![]()
To understand the structure and behaviour of flowers in their interactions with potential insect pollinators, we need to know how the insects are built, and something about their sensory abilities and behaviour. These are the subjects of this chapter and the two that follow it.
Differences in the mouth-parts and sensory capacities of insects provide the background against which the form, colours and scents of flowers can be interpreted (Chapter 2 and Chapter 6). The insect sense of touch depends on hair-like organs scattered over the body with varying density. The perception of chemicals in the vapour state (sense of smell) and in solution (sense of taste) comes through thin-walled discs in the cuticle, or thin-walled projecting or sunken hairs. These occur on the feet, the antennae and the mouth-parts. Nerve cells at the base of chemo-sensory hairs have processes running up inside each hair; these are exposed to the environment through a pore at the tip of the hair in the case of a taste-organ, but through hundreds of much smaller pores in the hair if the function is olfactory. The insect compound eye is a highly developed organ which can produce a detailed retinal image. Its facetted structure results in good ability to detect movement. Movement and length of contour seem to be important in resolving patterns. Most insects are sensitive to ultra-violet radiation but have little or no sensitivity to red, so that their visible spectrum covers the wavelengths 300 nm to 650 nm, compared with 400 nm to 750 nm in man (Burkhardt, 1964; Menzel, 1990). Many insects possess colour vision, and much work on this faculty has made use of the insects’ visits to flowers. Social bees have some ability to respond to sound (vibrations in the substratum or the atmosphere). (For further information, see the general account of insect senses in Imms [1947, in this series] and the textbook by Mordue et al. [1980] or the account in non-technical German or English by Barth [1982, 1985] which is related to pollination.)

Fig. 3.1 Wild cockroach (Ectobius pallidus, Dictyoptera), on sea. carrot (Daucus carota ssp. gummifer); Alderney.
Table 3.1 Orders of insects recorded as flower visitors.
APTERYGOTA
Collembola, springtails
Generalised mouth-parts. Feeding on pollen of Chrysosplenium and resting.
EXOPTERYGOTA
Dermaptera, earwigs
Mandibles like those of beetles. Hiding in and eating flowers of many species.
Dictyoptera, cockroaches
Ectobius licking nectar of Spiraea, Filipendula and Apiaceae (Fig. 3.1).
Psocoptera, booklice
Passing visitors.
Odonata, dragonflies, damselflies
Passing visitors.
Orthoptera, grasshoppers, crickets
Passing visitors.
Plecoptera, stoneflies
Mandibles like those of beetles. Feeding on pollen of Caltha, Helianthemum, Alchemilla, Rosa. Feeding on nectar of Listera ovata.
Hemiptera, bugs
Piercing and sucking mouth-parts. Aphididae: feeding on plant sap, sometimes in the flowers. Miridae (Capsidae): regular visitors for pollen from open flowers in Asteraceae, Apiaceae. Anthocoridae (Cimicidae): feeding on nectar from Salix etc. Lygaeidae: Lygaeus sucks juices, perhaps a minor pollinator of the pollen flower Adonis vernalis.
Neuroptera, lacewings
Biting mouth-parts. Recorded on Apiaceae, possibly feeding on nectar.
Mecoptera, scorpion-flies
Biting mouth-parts on long snout. Feeding on nectar of Apiaceae and Asteraceae and commonly on Polemonium caeruleum.
Trichoptera, caddis-flies
Mouth-parts adapted for liquid feeding. Feeding on nectar of Hedera helix, Listera ovata, Apiaceae, Valeriana officinalis, Nuphar spp.
Thysanoptera, thrips (note: thrips is both singular and plural) (Fig. 3. 2) Some species are pollinators.
| ENDOPTERYGOTA | ||
| Coleoptera | Lepidoptera | } of definite importance for pollination and |
| Diptera | Hymenoptera | dealt with separately in this book |
(Burkill, 1897; Müller, 1883; Knuth, 1906–1909; Willis & Burkill, 1895–1908; Hagerup, 1950a; Pigott, 1958; Porsch, 1957; Popham, 1961; Corbet, 1970; Borge Pettersson; MCFP)
The insects may be classified into three major groups. The most primitive of these comprises four orders with no trace of wings (Apterygota) and includes the Collembola, or springtails. The other insects are winged as adults and are divided into those in which the developing insect becomes gradually more like the adult at each moult (Exopterygota), and those in which the larva, usually very unlike the adult, enters a more or less dormant pupal phase during which the transformation to adult takes place (Endopterygota) (Table 3.1).
Apterygota and Exopterygota are incidental visitors to flowers or occasional consumers of nectar or pollen. They may sometimes transfer pollen, but their importance as pollinators must be negligible; exceptions to this are the stone-flies (Plecoptera), bugs (Hemiptera) and scorpion-flies (Mecoptera) as shown in Table 3.1, and the thrips, described separately below. Regular specialised flower visiting (other than by thrips) is recorded only in the four orders of the Endopterygota. Of these, the beetles (Coleoptera) and the flies (Diptera) are described in this chapter.
These minute insects have a pupal phase but are classed as Exopterygota because the wing buds are visible in the larval stages. They are usually 2 mm long, or less, and narrow-bodied (Fig. 3.2). Their four wings are each composed of a central shaft and a row of hairs to the front and back. Together with staphylinid beetles of similar size, they are popularly known as thunder-flies, as they enter houses in hot weather (and get behind picture-glass!). They have specialised asymmetric piercing and sucking mouth-parts and are sometimes pests of crops, either through feeding on them or by transmitting virus diseases. However, some species inhabit flowers and are specialised feeders on pollen, sucking out the contents of the grains after piercing them with their mouth-parts (Kirk, 1984b). Most thrips caught in flowers carry pollen grains, and the number of bristles on the body (which varies among species) affects the number of grains likely to be carried (Lewis, 1973). In a few cases, pollen-eating thrips may be significant as pollinators. For example, Hagerup (1950a) found that in the Faroes, where bees and butterflies are rare, thrips were pollinators of ling (Calluna vulgaris), cross-leaved heath (Erica tetralix) and common catsear (Hypochaeris radicata) (Hagerup & Hargerup, 1953). Some species go through their whole life-cycle in flowers. One example of this is Ceratothrips (Taeniothrips) ericae, which lives in Calluna flowers, where it often causes self- and cross-pollination. Strangely enough, species of these minute insects are regular pollinators of huge forest trees of the family Dipterocarpaceae in South-east Asia (see here and here). In addition, crop plants in which some degree of pollen transfer by thrips has been recorded are onion (Allium cepa), plum (Prunus domestica), French bean (Phaseolus vulgaris), flax (Linum usitatissimum), sugar beet (Beta vulgaris), pyrethrum (Tanacetum cinerariifolium) and cacao (Theobroma cacao) (Lewis, 1973).
The flower-visiting thrips show colour ‘preferences’. They respond more to the colours white (without ultraviolet, i.e. ‘bee blue-green’, see here), blue and yellow than to green, red, black or white-with-ultraviolet (‘bee-white’) (Kirk, 1984a). They also respond to the vapour of anisaldehyde, a flower scent (Kirk, 1985).
This is the largest order of insects, though not the largest in the British Isles. The beetles are not very important in flower pollination in cool-temperate climates but are more so in arid areas and in the moist tropics. In southern Africa different groups of the family Scarabaeidae, feeding on either pollen or nectar, are important in pollinating the rich annual flora of Namaqualand (including many Asteraceae) and also some species of Protea; other groups are probably pollinators too (Whitehead et al., 1987). In the moist tropics, they are of especial interest as pollinators of some primitive flowering plants and even some gymnosperms (Chapter 14), as well as particular groups such as palms, waterlilies and aroids (Chapter 10 & Chapter 11). The beetles are classified on structural and anatomical characters into two main sub-orders: Adephaga and Polyphaga. The first of these is mainly predatory and its members do not visit flowers. The second is much the larger group and includes the flower-devourers and the pollinators. Even so, beetles have primitive mouth-parts (Box 3.1).
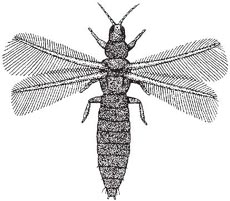
Fig. 3.2 A typical thrips (Thysanoptera): insect about 2mm long. (Derek Whiteley).
Fig. 3.3 Garden chafer, Phyllopertha horticola (Coleoptera: Scarabaeidae, subfam. Rutelinae), on hogweed, Heracleum sphondylium.

Fig.3.4 Soldier beetles, Rhagonycha fulva (Coleoptera: Cantharidae), on hogweed, Heracleum sphondylium.

Fig. 3.5 Oedemera nobilis (male) (Coleoptera: Oedemeridae) on mouse-ear hawkweed (Pilosella officinarum).
The European beetles listed as flower visitors by Kugler (1984) represents 18 genera. Some of these, and the families to which they belong, are listed in Table 3.2. In some instances, the nearest relatives of flower-frequenting (anthophilous) species are not known to visit flowers; on the other hand, in some whole genera or even families the adult beetles feed exclusively on flower foods (Müller, 1983). This habit has in some evolutionary lines of beetles than in others. The fossil records show that beetles were abundant during the Mesozoic; at least some must have been flower visitors in the times of the earliest angiosperms (see here) and probably earlier (see here). The present-day association of beetle pollination with primitive woody angiosperms such as Magnolia (Thien, 1974) and Calycanthus (Grant, 1950a) is probably ancient, going back to their evolutionary origins. Some families of flower-visiting beetles are found as fossils back into the Mesozoic (e.g. Elateridae, Nitidulidae, Cerambycidae). Others (e.g. Scarabaeidae, Oedemeridae, Cantharidae) probably arose in the burst of evolutionary radiation of flowers and insects in the Tertiary which saw the origin of bees, butterflies, and other specialised flower-visitors that now make up the majority of pollinators (see here Willemstein, 1987). In Calycanthus and some beetle-pollinated flowers described in Chapter 10 & Chapter 11 beetles eat nutritive tissues which, apparently, are provided especially for them.
Diagrams A–E show the mouth-parts of a cockroach, Blatta (Dictyoptera), illustrating the primitive condition in insects. The capital letters in the labels indicate abbreviations used in other figures; for ‘compass-points’ see Box 3.2. A, labrum-epipharynx; B, one mandible of pair; C, one maxilla of pair; D. hypopharynx; E, labium, with one each of paired appendages. Diagrams F and G show a beetle: F, side view of head, with parts shown in A to E attached in same sequence from ‘a’ to ‘b’ (distance ‘a’ to ‘b’ exaggerated); G, head from below, with paired parts shown singly (parts shown in A, B, C and E are visible, that shown in D is concealed) (G after Crowson, 1956).
The mandibles of beetles are the biting and chewing parts, while the maxillae and labium taste and manipulate the food; the labium also helps, in conjunction with the labrum, to form a chamber in which the food can be confined during mastication. Thus a beetle bites and chews the food while it is still outside the mouth, and its mouth-parts perform roughly the functions which in the mammal are performed by mouth, lips, jaws, tongue and, in some cases, fore-limbs.
Modifications towards flower feeding in beetles consist of a tilting upwards of the head which brings the mouth-parts forward, the development of the head behind the eyes to form an additional, neck-like region, transformation of the usually short and broad first segment of the thorax into a long and narrow segment, and a lengthening of the hairs on the lobes of the maxillae. These modifications increase the insects’ ability to reach sunken nectaries and lick up the nectar efficiently. The greatest depth to which European beetles can reach with their fore-parts for nectar is about 6 mm (in Strangalia). In South America, however, the genus Nemognatha is adapted to reach deep-seated nectar, having its maxillae modified to form a slender tube 12 mm long (Müller, 1883).
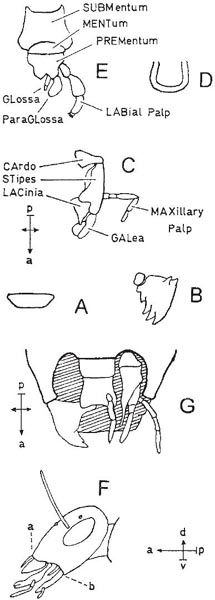
Table 3.2 Beetles recorded visiting flowers in Europe.

(Hobby, 1933; Kugler, 1984; Müller, 1883; Parmenter, 1956; MCFP)
Fig. 3.6 Flower beetles (Meligethes sp., Coleoptera: Nitidulidae), on wall rocket (Diplotaxis muralis).
Fig. 3.7 Longhorn beetle (Judolia cerambyciformis, Coleoptera: Cerambycidae) on bramble (Rubus fruticosus agg.).

Fig. 3.8 Leaf beetle, Cryptocephalus sp. (Coleoptera: Chrysomelidae) on flower of buttercup, Ranunculus sp.
Though the flower-visiting beetles are more active than many beetles with different habits they are usually less active than insects such as flies and Hymenoptera, and therefore less useful to the plants they visit except for those that are highly specialised to them. Beetles tend to protect themselves by their horny exterior and their repellent secretions rather than by flight, and may linger in the same flower or inflorescence for hours. In an assessment of the interactions between Oedemera species (Fig. 3.5) and flowers, Kugler (1984) found that they readily moved from flower to flower and that the beetles were well adapted to living on pollen and nectar and were effective pollinators of the flowers that they visited; however, they accounted for a tiny minority of insect visits to these flowers.
The small pollen beetles in the family Nitidulidae (Table 3.6) and small rove beetles (Staphylinidae), which may be abundant in many flowers, mainly creep about eating pollen and nectar, only sometimes causing pollination. However, Marsden-Jones (1935) proved that the pollen beetle Meligethes (Nitidulidae) moves between the flowers of lesser celandine (Ranunculus ficaria); he removed the beetles from all the flowers of certain plants and found that the flowers were again occupied by Meligethes a few hours later.
Adaptations against damage to the ovules would be expected in flowers specialised for beetle-pollination. Grant (1950b) suggested that this protection might be provided by the sinking of the ovules into the receptacle (by epigyny or perigyny) or by the close massing together of the flowers so that the ovaries cannot be reached, as in some of the more robustly constructed Asteraceae (see here).
In beetles, the sense of smell plays a large part in feeding and in finding a place for egg-laying. Fritz Knoll, in his study of the plant Arum nigrum (1926; see here), obtained evidence that dung-frequenting beetles found their way to its inflorescences by scent. The smell of this plant is produced by its spadix, and as the beetles flew past they suddenly turned in flight towards the spadices on coming within about 30 cm of them. They then either flew straight towards them, with repeated brief reversals of direction, or flew round, gradually getting nearer. These flight paths are indicative of the insects’ finding their way by scent; this becomes specially apparent when they are compared with the flight paths of insects finding their way to flowers by sight (see here).
Scents specifically associated with beetle-pollination (in preference to pollination by short-tongued insects in general) are found mainly in plants from outside Europe; examples are the spicy scent of certain crab-apples (Malus species), of Paeonia delavayi (a tree peony) and wintersweet (Chimonanthus praecox), and the smell of fermenting fruit produced by Calycanthus (Grant, 1950a).
Colour vision was demonstrated in some beetles by Schlegtendal (1934), using a method of investigation independent of feeding reactions. She found that Chrysomelid beetles could clearly distinguish yellow and orange from blue, and violet from green; dung-beetles (Geotrupes) could distinguish yellow, orange and violet from blue, and also yellow-green and light green from other colours. Species of Amphicoma (Scarabaeidae subfamily Glaphyrinae) in the eastern Mediterranean region are strongly associated with red bowl-shaped pollen-flowers (see here) and have been found to respond preferentially to red plastic cups, so these beetles are presumably sensitive to red (Dafni et al., 1990).
This is another major order of insects, and in the British Isles it substantially outnumbers the Coleoptera in species, with about 5,200 as against 3,700. Structurally the true flies are easily distinguished by their possession of only one pair of wings, the second pair being reduced to stalked knobs, the halteres or balancers. In the adult state the vast majority of species have well-developed wings and fly readily. Very many flies are flower visitors and the order is therefore an important one in flower pollination.
Nearly all flies have suctorial mouth-parts, and there are two main types of these; one type is adapted for penetrating the tissues of animals in order to imbibe their internal fluids, while the other lacks penetrating organs and is adapted for mopping up exposed liquids. The second is much the commoner and the piercing type has probably arisen repeatedly from the mopping-up type. Many of the flies that feed on exposed fluids can also eat small solid particles, including pollen grains. The ability of flies to take in solid particles through their tubular proboscides is dependent on the suspension of the particles in a liquid, and this is always available because saliva is conveyed nearly to the apex of the proboscis. In some groups of flies the proboscis has become elongated in adaptation to feeding at the longer-tubed flowers. The main organ of the mouth-parts of Diptera is the labium, with its labella which may correspond to the paraglossae of other orders (Box 3.1). In the mopping-up type, the labella collect the food and the labium conveys it to the mouth. Details of fly mouth-parts are given in Box 3.2–Box 3.6.
In Diptera the taste organs are chiefly in the region of the mouth, but they often also occur in the tarsi of the legs. When they taste food with their legs insects automatically begin to lower the proboscis in order to feed; it is, therefore, easy to investigate the sensitivity of the taste organs in the legs. In this way it has been found that the legs of blow-flies (Calliphora vomitoria or C. vicina) are 100 to 200 times more sensitive to the taste of cane sugar (sucrose) than the human tongue.
The scent organs of Diptera are found on the antennae. The thin-walled olfactory hairs were found by Liebermann (1925) to be either solitary on the surface, or sunk, singly or in groups, into pits. The pits, when large, may be partially subdivided and they are always oblique, the openings facing towards the apex of the antenna. The olfactory hairs occur only on the terminal joint of the three-jointed antennae. In the species investigated, the total number of olfactory hairs in the two antennae ranges from 300 in the male of Psila rosae to 9,260 in the male of Calliphora vomitoria. On average, there were more olfactory hairs on the males of dung- and carrion-frequenting species than on those of flower-frequenting species. The females were left out of this comparison because some species feed on flowers as adults but lay eggs in dead organic matter. In these, the females have more olfactory hairs than the males. Liebermann thought that the explanation of these differences lay in the fact that vision cannot play such a big part in finding decaying matter as it can in finding flowers.

Fig. 3.9 St Mark’s fly (Bibio marci, Diptera: Bibionidae), and a smaller species of Bibio on alexanders (Smyrnium olusatrum).
Most Diptera that visit flowers have large eyes with many ommatidia (facets), and it seems probable that at least all the higher Diptera have colour vision. The lesser house fly (Fannia canicularis) was included in Schlegtendal’s investigation (see here) and was found to have similar colour vision to that of the chrysomelid beetles.
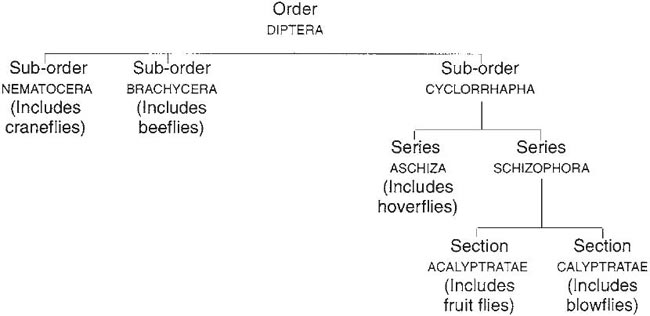
The following diagram shows how the Diptera are classified and the sequence (from left to right) in which they will now be described.
The flower visitors of the sub-order Nematocera comprise about eleven families. In general they are not very important pollinators, for most are very small and all except Culicidae have very short mouth-parts, even the relatively large Bibio (Fig. 3.9) and Tipulidae having a proboscis only a millimetre or two in length. Table 3.3 shows that most of the flowers visited by them are either flat or bowl-shaped and have well-exposed nectar, or are tubular but so small that the nectar is easily reached. (These small tubular flowers are frequently assembled into dense clusters to form ‘brush-blossoms’ [see here], or into a well-defined head or ‘capitulum’ [see here].) Nectarless flowers are presumably visited for their pollen. Nematocera appear, from the records of Willis & Burkill (1895–1908), to visit flowers chiefly for nectar, but these authors record that Bibionidae, Mycetophilidac and Scatopsidae eat pollen as well. Not unexpectedly, mosquitoes visit flowers at night (Parmenter, 1958; Corbet, 1970). Brantjes & Leemans (1976), in their study of Silene otites in the Netherlands, found that mosquitoes of both sexes were flower-visitors, drinking the nectar and causing pollination.
The very small insects of the remaining families of Nematocera visit a range of the flowers already mentioned and, in addition, those of some dwarf herbaceous plants of damp shady places that are particularly suited to them: Chrysosplenium species (golden saxifrage) and Adoxa moschatellina (moschatel) (Grensted, 1946; Hagerup, 1951; Kimmins, 1939; Knuth, 1906–1909; Parmenter, 1952b; Smart, 1943; Willis & Burkill, 1895–1908). Such flies are often involved in pollination systems that deceive them, for example, the relationship between lords and ladies (Arum maculatum) and the owl midges (family Psychodidae). Outside the British Isles, Nematocera are special pollinators of various plants. For example, mosquitoes pollinate a small North American
Both the piercing and the mopping-up types of mouth-parts are found in this sub-order. In Tipula (cranefly or daddylonglegs, family Tipulidae), shown here (after Hammond, 1874), the proboscis is quite short. The mouth-parts are borne on a tubular prolongation of the head (Diagrams A–C; note the compass-points shown: anterior, posterior, dorsal, ventral, left and right). The mandibles are missing and there is little of the maxillae apart from their very long, five-jointed palps (C); the remaining structures are the labium, with no palps, and the labrum. The labrum is a simple structure attached above the mouth (C). The labium, attached below the mouth (A), is more complex; it is partly covered with soft cuticle, but its short and stout basal portion is supported at the back by a sclerite (a hard plate) corresponding to the mentum of beetles. On the front is a furrow which leads out from the mouth, beneath the labrum, to the gap between the labella, where the salivary duct opens into the furrow (B). The bladdery labella (A,B,C) contain in their surface a number of sclerites between which they can be folded up; they are presumably kept in a distended condition by blood-pressure as in the blowfly (Calliphora), described in Box. 3.6. They are partly covered with a system of microscopic furrows called pseudotracheae (because of their resemblance to the tracheae, or breathing tubes, of insects) (A,D,E). The furrows are kept in shape by transverse, incomplete rings of hard chitin (E); there are four major pseudotracheae in Tipula (A,D) and numerous minor ones that lead into them. The pseudotracheal system acts as a filter by which liquids can be mopped up from a wet surface and solid particles left behind. The fluid is presumably drawn into the pseudotracheae by capillary action; the main pseudotracheae lead into the groove on the front of the labium (D), and the liquid is drawn into the mouth by the sucking action of the pharynx exerted through a tube formed by the labrum and the groove on the front of the labium. Tipula differs from most Diptera in the lack of a free tongue-like or lance-like hypopharynx with the salivary duct inside it and opening at its tip.
The piercing Nematocera are mosquitoes and gnats, black-flies and biting-midges. The largest of these are the mosquitoes, such as Culex and Anopheles. They have a long narrow labium with small, equally narrow labella; the labrum and hypopharynx are long and sword-like and are placed together to form a feeding tube which lies in the groove on the front of the labium. There are also two needle-like maxillae and, in the female, two mandibles of the same form. All these long slender organs are used by the female to pierce the skin of the food-animal. The male mouth-parts are not used for bloodsucking, the mandibles being absent. Thus the mosquitoes (and, similarly, the black-flies and biting-midges) lack the mopping-up type of labella of the cranefly, but the proboscis can be dipped into drops of nectar.
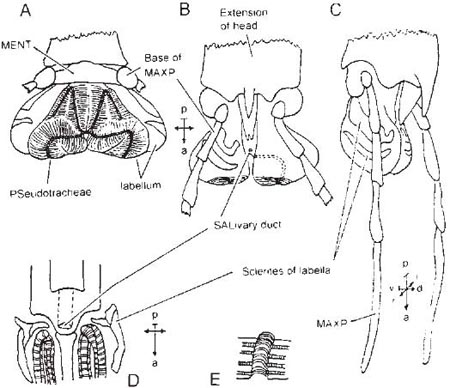
Table 3.3 Diptera-Nematocera and the flowers they visit in Britain.
| A. Flowers visited by Tipulidae and Culicidae | ||
| (craneflies and mosquitoes) | ||
| Silene otites | Spanish catchfly | small tubular flowers |
| Parnassia palustris | grass of Parnassus | nectar well exposed |
| Saxifraga hypnoides | mossy saxifrage | nectar well exposed |
| Filipendula ulmaria | meadowsweet | nectarless flowers |
| Potentilla palustris | marsh cinquefoil | nectar partly concealed |
| Euphorbia | spurge | nectar well exposed |
| Frangula alnus | alder buckthorn | nectar well exposed |
| Hedera helix | ivy | nectar well exposed |
| Apiaceae | umbellifers | nectar well exposed |
| Mentha aquatica | water mint | small tubular flowers |
| Valeriana dioica | marsh valerian | small tubular flowers |
| Valeriana officinalis | common valerian | small tubular flowers |
| Listera cordata | lesser twayblade | nectar well exposed |
| B. Flowers visited by Bibionidae | ||
| (St Mark’s flies – Bibio spp., and fever flies – Dilophus spp.) | ||
| Euphorbia | spurge | nectar well exposed |
| Acer pseudoplatanus | sycamore | nectar well exposed |
| Crataegus spp. | hawthorn | cup-shaped flowers |
| Sorbus aucuparia | rowan | cup-shaped flowers |
| Malus | apple | cup-shaped flowers |
| Apiaceae | umbellifers | nectar well exposed |
| Euonymus europaeus | spindle-tree | nectar well exposed |
(Records in section A from: Brantjes & Leemans, 1976; Corbet, 1970; Knuth, 1906–1909; Müller, 1883; Parmenter, 1952b; Willis & Burkill, 1895–1908; in section B from Drabble & Drabble, 1927; Knuth, 1906–1909; Parmenter, 1952b; Willis & Burkill, 1895–1908.)
orchid, Habenaria obtusata (Dexter, 1913; Thien, 1969a & b), and these and other Nematocera are the pollinators of the orchid genus Pterostylis (see here) and several of the other exotic insect-trapping or insect-deceiving plants mentioned in Chapter 10, as well as some members of the family Sterculiaceae, including the cocoa plant (see here) (Schumann, 1890–1893; Young et al., 1984).
Flower visitors in this suborder are spread over several families (Table 3.4). Distinctive types of flower-visitor are covered in five families described here.
Stratiomyiidae are medium-sized flies, with flattened and often brightly coloured bodies suggestive of hoverflies. However, they are rather slow in flight and usually frequent damp places and waterside habitats (Fig. 3.10). The proboscis is well-developed but rather short; it has large labella with numerous pseudotracheae, and thus functions like the proboscis of the cranefly. Knuth (1906–1909) records that Stratiomyiidae have been seen at the flowers of 22 different families. British records, however, are rather scarce. They visit Apiaceae freely and the small tubular flowers of mint (Mentha), but are also recorded at a number of Asteraceae, which they perhaps visit for pollen, since either the depth or the narrowness of the floral tubes in this family would probably prevent the flies from reaching the nectar.
The Empididae is a large family of small flies (Fig. 6.2) in which only exceptionally large species reach a length of 10 mm. They are predatory on other insects, chiefly Diptera, and have a rigid, piercing proboscis (Box. 3.3) which is also suitable for taking nectar from small tubular flowers, including the longer-tubed Asteraceae, such as knapweed (Centaurea) and Cirsium spp. (thistles). They are presumably effective pollinators of such flowers. The records of Willis & Burkill (1895–1908) indicate Empis as being much the most important genus of flower visitors in this family in Britain, having been seen at the flowers of about 50 species of plants, while Hobby & Smith (1961) list 20 species of flowers visited by the large species Empis tessellata (Fig. 3.11).
Fig. 3.10 Soldier fly (Chloromyia formosa, Diptera: Stratiomyiidae), on hogweed (Heracleum sphondylium).
The Bombyliidae include some of the most highly specialised flower-feeders in the Diptera. The genera Bombylius and Phthiria both have a long, slender, rigid proboscis normally held pointing forwards horizontally but having some flexibility in its joint with the head (Box. 3.3). Bombylius species (bee-flies, Plate 2f) are rather large and visit chiefly large, long-tubed flowers, but also some flowers with more exposed nectar (Table 3.5; see also Table 3.7).
Bombylius and Phthiria each have a proboscis similar to that of the Empididae but use it exclusively for feeding at flowers. Phthiria is a small insect, so cannot reach deep-seated nectar. Bombylius species, however, look like small bumblebees, and the larger ones have a proboscis 10–12 mm long.
Table 3.4 Alphabetical list of families of Diptera-Brachycera for which records of flower-visiting are available.
| Acroceridae | small-headed flies |
| Apioceridae | flower-loving flies |
| Asiliidae | robber-flies |
| Bombyliidae | bee-flies |
| Dolichopodidae | longheaded-flies |
| Empididae | empids |
| Lonchopteridae* | pointed wing-flies |
| Nemestrinidae | (South African) |
| Phoridae* | scuttle-flies |
| Rhagionidae | snipe-flies |
| Stratiomyiidae | soldier-flies |
| Tabanidae | horse-flies |
| Therevidae | stiletto-flies |
| (Disney, 1980; Knuth, 1906–1909; Whitehead et al., 1987; Willis & Burkill, 1895–1908) |
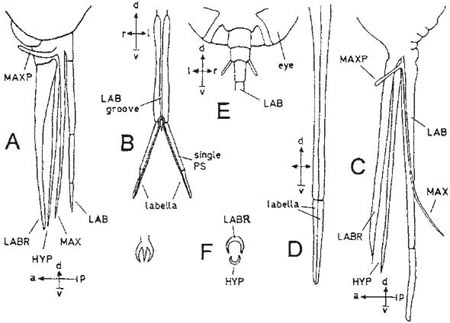
Diagram A shows the complete mouth-parts of Empis and B the labium and 3-toothed tip of the labrum. Diagrams C–F show Bombylius; as in Empis the feeding tube is formed by the labrum and hypopharynx (C–F), supported by the labium (seen from beneath in D). The folds at the base of the proboscis (C, E) allow some extension and retraction of the labium. The labella are long and narrow (D), forming an extension of the tube, and each carries only a few pseudotracheae, joined at the base (Becher, 1882; Gouin, 1949). The margins of the pseudotracheae are raised above the general surface of the labella (Peterson, 1916). The maxillae are fine bristles (C). Müller (1883) considered that the hypopharynx and labella were used for boring into soft tissues, as he had often seen these flies putting their tongues into the nectarless flowers of common St John’s wort. (Diagrams A–C after Peterson, 1916; F, D based on Kugler, 1955a; E after Gouin,
Bombylius usually hover when feeding but may sometimes hold the flower with the legs, thereby tilting the body if necessary so that the proboscis can enter the tube (Knoll, 1921; Simes, 1946). These flies move rapidly from flower to flower and are clearly highly developed nectar-feeders, though some are known to eat pollen as well (Knoll, 1921; Beattie, 1972). They are on the wing early in the year and may be quite important as pollinators of some spring flowers, although they are more or less ineffective as pollinators of wide-throated flowers such as ground ivy (Glechoma hederacea), even though they may visit them freely.
The long-tongued hovering mode has reached a remarkable level in the South African Tabanidae and Nemestrinidae. The proboscis is up to 47 mm and 70 mm long in these groups respectively, and it may be four times the length of the body. These insects have perhaps evolved to fill the niche of butterflies where these are absent (Vogel, 1954) (see here). The flowers they visit have their own distinctive characteristics (Whitehead et al., 1987).
The remaining families of the Brachycera, such as Dolichopodidae (Box. 3.12), are infrequently seen at flowers and they mainly visit those with exposed or slightly concealed nectar and small tubular flowers. The robber-flies (Asiliidae), large insects that are similar in their method of catching prey to Empididae, have been recorded at flowers of field scabious (Knautia arvensis) and bilberry (Vaccinium myrtillus), with relatively deep-seated nectar.
Knoll (1921), working in Dalmatia, tested the flower colour discrimination and preferences of Bombylius fuliginosus. By presenting a board with 16 contiguous squares of various shades of grey and one of blue-violet, like the flowers of the grape hyacinth (Muscari neglectum), he demonstrated that the flies could distinguish its violet colour from grey, for they paused only over the violet square. Flies that were visiting Cerastium litigiosum, a white flower similar to greater stitchwort (Table 3.5), approached flowers of all colours except red (poppies, which were ignored), but yellow only rarely.

Fig. 3.11 Empid fly (Empis tessellata, Diptera: Empididae) on flowers of hawthorn (Crataegus monogyna).
When foraging, bee-flies clearly used sight to find flowers, since they flew quickly and straight from flower to flower in the characteristic manner of visual flower seekers. Where flowers were scarce they would make wide sweeps over the ground, slowing down near attractive flowers and resuming their sweeps if the flower was unsuitable. They usually passed from one plant to another in a constant direction, even though at each plant they had gone all round the inflorescence (Fig. 3.12a), which suggests that they orientate by the sun. The flight-line was independent of wind direction, so scent was not involved. If the inflorescences of grape hyacinth were covered by inverted glass tubes, the flies pushed against the glass where it covered the flowers and not, even at this close range, at the source of the scent emanating from the end of the tube a little way below the inflorescence. When presented with scented and old scentless flowers, the flies sucked nectar from the the scented ones but only hastily alighted on scentless ones, suggesting that scent decided whether a proper feeding visit was made.
Table 3.5 Flowers visited by bee-flies, Bombylius.
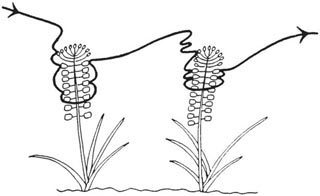
Fig. 3.12a Flight path of the beefly Bombylius medius (Diptera Bombyliidae) visiting Muscari comosum. After Knoll (1921).
The normal behaviour of the bee-flies in relation to Muscari was to approach very closely the light violet, sterile, nectarless flowers that top the inflorescence, and then to descend and feed at the lower, dark violet, fertile flowers (Fig. 3.12a). When inflorescences of the tassel hyacinth, Muscari comosum, which has violet sterile flowers and brown scentless fertile flowers, were placed in a habitat of M. neglectum, B. fuliginosus went to the sterile flowers repeatedly but did not find the fertile flowers. In its natural habitat, M. comosum was ignored by B. fuliginosus but it was a favourite flower of B. medius, which fed from it in the same way as B. fuliginosus did at M. neglectum.
This last sub-order of the Diptera is by far the largest and is itself subdivided into series (see here), the first of which to be dealt with is the Aschiza. This includes, apart from a few small families, the Syrphidae, the most important family of flower visitors among the Diptera.
The Syrphidae, or hoverflies (Fig. 2.16, Fig. 3.13, Fig. 3.14, 3. 16 & Fig. 3.17), comprise nearly 250 species in the British Isles. They get their popular name from their habit of remaining stationary in the air, a habit shared with Bombyliidae. Many of them are brightly coloured, while the darker species are often highly polished. The bright colouring often consists of a pattern of yellow and black which gives them a resemblance to wasps, or red and black, which again makes them resemble certain kinds of wasp, bee or ichneumon. Some species are stout and furry, and in their colouring closely mimic various species of bumblebees. Many species are to be found in sunny places but some are frequent in and on the edges of woods.

Fig. 3.12 ‘Long-headed flies’ (Dolichopus sp., Diptera: Dolichopodidae) on flower of creeping cinquefoil (Potentilla reptans).
The lower part of the head of Eristalis is produced into a conical snout (rostrum or fulcrum); this is partly membranous and partly hardened for support. Two of the supporting structures embedded in the front of the rostrum seem to be the basal parts of the maxillae. At the apex of the rostrum are the small laciniae and the larger palps of the maxillae (Diagram A). The rostrum is continued downwards by the labium (A, B), which is again partly membranous and partly hardened, with a contractile basal part and a main channelled part supported underneath by a sclerite (C). Emerging from the rostrum, and normally lying in the labial channel, are the usual labrum and hypopharynx, the latter housing the salivary duct (A, B) (Becher, 1882). The labella are well developed and have abundant pseudotracheae (B), similar to those of the blowfly Calliphora (Dimmock, 1881). (Diagrams are based on Müller, 1883; Peterson, 1916; and Gouin, 1949.)
Food suspended or dissolved in the saliva is sucked back into the mouth, often having first been taken up by the pseudotracheae. Gilbert (1981) noted that bristles are present on the labella, their length and number varying with the species. The food is drawn first into a canal formed by the labrum and hypopharynx when muscles in the labral part contract and widen the tube. When these muscles relax, this tube closes by its own elasticity. As this happens, muscles in the rostrum contract and dilate the next part of the food canal, so that the liquid is drawn into this. At the inner end of this region is the true mouth, hitherto kept shut by muscles. Relaxation of the rostral muscles closes the rostral passage while (contrarily) relaxation of the mouth muscles opens the mouth and so the food continues its journey (Schiemenz, 1957).
During feeding on nectar, the labella can either be spread out if the liquid surface is wide enough or pressed together in narrowly tubular flowers. Müller (1883) reported that in this case the labium is retracted sufficiently for the labrum and hypopharynx to protrude and suck the nectar directly. Gilbert (1981) noted that the nectar-specialists are all large species, possibly because these have heavier energy requirements than the small ones. The density of the pseudotracheae is correlated with the percentage of pollen taken in the diet, but the role of the pseudotracheae in pollen-feeding is not fully understood. In long-tongued hoverflies both the rostrum and the labium are lengthened, in contrast to Bombylius where only the labium is elongated and the proboscis cannot be folded.
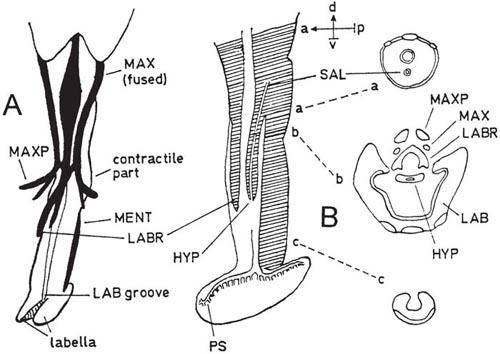
One of the first people to study the proboscis of hoverflies was Müller (1883) and one of the most recent was Schiemenz (1957). Both worked on species of Eristalis, and details are given in Box 3.4.
Hoverfly feeding behaviour has been the subject of much confusion and contradiction in the literature (Gilbert, 1981). Gilbert himself studied eight common hoverfly species at two dissimilar sites in Cambridge. He found considerable differences between species in the relative numbers seen feeding on pollen and nectar. Three were principally pollen feeders (Syrphus ribesii, Episyrphus balteatus and Melanostoma scalare), two fed more on nectar than pollen (Eristalis arbustorum and E. tenax) and three divided their time about equally between the two types of food (Metasyrphus corollae, Platycheirus albimanus and Syritta pipiens). These differences in habits are reflected in the form of the proboscis. The pollen feeders (those mentioned above, together with Meliscaeva auricollis) have a short thick proboscis with broad labella, the nectar feeders (which include also Sphaerophoria scripta and Platycheirus peltatus) have a long proboscis with relatively quite small labella with only short bristles, while the mixed feeders are intermediate or like the nectar feeders. Gilbert also found that, with one exception, male hoverflies took pollen less frequently than females of the same species, though newly emerged males may need pollen to provide the nutrients required for the production of sperm. Males of the mixed feeders spend a lot of time hovering and their feeding at sources of sugar presumably meets their energy needs for this activity.

Fig. 3.13 Drone fly (Eristalis cf. arbustorum, Diptera: Syrphidae) on hogweed (Heracleum sphondylium).

Fig. 3.14 Long-tongued hoverfly (Rhingia campestris, Diptera: Syrphidae), on herb robert (Geranium robertianum).
Pollen is usually taken directly from the anthers of the flower by rubbing them between the labella. Gilbert saw rapid movements in which the labial gutter seems to move up and down relative to the labral sucking tube; these are perhaps actuated by the contractile zone at the base of the labium (see Box 3.4). However, species of Melanostoma feeding on wind-pollinated plants with pivoted anthers grasp these with their forelegs, insert their labella into the anther cells and systematically clean them out (Holloway, 1976, in New Zealand; Stelleman & Meeuse, 1976, in the Netherlands). During feeding, hoverflies frequently pause to clean the face and proboscis and they consume the pollen thus collected. The forelegs clean the head, the middle legs and one another; the hind tibiae clean the abdomen. Intermittently during grooming, Eristalis tenax (Fig. 6.50) holds either the forelegs or the hind legs out beyond the body and scrapes the tip of the tibia and the tarsus of one leg against those of the other. This causes transfer of pollen to spirally grooved, pollen-retaining hairs on the tarsus.
Table 3.6 Flowers visited by long-tongued hoverflies (Syrphidae).
| A. Narrow-tubed flowers visited by Volucella (Kugler, 1955a) | ||
| Melilotus | melilot | Fabaceae-Faboideae |
| Trifolium spp. | clover | Fabaceae-Faboideae |
| Stachys | woundwort | Lamiaceae |
| Armeria | thrift | Plumbaginaceae |
| Knautia | scabious | Dipsacaceae |
| Centaurea | knapweed | Asteraceae |
| Cirsium | thistle | Asteraceae |
| B. Narrow-tubed flowers visited in Britain by Rhingia campestris | ||
| Viola spp. | violets | Violaceae |
| Silene dioica | red campion | Caryophyllaceae |
| Geranium robertianum | herb robert | Geraniaceae |
| Primula vulgaris | primrose | Primulaceae |
| Glechoma hederacea | ground ivy | Lamiaceae |
| Ajuga reptans | bugle | Lamiaceae |
| Hyacinthoides non-scripta | bluebell | Liliaceae |
Table 3.7 Flowers visited by some Conopidae as well as by Bombylius, Rhingia and bees.
| Species | Common name | Flower characteristics |
|---|---|---|
| Succisa pratensis | devil’s-bit scabious | flowers small, tubular |
| Scabiosa columbaria | small scabious | flowers small, tubular |
| Trifolium pratense | red clover | flowers large, tubular |
| Echium vulgare | viper’s bugloss | flowers large, tubular |
Sometimes the fly hovers and scrapes all its legs against one another as they dangle below the body; this brings pollen from the hind legs to the forelegs, whence it can be eaten (Holloway, 1976). E. tenax was found by Holloway to obtain all or nearly all the pollen it consumed indirectly by grooming in this way after visits to nectar-producing flowers. Its dense covering of hairs, including branched, plumose and curly-tipped types, is apparently related to this habit. This fly thus presents an interesting parallel with the honeybee. The grooming movements are the same as those used purely for cleaning in Melanostoma fasciatum.
Various views have been put forward as to the treatment of pollen gathered by hoverflies but, as undamaged grains have been found in the gut by Gilbert and others, it seems that they are not physically crushed or broken. Nutrients are probably extracted by enzymatic penetration of the pollen grains.
The proboscis length of six of the eight hoverflies in Gilbert’s (1981) study fell between 2 mm and 3.5 mm, while that of the other two, Eristalis arbustorum (Fig. 3.13) and E. tenax, was 5.36 mm and 7.85 mm respectively. Volucella (Fig. 2.13) has a similarly long proboscis, while that of Rhingia campestris (Fig. 3.14) measures 12 mm (Gilbert, 1981). All the Syrphidae can fold up the proboscis into a cavity under the beaked head; the size of this beak corresponds with the size of the proboscis, and it is therefore a conspicuous feature of flies such as Volucella and Rhingia (Plate 3a).
Gilbert found a very strong correlation between the proboscis-lengths that he measured and the depth of the flowers that the insects visited for nectar; the flower/insect relations at the upper limits of tube-length are as shown in Table 3.6. However, there are also records of visits by Rhingia to many small tubular flowers (Table 3.7) and some with exposed or only slightly concealed nectar. The flowers in these last groups belong to the families that appear to be most favoured by hoverflies in general: Ranunculaceae, Brassicaceae, Caryophyllaceae, Rosaceae, Apiaceae and Asteraceae. Gilbert found that the mixed feeders Episyrphus balteatus (Fig. 6.11b) and Syrphus ribesii relied for their pollen requirements particularly on the nectar-producing flowers of Apiaceae and Asteraceae.
Two English naturalists, who recorded visits by 123 species of flies, nearly all hoverflies, to flowers of 35 species of plants (Drabble & Drabble, 1917 and 1927), concluded that Asteraceae were especially favoured by hoverflies. However, they found inexplicable differences in attractiveness; thus autumn hawkbit (Leontodon autumnalis) was very attractive, and dandelion (Taraxacum officinale) and a hawkweed (Hieracium boreale) were well visited, but the similar flowers of nipplewort (Lapsana communis), common catsear (Hypochaeris radicata) and smooth hawksbeard (Crepis capillaris) were visited by only one or two species each. Only nipplewort, however, showed this lack of visits in the observations of Willis & Burkill (1895–1908).
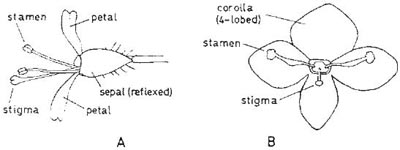
Fig. 3.15 A, flower of enchanter’s nightshade (Circaea lutetiana) from the side. B, flower of germander speedwell (Veronica chamaedrys) from the front. After Kugler, 1938.
Fig. 3.16 a–b, hoverflies (Diptera: Syrphidae) on flowers of Veronica chamaedrys. a, Baccha elongata. b, Melanostoma sp.
In some plants, there appears to be a degree of specialisation to hoverflies; examples are germander speedwell (Veronica chamaedrys) and enchanter’s nightshade (Circaea lutetiana) (Kugler, 1938). These are members of different families but have a similar arrangement of stamens and stigma (Fig. 3.15). In the speedwell, the corolla is bright blue and the two stamens are weak near the base. When an insect clings to one or both stamens they droop so that the underside of the insect’s body comes into contact with the stigma. The anthers also touch the underside of the insect so that repeated visits to different flowers of this species are likely to lead to cross-pollination (Fig. 3.16). Although hoverflies frequently alight on the stamens, they sometimes cling to the surface of the corolla, in which case they may avoid coming into contact with anthers and stigma. Small bees, however, which frequently visit the flowers, almost always alight on the stamens and are therefore more reliable pollinators than the hoverflies. The stamens of the white-petalled enchanter’s nightshade droop in the same way when an insect alights upon them, and pollination also takes place in the same way. In a natural woodland habitat Kugler found that Diptera were the only visitors to this flower, but in parkland where there was open ground near at hand small bees also visited it. The hoverflies best suited to these two flowers are small species which inhabit shady situations (those shown in Fig. 3.16 and Neoscia [Fig. 2.9] and Syritta species).
In England, the Drabbles found that germander speedwell and brooklime (Veronica beccabunga) received few visits from hoverflies but that these insects did operate the pollination mechanism.
The common British bindweeds (family Convolvulaceae) (Fig. 6.11) have been repeatedly noted as favourites of hoverflies, and especially of Rhingia, which has been recorded feeding on the pollen (Bennett, 1883; Drabble & Drabble, 1927; Verdcourt, 1948; Parmenter, 1948; Baker, 1957). A plant that is visited almost exclusively by the smaller hoverflies is small balsam (Impatiens parviflora), which has pale yellowish flowers and provides the insects with both nectar and pollen (Coombe, 1956).
Fig. 3.17 Hoverfly, Sphaerophoria ruepellii male (Diptera: Syrphidae), on flower of tormentil (Potentilla erecta).
The many reports of hoverflies feeding on the pollen of wind-pollinated plants have been listed by Stelleman & Meeuse (1976). This is an important habit of certain genera, the principal ones being Melanostoma and Platycheirus (Gilbert, 1981; Holloway, 1976; Stelleman & Meeuse, 1976). The grasses (Poaceae) are particularly important pollen sources for such hoverflies. For example, Drabble & Drabble (1927) found that Melanostoma mellinum ate the pollen of timothy grass (Phleum pratense) and cocksfoot (Dactylis glomerata) and apparently caused pollination. Melanostoma has also been seen feeding on the pollen of a sedge (Carex binervis) (MCFP). Hoverflies can effectively pollinate the apparently wind-pollinated ribwort plantain (Plantago lanceolata) (Stelleman & Meeuse, 1976). Another instance of pollen-feeding by a hoverfly involves the use of thoracic vibration to obtain pollen from flowers of Solanum (‘vibratory pollen-collection’, see here). The fly concerned, Volucella mexicana, is a mimic of a bee of the genus Xylocopa that exploits the flower in the same way (Buchmann, 1983).
Syrphidae that visit zygomorphic flowers such as those of Scrophulariaceae and Lamiaceae behave, apparently instinctively, in a manner suitable to the form of the flower, unlike most members of the fly families Muscidae and Calliphoridae, which tend to walk all over the flowers at random.
Flower-constancy is another attribute that is highly developed in Syrphidae, as demonstrated by Kugler (1950) in a habitat with abundant hoary alison (Berteroa incana, Brassicaceae) and scentless mayweed (Tripleurospermum inodorum, Asteraceae, named for its scentless leaves). One individual of Eristalis tenax visited 47 mayweed heads and two unopened yellow composite heads; it made nine approaches to other flowers, but none to Berteroa. Another E. tenax showed a similar constancy to Berteroa and did not approach Tripleurospermum.
Differential visitation by hoverflies was reported by Parmenter (1958). Yarrow (Achillea millefoliium, flowers white), common catsear (Hypochaeris radicata, yellow), and knapweed (Centaurea nigra, pinkish purple) grew in neighbouring patches of approximately equal size, and Eristalis arbustorum made 132 visits to yarrow, 34 to catsear and one to knapweed, whereas Helophilus parallelus made three visits to yarrow, none to catsear and 24 to knapweed.
The reaction of Eristalis tenax to colour was tested with artificial flowers containing sugar-water (Ilse, 1949). Yellow ‘flowers’ were presented among others with a range of shades of grey; although there were five grey flowers to each yellow one, many more visits were made to the yellow ones. This and other experiments showed that Eristalis had a general preference for yellow, in contrast to Bombylius, but that periods of training on several colours modified the choice (Kugler, 1950). Blue always had a low stimulatory effect and deep red was not distinguished from grey. Tests with models of a fixed area but varied length of outline showed that the flies could distinguish the models but had no inborn preference (Kugler, 1950).
The response of Eristalis tenax to scent was tested by adding artificial carnation scent to various flowers that the flies were constantly visiting in a natural habitat (Kugler, 1950). Only 2% of approaches to these resulted in normal visits, while 46% resulted in short visits (a normal approach followed by alighting and flying away) and 52% resulted in no visit. When yellow model flowers were used, treated either with sugar-water and flower scent or salt solution, they were equally attractive. However, with blue flowers with sugar-water or salt and scent, 83% of visits were to flowers with sugar; here, the increased (negative) effect of scent was probably caused by the low stimulatory value of the colour blue. It is interesting that scent can inhibit visits.
The Acalyptratae (see here) comprise about 280 genera in numerous families, one of which, Conopidae, will be dealt with on its own and the rest as a group. The Conopidae is a small family, some of whose members have a long proboscis (see Box 3.5). These Conopidae sometimes visit tubular flowers of the type favoured by other long-tongued insects (Table 3.7) but most of them show a partiality for flowers with exposed or easily reached nectar such as Asteraceae, Apiaceae and Rosaceae. The rare Leopoldius signatus is recorded in Britain only at ivy which has exposed nectar (Harper & Wood, 1957; Smith, 1959, 1961).
Of the remaining 39 families of the Acalyptratae, species belonging to 23 were recorded at flowers (Knuth, 1906–1909; Willis & Burkill, 1895–1908). These are mainly rather small flies with a short proboscis of the mopping up type (see Box 3.5), and many have the habit of waving their wings, often alternately, as they walk about. In some, the wings are banded or marbled (giving rise to the name ‘picture-wing flies’) (Fig. 3.18). Members of one of these families, the Trypetidae, lay eggs in the capitula of Asteraceae, so that although they may transfer pollen, the capitula are later damaged by the larvae. Three genera of Trypetidae are recorded as specialised flower visitors (Knuth, 1906–1909). Six families, including the flower-visiting Sepsidae, have the wing-waving habit but clear wings. Species of Sepsis, small dark brown flies with the first abdominal segment narrow, and resembling ants, are frequently seen on the umbels of Apiaceae, and this and some other genera have been recorded from a number of flowers. They have the mopping-up type of mouth-parts.
Sicus (in Conopidae) has a slender proboscis about 6 mm long, including the rostrum; the labium, 2.5 mm long, is directed forwards, while the terminal part, 3 mm long, made up of the greatly elongated labella fused at the base, can be directed downwards or, when out of use, folded right back underneath the labium. In Conops quadrifasciatus the proboscis is 4 mm long, with shorter labella (Diagrams A, B). Conops and Sicus feed on nectar, but are said not to eat pollen; the nectar flows between the labella, into the groove on the labium and then up a tube formed by the labrum and hypopharynx. Diagram C shows the mouth-parts of Sepsis (Sepsidae), which are more like those of Eristalis.
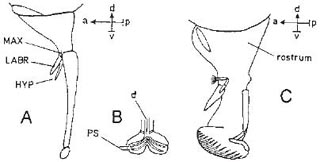
Fig. 3.18 ‘Picture-winged flies’ (Herina frondescentiae, Diptera: Otididae), on shrubby cinquefoil (Potentilla fruticosa).
The Lauxaniidae have been recorded at several rather diverse flowers by Willis & Burkill (1895–1908), while Chloropidae visit Apiaceae, Asteraceae and forget-me-not (Myosotis). Chloropid flower-visitors include the frit fly (Oscinella frit), the larvae of which are a pest of cereals, and Chlorops, a small yellowish fly with longitudinal black and yellow stripes on the thorax. Chloropidae (together with Chalcid wasps) are pollinators of the tiny flowers of the musk orchid (Herminium monorchis) which they visit for nectar (see here; Nilsson, 1979b). The flower-visiting genus Siphonella has a long proboscis similar to that of the hoverfly Eristalis. Members of several acalypterate families are attracted to the trap-flowers lords-and-ladies (Arum maculatum), large cuckoo pint (A. italicum subsp. neglectum) and Aristolochia (see Chapter 10). This seems to be especially the case with Drosophilidae, which breed in decaying fruit, carrion and excrement (among other things), and with Sphaeroceridae, which are often dung-frequenting (Knuth, 1906–1909; Grensted, 1947; Prime, 1954).
There are about 280 genera in the Calyptratae; the common flower-visiting members are grouped into seven families. These flies are generally larger than the Acalyptratae and are more important as flower visitors and pollinators, although they are nearly all unspecialised in feeding habits. In many genera the proboscis has ‘prestomal teeth’ between the labella which can be used for scraping. In all four families, feeding on both nectar and pollen is recorded.
Tachinidae (=Larvaevoridae) is a family of rather bristly flies, often greyish, whose larvae are internal parasites of insects and other arthropods. Much the commonest member of this family in Willis & Burkill’s (1895–1908) lists is Siphona geniculata which is only about 6 mm long, but has a slender proboscis twice as long as the head (Box 3.6). It visits plants with small tubular flowers and avoids certain flowers with exposed nectar favoured by less specialised Diptera. Prosena (Calirhoe) siberita, a fly about 10 mm long, is also long-tongued and visits various narrowly tubular flowers (Box 3.6). Eriothrix rufomaculatus, a small black and red fly, and Tachina (=Larvaevora=Echinomya) fera, a large coarsely bristly black and orange fly (Fig. 3.19), are also recorded on Asteraceae, Apiaceae, Mentha etc. T. fera has a proboscis 5–6 mm long and is found at tubular flowers more often than the shorter-tongued members of the family. Pollinating visits to the burnt orchid (Orchis ustulata) have been recorded for Tachina magnicornis (Vöth, 1984) and to Veratrum album (Liliaceae) for unspecified Tachinidae (Daumann, 1967). (Records of flower-visits by other members of the family are given by Colyer & Hammond, 1951; Andrews, 1953; Parmenter, 1941, 1952a & b; Grensted, 1946; Harper & Wood, 1957; and Willis & Burkill, 1895–1908.)
Eight of the 27 genera of Sarcophagidae and Calliphoridae were recorded at flowers by Willis & Burkill (1895–1908). Pollenia, Lucilia (green-bottles), Melinda (Onesia) and Sarcophaga (e.g. S. carnaria, the common flesh fly, a familiar large black and grey fly with red eyes) are predominantly flower-feeders, while Calliphora (blue-bottle or blowfly) (Fig. 3.20) and Cynomya feed chiefly on carrion and excrement but also visit flowers; Pollenia vespillo feeds at flowers and sap only (Kugler, 1955b). The Calliphoridae have a short proboscis (2–4 mm) and they visit flowers with well exposed nectar such as Apiaceae and yellow mountain saxifrage (Saxifraga aizoides) or small tubular flowers such as Asteraceae and alpine bistort (Persicaria vivipara) and water mint (Mentha aquatica). They also feed on the pollen of certain large tubular flowers, the nectar of which is inaccessible to them. Kugler (1956) found that Lucilia was sometimes constant in its visits to grass of Parnassus (Parnassia palustris) and other species in nature.

Fig. 3.19 Tachina fera (Diptera: Tachinidae) on ragwort (Senecio jacobaea).

Fig. 3.20 Blow-fly (Calliphora vomitoria, Diptera: Calliphoridae) feeding at ivy (Hedera helix).
Flies specialised for flower-visiting may have long and very slender tongues but attain this condition in different ways, as in Siphona (Diagrams A and B) and Prosena (C).
The proboscis of one calyptrate, the blowfly, Calliphora, is known in detail from the work of Graham-Smith (1930). The basic structure is the same as in the hoverflies and Sepsis (Box 3.4 and Box 3.5), but the maxillae are further reduced and only the palps remain (D, E). The way the feeding tube is formed is shown in sections a–d of Diagram F, corresponding to levels with the same letters in Diagram D. The edges of the labrum are grooved to receive the edges of the hypopharynx (Fb), but the tube ends just short of the end of the labium; here the labial groove is roofed over by pairs of interlocking folds arising from its edges so that the tube is continued forwards (Fc). The pseudotracheae are completely closed over when the labella are used for filtering, and the liquid passes into each pseudotrachea through short sloping passages at right angles to its length (J, K). The diameter of the pseudotracheae in Calliphora vicina ranges from .01 to .02 mm and the sloping lateral passages are .004 to .006 mm in diameter; supporting sclerites for these are seen in J (right) and K. Between the labella there are four rows of prestomal teeth (Fd, G, H).
Several different feeding positions of the labella are described by Graham-Smith. He fed films of drying milk and drying solutions of sugar containing Indian ink to the flies, and it was from the impressions left by their proboscides in these and other foods that he made his discoveries about these feeding positions. In the filtering position, the labella are inflated and spread horizontally on the food surface; they are also pressed together so that the labial tube has no connection with the food other than through the pseudotracheae (G). Other feeding positions allow the prestomal teeth to reach the food and are presumably not used for feeding at flowers. Filtering is entirely abandoned when the direct feeding position is adopted (H), as it is for viscid materials. The movements of the labella are produced by muscles acting on the furca (G, H) but the labella are inflated by blood pressure. When not in use, the proboscis is folded up and retracted into a recess in the head (E). The pseudotracheae and the tubes leading to the mouth can all be opened along one side; this may be of importance in allowing cleaning if the passages become blocked.
In the Scathophagidae only one genus (out of 22) appears to have been recorded at flowers in Britain; this is Scathophaga (=Scopeuma), which as larvae live in dung and as adults prey on insects, piercing them with their prestomal teeth. S. stercorarium (Fig. 3.21) is the common yellow dung fly, the males of which are golden-yellow furry insects. Scathophaga visits flowers to seek prey and to feed on pollen and nectar. Colyer & Hammond (1951) record it at blackthorn (Prunus spinosa), hawthorn and bramble, while Knuth (1906–1909) gave a long list, including many Ranunculaceae, Brassicaceae, Apiaceae and Asteraceae.
The very large families Anthomyiidae and Muscidae, and the family Fanniidae, together comprising about 80 genera and about 450 species in Britain, are the most important flower-visiting families among the Diptera apart from the hoverflies. Willis & Burkill’s (1895–1908) records cover about 28 genera. Examples are: Musca autumnalis (Fig. 3.22), a close relative of the house fly (M. domestica) with orange-yellow markings on the abdomen; Mesembrina meridiana, a large black fly; Dasyphora cyanella, Orthellia viridis and O. cornicina, green-bottles similar to Lucilia of the Calliphoridae; Polietes lardaria and Graphomya maculata (Fig. 3.23), two flies resembling the common flesh fly in their colouring, though this applies only to the female in Graphomya; and Fannia canicularis, the lesser house fly.
The anthomyiid, muscid and fannid flies do not appear to be specialised flower-visitors, but many of them are occasional or regular ones. Willis & Burkill (1895–1908) found that they visited species of thistle beside Cirsium arvense, which has the most accessible nectar, and recorded them feeding on pollen at flowers with inaccessible nectar. Totland (1993) found Muscidae and Anthomyiidae the commonest flower visitors at his site in the Norwegian mountains. The Drabbles recorded one species feeding on the pollen of grasses (cf. Syrphidae, see here).
These families of the Calyptratae comprise the great majority of the short-tongued Diptera that visit flowers. These flies are an important part of the insect fauna generally and they provide a greater proportion of the flower-visitors in north Europe (as in Britain and Norway) than in Germany, where bees and beetles are more abundant. At Scarborough (coast of north-east England), in summer, 61% of 1,800 individual visits to six species of plant were by short-tongued Diptera, as were 48% of visits to 27 species in spring (Willis & Burkill, 1895–1908). Willis & Burkill played down their importance as pollinators, in spite of their abundance, whereas Drabble & Drabble (1927) were convinced that they are effective pollinators, at least for Asteraceae. The less specialised flowers that are most visited by short-tongued Diptera are also those most visited by insects in general.

Fig. 3.22 Musca autumnalis (Diptera: Muscidae), a close relative of the housefly, on flower of coltsfoot (Tussilago farfara).

Fig. 3.23 Graphomya maculata (Diptera: Muscidae) on hogweed (Heracleum sphondylium).
Many of the flowers visited by short-tongued Diptera are sweet-scented, for example, meadowsweet (Filipendula ulmaria) and rowan (Sorbus aucuparia) with heavy scents, crab apple (Malus sylvestris) with a lighter scent, and sallows (Salix spp.), lady’s bedstraw (Galium verum), thrift (Armeria maritima) and white mignonette (Reseda alba), also with a light scent differing from that of crab apple but all much like each other (PFY). Some have a tang of stale dung or urine with their sweet scent, as in many umbellifers such as cow parsley (Anthriscus sylvestris), alexanders (Smyrnium olusatrum); the crucifers treacle mustard (Erysimum cheiranthoides), sweet alison (Lobularia maritima) and swede or rape (Brassica napus); some composites such as goldilocks (Aster linosyris); and, to some people, hawthorn (Crataegus monogyna). These scents, even to human noses, therefore show a correlation with the prevailing habits of these unspecialised flower visitors.
The responses of Calyptratae to the sensory stimuli offered by flowers are broadly similar to those of Syrphidae. Licbermann (1925) covered an isolated plant of cow-bane (Cicuta virosa, family Apiaceae) with large leaves and showed that the flies could find the flowers by scent alone. Flies of the genera Lucilia, Dexia, Pyrellia, Sarcophaga and others, when passing within 2 m downwind, turned towards it and flew to and fro, then alighted and crawled under the leaves to the flowers; on the windward side, none was deflected towards it. In another experiment, a plant of Cicuta was placed in a room behind a curtained window, which was then opened 5 cm. On the wall of the house near the window many flies that were resting in the sun became restless, and after some flying to and fro before the opening, flew through it and on to the flowers. Flies were also attracted from a distance, first settling on the wall and then entering the room. This shows that insects which are not actively seeking food may respond to stimuli associated with it.
Knoll (1926) found that flies that were attracted to Arum nigrum were attracted by the scent, as were the beetles; they were species normally associated with human excrement.
Kugler (1956) carried out experiments on calypterate flies hatched in captivity (naive flies). Lucilia, Calliphora and Sarcophaga more or less ignored flower-models (coloured discs) until scent was brought near them, whereupon visits and proboscis-reactions took place. When scent was placed on some of the models these were preferred. Both fragrant and excremental scents were used, but Calliphora and Sarcophaga preferred yellow models over brown-purple in the presence of sweet scents, and brown-purple over yellow and white in the presence of excremental scents.
Other experiments did not use naive insects. Captive Lucilia, presented with artificial flowers of the type used for Eristalis (Syrphidae, see here), made 62% of their visits to hawthorn-scented models and 38% to scentless models. If the smell of ammonia, associated with egg-laying, was substituted, the flies visited both models equally, showing no instinctive association of ammonia smell with food. Rewardless models smelling of ammonia were avoided, showing that the flies perceived the scent and learnt to associate it with the absence of food.
In experiments involving real flowers of scentless mayweed (Tripleurospermum inodorum) and artificial models, Kugler (1951) found that Lucilia responded in a similar way to Eristalis (Syrphidae, see here): visual stimuli were used to find the flowers from a distance, but olfactory cues triggered alighting. The reactions of the flies to shape, and to yellow, blue and grey colours were also the same as those of Eristalis (Kugler, 1955b, 1956), and they preferred the form of a funnel to a disc shape.
Calliphoridae and Syrphidae are visitors to the curious flowers of grass of Parnassus (Parnassia palustris). These have five green nectaries that produce scent, and five clusters of glistening knobs on stalks. Daumann (1932, 1935) found that the approach of the hoverflies to the flowers was visual but that alighting was induced by scent; the flies also located the individual nectaries by scent. Removal of the glistening knobs made no difference to the flies’ behaviour, and the knobs therefore appeared to be functionless. However, Kugler (1956), using naive Lucilia and hoverflies, showed that they were initially much more attracted to the knobs (see Fig. 3.24), but in successive tests were increasingly likely to touch the nectaries first. Experiments showed that the flies were attracted to small glistening objects placed on an artificial flower. Thus the knobs may, indeed, be ‘false nectaries’, as earlier supposed.
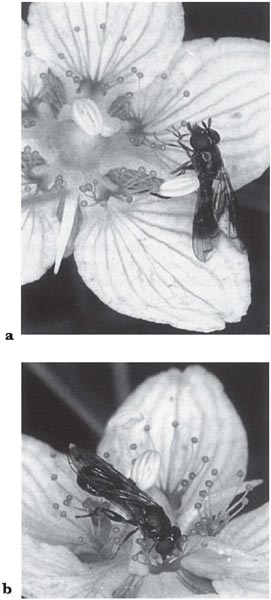
Fig. 3.24 a, b Hoverfly (Neoascia podagrica) on grass of Parnassus (Parnassia palustris); a, probing one of the false nectaries; b, sucking nectar from the disc of the receptacle.
With naive or resting flies, scent is mainly excitatory, inducing the insects to seek and alight on objects of appropriate colours, either yellow and white, as the colours of sweetly scented flowers most commonly suited to them, or brown and dark purple, as the colours of excrement and carrion, sometimes imitated by flowers (see Chapter 10). Sometimes scent may guide insects all the way to its source. However, with foraging flies the stimulus that elicits approach from a distance does not usually enable the insect to tell whether the flower is suitable for it. When it is close to the flower, different sensory perceptions come into play which determine whether a visit takes place. The reaction to a flower may be divided into three phases: response to a distant signal leading to approach; response to a short-range signal, leading either to alighting or flying away; and response to internal flower-signals, including the presence of food, leading to uptake of food. Sometimes alighting takes place before the response to the short-range signal. The first two stages, with a visual distant signal and an olfactory short-range signal, have been repeatedly noted in this chapter.
Diptera can distinguish colours when feeding, and in general tend to visit white, pink, yellow and green flowers most readily. Red-blindness is proved to exist in the blowfly (Calliphora) and the drone-fly (Eristalis tenax), and it probably occurs in the beefly as well. Visits to purple and blue flowers are commonly made only by the longer-tongued genera of the Brachycera and Cyclorrhapha, and all of these also visit flowers of other colours quite readily. The blue and purple flowers usually have more deeply seated nectar than flowers of other colours. These colour differences therefore help the insects to find the flowers most suited to them. The preferences of the insects may be inherited in some flies, for example the aversion of Bombylius fuliginosus to yellow, but learned in others (see remarks on trainability in the corresponding section of Chapter 4). Innate preferences for certain colours might be expected to have evolved once the association between particular colours and deep-seated or exposed nectar had become established.
Diptera with long tongues are scattered through the Brachycera and Cyclorrhapha; these flies can reach nectar not available to shorter-tongued insects and they show some diminution of attention to flowers with well exposed nectar. This is particularly true of Bombylius, Rhingia and Siphona and is a sign of their specialisation which has evolved independently.