Epidemiology of Infections
David B. Haslam
Infections in the newborn are often classified by their timing relative to birth and include congenital, perinatal, early-onset, and late-onset disease. These are clinically useful designations because the mechanisms of infection, etiologies, and outcomes are distinct at each stage. Congenital infection denotes infection acquired in utero. Such infections are generally caused by viral or other non-bacterial organisms and are often associated with injury to developing organs (see Chapter 131 ). Perinatal infection indicates acquisition around the time of delivery. Perinatally acquired organisms include both bacteria and viruses, some of which are the same as those causing congenital infection but often manifest with different features. Early-onset infection occurs in the 1st wk of life and is generally the consequence of infection caused by organisms acquired during the perinatal period. Late-onset infection occurs between 7 and 30 days of life and may include bacteria, viruses, or other organisms that are typically acquired in the postnatal period. Hospital-acquired infections typically occur beyond the 1st wk of life (see Chapter 130 ).
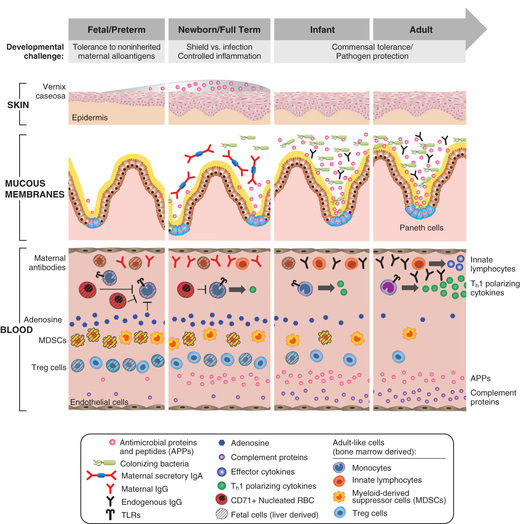
Neonates are uniquely prone to invasive disease because of their lack of fully responsive innate immunity (Fig. 129.1 ). Attenuated immune responses often result in minimal or nonspecific clinical manifestations, and effective treatment requires attention to subtle signs of infection. Compared to older infants, newborns are often treated empirically while awaiting results of laboratory investigations. Preterm infants are particularly susceptible to infection because of their decreased innate immune and barrier defenses and their prolonged stay in hospital settings.
Incidence and Epidemiology
Despite advances in maternal and neonatal care, infections remain a frequent and important cause of neonatal and infant morbidity and mortality. Up to 10% of infants have infections in the 1st mo of life. Newborn infection is more common in areas with limited access to healthcare than in areas with well-established healthcare infrastructure. The overall incidence of neonatal sepsis ranges from 1 to 5 cases per 1,000 live births. Estimated incidence rates vary based on the case definition and the population studied. Globally, neonatal sepsis and other severe infections were responsible for an estimated 430,000 neonatal deaths in 2013, accounting for approximately 15% of all neonatal deaths.
A number of bacterial and nonbacterial agents may infect newborns in the intrapartum or postpartum period (Table 129.1 ). Although herpes simplex virus (HSV), human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), and tuberculosis (TB) can each result in transplacental infection, the most common mode of transmission for these agents is intrapartum , during labor and delivery with passage through an infected birth canal (HIV, HSV, HBV), or postpartum , from contact with an infected mother or caretaker (TB) or with infected breast milk (HIV) (Fig. 129.2 and Table 129.2 ). Any microorganism inhabiting the genitourinary or lower gastrointestinal tract may cause intrapartum and postpartum infection. The most common bacteria are group B streptococcus (GBS), Escherichia coli, and Klebsiella spp. Salmonella spp. are common causes of gram-negative sepsis in developing countries; less common causes of bacterial infection in the United States include Citrobacter , enterococci, gonococci, Listeria monocytogenes , Streptococcus pneumoniae, and Haemophilus influenzae . The more common viruses are cytomegalovirus (CMV), HSV, enteroviruses, and HIV (Table 129.2 ).
Table 129.1
Nonbacterial Causes of Systemic Neonatal Infections
|
VIRUSES MYCOPLASMA FUNGI PROTOZOA |
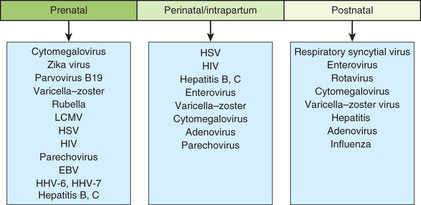
Table 129.2
Period of Transmission of Selected Viruses to the Fetus or Newborn Infant
| VIRUSES | CONGENITAL | NATAL | POSTNATAL |
|---|---|---|---|
| Adenovirus | + | + | + |
| Chikungunya | ++ | + | − |
| Cytomegalovirus | ++ | ++ | ++ |
| Dengue | ++ | − | − |
| Ebola virus | ++ | + | + |
| Echoviruses | + | + | + |
| Epstein-Barr | + | − | + |
| Hepatitis A | − | ++ | + |
| Hepatitis B | + | ++ | + |
| Hepatitis C | + | ++ | − |
| Herpes simplex | + | ++ | + |
| Herpesvirus-6 | + | − | + |
| Human immunodeficiency virus | + | ++ | + |
| Human parvovirus B19 | + | − | − |
| Influenza | (+) | − | + |
| Lymphocytic choriomeningitis virus | ++ | − | − |
| Measles | + | − | + |
| Mumps | + | − | − |
| Parechovirus | − | + | + |
| Polioviruses | + | + | + |
| Rubella | ++ | − | − |
| Smallpox | + | + | + |
| St. Louis encephalitis | (+) | − | (+) |
| Type B coxsackieviruses | + | + | + |
| Vaccinia | + | + | + |
| Varicella-zoster virus | ++ | + | + |
| West Nile virus | + | − | + |
| Western equine encephalitis | + | − | + |
| Zika virus | ++ | ? | (+) |
++ , Major demonstrated route; +, minor demonstrated route; (+), suggested route, few supporting data; −, route not demonstrated.
From Harrison GJ: Approach to infections in the fetus and newborn. In Cherry JD, Demmler-Harrison GJ, Kaplan SL, et al, editors: Feigin and Cherry's textbook of pediatric infectious diseases, ed 7, Philadelphia, 2014, Elsevier (Table 66.1, p 878).
Microorganisms causing pneumonia acquired during labor and delivery include GBS, gram-negative enteric aerobes, L. monocytogenes, genital Mycoplasma, Chlamydia trachomatis, CMV, HSV, and Candida spp. (Table 129.3 ).
Table 129.3
Etiologic Agents of Neonatal Pneumonia According to Timing of Acquisition
|
TRANSPLACENTAL PERINATAL POSTNATAL |
* More likely with mechanical ventilation or indwelling catheters, or after abdominal surgery.
The most common bacterial causes of neonatal meningitis are GBS, E. coli, and L. monocytogenes. S. pneumoniae, other streptococci, nontypable H. influenzae, both coagulase-positive and coagulase-negative staphylococci, Klebsiella, Enterobacter, Pseudomonas, Treponema pallidum, and Mycobacterium tuberculosis infection involving the central nervous system (CNS) may also result in meningitis.
Early- and Late-Onset Neonatal Infections
The terms early-onset infection and late-onset infection refer to the different ages at onset of infection in the neonatal period. Early-onset sepsis is defined as the onset of symptoms before 7 days of age, although some experts limit the definition to infections occurring within the 1st 72 hr of life. Late-onset sepsis is generally defined as the onset of symptoms at ≥7 days of age. Similar to early-onset sepsis, there is variability in the definition, ranging from an onset at >72 hr of life to ≥7 days of age. Early-onset infections are acquired before or during delivery (vertical mother-to-child transmission). Late-onset infections develop after delivery from organisms acquired in the hospital or the community. The age at onset depends on the timing of exposure and virulence of the infecting organism. Very-late-onset infections (onset after age 1 mo) may also occur, particularly in very-low-birthweight (VLBW) preterm infants or term infants requiring prolonged neonatal intensive care.
The incidence of neonatal bacterial sepsis varies from 1-4 per 1,000 live births, with geographic variation and changes over time. Studies suggest that term male infants have a higher incidence of sepsis than term females. This sex difference is less clear in preterm low-birthweight (LBW) infants. Attack rates of neonatal sepsis increase significantly in LBW infants in the presence of maternal chorioamnionitis, congenital immune defects, mutations of genes involved in the innate immune system, asplenia, galactosemia (E. coli), and malformations leading to high inocula of bacteria (e.g., obstructive uropathy).
Data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network documented rates of early-onset sepsis among almost 400,000 live births at Network centers. The overall rate of early-onset sepsis was 0.98 cases per 1,000 live births, with rates inversely related to birthweight: 401-1,500 g, 10.96 per 1,000 births; 1,501-2,500 g, 1.38/1,000; and >2,500 g, 0.57/1,000 (Table 129.4 ).
Table 129.4
Rates of Early-Onset Sepsis Per 1,000 Live Births*
| BIRTHWEIGHT (g) | ||||
|---|---|---|---|---|
| 401-1,500 | 1,501-2,500 | >2,500 | All | |
| All | 10.96 | 1.38 | 0.57 | 0.98 |
| Group B streptococci | 2.08 | 0.38 | 0.35 | 0.41 |
| Escherichia coli | 5.09 | 0.54 | 0.07 | 0.28 |
* NICHD Neonatal Research Network/CDC Surveillance Study of Early-Onset Sepsis.
Adapted from Stoll BJ, Hansen NI, Sanchez PJ, et al: Early onset neonatal sepsis: the burden of group B streptococcal and E. coli disease continues, Pediatrics 127(5):817–826, 2011.
The incidence of meningitis is 0.2-0.4 per 1,000 live births in newborn infants and is higher in preterm infants. Bacterial meningitis may be associated with sepsis or may occur as a local meningeal infection. Up to one third of VLBW infants with late-onset meningitis have negative blood culture results . The discordance between results of blood and cerebrospinal fluid (CSF) cultures suggests that meningitis may be underdiagnosed among VLBW infants and emphasizes the need for culture of CSF in VLBW infants when late-onset sepsis is suspected and in all infants who have positive blood culture results. Most neonates with sepsis presenting in the 1st day of life have a positive blood culture; analysis of CSF is usually deferred until the unstable cardiorespiratory status (shock, respiratory failure) has stabilized.
Pathogenesis
Early-Onset Infections
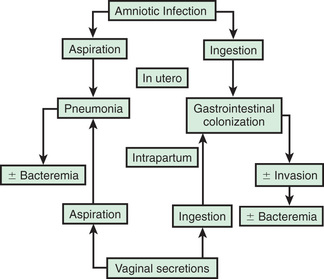
In most cases, the fetus or neonate is not exposed to potentially pathogenic bacteria until the membranes rupture and the infant passes through the birth canal and/or enters the extrauterine environment. The human birth canal is colonized with aerobic and anaerobic organisms that may result in ascending amniotic infection and/or colonization of the neonate at birth. Vertical transmission of bacterial agents that infect the amniotic fluid and vaginal canal may occur in utero or, more often, during labor and delivery (Fig. 129.3 ).
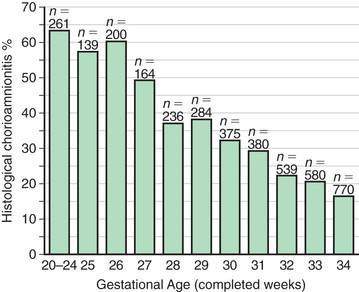
Chorioamnionitis results from microbial invasion of amniotic fluid, often as a result of prolonged rupture of the chorioamniotic membrane. Amniotic infection may also occur with apparently intact membranes or with a relatively brief duration of membrane rupture. The term chorioamnionitis refers to the clinical syndrome of intrauterine infection, which includes maternal fever, with or without local or systemic signs of chorioamnionitis (uterine tenderness, foul-smelling vaginal discharge/amniotic fluid, maternal leukocytosis, maternal and/or fetal tachycardia). Chorioamnionitis may also be asymptomatic, diagnosed only by amniotic fluid analysis or pathologic examination of the placenta. The rate of histologic chorioamnionitis is inversely related to gestational age at birth (Fig. 129.4 ) and directly related to duration of membrane rupture.
Chorioamnionitis was thought to result from infection of the amniotic fluid but is now better defined by the term intrauterine inflammation or infection at birth (Triple I) . This is defined by fetal tachycardia, maternal leukocytosis (>15,000 cells in the absence of corticosteroids), purulent fluid from the cervical os, biochemical or microbiologic amniotic fluid changes consistent with infection, and fever (≥39.0°C/10.2°F) (see Chapter 131.2 ).
Rupture of membranes for >24 hr was once considered prolonged because microscopic evidence of inflammation of the membranes is uniformly present when the duration of rupture exceeds 24 hr. At 18 hr of membrane rupture, however, the incidence of early-onset disease with group B streptococcus (GBS) increases significantly; 18 hr is the appropriate cutoff for increased risk of neonatal infection (see Chapter 211 ).
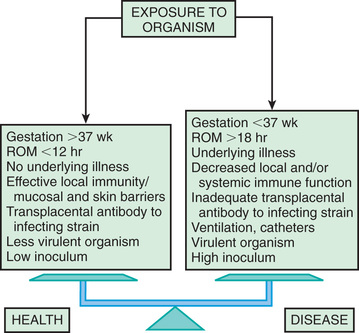
Bacterial colonization does not always result in disease. Factors influencing which colonized infant will experience disease are not well understood but include prematurity, underlying illness, invasive procedures, inoculum size, virulence of the infecting organism, genetic predisposition, the innate immune system, host response, and transplacental maternal antibodies (Fig. 129.5 ). Aspiration or ingestion of bacteria in amniotic fluid may lead to congenital pneumonia or systemic infection, with manifestations becoming apparent before delivery (fetal distress, tachycardia), at delivery (failure to breathe, respiratory distress, shock), or after a latent period of a few hours (respiratory distress, shock). Aspiration or ingestion of bacteria during the birth process may lead to infection after an interval of 1-2 days.
Resuscitation at birth, particularly if it involves endotracheal intubation, insertion of an umbilical vessel catheter, or both, is associated with an increased risk of bacterial infection. Explanations include the presence of infection at the time of birth or acquisition of infection during the invasive procedures associated with resuscitation.
Late-Onset Infections
After birth, neonates are exposed to infectious agents in the neonatal intensive care unit (NICU), the nursery, or in the community (including family). Postnatal infections may be transmitted by direct contact with hospital personnel, the mother, or other family members; from breast milk (HIV, CMV); or from inanimate sources such as contaminated equipment. The most common source of postnatal infections in hospitalized newborns is hand contamination of healthcare personnel, underscoring the importance of handwashing.
Most cases of meningitis result from hematogenous dissemination. Less often, meningitis results from contiguous spread as a result of contamination of open neural tube defects, congenital sinus tracts, or penetrating wounds from fetal scalp sampling or internal fetal electrocardiographic monitors. Cerebral abscess formation, ventriculitis, septic infarcts, hydrocephalus, and subdural effusions are complications of meningitis that occur more often in newborn infants than in older children. Metabolic factors, including hypoxia, acidosis, hypothermia, and inherited metabolic disorders (e.g., galactosemia), are likely to contribute to risk for and severity of neonatal sepsis
Infection in Premature Infants
The most important neonatal factor predisposing to infection is prematurity or LBW. Preterm LBW infants have a 3- to 10-fold higher incidence of infection than full-term normal-birthweight infants. Possible explanations include (1) maternal genital tract infection is considered to be an important cause of preterm labor, with an increased risk of vertical transmission to the newborn; (2) the frequency of intraamniotic infection is inversely related to gestational age (see Figs. 129.1 and 129.5 ); (3) premature infants have documented immune dysfunction; and (4) premature infants often require prolonged intravenous access, endotracheal intubation, or other invasive procedures that provide a portal of entry or impair barrier and clearance mechanisms, putting them at continued risk for hospital-acquired infections.
Clinical Manifestations
The maternal history provides important information about maternal exposures to infectious diseases, bacterial colonization, immunity (natural and acquired), and obstetric risk factors (prematurity, prolonged ruptured membranes, maternal chorioamnionitis). Signs and symptoms in the neonate are often subtle and nonspecific. Temperature instability, tachypnea, lethargy, and poor feeding are common initial signs and should raise suspicion for systemic or focal infection (Table 129.5 ).
Table 129.5
Initial Signs and Symptoms of Infection in Newborn Infants
|
GENERAL GASTROINTESTINAL SYSTEM RESPIRATORY SYSTEM RENAL SYSTEM CARDIOVASCULAR SYSTEM CENTRAL NERVOUS SYSTEM HEMATOLOGIC SYSTEM |
Bacterial Sepsis
Neonates with bacterial sepsis may have either nonspecific manifestations or focal signs of infection (Table 129.5 ), including temperature instability, hypotension, poor perfusion with pallor and mottled skin, metabolic acidosis, tachycardia or bradycardia, apnea, respiratory distress, grunting, cyanosis, irritability, lethargy, seizures, feeding intolerance, abdominal distention, jaundice, petechiae, purpura, and bleeding. Table 129.6 lists World Health Organization international criteria for bacterial sepsis. The initial manifestation may involve only limited symptomatology and only one system, such as apnea alone or tachypnea with retractions, or tachycardia, or the infant may present with an acute catastrophic manifestation with multiorgan dysfunction and shock. Infants should be reevaluated over time to determine whether the symptoms have progressed from mild to severe. Later complications of sepsis include respiratory failure, pulmonary hypertension, cardiac failure, shock, renal failure, liver dysfunction, cerebral edema or thrombosis, adrenal hemorrhage and/or insufficiency, bone marrow dysfunction (neutropenia, thrombocytopenia, anemia), and disseminated intravascular coagulopathy (DIC).
Table 129.6
Clinical Criteria for the Diagnosis of Sepsis in the International Setting
IMCI, Integrated Management of Childhood Illness; WHO, World Health Organization.
Adapted from WHO: Pocket book of hospital care for children: guidelines for the management of common childhood illnesses, ed 2, Geneva, 2013, WHO, pp 45–69. http://www.who.int/maternal_child_adolescent/documents/child_hospital_care/en/ .
A variety of noninfectious conditions can occur together with neonatal infection or can make the diagnosis of infection more difficult. Respiratory distress syndrome (RDS) secondary to surfactant deficiency can coexist with bacterial pneumonia. Because bacterial sepsis can be rapidly progressive, the physician must be alert to the signs and symptoms of possible infection and must initiate diagnostic evaluation and empirical therapy in a timely manner. The differential diagnosis of many of the signs and symptoms that suggest infection is extensive; noninfectious disorders must also be considered (Table 129.7 ).
Table 129.7
Serious Systemic Illness in Newborns: Differential Diagnosis of Neonatal Sepsis
Systemic Inflammatory Response Syndrome
The clinical manifestations of infection depend on the virulence of the infecting organism and the body's inflammatory response. The term systemic inflammatory response syndrome (SIRS) is most frequently used to describe this unique process of infection and the subsequent systemic response (see Chapters 88 ). In addition to infection, SIRS may result from trauma, hemorrhagic shock, other causes of ischemia, necrotizing enterocolitis, and pancreatitis.
Patients with SIRS have a spectrum of clinical symptoms that represent progressive stages of the pathologic process. In adults, SIRS is defined by the presence of 2 or more of the following: (1) fever or hypothermia, (2) tachycardia, (3) tachypnea, and (4) abnormal white blood cell (WBC) count or an increase in immature forms. In neonates and pediatric patients, SIRS manifests as temperature instability, respiratory dysfunction (altered gas exchange, hypoxemia, acute respiratory distress syndrome), cardiac dysfunction (tachycardia, delayed capillary refill, hypotension), and perfusion abnormalities (oliguria, metabolic acidosis) (Table 129.8 ). Increased vascular permeability results in capillary leak into peripheral tissues and the lungs, with resultant peripheral and pulmonary edema. DIC results in the more severely affected cases. The cascade of escalating tissue injury may lead to multisystem organ failure and death.
Table 129.8
Definitions of Systemic Inflammatory Respiratory Response Syndrome (SIRS) and Sepsis in Pediatric Patients
From Adams-Chapman I, Stoll BJ: Systemic inflammatory response syndrome, Semin Pediatr Infect Dis 12:5–16, 2001.
Temperature Instability
Fever or hypothermia may be the only initial manifestation of serious infection in newborns. However, only approximately 50% of infected newborn infants have a temperature >37.8°C (100°F) (axillary) (see Chapter 202 ). Fever in newborn infants does not always signify infection; it may be caused by increased ambient temperature, isolette or radiant warmer malfunction, dehydration, CNS disorders, hyperthyroidism, familial dysautonomia, or ectodermal dysplasia. A single temperature elevation is infrequently associated with infection; fever sustained over 1 hr is more likely to be caused by infection. Most febrile infected infants have additional signs compatible with infection, although a focus of infection is not always apparent. Acute febrile illnesses occurring later in the neonatal period may be caused by urinary tract infection, meningitis, pneumonia, osteomyelitis, or gastroenteritis, in addition to sepsis, thus underscoring the importance of a diagnostic evaluation that includes blood culture, urine culture, lumbar puncture (LP), and other studies as indicated. Many agents may cause these late infections, including HSV, enteroviruses, respiratory syncytial virus (RSV), and bacterial pathogens. In premature infants, hypothermia or temperature instability requiring increasing ambient (isolette, warmer) temperatures is more likely to accompany infection.
Respiratory and Cardiovascular Symptoms
Early signs and symptoms of pneumonia may be nonspecific, including poor feeding, lethargy, irritability, cyanosis, temperature instability, and the overall impression that the infant is not well. Respiratory symptoms of increasing severity are grunting, tachypnea, retractions, flaring of the alae nasi, cyanosis, apnea, and progressive respiratory failure. If the infant is premature, signs of progressive respiratory distress may be superimposed on RDS or bronchopulmonary dysplasia (BPD). For infants on mechanical ventilation, the need to increase ventilator support may indicate infection. Although a common finding in neonatal sepsis, tachycardia is nonspecific. Bradycardia may also occur. Poor perfusion and hypotension are more sensitive indicators of sepsis but tend to be late findings. In a prospective national surveillance study, 40% of neonates with sepsis required volume expansion, and 29% required vasopressor support.
Signs of pneumonia on physical examination, such as dullness to percussion, change in breath sounds, and the presence of rales or rhonchi, are very difficult to appreciate in a neonate. Radiographs of the chest may reveal new infiltrates or an effusion, but if the neonate has underlying RDS or BPD, it is very difficult to determine whether the radiographic changes represent a new process or worsening of the underlying disease.
The progression of neonatal pneumonia can be variable. Fulminant infection is most frequently associated with pyogenic organisms such as GBS (see Chapter 211 ). Onset may occur during the 1st hours or days of life, with the infant often manifesting rapidly progressive circulatory collapse and respiratory failure. With early-onset pneumonia in premature infants, the clinical course and chest radiographs may be indistinguishable from those with severe RDS.
In contrast to the rapid progression of pneumonia caused by pyogenic organisms, an indolent course may be seen in nonbacterial infection. The onset can be preceded by upper respiratory tract symptoms or conjunctivitis. The infant may demonstrate a nonproductive cough, and the degree of respiratory compromise is variable. Fever is usually absent or low grade, and radiographic examination of the chest shows focal or diffuse interstitial pneumonitis or hyperinflation. Infection is generally caused by C. trachomatis, CMV, Ureaplasma urealyticum, or one of the respiratory viruses. Rhinovirus has been reported to cause severe respiratory compromise in infants, particularly those who are preterm. Although Pneumocystis (carinii) jiroveci was implicated in the past, its etiologic role is now in doubt, except in newborns infected with HIV.
Conjunctivitis
Conjunctival infection is relatively common and may be caused by a variety of organisms. The presentation includes periorbital swelling, conjunctival injection, and purulent conjunctival drainage. C. trachomatis and Neisseria gonorrhea are common causes; other gram-positive and gram-negative organisms are occasionally involved. Pseudomonas aeruginosa is an important pathogen in hospitalized VLBW infants and may be a precursor to invasive disease. Viral infections (e.g., HSV, adenovirus) are occasionally seen. Recognition of HSV infection is important to prevent corneal injury and dissemination to systemic sites.
Skin and Soft Tissue Infection
Cutaneous manifestations of infection include omphalitis, cellulitis, mastitis, and subcutaneous abscesses. Pustules likely indicate the presence of staphylococcal infection but must be distinguished from the vesicular rash of HSV infection. Staphylococcal pustulosis results in larger, pus-filled lesions 1 mm in diameter and often scattered around the umbilicus, whereas HSV infection often appears as tiny vesicles in crops, often on the scalp. Ecthyma gangrenosum indicates infection with Pseudomonas spp. and is rare except in VLBW infants. The presence of small, salmon-pink papules suggests L. monocytogenes infection. Mucocutaneous lesions suggest Candida spp. (see Chapter 261.1 ). Petechiae and purpura may be the result of systemic viral or bacterial infection.
Omphalitis
Omphalitis is a neonatal infection resulting from unhygienic care of the umbilical cord, which continues to be a problem, particularly in developing countries. The umbilical stump is colonized by bacteria from the maternal genital tract and the environment (see Chapter 125 ). The necrotic tissue of the umbilical cord is an excellent medium for bacterial growth. Omphalitis may remain a localized infection or may spread to the abdominal wall, the peritoneum, the umbilical or portal vessels, and the liver. Abdominal wall cellulitis or necrotizing fasciitis , with associated sepsis and a high mortality rate, may develop in infants with omphalitis. Prompt diagnosis and treatment are necessary to avoid serious complications. Staphylococcus aureus and gram-negative organisms are common pathogens involved.
Tetanus
Neonatal tetanus remains a serious infection in resource-limited countries (see Chapter 238 ). It results from unclean delivery and unhygienic management of the umbilical cord in an infant born to a mother who has not been immunized against tetanus. The surveillance case definition of neonatal tetanus requires the ability of a newborn to suck at birth and for the 1st few days of life, followed by an inability to suck. Neonatal tetanus typically occurs in infants 5-7 days after birth (range: 3-24 days), difficulty swallowing, spasms, stiffness, seizures, and death. Bronchopneumonia , presumably resulting from aspiration, is a common complication and cause of death. Neonatal tetanus can be prevented by immunizing mothers before or during pregnancy and by ensuring a clean delivery, sterile cutting of the umbilical cord and proper cord care after birth.
Laboratory Findings
Maternal history and infant signs should guide diagnostic evaluation (Table 129.9 ). Additionally, signs of systemic infection in newborn infants may be unrevealing, so laboratory investigation plays a particularly important role in diagnosis. Cultures and cell counts are obtained from blood and urine. CSF should be sent for Gram stain, routine culture, cell count with differential, and protein/glucose concentrations. Surface swabs, blood, and CSF are often obtained for HSV testing. Except for culture and directed pathogen testing, no single laboratory test is completely reliable for diagnosis of invasive infection in the newborn. Complete blood count may demonstrate elevated or decreased WBC count, often with a shift toward more immature forms. Thrombocytopenia can be seen in systemic bacterial or viral infection. Hyponatremia, acidosis, and other electrolyte abnormalities can be seen. Hyperbilirubinemia is nonspecific but may be an indication of systemic infection. Elevated serum transaminases may be a clue to systemic HSV or enterovirus infection.
Table 129.9
Evaluation of a Newborn for Infection or Sepsis
|
HISTORY (SPECIFIC RISK FACTORS) Maternal infection during gestation or at parturition (type and duration of antimicrobial therapy): Maternal colonization with group B streptococci, Neisseria gonorrhoeae , herpes simplex Low gestational age/birthweight Age at onset (in utero, birth, early postnatal, late) EVIDENCE OF OTHER DISEASES * Congenital malformations (heart disease, neural tube defect) Respiratory tract disease (respiratory distress syndrome, aspiration) EVIDENCE OF FOCAL OR SYSTEMIC DISEASE LABORATORY STUDIES Evidence of Infection Culture from a normally sterile site (blood, CSF, other) Demonstration of a microorganism in tissue or fluid Molecular detection (blood, urine, CSF) by specific PCR and/or 16S ribosomal DNA Evidence of Inflammation Leukocytosis, increased immature/total neutrophil count ratio Acute-phase reactants: C-reactive protein, erythrocyte sedimentation rate, procalcitonin Cytokines: interleukin-6, interleukin-B, tumor necrosis factor Pleocytosis in CSF or synovial or pleural fluid Disseminated intravascular coagulation: fibrin degradation products, D-dimer Evidence of Multiorgan System Disease |
* Diseases that increase the risk of infection or may overlap with signs of sepsis.
CSF, Cerebrospinal fluid; PCR, polymerase chain reaction.
Various serum biomarkers have been investigated for their ability to identify infants with serious bacterial infection (SBI). An immature-to-total phagocyte count (I/T ratio) (≥0.2) has the best sensitivity of the neutrophil indices for predicting neonatal sepsis. After the newborn period, serum C-reactive protein (CRP) and procalcitonin have demonstrated reasonable sensitivity and specificity for SBI. CRP may be monitored in newborn infants to assess response to therapy. Their value in the initial diagnosis of sepsis in the newborn period has yet to be clarified, as does the value of these biomarkers in determining optimal length of empirical therapy in infants with negative cultures. Cytokines (both proinflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor-α and antiinflammatory cytokines such as IL-4 and IL-10), chemokines, and other biomarkers are increased in infected infants. Elevations of serum amyloid A and the cell surface antigen CD64 also have high sensitivity for identifying infants with sepsis. Chest radiography is generally not indicated in infants without signs of respiratory infection.
Table 129.9 and 129.10 list clinical features and laboratory parameters that are useful in the diagnosis of neonatal infection or sepsis.
Table 129.10
Culture-Based and Non–Culture-Based Diagnostics for Neonatal Sepsis
| CATEGORY | PARAMETER | OPTIMAL TIMING, VOLUME OF SPECIMEN, ROUTINE/INVESTIGATIONAL* | APPLICABILITY FOR NEONATAL SEPSIS |
|---|---|---|---|
| CULTURE BASED | |||
| Blood | Culture | >1 mL of whole blood, from 2 sites | Gold standard for bacteremia |
| CSF | Culture | When clinically feasible | Optimize antimicrobial therapy |
| Urine | Culture | >72 hr of life | Not useful for EOS; potential benefits for LOS |
| Tracheal aspirate | Culture | Neonates with endotracheal tube in place and signs of progressive respiratory distress | Usually reflects colonization |
| NON–CULTURE BASED | |||
| Immune function |
MHC II TNF-α |
Investigational Investigational |
Both decreased in chorioamnionitis and sepsis |
| Neutrophil indices |
Neutropenia Absolute neutrophil count Absolute immature neutrophil count |
After 12 hr of life Consider GA, delivery mode, altitude, arterial versus venous sampling, time since birth |
Neutropenia better predictor for sepsis than leukocytosis |
| Neutrophil markers | CD64 |
Elevated for 24 hr after infection Requires 50 µL blood Results within hours Investigational |
Cut points between 2.38 and 3.62 optimal sensitivity, specificity, and NPV for EOS |
| Platelet count | Thrombocytopenia and thrombocytosis | Late findings; slow to respond | Thrombocytopenia associated with fungal infection |
| CSF cell count | CSF WBC | Uninfected neonates: mean 10 cells/mm3 ; range up to 20 cells/mm3 | Does not predict culture-proven meningitis |
| CSF chemistries |
CSF protein CSF glucose |
Term <100 mg/dL Preterm higher; 70–80% of serum glucose |
Elevated in fungal meningitis Low glucose specific for bacterial meningitis |
| Acute phase reactants |
CRP Procalcitonin |
8–24 hr after infection 2–12 hr after infection |
Good NPV Better sensitivity but less specificity than CRP |
| Sepsis panels/scores |
After 24 hr of life Investigational |
Most useful for NPV and discontinuation of antimicrobial therapy | |
* Investigational refers to an assay or parameter that is undergoing evaluation for clinical use and applicability.
CRP, C-reactive protein; CSF, cerebrospinal fluid; EOS, early-onset sepsis; GA, gestational age; LOS, late-onset sepsis; MHC II, major histocompatibility complex class II; NPV, negative predictive value; TNF, tumor necrosis factor; WBC, white blood cell count.
From Shane AL, Stoll BJ. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis, Am J Perinatol 30(2):131–141, 2013.
General Approach to Management
In the absence of specific signs of focal infection, therapy for presumed infection in the neonate is often empirical and initiated on the basis of fever or hypothermia, listlessness, irritability, or apneic episodes. Antibiotics are chosen to cover the organisms typically causing neonatal sepsis, including GBS, gram-negative organisms, Listeria , and Enterococcus . Since the latter 2 organisms are intrinsically resistant to cephalosporins, ampicillin is generally included in the empirical treatment of infants with presumed neonatal infection (Table 129.11 ).
Table 129.11
Management and Prevention of Neonatal Sepsis
| CONDITION | THERAPY | ADDITIONAL CONSIDERATIONS |
|---|---|---|
| EMPIRICAL MANAGEMENT | ||
| Early-onset sepsis |
Ampicillin + aminoglycoside 10 days for bacteremia; 14 days for GBS and uncomplicated meningitis; extend to 21-28 days for complicated infections |
Consider a third-generation cephalosporin (cefotaxime preferred) or carbapenem for meningitis. Tailor therapy to pathogen. Consider discontinuation of therapy if pathogen not isolated. |
| Late-onset sepsis |
Vancomycin + aminoglycoside Duration dependent on pathogen and site |
Alternatives to vancomycin may be considered based on local epidemiology and clinical presentation. Aminoglycoside-based regimen preferred to cephalosporin given reduced risk of resistance. Consider cephalosporin if meningitis suspected. Consider a carbapenem if third-generation cephalosporin recently received. Consider amphotericin for fungal etiologies. Tailor therapy to pathogen. Consider discontinuation of therapy if pathogen not isolated. |
| NONANTIMICROBIAL TREATMENT STRATEGIES | ||
|
Recombinant G-CSF Recombinant GM-CSF |
Enhance neutrophil number and function, but no reduction in infection when administered as prophylaxis or improvement in survival when administered as therapy. | Insufficient evidence to support the clinical use of G-CSF or GM-CSF either as treatment or prophylaxis to prevent systemic infections. |
| IVIG | Augments antibody-dependent cytotoxicity and improves neutrophilic function, but no evidence that IVIG in suspected or proven sepsis reduces death. | Insufficient evidence from 10 RCTs or quasi-RCTs to support use in neonates with confirmed or suspected sepsis. |
| PREVENTION STRATEGIES | ||
| IAP | Administration of penicillin or ampicillin 4 hr before parturition |
Successfully reduces rates of EOS caused by GBS No effect on LOS GBS |
| Fluconazole prophylaxis | Administration of weight-based dosing to neonates <1,500 g | Most beneficial in NICUs with high baseline rates of invasive candidiasis |
| BLF supplementation with a probiotic, Lactobacillus rhamnosus (GG) | BLF is a human milk glycoprotein with a role in innate immune response. LGG enhances the activity of lactoferrin. |
BLF supplementation with and without LGG reduced the incidence of 1st LOS in 472 VLBW neonates in large randomized, double-blind RCT. Additional confirmatory studies warranted. |
BLF, Bovine lactoferrin supplementation; EOS, early-onset sepsis; GBS, group B streptococcus; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IAP, intrapartum antimicrobial prophylaxis; IVIG, intravenous immune globulin, LGG, Lactobacillus rhamnosus GG; LOS, late-onset sepsis; NICUs, neonatal intensive care units; RCTs, randomized controlled trials; VLBW, very-low-birthweight.
Created with data from Carr R, Modi N, Doré C: G-CSF and GM-CSF for treating or preventing neonatal infections, Cochrane Database Syst Rev (3):CD003066, 2003; Brocklehurst P, Farrell B, King A, et al; INIS Collaborative Group: Treatment of neonatal sepsis with intravenous immune globulin, N Engl J Med 365:1201–1211, 2011; and Manzoni P, Decembrino L, Stolfi I, et al; Italian Task Force for the Study and Prevention of Neonatal Fungal Infections; Italian Society of Neonatology: Lactoferrin and prevention of late-onset sepsis in the pre-term neonates, Early Hum Dev 86(Suppl 1):59–61, 2010.
Used with permission from Shane AL, Stoll BJ. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. Am J Perinatol 30(2):131–141, 2013.
An empirical regimen for suspected early-onset sepsis in a term or late preterm infant is ampicillin , 150 mg/kg/dose intravenously (IV) every 12 hr, and gentamicin , 4 mg/kg/dose IV every 24 hr. This has long been a standard regimen for early-onset sepsis and provides coverage for the most prevalent organisms, predominantly GBS and gram-negative ones. Ampicillin plus cefotaxime (if available) or cefepime may be substituted if the patient presents with infection after discharge from the nursery, or when infection with ampicillin-resistant E. coli is suspected. Ceftriaxone may be substituted if premature infants are ≥41 wk postconception age; it may be used in term infants if they are not receiving intravenous calcium or do not have hyperbilirubinemia. There is concern this regimen may be associated with higher rates of mortality in NICU patients compared to ampicillin and gentamicin. Alterations to the standard regimen may be appropriate in some circumstances, such as suspected infection with S. aureus , in which case vancomycin may be substituted for ampicillin, and in environments where infections from antibiotic-resistant bacteria are prevalent.
Herpes simplex virus infection may present without cutaneous signs, in the absence of maternal history of infection, and in mothers receiving suppressive antiviral therapy. Therefore, management of the ill newborn requires a high index of suspicion for HSV infection. Surface swabs, blood, and CSF are obtained for HSV culture or PCR, and empirical acyclovir is often recommended while the results of these studies are pending (see Chapters 202 and 279 ).
Systemic infection caused by Candida spp. is a concern in hospitalized infants, particularly VLBW infants with central venous access catheters and prior antibiotic use. Empirical therapy for fungal infection is generally not recommended unless the patient fails to respond to broad-spectrum antibiotic therapy.
Definitive therapy is based on identification and susceptibility of the offending organism. In almost all circumstances, the leas t broad antibiotic with activity against the organism is chosen. Duration of therapy depends on the organism and the site of infection. In neonates with culture-proven sepsis, the usual course of therapy is 10 days. Longer treatment courses may be warranted if a specific focus of infection is identified (e.g., meningitis, osteomyelitis, septic arthritis). Antimicrobial therapy should be altered based on the susceptibility profile of the pathogen isolated. In infants with a negative blood culture but a clinical status that remains concerning for a systemic infection, antibiotic therapy can be extended for as long as a total of 5 to 10 days. Sepsis is unlikely in these infants if they remain well and the blood culture is sterile at 48 hr. Empirical antibiotic therapy should be discontinued after 48 hr in these neonates.
Prevention
Intrapartum antibiotics are used to reduce vertical transmission of GBS (Table 129.12 ), as well as to lessen neonatal morbidity associated with preterm labor and preterm premature rupture of membranes (see Figs. 211.2 and 211.3 in Chapter 211 ). With introduction of selective intrapartum antibiotic prophylaxis to prevent perinatal transmission of GBS, rates of early-onset neonatal GBS infection in the United States declined from 1.7/1,000 live births to 0.25/1,000. Intrapartum chemoprophylaxis does not reduce the rates of late-onset GBS disease and has no effect on the rates of infection with non-GBS pathogens (see Chapter 211 ). Of concern is a possible increase in gram-negative infections (especially E. coli ) in VLBW and possibly term infants despite a reduction in early GBS sepsis by intrapartum antibiotics.
Table 129.12
Indications for Intrapartum Antibiotic Prophylaxis to Prevent Early-Onset GBS Disease
| INTRAPARTUM GBS PROPHYLAXIS INDICATED | INTRAPARTUM GBS PROPHYLAXIS NOT INDICATED |
|---|---|
| Previous infant with invasive GBS disease | Colonization with GBS during a previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) |
| GBS bacteriuria during any trimester of the current pregnancy | GBS bacteriuria during previous pregnancy (unless another indication for GBS prophylaxis is present for current pregnancy) |
| Positive GBS screening culture during current pregnancy (unless a cesarean delivery is performed before onset of labor or amniotic membrane rupture) | Cesarean delivery before onset of labor or amniotic membrane rupture, regardless of GBS colonization status or gestational age |
|
Unknown GBS status at the onset of labor (culture not done, incomplete, or results unknown) and any of the following: |
Negative vaginal and rectal GBS screening culture in late gestation during the current pregnancy, regardless of intrapartum risk factors |
* Recommendations for the use of intrapartum antibiotics for prevention of early-onset GBS disease in the setting of threatened preterm delivery are presented in Chapter 211 .
† If amnionitis is suspected, broad-spectrum antibiotic therapy that includes an agent known to be active against GBS should replace GBS prophylaxis.
‡ If intrapartum NAAT is negative for GBS but any other intrapartum risk factor (delivery at <37 wk gestation, amniotic membrane rupture ≥18 hr, or temperature ≥38.0°C/100.4°F) is present, intrapartum antibiotic prophylaxis is indicated.
GBS, Group B streptococcus; NAAT, nucleic acid amplification test.
From Verani J, McGee L, Schrag S: Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010, MMWR Recomm Rep 59(RR-10):1–36, 2010.
Aggressive management of suspected maternal chorioamnionitis with antibiotic therapy during labor, along with rapid delivery of the infant, reduces the risk of early-onset neonatal sepsis. Vertical transmission of GBS and early-onset GBS disease is significantly reduced by selective intrapartum chemoprophylaxis (see Fig. 211.4 ). A number of candidate GBS vaccines are currently being studied. Neonatal infection with Chlamydia can be prevented by identification and treatment of infected pregnant women (see Chapter 253 ). Mother-to-child transmission of HIV is significantly reduced by maternal antiretroviral therapy during pregnancy, labor, and delivery, by cesarean delivery before rupture of membranes, and by antiretroviral treatment of the infant after birth (see Chapter 302 ).
Prevention of congenital and perinatal infections predominantly focuses on maternal health. The Centers for Disease Control and Prevention (CDC) recommends the following screening tests and treatment when indicated:
- 1. All pregnant women should be offered voluntary and confidential HIV testing at the first prenatal visit, as early in pregnancy as possible. HIV screening should be part of routine prenatal testing, unless the mother declines testing (opt-out screening). For women at high risk of infection during pregnancy (multiple sexual partners or STIs during pregnancy, intravenous drug use, HIV-infected partners), repeat testing in the third trimester is recommended. Rapid HIV screening is indicated for any women who presents in labor with an undocumented HIV status, unless she declines testing.
- 2. A serologic test for syphilis should be performed on all pregnant women at the first prenatal visit. Repeat screenings early in the third trimester and again at delivery are recommended for women in whom syphilis test results in the first trimester were positive and for those at high risk for infection during pregnancy. Infants should not be discharged from the hospital unless the syphilis status of the mother has been determined at least once during pregnancy and preferably again at delivery.
- 3. Serologic testing for hepatitis B surface antigen (HBsAg) should be performed at the first prenatal visit, even if the woman has been previously vaccinated or tested. Women who were not screened prenatally, those who are at high risk for infection (multiple sexual partners, intravenous drug use, HBsAg-positive sex partner) and those with clinical hepatitis should be retested at the time of delivery.
- 4. A maternal genital culture for C. trachomatis should be performed at the first prenatal visit. Young women (<25 yr) and those at increased risk for infection (new or multiple partners during pregnancy) should be retested during the third trimester.
- 5. A maternal culture for Neisseria gonorrhoeae should be performed at the first prenatal visit. Those at high risk for infection should be retested in the third trimester.
- 6. All pregnant women at high risk for hepatitis C infection (intravenous drug use, blood transfusion or organ transplantation before 1992) should be screened for hepatitis C antibodies at the first prenatal visit.
- 7. Evidence does not support routine testing for bacterial vaginosis in pregnancy. For asymptomatic women at high risk for preterm delivery, testing may be considered. Symptomatic women should be tested and treated.
- 8. The CDC recommends universal screening for rectovaginal GBS colonization of all pregnant women at 35-37 wk gestation, and a screening-based approach to selective intrapartum antibiotic prophylaxis against GBS (Table 129.12 ) (see Figs. 211.2 and 211.3 ). Fig. 211.4 shows the approach to the infant born after intrapartum prophylaxis (see Chapter 211 ).