CHAPTER 99
Diseases Caused by Gram-Negative Enteric Bacteria, Pseudomonas, and Legionella
INFECTIONS CAUSED BY GRAM-NEGATIVE ENTERIC BACTERIA
GENERAL CONSIDERATIONS
Gram-negative bacilli (GNB) are normal components of the human colonic flora and/or a number of environmental habitats and can colonize mucosal and skin surfaces, especially in pts in long-term-care facilities and hospital settings. GNB cause a wide variety of infections involving diverse anatomic sites in both healthy and compromised hosts; extraintestinal infections due to Escherichia coli and, to a lesser degree, Klebsiella and Proteus species are most common. Isolation of GNB from any sterile site almost always implies infection, whereas isolation from nonsterile sites requires clinical correlation. Early appropriate antimicrobial therapy improves outcomes. Given worldwide increases in multi-drug-resistant GNB [e.g., due to extended-spectrum β-lactamases (ESBLs) and AmpC β-lactamases], combination empirical antimicrobial therapy pending susceptibility results may be appropriate for critically ill pts.
INFECTIONS CAUSED BY EXTRAINTESTINAL PATHOGENIC E. COLI (ExPEC)
In contrast to intestinal pathogenic E. coli, ExPEC strains are often found in the intestinal flora of healthy individuals but cause disease only when they enter a normally sterile extraintestinal site (e.g., the urinary tract, peritoneal cavity, or lungs). Most ExPEC strains have virulence factor profiles distinct from those of other commensal strains and from those of pathogenic strains that cause intestinal infections.
Clinical Manifestations
The clinical presentation depends in large part on the site of the body infected by ExPEC.
• Urinary tract infection: The urinary tract is the site most frequently infected by ExPEC; see Chap. 154 for more details. E. coli causes 85–95% of ~6–8 million episodes of acute uncomplicated UTI in premenopausal women.
• Abdominal and pelvic infection: The abdomen and pelvis represent the second most common site of infection by ExPEC, which may be isolated in the setting of a polymicrobial infection; see Chap. 90 for more details. Syndromes include peritonitis, intraabdominal abscesses, and cholangitis.
• Pneumonia: ExPEC is generally the third or fourth most commonly isolated GNB in hospital-acquired pneumonia and can be a common cause of pneumonia in pts residing in long-term-care facilities; see Chap. 141 for more details.
• Meningitis: E. coli is one of the two leading causes of neonatal meningitis (the other being group B Streptococcus). Strains with the K1 capsular serotype are generally involved.
• Cellulitis/musculoskeletal infection: E. coli often contributes to infection of decubitus ulcers and diabetic lower-extremity ulcers; cellulitis; and burn-site or surgical-site infections. Hematogenously acquired osteomyelitis, particularly vertebral, is more commonly caused by E. coli than is generally appreciated. See Chap. 93 for more details.
• Bacteremia: E. coli is one of the two most common blood isolates of clinical significance. E. coli bacteremia can arise from primary infection at any site, but originates most commonly from the urinary tract (50–67% of episodes) and next most commonly from the abdomen (25% of episodes). E. coli bacteremia is typically associated with sepsis. Endovascular infections are rare but have been described.
Diagnosis
ExPEC grows readily on standard media under either aerobic or anaerobic conditions. More than 90% of strains ferment lactose and are indole positive.
TREATMENT Extraintestinal Infections Caused by E. coli
• Rates of resistance to ampicillin, first-generation cephalosporins, trimethoprim-sulfamethoxazole (TMP-SMX), and fluoroquinolones are increasing. ESBLs are increasingly common in E. coli.
• Carbapenems and amikacin are the most predictably active agents overall, but carbapenemase-producing strains are on the rise.
• It is important to use the most appropriate narrower-spectrum agent whenever possible and to avoid treating colonized but uninfected pts, thus combating the increase in antibiotic resistance.
INFECTIONS CAUSED BY INTESTINAL PATHOGENIC E. COLI
Microbiology and Clinical Manifestations
At least five distinct pathotypes of intestinal pathogenic E. coli exist; see Chap. 91 for more details. As mentioned above, these strains are rarely encountered as part of the commensal flora in healthy individuals.
• Shiga toxin–producing E. coli (STEC)/enterohemorrhagic E. coli (EHEC): In addition to diarrhea, STEC/EHEC infection results in the hemolyticuremic syndrome in 2–8% of pts, particularly those who are very young or elderly.
– STEC/EHEC is associated with ingestion of contaminated food (e.g., undercooked ground beef, fresh produce) and water; person-to-person transmission (e.g., at day-care centers) is an important route for secondary spread.
– Disease can be caused by <102 colony-forming units (CFU) of STEC/EHEC.
– In contrast to the other pathotypes, STEC/EHEC (including E. coli O157:H7) causes infection more frequently in industrialized countries than in developing countries.
• Enterotoxigenic E. coli (ETEC): These strains are a major cause of endemic diarrhea among children residing in tropical and low-income countries and are the most common agent of traveler’s diarrhea; 106–1010 CFU are needed to cause disease.
• Enteropathogenic E. coli (EPEC): EPEC is an important cause of diarrhea among infants in developing countries.
• Enteroinvasive E. coli (EIEC): EIEC, an uncommon cause of diarrhea, produces inflammatory colitis (stools containing mucus, blood, and inflammatory cells) similar to that caused by Shigella and primarily affects children and travelers in developing countries; 108–1010 CFU are needed to cause disease.
• Enteroaggregative and diffusely adherent E. coli (EAEC): Initially described in young children in developing countries, more recent studies indicate that EAEC may be a common cause of prolonged, watery diarrhea in all age groups in industrialized countries.
Diagnosis
Specific diagnosis is usually unnecessary except when STEC/EHEC is involved. To detect the latter, testing for Shiga toxins or toxin genes is more sensitive, specific, and rapid than screening for E. coli strains that do not ferment sorbitol followed by serotyping for O157.
TREATMENT Intestinal Infections Caused by E. coli
• See Chap. 91 for more details. Replacement of water and electrolytes and avoidance of antibiotics in STEC/EHEC infection (since antibiotic use may increase the incidence of hemolytic-uremic syndrome) are indicated.
KLEBSIELLA INFECTIONS
Epidemiology
K. pneumoniae colonizes the colon in 5–35% of healthy individuals and, from a medical standpoint, is the most important Klebsiella species. K. oxytoca primarily causes infections in long-term-care and hospital settings. K. pneumoniae subspecies rhinoscleromatis, which causes rhinoscleroma, and K. pneumoniae subspecies ozaenae, which causes chronic atrophic rhinitis, infect pts in tropical climates.
Clinical Manifestations
As in other GNB infections, the clinical presentation depends on the infected anatomic site.
• Pneumonia: Klebsiella is an uncommon cause of community-acquired pneumonia, which occurs primarily in pts with underlying disease (e.g., alcoholism, diabetes, chronic obstructive pulmonary disease) and among residents of long-term-care facilities and hospitalized pts.
– The presentation is similar to that of pneumonia caused by other enteric GNB, with purulent sputum production and pulmonary infiltrates on CXR.
– Infection can progress to pulmonary necrosis, pleural effusion, and empyema.
• UTI: K. pneumoniae causes 1–2% of cases of uncomplicated cystitis and 5–17% of cases of complicated UTI.
• Abdominal infections: Klebsiella causes a spectrum of disease similar to that of E. coli, but with less frequent occurrence. Hypervirulent variants that contain capsular serotype K1 or K2 have become more common in the past decade.
• Bacteremia: Bacteremia can arise from a primary infection at any site; infections of the urinary tract, respiratory tract, and abdomen (especially hepatic abscess) each account for 15–30% of episodes.
• Other infections: Klebsiella cellulitis or soft tissue infection most frequently affects devitalized tissue and immunocompromised hosts. Klebsiella can also cause endophthalmitis, nosocomial sinusitis, and osteomyelitis.
Diagnosis
Klebsiellae usually ferment lactose, although the subspecies rhinoscleromatis and ozaenae are nonfermenters and are indole negative.
TREATMENT Klebsiella Infections
• Klebsiellae are resistant to ampicillin and ticarcillin.
– Resistance to third-generation cephalosporins is increasing, as is the frequency of ESBL-containing isolates.
– Fluoroquinolone resistance is increasing, especially among ESBL-containing strains.
• Empirical treatment of serious or health care–associated Klebsiella infections with amikacin or carbapenems is prudent; however, carbapenemase-producing strains are increasing in frequency. Optimal therapy for carbapenemase strains is unclear, but tigecycline, polymyxin B, and colistin are used most frequently on the basis of in vitro susceptibility profiles.
PROTEUS INFECTIONS
Epidemiology
P. mirabilis is part of the normal flora in 50% of healthy people and causes 90% of Proteus infections. P. vulgaris and P. penneri are isolated primarily from pts in hospitals and long-term care facilities.
Clinical Manifestations
Most Proteus infections arise from the urinary tract. Proteus species account for 1–2% of uncomplicated UTIs, 5% of hospital-acquired UTIs, and 10–15% of complicated UTIs (especially those associated with urinary catheters).
• Proteus produces high levels of urease that result in alkalinization of urine and ultimately in formation of struvite and carbonate-apatite calculi.
• Other sites of infection are uncommon but include pneumonia, abdominal infections, soft tissue infections, and bacteremia.
Diagnosis
Proteus strains are typically lactose negative, produce H2S, and exhibit swarming motility on agar plates. P. mirabilis is indole negative, whereas P. vulgaris and P. penneri are indole positive.
TREATMENT Proteus Infections
• P. mirabilis is susceptible to most agents except tetracycline, nitrofurantoin, polymyxin B, and tigecycline. Resistance to ampicillin, first-generation cephalosporins, and fluoroquinolones is increasing.
• P. vulgaris and P. penneri are more resistant; ~30% of P. vulgaris isolates have an inducible AmpC β-lactamase. Imipenem, fourth-generation cephalosporins, amikacin, and TMP-SMX exhibit excellent activity: 90–100% of Proteus isolates are susceptible.
INFECTIONS CAUSED BY OTHER GRAM-NEGATIVE ENTERIC PATHOGENS
• Enterobacter (e.g., E. cloacae, E. aerogenes), Acinetobacter (e.g., A. baumannii), Serratia (e.g., S. marcescens), and Citrobacter (e.g., C. freundii, C. koseri) usually cause nosocomial infections. Risk factors include immunosuppression, comorbid disease, prior antibiotic use, and ICU stays.
• Infections caused by Morganella (e.g., M. morganii) and Providencia (e.g., P. stuartii, P. rettgeri) resemble Proteus infections in terms of epidemiology, pathogenicity, and clinical manifestations but occur almost exclusively among persons in long-term-care facilities and, to a lesser degree, in hospitalized pts.
Clinical Manifestations
These organisms generally cause a spectrum of disease similar to that caused by other GNB, including pneumonia (particularly ventilator-associated), UTI (especially catheter-related), intravascular device–related infection, surgical-site infection, and abdominal infections.
• Citrobacter, Morganella, and Providencia infections are generally associated with UTIs.
• Acinetobacter has caused soft tissue and bone infections among soldiers with battlefield injuries and is a well-known pathogen in burn units.
TREATMENT Infections Caused by Other Gram-Negative Enteric Pathogens
• Significant antibiotic resistance among these organisms makes therapy challenging.
– Many of these organisms (e.g., Serratia, Providencia, Acinetobacter, Citrobacter, Enterobacter, Morganella) have a derepressible AmpC β-lactamase that results in resistance to third-generation cephalosporins, monobactams, and—in many cases—β-lactam/β-lactamase inhibitor combinations.
• Carbapenems and amikacin are most reliably active, and fourth-generation cephalosporins are active provided the organism does not express an ESBL. Susceptibility testing is essential. Some isolates may retain susceptibility only to colistin and polymyxin B.
AEROMONAS INFECTIONS
A. hydrophila causes >85% of Aeromonas infections. Aeromonas organisms proliferate in potable water, freshwater, and soil and are a putative cause of gastroenteritis. Aeromonas causes bacteremia and sepsis in infants and compromised hosts, especially those with cancer, hepatobiliary disease, trauma, or burns. The organisms can produce skin lesions similar to the ecthyma gangrenosum caused by Pseudomonas aeruginosa. Aeromonas causes nosocomial infections related to catheters, surgical incisions, and use of leeches.
TREATMENT Aeromonas Infections
• Aeromonas is usually susceptible to fluoroquinolones (e.g., ciprofloxacin at a dosage of 500 mg PO q12h or 400 mg IV q12h), third-generation cephalosporins, carbapenems, and aminoglycosides.
• Susceptibility testing is critical to guide therapy since Aeromonas can produce various β-lactamases, including carbapenemases.
INFECTIONS DUE TO PSEUDOMONAS AERUGINOSA AND RELATED ORGANISMS
The pseudomonads make up a set of gram-negative organisms unable to ferment lactose. This group includes three medically important genera—Pseudomonas, Burkholderia, and Stenotrophomonas—that typically cause opportunistic disease.
P. AERUGINOSA INFECTIONS
Microbiology
P. aeruginosa is a motile gram-negative rod that commonly produces green or bluish pigment and may have a mucoid appearance (which is particularly common in isolates from pts with cystic fibrosis). P. aeruginosa differs from enteric GNB in that it has a positive reaction in the oxidase test and does not ferment lactose.
Epidemiology
Because P. aeruginosa is found in most moist environments (e.g., in soil, in tap water, and on countertops), people routinely come into contact with the organism. The many factors that predispose to P. aeruginosa infection include disruption of cutaneous or mucosal barriers (e.g., due to burns or trauma), immunosuppression (e.g., due to neutropenia, AIDS, or diabetes), and disruption of the normal bacterial flora (e.g., due to broad-spectrum antibiotic therapy).
• P. aeruginosa is no longer a major cause of life-threatening bacteremia among pts with neutropenia or burn injury.
• P. aeruginosa bacteremia is currently most common among pts in the ICU.
Clinical Manifestations
P. aeruginosa can infect virtually all sites in the body but has a strong predilection for the lungs.
• Pneumonia: P. aeruginosa is considered a major cause of ventilator-associated pneumonia, although colonization may be difficult to distinguish from true infection.
– Clinically, most pts have a slowly progressive infiltrate, although progression is rapid in some cases. Infiltrates may become necrotic.
– It is unclear whether an invasive procedure (e.g., bronchoalveolar lavage, protected-brush sampling of distal airways) is superior to tracheal aspiration in obtaining samples for culture.
– Chronic respiratory infection with P. aeruginosa is associated with underlying or predisposing conditions (e.g., cystic fibrosis, bronchi-ectasis).
• Bacteremia: The presentation of P. aeruginosa bacteremia resembles that of sepsis in general.
– Pathognomonic skin lesions (ecthyma gangrenosum) that at first are painful, reddish, and maculopapular and later become black and necrotic may develop in pts with marked neutropenia or with HIV infection.
– Endovascular infections occur mostly in IV drug users and pts with prosthetic valves.
• Bone and joint infections: P. aeruginosa is an infrequent cause of bone and joint infections.
– Injection drug use (associated with sternoclavicular joint infections and vertebral osteomyelitis) and UTIs in the elderly (associated with vertebral osteomyelitis) are risk factors.
– Pseudomonas osteomyelitis of the foot most often follows puncture wounds through sneakers and most commonly affects children.
• CNS infections: CNS infections due to P. aeruginosa are relatively rare and are almost always secondary to a surgical procedure or head trauma.
• Eye infections: Keratitis and corneal ulcers can occur, usually resulting from trauma or surface injury by contact lenses. These infections are rapidly progressing entities that demand immediate therapeutic intervention. P. aeruginosa endophthalmitis secondary to bacteremia is a fulminant disease with severe pain, chemosis, decreased visual acuity, anterior uveitis, vitreous involvement, and panophthalmitis.
• Ear infections: In addition to mild swimmer’s ear, Pseudomonas ear infections can result in malignant otitis externa, a life-threatening infection that presents as severe ear pain and decreased hearing.
– Pts may develop cranial-nerve palsies or cavernous venous sinus thrombosis.
– Most ear infections due to P. aeruginosa occur in elderly diabetic pts.
• UTIs: UTIs due to P. aeruginosa usually result from a foreign body in the urinary tract, an obstruction in the genitourinary system, or urinary tract instrumentation or surgery.
• Skin and soft tissue infections: P. aeruginosa can cause a variety of dermatitides, including pyoderma gangrenosum in neutropenic pts, folliculitis, and other papular or vesicular lesions. Multiple outbreaks have been linked to whirlpools, spas, and swimming pools.
• Infections in pts with fever and neutropenia: P. aeruginosa is always targeted in empirical treatment of these pts, given high rates of infection in the past and high associated mortality rates.
• Infections in pts with AIDS: P. aeruginosa infections in pts with AIDS can be fatal even though the clinical presentation is not particularly severe.
– Pneumonia is the most common type of infection, with a high frequency of cavitary disease.
– Since the advent of antiretroviral therapy, P. aeruginosa infection has declined in incidence among these pts but still occurs.
TREATMENT P. aeruginosa Infections
• See Table 99-1 for antibiotic options and schedules.
TABLE 99-1 ANTIBIOTIC TREATMENT OF INFECTIONS DUE TO PSEUDOMONAS AERUGINOSA AND RELATED SPECIES
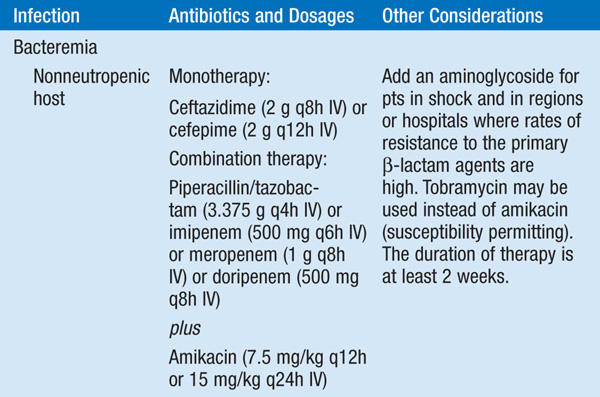
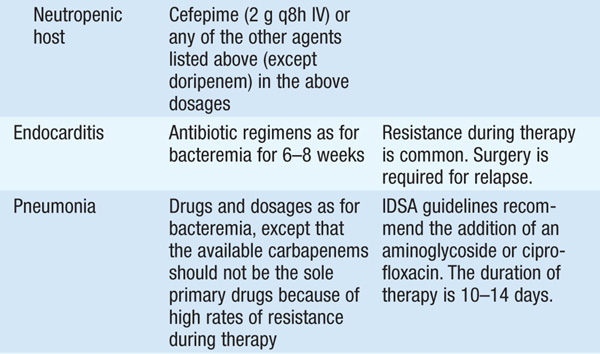
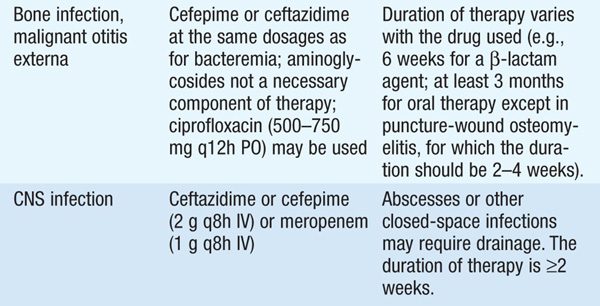
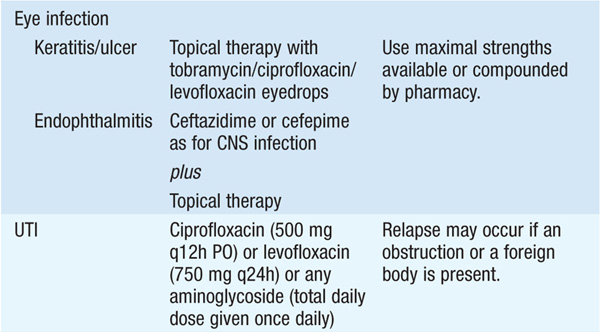
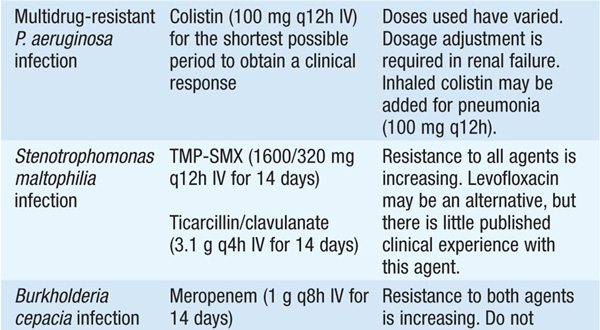

• Several observational studies indicate that a single modern antipseudomonal β-lactam agent to which the isolate is sensitive is as efficacious as combination therapy. However, if—in the local environment—the susceptibility to first-line agents is <80%, empirical combination therapy should be administered until isolate-specific susceptibility data are available.
INFECTIONS CAUSED BY BACTERIA RELATED TO PSEUDOMONAS SPECIES
Stenotrophomonas maltophilia
S. maltophilia is an opportunistic pathogen. Most infections occur in the setting of prior broad-spectrum antimicrobial therapy that has eradicated the normal flora in immunocompromised pts.
• S. maltophilia causes pneumonia, especially ventilator-associated pneumonia, with or without bacteremia.
• Central venous line infection (most often in cancer pts) and ecthyma gangrenosum in neutropenic pts have been described.
Burkholderia cepacia
This organism can colonize airways during broad-spectrum antimicrobial treatment and is a cause of ventilator-associated pneumonia, catheter-associated infection, and wound infection.
• B. cepacia is recognized as an antibiotic-resistant nosocomial pathogen in ICU pts.
• B. cepacia can cause a rapidly fatal syndrome of respiratory distress and septicemia (the “cepacia syndrome”) in pts with cystic fibrosis.
TREATMENT S. maltophilia and B. cepacia Infections
Intrinsic resistance to many antibiotics limits treatment. See Table 99-1 for recommended antibiotic regimens.
Miscellaneous Organisms
Melioidosis is endemic to Southeast Asia and is caused by B. pseudomallei. Glanders is associated with close contact with horses or other equines and is caused by B. mallei. These diseases present as acute or chronic pulmonary or extrapulmonary suppurative illnesses or as acute septicemia.
LEGIONELLA INFECTIONS
Microbiology
Legionellaceae are intracellular aerobic gram-negative bacilli that grow on buffered charcoal yeast extract (BCYE) agar. L. pneumophila causes 80–90% of cases of human Legionella disease and includes 16 serogroups; serogroups 1, 4, and 6 are most common.
Epidemiology
• Legionella is found in fresh water and human-constructed water sources. Outbreaks have been traced to drinking water systems and rarely to cooling towers.
• The organisms are transmitted to individuals primarily via aspiration but can also be transmitted by aerosolization and direct instillation into the lungs during respiratory tract manipulations.
• Legionella is the fourth most common cause of community-acquired pneumonia, accounting for 2–9% of cases. It causes 10–50% of cases of nosocomial pneumonia if the hospital’s water system is colonized with the organism.
• Pts who have chronic lung disease, who smoke, and/or who are elderly, immunosuppressed, or recently discharged from the hospital are at particularly high risk for disease.
Clinical Manifestations
Legionellosis manifests as either an acute, febrile, self-limited illness (Pontiac fever) or pneumonia (Legionnaires’ disease).
• Pontiac fever is a flulike illness with a 24- to 48-h incubation period. Malaise, fatigue, and myalgias occur in 97% of cases. Fever and headaches are also very common, but pneumonia does not develop. The disease is self-limited and does not require antimicrobial treatment. Recovery takes place in a few days.
• Legionnaires’ disease is more severe than other atypical pneumonias and is more likely to result in ICU admission.
– After a usual incubation period of 2–10 days, nonspecific symptoms (e.g., malaise, fatigue, headache, anorexia) develop and are followed by a cough that is usually mild and only slightly productive. Chest pain can be prominent.
– Radiologic findings are nonspecific, but pleural effusions are present in 28–63% of pts on hospital admission.
– Legionnaires’ disease is not readily distinguishable from pneumonia of other etiologies based on clinical manifestations, but diarrhea, confusion, temperatures >39°C, hyponatremia, increased aminotransferase levels, hematuria, hypophosphatemia, and elevated creatine phosphokinase levels are documented more frequently than in other pneumonias.
– Extrapulmonary infection results from hematogenous dissemination and most commonly affects the heart (e.g., myocarditis, pericarditis).
Diagnosis
The use of Legionella testing—especially the Legionella urinary antigen test—is recommended for all pts with community-acquired pneumonia.
• Sputum or bronchoscopy specimens can be subjected to direct fluorescent antibody (DFA) staining and culture.
– DFA testing is rapid and specific but is less sensitive than culture.
– Cultures on BCYE media (with antibiotics to suppress competing flora) require 3–5 days to become positive.
• Serologic confirmation requires comparison of acute- and convalescent-phase samples. Detection of the necessary 4-fold rise in titers often requires 12 weeks.
• Urinary antigen testing is rapid, inexpensive, easy to perform, second only to culture in terms of sensitivity, and highly specific. It is useful only for L. pneumophila serogroup 1, which causes 80% of disease cases.
– Urinary antigen is detectable 3 days after disease onset and generally disappears over 2 months, although positivity can be prolonged if the pt is receiving glucocorticoid therapy.
– The test is not affected by antibiotic administration.
TREATMENT Legionella Infections
• Newer macrolides (e.g., azithromycin at 500 mg/d PO, with consideration of doubling the first dose; or clarithromycin at 500 mg bid IV or PO) or fluoroquinolones (e.g., levofloxacin at 750 mg/d IV or 500 mg/d PO or moxifloxacin at 400 mg/d PO) are most effective.
– Rifampin (100–600 mg bid) combined with either class of drug is recommended in severe cases.
– Tetracyclines (doxycycline at 100 mg bid IV or PO) are alternatives.
• Immunocompetent hosts should receive 10–14 days of therapy, but immunocompromised hosts and pts with advanced disease should receive a 3-week course of treatment.
– A 5- to 10-day course of azithromycin is adequate because of this drug’s long half-life.
– A clinical response usually occurs within 3–5 days after the initiation of parenteral therapy, at which point oral therapy can be substituted.
Prognosis
Mortality rates approach 80% among immunocompromised pts who do not receive timely therapy. Among immunocompetent hosts, mortality can approach 31% without treatment but ranges from 0 to 11% with appropriate and timely therapy. Fatigue, weakness, and neurologic symptoms can persist for >1 year.

For a more detailed discussion, see Barlam TF, Kasper DL: Infections Due to the HACEK Group and Miscellaneous Gram-Negative Bacteria, Chap. 146, p. 1233; Sabria M, Yu VL: Legionella Infections, Chap. 147, p. 1236; Russo TA, Johnson JR: Diseases Caused by Gram-Negative Enteric Bacilli, Chap. 149, p. 1246; Paterson DL, Peleg AY: Acinetobacter Infections, Chap. 150, p. 1258; and Ramphal R: Infections Due to Pseudomonas Species and Related Organisms, Chap. 152, p. 1266, in HPIM-18.






