Urinary Tract Infections
Karen E. Jerardi, Elizabeth C. Jackson
Prevalence and Etiology
Urinary tract infections (UTIs) commonly occur in children of all ages, though the prevalence varies with age. UTIs are most common in children under age 1 yr; the prevalence of afebrile symptomatic UTIs in children over age 1 yr is ~8%; the prevalence in febrile infants is 7%. During the first yr of life, the male:female ratio is 2.8 : 5.4. Beyond 1-2 yr, there is a female preponderance, with a male:female ratio of 1 : 10. In males, most UTIs occur during the first year of life; UTIs are much more common in uncircumcised males, especially in the first yr of life, where the rate is 20% in febrile uncircumcised males under age 1 yr. In females, the first UTI usually occurs by the age of 5 yr, with peaks during infancy, toilet training, and onset of sexual activity.
UTIs are caused primarily by colonic bacteria. Escherichia coli (see Chapter 227 ) causes 54–67% of all UTIs, followed by Klebsiella spp. and Proteus spp., Enterococcus, and Pseudomonas (see Chapter 129 ). Other bacteria known to cause UTIs include Staphylococcus saprophyticus, group B streptococcus, and, less commonly, Staphylococcus aureus, Candida spp., and Salmonella spp.
UTIs have been considered a risk factor for the development of renal insufficiency or end-stage renal disease in children, although some have questioned the importance of UTI as an isolated risk factor, because only 2% of children with renal insufficiency report a history of UTI. Furthermore, many children receive antibiotics for fever without a specific diagnosis (e.g., treating a questionable otitis media), resulting in a partially treated UTI. Some children with end-stage renal disease diagnosed as reflux nephropathy actually have dysplasia associated with reflux rather than scarring caused by infection and reflux.
Clinical Manifestations and Classification
The two basic forms of UTIs (defined as symptoms and a positive culture) are pyelonephritis and cystitis. Focal pyelonephritis (lobar nephronia) and renal abscesses are less common.
Pyelonephritis
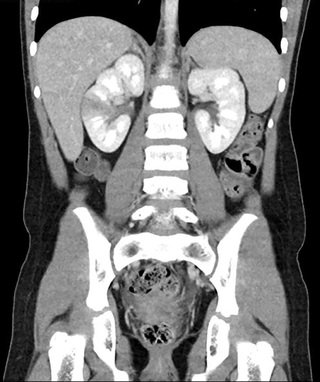
Pyelonephritis is characterized by any or all of the following: abdominal, back, or flank pain; fever; malaise; nausea; vomiting; and, occasionally, diarrhea. Fever may be the only manifestation; particular consideration should occur for a temperature > 39°C without another source lasting more than 24 hr for males and more than 48 hr for females . Newborns can show nonspecific symptoms, such as poor feeding, irritability, jaundice, and weight loss. Pyelonephritis is the most common serious bacterial infection in infants younger than 24 mo of age who have fever without an obvious focus (see Chapters 202 and 203 ). Involvement of the renal parenchyma is termed acute pyelonephritis (Figs. 553.1 and 553.2 ), whereas if there is no parenchymal involvement, the condition may be termed pyelitis. Acute pyelonephritis can result in renal injury, termed pyelonephritic scarring.
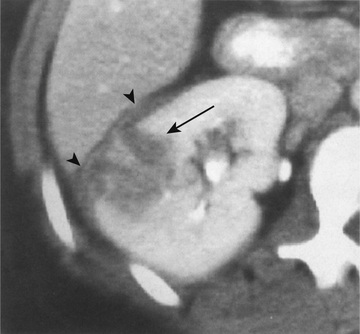
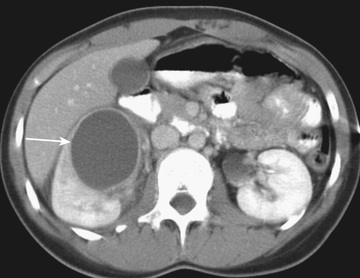
Acute lobar nephronia (acute lobar nephritis) is a localized renal parenchymal mass caused by acute focal infection without liquefaction; it more commonly occurs in older children. It may be an early stage in the development of a renal abscess (Fig. 553.3 ). Manifestations are identical to those of pyelonephritis and include fever and flank pain. The epidemiology of the causative organism is also similar to that of pyelonephritis. Renal abscess typically occurs following hematogenous spread with S. aureus or can occur following a pyelonephritic infection caused by the usual uropathogens. Most abscesses are unilateral and right sided and can affect children of all ages (Fig. 553.4 ). Both acute lobar nephronia and renal abscess are associated with an increased risk of renal scarring. Perinephric abscess (see Fig. 553.3 ) can occur secondary to contiguous infection in the perirenal area (e.g., vertebral osteomyelitis, psoas abscess) or pyelonephritis that dissects to the renal capsule. It differs from renal abscess in that it is diffuse throughout the capsule and is not walled off, although it can develop septations. As with renal abscesses, the most common organisms are S. aureus and E. coli. A perinephric abscess may not communicate with the collecting system, and, thus, abnormal findings may not be seen on urinalysis or culture.
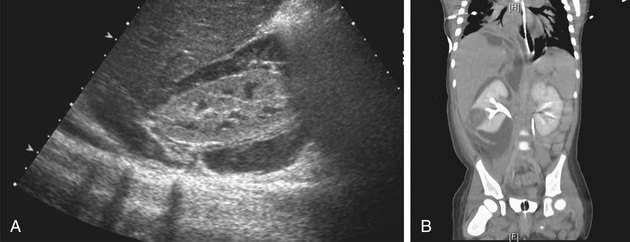
Xanthogranulomatous pyelonephritis is a rare type of renal infection characterized by granulomatous inflammation with giant cells and foamy histiocytes. It can manifest clinically as a renal mass or an acute or chronic infection. Renal calculi, obstruction, and infection with Proteus spp. or E. coli contribute to the development of this lesion, which usually requires total or partial nephrectomy.
Cystitis
Cystitis indicates that there is only bladder involvement; symptoms include dysuria, urgency, frequency, suprapubic pain, incontinence, and possibly malodorous urine. Cystitis does not cause high fever and does not result in renal injury. Malodorous urine is not specific for a UTI.
Acute hemorrhagic cystitis, though uncommon in children, is often caused by E. coli; it also has been attributed to adenovirus types 11 and 21. Adenovirus cystitis is more common in boys; it is self-limiting, with hematuria lasting approximately 4 days. Patients receiving immunosuppressive therapy (e.g., solid-organ or bone marrow transplantation) are at higher risk for hemorrhagic cystitis; adenoviruses and polyomaviruses (i.e., JC virus and BK virus) are important causes in immunocompromised populations (see Chapter 301 ). Other rare types of cystitis that may be confused with infection include eosinophilic cystitis or interstitial cystitis. Eosinophilic cystitis may present with hematuria, whereas interstitial cystitis may present with irritative voiding symptoms but a negative urine culture.
Pathogenesis and Pathology
Nearly all UTIs are ascending infections. The bacteria arise from the fecal flora, colonize the perineum, and enter the bladder via the urethra. In uncircumcised males, the bacterial pathogens arise from the flora beneath the prepuce. In some cases, the bacteria causing cystitis ascend to the kidney to cause pyelonephritis. Rarely, renal infection occurs by hematogenous spread, as in endocarditis or in some bacteremic neonates.
If bacteria ascend from the bladder to the kidney, acute pyelonephritis can occur. Normally, the simple and compound papillae in the kidney have an antireflux mechanism that prevents urine in the renal pelvis from entering the collecting tubules. However, some compound papillae, typically in the upper and lower poles of the kidney, allow intrarenal reflux. Infected urine stimulates an immunologic and inflammatory response, causing renal injury and scarring (Figs. 553.5 and 553.6 ). Children of any age with a febrile UTI can have acute pyelonephritis and subsequent renal scarring, but the risk is highest in those younger than 2 yr of age.
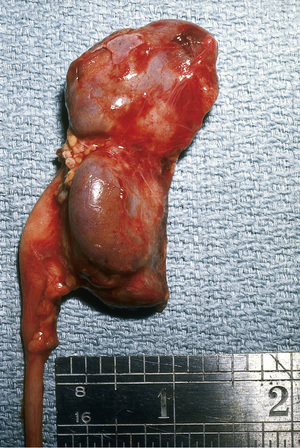
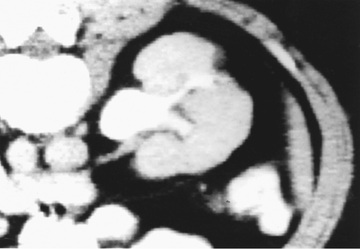
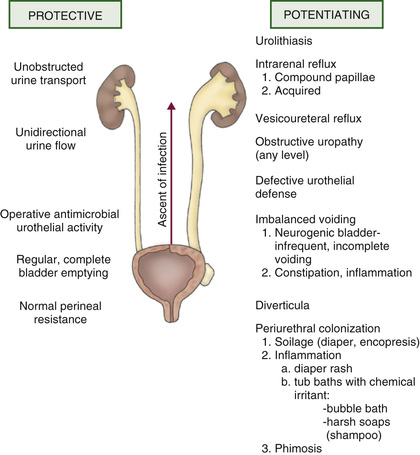
Table 553.1 and Fig. 553.7 outline the host risk factors for UTI. Vesicoureteral reflux (VUR) is discussed in Chapter 554 . If there is grade III, IV, or V VUR and a febrile UTI, 90% have evidence of acute pyelonephritis on renal scintigraphy or other imaging studies. In females, UTIs often occur at the onset of toilet training because of bowel–bladder dysfunction that occurs at that age. The child is trying to retain urine to stay dry, yet the bladder may have uninhibited contractions forcing urine out. The resulting high-pressure, turbulent urine flow and incomplete bladder emptying both increase the likelihood of bacteriuria. Bowel–bladder dysfunction can arise in school-age children who refuse to use the school bathroom, creating a state of urinary retention. Obstructive uropathy resulting in hydronephrosis increases the risk of UTI because of urinary stasis. Specifically, patients who require clean intermittent catheterization due to neurogenic bladder dysfunction are at high risk for UTI, often from organisms with more antibiotic resistance. Constipation with fecal impaction can increase the risk of UTI because it can cause bladder dysfunction.
Table 553.1
Risk Factors for Urinary Tract Infection
The pathogenesis of UTI is based in part on the presence of bacterial pili or fimbriae on the bacterial surface. There are two types of fimbriae, type I and type II. Type I fimbriae are found in most strains of E. coli. Because attachment to target cells can be blocked by D -mannose, these fimbriae are referred to as mannose sensitive. They have no role in pyelonephritis. The attachment of type II fimbriae is not inhibited by mannose, and these are known as mannose resistant. These fimbriae are expressed by only certain strains of E. coli. The receptor for type II fimbriae is a glycosphingolipid that is present on both the uroepithelial cell membrane and red blood cells. The Gal 1-4 Gal oligosaccharide fraction is the specific receptor. Because these fimbriae can agglutinate by P blood group erythrocytes, they are known as P fimbriae. Bacteria with P fimbriae are more likely to cause pyelonephritis. Between 76% and 94% of pyelonephritogenic strains of E. coli have P fimbriae, compared with 19–23% of cystitis strains.
Other host factors for UTI include anatomic abnormalities precluding normal micturition, such as a labial adhesion. This lesion acts as a barrier and causes vaginal voiding. A neuropathic bladder can predispose to UTIs if there is incomplete bladder emptying and/or detrusor–sphincter dyssynergia or a resultant need for frequent catheterization. Sexual activity is associated with UTIs in females, due to introduction of bacteria near the urinary tract that can be exacerbated in part because of urethral irritation and incomplete bladder emptying following intercourse. The incidence of UTI in infants who are breastfed is lower than in those fed with formula.
The first step in an E. coli UTI is attachment of the bacteria to the mannose receptor on the umbrella cells, the cells lining the bladder. These bladder-lining cells may take the E. coli into the cell, where the bacteria replicate in the nutrient-rich environment, forming intracellular bacterial communities (IBCs). Within the IBCs, some of the E. coli fail to divide, becoming filamentous. Part of the bladder's defense against infection is to shed the lining cells with the IBCs; however, some IBCs break out from the cell and repopulate the urine. The filamentous forms can evade attack by polymorphonuclear white blood cells (WBCs) in the urine. When the lining of the bladder is shed, E. coli may be taken up in the cells of the bladder wall, where they form quiescent intracellular reservoirs (QIRs). The dormant QIRs are completely protected from antibiotics and may be a source of recurrent infections. Much current research is attempting to prevent the initial attachment of bacteria to the bladder wall that can lead to the IBCs and QIRs.
Diagnosis
UTIs may be suspected based on symptoms or findings on urinalysis, or both; a urine culture is necessary for confirmation and appropriate therapy . There are several ways to obtain a urine sample; some are more accurate than others. In toilet-trained children, a midstream urine sample usually is satisfactory; the introitus should be cleaned before obtaining the specimen. In uncircumcised males, the prepuce must be retracted; if the prepuce is not retractable, a voided sample may be unreliable and contaminated with skin flora. According to the 2011 American Academy of Pediatrics (AAP) Clinical Guideline for children 2-24 mo, in children who are not toilet trained, a catheterized or suprapubic aspirate urine sample should be obtained. Alternatively, the application of an adhesive, sealed, sterile collection bag after disinfection of the skin of the genitals can be useful only if the urinalysis or culture is negative; the negative predictive value for urinalysis from a “bag” specimen is 99%. However, a positive culture can result from skin contamination, particularly in females and uncircumcised males. If treatment is planned immediately after obtaining the urine culture, a bagged specimen should not be the method because of a high rate of contamination, often with mixed organisms. A suprapubic aspirate generally is unnecessary.
Nitrites and leukocyte esterase are often positive in infected urine. Bacteria generally require 4 hr for metabolism of nitrates to nitrites. Nitrites may not be detected in cases of UTI if the organism does not convert nitrates to nitrites (most notably enterococcus) or if the child has urinary frequency, where there may not be enough time for the conversion to nitrites (Table 553.2 ). In febrile infants less than 60 days old, the presence of pyuria, nitrites, or leukocyte esterase has a high sensitivity and specificity for a UTI.
Table 553.2
From Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management: Clinical practice guideline. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 128:595-610, 2011.
Microscopic hematuria is common in acute cystitis, but microhematuria alone does not suggest UTI. WBC casts in the urinary sediment suggest renal involvement, but in practice these are rarely seen. If the child is asymptomatic and the urinalysis result is normal, it is unlikely that there is a UTI. However, if the child is symptomatic, a UTI is possible, even if the urinalysis result is negative, and the urine culture should be monitored.
Pyuria (leukocytes on urine microscopy) suggests infection, but infection can occur in the absence of pyuria; this finding is more confirmatory than diagnostic (see Table 553.2 ). A WBC count on urinalysis above 3-6 WBCs/high-power-field is indicative of infection with a likelihood ratio of 10 in a symptomatic child. Conversely, pyuria can be present without UTI. Asymptomatic bacteriuria can also have pyuria.
Sterile pyuria (positive leukocytes, negative culture) may occur in partially treated bacterial UTIs, viral infections, urolithiasis, renal tuberculosis, renal abscess, UTI in the presence of urinary obstruction, urethritis as a consequence of a sexually transmitted infection (see Chapter 146 ), inflammation near the ureter or bladder (appendicitis, Crohn disease), Kawasaki disease (Chapter 471.1 ), schistosomiasis, neoplasm, renal transplant rejection, or interstitial nephritis (eosinophils). Prompt plating of the urine sample for culture is important, because if the urine sits at room temperature for more than 60 min, overgrowth of a minor contaminant can suggest a UTI when the urine might not be infected. Refrigeration is a reliable method of storing the urine until it can be cultured.
If the culture shows > 50,000 colony-forming units/mL of a single pathogen (suprapubic or catheter sample) and the urinalysis has pyuria or bacteriuria in a symptomatic child, the child is considered to have a UTI. In a bag sample, if the urinalysis result is positive and the patient is symptomatic, a catheter sample should be obtained for culture.
With acute renal infection, leukocytosis and neutrophilia are noted on the complete blood count (CBC); an elevated serum erythrocyte sedimentation rate, procalcitonin level, and C-reactive protein are common. However, these are all nonspecific markers of inflammation, and their elevation does not prove that the child has acute pyelonephritis. Bacteremia in the setting of pyelonephritis is reported to occur in 3–20% of patients and is most common in infants less than 90 days old (with rates decreasing with increasing age in the first 90 days) and in any child with obstructive uropathy. For these high-risk groups, particularly if the patient appears to be ill at presentation, blood cultures should be drawn before starting antibiotics, if possible.
Among children 2-24 mo, risk factors for females include white race, age younger than 12 mo, temperature > 39°C (102.2°F), fever for longer than 2 days, and absence of another source of infection. Risk factors for males include nonblack race, temperature > 39°C (102.2°F), fever for longer than 24 hr, and absence of another source of infection. Atypical features include failure to respond within 48 hr of appropriate antibiotics, poor urine flow, an abdominal flank or suprapubic mass, non–E. coli pathogen, urosepsis, and an elevated creatinine level.
Imaging Findings
Imaging is not needed to make the clinical diagnosis of UTI or pyelonephritis. If there is concern about acute lobar nephronia or renal abscess, imaging should be considered. Ultrasound is the first-line type of imaging for screening and will likely demonstrate an enlarged kidney with a possible mass in the case of acute lobar nephronia or renal abscess. CT scan is more sensitive and specific for lobar nephronia and will typically show a wedge-shaped, lower-density area after contrast administration. The more frequent use of imaging in patients with possible pyelonephritis is thought to be contributing to the increasing frequency of acute lobar nephronia diagnoses.
Treatment
Acute cystitis should be treated promptly to prevent possible progression to pyelonephritis. If the symptoms are severe, presumptive treatment is started pending results of the culture. If the symptoms are mild or the diagnosis is doubtful, treatment can be delayed until the results of culture are known, and the culture can be repeated if the results are uncertain. If treatment is initiated before the results of a culture and sensitivities are available, a 3- to 5-day course of therapy with trimethoprim-sulfamethoxazole (TMP-SMX) (6-12 mg TMP/kg/day in 2 divided doses) or trimethoprim is effective against many strains of E. coli. Nitrofurantoin (5-7 mg/kg/24 hr in 3-4 divided doses) also is effective and has the advantage of being active against Klebsiella and Enterobacter organisms. Amoxicillin (50 mg/kg/24 hr in 2 divided doses) also may be effective as initial treatment but has a high rate of bacterial resistance.
In acute febrile UTIs , the clinical symptoms of UTI and pyelonephritis are difficult to differentiate. Given this, it is reasonable to consider that given the presence of systemic symptoms, the infection has likely progressed to the kidneys and the patient should be treated for pyelonephritis. A course of antibiotics for 7-14 days that is capable of reaching significant tissue levels is preferable for pyelonephritis; oral and parental routes are equally efficacious. Children who are dehydrated, are vomiting, are unable to drink fluids, have complicated infection, or in whom urosepsis is a possibility should be admitted to the hospital for intravenous (IV) rehydration and IV antibiotic therapy. Local antimicrobial sensitivity patterns should be considered when selecting empirical antibiotic treatment. For hospitalized children, parenteral treatment with ceftriaxone (50 mg/kg/24 hr, not to exceed 2 g) or cefepime (100 mg/kg/24 hr q 12 h) or cefotaxime (100-150 mg/kg/24 hr in 3-4 divided doses) (when available) is a reasonable choice until culture results are back to determine whether a narrower-spectrum antibiotic can be used. If prior urine culture results have grown resistant or atypical organisms, other antibiotic choices may be prudent on a case-by-case basis.
Oral 3rd-generation cephalosporins such as cefixime are as effective as parenteral ceftriaxone against a variety of Gram-negative organisms other than P. aeruginosa, and these medications are considered by some authorities to be the treatment of choice for oral outpatient therapy. Cephalexin may also be considered given the increasing resistance of Gram-negative organisms to amoxicillin. Nitrofurantoin should not be used routinely in children with a febrile UTI, because it does not achieve significant renal tissue levels. The oral fluoroquinolone ciprofloxacin is an alternative agent for resistant microorganisms, particularly P. aeruginosa, in patients older than 17 yr; however, evidence suggests that the potential side effects of fluoroquinolones should be weighed against the benefits of this antibiotic selection. It also has been used on occasion for short-course therapy in younger children with P. aeruginosa UTIs. Levofloxacin is an alternative quinolone with a good safety profile in children. However, clinical treatment with fluoroquinolones in children should be used with caution because of potential cartilage damage. In some children with a febrile UTI, intramuscular injection of a loading dose of ceftriaxone followed by oral therapy with a third-generation cephalosporin is effective. This may be especially useful in children with vomiting or to allow families time to obtain the oral medication. A repeat urine culture after the termination of treatment of a UTI is not routinely needed. Urine cultures are typically negative within 24 hr of initiation of antibiotic therapy, and therefore a urine culture during treatment is almost invariably negative.
Acute Lobar Nephronia, Renal Abscess, and Perinephric Abscess
Acute lobar nephronia is treated with the same antibiotics as pyelonephritis. The duration of treatment is recommended for 14-21 days; one study suggested higher treatment failure in the group treated for the shorter duration. Children with a renal or perirenal abscess or with infection in obstructed urinary tracts can require surgical or percutaneous drainage in addition to antibiotic therapy and other supportive measures (see Fig. 553.4 ). Percutaneous drainage is typically attempted prior to surgical intervention. Immediate percutaneous drainage has been recommended when the abscess is larger than 3-5 cm; however, some patients have been managed successfully with IV antibiotics only. A 48-hr trial of IV antibiotics first before surgical drainage may be warranted in otherwise stable children. Small abscesses, less than 3 cm, may initially be treated with antibiotics alone. Few studies address the role of oral antibiotic therapy for renal abscess. Traditionally, patients received 10-14 days of IV antibiotics, followed by 2-4 wk of oral antibiotic therapy targeted against the known organism (or the likely causes of E. coli and S. aureus if the organism was unknown). The increasing use of oral antibiotics for other serious infections (e.g., osteomyelitis) suggests that an earlier transition to oral therapy for renal abscess is likely feasible. Kidney loss is reported to occur in 10–20% of cases of renal abscess. Perinephric abscesses may be managed with IV antibiotics alone or with percutaneous drainage if the area is large, causing impaired kidney function. Identification of a causative organism can be an additional advantage of percutaneous drainage of a perinephric abscess because the infection may remain isolated from the collecting system based on the location.
Other Potential Treatment or Prevention Options
There is interest in probiotic therapy, which replaces urogenital flora, as well as cranberry juice to prevent UTIs. Studies are beginning in the United States with a non-uropathogenic E. coli called Nissle 1917 already available in Europe. These bacteria may inhibit growth of other bacteria. Cranberry juice may prevent bacterial adhesion and biofilm formation, hypothesized to be via proanthocyanidin (PAC). Currently there is insufficient evidence regarding the use of these therapies to reduce UTIs.
The main consequences of chronic renal damage caused by pyelonephritis are arterial hypertension and end-stage renal insufficiency; when they are found, they should be treated appropriately (see Chapters 472 and 550 ). Even without chronic renal damage, the consequences of infections include lost days from school and work, uncomfortable symptoms, and exposure to antibiotics that change the healthy microbiome.
Imaging Studies in Children With a Febrile UTI
The goal of imaging studies in children with a UTI is to identify anatomic abnormalities that predispose to infection, determine whether there is active renal involvement, and assess whether renal function is normal or at risk.
There are two historical approaches to imaging, the traditional “bottom-up” and “top-down” approaches.
- 1. The “bottom-up” method was a renal sonogram plus a voiding cystourethrogram (VCUG), which will identify upper and lower urinary tract abnormalities, including VUR, bladder–bowel dysfunction, and bladder abnormalities, such as a paraureteral diverticulum.
- 2.
The “top-down” approach was intended to reduce the number of VCUG examinations. It begins with a dimercaptosuccinic acid (DMSA) renal scan, to identify areas of acute pyelonephritis
(Fig. 553.8
). The DMSA scan in younger children generally requires sedation. On
DMSA, involved areas of the kidney are photopenic and the kidney is enlarged. Among children with a febrile UTI, approximately 50% have a positive DMSA scan; the proportion with acute pyelonephritis is 80–90% among those with dilating grades of reflux (III, IV, V). Of those with a positive scan, approximately 50% develop renal scarring in the areas of acute pyelonephritis. If the DMSA scan is positive, a VCUG is performed (Fig. 553.9
) because 90% of children with dilating reflux have a positive DMSA scan. If reflux is identified, treatment is based on the perceived long-term risk of the reflux to the child (see Chapter 554
).
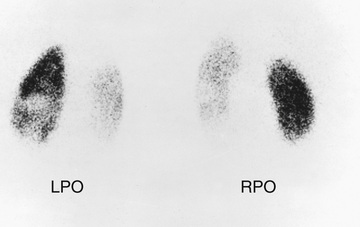
Fig. 553.8 DMSA renal scan showing bilateral photopenic areas indicating acute pyelonephritis and renal scarring. LPO, left posterior oblique; RPO, right posterior oblique.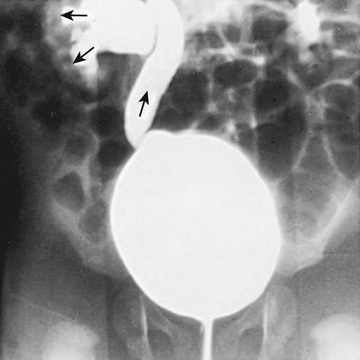
Fig. 553.9 Intrarenal reflux. VCUG in an infant boy with a history of a UTI. Note the right VUR with ureteral dilation, with opacification of the renal parenchyma representing intrarenal reflux.
The AAP practice parameter recommends initial ultrasound of the kidneys, ureters, and bladder for children 2-24 mo with a first episode of UTI. VCUG is indicated only if the ultrasound study indicates hydronephrosis, scarring or other findings suggestive of reflux or obstructive uropathy, or if the patient has other atypical complex features. Further, they recommend VCUG if the child has a recurrent febrile UTI (Table 553.3 ). This recommendation minimizing VCUG use highlights the importance of parental education to return for evaluation of subsequent fevers, so that the child can be promptly evaluated for a recurrent febrile UTI. The rate of renal scarring increases between days 2 and 3 of fever; this makes the prompt evaluation and appropriate treatment of a recurrent UTI important. The risk of scarring also increases with the number of episodes of pyelonephritis and with the grade of reflux.
Table 553.3
Guideline Recommendations for Diagnostic Evaluation Following a Febrile Urinary Tract Infection in Infants
| GUIDELINE | ULTRASONOGRAPHY | VCUG | LATE DMSA SCAN |
|---|---|---|---|
| National Institute for Health and Care Excellence (NICE)* | (see Table 553.4 ) | ||
| American Academy of Pediatrics | Yes | If abnormal ultrasonogram or febrile recurrence | No |
| Italian Society for Paediatric Nephrology (ISPN) | Yes | If abnormal ultrasonogram or if risk factors are present † | If abnormal ultrasonogram or VUR |
* Upper urinary tract dilation on ultrasonography, poor urinary flow, infection with organism other than E. coli , or family history of vesicoureteral reflux.
† Abnormal antenatal ultrasonogram of fetal urinary tract, family history of reflux, septicemia, renal failure, age younger than 6 mo in a male infant, likely family noncompliance, incomplete bladder emptying, no clinical response to appropriate antibiotic therapy within 72 hr, or infection with organism other than E. coli.
VCUG, voiding cystourethrogram; DMSA, dimercaptosuccinic acid; VUR, vesicoureteral reflux.
The AAP guidelines address only febrile infections in children between the ages of 2 and 24 mo. Given the discomfort associated with imaging, other causes of infection in children older than 2 yr of age, and unclear optimal management of reflux in other age-groups, shared decision making with parents or guardians and the child, when appropriate, should be considered for children outside the ages of the guideline.
In children with a history of cystitis (dysuria, urgency, frequency, suprapubic pain), imaging is usually unnecessary. Instead, assessment and treatment of bladder and bowel dysfunction is important. This evaluation is also recommended in recurrent upper tract infections.
The AAP recommendation has resulted in a significant decrease in the number of VCUGs performed. However, the pediatric urologic community has raised numerous concerns regarding the recommendations. One concern is that many primary care physicians may generalize these recommendations, which were intended for children 2-24 mo , to all children. Further, there is concern that the premise on which the AAP recommendations were made was that prophylaxis did not reduce the frequency of UTIs but that the Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) showed a significant reduction in febrile UTIs in children with reflux on prophylaxis. However, given that the rates of renal scarring were unchanged in those receiving prophylaxis, the AAP reaffirmed the recommendations in 2016.
Similarly, in 2007, the NICE (National Institute for Health and Clinical Excellence, UK) guidelines for diagnosis, management, and imaging after UTI were released (Table 553.4 ). These recommendations divided children into those younger than 6 mo, 6 mo to 3 yr, and older than 3 yr of age. An initial ultrasound is recommended for children younger than 6 mo, and a VCUG is recommended only in children younger than age 6 mo with atypical features (non-E. coli , significant family history), recurrent UTI, or abnormal ultrasound findings. For children age 6 mo to 3 yr, an ultrasound and VCUG are recommended for those patients with atypical features or recurrent UTIs. No imaging is suggested for first-time, typical UTIs in this age-group. These recommendations are controversial because the methodology was not based on evidence but on expert opinion. In addition, there was no retrospective or prospective assessment of the potential of this approach to identify significant uropathology. There is evidence that a significant number of children with uropathology would not have been identified under these guidelines.
Table 553.4
Recommended Imaging Schedule for Children With Urinary Tract Infection
| CHILD AGE AND TESTS | TYPE OF INFECTION | ||
|---|---|---|---|
| RESPONDS WELL TO TREATMENT WITHIN 48 HR | ATYPICAL INFECTION | RECURRENT INFECTION | |
| CHILDREN YOUNGER THAN 6 MO OLD | |||
| Ultrasound scan during acute infection | No | Yes | Yes |
| Ultrasound scan within 6 wk of infection | Yes | No | No |
| DMSA scan 4-6 mo after acute infection | No | Yes | Yes |
| Micturating cystograms | Consider if ultrasound scan abnormal | Yes | Yes |
| CHILDREN 6 MO TO 3 YR OLD | |||
| Ultrasound scan during acute infection | No | Yes | No |
| Ultrasound scan within 6 wk of infection | No | No | Yes |
| DMSA scan 4-6 mo after acute infection | No | Yes | Yes |
| Micturating cystograms | No | Not routine; consider if dilation on ultrasound, poor urine flow, non–E. coli infection, or family history of vesicoureteric reflux | |
| CHILDREN OLDER THAN AGE 3 YR | |||
| Ultrasound scan during acute infection | No | Yes | No |
| Ultrasound scan within 6 wk of infection | No | No | Yes |
| DMSA scan 4-6 mo after acute infection | No | Yes | Yes |
| Micturating cystograms | No | No | No |
DMSA, dimercaptosuccinic acid.
Adapted from National Institute for Health and Clinical Excellence. Urinary tract infection in children: diagnosis, treatment, and long-term management. NICE clinical guidelines, no. 54. London, 2007, RCOG Press, Tables 6-13, 6-14, and 6-15.
Prevention of Recurrences
In a child with recurrent UTIs, identification of predisposing factors is beneficial. Bowel and bladder dysfunction is a very important contributor to recurrent UTIs and is one of the main reasons for an increase in UTIs around the time of toilet training. Some children with UTIs may also have constipation (see Chapter 358.1 ). Behavioral modification, with treatment of constipation as described in Chapter 558 , often is effective. The first UTI gives the pediatrician a chance to investigate constipation. The Rome III criteria for constipation in the pediatric age-group standardize the definition of constipation.
Bladder dysfunction manifested by urgency, wetting, and especially “Vincent's curtsy” (females squat on their heels in response to an uninhibited bladder contraction) can predispose to UTI.
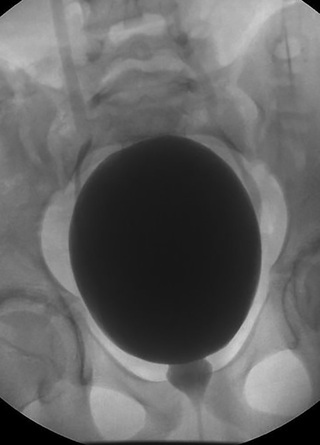
In toilet-trained children, a thorough history and use of urodynamic studies and measurement of postvoid residual volumes may be helpful in identifying children with bladder dysfunction that may contribute to UTIs. Tightening the pelvic floor during urination can sometimes be seen on a VCUG as a spinning-top urethra (Fig. 553.10 ). An ultrasound may document residual urine and possibly a thick bladder wall. Urodynamics may show an intermittent stream with increased activity in the pelvic floor muscles.
The RIUVR study was a randomized trial of TMP-SMX prophylaxis for patients with a history of UTI and diagnosed VUR. Although the UTI recurrence rate was decreased by half from 30% in the group not receiving prophylaxis to 15% in those receiving prophylaxis, rates of renal scarring were the same in both groups. Additionally, the rates of UTI caused by resistant organisms increased in the group receiving prophylaxis. Taken together, although the use of prophylaxis can decrease rates of recurrence, the increase in antibiotic resistance, need for daily medication in children, and no change in renal scarring prevent firm recommendations for prophylaxis. The AAP does not recommend routine use of antibiotic prophylaxis in children with a first episode of pyelonephritis in an otherwise anatomically normal urinary tract. Urologic conditions that can cause recurrent UTIs that might benefit from long-term antibiotic prophylaxis include neuropathic bladder, urinary tract stasis and obstruction, severe VUR (see Chapter 554 ), and urinary calculi.