CHAPTER 128
ST-Segment Elevation Myocardial Infarction (STEMI)
Early recognition and immediate treatment of acute MI are essential; diagnosis is based on characteristic history, ECG, and serum cardiac markers.
Symptoms
Chest pain similar to angina (Chap. 37) but more intense and persistent; not fully relieved by rest or nitroglycerin, often accompanied by nausea, sweating, apprehension. However, ~25% of MIs are clinically silent.
Physical Examination
Pallor, diaphoresis, tachycardia, S4, dyskinetic cardiac impulse may be present. If CHF exists, rales and S3 are present. Jugular venous distention is common in right ventricular infarction.
ECG
ST elevation, followed (if acute reperfusion is not achieved) by T-wave inversion, then Q-wave development over several hours (see Figs. 120-3 and 120-4).
Non-ST Elevation MI, or NSTEMI
ST depression followed by persistent ST-T-wave changes without Q-wave development. Comparison with old ECG helpful (see Chap. 129).
Cardiac Biomarkers
Cardiac-specific troponins T and I are highly specific for myocardial injury and are the preferred biochemical markers for diagnosis of acute MI. They remain elevated for 7–10 days. Creatine phosphokinase (CK) level rises within 4–8 h, peaks at 24 h, and returns to normal by 48–72 h. CK-MB isoenzyme is more specific for MI but may also be elevated with myocarditis or after electrical cardioversion. Total CK (but not CK-MB) rises (two- to threefold) after IM injection, vigorous exercise, or other skeletal muscle trauma. A ratio of CK-MB mass:CK activity ≥2.5 suggests acute MI. CK-MB peaks earlier (about 8 h) following acute reperfusion therapy (see below). Serum cardiac markers should be measured at presentation, 6–9 h later, and then at 12–24 h.
Noninvasive Imaging Techniques
Useful when diagnosis of MI is not clear. Echocardiography detects infarct-associated regional wall motion abnormalities (but cannot distinguish acute MI from a previous myocardial scar). Echo is also useful in detecting RV infarction, LV aneurysm, and LV thrombus. Myocardial perfusion imaging (thallium 201 or technetium 99m-sestamibi) is sensitive for regions of decreased perfusion, but is not specific for acute MI. MRI with delayed gadolinium enhancement accurately indicates regions of infarction, but is technically difficult to obtain in acutely ill pts.
INITIAL THERAPY Initial goals are to (1) quickly identify if pt is candidate for reperfusion therapy, (2) relieve pain, and (3) prevent/treat arrhythmias and mechanical complications.
• Aspirin should be administered immediately (162–325 mg chewed at presentation, then 162–325 mg PO qd), unless pt is aspirin-intolerant.
• Perform targeted history, exam, and ECG to identify STEMI (>1 mm ST elevation in two contiguous limb leads, ≥2 mm ST elevation in two contiguous precordial leads, or new LBBB) and appropriateness of reperfusion therapy [percutaneous coronary intervention (PCI) or IV fibrinolytic agent], which reduces infarct size, LV dysfunction, and mortality.
• Primary PCI is generally more effective than fibrinolysis and is preferred at experienced centers capable of performing the procedure rapidly (Fig. 128-1), especially when diagnosis is in doubt, cardiogenic shock is present, bleeding risk is increased, or symptoms have been present for >3 h.
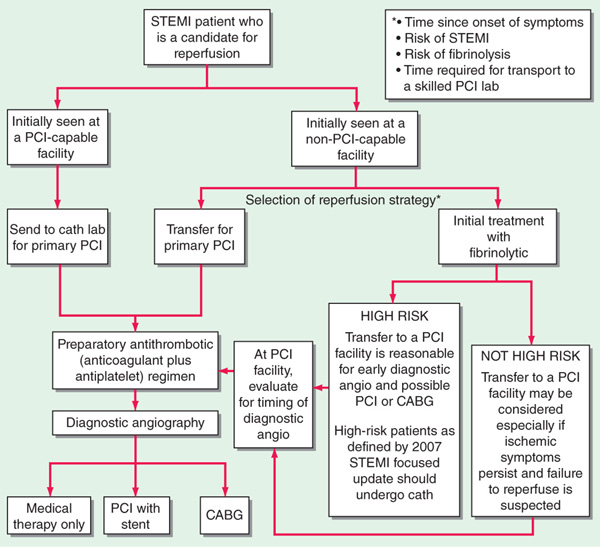
FIGURE 128-1 Reperfusion strategies in STEMI. [Adapted from Kushner FG et al: 2009 focused update of the ACC/AHA Guidelines for the Management of Patients with ST-Elevation Myocardial Infarction (updating the 2004 guideline and 2007 focused update): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 120:2271, 2009.]
• Proceed with IV fibrinolysis if PCI is not available or if logistics would delay PCI >1 h longer than fibrinolysis could be initiated (Fig. 128-1). Door-to-needle time should be <30 min for maximum benefit. Ensure absence of contraindications (Fig. 128-2) before administering fibrinolytic agent. Those treated within 1–3 h benefit most; can still be useful up to 12 h if chest pain is persistent or ST remains elevated in leads that have not developed new Q waves. Complications include bleeding, reperfusion arrhythmias, and, in case of streptokinase (SK), allergic reactions. Enoxaparin or heparin [60 U/kg (maximum 4000 U), then 12 (U/kg)/h (maximum 1000 U/h)] should be initiated with fibrinolytic agents (Fig. 128-2); maintain activated partial thromboplastin time (aPTT) at 1.5–2.0 × control (~50–70 s).
• If chest pain or ST elevation persists >90 min after fibrinolysis, consider referral for rescue PCI. Coronary angiography after fibrinolysis should also be considered for pts with recurrent angina or high-risk features (Fig. 128-2) including extensive ST elevation, signs of heart failure (rales, S3, jugular venous distension, LVEF ≤35%), or systolic BP <100 mmHg.
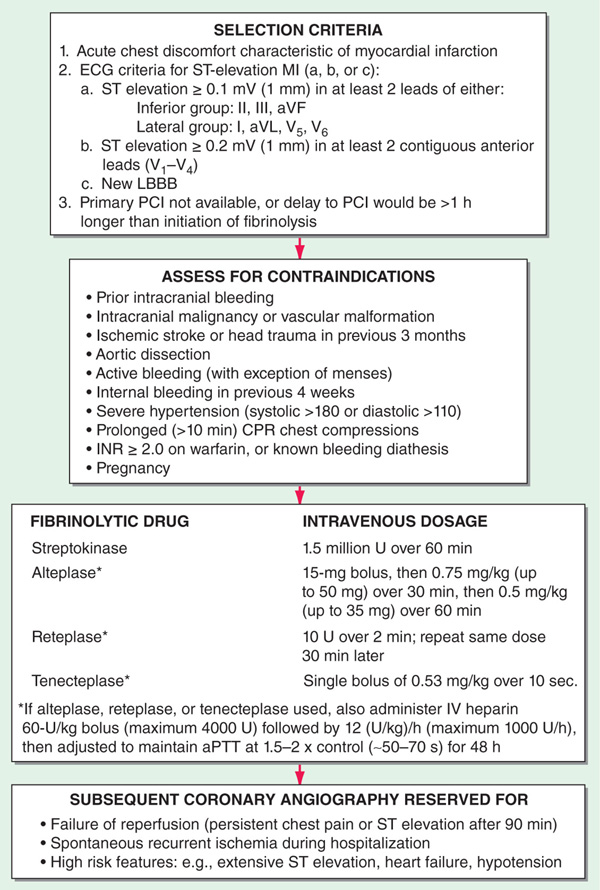
FIGURE 128-2 Algorithm for fibrinolytic therapy of acute STEMI.
The initial management of NSTEMI (non-Q MI) is different (Chap. 129). In particular, fibrinolytic therapy should not be administered.
ADDITIONAL STANDARD TREATMENT (Whether or not reperfusion therapy is undertaken):
• Hospitalize in CCU with continuous ECG monitoring.
• IV line for emergency arrhythmia treatment.
• Pain control: (1) Morphine sulfate 2–4 mg IV q5–10 min until pain is relieved or side effects develop [nausea, vomiting, respiratory depression (treat with naloxone 0.4–1.2 mg IV), hypotension (if bradycardic, treat with atropine 0.5 mg IV; otherwise use careful volume infusion)]; (2) nitroglycerin 0.3 mg SL if systolic bp >100 mmHg; for refractory pain: IV nitroglycerin (begin at 10 μg/min, titrate upward to maximum of 200 μg/min, monitoring bp closely); do not administer nitrates within 24 h of sildenafil or within 48 h of tadalafil (used for erectile dysfunction); (3) β-adrenergic antagonists (see below).
• Oxygen: 2–4 L/min by nasal cannula (if needed to maintain O2 saturation >90%).
• Mild sedation (e.g., diazepam 5 mg, oxazepam 15–30 mg, or lorazepam 0.5–2 mg PO three to four times daily).
• Soft diet and stool softeners (e.g., docusate sodium 100–200 mg/d).
• β-Adrenergic blockers (Chap. 126) reduce myocardial O2 consumption, limit infarct size, and reduce mortality. Especially useful in pts with hypertension, tachycardia, or persistent ischemic pain; contraindications include active CHF, systolic bp <95 mmHg, heart rate <50 beats/min, AV block, or history of bronchospasm. Consider IV (e.g., metoprolol 5 mg q2–5min to total dose of 15 mg) if pt is hypertensive. Otherwise, begin PO regimen (e.g., metoprolol tartrate 25–50 mg four times daily).
• Anticoagulation/antiplatelet agents: Pts who receive fibrinolytic therapy are begun on heparin and aspirin as indicated above. In absence of fibrinolytic therapy, administer aspirin, 160–325 mg qd, and low-dose heparin (5000 U SC q12h) or low-molecular-weight heparin (LMWH, e.g., enoxaparin 40 mg SC daily) for DVT prevention. Full-dose IV heparin (PTT 1.5-2 × control) or LMWH (e.g., enoxaparin 1 mg/kg SC q12h) followed by oral anticoagulants is recommended for pts with severe CHF, presence of ventricular thrombus by echocardiogram, or large dyskinetic region in acute anterior MI. If used, oral anticoagulants are continued for 3–6 months, then replaced by aspirin. The addition of a P2Y12 platelet receptor antagonist after STEMI (e.g., clopidogrel 75 mg daily) reduces future adverse cardiac events whether or not fibrinolysis or PCI are undertaken.
• ACE inhibitors reduce mortality in pts following acute MI and should be prescribed within 24 h of hospitalization for pts with STEMI—e.g., captopril (6.25 mg PO test dose advanced to 50 mg PO tid). ACE inhibitors should be continued indefinitely after discharge in pts with CHF or those with asymptomatic LV dysfunction (ejection fraction ≤40%); if pt is ACE inhibitor intolerant, use ARB (e.g., valsartan or candesartan).
• Aldosterone antagonists (spironolactone or eplerenone 25–50 mg daily) further reduce mortality in pts with LVEF <40% and either symptomatic heart failure or diabetes; do not use in pts with advanced renal insufficiency (e.g., creatinine ≥2.5 mg/dL) or hyperkalemia.
• Serum magnesium level should be measured and repleted if necessary to reduce risk of arrhythmias.
COMPLICATIONS
(For arrhythmias, see also Chaps. 131 and 132)
Ventricular Arrhythmias
Isolated ventricular premature beats (VPBs) occur frequently. Precipitating factors should be corrected [hypoxemia, acidosis, hypokalemia (maintain serum K+ ~4.5 mmol/L), hypercalcemia, hypomagnesemia, CHF, arrhythmogenic drugs]. Routine beta blocker administration (see above) diminishes ventricular ectopy. Other in-hospital antiarrhythmic therapy should be reserved for pts with sustained ventricular arrhythmias.
Ventricular Tachycardia
If hemodynamically unstable, perform immediate electrical countershock (unsynchronized discharge of 200–300 J or 50% less if using biphasic device). If hemodynamically tolerated, use IV amiodarone (bolus of 150 mg over 10 min, then infusion of 1.0 mg/min for 6 h, then 0.5 mg/min).
Ventricular Fibrillation (VF)
VF requires immediate defibrillation (200–400 J). If unsuccessful, initiate cardiopulmonary resuscitation (CPR) and standard resuscitative measures (Chap. 11). Ventricular arrhythmias that appear several days or weeks following MI often reflect pump failure and may warrant invasive electrophysiologic study and implantation of a cardioverter defibrillator (ICD).
Accelerated Idioventricular Rhythm
Wide QRS complex, regular rhythm, rate 60–100 beats/min, is common and usually benign; if it causes hypotension, treat with atropine 0.6 mg IV.
Supraventricular Arrhythmias
Sinus tachycardia may result from heart failure, hypoxemia, pain, fever, pericarditis, hypovolemia, administered drugs. If no cause is identified, suppressive beta blocker therapy may be beneficial to reduce myocardial oxygen demand. Other supraventricular arrhythmias (paroxysmal supraventricular tachycardia, atrial flutter, and fibrillation) are often secondary to heart failure. If hemodynamically unstable, proceed with electrical cardio-version. In absence of acute heart failure, suppressive alternatives include beta blockers, verapamil, or diltiazem (Chap. 132).
Bradyarrhythmias and AV Block
(See Chap. 131) In inferior MI, usually represent heightened vagal tone or discrete AV nodal ischemia. If hemodynamically compromised (CHF, hypotension, emergence of ventricular arrhythmias), treat with atropine 0.5 mg IV q5min (up to 2 mg). If no response, use temporary external or transvenous pacemaker. Isoproterenol should be avoided. In anterior MI, AV conduction defects usually reflect extensive tissue necrosis. Consider temporary external or transvenous pacemaker for (1) complete heart block, (2) Mobitz type II block (Chap. 131), (3) new bifascicular block (LBBB, RBBB + left anterior hemiblock, RBBB + left posterior hemiblock), (4) any bradyarrhythmia associated with hypotension or CHF.
Heart Failure
CHF may result from systolic “pump” dysfunction, increased LV diastolic “stiffness,” and/or acute mechanical complications.
Symptoms Dyspnea, orthopnea, tachycardia.
Examination Jugular venous distention, S3 and S4 gallop, pulmonary rales; systolic murmur if acute mitral regurgitation or ventricular septal defect (VSD) has developed.
Initial therapy includes diuretics (begin with furosemide 10–20 mg IV), inhaled O2, and vasodilators, particularly nitrates [PO, topical, or IV (Chap. 133) unless pt is hypotensive (systolic bp <100 mmHg)]; digitalis is usually of little benefit in acute MI unless supraventricular arrhythmias are present. Diuretic, vasodilator, and inotropic therapy (Table 128-1) may be guided by invasive hemodynamic monitoring (Swan-Ganz pulmonary artery catheter, arterial line), particularly in pts with accompanying hypotension (Table 128-2; Fig. 128-3). In acute MI, optimal pulmonary capillary wedge pressure (PCW) is 15–20 mmHg; in the absence of hypotension, PCW >20 mmHg is treated with diuretic plus vasodilator therapy [IV nitroglycerin (begin at 10 μg/min) or nitroprusside (begin at 0.5 μg/kg per min)] and titrated to optimize bp, PCW, and systemic vascular resistance (SVR).
TABLE 128-1 IV VASODILATORS AND INOTROPIC DRUGS USED IN ACUTE MI
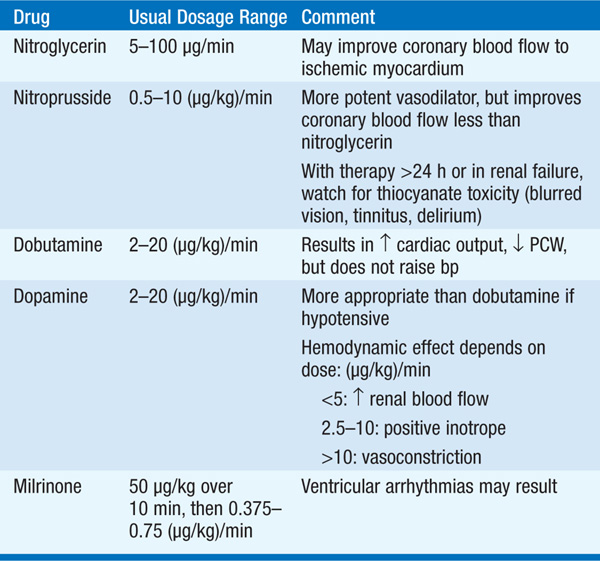
TABLE 128-2 HEMODYNAMIC COMPLICATIONS IN ACUTE MI
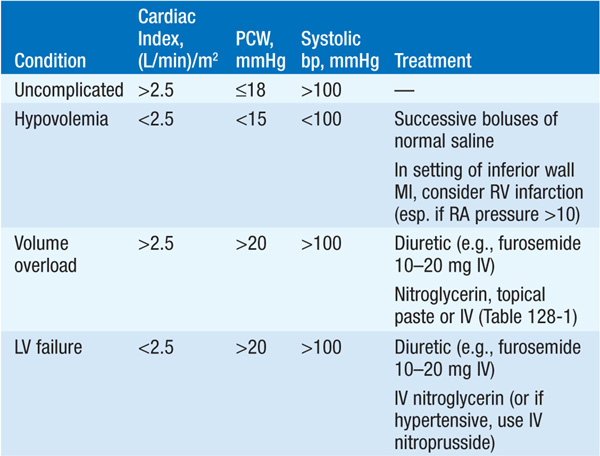

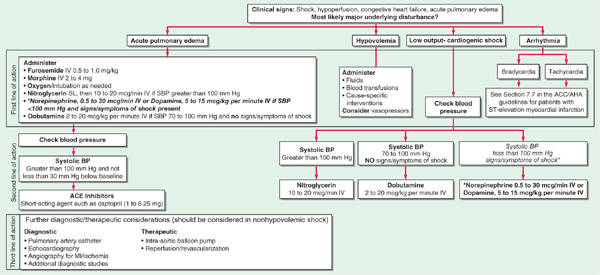
FIGURE 128-3 Emergency management of cardiogenic shock and pulmonary edema. ACE, angiotensin converting-enzyme; BP, blood pressure; MI, myocardial infarction. [Modified from Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 7: The era of reperfusion: Section 1: Acute coronary syndromes (acute myocardial infarction). The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation 102:I172, 2000.]
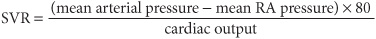
Normal SVR = 900 – 1350 dyne•s/cm5. If PCW >20 mmHg and pt is hypotensive (Table 128-2 and Fig. 128-3), evaluate for VSD or acute mitral regurgitation, consider dobutamine [begin at 1–2 (μg/kg)/min], titrate upward to maximum of 10 (μg/kg)/min; beware of drug-induced tachycardia or ventricular ectopy.
After stabilization on parenteral vasodilator therapy, oral therapy follows with an ACE inhibitor or an ARB (Chap. 133). Consider addition of long-term aldosterone antagonist (spironolactone 25–50 mg daily or eplerenone 25–50 mg daily) to ACE inhibitor if LVEF ≤40% or symptomatic heart failure or diabetes are present—do not use if renal insufficiency or hyperkalemia are present.
Cardiogenic Shock
(See Chap. 12) Severe LV failure with hypotension (bp <90 mmHg) and elevated PCW (>20 mmHg), accompanied by oliguria (<20 mL/h), peripheral vasoconstriction, dulled sensorium, and metabolic acidosis.
Swan-Ganz catheter and intraarterial bp monitoring are not always essential but may be helpful; aim for mean PCW of 18–20 mmHg with adjustment of volume (diuretics or infusion) as needed. Vasopressors [e.g., dopamine (Table 128-1)] and/or intraaortic balloon counterpulsation may be necessary to maintain systolic bp >90 mmHg and reduce PCW. Administer high concentration of O2 by mask; if pulmonary edema coexists, consider bilateral positive airway pressure (BiPAP) or intubation and mechanical ventilation. Acute mechanical complications (see below) should be sought and promptly treated.
If cardiogenic shock develops within 36 h of acute STEMI, reperfusion by PCI or coronary artery bypass grafting (CABG) may markedly improve LV function.
Hypotension
May also result from right ventricular MI, which should be suspected in inferior or posterior MI, if jugular venous distention and elevation of right-heart pressures predominate (rales are typically absent and PCW may be normal); right-sided ECG leads typically show ST elevation, and echocardiography may confirm diagnosis. Treatment consists of volume infusion. Noncardiac causes of hypotension should be considered: hypovolemia, acute arrhythmia, or sepsis.
Acute Mechanical Complications
Ventricular septal rupture and acute mitral regurgitation due to papillary muscle ischemia/infarct develop during the first week following MI and are characterized by sudden onset of CHF and new systolic murmur. Echocardiography and Doppler interrogation can confirm presence of these complications. PCW tracings may show large v waves in either condition, but an oxygen “step-up” as the catheter is advanced from right atrium to right ventricle suggests septal rupture.
Acute medical therapy of these conditions includes vasodilator therapy (IV nitroprusside: begin at 10 μg/min and titrate to maintain systolic bp ~100 mmHg); intraaortic balloon pump may be required to maintain cardiac output. Mechanical correction is the definitive therapy. Acute ventricular free-wall rupture presents with sudden loss of bp, pulse, and consciousness, while ECG shows an intact rhythm (pulseless electrical activity); emergent surgical repair is crucial, and mortality is high.
Pericarditis
Characterized by pleuritic, positional pain and pericardial rub (Chap. 125); atrial arrhythmias are common; must be distinguished from recurrent angina. Often responds to aspirin, 650 mg PO qid. Anticoagulants should be avoided when pericarditis is suspected to avoid development of tamponade.
Ventricular Aneurysm
Localized “bulge” of LV chamber due to infarcted myocardium. True aneurysms consist of scar tissue and do not rupture. However, complications include CHF, ventricular arrhythmias, and thrombus formation. Typically an aneurysm is confirmed by echocardiography or by left ventriculography. The presence of thrombus within the aneurysm, or a large aneurysmal segment due to anterior MI, warrants oral anticoagulation with warfarin for 3–6 months.
Pseudoaneurysm is a form of cardiac rupture contained by a local area of pericardium and organized thrombus; direct communication with the LV cavity is present; surgical repair usually necessary to prevent rupture.
Recurrent Angina
Usually associated with transient ST-T wave changes; signals high incidence of reinfarction; when it occurs in early post-MI period, proceed directly to coronary arteriography, to identify those who would benefit from PCI or CABG.
SECONDARY PREVENTION
For pts who have not already undergone coronary angiography and PCI, submaximal exercise testing should be performed prior to or soon after discharge. A positive test in certain subgroups (angina at a low workload, a large region of provocable ischemia, or provocable ischemia with a reduced LVEF) suggests need for cardiac catheterization to evaluate myocardium at risk of recurrent infarction. Beta blockers (e.g., metoprolol, 25–200 mg daily) should be prescribed routinely for at least 2 years following acute MI, unless contraindications present (asthma, CHF, bradycardia, “brittle” diabetes). Continue oral antiplatelet agents (e.g., aspirin 81–325 mg daily, clopidogrel 75 mg daily) to reduce incidence of reinfarction. If LVEF ≤40%, an ACE inhibitor (e.g., captopril 6.25 mg PO tid, advanced to target dose of 50 mg PO tid) or ARB (if ACE inhibitor is not tolerated) should be used indefinitely. Consider addition of aldosterone antagonist (see “Heart Failure,” above).
Modification of cardiac risk factors must be encouraged: discontinue smoking; control hypertension, diabetes, and serum lipids (typically atorvastatin 80 mg daily in immediate post-MI period—see Chap. 189); and pursue graduated exercise.

For a more detailed discussion, see Antman EM, Loscalzo J: ST-Segment Elevation Myocardial Infarction, Chap. 245, p. 2021; and Hochman JS, Ingbar DH: Cardiogenic Shock and Pulmonary Edema, Chap. 272, p. 2232, in HPIM-18.







