Fever Without a Focus in the Neonate and Young Infant
Laura Brower, Samir S. Shah
Fever is a common reason for neonates and young infants to undergo medical evaluation in the hospital or ambulatory setting. For this age-group (0-3 mo), fever without a focus refers to a rectal temperature of 38°C (100.4°F) or greater, without other presenting signs or symptoms. The evaluation of these patients can be challenging because of the difficulty distinguishing between a serious infection (bacterial or viral) and a self-limited viral illness. The etiology and evaluation of fever without a focus depend on the age of the child. Three age-groups are typically considered: neonates 0-28 days, young infants 29-90 days, and children 3-36 mo. This chapter focuses on neonates and young infants.
Etiology and Epidemiology
Serious bacterial infection (SBI) occurs in 7% to 13% of neonates and young infants with fever. In this group, the most common SBIs are urinary tract infection (UTI; 5–13%), bacteremia (1–2%) and meningitis (0.2–0.5%). Escherichia coli is the most common organism causing SBI, followed by group B streptococcus (GBS). The decrease in GBS infections is related to increased screening of pregnant women and use of intrapartum antibiotic prophylaxis. Other, less common organisms include Klebsiella spp., Enterococcus spp., Streptococcus pneumoniae , Neisseria meningitidis , and Staphylococcus aureus (Table 202.1 ). Listeria monocytogenes is a rare cause of neonatal infections, potentially related to changes in public health education and improvements in food safety. Additional details about specific bacteria are available in the following chapters: Escherichia coli (Chapter 227 ), GBS (Chapter 211 ), Streptococcus pneumoniae (Chapter 209 ), Neisseria meningitidis (Chapter 218 ), Staphylococcus aureus (Chapter 208.1 ), and Listeria monocytogenes (Chapter 215 ). Specific bacterial infections that can present with fever in this age-group, although often with symptoms other than isolated fever, include pneumonia (Chapter 428 ), gastroenteritis (Chapter 366 ), osteomyelitis (Chapter 704 ), septic arthritis (Chapter 705 ), omphalitis (Chapter 125 ), cellulitis, and other skin and soft tissue infections (Chapter 685 ).
Table 202.1
Bacterial Pathogens in Neonates and Young Infants With Urinary Tract Infection, Bacteremia, or Meningitis
| FREQUENCY | URINARY TRACT INFECTION | BACTEREMIA AND MENINGITIS |
|---|---|---|
| Common | Escherichia coli |
Escherichia coli Group B streptococcus |
| Less common |
Klebsiella spp. Enterococcus spp. |
Streptococcus pneumoniae Staphylococcus aureus Klebsiella spp. |
| Rare | Group B streptococcus | |
|
Staphylococcus aureus Pseudomonas aeruginosa Enterobacter spp. Citrobacter spp. Proteus mirabilis |
Listeria monocytogenes Neisseria meningitidis Salmonella spp. Enterobacter spp. Enterococcus spp. Cronobacter sakazakii |
Herpes simplex virus (HSV ) infections (Chapter 279 ) should also be considered in febrile neonates <28 days old, particularly given the high rate of mortality and significant morbidity among survivors. Neonatal HSV is rare, with a prevalence of 0.2–0.3% among febrile neonates. Most of these infections are caused by HSV type 2, though HSV type 1 can also cause neonatal infection. Neonates with disseminated disease and skin, eye, and mouth (SEM) disease typically present at 5-12 days of life. Neonates with central nervous system (CNS) disease generally present at 16-19 days. Perinatally acquired HSV may occasionally manifests beyond 28 days of age, although some of these later-onset cases may represent postnatal acquisition.
In febrile infants who appear well, viral illnesses are much more common than bacterial or serious viral infections. The most common viruses include respiratory syncytial virus (RSV ; Chapter 287 ), enteroviruses (Chapter 277 ), influenza viruses (Chapter 285 ), parainfluenza viruses (Chapter 286 ), human metapneumovirus (Chapter 288 ), adenovirus (Chapter 289 ), parechoviruses (Chapter 277 ), and rhinovirus (Chapter 290 ).
Clinical Manifestations
In neonates and young infants, bacterial and viral infections can present with isolated fever or nonspecific symptoms, making diagnosis of serious illnesses challenging. Some neonates and young infants will have signs of systemic illness at presentation, including abnormal temperature (hypothermia <36°C [96.8°F], fever ≥38°C [100.4°F), abnormal respiratory examination (tachypnea >60 breaths/min, respiratory distress, apnea), abnormal circulatory examination (tachycardia >180 beats/min, delayed capillary refill >3 sec, weak or bounding pulses), abnormal abdominal examination, abnormal neurologic examination (lethargy, irritability, alterations in tone), or abnormal skin examination (rash, petechiae, cyanosis). Infants with septic arthritis or osteomyelitis may appear well except for signs around the involved joint or bone or may only manifest with pseudoparalysis (disuse) and paradoxical irritability (pain when attempting to comfort the child).
Diagnosis
No consensus exists on the diagnosis and empirical treatment of febrile neonates and young infants. Traditionally, all neonates <60 or <90 days of age were hospitalized; underwent laboratory evaluation of the blood, urine, and cerebrospinal fluid (CSF); and received empirical antibiotics. Additionally, some patients had stool cultures, chest radiographs, HSV evaluation, and/or received empirical antiviral agents. Under this approach, many infants without SBI or serious viral infection received evaluation, treatment, and hospitalization. Protocols were subsequently developed to identify infants at lower risk of SBI, who may be managed outside the hospital setting. The 3 most widely used are the Rochester, Philadelphia, and Boston criteria (Table 202.2 ). Clinical prediction rules are further discussed later in the Other Diagnostic Studies section. Despite these protocols, substantial variation continues to exist in the approach to and management of the febrile infant. It must be emphasized that these criteria apply to the well-appearing child; those who appear critically ill (septic) require prompt evaluation, resuscitation, and empirical antibiotic therapy (within 1 hr).
Table 202.2
Protocols to Identify Febrile Infants at Low Risk of Serious Bacterial Infection (SBI)
DTaP, Diphtheria-tetanus-pertussis; WBC, white blood cell; CSF, cerebrospinal fluid; IV, intravenous; SBI, serious bacterial infection; hpf, high-power field
Many experts advocate that all neonates ≤28 days old undergo a complete evaluation for serious infection, receive empirical antimicrobials, and be hospitalized. Of the 3 widely used criteria, only the Rochester criteria allow neonates ≤28 days to be designated as “low risk” and managed outside the hospital without antimicrobials. In one study, <1% of low-risk infants ≤28 days old had SBI; however, in another study applying the Boston and Philadelphia criteria to neonates, 3–4% of those classified as low risk had SBI.
Young febrile infants ≥29 days old who appear ill (with signs of systemic illness) require complete evaluation for SBI, including antimicrobials and hospitalization; however, well-appearing infants can be managed safely as outpatients using low-risk criteria as indicated in Table 202.2 . In each of these approaches, infants must have a normal physical examination, must be able to reliably obtain close follow-up, and must meet certain laboratory and/or radiographic criteria. Based on these protocols, all infants following the Boston or Philadelphia criteria would undergo lumbar puncture (LP), whereas low-risk infants following the Rochester criteria would not. There is substantial variation in clinical practice in the performance of LPs in well-appearing infants >28 days. Clinicians should consider multiple factors, including the home situation and ability to contact the family, when deciding about LP in this age-group.
In addition, approximately 35% of infants with bacterial meningitis do not have a positive blood culture.
The protocols discussed in Table 202.2 were initially developed for use in the emergency department (ED). Infants evaluated in the office setting may warrant a different approach when a relationship between the physician and family already exists to facilitate clear communication and timely follow-up. In one large study of febrile infants <3 mo old who were initially evaluated for fever in the office setting, clinicians hospitalized only 36% of infants but initiated antibiotics in 61 of the 63 infants with bacteremia or bacterial meningitis. These findings suggest that, with very close follow-up (including multiple in person visits or frequent contacts by telephone), some febrile infants perceived to be at low risk for invasive bacterial infection (IBI ; bacteremia and meningitis), on the basis of history, physical examination, and normal but limited laboratory testing, can be managed in an office-based setting. It is important to note that 3% of infants with SBI did not initially receive empirical antibiotics, necessitating careful consideration of risks and benefits of selective rather than universal testing and empirical antibiotic treatment of febrile infants evaluated in the office setting.
Viral Respiratory Illness
Several studies have demonstrated a decreased risk of SBI in infants with positive testing for influenza or RSV, although the risk of UTI remains significant. In one prospective study, the risk of SBI in neonates <28 days old was not altered by RSV status. Given these data, young febrile infants with bronchiolitis may not require LP, particularly if they can be closely observed or have close follow-up.
Urinary Tract Infection and Bacterial Meningitis
Traditionally, infants with abnormal findings on urinalysis (UA) would undergo complete evaluation for infection, including LP. In well-appearing infants >28 days old with an abnormal UA, some evidence suggests that the risk of bacterial meningitis is extremely low, <0.5%. For neonates 0-28 days, the risk of concomitant bacterial meningitis with UTI is 1–2%.
CSF pleocytosis in the absence of bacterial meningitis (i.e., sterile pleocytosis ) has been reported in infants with UTI. The cause is uncertain, with some studies attributing this phenomenon to traumatic LPs or undetected viral infection rather than inflammation in the context of systemic illness.
Laboratory Diagnosis
Complete Blood Count
The peripheral complete blood cell count (CBC) and differential are frequently obtained by providers when evaluating febrile neonates and infants. The white blood cell (WBC) count alone cannot accurately predict SBI risk. In one series, isolated use of the WBC cutoffs in the Rochester criteria, outside 5-15,000 WBCs/mm3 , would miss at least 33% of infants with bacteremia and 40% of those with meningitis. A prospective study found no increased risk of SBI in febrile, well-appearing infants with leukopenia (WBC count <5,000/mm3 ). The WBC count combined with other factors may help determine an infant's risk of SBI, but it should not be used in isolation to predict infection risk.
Blood Culture
The ability to identify pathogens in the blood depends on the volume of blood, the timing of the blood culture in relation to antimicrobial administration, and to a lesser degree, on the number of blood cultures obtained. A negative blood culture does not eliminate the risk of bacterial meningitis; in one study, 38% of infants with culture-proven bacterial meningitis had negative blood cultures. For additional information on the time to positivity of blood cultures in neonates and young infants, see “Discharge from the Hospital,” later.
Urinalysis
Different methods can assist in making a presumptive diagnosis of UTI while awaiting results of a urine culture. Traditional UA consists of dipstick biochemical analysis of urine for nitrites or leukocyte esterase (LE) and microscopic examination of the urine for WBCs and bacteria. One study found that the traditional UA had a higher negative predictive value (NPV) than dipstick alone (99.2% vs 98.7%), but that dipstick alone had a higher positive predictive value (PPV, 66.8% for dipstick alone vs 51.2% for traditional UA). Enhanced UA includes hemocytometer cell count (to decrease variability of urine cell counts) and Gram stain on uncentrifuged urine. The enhanced UA has a higher sensitivity but comparable specificity to traditional UA. However, the enhanced UA has not been studied in the most common protocols for evaluation of the febrile infant, and many institutions/office practices do not perform this test.
Cerebrospinal Fluid
CSF evaluation consists of culture and Gram stain, cell count, glucose and protein. Polymerase chain reaction (PCR) testing may also be sent based on the clinical scenario, usually for enterovirus or HSV. Normal CSF parameters vary by age of the infant and should be interpreted in combination with other clinical and historical risk factors, given that some infants with normal CSF parameters may have CNS infections (Table 202.3 ). The CSF Gram stain can be a useful adjunct to other CSF parameters given the high specificity of the test (99.3–99.9%; i.e., relatively few false-positive results), although the range of reported sensitivity is much broader (67–94.1%).
Table 202.3
| CSF WHITE BLOOD CELL COUNTS | CELLS/mm3 |
|---|---|
| Upper limit of normal by age* | |
| 1-28 days | 18 |
| 29-60 days | 8.5 |
| 61-90 days | 8.5 |
| 90th percentile by age † | |
| 0-7 days | 26 |
| 8-28 days | 8–9 |
| 29-56 days | 6–8 |
| 95th percentile by age ‡ | |
| 0-28 days | 19 |
| 29-56 days | 9 |
| CSF Protein | mg/dL |
| Upper limit of normal by age* | |
| 1-28 days | 131 |
| 29-60 days | 105.5 |
| 61-90 days | 71 |
| 90th percentile by age † | |
| 0-7 days | 153 |
| 8-28 days | 84–106 |
| 29-56 days | 84–105 |
| 95th percentile by age § | |
| 0-14 days | 132 |
| 15-28 days | 100 |
| 29-42 days | 89 |
| 43-56 days | 83 |
| CSF Glucose | mg/dL |
| Lower limit of normal by age* | |
| 1-28 days | 30 |
| 29-60 days | 30.5 |
| 61-90 days | 33.5 |
| 10th percentile for infants 0-56 days † | 38–43 |
* Data from Byington CL, Kendrick J, Sheng X: Normative cerebrospinal fluid profiles in febrile infants, J Pediatr 158(1):130–134, 2011. All infants had nontraumatic lumbar puncture (LP) and no evidence of bacterial or viral infection.
† Data from Chadwick SL, Wilson JW, Levin JE, Martin JM: Cerebrospinal fluid characteristics of infants who present to the emergency department with fever: establishing normal values by week of age, Pediatr Infect Dis J 30(4):e63–e67, 2011. All infants were excluded if they had identified viral or bacterial meningitis, positive blood or urine cultures, a ventriculoperitoneal shunt, recent neurosurgery/antibiotics/seizure, or a traumatic LP.
‡ Data from Kestenbaum LA, Ebberson J, Zorc JJ, et al: Defining cerebrospinal fluid white blood cell count reference values in neonates and young infants, Pediatrics 125(2):257–264, 2010. Infants were excluded for traumatic LP, serious bacterial infection, congenital infection, seizure, presence of ventricular shunt, or positive CSF testing for enterovirus.
§ Data from Shah SS, Ebberson J, Kestenbaum LA, et al: Age-specific reference values for cerebrospinal fluid protein concentration in neonates and young infants, J Hosp Med 6(1):22–27, 2011. Infants were excluded for traumatic LP, serious bacterial infection, congenital infection, seizure, presence of a ventricular shunt, positive CSF testing for enterovirus, or elevated serum bilirubin.
The interpretation of CSF can be challenging in the setting of a traumatic LP, where the CSF is contaminated with peripheral blood. Some clinicians assume a ratio of WBCs to red blood cells (RBCs) of 1 : 500 in the CSF. Others advocate calculating the expected CSF WBCs based on the peripheral blood WBCs and RBCs and then using the observed-to-predicted ratio of CSF WBCs to aid in the identification of bacterial meningitis. This calculation assumes that the ratio of WBCs to RBCs in the peripheral blood remains constant after introduction into the CSF. The formula is:
Predicted CSF WBCs=CSF RBCs×(Peripheral blood WBCs/ Peripheral blood RBCs)
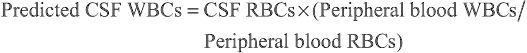
One retrospective cohort study concluded that an observed/predicted CSF WBC ratio of ≤0.01 was helpful in predicting the absence of bacterial meningitis; however, another retrospective cohort study and one case series of traumatic LPs concluded that adjustment of CSF WBC count does not improve the accuracy of diagnosis of meningitis in patients with traumatic LPs. Clinicians may consider hospitalization and empirical antimicrobials in patients with traumatic LPs (per the Philadelphia criteria) given the challenge of interpreting the CSF WBC count when there is blood contamination of the specimen.
Treatment with antibiotics prior to LP can complicate the interpretation of CSF parameters. CSF cultures are negative relatively rapidly after antibiotic administration, within 2 hr for N. meningitidis and 4-24 hr for S. pneumoniae . In patients with bacterial meningitis, CSF glucose increases to normal range, usually within 4-24 hr of antibiotic administration, while CSF protein concentrations, despite decreasing, remain abnormal for >24 hr after antibiotic administration. Changes in CSF WBC count and absolute neutrophil count (ANC) are minimal in the 1st 24 hr of antibiotic therapy. Therefore, CSF findings can provide relevant management information even in the setting of antibiotic administration before LP. Multiplex PCR testing for common bacterial pathogens should not be affected by prior antibiotic therapy.
Herpes Simplex Virus Testing
No consensus exists on which neonates should be tested and empirically treated for HSV infection. Historical and clinical features that should raise concern for HSV include exposure to individuals infected with HSV, particularly mothers with primary HSV infections or first-time genital infections, seizure or abnormal neurologic examination, vesicular rash, ill appearance, apnea, hypothermia, petechial rash/excessive bleeding, or a history of a scalp electrode. However, neonates with HSV can present without any high-risk clinical or historical features, particularly with early isolated CNS disease. Published approaches to neonatal HSV include (1) testing and empirical treatment of all neonates <21 days old who are evaluated for infection; (2) testing and empirical treatment of neonates with the presence of high-risk clinical features for HSV; and (3) testing and empirical treatment for all neonates with high-risk features plus testing the CSF of all neonates <21 days old while deferring empirical acyclovir in those without high-risk features, unless the CSF HSV PCR test is positive.
The American Academy of Pediatrics (AAP) Committee on Infectious Diseases recommends that neonates undergoing evaluation for HSV have the following laboratory studies performed: surface cultures of the mouth, conjunctiva, nasopharynx, rectum, and any vesicles; CSF PCR (sensitivity: 75–100%); whole blood PCR; and serum levels of alanine transaminase (ALT). HSV PCR testing of the mouth, conjunctiva, nasopharynx, rectum, and vesicles has been shown to be more sensitive than culture, with comparable specificity, although no direct comparisons have been performed in neonates.
Enterovirus Testing
Enterovirus is a common and typically benign cause of fever in febrile infants, although it can be difficult to distinguish from SBI on initial presentation. Enterovirus PCR testing of the CSF is a sensitive and rapid means to diagnose infection. One retrospective study of patients with CSF enterovirus testing found no cases of bacterial meningitis in patients with positive enterovirus PCR; this study did not include neonates ≤28 days old. Several studies have demonstrated shorter length of stay, fewer antibiotics, and lower cost among infants with positive CSF enterovirus test results. These results suggest that during local enterovirus seasons, and if PCR testing is available, testing for enterovirus may be of benefit in the evaluation of febrile infants and neonates. Some centers have implemented multiplex PCR panels, which permit testing for multiple viruses, including enterovirus and HSV (and bacteria), simultaneously.
Other Diagnostic Studies
Investigations have examined the utility of inflammatory markers such as C-reactive protein (CRP) and serum procalcitonin (PCT) in the diagnosis of SBI and, more specifically, IBI (bacteremia and meningitis). One meta-analysis reported that PCT is superior to WBC count and CRP for the detection of IBI in children <3 yr old, whereas another found that PCT was inferior to prediction rules in identifying SBI in young infants. A prospective multicenter cohort study of febrile infants 7-91 days old determined that the PCT was better at identifying patients with IBI than CRP, WBC count, or ANC. Building on these results, clinical prediction rules for febrile infants, such as the Step-by-Step approach, incorporate PCT (≥0.5 ng/mL) and CRP (>20 mg/L), along with age ≤21 days, ill appearance, ANC >10,000/mm3 , and pyuria in a stepwise approach to determine which patients are high risk for IBI; only 0.7% of infants who met none of those criteria had IBI.
As previously described, older infants with positive RSV and influenza testing have a very low risk of SBI beyond UTI. One large case-based survey demonstrated decreased admission rates and antibiotic use for infants with positive respiratory viral tests, and another study demonstrated that implementation of a care algorithm incorporating viral testing led to shorter length of stay and antibiotic course.
Chest radiographs are unlikely to be clinically useful in the evaluation of the febrile infant without respiratory symptoms. Studies that have examined routine use of radiographs have found limited utility because in infants without respiratory symptoms, most results will be normal, and abnormal results can be difficult to interpret.
Treatment
Antimicrobials
Neonates and infants hospitalized for evaluation for SBI should receive antimicrobial therapy. Commonly used regimens include (1) a third-generation cephalosporin (typically cefepime), (2) a third-generation cephalosporin and ampicillin, or (3) an aminoglycoside and ampicillin.
Ampicillin is the preferred treatment of GBS and covers L. monocytogenes and many Enterococcus spp. For neonates 0-28 days, options 2 or 3 have been recommended, given the risk of L. monocytogenes . For young infants >28 days, option 1 (third-generation cephalosporin: ceftriaxone) can be a reasonable choice. For ill-appearing infants or those with positive CSF Gram stains, additional antibiotics may include vancomycin or broad-spectrum antibiotics such as carbapenems. Local epidemiology and resistance patterns may assist in these choices. Neonates with concern for HSV should be empirically treated with high-dose acyclovir (60 mg/kg/day).
Treatment duration and route of antimicrobial administration depend on the infection. Additional details based on specific infections and organisms are available in the following chapters: meningitis (Chapter 129 ), urinary tract infection (Chapter 553 ), Escherichia coli (Chapter 227 ), GBS (Chapter 211 ), and HSV (Chapter 279 ).
Discharge From the Hospital
Traditionally, infants remained in the hospital receiving antimicrobial therapy until bacterial cultures were negative for 48 hr or even longer. Multiple studies have suggested that shorter culture observation periods (i.e., 24 or 36 hr) may be reasonable since most pathogens in the blood grow within this time frame when automated blood culture monitoring systems are used. In one multicenter retrospective cross-sectional study, 91% of blood cultures were positive by 24 hr and 96% by 36 hr. Fewer studies have evaluated the time to positivity of CSF and urine cultures, but in one large study of febrile infants 28-90 days old, all positive CSF cultures grew within 24 hr (median time to positivity, 18 hr). For blood cultures, 1.3% grew after 24 hr (median time to positivity, 16 hr), and for urine cultures, 0.9% grew after 24 hr (median time to positivity, 16 hr). For neonates undergoing evaluation for HSV, it is reasonable to await results of HSV testing before discharge to home. For patients with identified bacterial infections or HSV infections, the duration of the hospital stay will be determined by the specific pathogen and site of infection.
Prognosis
Most well-appearing neonates and young infants with fever recover completely and relatively quickly, depending on the etiology of the fever. Most infection-related mortality and long-term morbidity results from HSV infection and bacterial meningitis. For HSV, reported mortality rates for range from 27–31% for disseminated disease and 4–6% for CNS disease. Of those who survive, 83% of patients with disseminated disease and 31% of those with CNS disease will have normal development at 12 mo old. The mortality of bacterial meningitis varies by pathogen, but ranges from 4–15%. In one study of children who had meningitis as infants, 84% had normal development at age 5 yr.