Congenital and Perinatal Infections
Felicia A. Scaggs Huang, Rebecca C. Brady
Infections are a frequent and important cause of neonatal morbidity and mortality. Congenital or intrauterine infections (i.e., those transmitted across the placenta) and perinatal infections (i.e., those transmitted from the mother to the fetus or newborn infant during the birth process) represent 2 major routes of neonatal infection.
Congenital Infections
Felicia A. Scaggs Huang, Rebecca C. Brady
As many as 2% of fetuses are infected in utero; disease can be acquired prenatally from a wide variety of etiologic agents, including bacteria, viruses, fungi, and protozoa. Clinical manifestations can range from asymptomatic or subclinical to life-threatening disease. History and physical examination findings provide insight into the best approach for this immunologically immature population. (See Fig. 129.2 and Table 129.2 in Chapter 129 .)
General Approach
Infectious as well as noninfectious processes, such as underlying congenital heart disease, genetic disorders, and inborn errors of metabolism, should be considered in the differential diagnosis of congenital and perinatal infections. Because maternal infection is a prerequisite for infection in the fetus, a thorough history is essential to assess the mother for her symptoms, travel, diet, medication use, occupational exposures, and any sexually transmitted infections (STIs) during pregnancy. Clinical manifestations are varied and overlap for many of the pathogens causing intrauterine infection. Laboratory testing and/or radiologic imaging is often required to confirm the diagnosis. Treatment depends on the specific pathogen and can range from symptomatic management with close follow-up for long-term sequelae to targeted antimicrobial therapy.
Pathogenesis
The route and timing of infection can provide helpful clues as to the potential infectious etiology (Fig. 131.1 and Table 131.1 ). First-trimester infection may alter embryogenesis and result in malformations of the heart and eyes, as seen in congenital rubella syndrome. Third-trimester infection (e.g., congenital toxoplasmosis) can result in active infection with signs of hepatomegaly, splenomegaly, and generalized lymphadenopathy at birth. Infections that occur late in gestation (e.g., congenital syphilis) may lead to a delay in clinical manifestations until weeks to years after birth.
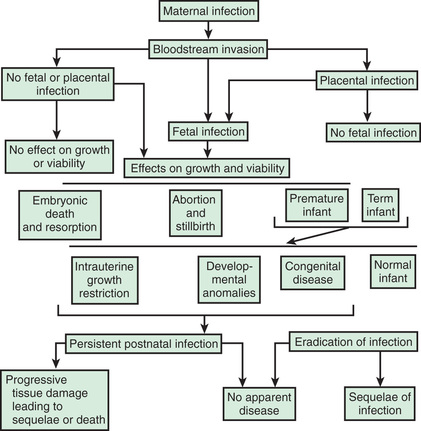
Table 131.1
Specific Agents in Effects of Transplacental Fetal Infection on the Fetus and Newborn Infant
| ORGANISM | DISEASE | ||||
|---|---|---|---|---|---|
| Prematurity | Intrauterine Growth Restriction/Low Birthweight | Developmental Anomalies | Congenital Disease | Persistent Postnatal Infection | |
| Viruses |
CMV HSV Rubeola Smallpox HBV HIV* |
CMV Rubella VZV* HIV* |
CMV Rubella VZV Coxsackievirus B* HIV* Zika |
CMV Rubella VZV HSV Mumps* Rubeola Vaccinia Smallpox Coxsackievirus B Poliovirus HBV HIV LCV Parvovirus |
CMV Rubella VZV HSV HBV HIV Zika |
| Bacteria |
Treponema pallidum Mycobacterium tuberculosis Listeria monocytogenes Campylobacter fetus Salmonella typhi |
T. pallidum M. tuberculosis L. monocytogenes C. fetus S. typhi Borrelia burgdorferi |
T. pallidum M. tuberculosis |
||
| Protozoa |
Toxoplasma gondii Plasmodium * Trypanosoma cruzi |
T. gondii Plasmodium T. cruzi |
T. gondii Plasmodium T. cruzi |
T. gondii Plasmodium |
|
* Association of effect with infection has been suggested and is under consideration.
CMV , Cytomegalovirus; HBV , hepatitis B virus; HIV , human immunodeficiency virus; HSV , herpes simplex virus; LCV , lymphocytic choriomeningitis virus; VZV , varicella-zoster virus.
From Maldonado YA, Nizet V, Klein JO, et al: Current concepts of infections of the fetus and newborn infant. In Wilson CB, Nizet V, Maldonado Y, et al, editors: Remington and Klein's infectious diseases of the fetus and newborn, ed 8, Philadelphia, 2016, Elsevier (Table 1-5).
Intrauterine infection from cytomegalovirus (CMV), Treponema pallidum , Toxoplasma gondii , rubella virus, varicella-zoster virus (VZV), and human parvovirus B19 may cause minimal or no symptoms in the mother but still may be transmitted across the placenta to the fetus. The presence of maternal antibodies to rubella prevents infection, but transmission of CMV can occur despite preexisting antibodies. Regardless of the mother's immune status, the placenta may act as a barrier, and the fetus may or may not be infected. If infection occurs, signs may or may not be noted in the fetus during pregnancy. Infection can result in spontaneous abortion, congenital malformation, intrauterine growth restriction (IUGR), premature birth, stillbirth, acute or delayed disease in the neonate, or asymptomatic persistent infection with sequelae later in life.
Clinical Manifestations
The clinical manifestations of intrauterine infections can range from asymptomatic to severe multiorgan system complications. For some agents (e.g., CMV, T. pallidum ), ongoing injury after birth leads to late sequelae. The specific clinical signs in the newborn period are usually not sufficient to make a definitive diagnosis but are useful to guide more specific laboratory testing. Symptomatic congenital infections often affect the central nervous system (CNS; brain and eyes) and the reticuloendothelial system (RES; bone marrow, liver, and spleen). Table 131.2 presents the clinical manifestations of some specific congenital infections. Congenital Zika virus infection has features that are rarely seen with other congenital infections (Table 131.3 ). No hematologic or hepatic laboratory abnormalities have been documented in infants with congenital Zika virus infection. Table 131.4 provides late sequelae of some congenital infections.
Table 131.2
Clinical Manifestations of Specific Neonatal Infections Acquired in Utero or at Delivery
| Rubella Virus | Cytomegalovirus | Toxoplasma gondii | Herpes Simplex Virus | Treponema pallidum | Enteroviruses |
|---|---|---|---|---|---|
|
Hepatosplenomegaly Jaundice Pneumonitis Petechiae or purpura Meningoencephalitis Hydrocephalus Adenopathy Hearing deficits Myocarditis Congenital defects* Bone lesions* Glaucoma* Chorioretinitis or retinopathy* Cataracts* Microphthalmia |
Hepatosplenomegaly Jaundice Pneumonitis Petechiae or purpura Meningoencephalitis Hydrocephalus Microcephaly* Intracranial calcifications* Hearing deficits Chorioretinitis or retinopathy Optic atrophy |
Hepatosplenomegaly Jaundice Pneumonitis Petechiae or purpura Meningoencephalitis Hydrocephalus* Microcephaly Maculopapular exanthems Intracranial calcifications* Myocarditis Bone lesions Chorioretinitis or retinopathy* Cataracts Optic atrophy Microphthalmia Uveitis |
Hepatosplenomegaly Jaundice Pneumonitis Petechiae or purpura Meningoencephalitis Hydrocephalus Microcephaly Maculopapular exanthems Vesicles* Myocarditis Chorioretinitis or retinopathy Cataracts Conjunctivitis or keratoconjunctivitis* |
Hepatosplenomegaly Jaundice Pneumonitis Petechiae or purpura Meningoencephalitis Adenopathy Maculopapular exanthems* Bone lesions* Glaucoma Chorioretinitis or retinopathy Uveitis |
Hepatosplenomegaly Jaundice Pneumonitis Petechiae or purpura Meningoencephalitis Adenopathy Maculopapular exanthems Paralysis* Myocarditis* Conjunctivitis or keratoconjunctivitis |
* Has special diagnostic significance for this infection
From Maldonado YA, Nizet V, Klein JO, et al: Current concepts of infections of the fetus and newborn infant. In Wilson CB, Nizet V, Maldonado Y, et al, editors: Remington and Klein's infectious diseases of the fetus and newborn, ed 8, Philadelphia, 2016, Elsevier (Table 1-6).
Table 131.3
HIV , Human immunodeficiency virus; VZV , varicella-zoster virus.
From Maldonado YA, Nizet V, Klein JO, et al: Current concepts of infections of the fetus and newborn infant. In Wilson CB, Nizet V, Maldonado Y, et al, editors: Remington and Klein's infectious diseases of the fetus and newborn, ed 8, Philadelphia, 2016, Elsevier (Table 1-7).
Diagnosis
During Pregnancy
The presence of IUGR or a physical abnormality on a prenatal fetal ultrasound raises concern for a congenital infection. The well-known acronym TORCH — T oxoplasma gondii , O ther (Treponema pallidum , human parvovirus B19, HIV, Zika virus, others), R ubella, C ytomegalovirus, and H erpes simplex virus (HSV)—is a useful mnemonic. However, the routine ordering of TORCH serology panels is not recommended because the presence of a TORCH agent IgG antibody in the mother indicates past infection but does not establish if the infection occurred during pregnancy. Maternal IgM titers to specific pathogens are only moderately sensitive, and a negative result cannot be used to exclude infection.
In certain cases, a fetal blood sample with cordocentesis can be obtained and tested for total and pathogen-specific IgM assays, polymerase chain reaction assays (PCRs), or cultures. A total IgM concentration is a helpful screening test because a normal fetal IgM is <5 mg/dL, so any elevation in total IgM may indicate an underlying infection. A positive pathogen-specific IgM test is strongly suggestive of infection, but a negative test does not rule out the organism as the cause of the fetopathy. Amniotic fluid can also be obtained and sent for PCR or culture. The presence of CMV, T. gondii , or human parvovirus B19 in amniotic fluid indicates the fetus likely is infected but cannot establish the severity of disease. Although HSV is included in the TORCH acronym, it is rarely isolated from amniotic fluid and is rarely transmitted across the placenta from mother to fetus. Fetal blood can be collected to test for human parvovirus B19 IgM and PCR.
Newborn Infant
When a congenital infection is suspected because clinical signs are present, a complete blood count with differential and platelet count along with measurements of transaminases and total/direct bilirubin are routinely performed. Additional evaluations may include a dilated funduscopic examination, auditory brainstem response (ABR) for those failing the newborn hearing screen, and CNS imaging. If available, pathologic examination of the placenta may be informative. Infectious diseases consultation is valuable in guiding the evaluation.
Neonatal antibody titers for specific pathogens are often difficult to interpret because IgG is acquired from the mother by transplacental passage, and a positive result may reflect the mother's past infection and not infection of the newborn. Neonatal IgM antibody titers to specific pathogens have high specificity and only moderate sensitivity; a negative result cannot be used to exclude infection. Paired maternal and fetal-neonatal IgG antibody titers showing higher or rising infant IgG antibodies can diagnose some congenital infections (e.g., syphilis). Total cord blood IgM and IgA are not actively transported across the placenta to the fetus and are not specific for intrauterine infection.
Although viral culture has long been considered the standard for CMV and other viral infections, PCR is sensitive, specific, and now widely accepted. The Palo Alto Medical Foundation Toxoplasma Serology Laboratory (PAMF-TSL; Palo Alto, CA: www.pamf.org/serology/ ; telephone: (650) 853-4828; e-mail: toxolab@pamf.org ) offers specialized tests and physician experts to aid in the diagnosis of congenital toxoplasmosis. If there is concern for congenital Zika virus infection, healthcare providers should refer to the Centers for Disease Control and Prevention (CDC) Guidance for US Laboratories Testing for Zika Virus Infection (www.cdc.gov/zika/laboratorie/lab-guidance.html ) to assist in collecting and sending appropriate laboratory tests from the mother, newborn infant, placenta, and umbilical cord. Currently, testing for Zika virus with real-time reverse-transcription PCR (rRT-PCR) and IgM enzyme-linked immunosorbent assay (ELISA) from neonatal urine and serum specimens is recommended. However, the most reliable method of testing has not been established. In endemic areas, this workup should be done within 2 days of delivery because it is difficult to distinguish congenital from postnatal infection if testing is done later.
Specific Infectious Agents
Important congenital infections include more than the TORCH agents. The following is a list of pathogens that may be transmitted across the placenta and the respective chapters where they are discussed in more detail, including treatment.
Viruses
- Cytomegalovirus (Chapter 282 )
- Hepatitis B (Chapter 385 )
- Hepatitis C (Chapter 385 )
- Herpes simplex virus (Chapter 279 )
- Human immunodeficiency virus (Chapter 302 )
- Human parvovirus B19 (Chapter 278 )
- Lymphocytic choriomeningitis virus (Chapter 298 )
- Rubella (Chapter 274 )
- Varicella-zoster virus (Chapter 280 )
- Zika virus (Chapter 294.12 )
Perinatal Infections
Felicia A. Scaggs Huang, Rebecca C. Brady
Perinatal infections are defined as those that are transmitted from the mother to the fetus or newborn infant during the birth process. Despite recommended universal screening of pregnant women for Chlamydia trachomatis and gonorrhea, transmission to the newborn still occurs. In addition to these STIs, other bacteria, viruses, and Candida spp. may cause perinatal infections. Similar to congenital infections, their presentation can range from asymptomatic to a sepsis-like syndrome.
General Approach
The general approach is similar to that for congenital infections and includes a detailed maternal history and a careful examination of the newborn ( see Chapter 129 ). Many clinical syndromes overlap, and therefore laboratory testing is usually required to establish a specific microbiologic etiology and guide management decisions.
Pathogenesis
The human birth canal is colonized with aerobic and anaerobic bacteria. Ascending amniotic infection may occur with either apparently intact membranes or relatively brief duration of membrane rupture. Infectious agents can also be acquired as the newborn infant passes through the vaginal canal. This acquisition may result in either colonization or disease. Factors influencing which colonized infants will experience disease are not well understood but include prematurity, underlying illness, invasive procedures, inoculum size, virulence of the infecting organism, genetic predisposition, the innate immune system, host response, and transplacental maternal antibodies.
Chorioamnionitis has been historically used to refer to microbial invasion of the amniotic fluid, often as a result of prolonged rupture of the chorioamniotic membrane for >18 hr. The term chorioamnionitis is confusing because it does not convey the spectrum of inflammatory or infectious diseases, it leaves out other intrauterine components that can be involved (e.g., decidua), and it results in significant variability in clinical practice, with the potential for a significant number of well newborns being exposed to antimicrobial agents. The term intrauterine inflammation or infection at birth , abbreviated as Triple I , has become more accepted because of the heterogeneous nature of conditions that can affect the mother and neonate (Table 131.5 ). Regardless of the definition used, prematurity (<37 wk) is associated with a greater risk of early-onset sepsis, especially with group B streptococcus.
Table 131.5
Classification of Triple I and Isolated Maternal Fever
| TERMINOLOGY | FEATURES |
|---|---|
| Isolated maternal fever |
Maternal oral temperature ≥39°C is considered a “documented fever.” If the oral temperature is ≥38°C but ≤39°C, repeat the measurement in 30 min. If the repeat value is ≥38°C, it is considered a “documented fever.” |
| Suspected Triple I | Fever without a clear source with any of the following: |
| Confirmed Triple I | All the above (from suspected Triple I) with any of the following: |
Triple I, Intrauterine inflammation or infection at birth; WBC, white blood cell count.
Adapted from Higgins RD, Saade G; Chorioamnionitis Workshop participants: Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop, Obstet Gynecol 127(3):426–436, 2016.
Aspiration or ingestion of bacteria in amniotic fluid may lead to congenital pneumonia or systemic infection, with manifestations becoming apparent before delivery (fetal distress, tachycardia), at delivery (failure to breathe, respiratory distress, shock), or after a latent period of a few hours (respiratory distress, shock). Aspiration or ingestion of bacteria during the birth process may lead to infection after an interval of 1-2 days.
Clinical Manifestations
Most perinatal infections present clinically during the 1st mo of life. Initial signs and symptoms may be either nonspecific or focal (see Chapter 129 ). Additional information on specific infectious agents and their management are reviewed in the chapters indicated below.
Specific Infectious Agents
Bacteria
- Chlamydia trachomatis (Chapter 253 )
- Escherichia coli (Chapter 227 )
- Genital mycoplasmas (Chapter 251 )
- Group B streptococci (Chapter 211 )
- Neisseria gonorrhoeae (Chapter 219 )
- Syphilis (Treponema pallidum ) (Chapter 245 )
Viruses
- Cytomegalovirus (Chapter 282 )
- Enteroviruses (Chapter 277 )
- Hepatitis B (Chapter 385 )
- Herpes simplex virus (Chapter 279 )
- Human immunodeficiency virus (Chapter 302 )
Diagnosis
The maternal history provides important information about maternal exposures to infectious diseases, bacterial colonization, immunity (natural and acquired), and obstetric risk factors (prematurity, prolonged ruptured membranes, chorioamnionitis). STIs acquired by a pregnant woman, including syphilis, N. gonorrhoeae , and C. trachomatis, have the potential for perinatal transmission.
Neonates with perinatal infections often present with nonspecific symptoms and signs; therefore the general diagnostic evaluation for the ill neonate as discussed in Chapter 202 should be followed. Table 131.6 provides a summary of laboratory tests that are useful to diagnose specific perinatal infections.
Table 131.6
Laboratory Tests in the Diagnosis of Specific Perinatal Infections
| INFECTIOUS AGENT | ACCEPTABLE SPECIMEN(S) FROM INFANT UNLESS OTHERWISE INDICATED | LABORATORY TEST |
|---|---|---|
| Chlamydia trachomatis | Conjunctiva, nasopharyngeal swab, tracheal aspirate |
Culture using special transport media Nucleic acid amplification tests (NAATs) are not FDA-approved for specimens from neonates.* |
| Genital mycoplasmas (Mycoplasma hominis , M. genitalium , Ureaplasma urealyticum ) | Tracheal aspirate, blood, or cerebrospinal fluid (CSF) |
Culture using special transport media Real-time polymerase chain reactions (PCRs) |
| Neisseria gonorrhoeae | Conjunctiva, blood, CSF, or synovial fluid |
Finding gram-negative intracellular diplococci on Gram stain is suggestive. Culture on special media establishes the diagnosis. |
| Syphilis (Treponema pallidum ) | Serum (mother) | Rapid plasma reagin (RPR) and if reactive, a specific treponemal test † |
| Serum | RPR | |
| CSF | Venereal Disease Research Laboratories (VDRL) | |
| Cytomegalovirus | Urine, saliva, blood, or CSF |
PCR for detection of CMV DNA Obtain within 2-4 wk of birth. |
| Enteroviruses | Blood, nasopharyngeal swab, throat swab, conjunctival swab, tracheal aspirate, urine, stool, rectal swab, or CSF |
PCR Cell culture (sensitivity depends on serotype and cell lines used) |
| Hepatitis B | Serum (mother) | Hepatitis B surface antigen (HBsAg) |
| Serum | If mother's HBsAg is positive, at age 9 mo, test the infant for HBsAg and hepatitis B surface antibody. | |
| Herpes simplex viruses 1 and 2 | Conjunctiva, skin vesicle scraping, whole blood, or mouth vesicles | PCR or cell culture |
| CSF | PCR | |
| “Surface cultures” (mouth, nasopharynx, conjunctiva, and anus) | PCR or cell culture | |
| Human immunodeficiency virus (HIV) | Serum (mother) | Fourth-generation HIV antigen/antibody test |
| Whole blood | HIV DNA PCR | |
| Candida species | Blood, skin biopsy, or CSF | Culture |
| Zika virus | Blood, urine, CSF |
NAT and serum IgM NAT may be falsely negative IgG antibodies may reflect maternal exposure Antibodies may cross react with other flaviviruses |
* Published evaluations of NAATs for these indications are limited, but sensitivity and specificity is expected to be at least as high as those for culture. FDA, U.S. Food and Drug Administration.
† Treponemal tests include the T. pallidum particle agglutination (TP-PA) test, T. pallidum enzyme immunoassay (TP-EIA), T. pallidum chemiluminescent assay (TP-CIA), and fluorescent treponemal antibody absorption (FTA-ABS) test.