Community-Acquired Pneumonia
Matthew S. Kelly, Thomas J. Sandora
Epidemiology
Pneumonia, defined as inflammation of the lung parenchyma, is the leading infectious cause of death globally among children younger than 5 yr, accounting for an estimated 920,000 deaths each year (Fig. 428.1 ). Pneumonia mortality is closely linked to poverty. More than 99% of pneumonia deaths are in low- and middle-income countries, with the highest pneumonia mortality rate occurring in poorly developed countries in Africa and South Asia (Table 428.1 ).
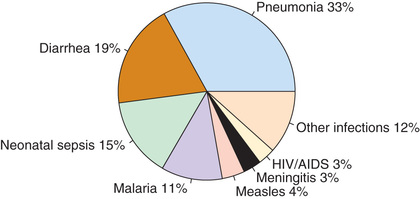
Table 428.1
From United Nations Children's Fund: One is too many—ending child deaths from pneumonia and diarrhoea. https://data.unicef.org/resources/one-many-ending-child-deaths-pneumonia-diarrhoea/ . Accessed January 21, 2017.
In the United States, mortality from pneumonia in children declined by 97% between 1939 and 1996. This decline likely resulted from the development of antibiotics and vaccines and the expansion of medical insurance coverage for children. Effective vaccines against measles (see Chapter 273 ) and pertussis (see Chapter 224 ) contributed to the decline in pneumonia-related mortality during the 20th century. Haemophilus influenzae type b (see Chapter 221 ) was also an important cause of bacterial pneumonia in young children but became uncommon following licensure of a conjugate vaccine in 1987. The introduction of pneumococcal conjugate vaccines (PCVs) (see Chapter 209 ) has been an important contributor to the further reductions in pneumonia-related mortality achieved over the past 15 yr.
Etiology
Although most cases of pneumonia are caused by microorganisms, noninfectious causes include aspiration (of food or gastric acid, foreign bodies, hydrocarbons, and lipoid substances), hypersensitivity reactions, and drug- or radiation-induced pneumonitis (see Chapter 427 ). The cause of pneumonia in an individual patient is often difficult to determine because direct sampling of lung tissue is invasive and rarely performed. Bacterial cultures of sputum or upper respiratory tract samples from children typically do not accurately reflect the cause of lower respiratory tract infection. Streptococcus pneumoniae (pneumococcus) is the most common bacterial pathogen in children 3 wk to 4 yr of age, whereas Mycoplasma pneumoniae and Chlamydophila pneumoniae are the most frequent bacterial pathogens in children age 5 yr and older. In addition to pneumococcus, other bacterial causes of pneumonia in previously healthy children in the United States include group A streptococcus (Streptococcus pyogenes ; see Chapter 210 ) and Staphylococcus aureus (see Chapter 208.1 ) (Tables 428.2 , 428.3 , and 428.4 ). S. aureus pneumonia often complicates an illness caused by influenza viruses.
Table 428.2
Causes of Infectious Pneumonia
| BACTERIAL | |
|---|---|
| COMMON | |
| Streptococcus pneumoniae | Consolidation, empyema |
| Group B streptococci | Neonates |
| Group A streptococci | Empyema |
| Staphylococcus aureus | Pneumatoceles, empyema; infants; nosocomial pneumonia |
| Mycoplasma pneumoniae * | Adolescents; summer–fall epidemics |
| Chlamydophila pneumoniae * | Adolescents |
| Chlamydia trachomatis | Infants |
| Mixed anaerobes | Aspiration pneumonia |
| Gram-negative enterics | Nosocomial pneumonia |
| UNCOMMON | |
| Haemophilus influenzae type b | Unimmunized |
| Moraxella catarrhalis | |
| Neisseria meningitidis | |
| Francisella tularensis | Animal, tick, fly contact; bioterrorism |
| Nocardia species | Immunocompromised patients |
| Chlamydophila psittaci * | Bird contact (especially parakeets) |
| Yersinia pestis (plague) | Rat contact; bioterrorism |
| Legionella species* | Exposure to contaminated water; nosocomial |
| Coxiella burnetii * (Q fever) | Animal (goat, sheep, cattle) exposure |
| VIRAL | |
| COMMON | |
| Respiratory syncytial virus | Bronchiolitis |
| Parainfluenza types 1-4 | Croup |
| Influenza A, B | High fever; winter months |
| Adenovirus | Can be severe; often occurs between January and April |
| Human metapneumovirus | Similar to respiratory syncytial virus |
| UNCOMMON | |
| Rhinovirus | Rhinorrhea |
| Enterovirus | Neonates |
| Herpes simplex | Neonates, immunocompromised persons |
| Cytomegalovirus | Infants; immunocompromised persons (particularly HIV-infected infants) |
| Measles | Rash, coryza, conjunctivitis |
| Varicella | Unimmunized; immunocompromised persons |
| Hantavirus | Southwestern United States, rodents |
| Coronaviruses [severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS)] | Asia, Arabian Peninsula |
| FUNGAL | |
| Histoplasma capsulatum | Ohio/Mississippi River valley; bird, bat contact |
| Blastomyces dermatitidis | Ohio/Mississippi River valley |
| Coccidioides immitis | Southwestern United States, Great Lakes states |
| Cryptococcus neoformans and C. gattii | Bird contact; immunocompromised; Northwestern United States (C. gattii) |
| Aspergillus species | Immunocompromised persons; nodular lung infection |
| Mucormycosis | Immunocompromised persons |
| Pneumocystis jiroveci | Immunocompromised persons (particularly HIV-infected infants); steroids |
| RICKETTSIAL | |
| Rickettsia rickettsiae | Tick bite |
| MYCOBACTERIAL | |
| Mycobacterium tuberculosis | Travel to endemic region; exposure to high-risk persons |
| Mycobacterium avium complex | Immunocompromised (particularly HIV-infected) persons |
| Other non-tuberculous mycobacteria | Immunocompromised persons; cystic fibrosis |
| PARASITIC | |
| Various parasites (e.g., Ascaris , Strongyloides species) | Eosinophilic pneumonia |
* Atypical pneumonia syndrome; may have extrapulmonary manifestations, low-grade fever, patchy diffuse infiltrates, and poor response to β-lactam antibiotics.
Adapted from Kliegman RM, Greenbaum LA, Lye PS: Practical strategies in pediatric diagnosis & therapy , ed 2, Philadelphia, 2004, Elsevier, p. 29.
Table 428.3
| AGE GROUP | FREQUENT PATHOGENS (IN ORDER OF FREQUENCY) |
|---|---|
| Neonates (<3 wk) | Group B streptococcus, Escherichia coli, other Gram-negative bacilli, Streptococcus pneumoniae, Haemophilus influenzae (type b,* nontypeable) |
| 3 wk-3 mo | Respiratory syncytial virus, other respiratory viruses (rhinoviruses, parainfluenza viruses, influenza viruses, human metapneumovirus, adenovirus), S. pneumoniae, H. influenzae (type b,* nontypeable); if patient is afebrile, consider Chlamydia trachomatis |
| 4 mo-4 yr | Respiratory syncytial virus, other respiratory viruses (rhinoviruses, parainfluenza viruses, influenza viruses, human metapneumovirus, adenovirus), S. pneumoniae, H. influenzae (type b,* nontypeable), Mycoplasma pneumoniae, group A streptococcus |
| ≥5 yr | M. pneumoniae, S. pneumoniae, Chlamydophila pneumoniae, H. influenzae (type b,* nontypeable), influenza viruses, adenovirus, other respiratory viruses, Legionella pneumophila |
* H. influenzae type b is uncommon with routine immunization.
Adapted from Kliegman RM, Marcdante KJ, Jenson HJ, et al: Nelson essentials of pediatrics , ed 5, Philadelphia, 2006, Elsevier, p. 507.
Table 428.4
Pneumonia: Etiology Suggested by Exposure History
| EXPOSURE HISTORY | INFECTIOUS AGENT |
|---|---|
| Exposure to concurrent illness in school dormitory or household setting | Neisseria meningitidis, Mycoplasma pneumoniae |
| ENVIRONMENTAL EXPOSURES | |
| Exposure to contaminated aerosols (e.g., air coolers, hospital water supply) | Legionnaires' disease |
| Exposure to goat hair, raw wool, animal hides | Anthrax |
| Ingestion of unpasteurized milk | Brucellosis |
| Exposure to bat droppings (caving) or dust from soil enriched with bird droppings | Histoplasmosis |
| Exposure to water contaminated with animal urine | Leptospirosis |
| Exposure to rodent droppings, urine, saliva | Hantavirus |
| Potential bioterrorism exposure | Anthrax, plague, tularemia |
| ZOONOTIC EXPOSURES | |
| Employment as abattoir work or veterinarian | Brucellosis |
| Exposure to cattle, goats, pigs | Anthrax, brucellosis |
| Exposure to ground squirrels, chipmunks, rabbits, prairie dogs, rats in Africa or southwestern United States | Plague |
| Hunting or exposure to rabbits, foxes, squirrels | Tularemia |
| Bites from flies or ticks | Tularemia |
| Exposure to birds (parrots, budgerigars, cockatoos, pigeons, turkeys) | Psittacosis |
| Exposure to infected dogs and cats | Pasteurella multocida, Q fever (Coxiella burnetii) |
| Exposure to infected goats, cattle, sheep, domestic animals, and their secretions (milk, amniotic fluid, placenta, feces) | Q fever (C. burnetii) |
| TRAVEL EXPOSURES | |
| Residence in or travel to San Joaquin Valley, southern California, southwestern Texas, southern Arizona, New Mexico | Coccidioidomycosis |
| Residence in or travel to Mississippi or Ohio river valleys, Great Lakes States, Caribbean, Central America, or Africa | Histoplasmosis, blastomycosis |
| Residence in or travel to southern China | SARS, avian influenza |
| Residence in or travel to Arabian peninsula | MERS-CoV |
| Residence in or travel to Southeast Asia | Paragonimiasis, melioidosis |
| Residence in or travel to West Indies, Australia, or Guam | Melioidosis |
MERS-CoV, Middle East respiratory syndrome coronavirus; SARS, severe acute respiratory syndrome.
From Ellison RT III, Donowitz GR: Acute pneumonia, In Bennett JE, Dolin R, Blaser MJ, editors: Mandell, Douglas, and Bennett's principles and practice of infectious diseases , ed 8, vol 1, Philadelphia, 2015, Elsevier, Table 69.3, p. 828.
S. pneumoniae, H. influenzae, and S. aureus are the major causes of hospitalization and death from bacterial pneumonia among children in developing countries, although in children with HIV infection, Mycobacterium tuberculosis (see Chapter 242 ), non-tuberculous mycobacteria (see Chapter 244 ), Salmonella (see Chapter 225 ), Escherichia coli (see Chapter 227 ), Pneumocystis jiroveci (see Chapter 271 ), and cytomegalovirus (see Chapter 282 ) must be considered. The incidence of pneumonia caused by H. influenzae or S. pneumoniae has been significantly reduced in areas where routine immunization has been implemented.
Viral pathogens are the most common causes of lower respiratory tract infections in infants and children older than 1 mo but younger than 5 yr of age. Viruses can be detected in 40–80% of children with pneumonia using molecular diagnostic methods (e.g., polymerase chain reaction [PCR]). Of the respiratory viruses, respiratory syncytial virus (RSV; see Chapter 287 ) and rhinoviruses (see Chapter 290 ) are the most commonly identified pathogens, especially in children younger than 2 yr of age. However, the role of rhinoviruses in severe lower respiratory tract infection remains unclear as these viruses are frequently detected with co-infecting pathogens and among asymptomatic children. Other common viruses causing pneumonia include influenza viruses (see Chapter 285 ), human metapneumovirus (see Chapter 288 ), parainfluenza viruses (see Chapter 286 ), adenoviruses (see Chapter 289 ), and enteroviruses (see Chapter 277 ). Infection with more than one respiratory virus occurs in up to 20% of cases. The age of the patient can suggest the likely pathogens (see Table 428.3 ).
Lower respiratory tract viral infections are much more common in the fall and winter in both the northern and southern hemispheres in relation to the seasonal epidemics of respiratory viruses that occur each year. The typical pattern of these epidemics usually begins in the fall, when parainfluenza virus infections appear and most often manifest as croup. Later in winter, RSV, human metapneumovirus, and influenza viruses cause widespread infection, including upper respiratory tract infections, bronchiolitis, and pneumonia. RSV is particularly severe among infants and young children, whereas influenza viruses cause disease and excess hospitalization for acute respiratory illness in all age groups. Knowledge of the prevailing viruses circulating in the community may lead to a presumptive initial diagnosis.
Immunization status is relevant because children fully immunized against H. influenzae type b and S. pneumoniae are less likely to have pneumonia caused by these pathogens. Children who are immunocompromised or who have certain medical comorbidities may be at risk for specific pathogens, such as Pseudomonas spp. in patients with cystic fibrosis (see Chapter 432 ).
Pathogenesis
The lower respiratory tract possesses a number of defense mechanisms against infection, including mucociliary clearance, macrophages and secretory immunoglobulin A, and clearing of the airways by coughing. Previously, it was believed that the lower respiratory tract was—in the absence of infection—kept sterile by these mechanisms, supported primarily by culture-based studies. However, recent use of culture-independent techniques, including high-throughput sequencing methods, suggests that the lower respiratory tract contains diverse microbial communities. These data have challenged the traditional model of pneumonia pathogenesis that maintained that pneumonia was the result of invasion of the sterile lower respiratory tract by a single pathogen. More recent conceptual models postulate that pneumonia results from disruption of a complex lower respiratory ecosystem that is the site of dynamic interactions between potential pneumonia pathogens, resident microbial communities, and host immune defenses.
Viral pneumonia usually results from spread of infection along the airways, accompanied by direct injury of the respiratory epithelium, which results in airway obstruction from swelling, abnormal secretions, and cellular debris. The small caliber of airways in young infants makes such patients particularly susceptible to severe infection. Atelectasis, interstitial edema, and hypoxemia from ventilation–perfusion mismatch often accompany airway obstruction. Viral infection of the respiratory tract can also predispose to secondary bacterial infection by disturbing normal host defense mechanisms, altering secretions, and through disruptions in the respiratory microbiota.
Bacterial pneumonia most often occurs when respiratory tract organisms colonize the trachea and subsequently gain access to the lungs, but pneumonia may also result from direct seeding of lung tissue after bacteremia. When bacterial infection is established in the lung parenchyma, the pathologic process varies according to the invading organism. M. pneumoniae (see Chapter 250 ) attaches to the respiratory epithelium, inhibits ciliary action, and leads to cellular destruction and an inflammatory response in the submucosa. As the infection progresses, sloughed cellular debris, inflammatory cells, and mucus cause airway obstruction, with spread of infection occurring along the bronchial tree, as is seen in viral pneumonia. S. pneumoniae produces local edema that aids in the proliferation of organisms and their spread into adjacent portions of lung, often resulting in the characteristic focal lobar involvement. Group A streptococcus lower respiratory tract infection typically results in more diffuse lung involvement with interstitial pneumonia. The pathology includes necrosis of tracheobronchial mucosa; formation of large amounts of exudate, edema, and local hemorrhage, with extension into the interalveolar septa; and involvement of lymphatic vessels with frequent pleural involvement. S. aureus pneumonia manifests as confluent bronchopneumonia, which is often unilateral and characterized by the presence of extensive areas of hemorrhagic necrosis and irregular areas of cavitation of the lung parenchyma, resulting in pneumatoceles, empyema, and, at times, bronchopulmonary fistulas.
Recurrent pneumonia is defined as 2 or more episodes in a single year or 3 or more episodes ever, with radiographic clearing between occurrences. An underlying disorder should be considered if a child experiences recurrent pneumonia (Table 428.5 ).
Clinical Manifestations
Pneumonia is frequently preceded by several days of symptoms of an upper respiratory tract infection, typically rhinitis and cough. In viral pneumonia, fever is usually present but temperatures are generally lower than in bacterial pneumonia. Tachypnea is the most consistent clinical manifestation of pneumonia. Increased work of breathing accompanied by intercostal, subcostal, and suprasternal retractions, nasal flaring, and use of accessory muscles is common. Severe infection may be accompanied by cyanosis and lethargy, especially in infants. Auscultation of the chest may reveal crackles and wheezing, but it is often difficult to localize the source of these adventitious sounds in very young children with hyperresonant chests. It is often not possible to distinguish viral pneumonia (especially adenovirus) clinically from disease caused by Mycoplasma and other bacterial pathogens.
Bacterial pneumonia in adults and older children typically begins suddenly with high fever, cough, and chest pain. Other symptoms that may be seen include drowsiness with intermittent periods of restlessness; rapid respirations; anxiety; and, occasionally, delirium. In many children, splinting on the affected side to minimize pleuritic pain and improve ventilation is noted; such children may lie on one side with the knees drawn up to the chest.
Physical findings depend on the stage of pneumonia. Early in the course of illness, diminished breath sounds, scattered crackles, and rhonchi are commonly heard over the affected lung field. With the development of increasing consolidation or complications of pneumonia such as pleural effusion or empyema, dullness on percussion is noted and breath sounds may be diminished. A lag in respiratory excursion often occurs on the affected side. Abdominal distention may be prominent because of gastric dilation from swallowed air or ileus. Abdominal pain is common in lower-lobe pneumonia. The liver may seem enlarged because of downward displacement of the diaphragm secondary to hyperinflation of the lungs or superimposed congestive heart failure.
Symptoms described in adults with pneumococcal pneumonia may be noted in older children but are rarely observed in infants and young children, in whom the clinical pattern is considerably more variable. In infants, there may be a prodrome of upper respiratory tract infection and poor feeding, leading to the abrupt onset of fever, restlessness, apprehension, and respiratory distress. These infants typically appear ill, with respiratory distress manifested as grunting; nasal flaring; retractions of the supraclavicular, intercostal, and subcostal areas; tachypnea; tachycardia; air hunger; and often cyanosis. Auscultation may be misleading, particularly in young infants, with meager findings disproportionate to the degree of tachypnea. Some infants with bacterial pneumonia may have associated gastrointestinal disturbances characterized by vomiting, anorexia, diarrhea, and abdominal distention secondary to a paralytic ileus. Rapid progression of symptoms is characteristic in the most severe cases of bacterial pneumonia.
Diagnosis
In 2011, the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA) published clinical practice guidelines for community-acquired pneumonia in children older than 3 mo of age. These evidence-based guidelines provide recommendations for diagnostic testing and treatment of previously healthy children with pneumonia in both outpatient and inpatient settings.
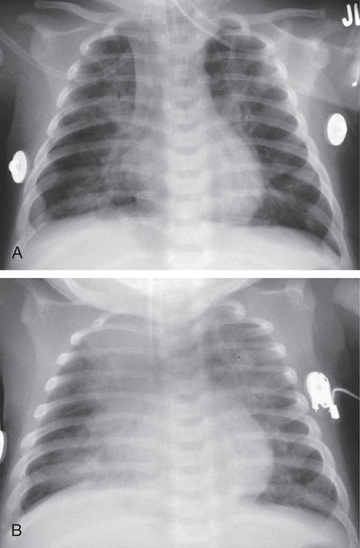
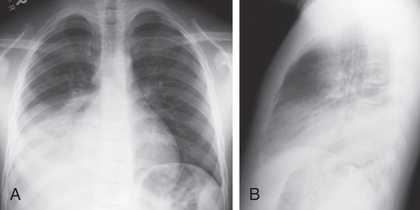
An infiltrate on chest radiograph (posteroanterior and lateral views) supports the diagnosis of pneumonia; images may also identify a complication such as a pleural effusion or empyema. Viral pneumonia is usually characterized by hyperinflation with bilateral interstitial infiltrates and peribronchial cuffing (Fig. 428.2 ). Confluent lobar consolidation is typically seen with pneumococcal pneumonia (Fig. 428.3 ). The radiographic appearance alone does not accurately identify pneumonia etiology, and other clinical features of the illness must be considered. Repeat chest radiographs are not required for proof of cure for patients with uncomplicated pneumonia. Moreover, current PIDS–IDSA guidelines do not recommend that a chest radiograph be performed for children with suspected pneumonia (cough, fever, localized crackles, or decreased breath sounds) who are well enough to be managed as outpatients because imaging in this context only rarely changes management.
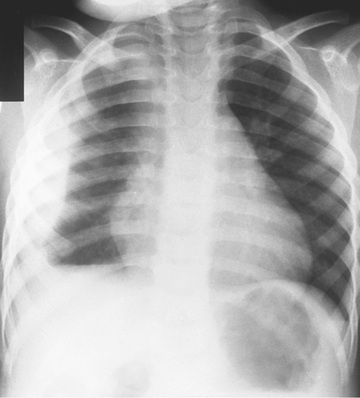
Point-of-care use of portable or handheld ultrasonography is highly sensitive and specific in diagnosing pneumonia in children by determining lung consolidations and air bronchograms or effusions (Fig. 428.4 ). However, the reliability of this imaging modality for pneumonia diagnosis is highly user-dependent, which has limited its widespread use.

The peripheral white blood cell (WBC) count can be useful in differentiating viral from bacterial pneumonia. In viral pneumonia, the WBC count can be normal or elevated but is usually not higher than 20,000/mm3 , with a lymphocyte predominance. Bacterial pneumonia is often associated with an elevated WBC count, in the range of 15,000-40,000/mm3 , and a predominance of polymorphonuclear leukocytes. A large pleural effusion, lobar consolidation, and a high fever at the onset of the illness are also suggestive of a bacterial etiology. Atypical pneumonia caused by C. pneumoniae or M. pneumoniae is difficult to distinguish from pneumococcal pneumonia on the basis of radiographic and laboratory findings; although pneumococcal pneumonia is associated with a higher WBC count, erythrocyte sedimentation rate, procalcitonin, and C-reactive protein level, there is considerable overlap.
The definitive diagnosis of a viral infection rests on the detection of the viral genome or antigen in respiratory tract secretions. Reliable PCR assays are widely available for the rapid detection of many respiratory viruses, including RSV, parainfluenza, influenza, human metapneumovirus, adenovirus, enterovirus, and rhinovirus. Serologic techniques can also be used to diagnose a recent respiratory viral infection but generally require testing of acute and convalescent serum samples for a rise in antibodies to a specific virus. This diagnostic technique is laborious, slow, and not generally clinically useful because the infection usually has resolved by the time it is confirmed serologically. Serologic testing may be valuable as an epidemiologic tool to define the incidence and prevalence of the various respiratory viral pathogens.
The definitive diagnosis of a typical bacterial infection requires isolation of an organism from the blood, pleural fluid, or lung. Culture of sputum is of little value in the diagnosis of pneumonia in young children, while percutaneous lung aspiration is invasive and not routinely performed. Blood culture is positive in only 10% of children with pneumococcal pneumonia and is not recommended for nontoxic-appearing children treated as outpatients. Blood cultures are recommended for children who fail to improve or have clinical deterioration, have complicated pneumonia (Table 428.6 ), or require hospitalization. Urinary antigen tests should not be used to diagnose pneumonia caused by S. pneumoniae in children because of a high rate of false positives resulting from nasopharyngeal carriage. Pertussis infection can be diagnosed by PCR or culture of a nasopharyngeal specimen; although culture is considered the gold standard for pertussis diagnosis, it is less sensitive than the available PCR assays. Acute infection caused by M. pneumoniae can be diagnosed on the basis of a PCR test result from a respiratory specimen or seroconversion in an immunoglobulin G assay. Cold agglutinins at titers > 1 : 64 are also found in the blood of roughly half of patients with M. pneumoniae infections; however, cold agglutinins are nonspecific because other pathogens such as influenza viruses may also cause increases. Serologic evidence, such as antistreptolysin O and anti-DNase B titers, may also be useful in the diagnosis of group A streptococcal pneumonia.
Table 428.6
Factors Suggesting Need for Hospitalization of Children With Pneumonia
|
Moderate to severe respiratory distress Hypoxemia (oxygen saturation <90% breathing room air, sea level) Complicated pneumonia* Sickle cell anemia with acute chest syndrome Vomiting or inability to tolerate oral fluids or medications No response to appropriate oral antibiotic therapy Social factors (e.g., inability of caregivers to administer medications at home or follow-up appropriately) |
* Pleural effusion, empyema, abscess, bronchopleural fistula, necrotizing pneumonia, acute respiratory distress syndrome, extrapulmonary infection (meningitis, arthritis, pericarditis, osteomyelitis, endocarditis), hemolytic uremic syndrome, or sepsis.
Adapted from Baltimore RS: Pneumonia. In Jenson HB, Baltimore RS, editors: Pediatric infectious diseases: principles and practice, Philadelphia, 2002, WB Saunders, p. 801.
There is a great deal of interest in developing a non-invasive diagnostic test that can accurately differentiate children with bacterial versus viral causes of pneumonia. Various biomarkers, including C-reactive protein, procalcitonin, lipocalin-2, and tumor necrosis factor-related apoptosis-inducing ligand, have been evaluated for their ability to differentiate these pneumonia etiologies. For many of these biomarkers, values differ in children with bacterial compared with viral causes of pneumonia, but the reliability of these tests is not sufficiently high to justify routine use. Studies of these biomarkers have also been hampered by the lack of a gold standard for determining pneumonia etiology and the relatively frequent occurrence of viral–bacterial co-infections. Patient peripheral cell gene expression patterns determined by microarray reverse transcription PCR is an emerging technology that may help differentiate bacterial from viral causes of pneumonia, although further study is needed.
Treatment
Treatment of suspected bacterial pneumonia is based on the presumptive cause and the age and clinical appearance of the child. For mildly ill children who do not require hospitalization, amoxicillin is recommended. With the emergence of penicillin-resistant pneumococci, high doses of amoxicillin (90 mg/kg/day orally divided twice daily) should be prescribed unless local data indicate a low prevalence of resistance (Table 428.7 ). Therapeutic alternatives include cefuroxime and amoxicillin/clavulanate. For school-aged children and adolescents or when infection with M. pneumoniae or C. pneumoniae is suspected, a macrolide antibiotic is an appropriate choice for outpatient management. Azithromycin is generally preferred, while clarithromycin or doxycycline (for children 8 yr or older) are alternatives. For adolescents, a respiratory fluoroquinolone (levofloxacin, moxifloxacin) may also be considered as an alternative if there are contraindications to other agents.
Table 428.7
Doses for oral therapy should not exceed adult doses.
a Clindamycin resistance appears to be increasing in certain geographic areas among S. pneumoniae And S. aureus infections.
b For β-lactam–allergic children.
AUC, area under the time vs. serum concentration curve; MIC, minimum inhibitory concentration.
From Bradley JS, Byington CL, Shah SS, et al: The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America, Clin Infect Dis 53(7):617–630, 2011, Table 5, pp. 623–624.
The empiric treatment of suspected bacterial pneumonia in a hospitalized child requires an approach based on local epidemiology, the immunization status of the child, and the clinical manifestations at the time of presentation. In areas without substantial high-level penicillin resistance among S. pneumoniae , children who are fully immunized against H. influenzae type b and S. pneumoniae and are not severely ill should receive ampicillin or penicillin G. For children who do not meet these criteria, ceftriaxone or cefotaxime may be used. If clinical features suggest staphylococcal pneumonia (pneumatoceles, empyema), initial antimicrobial therapy should also include vancomycin or clindamycin. Moreover, if infection with M. pneumoniae or C. pneumoniae is suspected, a macrolide antibiotic should be included in the treatment regimen.
If viral pneumonia is suspected, it is reasonable to withhold antibiotic therapy, especially for preschool-aged patients who are mildly ill, have clinical evidence suggesting viral infection, and are in no respiratory distress. However, up to 30% of patients with known viral infection, particularly influenza viruses, may have coexisting bacterial pathogens. Therefore, if the decision is made to withhold antibiotic therapy on the basis of presumptive diagnosis of a viral infection, deterioration in clinical status should signal the possibility of superimposed bacterial infection, and antibiotic therapy should be initiated.
Table 428.7 notes the indications for admission to a hospital. Hospitalized children should receive supportive care and may require intravenous fluids; respiratory support, including supplemental oxygen, continuous positive airway pressure (CPAP), or mechanical ventilation; or vasoactive medications for hypotension or sepsis physiology.
The optimal duration of antibiotic treatment for pneumonia has not been well-established in controlled studies. However, antibiotics should generally be continued until the patient has been afebrile for 72 hr, and the total duration should not be less than 10 days (or 5 days if azithromycin is used). Shorter courses (5-7 days) may also be effective, particularly for children managed on an outpatient basis, but further study is needed. Available data do not support prolonged courses of treatment for uncomplicated pneumonia. Preliminary studies suggest that a reduction of previously elevated serum procalcitonin levels to an absolute level (0.1-0.25 µg/L) may help determine when to stop treatment.
Despite substantial gains over the past 15 yr, in developing countries less than two-thirds of children with symptoms of pneumonia are taken to an appropriate caregiver, and fewer than half receive antibiotics. The World Health Organization and other international groups have developed systems to train mothers and local healthcare providers in the recognition and appropriate antibiotic treatment of pneumonia. In addition to antibiotics, oral zinc (10 mg/day for < 12 mo, 20 mg/day for ≥ 12 mo given for 7 days) may reduce mortality among children in developing countries with clinically defined severe pneumonia. Bubble CPAP improves mortality from pneumonia with hypoxemia compared with standard oxygen therapy in settings without access to ventilator-derived CPAP or mechanical ventilation.
Prognosis
Typically, patients with uncomplicated community-acquired bacterial pneumonia show response to therapy, with improvement in clinical symptoms (fever, cough, tachypnea, chest pain), within 48-72 hr of initiation of antibiotics. Radiographic evidence of improvement lags substantially behind clinical improvement. A number of possibilities must be considered when a patient does not improve with appropriate antibiotic therapy: (1) complications, such as pleural effusion or empyema (see Table 428.6 ); (2) bacterial resistance; (3) nonbacterial etiologies such as viruses or fungi and aspiration of foreign bodies or food; (4) bronchial obstruction from endobronchial lesions, foreign body, or mucous plugs; (5) preexisting diseases such as immunodeficiencies, ciliary dyskinesia, cystic fibrosis, pulmonary sequestration, or congenital pulmonary airway malformation; and (6) other noninfectious causes (including bronchiolitis obliterans, hypersensitivity pneumonitis, eosinophilic pneumonia, and granulomatosis with polyangiitis, formerly called Wegener granulomatosis). A chest radiograph is the first step in determining the reason for a lack of response to initial treatment. Bronchoalveolar lavage may be indicated in children with respiratory failure; high-resolution CT scans may better identify complications or an anatomic reason for a poor response to therapy.
Mortality from community-acquired pneumonia in developed countries is rare, and most children with pneumonia do not experience long-term pulmonary sequelae. Some data suggest that up to 45% of children have symptoms of asthma 5 yr after hospitalization for pneumonia; this finding may reflect either undiagnosed asthma at the time of presentation or a propensity for development of asthma after pneumonia.
Complications
Complications of pneumonia (see Table 428.6 ) are usually the result of direct spread of bacterial infection within the thoracic cavity (pleural effusion, empyema, and pericarditis) or bacteremia and hematologic spread (Fig. 428.5 ). Meningitis, endocarditis, suppurative arthritis, and osteomyelitis are rare complications of hematologic spread of pneumococcal or H. influenzae type b infection.
S. aureus, S. pneumoniae, and S. pyogenes are the most common causes of parapneumonic effusions and empyema. Nonetheless many effusions that complicate bacterial pneumonia are sterile. Analysis of pleural fluid parameters, including pH, glucose, protein, and lactate dehydrogenase, can differentiate transudative from exudative effusions (Table 428.8 ). However, current PIDS–IDSA guidelines do not recommend that these tests be performed because this distinction rarely changes management. Pleural fluid should be sent for Gram stain, and bacterial culture as this may identify the bacterial cause of pneumonia. Molecular methods, including bacterial species-specific PCR assays or sequencing of the bacterial 16S ribosomal RNA gene, detect bacterial DNA and can often determine the bacterial etiology of the effusion if the culture is negative, particularly if the pleural fluid sample was obtained after initiation of antibiotics. A pleural fluid WBC count with differential may be helpful if there is suspicion for pulmonary tuberculosis or a noninfectious etiology for the pleural effusion, such as malignancy.
Table 428.8
LDH, lactate dehydrogenase.
From Septimus EJ: Pleural effusion and empyema, In Bennett JE, Dolin R, Blaser MJ, editors: Mandell, Douglas, and Bennett's principles and practice of infectious diseases , ed 8, vol 1, Philadelphia, 2015, Elsevier, Table 70-1, p. 851.
Small (<1 cm on lateral decubitus radiograph), free-flowing parapneumonic effusions often do not require drainage but respond to appropriate antibiotic therapy. Larger effusions should typically be drained, particularly if the effusion is purulent (empyema) or associated with respiratory distress. Chest ultrasound, or alternatively CT, may be helpful in determining whether loculations are present. The mainstays of therapy include antibiotic therapy and drainage by tube thoracostomy with the instillation of fibrinolytic agents (urokinase, streptokinase, tissue plasminogen activator). Video-assisted thoracoscopy is a less often employed alternative that enables debridement or lysis of adhesions and drainage of loculated areas of pus. Early diagnosis and intervention, particularly with fibrinolysis or less often video-assisted thoracoscopy, may obviate the need for thoracotomy and open debridement.
Prevention
The introduction of PCVs resulted in a substantial reduction in the incidence of pneumonia hospitalizations among children. The annual rate of all-cause pneumonia hospitalization among children younger than 2 yr of age in the United States was 12.5 per 1,000 children during the period from 1997 to 1999. In 2000, 7-valent pneumococcal conjugate vaccine (PCV7) was licensed and recommended. In 2006, the pneumonia hospitalization rate in this age group was 8.1 per 1,000 children, a 35% decrease from the pre-vaccine rate. In 2010, 13-valent pneumococcal conjugate vaccine (PCV13) was licensed in the United States. Early data indicate that introduction of this vaccine resulted in a 16–27% further reduction in pneumonia hospitalizations among children relative to the post-PCV7 era.
Influenza vaccine may also prevent pneumonia hospitalizations among children and should be administered to all children >6 mo of age. For infants <6 mo of age, household contacts and other primary caregivers should be immunized. Maintaining high rates of vaccination for H. influenzae type b, pertussis, and measles remains important for the prevention of pneumonia from these causes. Several RSV vaccines are currently under development; introduction of an effective vaccine against RSV would be anticipated to substantially reduce pneumonia incidence among children, particularly young infants.