trough levels are drawn at designated time periods; see p. 21.)
Methods of Blood Collection
There are three general methods for obtaining blood: venous, arterial, and skin puncture. Blood collected from these sites differs in several important aspects. For example, arterial blood is oxygenated by the lungs and pumped from the heart to body organs and tissues. It is essentially uniform in its composition throughout the body. Venous blood composition varies depending on the metabolic activity of the organ or tissue being perfused. Venous blood is oxygen-deficient in comparison to arterial blood. Variations between arterial and venous blood are often seen in measurements of pH, CO2 concentration, and glucose, lactic acid, and ammonia levels. On the other hand, blood obtained by skin puncture is a mixture of arterial and venous blood. Skin puncture blood also includes intracellular and interstitial fluid. By far the most common access for blood withdrawal is venous puncture.
Venous Puncture
Background Information
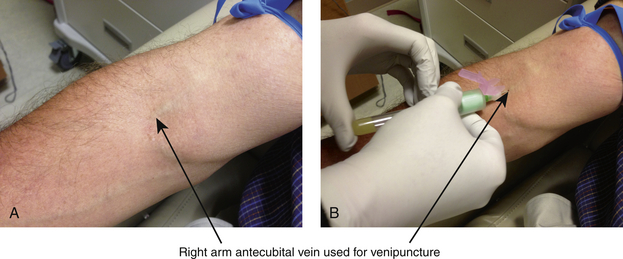
The ease of obtaining venous blood makes this the primary source of blood collection. It is relatively free of any complications. Venipuncture is usually obtained by drawing a specimen of blood from a superficial vein (Figure 2-1). The site most often used is the antecubital fossa of the arm because there are several large superficial veins at that location. The basilic, cephalic, and median cubital veins are the most commonly used sites. Veins of the wrist or hand can also be used. When venous puncture cannot be performed on the upper extremities, the femoral vein is the most easily accessible for puncture.
Collection Tubes
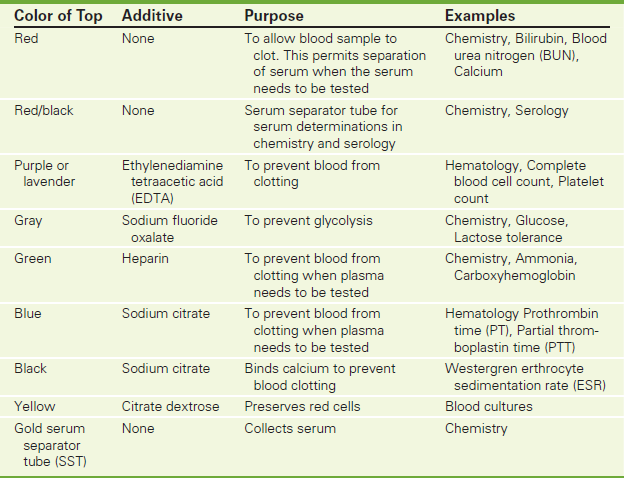
Venipuncture is usually accomplished using needles attached to glass tubes under specified vacuum. A needle and a syringe can also be used to collect the blood sample and then to inject it into the appropriate tube. Tubes come in various sizes (2, 3, 5, 7, 10, and 15 mL). The rubber stoppers are color-coded to distinguish whether the tube is a plain tube (e.g., no preservatives or anticoagulants added), whether the tube contains a specific anticoagulant (such as heparin, oxalate, citrate, or ethylenediamine tetraacetic acid salts [EDTA]), or whether the tube is chemically clean (e.g., iron determination). Depending on the tests needed, the analysis is performed on whole blood, serum, or plasma. A centrifuge is used to separate the blood components and to obtain either serum or plasma. Whole blood collected without anticoagulant clots, and serum can be separated out for testing. Whole blood collected with an anticoagulant prevents clotting, and plasma can be tested. Plasma contains fibrinogen, which is missing from serum.
The selection of the color-coded tube is based on the requirements of the test. Charts are available from the laboratory that indicate the type of tube needed for a particular blood test. Colors and amount of blood required may vary according to the laboratory. A representative chart is shown in Table 2-1.
The recommended order of draw must be followed when collecting multiple tubes of blood. Specimens should be drawn into nonadditive tubes (e.g., red top) before tubes are drawn that contain additives. The tubes should be filled in the following order:
Technique
Before
• Identify the patient. Assemble all equipment and supplies and put on gloves (Figure 2-2).
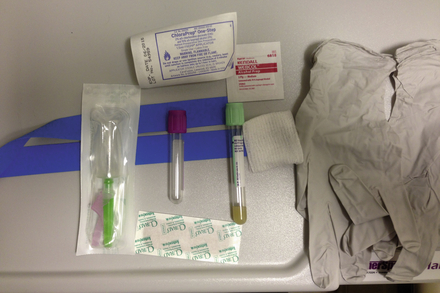
Figure 2-2 Supplies for venipuncture: tourniquet, Vacutainer and needle, specimen tubes, skin preparation antiseptics, protective gloves, gauze, Band-Aid.
![]() Explain the procedure to the patient. Explain that mild, brief discomfort may result from the needlestick.
Explain the procedure to the patient. Explain that mild, brief discomfort may result from the needlestick.
• If fasting is required, verify that this requirement has been followed.
During
• Position the patient properly for easy access to the antecubital fossa.
• Ask the patient to make a fist to distend the veins.
• Select a vein for venipuncture.
• Apply a tourniquet several inches above the puncture site.
• Cleanse the venipuncture site with an antiseptic solution (such as chlorhexidine, 70% isopropyl alcohol, or Betadine). Allow the area to dry.
• Perform the venipuncture by entering the skin with the needle bevel up and the needle at approximately a 15-degree angle to the skin.
• If using a Vacutainer, ease the tube forward in the holder as soon as the needle is in the vein. When the tube is filled, remove it. Another tube can then be inserted into the holder. If using a syringe, pull back on the barrel with slow, even tension as blood fills the syringe. Transfer the blood to the appropriate color tubes. Butterfly needles can also be used for collection.
After
• After the blood is drawn, place a cotton ball over the site. Withdraw the needle and apply pressure to the site. A Band-Aid applied over the cotton ball usually stops the bleeding.
• Discard the needle in an appropriate receptacle to prevent inadvertent needle sticks (Figure 2-3).
• Mix tubes with the additives by gently rolling the tubes. Do not vigorously shake the tubes. Specimens collected in the syringe should be transferred to appropriate test tube containers.
• Properly dispose of contaminated materials, syringes, and cotton balls.
• Initial the label and record the date and time of blood collection. Attach a label to each vial of blood.
• Arrange for prompt delivery of the blood specimen to the laboratory.
• If the patient fasted before the test, remove diet restrictions as per physician recommendations.
Potential Complications
• Bleeding. After the specimen is drawn, apply pressure or a pressure dressing to the venipuncture site. Assess the venipuncture site for bleeding.
• Hematoma. Hematomas can form under the skin when the vein continues to leak blood. This results in a large, bruised area. This can usually be prevented by applying pressure to the venipuncture site until clotting occurs. If a hematoma does occur, reabsorption of the blood can be enhanced by the application of warm compresses.
• Infection. Instruct the patient to assess the venipuncture site for redness, pain, swelling, or tenderness. This is more common in immunocompromised patients or patients who have had lymph node dissection above the venipuncture site.
• Dizziness and fainting. If this occurs, prevent injury by helping the patient to a sitting or reclining position. Lowering the head between the knees or using smelling salts can also help.
Preventing Interfering Factors
• Hemolysis may result from vigorous shaking of a blood specimen. This may invalidate test results.
• Collect the blood specimen from the arm without an intravenous (IV) device if possible. IV infusion can influence test results. If it is necessary to draw blood from the arm with an IV device, never draw blood above the IV needle site. Satisfactory samples may be obtained by drawing the blood below the IV needle after turning the IV infusion off for 2 minutes before the venipuncture. Select a vein other than the one with the IV device and draw 5 mL of blood. Discard this sample before drawing blood for analysis.
• Do not use the arm with the dialysis arteriovenous fistula for a venipuncture unless the physician specifically authorizes it.
• Because of the risk for cellulitis, specimens should not be taken from the side on which an axillary lymph node dissection has been performed.
• To obtain valid results, do not fasten the tourniquet for longer than 1 minute. Prolonged tourniquet application can cause stasis, localized acidemia, and hemoconcentration.
Drawing Blood from an Indwelling Venous Catheter
Follow the institutional guidelines for drawing blood from an indwelling venous catheter, such as a central venous catheter or a peripherally inserted central catheter. Guidelines will specify the amount of blood to be drawn from the catheter and discarded before blood is collected for laboratory studies. The guidelines also indicate the amount and type of solution needed to flush the catheter to prevent it from being clogged by blood.
Arterial Puncture
Background Information
Arterial blood is used to measure oxygen, CO2, and pH. These are often referred to as arterial blood gases (ABGs) and are described on p. 109. If a patient will require frequent sampling, an indwelling arterial catheter is usually placed. Arterial puncture is used for single or infrequent sampling.
Arterial punctures are more difficult to perform than venipuncture. They also cause a significant amount of patient discomfort. The brachial and radial arteries are the arteries most often used for arterial puncture. The femoral artery is usually avoided because bleeding occurs more often after the procedure and may not be noted because it is hidden by bed covers. Large amounts of blood could be lost before the problem is detected.
Technique
Before
![]() Explain the procedure to the patient. Inform the patient why this blood test is necessary. Tell the patient that the test causes more discomfort than a venipuncture.
Explain the procedure to the patient. Inform the patient why this blood test is necessary. Tell the patient that the test causes more discomfort than a venipuncture.
• Notify the laboratory before drawing arterial blood samples so the necessary equipment can be calibrated before the blood sample arrives.
• Perform the Allen test to assess collateral circulation before performing the arterial puncture on the radial artery. To perform the Allen test, make the patient's hand blanch by obliterating both the radial and ulnar pulses. Then release the pressure over the ulnar artery only. If flow through the ulnar artery is good, flushing will be observed immediately. The Allen test is then positive and the radial artery can be used for venipuncture. If the Allen test is negative (no flushing), repeat it on the other arm. If the results are negative in both arms, choose another artery for puncture. The Allen test is important because it ensures collateral circulation to the hand if thrombosis of the radial artery occurs after the puncture.
• Assemble appropriate equipment and specimen container for the specimen. Put on protective gloves.
During
• Cleanse the arterial site with 70% isopropyl alcohol. Allow the site to dry.
• Attach a 20-gauge needle to a syringe containing approximately 0.2 mL of heparin. Insert the needle at a 45- to 60-degree angle into the skin over the palpable artery (Figure 2-4).
• After drawing approximately 3 to 5 mL of blood, remove the needle and apply pressure to the arterial site for 3 to 5 minutes. Expel any air bubbles in the syringe and activate the protective cover.
• Cap the syringe and gently rotate to mix the blood and the heparin.
After
• Indicate on the laboratory slip if the patient is receiving any oxygen therapy or is attached to a ventilator.
• Place the arterial blood on ice and immediately take it to the chemistry laboratory for analysis.
• If the patient has an abnormal clotting time or is taking anticoagulants, apply pressure for approximately 15 minutes. A pressure dressing is usually applied.
Potential Complications
• Arterial thrombosis. Thrombosis can result and impair arterial circulation. This can result in ischemia or necrosis of tissue on the extremity.
• Hematoma formation. Pressure must be applied to the arterial puncture site for at least 3 to 5 minutes to prevent hematoma formation (longer if the patient is anticoagulated). If a hematoma results, warm compresses will enhance absorption of the blood.
• Bleeding. The site must be carefully assessed for bleeding. An arterial puncture can cause rapid bleeding. This is especially important if the patient has an abnormal clotting time or is taking anticoagulants.
Skin Puncture
Background Information
Skin puncture (sometimes called capillary puncture) is the method of choice for obtaining blood from pediatric patients, especially infants, because large amounts of blood required for repeated venipuncture could result in anemia. However, skin punctures are also used in adult patients.
Common puncture sites include the fingertips, earlobes, and heel surfaces. The fingertips are often used in adults and small children. The heel is the most commonly used site for infants. The earlobe can be used to obtain blood in adults and older pediatric patients. The earlobe can also be used to obtain arterialized capillary blood as a possible substitute for arterial blood in determining the pH, PCO2, and PO2.
With changes in health care economics and delivery, the use of skin punctures will probably increase. Blood monitoring will be increasingly performed in outpatient settings. Clinical laboratory tests will be performed more frequently at the bedside using a skin puncture.
Technique
Before
![]() Identify the patient using two separate identifiers. Explain the procedure to the patient and/or family. Assemble all supplies. Put on gloves.
Identify the patient using two separate identifiers. Explain the procedure to the patient and/or family. Assemble all supplies. Put on gloves.
• Select an appropriate puncture site. For infants, the lateral or medial heel surface is commonly used. For older infants, children, or adults, the lateral aspect of the second, third, or fourth fingertip may be used to avoid the central tip of the fingers where the nerve supply is more dense.
During
• Warm the puncture site with a warm, moist towel to increase blood flow.
• Cleanse the puncture site with 70% isopropyl alcohol. Allow the site to dry.
• Make the puncture with a sterile lancet or skin puncture device.
• Discard the first drop of blood by wiping it away with a sterile pad.
• Do not milk the site, as this may hemolyze the specimen and introduce excess tissue fluid. Also avoid using excess pressure on the fingers during blood collection. This may cause hemolysis of the sample.
• Collect the specimen in capillary tubes or on special filter papers.
• If using capillary tubes, seal the capillary tubes by inserting clay into the end of the micropipette.
Potential Complications
• Infection. Assess the skin puncture site for redness, swelling, pain, or tenderness. Although this is a serious complication, the incidence is very low.
• Hematoma and bruising. Check the skin puncture site for discoloration, bruising, or swelling. Look for bleeding onto the skin. Avoid frequent skin punctures or excessive squeezing of the tissue during blood collection to prevent this problem.
Timing of Blood Collection
Although many blood specimens can be obtained randomly, some must be drawn at specific times. For example, lipoproteins (see p. 342) should be drawn after a 12- to 14-hour fast (except for water), because food can alter lipoprotein values. Because glucose levels are related to food intake, fasting blood glucose specimens require an 8-hour fast. Glucose tolerance tests (see p. 261) require a fasting blood glucose level and a glucose level drawn at 30 minutes, 1 hour, 2 hours, 3 hours, and sometimes 4 hours after glucose administration.
Specimens for therapeutic drug monitoring (see p. 211) must be obtained at specific times determined by the method of drug delivery (e.g., IV or oral), dosage interval, absorption characteristics of the drug, and half-life of the drug. Drug monitoring is especially important in patients taking medications (such as antiarrhythmics, bronchodilators, antibiotics, anticonvulsants, and cardiotonics), because the margin of safety between therapeutic and toxic levels may be narrow. Blood levels can be taken at the drug's peak level (highest concentration) or at the drug's trough level (lowest concentration). Peak levels are useful when testing for toxicity, and trough levels are useful for demonstrating a satisfactory therapeutic level.
Transport and Processing of Blood Specimens
Once blood specimens are obtained, they should be promptly transported to the laboratory. Because the blood cells continue to live in the collection tubes, they will metabolize some of the components in the blood. This can result in alterations in the concentration of some blood components before analysis in the laboratory. Therefore blood specimens should be delivered to the laboratory for processing within 1 hour, depending on the test. Stat specimens should be delivered immediately after being drawn. Laboratories have written criteria for rejecting a specimen as unsuitable for testing. Box 2-1 lists common reasons for rejecting a blood specimen.
In general, specimens should be tested within 1 hour of collection. If this is not possible, the sample may need to be refrigerated or frozen depending on the compound for testing. Some blood specimens must be sent by mail or special courier from physicians' offices or small hospitals to large reference laboratories. As a result, delays of 24 hours may occur before specimen analysis.
After testing, the remainder of the blood sample should be saved by the laboratory along with the original sample for 24 hours to be retested if needed to verify discrepant results. These samples can also be used for additional (“add-on”) tests ordered by the physician while avoiding additional venipunctures. With retesting or “add-on” requests, the stability of the requested serum constituent becomes an important consideration.
Multiphasic screening machines can perform many blood tests quickly and simultaneously using a very small blood sample. An example is the Astra-7 or Chem-7, which usually includes the following seven studies: sodium, potassium, chloride, CO2 content, BUN, creatinine, and glucose. See Appendix C for a listing of common panels. The basic metabolic panel and comprehensive metabolic panel have replaced the Chem-7 and Astra panels. These changes are the result of the recent federal guidelines to standardize the nomenclature for chemistry panels. The advantage of these machines is that results are available quickly and the cost is cheaper when compared to performing each test individually.
Reporting of Results
Although accuracy and processing are the prerequisites of good laboratory practice, timeliness in reporting results is essential. To be clinically useful, a test result must be reported promptly. Delays in reporting a result can make the data useless and potentially could be life-threatening to the patient. Verbal reporting of result to the clinician should be provided by a licensed health care provider after using two patient identifiers (e.g., patient's name and medical record number/date of birth). A “read-back” of the information from the clinician should also occur.
The report must also be entered in the appropriate medical record and must be presented in a manner that is clear and easily interpreted. A listing of the patient's medications will help with test result interpretation.
The results should include the test results, reporting units, and reference ranges. It is important to note that normal ranges for laboratory tests vary from institution to institution. Often serial listing of results is useful for tests in which trends and values make interpretation easier. Comments may be added to help interpret test results; for example, the technologist would indicate if the sample was hemolyzed.
Because acronyms are used to shorten test names, these code names must be understood for proper interpretation. For example, the acronym LAP could stand for leucine amino peptidase or for leukocyte alkaline phosphatase.
Proper reporting of a “critical” or “panic” value is essential. These values are results well outside the usual range of normal and generally require immediate intervention. Common examples are shown in Box 2-2. If these results were phoned to a physician or nurse, verification of this notification must be properly documented.
Acetylcholine Receptor Antibody Panel (AChR Ab, Anti–AChR Antibody)
Indications
Antibodies to AChR are used to diagnose acquired myasthenia gravis (MG) and also to monitor patient response to immunosuppressive therapy.
Test Explanation
These antibodies may cause blocks in neuromuscular transmission by interfering with the binding of acetylcholine (ACh) to ACh receptor (AChR) sites on the muscle membrane, thereby preventing muscle contraction. It is this phenomenon that characterizes myasthenia gravis (MG). Myasthenia gravis (MG) is an autoimmune disease usually caused by antibodies that block or destroy receptors for the neurotransmitter acetylcholine, leading to muscle weakness and fatigue. Antibodies to AChR occur in more than 85% to 90% of patients with acquired MG, and 63% of patients with only ocular MG have elevated levels. The presence of these antibodies is virtually diagnostic of MG, but a negative test does not exclude the disease. The measured titers do not correspond well with the severity of MG. In an individual patient with MG, however, antibody levels are particularly useful in monitoring response to immunosuppressive or plasmapheresis therapy. As the patient improves, antibody titer decreases.
In adults with MG, there is at least a 20% occurrence of thymoma or other neoplasm. Neoplasms are an endogenous source of the antigens driving production of AChR autoantibodies. Among patients who have a thymoma, 59% have MG. Because congenital MG is not an autoimmune disease, this antibody test is not helpful in the diagnosis of congenital MG.
There are several AChR antibodies that can be associated with MG binding, blocking, and modulating antibodies. The AChR-binding antibody can activate complement and lead to loss of AChR. The AChR-binding antibody is most commonly used. The AChR-modulating antibody causes receptor endocytosis resulting in loss of AChR expression, which correlates most closely with clinical severity of disease. It is most sensitive. A positive modulating antibody test may indicate subclinical MG, contraindicating the use of curare-like drugs during surgery. Approximately 10% to 15% of individuals with confirmed myasthenia gravis have no measurable binding, blocking, or modulating antibodies. The AChR-blocking antibody may impair binding of acetylcholine to the receptor, leading to poor muscle contraction. It is the least sensitive test (positive in only 61% of patients with MG), but it can be quantified more accurately. The blocking and modulating antibodies are not often positive for about 1 year after onset of MG symptoms.
The most commonly used method for the detection of these AChR antibodies is the Quantitative Radioimmunoassay/Semi-Quantitative Radioreceptor Assay.
Anti-Striated Muscle Antibody (Striated Muscle Antibody, IgG) titers greater than or equal to 1:80 are suggestive of myasthenia. This antibody is detectable in 30% to 40% of anti-AChR-negative patients (particularly those with bulbar symptoms only). However, striated muscle antibody can be found in rheumatic fever, myocardial infarction, and a variety of post-cardiotomy states.
Interfering Factors
• False-positive results may occur in patients with amyotrophic lateral sclerosis who have been treated with cobra venom.
• False-positive results may be seen in patients with penicillamine-induced or Lambert-Eaton myasthenic syndromes.
• Patients with autoimmune liver disease may have elevated results.
![]() The use of muscle relaxant drugs (metocurine and succinylcholine) or penicillamine may cause false-positive results.
The use of muscle relaxant drugs (metocurine and succinylcholine) or penicillamine may cause false-positive results.
![]() Immunosuppressive drugs may suppress the formation of these antibodies in patients with subclinical MG.
Immunosuppressive drugs may suppress the formation of these antibodies in patients with subclinical MG.
Related Tests
Cholinesterase (p. 159). Patients with an acquired or congenital deficiency of this enzyme will experience acute MG-like muscle paralysis when a depolarizing agent, such as succinylcholine, is used for anesthesia induction.
Electromyography (p. 554). Repetitive stimulation or single-fiber electromyogram is positive in 90% of MG patients.
Chest x-ray (p. 1014) or chest CT (p. 1029). These tests are used to identify thymoma.
Acid Phosphatase (Prostatic Acid Phosphatase [PAP], Tartrate-Resistant Acid Phosphatase [TRAP])
Indications
Total acid phosphatase and specifically the PAP isoenzyme is primarily used to document rape cases. It was used in the diagnosis of prostate cancer, but has been replaced by the use of prostate specific antigen (PSA, p. 420). Otherwise this test has very little clinical usefulness.
Test Explanation
Acid phosphatase is found in many tissues, including liver, red blood cells, bone marrow, and platelets. The highest levels are found in the prostate gland—the PAP isoenzyme. Usually (but not always) elevated levels are seen in patients with prostatic cancer, especially if it has metastasized beyond the capsule to other parts of the body.
Because acid phosphatase is also found at high concentrations in seminal fluid, this test can be performed on vaginal secretions to investigate alleged rape. This is now the primary use of PAP testing. High levels of acid phosphatase also exist in white blood cells (mostly monocytes and lymphocytes). They are helpful in determining the clinical course of patients with lymphoproliferative diseases and hairy cell leukemia. Acid phosphatase is a lysosomal enzyme; therefore lysosomal storage diseases (such as Gaucher disease and Niemann-Pick disease) are associated with elevated levels.
Interfering Factors
• Falsely high levels of acid phosphatase (and specifically PAP) may occur in males after a digital rectal examination or after instrumentation of the prostate (e.g., cystoscopy) because of prostatic stimulation. Elevated levels of 25% to 50% may occur for up to 48 hours after prostate manipulation. The test should be repeated if elevated levels occur after a rectal or prostate examination.
• Alkaline and acid phosphatase are very similar enzymes that function at different pH levels. Any condition associated with very high levels of alkaline phosphatase may falsely indicate high acid phosphatase levels.
![]() Drugs that may cause increased levels of acid phosphatase include alglucerase, androgens (in females), and clofibrate (Atromid-S).
Drugs that may cause increased levels of acid phosphatase include alglucerase, androgens (in females), and clofibrate (Atromid-S).
![]() Drugs that may cause decreased levels include alcohol, fluorides, heparin, oxalates, and phosphates.
Drugs that may cause decreased levels include alcohol, fluorides, heparin, oxalates, and phosphates.
Procedure and Patient Care
After
• Apply pressure or a pressure dressing to the venipuncture site.
• Assess the venipuncture site for bleeding.
• Promptly deliver the specimen to the laboratory.
• Do not leave the specimen at room temperature for 1 hour or longer; the enzyme is heat and pH sensitive, and acid phosphatase activity will decrease. Once the specimen is received by the laboratory, the use of a preservative and prompt refrigeration are important.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Acid phosphatase and specifically PAP exist in the lysosomes of prostate cells. Diseases affecting prostate tissue will destroy those cells, and the lysosomal contents will spill into the bloodstream, where they will be detected.
Because acid phosphatase exists in the lysosomes of the bone marrow, diseases affecting the bone will be associated with elevated blood levels.
Because acid phosphatase exists in the lysosomes of blood cells, diseases affecting blood cells will be associated with elevated blood levels.
Lysosomal disorders (e.g., Gaucher disease): Because acid phosphatase exists in the lysosomes of many tissues affected by these diseases, elevated blood levels can be expected.
Activated Clotting Time (ACT, Activated Coagulation Time)
Indications
The ACT is primarily used to measure the anticoagulant effect of heparin or other direct thrombin inhibitors during cardiac angioplasty, hemodialysis, and cardiopulmonary bypass (CPB) surgery.
Test Explanation
This test measures the time for whole blood to clot after the addition of particulate activators. Like the activated partial thromboplastin time (aPTT, p. 383), it measures the ability of the intrinsic pathway (reaction 1) to begin clot formation by activating factor XII (see Figure 2-12, p. 167). By checking the blood clotting status with ACT, the response to unfractionated heparin therapy can be easily and rapidly monitored. Equally important is the use of the ACT in determining the appropriate dose of protamine sulfate required to reverse the effect of heparin on completion of surgical procedures and hemodialysis.
Both the aPTT and the ACT can be used to monitor heparin therapy in patients on CPB. However, the ACT has several advantages over the aPTT. First, the ACT is more accurate than the aPTT when high doses of heparin are used for anticoagulation. This makes it especially useful during clinical situations requiring high-dose heparin, such as during CPB when high-dose anticoagulation is necessary at levels 10 times those used for venous thrombosis. The aPTT is not measurable at these high doses. The accepted goal for the ACT is 400-480 seconds during CPB.
Second, the ACT is not only less expensive, but it is also more easily and rapidly performed than the aPTT, which is time consuming and requires full laboratory facilities. The ACT can be performed at the bedside. This provides immediate information on which further therapeutic anticoagulation decisions can be based. The capability to perform the ACT at the “point of care” makes the ACT particularly useful for patients requiring angioplasty, hemodialysis, and CPB.
A nomogram adjusted to the patient's baseline ACT is often used as a guide to reach the desired level of anticoagulation during these procedures. This same nomogram is used in determining the dose of protamine to be administered to neutralize the heparin when a return to normal coagulation is desired on completion of these procedures. The ACT is used in determining when it is safe to remove the vascular access after these procedures. The modified ACT test requires a smaller-volume blood specimen, automated blood sampling, standardized blood/reagent mixing, and faster clotting time results than the conventional ACT. The modified ACT is now being used more frequently.
Interfering Factors
• The ACT is affected by several biologic variables, including hypothermia, hemodilution, and platelet number and function.
• Factors affecting the pharmacokinetics of heparin (e.g., kidney or liver disease) and heparin resistance due to antithrombin deficiency and contact factor deficiencies can affect ACT measurements.
• A partially or completely occluded specimen can increase ACT measurements.
Procedure and Patient Care
During
• Less than 1 mL of blood is collected into a commercial container. This container is then placed into a whole blood microcoagulation analyzer at the bedside. When a clot is formed, the ACT value is displayed on the machine's panel.
• If the patient is receiving a continuous heparin drip, the blood sample is obtained from the arm without the intravenous catheter.
After
• Apply pressure to the venipuncture site. Remember that the bleeding time will be prolonged because of anticoagulation therapy.
• Assess the patient to detect possible bleeding. Check for blood in the urine and all other excretions and assess the patient for bruises, petechiae, and low back pain.
• For clinical significance, the test results must be correlated with the time of heparin administration. A clinical flow sheet is used to list the test results with the time and route of heparin administration.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Heparin administration: Heparin, along with antithrombin III, interrupts in the action of several coagulation proteins (except factor VII). As a result, the intrinsic pathway of coagulation is inhibited. This pathway is measured by the ACT and is therefore prolonged.
Clotting factor deficiencies: Deficiencies in any clotting factor associated with the intrinsic pathway will be associated with prolonged ACT.
Cirrhosis of the liver: Coagulation factors are proteins that are synthesized in the liver. Liver pathology therefore is associated with a reduction in coagulation factors; this prolongs the time required for the reactions of the intrinsic pathway and prolongs the ACT.
Coumadin administration: Deficiencies in the vitamin K clotting factors associated with the intrinsic pathway will cause a prolonged ACT.
Lupus inhibitor: Lupus inhibitors are autoantibodies against components involved in the activation of the coagulation cascade and thus prolong the ACT.
Related Tests
Partial Thromboplastin Time (PTT) (p. 383). The PTT is another test used to evaluate the intrinsic pathway of secondary hemostasis. It, too, is commonly used to monitor heparin therapy.
Prothrombin Time (p. 434). The PT is used to evaluate the extrinsic and common pathways of secondary hemostasis.
Coagulating Factor Concentration (p. 163). This is a quantitative measurement of specific coagulation factors.
Adrenal Steroid Precursors (Androstenediones [AD], Dehydroepiandrosterone [DHEA], Dehydroepiandrosterone Sulfate [DHEA S], 11-Deoxycortisol, 17-Hydroxyprogesterone, 17-Hydroxypregnenolone, Pregnenolone)
Test Explanation
Androstenediones (ADs, DHEA, and the sulfuric ester, DHEA S) are precursors of testosterone and estrone, and are made in the gonads and the adrenal gland. 11-Deoxycortisol, 17-hydroxyprogesterone, 17-hydroxypregnenolone, and pregnenolone are precursors of cortisol. ACTH stimulates their adrenal secretion. Children with congenital adrenal hyperplasia (CAH) have genetic mutations that cause deficiencies in the enzymes involved in the synthesis of cortisol, testosterone, aldosterone, and estrone. When defects in enzyme synthesis occur along the path of hormone synthesis, the listed precursors exists in levels that exceed normal through the increased stimulation of ACTH. In most cases, CAH is a genetic autosomal recessive disorder.
The symptoms of this disorder depend on which steroids are overproduced and which are deficient. As a result, CAH may present with various symptoms, including virilization of the affected female infant, signs of androgen excess in males and females, signs of sex hormone deficiency in males and females, salt-wasting crisis secondary to cortisol and aldosterone deficiency, or hormonal hypertension caused by increased mineralocorticoids. A milder, nonclassic form of CAH is characterized by premature puberty, acne, hirsutism, menstrual irregularity, and infertility.
These same precursors can occur in adults because of adrenal or gonadal tumors that produce one of these precursors. Patients with polycystic ovary (Stein-Leventhal) syndrome have particularly elevated levels of ADs. Levels of DHEA S are particularly high in patients with adrenal carcinoma.
In patients suspected of CAH, testing for a panel of steroids involved in the cortisol biosynthesis pathway may be performed to establish the specific enzyme deficiency. In most cases, basal concentrations within the normal reference interval rule out CAH. The ratio of the precursor to the final pathway product (with and without ACTH stimulation) may be used to diagnose which enzyme is deficient.
Testing is performed by Quantitative High Performance Liquid Chromatography-Tandem Mass Spectrometry. Results vary considerably based on testing method.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Adrenal tumor: Some tumors make large amounts of androstenediones, which is then converted by the ovaries and fatty tissue to testosterone and estrogen. The relatively high level of testosterone causes the virilizing signs.
Congenital adrenal hyperplasia: This disease is characterized by enzyme defects that prevent conversion of androstenediones to cortisol. Androstenediones levels are increased.
Ectopic ACTH-producing tumors,
Cushing syndrome: Large amounts of hormones, including androstenediones, are made in the adrenal gland.
Adrenocorticotropic Hormone (ACTH, Corticotropin)
Indications
The serum ACTH study is a test of anterior pituitary gland function that affords the greatest insight into the causes of either Cushing syndrome (overproduction of cortisol) or Addison disease (underproduction of cortisol).
Test Explanation
An elaborate feedback mechanism for cortisol coordinates the function of the hypothalamus, pituitary gland, and adrenal glands. ACTH is an important aspect of this mechanism. Corticotropin-releasing hormone (CRH) is made in the hypothalamus. This stimulates ACTH production in the anterior pituitary gland, which in turn stimulates the adrenal cortex to produce cortisol. The rising levels of cortisol act as negative feedback and curtail further production of CRH and ACTH.
In the patient with Cushing syndrome, an elevated ACTH level can be caused by a pituitary ACTH-producing tumor or a nonpituitary (ectopic) ACTH-producing tumor, usually in the lung, pancreas, thymus, or ovary. ACTH levels greater than 200 pg/mL usually indicate ectopic ACTH production. If the ACTH level is below normal in a patient with Cushing syndrome, an adrenal adenoma or carcinoma is probably the cause of the hyperfunction (Table 2-2).
TABLE 2-2
Cortisol/ACTH Levels in Diagnosis of Adrenal Dysfunction
| Disease | Cortisol Level | ACTH Level |
| Cushing syndrome Adrenal micronodular hyperplasia Adrenal tumor (adenoma, cancer) |
High | Low |
| Cushing syndrome Cushing disease (ACTH-producing pituitary tumor )Ectopic ACTH-producing tumor (e.g., lung cancer) |
High | High |
| Addison disease Adrenal gland failure (e.g., infarction, hemorrhage, congenital adrenal hyperplasia) |
Low | High |
| Hypopituitarism | Low | Low |
In patients with Addison disease, an elevated ACTH level indicates primary adrenal gland failure, as in adrenal gland destruction caused by infarction, hemorrhage, or autoimmunity; surgical removal of the adrenal gland; congenital enzyme deficiency; or adrenal suppression after prolonged ingestion of exogenous steroids. If the ACTH level is below normal in a patient with adrenal insufficiency, hypopituitarism is most probably the cause of the hypofunction (see Table 2-2).
ACTH can be directly measured by Quantitative Chemiluminescent Immunoassay. ACTH levels exhibit diurnal variations that correspond to cortisol levels. Levels in evening (8 PM to 10 PM) samples are usually one half to two thirds those of morning (4 AM to 8 AM) specimens. This diurnal variation is lost when disease (especially neoplasm) affects the pituitary or adrenal glands. Likewise stress can blunt or eliminate this normal diurnal variation.
ACTH is measured in amniotic fluid when anencephaly is suspected. Decreased levels are noted in anencephalic fetuses (see discussion of amniocentesis on p. 632).
Interfering Factors
• Stress (trauma, pyrogen, hypoglycemia), menses, and pregnancy cause increased levels of cortisol. This is accomplished through elevation of ACTH.
![]() Recently administered radioisotope scans can affect levels measured by radioimmunoassay or immunoradiometry.
Recently administered radioisotope scans can affect levels measured by radioimmunoassay or immunoradiometry.
![]() Drugs that may cause increased levels include aminoglutethimide, amphetamines, estrogens, ethanol, insulin, levodopa, metyrapone, spironolactone, and vasopressin.
Drugs that may cause increased levels include aminoglutethimide, amphetamines, estrogens, ethanol, insulin, levodopa, metyrapone, spironolactone, and vasopressin.
![]() Exogenously administered corticosteroids decrease ACTH levels.
Exogenously administered corticosteroids decrease ACTH levels.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient. Allow plenty of time to answer questions so the patient's stress is diminished as much as possible.
Explain the procedure to the patient. Allow plenty of time to answer questions so the patient's stress is diminished as much as possible.
![]() Keep the patient on nothing by mouth (NPO) status after midnight the day of the test.
Keep the patient on nothing by mouth (NPO) status after midnight the day of the test.
• Evaluate the patient for stress factors that could invalidate the test results.
• Evaluate the patient for sleep pattern abnormalities. With a normal sleep pattern, the ACTH level is highest between 4 AM and 8 AM and lowest around 9 PM.
• Assess the patient for self-administration of drugs that could affect test results.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Addison disease (primary adrenal insufficiency),
Adrenogenital syndrome (congenital adrenal hyperplasia):
The adrenal glands are not making enough cortisol for the body's needs. The reduced serum cortisol level is a strong stimulus to pituitary production of ACTH.
Related Tests
Cortisol, Blood, and Urine (pp. 179 and 920). Cortisol is a hormone produced by the adrenal gland and is the main determinant of Cushing syndrome (overproduction) or Addison disease (underproduction).
Adrenocorticotropic Hormone (ACTH) Stimulation (p. 34). This test is used to determine the cause of adrenal insufficiency.
Dexamethasone Suppression (p. 204). This test is used to determine the cause of Cushing syndrome.
Adrenocortotropic Hormone Stimulation With Metyrapone (p. 36). This test is used to determine the cause of Cushing syndrome.
Adrenocorticotropic Hormone Stimulation (ACTH Stimulation With Cosyntropin, Cortisol Stimulation)
Indications
This test evaluates the ability of the adrenal gland to respond to ACTH administration. It is useful in evaluating the cause of adrenal insufficiency and also in evaluating patients with cushingoid symptoms.
Test Explanation
This test is performed on patients found to have adrenal insufficiency. An increase in plasma cortisol levels after the infusion of an ACTH-like drug indicates that the adrenal gland is normal and capable of functioning if stimulated. In that case the cause of adrenal insufficiency would lie within the pituitary gland (hypopituitarism, which is called secondary adrenal insufficiency). If little or no rise in cortisol levels occurs after the administration of the ACTH-like drug, the adrenal gland is the source of the problem and cannot secrete cortisol. This is called primary adrenal insufficiency (Addison disease), which may be caused by adrenal hemorrhage, infarction, autoimmunity, metastatic tumor, surgical removal of the adrenal glands, or congenital adrenal enzyme deficiency.
This test can also be used to evaluate patients with Cushing syndrome. Patients with Cushing syndrome caused by bilateral adrenal hyperplasia have an exaggerated cortisol elevation in response to the administration of the ACTH-like drug. Those experiencing Cushing syndrome as a result of hyperfunctioning adrenal tumors (which are usually autonomous and relatively insensitive to ACTH) have little or no increase in cortisol levels over baseline values.
Cosyntropin (Cortrosyn) is a synthetic subunit of ACTH that has the same corticosteroid-stimulating effect as endogenous ACTH in healthy persons. During this test, cosyntropin is administered to the patient, and the ability of the adrenal gland to respond is measured by plasma cortisol levels.
The rapid stimulation test is only a screening test. A normal response excludes adrenal insufficiency. An abnormal response, however, requires a 1- to 3-day prolonged ACTH stimulation test to differentiate primary insufficiency from secondary insufficiency. It should be noted that the adrenal gland can also be stimulated by insulin-induced hypoglycemia as a stressing agent. When insulin is the stimulant, cortisol and glucose levels are measured.
Procedure and Patient Care
During
Rapid Test
• Obtain a baseline plasma cortisol level less than 30 minutes before cosyntropin administration.
• Administer an intravenous (IV) injection of cosyntropin over a 2-minute period. An intramuscular (IM) injection may also be used.
• Measure plasma cortisol levels 30 and 60 minutes after drug administration. Serum or heparinized blood is acceptable.
Test Results and Clinical Significance
 Normal or Below-Normal Response
Normal or Below-Normal Response
Cushing syndrome: Adrenal adenoma, adrenal carcinoma, ACTH-producing tumor, chronic steroid ingestion.
Adrenal insufficiency: Primary adrenal insufficiency (Addison disease) caused by adrenal infarction, hemorrhage, infection, or metastatic tumor to adrenal gland.
Congenital enzyme adrenal insufficiency, surgical removal of adrenal gland, and ingestion of drugs, such as mitotane, metyrapone, or aminoglutethimide.
Related Tests
Cortisol (p. 179). Cortisol is a hormone produced by the adrenal gland and is the main determinant of Cushing syndrome (overproduction) or Addison disease (underproduction).
Adrenocorticotropic Hormone (ACTH) (p. 31). This test is used to determine the cause of Cushing syndrome or Addison disease.
Dexamethasone Suppression (p. 204). This test is used to determine the cause of Cushing syndrome.
Adrenocortotropic Hormone Stimulation With Metyrapone (see following test). This test is used to determine the cause of Cushing syndrome.
Adrenocorticotropic Hormone Stimulation With Metyrapone (Metyrapone, ACTH Stimulation With Metyrapone)
Indications
This test is useful in differentiating adrenal hyperplasia from a primary adrenal tumor by determining whether the pituitary-adrenal feedback mechanism is intact.
Test Explanation
Metyrapone (Metopirone) is a potent blocker of an enzyme involved in cortisol production. Cortisol production is therefore reduced. When this drug is given, the resulting decrease in cortisol production should stimulate pituitary secretion of adrenocorticotropic hormone (ACTH) by way of a negative-feedback mechanism. Cortisol itself cannot be synthesized because of the metyrapone inhibition at the 11-beta-hydroxylation step, but an abundance of cortisol precursors (11-deoxycortisol and OCHS) will be formed. These cortisol precursors can be detected in the urine or in the blood. This test is similar to the ACTH stimulation test.
In patients with adrenal hyperplasia caused by pituitary overproduction of ACTH, the cortisol precursors are greatly increased. This is because the normal adrenal-pituitary feedback response mechanism is still intact. No response to metyrapone occurs in patients with Cushing syndrome resulting from adrenal adenoma or carcinoma, because the tumors are autonomous and therefore insensitive to changes in ACTH secretion. This test has no significant advantage over the ACTH stimulation test in the differential diagnosis of Cushing disease.
This test is also used to evaluate the pituitary reserve capacity to produce ACTH. It can document that adrenal insufficiency exists as a result of pituitary disease (secondary adrenal insufficiency) rather than primary adrenal pathology. This test should not be performed if primary adrenal insufficiency is likely. A severe, life-threatening adrenal crisis could be precipitated. A normal response to ACTH should be demonstrated before metyrapone is given.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Obtain a baseline 24-hour urine specimen for 17-OCHS level for the urine test.
• Obtain a baseline cortisol level (see p. 179) for the blood test.
After
• Assess the patient for impending signs of addisonian crisis (muscle weakness, mental and emotional changes, anorexia, nausea, vomiting, hypotension, hyperkalemia, vascular collapse).
• Note that addisonian crisis is a medical emergency that must be treated vigorously. Basically, the immediate treatment includes replenishing steroids, reversing shock, and restoring blood circulation.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Adrenal hyperplasia: Cortisol precursors will be significantly increased as a result of accentuating the ACTH effect.
Adrenal tumor: Tumors are autonomous and are not affected by inhibitory or stimulatory feedback. There is no apparent change in cortisol precursors.
Ectopic ACTH syndrome: This syndrome occurs when neoplasms (usually lung cancer) produce ACTH without regard to regulatory mechanisms. There is no apparent change in cortisol precursors.
Secondary adrenal insufficiency: There will be no significant change in cortisol precursors, because there is no pituitary function to stimulate the production of ACTH.
Related Tests
Adrenocorticotropic Hormone (ACTH) (p. 31). This is a direct measurement of ACTH, which is used in the evaluation of Cushing syndrome and Addison disease.
Adrenocorticotropic Hormone (ACTH) Stimulation (p. 34). This test is used similarly to the metyrapone test for evaluation of Addison disease and Cushing syndrome.
Age-Related Macular Degeneration Risk Analysis (Y402H and A69S)
Indications
This test is used for risk assessment and as supportive documentation of macular degeneration.
Test Explanation
Age-related macular degeneration (ARMD) is recognized as a leading cause of blindness in the United States. Blurred or distorted vision and difficulty adjusting to dim light are common symptoms. ARMD, both wet and dry types, is considered a multifactorial disorder, as it is thought to develop because of the interplay among environmental (smoking), genetic (gender, ethnicity) risk, and protective (antioxidants) factors. At least two genetic variants (Y402H and A69S) have been found to be associated with an increased risk for ARMD. The Y402H and A69S variant genetic variants are common polymorphisms in ARMD. An individual with two copies of the Y402H variant in the gene CFH and two copies of the A69S variant in the gene LOC387715 has an approximate 60-fold increased risk for ARMD. This is significant given how common ARMD is in the general population.
This information can be clinically useful when making medical management decisions (e.g., the use of inflammatory markers) and emphasizing to patients the benefits of smoking cessation and dietary modification. In some cases, genotype information may also assist with clinical diagnosis.
Alanine Aminotransferase (ALT, formerly Serum Glutamic-Pyruvic Transaminase [SGPT])
Indications
This test is used to identify hepatocellular diseases of the liver. It is also an accurate monitor of improvement or worsening of these diseases. In jaundiced patients an abnormal alanine aminotransferase (ALT) will incriminate the liver rather than red blood cell (RBC) hemolysis as a source of the jaundice.
Test Explanation
ALT is found predominantly in the liver; lesser quantities are found in the kidneys, heart, and skeletal muscle. Injury or disease affecting the liver parenchyma will cause a release of this hepatocellular enzyme into the bloodstream, thus elevating serum ALT levels. Most ALT elevations are caused by liver dysfunction. Therefore this enzyme is not only sensitive but also quite specific for hepatocellular disease. In hepatocellular disease other than viral hepatitis the ALT/AST (aspartate aminotransferase) ratio (DeRitis ratio) is less than 1. In viral hepatitis the ratio is greater than 1. This is helpful in the diagnosis of viral hepatitis.
Interfering Factors
![]() Previous intramuscular (IM) injections may cause elevated levels.
Previous intramuscular (IM) injections may cause elevated levels.
![]() Drugs that may cause increased ALT levels include acetaminophen, allopurinol, aminosalicylic acid, ampicillin, azathioprine, carbamazepine, cephalosporins, chlordiazepoxide, chlorpropamide, clofibrate, cloxacillin, codeine, dicumarol, indomethacin, isoniazid (INH), methotrexate, methyldopa, nafcillin, nalidixic acid, nitrofurantoin, oral contraceptives, oxacillin, phenothiazines, phenylbutazone, phenytoin, procainamide, propoxyphene, propranolol, quinidine, salicylates, tetracyclines, and verapamil.
Drugs that may cause increased ALT levels include acetaminophen, allopurinol, aminosalicylic acid, ampicillin, azathioprine, carbamazepine, cephalosporins, chlordiazepoxide, chlorpropamide, clofibrate, cloxacillin, codeine, dicumarol, indomethacin, isoniazid (INH), methotrexate, methyldopa, nafcillin, nalidixic acid, nitrofurantoin, oral contraceptives, oxacillin, phenothiazines, phenylbutazone, phenytoin, procainamide, propoxyphene, propranolol, quinidine, salicylates, tetracyclines, and verapamil.
Related Tests
Aspartate Aminotransferase (AST) (p. 119). This is another enzyme existing predominantly in the liver.
Gamma-Glutamyl Transpeptidase (GGTP) (p. 246). This is another enzyme predominantly existing in the liver.
Alkaline Phosphatase (p. 47). This is another enzyme existing predominantly in the liver.
5′-Nucleotidase (p. 376). This is another enzyme existing predominantly in the liver.
Creatine Kinase (CK) (p. 186). This enzyme is used similarly to AST and exists predominantly in heart and skeletal muscle.
Lactic Dehydrogenase (LDH) (p. 329). This is an intracellular enzyme used to support the diagnosis of injury or disease involving the heart, liver, RBCs, kidneys, skeletal muscle, brain, and lungs.
Leucine Aminopeptidase (p. 337). This enzyme is specific to the hepatobiliary system. Diseases affecting that system will cause elevation of this enzyme.