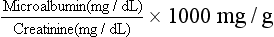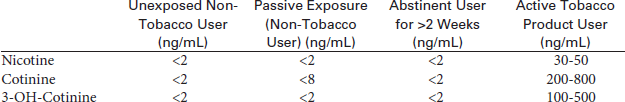Urine Studies
Overview
Urine is derived from filtration of the blood by the nephrons in the kidney. Blood enters the kidney through the renal artery and passes into small capillaries in the glomerulus. There, solute and water are filtered through the capillary and into Bowman capsule. This fluid progressively passes through the capsule and into the renal tubule. More capillaries surround the tubule, and water and other solutes can pass through the tubule into and out of the capillaries according to the body's needs. Within the renal medulla, the collecting system collects all the urine from each nephron and transports it to the renal pelvis. The urine then passes through the ureters and into the bladder. At micturition (voiding), the urine passes through the urethra and out of the body.
Urine is nearly all water, with a small percentage of solutes. All end products of metabolism and all potentially harmful materials are excreted in the urine to maintain normal acid/base balance, fluid and electrolyte balance, and homeostasis.
In general, the urine reflects the blood level for any analyte. If the blood level is elevated and the kidneys are working well, the urine level for that same product can be expected to be high. If the urine level is not high, the kidneys may be diseased, resulting in high levels in the blood. In some instances, certain blood solute products are not filtered from the blood unless “threshold” levels of the solute are exceeded. For example, glucose is not excreted by the kidney unless blood levels exceed approximately 180 mg/dL.
Reasons for Obtaining Urine Specimens
The urine specimen has been referred to as a “fluid biopsy” of the urinary tract. It is usually painlessly obtained, and it provides a great deal of information quickly and economically. Like other specimen tests, urine tests must be carefully performed and properly controlled. Most urine tests are performed for one of the following reasons:
1. To diagnose renal or urinary tract disease (e.g., proteinuria may indicate glomerulonephritis).
2. To monitor renal or urinary tract disease (e.g., urine cultures may be used to monitor the effectiveness of antibiotic therapy for urinary tract infections).
3. To detect metabolic or systemic diseases not directly related to the kidneys (e.g., glucose in the urine may be indicative of diabetes mellitus or Cushing syndrome).
Although blood tests provide valuable information about the body, urinalysis may be preferred for several reasons:
1. Identification of urinary tract infection (UTI) requires a urine specimen.
2. A 24-hour urine collection will reflect homeostasis and disease better than a blood specimen obtained at a random moment of the day.
3. Some products are rapidly cleared by the kidneys and may not be apparent in the blood (e.g., Bence-Jones protein). Results of a blood test may be normal while urinalysis indicates the presence of these products.
4. The serum product being tested may be affected by renal clearance (e.g., sodium). Therefore a urine specimen to measure the sodium concentration will add significant additional information to a serum sodium level.
5. Urine testing is easily performed and does not require an invasive skin puncture.
6. Many urine tests are cheaper than blood tests. The urine test may be less accurate or only qualitative, but that may be all that is needed.
Types of Urine Specimens
The type of urine specimen collected and the collection procedure depend on the test ordered. There are five basic types of urine specimens. In addition, other body fluids can be evaluated to determine whether they contain urine.
First Morning Specimen
To collect a first morning specimen, the patient voids before going to bed. Immediately on rising, the patient collects a urine specimen. The benefits of a first morning specimen are multiple. First, the urine in the bladder overnight represents all of the urine for the previous 6 to 8 hours. Unlike a random spot urine sample, it is a more accurate reflection of the patient's 24-hour urine. Second, postural changes that may affect the urine can be avoided by obtaining the urine specimen immediately on arising. Third, diurnal variations may affect test results. Collecting the first morning specimen allows one to factor in the timing of the testing. Finally, because the urine has been retained in the bladder during a relative overnight fast, it is concentrated, and testing is more likely to detect positive findings. This specimen is ideal for detecting substances such as proteins and nitrates, and is often used to confirm a diagnosis of orthostatic proteinuria.
Although the first morning specimen is frequently the specimen of choice, it is not the most convenient to obtain. It requires that the patient be given instructions and the collection container at least 1 day before the specimen is needed. In addition, the specimen must be preserved if it is not going to be delivered to the laboratory within 2 hours of collection.
Random Urine Specimen
Random urine specimens are usually obtained during daytime hours and without any prior patient preparation. For ease and convenience, routine screening is most often performed on a random specimen. Random testing is usually performed when the substance to be tested does not have significant diurnal variation and its normal concentration is adequate to be detected in a small volume of urine. Random urine is also the specimen of choice for illegal drug screening. This avoids patient tampering with results or changing behavior in anticipation of testing.
Timed Urine Collection
Because substances such as hormones, proteins, and electrolytes are variably excreted over 24 hours, and because of the effects of exercise, posture, hydration, and body metabolism on excretion rates, quantitative urine tests often require a timed collection. These time periods may range anywhere from 2 to 24 hours. Timed collections are of two types. One type includes urine specimens collected at a predetermined time. For example, glucose is often measured 2 hours after a meal (postprandial), because that is when the urine is expected to contain the maximum glucose level. A 2-hour postprandial specimen can be collected after any meal. The second type includes specimens collected at a specific time of day. For example, a specimen for urobilinogen testing is best collected between 2:00 PM and 4:00 PM, when bilinogen is maximally excreted. Depending on the substance being measured and the type of collection, a preservative may be needed to ensure stability throughout the collection period. In addition, certain foods and drugs may need to be avoided during the collection period. Box 11-1 lists some of the more common errors in collecting timed urine specimens.
To collect a timed specimen, the patient is instructed to void and discard the first specimen. This is noted as the start time of the test. All subsequent urine is saved in a special container for the designated period of time. At the end of the specified time period, the patient voids and adds this urine to the specimen container, completing the collection process. (For example, see 24-Hour Urine Collection, p. 906).
Double-Voided Specimen
This collection method is performed to obtain and evaluate fresh urine. To obtain this specimen, the patient first empties the bladder. Shortly thereafter, the patient voids again. The second specimen in the double-voided specimen is the freshest urine and is used for testing. It accurately reflects blood concentrations at that particular time.
Urine Specimen for Culture and Sensitivity
This specimen is collected for examination of bacteria. The specimen must be collected in a sterile container as aseptically as possible. This requires meticulous cleansing of the urinary meatus with an antiseptic preparation to reduce contamination of the specimen by external organisms. A midstream collection technique will cleanse the urethral canal of contaminant bacteria. The specimen should be cultured within 1 hour of collection.
Other Body Fluids
Body fluids can be tested for blood urea nitrogen (BUN) and creatinine to determine whether the fluid is urine. This is done commonly after pelvic surgery. Abdominal fluid serous drainage can look like urine. If the BUN and creatinine concentrations in that fluid are the same as in serum, the fluid is considered to be serous drainage or ascites. If, however, the concentration of BUN and creatinine in the fluid is more than three times that in serum, the fluid is urine. This testing is also helpful in obstetrics to differentiate amniotic fluid from urine.
Collection Methods
Collection methods vary from those requiring no patient preparation to invasive-type procedures. The reason for the test and the clinical situation determine the appropriate collection method.
Common Collection Methods
Routine Void Specimen
A routine void specimen requires no preparation and is collected by having the patient urinate into an appropriate nonsterile container. Random and first morning specimens are collected in this manner.
Midstream and Clean-Catch Specimens
If a culture and sensitivity study is required or if the specimen is likely to be contaminated by vaginal discharge or bleeding, a clean-catch or midstream specimen is collected. For a clean-catch specimen, meticulous cleansing of the urinary meatus with an antiseptic preparation is necessary to reduce contamination of the specimen by external organisms. In male patients, the foreskin is retracted and the meatus cleansed. Then the cleansing agent must be carefully removed, because it may inhibit growth of any bacteria in the specimen, which would affect the culture and sensitivity determination. For a midstream collection, the patient begins to urinate into a bedpan, urinal, or toilet, then stops. This washes the urine out of the distal urethra. The patient voids 3 to 4 ounces of urine into a sterile container, which is then capped, and the patient is allowed to finish voiding.
24-Hour Urine Collection
The patient is instructed to void and discard the first specimen (e.g., at 8:00 AM on day 1). This is noted as the start time of the 24-hour collection. The patient collects all urine voided up to and including that at 8:00 AM the following morning (day 2). In the laboratory, the total volume of the sample is recorded. After the specimen is thoroughly mixed, a measured sample is withdrawn for analysis. See Box 11-2.
If any urine is removed or discarded during a timed collection, the entire timed collection is invalid. Twenty-four-hour urine collections are more accurate than specimens collected over a shorter time. Some analytes are excreted at different rates throughout the day or night, and random specimens may miss the time of maximal excretion. Also, because greater concentrations of an analyte are present in a 24-hour collection, the chance of a false-negative result is reduced.
Special Collection Methods
Special collection methods are indicated when a specimen cannot be obtained by the more common techniques.
Urethral Catheterization
A urine specimen can be obtained by inserting a sterile catheter through the urethra into the bladder. Although catheterization may cause infection, this collection method is used when patients are unable to void or cannot void when the specimen is required (e.g., during trauma).
In patients with an indwelling urinary catheter in place, a specimen is obtained by attaching a syringe to the catheter at a point distal to the sleeve leading to the balloon. Many tubes have an access (sampling port) area for this type of collection technique. Urine is aspirated and placed in a sterile urine container. (Usually the catheter tubing distal to the puncture site needs to be clamped for 15 to 30 minutes before the aspiration of urine to allow urine to fill the tubing. After the specimen is withdrawn, the clamp is removed.) The urine that accumulates in a plastic reservoir bag should never be used for a urine test.
Suprapubic Aspiration
In suprapubic aspiration, urine is collected directly from the bladder by inserting a needle through the abdominal wall and into the bladder. The urine is aspirated into a syringe and sent for analysis. This method is mainly used to obtain urine for anaerobic culture, when specimen contamination is unavoidable, and in infants and young children. Complications are rare.
Pediatric Collections
Urine specimens from infants and young children are often collected using a pediatric collection bag. This clear, pliable, polyethylene bag has a hypoallergenic skin-adhesive backing around the opening. The perineal skin is cleansed and dried before the specimen bag is applied to the skin. The bag is placed over the penis in male children and around the vagina (excluding the rectum) in female children. Once the bag is in place, the patient is checked every 15 minutes until the urine is collected. The specimen bag should be removed as soon as the urine is collected. Bags may be folded and self-sealed for transportation. If a 24-hour specimen is needed, a tube is attached to the bag and connected to a storage container. This avoids repeated skin preparation and reapplication of adhesive to the child's sensitive skin.
Transport, Storage, and Preservation
Disposable plastic containers (100- to 200-mL capacity) with lids are sufficient for most routine urine tests. Screw-top containers are preferred because they are less likely to leak during transportation. Wax-coated cardboard containers should not be used because of the possibility of contaminating the specimen with fatty material. Sterile kits are available for bacterial cultures. Kits usually contain a disposable plastic urine container and cleansing pads.
Rigid, brown, light-resistant plastic containers (approximately 3000-mL capacity) are suitable for most 12- and 24-hour urine collections. These containers have a wide mouth and a leak-proof screw cap. Preservatives may be added to these containers. One-gallon glass jugs may also be used.
Specimen containers must be correctly labeled. Labels should not be placed on the lid, because when the lid is removed the specimen is unlabeled. The patient identification label should be placed directly on the container.
Specimens should be promptly transported to the laboratory. If this is not possible and specimen transportation will be delayed 2 hours or longer, precautions need to be taken to preserve the integrity of the specimen. A variety of changes can occur in an unpreserved specimen. Physical, chemical, and microscopic examinations can all be affected by oxidation, precipitation, and overgrowth of bacteria. Therefore appropriate handling and storage are necessary to ensure that changes do not occur and that accurate results are obtained. Laboratories have written criteria describing when to reject a urine specimen as unsuitable for testing. Box 11-3 lists common criteria for rejecting a urine specimen.
Many analytes require preservatives to maintain viability during the collection period. The proper preservative depends on the type of collection, the delay before testing, and the tests to be performed. No single urine preservative suits all testing requirements. Some analytes require an acidic pH for stability; others are stable in an alkaline pH. For example, acetic acid can be used as a preservative to maintain acidity. Sodium carbonate may be used to maintain alkalinity. Boric acid may be used to inhibit bacterial multiplication. Some analytes are best preserved by refrigeration, which is the easiest means of preserving many urine specimens. If possible, all timed urine specimens should be refrigerated or on ice throughout the collection period. Foley catheter bags can be placed in a basin of ice. Timed specimens may also require the addition of a chemical preservative. For example, sodium fluoride is used to preserve glucose in a 24-hour urine collection. Some analytes need to be protected from light by using a dark collection container or by wrapping the container with foil. Urine for the evaluation of tumor cells may be collected into a container with alcohol. Fixatives (e.g., Saccomanno) also can be used to preserve cytologic specimens.
Collection preservatives may differ among laboratories, depending on (1) testing methods, (2) units of measurement, (3) how often the test is performed, (4) time delays, or (5) transportation to reference laboratories.
Urine Reagent Strips
The urine reagent strip has replaced many complicated individual chemical analyses for determination of various components in the urine. For example, estimation of glucose, albumin, hemoglobin, and bile concentrations, as well as urinary pH, specific gravity, protein, ketone bodies, nitrates, and leukocyte esterase, can be easily determined using a dipstick. Dipsticks are small strips of paper impregnated with a chemical that reacts to products in the urine by changing color. The color correlates with concentrations of the analyte in the urine. Many tests can be performed with one dipstick.
This method of testing involves dipping a “fresh” (not outdated) reagent strip or dipstick into urine and observing the color change on the strip. The color is compared with the color chart on the bottle of reagent strips at the exact time indicated. Dipstick testing is accurate and somewhat quantitative. However, a large number of products in a urine specimen can cause false-positive or false-negative results. Dipstick testing is considered preliminary or for screening. Often more definitive and quantitative studies are necessary to confirm the results.
Reporting of Results
Accurate results depend on appropriate collection, transport, storage, and preservation of the urine specimen. To be clinically useful, test results must be promptly reported, because delays can make the data useless. The report must also be delivered to the appropriate medical record keeper and must be presented in a manner that is clear and easily interpreted.
The report should include the test results, reporting units, and reference ranges. Reference ranges vary from institution to institution. Comments may be included to help interpret results. For example, the technologist may note that the urine specific gravity is too low for proper interpretation of results. Proper reporting of “critical” or “panic” values (well outside the usual range of normal) is essential because such results generally require immediate intervention. If these results are called in to a physician or nurse, verification of notification must be properly documented.
Amylase, Urine
Indications
The urine amylase concentration is used to assist in making the diagnosis of pancreatitis, although other nonpancreatic diseases can also cause elevated urine amylase levels. Urine amylase levels rise later than blood amylase levels. Several days after onset of the disease process, serum amylase levels may be normal while urine levels are significantly elevated. Urine amylase concentration is particularly useful in detecting pancreatitis late in the disease course.
Test Explanation
Amylase is normally secreted from the pancreatic acinar cells into the pancreatic duct and then into the duodenum. Once in the intestine, it aids catabolism of carbohydrates to their component simple sugars. Destruction of acinar cells (as in pancreatitis) or obstruction to the pancreatic duct flow (as in pancreatic carcinoma) causes outpouring of this enzyme into the bloodstream.
Because the kidneys rapidly clear amylase, disorders that affect the pancreas cause elevated amylase levels in the urine. Serum levels of amylase rise transiently but usually return to normal 1 to 2 days after resolution of the acute phase of disease. Levels of amylase in the urine, however, remain elevated 5 to 7 days after onset of disease. This is an important indicator of pancreatitis in patients who have had symptoms for 3 days or longer.
As with serum amylase (p. 61), urine amylase is sensitive but not specific for pancreatic disorders. Other diseases, such as parotiditis (mumps), cholecystitis, perforated bowel, penetrating peptic ulcer, ectopic pregnancy, and renal infarction, can cause elevated urine levels; however, urine levels are usually highest with pancreatitis. A comparison of the renal clearance ratio of amylase to creatinine provides more specific diagnostic information than either the urine amylase level or the serum amylase level alone. When the amylase/creatinine clearance ratio is 5% or more, the diagnosis of pancreatitis can be made with certainty. A ratio less than 5% in a patient with elevated serum and urine amylase levels is indicative of nonpancreatic pathologic conditions (e.g., perforated bowel, macroamylasemia).
Interfering Factors
• Intravenous dextrose solutions can cause a false-negative result.
![]() Drugs that may cause increased amylase levels include aminosalicylic acid, aspirin, azathioprine, corticosteroids, dexamethasone, ethyl alcohol, glucocorticoids, iodine-containing contrast media, loop diuretics (e.g., furosemide), methyldopa, narcotic analgesics, oral contraceptives, and prednisone.
Drugs that may cause increased amylase levels include aminosalicylic acid, aspirin, azathioprine, corticosteroids, dexamethasone, ethyl alcohol, glucocorticoids, iodine-containing contrast media, loop diuretics (e.g., furosemide), methyldopa, narcotic analgesics, oral contraceptives, and prednisone.
![]() Drugs that may cause decreased levels include citrates, glucose, and oxalates.
Drugs that may cause decreased levels include citrates, glucose, and oxalates.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Chronic relapsing pancreatitis:
Damage to pancreatic acinar cells (as in pancreatitis) causes outpouring of amylase into the intrapancreatic lymph system and the free peritoneum. Blood vessels draining the free peritoneum and absorbing the lymph pick up the excess amylase. The amylase is then cleared by the kidneys, and urine levels rise. Amylase clearance can be expected to be greater than 5.
Penetrating peptic ulcer into the pancreas,
In patients with perforated peptic ulcer, necrotic bowel, perforated bowel, or duodenal obstruction, amylase leaks out of the gut and into the free peritoneal cavity. The amylase is picked up by the blood and lymphatic vessels of the peritoneum. The amylase is cleared by the kidneys, and urine levels rise. Amylase clearance is between 2 and 5.
Bence-Jones Protein (Free Kappa and Lambda Light Chains)
Indications
The detection of Bence-Jones protein in the urine most commonly indicates multiple myeloma (especially when the urine levels are high). The test is used to detect and monitor the treatment and clinical course of multiple myeloma and other similar diseases.
Test Explanation
Bence-Jones proteins are monoclonal light-chain portions of immunoglobulins found in 75% of the patients with multiple myeloma. These proteins are made most notably by the plasma cells in these patients. They also may be associated with tumor metastases to the bone, chronic lymphocytic leukemias, lymphoma, macroglobulinemia, and amyloidosis.
Immunoglobulin light chains are usually cleared from blood through the renal glomeruli and reabsorbed in the proximal tubules so that urine light-chain concentrations are very low or undetectable. The production of large amounts of monoclonal light chains, however, can overwhelm this reabsorption mechanism. Because the Bence-Jones protein is rapidly cleared from the blood by the kidney, it may be very difficult to detect in the blood; therefore urine is used for this study. Normally urine should contain no Bence-Jones proteins.
Routine urine testing for proteins using reagent strips often does not reflect the type or amount of proteins in the urine. In fact, the strip may show a completely negative result despite large amounts of Bence-Jones globulins in the urine. Proteins in the urine are best identified by protein electrophoresis of the urine. With this method, the proteins are separated based on size and electrical charge in an electric field when the urine specimen is applied to a gel plate. Once the various proteins are separated, antisera to specific proteins can be added to the gel and specific precipitin arcs can be identified and quantified (immunofixation). Monitoring the urine M-spike is especially useful in patients with light-chain multiple myeloma in whom the serum M-spike may be very small or absent, but in whom the urine M-spike is large.
Test Results and Clinical Significance
Increased Levels
Multiple myeloma (plasmacytoma): Only about 2% of patients with myeloma do not produce Bence-Jones protein. Detection of Bence-Jones protein at high levels (>60 mg/L) is most common with this malignant disease.
Metastatic colon, breast, lung, or prostate cancer:
Several neoplastic disorders are associated with monoclonal gammaglobulinopathies. Some can produce Bence-Jones protein.
Amyloidosis: Primary amyloidosis can produce immunoglobulin light chains similar to those of Bence-Jones protein.
Waldenström macroglobulinemia: This malignant lymphoproliferative disease is highlighted by lymphadenopathy, hepatosplenomegaly, anemia, hyperviscosity, and Bence-Jones proteinuria (about 20% of the patients).
11 Beta-Prostaglandin F(2) Alpha, Urine
Indications
Measurement of 11 beta-prostaglandin F(2) alpha in urine is useful in the evaluation of patients suspected of having systemic mastocytosis (systemic mast-cell disease [SMCD]).
Test Explanation
SMCD is characterized by mast cell infiltration of extracutaneous organs (usually the bone marrow). Focal mast cell lesions in the bone marrow are found in approximately 90% of adult patients with systemic mastocytosis.
Prostaglandin D(2) (PGD2) is generated by human mast cells, activated alveolar macrophages, and platelets. There are a large number of metabolic products of PGD(2), the most abundant is 11 beta-prostaglandin F2 alpha. Although the most definitive test for systemic mast cell disease is bone marrow biopsy (p. 712), measurement of mast cell mediators like beta prostaglandin in urine is advised for the initial evaluation of suspected cases. Elevated levels of 11 beta-prostaglandin F(2) alpha in urine are not specific for systemic mast cell disease and may be found in patients with angioedema, diffuse urticaria, or myeloproliferative diseases in the absence of diffuse mast cell proliferation.
Testing is most commonly performed using a commercially available alpha EIA kit.
Bladder Cancer Markers (Bladder Tumor Antigen [BTA], Nuclear Matrix Protein 22 [NMP22])
Indications
This test is performed on patients who have had a transurethral resection of a superficial bladder cancer to predict or identify tumor recurrence.
Test Explanation
The recurrence rate for superficial bladder cancers that have been resected by transurethral cystoscopy is high. Surveillance testing requires frequent urine testing for cytology and frequent cystoscopic evaluations. The use of bladder tumor markers may provide an easier and cheaper method of diagnosing recurrent bladder cancer that also improves accuracy.
Bladder Tumor Antigen (BTA) and Nuclear Matrix Protein 22 (NMP22) are proteins produced by bladder tumor cells and deposited into the urine. Normally, none or very low levels of these proteins are found in the urine. When levels of bladder cancer tumor markers are normal, cystoscopy rarely yields positive results. When these markers are elevated, bladder tumor recurrence is strongly suspected and cystoscopy is indicated to confirm bladder cancer recurrence.
NMP22 may also be a good screening test for patients at increased risk for developing bladder cancer. However, these markers can be elevated in other circumstances (recent urologic surgery, urinary tract infection, or calculi). Cancers involving the ureters and renal pelvis may also be associated with increased BTA and NMP22.
Bladder cancer cells have been found to exhibit aneuploidy (gene amplifications on chromosomes 3, 7, and 17, and the loss of the 9p21 locus on chromosome 9). Using DNA probes, through fluorescence in situ hybridization (FISH), these chromosomal abnormalities can be identified with great accuracy. FISH can be performed on cells isolated in a fresh urine specimen or cells available on a ThinPrep slide (similar to Pap tests [see p. 743]). When these chromosomal abnormalities are present, fluorescent staining will be obvious using a fluorescence microscope.
Although not actually a tumor marker, a cytology test is available that can be used in the early detection of bladder cancer recurrence. It is an immunocytofluorescence technique based on a patented cocktail of three monoclonal antibodies labeled with fluorescence markers. These antibodies bind to two antigens: a mucin glycoprotein and a carcinoembryonic antigen (CEA). These antigens are expressed by tumor cells found in bladder cancer patients and exfoliated in the urine.
Bone Turnover Markers (BTMs, N-Telopeptide [NTx], Bone Collagen Equivalents [BCEs], Osteocalcin [Bone G1a Protein, BGP, Osteocalc], Pyridinium [PYD] Crosslinks, Bone-Specific Alkaline Phosphatase [BSAP], Amino-Terminal Propeptide of Type 1 Procollagen [P1 NP], C-Telopeptide [CTx])
Normal Findings
Indications
N-telopeptide, bone specific alkaline phosphatase, pyridinium, and osteocalcin are rapid biochemical markers of bone turnover and are used to monitor treatment for osteoporosis.
Test Explanation
With the increased use of bone density scans (see p. 1002), osteoporosis can now be diagnosed and treated more easily. This has prompted an interest in biochemical markers of bone metabolism. Bone is continuously being turned over—bone resorption by osteoclasts and bone formation by osteoblasts. Osteoporosis is a common disease of postmenopausal women and is associated with increased bone resorption and decreased bone formation. The result is thin and weak bones that are prone to fracture. The same process is now becoming increasingly recognized in elderly men, as well. Early diagnosis allows therapeutic intervention to prevent bone fracture.
Bone mineral density studies are valuable tools in the identification of osteoporosis; however, they cannot recognize small changes in bone metabolism. Although bone density studies can be used to monitor the effectiveness of therapy, it takes years to detect measurable changes in bone density. Bone turnover markers (BTM), however, can identify significant improvement in a few months after instituting successful therapy. Furthermore, the cost of bone density studies limits the feasibility of performing this test as frequently as may be required to monitor treatment.
Because the levels of BTMs vary according to the time of day and bone volume, these studies are not widely used or helpful in screening for detection of osteoporosis. Their use is in determining the effect of treatment as these markers are compared to pretreatment levels. Levels will decline with the use of antiresorption drugs (such as estrogen, biphosphonates, calcitonin, and raloxifene). BTMs have shown to be accurately predictive of early improvement in bone mineral density and antifracture treatment efficacy. BTMs are also useful in documenting treatment compliance.
“N-” and “C-” telopeptides (NTx) are protein fragments used in type 1 collagen that make up nearly 90% of the bone matrix. The “C” and “N” terminals of these proteins are cross-linked to provide tensile strength to the bone. When bone is broken down, CTx and NTx are released into the bloodstream and excreted in the urine. Serum levels of these fragments have been shown to correlate well with urine measurements normalized to creatinine. Measurements of these fragments show early response to antiresorptive therapy (within 3 to 6 months) and are good indicators of bone resorption. Normal levels can vary with method of testing.
Amino-terminal propeptide of type I procollagen (P1NP), like NTx, is directly proportional to the amount of new collagen produced by osteoblasts. Concentrations are increased in patients with various bone diseases and therapies characterized by increased osteoblastic activity. P1NP is the most effective marker of bone formation and is particularly useful for monitoring bone formation therapies and antiresorptive therapies.
Osteocalcin, or bone G1a protein (BGP), is a noncollagenous protein in the bone and is made by osteoblasts. It enters the circulation during bone resorption as well as bone formation and is a good indicator of bone metabolism. Serum levels of BGP correlate with bone formation and destruction (turnover). Increased levels are associated with increased bone mineral density loss. BGP is a vitamin K–dependent protein. A reduced vitamin K intake is associated with reduced BGP levels. This probably explains the pathophysiology of vitamin K–dependent deficiency osteoporosis.
Pyridinium (PyD) crosslinks are formed during maturation of the type 1 collagen during bone formation. During bone resorption, these pyridinium crosslinks are released into the circulation.
Bone Specific Alkaline Phosphatase (BSAP) is an isoenzyme of alkaline phosphate (p. 47) and is found in the cell membrane of the osteoblast. It is, therefore, an indicator of the metabolic status of osteoblasts and bone formation.
These BTMs cannot indicate the risk for bone fracture nearly as well as a bone density measurement scan. These markers can be used to monitor the activity and treatment of Paget disease, hyperparathyroidism, and bone metastasis.
BTMs are normally high in children because of increased bone resorption associated with growth and remodeling of the ends of the long bones. The levels reach a peak at about age 14, and then gradually decline to adult values. Because estrogen is a strong inhibitor of osteoclastic (bone resorption) activity, loss of bone density begins soon after menopause begins. Marker levels therefore rise after menopause. Most urinary assays are correlated with creatinine excretion for normalization.
Interfering Factors
• Measurements of these urinary markers can differ by as much as 30% in one person even on the same day. Collecting double-voided specimens in the morning can minimize variability.
• Osteocalcin production is dependent on the availability of vitamins D, C, and K.
![]() Drugs taken for bodybuilding treatments, such as testosterone, can cause reduced levels of NTx.
Drugs taken for bodybuilding treatments, such as testosterone, can cause reduced levels of NTx.
Chloride, Urine (CI)
Indications
This test is used with other urinary electrolytes to indicate the state of electrolyte or acid/base imbalance.
Test Explanation
Chloride is the major extracellular anion. Its main purpose is to maintain electrical neutrality, mostly as a salt with sodium. It follows sodium (cation) losses and accompanies sodium excesses to maintain electrical neutrality. For example, when aldosterone encourages sodium reabsorption, chloride follows to maintain electrical neutrality. Because water moves with sodium and chloride, chloride also affects water balance. Finally, chloride serves as a buffer to assist in acid-base balance. As carbon dioxide (and H cation) increases, bicarbonate must move from the intracellular space to the extracellular space. To maintain electrical neutrality, chloride shifts back into the cell.
A 24-hour urine collection for chloride is useful to evaluate the electrolyte composition of urine and to help determine acid-base imbalances. It is also useful to evaluate the effectiveness of diets with restricted salt (sodium chloride). If sodium and chloride levels are high, the patient is not complying with the diet.
Cortisol, Urine (Hydrocortisone, Urine Cortisol, Free Cortisol)
Indications
This test, a measure of urinary cortisol, is performed in patients with suspected hyperfunction or hypofunction of the adrenal gland.
Test Explanation
An elaborate feedback mechanism for cortisol exists to coordinate the function of the hypothalamus, pituitary gland, and adrenal glands. Corticotropin-releasing hormone (CRH) is made in the hypothalamus. This stimulates adrenocorticotropic hormone (ACTH) production in the anterior pituitary gland. ACTH, in turn, stimulates the adrenal cortex to produce cortisol. The rising levels of cortisol act as a negative feedback and curtail further production of CRH and ACTH. Free or unconjugated cortisol is filtered by the kidneys and excreted in the urine. Elevated urine levels reflect elevated serum cortisol levels.
Cortisol is a potent glucocorticoid released from the adrenal cortex. This hormone affects the metabolism of carbohydrates, proteins, and fats. It has an especially profound effect on glucose serum levels. Cortisol tends to increase glucose by stimulating gluconeogenesis from glucose stores. It also inhibits the effect of insulin and thereby inhibits glucose transport into the cells.
Interfering Factors
• Pregnancy causes increased cortisol levels.
• Physical and emotional stress can elevate cortisol levels.
• Stress is stimulatory to the pituitary-cortical mechanism, which thereby stimulates cortisol production.
![]() Drugs that may cause increased levels include danazol, hydrocortisone, oral contraceptives, and spironolactone.
Drugs that may cause increased levels include danazol, hydrocortisone, oral contraceptives, and spironolactone.
![]() Drugs that may cause decreased levels include dexamethasone, ethacrynic acid, ketoconazole, and thiazides.
Drugs that may cause decreased levels include dexamethasone, ethacrynic acid, ketoconazole, and thiazides.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Ectopic ACTH-producing tumors,
ACTH is overproduced as a result of neoplastic overproduction of ACTH in the pituitary gland or elsewhere in the body by an ACTH-producing cancer. Stress is a potent stimulus to ACTH production. Cortisol levels rise as a result.
Cushing syndrome (adrenal adenoma or carcinoma): Neoplasm produces cortisol without regard to the normal feedback mechanism.
Hyperthyroidism: Metabolic rate is increased and cortisol levels rise accordingly to maintain elevated glucose needs.
Obesity: All sterols are increased in the obese, perhaps because fatty tissue may act as a depository or location of synthesis.
 Decreased Levels
Decreased Levels
Adrenal hyperplasia: Congenital absence of important enzymes in the synthesis of cortisol prevents adequate serum levels.
Addison disease: As a result of hypofunctioning of the adrenal gland, cortisol levels drop.
Hypopituitarism: ACTH is not produced by the pituitary gland destroyed by disease, neoplasm, or ischemia. The adrenal gland is not stimulated to produce cortisol.
Hypothyroidism: Normal cortisol levels are not required to maintain the reduced metabolic rate in patients with hypothyroidism.
Related Tests
Adrenocorticotropic Hormone Stimulation (p. 34). This test is used to evaluate the differential diagnosis of Cushing syndrome or Addison disease.
Adrenocorticotropic Hormone (p. 31). The serum ACTH study is a test of anterior pituitary gland function that affords the greatest insight into the causes of Cushing syndrome (overproduction of cortisol) and Addison disease (underproduction of cortisol).
Cortisol, Blood (p. 179). This is direct measurement of cortisol blood level.
Delta-Aminolevulinic Acid (Aminolevulinic Acid [ALA], δ-ALA)
Indications
This test is used to diagnose porphyria, and in the evaluation of subclinical forms of lead poisoning in children.
Test Explanation
As the basic precursor for the porphyrins (p. 940), delta-ALA is needed for the normal production of porphobilinogen, which ultimately leads to heme synthesis in erythroid cells. Heme is used in the synthesis of hemoglobin. Genetic disorders (e.g., porphyria) are associated with lack of a particular enzyme vital to heme metabolism. These disorders are characterized by accumulation of porphyrin products in the liver or RBCs. The liver porphyrias are much more common. Symptoms of liver porphyrias include abdominal pain, neuromuscular signs and symptoms, constipation and, occasionally, psychotic behavior. This group of disorders results from enzymatic deficiency in synthesis of heme (a portion of hemoglobin). Acute intermittent porphyria (AIP) is the most common form of liver porphyria and is caused by a deficiency in uroporphyrinogen-1-synthase (also called porphobilinogen deaminase).
Most patients with AIP have no symptoms (latent phase) until the acute phase is precipitated by medication or some other factor (see Box 2-20, p. 517). The acute phase is characterized by abdominal and muscular pain, nausea, vomiting, hypertension, mental symptoms (e.g., anxiety, insomnia, hallucinations, paranoia), sensory loss, and urinary retention. Hemolytic anemia also may develop during the acute phase. These acute symptoms are associated with increased serum and urine levels of porphyrin precursors (aminolevulinic acid, porphyrins, and porphobilinogens).
In lead intoxication, heme synthesis is similarly diminished by the inhibition of ALA dehydrase. This enzyme assists in the conversion of ALA to porphobilinogen. As a result of lead poisoning, ALA accumulates in the blood and urine.
Procedure and Patient Care
During
• See Box 11-2, Guidelines for a 24-Hour Urine Collection, p. 907.
• Keep the urine in a light-resistant container with a preservative.
• If the patient has a Foley catheter in place, cover the drainage bag to prevent exposure to light.
![]() Encourage the patient to drink fluids during the 24 hours unless contraindicated for medical reasons.
Encourage the patient to drink fluids during the 24 hours unless contraindicated for medical reasons.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Porphyria (acute intermittent, variegate, and coproporphyria): During the acute phase, porphyrin precursors (including ALA) accumulate in the blood and urine.
Lead intoxication: Chronic lead intoxication may be associated with increased ALA, which accumulates in the blood and urine.
Related Tests
Uroporphyrinogen-1-Synthase (p. 516). This test is used to identify persons at risk for development of porphyria, and to diagnose porphyria in the acute and latent stages.
Porphyrins and Porphobilinogens, (p. 940). This is a quantitative measurement of porphyrins and porphobilinogen in the urine. Helps to define a porphyrin pattern that can classify the type of porphyria.
Glucose, Urine (Urine Sugar)
Indications
Testing for glucose in the urine is part of routine urinalysis. If present, it reflects the degree of glucose elevation in the blood. Urine glucose tests are also used to monitor the effectiveness of therapy for diabetes mellitus.
Test Explanation
A qualitative glucose test is part of routine urinalysis. This screening test for the presence of glucose within the urine may indicate the likelihood of diabetes mellitus or other causes of glucose intolerance (see Glucose, p. 253). This diagnosis must be confirmed by other tests (e.g., fasting glucose, glucose tolerance, glycosylated hemoglobin). Urine glucose tests may be used to monitor the effectiveness of diabetes therapy; however, today this is largely supplanted today by fingerstick determinations of blood glucose levels.
In patients with diabetes that is not well controlled with hypoglycemic agents, blood glucose levels can become very high. Normally, glucose is filtered from the blood by the glomeruli of the kidney. In the glomerular filtrate, the glucose concentration is the same as in the blood. Normally, all of the glucose is reabsorbed in the proximal renal tubules. When the blood glucose level exceeds the capability of the renal threshold to reabsorb the glucose (about 180 mg/dL), it begins to spill over into the urine (glycosuria). As the blood glucose level increases, the amount of glucose spilling into the urine also increases.
Glucosuria may occur immediately after eating a high-carbohydrate meal, and in patients with otherwise normal glucose levels or prediabetic patients receiving dextrose-containing intravenous (IV) fluids. Further, glucosuria does not always indicate diabetes but can occur normally or in diseases that affect the renal tubule or in genetic defects in metabolism and excretion of glucose. In these diseases, the renal threshold for glucose is abnormally low. Despite a normal blood glucose concentration, the kidney cannot reabsorb the normal glucose load. As a result, surplus glucose is spilled into the urine. In these patients, results of glucose tolerance tests are normal. Patients with acute severe physical stress or injury can have a transient glucosuria caused by normal compensatory endocrine-mediated responses.
Interfering Factors
• Any substance that can reduce copper in the Clinitest can produce false-positive results. This may include other sugars (e.g., galactose, fructose, lactose).
![]() Drugs that may cause false-positive results with reagent tablets (e.g., Clinitest) but not with enzyme-impregnated strips (Clinistix, Tes-Tape) include acetylsalicylic acid, aminosalicylic acid, ascorbic acid, cephalothin, chloral hydrate, nitrofurantoin, streptomycin, and sulfonamides.
Drugs that may cause false-positive results with reagent tablets (e.g., Clinitest) but not with enzyme-impregnated strips (Clinistix, Tes-Tape) include acetylsalicylic acid, aminosalicylic acid, ascorbic acid, cephalothin, chloral hydrate, nitrofurantoin, streptomycin, and sulfonamides.
![]() Drugs that may cause false-negative tests include ascorbic acid (Clinistix, Tes-Tape), levodopa (Clinistix), and phenazopyridine (Clinistix, Tes-Tape).
Drugs that may cause false-negative tests include ascorbic acid (Clinistix, Tes-Tape), levodopa (Clinistix), and phenazopyridine (Clinistix, Tes-Tape).
![]() Drugs that may increase urine glucose levels include aminosalicylic acid, cephalosporins, chloral hydrate, chloramphenicol, dextrothyroxine, diazoxide, diuretics (loop and thiazide), estrogen, glucose infusions, isoniazid, levodopa, lithium, nafcillin, nalidixic acid, and nicotinic acid (large doses).
Drugs that may increase urine glucose levels include aminosalicylic acid, cephalosporins, chloral hydrate, chloramphenicol, dextrothyroxine, diazoxide, diuretics (loop and thiazide), estrogen, glucose infusions, isoniazid, levodopa, lithium, nafcillin, nalidixic acid, and nicotinic acid (large doses).
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Read the directions on the bottle or container of reagent strips.
• Check the expiration date on the bottle before use.
![]() Inform the patient that urine tests for glucose may be performed at specified times during the day, generally before meals and at bedtime, and that test results may be used to help determine insulin requirements.
Inform the patient that urine tests for glucose may be performed at specified times during the day, generally before meals and at bedtime, and that test results may be used to help determine insulin requirements.
During
• Because accuracy is necessary, collect a “fresh” urine specimen. Stagnant urine that has been in the bladder for several hours will not accurately reflect the serum glucose level at testing.
• Preferably, obtain a double-voided specimen by the following method:
1. Collect a urine specimen 30 to 40 minutes before the time the urine specimen is actually needed.
2. Discard this first specimen.
3. Give the patient a glass of water to drink.
4. At the required time, obtain a second specimen to be tested for glucose.
![]() Inform the patient that testing for glucose can be easily performed using enzyme tests such as Clinistix, Diastix, or Tes-Tape.
Inform the patient that testing for glucose can be easily performed using enzyme tests such as Clinistix, Diastix, or Tes-Tape.
• If a 24-hour specimen is required, refrigerate the urine during the collection period. See Box 11-2, Guidelines for a 24-Hour Urine Collection, p. 907.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Diabetes mellitus and other causes of hyperglycemia
Pregnancy: Glycosuria is common in pregnant women. Persistent and significantly high levels may indicate gestational diabetes or other obstetric illness. Also, lactosuria is common in nursing women. Lactose is a reducing substance that may cause false-positive results for glucose, depending on the method of testing.
Renal glycosuria: It can occur normally or in patients with diseases that affect the renal tubule. It can also result from genetic defects in the metabolism and excretion of glucose. In these diseases, the renal threshold for glucose is abnormally low. Despite a normal blood glucose level, the kidney cannot reabsorb the glucose it should. As a result, the surplus glucose is spilled into the urine.
Fanconi syndrome: Associated with transport defects in the proximal renal tubules, causing glycosuria, this genetic defect can also affect the metabolism and excretion of amino acids and electrolytes.
Hereditary defects in metabolism of other reducing substances (e.g., galactose, fructose, pentose): These reducing substances may cause false-positive tests for glucose, depending on the method of testing.
Increased intracranial pressure (e.g., from tumors, hemorrhage): The pathophysiology for this observation is not well defined, although many theories exist.
Nephrotoxic chemicals (e.g., carbon monoxide, mercury, lead): These chemicals injure the kidney and lower the renal threshold.
Related Tests
Glucose (p. 253). This is the main screening test for diagnosis of diabetes.
Glycosylated Hemoglobin (p. 266). This is an accurate method for indicating glucose tolerance in the recent past.
Glucose Tolerance (p. 261). This is a test of a patient's capability to handle a glucose load.
Timed Postprandial Glucose (p. 257). This is a timed glucose measurement after a carbohydrate meal.
Glucagon (p. 251). This is a direct measurement of glucagon, which acts to increase glucose in the blood.
Insulin Assay (p. 315). This is a direct measurement of insulin, which acts to decrease glucose in the blood.
17-Hydroxycorticosteroids (17-OCHS)
Indications
This urine study is used to assess adrenocortical function by measuring the cortisol metabolites (17-OCHS) in a 24-hour urine collection.
Test Explanation
Elevated levels of 17-OCHS are noted in patients with adrenal hyperfunction (Cushing syndrome), whether the condition is caused by a pituitary or adrenal tumor, bilateral adrenal hyperplasia, or ectopic tumors producing adrenocorticotropic hormone (ACTH). Low levels of 17-OCHS are seen in patients with adrenal hypofunction (Addison disease) as a result of destruction of the adrenal glands (by hemorrhage, infarction, metastatic tumor, or autoimmunity), surgical removal of an adrenal gland without appropriate steroid replacement, congenital enzyme deficiency, hypopituitarism, or adrenal suppression after prolonged exogenous steroid ingestion.
Testing the urine for this hormone metabolite is an indirect measure of adrenal function. Urine and plasma levels of cortisol (see p. 920 and p. 179, respectively) provide a much more accurate measurement of adrenal function. Because excretion of cortisol metabolites follows a diurnal variation, 24-hour urine collection is necessary.
Interfering Factors
• Emotional and physical stress (e.g., infection) and licorice ingestion may cause increased adrenal activity.
![]() Drugs that may cause increased 17-OCHS levels include acetazolamide, chloral hydrate, chlorpromazine, colchicine, erythromycin, meprobamate, paraldehyde, quinidine, quinine, and spironolactone.
Drugs that may cause increased 17-OCHS levels include acetazolamide, chloral hydrate, chlorpromazine, colchicine, erythromycin, meprobamate, paraldehyde, quinidine, quinine, and spironolactone.
![]() Drugs that may cause decreased levels include estrogen, oral contraceptives, phenothiazines, and reserpine.
Drugs that may cause decreased levels include estrogen, oral contraceptives, phenothiazines, and reserpine.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Ectopic ACTH-producing tumors:
Overproduction of ACTH results from ACTH-producing cancers in the pituitary gland or elsewhere in the body.
Stress: Stress is a potent stimulus to ACTH production. Cortisol and 17-OCHS levels rise as a result.
Cushing syndrome (adrenal adenoma or carcinoma): The neoplasm produces cortisol without regard to the normal feedback mechanism, and 17-OCHS levels rise.
Hyperthyroidism: Metabolic rate is increased, and cortisol and 17-OCHS levels rise accordingly to maintain the elevated glucose needs.
Obesity: All sterols are increased in obese patients, perhaps because fatty tissue acts as a depository or location of synthesis.
 Decreased Levels
Decreased Levels
Adrenal hyperplasia (adrenogenital syndrome): Congenital absence of important enzymes in the cortisol synthesis process prevents adequate serum and urine levels.
Addison disease due to adrenal infarction, adrenal hemorrhage, surgical removal of the adrenal glands, congenital enzyme deficiency, or adrenal suppression from steroid therapy: As a result of hypofunctioning of the adrenal gland, cortisol and 17-OCHS levels are decreased.
Hypopituitarism: ACTH is not produced by the pituitary gland destroyed by disease, neoplasm, or ischemia. The adrenal glands are not stimulated to produce cortisol and 17-OCHS.
Hypothyroidism: Normal cortisol levels are not required to maintain the reduced metabolic rate in patients with hypothyroidism. Cortisol and 17-OCHS levels are decreased.
Related Test
Cortisol, Blood (p. 179). This test is a measure of serum cortisol and is performed in patients with suspected adrenal gland hyperfunction or hypofunction.
5-Hydroxindoleacetic Acid (5-HIAA)
Indications
This test is used to identify patients with carcinoid tumor and to monitor their therapy.
Test Explanation
Quantitative analysis of urine 5-HIAA is performed to detect and monitor the clinical course of carcinoid tumors. Carcinoid tumors are serotonin-secreting tumors that may grow in the appendix, intestine, lung, or any tissue derived from the neuroectoderm. These tumors contain argentaffin-staining (enteroendocrine) cells, which produce serotonin and other powerful neurohormones that are metabolized by the liver to 5-HIAA and excreted in the urine. These powerful neurohormones are responsible for the clinical symptoms (e.g., bronchospasm, flushing, diarrhea) of carcinoid syndrome. This test is used not only to identify carcinoid tumors but also to reevaluate known tumors by means of serial levels of urinary 5-HIAA. Increasing levels of 5-HIAA indicate progression of tumor; decreasing levels indicate a therapeutic response to antineoplastic therapy.
Interfering Factors
• Bananas, plantain, pineapple, kiwi, walnuts, plums, pecans, and avocados can factitiously elevate 5-HIAA levels.
![]() Drugs that may cause increased 5-HIAA levels include acetanilid, acetophenetidin, glyceryl guaiacolate, methocarbamol, acetaminophen, and reserpine.
Drugs that may cause increased 5-HIAA levels include acetanilid, acetophenetidin, glyceryl guaiacolate, methocarbamol, acetaminophen, and reserpine.
![]() Drugs that may cause decreased levels include aspirin, chlorpromazine, ethyl alcohol, heparin, imipramine, isoniazid, levodopa, methenamine, methyldopa, monoamine oxidase (MAO) inhibitors, phenothiazines, promethazine, and tricyclic antidepressants.
Drugs that may cause decreased levels include aspirin, chlorpromazine, ethyl alcohol, heparin, imipramine, isoniazid, levodopa, methenamine, methyldopa, monoamine oxidase (MAO) inhibitors, phenothiazines, promethazine, and tricyclic antidepressants.
Test Results and Clinical Significance
17-Ketosteroid (17-KS)
Indications
This urine test is performed to assist in evaluation of adrenal cortex function, especially as it relates to androgenic function. It is especially useful for evaluation and monitoring of adrenal hyperplasia (adrenogenital syndrome) and adrenal tumors.
Test Explanation
This urine test is used to measure adrenocortical function by measuring 17-ketosteroids (17-KSs) in the urine. 17-KSs are metabolites of testosterone and other androgenic sex hormones. The principal 17-KS is dehydroepiandrosterone (DHEA). In men, approximately one third of the hormone metabolites come from testosterone, produced in the testes, and two thirds come from other androgenic hormones, produced in the adrenal cortex. In women and children, almost all 17-KSs are nontestosterone androgenic hormones, produced in the adrenal cortex. Therefore this test is useful in diagnosing adrenocortical dysfunction. It is important to note that 17-KSs are not metabolites of cortisol and do not reflect levels of cortisol production. Elevated 17-KS levels are frequently noted in congenital adrenal hyperplasia and androgenic tumors of the adrenal glands. In these diseases, excess steroid synthesis is of the “noncortisol” androgenic sterols. These diseases frequently cause virilization syndromes. Testicular tumors rarely cause elevated 17-KS levels.
Low levels of 17-KSs have little clinical significance, because of the inaccuracy of determining low levels. The most common cause of low 17-KS levels is stress. During stress, the adrenal glands produce less androgen and more cortisol. In this regard, low 17-KS levels may reflect states of good health.
Interfering Factors
• Stress may decrease adrenal androgenic activity.
![]() Drugs that may cause increased 17-KS levels include antibiotics, chloramphenicol, chlorpromazine, dexamethasone, meprobamate, phenothiazines, quinidine, secobarbital, and spironolactone.
Drugs that may cause increased 17-KS levels include antibiotics, chloramphenicol, chlorpromazine, dexamethasone, meprobamate, phenothiazines, quinidine, secobarbital, and spironolactone.
![]() Drugs that may cause decreased levels include estrogen, oral contraceptives, probenecid, promazine, reserpine, salicylates (prolonged use), and thiazide diuretics.
Drugs that may cause decreased levels include estrogen, oral contraceptives, probenecid, promazine, reserpine, salicylates (prolonged use), and thiazide diuretics.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Congenital adrenal hyperplasia: In congenital hyperplasia, an enzyme defect results in underproduction of cortisol. By the normal feedback mechanism, ACTH is maximally produced. The result is maximum noncortisol adrenal (androgenic) sterol production. Levels of 17-KS are therefore elevated. This often causes masculinizing syndrome in female patients and precocious puberty in male patients. Congenital adrenal hyperplasia is the most common cause of elevated 17-KS levels in children.
Pregnancy: Pregnancy is associated with slightly higher levels of androgens. 17-KS levels are therefore elevated.
ACTH-secreting ectopic tumors,
ACTH stimulates adrenal cortisol and, to a lesser degree, androgenic sterol production. 17-KS levels are therefore elevated in these three clinical situations.
Testosterone-secreting or androgenic-secreting tumors of the adrenal glands, ovaries, or testes: These tumors are most often associated with elevated 17-KS levels in adults and can produce very high androgen levels. 17-KS levels also can be very high. Adrenal androgenic (mostly DHEA)–producing cancers or adenomas also can produce very high levels of 17-KS.
Cushing syndrome: 17-KS production varies depending on the cause of adrenal overproduction.
Stein-Leventhal syndrome: This masculinizing syndrome is not well understood. Elevated 17-KS levels have been noted.
 Decreased Levels
Decreased Levels
In serious illness, the adrenal glands produce more cortisol and less androgenic hormone. 17-KS levels are therefore low.
Addison disease: With diminished adrenal function, production of androgenic hormones is reduced. 17-KS levels are therefore low.
Hypogonadism (Klinefelter syndrome),
Hypopituitarism: Reduced production of ACTH reduces the activity of the adrenal cortex. 17-KS levels are therefore low.
Related Test
17-Hydroxycorticosteroids (p. 926). This urine test measures the metabolites of cortisol and function of the adrenal cortex.
Microalbumin (MA)
Indication
This test is used as an indicator of complications (kidney, heart, or small vessels) of diabetes. Often it is the first indicator of renal disease.
Test Explanation
Microalbuminuria refers to an albumin concentration in the urine that is greater than normal, but not detectable with routine protein testing. Normally, only small amounts of albumin are filtered through the renal glomeruli, and that small quantity can be reabsorbed by the renal tubules. However, when the increased glomerular permeability of albumin overcomes tubular reabsorption capability, albumin is spilled in the urine. Preceding this stage of a disease is a period where there is only a very small amount of albumin (microalbuminuria) that would normally go undetected. Therefore MA is an early indication of renal disease.
For the diabetic patient, the amount of albumin in the urine is related to duration of the disease and the degree of glycemic control. MA is the earliest indicator for the development of diabetic complications (nephropathy, cardiovascular disease [CVD], and hypertension). MA can identify diabetic nephropathy 5 years before routine protein urine tests. Diabetics with elevated MA have a 5- to 10-fold increase in the occurrence of CVD mortality, retinopathy, and end-stage kidney disease.
It is recommended that all diabetics older than the age of 12 be screened annually for MA. This can be done on a spot urine specimen using a semiquantitative Micral Urine Test Strip. If MA is present, the test should be repeated two more times. If two of three MA urine tests are positive, a quantitative measurement using a 24-hour urine specimen should be performed.
The presence of MA in nondiabetics is an early indicator of lower life expectancy because of CVD and hypertension. Nondiabetic nephropathies may also be associated with microalbuminuria. Life insurance underwriters are increasingly using MA testing to indicate life expectancy.
Because MA levels may be affected by hydration status, the MA/creatinine ratio can be calculated. This is obtained by determining the ratio of urinary microalbumin to urinary creatinine (an indicator of urine concentration). The ratio is calculated as follows:
Procedure and Patient Care
During
• Collect a fresh urine specimen in a urine container.
• If the urine specimen contains vaginal discharge or bleeding, a clean-catch or midstream specimen will be needed (see p. 906).
• Ensure that the urine sample is at room temperature for testing.
• If using a Micral Urine Test Strip:
1. Dip the test strip into the urine for 5 seconds.
2. Allow the strip to dry for 1 minute.
3. Compare the strip with the color scale on the label. The concentration of the red color is proportional to the amount of MA in the patient's sample.
• For quantification of MA, a 10-mL random sample or a portion of a 24-hour urine specimen is obtained. No preservative is used during the 24-hour collection.
Microglobulin (Beta-2 Microglobulin [B2M], Alpha 1 Microglobulin, and Retinol-Binding Protein)
Indications
This test is used to evaluate patients with malignancies, chronic infections, inflammatory diseases, and renal diseases.
Test Explanation
Beta-2 microglobulin (B2M) is a protein found on the surface of all cells. It is an HLA major histocompatibility antigen that exists in increased numbers on the cell surface and particularly on lymphatic cells. Production of this protein increases with cell turnover. B2M is increased in patients with malignancies (especially B-cell lymphoma, leukemia, or multiple myeloma), chronic infections, and in patients with chronic severe inflammatory diseases. It is an accurate measurement of myeloma tumor disease activity, stage of disease, and prognosis and, as such, is an important tumor marker. This tumor marker is best determined in the blood.
B2M, alpha 1 microglobulin, and retinol-binding proteins pass freely through glomerular membranes and are near completely reabsorbed by renal proximal tubules cells. Because of extensive tubular reabsorption, under normal conditions very little of these proteins appear in the final excreted urine. Therefore an increase in the urinary excretion of these proteins indicates proximal tubule disease or toxicity and/or impaired proximal tubular function. In patients with a urinary tract infection, these proteins indicate pyelonephritis. These proteins are helpful in differentiating glomerular from tubular renal disease. In patients with aminoglycoside toxicity, heavy metal nephrotoxicity, or tubular disease, protein urine levels are elevated. Excretion is increased 100 to 1000 times normal levels in cadmium-exposed workers. This test is used to monitor these workers. Periodic testing is performed on these patients to detect kidney disease at its earliest stage. To date, there are no convincing studies to indicate that one protein has better clinical utility than the other.
B2M is particularly helpful in the differential diagnosis of renal disease. If blood and urine levels are obtained simultaneously, one can differentiate glomerular from tubular disease. In glomerular disease, because of poor glomerular filtration, blood levels are high and urine levels are low. In tubular disease, because of poor tubular reabsorption, the blood levels are low and urine levels are high. Blood levels increase early in kidney transplant rejection.
Urinary excretion of these proteins can be determined from either a 24-hour collection or from a random urine collection. The 24-hour collection is traditionally considered the gold standard. For random or spot collections, the concentration of alpha-1-microglobulin is divided by the urinary creatinine concentration. This corrected value adjusts alpha-1-microglobulin for variabilities in urine concentration.
Increased CSF levels of B2M indicate central nervous system involvement with leukemia, lymphoma, HIV, or multiple sclerosis.
Quantitative chemiluminescent immunoassay or nephelometry methods are used to identify these proteins in the urine/serum.
Procedure and Patient Care
During
Urine
![]() See Box 11-2, Guidelines for a 24-Hour Collection, p. 907.
See Box 11-2, Guidelines for a 24-Hour Collection, p. 907.
![]() Encourage the patient to drink fluids during the 24 hours unless this is contraindicated for medical purposes.
Encourage the patient to drink fluids during the 24 hours unless this is contraindicated for medical purposes.
• If a single random urine collection is requested, collect specimen for protein and creatinine testing to adjust for urine concentration.
Test Results and Clinical Implications
 Increased Urine Levels
Increased Urine Levels
Nicotine and Metabolites (Nicotine, Cotinine, 3-Hydroxy-Cotinine, Nornicotine, Anabasine)
Indications
This test is used to document tobacco use. It is used to assess compliance with smoking cessation programs and qualify for surgical procedures. It is also used by insurance companies to determine if the applicant is a smoker.
Test Explanation
Nicotine is metabolized into cotinine and 3-hydroxy-cotinine which are measurable in urine and serum. The word “cotinine” is actually an anagram of “nicotine”—the eight letters are rearranged. In addition to nicotine and metabolites, tobacco products also contain other alkaloids (anabasine and nornicotine). The purpose of this testing is to differentiate patient tobacco use as the following:
Cotinine and 3-hydroxy-cotinine have an in vivo half-life of approximately 20 hours, and are typically detectable from several days to up to 1 week after the use of tobacco. Because the level of these metabolites in the blood is proportionate to the amount of exposure to tobacco smoke, it is a valuable indicator of tobacco smoke exposure. Nicotine and its metabolites can be measured in the serum, urine, or other biofluids (most commonly the saliva). Cotinine is found in urine from 2 to 4 days after tobacco use. Serum/plasma testing is required when a valid urine specimen cannot be obtained (anuretic or dialysis patient) or to detect recent use (within the past 2 weeks). Blood cotinine will increase no matter how the tobacco is used (smoke, chew, dip, or snuff products). Nicotine levels have an in vivo half-life of approximately 2 hours, which is too short to be useful as a marker of smoking status.
Anabasine (only measured in the urine) is present in tobacco products, but not nicotine replacement therapies. Nicotine, cotinine, 3-hydroxy-cotinine, and nornicotine will also be elevated by the use of any of the nicotine replacement gum, patch, or pill products. The presence of anabasine >10 ng/mL or nornicotine >30 ng/mL in urine indicates current tobacco use, irrespective of whether the subject is on nicotine replacement therapy. The presence of nornicotine without anabasine is consistent with use of nicotine replacement products. Heavy tobacco users who abstain from tobacco for 2 weeks exhibit urine nicotine values <30 ng/mL, cotinine <50 ng/mL, anabasine <3 ng/mL, and nornicotine <2 ng/mL. Passive exposure to tobacco smoke can cause accumulation of nicotine metabolites in nontobacco users. Urine cotinine has been observed to accumulate up to 20 ng/mL from passive exposure. Neither anabasine nor nornicotine accumulates from passive exposure.
For smokers, another method of determining tobacco use is expired carbon monoxide. Again, a relatively short half-life (approximately 4 hours) limits the reliability and accuracy. Furthermore, carbon monoxide testing is unable to detect the use of smokeless tobacco.
Urine and salivary cotinine levels are less reliable. Nicotine and metabolite levels will vary by the amount of tobacco used, the use of a filter, the depth of the inhalation, and the size, gender, and weight of the person being tested. Because hydration status and renal function may affect urinary cotinine results, a spot urine cotinine test is always accompanied by a spot urine creatinine.
Quantification of urine nicotine and metabolites while a patient is actively using a tobacco product is useful to define the concentrations that a patient achieves through self-administration of tobacco. The nicotine replacement dose can then be tailored to achieve the same concentrations early in treatment to assure adequate nicotine replacement so the patient may avoid the strong craving he or she may experience early in the withdrawal phase.
Nicotine and metabolites can be accurately quantified with various laboratory methods, including high performance liquid chromatography, gas chromatography/mass spectroscopy, enzyme immunoassay (EIA), and enzyme-linked immunosorbent immunoassay (ELISA). Qualitative assays (including EIA and ELISA) are relatively easy to perform on urine and saliva, but are less accurate than the blood measurement. Absolute laboratory normal values may vary depending on the method of testing.
Osmolality, Urine
Indications
This test is used to evaluate fluid and electrolyte abnormalities. It is an accurate determination of the kidney's concentrating capabilities. It is also used to investigate antidiuretic hormone (ADH) abnormalities (e.g., diabetes insipidus) and the syndrome of inappropriate ADH (SIADH) secretion.
Test Explanation
Osmolality is the measurement of the number of dissolved particles in a solution. It is a more exact measurement of urine concentration than specific gravity because specific gravity depends on the number and precise nature of the particles in the urine. Specific gravity also requires correction for the presence of glucose or protein, as well as for temperature; in contrast, osmolality depends only on the number of particles of solute in a unit of solution. Osmolality also can be measured over a wider range than specific gravity and with greater accuracy.
Osmolality is used in the precise evaluation of the concentrating and diluting abilities of the kidney. With normal fluid intake and normal diet, a patient will produce urine of about 500 to 850 mOsm/kg water. The normal kidney can concentrate urine to 800 to 1400 mOsm/kg. With excess fluid intake, a minimal osmolality of 40 to 80 mOsm/kg can be obtained. With dehydration, the urine osmolality should be three to four times the plasma osmolality.
Osmolality is used in the evaluation of kidney function and the ability to excrete ammonium salts. Osmolality may be used as part of the urinalysis when the patient has glycosuria or proteinuria or has had tests that use radiopaque substances. In these situations, the urine osmolar gap increases because of other organic osmolar particles. The urine osmolar gap is the sum of all the particles predicted or calculated to be in the urine (electrolytes, urea, and glucose) compared with the actual measurement of the osmolality. The predicted/calculated urine osmolality can then be determined by urine levels of sodium, potassium, glucose, and urea nitrogen:
Normally the osmolar gap is 80 to 100 mOsm/kg of H2O. The urine osmolality is more easily interpreted when the serum osmolality (see p. 378) is simultaneously performed. More information concerning the state of renal water handling or abnormalities of urine dilution or concentration can be obtained if urinary osmolality is compared with serum osmolality and urine electrolyte studies are performed. Normally the ratio of urine osmolality to serum osmolality is 1.0 to 3.0, reflecting a wide range of urine osmolality.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Tell the patient that no special preparation is necessary for a random urine specimen.
Tell the patient that no special preparation is necessary for a random urine specimen.
![]() Inform the patient that preparation for a fasting urine specimen may require ingestion of a high-protein diet for 3 days before the test.
Inform the patient that preparation for a fasting urine specimen may require ingestion of a high-protein diet for 3 days before the test.
![]() Instruct the patient to eat a dry supper the evening before the test and to drink no fluids until the test is completed the next morning.
Instruct the patient to eat a dry supper the evening before the test and to drink no fluids until the test is completed the next morning.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Syndrome of inappropriate antidiuretic hormone (SIADH) secretion: Several illnesses can produce SIADH secretion. ADH is inappropriately secreted despite factors that normally would inhibit its secretion. As a result, large quantities of water are reabsorbed by the kidney. Less free water is excreted, and the urine osmolality rises.
Paraneoplastic syndromes associated with carcinoma (e.g., lung, breast, colon): These cancers act as an autonomous ectopic source for secretion of ADH. The pathophysiology is the same as is described for SIADH.
Shock: The normal physiologic response to shock is to minimize the loss of free body water. The kidneys therefore absorb all the free water possible. Urine osmolality rises.
 Decreased Levels
Decreased Levels
Related Tests
Serum Osmolality (p. 378). This is a measurement of osmolality of the serum. When combined with urine osmolality, more accurate interpretation of either test is possible.
Antidiuretic Hormone (p. 73). This provides a direct measurement of ADH in the blood.
Antidiuretic Hormone Suppression (p. 76). This test is helpful in evaluation of ADH abnormalities.
Porphyrins and Porphobilinogens (Uroporphyrins, Coproporphyrin, Free Erythrocyte Protoporphyrin [FEP])
Indications
This test is a quantitative measurement of porphyrins and porphobilinogen. It is used along with aminolevulinic acid (ALA) to identify the various forms of porphyria.
Test Explanation
Porphyria is a group of genetic disorders associated with enzyme deficiencies involved with porphyrin synthesis or metabolism. Porphyrins (e.g., uroporphyrin, coproporphyrin) and porphobilinogens are important building blocks in the synthesis of heme. Heme is incorporated into hemoglobin within the erythroid cells. Porphyrias are classified according to location of the accumulation of the porphyrin precursors. In most forms of porphyria, increased levels of porphyrins and porphobilinogen are found in the urine. Heavy metal (lead) intoxication is also associated with increased porphyrins in the urine.
Variable symptoms are associated with different types of porphyrias. Erythropoietic porphyria is associated with photosensitivity of the eyes and skin. Intermittent porphyria and, less often, variegate and hereditary coproporphyria are associated with abdominal pain and neurologic symptoms. Heavy metal (e.g., lead) intoxication is also associated with increased porphyrins in the urine. Certain drugs can induce porphyria and cause elevated porphyrin levels in the urine (see Box 2-20, p. 517). This test is a quantitative analysis of urinary porphyrins and porphobilinogens. If porphyrins are present, the urine may be colored amber red or burgundy, or even darker after standing in the light.
Urine tests for porphyrins are not as accurate as plasma measurements and pattern identification for the various forms of porphyria. They are accurate, however, in screening for porphyria, especially the intermittent variety. Porphyrin fractionation of erythrocytes and of plasma provides specific assays for primary red blood cell (RBC) porphyrins. These assays are predominantly used to differentiate the various forms of congenital porphyrias. Plasma measurement of free erythrocyte protoporphyrin (FEP) is helpful in the diagnosis of iron deficiency anemia or lead intoxication. In these latter diseases, a small amount of excess porphyrin remains in the RBC after heme synthesis. This is measured as FEP.
Although this test can also be done on a fresh stool specimen, random and 24-hour urine collections are more accurate. Colorimetric methods (or spectrophotometry) are used most often. Porphyrin fractionation of the various types of porphyrins within the urine allows identification of patterns commonly associated with the various porphyrias. High-performance liquid chromatography is used for that. The diagnosis of porphyria and interpretation of test results are difficult. The American Porphyria Foundation can provide assistance to health care providers in this area.
Procedure and Patient Care
During
Porphyrins
• See Box 11-2, Guidelines for a 24-Hour Urine Collection, p. 907.
![]() Instruct the patient to avoid alcohol use during the collection period.
Instruct the patient to avoid alcohol use during the collection period.
• Keep the specimen on ice or refrigerated during the 24 hours.
• Keep the urine in a light-resistant specimen bottle with a preservative to prevent degradation of the light-sensitive porphyrin.
![]() Encourage the patient to drink fluids during the 24 hours unless contraindicated for medical reasons.
Encourage the patient to drink fluids during the 24 hours unless contraindicated for medical reasons.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Porphyrias
Acute intermittent porphyria: Porphobilinogen and to a lesser degree porphyrin levels are elevated during the acute phase. No real increase is noted during the latent phases.
Congenital erythropoietic porphyria: Porphobilinogen level is elevated.
Hereditary coproporphyria: Coproporphyrin and porphobilinogen levels are elevated.
Variegate porphyria: In acute episodes, porphobilinogen and ALA (p. 922) levels are elevated.
Lead poisoning: ALA level is most significantly elevated; porphyrin levels are slightly elevated.