Miscellaneous Studies
Overview
We have tried to organize a multitude of diverse diagnostic tests into groups based on the specimen on which the test was performed and the method of testing. This led to the development of chapters as presented in this text. However, a few tests could not readily be appropriated to any chapter. Therefore this chapter was created to include these important tests. There are no commonalities associated with these tests. All are described separately and in detail.
Indications
Skin testing is the most commonly used and easiest method of identifying patients who suffer from allergies. Furthermore, it is a method by which a specific allergen can be determined.
Test Explanation
When properly performed, skin testing is considered to be the most convenient and least expensive test for detecting allergic reactions. Since the early 1900s, skin testing has been a common practice for establishing a diagnosis of allergy by reexposure of the individual to a specific allergen. Skin testing provides useful confirmatory evidence when a diagnosis of allergy is suspected on clinical grounds. The simplicity, rapidity, low costs, sensitivity, and specificity explain the crucial position skin testing has in allergy testing.
In an allergic patient, an immediate wheal (small swelling, as from an insect bite) and flare (red, inflammed area) reaction follows injection of the specific allergen (that substance to which the person is allergic). This reaction is initiated by immunoglobulin E (IgE) antibodies and is mediated primarily by histamine secreted from mast cells. This usually occurs in about 5 minutes and peaks at 30 minutes. In some patients a “late-phase reaction” occurs; this is highlighted by antibody and cellular infiltration into the area that usually occurs within 1 to 2 hours.
There are three commonly accepted methods of injecting the allergen into the skin. The first method is called the prick-puncture test or scratch test. In this method, the allergen is injected into the epidermis. Life-threatening anaphylaxis reactions have not been reported with this method. The second method is called the intradermal test. Here the allergen is injected into the dermis (creating a skin wheal). Large local reactions and anaphylaxis have been reported with this latter method. For these two tests, the allergen placement part of the test takes about 5 to 10 minutes. The third method is called the patch test. This takes much longer because the patient must wear the patch for 48 hours to see if there is a delayed allergic reaction. With this method, needles are not used. Instead, an allergen is applied to a patch that is placed on the skin. It is usually done to detect whether a particular substance (e.g., latex, medications, fragrances, preservatives, hair dyes, metals, resins) is causing an allergic skin irritation, such as contact dermatitis.
Patients with dermographism (nonallergic response of redness and swelling of the skin at the site of any stimulation) develop a skin wheal with any skin irritation, even if nonallergic. In these patients, a false-positive reaction can occur with skin testing. To eliminate these sort of false positives, a “negative control” substance consisting of just the diluent without an allergen is injected at the same time as the other skin tests are performed. Patients who are immunosuppressed because of concurrent disease or medicines may have a blunted skin reaction even in the face of allergy. This would cause false-negative results. To avoid false negatives, a “positive control” substance consisting of a histamine analogue is also injected into the forearm at the time of skin testing. This will cause a wheal and flare response even in the nonallergic patient, unless the patient is immunosuppressed.
For inhalant allergens, skin tests are extremely accurate. However, they are less reliable for food allergies, latex allergies, drug sensitivity, and occupational allergies. Although there is considerable variability in accuracy of skin testing because of poor injection techniques, when performed correctly, skin testing represents one of the major tools in the diagnosis of allergy.
Interfering Factors
• False-positive results may occur in patients with dermographism.
• False-positive results may occur if the patient has a reaction to the diluent used to preserve the extract.
• False-negative results may be caused by poor-quality allergen extracts, diseases that attenuate the immune response, or improper technique.
• Infants and the elderly may have decreased skin reactivity.
![]() Drugs that may decrease the immune response (size of wheal and flare) of skin testing include angiotensin-converting enzyme (ACE) inhibitors, beta blockers, corticosteroids, nifedipine, and theophylline.
Drugs that may decrease the immune response (size of wheal and flare) of skin testing include angiotensin-converting enzyme (ACE) inhibitors, beta blockers, corticosteroids, nifedipine, and theophylline.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Observe the following skin-testing precautions:
1. Be sure that a physician is immediately available.
2. Evaluate the patient for dermographism.
3. Have medications and equipment available to handle anaphylaxis.
4. Proceed with caution in patients with current allergic symptoms.
5. Pay great attention to the technique chosen for the skin test in order to get accurate results.
6. Avoid bleeding caused by injection.
• Obtain a history to evaluate the risk of anaphylaxis.
• Identify any immunosuppressive medications the patient may be taking.
• Evaluate the patient for dermographism by rubbing the skin with a pencil eraser and looking for a wheal at the site of irritation.
• Draw up 0.05 mL of 1:1000 aqueous epinephrine into a syringe before testing in the event of an exaggerated allergic reaction.
• A negative prick-puncture test should be performed before an intradermal test.
During
Prick-Puncture Method (Scratch Test)
• A drop of the allergen solution is placed onto the volar surface of the forearm or back after cleaning the area.
• A 25-gauge needle is passed through the droplet and inserted into the epidermal space at an angle with the bevel facing up.
• The skin is lifted up and the fluid is allowed to seep in. Excess fluid is wiped off after about a minute.
Intradermal Method
• With a 25-gauge needle, the allergen solution is injected into the dermis by creating a skin wheal. In this method, the bevel of the needle faces downward. A volume of between 0.01 and 0.05 mL is injected.
• In general, the allergen solution is diluted 100- to 1000-fold before injection.
Patch Method
• Clean the skin area (usually back or arm).
• Apply the patches to the skin (as many as 20-30 can be applied).
• Instruct the patient to wear the patches for 48 hours. Tell the patient to avoid bathing or activities that cause heavy sweating.
• Tell the patient the patches will be removed at the doctor's office. Irritated skin at a patch site may indicate an allergy.
After
• Document allergen solution, location, and patient reaction.
• Evaluate the patient for exaggerated allergic response.
• In the event of a systemic reaction, a tourniquet should be placed above the testing site and epinephrine should be administered subcutaneously.
• With a pen, encircle the area of testing and mark the allergen used.
• Read the skin test at the appropriate time.
• Skin tests are read when the reaction is mature, after about 15 to 20 minutes. Both the largest and smallest diameter of the wheal is determined. The measurements (in millimeters) are averaged.
• The flare is measured in the same manner.
• Observe the patient for 20 to 30 minutes before discharge.
Related Test
Allergy Blood Testing (p. 49). Allergy blood testing is an alternative to allergy skin testing in diagnosing allergy as a cause of a particular symptom complex. It is also useful in identifying the particular allergen affecting a patient. It is particularly helpful when allergy skin testing is contraindicated.
Bioterrorism Infectious Agents Testing (Botulism, Anthrax, Hemorrhagic Fever, Plague, Smallpox, Tularemia, Brucellosis)
Indications
These tests are indicated if terrorism is suspected because of suspicious illness, or some other type of evidence.
Test Explanation
Infectious agents used in bioterrorism are many and it would be difficult to discuss each possible agent. This test discusses those agents that humans are most likely to be exposed to in war or in a civilian terrorist attack. Please refer to Table 13-1 for specifics of each agent. All documented cases must be reported to the Department of Public Health.
Botulism Infection
The botulinum toxin produced by Clostridia botulinum, a spore-forming anaerobic bacterium, causes the symptoms associated with botulism. The gastrointestinal (GI) tract is the usual port of entry through the ingestion the toxin itself, C. botulinum spores, or the actual bacterium. Ingestion of the toxin produces symptoms almost immediately. Symptoms may be delayed if the spores or the bacterium are ingested. Common sources of C. botulinum include undercooked meat or sauces exposed to room temperature for prolonged periods. This bacterium can be inhaled by handling the same food or by open wound contamination of soil that contains C. botulinum.
The toxin binds irreversibly to the presynaptic nerve terminal at the neuro-muscular junction and prevents the release of acetylcholine necessary for normal muscular function. As a result, one may experience bulbar palsies causing blurred vision, dysphagia, dysarthria and skeletal muscle weakness progressing to flaccid paralysis. Symptoms begin 6 to 12 hours after ingestion of the contaminated food or approximately 1 week after wound contamination. The test used to diagnose this disease involves the identification of the toxin in the blood, stool, or vomitus of the affected individual. The food itself can also be tested. The toxin can be identified by the biologic Mouse Neutralization test. C. botulinum can also be cultured in an anaerobic environment from the stool or from contaminated food.
Treatment involves mechanical support of ventilation and nutrition. The use of botulinum antitoxin that can be obtained from the Centers for Disease Control and Prevention (CDC) is the mainstay of treatment. This antitoxin presents a risk of “serum sickness” in nearly one quarter of the patients who receive it.
Anthrax
Anthrax is caused by Bacillus anthracis, which is a spore forming gram-positive rod. The organism is widely distributed in the soil and, under natural conditions, grazing animals can become infected and pass it on to those working in close contact with grazing animal products (meat, wool, or hides). It can be contracted by eating undercooked meat or inhaled from animal products (such as wool) or by inhaling the spores. Once inhaled, it is uniformly fatal without treatment. Cutaneous anthrax occurs from contact with contaminated meat, wool, hides, or leather from infected animals.
There are three forms of the disease: cutaneous, gastrointestinal, and pulmonary. Symptoms include fever, malaise, fatigue progressing to cutaneous lesions, or pulmonary failure. Symptoms occur about 2 to 6 days after exposure.
Culturing the organism in sheep blood agar makes the diagnosis. Appropriate specimens for culture would be stool, blood, sputum, or the cutaneous vesicle. Treatment for this disease is early institution of antibiotics and supportive care.
Hemorrhagic Fever (Yellow Fever)
This disease complex has many causative virus families including arenavirus, bunyavirus (including Hantavirus), Filovirus (including Ebola), and flavivirus. Symptoms include fever, thrombocytopenia, shock, multiorgan failure, lung edema, and jaundice. Symptoms develop 4 to 21 days after a mosquito or rodent bite (depending on the disease). This disease is contagious and patients with suspicious symptoms should be quarantined.
The diagnosis is determined by clinical evaluation. However, viral cultures with polymerase chain reaction (PCR) identification, serology, and immunohistochemistry of tissue specimens are possible. There is no specific treatment other than aggressive medical therapy and support of organ failure.
Plague
This disease is caused by the gram-negative coccobacillus Yersinia pestis. It is transmitted to humans primarily by the bite of fleas or contact with other human bodily fluids. It has three forms: bubonic (enlarged lymph nodes), septicemic (blood-borne), and pneumonic (aerosol). Pneumonic is, by far, the deadliest form of the infection. Symptoms may include fever, chills, weakness, enlarged lymph nodes, or bacterial pneumonia and respiratory failure.
The diagnosis is made by culture of the blood, sputum, or lymph node aspirate. This disease complex can be treated with antibiotics when started early in the course of the disease. Early testing and diagnosis affects patient outcome. The risk for bioterrorism is weapon attack or spread by aerosol transmission.
Brucellosis
This disease is caused by Brucella abortus, B. suis, B. melitensis, or B. canis. It is contracted by ingestion of contaminated milk products (especially goat's milk), direct puncture of the skin (by butchers and farmers), or by inhalation. This multisystem disease is characterized by acute or insidious onset of fever, night sweats, undue fatigue, anorexia, weight loss, headache, and arthralgia. Hepatomegaly, splenomegaly, and spondylitis are also common. Brucella can be cultured from a blood, sputum, or food specimen. Serology testing is also possible. Diagnosis is confirmed by a fourfold or greater rise in Brucella agglutination titer between acute- and convalescent-phase serum specimens obtained greater than or equal to 2 weeks apart and studied at the same laboratory. Demonstration by immunofluorescence of a Brucella organism in a clinical specimen is another method of diagnosis. Infections are usually treated with antibiotics.
Smallpox
Smallpox is a serious, contagious, and sometimes fatal infectious disease caused by the variola virus (a deoxyribonucleic acid [DNA] virus). There is no specific treatment for smallpox disease, and the only prevention is vaccination. There are two clinical forms of smallpox. Variola major is the severe and most common form of smallpox, with a more extensive rash and higher fever. Variola minor is a less common presentation of smallpox and a much less severe disease. The disease has been eradicated after a successful worldwide vaccination program. It is very easily spread and is therefore considered a potential bioterrorism weapon. It has the potential to cause widespread disease and death that could devastate a whole city or region.
The first symptoms of smallpox include fever, malaise, head and body aches, and sometimes vomiting. Next a rash occurs in the mouth and then on the skin. This rash proceeds to become pustular. As the pustules dry up and scab, the patient is no longer contagious.
Viral culture, serology, immunohistochemistry, or electron microscopy can make the diagnosis. The best specimen is the vesicular rash. While there is no treatment for the disease, vaccination is available and is offered to all those at risk for bioterrorism.
Tularemia
This disease is caused by a gram-negative bacterium called Francisella tularensis. It is contracted by drinking contaminated water or eating vegetation contaminated by infected animals. It can be aerosolized and can contaminate the air or drinking water supplies. When it enters through the skin by an insect bite, tularemia can be recognized by the presence of a lesion and swollen glands. Ingestion of the organism may produce a throat infection, intestinal pain, diarrhea, and vomiting. Symptoms generally appear between 2 and 10 days, but usually 3 days after exposure.
Inhalation of the organism may produce a fever alone or fever combined with a pneumonia-like illness that is difficult to distinguish from influenza or other atypical pneumonias. Diagnosis is made by culture of the blood, sputum, or stool. Although tularemia can be life threatening, most infections can be successfully treated with antibiotics.
Procedure and Patient Care
Before
• Follow guidelines for safe contact with the patient, who can be highly infectious.
• Maintain strict adherence to all procedures in regard to isolation or contamination of the specimen.
• Biohazard precautions are to be taken with each patient and specimen.
• Laboratory personnel must strictly adhere to all standard precautions and transmission principles.
During
• If an enema is used to obtain a botulinum stool specimen, use sterile water. Saline can negate results.
• Send enough blood for adequate testing. Usually two red-top tubes are adequate. It is best to send the blood specimens on ice.
• If food is sent for testing, it should be sent in its original containers.
• For anthrax or smallpox testing of a cutaneous lesion, soak one or two culture swabs with fluid from a previously unopened lesion.
Indications
Because molecular genomic studies measure the quantity of specific breast cancer–related genes, they can help predict the possibility of cancer susceptibility to chemotherapy. They also provide a powerful indicator of the likelihood of breast cancer recurrence (local and metastatic) after primary breast cancer surgery.
Test Explanation
Genomic testing using either Oncotype DX or MammaPrint is a clinically validated, multigene assay that provides a quantitative assessment of the likelihood of distant breast cancer recurrence and also assesses the benefit from certain types of chemotherapy in newly diagnosed breast cancer patients. In early-stage invasive breast cancer, the evaluation of the likelihood of distant recurrence is usually based on multiple pathologic factors, such as nodal status, tumor size and grade, estrogen and progesterone receptors, and HER-2 status (see p. 717). However, these factors are often inaccurate and cannot quantify the recurrence risk sufficiently to provide significant insight into the risks and benefits of adjuvant chemotherapy. Genomic testing is designed to provide quantitative data to assist in clinical decision making regarding the use of adjuvant systemic therapies.
The Oncotype DX rtPCR assay—performed using formalin-fixed, paraffin-embedded tumor tissue—analyzes the expression of a panel of 21 genes (16 tumor-related genes and 5 reference genes) and provides the results as a recurrence score (0 to 100). The gene panel was selected and the recurrence score calculation derived through extensive laboratory testing followed by appropriate corroboration with multiple clinical studies in which Oncotype's predictability was validated. The MammaPrint, using microarray assay on fresh-frozen breast cancer tissue, analyzes the expression of 70 prognostic genes. A 5-gene IHC assay, the Mammostrat, uses monoclonal antibody biomarkers and a diagnostic algorithm with fresh-frozen cancer tissue. Molecular genomics is sensitive, specific, and highly reproducible and has a wide dynamic range.
Patients whose tumor genomics have low recurrence scores have only a slight chance of recurrence and derive minimal or no benefit from chemotherapy. Patients with tumors that have high recurrence scores have a significant chance of recurrence and can experience considerable benefit from chemotherapy. At present, genomic testing is intended for newly diagnosed patients whose breast cancer is stage I or II, node negative, HER-2/neu negative, and estrogen receptor positive. Clinical studies in other populations are currently underway.
Procedure and Patient Care
Before
![]() Explain the significance of the prognostic data available for the patient's tumor.
Explain the significance of the prognostic data available for the patient's tumor.
![]() Explain the benefits of genomics in helping the physician and the patient make appropriate decisions regarding the use of adjuvant chemotherapy.
Explain the benefits of genomics in helping the physician and the patient make appropriate decisions regarding the use of adjuvant chemotherapy.
• Provide the patient with emotional support through the postoperative period.
• Ensure that the patient's insurance will cover this expensive testing.
Related Tests
Estrogen/Progesterone Receptor Assay (pp. 728 and 750, respectively). These are also prognostic indicators for breast cancer.
HER-2/neu (p. 717). This is a breast cancer prognosticator and target for monoclonal therapy.
Indications
This still-experimental test is performed to evaluate the sensitivity of a patient's cancer cells to anticancer drugs.
Test Explanation
Cell culture drug resistance testing (CCDRT) refers to testing the reaction of a patient's own cancer cells in the laboratory to drugs that may be used to treat the patient's cancer. The idea is to identify which drugs are more likely to work and which drugs are less likely to work. By avoiding the latter and choosing from among the former, the patient's probability of benefiting from the chemotherapy may be improved. There are multiple tests available for drug sensitivity testing, but all have four common steps. Cancer cells from the patient's tumor must be obtained and isolated. The cells are then isolated with various potentially therapeutic drugs. Assessment of cell survival is then performed and the results are provided. Based on those results, the clinician can recommend more appropriate chemotherapy for a particular cancer. In most cases, this testing is used for patients with refractory or recurrent epithelial tumors (usually breast or ovarian cancer).
Indications
CVS is performed in women whose unborn child may be at risk for a life-threatening or life-altering genetic defect. This includes women who (1) are older than 35 years at the time of pregnancy, (2) have had frequent spontaneous abortions, (3) have had previous pregnancies with fetuses or infants with chromosomal or genetic defects (e.g., Down syndrome), (4) have a genetic defect themselves (e.g., hemoglobinopathy), or (5) have increased fetal nuchal transparency or other abnormal ultrasound finding.
Test Explanation
CVS can be performed at 8 to 12 weeks of gestation for early detection of genetic and biochemical disorders. Because CVS detects congenital defects early, first-trimester therapeutic abortions can be performed if indicated and desired.
A sample of chorionic villi from the chorion frondosum, which is the trophoblastic origin of the placenta, is obtained for analysis. These villi in the chorion frondosum are present from 8 to 12 weeks on and reflect fetal chromosome, enzyme, and deoxyribonucleic acid (DNA) content. This permits much earlier diagnosis of prenatal problems than with amniocentesis, which cannot be done before 14 to 16 weeks. Further, the cells derived by CVS are more easily cultured for karyotyping (determination of chromosomal and genetic abnormalities). Although amniocentesis is the safer procedure, the cells obtained take longer to grow in culture, which further adds to the delay in obtaining results. At this later point, therapeutic abortion for severe genetic defects is more difficult.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient. Encourage patient to have someone accompany her to the appointment for emotional support and to drive home afterward.
Explain the procedure to the patient. Encourage patient to have someone accompany her to the appointment for emotional support and to drive home afterward.
• Ensure that signed consent for the procedure has been obtained.
![]() Tell the patient that no food or fluid restrictions are necessary.
Tell the patient that no food or fluid restrictions are necessary.
![]() Encourage the patient to drink at least 1 to 2 glasses of fluid before the test.
Encourage the patient to drink at least 1 to 2 glasses of fluid before the test.
![]() Instruct the patient not to urinate for several hours before the test. A full bladder is an excellent reference point for pelvic ultrasound.
Instruct the patient not to urinate for several hours before the test. A full bladder is an excellent reference point for pelvic ultrasound.
• Assess the vital signs of the mother and fetal heart rate before the test, and again during and on completion of the test.
During
• Note the following procedural steps:
1. The patient is placed in the lithotomy position, and a sterile speculum is placed into the previously cleansed vagina to visualize the cervix.
2. A cannula is inserted into the cervix and uterine cavity (Figure 13-1).
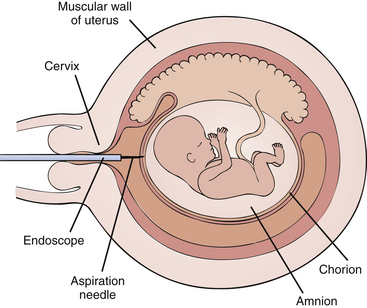
Figure 13-1 Chorionic villus sampling (CVS). Diagram of an 8-week pregnancy showing endoscopic aspiration of extraplacental villi.
3. Under ultrasound guidance, the cannula is rotated to the site of the developing placenta.
4. A syringe is attached, and suction is applied to obtain three or more villous samples to ensure sufficient tissue for accurate sampling.
5. If ultrasound indicates that the trophoblastic tissue is remote from the cervix, a transabdominal approach similar to that described for amniocentesis (p. 632) may be used.
• This procedure is performed by an obstetrician in approximately 30 minutes.
![]() Inform the patient that discomfort associated with this test is similar to that of a Papanicolaou test (Pap test).
Inform the patient that discomfort associated with this test is similar to that of a Papanicolaou test (Pap test).
After
• Some mothers with Rh-negative blood may receive Rho(D) immune globulin (RhoGAM) because of the risk for development of maternal antibodies to the fetal blood cells, which could threaten fetal well-being.
• Monitor vital signs, and check the mother for signs of bleeding.
• Schedule an ultrasound in 2 to 4 days to affirm continued viability of the fetus.
• Assess the vaginal area for discharge and drainage; note the color and amount.
![]() Assess and educate the patient concerning signs of spontaneous abortion (e.g., cramps, bleeding) and endometrial infection (e.g., vaginal discharge, fever, crampy abdominal pain).
Assess and educate the patient concerning signs of spontaneous abortion (e.g., cramps, bleeding) and endometrial infection (e.g., vaginal discharge, fever, crampy abdominal pain).
![]() Inform the patient how to obtain the results from the physician. Be sure she understands that the results are usually not available for several weeks (although they may be available much sooner if the test is performed at a major medical center). If results are unclear, amniocentesis may be needed.
Inform the patient how to obtain the results from the physician. Be sure she understands that the results are usually not available for several weeks (although they may be available much sooner if the test is performed at a major medical center). If results are unclear, amniocentesis may be needed.
![]() Inform the patient about genetic counseling services if needed to help understand the results or make a decision regarding a problem.
Inform the patient about genetic counseling services if needed to help understand the results or make a decision regarding a problem.
Test Results and Clinical Significance
Chromosomal, genetic, and biochemical disorders: Many chromosomal and genetic defects are identified by karyotyping and genetic mapping. Genetic counseling is a vital part of this sort of testing. If therapeutic abortion is an option, the religious, moral, and ethical aspects of this decision need to be considered.
Related Tests
Obstetric Ultrasonography (p. 887). This test is used to localize trophoblastic tissue.
Amniocentesis (p. 632). Used to indicate fetal well-being and allows tissue sampling for karyotyping and genetic mapping.
Fetoscopy (p. 612). During this test, tissue can be obtained for karyotyping and genetic mapping.
Fetal Nonstress Test (p. 569). This test is used to evaluate the viability of the fetus before, during, and after CVS.
Indications
This test is performed to diagnose disease affecting the posterior eye including the retina, choroid, and optic nerve. It is also used to monitor disease progression and treatment.
Test Explanation
With the use of fluorescein angiography, the patency and integrity of the retinal circulation can be determined. It involves injection of sodium fluorescein into the systemic circulation followed by timed-interval photographs performed with a fundus camera. The timed images are then reviewed for specific patterns indicative of disease states. The test is often repeated at intervals to monitor treatment or disease progression.
Fluorescein is a member of the triphenylmethane dyes. When the fluorescein molecules absorb light toward the end of the blue spectrum (465 to 490 nm), the molecules transfer from a basal state to an excited state. In doing so, light of a different wavelength (450 to 465 nm—the yellow-green end of the light spectrum) is emitted. This light emission is then recorded by a specialized camera where very little light outside the blue spectrum is allowed to enter. The camera also has a filter that limits recording of light other than the yellow to green range. With digital technology, color photographs can be obtained at specified times after dye injections. With this technique, baseline photographs are taken prior to fluorescein injection. A 6-second bolus injection of approximately 5 mL of sodium fluorescein is made into a vein in the upper extremity. Photos are taken 10 seconds later and approximately once every second for about 20 seconds, then less often. A delayed image is obtained at 5 and 10 minutes. Some physicians like to see a 15-minute image as well. Normal circulatory filling times are approximate:
0 seconds: Injection of fluorescein
9.5 seconds: Posterior ciliary arteries
10 seconds: Choroidal flush (or pre-arterial phase)
10 to 12 seconds: Retinal arterial stage
13 seconds: Capillary transition stage
14 to 15 seconds: Early venous stage (or lamellar stage, arterial-venous stage)
16 to 17 seconds: Venous stage
Fluorescein enters the ocular circulation from the internal carotid artery via the ophthalmic artery. The ophthalmic artery supplies the choroid via the short posterior ciliary arteries and the retina via the central retinal artery. However, the route to the choroid is typically less circuitous than the route to the retina. This accounts for the short delay between the “choroidal flush” and retinal filling. Pathologic changes are recognized by the detection of either hyperfluorescence or hypofluorescence. Among the common groups of ophthalmologic disease, fluorescein angiography can detect diabetic retinopathy, vein occlusions, retinal artery occlusions, edema of the optic disc, and tumors.
Fluorescein angiography is often done to follow the course of a disease such as diabetes—a disease that can cause the blood vessels of the retina to leak blood or fluid. Age-related macular degeneration is another disease that can cause the blood vessels of the retina to leak blood or fluid. Both of these abnormalities can be treated with a laser to help prevent loss of vision, and treatment results can be monitored using fluorescein angiography.
The test is performed and interpreted by an ophthalmologist, usually in the office setting. Results are available in less than 30 minutes.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Reinforce the need for the patient to remain still during the few seconds following fluorescein injection.
Reinforce the need for the patient to remain still during the few seconds following fluorescein injection.
• Obtain an ocular history of cataracts, prior retinal surgery, or other disease that may inhibit photography.
![]() Instruct the patient to remove any ocular lenses.
Instruct the patient to remove any ocular lenses.
![]() Inform the patient that there are no dietary restrictions.
Inform the patient that there are no dietary restrictions.
• Pupil dilatation can improve access to the posterior eye. If ordered, administer appropriate mydriatic medications. Note, however, that these medications are contraindicated for patients with glaucoma as they may dangerously increase ocular pressures.
During
Note the following procedural steps:
1. The patient is positioned in the fundus camera with the chin on the bar.
2. The patient is told to pick a spot in the far distance and concentrate on that spot during the examination.
3. Intravenous access is obtained.
4. Fluorescein dye is injected with the assistance of an autoinjector.
5. Photographs are taken by the ophthalmologist at timed intervals.
• This test is performed and interpreted by an ophthalmologist, usually in the office setting. Results are available in less than 30 minutes.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Genetic Testing (Breast Cancer [BRCA] and Ovarian Cancer, Colon Cancer, Cardiovascular Disease, Tay-Sachs Disease, Cystic Fibrosis, Melanoma, Hemochromatosis, Thyroid Cancer, Paternity [Parentage Analysis] and Forensic Genetic Testing)
Indications
Genetic testing is used to identify a predisposition to disease, establish the presence of a disease, establish or refute paternity, or to provide forensic evidence used in criminal investigations.
Test Explanation
As research progresses and the Human Genome Project provides more information, precise and accurate methods of identification of normal and mutated genes are becoming more common. The use of gene amplification methods has contributed to the explosion of genetic information in regard to disease propensity. These exquisite and sensitive laboratory methods are revolutionizing medicine and the courtrooms. Tests for defective genes known to be associated with certain diseases are now commonly used in screening populations of people who have certain phenotypes and family history compatible with a genetic mutation. Genetic testing is done in addition to a family history (pedigree). Whereas a family history is not always reliable, accurate, or available, genetic testing is very accurate in its determination of risks. Preventive medicine or surgery can be provided to eliminate disease development. Reproductive counseling and pregnancy prevention can preclude the conception of children who are likely to suffer the consequence of disease. Paternity and forensic genetic testing can accurately place responsibility, guilt, and innocence.
The ethics and disadvantages to this genetic testing are presently being discussed. Patients may face financial discrimination for health or life insurance or employment if the results are positive. The Health Insurance Portability and Accountability Act (HIPAA) protects patients from discrimination based on genetic information. This testing may be expensive and not covered by insurance. The information obtained by testing may cause great emotional turmoil in affected individuals or their family. The information obtained by medical genetic testing should be shared with the patient only. If the patient chooses to allow others to know the information, the patient must direct that release of information. Voluntary genetic testing should always be associated with aggressive counseling and support. Because of the potential changes in life for other family members, each person receiving the genetic information must be counseled separately.
Breast Cancer and Ovarian Cancer Genetic Testing
Inherited mutations in BRCA (BReast CAncer) genes indicate an increased susceptibility for development of breast cancer. The two genes in which mutations are most commonly seen are BRCA1 and BRCA2. The BRCA1 gene exists on chromosome 17. BRCA 2 is on chromosome 13. These genes encode tumor suppressor proteins. More than half of the women who inherit mutations will develop breast cancer by the age of 50 compared with less than 2% of women without the genetic defect. See Box 13-1 for screening recommendations for those with BRCA mutations.
The BRCA genes also confer an increased susceptibility for ovarian cancer. In the normal population, less than 2% of women develop ovarian cancer by age 70. Of women with mutations of the BRCA1 gene, 44% develop ovarian cancer by that age. Ovarian cancer is less commonly associated with the BRCA2 gene (20%). Furthermore, a woman who has already had breast cancer and who has a BRCA mutation has a 65% chance of developing a contralateral breast cancer in her lifetime (compared with less than 15% of women without the genetic defect). The woman with breast cancer and a BRCA genetic defect has a 10 times greater risk of developing ovarian cancer as a second primary cancer when compared with similar women without the mutated form of the gene. See Box 13-2 for ovarian cancer screening for those with BRCA mutations.
These mutations have an autosomal dominant inheritance pattern, indicating that women who inherit just one genetic defect can develop the phenotypic cancers. Men with BRCA genetic mutations (most commonly BRCA2) are at an increased risk for the development of breast, prostate, and colon cancer. In addition, they can pass the mutation to their daughters. Because BRCA is an autosomal dominant gene, 50% of the children are at risk. See Table 13-2 for determining who should be tested for BRCA mutations.
TABLE 13-2
Who Should Be Tested for BRCA Mutations?
| Patient With Breast Cancer | Family History (With at Least One Characteristic) |
| Diagnosed <40 years of age. | No other family history |
| Diagnosed around 50 years of age with two primary breast cancers | One relative around 50 years of age with breast cancer One relative with ovarian cancer |
| Diagnosed at any age | Two relatives with ovarian cancer Two relatives with breast cancer Male with breast cancer Personal history of ovarian cancer Ashkenazi Jewish heritage First- or second-degree relative with BRCA mutation |
| Male breast cancer at any age | One relative with breast cancer or ovarian cancer Ashkenazi Jewish heritage First- or second-degree relative with BRCA mutation |
The value of testing a select group of women who may be at high risk for BRCA genetic mutations includes:
1. Identification of those who are at high risk for developing breast or ovarian cancer
2. Consideration of interventions for those who test positive for BRCA mutations (e.g., prophylactic mastectomy and/or oophorectomy, or chemoprevention with tamoxifen)
3. Adoption of aggressive screening surveillance testing, which includes the following:
• Breast: Physical examination, mammography (see p. 1043) starting at age 25, and semiannual breast MRI imaging
• Ovary: Transvaginal ultrasound (see p. 887) starting at age 25
• Semiannual CA-125 (see p. 134) testing starting at age 25
4. Estimation of potential for passing the mutated BRCA gene to offspring
The method of testing includes obtaining a blood sample from a patient who has breast or ovarian cancer. Through reverse-transcriptase polymerase chain reaction (RT-PCR) amplification, the deoxyribonucleic acid (DNA) is sequenced and amplified for quantitation. If results are positive, blood samples of other family members are specifically tested for that particular genetic mutation only. Therefore, testing is expensive for the first person examined because the search is for any number of potential genetic mutations. However, for the other family members, it is much less expensive because the search has been narrowed to only a single genetic mutation.
Colon Cancer Genetic Testing
Two common forms of colon cancer are associated with a strong familial link. The first is familial adenomatous polyposis (FAP). These patients present with hundreds of polyps in their colon—one or two of which degenerate into cancer. The second type is hereditary nonpolyposis colorectal cancer (HNPCC). HNPCC is also known as the Lynch syndrome. These patients are more difficult to recognize because they do not have polyps; colon cancers develop de novo.
FAP is caused by a genetic mutation in the 5 q 21-22 (APC) gene on chromosome 5. Like BRCA genes, these genes are responsible for the synthesis of tumor-suppressor proteins. HNPCC is associated most often with mutations (defective DNA mismatch repair) of MLH 1, MLH 2, and MLH 6 genes. These genes are on chromosome 5 and are important for genome stability (prevention of chromosomal breakage and exchange). HNPCC is associated with several other cancers (Table 13-3), especially endometrial cancer.
These genetic defects are autosomal dominant, indicating that a person with just one defective gene can develop any of the phenotypic cancers. Furthermore, their children have a 50% chance of receiving the genetic mutation with its inherent cancer risks from the affected parent. Characteristics of FAP or HNPCC include:
1. Early-onset colorectal cancer (usually before the age of 50)
2. Polyps in large numbers (FAP only)
3. Cancer in the proximal colon
4. Cancers that tend to be more aggressive
A family member meeting the following criteria should consider genetic testing:
1. A family must have three (two first-degree) relatives with colorectal cancer
2. At least two generations of the family must be affected
3. Colorectal cancer must be found in at least one individual under the age of 50
The value of testing a family who may be at high risk for a genetic mutations includes:
1. Identification of those who are at high risk for developing colorectal or other cancers
2. Consideration of interventions for those who test positive for APC or MLH mutations (e.g., prophylactic proctocolectomy and/or hysterectomy, or chemoprevention with nonsteroidal antiinflammatory drugs [NSAIDs], which have been shown to reduce the incidence of colon polyps and cancers)
3. Adoption of aggressive screening surveillance testing, which includes:
• Colon: Annual colonoscopy (p. 591) starting at age of 25
• Uterus: Transvaginal ultrasound (see p. 887) and endometrial biopsy starting at age 25
4. Estimation of potential for passing the mutated APC or MLH gene to offspring
The laboratory methods of genetic testing are similar to those described for BRCA testing discussed previously.
Cardiovascular Disease Genetic Testing
Because half of all patients with cardiovascular disease (CVD) do not have the traditional risk factors (cholesterol, obesity, diabetes, and high blood pressure), these factors alone may fall short in the identification of patients at high risk for cardiac disease. Although a family history is helpful in identifying families at risk for CVD, genetic testing is more accurate and—if confirmed—more predictive among individuals in such a family. The angiotensinogen (AGT) gene demonstrates the strongest and most consistent associations with CVD. This gene is on chromosome 1. This is an autosomal recessive gene. When a patient has just one AGT mutation, the risk for CVD is moderately elevated. When an individual has two AGT genetic mutations, the risk for CVD is nearly triple that of the general population. These patients have early age onset of hypertension, myocardial infarction (MI), and hypertrophic cardiomyopathy. With genetic testing of individuals in families in which CVD is predominant, early therapeutic interventions (e.g., aggressive lipid-lowering agents and aggressive use of antihypertensives) may preclude disease.
Mutations in sarcomeric genes cause early-onset cardiac channelopathies and cardiomyopathies. These are rare but potentially lethal heart conditions that include long QT syndrome (LQTS), catecholaminergic polymorphic ventricular tachycardia (CPVT), hypertrophic cardiomyopathy (HCM), arrhythmogenic right ventricular cardiomyopathy, and dilated cardiomyopathy (DCM). Patients with a sarcomeric gene mutation are nearly three times more likely to suffer an adverse cardiac outcome (cardiovascular death, nonfatal ischemic stroke, or progression to severe heart failure). Identifying patients with these genetic mutations can help diagnose a patient's disease, guide treatment options, and determine whether family members are at risk.
Tay-Sachs Disease Genetic Testing
Tay-Sachs disease is characterized by the onset of severe mental and developmental retardation in the first few months of life. Affected children become totally debilitated by 2 to 5 years of age and die by age 5 to 8. Another form of the same disease is “late-onset Tay-Sachs” or chronic GM2, also known as gangliosidosis. The basic defect in affected children is a mutation in the hexosaminidase gene, which is on chromosome 15. This gene is responsible for the synthesis of hexosaminidase [HEX] (p. 290), an enzyme that normally breaks down a fatty substance called GM2 gangliosides. When this enzyme is not present in sufficient quantities, gangliosides build up in the nervous system and cause the debilitation characteristic of this disease. Ashkenazi (Eastern European) Jews and non-Jewish French Canadians, particularly those in the Cajun population in Louisiana, are affected most. This gene is an autosomal recessive gene. Carriers have one defective gene. Affected individuals have both genes defective. A “carrier couple” has a 25% chance of having a child affected with the disease.
At present, there is no treatment for the disease. It is important to identify carriers so that reproductive counseling can be provided. Hexosaminidase protein testing (p. 290) has been extremely effective for identification of carriers and affected individuals. However, sometimes the results of HEX protein tests are inconclusive or uncertain. Furthermore, genetic testing is used to diagnose “late-onset” Tay-Sachs. Both the test for the protein and that for the gene mutation are performed on a blood sample or on chorionic villus samples obtained during amniocentesis (p. 632). Genetic testing is performed using amino acid sequencing and comparison.
Cystic Fibrosis Genetic Testing
Cystic fibrosis (CF) is caused by a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. This gene encodes the synthesis of a protein that serves as a channel through which chloride enters and leaves cells. A mutation in this gene alters the cell's capability to regulate the chloride (and therefore sodium) transport. As a result, the lungs and digestive tract of CF patients fill with thick mucus. As bacteria invade their mucus-filled lungs, CF patients experience frequent lung infection. As mucus blocks the pancreas, inefficient digestion results.
There are thousands of potential mutations that are fatally deleterious to the CFTR gene. However, the most common mutation that accounts for 70% of the CF cases is known as the Delta AF508. Currently more than 30 genetic mutations can be recognized to cause CF, and these account for 90% of the cases.
The CFTR gene is an autosomal recessive gene located on chromosome 7. A carrier has one mutated gene. The person affected by CF has both defective genes. Genetic testing is now used to identify carriers of CF and identify neonates with the disease, and detecting fetal disease during pregnancy. The sweat chloride test (p. 678) is a more easily performed and cheaper way to diagnose the disease in affected children. Therefore the use of genetic testing for CF is often limited to those with a family history of CF, partners of patients with CF, and pregnant couples with a family history of CF. The main purpose of CF genetic testing is to identify carriers who could conceive a child with CF.
It is important to recognize that not all patients who have the CF genetic mutation will develop the disease. Further, because only a few mutations that may cause CF can be detected, a negative test does not necessarily eliminate the possibility of being affected by the diseases.
Genetic testing can be performed on blood samples or on samples taken during chorionic villus sampling (CVS) (p. 1088) or during amniocentesis (p. 632). Polymerase chain reaction (PCR) is used to amplify the locus for the CTFR gene. Amplification products are then hybridized to probes for the 36 most common CFTR-related mutations, using a line probe assay. Several laboratory methods are used to separate out the sequences for study.
Melanoma Genetic Testing
Recent progress in the genetics of cutaneous melanoma has led to the identification of two melanoma susceptibility genes: the tumor suppressor gene CDKN2A encoding the p16 protein on chromosome 9p21 and the CDK4 gene, on chromosome 12q13. The p16 genetic mutation is by far the most common form of hereditary melanoma. Characteristics of familial melanoma include frequent multiple primary melanomas, early age of onset of first melanoma, and frequently the presence of atypical or dysplastic nevi (moles). Family members with the following characteristics may consider testing for p16 genetic mutations:
• Multiple diagnoses of primary melanoma
• Two or more family members with melanoma
• Melanoma and pancreatic cancer
• Melanoma and a personal/family history of multiple atypical nevi
• Relatives of a patient with a confirmed p16 genetic mutation
Approximately 20% to 40% of families with three or more affected first-degree relatives show inheritance of mutations in the p16 gene. Fifteen percent of patients with multiple melanoma will have a p16 mutation. The average age at diagnosis is 35 years for those with a mutation in p16 versus 57 years in the general population. Carriers of the p16 gene mutation also have an increased risk for pancreatic cancer.
Once a p16 mutation is identified, education of all family members about the need for sun protection is essential. Commencing at the age of 10 years, family members should have a baseline skin examination with characterization of moles. It is recommended that an appropriately trained health care provider carry out skin examinations every 6 to 12 months. A monthly self-examination or examination by parent, partner, or family member should also be performed. Individuals should be taught about routine self-examination in the hope that this will prompt earlier diagnosis and removal of melanomas. The significance of change in shape and size of pigmented lesions should be understood, and the rules regarding asymmetry, border, color, and diameter (i.e., the ABCD rules) are often helpful in this regard.
Hemochromatosis Genetic Testing
The diagnosis of hemochromatosis is traditionally made by using serum iron studies. When hereditary hemochromatosis is suspected, mutation analysis of the hemochromatosis-associated HFE genes (C282Y and H63D) is done. Hereditary hemochromatosis (HH), an iron overload disorder considered to be the most common inherited disease in Caucasians, affects 1 in 500 individuals. Increased intestinal iron absorption and intracellular iron accumulation lead to progressive damage of the liver, heart, pancreas, joints, reproductive organs, and endocrine glands. Without therapy, males may develop symptoms between 40 and 60 years of age and women after menopause.
A large, but as yet undefined, fraction of homozygotes for this disease do not develop clinical symptoms (i.e., penetrance is low). Patients with symptoms and early biochemical signs of iron overload consistent with hereditary hemochromatosis should be tested. Relatives of individuals with hereditary hemochromatosis should also be studied. HFE genotyping could improve disease outcomes of the disease. Serum iron markers are monitored at more frequent intervals if an HFE mutation is detected and phlebotomy therapy is initiated earlier. Early initiation of phlebotomy therapy reduces the frequency or severity of hemochromatosis-related symptoms and organ damage.
Thyroid Cancer Genetic Testing
The RET proto-oncogene, located on chromosome subband 10 q11.2, encodes a receptor tyrosine kinase expressed in tissues and tumors derived from neural crest. Genetic testing for RET germline mutation has shown 100% sensitivity and specificity for identifying those at risk for developing inherited medullary thyroid cancer (multiple endocrine neoplasia [MEN] 2A, MEN 2B, or familial medullary thyroid carcinoma [FMTC]).
Use of the genetic assay allows earlier and more definitive identification and clinical management of those with a familial risk for medullary thyroid cancer. Medullary thyroid carcinoma is surgically curable if detected before it has spread to regional lymph nodes. However, lymph node involvement at diagnosis may be found in up to 75% of patients for whom a thyroid nodule is the first sign of disease. Thus there is an emphasis on early detection and intervention in families, which are affected by the familial cancer syndromes of MEN types 2A and 2B and FMTC, which account for one fourth of medullary thyroid cancer.
After genetic counseling, most family members who test positive undergo surgery to remove the thyroid gland. First-degree relatives of those with medullary thyroid carcinoma that appears to be sporadic in origin also undergo testing to verify that the patient's tumor is not caused by an inheritable form of this disease. RET testing is considered the standard of care in MEN 2 families because clinical decisions are made based on the results of such gene testing.
Paternity Genetic Testing (Parentage Analysis)
Deoxyribonucleic acid (DNA) testing is the most accurate form of testing to prove or exclude paternity when the identity of the biologic father of a child is in doubt. By comparing DNA characteristic of the mother and child, it is possible to determine characteristics that the child inherited from the biologic mother. Thus any remaining DNA must have come from the biologic father. If the DNA from the tested man is found to contain these paternal characteristics, then the probability of paternity can be determined. Testing is 99% accurate. However, in cases when the suspected fathers are close siblings, differentiation cannot be as certain.
Several particular regions (short tandem repeats [STRs]) of several chromosomes are copied by PCR. Frequency of repeated sequencing is then measured, usually by electrophoresis. The number of repeat sequences on the STR varies by individual. Testing is so reliable that it is admissible in court. Testing can be done on a mouth swab, blood, or CVS sample. Results are usually available in 1 to 3 weeks.
Many parents are given misinformation at the time of twin births regarding whether the twins are identical or fraternal. DNA samples from siblings can be analyzed in a manner described to indicate twinship. Again, these tests are 99% accurate.
Unfortunately, prenatal testing of the fetal components for paternity testing requires invasive testing such as chorionic villus sampling or amniocentesis. There are times, particularly in circumstances of rape, when early pregnancy paternity identification is desired. Noninvasive prenatal paternity testing can now be performed accurately by extracting and amplifying fetal chromosome alleles from maternal blood. This is a difficult process because “cell-free maternal DNA” quickly degrades fetal DNA. Now with the addition of cell stabilizers to maternal blood, cell-free maternal DNA is minimized and fetal DNA can be obtained. By using single nucleotide polymorphisms to distinguish fetal DNA from maternal DNA, an accurate prediction of paternity can be made.
Forensic Genetic Testing
Forensic DNA testing is used with increasing frequency in today's courtrooms because of its accuracy. In a courtroom, the reliability of the evidence can protect the individual and society as a whole. Further, DNA testing can be so conclusive that it often motivates plea bargaining and thereby reduces court time. It can quickly establish guilt or innocence beyond a reasonable doubt. Like paternity testing, forensic DNA testing is based on the fact that each individual is genetically different (except for twins). Through the use of PMR chemical probes, or through restriction length polymorphism methods, the DNA content of a person can be determined from nearly any body part. Furthermore, because DNA does not change or deteriorate even after death, testing can be performed on any body part, cadaver, or live person. Specimens considered adequate for DNA testing include blood, teeth, semen, saliva, bone, nails, skin scrapings, and hair. Forensic testing is also used for body identification. In time, central Federal Bureau of Investigation (FBI) data recording methods may allow for the collation of DNA data similar to the database of hundreds of millions of fingerprints on file.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Tell the patient that no fasting is required.
Tell the patient that no fasting is required.
![]() It is recommended that all patients who undergo testing should receive genetic counseling.
It is recommended that all patients who undergo testing should receive genetic counseling.
![]() Tell the patient the time it will take to have the results back.
Tell the patient the time it will take to have the results back.
![]() Inform the patient of the high costs of genetic testing and that it may not be covered by all medical insurance plans.
Inform the patient of the high costs of genetic testing and that it may not be covered by all medical insurance plans.
During
• Obtain the specimen in a manner provided by the specialized testing laboratory.
Blood is collected in a lavender-top tube. Cord blood can be used for infants.
Buccal swab: A cotton swab is placed between the lower cheek and gums. It is twisted and then placed on a special paper or in a special container. Usually two to four swabs are requested.
Amniotic fluid: At least 20 mL of fluid is preferred.
Chorionic villus sampling: 10 mg of cleaned villi are sent as prescribed by the testing laboratory.
Product of conception: 10 mg of placental tissue is preserved in a sterile medium.
Other body parts: As much tissue as is available is sent for testing.
After
• Document the procedure and the patient's response.
• Apply pressure or a pressure dressing to the venipuncture site.
![]() Be sure that the patient has an appointment scheduled for obtaining the results. It is very upsetting for a patient and family to wait for the results.
Be sure that the patient has an appointment scheduled for obtaining the results. It is very upsetting for a patient and family to wait for the results.
![]() Arrangements should be made to ensure genetic and emotional counseling after abnormal results are obtained.
Arrangements should be made to ensure genetic and emotional counseling after abnormal results are obtained.
Test Results and Clinical Significance
Genetic carrier state: These people carry one autosomal genetic recessive gene mutation. They themselves rarely have any abnormal phenotype (disease characteristics). However, if a child is conceived with a similar carrier, the child has a 25% chance of having the disease.
Affected state: These individuals have the phenotype demonstrating the genetic defect. This can occur if the person has either one autosomal dominant gene or two autosomal recessive genes. These people may not live long enough to have children of their own.
Related Tests
Sweat Electrolytes (p. 678). This is the definitive test to diagnose CF.
Hexosaminidase A (p. 290). This is the definitive test to diagnose Tay-Sachs disease.
Mammography (p. 1043). This is the most commonly used test to screen for breast cancer.
CA-125 Tumor Marker (p. 134). This is a commonly used test to screen high-risk patients for ovarian cancer.
Helicobacter pylori Testing (Campylobacter pylori, Anti–Helicobacter pylori Immunoglobulin G [IgG] Antibody, Campylobacter-Like Organism [CLO] Test, Rapid Urease Test, H. pylori Antigen Stool Test, Urea Breath Test [UBT, H. pylori breath test])
Indications
This test is used to detect Helicobacter pylori infections. It is indicated in patients who are suspected of having peptic ulcers (active or past history), gastric MALT lymphoma, melena, hematemesis, weight loss, persistent vomiting, dysphagia, or anemia.
Test Explanation
H. pylori, a bacterium is a gram-negative (p. 704) bacillus that infects the mucus overlying the gastric mucosa and the mucosa cells that line the stomach. It is a major risk factor for gastric and duodenal ulcers, chronic gastritis, or even ulcerative esophagitis. It is also a class I gastric carcinogen. Gastric colonization by this organism has been reported in about 90% to 95% of patients with a duodenal ulcer, 60% to 70% of patients with a gastric ulcer, and about 20% to 25% of patients with gastric cancer. Although some infected patients are asymptomatic, most individuals develop peptic symptoms within 2 weeks of exposure.
Approximately 10% of healthy persons younger than 30 years of age have H. pylori without disease or symptoms. Gastric “colonization” increases with age, with people older than age 60 years having rates at a percentage similar to their age. Testing should only be performed on symptomatic patients because a large percentage of Helicobacter pylori–colonized individuals would have positive results. All patients who test positive for H. pylori should be treated with aggressive antibiotics.
There are several methods of detecting the presence of this organism (Table 13-4). A single gold standard test does not exist. The organism can be cultured from a specimen of mucus obtained through a gastroscope (see p. 608). The specimen is plated on an enriched medium (such as chocolate or Skirrow's medium) and incubated for 5 to 7 days at 37° C. Although the delay in diagnosis is not preferred, culture can provide sensitivities for antibiotic therapy choices.
TABLE 13-4
Tests Commonly Used to Detect Helicobacter pylori Infection
| Test | Advantages |
| Invasive (Specimen Obtained by Endoscopy) Culture Urease Noninvasive Serology C13 urea breath |
Can determine antibody sensitivity Quick and simple Convenient and inexpensive Safer and less expensive than endoscopy |
The organism can also be detected on histology of a gastric mucosal biopsy (from the antrum and greater curvature of the corpus) using Gram, silver, Giemsa, or acridine orange stains or by immunofluorescence or immunoperoxidase methods. It may be several weeks before the results are available from cultures or extensive histology. It is preferable to start treatment before that time on a patient with symptomatic or active ulcer disease. For that reason, rapid urease testing for H. pylori is available. H. pylori is capable of breaking down high quantities of urea because of its capability to produce great amounts of an enzyme called urease, which can be found in the lining of the stomach of infected patients. In the rapid urease test, a small piece of gastric mucosa (obtained through gastroscopy) is placed onto a specialized testing gel/agar containing a pH indicator. If H. pylori organisms are present in the gastric mucosa, the urease (made by the H. pylori) will change the pH and the color of the test material. Results are available in 3 hours.
A breath test is also available for the detection of H. pylori. It is may be used as first-line testing in symptomatic patients. In the breath test, radioactive carbon urea (13C urea) is administered orally. The urea is absorbed through the gastric mucosa, where, if H. pylori is present, the 13C urea is converted to ammonia and 13CO2. The 13CO2 is then taken up by the capillaries in the stomach wall and delivered to the lungs. There the 13CO2 is exhaled and will be detected in the exhaled breath. The breath test is very reliable but is expensive and labor laden.
Although H. pylori does not survive in the stool, an enzyme-linked immunosorbent assay (EIA) using a polyclonal anti–H. pylori capture antibody can detect the presence of H. pylori antigen in a fresh stool specimen. Stool testing is very accurate. Stool tests are mostly used in monitoring the eradication of Helicobacter pylori after therapy.
Serologic testing is an inexpensive and noninvasive method of diagnosis of H. pylori infection. It is also used as a supportive diagnostic in which no preparation or abstinence from antacids is required. It is the least sensitive of the H. pylori tests. The IgG anti–H. pylori antibody is most commonly used. It becomes elevated 2 months after infection and stays elevated for more than a year after treatment. The IgA anti–H. pylori antibody, like IgG, becomes elevated 2 months after infection but decreases 3 to 4 weeks after treatment. The IgM anti–H. pylori antibody is the first to become elevated (about 3 to 4 weeks after infection) and is not detected 2 to 3 months after treatment. These antibody titers are fast becoming the gold standard for H. pylori detection. These antibodies can be detected with use of a small amount of blood obtained by fingerstick. Serologic testing is often used several months after treatment to document eradication of H. pylori infection. Serologic testing is also used to corroborate the findings of other H. pylori testing methods. Because serology may lack specificity, nonserologic tests described in the preceding paragraphs can be used to confirm Helicobacter pylori infection.
Interfering Factors
• H. pylori can be transmitted by contaminated endoscopic equipment during endoscopic procedures.
• Sensitivity can be reduced in patients who are actively bleeding from ulcers.
![]() Rapid urease tests can be falsely negative if the patient uses antacid therapy within the week before testing.
Rapid urease tests can be falsely negative if the patient uses antacid therapy within the week before testing.
![]() Bismuth (Pepto Bismol) or sucralfate (Carafate) will suppress mucosal uptake of the urea and interfere with test results.
Bismuth (Pepto Bismol) or sucralfate (Carafate) will suppress mucosal uptake of the urea and interfere with test results.
![]() The concomitant use of a proton pump inhibitor, such as Prilosec, Nexium, Prevacid, or Protonix, will also inhibit urea absorption and diminish the sensitivity of all testing methods.
The concomitant use of a proton pump inhibitor, such as Prilosec, Nexium, Prevacid, or Protonix, will also inhibit urea absorption and diminish the sensitivity of all testing methods.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Tell the patient that no fasting is required for the blood test.
Tell the patient that no fasting is required for the blood test.
• If a biopsy or culture will be obtained by endoscopy, see discussion of esophagogastroduodenoscopy (EGD) on p. 608.
• If culture is to be performed, be sure the patient has not had any antibiotic, antacid, or bismuth treatment for 5 to 14 days before the endoscopy.
During
• Collect a venous blood sample according to the protocol of the laboratory performing the test.
• A gastric or duodenal biopsy or specimen of mucus can be obtained by endoscopy. Keep the specimen moist by the addition of 2 to 5 mL of sterile saline solution or other wetting agent as required by the laboratory. Place in a sterile container. Minimize transport time for cultures.
• Follow the following steps for the Breath Test:
1. Verify that female patients are not pregnant.
2. Give a dose of radioactive 14C or nonradioactive 13C urea by mouth. Follow the guidelines of the laboratory.
3. Follow all the testing precautions for handling radioactive pharmaceuticals.
4. Several minutes after the patient has swallowed the carbon dose, provide the patient with 2 oz of water.
5. Breath samples are collected in any one of a number of gas collection devices depending on how and when the sample will be analyzed.
After
• Apply pressure or a pressure dressing to the venipuncture site.
• Assess the venipuncture site for bleeding.
• If endoscopy was used to obtain a culture, see procedure for esophagogastroduodenoscopy on p. 608. The specimen should be transported to the laboratory within 30 minutes after collection.
Related Tests
Gastrin (p. 248). This test is a measure of serum gastrin. This hormone stimulates gastric acid secretion. Oversecretion can cause recurrent peptic ulcers. The initial symptoms may be similar to those of chronic H. pylori infection.
Esophagogastroduodenoscopy (p. 608). This endoscopic procedure is used to directly biopsy the gastric mucosa for definitive H. pylori identification.
Indications
Laboratory genetics is used to identify a broad range of diseases and predisposition to diseases. Its use is extensive and growing daily in the field of laboratory medicine.
Test Explanation
Genetic laboratory testing has become a vital part of identifying diseases of inborn errors in metabolism, such as phenylketonuria (PKU). These genetic laboratory tests have also proved to be helpful in the identification, classification, and prognostication of many oncologic diseases, such as leukemias. The heredity of diseases can be more accurately traced with the use of laboratory genetics.
There are many different laboratory methods used in genetic testing and each is particularly helpful for study of a particular disease. It is not the intent of this manual to explain the details of commonly used genetic laboratory methods. However, it is important to be aware of the availability and ability of genetic laboratory testing in clinical medicine.
Molecular genetics is used to detect mutation carriers, diagnose genetic disorders, test at-risk fetuses, and identify patients at high risk of developing adult-onset conditions (such as Huntington disease or familial cancers). In addition, full-gene analysis is available for diseases such as cystic fibrosis, beta globin, and hereditary hemorrhagic telangiectasia. Once a mutation is identified in a family, a family-specific mutation micro array testing can be performed.
Biochemical genetics is frequently used to diagnose one of many metabolic disorders that affect the body's ability to produce or break down amino acids, organic acids, and fatty acids. Early identification of such a metabolic disorder may prevent serious health problems, as well as death. Biochemical genetic testing can be used as a supplemental newborn screening for inborn errors of metabolism (e.g., PKU, creatine, tyrosine disorders). Biochemical genetics is also helpful in the evaluation of malabsorption syndromes. For some of these disorders, more precise DNA testing for causative mutations is also available. Biochemical testing can differentiate heterozygous carriers from non-carriers of genes by metabolite and enzymatic analysis of physiologic fluids and tissues.
Cytogenetics is used to identify chromosome disorders that cause spontaneous abortions, congenital malformations, mental retardation, or infertility. It is used to evaluate women with gonadal dysgenesis and couples with repeated spontaneous miscarriages. Additionally, the field of cytogenetics is very important in the diagnosis and classification of leukemias, lymphomas, myeloma, and myeloproliferative diseases. This laboratory method also helps with decisions about treatment and monitoring disease status and recovery.
Fluorescence in situ hybridization (FISH) testing uses genomic microarray probes to identify well-characterized hereditary genetic microdeletion, microduplication, or rearrangement inherited disorders (such as DiGeorge syndrome). It is also helpful in the evaluation of oncology specimens (see Breast Cancer Tumor Analysis, p. 717). Many disease-specific FISH panels target subtelomeric and pericentromeric sites and locations of known microdeletion syndromes. FISH testing can assist in the diagnosis and monitoring of patients with cancer (such as breast, leukemia, and lymphomas). It can help determine the specific type of cancer present, predict disease course, and determine a course of treatment.
Microarray genetic testing can identify diseases associated with oligonucleotide and SNP-based genetic diseases. Single nucleotide polymorphisms (SNP, snips, or snippets) are variations in the genetic code at a specific point on the DNA. Like cytogenetic techniques, microarray analysis identifies unbalanced chromosomal abnormalities (loss and/or gain of DNA) in patients with unexplained abnormal phenotypes. Examples include persons with mental retardation, developmental delay, dysmorphic features, congenital anomalies, and autism. In addition, the SNP-based array will also identify long contiguous stretches of homozygosity, which may suggest an increased likelihood for a recessive condition or uniparental disomy.
Microarray FISH testing is also used to determine the presence of a genetic deletion/duplication in a family with a known inheritable disease. FISH testing is used to determine ploidy status of newborns or of cancers. FISH techniques are often used in the evaluation of amniotic fluid, products of conception, and chorionic villi.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient
Explain the procedure to the patient
• When testing for inheritable diseases, obtain the services of a licensed genetic counselor to inform the patient and family of the testing methods and potential results. The counselor will also provide the patient and family with potential actions that may need to be taken if the results are positive.
Test Results and Clinical Significance
Indications
The indications for MRI change constantly as new uses for this technique are discovered. Its most important indications include evaluation of the central nervous system (CNS), neck and back, bones and joints, heart, and the breasts.
Test Explanation
MRI is a noninvasive diagnostic scanning technique that provides valuable information about the body's anatomy by placing the patient in a magnetic field. MRI is based on how hydrogen atoms behave when they are placed in a magnetic field and then disturbed by radiofrequency signals. The unique feature about MRI is that it does not require exposure to ionizing radiation. MRI has several advantages over computed tomography (CT) scanning, including the following:
• MRI provides better contrast between normal tissue and pathologic tissue.
• Obscuring bone artifacts that occur in CT scanning do not occur in MRI scanning.
• Because rapidly flowing blood appears dark, which results from its quick motion, many blood vessels appear as dark lumens. This provides a natural contrast between the blood vessels and other tissues when using MRI.
• Because spatial information depends only on how the magnetic fields are varied in space, it is possible to image the transverse, sagittal, and coronal planes directly with MRI.
MRI is useful in the evaluation of the following areas:
• Head and surrounding structures (see Figure 13-2)
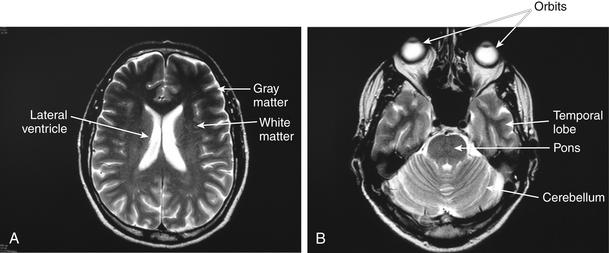
Figure 13-2 A and B, Normal MRI of the upper and lower brain levels.
Note that gray matter is portrayed light and white matter is portrayed dark.
• Spinal cord and surrounding structures (see Figure 13-3)

Figure 13-3 MRI of the spine demonstrating a herniated disk between lumbar vertebrae 4 and sacral vertebrae 1 compressing the spinal cord.
• Face and surrounding structures
An important advantage of MRI is that serial studies can be performed on the patient without any health risk. This is useful in assessing the response of cancer to radiotherapy and chemotherapy. A major disadvantage of MRI is that patient eligibility is reduced in comparison to CT scanning. For example, examination of patients requiring cardiac monitoring or having metal implants, metal joint replacements, pins for open reduction of fractures, pacemakers, or cerebral aneurysm clips will result in image degradation and may endanger the patient.
An MRI of the brain (Figure 13-2) and meninges is particularly accurate in identifying benign and malignant neoplasms. It is able to identify and quantify brain edema, ventricular compression, hydrocephalus, and brain herniation. Intracranial hemorrhage can also be seen on MRI. Magnetic resonance spectroscopy (MRS) is a noninvasive procedure that generates high-resolution clinical images based on the distribution of chemicals in the body. This is particularly useful in the brain, where certain chemical metabolites will enhance the image of a high-grade malignancy. MR spectroscopy has also been used to assess chemical abnormalities in the brain associated with HIV infection without having to perform a brain biopsy. This procedure has been used in a wide variety of disorders, including stroke, head injury, coma, Alzheimer disease, and multiple sclerosis.
MRI has revolutionized the practice of orthopedic surgery. It is particularly helpful in the determination of anatomic changes in muscle and joints (particularly knee and shoulder).
Magnetic resonance angiography (MRA) is a noninvasive procedure for viewing possible blockages in arteries. MRA has been useful in evaluation of the extracranial carotid artery and large-caliber intracranial arterial and venous structures. Cardiac abnormalities, aortic aneurysm, and anatomic variants can be identified. This procedure also has proved useful in the noninvasive detection of intracranial aneurysms and vascular malformations, and especially in renal artery stenosis. Coronary angiography with the resolution of most magnets is sufficient for the detection of stenosis in the large coronary arteries or venous bypass grafts but is inadequate for the detection of stenosis in smaller branches of the coronary tree.
MRI of the breast has expanded significantly over the past few years. With examiner experience, this procedure is more sensitive and specific than mammography or ultrasonography of the breast. Furthermore, lesions that previously were difficult to visualize (e.g., those close to the chest wall) are easily seen with this technique. MRI is fast becoming a reliable technique for breast imaging. MRI of the breast is used for accurate localized staging of breast cancer by demonstrating an excellent three-dimensional image of a cancer and high sensitivity for other smaller synchronously occurring breast cancers that are missed on mammography. MRI of the breast is helpful for preoperative surgical staging and the identification of postoperative positive margins. MRI of the breast can demonstrate response of a primary breast cancer to chemohormonal therapy. This study is particularly helpful in differentiating postoperative scar tissue from breast cancer recurrence. MRI of the breast is the most accurate method of determining fracture of a breast implant. Most breast protocols use a dynamic contrast enhancement pattern on fat suppressed images. Most protocols use gadolinium contrast agents. Cancers tend to enhance more rapidly than benign lesions. The washout of the contrast agent is slower than benign tumors. Interpretive radiologists use both the anatomic changes of breast tumors and gadolinium enhancement washout curves to differentiate benign from malignant tumors.
With the addition of a needle-guiding system to the MRI, breast tumors can be non-operatively and accurately localized and also biopsied. MRI of the breast is expensive and labor intensive. For that reason, it is not an effective screening tool, except for women who are at extremely high risk for the development of breast cancer.
Significant improvement in MRI of the heart and great vessels has moved this noninvasive diagnostic procedure into the mainstream of clinical cardiology. Cardiac MRI already is considered the procedure of choice in the evaluation of pericardial disease and intracardiac and pericardiac masses; for imaging the right ventricle and pulmonary vessels; and for assessing many forms of congenital heart disease, especially after corrective surgery. There is increasing support for the use of MRI in the assessment of ischemic heart disease. The ventricle size, shape, and blood volumes can be evaluated. Cardiac valvular abnormalities, cardiac septal defects, and suspected intracardiac or pericardiac masses or thrombi can be identified. Pericardial disease (e.g., pericarditis or effusion) is easily identified. Ventricular muscle changes from ischemia or infarction can be determined. Finally advanced MRI techniques are able to evaluate the coronary vessels directly.
Phase-contrast magnetic resonance imaging (PC-MRI) of the heart quantifies velocity and blood flow in the great arteries. Measurements of blood flow in the aorta and pulmonary trunk produce a wealth of information, including cardiac outputs of the left and right ventricles, regurgitant volumes and fraction of the aortic and pulmonary valves, and shunt ratio. Regurgitant fraction is a particularly important parameter that determines the need for valvular repair or replacement. Shunt ratio is an important parameter for evaluating the need for closing shunt lesions caused by atrial septal defects and ventricular septal defects. Velocity of moving blood is related to the pressure gradients. This relationship is used to estimate pressure gradient across stenotic cardiovascular lesions.
A combined diagnostic session of cine MRI for morphology and function, first past perfusion MRI, and late enhancement MRI to assess the heart viability is feasible in less than an hour and answers most of the relevant questions clinicians have regarding heart function and coronary patency. Stress cardiac MRI can be performed using nitrates, dobutamine, and adenosine. When beta blockers are added to EKG gating, cardiac volumes and images can be better portrayed.
Magnetic resonance cholangiopancreatography (MRCP) allows noninvasive imaging of the biliary tree, gallbladder, pancreas, and pancreatic duct. It is used to:
• Identify pancreatobiliary tumors, stones, inflammation or infection.
• Evaluate patients with pancreatitis to detect the underlying cause.
• Help in the diagnosis of unexplained abdominal pain.
• Provide a less invasive alternative to endoscopic retrograde cholangiopancreatography (ERCP).
Unlike ERCP, MRCP is not a therapeutic procedure in which papillotomy or sphincterotomy can be performed in the event that these ducts are obstructed. Indications for the use of MRCP include unsuccessful or contraindicated ERCP; patient preference for noninvasive imaging; patients considered to be at low risk of having pancreatic or biliary disease; patients in which the need for therapeutic ERCP is considered unlikely; and those with a suspected neoplastic cause for pancreatic or biliary obstruction. Complication rates are much lower for MRCP than ERCP.
MRI of the liver has improved significantly with the use of gadolinium-like contrast agent called gadoxetate (Eovist). Imaging with this agent provides extremely sharp imaging where liver and biliary tumors smaller than a centimeter can be identified. Contrast between tissues can be created by the development of the magnetic fields. However, there are multiple gadolinium-based contrast agents available to enhance MRI imaging/contrast.
Magnetic resonance enterography (MRE) is used to identify inflammatory bowel disease. It is also helpful in determining extra luminal bowel pathology. MRI is an effective tool in liver imaging and in the staging of known prostate cancers.
One of the most common uses is MRI of the cervical or lumbar spine (Figure 13-3). The main purpose of this test is to determine the cause of neck or back pain, respectively. The MRI is the most accurate test to identify herniated disk disease. Using different MRI protocols, an MRI myelogram can be performed where the spinal fluid appears white and the solid tissue (disks/nerves) appears dark. Herniated disks are easily seen and graded as to their compression on the nerves. Furthermore, MRI of the spine is able to identify subtle changes associated with early infiltrating diseases such as metastatic cancer. An upright MRI can scan patients in any position. The upright MRI can scan patients in their positions of symptoms (such as pain or numbness) including weight-bearing positions, such as sitting, standing, or bending. The upright MRI can provide diagnostic images of the cervical spine, lumbar spine, and the joints over their full range of motion (such as cervical flexion/extension). The front-open and top-open design of the upright MRI nearly eliminates possible claustrophobia and accommodates larger patients.
Potential Complications
• Gadolinium-based contrast agents (gadopentetate dimeglumine [Magnevist], gadobenate dimeglumine [MultiHance], gadodiamide [Omniscan], gadoversetamide [OptiMARK], gadoteridol [ProHance]) have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). A creatinine, BUN, and/or estimated GFR (p. 193) may be obtained, especially in adults over the age of 60.
Contraindications
• Patients who are extremely obese, usually more than 300 lb
• Patients who are confused or agitated
• Patients who are claustrophobic, if an enclosed scanner is used. This can be overcome with the administration of anti-anxiety medication.
• Patients who are unstable and require continuous life support equipment, because most monitoring equipment cannot be used inside the scanner room. Magnet-adaptive equipment is becoming available for use in the MRI scanner room.
• Patients with implantable metal objects (e.g., pacemakers, cardioverter defibrillators, extensive cardiac stents, infusion pumps, aneurysm clips, inner ear implants, metal fragments in one or both eyes), because the magnet may move the object in the body and injure the patient. Piercings, braces, and retainers need to be removed.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Inform the patient that there is no exposure to radiation.
Inform the patient that there is no exposure to radiation.
• Obtain informed consent if required by the institution.
![]() Tell the patient that he or she can drive without assistance after the procedure unless anti-anxiety medications are administered to treat claustrophobia.
Tell the patient that he or she can drive without assistance after the procedure unless anti-anxiety medications are administered to treat claustrophobia.
![]() Tell parents of young patients that they may read or talk to a child in the scanning room during the procedure. There is no risk of radiation from the procedure.
Tell parents of young patients that they may read or talk to a child in the scanning room during the procedure. There is no risk of radiation from the procedure.
• Assess the patient for any contraindications for testing (e.g., aneurysm clips).
![]() If available, show the patient a picture of the scanning machine (Figure 13-4) and encourage verbalization of anxieties. Some patients may experience claustrophobia. Anti-anxiety medications may be helpful for those with mild claustrophobia. If possible, an open MRI system can be used for these patients.
If available, show the patient a picture of the scanning machine (Figure 13-4) and encourage verbalization of anxieties. Some patients may experience claustrophobia. Anti-anxiety medications may be helpful for those with mild claustrophobia. If possible, an open MRI system can be used for these patients.

Figure 13-4 Siemens MRI. Note spacious short tube combining high-quality imaging without concerns for claustrophibia.
![]() Tell patients that a microphone within the MRI tube allows them to communicate with personnel performing the study (Figure 13-5).
Tell patients that a microphone within the MRI tube allows them to communicate with personnel performing the study (Figure 13-5).
![]() Instruct the patient to remove all metal objects (e.g., dental bridges, jewelry, hair clips, belts), because they will create artifacts on the scan. The magnetic field can damage watches and credit cards. Also, movement of metal objects within the magnetic field can be detrimental to patients or staff within the field.
Instruct the patient to remove all metal objects (e.g., dental bridges, jewelry, hair clips, belts), because they will create artifacts on the scan. The magnetic field can damage watches and credit cards. Also, movement of metal objects within the magnetic field can be detrimental to patients or staff within the field.
![]() Tell the patient wearing a nicotine patch (or any other patch with a metallic foil backing) to remove it. These patches can become intensely hot during the MRI and can cause burns.
Tell the patient wearing a nicotine patch (or any other patch with a metallic foil backing) to remove it. These patches can become intensely hot during the MRI and can cause burns.
![]() Inform the patient that he or she will be required to remain motionless during this study. Any movement can cause artifacts on the scan.
Inform the patient that he or she will be required to remain motionless during this study. Any movement can cause artifacts on the scan.
![]() Tell the patient that during the procedure he or she may hear a thumping sound. Earplugs are available if the patient wishes to use them.
Tell the patient that during the procedure he or she may hear a thumping sound. Earplugs are available if the patient wishes to use them.
![]() Inform the patient that fluid or food restrictions may be required before abdominal MRI.
Inform the patient that fluid or food restrictions may be required before abdominal MRI.
![]() For comfort, instruct the patient to empty the bladder before the test.
For comfort, instruct the patient to empty the bladder before the test.
During
• Note the following procedural steps:
1. The patient lies on a platform that slides into a tube containing the cylinder-shaped tubular magnet.
2. For cardiac MRI, EKG leads are applied (p. 544).
3. The patient is instructed to lie very still during the procedure. The patient may be asked to stop breathing for short periods of time.
4. During the scan, the patient can talk to and hear the staff via microphone or earphones placed in the scanner.
5. A contrast medium called gadolinium is a paramagnetic enhancement agent that crosses the blood-brain barrier. It is especially useful for distinguishing hypermetabolic abnormalities like tumors. If this is to be administered, approximately 10 to 15 mL is injected in a vein. Imaging can begin shortly after the injection. No dietary restrictions are necessary before using this agent.
• Note that a qualified radiologic technologist performs this procedure in approximately 30 minutes to several hours.
![]() Tell the patient that the only discomfort associated with this procedure may be lying still on a hard surface and a possible tingling sensation in teeth containing metal fillings. Also, an injection may be needed for administration of the contrast medium.
Tell the patient that the only discomfort associated with this procedure may be lying still on a hard surface and a possible tingling sensation in teeth containing metal fillings. Also, an injection may be needed for administration of the contrast medium.
Test Results and Clinical Significance
Brain
Cerebral tumor: Natural contrast can be accentuated by varying the MRI coil. Brain tumors can be specifically diagnosed. On T1-weighted images, tumors are radiolucent (dark), whereas on T2-weighted images they are radiopaque (white). MRI is particularly useful in evaluating the pituitary gland. With gadolinium, primary brain tumors light up quickly.
Aneurysm: This condition is evident as compression of normal brain tissue by an enlarged vascular abnormality that is made more apparent with gadolinium. Bleeding or edema may be present with aneurysmal leak.
Arteriovenous (AV) malformation: MRA is useful in this problem. Large AV malformations can be seen with regular MRI as large radiolucent masses in the brain tissue.
MRI can demonstrate intracranial hemorrhage, abscess, or atrophy.
Degenerating diseases (e.g., multiple sclerosis, hypoxic encephalopathy, encephalomyelitis): Specific characteristics of these diseases can be detected with MRI.
Hydrocephalus: This condition is evident as tremendous enlargement of the ventricular system of the brain.
Heart
Coronary occlusive disease: With the addition of gadolinium, stenosis in large coronary vessels can be detected. With the use of “chemical stress” testing, stenosis in smaller coronary vessels can be identified.
Valvular heart disease: With the use of PC-MRI, cardiac valve function can be assessed.
Intracardiac and pericardiac masses: Tumors and clots can easily be seen.
Ventricular dilatation and hypertrophy: From these images, ventricular volumes can be calculated.
Breast
Cancer: For high-risk patients, MRI of the breast is useful for asymptomatic women. MRI of the breast is helpful in identifying second synchronously occurring breast cancers. Images obtained from the MRI can help the surgeon plan local excision of the cancer. The extent of the ductal carcinoma in situ (DCIS) can be well demonstrated with MRI.
Implant disruption: MRI is the most accurate test to indicate disruption of a foreign breast implant.
Benign tumors: With a fairly high degree of specificity, MRI may be able to separate benign from malignant tumors. However, biopsy is always required.
Other
Herniated lumbar and cervical disks: MRI is very sensitive for detection of these abnormalities. It is the diagnostic test of choice. MRI not only can determine disk herniation, but also demonstrate consequential nerve compression.
Tumor (primary or metastatic): MRI is especially useful for detection of liver, lung, and soft-tissue lesions.
Joint disorders: MRI is especially useful for evaluation of knee and shoulder injuries.
Destructive lesion of bone: With multiple-weighted images, tumors, osteomyelitis, and other destructive diseases of bone, and especially the spine, can be well demonstrated.
Vascular disease: Occlusive disease can be identified in the vessels of brain, chest, abdomen, and extremities.
Related Test
Computed Tomography (CT) (p. 1020). MRI and CT are not mutually exclusive. However, there are several instances in which CT scan is preferable to MRI. In most institutions, CT scanning is more readily available, especially for the patient requiring emergency imaging.
Indications
Oximetry is used to monitor arterial O2 saturation levels (SaO2) in patients at risk for hypoxemia. This includes patients who are undergoing surgery, cardiac stress testing, mechanical ventilation, heavy sedation, or lung function testing, or who have multiple trauma. It is also used as an indicator of partial pressure of oxygen (Po2) in patients who may experience hypoventilation, sleep apnea, or dyspnea. This test is commonly used to titrate O2 levels in hospitalized patients.
Test Explanation
Oximetry is a noninvasive method of monitoring Sao2 (i.e., ratio of oxygenated hemoglobin to total hemoglobin). Sao2 is expressed as a percentage; for example, Sao2 of 95% indicates that 95% of the total hemoglobin attachments for O2 have O2 attached to them. Sao2 is an accurate approximation of O2 saturation obtained from arterial blood gas study (see p. 109). By correlating Sao2 with the patient's physiologic status, a close estimate of Po2 can be obtained.
Oximetry is typically used to monitor oxygenation status during the perioperative period and in patients receiving heavy sedation or mechanical ventilation. This test is also used in clinical situations such as pulmonary rehabilitation programs, stress testing, and sleep laboratories. Oximetry can be used to monitor response to drug therapy (e.g., theophylline). Pulse oximetry is constantly monitored during the perioperative period, and the results are one of the discharge criteria used in the postanesthesia unit.
Fetal oxygen saturation monitoring (FSpo2) is very useful in the monitoring of fetal well-being during delivery. When the fetal heart rate becomes significantly abnormal (nonreassuring), C-section is often performed because of concern for fetal well-being. However, with FSpO2, an accurate measure of fetal O2 saturation can be determined and, if normal, C-section can be avoided. The technology is based on the same principle as adult pulse oximetry except that the machine is far more sensitive to accurately read saturations of less than 70%. After membranes are ruptured, and if the baby is in vertex position with good cervical dilatation, a specialized probe can be placed on the temple or cheek of the fetus for FSpO2 monitoring. Expertise is required for appropriate placement of the sensor. The O2 saturation is displayed on a monitor screen as a percentage. The normal O2 saturation for a baby in the womb, receiving oxygenated blood from the placenta, is usually between 30% and 70%. When FSpO2 is less than 30% for several minutes, there is marked and progressive deterioration in fetal well-being as hypoxia and acidemia progress.
O2 levels can also be measured in various body tissues. For example, monitors that continuously measure tissue O2 partial pressures are attached to a small catheter placed in the brain, heart, or peripheral muscle. Brain tissue oxygen testing and monitoring is the most common use of this technology. Used to monitor the condition of the brain following severe head trauma, it is a measure of cerebral blood flow and pulmonary oxygenation. It is more accurate than intracranial pressure in indicating brain injury.
Interfering Factors
• Extreme vasoconstriction diminishes blood flow to the peripheral vessels, which decreases the accuracy of oximetry.
• Extreme alteration in temperature may diminish the accuracy of oximetry.
• Oximetry cannot differentiate carboxyhemoglobin saturation from O2 saturation. Therefore, in cases of suspected smoke or carbon monoxide (CO) inhalation, oximetry should not be used to monitor oxygenation. The levels will be falsely elevated.
• Digital motion can alter accurate readings.
• Severe anemia affects the accuracy of comparison of oximetry and Po2 levels.
• Fingernail polish and fake nails will interrupt digital readings. The earlobe can be used as an alternative.
Procedure and Patient Care
During
• Rub the patient's fingertip or, if the ear will be used, earlobe or pinna (upper portion of the ear) to increase blood flow.
• Clip the monitoring probe or sensor to the finger or ear. A beam of light passes through the tissue, and the sensor measures the amount of light the tissue absorbs (Figure 13-6).

Figure 13-6 Oximetry. A, The pulse oximeter passes a beam of light from a light-emitting diode through a vascular bed to a photodetector. The amount of light absorbed by the oxygen-saturated hemoglobin is measured by the sensor to determine the oxygen saturation level. B, Pulse oximeter displays oxygen saturation and pulse rate.
• This study is usually performed by a nurse's aide or nurse at the patient's bedside in a few seconds.
![]() Tell the patient that no discomfort is associated with this study.
Tell the patient that no discomfort is associated with this study.
Test Results and Clinical Significance
 Decreased Levels
Decreased Levels
Inadequate O2 in inspired air (suffocation):
Atelectasis, mucus plug, bronchospasm, pneumothorax, pulmonary edema, acute respiratory distress syndrome, restrictive lung disease: Non-aerated portions of the lung are still perfused with unoxygenated blood. This blood returns to the heart with little or no oxygen. The O2 content is diluted, and oximetry values diminish.
Atrial or ventricular cardiac septal defects: Unoxygenated blood gains access to oxygenated blood by direct shunting. By dilution, the O2 content of the mixed blood returning to the heart is lowered, as is that of arterial blood.
Severe hypoventilation states (e.g., oversedation, neurologic somnolence): Without air exchange, Po2 levels decrease.
Pulmonary emboli: When ventilation is reduced, oximetry values diminish.
Related Tests
O2 Saturation (p. 109). This is an indication of the percentage of hemoglobin saturated with O2. This is part of arterial blood gas measurements.
Po2 (p. 109). This is a measure of the tension (pressure [P]) of oxygen dissolved in the plasma. This pressure determines the force of O2 to diffuse across the alveolocapillary membrane. This is part of arterial blood gas measurements.
O2 Content (p. 109). This is a calculated number that represents the amount of O2 in the blood.
Indications
The primary reasons for performing pulmonary function studies include the following:
1. Preoperative evaluation of the lungs and pulmonary reserve. When planned thoracic surgery will result in loss of functional pulmonary tissue, as in lobectomy (removal of part of a lung) or pneumonectomy (removal of an entire lung), a significant risk of pulmonary failure exists if the preoperative pulmonary function is already severely compromised by other diseases, such as chronic obstructive pulmonary disease (COPD).
2. Evaluation of response to bronchodilator therapy. In some patients with a spastic component to COPD, long-term use of bronchodilators may be useful. Pulmonary function studies performed before and after the use of bronchodilators will identify this group of patients.
3. Differentiation between restrictive and obstructive forms of chronic pulmonary disease. Restrictive defects (e.g., pulmonary fibrosis, tumors, chest wall trauma) occur when ventilation is disturbed by limitation of chest expansion. Inspiration is primarily affected. Obstructive defects (e.g., emphysema, bronchitis, asthma) occur when ventilation is disturbed by increased airway resistance. Expiration is primarily affected.
4. Determination of the diffusing capacity of the lungs (DL). Rates are based on the difference in concentration of gases in inspired and expired air.
5. Performance of inhalation tests in patients with inhalation allergies.
Test Explanation
Pulmonary function tests are performed to detect abnormalities in respiratory function and to determine the extent of pulmonary abnormality. Pulmonary function tests routinely include spirometry, measurement of airflow rates, and calculation of lung volumes and capacities. Gas diffusion and inhalation tests (bronchial provocation) are also performed when requested, but not routinely. Exercise pulmonary stress testing can also be performed to provide data concerning pulmonary reserve. During this staged test, the patient performs an aerobic function such as stationary biking or walking on a treadmill.
Spirometry is performed first. A spirometer is a machine that can measure air volumes. When a time element is added to the tracing, airflow rates can be determined. Based on age, height, weight, race, and sex, normal values for volumes and flow rates can be predicted. Values greater than 80% of predicted values are considered normal. Spirometry provides information about obstruction or restriction of airflow. Spirometry supports the diagnosis of COPD and chronic restrictive pulmonary disease (CRPD).
Measurement of airflow rates provides information about airway obstruction. This portion of the study adds a time element to spirometry. When flow is plotted on the Y axis and volume is plotted on the X axis, flow/volume curves (isoflow loops) can be drawn when the patient is asked to maximally inhale, then forcefully exhale while being timed. The shape of the curve can be interpreted to identify and quantify airway obstruction. If airflow rates are significantly diminished (<60% of normal) or if requested by the physician, the test can be repeated after bronchodilators are administered by nebulizer. If the airflow rates improve by 20%, use of bronchodilators may be recommended for the patient. Emphysema or restrictive lung disease usually does not improve with bronchodilator therapy. Patients with an asthmatic component to COPD will benefit from bronchodilators.
Measurement of lung capacity (combination of two or more measurements of lung volume) can be performed using nitrogen or helium washout techniques. This provides further information about air trapping within the lung.
Gas exchange studies measure the diffusing capacity of the lung (DL), that is, the amount of gas exchanged across the alveolar-capillary membrane per minute. Most laboratories use carbon monoxide (CO) to measure DL, because CO has a great affinity for hemoglobin and only a small concentration is needed. Because of this affinity of hemoglobin for CO, the only limiting factor to the transfer of the gas is its rate of diffusion across the alveolar-capillary membrane (which is what is measured). Gas exchange is abnormal in congestive heart failure, pneumonia, and other diseases that fill the alveoli with fluid or exudate. Any disease that causes deposition of material in the interstitium of the lung (e.g., acute respiratory distress syndrome [ARDS], collagen-vascular disease, Goodpasture syndrome, pulmonary fibrosis) will decrease gas exchange.
Pulmonary function tests routinely include the following:
Forced vital capacity (FVC): Amount of air that can be forcefully expelled from a maximally inflated lung position. Less than expected values occur in obstructive and restrictive pulmonary diseases.
Forced expiratory volume in 1 second (FEV1): Volume of air expelled during the first second of FVC. In obstructive pulmonary disease, airways are narrowed and resistance to flow is high. Therefore not so much air can be expelled in 1 second, and FEV1 is less than the predicted value. In restrictive lung disease, FEV1 is decreased because the amount of air originally inhaled is low, not because of airway resistance. Therefore the FEV1/FVC ratio should be measured. In restrictive lung disease a normal value is 80%, and in obstructive lung disease this ratio is considerably less. The FEV1 value will reliably improve with bronchodilator therapy if a spastic component to obstructive pulmonary disease exists.
Maximal midexpiratory flow (MMEF) or forced midexpiratory flow: Maximal rate of airflow through the pulmonary tree during forced expiration. This test is independent of the patient's effort or cooperation. MMEF volumes are lower than expected in obstructive pulmonary diseases and normal in restrictive pulmonary diseases.
Maximal volume ventilation (MVV) (formerly, maximal breathing capacity): Maximal volume of air that a patient can breathe in and out during 1 minute. It is less than the expected value in both restrictive and obstructive pulmonary disease.
A comprehensive pulmonary function study also may include evaluation of the following lung volumes and lung capacities (Figure 13-7):
Tidal volume (TV or VT): Volume of air inspired and expired with each normal respiration.
Inspiratory reserve volume (IRV): Maximal volume of air that can be inspired from end of normal inspiration. It represents forced inspiration over and beyond VT.
Expiratory reserve volume (ERV): Maximal volume of air that can be exhaled after normal expiration.
Residual volume (RV): Volume of air remaining in the lungs following forced expiration.
Inspiratory capacity (IC): Maximal amount of air that can be inspired after normal expiration.
Functional residual capacity (FRC): Amount of air left in the lungs after normal expiration.
Vital capacity (VC): Maximal amount of air that can be expired after maximal inspiration.
Total lung capacity (TLC): Volume to which the lungs can be expanded with greatest inspiratory effort.
Minute volume (MV), or minute ventilation: Volume of air inhaled and exhaled per minute.
Dead space: Part of VT that does not participate in alveolar gas exchange. Includes air within the trachea.
Forced expiratory flow (FEF): Portion of airflow curve most affected by airway obstruction.
FEF200-1200: Rate of expired air between 200 mL and 1200 mL during FVC.
FEF25-75: Rate of expired air between 25% and 75% of flow during FVC.
Peak inspiratory flow rate (PIFR): Flow rate of inspired air during maximum inspiration. It indicates large (trachea and bronchi) airway disease.
Peak expiratory flow rate (PEFR): Maximum airflow rate during forced expiration.
Spirometry is the standard method for measuring most relative lung volumes; however, it is incapable of providing information about absolute volumes of air in the lung. Thus a different approach is required to measure residual volume, functional residual capacity, and total lung capacity. Two of the most common methods of obtaining information about these volumes are body plethysmography and gas dilution tests.
In body plethysmography, the patient sits inside an airtight box, inhales or exhales to a particular volume (usually functional residual capacity, FRC), and then a shutter drops across the breathing tube. The subject makes respiratory efforts against the closed shutter. Changes in total lung volumes can be easily measured instead of calculated. From those values, assuming pressures in the box are stable, airway resistance and lung compliance can be measured. Body plethysmography is particularly appropriate for patients who have airspaces within the lung that do not communicate with the bronchial tree.
Gas dilution or gas exchange studies measure the diffusing capacity of the lung (DL) (i.e., the amount of gas exchanged across the alveolar-capillary membrane per minute). Gases like helium have densities lower than air. These gases are not affected by turbulent airflow. As a result, the use of helium provides an extremely accurate method of measuring even the most minimal airway resistance existing in small airways. This is used to test volume of isoflow (VisoV) that is helpful in identifying early obstructive changes.
Procedure and Patient Care
Before
![]() Explain the test to the patient.
Explain the test to the patient.
![]() Inform the patient that cooperation is necessary for accurate results.
Inform the patient that cooperation is necessary for accurate results.
![]() Instruct the patient not to use any bronchodilators (if requested by health care provider) or to smoke for 6 hours before this test.
Instruct the patient not to use any bronchodilators (if requested by health care provider) or to smoke for 6 hours before this test.
• The use of small-dose meter inhalers and aerosol therapy may be withheld before this study. Verify with the health care provider.
• Measure and record the patient's height and weight before this study to determine predicted values.
• List on the laboratory slip any medications the patient is taking.
During
Spirometry and Airflow Rates
1. The unsedated patient is taken to the pulmonary function laboratory.
2. The patient breathes through a sterile mouthpiece into a spirometer, which measures and records the values.
3. The patient is asked to inhale as deeply as possible and then forcibly exhale as much air as possible. This is repeated several (usually two to three) times. The two best values are used for calculations. This test may be repeated with bronchodilators if values are deficient.
4. The machine computes FVC, FEV1, FEV1/FVC, PIFR, PEFR, and MMEF.
5. The patient is asked to breathe in and out as deeply and frequently as possible for 15 seconds. The total volume breathed is recorded and multiplied by 4 to obtain MVV.
6. The patient is asked to breathe in and out normally into the spirometer and then exhale forcibly from the end-tidal volume expiration point, to measure ERV.
7. The patient is asked to breathe in and out normally into the spirometer and then inhale forcibly from the end-tidal volume expiration point, to measure IC.
8. The patient is asked to breathe in and out maximally (but not forced), to measure VC and calculated TLC.
Inhalation Tests (Bronchial Provocation Studies)
1. These tests may be performed during pulmonary function studies to establish a cause-and-effect relationship in some patients with inhalant allergies.
2. The Provocholine challenge test is typically used to detect the presence of hyperactive airway disease. This test is not indicated in patients with asthma.
3. Care is taken during this challenge test to reverse any severe bronchospasm with prompt administration of an inhalant bronchodilator (e.g., isoproterenol).
Test Results and Clinical Significance
Interstitial lung diseases are highlighted by perialveolar inflammation followed by fibrosis. Asbestosis, ARDS, radiation fibrosis, collagen-vascular diseases, Goodpasture disease, amyloidosis, sarcoidosis, and end-stage hypersensitivity pneumonitis are some of the more common etiologic factors. Usually the FEV1/FVC ratio is normal. Lung volumes and capacities are reduced. Hypoxemia is common. Diffusing capacity is markedly reduced.
Tumor: Cancers of the peripheral small bronchi may not cause any changes in pulmonary function studies. Tumors of the trachea (rare) and large bronchi (common) cause a reduction in PIFR.
Chest wall trauma: Fractured ribs or recent surgery inhibits a patient's ability to fully cooperate with pulmonary requirements. As a result, most lung volumes and capacities will be reduced.
Patients with COPD can be expected to have reduced airflow rates (FEV1, FEF25-75, FEF200-1200) and abnormal airflow curves (loops). RV and ERV are increased. VC is reduced. In patients with asthma, this is reversible to a large degree with the use of bronchodilators.
Inhalant pneumonitis (e.g., farmer's lung, miner's lung): These patients have reduced lung volumes, impaired diffusing capacity, and exercise-induced hypoxemia. There is little airflow rate abnormality.
Post-pneumonectomy: As expected, lung volumes and capacities are reduced. With no preexisting obstructive disease, no changes in airflow rates would be expected.
Bronchiectasis: These patients, with chronic and recurrent bronchiole infection pockets, have reduced airflow rates (FEV1, FEF25-75, FEF200-1200) and abnormal airflow curves (loops), which may be reversible. They also may have some reaction to methacholine challenge.
Airway infection: Patients with acute bronchitis may experience transient airflow obstruction, as determined by reduced airflow rates (FEV1, FEF25-75, FEF200-1200) and abnormal airflow curves (loops), which return to normal when the infection has resolved.
Pneumonia: These patients may have reduced lung volumes and capacities. Without other concurrent lung disease, there is no airflow obstruction. Diffusing capacity is impaired.
Neuromuscular disease: Patients with impaired muscle strength because of neuromuscular diseases (e.g., multiple sclerosis, myasthenia gravis) have reduced lung volumes and capacities.
Hypersensitivity bronchospasm: These patients have reversible airway obstruction (FEV1, FEF25-75, FEF200-1200) and abnormal airflow curves (loops) when induced by methacholine challenge. Airflow rates are reduced. Lung volumes may also be affected.
Related Tests
Arterial Blood Gases (p. 109). Measurements of arterial blood O2 and CO2 pressure and content are useful in determining pulmonary function and calculating lung volumes and capacities.
Chest X-Ray (p. 1014). Pulmonary function can, to a small degree, be assessed with x-ray films of the chest. Hyperexpanded lungs are evidence of chronic obstructive airway disease. Pulmonary fibrosis, pneumonia, and bronchiectasis may also be evident.
Sleep Studies (Polysomnography [PSG], Multiple Sleep Latency Tests [MSLT], Multiple Wake Test [MWT])
Indications
Sleep studies are indicated in any person who snores excessively; experiences narcolepsy, excessive daytime sleeping, or insomnia; or has motor spasms while sleeping; and in patients with documented cardiac rhythm disturbances limited to sleep time.
Test Explanation
There are many types of sleep disorders. Most, however, are associated with impaired nighttime sleep and excessive daytime drowsiness. Sleep disorders can be caused by alterations in sleep times (e.g., night-shift workers), medications (stimulants), or psychiatric problems (e.g., depression, mania). In general, sleep disorders can be categorized as follows:
• Dyssomnia: Includes insomnia, sleep apnea, narcolepsy, and restless leg syndrome
• Parasomnia: Includes sleep walking, sleep talking, sleep terrors, and rapid eye movement disorders
Sleep studies can identify the cause of the sleep disorders and indicate appropriate treatment. Sleep studies include polysomnography (PSG) and testing for wakefulness and sleepiness. A full PSG would include:
• Electroencephalography: Limited to two or more channels (see p. 549)
• Electrooculography: Documents eye movements (see Electronystagmography, p. 557)
• Electromyography: Demonstrates muscle movement, usually of the chin and legs (see p. 554)
• Electrocardiography: EKG (see p. 544)
• Chest impedance: Monitors chest wall movement and respirations
• Airflow monitors: Measures amount of airflow in and out of the mouth and nose
• CO2 monitor: Measures expiratory CO2 levels
• Pulse oximetry: Monitors tissue oxygen levels (see p. 1114)
• Sound sensors: Used to document snoring sounds
• Audio/video recordings: Used to document restless motions and fitfulness
• Esophageal pH probe: Used only if gastroesophageal reflux is considered to be a cause of paroxysmal nocturnal dyspnea and coughing (see p. 691)
On occasions when sleep apnea alone is suspected, a four-channel PSG is performed. This more simplified test includes the electrocardiogram (EKG), chest impedance, airflow monitor, and O2 oximetry. Video and/or audio recordings are performed also. Often a sleep-screening study is performed to see if full sleep studies are indicated. This is done by using pulse oximetry during sleep. If no hypoxia occurs, significant sleep apnea would be rare and full studies are not indicated.
Sleep apnea can be obstructive or central. Obstructive apnea is by far the most common and is caused by muscle relaxation of the posterior pharyngeal muscles. Breathing stops for 10 to 40 seconds. Central sleep apnea is highlighted by simple cessation of breathing rather than obstructed airway. Primary cardiac events that lead to significant and transient reduction in cardiac output can also cause apnea. Apnea from either cause is associated with increase in heart rate, decreased O2 levels, change in brain waves, and increased expiratory CO2. Obstructive apnea is also associated with progressively diminished airflow.
Narcolepsy is a frequent and irresistible need for sleep during daytime hours. Sleepiness can occur even during conversation or driving. Sleep studies can diagnose narcolepsy.
The restless leg syndrome is associated with an acute sensation of discomfort during periods of inactivity. It is difficult for affected patients to fall asleep and to stay asleep. Video monitoring identifies periodic limb movement—jerking of the legs associated with electroencephalogram (EEG) evidence of sleep interruption.
Parasomnias include sleepwalking and sleep-talking. Sleep terrors, associated with sudden awakening with screaming or fighting to escape a terrifying dream that the patient cannot recall, is another example of this sleep disorder.
Another sleep disorder is called rapid eye movement (REM) disorder. Normally during REM sleep, one experiences varying degrees of muscle paralysis. However, patients with REM disorders do not. They may act out their dreams in a way that varies from calling out to violent behavior. These patients can vividly recall their dreams.
Insomnia is an inability to sleep. Although it is the most common form of sleep disorder, it is usually acute and short-lived. However, when it is persistent, a sleep study is indicated. Often the pretest questionnaire can pinpoint stress or restless leg syndrome.
During a sleep study, electrodes for the EKG, EEG, electrooculography, and electromyography are applied. The chest impedance belt monitors are also placed. Under audiovisual monitoring the patient is placed in a comfortable room and sleeps. During sleep, information is synchronously gathered. The various stages of sleep architecture are determined by the EEG, and the physiologic changes during each stage are documented. By the use of the EEG, five stages of sleep can be identified (Table 13-5). The sleep study will be repeated after the patient has been using CPAP or a dental fixture for therapy. On therapy, no sleep apnea should be noted. If the sleep apnea is significant on the first night of study, a “split study” can be performed where the sleep is interrupted after 4 hours and a CPAP machine is provided for the next 4 hours. During that time, appropriate CPAP settings are calibrated to reduce apneic episodes and, at the same time, minimize uncomfortable side effects.
Testing for obstructive sleep apnea is performed in a specially constructed sleep laboratory. This is a well-insulated room in which external sounds are blocked and room temperature is easily controlled. It is performed by a certified sleep technologist and interpreted by a physician trained in sleep disorders. The study is usually completed in one night, although occasionally two nights are required. A second day is often required to administer the multiple sleep latency test (MSLT) or the multiple wake test (MWT). The MSLT is a measure of the patient's ability to sleep during a series of structured naps. The MWT is a measure of the patient's ability to not fall asleep during a period of what should be wakefulness. These tests are used to diagnose narcolepsy that follows a night of inadequate sleep. These tests can also be used to determine the success of therapy for sleep disorders.
These tests can also be used to determine the success of therapy for sleep disorders. The sleep study can be repeated after the patient has started using CPAP or a dental fixture for therapy. While on therapy, no sleep apnea should be noted. Because of the expense and the psycho-emotional difficulties associated with testing in a sleep laboratory, there has been significant growth in unattended home sleep studies. The patient is attached to a multichannel monitor by a sleep technician as previously described. The technician does not remain in attendance. The monitoring device records key data so that a sleep disorder can be identified.
Actigraphy can be used to determine sleep patterns and circadian rhythms. A sleep actigraph is a simple device that is worn like a wrist watch. It can be used during normal activities (except swimming or bathing) for several days and nights. It does not require an overnight stay at a sleep center. Doctors can use actigraphy to help diagnose sleep disorders, including circadian rhythm disorders, such as jet lag and shift work disorders. This test can also detect how well sleep treatments are working. Actigraphy can be used with PSG or alone. In some cases, it can replace the need for PSG.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Tell the patient that caffeine products should be avoided for several days before testing as they may delay onset of sleep.
Tell the patient that caffeine products should be avoided for several days before testing as they may delay onset of sleep.
• Sedatives are prohibited as they will alter usual sleep patterns.
![]() Reassure the patient that monitoring equipment will not interrupt the sleeping pattern.
Reassure the patient that monitoring equipment will not interrupt the sleeping pattern.
• Allow the patient to express concerns about videotaping and other forms of monitoring.
• Several sleep rating questionnaires are completed by both the patient and his or her sleeping partner.
During
• Electrodes for EKG, EEG, electrooculography, and electromyography are applied. Excessive hair may need to be shaved in male patients.
• Airflow, oximetry, and impedance monitors are applied.
• Once the patient is comfortable, he or she is allowed to sleep.
• The lights are turned off and monitoring begins before the patient is asleep.
Test Results and Clinical Significance
Obstructive sleep apnea: Patients experience apneic episodes for 10 seconds or more. They experience synchronous periods of O2 desaturation and experience sleep disturbances on EEG, increase in cardiac rate, and decreased airflow.
Central sleep apnea: Patients do not have the stimulus to breathe during the apneic episode. Otherwise, the findings are nearly the same as for obstructive sleep apnea. Snoring and chest impedance extremes are absent. Cardiac arrhythmia may be observed.
Insomnia: These patients demonstrate a delay in falling asleep. They may also show evidence of restless leg syndrome.
Narcolepsy: These patients will demonstrate EEG changes compatible with sleep rather than napping. The time in which they fall asleep is less than 5 minutes on repeated napping.
Restless leg syndrome: These patients will experience excessive extremity motion before and after sleep.
Parasomnia: These patients may demonstrate sleepwalking or phonating.
REM disorder: These patients may sleep restlessly and move about as if fighting or escaping terror.
Indications
Tuberculin testing is performed for persons who are:
1. Suspected of having active TB (e.g., patients with suspicious chest x-ray findings, productive cough with negative routine cultures, hemoptysis, or undetermined weight loss)
2. At increased risk for progression to active TB
3. At increased risk for latent TB infection (LTBI) (e.g., health care workers, recent transplant organ recipients, HIV patients, recent immigrants, IV drug abusers, or those in close contact with someone known to have TB)
4. At low risk for LTBI, but are tested for other reasons (e.g., entrance to college)
Test Explanation
Purified protein derivative (PPD) of the tubercle bacillus is injected intradermally. If the patient is infected with or has been exposed to TB (whether active or dormant), lymphocytes will recognize the PPD antigen and cause a local inflammatory reaction (Boxes 13-3 and 13-4). Although this test is used to detect TB infection, results do not indicate whether the infection is active or dormant. If test results are negative but the physician strongly suspects TB, testing with “second-strength” PPD can be performed. If these test results are negative, the patient has not been exposed to TB (see p. 768 for tuberculosis culture). Results of PPD skin testing usually become positive 6 weeks after infection. Once positive, the reaction usually persists for life. Box 13-5 lists patients in whom test results may revert to negative or fail to become positive.
The PPD test also can be used as part of a series of skin tests performed to assess the immune system. If the immune system is nonfunctioning because of poor nutrition or chronic illness (e.g., neoplasia, infection, AIDS), PPD test results will be negative despite active or dormant TB infection. Other pathogens used in skin tests to test immune function include Candida, mumps virus, and Trichophyton, organisms most people in the United States have been exposed to. It has been well established that any surgery is associated with greater mortality in patients with negative skin tests than in patients who react to these common pathogen skin tests. Box 13-6 lists skin tests for other diseases.
There is now an alternative to skin testing. For example, the QuantiFERON-TB Gold Test (QFT) is a blood test used as an aid in diagnosing Mycobacterium tuberculosis infection (see Tuberculosis Testing, p. 770).
Laboratory testing for TB is usually performed as part of routine prenatal evaluation in pregnant women. Often this may be the mother's first contact with the health care system in several years.
PPD testing is associated with no complications, except in patients known to have active TB or who have been vaccinated against TB. In these patients, local reaction may be so severe as to cause complete skin slough, requiring surgical care. PPD testing will not cause active TB because the test solution contains no live organisms.
Contraindications
Interfering Factors
Procedure and Patient Care
During
• Prepare the volar (inner) forearm with alcohol, and allow it to dry.
• Intradermally inject PPD (Figure 13-8). A skin wheal (nearly 1 cm) should develop.
• Circle the area with indelible ink. Do not cover with a Band-Aid.
After
• Have a health care professional read the results in 48 to 72 hours.
• Examine the test site for induration (hardening), and encircle the area of induration. Measure the area of induration (not redness) in millimeters.
• If the test results are positive, ensure that the physician is notified and the patient is given appropriate treatment.
• If the test results are positive, check the patient's arm 4 to 5 days after the test to be certain that a severe skin reaction has not occurred.
Test Results and Clinical Significance
Negative Results
Possible immunoincompetence in chronically ill patients: Immunocompromised patients and patients who have not been exposed to TB will not react to PPD. In other patients, positive results can revert to negative (see Box 13-5). Immunocompromised patients will not respond to other common pathogens.
Related Tests
Acid-Fast Bacilli Smear (p. 706). This smear (usually sputum) is used to support the diagnosis of TB. By itself it cannot confirm a diagnosis of TB. This smear test is also used to monitor treatment for TB.
Chest X-Ray (p. 1014). Because TB is most usually infective to the lungs, as a result of inhalation of airborne infectious material, the chest x-ray film often demonstrates the results (Ghon complex) of acute granulomatous infection.
Tuberculosis Culture (p. 768). This is the only way to confirm a diagnosis of TB. When TB is grown from culture of a specimen, the diagnosis of TB can be made and treatment based on drug sensitivities can be started.
Tuberculosis Testing (p. 770). This is a blood test that can identify active and latent TB infection.
Indications
This test is used to detect Helicobacter pylori (H. pylori) infections. It is indicated in patients who have recurrent or chronic gastric or duodenal ulceration or inflammation. When the H. pylori infection is successfully treated, the ulcer or inflammation will usually heal.
Test Explanation
H. pylori is a bacterium that can be found in the mucus overlying the gastric mucosa and in the mucosa (cells that line the stomach). It is a risk factor for gastric and duodenal ulcers, chronic gastritis, or even ulcerative esophagitis. This gram-negative bacillus is also a class I gastric carcinogen. Gastric colonization by this organism has been reported in about 90% to 95% of patients with a duodenal ulcer; in 60% to 70% of patients with a gastric ulcer; and in about 20% to 25% of patients with gastric cancer. There are several serologic and microscopic methods of detecting H. pylori (see Helicobacter pylori Testing, p. 1101).
The UBT is the noninvasive test of choice for diagnosis of H. pylori infection. It is based on the capability of H. pylori to metabolize urea to CO2 because of the organism's capability to produce a large amount of urease. In the breath test, carbon (13C) labeled urea is administered orally. The urea is then absorbed through the gastric mucosa. If H. pylori is present, the urea will be converted to 13CO2. The 13CO2 is then taken up by the capillaries in the stomach wall and delivered to the lungs where it is exhaled. The labeled carbon can be measured by gas chromatography or a mass spectrometer.
This test has been simplified to the point that two breath samples collected before and 30 minutes after the ingestion of urea in a liquid form suffice to provide reliable diagnostic information. Labeling urea with 13C is becoming increasingly popular because it is a nonradioactive isotope of 14C and is innocuous. It can be safely used in children and women of childbearing age.
Interfering Factors
• Dietary constituents with a natural abundance of 13C, such as maize, cane, and corn flour, can cause increased levels.
![]() Bismuth (Pepto Bismol) or sucralfate (Carafate) will suppress mucosal uptake of the urea and interfere with test results.
Bismuth (Pepto Bismol) or sucralfate (Carafate) will suppress mucosal uptake of the urea and interfere with test results.
![]() The concomitant use of a proton pump inhibitor, such as Prilosec, Nexium, Prevacid, or Protonix, will also inhibit urea absorption.
The concomitant use of a proton pump inhibitor, such as Prilosec, Nexium, Prevacid, or Protonix, will also inhibit urea absorption.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Instruct the patient to abstain from oral intake for 6 hours before testing.
Instruct the patient to abstain from oral intake for 6 hours before testing.
• If radioactive carbon (rare) is being used, be sure that female patients are not pregnant.
![]() When providing the isotopic urea to the patient, instruct the patient as to proper administration (per local laboratory routine).
When providing the isotopic urea to the patient, instruct the patient as to proper administration (per local laboratory routine).