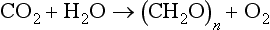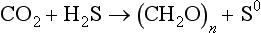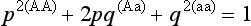Evolution and the Biosphere
Although physical and chemical factors were exclusively responsible for affecting the distributions of elements 4.5 billion years ago when Earth formed (see Chapter 2), this is no longer true. Ever since life originated on Earth more than 3.5 Gyr (Gyr=109 years) ago, biological processes have become increasingly important in determining the distribution of elements and the compounds into which they are incorporated. To understand the basic features of the Earth system and the functioning of biogeochemical cycles, it is necessary to know something about the chemistry of living organisms, how organisms derive energy, and how they influence and are influenced by the physico-chemical states of the environments in which they live. In this chapter we discuss how life originated in a non-living world, the biochemical machinery of living organisms, the mechanisms that have governed the evolution of life during the past 3.5 Gyr, the ecological organization of the living world, and some of the major impacts organisms have had on the development and continued functioning of Earth’s processes. In addition, we present an overview of some of the important roles organisms play in the functioning of biogeochemical cycles.
3.1 The Origin of Life on Earth
People have sought to explain the origin of organisms since at least the beginning of historical times. At the time of the Renaissance most Europeans believed that living organisms developed from non-living materials, i.e. that maggots could be “generated” by allowing meat to decay. The careful experiments of the Italian naturalist Francesco Redi (1626–1698) on decaying meat temporarily set to rest this belief. But the theory of spontaneous generation of life gained many new adherents with the discovery of microbes by Anton van Leeuwenhoek (1632–1723), and by experiments showing that mixtures, such as boiled aqueous extracts of meat, developed microorganisms even after prolonged heating in sealed vessels. Scientists reasonably, but incorrectly, inferred that the microorganisms were developing spontaneously from these non-living materials rather than, as was actually the case, from heat-resistant bacterial spores that survived the boiling temperatures.
Scientists do not believe that life is arising from non-life on Earth today, but, if life originated on Earth, as it apparently did, it must have developed from non-living materials. Current scientific views of when and how life might have originated and evolved are based upon imaginative chemical experiments in the laboratory, combined with studies of the fossil record and ways of dating events in the remote past.
Early students of the origin of life were misled because they believed that Earth was very young, in part because no methods were available for dating ancient events. Today, suitable methods exist for determining the age of materials that are billions of years old, and the fossil record of ancient organisms has vastly improved. The evolution of living organisms was in part possible because of important changes in the physical environment, but living organisms in turn caused profound changes in the physical nature of the Earth and its atmosphere. Ever since the evolution of life, living organisms have been major participants in biogeochemical cycles. This biological activity, which continues today, is being increasingly influenced by human activities.
3.1.1 Synthesis of Organic Molecules on the Primeval Earth
We are uncertain just how life originated, but a reasonable first step in the development of organisms was the non-biological synthesis of compounds of carbon upon which all living systems are based. There is considerable evidence indicating that these compounds can be formed abiologically. For example, one group of meteorites, the carbonaceous chondrites, contains organic matter, which was presumably formed in intergalactic space. Some of these meteorites contain a variety of organic constituents, including sugars, organic acids, and amino acids. The fact that the amino acids in meteorites contain equal portions of the D and L stereo-isomers (see Section 3.2) provides evidence that these organic materials were produced abiotically, or at least were not contaminants derived from Earth, where organisms produce primarily L-amino acids.
Stanley Miller (1953) conducted pioneering experiments that had a profound influence on subsequent thinking. He set out to determine if organic chemicals could be formed from water and the various gases that were presumed to have existed in the atmosphere 4 Gyr ago. He used an atmosphere containing methane, ammonia, and hydrogen as its principal constituents of biological interest. This atmosphere differed from the current one by lacking oxygen, carbon dioxide, and molecular nitrogen. The experiments were conducted in a glass-enclosed system (Fig. 3-1). Water, representing the sea, was contained in one flask which was connected to a condenser; the other part, representing the atmosphere, contained the gases. Electrodes inserted into the atmosphere were used to produce sparks to simulate lightning. In a relatively short time of operation, amino acids and a variety of other organic substances were produced under these anaerobic conditions. The energy source for his experiments, electric discharges, is destructive of life as we know it, and is not used today by any biological systems. Other energy sources probably powered the evolution of life.

Fig. 3-1 Schematic of the apparatus Stanley Miller used to demonstrate formation of amino acids from simple inorganic compounds under conditions similar to those of the early Earth.
An alternative view of Earth’s early atmosphere was advanced by Rubey (1951, 1955). He proposed that the early atmosphere was strongly affected by volcanic outgassing, which released carbon dioxide, nitrogen, sulfur compounds such as hydrogen sulfide, methane, hydrogen, and water. In his view, the early atmosphere was very similar to that of present-day Earth except that it was anaerobic. Rubey’s view, which is supported by chemical analyses of the oceans and atmosphere, has gained wide acceptance among evolutionists and has stimulated research to determine whether organic compounds can be synthesized abiotically under such atmospheric conditions. The results from these experiments, which have been discussed by Chang et al. (1983), confirm that organic compounds can be synthesized by many combinations of these gases, again supporting the plausibility of prebiotic organic synthesis. Others are investigating the abiotic synthesis of organic compounds such as amino acids under high hydrostatic pressure and temperature (Amend and Shock, 1998). This approach is consistent with the view increasingly favored by some biologists, that life may have arisen in marine hydrothermal vents. Organic compounds can be produced under so many environmental conditions that the current question is – which of the many possible conditions were the ones actually found on the early Earth?
Although much is known about how the organic compounds might have been synthesized abiogenically, scientists still have a poor understanding of how those molecules were assembled to form the first living organisms. All organisms, even the simplest of them, are extremely complex structures that carry out a rich variety of chemical and physical processes. And they are able to precisely control exchanges of materials between themselves and the environment. Thus, it is not surprising that no one has produced living organisms or functioning prototypes of them in the laboratory.
3.2 The Machinery of Life
Even though we don’t know how life arose, we can describe in great detail its physical and chemical structure. But simple descriptions of the structure of living organisms do not enable us to define exactly what life is. One way to define life is to state what appear to be the simplest requirements for life as we know it. They include: (a) the presence of a semipermeable membrane to control the passage of materials into and out of the compartments (cells) of which all living organisms are composed; (b) chemical machinery for the synthesis and degradation of essential molecules; (c) genetic material that encodes the synthesis of the molecules required to catalyze those syntheses and degradations; (d) sufficient structure to prevent unwanted reactions from occurring; and (e) machinery to duplicate all the above capabilities in the formation of a new organism (i.e. reproduction).
We do not know the minimum number of molecules required to perform these activities. Viruses, on the border of life, are non-cellular, and are able to multiply only by utilizing the complex genetic and structural machinery of more complex cellular organisms, which they parasitize. The largest viruses contain enough genetic material to encode only about 100 different proteins. The smallest known organisms that are able to live independently are single-celled bacterial parasites called the Rickettsiae that contain enough genes to encode about 200–400 proteins. Whether smaller independently living organisms could have survived prior to the evolution of more complex forms of life is not known.
3.2.1 The Cellular Structure of Life
The unit of construction of all living organisms on Earth is the cell. Some organisms consist of a single cell; others contain many cells. Cells range in size from less than 1 µm (10−6 m) to more than 500 µm in diameter. All cells have the same basic structures: a bounding cell membrane, a nucleus or nuclear material, and cytoplasm in which most biochemical reactions take place.
3.2.2 The Chemical Basis of Life
To build their structures and to carry out the myriad biochemical reactions that take place within their cells, organisms need a source of energy. The needed energy is obtained via biochemical pathways driven either by sunlight or by energy contained in reduced chemical compounds.
Life is based on interactions among a set of large organic molecules, each of which is assembled from smaller molecules. In addition to carbon and hydrogen, most naturally occurring organic molecules contain one or more of four key elements, all of which are from the second and third period of the periodic table: N, O, P, and S. Carbon atoms form most of the skeleton of these molecules. Bonding among carbon atoms can lead to very large molecules with a variety of structures. Hydrogen may share one or more of the valence electrons of carbon and may also participate in hydrogen bonds. Hydrogen bonds also involve nitrogen and oxygen and are important in determining the structures of many molecules.
Most organo-phosphorus compounds are phosphates, R-PO4. The bond energy of the phosphate to the rest of the molecule is central to the flow of energy in all metabolic processes. Sulfur, found largely as sulfide, is central to providing three-dimensional rigidity by uniting parts of a molecule by the disulfide (—S—S—) bridge.
Amino acids, the building blocks of giant protein molecules have a carboxyl group and an amino group attached to the same carbon atom. A protein is a linear polymer of amino acids combined by peptide linkages. Twenty different amino acids are common in proteins. Their side chains, which have a variety of chemical properties, control the shapes and functions of proteins. Some of these side chains are hydrophobic, others are hydrophilic, and still others occur either on the surface or the interiors of proteins.
Carbohydrates form a diverse group of compounds that share an approximate formula (CH2O)n. The major categories of carbohydrates are monosaccharides (simple sugars); oligosaccharides (small numbers of simple sugars linked together); and polysaccharides (very large molecules), among which are starches, glycogen, cellulose, and other important compounds. Derivative carbohydrates, such as sugar phosphates and amino sugars, contain additional elements. Important amino sugars are chitin, the principal structural carbohydrate in insect skeletons and cell walls of fungi, and cartilage.
Lipids are insoluble in water and release large amounts of energy when they are metabolized. The lipids of Eubacteria and Eucarya are composed of two principal building blocks, fatty acids, and glycerol. Three fatty acids (carboxylic acids with long hydrocarbon tails) combine with one molecule of glycerol by ester linkages to form a triglyceride. More complex lipids are formed by the addition of other groups, the most important of which contain phosphorus. The lipids of Archaea are quite different in that they do not contain fatty acids.
Deoxyribonucleic acid (DNA) is the genetic material of all organisms, including plants, animals, and microorganisms. (Some viruses lack DNA, but use RNA (ribonucleic acid) in its place.) DNA, which carries all the hereditary information of the organism, is replicated and passed from parent to offspring. RNA is formed on a DNA template in the nucleus of a cell. The RNA carries the genetic information to the cytoplasm where it is used to produce proteins on the ribosomes. The specific proteins formed include enzymes which carry out the characteristic activities of the organism. Both RNA and DNA are formed from monomers, called nucleotides, each of which consists of a simple sugar, a phosphate group, and a nitrogen-containing base. The complex structure of DNA is founded on only four bases. A tremendous wealth of information is contained in the precise ordering of these bases to form the genetic code.
The major classes of macromolecules and their subunits are described more fully in the appendix that follows this chapter.
3.2.3 The Energetics of Living Organisms
To maintain themselves, to grow, and to reproduce, all organisms must obtain raw materials and energy from the environment. The raw materials – chemicals – are digested, and the products are used to build large carbon-based molecules. The energy obtained from chemical digestion is used to power the synthetic reactions. These conversions of matter and energy are called metabolism. Organisms can be viewed as devices for capturing, processing, and converting matter and energy from one form to another.
The energy obtained from chemical digestion is stored in cells in the short term in the form of ATP (adenosine triphosphate) (Fig. 3-2). ATP contains high-energy bonds in its triphosphate group. When it is hydrolyzed to form ADP (adenosine diphosphate) and H2O, 30 kJ/mol of energy is released. In cellular metabolism, this energy is used to carry out synthetic reactions for the production of DNA, proteins, carbohydrates, and all other cellular materials.
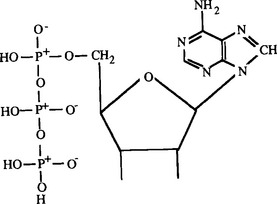
Fig. 3-2 Chemical diagram of ATP (adenosine triphosphate). The three functional groups are the base adenosine (upper right), a five-carbon ribose sugar (middle), and three molecules of phosphate (left). Lines at bottom of sugar ring indicate hydroxyl groups.
The energy stored in ATP is released during reactions catalyzed by proteins called enzymes. Enzymes typically have molecular weights of about 30000, but they vary greatly in size. Enzymes function by lowering the activation energy for reactions by complexing with the substrate for the reactions. In the absence of enzymes, many vital cellular reactions would not take place at all or would proceed much more slowly than they do.
Organisms obtain their energy either by oxidizing preformed organic molecules (heterotrophs = “other feeders”) or by using an external source of energy, such as sunlight or chemically reduced matter, to drive the synthesis of large, energy-rich molecules (autotrophs = “self-feeders”).
3.2.3.1 Energy generation by heterotrophic organisms
Heterotrophic organisms obtain their energy by oxidizing reduced organic compounds. In the absence of oxygen, organisms may oxidize organic compounds either by fermentation or by anaerobic respiration. In contrast, aerobic organisms can function only in oxygenated environments. Anaerobic processes probably evolved early in Earth’s history, before oxygen was readily available.
3.2.3.1.1 Fermentation.
Many bacteria live in anaerobic environments where they ferment carbohydrates to produce substances such as ethanol, propionic acid, butyric acid, formate, hydrogen gas, and carbon dioxide, depending on the species and its enzymes, as well as the substrate (Perry and Staley, 1997). One example of this is the fermentation carried out by lactic acid bacteria. In this process sugars are first oxidized anaerobically to form pyruvic acid. In this process ATP is produced during one of the steps (Fig. 3-3).
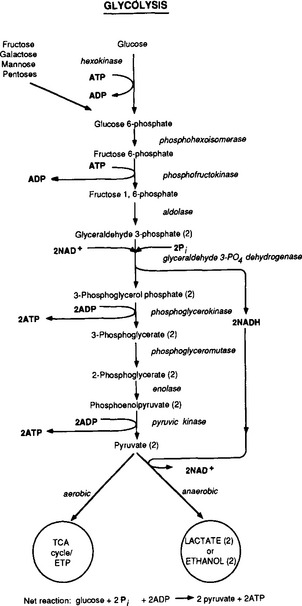
Fig. 3-3 The biochemical pathway of glycolysis, which obtains energy from the breakdown of 6-carbon sugars to a pair of 3-carbon pyruvate molecules. Enzymes at each reaction in the sequence are in italics. The energy generated is stored in the form of ATP (see Fig. 3-2).
3.2.3.1.2 Anaerobic respiration.
Some microbes carry out other chemical transformations in anaerobic freshwater and marine sediments and in waterlogged soils. Anaerobic bacteria use substances such as nitrate, iron and manganese oxides, sulfate, and carbon dioxide as the ultimate electron acceptors in the absence of oxygen. In typical marine sediments (Table 3-1) a gradient exists in which aerobic respiration occurs in the surficial sediments. The oxygen is depleted in the underlying sediments so aerobic respiration is replaced by anaerobic respiration. These include nitrate reduction (denitrification), iron and manganese reduction, sulfate reduction, and carbon dioxide reduction (methanogenesis) in progressively deeper sediment layers. Some microorganisms are able to move vertically in the gradient to take advantage of local availability of electron acceptors.
3.2.3.1.3 Aerobic respiration.
Many organisms carry out aerobic respiration in which enzymes remove electrons from organic compounds and pass them through a chain of carriers including flavoproteins and cytochromes located in intracellular membranes (Fig. 3-4) until finally they are used to reduce oxygen to produce water. ATP is produced by an enzyme called ATPase, that is located in the cell membrane, and the process is driven by a proton gradient across the membrane.
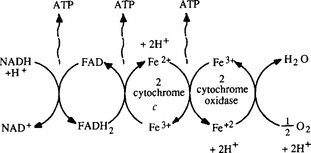
Fig. 3-4 Electron transport process schematic, showing coupled series of oxidation–reduction reactions that terminate with the reduction of molecular oxygen to water. The three molecules of ATP shown are generated by an enzyme called ATPase which is located in the cell membrane and forms ATP from a proton gradient created across the membrane.
If aerobic respiration continues to completion, all of the organic material is oxidized to form carbon dioxide and water. Much more energy is potentially available in this process than in fermentations because the organic compound is completely oxidized to inorganic constituents. For example, in lactic acid fermentation, a net gain of only two ATP molecules results from each molecule of glucose degraded. In contrast, 36 ATP molecules can be generated per molecule of glucose during aerobic respiration.
Some bacteria generate energy by the oxidation of reduced inorganic compounds, using special enzymes that allow them to remove electrons from these materials. The electrons are passed through electron transport systems in which a proton gradient is formed and ATP is generated by membrane-bound ATPases. For example, nitrifying bacteria oxidize ammonia to nitrite or nitrite to nitrate; sulfur bacteria oxidize hydrogen sulfide or sulfur to sulfur or sulfate; iron bacteria oxidize reduced ferrous iron to form hematite; hydrogen bacteria use hydrogen gas as an energy source. Some of these bacteria are aerobic; others are anaerobic. For example, some hydrogen-oxidizing bacteria use sulfate as an electron acceptor.
3.2.3.2 Energy generation by autotrophic organisms
Autotrophic organisms derive their carbon from carbon dioxide. They use either light or an inorganic chemical as their energy source. The dominant form of autotrophy is photosynthesis, a process that uses light as an energy source. Photosynthetic organisms produce special pigments such as chlorophyll a that absorb light in internal cell membranes. When a phototroph is illuminated, photons cause electrons to be emitted from chlorophyll a. These electrons are then passed through an electron transport chain in a membrane. Protons are released across the membrane as in respiration, and they produce ATP via ATPases.
Photoautotrophic organisms, such as algae, cyanobacteria, and plants, all contain chlorophyll a and obtain energy by a process known as oxygenic photosynthesis. The overall chemical reaction of this process is:
where (CH2O)n refers to organic material. In this reaction, the oxygen is derived from water. The sequence of reactions by which plants, algae, and cyanobacteria fix carbon dioxide is referred to as the Calvin-Benson cycle (Fig. 3-5).
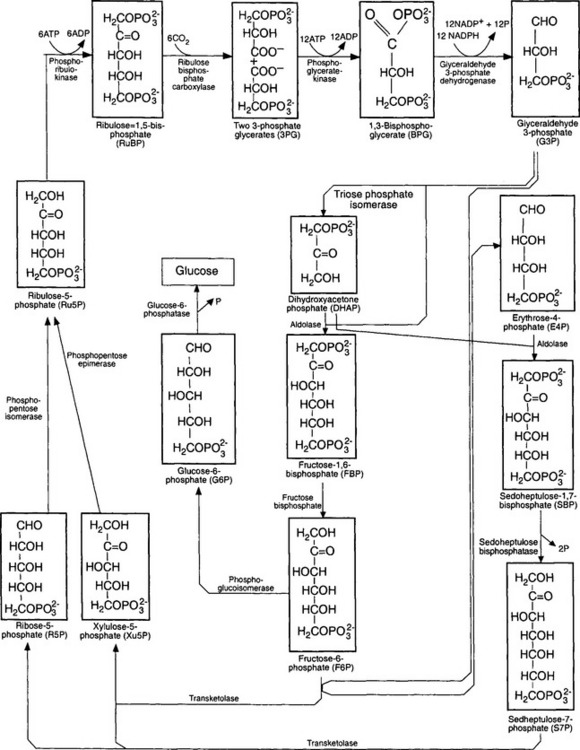
Fig. 3-5 The Calvin–Benson cycle. This is the series of reactions by which CO2 is fixed into organic material.
However, many photosynthetic bacteria, such as purple sulfur and green sulfur bacteria contain special bacteriochlorophyll compounds (not chlorophyll a) and carry out anoxygenic photosynthesis without producing oxygen:
Purple sulfur bacteria fix carbon dioxide using the Calvin-Benson cycle, but green sulfur bacteria use a completely different pathway, the reverse tricarboxylic acid cycle. Other photosynthetic bacteria use still different pathways for CO2 fixation (Perry and Staley, 1997).
Carbon dioxide can also be fixed by bacteria that use inorganic chemicals as an energy source, a process called chemoautotrophy. For example, sulfur-oxidizing bacteria obtain energy by oxidizing hydrogen sulfide or elemental sulfur as an energy source and produce sulfate as an end product. They fix carbon dioxide using the Calvin-Benson cycle. Methanogens fix CO2 using an entirely different pathway. In addition, acetogenic bacteria fix carbon dioxide and produce acetic acid (as well as cell material). Both acetogens and chemoautotrophic methanogens use hydrogen gas as an energy source.
3.2.3.3 Molecules synthesized by organisms
Using the energy obtained from photosynthesis, chemoautotrophy, and heterotrophy, organisms synthesize an amazing variety of molecules. For the purpose of understanding the roles of biochemical syntheses carried out by organisms, we can group this enormous variety of molecules into a rather limited number of groups according to their functions. Basically molecules are used for (a) cell structure; (b) metabolism, energy storage, and energy transfer; (c) information storage and information transfer; (d) modifiers of other chemicals (enzymes); and (e) defense against predators, parasites, and competitors. A given molecule may, of course, serve more than one function. Because of the functional requirements of molecules for these different purposes, some types are important in biogeochemical cycles; others are not, as will be described later. The key factors influencing the biogeochemical significance of a molecule are its per capita rate of production, the abundance of its producers, its rate of chemical decomposition, the nature of its degradation products, and the mobility of the molecule in its original and transformed states.
3.3 Evolutionary Mechanisms
Biological evolution is a change over time in the genetic composition of members of a population of organisms that are mating with one another. Thus, evolution is a population process, not a change that happens to individuals; to understand evolutionary changes “population thinking” is required. Changes that happen over a small number of generations constitute microevolution. Changes that take centuries, millennia, or longer to be completed are called macroevolution.
Much of what we know about the history of life on Earth comes from fossils, the preserved remains of organisms or impressions of organisms in materials that eventually became rocks. Although fossils have been known since prehistoric times, their significance as the remains or traces of early life forms was not appreciated until the Renaissance. The careful studies of William Smith (1769–1839), an English geologist, led to the recognition that certain identifiable strata in sedimentary rocks always contained the same types of fossils. Paleontologists also noted that the lowest strata with fossils contained fewer types of organisms and ones that were simpler in structure than strata closer to the surface. In the most ancient strata there is no evidence at all for living organisms, but rocks dated at 3.5 Gyr of age contain spherical carbon-containing structures approximately the size of modern prokaryotic cells, fossils of filamentous bacteria, and finely stratified undulating sediments (Fig. 3-6), thought to be fossilized stromatolites (Hofmann and Schopf, 1983). Microbial fossils are now known from deposits older than 3.5 Gyr of age, indicating that the first microorganisms had evolved within 1 Gyr after the formation of Earth (Awramik et al., 1983).
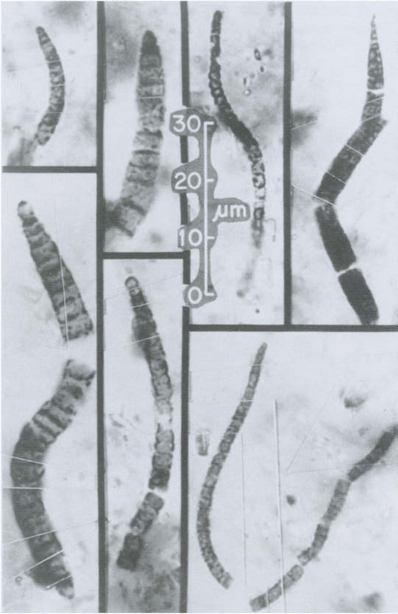
Fig. 3-6 Microfossils of several filamentous microorganisms. Bitter Springs Formation, Central Australia. Dated at 0.85 Gyr. (Courtesy William Schopf.)
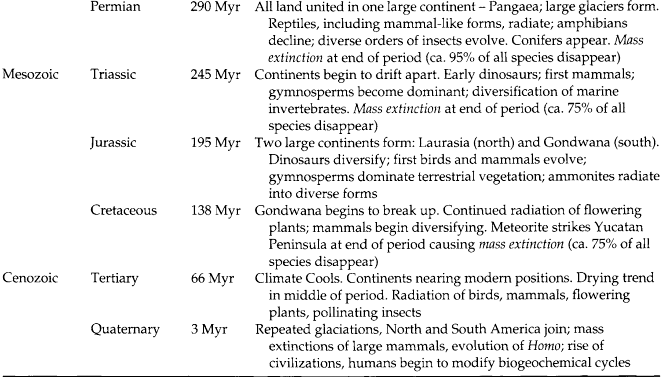
The major periods during Earth’s evolution were originally demarcated by fossils in strata of different ages, even though the ages of the strata had not been determined (Table 3-2). The Hadean eon, which extended from the time of the origin of Earth to about 3.9 Gyr ago, was a period during which Earth’s crust formed. So much debris hit the surface of Earth at that time, and so much crustal movement has occurred since then, that no rocks survive on the surface from this eon. During the Archaean eon, which extended from about 3.9 to 2.6 Gyr ago, more stable crustal features developed and life evolved. The major metabolic patterns of living organisms also evolved during this eon, including fermentation, photosynthesis, and the ability of cells to convert atmospheric nitrogen into a useful form (i.e., nitrogen fixation). The Proterozoic eon extended from 2.6 Gyr ago to about 0.6 Gyr ago. During this time new cell types and sexual reproduction evolved, leading to the evolution of plants and animals.
The fossil record illustrates that most changes in lineages of organisms were gradual. Even the most rapid ones required millions of years for their realization. Species persist, on average, for only a few million years, and the course of biological evolution has been interrupted many times by periods of mass extinction. After each of these episodes, the diversity of life has rebounded, but several million years or more were required before the original diversity of life was reestablished or exceeded. After periods of mass extinction, the groups of organisms that became dominant usually differed from those that dominated the biota prior to the extinction episode. Rates of evolutionary change have been very uneven. Many species experienced long periods of stasis, during which they changed very little. Periods of stasis were repeatedly interrupted by periods of rapid evolutionary change, but not all lineages underwent rapid evolution at the same time.
Because biological evolution is a change over time in the genetic composition of members of a population, evolutionary biologists attempt to measure of genetic variability and how it changes. The genetic constitution governing a heritable trait is called its genotype. A population evolves when individuals with different genotypes survive or reproduce at different rates, but agents of evolution do not act directly on a genotype. They act on the physical expression of an organism’s genotype – its phenotype. Not all phenotypic variation is governed by genotypes. For example, the leaves on a tree or shrub are normally genetically identical, but they may differ dramatically in size and shape as a result of differences in the amount of wind and sunlight to which they are exposed when they expand. Such environmentally induced variation is ecologically important, but only traits that are, at least in part, heritable, determine the direction of evolution.
Individuals of most species have two sets of chromosomes, the structures on which the genes are linearly arranged, in their cells. One chromosome is derived from their maternal parent and one from their paternal parent. A gene occupies a particular place (locus) on a chromosome, and different forms of a gene – called alleles – are present in most populations. An individual may have the same or different alleles for a particular gene on the chromosomes it inherited from its maternal and paternal parents. The alleles of a particular gene are designated by variations of a single label, i.e. A and a, or A1, A2, and A3. The percentages of different alleles at each locus describe the genetic structure of a population.
An equilibrium population, one that is not changing genetically, persists under conditions that were discovered independently in 1908 by the British mathematician G. H. Hardy and the German physician W. Weinberg. The Hardy-Weinberg Rule, which is the foundation of population genetics, consists of three assumptions and two major mathematical results. The assumptions are that the population is very large, that mating is random, and that no
agents of evolution are acting on the population. If these conditions hold, the frequencies of alleles at a locus will remain constant from generation to generation, and, after one generation of random mating, the genotypic frequencies will remain in the proportions
where p is the frequency of allele A in the population and q is the frequency of allele a in the population, provided that the gene has only two allelic forms, A and a. The mathematical derivation of these results, which need not concern us here, is presented in all elementary biology texts.
It is clear that the conditions necessary for the Hardy-Weinberg Rule to apply are rarely met in nature. Populations are often small, mating is often nonrandom with respect to genotype (likes mate with likes), and evolutionary agents typically are acting on populations. The rule is nonetheless important because significant deviations of genotype frequencies in a population from Hardy-Weinberg expectations are evidence that an agent of evolution is in action.
Sexual reproduction, by bringing together the genetic material from two parents, greatly increases the amount of genetic variation present within a population. In a population that reproduces asexually, offspring are genetically identical to their parents unless genes in the parents have mutated. Sexual recombination, on the other hand, generates an enormous variety of genotypic combinations upon which the agents of evolution can act. Therefore, sexual recombination increases the evolutionary potential of a population, but sexually reproducing populations do not necessarily evolve rapidly.
3.3.1 Evolutionary Agents
Evolutionary agents are forces that change allele and genotype frequencies in populations, causing them to deviate from Hardy-Weinberg expectations. The known evolutionary agents are mutation, gene flow, genetic drift, nonrandom mating, and natural selection.
3.3.1.1 Mutation
Mutation is a stable, heritable change of a gene from one allele to another, which both creates and maintains genetic variability in populations. Most mutations adversely affect the survival and reproductive success of their bearers, but if the physical or biological environment changes, previously neutral or harmful alleles may become beneficial. Mutation rates typically are very low, but they are sufficient to create considerable genetic variation over many generations.
3.3.1.2 Gene flow
When individuals migrate to, and then breed in a new location, they may add new alleles to a population or may change the frequencies of alleles already present. Similarly, emigrants may remove alleles or may change the frequencies of alleles in the populations from which they departed.
3.3.1.3 Genetic drift
Genetic drift is alteration of allele frequencies in small populations by chance events. During times when large populations are reduced to small numbers of individuals, genetic variation is likely to be lost by chance. Genetic drift also occurs when a small number of individuals colonize a new region. The pioneers are unlikely to have all the alleles found in the source population and the frequencies of the ones they do have are likely to differ from those in the source population.
3.3.1.4 Nonrandom mating
This results when individuals with certain genotypes mate more often with individuals of either the same or different genotypes than would be expected on a random basis. Self-fertilization, which is common among many groups of organisms, particularly plants, is an extreme form of nonrandom mating. Another common type of nonrandom mating is preferential mating by females with certain males.
3.3.1.5 Natural selection
Because not all individuals in a population survive and reproduce equally well in a particular environment, some individuals contribute more offspring to subsequent generations than do other individuals. Such differential contribution of offspring resulting from variations in heritable traits was called natural selection by Charles Darwin. Natural selection is especially important because it is the only evolutionary agent that adapts organisms to their environments.
Natural selection can produce several different outcomes. It may (1) preserve the genetic characteristics of a population by favoring average individuals (stabilizing selection), (2) change the characteristics of a population by favoring individuals that vary in one direction from the population mean (directional selection), or (3) change the characteristics of a population by favoring individuals that vary in opposite directions from the population mean (disruptive selection). Stabilizing selection is the norm because most populations are not evolving rapidly most of the time, but evolutionary changes depend on directional or disruptive selection.
3.3.2 Catastrophic Events and the Course of Evolution
Although natural selection is the only evolutionary agent that adapts organisms to their environments, the course of evolution has been profoundly influenced by major environmental changes, some of which had catastrophic effects. Some of these events resulted from Earth’s internal processes, such as the activity of volcanoes and the shifting and colliding of continents. Others were the result of external events, such as collision of meteorites with Earth.
The movement of Earth’s crustal plates and the continents they contain – continental drift – has had enormous effects on climate, sea levels, and the distributions of organisms. Mass extinctions of organisms have usually accompanied major drops in sea levels. The collision of all the continents to form the gigantic landmass called Pangaea about 260 million years ago, triggered massive volcanic eruptions. The volcanoes ejected enough ash into Earth’s atmosphere to significantly reduce sunlight penetration and trigger massive glaciation. The result was the extinction of about 90% of all species on Earth, both terrestrial and marine.
Through much of its history, Earth’s climate was much warmer than it is today, and temperatures decreased more slowly toward the poles. At other times, Earth was colder than it is today, and massive glaciers formed at high latitudes. We live in one of the colder periods of Earth’s history.
External events have also triggered important changes. At least 30 meteorites hit Earth each year, but collisions with very large meteorites are very rare. One, about 10 km in diameter that collided with Earth 65 million years ago, caused massive firestorms and tidal waves and triggered the extinction of many species of marine organisms and all terrestrial animals larger than about 25 kg in body weight.
3.3.3 The Biological Consequences of Evolution
All living organisms are descendants of a lineage of unicellular organisms that lived almost four billion years ago. Over the course of evolution, living organisms stored greater quantities of information and evolved increasingly complex mechanisms for using it. But if the evolution of complexity were the entire story, only one kind of organism might exist on Earth today. Instead, Earth is populated by many millions of genetically different kinds of organisms, called species, that rarely or never interbreed with one another.
For two species to form from a single one, the ancestral population must become divided into two or more separate populations among which gene exchange does not occur. Over time, genetic differences may accumulate as the separated groups adapt to the particular environments in which they live. If enough differences accumulate, individuals of one population may not be able to breed with individuals of the other if their ranges subsequently overlap. They will have become different species.
Gene flow among members of a population may be interrupted in several ways. A common method is division of the population by a barrier, such as a water gap for terrestrial organisms, dry land for aquatic organisms, and mountains. Barriers can form when continents drift, sea levels rise and fall, or climates change. Populations separated in these ways are typically large initially. They evolve differences because the places in which they live are or become different. Alternatively, gene flow may be interrupted if some members of a population cross an existing barrier and form a new isolated population.
Gene flow among members of a population may also be interrupted in the absence of geographical separation. The most common means is polyploidy, a duplication of the number of chromosomes in the cells of its members. Polyploidy can arise in two ways. One way is the accidental production during cell division of cells having four (tetraploid) rather than the normal two (diploid) sets of chromosomes. Tetraploid individuals usually cannot produce fertile offspring by mating with diploids because their chromosomes do not pair properly during cell division, but they can mate with one another to form a new evolutionary lineage. A polyploid species can also be produced when individuals of two different species, whose chromosomes do not pair properly during cell division, interbreed. The resulting individuals are usually sterile, but they may be able to reproduce asexually. After many generations, their descendants may eventually become fertile as a result of further chromosome duplication. Speciation by means of polyploidy is very rare among animals but has been very important in the evolution of flowering plants. More than half of all species of flowering plants are polyploids.
Repeated splitting of lineages of organisms into separate species has resulted in the great richness and diversity of life that lived in the past and is found on Earth today. Earth would be very different if speciation had been a rare event during the life’s history. Biogeochemical cycles are influenced by millions of species, each adapted to live under a particular range of conditions and to use environmental resources in a particular way.
3.4 The Diversity of Living Organisms
Based on the structure of their cells, organisms can be grouped into two broad categories. Prokaryotic (meaning “before-nucleus”) organisms have structurally simple cells. Their DNA is not bound by a nuclear membrane (Fig. 3-7), and they typically have a single, circular chromosome. By contrast, the cells of eukaryotic organisms (meaning “true nucleus”) have a nuclear membrane that surrounds their chromosomes (Fig. 3-8). Typically each eukaryotic cell has two sets of chromosomes, one set derived from each parent. Eukaryotic cells also contain additional membrane-bound structures outside the nucleus. The most important of these are mitochondria, the sites of aerobic respiration, and – among photosynthetic plants – chloroplasts, the centers of photosynthetic activity.
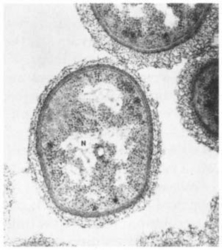
Fig. 3-7 A thin section through a prokaryotic cell. Note that the nuclear material (N) is not bound by a membrane, but is free in the cytoplasm. Mitochondria and other intracytoplasmic structures are absent. (Reprinted with permission from J. J. Cardamone, Jr., Univ. of Pittsburgh/Biological Photo Service.)
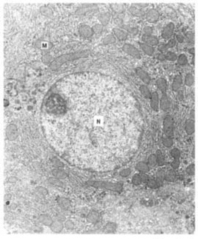
Fig. 3-8 A thin section showing a eukaryotic cell. Note the nucleus (N) is bound by a nuclear membrane. In the cytoplasm of the cell are many mitochondria (M) and intracytoplasmic membranes. (Reprinted with permission from Richard Rodewald, Univ. of Virginia/Biological Photo Service.)
Although many prokaryotic organisms are single-celled (unicellular), some exist as multicellular filaments or collections of cells. Eukaryotic organisms may be unicellular or multicellular. Most eukaryotic cells are at least 5 µm in diameter, but many are much larger. The cells of most prokaryotes are small, ranging from 0.2 to 1 µm in diameter, but a few are much larger.
Each cell of a prokaryotic organism typically divides by an asexual process, after duplication of its chromosome, to give rise to two essentially identical daughter cells. Gene exchange among prokaryotes occurs in some species when two cells of different mating types come into contact or by virus-mediated transfers. Typically, only part of the genetic material of a cell is transferred to a recipient cell. Most eukaryotic organisms undergo a sexual process during reproduction in which the genetic material is duplicated and a complete set is transferred to the recipient cell.
Because most complex organisms require oxygen for growth, multicellular organisms probably did not evolve until after the appearance of oxygen on Earth, i.e. not before some 2 Gyr ago. Indeed, even though oxygen might have been produced prior to 2 Gyr ago, considerable time would have been required before atmospheric concentrations of oxygen became substantial. Initially oxygen would have reacted with reduced compounds such as iron; only after these more reduced forms were fully oxidized would oxygen have accumulated to a significant level. Moreover, when oxygen first accumulated on Earth, it would have been toxic to nearly all organisms, just as O2 kills most organisms that live in environments lacking oxygen today. This may have been the first major event of biologically caused pollution, but it provided conditions that favored both the ability to tolerate oxygen and the ability to use it in aerobic respiration. Prokaryotes predominated from about 3.5 Gyr ago to about 0.6 Gyr ago, or three-quarters of the time life has existed. The tremendous variety of eukaryotic organisms originated and evolved largely during the last 0.6 Gyr, after Earth’s atmosphere became oxygenated.
3.4.1 The Classification of Organisms
To help us comprehend the incredible diversity of life, biologists have developed a system of classifying organisms. In that system, first proposed by the Swedish biologist Carolus Linnaeus in 1758, each species, for example, Homo sapiens, is given two names. The first name identifies the genus (plural, genera), a group of closely related species; the second designates the species. Because this system of binomial nomenclature has been universally adopted, scientists throughout the world are able to use a single name for a particular species of organism.
Species and genera are, in turn, grouped into larger units in a hierarchical system. The units in the system cluster organisms that are believed to share a common ancestor, that is, the classification system attempts to represent evolutionary relationships among organisms. Members of a genus share a relatively recent ancestor. The most recent common ancestor of members of larger taxonomic units lived in the more distant past.
For two reasons, determining evolutionary relationships among organisms is a difficult task. First, the fossil record is incomplete; only a small fraction of species that have ever lived on Earth have left a fossil record, and typically only hard body parts are preserved. Second, organisms are so variable in their morphological and physiological features that they share few traits with which they can be compared. However, an important evolutionary record is preserved in the sequences of certain macromolecules that are shared by all living organisms. The most important of these universally shared molecules is ribosomal RNA (rRNA). The RNA of ribosomes, which carry out protein synthesis, contains a small and a large subunit. A widely accepted phylogeny of all life on Earth has been erected by comparing the structure of RNA in the small subunit. In the classification system based on these data, organisms are grouped into three domains, the Bacteria, the Archaea, and the Eucarya (Table 3-3).
Table 3-3
Major phylogenetic groups of living organisms
| Taxon | Representatives |
| Domain Bacteria phyla: | Proteobacteria, Cyanobacteria, Gram-positive Bacteria, Chlamydia, Spirochaetes, Planctomycetes, Green sulphur bacteria, Green filamentous bacteria |
| Domain Archaea phyla: | Verrucomicrobia, Cytophaga-Flavobacterium, Deinococci Methanogens, Extreme Halophiles, Thermoplasmas, Hyperthermophiles |
| Domain EukaryaKingdom ProtistaPhylum Mastigophora | Flagellates |
| Phylum Sarcodina | Amebas and their relatives |
| Phylum Actinopodia | Actinopods |
| Phylum Foraminifera | Foraminiferans |
| Phylum Sporozoa | Ameboid parasites |
| Phylum Ciliophora | Ciliates |
| Phylum Apicomplexa | Apicomplexans |
| Phylum Pyrrophyta | Dinoglagellates |
| Phylum Chrysophyta | Diatoms |
| Phylum Phaeophyta | Brown algae |
| Phylum Rhodophyta | Red algae |
| Phylum Chlorophyta | Green algae |
| Kingdom Plantae | |
| Phylum Hepatophyta | Liverworts |
| Phylum Anthocerophyta | Hornworts |
| Phylum Broyphyta | Mosses |
| Phylum Lycophyta | Club mosses |
| Phylum Sphenophyta | Horsetails |
| Phylum Pterophyta | Ferns |
| Phylum Cycadophyta | Cycads |
| Phylum Ginkgophyta | Ginkgos |
| Phylum Gnetophyta | Gnetum, Ephedras |
| Phylum Coniferophyta | Conifers |
| Phylum Angiospermae | Flowering plants |
| Kingdom Fungi | |
| Phylum Chytridiomycota | Water molds |
| Phylum Zygomycota | Bread molds |
| Phylum Basidiomycota | Mushrooms, rusts, smuts |
| Phylum Ascomycota | Yeasts, sac fungi |
| Kingdom Animalia (only the major phyla are listed) | |
| Phylum Porifera | Sponges |
| Phylum Cnidaria | Hydras, jellyfish, corals |
| Phylum Ctenophora | Comb jellies |
| Phylum Platyhelminthes | Flatworm |
| Phylum Rotifera | Rotifers |
| Phylum Nematoda | Round worms |
| Phylum Mollusca | Chitons, snails, clams, squids, octopi |
| Phylum Annelida | Segmented worms |
| Phylum Arthropoda | Scorpions, spiders, crabs, insects, millipedes, centipedes |
| Phylum Bryozoa | Moss animals |
| Phylum Brachiopoda | Brachiopods |
| Phylum Echinodermata | Sea lilies, seastars, sea urchins, sand dollars, sea cucumbers |
| Phylum Chordata | Tunicates, sharks, bony fishes, amphibians, reptiles, birds, mammals |
Members of the domains Bacteria and Archaea are prokaryotic. Their cells lack the complex, membrane-bound structures found in cells of the third domain. Approximately half the mass of living organisms on Earth consists of prokaryotic organisms (Whitman et al., 1998). Bacteria are divided into more than a dozen major groups, such as the Gram positive bacteria, Spirochetes, Chlamydia, Proteobacteria, Green filamentous bacteria, and Planctomycetes, as well as others. These organisms, which are found in nearly all soils and aquatic environments, include the medically important pathogenic bacteria. Almost all contain a cell wall macromolecule – peptidoglycan – whose synthesis is inhibited by penicillin. Processes carried out by members of this group include nitrogen fixation, denitrification, nitrification, anoxygenic photosynthesis, sulfate reduction, sulfur and sulfide oxidation, acetogenesis, and methane oxidation. Members of the group also degrade toxic compounds such as aliphatic and polycyclic aromatic hydrocarbons and halogenated organic compounds such as chlorinated phenols.
The Archaea differ from the Bacteria in that they lack peptidoglycan and contain special membranes that lack the fatty acids found in Bacteria and Eucarya. The Archaea also are characterized by their unusual habitats and metabolic processes. Some of them, which can grow at temperatures in excess of 100°C, live in hot springs and marine hydrothermal vents. Others grow in saturated brines. Another group is noted for its unique metabolic capability to produce methane gas (methanogenic) from hydrogen and carbon dioxide as well as methanol or acetic acid. These methanogens are obligate anaerobes that live in reduced aquatic sediments, soils, and in the intestinal tracts of animals such as ruminants.
Members of the other domain – Eucarya – have more complex cells with nuclei and elaborate cellular compartments. They are classified into four kingdoms – Protista, Plantae, Fungi, and Animalia.
Protists are a heterogeneous group of microorganisms that either have a single cell or are groups of similar cells joined together. Some are autotrophs, others are ingestive heterotrophs, and still others are heterotrophs. One phylum (Sporozoa) consists of nonmotile forms, but members of the others move by means of ameboid motion, ciliary action, or flagella. Many marine species (foraminifera) secrete skeletons of calcium carbonate and their remains are the major contributors to the formation of limestone. Others (radiolarians) secrete glassy siliceous skeletons that are the principal components of sediments under many tropical seas.
Fungi are mostly multicellular, heterotrophic organisms that absorb their food. Most species are saprophytes (living on dead matter) and they produce, at some time in their life cycles, characteristic and often complex reproductive structures that differentiate them from protists, plants, or animals. Some are parasites. Fungal cell walls contain a variety of polysaccharides, often including cellulose and, in some species, chitin. Lichens are composite organisms that consist of a meshwork of a fungus and some photosynthetic organism, either an alga or a cyanobacterium.
Plants range in size from single-celled forms to large trees and vines. The Rhodophyta, Chlorophyta, Pyrrophyta, Chrysophyta, and Phaeophyta, collectively known as algae, abound in fresh and marine waters and on moist terrestrial substrates. They account for more than one-fourth of the photosynthesis occurring on Earth. They use a variety of photosynthetic pigments, apparently adaptations to the very different light regimes found at different depths in aquatic environments. They synthesize a number of different molecules for the storage of their food reserves (starch, fats, and oils). The materials used in the construction of their cell walls are also highly varied (cellulose, pectin substances, silica, lignin, mucilage, and calcium carbonate). The largest species may attain lengths in excess of 35 m, but the cells of even
the largest species are not differentiated into distinct types. The large blades of marine kelps, for example, consist primarily of thin sheets of identical cells.
Mosses and liverworts (Bryophyta) are more complex than algae. Some of the larger species have structures that superficially appear similar to roots, stems and leaves, but they lack the internal conducting systems present in the vascular plants (Tracheophyta). Internal transport systems (vascular systems) make possible the large sizes of terrestrial plants where the soil is the source of some requisites (water, mineral nutrients) and the air is the source of others (CO2, sunlight). The different groups of vascular plants are characterized primarily by their methods of reproduction. Vascular plants are the source of all wood.
More than one million species of animals (kingdom Animalia) have been described by scientists. Estimates of the true number range to higher than 30 million because most species of insects and other arthropods are as yet not described. Because they are heterotrophs, animals represent less biomass than the autotrophs upon which they depend for their food, but because many of them construct sturdy skeletons that are durable and resistant to degradation, they are important contributors to biogeochemical cycles. And, of course, the species producing the largest contributions to and perturbations of biogeochemical cycles is an animal – Homo sapiens.
3.5 The Ecological Organization of the Living World
Ecology is the study of the distribution and abundance of organisms, their interrelationships, and the communities of which they are a part. Ecological investigations range from the study of the behavior of individuals in response to their environments, to the flow of energy and matter through organisms and the environment at regional to global scales. Here we focus on ecosystems – spatially explicit units that include all organisms that live there and relevant components of their abiotic environment – because the consequences of interactions of organisms with the physical environment at large scales are the subject matter of this book.
The bodies of living organisms are excellent sources of energy-rich molecules that can be used to fuel the machinery of other organisms. Since early in the evolution of life, some organisms have obtained their energy and materials by consuming others. The major dynamics of the living world today are dominated by eating and being eaten. Even though most species interact strongly with only a small proportion of species living in the same area, a rich network of connections exists in all ecosystems. Ecologists devote much of their efforts to studying the webs of connections that form the bases of ecological communities.
3.5.1 Energy Flow in Ecosystems
The entry of materials and energy into the living world is largely via photosynthesis. Except for a few ecosystems (deep-sea thermal systems, deep-Earth microbial communities) in which solar energy is not the main energy source, almost all energy utilized by organisms comes from (or once came from) the sun. The fraction of solar energy falling on Earth’s surface that is used in photosynthesis is small. Earth intercepts 5 × 1024 J of energy per year from the sun, only about 3 × 1020 J of which are captured by photosynthesis. Chlorophyll a and its associated pigments absorb only a small fraction of the total energy present in sunlight. Most incident solar radiation is reflected from Earth’s surface. Much is converted to heat, some of which is used to evaporate water and, hence, to drive the global hydrologic cycle.
The organisms that obtain their energy from the same general source constitute a trophic level. The major trophic levels in ecological communities are photosynthesizers (primary producers), herbivores (eaters of plants or parts of them), primary carnivores (eaters of herbivores), secondary carnivores (eaters of primary carnivores), tertiary carnivores, and detritivores (eaters of the dead remains of once living organisms) (Fig. 3-9). A sequence of linkages in which a plant is eaten by an herbivore, which is in turn eaten by a primary carnivore, and so on, is called a food chain. Because most species in a community eat and are eaten by more than one species, food chains are interconnected to form food webs.
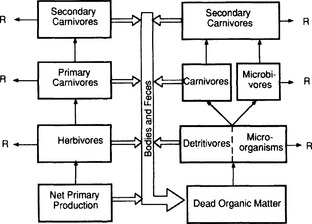
Fig. 3-9 Trophic levels in ecosystems. Thin arrows show flow of energy up the food chain (through living biomass) and the broad arrows show the complementary flow of dead organic matter (detritus) back down. R indicates respiration.
Trophic levels are imprecise categories; many organisms, such as human beings, obtain their energy from two or more trophic levels.
The total amount of energy that plants assimilate by photosynthesis is called gross primary production. The production that remains after subtracting the energy that plants use for maintenance and building tissues is called net primary production. The global distribution of net primary production in Earth’s major ecological zones is shown in Table 3-4. Oceans, despite their much larger surface area, contribute less than half of Earth’s net primary production because surface waters, where photosynthesis can take place, are highly deficient in nutrients over most of the oceans. Oceanic production is concentrated in coastal areas, especially where upwelling of deep water brings nutrients to the surface. On land, photosynthesis is often limited by dryness, cold temperatures, and nutrient shortages, which is why subtropical and tropical areas contribute much more than their proportional share to global primary production. Productivity also changes in response to disturbances, such as fires, volcanic eruptions, and severe storms.
Table 3-4
Net annual primary production of Earth’s major ecological zones a
| Zone | Area (106 km2) | Primary production of carbon (Pg)b |
| Terrestrial | 133.9 | |
| Tropical evergreen forests | 17.8 | |
| Broadleaf deciduous forests | 1.5 | |
| Mixed broadleaf/needle forests | 3.1 | |
| Coniferous forests | 4.5 | |
| Savannahs | 16.8 | |
| Grasslands | 2.4 | |
| Deserts | 1.5 | |
| Tundra | 0.8 | |
| Cultivated areas | 8.0 | |
| Total terrestrial | 56.4 | |
| Marine | 361.0 | 48.5 |
| Global total | 510.3 | 104.9 |
aAfter Field, Behrenfeld, Randerson and Falkowski, Science 281, 237–240, 1998.
A shortage of any of some two dozen chemical elements that are essential for the growth of organisms can reduce ecosystem productivity, but phosphorus and nitrogen are often the most limiting nutrients, which is why these two elements are standard components of commercial fertilizers. Phosphorus is often limiting because it moves through soil pores and aquatic
ecosystems in the form of phosphate salts or organic phosphate compounds dissolved in water. If oxygen is present and pH is neutral or alkaline, phosphate complexes with calcium or iron and become immobile.
Much of the energy captured by an organism is used to support its basic metabolism and is dissipated as heat. Only the energy content of an organism’s net production – its growth plus reproduction – is available to organisms at the next trophic level. Moreover, much of the energy potentially available to organisms in the next higher trophic level is not captured by them. Many organisms or parts of them die and are consumed by detritivores, which dominate the flow of energy from green plants and are the primary pathway for return of nutrients in a form useful for assimilation by green plants.
On average, only about 10% of the energy captured by one trophic level is taken in by the next level, but there are wide variations around this overall average. For example, the small, unicellular algae that dominate marine photosynthesis have high growth and cell division rates, possess easy-to-digest tissues, and can be eaten by very small animals. As a result, aquatic herbivores are able to consume about 51% of the primary production of oceans and lakes (Cyr and Pace, 1993). In many marine areas there may be, for parts of the year, a larger standing crop of herbivores than of the plants upon which they feed, due to the very high algal growth and reproduction rates. Similarly, in grasslands, where plants allocate most of their net production to easily digested tissues, mammals may consume 30 to 40% of the annual net above-ground net primary production; insects may consume an additional 5 to 15%. By contrast, in forested ecosystems, where plants allocate much of their energy to production of wood, which is difficult for most organisms to digest, less than 3% of net primary production may be consumed by herbivores.
The consequences of the massive “loss” of energy accompanying passage from one trophic level to another also include the fact that organisms low in the trophic ladder tend to dominate the cycling of elements through the biosphere. This is especially true on land where vascular plants dominate both the physical structure and the flow of energy in ecosystems. In the oceans, because the major photosynthesizers are small and structurally simple, the cycling of many elements is much more strongly influenced by herbivores and carnivores (Longhurst, 1998).
3.5.2 Elemental Cycles in Ecosystems
Energy is not recycled in ecosystems because at each transformation much of it is dissipated as heat, a form of energy that cannot be used by organisms to power their metabolism. Chemical elements, on the other hand, are not lost when they are transferred among organisms. Instead, they cycle through organisms and the environment. The quantities of carbon, nitrogen, phosphorus, calcium, sodium, sulfur, hydrogen, and oxygen, together with smaller amounts of other chemical elements, that are available to organisms are strongly influenced by how organisms obtain them, how long they hold on to them, and what they do with them while they have them.
The basic principles that govern the behavior of nutrients also govern the behavior of toxic materials in the environment, but some contaminants, such as metals and organic compounds, build up to very high concentrations in organisms via biomagnification. Any substance that is retained more efficiently than carbon or nutrients is likely to increase in concentration in an organism’s tissues. At each step in a food chain, the concentration of such a contaminant increases in the consumers’ tissues. The most serious contaminants whose concentrations have been greatly biomagnified are mercury and organochlorine compounds (pesticides such as DDT and chlordane, PCBs, chloromethanes). Microscopic photosynthetic aquatic organisms can concentrate PCBs up to 100000 times more than in the surrounding water.
3.6 The Impact of Life on Biogeochemical Cycles
Organisms are involved in many transformations that have important environmental consequences. The activities of microbes are especially diverse and important. Pesticide degradation, cellulose decomposition, toxin production, deposition of limestone, rock and mineral weathering, formation of sulfur deposits, production of antibiotics, and methyl mercury formation are examples of the many processes of ecological and geochemical significance that are mediated, at least in part, by microorganisms (Table 3-5). Many further details of important transformations are brought out in the chapters on the cycles of specific elements.
Table 3-5
Elemental cycles and selected important transformations in which organisms play roles
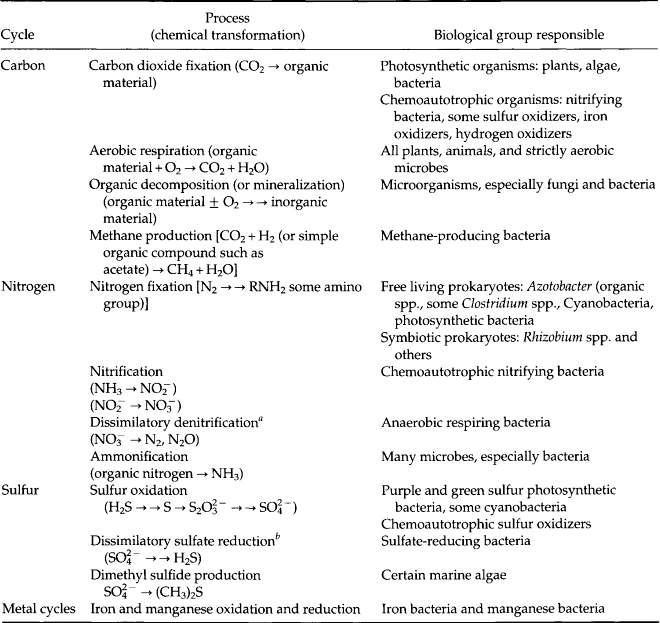
aAssimilatory denitrifiers reduce nitrate to the amino acid level where it is incorporated into protein. Many plants and bacteria can do this and, therefore, use nitrate as a nitrogen source.
bAssimilatory sulfate reducers reduce sulfate to the sulfhydryl level where it is incorporated into the sulfur amino acids of protein. Many plants and bacteria can do this.
3.6.1 The Hydrologic Cycle
The major reservoirs of water on Earth are the oceans. The hydrologic cycle is driven primarily by evaporation of water from the oceans, lakes,
and rivers. However, land plants also influence the hydrologic cycle by taking up water through their root systems. Impurities in the water provide nutrients for plant growth and water is a raw material for photosynthesis. Excess water escapes to the atmosphere through tiny openings in the leaves stomata, a process known as evapotranspiration.
The synthesis of 1 kg of dry plant biomass requires the evapotranspiration of about 300 L of water, although smaller amounts of water are needed by some plants such as desert cacti. Approximately one-third of the annual continental rainfall (100 cm/yr) is returned to the atmosphere by evapotranspiration. Although it accounts for only about 10–15% of global evaporation, plant evapotranspiration can play a major role in local climates. For example, a molecule of water falling on the upper Amazon Basin is recycled on average five times during its eventual return to the Atlantic Ocean.
3.6.2 The Carbon Cycle
As described in Chapter 11, fast fluxes in the carbon cycle are driven by removal of carbon dioxide from the environment during photosynthesis by plants, algae, and cyanobacteria, which are the major photosynthesizers in most environments. Chemoautotrophic bacteria, which are also important primary producers, play a major role in carbon fixation in environments lacking light. Carbon dioxide is returned to the environment as a byproduct of the respiration of all organisms, including photosynthesizers during night-time hours. Organic decomposition, which also produces carbon dioxide and water and consumes oxygen, is carried out by saprophytic fungi and bacteria that obtain the organic material from non-living sources, such as dead trees, and other non-living plant and animal material. Animals, too, are important decomposers of plant and animal organic material.
The methane-producing bacteria that derive their energy from the oxidation of simple organic compounds such as methanol and acetate, release large quantities of methane. They are responsible for swamp gas (methane) production in the sediments of aquatic habitats. Some reside as symbionts in the rumen of cattle and hindguts of termites, whose metabolism releases large quantities of methane to the atmosphere.
3.6.3 The Nitrogen Cycle
Nitrogen, despite its abundance on the atmosphere, is the element that most commonly limits ecosystem productivity (Vitousek and Howarth, 1991). Atmospheric nitrogen, N2, cannot be used by most organisms. To enter an ecosystem, N2 must be “fixed” as ammonia, nitrate, nitrite, or an organic compound of nitrogen. Small amounts of nitrogen are fixed by lightning and a small amount of ammonia is vented into the atmosphere by volcanoes, but most nitrogen in the biosphere is fixed by organisms – by cyanobacteria in aquatic systems and by symbiotic bacteria associated with the roots of certain plants in terrestrial ecosystems. Nitrogen is lost rapidly from ecosystems via groundwater, vaporization of ammonia, and denitrification, but it is released slowly from decaying organic matter. Thus, nitrogen is typically limiting because it is lost rapidly but is fixed and recycled slowly. Humans have roughly doubled the input of fixed nitrogen to the biosphere, primarily by combustion of fossil fuels and industrial fixation of nitrogen for use as fertilizer (Vitousek et al., 1997). Managing the nitrogen cycle is certain to be a major problem for human society in the foreseeable future.
Dissimilatory denitrification occurs anaerobically and is mediated by bacteria that use nitrate in place of oxygen as an acceptor of electrons during respiration. The result is the formation of molecular nitrogen and nitrous oxide. The nitrous oxide plays a role in the chemistry of stratospheric ozone, and is, therefore, extremely important biogeochemically. These bacteria derive energy from the anaerobic oxidation of organic compounds. Many organisms, especially bacteria, decompose organic material with the release of ammonia, a process referred to as ammonification.
3.6.4 The Sulfur Cycle
Reduced sulfur compounds serve as hydrogen donors for anoxygenic photosynthetic bacteria such as the green and purple sulfur bacteria and some cyanobacteria. In contrast, chemoautotrophic sulfur bacteria obtain energy from the oxidation of reduced sulfur compounds including hydrogen sulfide, sulfur, and thiosulfate. As with the nitrifying bacteria, these bacteria are primarily aerobic and use carbon dioxide as their source of carbon. The ultimate product of their metabolism is sulfuric acid. These bacteria are responsible for the production of acid mine waters in areas where strip mining has exposed pyrite minerals to rainfall and oxygen. Some of these bacteria can grow at pH values as low as 1.0; pH values of 3.0 and 4.0 are common in runoff streams from mining areas. Fish cannot live in these waters and most plants cannot grow in such highly acidic soils.
Dissimilatory sulfate reducers such as Desulfovibrio derive their energy from the anaerobic oxidation of organic compounds such as lactic acid and acetic acid. Sulfate is reduced and large amounts of hydrogen sulfide are generated in this process. The black sediments of aquatic habitats that smell of sulfide are due to the activities of these bacteria. The black coloration is caused by the formation of metal sulfides, primarily iron sulfide. These bacteria are especially important in marine habitats because of the high concentrations of sulfate that exists there.
Dimethylsulfide (DMS) is the major volatile sulfur compound of biogenic origin emitted from the oceans into the atmosphere. It is estimated that the annual global sea-to-air flux is 15–40 million metric tons of sulfur per year. DMS is produced by the enzymatic cleavage of dimethylpropiothetin (DMPT). The function of DMPT in these algae is uncertain, but there is strong evidence that it may function as a very effective osmoregulator (Andreae and Bernard, 1984; Vairavamurthy et al., 1985). The dipolar ionic nature of DMPT gives the molecule a very low membrane permeability. The osmotic role of DMPT is also suggested by the fact that most freshwater algae produce little or no DMS, although cyanobacteria do. The dimethylsulfide produced by marine algae reacts in the atmosphere to form sulfuric acid as well as ammonium sulfate, ammonium bisulfate, and methane sulfonic acid, all of which have low vapor pressures in the atmosphere and can condense to form aerosol particles. These particles can affect climate by changing the reflective properties of the marine atmosphere and by providing particles on which cloud droplets can nucleate. (See Chapters 7 and 17 for more details.)
3.6.5 The Phosphorus Cycle
Phosphorus is not an abundant constituent of the biosphere, but it is an essential component of living organisms as a component of nucleic acids and high-energy compounds such as ATP. Organisms have evolved mechanisms for concentrating phosphorus, which is often a limiting nutrient, from soil and water. In freshwaters, algal blooms are frequently controlled by the availability of phosphate. Microorganisms are able to store phosphate as a polymer inside their cells, which they use during periods of phosphorus limitation.
3.6.6 The Metal Cycles
Many bacteria can oxidize and reduce metals and metallic ions. Some can even derive energy from those oxidative processes. For example, iron oxidizers, such as species of Thiobacillus, can grow on reduced iron compounds and obtain energy from their oxidation if the pH is sufficiently low, as in pyrite oxidation in acid mine waters. No chemoautotrophs are known to be able to derive energy from the oxidation of manganese, but many heterotrophic bacteria can do so. Many bacteria are also involved in the deposition of oxidized iron and manganese compounds, thereby immobilizing these elements. Trace amounts of metals can be very important to organisms either through their toxic effects or the roles that metals play in enzymes and energy transfer compounds.
3.6.7 Long-term Influences of Living Organisms on Earth’s Atmosphere
The long-term influences of living organisms on Earth’s atmosphere have been enormous. The biological inputs of major atmospheric gases is given in Table 3-6. Had Earth remained lifeless, concentrations of carbon dioxide in the atmosphere probably would have remained very high, and the temperature would be very different from that of today. Oxygen would have slowly increased due to the splitting of water by sunlight, but it would not have risen above 1% of its present concentration. The atmosphere of Earth also differs in the chemical interactions taking place in it. If life were to disappear today, nitrogen in the atmosphere would eventually be transformed into nitrate, which would be transferred to the oceans, lowering their pH considerably from present values (Hutchinson, 1944).
Table 3-6
Biological sources of Earth’s major atmospheric gases a
| Gas | Principal biological source | Residence time in the atmosphere |
| Nitrogen | Bacteria | 107 to 109 years |
| Oxygen | Photosynthesis | Thousands of years |
| Carbon dioxide | Organism respiration, fuel combustion | About 100 years |
| Carbon monoxide | Bacterial processes, incomplete combustion | A few months |
| Methane | Bacteria in anaerobic environments | A few years |
| Nitrous oxide | Bacteria and fungi | About 100 years |
| Ammonia | Bacteria and fungi | A few days |
| NOx | Reaction of pollutants in sunlight | A few days |
| Hydrogen sulfide | Anaerobic bacteria | A few days |
| Hydrogen | Photosynthetic bacteria, methane oxidation | A few years |
aModified from Lovelock and Margulis (1974).
The mean temperature of Earth is a result of input of energy from the sun and loss of energy by emission of radiation. The input of energy is a function of the reflectivity (albedo) of Earth. Ice reflects 80–95% of incident light, dry grassland 30–40%, and a conifer forest 10–15%. Therefore seasonal changes in vegetation substantially alter the amount of radiation absorbed by the surface. Major changes in vegetation due to human activity have the same effect. The surface temperature of Earth also depends on atmospheric concentrations of carbon dioxide, nitrous oxide, and methane, greenhouse gases whose concentrations are influenced by the biosphere.
3.6.8 Evolutionary Perspectives on Gaia
It is now generally appreciated that the chemical disequilibrium of Earth’s atmosphere is due to the activities of living organisms, but acceptance of this view is surprisingly recent. Indeed, until 1974, when James Lovelock and Lynn Margulis built on Vernadsky’s theories and proposed the Gaia hypothesis, most scientists believed that living organisms were buffeted by powerful physical forces that they were unable to influence. The form in which the Gaia hypothesis was first advanced (Lovelock, 1979), proposed that living organisms evolved certain traits because those traits stabilized the composition of the atmosphere, thereby maintaining conditions favorable for life. In response to various criticisms, Lovelock subsequently modified his views. The modified form of Gaia postulates only that the activities of living organisms have had, and continue to have, major effects on the composition of the atmosphere. It does not specify why organisms evolved those features.
Debates over the validity of the Gaia hypothesis have focused on two very different issues. One concerns the degree to which the composition of the atmosphere and, hence, Earth’s climate, have actually been precisely regulated. This is an empirical issue that can be and is being resolved by accumulating detailed data on temporal changes in Earth’s atmosphere and climate. Fluctuations have occurred, but they have been less than would have been occurred without the involvement of life.
The second issue, which is more relevant to the focus of this chapter, concerns the evolutionary basis of the profound influences living organisms have on Earth’s atmosphere. In part this debate continues because of a widespread failure to appreciate limitations on the mechanisms of evolutionary change. For adaptive evolutionary change to happen, an allele must confer benefits to its possessors when the allele is present in only a tiny fraction of members of a population. In other words, an allele must be able to increase in frequency when it is rare.
The severe limitation that this requirement imposes is made clear by considering one of Lovelock’s most famous and provocative examples – Daisy World. Lovelock proposed that Earth’s temperature could have been maintained at a relatively constant level despite a steady reduction in solar energy input over the eons by a change in Earth’s albedo caused by a change from a landscape dominated by, say, white daisies to one dominated by dark daisies. Calculations of the potential consequences of changes in the reflective properties of dominant organisms on Earth’s temperature are not controversial. The evolutionary problem is to identify what would favor dark daisies when they were rare. Clearly, when dark daisies are rare, they would have an utterly trivial influence of Earth’s albedo.
An answer follows from noting that in a cooling world, a dark daisy might well benefit directly (increased photosynthetic rate) by being warmer. If so, dark daisies could out-produce their white associates and, over many generations, come to dominate the daisy population. The end result would be a significant change in Earth’s albedo, but the change would be a byproduct of benefits accruing to individual daisies from their color. This example illustrates why most evolutionary biologists reject the view that organisms evolved traits because of their effects on the atmosphere, while accepting that the activities of organisms do exert powerful influences on the maintenance of Earth’s atmosphere.
3.7 How Biogeochemical Cycles Affect Life
Geochemical conditions on Earth have profound effects on living organisms. Our anthropocentric view knows that life flourishes at neutral pH, temperature ranges from freezing to 40°C, and pressures of about 1 atm. However, microbial life occurs at much more extreme conditions as well. In addition, organisms are influenced by changes in biogeochemical cycles.
3.7.1 Extreme Environments
Living organisms require water, an energy source, and the necessary nutrients to make cellular material for growth. Some places on Earth do not provide combinations of these resources necessary for organisms to survive. For example, some areas of the Atacama Desert in Peru have received no rain for more than 30 years (E. I. Friedmann, personal communication) and harbor no life. Likewise, living organisms are not known to grow at temperatures exceeding about 115°C and below about –10°C (Morita, 1975). Other physico-chemical constraints on life include high and low pH, high salinity, and high radiation. The pH range for microbial growth is remarkably broad, extending from about pH 1 to more than 12. Some Archaea are able to grow in saturated brines. Other microorganisms, such as the bacterial genus Deinococcus, can survive high doses of UV and radioisotopic radiation. Furthermore, some environments, such as acid hot springs, that have stressful combinations of these limiting factors, nonetheless support life. Thus, microorganisms have evolved to live in a wide range of environments on Earth, many of which are inhospitable to more complex organisms.
3.7.2 Response of Ecosystems to Changes in Biogeochemical Cycles and Climate
Perturbation of normal geochemical cycles is increasing as a result of human activities. One example is the ozone hole in the stratosphere centered over the North and South Poles. The ozone layer, which absorbs ultraviolet radiation in the stratosphere, is formed by the reaction of oxygen with sunlight. Stratospheric ozone concentrations are decreasing because chlorofluorocarbon (CFC) compounds used as refrigerants and air conditioners (such as Freon) are increasingly released to the atmosphere where they are converted into fluorine, chlorine, and carbon by ultraviolet light in the stratosphere. Chlorine interacts with ozone to convert it to oxygen. Chlorine is not degraded in this process, so it can cause the destruction of many additional ozone molecules.
The increase in fossil fuel burning since the industrial revolution, coupled with deforestation, has resulted in increased concentrations of carbon dioxide in the atmosphere. Increased concentrations of carbon dioxide affect plant and animal life both directly and indirectly. Directly, higher atmospheric carbon dioxide concentrations can, if other factors are not limiting, stimulate photosynthesis, and hence, plant growth rates (Oechel and Strain, 1985). This “fertilizing” effect is most pronounced in agricultural systems in which levels of water and mineral nutrients are highly favorable (Bazzaz, 1990; Mooney et al., 1991). Natural ecosystems in which rates of photosynthesis are limited by moisture, temperature, or low levels of soil nutrients, may experience only brief or minor increases in productivity (Field et al., 1992; Mellilo et al., 1993; Parton et al., 1995; Schimel, 1995). However, in combination with higher temperatures and longer growing seasons, carbon dioxide enrichment may result in increased rates of photosynthesis and increased rates of storage of carbon in forested ecosystems, as is currently happening in temperate and tropical forests in the New World (Fan et al., 1998; Phillips et al., 1998).
Increases in concentrations of carbon dioxide within projected ranges are not expected to have direct effects on animals. However, changes in plant productivity and chemical composition of plant tissues that are caused by carbon dioxide enrichment, alter the palatability of plant tissues, and, hence, population dynamics of herbivores and the carnivores that feed on them (Ayres, 1993; Fajer et al., 1992).
Increases in temperature can affect natural ecosystems by changing the spatial distribution of climates and, hence, ecosystems. For example, the species living in the major vegetation regions in eastern North America are predicted to be displaced northward by 100 to 1000 km (Overpeck et al., 1991). Thus, pines, oaks, birch, spruce, and non-grass prairie plants must either undergo major shifts in ranges or face extinction. Furthermore, the period of time over which climate warming is projected to occur is on the order of hundreds rather than thousands of years as occurred during the most recent postglacial period. Plant species differ in their abilities to adapt to and respond to rapid changes in climate and habitats. Species that have broad ranges, good dispersal mechanisms, and broad habitat tolerances are expected to handle the changes better than species with more restrictive requirements and poorer dispersal abilities. Similar arguments apply to animals. Animals that are judged to be most susceptible include local endemic species, rare species, top carnivores, migratory species, and species that depend on highly specific plant resources (Terborgh and Winter, 1980).
Species interactions and community structure will also be influenced by major climate changes, but details are very difficult to predict. The paleoecological record indicates that the species that composed the communities of organisms did not simply shift their ranges together during the cooling that accompanied glacial expansion or the warming that followed the retreat of the glaciers. Instead, species responded individualistically. As a result, past ecological communities were characterized by mixtures of species that differ from any found on Earth today. The composition of future ecological communities is certain to hold many surprises, not all of which are likely to be favorable from a human perspective.
Many types of organic molecules are basic constituents of living organisms or are produced by them. Besides carbon and hydrogen, most naturally occurring organic molecules contain one or more of four key elements, all of which are from the second and third period of the periodic table: N, O, P, and S. Carbon atoms form most of the skeleton of these molecules. Bonding of carbon can lead to very large molecules with a variety of structures. Hydrogen may share one or more of the valence electrons of carbon and may also participate in hydrogen bonds. Hydrogen bonds also involve nitrogen and oxygen and are important in determining the structures of DNA and many other molecules. Much of the nitrogen is found in amino (—NH2) groups, which provide the possibility of basic behavior as well as polarity, hydrophilic behavior, and in some cases, water solubility. Oxygen, in carbonyl (C=0), carboxyl (—COOH), and hydroxyl groups (—OH), provides acidic and hydrophilic character.
Most organo-phosphorus compounds are phosphates, R–PO4. The bond energy of the phosphate to the rest of the molecule is central to the flow of energy in all metabolic processes. Sulfur, found largely as sulfide, is central to providing three dimensional rigidity by uniting parts of a molecule by the disulfide (—S—S—) bridge.
Amino acids, the building blocks of giant protein molecules have a carboxyl group and an amino group attached to the same carbon atom (Fig. 3-10). A protein is a linear polymer of amino acids combined by peptide linkages. Twenty different amino acids are common in proteins. Their side chains, which have a variety of chemical properties, control the shapes and functions of proteins. Some of these side chains are hydrophobic (Fig. 3-10a), others are hydrophilic (Fig. 3.10b), and still others (Figure 3.10c) occur either on the surface or the interiors of proteins.
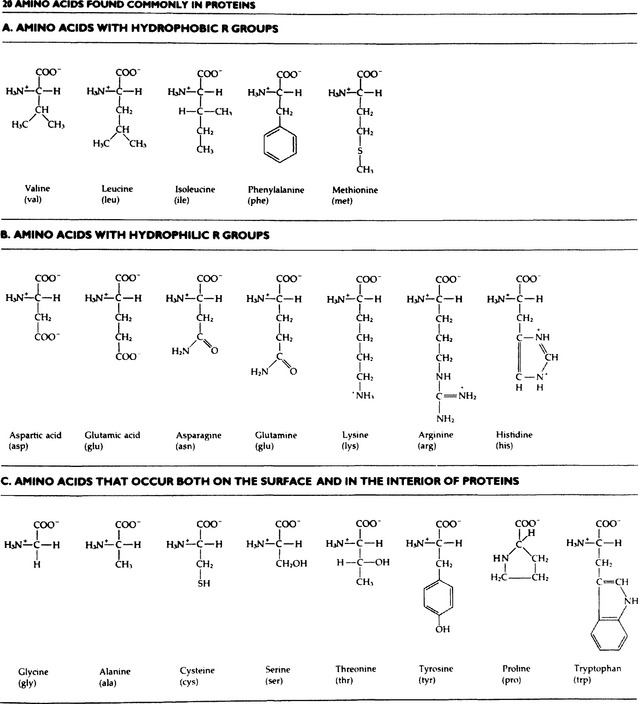
Fig. 3-10 The 20 protein amino acids divided by R group character as (a) hydrophobic, (b) hydrophilic, and (c) mixed. (Reprinted with permission from W. K. Purves and G. H. Orians, “Life: The Science of Biology,” pp. 63–81, Copyright © 1987 by Sinauer Associates, Inc., Sunderland, MA.)
Carbohydrates form a diverse group of compounds (Fig. 3-11) that share an approximate formula (CH2O)n. The major categories of carbohydrates are monosaccharides (simple sugars, Fig. 3-11a); oligosaccharides (small numbers of simple sugars linked together, Fig. 3-11b); and polysaccharides (very large molecules, Fig. 3-11c), among which are starches, glycogen, cellulose, and other important compounds. Derivative carbohydrates, such as sugar phosphates and amino sugars, contain additional elements. Important amino sugars are cartilage and chitin, the principal structural carbohydrate in insect skeletons and cell walls of fungi.
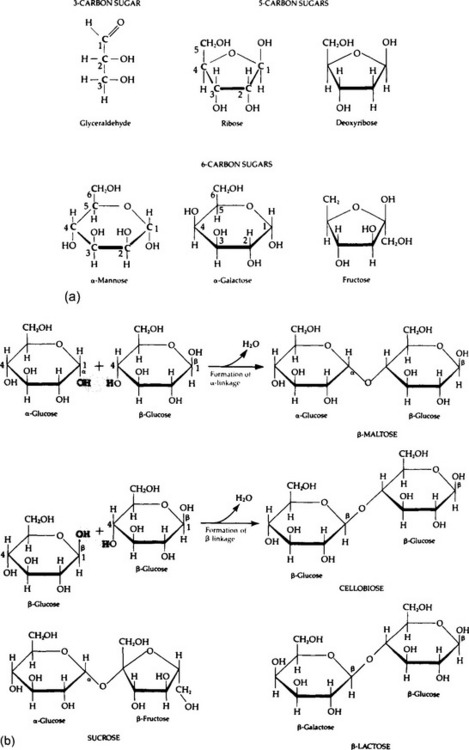
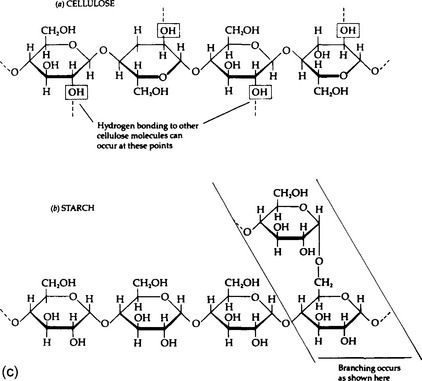
Fig. 3-11 Carbohydrates in (a) 3-, 5-, and 6-carbon sugars (monosaccharides), (b) oligosaccharides, and (c) polysaccharides. (Reprinted with permission from W. K. Purves and G. H. Orians, “Life: The Science of Biology,” pp. 63–81, Copyright © 1987 by Sinauer Associates, Inc., Sunderland, MA.)
Lipids (Fig. 3-12) are insoluble in water and release large amounts of energy when they are metabolized. Lipids are composed of two principal building blocks, fatty acids (Fig. 3-12a), and glycerol (Fig. 3-12b). Three fatty acids (carboxylic acids with long hydrocarbon tails) combine with one molecule of glycerol to form a triglyceride. More complex lipids are formed by the addition of other groups, the most important of which contain phosphorus (Fig. 3.12c).
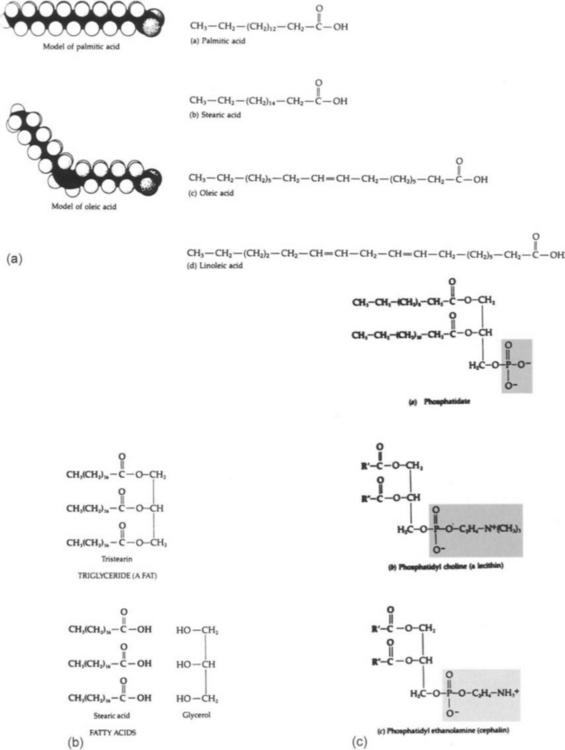
Fig. 3-12 Lipids consist of a triglyceride, three fatty acids such as those in (a) joined to glycerol (b). Other lipids include other functional groups such as phosphate derivatives (c). (Reprinted with permission from W. K. Purves and G. H. Orians, “Life: The Science of Biology,” pp. 63–81, Copyright © 1987 by Sinauer Associates, Inc., Sunderland, MA.)
Deoxyribonucleic acid (DNA, Fig. 3-13) is the genetic material of all organisms, including plants, animals, and microorganisms. (Some viruses lack DNA, but use RNA (ribonucleic acid) in its place.) DNA carries all the hereditary information of the organism and is therefore replicated and passed from parent to offspring. RNA is formed on DNA in the nucleus of the cell. The RNA carries the genetic information to the cytoplasm where it is used to produce proteins on the ribosomes. The specific proteins formed include enzymes which carry out the characteristic activities of the organism. Both RNA and DNA are formed from monomers, called nucleotides, each of which consists of a simple sugar, a phosphate group, and a nitrogen-containing base. The complex structure of DNA is founded on only four bases. A tremendous wealth of information is contained in the precise ordering of these bases to form the genetic code.
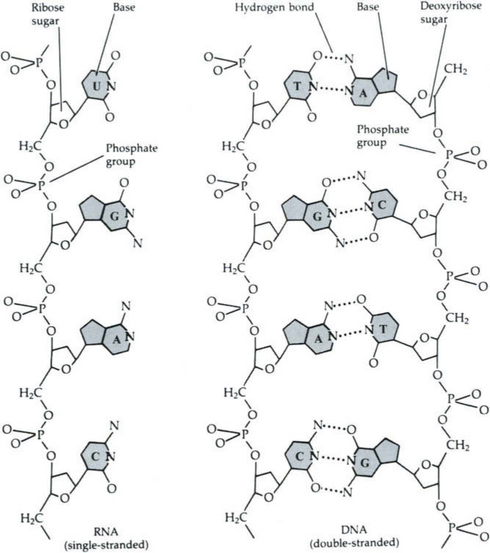
Fig. 3-13 RNA and DNA, the carriers of genetic information. Bases are denoted as A = adenine, T = thymine, G = guanine, C = cytosine, and U = uracil. Note that RNA contains U where DNA contains T. (Reprinted with permission from W. K. Purves and G. H. Orians, “Life: The Science of Biology,” pp. 63–81, Copyright © 1987 by Sinauer Associates, Inc., Sunderland, MA.)
Amend, J. P., Shock, E. L. Energetics of amino acid synthesis in hydrothermal ecosystems. Science. 1998; 281:1659–1662.
Andreae, M. O., Bernard, W. R. The marine chemistry of dimethyl sulfide. Marine Chem. 1984; 14:267–269.
Awramik, S. M., Schopf, J. W., Walter, M. R. Filamentous fossil bacteria 3. 5 Ö 109 years old from the Archaen of Western Australia. Precambrian Res. 1983; 20:357–374.
Ayers, M. P. Plant defense, herbivory, and climate change. In: Kareiva P. M., Kingsolver J. G., Huey R. B., eds. Biotic Interactions and Global Change. New York: Sinauer Associates; 1993:75–94.
Bazzaz, F. A. The response of natural ecosystems to the rising CO2 levels. Annu. Rev. Ecol. System. 1990; 21:167–196.
Chang, S., Desmarias, D., Mack, R., Miller, S. L., Strathern, G. E. Prebiotic organic synthesis and the origin of life. In: Schopf J. W., ed. Earth’s Earliest Biosphere, Its Origin and Evolution. Sunderland, MA: Princeton University Press; 1983:53–88.
Cyr, H., Pace, M. L. Magnitude and patterns of herbivory in aquatic and terrestrial ecosystems. Nature. 1993; 36:148–150.
Fajer, E. D., Bowers, M. D., Bazzaz, F. A. The effects of nutrients and enriched CO2 environments on production of carbon-based allelochemicals in Plantago: a test of the carbon/nutrient balance hypothesis. Am. Natur. 1992; 140:707–723.
Fan, S., Gloor, M., Mahlman, J., Pacala, S., Sarmiento, J., Takahashi, T., Tans, P. A large terrestrial carbon sink in North America implied by atmospheric and oceanic carbon dioxide data and models. Science. 1998; 282:442–446.
Field, C. B., Chapin, F. S., III., Matson, P. A., Mooney, H. A. Responses of terrestrial ecosystems to the changing atmosphere: a resource-based approach. Annu. Rev. Ecol. System. 1992; 23:201–235.
Hofmann, H. J., Schopf, J. W. Early Proterozoic microfossils. In: Schopf J. W., ed. Earth’s Earliest Biosphere, Its Origin and Evolution. Princeton, New Jersey: Princeton University Press; 1983:321–359.
Hutchinson, G. E. Nitrogen in the biogeochemistry of the atmosphere. Am. Scient. 1944; 32:178–195.
Longhurst, A. Ecological Geography of the Sea. Princeton, New Jersey: Academic Press; 1998.
Lovelock, J. E. Gaia. A New Look at Life on Earth. London: Oxford University Press; 1979.
Lovelock, J. E., Margulis, L. Atmospheric homoeostatis by and for the biosphere: the Gaia hypothesis. Tellus. 1974; 26:1–10.
Melillo, J. M., McGuire, A. D., Kicklighter, D. W., Moore, B., III., Vorosmarty, C. J., Schloss, A. L. Global climate change and terrestrial net production. Nature. 1993; 363:234–240.
Miller, S. L. A production of amino acids under possible primitive Earth conditions. Science. 1953; 117:528–529.
Mooney, H. A., Drake, B. G., Luxmoore, R. J., Oechel, W. C., Pitelka, L. F. Predicting ecosystem responses to elevated CO2 concentrations. Bioscience. 1991; 41:96–104.
Morita, R. Y. Psychrophilic bacteria. Bacteriol. Rev. 1975; 39:144–167.
Oechel, W. C., Strain, B. R. Native species responses to increased carbon dioxide concentration. In: Strain B. R., Cure J. D., eds. Direct Effects of Increasing Carbon Dioxide on Vegetation. Springfield, VA: U. S. Department of Energy; 1985:117–154.
Overpeck, J. T., Bartlein, P. J., Webb, T., III. Potential magnitude of future vegetation change in eastern North America: Comparisons with the past. Science. 1991; 254:692–695.
Parton, W. J., Scurlock, J. M. O., Ojima, D. S., Schimel, D. S., Hall, D. O., SCOPEGRAM group members. Impact of climate change on grassland production and soil carbon worldwide. Glob. Change Biol. 1995; 1:13–22.
Perry, J. J., Staley, J. T. Microbiology: Dynamics and Diversity. Saunders College Publishing; 1997.
Phillips, O. L., Malhi, Y., Higuchi, N., Laurance, W. F., Nuñez, P. V., Vasquez, R. M., Laurance, S. G., Ferreria, L. V., Stern, M., Brown, S., Grace, J. Changes in the carbon balance of tropical forests: evidence from long-term plots. Science. 1998; 282:4398–4442.
Rubey, W. W. Geological history of sea water. An attempt to state the problem. Geol. Soc. Am. Bull. 1951; 62:1111–1148.
Rubey, W. W. Development of the hydrosphere and atmosphere, with special reference to probable composition of the early atmosphere. In: Poldenvaart A., ed. Crust of the Earth. Ft. Worth, TX: Geological Society of America; 1955:631–650.
Schimel, D. S. Terrestrial ecosystems and the carbon cycle. Glob. Change Biol. 1995; 1:77–91.
Terborgh, J., Winter, B. Some causes of extinction. In: Soulé M. E., Wilcox B. A., eds. Conservation Biology: An Evolutionary-Ecological Perspective. New York: Sinauer Associates; 1980:119–133.
Vairavamurthy, A., Andreae, M. O., Iverson, R. L. Biosynthesis of dimethylpropionthetin by Hymenomnonas carterae in relation to sulfur source and salinity variations. Limnol. Oceanogr. 1985; 30:59–70.
Vitousek, P. M., Howarth, R. W. Nitrogen limitation on land and in the sea. How can it occur? Biogeochemistry. 1991; 13:87–115.
Vitousek, P. M., Aber, J. D., Howarth, R. W., Likens, G. E., Matson, P. A., Schindler, D. W., Schlesinger, W. H., Tilman, D. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Apps. 1997; 7:737–750.
Whitman, W. B., Coleman, D. C., Wiebe, W. J., Prokaryotes: the unseen majority. Proc. Natl Acad. Sci. USA; 95. 1998:6578–6583.